Abstract
Huanglongbing (HLB) disease has caused a severe decline in citrus production globally over the past decade. There is a need for improved nutrient regimens to better manage the productivity of HLB-affected trees, as current guidelines are based on healthy trees. The aim of this study was to evaluate the effects of different fertilizer application methods and rates with different planting densities on HLB-affected citrus root and soil health. Plant material consisted of ‘Ray Ruby’ (Citrus × paradisi) grapefruit trees grafted on ‘Kuharske’ citrange (Citrus × sinensis × Citrus trifoliata). The study consisted of 4 foliar fertilizer treatments, which included 0×, 1.5×, 3× and 6× the University of Florida Institute of Food and Agriculture (UF/IFAS) recommended guidelines for B, Mn and Zn. Additionally, 2 ground-applied fertilizer treatments were used, specifically controlled-release fertilizer (CRF1): 12−3−14 + B, Fe, Mn and Zn micronutrients at 1× UF/IFAS recommendation, and (CRF2): 12−3−14 + 2× Mg + 3× B, Fe, Mn and Zn micronutrients, with micronutrients applied as sulfur-coated products. The planting densities implemented were low (300 trees ha−1), medium (440 trees ha−1) and high (975 trees ha−1). The CRF fertilizer resulted in greater soil nutrient concentrations through all of the time sampling points, with significant differences in soil Zn and Mn. Grapefruit treated with ground-applied CRF2 and 3× foliar fertilizers resulted in the greatest bacterial alpha and beta diversity in the rhizosphere. Significantly greater abundances of Rhizobiales and Vicinamibacterales were found in the grapefruit rhizosphere of trees treated with 0× UF/IFAS foliar fertilizer compared to higher doses of foliar fertilizers.
1. Introduction
Much citrus production worldwide has significantly declined due to a disease known as huanglongbing (HLB, or citrus greening) [1]. In Florida, citrus production has been reduced by more than 70% since HLB was first detected in 2005 [2,3,4]. The disease is associated with Candidatus Liberibacter asiaticus (CLas), bacteria transmitted to tree hosts by a vector called the Asian Citrus Psyllid (Diaphorina citri, ACP) [5,6,7]. As the bacteria colonize the sieve tubes within the phloem, callose deposition occurs, resulting in the plugging of the phloem, thus inhibiting nutrient uptake [1]. Symptoms following infection can include leaf chlorosis, leaf drop, reduced canopy density, smaller fruit size, root dieback, lack of juice quality and reduced yield [8]. Grapefruit, in particular, are more prone to root dieback when infected with HLB compared to specialty citrus, such as lemon and lime [9]. Most of the grapefruit production in Florida takes place in the Indian River district, a 200-mile-long area that borders the Atlantic Ocean. Since the introduction of HLB, grapefruit production has undergone an 85% decline [10].
There are several approaches toward dealing with HLB and alleviating symptoms, including the development of disease-tolerant rootstocks [7,11], the implementation of vector control [12] and the use of soil amendments [13] to improve soil health. The effect of different methods and rates of fertilizer application on citrus health and yield have also been studied [4,14,15,16]. There are several ways of applying nutrients to citrus: foliar applications, ground-applied granular fertilizers, fertigation and banding [14]. The mobility of nutrients in soil and plant tissues is a notable factor in deciding which application method is most appropriate [15]. For instance, when applying micronutrients (generally performed in smaller quantities), the efficiency of root uptake may be compromised when soil conditions are not favorable, and thus the foliar application of micronutrients may serve as a better alternative due to greater efficiency [17]. When applying micronutrients to the ground, variation in soil characteristics, such as soil pH, drainage and moisture-holding capacity, can significantly impact mobility, solubility and the root uptake of nutrients [15].
The development of improved nutrient guidelines has proven to be beneficial since most of the established guidelines are based on healthy noninfected citrus trees rather than HLB-affected trees [18]. Several studies have examined the effects of various fertilizer types and rates on citrus health and yield [4,9,19,20,21,22]. The fruit yield increased in ‘Valencia’ sweet orange trees (Citrus × sinensis) when evaluating varying rates of manganese (Mn) via foliar application at 3× the recommended rate [23]. Additionally, when examining the effect of foliar application rates on mandarins, increased rates of boron (B) and zinc (Zn) above the recommended guidelines resulted in greater fruit yield and quality [24]. The effect of controlled-release fertilizer (CRF) formulations has also been tested on HLB-affected citrus, with CRF treatments resulting in a greater concentration of nitrogen (N), calcium (Ca), sulfur (S) and B in leaves compared to soluble dry granular fertilizers [10]. Furthermore, [25] found that CRF formulations resulted in significantly high yields in HLB-affected sweet orange trees relative to conventional fertilizer sources.
The application of various types and rates of fertilizers may also have subsequent effects on the microbial communities in the soil, notably those that reside within the rhizosphere (a portion of the soil that encompasses the roots of plants). When studying the effects of various N fertilizer treatments on wheat health and rhizosphere composition, Ref. [26] found that the abundance of dominant soil bacteria was significantly altered through changes in pH from long-term fertilization. Similarly, Ref. [27] found that tea orchards treated with a long-term application of organic fertilizer resulted in significant shifts in soil pH correlated with substantial differences in rhizosphere bacterial diversity. Potential shifts in rhizosphere community composition from fertilizer may be crucial for plant health, as they consist of plant-growth-promoting rhizobacteria (PGPR), which can improve plant growth through symbiotic relationships [28]. Some bacteria, such as Bacillus subtilis, can assist plant hosts directly in the defense against pathogens [29]. Furthermore, PGPR can make plant essential nutrients available for root uptake, as they are capable of solubilizing nutrients into forms that can be utilized by plants [30]. Further insight into the interactions shared between fertilizer regimens, rhizosphere microbial composition and root health is still needed, specifically toward citrus. This study aimed to determine the impact of foliar and ground-applied fertilizer treatments on grapefruit root health and rhizosphere composition at varying planting densities. It is predicted that grapefruit treated with greater nutrient concentrations will result in a more diverse rhizosphere bacterial community composition.
2. Results
2.1. Soil Nutrient Concentrations
Soil nutrient concentrations were influenced by planting density treatments, but no clear patterns were established (Table 1). In September 2020, trees planted in a high density (975 trees ha−1) had significantly greater soil Mg (20%, Figure 1A), whereas no significant differences were observed for Ca (Figure 2A). In September 2020, trees planted in a high density had significantly greater soil Zn (31%, Figure 3A) than trees planted in a medium density (440 trees ha−1). However, trees planted in a medium density had significantly greater soil P (17%, Figure 1A) compared to trees planted in a high density. In January 2021, trees planted in a low density (300 trees ha−1) had significantly greater soil Zn (23%, Figure 3A) compared to trees planted in a high density. In May 2021, trees planted in a high density had significantly greater soil Mn (29%, Figure 3A) and Zn (28%, Figure 3A) compared to trees planted in a low density. In September 2021, trees planted in a high density had significantly greater soil P (25%, Figure 1A) and B (14%, Figure 4A) compared to trees planted in a medium density. In January 2022, trees planted in a high density had significantly greater soil Zn (36%, Figure 3A) compared to trees planted in a medium density.

Table 1.
Corresponding p-values and r values (Pearson’s coefficient) of correlations examined between soil nutrient concentrations and planting density treatments from all time points of sampling, which include September 2020, January 2021, May 2021, September 2021 and January 2022.
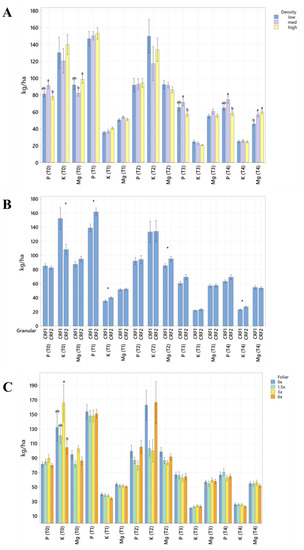
Figure 1.
Soil macronutrient concentrations of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA, in response to (A) planting density, (B) ground-applied fertilizer and (C) foliar fertilizer treatments during September 2020 (T0), January 2021 (T1), May 2021 (T2), September 2021 (T3) and January 2022 (T4). Bars are ± standard deviation of the mean. Treatments with * and different letters were considered to be significantly different (p < 0.05).
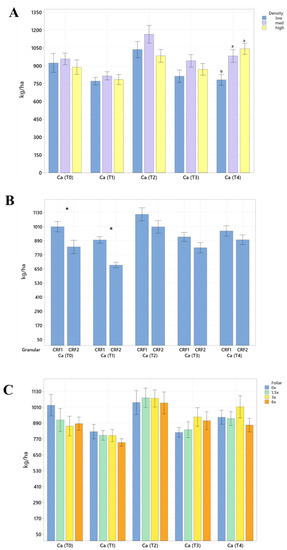
Figure 2.
Soil Ca concentrations of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA, in response to (A) planting density, (B) ground-applied fertilizer and (C) foliar fertilizer treatments during September 2020 (T0), January 2021 (T1), May 2021 (T2), September 2021 (T3) and January 2022 (T4). Bars are ± standard deviation of the mean. Treatments with * and different letters were considered to be significantly different (p < 0.05).
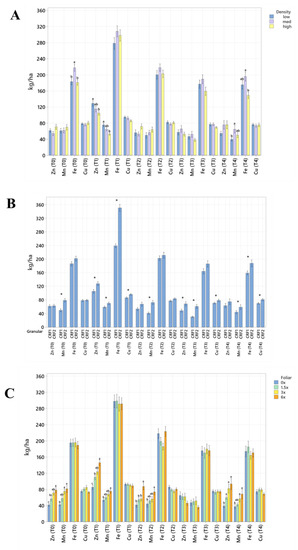
Figure 3.
Soil micronutrient concentrations of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA, in response to (A) planting density, (B) ground-applied fertilizer and (C) foliar fertilizer treatments during September 2020 (T0), January 2021 (T1), May 2021 (T2), September 2021 (T3) and January 2022 (T4). Bars are ± standard deviation of the mean. Treatments with * and different letters were considered to be significantly different (p < 0.05).
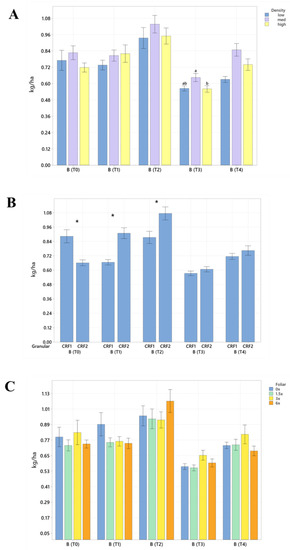
Figure 4.
Soil boron concentrations of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA, in response to (A) planting density, (B) ground-applied fertilizer and (C) foliar fertilizer treatments during September 2020 (T0), January 2021 (T1), May 2021 (T2), September 2021 (T3) and January 2022 (T4). Bars are ± standard deviation of the mean. Treatments with * and different letters were considered to be significantly different (p < 0.05).
Ground-applied CRF treatments influenced soil nutrient concentrations; however, similarly to what was reported for planting density, no patterns were established (Table 2). Soil samples fertilized with CRF1 treatment had significantly greater K (41%, Figure 1B) and B (34%, Figure 4B) than those fertilized with the CRF2 treatment in September 2020. However, soil receiving CRF2 treatment had significantly greater Mn (56%, Figure 3B) than the CRF1 treatment. In January 2021, soil receiving CRF2 treatment had significantly greater P (16%, Figure 1B) and Zn (21%, Figure 3B) than those fertilized with CRF1. In May 2021, soil fertilized with the CRF2 treatment had significantly greater Mg (11%, Figure 1B), Mn (75%, Figure 3B), Zn (26%, Figure 3B) and B (23%, Figure 4B) than those fertilized with CRF1. In September 2021, soil fertilized with CRF2 had significantly greater Zn (41%, Figure 3B), Mn (100%, Figure 3B) and Cu (11%, Figure 3B) than those fertilized with CRF2. In January 2022, soil fertilized with CRF2 had significantly greater B (23%, Figure 4B) than soil fertilized with CRF1. No significant differences were detected for Ca (Figure 2B).

Table 2.
Corresponding p-values and r values (Pearson’s coefficient) of correlations examined between soil nutrient concentrations and ground-applied CRF treatments from all time points of sampling, which include September 2020, January 2021, May 2021, September 2021 and January 2022.
As previously reported for planting densities and ground-applied fertilizers, foliar fertilizer treatments affected soil nutrient concentrations. However, no clear trends were observed (Table 3) and no significant differences were detected in soil macronutrients, except for K in September 2020 (Figure 1C). Soil samples from 6× foliar treatments had significantly greater Zn (88% and 39%, Figure 3C) and Mn (99% and 47%, Figure 3C) concentrations than treatments fertilized with 0× and 1.5× foliar spray in September 2020. In January 2021, soil receiving 6× foliar sprays had significantly greater Zn (71% and 32.71%, Figure 3C) and Mn (41% and 26%, Figure 3C) concentrations than 0× and 1.5× foliar treatments. In May 2021, soil receiving 6× foliar sprays had significantly greater Zn (110% and 60%, Figure 3C) concentrations than those fertilized with 0× and 1.5× sprays. In January 2022, soil receiving 6× foliar sprays had significantly greater Zn (110%, 30% and 54%, Figure 3C) concentrations than those fertilized with 0×, 1.5× and 3× foliar sprays. Neither Ca or B showed significant differences (Figure 2C and Figure 4C).

Table 3.
Corresponding p-values and r values (Pearson’s coefficient) of correlations examined between soil nutrient concentrations and foliar fertilizer treatments from all time points of sampling, which include September 2020, January 2021, May 2021, September 2021 and January 2022.
2.2. Root Nutrient Concentrations and Size
Root nutrient concentrations and size were significantly affected by planting density. During September 2020, trees planted in a high density resulted in significantly greater total root length (60%, Table 4 and Table 5) than trees planted in a low density. In September 2021, the root concentrations of Mg (52%, Figure 5A) were significantly greater in trees planted in a high density than those planted in a low density. In May 2021, the root concentrations of N (18%, Figure 6A), Mn (73%, Figure 7A) and Zn (59%, Figure 7A) were significantly greater in trees planted in a high density compared to the trees planted in a medium density. Additionally, B was significantly higher in high-density plantings compared to those in a low planting density (45%, Figure 8A). In January 2022, trees planted in a high density had significantly greater Zn (59%, Figure 7A) and Mn (73%, Figure 7A) in their roots than those grown in a medium density. No significant differences were observed in root density in response to planting density (Figure 9A).

Table 4.
Total root length of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA, and treated with ground-applied fertilizer, foliar fertilizer and different planting densities from September 2020 to January 2022.

Table 5.
p-values obtained via a three-way ANOVA followed by Tukey’s post hoc test, corresponding to the total root length of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA, and treated with ground-applied fertilizer, foliar fertilizer and different planting densities from September 2020 to January 2022. Bold values were considered to be significantly different (p < 0.05).
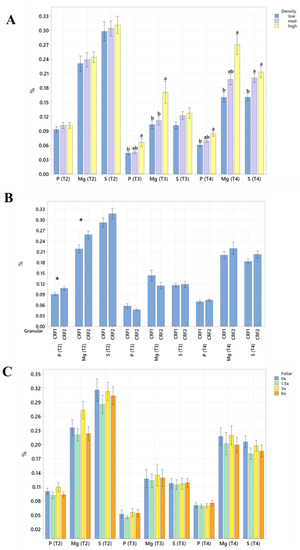
Figure 5.
Root P, Mg and S concentrations of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA. Graphs indicate response to (A) planting density, (B) ground-applied fertilizer and (C) foliar fertilizer treatments during May 2021 (T2), September 2021 (T3) and January 2022 (T4). Bars are ± standard deviation of the mean. Treatments with * and different letters were considered to be significantly different (p < 0.05).
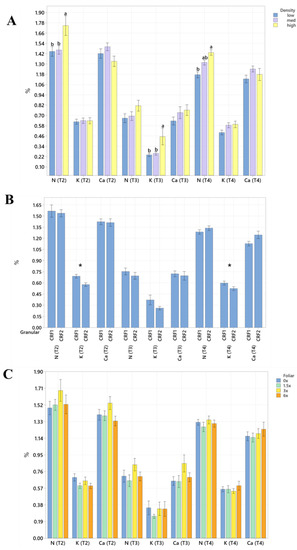
Figure 6.
Root N, K and Ca concentrations of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA. Graphs indicate response to (A) planting density, (B) ground-applied fertilizer and (C) foliar fertilizer treatments during May 2021 (T2), September 2021 (T3) and January 2022 (T4). Bars are ± standard deviation of the mean. Treatments with * and different letters were considered to be significantly different (p < 0.05).
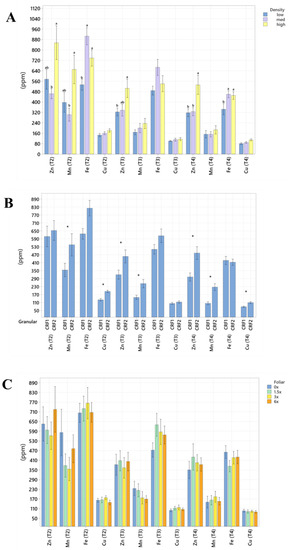
Figure 7.
Root micronutrient concentrations of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA. Graphs indicate response to (A) planting density, (B) ground-applied fertilizer and (C) foliar fertilizer treatments during May 2021 (T2), September 2021 (T3) and January 2022 (T4). Bars are ± standard deviation of the mean, and treatments with * and different letters were considered to be significantly different (p < 0.05).
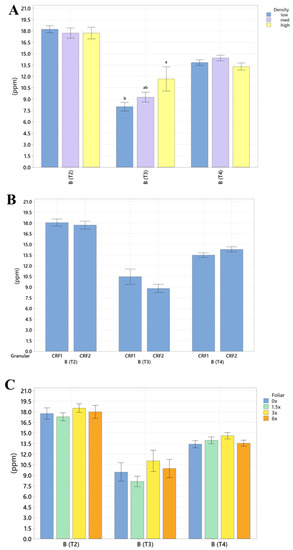
Figure 8.
Root B concentrations of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA. Graphs indicate response to (A) planting density, (B) ground-applied fertilizer and (C) foliar fertilizer treatments during May 2021 (T2), September 2021 (T3) and January 2022 (T4). Bars are ± standard deviation of the mean, and treatments with different letters were considered to be significantly different (p < 0.05).
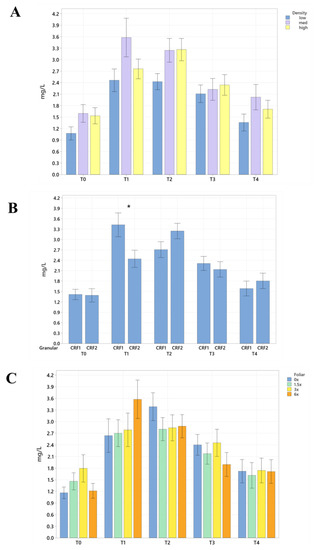
Figure 9.
Root density of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA, in response to (A) planting density, (B) ground-applied fertilizer and (C) foliar fertilizer treatments during September 2020 (T0), January 2021 (T1), May 2021 (T2), September 2021 (T3) and January 2022 (T4). Bars are ± standard deviation of the mean, and treatments with * were considered to be significantly different (p < 0.05).
Root nutrient concentrations and measurements were significantly affected by ground-applied CRF treatments. In September 2020, grapefruit treated with the CRF2 treatment resulted in roots with a significantly greater root density (40%, Figure 9B) and grapefruit fertilized with the CRF1 treatment was significantly greater than those fertilized with the CRF2. In May 2021, the root concentrations of K (17.32%, Figure 6B) were significantly greater in trees fertilized with the CRF1 treatment compared to the CRF2. However, the root concentrations of P (11%, Figure 5B) and Mg (17%, Figure 5B) were significantly greater in trees fertilized with the CRF2 treatment compared to the CRF1. Additionally, the total root volume (122%, Table 6 and Table 7) and total root area (63%, Table 8 and Table 9) of grapefruit fertilized with the CRF2 treatment were significantly greater than those fertilized with the CRF1. In September 2021, the root concentrations of Mn (52%, Figure 7B) and Zn (35%, Figure 7B) were significantly greater in trees fertilized with CRF2 compared to CRF1. In January 2022, the root concentrations of P (20%, Figure 5B) and Mg (17%, Figure 5B) were significantly greater in trees fertilized with CRF2 compared to CRF1. However, the root concentrations of K (17%, Figure 6B) were significantly greater in trees fertilized with CRF1 compared to CRF2. Ground-applied fertilizer treatments were not observed to have a significant effect on the root B concentrations at all sampling times (Figure 8B). No significant differences were observed in root nutrient concentrations in response to foliar fertilizer treatments (Figure 5C, Figure 6C, Figure 7C and Figure 8C); additionally, no significant difference in root density in response to foliar fertilizer treatments was observed (Figure 9C).

Table 6.
Total root volume of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA, and treated with ground-applied fertilizer, foliar fertilizer and different planting densities from September 2020 to January 2022.

Table 7.
p-values obtained via a three-way ANOVA followed by Tukey’s post hoc test, corresponding to the total root volume of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA, and treated with ground-applied fertilizer, foliar fertilizer and different planting densities from September 2020 to January 2022.

Table 8.
Total root area of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA, and treated with ground-applied fertilizer, foliar fertilizer and different planting densities from September 2020 to January 2022.

Table 9.
p-values obtained via a three-way ANOVA followed by Tukey’s post hoc test, corresponding to the total root area of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA, and treated with ground-applied fertilizer, foliar fertilizer and different planting densities from September 2020 to January 2022. Bold values were considered to be significantly different (p < 0.05).
2.3. Rhizosphere Microbiome Diversity
Data were log-transformed to reduce error rates caused by rarefaction and later utilized for alpha and beta diversity analyses of the rhizosphere bacterial community through the R package “Phyloseq” v1.24.0 (McMurdie and Holmes 2013). Rhizosphere bacterial alpha diversity did vary according to treatments according to the Shannon index. Grapefruit trees treated with CRF 2 had a greater bacterial alpha diversity than those treated with CRF 1 (Figure 10A). Grapefruit trees treated with 3× foliar fertilizer had a greater bacterial alpha diversity compared to those treated with 0×, 1.5× and 6× foliar fertilizer (Figure 11A).
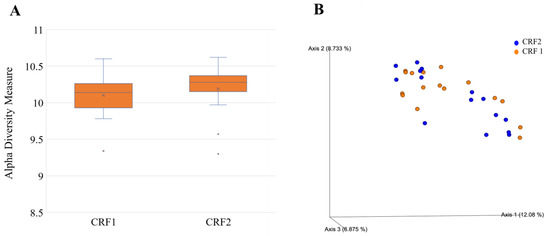
Figure 10.
Alpha (A) and beta diversity (B) in rhizosphere bacteria of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA, and grown with different ground-applied fertilizer treatments. Alpha diversity was measured using the Shannon index of rhizosphere bacteria among treatments. Plotted in Figure (A) are boxes (interquartile), the median (line within each box), the mean (× within each box), and whiskers (lowest and greatest values). Principal coordinates analysis (PCoA) based on the Bray–Curtis dissimilarity matrix of rhizosphere bacterial samples can be found in Figure (B), where colors indicate treatment and include CRF1 (orange) and CRF2 (blue).
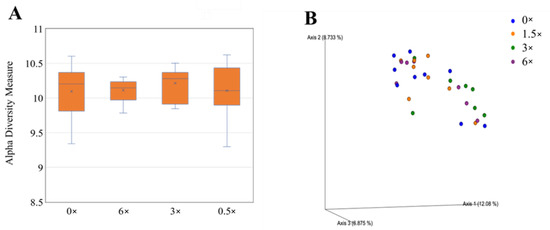
Figure 11.
Alpha (A) and beta diversity (B) in rhizosphere bacteria of ‘Ray Ruby’ grapefruit grafted on ‘Kuharske’ citrange planted in flatwood soils located in Fort Pierce, FL, USA, and treated with various doses of foliar fertilizer applications. Alpha diversity was measured using the Shannon index of rhizosphere bacteria among treatments. Plotted in Figure (A) are boxes (interquartile), the median (line within each box), the mean (× within each box), and whiskers (lowest and greatest values). Principal coordinates analysis (PCoA) based on the Bray–Curtis dissimilarity matrix of rhizosphere bacterial samples can be found in Figure (B), where colors indicate treatment and include 0× (blue), 1.5× (orange), 3× (green) and 6× (purple) the UF/IFAS recommended.
Beta diversity analyses included principal coordinate analysis (PCoA) on Bray–Curtis distances. An ANOSIM test was performed to determine significant differences in beta diversity between treatments. Rhizosphere bacterial beta diversity did vary according to treatments according to the Shannon index. Grapefruit trees treated with CRF 2 had greater bacterial beta diversity than those treated with CRF 1 (Figure 10B). Grapefruit trees treated with 3× foliar fertilizer had greater bacterial beta diversity compared to those treated with 0×, 1.5× and 6× foliar (Figure 11B).
There was variation in the relative abundance of bacterial taxonomic orders among treatments. Grapefruit trees treated with 0× foliar fertilizer had a rhizosphere bacterial community with a significantly greater abundance of Reyranellales (p < 0.05), Rhizobiales (p < 0.05) and Rickettsiales (p < 0.05) compared to those from the 1.5× foliar treatment. Additionally, grapefruit trees at 0× foliar fertilizer had a rhizosphere bacterial community with a significantly greater abundance of Nitrosotaleales (p < 0.05), Vicinamibacterales (p < 0.05), Tistrellales (p < 0.05) and Solirubrobacterales (p < 0.05) compared to those from the 3× foliar fertilizer treatment. Furthermore, grapefruit trees with 0× foliar fertilizer had a rhizosphere bacterial community with a significantly greater abundance of Nitrososphaerales (p < 0.05), Clostridiales (p < 0.05), Caulobacterales (p < 0.05) and Rickettsiales (p < 0.05) compared to 6× foliar treatment.
3. Discussion
Planting density affected root nutrient concentrations and root size, with notably significantly greater root concentrations of Mn and Zn concentrations in a high density than in a medium density in May 2021 and January 2022. When planting grapefruit in higher densities, the greater presence of roots within a given soil area may have allowed for greater root interception of essential nutrients. Similarly, Gezahegn et al. found that closely spaced fava bean plants had increased root elongation, increasing moisture and nutrient uptake [31]. Additionally, the greater presence and activity of grapefruit roots from high-density plantings may also increase the amount of root exudates in these soils, potentially contributing to observed increases in root concentrations of Mn and Zn. Root exudates function to recruit microbes from the bulk soil to the rhizosphere for a multitude of tasks, whereby some of which range from plant defense to nutrient acquisition [32,33]. The type and amount of root exudates released vary according to the plant’s growth stage and the root characteristics, such as root physiology and morphology [34,35].
Changes in root nutrient concentrations and measured parameters were more influenced by ground-applied CRF treatments than foliar fertilizer treatments, due to application sites being closer to the root zone than foliar application sites in the canopy. Long-term ground-applied CRF use can have more pronounced effects on soil characteristics, such as pH (commonly increased soil acidification), when compared to foliar fertilizers, affecting the availability of plant essential nutrients [36,37]. Furthermore, long-term excessive fertilizer applications have also been shown to alter total organic carbon (TOC), basic cation content and soil physical properties, further emphasizing the effect of ground-applied CRF on overall root health [38,39]. The phenology of citrus trees with several vegetive flushes during the year, in combination with the sub-tropical weather of Florida (with an abundance of rain during the summer and fall seasons) and the deterioration in tree health caused by HLB disease, may have contributed toward the inconsistency in nutrient concentrations, and the lack of observed patterns during the time periods of the study.
When comparing the impact of ground-applied CRF on root health, the CRF2 treatment had an overall greater beneficial effect compared to the CRF1 treatment, notably in May 2021, with significant increases in the total root area and total root volume of grapefruit. Similarly, [40] found that 2× the dose of micronutrients (Mn, Zn and B) in HLB-affected sweet orange trees led to a higher median root lifespan. Greater concentrations of micronutrients (3× B, Fe, Mn and Zn) in the CRF2 treatment may have also contributed toward better root health. Nutrient supply is imperative to disease control because nutrients influence plant resistance, pathogen vigor, growth and associated factors [15].
Both foliar and ground-applied fertilizer treatments had a greater impact on soil micronutrient concentrations compared to planting density at all timepoints, as confirmed through Pearson correlation coefficients. This was expected, as micronutrients were the main component that changed through the foliar and ground-applied fertilizer treatments.
Excess amounts of micronutrients provided by the CRF2 treatment may have improved HLB-affected root health by reducing the activity of pathogenic organisms, such as CLas. Greater concentrations of micronutrients, such as Mn and Zn, have been shown to exhibit antimicrobial functions [41]. For instance, Zn has been shown to exhibit host-pathogen interactions, as increased concentrations have been shown to suppress the growth of potential phytopathogens [42,43], thus promoting soil and plant health. Higher doses of foliar Mn in HLB-affected trees reduced symptom severity [16].
Significantly greater abundances of Rhizobiales and Vicinamibacterales may have been recruited from excess root exudates released from stressed HLB-affected grapefruit treated with 0× UF/IFAS foliar fertilizer compared to the other trees treated with higher doses of foliar fertilizers. Rhizobiales are a bacterial order of interest as they can interact with host plants to produce auxins, vitamins and N fixation, and protect the plants against stress [44]. Additionally, Vicinamibacterales have also been classified as PGPR, as they have been associated with increased plant available nutrient concentrations (specifically N and P) in rice rhizosphere soil [45]. Typically, greater amounts of exudates are released from roots during periods of stress for the purpose of recruiting microorganisms, specifically plant-growth-promoting rhizobacteria (PGPR) to assist in the acquisition of plant essential resources that would otherwise be unavailable [46,47,48]. Similarly, host plants grown under nutrient-deficient conditions have been shown to increase the synthesis of a root exudate known as Strigolactones to promote mycorrhizal fungal recruitment for nutrient acquisition [49,50]. Moreover, flavonoids released from the roots of nutrient-deficient legumes have been shown to stimulate bacterial root infection, leading to the establishment of nodules that promote N fixation [51].
4. Materials and Methods
4.1. Experimental Design
The study site is located at the University of Florida Institute of Food and Agricultural Sciences (UF/IFAS) Indian River Research and Education Center in Fort Pierce, FL (latitude 27.435342°, longitude -80.445197°, altitude 10 m). Plant material consisted of ‘Ray Ruby’ grapefruit trees (Citrus × paradisi) grafted on ‘Kuharske’ citrange (Citrus × sinensis × Citrus trifoliata). Trees were planted in September 2013 in flatwood soils (Pineda sands classified as loamy, siliceous, active, hyperthermic Arenic Glossaqualfs) which are poorly drained and consist of 96% sand, 2.5% silt and 1.5% clay and have an argillic soil layer at 90 cm below the soil surface. The average soil pH was 5.8 and the cation exchange capacity (CEC) was 3.5 cmol kg−1. Trees were grown on raised beds roughly 1 m tall to facilitate drainage, with swales 15 m between beds. Irrigation was delivered using 39.7 L h−1 microjet sprinklers (Maxijet, Dundee, FL, USA). The original experiment, which only focused on aboveground parameters, has been described in [9] and was arranged in a split-split-plot design with three factors, each consisting of plant densities, ground-applied controlled-release fertilizers (applied in February, May and September) and foliar-applied fertilizer combinations (applied in March, June and October). The three planting densities implemented were low (300 trees ha−1), medium (440 trees ha−1) and high (975 trees ha−1). The study consisted of two ground-applied fertilizer treatments, specifically, controlled-release fertilizer blends 1 (CRF1): 12.00N-1.31P-11.62K and micronutrients at 1× the UF/IFAS recommendation with micronutrients as sulfates (12-3-14 1× Micro) and CRF2: enhanced 12.00N-1.31P-11.62K with 2× Mg and 2.5× the UF/IFAS recommendation with micronutrients as sulfur-coated products (#12-3-14 2.5× Micro, Table 10 and Table 11). Additionally, there were four foliar fertilizer treatments, which included 0×, 1.5×, 3× and 6× the UF/IFAS recommendation [14]. Root and soil parameter sampling was performed every 4 months from September 2020 to January 2022. Rhizosphere samples were collected in January 2021.

Table 10.
Details about treatment factors and arrangements for experimental design.

Table 11.
Controlled-release fertilizer formula.
4.2. Soil Nutrient Analysis
A soil auger (One-Piece Auger model #400.48, AMS, Inc., American Falls, ID) 7 cm in diameter and 10 cm in depth was used to collect soil samples for nutrient concentrations. A single core was taken within the irrigated zone from one tree per experimental plot (total of 96) across eight experimental blocks. Soil samples were analyzed for extractable N, P, K, Mg, Ca, S, B, Zn, Mn, Fe and Cu.
Soil samples were dried overnight at 80 ℃, and nutrient concentrations were determined using Mehlich III extraction [52]. A total of 25 mL of Mehlich III extractant solution (0.2 M CH3COOH + 0.015 M NH4F + 0.013 M HNO3 + 0.001 M EDTA + 0.25 M H4NO3) was pipetted into extraction tubes containing 2.5 ± 0.05 g of soil. Soil nutrient concentrations were measured using inductively coupled plasma optical emission spectroscopy (ICP-OES, Spectro Ciros CCD, Fitzburg, MA, USA) [14].
4.3. Root Parameter Analysis
Root parameters were measured using a minirhizotron system (CID Bio-Science CI-602, CID Bioscience, Inc. Camas, WA, USA). Each tube consisted of three scannable windows at different depths (0–19 cm, 19–39 cm and 39–59 cm), and all three windows in every tube were scanned for each sampling point. After images were acquired, average root length and density were calculated using commercial software (RootSnap™ Version 1.3.2.25, CID Bio-Science, Camas, WA, USA).
4.4. Deoxyribonucleic Acid (DNA) Isolation and Quantification
Rhizosphere samples were taken shortly after bulk soil sampling, which consisted of rhizosphere soil (located around the roots) being lightly shaken from the roots and placed in 50 mL sterile tubes. Approximately, 50 g of the soil was collected and stored at −20 °C before DNA extraction. Approximately, 15 mL of 1× sterile phosphate-buffered saline (800 mL distilled water, 8 g NaCl, 0.2 g KCl, 1.44 g Na2PO4 and 0.24 g KH2PO4) was added to the sample and shaken by hand for 15 s. Roots were removed with forceps and discarded, the remaining soil was centrifuged at 3000 g for 15 min and the supernatant was discarded. Soil DNA was extracted from 0.25 g of the soil pellet using the DNeasy PowerSoil Kit (Qiagen Inc., Germantown, MD, USA) according to the manufacturer’s instructions. A fluorometer (Qubit, Thermofisher Scientific, Wilmington, NC, USA) was used to quantify the extracted DNA and determine whether the DNA was concentrated enough for sequencing (>1 ng/µL). Rhizosphere DNA was amplified with 515Fa/926R universal bacterial [53] primers and sequenced at the Genomics and Microbiome Core Facility at Rush University, Chicago, IL, USA.
4.5. Rhizosphere Microbiome Diversity and Statistical Analysis
After sequencing, bioinformatic data were processed using DADA2 [54] within the Qiime 2 [55] package. Raw sequences were demultiplexed. DADA2 was used to filter chimeras, primers and adapters and assemble pair-ended sequences. Taxonomy was assigned to amplicon sequence variants (ASVs) with the reference dataset SILVA 128 database for 16S rRNA using a naïve Bayes classifier in Qiime2 [56]. Alpha and beta diversity analyses of the bacterial community were performed on log-normalized data to avoid an increase in error rates due to rarefaction [57] with the R package “Phyloseq” v1.24.0 [58]. Alpha diversity analyses included the number of observed ASVs (Shannon index), whereas beta diversity analyses included principal coordinate analysis (PCoA) on weighted UniFrac distances. A two-way analysis of similarities (ANOSIM) test was performed to determine significant differences in beta diversity between treatments.
4.6. Plant and Soil Data Statistical Analysis
An analysis of variance (three-way ANOVA) was performed using statistical software (R Version 3.6.0, RStudio, Boston, MA, USA). The main effect means were separated using Tukey’s honestly significant difference post hoc test. Differences were considered to be significant when p-values were less than or equal to 0.05. Additionally, Pearson correlation coefficients were computed.
5. Conclusions
In this study, we examined the impact of planting densities, ground-applied controlled-release fertilizers and foliar fertilizer dosages on both soil and plant parameters, including rhizosphere community composition. The use of the CRF2 ground-applied fertilizer resulted in higher soil nutrient concentrations through all time points of the study, notably with significant differences in Zn and Mn. Additionally, increased micronutrient concentrations provided by the CRF2 ground-applied fertilizer may have provided additional nutrients required to assist the root health of HLB-affected grapefruit trees. Furthermore, HLB-affected grapefruit at 0× the UF/IFAS recommended foliar fertilizer resulted in a significantly greater abundance of Vicinamibacterales and Rhizobiales, whereby both of which are PGPR that may have been recruited to assist grapefruit health under nutrient-deprived conditions.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by J.M.S. and J.-P.F. The first draft of the manuscript was written by J.M.S. and all authors commented on the previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the UF/IFAS Citrus Initiative, U.S. Department of Agriculture (USDA)-APHIS MAC, agreement no. AP19PPQSandT00C116. This work was also supported by the U.S. Department of Agriculture National Institute of Food and Agriculture, Hatch project #FLA-IRC-005743.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study have been deposited to NCBI. The BioProject accession number is PRJNA937287.
Acknowledgments
We thank Tom James and Randy Burton for the treatment application and research field maintenance.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
References
- Bodaghi, S.; Meyering, B.; Pugina, G.; Bowman, K.D.; Albrecht, U. Different Sweet Orange–Rootstock Combinations Infected by Candidatus Liberibacter asiaticus under Greenhouse Conditions: Effects on the Scion. HortScience 2022, 57, 56–64. [Google Scholar] [CrossRef]
- Albrecht, U.; Mccollum, G.; Bowman, K. Influence of rootstock variety on Huanglongbing disease development in field-grown sweet orange (Citrus sinensis [L.] Osbeck) trees. Sci. Hortic. 2012, 138, 71–80. [Google Scholar] [CrossRef]
- Kwakye, S.; Kadyampakeni, D.; Morgan, K.; Vashisth, T.; Wright, A. Effects of Iron Rates on Growth and Development of Young Huanglongbing-affected Citrus Trees in Florida. HortScience 2022, 57, 1092–1098. [Google Scholar] [CrossRef]
- Hallman, L.M.; Kadyampakeni, D.M.; Ferrarezi, R.S.; Wright, A.L.; Ritenour, M.A.; Johnson, E.G.; Rossi, L. Impact of Ground Applied Micronutrients on Root Growth and Fruit Yield of Severely Huanglongbing-Affected Grapefruit Trees. Horticulturae 2022, 8, 763. [Google Scholar] [CrossRef]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Association of ‘CandidatusLiberibacter asiaticus’ root infection, but not phloem plugging with root loss on huanglongbing-affected trees prior to appearance of foliar symptoms. Plant Pathol. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, J.; Deng, X. Historical Perspectives, Management, and Current Research of Citrus HLB in Guangdong Province of China, Where the Disease has been Endemic for Over a Hundred Years. Phytopathology® 2018, 108, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Zapien-Macias, J.M.; Ferrarezi, R.S.; Spyke, P.D.; Castle, W.S.; Gmitter, F.G.; Grosser, J.W.; Rossi, L. Early Performance of Recently Released Rootstocks with Grapefruit, Navel Orange, and Mandarin Scions under Endemic Huanglongbing Conditions in Florida. Horticulturae 2022, 8, 1027. [Google Scholar] [CrossRef]
- Bowman, K.D.; Mccollum, G.; Albrecht, U. Performance of ‘Valencia’ orange (Citrus sinensis [L.] Osbeck) on 17 rootstocks in a trial severely affected by huanglongbing. Sci. Hortic. 2016, 201, 355–361. [Google Scholar] [CrossRef]
- Phuyal, D.; Nogueira, T.A.R.; Jani, A.D.; Kadyampakeni, D.M.; Morgan, K.T.; Ferrarezi, R.S. ‘Ray Ruby’ Grapefruit Affected by Huanglongbing I. Planting Density and Soil Nutrient Management. HortScience 2020, 55, 1411–1419. [Google Scholar] [CrossRef]
- Hallman, L.M.; Kadyampakeni, D.M.; Fox, J.-P.; Wright, A.L.; Rossi, L. Root-Shoot Nutrient Dynamics of Huanglongbing-Affected Grapefruit Trees. Plants 2022, 11, 3226. [Google Scholar] [CrossRef]
- Kunwar, S.; Meyering, B.; Grosser, J.; Gmitter, F.G.; Castle, W.S.; Albrecht, U. Field performance of ‘Valencia’ orange trees on diploid and tetraploid rootstocks in different huanglongbing-endemic growing environments. Sci. Hortic. 2022, 309, 111635. [Google Scholar] [CrossRef]
- Cocuzza, G.E.M.; Alberto, U.; Hernández-Suárez, E.; Siverio, F.; Di Silvestro, S.; Tena, A.; Carmelo, R. A review on Trioza erytreae (African citrus psyllid), now in mainland Europe, and its potential risk as vector of huanglongbing (HLB) in citrus. J. Pest Sci. 2017, 90, 1–17. [Google Scholar] [CrossRef]
- Li, B.; Wang, S.; Zhang, Y.; Qiu, D. Acid Soil Improvement Enhances Disease Tolerance in Citrus Infected by Candidatus Liberibacter asiaticus. Int. J. Mol. Sci. 2020, 21, 3614. [Google Scholar] [CrossRef] [PubMed]
- Kadyampakeni, D.M.; Morgan, K.T.; Nkedi-Kizza, P.; Kasozi, G.N. Nutrient Management Options for Florida Citrus: A Review of NPK Application and Analytical Methods. J. Plant Nutr. 2015, 38, 568–583. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Chinyukwi, T. Are macronutrients and micronutrients therapeutic for restoring performance of trees affected by citrus greening? A discussion of current practices and future research opportunities. J. Plant Nutr. 2022, 44, 2949–2969. [Google Scholar] [CrossRef]
- Zambon, F.T.; Kadyampakeni, D.M.; Grosser, J.W. Ground Application of Overdoses of Manganese Have a Therapeutic Effect on Sweet Orange Trees Infected with Candidatus Liberibacter asiaticus. HortScience 2019, 54, 1077–1086. [Google Scholar] [CrossRef]
- Atta, A.A.; Morgan, K.T.; Kadyampakeni, D.M.; Mahmoud, K.A. The Effect of Foliar and Ground-Applied Essential Nutrients on Huanglongbing-Affected Mature Citrus Trees. Plants 2021, 10, 925. [Google Scholar] [CrossRef]
- Ghimire, L.; Grosser, J.; Vashisth, T. Differences in Nutrient Uptake Can Influence the Performance of Citrus Rootstocks under Huanglongbing Conditions. HortScience 2023, 58, 40–46. [Google Scholar] [CrossRef]
- Esteves, E.; Kadyampakeni, D.M.; Zambon, F.; Ferrarezi, R.S.; Maltais-Landry, G. Magnesium fertilization has a greater impact on soil and leaf nutrient concentrations than nitrogen or calcium fertilization in Florida orange production. Nutr. Cycl. Agroecosystems 2022, 122, 73–87. [Google Scholar] [CrossRef]
- Morgan, K.T.; Wheaton, T.A.; Castle, W.S.; Parsons, L.R. Response of Young and Maturing Citrus Trees Grown on a Sandy Soil to Irrigation Scheduling, Nitrogen Fertilizer Rate, and Nitrogen Application Method. HortScience 2009, 44, 145–150. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Jani, A.D.; James, H.T.; Gil, C.; Ritenour, M.A.; Wright, A.L. Sweet Orange Orchard Architecture Design, Fertilizer, and Irrigation Management Strategies under Huanglongbing-endemic Conditions in the Indian River Citrus District. HortScience 2020, 55, 2028–2036. [Google Scholar] [CrossRef]
- Phuyal, D.; Nogueira, T.A.R.; Jani, A.D.; Kadyampakeni, D.M.; Morgan, K.T.; Ferrarezi, R.S. ‘Ray Ruby’ Grapefruit Affected by Huanglongbing II. Planting Density, Soil, and Foliar Nutrient Management. HortScience 2020, 55, 1420–1432. [Google Scholar] [CrossRef]
- Morgan, K.T.; Rouse, R.E.; Ebel, R.C. Foliar Applications of Essential Nutrients on Growth and Yield of ‘Valencia’ Sweet Orange Infected with Huanglongbing. HortScience 2016, 51, 1482–1493. [Google Scholar] [CrossRef]
- Al-Obeed, R.S.; Ahmed, M.A.-A.; Kassem, H.A.; Al-Saif, A.M. Improvement of “Kinnow” mandarin fruit productivity and quality by urea, boron and zinc foliar spray. J. Plant Nutr. 2018, 41, 609–618. [Google Scholar] [CrossRef]
- Vashisth, T.; Grosse, J. Comparison of Controlled Release Fertilizer (CRF) for Newly Planted Sweet Orange Trees under Huanglongbing Prevalent Conditions. J. Hortic. 2018, 5, 244. [Google Scholar] [CrossRef]
- Ren, N.; Wang, Y.; Ye, Y.; Zhao, Y.; Huang, Y.; Fu, W.; Chu, X. Effects of Continuous Nitrogen Fertilizer Application on the Diversity and Composition of Rhizosphere Soil Bacteria. Front. Microbiol. 2020, 11, 1948. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Lin, M.; Zhou, H.; Wu, H.; Li, Z.; Lin, W. The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS ONE 2019, 14, e0217018. [Google Scholar] [CrossRef]
- Malik, L.; Sanaullah, M.; Mahmood, F.; Hussain, S.; Siddique, M.H.; Anwar, F.; Shahzad, T. Unlocking the potential of co-applied biochar and plant growth-promoting rhizobacteria (PGPR) for sustainable agriculture under stress conditions. Chem. Biol. Technol. Agric. 2022, 9, 58. [Google Scholar] [CrossRef]
- Samaras, A.; Kamou, N.; Tzelepis, G.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Karaoglanidis, G.S. Root Transcriptional and Metabolic Dynamics Induced by the Plant Growth Promoting Rhizobacterium (PGPR) Bacillus subtilis Mbi600 on Cucumber Plants. Plants 2022, 11, 1218. [Google Scholar] [CrossRef]
- Zeng, Q.; Ding, X.; Wang, J.; Han, X.; Iqbal, H.M.N.; Bilal, M. Insight into soil nitrogen and phosphorus availability and agricultural sustainability by plant growth-promoting rhizobacteria. Environ. Sci. Pollut. Res. 2022, 29, 45089–45106. [Google Scholar] [CrossRef]
- Gezahegn, A.M.; Tesfaye, K.; Sharma, J.J.; Belel, M.D. Determination of optimum plant density for faba bean (Vicia faba L.) on vertisols at Haramaya, Eastern Ethiopia. Cogent Food Agric. 2016, 2, 1224485. [Google Scholar] [CrossRef]
- Rolfe, S.A.; Griffiths, J.; Ton, J. Crying out for help with root exudates: Adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr. Opin. Microbiol. 2019, 49, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Langridge, H.; Straathof, A.L.; Muhamadali, H.; Hollywood, K.A.; Goodacre, R.; Vries, F.T. Root functional traits explain root exudation rate and composition across a range of grassland species. J. Ecol. 2022, 110, 21–33. [Google Scholar] [CrossRef]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 2019, 7, 136. [Google Scholar] [CrossRef]
- Preece, C.; Peñuelas, J. A Return to the Wild: Root Exudates and Food Security. Trends Plant Sci. 2020, 25, 14–21. [Google Scholar] [CrossRef]
- Hartemink, A.E.; Barrow, N.J. Soil pH–nutrient relationships: The diagram. Plant Soil 2023. [Google Scholar] [CrossRef]
- Kumar Bhatt, M.; Labanya, R.; Joshi, H.C. Influence of Long-term Chemical fertilizers and Organic Manures on Soil Fertility—A Review. Univers. J. Agric. Res. 2019, 7, 177–188. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Yang, Y.; Gao, B.; Wan, Y.; Li, Y.C.; Cheng, D. Activated-Lignite-Based Super Large Granular Slow-Release Fertilizers Improve Apple Tree Growth: Synthesis, Characterizations, and Laboratory and Field Evaluations. J. Agric. Food Chem. 2017, 65, 5879–5889. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Naresh, R.K.; Mandal, A.; Walia, M.K.; Gupta, R.K.; Singh, R.; Dhaliwal, M.K. Effect of manures and fertilizers on soil physical properties, build-up of macro and micronutrients and uptake in soil under different cropping systems: A review. J. Plant Nutr. 2019, 42, 2873–2900. [Google Scholar] [CrossRef]
- Atta, A.A.; Morgan, K.T.; Hamido, S.A.; Kadyampakeni, D.M. Effect of Essential Nutrients on Roots Growth and Lifespan of Huanglongbing Affected Citrus Trees. Plants 2020, 9, 483. [Google Scholar] [CrossRef]
- Da Silva, J.R.; De Alvarenga, F.V.; Boaretto, R.M.; Lopes, J.R.S.; Quaggio, J.A.; Coletta Filho, H.D.; Mattos, D. Following the effects of micronutrient supply in HLB-infected trees: Plant responses and ‘Candidatus Liberibacter asiaticus’ acquisition by the Asian citrus psyllid. Trop. Plant Pathol. 2020, 45, 597–610. [Google Scholar] [CrossRef]
- Wu, D.; Ma, Y.; Yang, T.; Gao, G.; Wang, D.; Guo, X.; Chu, H. Phosphorus and Zinc Are Strongly Associated with Belowground Fungal Communities in Wheat Field under Long-Term Fertilization. Microbiol. Spectr. 2022, 10, e00110-22. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Johnson, E.G.; Myers, M.E.; Young, M.; Rajasekaran, P.; Das, S.; Santra, S. Potential of Nano-Formulated Zinc Oxide for Control of Citrus Canker on Grapefruit Trees. Plant Dis. 2016, 100, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Erlacher, A.; Cernava, T.; Cardinale, M.; Soh, J.; Sensen, C.; Grube, M.; Berg, G. Rhizobiales as functional and endosymbiontic members in the lichen symbiosis of Lobaria pulmonaria L. Front. Microbiol. 2015, 6, 53. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.; Qiu, Z.; Singh, B. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar]
- Vives-Peris, V.; Molina, L.; Segura, A.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates from citrus plants subjected to abiotic stress conditions have a positive effect on rhizobacteria. J. Plant Physiol. 2018, 228, 208–217. [Google Scholar] [CrossRef]
- Lombardi, N.; Vitale, S.; Turrà, D.; Reverberi, M.; Fanelli, C.; Vinale, F.; Marra, R.; Ruocco, M.; Pascale, A.; D’Errico, G.; et al. Root Exudates of Stressed Plants Stimulate and Attract Trichoderma Soil Fungi. Mol. Plant-Microbe Interact. 2018, 31, 982–994. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Pozo, M.J.; García-Garrido, J.M. Strigolactones: A cry for help in the rhizosphere. Botany 2011, 89, 513–522. [Google Scholar] [CrossRef]
- Kim, B.; Westerhuis, J.; Smilde, A.; Flokova, K.; Suleiman, A.; Kuramae, E.; Bouwmeester, H.; Zancarini, A. Effect of strigolactones on recruitment of the rice root-associated microbiome. FEMS Microbiol. Ecol. 2022, 98, fiac010. [Google Scholar]
- Dong, W.; Song, Y. The Significance of Flavonoids in the Process of Biological Nitrogen Fixation. Int. J. Mol. Sci. 2020, 21, 5926. [Google Scholar] [CrossRef] [PubMed]
- Mylavarapu, R.; Obreza, T.; Morgan, K.; Hochmuth, G.; Nair, V.; Wright, A. Extraction of Soil Nutrients Using Mehlich-3 Reagent for Acid-Mineral Soils of Florida. EDIS 2014, 2014. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Joseph, C.M.L.; Allen, G.; Benson, A.K.; Mills, D.A. Next-Generation Sequencing Reveals Significant Bacterial Diversity of Botrytized Wine. PLoS ONE 2012, 7, e36357. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Waste Not, Want Not: Why Rarefying Microbiome Data Is Inadmissible. PLoS Comput. Biol. 2014, 10, e1003531. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).