The Effect of Irrigation and Humic Acid on the Plant Yield and Quality of Sweet Basil (Ocimum basilicum L.) with Mulching Application under Semi-Arid Ecological Conditions
Abstract
1. Introduction
2. Results
2.1. The Influence of Irrigation and Humic Acid on the Morpho-Physiological and Biochemical Traits
2.1.1. Morpho-Physiological Traits
2.1.2. Biochemical Traits
2.2. The Influence of Irrigation and Humic Acid on the Morpho-Physiological and Biochemical Traits with the Effect of Soil Mulching
2.2.1. Morpho-Physiological Traits
2.2.2. Biochemical Traits
3. Discussion
3.1. The Influence of Irrigation and Humic Acid on Morpho-Physiological and Biochemical Traits
3.1.1. Morpho-Physiological Traits
3.1.2. Biochemical Traits
3.2. The Influence of Irrigation and Humic Acid on Morpho-Physiological and Biochemical Traits with the Effect of Soil Mulching
3.2.1. Morpho-Physiological Traits
3.2.2. Biochemical Traits
4. Materials and Methods
4.1. Site Conditions
4.2. Field Experiments
4.3. Experimental Procedure
4.3.1. Morpho-Physiological Traits
4.3.2. Essential Oil Content
4.3.3. Composition of Essential Oil
4.3.4. Plant Protein Content (%)
4.4. Statistical Analysis
5. Conclusions
- (1)
- The first trial was conducted under various drip IRLs and HADs without the use of SM. The highest mean values of morpho-physiological traits were obtained at IRL 100 and IRL 75 in both years. The mean EOR of basil was highest at IRL 25. FHY and DLY were affected by HAD, and the highest mean values were obtained at HAD 20 and HAD 40.
- (2)
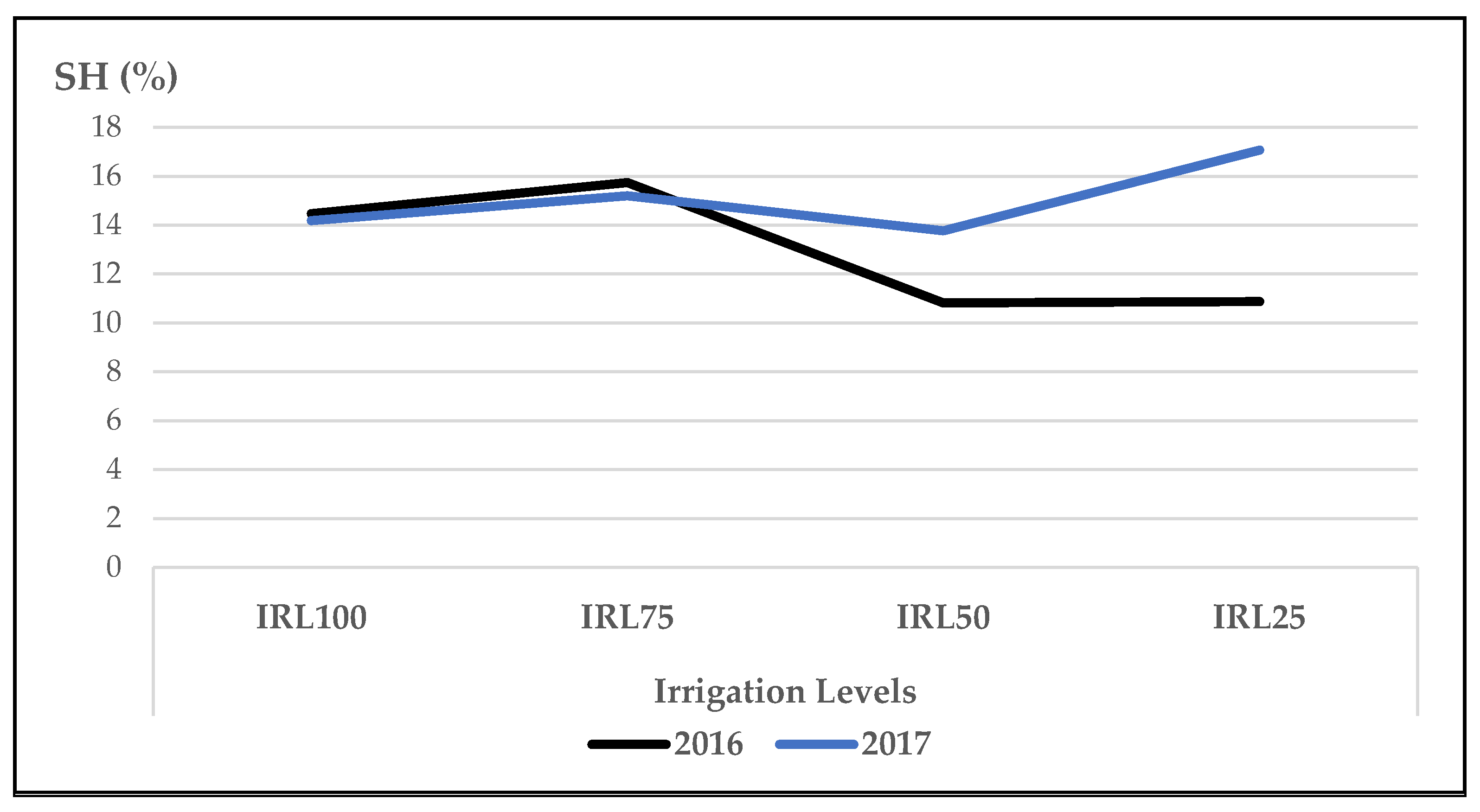
- For the biochemical traits without the use of SM, the mean content of linalool at IRL 100 and IRL 75 and 1,8-cineole at IRL 100 were highest in both years. The mean contents of oxygenated monoterpene hydrocarbons at IRL 100 and IRL 75 and sesquiterpene hydrocarbons at IRL 75 in the first year were higher than the previous IRL.
- (3)
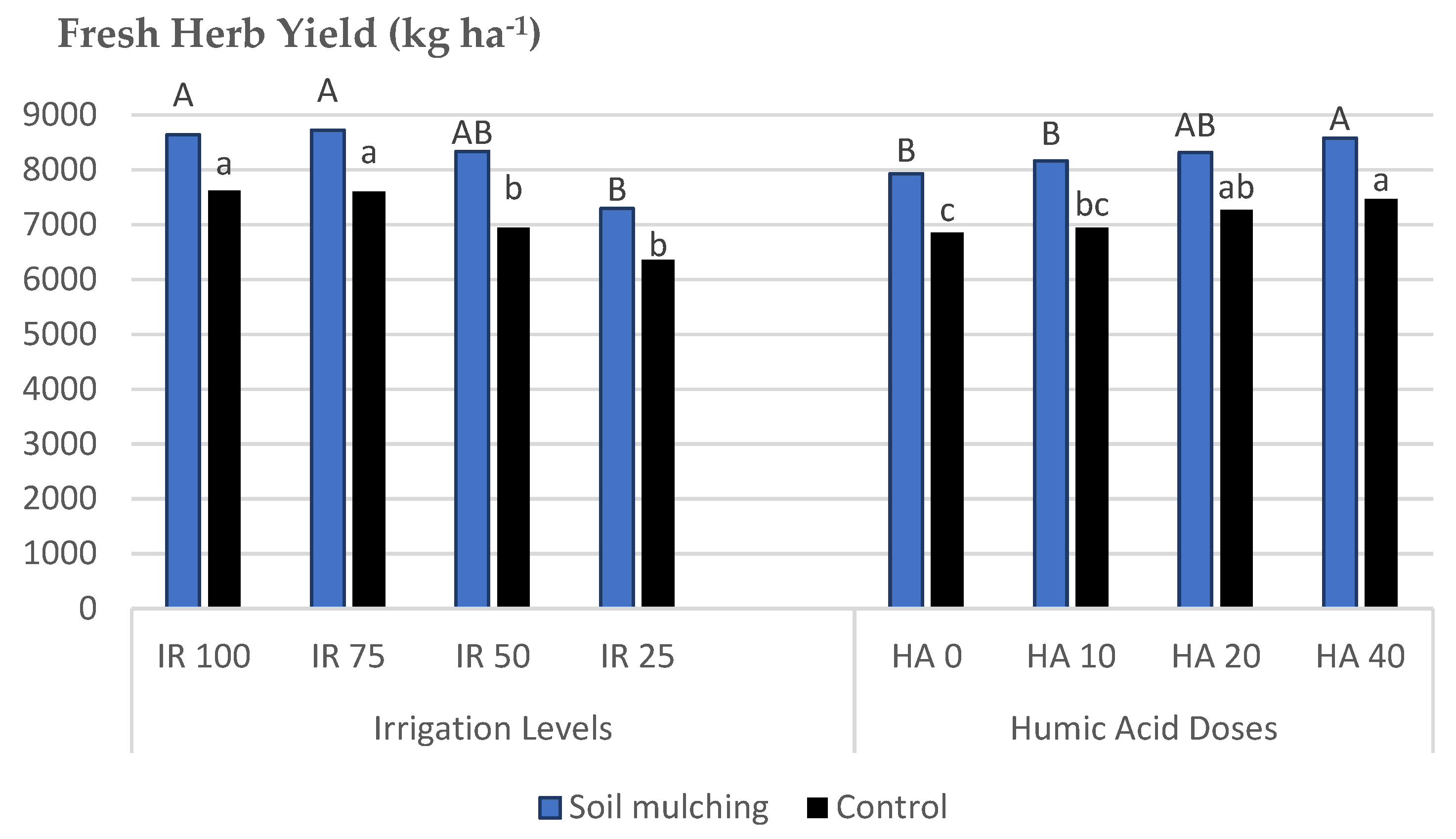
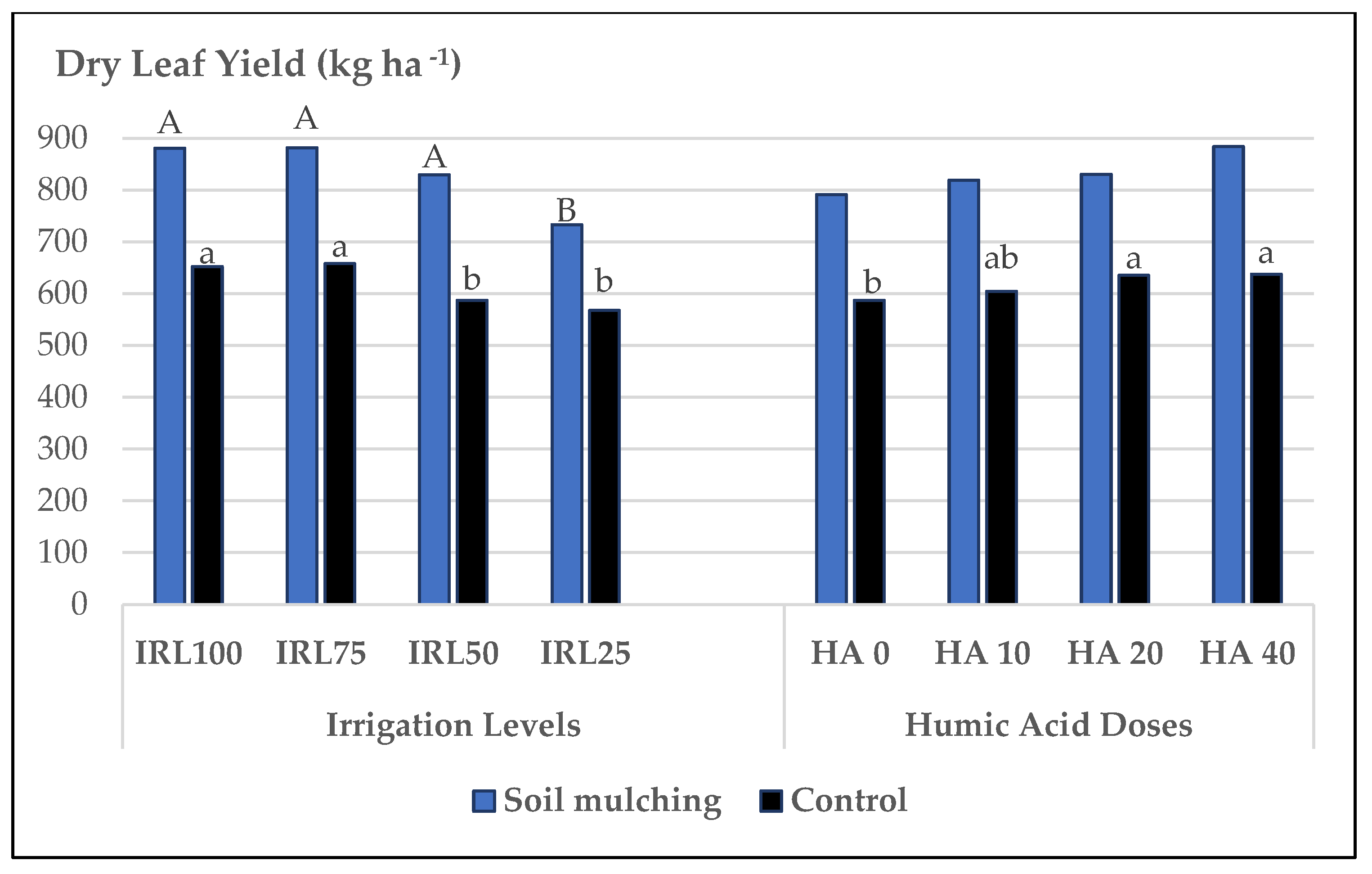
- The second trial was conducted under different drip IRLs and HADs with the use of SM. The mean values of plant height (PH), fresh herb yield (FHY), dry herb yield (DHY), dry leaf yield (DLY), and essential oil yield (EOY) belonging to SM application were higher compared to the plants cultivated without SM. The highest mean values for FHY were obtained at HAD 40 and HAD 20, whereas, without SM, the HA applications were insignificant. SM increased the PH up to +8.8–13.5%, FHY to +11.7–16.7%, and the DLY to +22.5–29.2% compared to the plots without SM. Under SM conditions, higher FHY and DLY were obtained at IRL 50 and higher EOY at IRL 75 and IRL 50. Irrigation at 50% field capacity may decrease water consumption up to 50% compared to the plants cultivated without SM.
- (4)
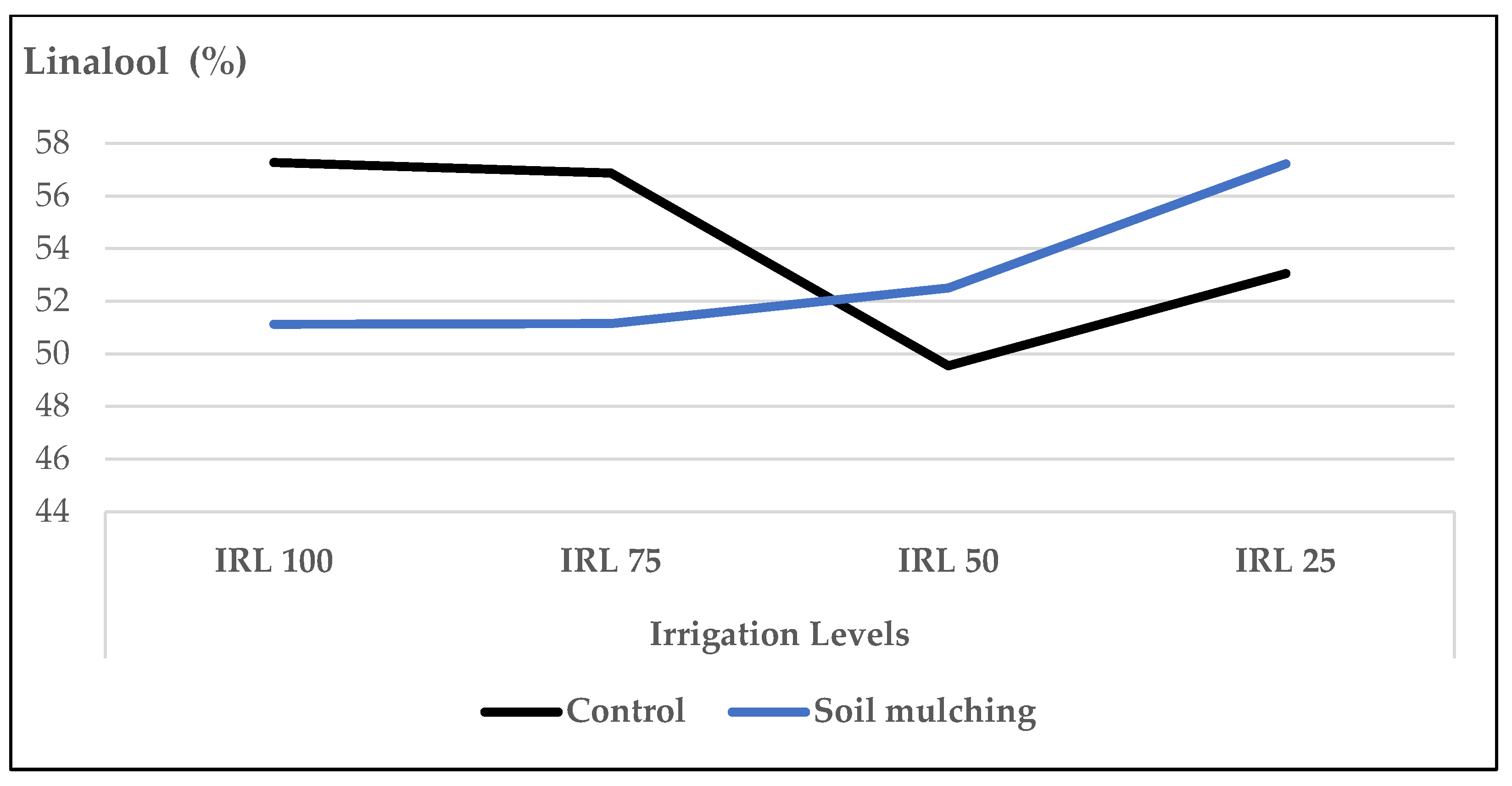
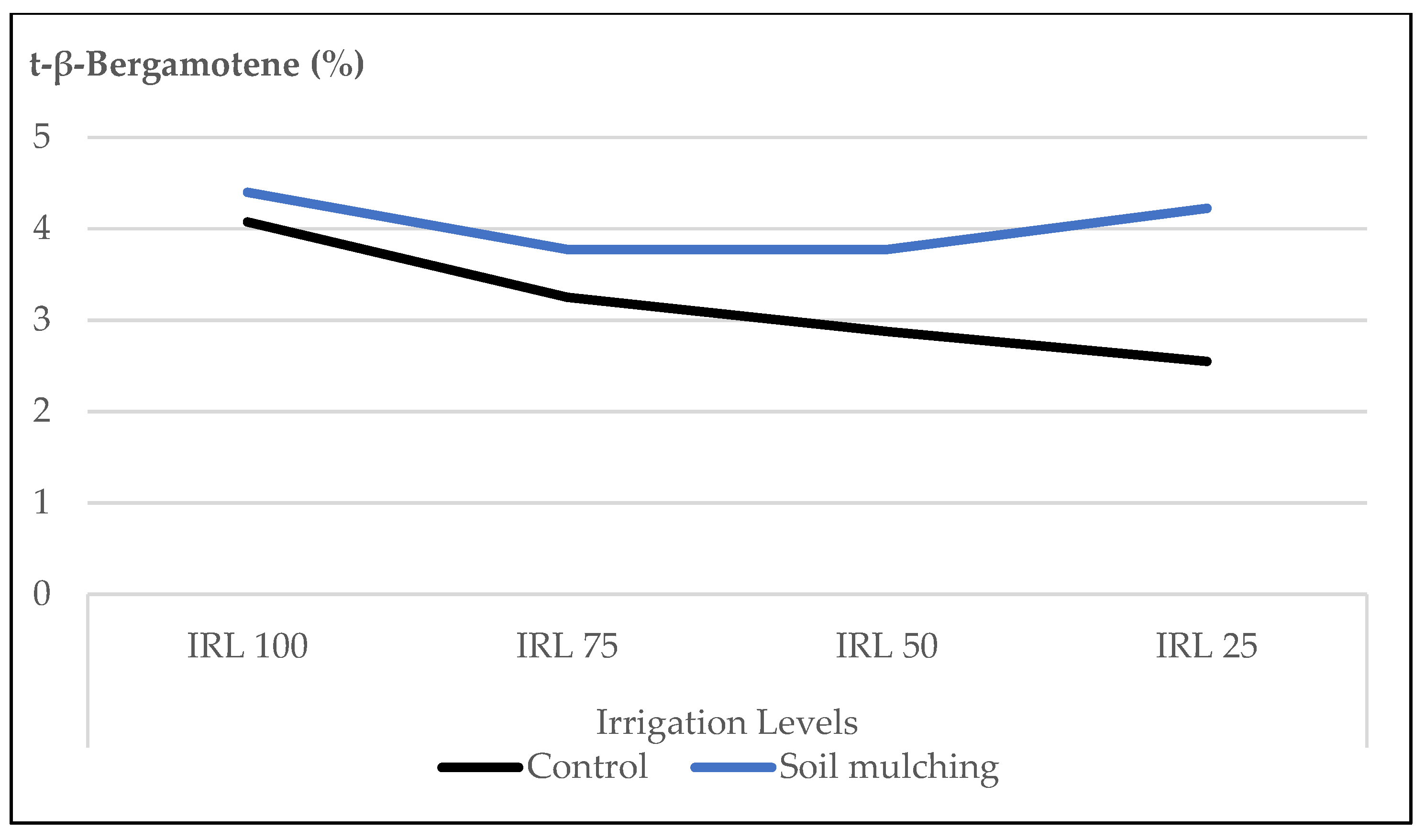
- Under SM conditions, the ratio of linalool, 1,8-cineole, and (E)-β-bergamotene increased at HAD 40, IRL 25, and IRL 50 which not only preserved the irrigation water up to 50–75% but also produced higher ratios of the main essential oil compounds. With the use of SM, it was also observed that HA worked more efficiently. The increase in the essential oil compounds at IRL 50 and IRL 25 was more pronounced at the dose of HA 40.
- (5)
- The HA used in this study improved FHY and DLY at HAD 20 and HAD 40, the yield values were significantly affected by HAD. Similarly, it increased the main essential oil compounds, especially at HAD 40 and IRL 50 under SM conditions. Because of these results, HA can be considered a plant biostimulant, which was defined by the 2018 Farm Bill (https://bpia.org/solutions-provided-by-biological-products-biostimulants/) and the regulation of (EU) 2019/1009 which defined it as the improvement of yield and quality traits (http://data.europa.eu/eli/reg/2019/1009/oj).
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çetin, Ö.; Yildirim, O.; Uygan, D.; Boyaci, H. Irrigation scheduling of drip-irrigated tomatoes using class A pan evaporation. Turk. J. Agric. For. 2002, 26, 171–178. [Google Scholar]
- Wang, H.; Wang, N.; Quan, H.; Zhang, F.; Fan, J.; Feng, H.; Xiang, Y. Yield and water productivity of crops, vegetables and fruits under subsurface drip irrigation: A global meta-analysis. Agric. Water Manag. 2022, 269, 107645. [Google Scholar] [CrossRef]
- Karlberg, L.; de Vries, F.W.P. Exploring potentials and constraints of low-cost drip irrigation with saline water in sub-Saharan Africa. Phys. Chem. Earth Parts A/B/C 2004, 29, 1035–1042. [Google Scholar] [CrossRef]
- Wang, D.; Li, G.; Mo, Y.; Cai, M.; Bian, X. Effect of planting date on accumulated temperature and maize growth under mulched drip irrigation in a middle-latitude area with frequent chilling injury. Sustainability 2017, 9, 1500. [Google Scholar] [CrossRef]
- Tang, M.; Li, H.; Zhang, C.; Zhao, X.; Gao, X.; Wu, P. Mulching measures improve soil moisture in rain-fed jujube (Ziziphus jujuba Mill.) orchards in the loess hilly region of China. Sustainability 2021, 13, 610. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, A.; Liu, F.; Niu, W.; Siddique, K.H. Effect of film mulching on crop yield and water use efficiency in drip irrigation systems: A meta-analysis. Soil Tillage Res. 2022, 221, 105392. [Google Scholar] [CrossRef]
- Loughrin, J.H.; Kasperbauer, M.J. Light reflected from colored mulches affects aroma and phenol content of sweet basil (Ocimum basilicum L.) leaves. J. Agric. Food Chem. 2001, 49, 1331–1335. [Google Scholar] [CrossRef]
- Wei, K.; Zhang, J.H.; Wang, Q.J.; Chen, Y.; Guo, Y.; Sun, Y. Effects of potassium humate on cotton (Gossypium hirsutum L.) growth and yield and soil salinity under film-mulched drip irrigation with brackish water in northwest china. Appl. Ecol. Environ. Res. 2021, 19, 3879–3895. [Google Scholar] [CrossRef]
- Thakur, M.; Kumar, R. Mulching: Boosting crop productivity and improving soil environment in herbal plants. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100287. [Google Scholar] [CrossRef]
- Palada, M.C.; Crossman, S.M.A.; Kowalski, J.A.; Collingwood, C.D. Evaluation of organic and synthetic mulches for basil production under drip irrigation. J. Herbs Spices Med. Plants 2008, 6, 39–48. [Google Scholar] [CrossRef]
- Delfine, S.; Velikova, V.B.; Mastrodonato, F. Soil-Mulching Influence on Spearmint Oil Yield, Ecophysiological Activities and Essential-Oil Content in Rainfed Environment of Southern Italy. Agronomy 2022, 12, 1521. [Google Scholar] [CrossRef]
- Cuello, J.P.; Hwang, H.Y.; Gutierrez, J.; Kim, S.Y.; Kim, P.J. Impact of plastic film mulching on increasing greenhouse gas emissions in temperate upland soil during maize cultivation. Appl. Soil Ecol. 2015, 91, 48–57. [Google Scholar] [CrossRef]
- Baydar, H. Tıbbi ve Aromatik Bitkiler Bilimi ve Teknolojisi, 4th ed.; Süleyman Demirel Üniversitesi: Isparta, Türkiye, 2013; Volume 51013, pp. 206–208. [Google Scholar]
- Ramos, R.S.; Rodrigues, A.B.L.; Almeida, S.S.M.S. Preliminary study of the extract of the barks of Licania macrophylla Benth: Phytochemicals and toxicological aspects. Biota Amaz. 2014, 4, 94–99. [Google Scholar] [CrossRef]
- Harley, M.M.; Paton, A.; Harley, R.M.; Cade, P.G. Pollen morphological studies in tribe Ocimeae (Nepetoideae: Labiatae): I. Ocimum L. Grana 1992, 31, 161–176. [Google Scholar] [CrossRef]
- Asadollahi, A.; Mirza, M.; Abbaszadeh, B.; Azizpour, S.; Keshavarzi, A. Comparison of essential oil from leaves and inflorescence of three basil (Ocimum basilicum L.) populations under drought stress. Int. J. Agron. Plant Prod. 2013, 4, 2764–2767. [Google Scholar]
- Omidbeigi, R. Production and Processing of Medicinal Plants; Astan Ghods Razavi Press: Tehran, Iran, 2000; pp. 99–104. [Google Scholar]
- Gürgan, M.; Adiloğlu, S. Increasing concentrations of iron fertilizer affect antibacterial activity of basil (Ocimum basilicum L.). Ind. Crops Prod. 2021, 170, 113768. [Google Scholar] [CrossRef]
- Mpiana, P.T.; Ngoy, E.M.; Kilembe, J.T.; Kabengele, C.N.; Matondo, A.; Inkoto, C.L.; Lengbiye, E.; Mwanangombo, D.T.; Tshibangu, D.S.-T.; Ngbolua, K.-T.-N.J.-P.; et al. Ocimum basilicum as a Potential Anti-COVID-19 Plant: Review on the Antiviral Activity and Molecular Docking of Some of Its Molecules with the SARS-CoV-2 Main Protease (MPRO). In Ocimum basilicum: Taxonomy, Cultivation and Uses; Nova Science Publishers: Hauppauge, NY, USA, 2021; pp. 73–111. [Google Scholar]
- Brandão, L.B.; Santos, L.L.; Martins, R.L.; Rodrigues, A.B.L.; da Costa, A.L.P.; Faustino, C.G.; de Almeida, S. The potential effects of species Ocimum basilicum L. on health: A review of the chemical and biological studies. Pharmacogn. Rev. 2022, 16, 23. [Google Scholar] [CrossRef]
- Dhama, K.; Sharun, K.; Gugjoo, M.B.; Tiwari, R.; Alagawany, M.; Iqbal Yatoo, M.; Thakur, P.; Iqbal, H.M.; Chaicumpa, W.; Michalak, I.; et al. A comprehensive review on chemical profile and pharmacological activities of Ocimum basilicum. Food Rev. Int. 2021, 1–29. [Google Scholar] [CrossRef]
- Faiza Fedoul, F.; Meddah, B.; Larouci, M.; Tir Touil, A.; Merazi, Y.; Bekhti, N.; Piras, A.; Falconieri, D.; Cakmak, Y.S. Medicinal Applications, Chemical Compositions, and Biological Effects of Algerian Ocimum basilicum L. var Genovese with the Conversion of Experimental Doses to Humans. J. Appl. Biotechnol. Rep. 2022, 9, 671–683. [Google Scholar]
- Davies, G.; Ghabbour, E.A.; Steelink, C. Humic acids: Marvelous products of soil chemistry. J. Chem. Educ. 2001, 78, 1609. [Google Scholar] [CrossRef]
- Senn, T.L.; Kingman, A.R. A review of humus and humic acids. Res. Ser. 1973, 145, 1–5. [Google Scholar]
- Ekren, S.; Sönmez, Ç.; Özçakal, E.; Kurttaş, Y.S.K.; Bayram, E.; Gürgülü, H. The effect of different irrigation water levels on yield and quality characteristics of purple basil (Ocimum basilicum L.). Agric. Water Manag. 2012, 109, 155–161. [Google Scholar] [CrossRef]
- Forouzandeh, M.; Fanoudi, M.; Arazmjou, E.; Tabiei, H. Effect of drought stress and types of fertilizers on the quantity and quality of medicinal plant Basil (Ocimum basilicum L.). Indian J. Innov. Dev. 2012, 1, 696–699. [Google Scholar]
- Simon, J.E.; Reiss-Bubenheim, D.; Joly, R.J.; Charles, D.J. Water stress-induced alterations in essential oil content and composition of sweet basil. J. Essent. Oil Res. 1992, 4, 71–75. [Google Scholar] [CrossRef]
- Khalid, K.A. Influence of water stress on growth, essential oil, and chemical composition of herbs [Ocimum sp.]. Int. Agrophysics 2006, 20, 289–296. [Google Scholar]
- Sirousmehr, A.; Arbabi, J.; Asgharipour, M.R. Effect of drought stress levels and organic manures on yield, essential oil content and some morphological characteristics of sweet basil (Ocimum basilicum). Adv. Environ. Biol. 2014, 8, 880–885. [Google Scholar]
- Ameri, A.; Aminifard, M.H.; Fatemi, H.; Aroiee, H. Response of growth and yield of Ocimum basilicum with application of humic acid. Angew. Biol. Forsch. 2013, 1, 1. [Google Scholar]
- Zhang, X.; Schmidt, R.E. The impact of growth regulators on alphatocopherol status of water-stressed Poa pratensis L. Int. Turfgrass. Soc. Res. J. 1997, 8, 1364–2137. [Google Scholar]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Madende, M.; Hayes, M. Fish By-Product Use as Biostimulants: An Overview of the Current State of the Art, Including Relevant Legislation and Regulations within the EU and USA. Molecules 2020, 25, 1122. [Google Scholar] [CrossRef] [PubMed]
- Boguta, P.; Sokołowska, Z. Interactions of humic acids with metal ions. Acta Agrophysica 2022, 2, 1–113. [Google Scholar]
- Singh, M. Effect of nitrogen and irrigation on the yield and quality of sweet basil (Ocimum basilicum L.). J. Spices Aromat. Crops 2002, 11, 151–154. [Google Scholar]
- Nonami, H.; Boyer, J.S. Turgor and growth at low water potentials. Plant Physiol. 1989, 89, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Sorial, M.E.; El-Gamal, S.M.; Gendy, A.A. Response of sweet basil to jasmonic acid application in relation to different water supplies. Biosci. Res. 2010, 7, 39–47. [Google Scholar]
- Pazoki, A. Effects of plant growth promoting rhizobacteria (PGPR) and humic acid on yield and yield components of basil (Ocimum basilicum L.) under drought stress in Qom region. J. Agroecol. 2016, 6, 60–80. [Google Scholar]
- Chaski, C.; Petropoulos, S.A. The Effects of Biostimulant Application on Growth Parameters of Lettuce Plants Grown under Deficit Irrigation Conditions. Biol. Life Sci. Forum 2022, 16, 4. [Google Scholar]
- Al-Gabbiesh, A.; Kleinwächter, M.; Selmar, D. Influencing the contents of secondary metabolites in spice and medicinal plants by deliberately applying drought stress during their cultivation. Jordan J. Biol. Sci. 2015, 147, 1–10. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Stress enhances the synthesis of secondary plant products: The impact of stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef]
- Osuagwu, G.G.E.; Edeoga, H.O.; Osuagwu, A.N. The influence of water stress (drought) on the mineral and vitamin potential of the leaves of Ocimum gratissimum L. Recent Res. Sci. Technol. 2010, 2, 27–33. [Google Scholar]
- Khaled, H.; Fawy, H.A. Effect of different levels of humic acids on the nutrient content, plant growth, and soil properties under conditions of salinity. Soil Water Res. 2011, 6, 21–29. [Google Scholar] [CrossRef]
- Dehsheikh, A.B.; Sourestani, M.M.; Zolfaghari, M.; Enayatizamir, N. Changes in soil microbial activity, essential oil quantity, and quality of Thai basil as response to biofertilizers and humic acid. J. Clean. Prod. 2020, 256, 120439. [Google Scholar] [CrossRef]
- Fattahi, S.; Khodabakhshzadeh, A.; Khazaei, I.; Rostami, G. Effects of biofertilizers on the growth, physiological parameters, and essential oil content of basil (Ocimum basilicum L.). J. BioScience Biotechnol. 2019, 8, 59–67. [Google Scholar]
- Rasouli, F.; Nasiri, Y.; Asadi, M.; Hassanpouraghdam, M.B.; Golestaneh, S.; Pirsarandib, Y. Fertilizer type and humic acid improve the growth responses, nutrient uptake, and essential oil content on Coriandrum sativum L. Sci. Rep. 2022, 12, 1–12. [Google Scholar]
- El-Ghamry, A.M.; Abd El-Hai, K.M.; Ghoneem, K.M. Amino and humic acids promote growth, yield and disease resistance of Faba bean cultivated in clayey soil. Aust. J. Basic Appl. Sci. 2009, 3, 731–739. [Google Scholar]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Basciftci, Z.B.; Olgun, M.; Yalcin, M.; Korkmazer, H.; Arpacioglu, N.G.A.; Aydin, D.; Sezer, O.; Ardic, M.; Erkara, I.P.; Koyuncu, O. Long-Term Analysis On Climate-Drought-Yield Relationship; Eskisehir Case Study. J. Appl. Biol. Sci. 2021, 15, 20–36. [Google Scholar]
- Youssef, M.A.; AL-Huqail, A.A.; Ali, E.F.; Majrashi, A. Organic Amendment and Mulching Enhanced the Growth and Fruit Quality of Squash Plants (Cucurbita pepo L.) Grown on Silty Loam Soils. Horticulturae 2021, 7, 269. [Google Scholar] [CrossRef]
- Alakashy, Z.H.; Al-Bedairi, N.A. Effect of three varieties of basil and the nitrogen source in essential oil contents. Euphrates J. Agric. Sci. 2020, 12, 1–6. [Google Scholar]
- Pandey, V.; Patel, A.; Patra, D.D. Integrated nutrient regimes ameliorate crop productivity, nutritive value, antioxidant activity and volatiles in basil (Ocimum basilicum L.). Ind. Crops Prod. 2009, 87, 124–131. [Google Scholar] [CrossRef]
- Rowell, D.R. Soil Science: Methods and Applications; Longman: Harlow, UK, 1996. [Google Scholar]
- Walkley, A.; Black, L.A. An examination of the Degtjareff method for determining soil organic metter and a proposed madification of the chramic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Olsen, S.R.; Dean, L.A. Phosphorus. In Methods of Soil Analysis, 1st ed.; Black, C.A., Ed.; Part 2. Chemical and Microbiological Properties; Agronomy Series No. 9 (Part 2); American Society of Agronomy, Inc.: Madison, WI, USA, 1965; pp. 1035–1049. [Google Scholar]
- Lindsay, W.L.; Norwell, W.A. Development of a DTPA soil test for Zn, Fe, Mn and Cd. J. Soil Sci. Soc. Am. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Bauder, T.A.; Waskom, R.M.; Davis, J.G. Irrigation Water Quality Criteria; No. 0.506; Colorado State University Extension Factsheet: Fort Collins, CO, USA, 2014. [Google Scholar]
- Zheljazkov, V.D.; Callahan, A.; Cantrell, C.L. Yield and oil composition of 38 basil (Ocimum basilicum L.) accessions grown in Mississippi. J. Agric. Food Chem. 2007, 56, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, N.; Kravchik, M.; Dudai, N. Salinity-induced changes in essential oil, pigments and salts accumulation in sweet basil (Ocimum basilicum) in relation to alterations of morphological development. Ann. Appl. Biol. 2010, 156, 167–177. [Google Scholar] [CrossRef]
- Enteshari, S.; Hajbagheri, S. Effects of mycorrhizal fungi on some physiological characteristics of salt stressed Ocimum basilicum L. Iran. J. Plant Physiol. 2011, 1, 215–222. [Google Scholar]
- Anonymous. TKI Humas. Available online: https://tkihumas.tki.gov.tr/ (accessed on 13 February 2023).
- Gulmezoglu, N.; İzci, E. Ionic responses of bean (Phaseolus vulgaris L.) plants under salinity stress and humic acid applications. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1317–1331. [Google Scholar] [CrossRef]
- Telci, İ. Determination of suitable harvesting height in basil (Ocimum basilicum L.) genotypes. J. Agric. Fac. Gaziosmanpasa Univ. 2005, 22, 77–83. [Google Scholar]
- Marotti, M.; Piccaglia, R.; Giovanelli, E. Differences in essential oil composition of basil (Ocimum basilicum L.) Italian cultivars related to morphological characteristics. J. Agric. Food Chem. 1996, 44, 3926–3929. [Google Scholar] [CrossRef]
- Wichtel, M. Die Pharmakognostich-Chemische Analyse; Band 12; Akademische Verlagsgesselschaft: Frankfurt am Main, Germany, 1971; p. 12. [Google Scholar]
- Aytaç, Z.; Gülbandılar, A.; Kürkçüoğlu, M. Humic Acid Improves Plant Yield, Antimicrobial Activity and Essential Oil Composition of Oregano (Origanum vulgare L. subsp. hirtum (Link.) Ietswaart). Agronomy 2022, 12, 2086. [Google Scholar] [CrossRef]
- Brennan, R.F.; Bolland, M.D.A. Comparing the nitrogen and potassium requirements of canola and wheat for yield and grain quality. J. Plant Nutr. 2009, 32, 2008–2026. [Google Scholar] [CrossRef]






| PH | NB | FHY | DHY | DLY | CV | EOR | EOY | PR | |
|---|---|---|---|---|---|---|---|---|---|
| IRL 1 | |||||||||
| IRL 100 | 34.79 a # | 11.41 | 7625.1 a | 1124.2 a | 652.2 a | 46.83 | 0.48 b | 3.1 c | 22.64 ab |
| IRL 75 | 35.46 a | 10.60 | 7605.9 a | 1111.5 a | 657.8 a | 46.72 | 0.54 b | 3.5 ab | 23.20 a |
| IRL 50 | 32.85 ab | 10.01 | 6944.4 b | 927.8 b | 586.9 b | 44.45 | 0.56 b | 3.2 bc | 21.52 c |
| IRL 25 | 31.78 b | 9.22 | 6363.5 b | 847.6 b | 567.5 b | 45.65 | 0.66 a | 3.7 a | 21.86 bc |
| HAD 2 | |||||||||
| HA 0 | 34.29 | 9.77 | 6852.6 c | 966.2 | 587.0 b | 46.13 | 0.56 | 3.3 | 21.91 |
| HA 10 | 33.82 | 10.11 | 6945.3 bc | 985.6 | 604.5 ab | 45.13 | 0.55 | 3.3 | 22.21 |
| HA 20 | 33.43 | 10.35 | 7268.3 ab | 1015.8 | 635.3 a | 46.46 | 0.58 | 3.6 | 22.40 |
| HA 40 | 33.35 | 11.00 | 7472.7 a | 1043.4 | 637.5 a | 45.93 | 0.54 | 3.4 | 22.69 |
| Means | 33.72 | 10.31 | 7134.72 | 1002.7 | 616.1 | 45.9 | 0.56 | 3.4 | 22.30 |
| IRL | ** | ns | ** | ** | * | ns | ** | * | ** |
| HAD | ns 3 | ns | * | ns | * | ns | ns | ns | ns |
| HAD × IRL | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Traits | PH | NB | FHY | DHY | DLY | CV | EOR | EOY | PR |
|---|---|---|---|---|---|---|---|---|---|
| IRL 1 | |||||||||
| IRL 100 | 34.07 a # | 9.39 a | 7139.3 a | 979.1 a | 566.2 a | 46.17 a | 0.60 c | 3.2 | 21.81 a |
| IRL 75 | 34.94 a | 8.94 ab | 7064.8 ab | 967.0 a | 565.0 a | 46.07 a | 0.61 bc | 3.5 | 21.04 ab |
| IRL 50 | 33.48 ab | 8.34 b | 6274.0 bc | 816.2 b | 447.5 b | 45.19 ab | 0.70 ab | 3.2 | 20.21 bc |
| IRL 25 | 30.62 b | 8.22 b | 5999.7 c | 763.3 b | 420.4 b | 44.43 b | 0.75 a | 3.2 | 19.24 c |
| HAD 2 | |||||||||
| HA 0 | 33.18 | 8.69 | 6511.6 | 868.8 | 501.2 | 44.26 | 0.69 | 3.3 ab | 20.58 |
| HA 10 | 32.24 | 8.73 | 6505.6 | 869.4 | 506.0 | 45.92 | 0.70 | 3.5 a | 20.71 |
| HA 20 | 34.16 | 8.93 | 6943.4 | 928.7 | 528.6 | 45.50 | 0.63 | 3.3 ab | 20.49 |
| HA 40 | 33.53 | 8.55 | 6517.1 | 858.9 | 463.3 | 46.18 | 0.64 | 2.9 b | 20.52 |
| Means | 33.28 | 8.73 | 6619.43 | 881.45 | 499.78 | 45.47 | 0.67 | 3.25 | 20.58 |
| IRL | ** | * | ** | ** | ** | * | * | ns | ** |
| HAD | ns 3 | ns | ns | ns | ns | ns | ns | * | ns |
| HAD × IRL | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| IRL | IRL 100 | IRL 75 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compounds (%) | RRI | 2016 | SE (±) | 2017 | SE (±) | 2016 | SE (±) | 2017 | SE (±) | IM |
| 1,8-cineole | 1213 | 3.83 | 0.94 | 3.98 | 0.36 | 3.15 | 0.64 | 2.75 | 1.24 | tR, |
| (E)-β-ocimene | 1246 | 0.80 | 0.55 | 0.95 | 0.26 | 0.55 | 0.26 | 0.80 | 0.53 | tR, MS |
| camphor | 1532 | 0.21 | 0.02 | 0.65 | 0.41 | 0.30 | 0.12 | 0.33 | 0.26 | tR, MS |
| linalool | 1553 | 57.28 | 4.73 | 57.53 | 5.72 | 56.88 | 9.01 | 59.50 | 5.57 | tR, MS |
| (E)-β-bergamotene | 1594 | 4.08 | 0.78 | 5.48 | 1.58 | 3.25 | 1.19 | 4.50 | 1.18 | MS |
| *α-guaiene elemene | 1597–1607 | 1.73 | 0.35 | 1.45 | 0.34 | 2.18 | 1.09 | 1.83 | 0.80 | MS |
| β -caryophyllene | 1612 | 1.73 | 0.62 | 1.10 | 0.43 | 1.98 | 1.12 | 1.73 | 0.95 | tR, MS |
| germacrene D | 1726 | 2.88 | 0.34 | 2.20 | 0.36 | 3.93 | 1.87 | 2.73 | 1.01 | MS |
| γ-guaiene | 1718 | 1.20 | 0.14 | 1.20 | 0.26 | 1.63 | 0.92 | 1.50 | 0.62 | MS |
| bicyclogermacrene | 1755 | 1.18 | 0.10 | 0.88 | 0.17 | 1.10 | 0.27 | 1.08 | 0.45 | tR, MS |
| γ-cadinene | 1776 | 1.70 | 0.22 | 1.88 | 0.55 | 1.68 | 0.36 | 1.83 | 0.72 | MS |
| (Z)-methyl. cinnamate | 1980 | 2.03 | 0.10 | - | - | 2.05 | 0.39 | - | - | MS |
| (E)-methyl cinnamate | 2100 | 9.53 | 2.18 | - | - | 9.13 | 1.52 | - | - | MS |
| cubenole | 2080 | 0.60 | 0.08 | 0.80 | 0.08 | 0.58 | 0.10 | 0.75 | 0.12 | MS |
| eugenol | 2186 | 3.83 | 0.68 | 9.90 | 1.36 | 2.53 | 0.42 | 9.48 | 1.52 | tR, MS |
| T-cadinol | 2187 | 4.83 | 0.28 | 5.00 | 1.61 | 4.70 | 0.74 | 4.88 | 1.61 | MS |
| OMH | 61.31 | 62.16 | 60.33 | 62.58 | ||||||
| MH | 0.80 | 0,95 | 0.55 | 0,80 | ||||||
| SH | 14.48 | 14.19 | 15.75 | 15.20 | ||||||
| OSH | 5.43 | 5.80 | 5.28 | 5.63 | ||||||
| O | 15.39 | 9.90 | 13.71 | 9.48 | ||||||
| Total | 97.40 | 93.00 | 95.62 | 93.69 | ||||||
| IRL | IRL 50 | IRL 25 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compounds (%) | RRI | 2016 | SE (±) | 2017 | SE (±) | 2016 | SE (±) | 2017 | SE (±) | IM |
| 1,8-cineole | 1213 | 3.40 | 0.86 | 3.58 | 0.34 | 2.58 | 0.80 | 2.68 | 0.6 | tR, MS |
| (E)-β-ocimene | 1246 | 0.63 | 0.21 | 1.00 | 0.42 | 0.55 | 0.13 | 0.90 | 0.41 | tR, MS |
| camphor | 1532 | 0.30 | 0.14 | 0.68 | 0.41 | 0.23 | 0.10 | 0.55 | 0.13 | tR, MS |
| linalool | 1553 | 49.55 | 6.41 | 54.95 | 4.02 | 53.05 | 4.88 | 56.70 | 4.19 | tR, MS |
| (E)-β-bergamotene | 1594 | 2.88 | 0.96 | 4.48 | 0.55 | 2.55 | 0.44 | 5.85 | 1.36 | MS |
| *α-guaiene/elemene | 1597–1607 | 1.25 | 0.24 | 1.35 | 0.37 | 1.45 | 0.58 | 1.83 | 0.43 | MS |
| β -caryophyllene | 1612 | 1.40 | 0.72 | 1.78 | 1.65 | 1.20 | 0.78 | 1.28 | 0.38 | tR, MS |
| germacrene D | 1726 | 2.35 | 0.50 | 2.28 | 0.62 | 2.40 | 0.94 | 3.13 | 0.90 | MS |
| γ-guaiene | 1718 | 0.88 | 0.15 | 1.18 | 0.34 | 1.15 | 0.48 | 1.60 | 0.44 | MS |
| bicyclogermacrene | 1755 | 0.78 | 0.15 | 1.08 | 0.48 | 0.88 | 0.10 | 1.13 | 0.22 | tR, MS |
| γ-cadinene | 1776 | 1.30 | 0.08 | 1.65 | 0.19 | 1.25 | 0.24 | 2.28 | 0.42 | MS |
| (Z)-methyl cinnamate | 1980 | 1.70 | 0.18 | - | - | 3.03 | 0.41 | - | - | MS |
| (E)-methyl cinnamate | 2100 | 14.75 | 2.63 | - | - | 13.63 | 3.80 | - | - | MS |
| cubenole | 2080 | 0.53 | 0.05 | 1.05 | 0.52 | 0.48 | 0.05 | 0.83 | 0.43 | MS |
| eugenol | 2186 | 4.78 | 0.22 | 10.80 | 1.21 | 2.98 | 1.72 | 8.48 | 3.37 | tR, MS |
| T-cadinol | 2187 | 3.98 | 0.53 | 4.38 | 0.29 | 3.65 | 0.64 | 5.60 | 1.12 | MS |
| OMH | 53.25 | 59.20 | 55.85 | 59.93 | ||||||
| MH | 0.63 | 1.00 | 0.55 | 0.90 | ||||||
| SH | 10.83 | 13.78 | 10.88 | 17.08 | ||||||
| OSH | 4.50 | 5.43 | 4.13 | 6.43 | ||||||
| O | 21,23 | 10.80 | 19,63 | 8.48 | ||||||
| Total | 90.42 | 90.20 | 91.03 | 92.81 | ||||||
| Soil Mulching Plots | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Traits | PH | NB | FHY | DHY | DLY | CA | EOR | EOY | PR |
| IRL 1 HAD 2 | ** * | * ns 3 | ** * | ns ns | * ns | ns * | ns ns | * ns | ns ns |
| IRL × HAD | * | ns | * | ns | ns | ns | ns | ns | ** |
| control plots | |||||||||
| IRL HAD | ** ns | ns ns | ** * | ** ns | * * | ns ns | ** ns | * ns | * ns |
| IRL × HAD | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| PH | NB | FHY | DHY | DLY | CV | EOR | EOY | PR | |
|---|---|---|---|---|---|---|---|---|---|
| CP 2016 | 33.72 | 10.31 | 7134.7 | 1002.7 | 616.1 | 45.9 | 0.56 | 3.4 | 22.30 |
| SM 2016 | 37.88 | 10.65 | 8249.2 | 1177.6 | 831.2 | 44.4 | 0.48 | 3.9 | 22.56 |
| CP 2017 | 33.28 | 8.73 | 6619.4 | 881.4 | 499.8 | 45.5 | 0.67 | 3.25 | 20.58 |
| IRL | IRL 100 | IRL 75 | IRL 50 | IRL 25 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAD (L HA ha−1) | 0 | 10 | 20 | 40 | 0 | 10 | 20 | 40 | 0 | 10 | 20 | 40 | 0 | 10 | 20 | 40 |
| Compounds (%) | ||||||||||||||||
| 1,8-cineole | 2.5 | 4.5 | 3.8 | 4.5 | 2.8 | 2.9 | 2.8 | 4.1 | 3.4 | 3.0 | 4.6 | 2.6 | 3.2 | 3.3 | 2.1 | 1.7 |
| (E)-β-ocimene | 0.4 | 0.7 | 1.6 | 0.5 | 0.7 | 0.2 | 0.5 | 0.8 | 0.4 | 0.5 | 0.8 | 0.8 | 0.4 | 0.7 | 0.6 | 0.5 |
| camphor | 0.2 | 0.2 | 0.2 | 0.25 | 0.4 | 0.2 | 0.4 | 0.2 | 0.5 | 0.2 | 0.3 | 0.2 | 0.2 | 0.3 | 0.3 | 0.1 |
| linalool | 50.6 | 60.2 | 57.3 | 61.0 | 52.8 | 471 | 59.6 | 68.0 | 58.1 | 50.8 | 45.2 | 44.1 | 56.7 | 57.7 | 49.9 | 47.9 |
| (E)-β-bergamotene | 3.1 | 4.1 | 4.1 | 5.0 | 3.0 | 2.4 | 5.0 | 2.6 | 3.6 | 3.8 | 2.0 | 2.1 | 2.3 | 3.2 | 2.3 | 2.4 |
| *α-guaiene/ β-elemene | 1.3 | 1.9 | 2.1 | 1.6 | 1.6 | 3.8 | 1.5 | 1.8 | 1.1 | 1.0 | 1.5 | 1.4 | 1.9 | 2.0 | 1.0 | 0.9 |
| Β-caryophyllene | 0.9 | 1.9 | 1.7 | 2.4 | 1.0 | 3.3 | 1.1 | 2.5 | 0.9 | 0.7 | 2.2 | 1.8 | 2.1 | 1.6 | 0.6 | 0.5 |
| germacrene D | 2.4 | 3.0 | 2.9 | 3.2 | 2.7 | 6.7 | 2.9 | 3.4 | 2.3 | 1.7 | 2.9 | 2.5 | 3.1 | 3.3 | 1.8 | 1.4 |
| γ-guaiene | 1.0 | 1.3 | 1.2 | 1.3 | 1.2 | 3.0 | 1.1 | 1.2 | 0.7 | 0.8 | 1.0 | 1.0 | 1.4 | 1.7 | 0.8 | 0.7 |
| bicyclogermacrene | 1.1 | 13 | 1.2 | 1.1 | 1.0 | 1.5 | 0.9 | 1.0 | 0.6 | 0.7 | 0.9 | 0.9 | 1.0 | 0.9 | 0.8 | 0.8 |
| γ-cadinene | 1.4 | 1.8 | 1.7 | 1.9 | 1.6 | 2.2 | 1.5 | 1.4 | 1.4 | 1.3 | 1.3 | 1.2 | 1.4 | 1.5 | 1.1 | 1.0 |
| (Z)-methyl. cinnamate | 2.1 | 2.0 | 1.9 | 2.1 | 1.7 | 2 | 2.6 | 1.9 | 1.8 | 1.5 | 1.6 | 1.9 | 2.9 | 2.5 | 3.3 | 3.4 |
| cubenole | 0.5 | 0.6 | 0.6 | 0.7 | 0.5 | 0.7 | 0.6 | 0.5 | 0.6 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.4 | 0.5 |
| (E)-methyl. cinnamate | 10.5 | 8.6 | 12.0 | 7.0 | 9.1 | 10 | 10.4 | 7 | 11.0 | 15.0 | 12 | 10 | 10.3 | 10.4 | 16.4 | 17.4 |
| eugenol | 3.4 | 4.7 | 4 | 3.2 | 2.4 | 2.5 | 2.1 | 3.1 | 4.7 | 5.0 | 4.5 | 4.9 | 4.9 | 3.7 | 2.4 | 0.9 |
| T-cadinol | 4.5 | 4.7 | 5 | 5.1 | 4.3 | 5.8 | 4.5 | 4.2 | 4.7 | 3.7 | 3.5 | 4.0 | 3.4 | 4.6 | 3.2 | 3.4 |
| IRL | IRL 100 | IRL 75 | IRL50 | IRL25 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAD (L HA ha−1) | 0 | 10 | 20 | 40 | 0 | 10 | 20 | 40 | 0 | 10 | 20 | 40 | 0 | 10 | 20 | 40 |
| Compounds (%) | ||||||||||||||||
| 1.8-cineole | 2.8 | 3.8 | 4.4 | 2.9 | 2.5 | 3.0 | 2.7 | 1.6 | 3.5 | 3.6 | 2.7 | 3.8 | 2.3 | 4.0 | 2.5 | 3.9 |
| linalool | 47.7 | 46.8 | 60.5 | 49.5 | 47.5 | 60.5 | 54.3 | 42.3 | 60.7 | 44.7 | 42.5 | 62.1 | 59.3 | 54.5 | 53.5 | 61.6 |
| (E)-β-bergamotene | 3.1 | 4.6 | 4.8 | 5.1 | 3.9 | 4.2 | 4.0 | 3.0 | 5.2 | 3.1 | 1.4 | 5.4 | 3.6 | 3.8 | 4.4 | 5.1 |
| *α-guaiene/ β-elemene | 1.1 | 1.6 | 1.3 | 1.1 | 0.9 | 1.3 | 1.5 | 0.8 | 1.7 | 1.2 | 1.0 | 1.8 | 1.2 | 0.8 | 1.6 | 1.3 |
| Β-caryophyllene | 1.0 | 1.8 | 0.8 | 0.8 | 0.6 | 1.1 | 1.5 | 0.5 | 1.2 | 0.8 | 0.6 | 1.4 | 0.8 | 0.7 | 0.9 | 0.9 |
| germacrene D | 2.3 | 2.7 | 2.6 | 1.9 | 1.5 | 3.1 | 2.7 | 1.6 | 3.4 | 2.2 | 1.7 | 3.3 | 2.1 | 1.6 | 2.3 | 2.3 |
| γ-guaiene | 0.9 | 1.8 | 1.1 | 1.0 | 0.7 | 1.1 | 1.1 | 0.7 | 1.5 | 1.0 | 1.3 | 1.7 | 1.8 | 0.6 | 1.1 | 1.0 |
| bicyclogermacrene | 0.6 | 0.7 | 0.9 | 0.8 | 0.7 | 1.1 | 0.9 | 0.7 | 1.3 | 1.0 | 0.6 | 1.3 | 1.2 | 0.6 | 1.0 | 0.9 |
| γ-cadinene | 1.2 | 1.8 | 1.5 | 1.6 | 1.4 | 1.6 | 1.7 | 1.1 | 1.8 | 1.5 | 0.9 | 2.1 | 1.4 | 1.1 | 1.6 | 1.6 |
| (Z)-methyl. cinnamate | 2.7 | 1.7 | 1.0 | 1.5 | 3.0 | 2.1 | 1.4 | 3.3 | 0.2 | 1.9 | 4.4 | 2.7 | - | 2.1 | 1.9 | 1.5 |
| cubenole | 0.5 | 0.9 | 0.5 | 0.6 | 0.6 | 0.5 | 0.6 | 0.6 | 0.6 | 0.5 | 0.6 | 0.5 | 0.6 | 0.7 | 0.5 | 0.7 |
| (E)-methyl. cinnamate | 27.2 | 4.0 | 9.5 | 14.9 | 28.2 | 7.6 | 12.0 | 34.4 | 2.7 | 15.3 | 35.4 | 0.2 | - | 17.2 | 17.7 | 5.6 |
| eugenol | 2.4 | 6.8 | 3.1 | 3.0 | 1.1 | 3.3 | 3.8 | 1.9 | 3.7 | 3.8 | 4.4 | 5.2 | 2.1 | 2.0 | 3.1 | 3.9 |
| T-cadinol | 3.2 | 5.6 | 4.3 | 3.9 | 3.4 | 4.7 | 4.4 | 3.0 | 4.7 | 4.4 | 3.9 | 5.0 | 3.6 | 2.2 | 4.2 | 4.3 |
| Rainfall (mm) | Humidity (%) | Temperature (°C) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | Long Term | 2016 | 2017 | Long Term | 2016 | 2017 | Long Term | |
| March | 40.6 | 16.2 | 30.3 | 70.3 | 68.7 | 65.1 | 7.5 | 7.6 | 5.1 |
| April | 30.6 | 62.0 | 42.2 | 64.5 | 66.9 | 62.7 | 12.9 | 9.6 | 9.9 |
| May | 44.4 | 50.8 | 41.6 | 74.2 | 73.0 | 60.8 | 14.1 | 14.4 | 15.0 |
| June | 7.0 | 44.8 | 31.1 | 62.1 | 73.4 | 57.2 | 21.0 | 19.1 | 19.2 |
| July | 12.0 | 13.4 | 12.4 | 58.3 | 59.5 | 53.0 | 22.8 | 23.1 | 22.2 |
| August | 26.4 | 31.4 | 13.0 | 66.0 | 67.3 | 54.6 | 22.8 | 22.0 | 22.0 |
| September | 31.1 | 3.0 | 18.1 | 67.1 | 57.0 | 58.2 | 17.8 | 19.6 | 17.3 |
| Total/mean | 192.1 | 221.6 | 188.7 | 66.0 | 66.5 | 58.8 | 16.9 | 16.4 | 15.8 |
| 2016 | 2017 | |
|---|---|---|
| EC(dS/m) | 0.060 | 0.045 |
| pH | 7.78 | 8.14 |
| CaCO3 (%) | 6.7 | 5.9 |
| Organic matter (%) | 2.80 | 0.31 |
| Available P2O5 (kg ha−1) | 0.736 | 0.542 |
| Available K2O (kg ha−1) | 24.10 | 48.11 |
| Fe (mg kg−1) | 4.87 | 2.82 |
| Zn (mg kg−1) | 0.64 | 0.51 |
| Mn (mg kg−1) | 28.98 | 15.30 |
| Cu (mg kg−1) | 2.45 | 2.38 |
| 2016 | 2017 | |
|---|---|---|
| Cations | meq/lt | meq/lt |
| Sodium | 1.48 | 1.27 |
| Potassium | 0.13 | 0.30 |
| Calcium | 3.54 | 3.33 |
| Magnesium | 4.46 | 5.27 |
| Total | 9.61 | 10.16 |
| Anions | meq/lt | meq/lt |
| Carbonate | 0.00 | 0.00 |
| Bicarbonate | 5.40 | 5.00 |
| Chlorine | 0.20 | 0.20 |
| Sulfate | 4.01 | 4.96 |
| Total | 9.61 | 10.16 |
| pH of water | 7.98 | 7.83 |
| EC (dS/m at 25 °C) | 0.803 | 0.836 |
| Hardness (French hardness) | 0.74 | 0.61 |
| Properties | Unit | Values |
|---|---|---|
| pH | - | 11.8 |
| EC | (dS m−1) | 5.6 |
| HA + FA | (%) | 12.0 |
| Organic Matter | (%) | 5.0 |
| K | (%) | 1.60 |
| Na | (%) | 0.49 |
| N | (%) | 0.23 |
| Ca | (mg kg−1) | 5228 |
| Mg | (mg kg−1) | 680 |
| Fe | (mg kg−1) | 550 |
| P | (mg kg−1) | 72 |
| Cu | (mg kg−1) | 2.4 |
| Mn | (mg kg−1) | 6.2 |
| Zn | (mg kg−1) | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayarer, M.; Aytaç, Z.; Kürkçüoğlu, M. The Effect of Irrigation and Humic Acid on the Plant Yield and Quality of Sweet Basil (Ocimum basilicum L.) with Mulching Application under Semi-Arid Ecological Conditions. Plants 2023, 12, 1522. https://doi.org/10.3390/plants12071522
Sayarer M, Aytaç Z, Kürkçüoğlu M. The Effect of Irrigation and Humic Acid on the Plant Yield and Quality of Sweet Basil (Ocimum basilicum L.) with Mulching Application under Semi-Arid Ecological Conditions. Plants. 2023; 12(7):1522. https://doi.org/10.3390/plants12071522
Chicago/Turabian StyleSayarer, Melike, Zehra Aytaç, and Mine Kürkçüoğlu. 2023. "The Effect of Irrigation and Humic Acid on the Plant Yield and Quality of Sweet Basil (Ocimum basilicum L.) with Mulching Application under Semi-Arid Ecological Conditions" Plants 12, no. 7: 1522. https://doi.org/10.3390/plants12071522
APA StyleSayarer, M., Aytaç, Z., & Kürkçüoğlu, M. (2023). The Effect of Irrigation and Humic Acid on the Plant Yield and Quality of Sweet Basil (Ocimum basilicum L.) with Mulching Application under Semi-Arid Ecological Conditions. Plants, 12(7), 1522. https://doi.org/10.3390/plants12071522





