Abstract
To look in-depth into the phytochemical and pharmacological properties of Taiwan juniper, this study investigated the chemical profiles and anti-lymphangiogenic activity of Juniperus chinensis var. tsukusiensis. In this study, four new sesquiterpenes, 12-acetoxywiddrol (1), cedrol-13-al (2), α-corocalen-15-oic acid (3), 1,3,5-bisaoltrien-10-hydroperoxy-11-ol (4), one new diterpene, 1β,2β-epoxy-9α-hydroxy-8(14),11-totaradiene-3,13-dione (5), and thirty-three known terpenoids were successfully isolated from the heartwood of J. chinensis var. tsukusiensis. The structures of all isolates were determined through the analysis of physical data (including appearance, UV, IR, and optical rotation) and spectroscopic data (including 1D, 2D NMR, and HRESIMS). Thirty-four compounds were evaluated for their anti-lymphangiogenic effects in human lymphatic endothelial cells (LECs). Among them, totarolone (6) displayed the most potent anti-lymphangiogenic activity by suppressing cell growth (IC50 = 6 ± 1 µM) of LECs. Moreover, 3β-hydroxytotarol (7), 7-oxototarol (8), and 1-oxo-3β-hydroxytotarol (9) showed moderate growth-inhibitory effects on LECs with IC50 values of 29 ± 1, 28 ± 1, and 45 ± 2 µM, respectively. Totarolone (6) also induced a significant concentration-dependent inhibition of LEC tube formation (IC50 = 9.3 ± 2.5 µM) without cytotoxicity. The structure–activity relationship discussion of aromatic totarane-type diterpenes against lymphangiogenesis of LECs is also included in this study. Altogether, our findings unveiled the promising potential of J. chinensis var. tsukusiensis in developing therapeutics targeting tumor lymphangiogenesis.
1. Introduction
Lymphangiogenesis is the process of new lymphatic vessels developing from pre-existing lymphatic vasculature. It plays a role in diverse physiological scenarios, such as maintaining homeostasis, supporting the immune system, contributing to embryonic development, and aiding in wound healing. Conversely, in pathological contexts, this process is often associated with issues like organ graft rejection, lymphedema, and even cancer spread [1]. Based on the annual statistics reported by the World Health Organization, cancer is a primary contributor to mortality and a significant impediment to raising life expectancy in every nation [2]. It is worth noting that around 90% of cancer-related deaths are caused by metastatic tumor spread. Tumor lymphangiogenesis has consequently emerged as a pivotal prognostic role for cancer patients [3]. Given this understanding, developing targeted cancer therapies with anti-lymphangiogenic activity is a promising strategy to enhance patient survival rates.
The Juniperus species (Cupressaceae) are coniferous plants encompassing evergreen trees and shrubs, widely distributed throughout the cold temperate regions of the Northern Hemisphere to Tropical Africa [4]. The aromatic nature of Juniperus is due to pine oil and resin within the plant. These compounds contribute to the unique scent and flavor associated with juniper berries. Furthermore, the high resin content makes Juniperus wood more resistant to decay and infestation, making it a valuable material in woodworking projects. Some characteristic phytochemicals have been isolated from Juniperus, including acetophenones, monoterpenes, sesquiterpenes, diterpenes, flavonoids, lignans, and phenylpropanoides, mainly terpenoids [5,6,7,8,9,10,11,12,13,14,15,16,17]. The representative terpenoids in Juniperus include α-pinene, camphene, β-pinene, sabinene, myrcene, limonene, imbricatolic acid, junicedral, trans-communic acid, and isocupressic acid [18,19]. The cytotoxic lignan compounds, podophyllotoxin and deoxypodophyllotoxin, could also be found in some Juniperus species [20]. One of the most famous Juniperus plants, J. communis Linn., is widely used as a diuretic, antiseptic, leucorrhea, and treating gastrointestinal problems in folk medicine [4,21]. It also presents various pharmacological potentials for anti-inflammatory [22,23,24,25], antifungal [24,25,26], hepatoprotective [18,27], neuroprotective [19], anti-diabetic and anti-hyperlipidemic [28,29], and anti-proliferative activities [30,31,32,33,34]. Due to the distinctive phytochemical composition and promising pharmacological activities exhibited by Juniperus plants, our interest in researching them has been sparked. Our previous studies on the J. chinensis var. tsukusiensis revealed some characteristic Juniperus sesquiterpenoids [35,36]. However, the biological activity of J. chinensis var. tsukusiensis has never been studied, which prompted us to further explore its bioactive ingredients.
In this study, a series of isolation and purification procedures were implemented, resulting in five new compounds (1–5) (Figure 1) and thirty-three known terpenoids (6–38) from the heartwood of J. chinensis var. tsukusiensis. Terpenoids are renowned for their cytotoxic effects on various types of tumors. Interestingly, in the previous investigation, it was discovered that diterpenes possess anti-lymphangiogenic properties, which is a new concept in targeting tumor cell metastasis [37,38,39]. To further explore the relationship between terpenoids and their anti-lymphangiogenic activity, as well as to expand the pharmacological profile of terpenoids, we evaluated the anti-lymphangiogenic activity of the isolated compounds in our study. Among these compounds, 6, 7, 8, and 9 demonstrated significant anti-lymphangiogenic activity.
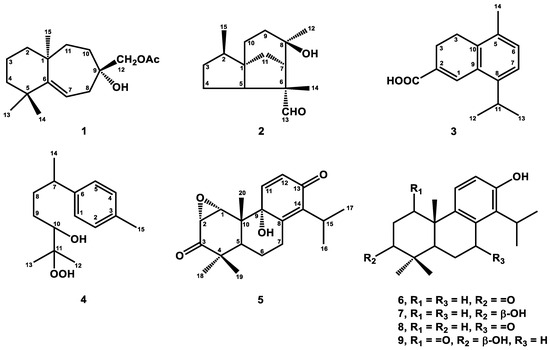
Figure 1.
Structures of new compounds (1–5) and bioactive compounds (6–9).
2. Results
2.1. Structure Elucidation
Compound 1 was obtained as a colorless, viscous oil with optical rotation. Its molecular formula was determined as C17H28O3 from HRESIMS data (m/z 281.2101 [M+H]+ (calcd. for 281.2117)), implying four degrees of unsaturation. The infrared spectroscopy (IR) spectrum showed typical absorptions of C=O (1742 cm−1) for the ester group and hydroxy group (3433 cm−1). The 1H NMR spectrum (Table 1) displayed signals of four singlet methyl groups at δH 1.04 (3H, s, H-14), 1.07 (3H, s, H-13), 1.20 (3H, s, H-15), and 2.10 (3H, s, OAc), one oxymethylene at δH 3.99 (2H, s, H-12), and one trisubstituted olefinic proton at δH 5.41 (1H, dd, J = 8.8, 6.0 Hz, H-7). The 13C NMR (Table 2) and DEPT spectra showed seventeen resonances comprising four methyls, seven methylenes, one methine, and five quaternary carbons. From the 1H and 13C NMR spectra, one C=O group (δC 171.3, C-16) and one C=C unit [δC 116.3 (C-7), 155.2 (C-6)] accounted for two of four degrees of unsaturation. Thus, compound 1 was suggested to be a bicyclic sesquiterpene with an acetyl group. The existence of the acetoxy methyl group was confirmed by the HMBC correlation (Figure 2) from H-17 to C-16 and the absorption of the ester group (1742 cm−1) in the IR spectrum. Comparing the 1H and 13C NMR data of 1 to those of the literature compound, widdrol [6], they shared similar structures, except for the oxidation of C-12 and an additional acetyl group in 1. Further HMBC correlation (Figure 2) between H-12 and C-16 allowed the acetoxy group to be located at C-12. The relative stereochemistry of 1 was assigned using the information provided by the NOESY spectrum (Figure 2) and compared with the literature compound, widdrol [6]. The NOESY correlations (Figure 2) between H-13/H-8α and H-15, and H-12/H-8β, confirmed that OH-9, H-13, and H-15 were in the same phase, while H-12 and H-14 were on the same side. Thus, compound 1 was determined and named 12-acetoxywiddrol.

Table 1.
1H NMR data of compounds 1–5 in CDCl3.

Table 2.
13C NMR data of compounds 1–5 in CDCl3.
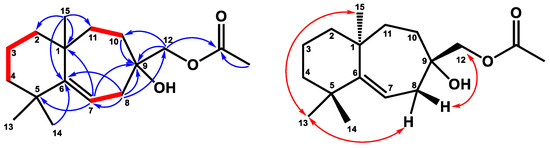
Figure 2.
Key 1H–-1H COSY (━), HMBC (H→C), and NOESY (↔) correlations of compound 1.
Compound 2 was isolated as an optical, viscid oil, and displayed a pseudo-molecular ion at m/z 237.1848 [M+H]+ (calcd. for C15H25O2, 237.1855) by HRFABMS with four degrees of unsaturation. Its IR spectrum showed hydroxy and aldehyde groups at 3403 cm−1 and 1711 cm−1, respectively. The 1H NMR data (Table 1) depicted signals of three methyls at δH 0.84 (3H, d, J = 6.9 Hz, H-15), 1.31 (3H, s, H-12), and 1.41 (3H, s, H-14), and an aldehyde group with low field chemical shift at δH 9.42 (1H, s, H-1). Further 13C NMR (Table 2) and DEPT spectra identified three methyls, five methylenes, three methines, and four quaternary carbons, including one oxygenated quaternary carbon (δC 73.8) and one aldehyde (δC 205.6). According to the above data, the structure of 2 was assumed to be a three-membered ring sesquiterpene with an aldehyde group. A detailed comparison of the NMR data of 2 to those of 8β,13-dihydroxycedrane [40] revealed that these two compounds were structure analogous, except for the hydroxymethyl group of 8β,13-dihydroxycedrane was changed to an aldehyde group in compound 2. The HMBC correlations (Figure 3) from H-14 to C-5 (δC 58.0), C-6 (δC 57.6), C-7 (δC 53.3), C-13 (δC 205.6), and H-13 (δH 9.42, 3H, s, H-13) to C-6 indicated that the aldehyde group was located at C-6. In the HMBC spectrum, the correlations of H-12/C-7, C-8 (δC 73.8), and C-9 (δC 34.6) verified the hydroxy group was attached at C-8. The planar structure of 2 was further supported by HMBC and COSY correlations, as shown in Figure 2. Further NOESY correlations (Figure 3) between H-15/H-10, H-14/H-5, H-9, and H-12/H-13 supported the relative configuration of 2 was the same as 8β,13-dihydroxycedrane [40]. Based on the above data, the structure of compound 2 was determined and named cedrol-13-al.
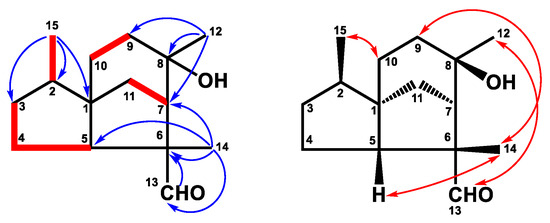
Figure 3.
Key 1H–1H COSY (━), HMBC (H→C), and NOESY (↔) correlations of compound 2.
Compound 3 was yielded as a whitish solid. Analysis by HREIMS of 3 indicated a molecular formula of C15H18O2, representing seven IHDs. The UV spectrum showed maximum absorptions at 213, 231, and 295 nm. The IR absorption bands at 1622, 1600, and 1485 cm−1, as well as the observation of featuring carbon resonances (Table 2), confirmed the existence of an aromatic ring [δC 122.9 (C-6), 129.1 (C-9), 132.0 (C-7), 132.6 (C-5), 136.0 (C-10), 144.6 (C-8)] and a double bond [δC 127.7 (C-2), 135.3 (C-1)]. The carboxylic acid group was observed both in IR (1682 cm−1) and 13C NMR [δC 172.3 (C-15)] spectra. The IR spectrum also revealed the double bond (1622 cm−1) and aromatic ring (1600 and 1485 cm−1). The 1H NMR spectrum (Table 1) of 3 exhibited one methyl group bearing with an aromatic ring at δH 2.26 (3H, s, H-14), an isopropyl group at δH 1.23 (6H, d, J = 6.8 Hz, H-12 and H-13), 3.33 (1H, sept, J = 6.8 Hz, H-11), an ortho-coupling aromatic ring at δH 7.07 (1H, d, J = 8.0 Hz, H-6), 7.12 (1H, d, J = 8.0 Hz, H-7), a trisubstituted olefinic proton at δH 8.03 (1H, s, H-1), and two methylene groups at δH 2.55 (2H, t, J = 8.0 Hz, H-3), 2.79 (2H, t, J = 8.0 Hz, H-4). The HMBC experiment (Figure 4) for 3 revealed correlations between H-1/C-2, C-8, C-9, and C-10, and H-3/C-1, C-4, and C-10, confirming the 3,4-dihydronaphthalene skeleton of 3. The HMBC correlations between H-14/C-5, C-6, and C-10 verified the methyl group (C-14) was attached to C-5 (Figure 4). The isopropyl group was located at C-8 based on the HMBC correlations between H-11/C-7 and H-13/C-8 (Figure 4). Finally, the carboxylic acid group gave cross-peak to a correlation with H-1, suggesting the carboxylic acid group was bearing with C-2. Therefore, the structure of 3 was defined as shown and named α-corocalen-15-oic acid.
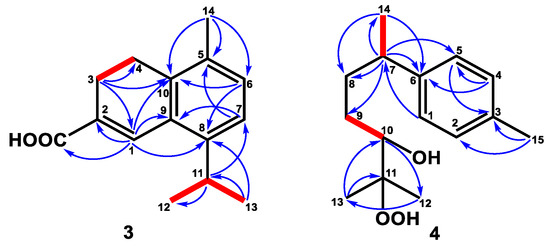
Figure 4.
Key 1H–1H COSY (━) and HMBC (H→C) correlations of compounds 3 and 4.
Compound 4 was purified as a colorless, viscous oil. Its molecular formula of C15H24O3 was determined by HRESIMS (m/z 253.1802, calcd. for 253.1804), indicating four degrees of unsaturation. Its IR spectrum depicted a hydroxy group at 3410 cm−1. The 13C NMR (Table 2) and DEPT spectra identified four methyls [δC 20.9 (C-15), 22.4 (C-14), 23.1 (C-12), 26.5 (C-13)], an aromatic ring [δC 126.8 (C-5), 129.1 (C-4), 135.3 (C-3), 144.4 (C-6)], one oxymethine [δC 78.8 (C-10)], one oxygenated quaternary carbon [δC 73.1 (C-11)], two methylenes [δC 29.9 (C-9), 35.5 (C-8)], and one methine [δC 39.7 (C-7)]. Further 1H NMR spectrum (Table 1) confirmed the substituted pattern of the para-substituted aromatic ring [δH 7.05 (2H, d, J = 7.8 Hz, H-1 and H-5), 7.06 (2H, d, J = 7.8 Hz, H-2 and H-4)] attaching with a low field methyl group [δH 2.29 (3H, s, H-15)]. The COSY correlation (Figure 4) between H-7/H-14 and H-9/H-10 revealed the existence of fragments C-7–C-14 and C-9–C-10, respectively. The cross-peak between H-14 (δH 1.21, d, J = 7.2 Hz)/C-7, C-8 (δC 35.5), H-7 (δH 2.63, sex, J = 7.2 Hz)/C-8, C-9, H-8β (δH 1.58, m)/C-9, C-10, and H-13 (δH 1.11, s)/C-10, C-11, and C-12 in the HMBC spectrum (Figure 4) suggested the existence of the side-chain group. Further analysis of the HMBC correlations (Figure 4) between H-7/C-1, C-5, and C-6 showed that the location of the side-chain group was connected with the aromatic ring at C-6. According to the carbon and HRESIMS spectra, compound 4 contained two oxygenated carbons (C-10 and C-11) with three oxygen atoms, implying the existence of a hydroxy and a hydroperoxy group. Based on the chemical shift of H-10 (δH 3.26), the hydroxy group was attached to C-10, and the hydroperoxy group was connected with C-11, respectively. The structure of 4 was accordingly confirmed and named 1,3,5-bisaoltrien-10-hydroperoxy-11-ol.
Compound 5 was obtained as a colorless crystal with optical rotation. A molecular formula of C20H26O4 was determined for this compound by HRESIMS (m/z 331.1906, calcd. for 331.1909), with eight degrees of unsaturation. The IR spectrum of 5 showed IR absorption bands at 3417 cm−1 for a hydroxy group, at 1699 cm−1 for one carbonyl group [δC 211.0 (C-3)], and at 1669 cm−1 for one conjugated carbonyl group [δC 184.6 (C-13)], which was supported by analysis of the 13C NMR spectrum (Table 2). The IR spectrum also revealed the presence of C=C double bonds (1631, 990, 840 cm−1). The 1H NMR spectrum of 5 exhibited three singlet methyl groups [δH 0.71 (3H, s, H-20), 0.98 (3H, s, H-19), and 1.20 (3H, s, H-18)], an isopropyl group [δH 1.21 (3H, d, J = 7.2 Hz, H-17), 1.23 (3H, d, J = 7.2 Hz, H-16)], and 3.22 (1H, sept, J = 7.2 Hz, H-15)], two oxymethines [δH 3.38 (1H, d, J = 4.6 Hz, H-2), 3.64 (1H, d, J = 4.6 Hz, H-1)], and double-bond signals [δH 6.28 (1H, d, J = 10.0 Hz, H-12), 7.01 (1H, d, J = 10.0 Hz, H-11)]. The 13C NMR and DEPT spectra indicated the presence of five methyls [δC 13.9 (C-20), 20.8 (C-19), 21.4 (C-17), 21.5 (C-16), 28.4 (C-18)], two oxymethines with epoxy ring [δC 54.5 (C-2), 60.9 (C-1)], one oxygenated quaternary carbon [δC 74.2 (C-9)], two pairs of double bonds [δC 131.3 (C-12)/144.9 (C-11), 144.0 (C-14)/153.7 (C-8)], two carbonyl groups [δC 184.6 (C-13), 211.0 (C-3)], two quaternary carbon [δC 44.8 (C-4), 46.7 (C-10)], two methines [δC 26.6 (C-15), 38.6 (C-55)], and two methylenes [δC 24.0 (C-6), 26.6 (C-7)]. From the above spectroscopic data, 5 was assumed to be a totarane diterpene similar to totarolone [41], except for an additional epoxy group at C-1/C-2, a hydroxy group at C-9, and the aromatic ring in totarolone was changed to a cyclohexa-2,5-dien-1-one ring in 5. The HMBC correlations (Figure 5) between H-20/C-1; H-1/C-2, C-5, and C-10; and H-2/C-3, C-4 indicated C-1 and C-2 were connected with epoxy group. Moreover, the cis disposition of H-1/H-2 was confirmed by the small coupling constant of H-1/H-2 (J = 4.6 Hz). The hydroxy group was located at C-9 from HMBC correlations of H20/C-9 and H-12/C-9. The existence of the cyclohexa-2,5-dien-1-one unit was verified based on the HMBC correlations between H-11/C-8, C-13 and H-12/C-9, C-14. The NOESY correlation (Figure 5) between H-19/H-6β, H-20 and H-5/H-6α, H-18, indicating H-19 and H-20 were in axial position. The NOESY correlations of H-1/H-2 and H-11 suggested that H-1, H-2, and H-11 were in the same phase (Figure 5). Additionally, the epoxy group and OH-9 were at the α-position based on the ring junction afforded by NOESY correlations. Thus, the structure of compound 5 was elucidated as 1β,2β-epoxy-9α-hydroxy-8(14),11-totaradiene-3,13-dione.
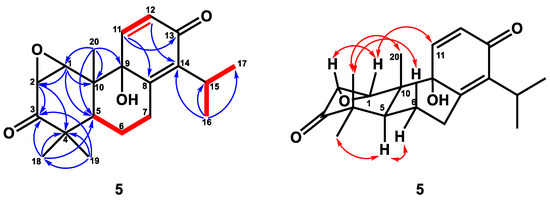
Figure 5.
Key 1H–1H COSY (━), HMBC (H→C), and NOESY (↔) correlations of compound 5.
By comparing the spectroscopic data ([a]D, UV, IR, NMR, and MS) of known compounds with the literature data, the known diterpenes were identified to be totarolone (6) [41], 3β-hydroxytotarol (7) [42], 7-oxototarol (8) [41], and 1-oxo-3β-hydroxytotarol (9) [41], 5,6-dehydrosugiol methyl ether (10) [43], sandaracopimaric acid (11) [35], 3,18-dihydroxypimara-8(14),15-diene (12) [44], secoabietane dialdehyde (13) [43], communic acid (14) [43], and (12R, 13S)-dihtdroxylabda-8(17),14-dien-19-oic acid (15) [45]. Additionally, the known sesquiterpenes were identified to be 2-himachalen-6-ol (16) [36], 3-himachalen-6-ol (17) [36], chinensiol (18) [36], ar-himachalene (19) [36], 12-hydroxy-α-longipinene (20) [35], 15-hydroxyacora-4(14),8-diene (21) [35], cedrol (22) [35], 8α,12-cedranediol (23) [46], 8-cedren-13-ol (24) [8], epicedrane-diol (25) [47], cedrol-13-oic acid (26) [48], 13-acetoxycedrol (27) [48], widdrol (28) [35], (+)-α-bisabolol (29) [7], sesquithuriferol (30) [8], 15-hydroxyallo-cedrol (31) [7], thujopsenal (32) [35], hinokiic acid (33) [35], mayurone (34) [49], 4α,5α-epoxymayurone (35) [50], 8β-hydroxythujopsan-9-one (36) [51], nootkatone (37) [52], and γ-cuparanol (38) [53].
2.2. Anti-Lymphangiogenic Effects of Compounds Isolated from J. chinensis var. tsukusiensis
The role of lymphangiogenesis in tumor development, progression, and metastasis has been well-documented in various human cancers. Therefore, our study focused on examining the anti-lymphangiogenic effects of 34 compounds in human lymphatic endothelial cells (LECs). As shown in Figure 6, compounds 6–9 showed growth-inhibitory effects on LECs (the percentage of LECs growth < 60%). These active isolates were further evaluated for their IC50 values of anti-lymphangiogenic activity (Table 3). As shown in Table 3, totarolone (6) exhibited the most potent anti-lymphangiogenic activity by suppressing LEC growth (IC50 = 6 ± 1 µM), with rapamycin as the positive control. 3β-Hydroxytotarol (7), 7-oxototarol (8), and 1-oxo-3β-hydroxytotarol (9) showed moderate growth-inhibitory effects on LECs with IC50 values of 29 ± 1, 28 ± 1, and 45± 2 µM, respectively.
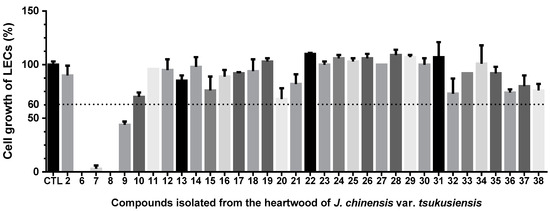
Figure 6.
Anti-lymphangiogenic effects of compounds in human LECs. LECs were treated with the indicated compounds at a concentration of 50 μM for 48 h, and anti-lymphangiogenic effects were elucidated in a cell growth assay (n = 3). Data were expressed as the mean ± SEM.

Table 3.
Anti-lymphangiogenic activity of selected compounds in human LECs.
To confirm the anti-lymphangiogenic effects of the active compounds, we proceeded with the capillary tube formation assay. As illustrated in Figure 7, compound 6 induced the promising anti-lymphangiogenesis property by disrupting LECs tube formation in a concentration-dependent manner (IC50 = 9.3 ± 2.5 µM). Furthermore, it was observed that compound 6 did not increase the levels of lactate dehydrogenase (LDH) in LECs. The results indicate that compound 6 exerts significant anti-lymphangiogenic effects without cytotoxic fashion.
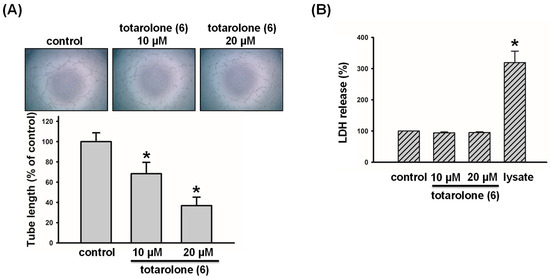
Figure 7.
Effects of compound 6 on tube formation and cytotoxicity in human LECs. (A) Cells were treated with compound 6 (10 and 20 μM) for 8 h, and tubular morphogenesis was recorded by an inverted phase-contrast microscope (n = 3). ImageJ 1.54a software was used to quantify the length of capillary-like tubes. (B) LECs were treated with compound 6 (10 and 20 μM), then the cytotoxicity was evaluated by the LDH assay (n = 3). Data are expressed as the mean ± SEM. * p < 0.05 compared with the control group.
3. Discussion
In this study, compounds isolated from the heartwood of J. chinensis var. tsukusiensis can be categorized into sesquiterpenoids and diterpenoids. Among the tested compounds, only aromatic totarane-type diterpenes depicted anti-lymphangiogenic effects in human LECs. An in-depth discussion of the structure–activity relationship (SAR) in tested compounds was raised. Totarolone (6) showed better anti-lymphangiogenic activity than 7-oxototarol (8), suggesting that the substituted position of the carbonyl group is crucial for anti-lymphangiogenic activity. Surprisingly, 3β-hydroxytotarol (7), a compound reduction by the 3-carbonyl group in totarolone (6), exhibits lower anti-lymphangiogenic activity than totarolone (6). Moreover, 3β-hydroxytotarol (7) provided better anti-lymphangiogenic activity than 1-oxo-3β-hydroxytotarol (9), implying that 3-carbonyl substituent on aromatic totarane-type diterpene may attenuate the anti-lymphangiogenic activity.
4. Material and Methods
4.1. General Experiment Procedures
Optical rotations were measured on a Jasco DIP-1000 Digital polarimeter (Jasco, Kyoto, Japan), and IR spectra (neat) were acquired with a Perkin-Elmer 983G spectrometer (Perkin-Elmer, Waltham, MA, USA). The 1D (1H, 13C, DEPT) and 2D (COSY, NOESY, HSQC, HMBC) NMR spectra were obtained from a Burcher DMX-300 spectrometer (Bruker Inc., Bremen, Germany) operated at 300 (1H) and 75 MHz (13C), Varian Unityplus-400 spectrometer (Varian, Inc. Vacuum Technologies, Lexington, MA, USA) operated at 400 (1H) and 100 MHz (13C). The HRESIMS data were generated at the Mass Spectrometry Laboratory by Orbitrap QE Plus Mass Spectrometry (Thermo Fisher Scientific, Inc., Bremen, Germany). Extracts were chromatographed on silica gel (70–230 mesh, 230–400 mesh, Merk, Darmstadt, Germany, ASTM) and purified with a semipreparative normal-phase HPLC column (Merck LichroCART 250 mm × 10 mm, 7 µm, LiChrosorb Si 60, Merck, Darmstadt, Germany) taken on LDC Analytical-III.
4.2. Plant Material
The heartwood of Juniperus chinensis Linn. var. tsukusiensis Masam. was collected in Chingshui Mountain, Hualien, Taiwan, in October 1990 and identified by Dr. Sheng-yYou Lu at the Taiwan Forestry Research Institute.
4.3. Extraction and Isolation
The dried heartwood of J. chinensis var. tsukusiensis (4 kg) was extracted at room temperature with MeOH (80 L) four times (4 days for each time) to yield an MeOH extract that was partitioned between n-hexane and H2O (1:1) to provide an n-hexane-soluble (230 g) and an H2O-soluble layer. The n-hexane-soluble layer was subjected to column chromatography (silica gel; 2.3 kg; started from 100% n-hexane, then washed with EtOAc in gradient mode, and finally washed with 100% EtOAc, 100% acetone, and 100% methanol, respectively) to yield 15 subfractions (Fr. A–~Fr. O). Fr. J was subjected to an HPLC column (silica gel; n-hexane/dichloromethane/EtOAc = 75/5/20) to obtain compound 2 (21.3 mg). Fr. L was separated with an HPLC column (silica gel; n-hexane/dichloromethane/EtOAc = 60/20/20) to gain compounds 4 (3.4 mg) and 5 (4.0 mg). Fr. M was purified on an HPLC column (silica gel; n-hexane/dichloromethane/EtOAc = 40/20/40) to furnish compound 1 (11.5 mg). Fr. N was re-chromatographed on an HPLC column (silica gel; n-hexane/dichloromethane/EtOAc = 20/20/60) to afford compound 3 (3.2 mg). The purification process of known compounds can be retrieved from the Supplementary Information (Table S1).
4.4. Spectroscopic Data of Compounds
4.4.1. 12-Acetoxywiddrol (1)
4.4.2. Cedrol-13-al (2)
4.4.3. α-Corocalen-15-oic Acid (3)
4.4.4. 1,3,5-Bisaoltrien-10-hydroperoxy-11-ol (4)
4.4.5. 1β,2β-Epoxy-9α-hydroxy-8(14),11-totaradiene-3,13-dione (5)
4.5. Anti-Lymphangiogenic Assay
The methods employed for cell culture, cell growth, tube formation, and cytotoxicity assessments of human lymphatic endothelial cells were consistent with our previous work [54].
5. Conclusions
Phytochemical investigation of the heartwood of J. chinensis var. tsukusiensis led to 38 compounds, including 5 new compounds and 33 known compounds in this study. The chemical components of Juniperus species have been well-studied; however, more studies focus on J. communis rather than J. chinensis var. tsukusiensis. The phytochemical findings in this study not only contribute to identifying additional natural sources of terpenoids but also enhance our understanding of the chemical profiles of J. chinensis var. tsukusiensis. Additionally, 34 isolates from J. chinensis var. tsukusiensis screened their anti-lymphangiogenic effects in human lymphatic endothelial cells. It is noticeable that this is the first report on the anti-lymphangiogenic activities of J. chinensis var. tsukusiensis and demonstrates the potential of totarolone (6), 3β-hydroxytotarol (7), 7-oxototarol (8), and 1-oxo-3β-hydroxytotarol (9) to develop therapeutics against tumor lymphangiogenesis.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants12223828/s1, including Table S1: Isolation and purification methods of known compounds isolated from J. chinensis var. tsukusiensis; Figure S1–S15: The phytochemical spectra of compounds 1–5.
Author Contributions
Conceptualization, Y.-H.K.; methodology, S.-W.W. and Y.-H.K.; formal analysis, H.-C.W., S.-W.W., T.-H.L. and Y.-H.K.; investigation, H.-C.W., L.-L.S., C.-Y.H., S.-W.W. and Y.-H.K.; resources, Y.-H.K.; data curation, Y.-H.K.; writing—original draft preparation, H.-C.W.; writing—review and editing, S.-W.W., T.-H.L., P.-J.S. and Y.-H.K.; visualization, H.-C.W., L.-L.S., C.-Y.H. and S.-W.W.; supervision, Y.-H.K.; project administration, Y.-H.K.; funding acquisition, Y.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a China Medical University grant in Taichung, Taiwan (CMU110-Z-08 and CMU109-AWARD-02 to Y.-H.K.), “Chinese Medicine Research Center, China Medical University, Taichung, Taiwan” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taipei, Taiwan (CMRC-CHM-2-1 to Y.-H.K.), and by the National Science and Technology Council, Taiwan (NSTC 112-2320-B-038-015-MY3 to H.-C.W.).
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, L.C.; Detmar, M. Tumor lymphangiogenesis and new drug development. Adv. Drug Deliv. Rev. 2016, 99, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus communis L. as a nutraceutical in human and veterinary medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, J.; He, F.; Fu, W.; Tang, B.; Bin, Y.; Fang, M.; Wu, Z.; Qiu, Y. Anti-inflammatory sesquiterpenoids from the heartwood of Juniperus formosana Hayata. Fitoterapia 2022, 157, 105105. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, Y.O.; Salabarria, I.S.; Collado, I.G.; Hernández-Galán, R. The antifungal activity of widdrol and its biotransformation by Colletotrichum gloeosporioides (penz.) Penz. & Sacc. and Botrytis cinerea Pers.: Fr. J. Agric. Food Chem. 2006, 54, 7517–7521. [Google Scholar] [CrossRef]
- Nuñez, Y.O.; Salabarria, I.S.; Collado, I.G.; Hernández-Galán, R. Sesquiterpenes from the wood of Juniperus lucayana. Phytochemistry 2007, 68, 2409–2414. [Google Scholar] [CrossRef]
- Barrero, A.F.; Quílez del Moral, J.F.; Lara, A.; Herrador, M.M. Antimicrobial activity of sesquiterpenes from the essential oil of Juniperus thurifera wood. Planta Med. 2005, 71, 67–71. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Silva, A.M.S. The chemical composition of the Juniperus genus (1970–2004). In Recent Progress in Medicinal Plants; Studium Press LLC: Houston, TX, USA, 2007; Volume 16, pp. 401–522. [Google Scholar] [CrossRef]
- De Marino, S.; Cattaneo, F.; Festa, C.; Zollo, F.; Iaccio, A.; Ammendola, R.; Incollingo, F.; Iorizzi, M. Imbricatolic acid from Juniperus communis L. prevents cell cycle progression in CaLu-6 cells. Planta Med. 2011, 77, 1822–1828. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, J.Y.; Fu, W.H.; Liu, S.Z.; Xie, S.Z.; Wang, Z.; Qiu, Y.K. Two new troponoides with anti-inflammatory activity from the stems of Juniperus formosana Hayata. Nat. Prod. Res. 2021, 35, 4901–4906. [Google Scholar] [CrossRef]
- Al Groshi, A.; Jasim, H.A.; Evans, A.R.; Ismail, F.M.D.; Dempster, N.M.; Nahar, L.; Sarker, S.D. Growth inhibitory activity of biflavonoids and diterpenoids from the leaves of the Libyan Juniperus phoenicea against human cancer cells. Phytother. Res. 2019, 33, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Quílez del Moral, J.F.; Herrador, M.M.; Akssira, M.; Bennamara, A.; Akkad, S.; Aitigri, M. Oxygenated diterpenes and other constituents from Moroccan Juniperus phoenicea and Juniperus thurifera var. africana. Phytochemistry 2004, 65, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Janar, J.; Nugroho, A.E.; Wong, C.P.; Hirasawa, Y.; Kaneda, T.; Shirota, O.; Morita, H. Sabiperones A-F, new diterpenoids from Juniperus sabina. Chem. Pharm. Bull. 2012, 60, 154–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Comte, G.; Allais, D.P.; Chulia, A.J.; Vercauteren, J.; Pinaud, N. Three phenylpropanoids from Juniperus phœnicea. Phytochemistry 1997, 44, 1169–1173. [Google Scholar] [CrossRef]
- Comte, G.; Chulia, A.J.; Vercauteren, J.; Allais, D.P. Phenylpropane glycosides from Juniperus phœnicea. Planta Med. 1996, 62, 88–89. [Google Scholar] [CrossRef]
- Inatomi, Y.; Murata, H.; Inada, A.; Nakanishi, T.; Lang, F.A.; Murata, J.; Iinuma, M. New glycosides of acetophenone derivatives and phenylpropanoids from Juniperus occidentalis. J. Nat. Med. 2013, 67, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Ved, A.; Gupta, A.; Rawat, A.K. Antioxidant and hepatoprotective potential of phenol-rich fraction of Juniperus communis Linn. leaves. Pharmacogn. Mag. 2017, 13, 108–113. [Google Scholar]
- Bais, S.; Gill, N.S.; Kumar, N. Neuroprotective effect of Juniperus communis on chlorpromazine induced parkinson disease in animal model. Chin. J. Biol. 2015, 2015, 542542. [Google Scholar] [CrossRef]
- Renouard, S.; Lopez, T.; Hendrawati, O.; Dupre, P.; Doussot, J.; Falguieres, A.; Ferroud, C.; Hagege, D.; Lamblin, F.; Laine, E.; et al. Podophyllotoxin and deoxypodophyllotoxin in Juniperus bermudiana and 12 other Juniperus species: Optimization of extraction, method validation, and quantification. J. Agric. Food Chem. 2011, 59, 8101–8107. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Flores-Félix, J.D.; Coutinho, P.; Alves, G.; Silva, L.R. Zimbro (Juniperus communis L.) as a promising source of bioactive compounds and biomedical activities: A review on recent trends. Int. J. Mol. Sci. 2022, 23, 3197. [Google Scholar] [CrossRef]
- Bais, S.; Gill, N.S.; Rana, N.; Shandil, S. A phytopharmacological review on a medicinal plant: Juniperus communis. Int. Sch. Res. Not. 2014, 2014, 634723. [Google Scholar] [CrossRef] [PubMed]
- Tunón, H.; Olavsdotter, C.; Bohlin, L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. J. Ethnopharmacol. 1995, 48, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Pepeljnjak, S.; Kosalec, I.; Kalodera, Z.; Blazević, N. Antimicrobial activity of juniper berry essential oil (Juniperus communis L., Cupressaceae). Acta Pharm. 2005, 55, 417–422. [Google Scholar]
- Fierascu, I.; Ungureanu, C.; Avramescu, S.M.; Cimpeanu, C.; Georgescu, M.I.; Fierascu, R.C.; Ortan, A.; Sutan, A.N.; Anuta, V.; Zanfirescu, A.; et al. Genoprotective, antioxidant, antifungal and anti-inflammatory evaluation of hydroalcoholic extract of wild-growing Juniperus communis L. (Cupressaceae) native to Romanian southern sub-Carpathian hills. BMC Complement. Altern. Med. 2018, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Abbassy, M.A.; Marei, G.I.K. Antifungal and chemical composition of essential oils of Juniperus communis L. and Thymus vulgaris L. against two phytopathogenic fungi. J. Appl. Sci. Res. 2013, 9, 4584–4588. [Google Scholar]
- Garg, G.P. Screening and evaluation of pharmacognostic, phytochemical and hepatoprotective activity of J. communis L. stems. Int. J. Pharma Bio Sci. 2010, 1, 17–23. [Google Scholar]
- Sánchez de Medina, F.; Gámez, M.J.; Jiménez, I.; Jiménez, J.; Osuna, J.I.; Zarzuelo, A. Hypoglycemic activity of juniper “berries”. Planta Med. 1994, 60, 197–200. [Google Scholar] [CrossRef]
- Bais, S.; Patel, N.J. In vitro anti diabetic and anti obesity effect of J. communis extract on 3T3L1 mouse adipocytes: A possible role of MAPK/ERK activation. Obes. Med. 2020, 18, 100219. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsiao, C.Y.; Lee, S.C.; Huang, X.F.; Chang, K.F.; Lee, M.S.; Hsieh, M.C.; Tsai, N.M. Suppression of oral cancer by induction of cell cycle arrest and apoptosis using Juniperus communis extract. Biosci. Rep. 2020, 40, BSR20202083. [Google Scholar] [CrossRef]
- Li, C.Y.; Lee, S.C.; Lai, W.L.; Chang, K.F.; Huang, X.F.; Hung, P.Y.; Lee, C.P.; Hsieh, M.C.; Tsai, N.M. Cell cycle arrest and apoptosis induction by Juniperus communis extract in esophageal squamous cell carcinoma through activation of p53-induced apoptosis pathway. Food Sci. Nutr. 2021, 9, 1088–1098. [Google Scholar] [CrossRef]
- Sahin Yaglioglu, A.; Eser, F. Screening of some Juniperus extracts for the phenolic compounds and their antiproliferative activities. S. Afr. J. Bot. 2017, 113, 29–33. [Google Scholar] [CrossRef]
- van Slambrouck, S.; Daniels, A.L.; Hooten, C.J.; Brock, S.L.; Jenkins, A.R.; Ogasawara, M.A.; Baker, J.M.; Adkins, G.; Elias, E.M.; Agustin, V.J.; et al. Effects of crude aqueous medicinal plant extracts on growth and invasion of breast cancer cells. Oncol. Rep. 2007, 17, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.C.; Huang, R.L.; Huang, X.F.; Chang, K.F.; Lee, C.J.; Hsiao, C.Y.; Lee, S.C.; Tsai, N.M. Evaluation of anticancer effects of Juniperus communis extract on hepatocellular carcinoma cells in vitro and in vivo. Biosci. Rep. 2021, 41, BSR20211143. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Shiu, L.L. Two new sesquiterpenes, 12-hydroxy-α-longipinene (I) and 15- hydroxyacora-4(14),8-diene (II), from the heartwood of Juniperus chinensis Linn. var. tsukusiensis Masam. Chem. Pharm. Bull. 1996, 44, 1758–1760. [Google Scholar] [CrossRef][Green Version]
- Shiu, L.L.; Chen, W.C.; Kuo, Y.H. Five new cis-himachalane-type sesquiterpenes from the heartwood of Juniperus chinensis var. tsukusiensis. Chem. Pharm. Bull. 1999, 47, 557–560. [Google Scholar] [CrossRef][Green Version]
- Chang, F.R.; Wang, S.W.; Chen, S.R.; Lee, C.Y.; Sheu, J.H.; Cheng, Y.B. Aleuritin, a novel dinor-diterpenoid from the twigs of Aleurites moluccanus with an anti-lymphangiogenic effect. Org. Biomol. Chem. 2020, 18, 7892–7898. [Google Scholar] [CrossRef]
- Jeong, D.; Watari, K.; Shirouzu, T.; Ono, M.; Koizumi, K.; Saiki, I.; Kim, Y.C.; Tanaka, C.; Higuchi, R.; Miyamoto, T. Studies on lymphangiogenesis inhibitors from Korean and Japanese crude drugs. Biol. Pharm. Bull. 2013, 36, 152–157. [Google Scholar] [CrossRef]
- Lee, T.H.; Hsieh, C.L.; Wu, H.C.; Wang, S.W.; Yu, C.L.; Hsiao, G.; Cheng, M.J.; Hsieh, W.T.; Kuo, Y.H. Anti-lymphangiogenic diterpenes from the bark of Calocedrus macrolepis var. formosana. J. Food Drug Anal. 2021, 29, 606–621. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Yang, I.C.; Chen, C.S.; Lin, Y.T. Five new sesquiterpenes from the heartwood of Juniperus Squamata Lamb. J. Chin. Chem. Soc. 1987, 34, 125–134. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chen, W.C. Three new diterpenes, 1,3-dioxototarol, isototarolenone, and 1-oxo-3β-hydroxytotarol, from the roots of Juniperus chinensis Linn. Chem. Pharm. Bull. 1994, 42, 1774–1776. [Google Scholar] [CrossRef][Green Version]
- Lin, T.C.; Fang, J.M.; Cheng, Y.S. Terpenes and lignans from leaves of Chamaecyparis formosensis. Phytochemistry 1999, 51, 793–801. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Ming Tsang, Y. Dehydroabietane diterpenes from Juniperus formosana hay. var. concolor hay. Phytochemistry 1996, 42, 779–781. [Google Scholar] [CrossRef]
- Feliciano, A.S.; López, J.L.; Medarde, M.; del Corral, J.M.M.; de Pascual-Teresa, B.; Puebla, P. Thuriferic acid. A novel lignan type from Juniperus thurifera L. Tetrahedron 1988, 44, 7255–7260. [Google Scholar] [CrossRef]
- Huang, T.; Ying, S.H.; Li, J.Y.; Chen, H.W.; Zang, Y.; Wang, W.X.; Li, J.; Xiong, J.; Hu, J.F. Phytochemical and biological studies on rare and endangered plants endemic to China. Part XV. Structurally diverse diterpenoids and sesquiterpenoids from the vulnerable conifer Pseudotsuga sinensis. Phytochemistry 2020, 169, 112184. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Liang, G.Y.; Sy, L.K. Terpenoids from the seeds of Artemisia annua. Phytochemistry 2003, 64, 303–323. [Google Scholar] [CrossRef]
- Fang, J.M.; Chen, Y.C.; Wang, B.W.; Cheng, Y.S. Terpenes from heartwood of Juniperus chinensis. Phytochemistry 1996, 41, 1361–1365. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Pu, F.S.; Lin, Y.T. The structure of epicedranediol. J. Chin. Chem. Soc. 1977, 24, 141–142. [Google Scholar] [CrossRef]
- Chetty, G.L.; Dev, S. Mayurone, A C14-sesquiterpene ketone. Tetrahedron Lett. 1965, 6, 3773–3776. [Google Scholar] [CrossRef]
- Cool, L.G.; Jiang, K. Thujopsene- and cis-muurolane-related sesquiterpenoids from Cupressus bakeri. Phytochemistry 1995, 40, 177–181. [Google Scholar] [CrossRef]
- Nagahama, S.; Tazaki, M. Terpenoids. IX. Permanganate oxidation of Thujopsene. neutral products. Bull. Chem. Soc. Jpn. 1987, 60, 4175–4177. [Google Scholar] [CrossRef]
- Schneider, I.; Gibbons, S.; Bucar, F. Inhibitory activity of Juniperus communis on 12(S)-HETE production in human platelets. Planta Med. 2004, 70, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Itô, S.; Endo, K.; Honma, H.; Ota, K. New constituents of Thujopsis dolabrata. Tetrahedron Lett. 1965, 6, 3777–3781. [Google Scholar] [CrossRef]
- Tai, H.C.; Lee, T.H.; Tang, C.H.; Chen, L.P.; Chen, W.C.; Lee, M.S.; Chen, P.C.; Lin, C.Y.; Chi, C.W.; Chen, Y.J.; et al. Phomaketide A inhibits lymphangiogenesis in human lymphatic endothelial cells. Mar. Drugs 2019, 17, 215. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).