Early Effects of Mycorrhizal Fungal Inoculum and Fertilizer on Morphological and Physiological Variables of Nursery-Grown Nothofagus alessandrii Plants
Abstract
1. Introduction
2. Results
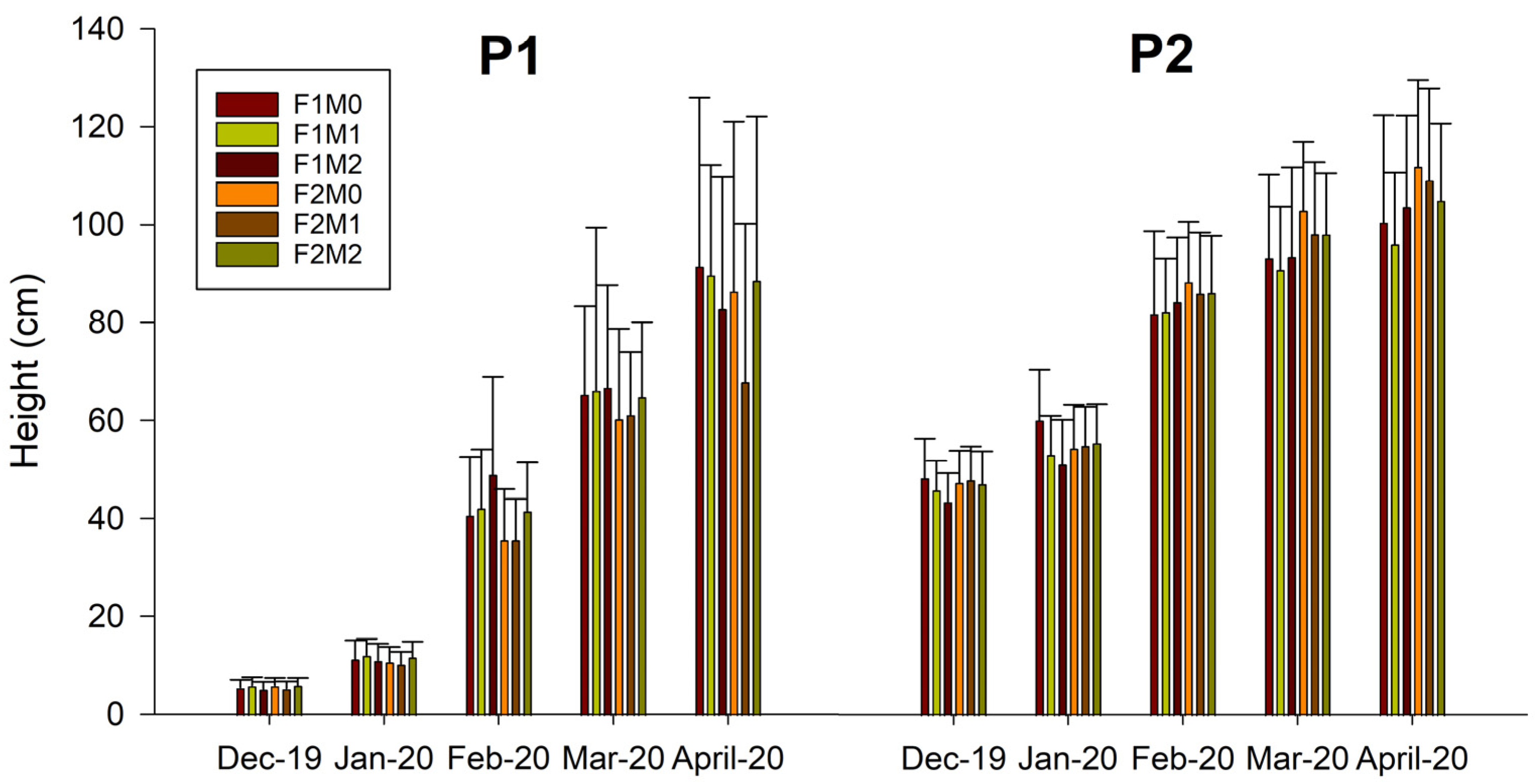
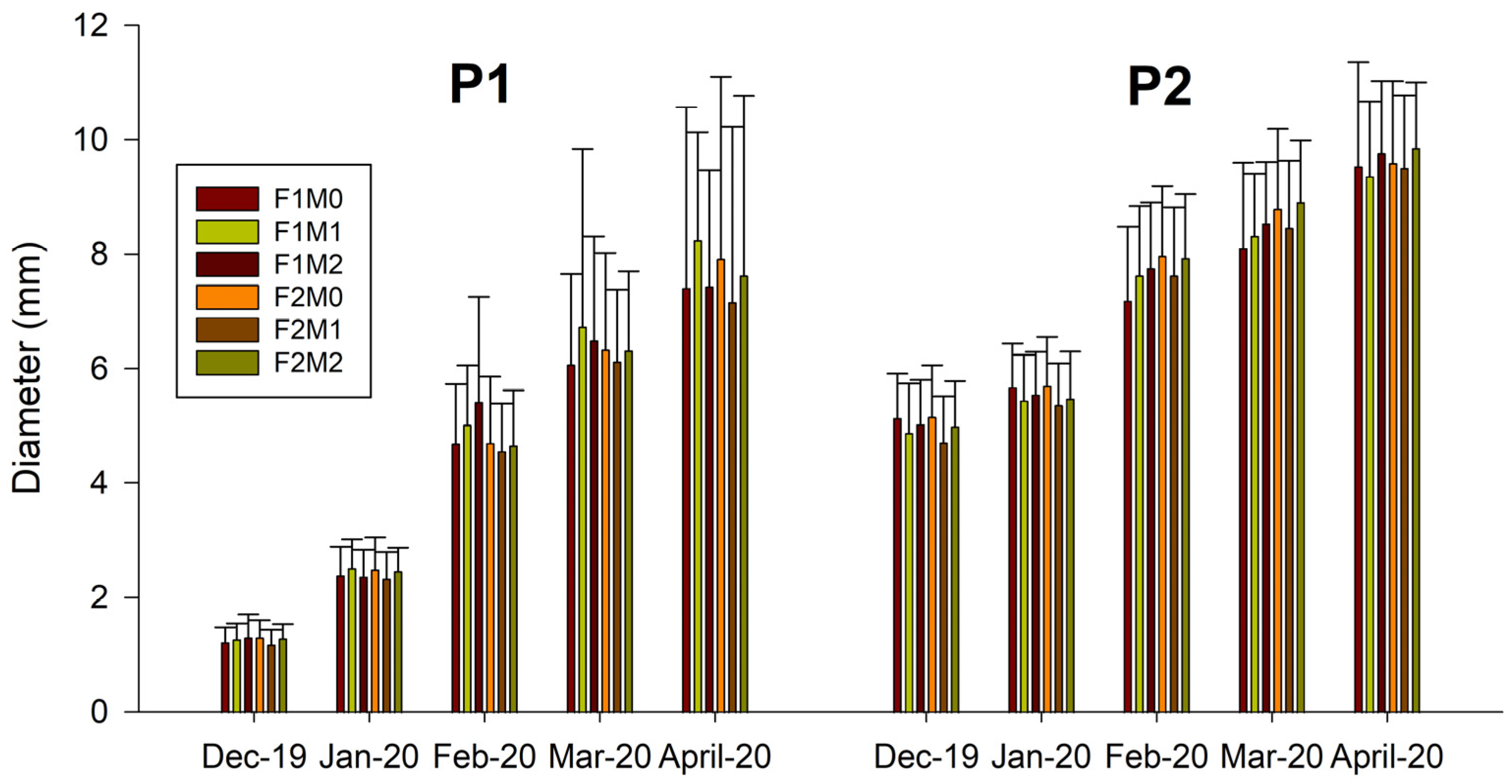
2.1. Morphological Variables
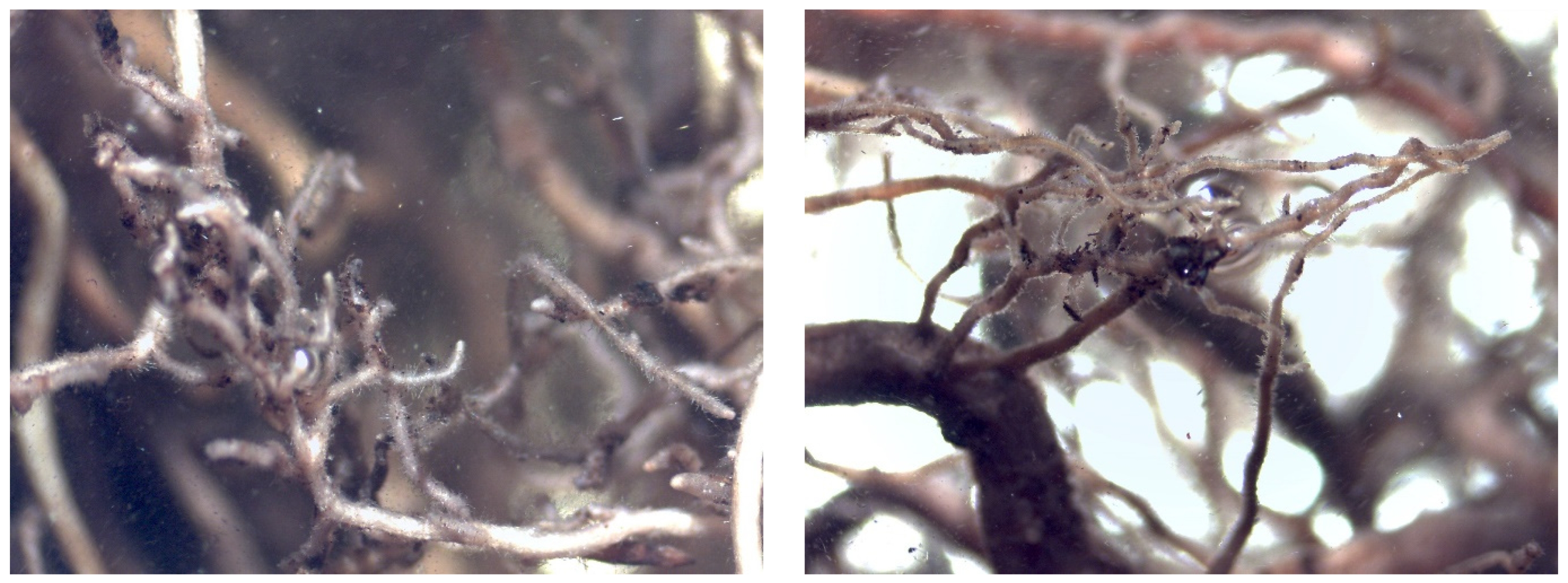
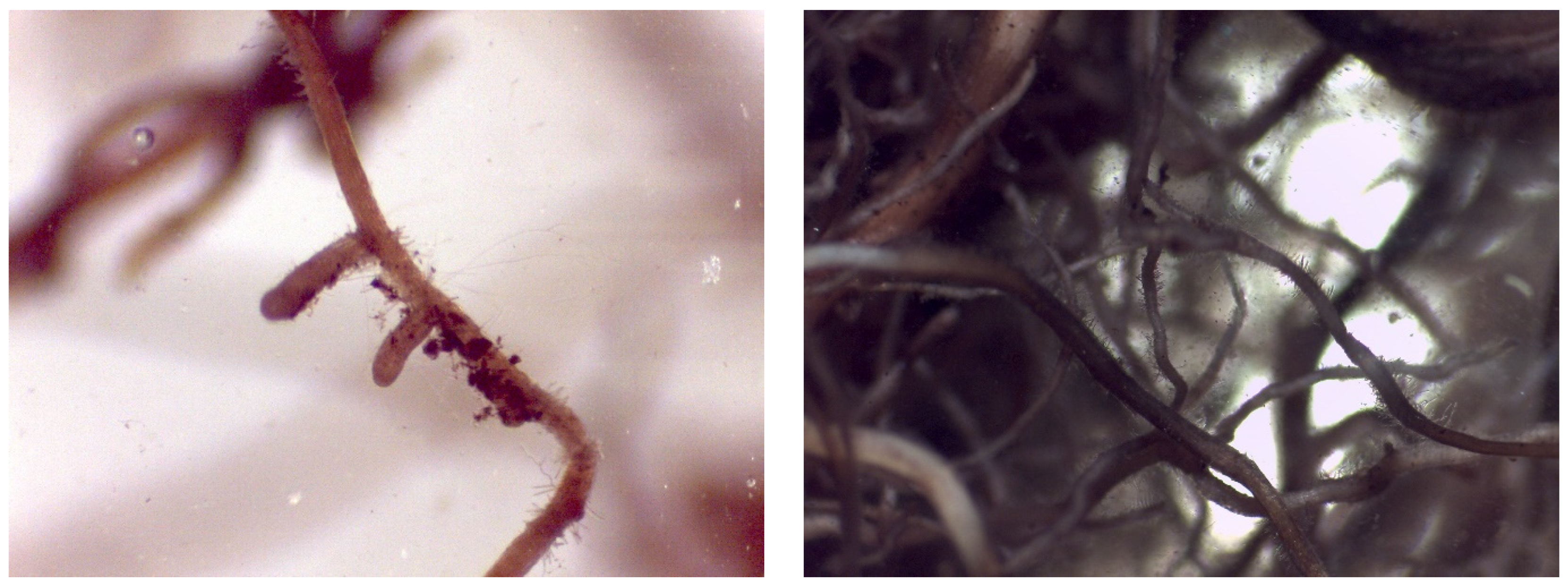
Mycorrhizal Analysis
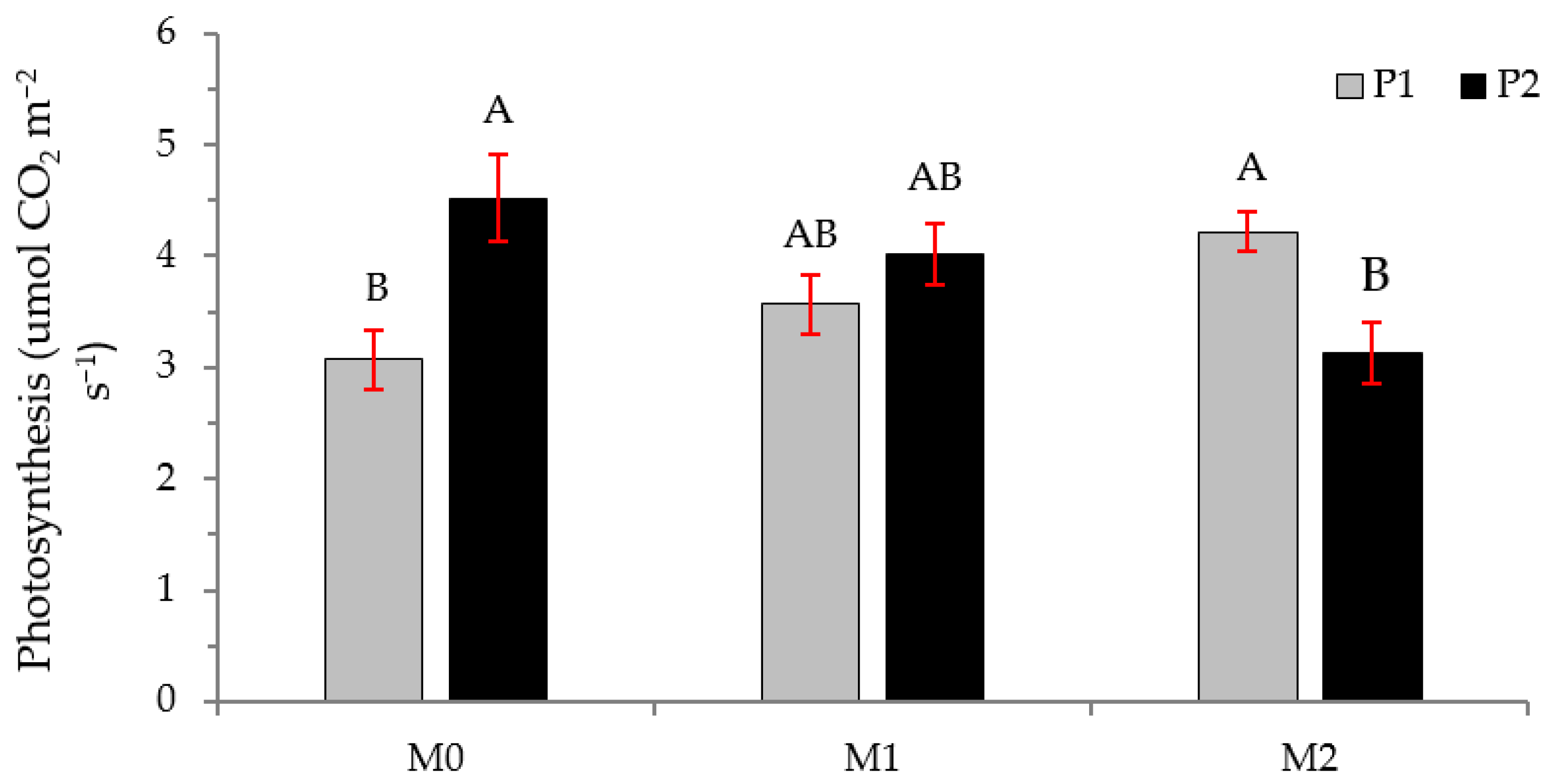
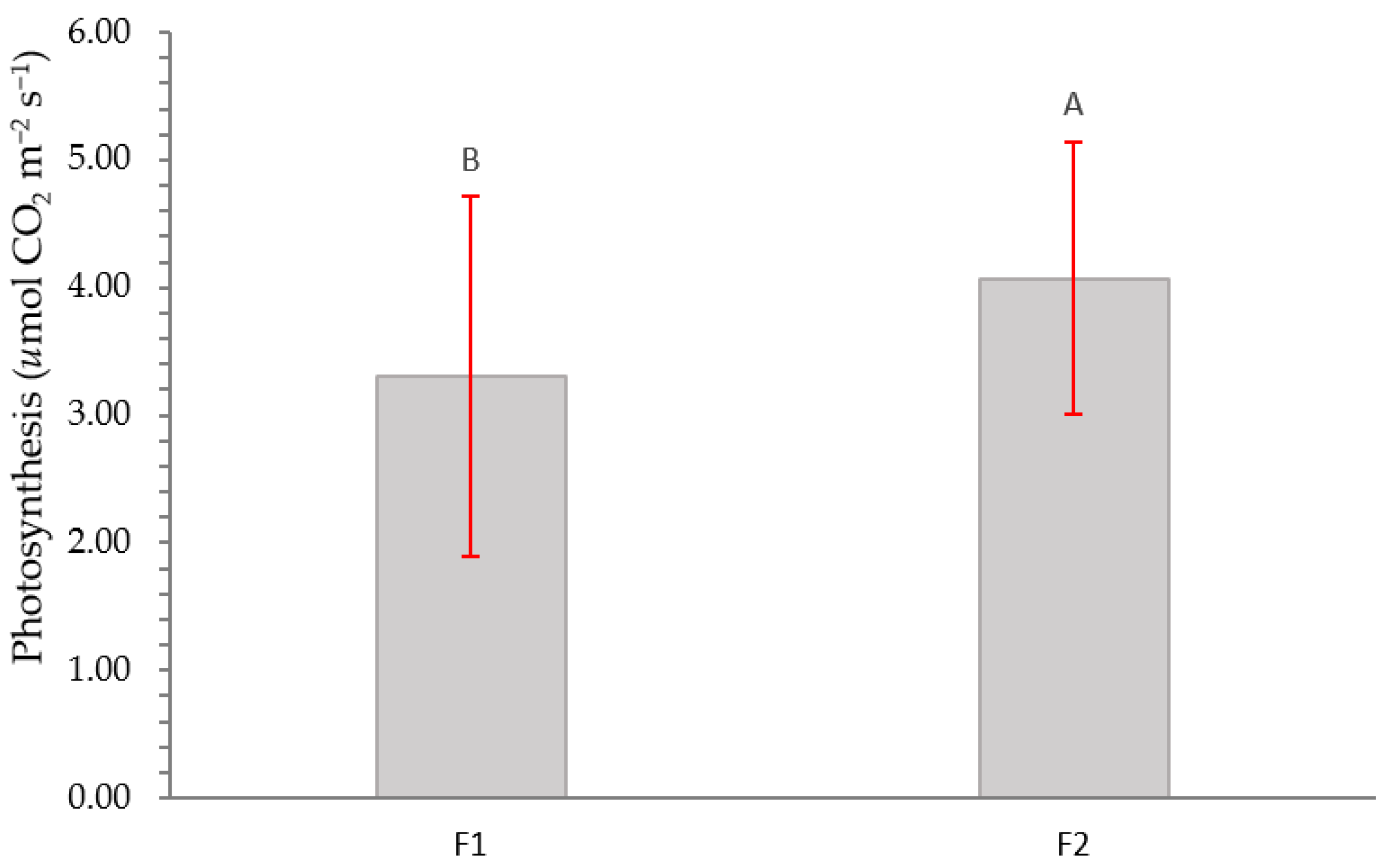
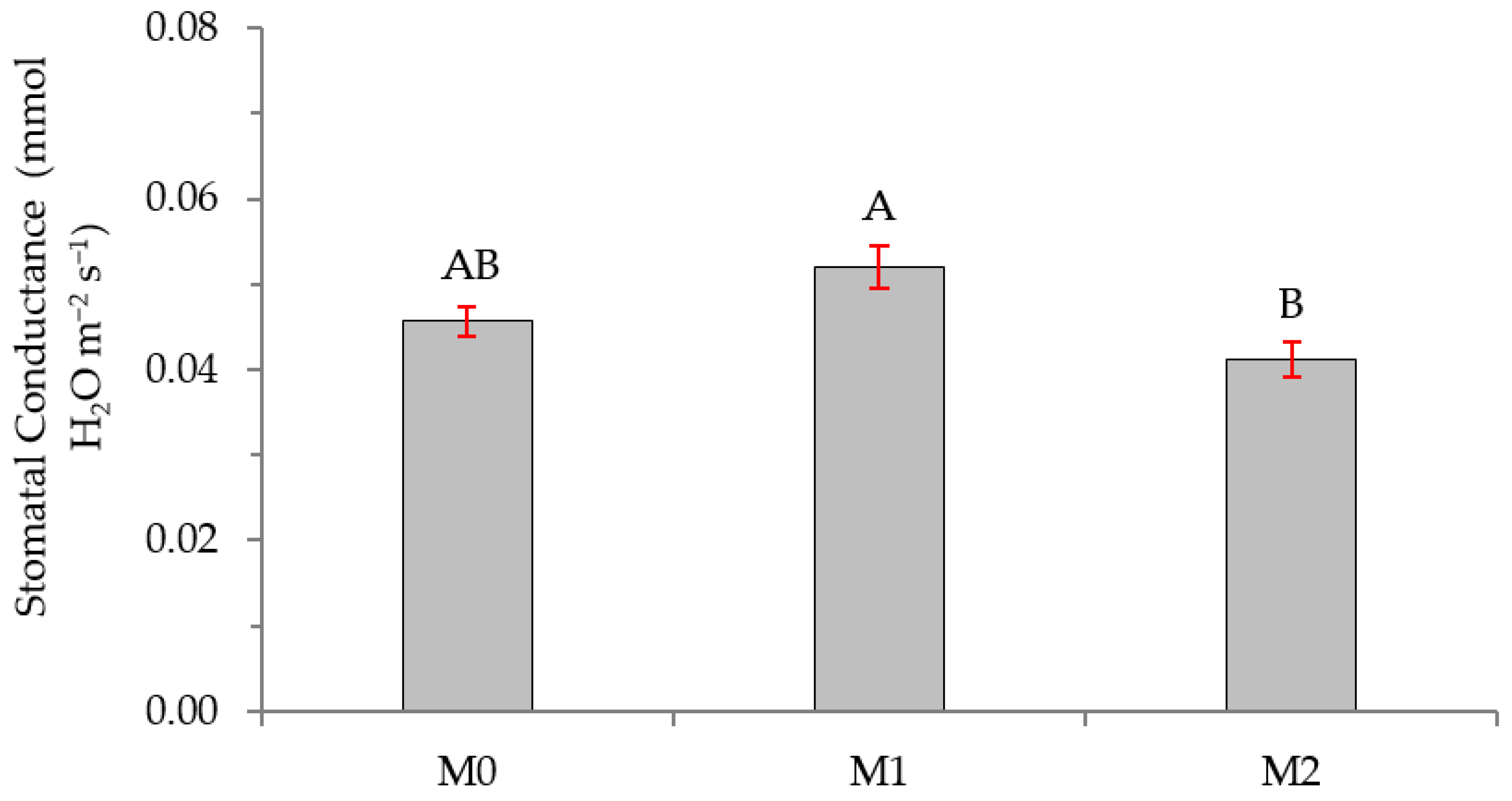
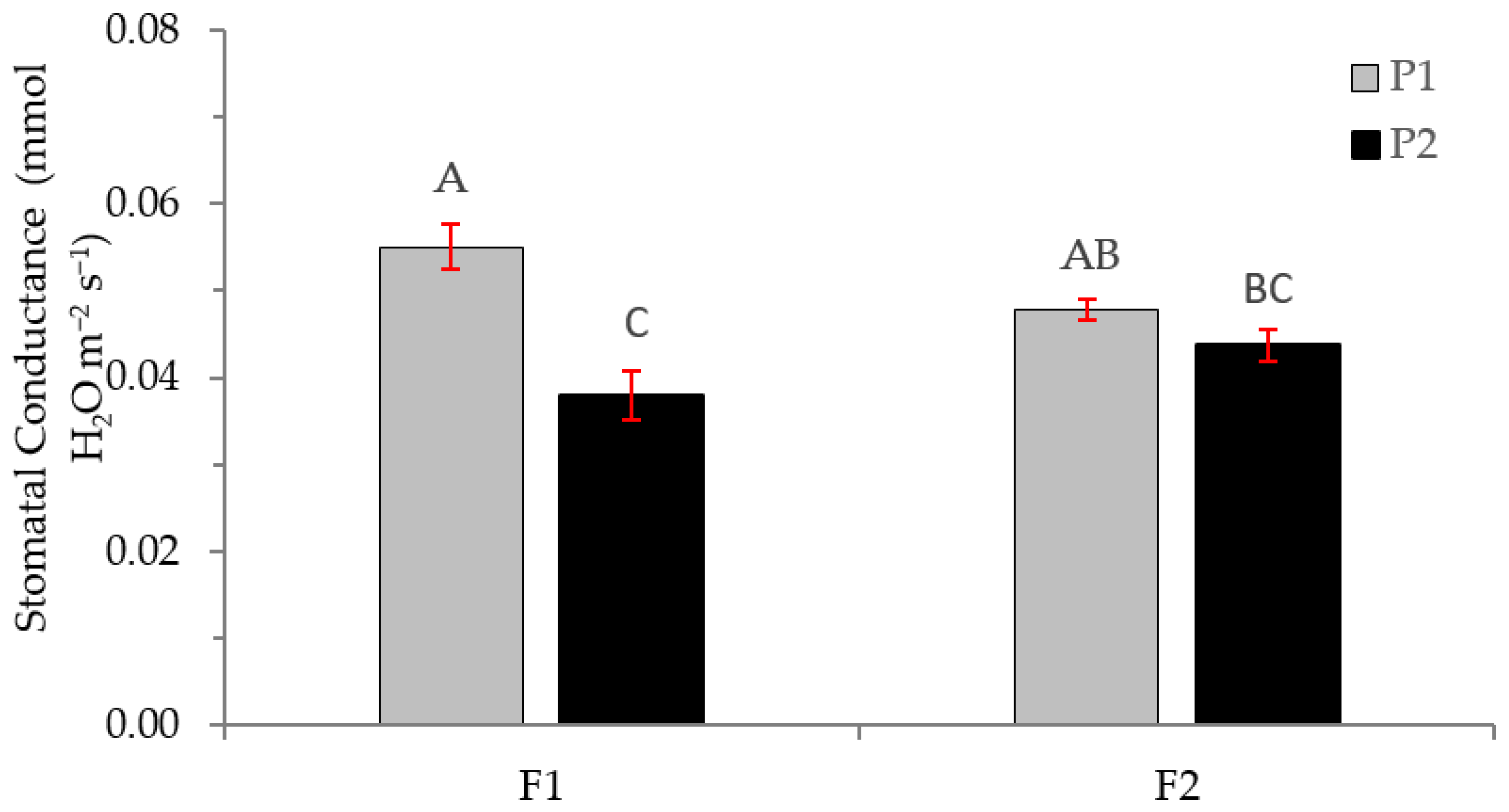
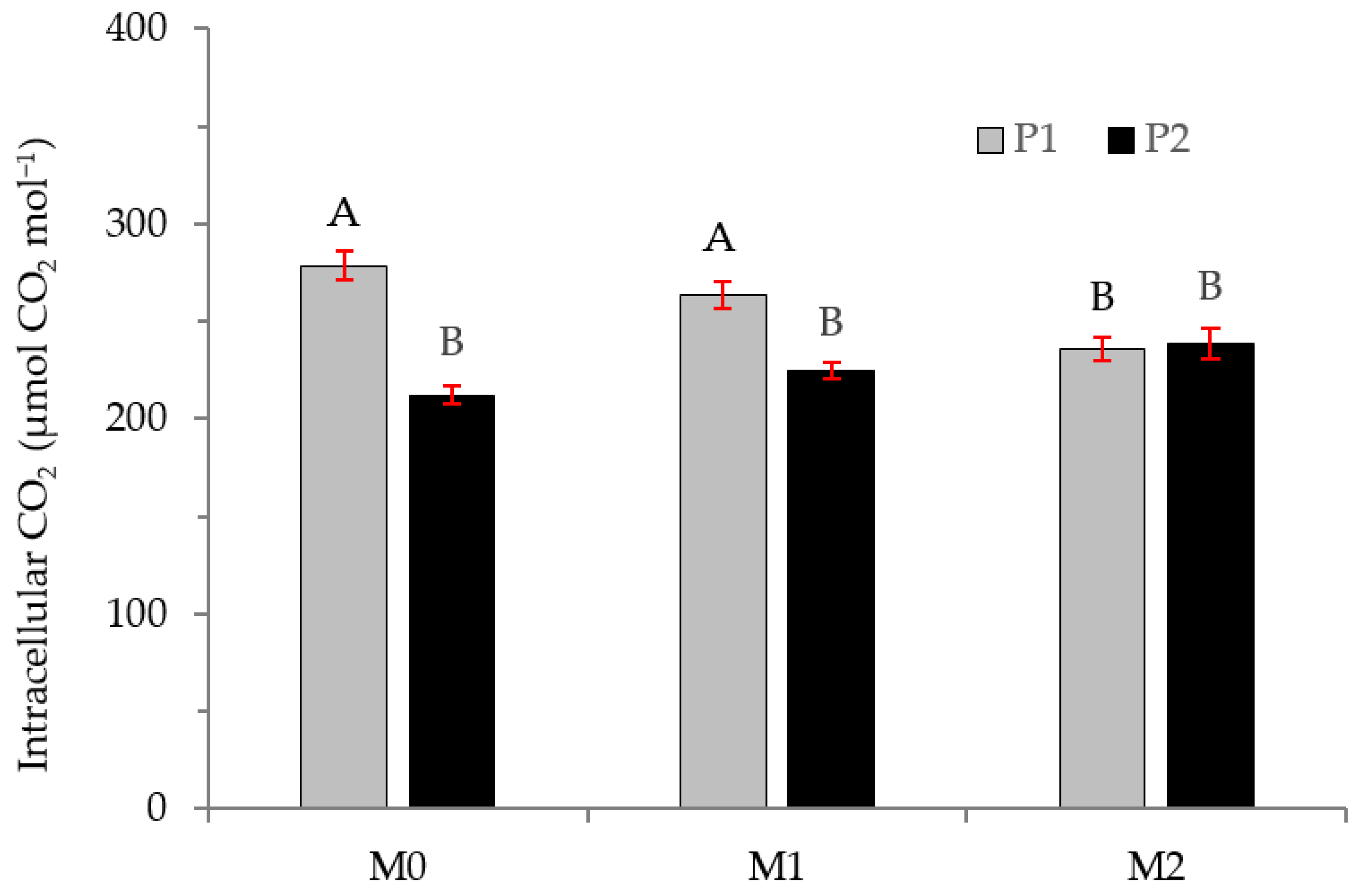
2.2. Physiological Variables
3. Discussion
3.1. Morphological Variables
Application of MFI
3.2. Physiological Variables
4. Materials and Methods
4.1. Plant Production
Fertilization Treatments
4.2. Mycelium Production and Inoculation
4.3. Evaluation of Morphological Variables
4.4. Evaluation of Physiological Variables
4.5. Evaluation of Mycorrhization
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luebert, F.; Pliscoff, P. Sinopsis Bioclimática y Vegetacional de Chile, 2nd ed.; Editorial Universitaria: Santiago de Chile, Chile, 2017; p. 381. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, M.T.K.; Riveros, M.; Peñaloza, A.; Cavieres, L.; Faggi, A.M. Phytogeographic relationships and regional richness patterns of the cool temperate rainforest flora of southern South America. In High-Latitude Rainforests and Associated Ecosystems of the West Coasts of the Americas: Climate, Hydrology, Ecology and Conservation; Lawford, R.G., Alaback, P.B., Fuentes, E., Eds.; Springer: New York, NY, USA, 1996; pp. 134–172. [Google Scholar]
- Gajardo, R. Sistema Básico de Clasificación de la Vegetación Nativa Chilena; Universidad de Chile y Corporación Nacional Forestal (CONAF): Santiago, Chile, 1983; p. 315. [Google Scholar]
- San Martín, J.; Sepúlveda, C. Diagnóstico del estado actual de los fragmentos de Nothofagus alessandrii, ruil, Fagaceae (=Nothofagaceae), de la Región del Maule, Chile Central; Informe técnico. Comisión Nacional del Medioambiente (CONAMA): Talca, Chile, 2002; p. 45. [Google Scholar]
- Olivares, P.; San Martín, J.; Santelices, R. Ruil (Nothofagus alessandrii): Estado del Conocimiento y Desafíos Para su Conservación; Comisión Nacional del Medioambiente (CONAMA): Talca, Chile, 2005; p. 55. [Google Scholar]
- Burgos, A.; Grez, A.A.; Bustamante, R.O. Seed production, pre-dispersal seed predation and germination of Nothofagus glauca (Nothofagaceae) in a temperate fragmented forest in Chile. For. Ecol. Manag. 2008, 255, 1226–1233. [Google Scholar] [CrossRef]
- Serra, M.T.; Gajardo, R.; Cabello, A. Programa de Protección y Recuperación de la Flora Nativa de Chile, Ficha Técnica de Especies Amenazadas: Nothofagus Alessandrii Espinosa “Ruil” (Fagaceae), Especie en Peligro; Corporación Nacional Forestal (CONAF): Santiago, Chile, 1986; p. 32. [Google Scholar]
- Knapp, M.; Stöckler, K.; Havell, D.; Delsuc, F.; Sebastiani, F.; Lockhart, P.J. Relaxed Molecular Clock Provides Evidence for Long-Distance Dispersal of Nothofagus (Southern Beech). PLoS Biol. 2005, 3, e14. [Google Scholar] [CrossRef]
- Santelices, R.; Drake, F.; Mena, C.; Ordenes, R.; Navarro-Cerrillo, R.M. Current and potential distribution areas for Nothofagus alessandrii, an endangered tree species from central Chile. Cienc. Investig. Agrar. 2012, 39, 521–531. [Google Scholar] [CrossRef]
- Benoit, I. Libro Rojo de la Flora Terrestre de Chile; Corporación Nacional Forestal: Santiago, Chile, 1989; p. 157. [Google Scholar]
- Hechenleitner, P.; Gardner, M.; Thomas, P.; Echeverría, C.; Escobar, B.; Brownless, P.; Martínez, C. Plantas Amenazadas del Centro-sur de Chile. Distribución, Conservación y Propagación, Primera Edición ed; Universidad Austral de Chile y Real Jardín Botánico de Edimburgo: Valdivia, Chile, 2005; p. 188. [Google Scholar]
- Barstow, M.; Echeverría, C.; Baldwin, H.; Rivers, M.C. Nothofagus Alessandrii. The IUCN Red List of Threatened Species 2017: E.T32033A2808995. Available online: http://www.iucnredlist.org/details/32033/0 (accessed on 1 March 2018).
- Donoso, C.; Landaeta, E. Ruil (Nothofagus alessandrii), a threatened Chilean tree species. Environ. Conserv. 1983, 10, 159–162. [Google Scholar] [CrossRef]
- Bustamante, R.O.; Castor, C. The decline of an endangered temperate ecosystem: The ruil (Nothofagus alessandrii) forest in central Chile. Biodivers Conserv. 1998, 7, 1607–1626. [Google Scholar] [CrossRef]
- Bustamante, R.; Grez, A. Consecuencias ecológicas de la fragmentación de los bosques nativos. Ambiente y Desarro. 1995, 11, 58–63. [Google Scholar]
- Bustamante, R.; Simonetti, J. Is Pinus radiata invading the native vegetation in Central Chile? Demographic responses in a fragmented forest. Biol. Invasions 2005, 7, 243–249. [Google Scholar] [CrossRef]
- San Martín, J.; Santelices, R.; Henríquez, R. Nothofagus alessandrii Espinosa, Ruil. Familia: Nothofagaceae. In Las Especies Arbóreas de los Bosques Templados de Chile y Argentina: Autoecología; Edición, S., Donoso, C., Eds.; Marisa Cuneo Ediciones: Valdivia, Chile, 2013; pp. 391–401. [Google Scholar]
- González, M.; Becerra-Rodas, C.; Molina, I.; Lara, A.; Little, C. Restauración de bosques de Rui: Línea base, desarrollo e implementación del plan de restauración en predio El Desprecio, Región del Maule. In Los Bosques Relictos de Ruíl: Ecología, Biodiversidad, Conservación y Restauración; San Martín, J., Ed.; INFOR: Talca, Chile, 2022; pp. 461–476. [Google Scholar]
- Quiroz, I. Técnicas de restauración ecológica con ruil. In Los Bosques Relictos de Ruíl: Ecología, Biodiversidad, Conservación y Restauración; San Martín, J., Ed.; INFOR: Talca, Chile, 2022; pp. 477–494. [Google Scholar]
- Acevedo, M.; Alvarez, C.; Cartes, E.; Dumroese, R.K.; Gonzalez, M. Production and establishment techniques for the restoration of Nothofagus alessandrii, an endangered keystone species in a Mediterranean forest. New For. 2020, 51, 159–174. [Google Scholar] [CrossRef]
- Santelices, R.; Navarro-Cerrillo, R.M.; Drake, F. Caracterización del material forestal de reproducción de cinco procedencias de Nothofagus alessandrii Espinosa una especie en peligro de extinción. Interciencia 2009, 34, 113–119. [Google Scholar]
- Santelices, R.; Navarro-Cerrillo, R.M.; Drake, F. Propagation and seedling cultivation of the endemic species Nothofagus alessandrii Espinosa in Central Chile. Restor. Ecol. 2011, 19, 177–185. [Google Scholar] [CrossRef]
- Santelices, R.; Espinoza, S.; Cabrera, A. Effect of four levels of shade on survival, morphology and chlorophyll fluorescence of Nothofagus alessandrii container-grown seedlings. Iforest—Biogeosciences For. 2015, 8, 638–641. [Google Scholar] [CrossRef]
- Pera, J.; Alvarez, I.; Parlade, J. Eficacia del inóculo miceliar de 17 especies de hongos ectomicorrícicos para la micorrización controlada de: Pinus pinaster, Pinus radiata y Pseudotsuga menziessii, en contenedor. Investig. Agraria. Sist. Y Recur. For. 1998, 7, 139–153. [Google Scholar]
- Palacios, Y.M.; Palfner, G.; Hernández, C.E. The ectomycorrhizal community in a chronosequence of Pinus radiata (Pinophyta: Pinaceae) of the transitional Mediterranean-temperate climatic zone of central Chile. Rev. Chil. Hist. Nat. 2012, 85, 61–71. [Google Scholar] [CrossRef]
- Kammerbauer, H.; Agerer, R.; Sandermann, H. Studies on ectomycorrhiza. Trees 1989, 3, 78–84. [Google Scholar] [CrossRef]
- Annesi, T.; Motta, E. Hebeloma inoculation on Norway spruce seedlings in solarized and infested soil. Eur. J. For. Pathol. 1998, 28, 159–166. [Google Scholar] [CrossRef]
- Siemens, J.A.; Calvo-Polanco, M.; Zwiazek, J.J. Hebeloma crustuliniforme facilitates ammonium and nitrate assimilation in trembling aspen (Populus tremuloides) seedlings. Tree Physiol. 2011, 31, 1238–1250. [Google Scholar] [CrossRef]
- van Galen, L.G.; Lord, J.M.; Orlovich, D.A.; Larcombe, M.J. Restoration of southern hemisphere beech (Nothofagaceae) forests: A meta-analysis. Restor. Ecol. 2021, 29, e13333. [Google Scholar] [CrossRef]
- Godoy, R.; Palfner, G. Ectomicorrizas en Nothofagus alpina (Poepp. & Endl.) Oerst. y N. dombeyi (Mirb. Oerst.) del sur de Chile. Boletín Micológico 1997, 12, 55–61. [Google Scholar]
- Palfner, G. Taxonomische Studien an Ektomykorrhizen aus den Nothofagus-Wäldern Mittelsüdchiles; Bibliotheca Mycologica: Berlin, Germany, 2001; p. 243. [Google Scholar]
- Diehl, P. Indicadores de Conservación de Nitrógeno y Fósforoen Especies Arbóreas de la Región Andino-Patagónica. Ph.D. Thesis, Universidad Nacional del Comahue, Centro Regional Universitario Bariloche, Bariloche, Argentina, 2006. [Google Scholar]
- Diehl, P.; Mazzarino, M.; Fontenla, S. Plant limiting nutrientsin Andean–Patagonian woody species: Effects of interannual rain-fall variation, soil fertility and mycorrhizal infection. Forest Ecol. Manag. 2008, 255, 2973–2980. [Google Scholar] [CrossRef]
- Nouhra, E.; Palfner, G.; Kuhar, F.; Pastor, N.; Smith, M. Ectomycorrhizal Fungi in South America: Their Diversity in Past, Present and Future Research. In Mycorrhizal Fungi in South America; Pagano, M., Lugo, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 73–95. [Google Scholar]
- Villar-Salvador, P. Importancia de la calidad de planta en los proyectos de revegetación. In Restauración de Ecosistemas Mediterráneos; Rey-Benayas, J.M., Espigares-Pinilla, T., Nicolau-Ibarra, J.M., Eds.; Universidad de Alcalá: Alcalá de Henares, España, 2003; pp. 65–86. [Google Scholar]
- Garrido, N. Agaricales s.l. und ihre Mykorrizen in den Nothofagus-Wäldern Mittelchiles; Bibliotheca Mycologica, J., Ed.; Cramer: Berlin, Germany, 1988; p. 528. [Google Scholar]
- Palfner, G.; Casanova, A.; Salazar, V.; Riquelme, A.; Santelices, R. Hongos no liquenizados: Diversidad, funciones, conservación y usos. In Los Bosques Relictos de Ruil: Ecología, Biodiversidad, Conservación y Restauración; San Martín, J., Ed.; INFOR: Talca, Chile, 2022; pp. 245–272. [Google Scholar]
- Navarro-Cerrillo, R.; Villar-Salvador, P.; Del Campo, A. Morfología y establecimiento de los plantones. In Calidad de Planta Forestal Para la Restauración en Ambientes Mediterráneos, Estado Actual de Conocimientos; Cortina, J., Peñuelas, J., Puértolas, J., Savé, R., Vilagrosa, A., Eds.; Ministerio del Medioambiente, Dirección General Para la Biodiversidad: Madrid, España, 2006; pp. 67–88. [Google Scholar]
- Thomson, S. Seedling morphological evaluation—What you can tell by looking. In Evaluating Seedling Quality: Principles, Procedures, and Predictive Abilities of Major Tests, Proceedings of the Workshop Held 16–18 October 1984; Durvea, M.L., Ed.; Forest Research Laboratory, Oregon State University: Corvallis, OR, USA, 1985; pp. 59–70. [Google Scholar]
- Mexal, J.; Landis, T.D. Target Seedling Concepts: Height and Diameter. In Target Seedling Symposium, Proceedings of the Western Forest Nursery Association, Roseburg, OR, USA, 13–17 August 1990; Rose, R., Campbell, S.J., Landis, T.D., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1990; pp. 13–17. [Google Scholar]
- Malik, V.; Timmer, V.R. Biomass partitioning and nitrogen retranslocation in black spruce seedlings on competitive mixed wood sites: A bioassay study. Can. J. For. Res. 1998, 28, 206–215. [Google Scholar] [CrossRef]
- Floistad, I.S.; Kohmann, K. Influence of nutrient supply on spring frost hardiness and time of bud break in Norway spruce (Picea abies (L.) Karst.) seedlings. New For. 2004, 27, 1–11. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Page-Dumroese, D.S.; Salifu, K.F.; Jacobs, D.F. Exponential fertilization of Pinus monticola seedlings: Nutrient uptake efficiency, leaching fractions, and early outplanting performance. Can. J. For. Res. 2005, 35, 2961–2967. [Google Scholar] [CrossRef]
- Salifu, K.F.; Jacobs, D.F. Characterizing fertility targets and multi-element interactions in nursery culture of Quercus rubra seedlings. Ann For. Sci. 2006, 63, 231–237. [Google Scholar] [CrossRef]
- Puértolas, J.; Gil, L.; Pardos, J.A. Effects of nutritional status and seedling size on field performance of Pinus halepensis planted on former arable land in the Mediterranean basin. Forestry Int. J. For. Res. 2003, 76, 159–168. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Planelles, R.; Enríquez, E.; Rubira, J.P. Nursery cultivation regimes, plant functional attributes, and field performance relationships in the Mediterranean oak Quercus ilex L. For. Ecol. Manag. 2004, 196, 257–266. [Google Scholar] [CrossRef]
- Oliet, J.A.; Planelles, R.; Artero, F.; Valverde, R.; Jacobs, D.F.; Segura, M.L. Field performance of Pinus halepensis planted in Mediterranean arid conditions: Relative influence of seedling morphology and mineral nutrition. New For. 2009, 37, 313–331. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Importance of root growth in overcoming planting stress. New For. 2005, 30, 273–294. [Google Scholar] [CrossRef]
- del Campo, A.D.; Navarro, R.M.; Ceacero, C.J. Seedling quality and field performance of commercial stocklots of containerized holm oak (Quercus ilex) in Mediterranean Spain: An approach for establishing a quality standard. New For. 2010, 39, 19. [Google Scholar] [CrossRef]
- Trubat, R.; Cortina, J.; Vilagrosa, A. Nutrient deprivation improves field performance of woody seedlings in a degraded semi-arid shrubland. Ecol. Eng. 2011, 37, 1164–1173. [Google Scholar] [CrossRef]
- Trubat, R.; Cortina, J.; Vilagrosa, A. Short-term nitrogen deprivation increases field performance in nursery seedlings of Mediterranean woody species. J. Arid. Environ. 2008, 72, 879–890. [Google Scholar] [CrossRef]
- Cortina, J.; Vilagrosa, A.; Trubat, R. The role of nutrients for improving seedling quality in drylands. New For. 2013, 44, 719–732. [Google Scholar] [CrossRef]
- Hernández, E.I.; Vilagrosa, A.; Luis, V.C.; Llorca, M.; Chirino, E.; Vallejo, V.R. Root hydraulic conductance, gas exchange and leaf water potential in seedlings of Pistacia lentiscus L. and Quercus suber L. grown under different fertilization and light regimes. Environ. Exp. Bot. 2009, 67, 269–276. [Google Scholar] [CrossRef]
- Santelices, R.; Navarro-Cerrillo, R.; Drake, F.; Mena, C. Efecto de la cobertura y de la fertilización en el desarrollo de plantas de Nothofagus alessandrii cultivadas en contenedor. Bosque 2011, 32, 85–88. [Google Scholar] [CrossRef]
- INN (INdN). NCH2957/0 Madera. Material de Propagación de Uso Forestal. Parte 0: Producción y Comercialización; INN (Instituto Nacional de Normalización): Santiago, Chile, 2005; p. 13. [Google Scholar]
- Ritchie, G.; Landis, T.; Dumroese, R.; Haase, D. Chapter 2: Assessing plant quality. In The Container Tree Nursery Manual, Volume 7. Seedling Processing, Storage, and Outplanting; Landis, T.D., Haase, D.L., Eds.; USDA Forest Service: Washington, DC, USA, 2010; pp. 17–81. [Google Scholar]
- South, D.B.; Rakestraw, J.L.; Lowerts, G.A. Early gains from planting large-diameter seedlings and intensive management are additive for loblolly pine. New For. 2001, 22, 97–110. [Google Scholar] [CrossRef]
- Salto, C.S.; Sagadin, M.B.; Luna, C.M.; Oberschelp, G.P.J.; Harrand, L.; Cabello, M.N. Interactions between mineral fertilization and arbuscular mycorrhizal fungi improve nursery growth and drought tolerance of Prosopis alba seedlings. Agroforest Syst. 2020, 94, 103–111. [Google Scholar] [CrossRef]
- Salcido, S.; Prieto, J.L.; Santana, E.; Chávez, J. Pinus greggii engelm.: Respuesta a la inoculación micorrícica controlada y a la fertilización en vivero. Agrociencia 2021, 55, 273–290. [Google Scholar] [CrossRef]
- Klironomos, J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Bustos, F.; González, M.; Donoso, P.; Gerding, V.; Donoso, C.; Escobar, B. Efectos de distintas dosis de fertilizante de liberación controlada (Osmocote®) en el desarrollo de plantas de coigüe, raulí y ulmo. Bosque 2008, 29, 155–161. [Google Scholar] [CrossRef]
- Dexheimer, J.; Pargney, J.C. Comparative anatomy of the host-fungus interface in mycorrhizas. Experientia 1991, 47, 312–321. [Google Scholar] [CrossRef]
- Ditengou, F.; Lapeyrie, F. Hypaphorine from the ectomycorrhizal fungus Pisolithus tinctorius counteracts activities of indole-3-acetic acid and ethylene but not synthetic auxins in eucalypt seedlings. Mol. Plant-Microbe Interact. 2000, 13, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Horan, D.; Chilvers, G.; Lapeyrie, F. Time sequence of the infection process in eucalypt ectomycorrhizas. New Phytol. 1988, 109, 451–458. [Google Scholar] [CrossRef]
- Felten, J.; Kohler, A.; Morin, E.; Bhalerao, R.; Palme, K.; Martin, F.S.; Ditengou, F.; Legué, V. The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through auxin transport and signaling. Plant Physiol. 2009, 151, 1991–2005. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, P.; Legué, V.; Vayssières, A.; Martin, F.; Tuskan, G.A.; Kalluri, U.C. Involvement of auxin pathways in modulating root architecture during beneficial plant-microorganism interactions. Plant Cell Environ. 2013, 36, 909–919. [Google Scholar] [CrossRef]
- Vayssières, A.; Pěnčík, A.; Felten, J.; Kohler, A.; Ljung, K.; Martin, F.; Legué, V. Development of the Poplar-Laccaria bicolor Ectomycorrhiza Modifies Root Auxin Metabolism, Signaling, and Response. Plant Physiol. 2015, 169, 890–902. [Google Scholar] [CrossRef]
- Boyle, C.; Robertson, W.; Salonius, P. Use of slurries of mycorrhizal fungi as inoculum for commercial tree seedling nurseries. Can. J. For. Res. 1987, 17, 1480–1486. [Google Scholar] [CrossRef]
- Carrillo, C. Técnicas de Micorrización En Vivero Con Hongos Ectomicorrícicos. Experiencias Realizadas en el Centro Nacional de Mejora Forestal “El Serranillo”; Tercer curso avanzado de viveros y producción de planta forestal: Guadalajara, España, 2000; p. 19. [Google Scholar]
- Castellano, M.A.; Trappe, J.M.; Molina, R. Inoculation of container-grown Douglas-fir seedlings with basidiospores of Rhizopogon vinicolor and R. colossus: Effects of fertility and spore application rate. Can. J. For. Res. 1985, 15, 10–13. [Google Scholar] [CrossRef]
- Khasa, P.D.; Sigler, L.; Chakravarty, P.; Dancik, B.P.; Erickson, L.; Mc Curdy, D. Effect of fertilization on growth and ectomycorrhizal development of container-grown and bare-root nursery conifer seedlings. New For. 2001, 22, 179–197. [Google Scholar] [CrossRef]
- Hilszcanska, D.; Sierota, Z. Persistence of ectomycorrhizas by Thelephora terrestres on outplanted Scots pine seedlings. Acta Mycol. 2006, 41, 313–318. [Google Scholar] [CrossRef]
- Dixon, R.K.; Behrns, G.T.; Garrett, H.E.; Cox, G.S.; Sander, I.L. Synthesis of Ectomycorrhizae on Container-Grown Oak Seedlings. South. J. Appl. For. 1985, 9, 95–99. [Google Scholar] [CrossRef]
- Landis, T.D. Containers: Types and functions. Agric. Handbk. 674. In The Containers Tree Nursery Manual, Volumen 2; Landis, T.D., Tinus, R.W., McDonald, S.E., Barnett, J.P., Eds.; USDA Forest Service: Washington, DC, USA, 1990; pp. 1–39. [Google Scholar]
- Honrubia, M.; Torres, P.; Díaz, G.; Cano, A. Manual Para Micorrizar Plantas En Viveros Forestales; Instituto para la Conservación de la Naturaleza (ICONA), Ministerio de Agricultura, Pesca y Alimentación: Murcia, España, 1992; p. 44. [Google Scholar]
- Davies, F.T., Jr.; Svenson, S.E.; Cole, J.C.; Phavaphutanon, L.; Duray, S.A.; Olalde-Portugal, V.; Meier, C.E.; Bo, S.H. Non-nutritional stress acclimation of mycorrhizal woody plants exposed to drought. Tree Physiol. 1996, 16, 985–993. [Google Scholar] [CrossRef]
- Singer, R. Mycoflora Australis; J. Cramer: Lehre, Germany, 1969. [Google Scholar]
- Torres-Díaz, C.; Valladares, M.A.; Acuña-Rodríguez, I.S.; Ballesteros, G.I.; Barrera, A.; Atala, C.; Molina-Montenegro, M.A. Symbiotic Interaction Enhances the Recovery of Endangered Tree Species in the Fragmented Maulino Forest. Front. Plant Sci. 2021, 12, 663017. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, R.; Alberdi, M.; Reyes-Díaz, M.; Olivares, E.; Hess, S.; Bravo, L.A.; Corcuera, L.J. Seasonal changes in the photosynthetic performance of two evergreen Nothofagus species in south central Chile. Rev. Chil. De Hist. Nat. 2006, 79, 489–504. [Google Scholar] [CrossRef]
- Sebastiana, M.; da Silva, A.B.; Matos, A.R.; Alcântara, A.; Silvestre, S.; Malhó, R. Ectomycorrhizal inoculation with Pisolithus tinctorius reduces stress induced by drought in cork oak. Mycorrhiza 2018, 28, 247–258. [Google Scholar] [CrossRef]
- Morte, A.; Dieste, C.; Díaz, G.; Gutiérrez, A.; Navarro, A.; Honrubia, M. Production of Terfezia olbiensis mycelial inoculum in a bioreactor. In Proceedings of the 1st Symp Champignons Hypoges du Basin Mediterraneen, Rabat, Morocco, 6–8 April 2004. [Google Scholar]
- Ortega, U.; Duñabeitia, M.; Menendez, S.; Gonzalez-Murua, C.; Majada, J. Effectiveness of mycorrhizal inoculation in the nursery on growth and water relations of Pinus radiata in different water regimes. Tree Physiol. 2004, 24, 65–73. [Google Scholar] [CrossRef]
- Baltruschat, H.; Fodor, J.; Harrach, B.D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.H.; Schäfer, P.; Schwarczinger, I.; et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008, 180, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Cartes Rodríguez, E.; Acevedo Tapia, M.; González Ortega, M.; Álvarez, C.; García Rivas, E.; Mena Marín, P.P. Manual de Manejo de Riego y Fertilización en Viveros de Plantas a Raíz Cubierta; Instituto Fotestal (INFOR): Santiago, Chile, 2019. [Google Scholar]
- Landis, T.D. Mineral nutrients and fertilization. In The Container Tree Nursery Manual, Volume 4. Agric. Handbk. 674; Landis, T.D., Tinus, R.W., McDonald, S.E., Barnett, J.P., Eds.; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1989; pp. 1–67. [Google Scholar]
- Arenas, F.; Navarro-Ródenas, A.; Chávez, D.; Gutiérrez, A.; Pérez-Gilabert, M.; Morte, A. Mycelium of Terfezia claveryi as inoculum source to produce desert truffle mycorrhizal plants. Mycorrhiza 2018, 28, 691–701. [Google Scholar] [CrossRef]
- Iverson, R.D. Planting-Stock Selection: Meeting Biological Needs and Operational Realities. In Forestry Nursery Manual: Production of Bareroot Seedlings; Duryea, M.L., Landis, T.D., Perry, C.R., Eds.; Springer: Dordrecht, The Netherlands, 1984; pp. 261–266. [Google Scholar]

| Treatment | Roots Biomass (gr) | Stems Biomass (gr) | Leaves Biomass (gr) | Total Biomass (gr) |
|---|---|---|---|---|
| P2F1M0 | 10.19 ± 3.74 | 19.65 ± 6.62 | 7.52 ± 3.28 | 37.37 |
| P2F1M1 | 11.71 ± 2.85 | 21.94 ± 5.03 | 9.03 ± 2.37 | 42.68 |
| P2F1M2 | 11.09 ± 3.98 | 19.97 ± 4.72 | 8.28 ± 2.69 | 39.34 |
| P2F2M0 | 10.49 ± 2.20 | 21.86 ± 4.75 | 8.17 ± 2.87 | 40.52 |
| P2F2M1 | 9.53 ± 3.74 | 18.41 ± 6.83 | 7.34 ± 3.99 | 35.28 |
| P2F2M2 | 10.21 ± 3.73 | 19.97 ± 6.44 | 7.78 ± 3.18 | 37.96 |
| P1F1M0 | 5.12 ± 2.23 | 9.50 ± 3.41 | 6.34 ± 2.17 | 20.96 |
| P1F1M1 | 4.45 ± 2.38 | 8.51 ± 3.92 | 5.76 ± 2.42 | 18.72 |
| P1F1M2 | 4.42 ± 2.44 | 8.00 ± 3.66 | 5.54 ± 2.25 | 17.96 |
| P1F2M0 | 3.99 ± 2.03 | 8.26 ± 3.95 | 5.71 ± 2.54 | 17.97 |
| P1F2M1 | 3.42 ± 1.79 | 7.13 ± 3.42 | 5.00 ± 2.09 | 15.55 |
| P1F2M2 | 3.35 ± 1.85 | 7.12 ± 3.75 | 4.88 ± 2.23 | 15.35 |
| Index | P1F1M0 | P1F1M1 | P1F1M2 | P1F2M0 | P1F2M1 | P1F2M2 |
|---|---|---|---|---|---|---|
| SI | 12.51 ± 3.32 | 11.07 ± 2.55 | 11.13 ± 2.73 | 11.05 ± 2.99 | 11.82 ± 2.20 | 8.50 ± 3.65 |
| SRI | 3.17 ± 0.68 | 2.78 ± 0.89 | 2.81 ± 0.85 | 3.39 ± 0.36 | 3.98 ± 0.95 | 3.91 ± 0.87 |
| P2F1M0 | P2F1M1 | P2F1M2 | P2F2M0 | P2F2M1 | P2F2M2 | |
| SI | 9.86 ± 1.92 | 9.39 ± 1.65 | 9.49 ± 1.93 | 9.63 ± 1.62 | 9.87 ± 1.88 | 9.46 ± 1.70 |
| SRI | 2.52 ± 0.58 | 2.28 ± 0.39 | 2.99 ± 0.58 | 3.08 ± 0.68 | 3.19 ± 0.79 | 2.77 ± 0.46 |
| Fertilization Scheme | Total N (mg L−1) | N–NO3− (mg L−1) | N–NH4+ (mg L−1) | P (mg L−1) | K (mg L−1) | Ca (mg L−1) | Mg (mg L−1) |
|---|---|---|---|---|---|---|---|
| F1 | 50 | 25 | 25 | 25 | 32.4 | 15 | 12.5 |
| F2 | 200 | 100 | 100 | 100 | 127 | 60 | 50 |
| Fertilization Scheme | S (mg L−1) | Fe (mg L−1) | Mn (mg L−1) | Cu (mg L−1) | Zn (mg L−1) | Mo (mg L−1) | B (mg L−1) |
| F1 | 17.2 | 2.5 | 2.5 | 0.5 | 2.5 | 0.5 | 0.5 |
| F2 | 79.7 | 10 | 10 | 2 | 10 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Ariza, A.M.; Silva-Flores, P.; González-Ortega, M.; Acevedo-Tapia, M.; Cartes-Rodríguez, E.; Palfner, G.; Ramos, P.; Santelices-Moya, R.E. Early Effects of Mycorrhizal Fungal Inoculum and Fertilizer on Morphological and Physiological Variables of Nursery-Grown Nothofagus alessandrii Plants. Plants 2023, 12, 1521. https://doi.org/10.3390/plants12071521
Cabrera-Ariza AM, Silva-Flores P, González-Ortega M, Acevedo-Tapia M, Cartes-Rodríguez E, Palfner G, Ramos P, Santelices-Moya RE. Early Effects of Mycorrhizal Fungal Inoculum and Fertilizer on Morphological and Physiological Variables of Nursery-Grown Nothofagus alessandrii Plants. Plants. 2023; 12(7):1521. https://doi.org/10.3390/plants12071521
Chicago/Turabian StyleCabrera-Ariza, Antonio M., Patricia Silva-Flores, Marta González-Ortega, Manuel Acevedo-Tapia, Eduardo Cartes-Rodríguez, Götz Palfner, Patricio Ramos, and Rómulo E. Santelices-Moya. 2023. "Early Effects of Mycorrhizal Fungal Inoculum and Fertilizer on Morphological and Physiological Variables of Nursery-Grown Nothofagus alessandrii Plants" Plants 12, no. 7: 1521. https://doi.org/10.3390/plants12071521
APA StyleCabrera-Ariza, A. M., Silva-Flores, P., González-Ortega, M., Acevedo-Tapia, M., Cartes-Rodríguez, E., Palfner, G., Ramos, P., & Santelices-Moya, R. E. (2023). Early Effects of Mycorrhizal Fungal Inoculum and Fertilizer on Morphological and Physiological Variables of Nursery-Grown Nothofagus alessandrii Plants. Plants, 12(7), 1521. https://doi.org/10.3390/plants12071521






