Abstract
Since the early 20th century, Iris maackii (Iridaceae) has been considered a synonym of I. laevigata, a synonym of I. pseudacorus, or an accepted species. The current concept of I. maackii in the literature and databases is often applied to yellow-flowered plants with prominently veined rosette leaves, which are diagnostic features of I. pseudacorus growing in Northeast Asia. Therefore, the objective was to clarify the taxonomic identity of I. maackii. This study is based on a critical examination of the literature, on the observed morphological characters in the holotype of I. maackii, and on a morphological comparison of I. maackii with living plants of I. laevigata and I. pseudacorus. Additionally, a morphometric comparison of the seed characters was carried out to clarify the morphological distinction among I. maackii, I. laevigata, and I. pseudacorus. A careful study demonstrated that the rosette leaf texture and the morphology of the flowering stem, fruit, and seeds of I. maackii are identical to or within the variation range of I. laevigata. Thus, I. maackii is morphologically non-distinct from I. laevigata and should be recognized as a taxonomic synonym of the latter. An image of the holotype of I. maackii is provided along with detailed illustrations of I. laevigata and I. pseudacorus.
1. Introduction
Richard Maack, an explorer and naturalist, took part in an expedition up the Ussuri River in June–August 1859 [1]. The botanical material from that expedition was treated by Eduard August von Regel in St. Petersburg [2]. In particular, Maack collected a fruiting specimen (Figure 1) from marshes on the Chinese left bank of the Ussuri River upstream of Shang-Ong (currently known as Hutou, northeastern Heilongjiang Province, China), opposite the mouth of the Iman River (currently known as the Bol’shaya Ussurka River, Primorsky Krai, Russia), on 15 July (27 July, according to the new calendar), 1859 [1]. Originally, Regel [2] (p. 148) identified this specimen as Iris pseudacorus L. However, Carl Johann Maximowicz described I. maackii Maxim. on the basis of Maack’s specimen [3].

Figure 1.
Holotype of Iris maackii (LE01010783) (included with the permission of the curator).
The usage of the name I. maackii varies in the literature and databases. In fact, after being described, it was considered a synonym of I. laevigata Fisch. [4,5,6,7,8,9,10,11,12,13], or as a synonym of I. pseudacorus [14]. Currently, I. maackii is considered an accepted species native to Northeast Asia [15,16,17,18,19,20,21,22,23,24].
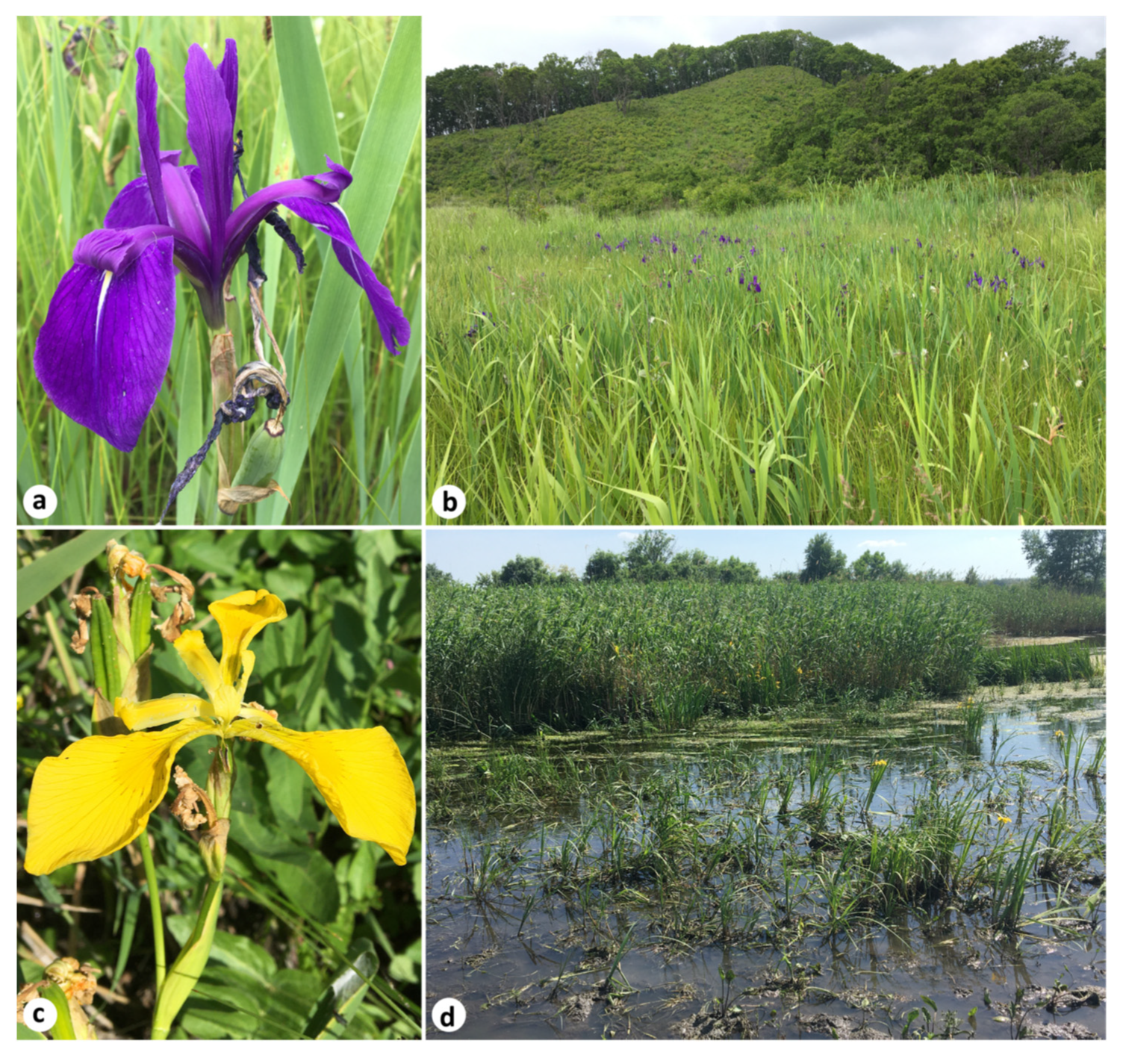
Iris laevigata and I. pseudacorus are ornamental, wetland-associated, herbaceous perennials belonging to I. ser. Laevigatae (Diels) G.H.M.Lawr., according to the conservative taxonomy of Iris [8,9,25,26]. To the best of my knowledge, the blue-flowered I. laevigata (Figure 2a,b) is native to Northeast Asia, i.e., to the Russian Far East, northeastern China (Heilongjiang and Jilin provinces), the Korean Peninsula, and Japan (Hokkaido and Honshu islands). The natural distribution of I. pseudacorus, long known as the “yellow iris” and “yellow flag” (Figure 2c,d), covers Europe and extends to Western Siberia, Western Asia, and the northern fringe of Africa [27,28].

Figure 2.
Images of the Iris species studied: a flower (a) and a habitat (b) of I. laevigata on a floating mat near Rudnev Bay, Primorsky Krai, Russia (42°55′10″ N 132°28′40″ E); a flower (c) and a habitat (d) of I. pseudacorus in the Tuzlov River, Rostov Oblast, Russia (47°28′15″ N 39°27′59″ E). Photos by the author.
Regarding I. pseudacorus, it is necessary to pay special attention to some of its biological characteristics. On the one hand, due to the ability of I. pseudacorus to remove pollutants from water [27,29,30,31] and soil [32], it has been suggested to be used as an available and economically efficient species for phytoremediation. On the other hand, since I. pseudacorus has been extensively cultivated and naturalized, it is becoming highly invasive in North America, in the southern half of South America, southern South Africa, southeastern Australia, and New Zealand [21,33]. However, I. maackii is currently illustrated with images of I. pseudacorus and, therefore, these taxa are actually considered to be identical [34,35,36,37,38,39]. For this reason, the study of the morphological characters of I. maackii, as determined by its nomenclatural type, will contribute to the understanding of its taxonomy. This circumstance can undoubtedly improve the monitoring of the species’ invasion and help adjust the biocontrol program for I. pseudacorus [40,41,42].
This study aims to clarify the taxonomic identity of I. maackii in order to disentangle the confusion around this name. A comparison of rosette leaves, flowering stems, fruits, and seed morphology among I. maackii, I. laevigata, and I. pseudacorus, including data from the literature and field surveys, is presented. Detailed morphological illustrations of I. laevigata and I. pseudacorus based on complete material collected by the author are provided.
2. Materials and Methods
2.1. Plant Material and Morphological Study
Iris maackii was examined based on a single specimen (LE01010783!; Figure 1) that is a holotype of the name, consisting of two rosette leaf fragments and the upper part of the flowering stem, bearing mainly immature fruit. For plant morphology, this specimen was re-examined and 20 morphological characters, including 14 quantitative and 6 qualitative, were selected. These characters are listed in detail in Table 1. The author collected a total of 90 individuals of I. laevigata from a wild locality in the vicinity of Shtykovo Village (43°21′35″ N 132°22′1″ E, Primorsky Krai, Russia) on 27 June 2021 (Table S1). From 22 July to 3 August 2021, 63 individuals of I. pseudacorus were measured directly in the living collection of the Botanical Garden-Institute (BGI, Vladivostok, Russia). The measurements were taken during fruiting.

Table 1.
Morphological characters examined.
For the seed morphology, material from eight collection sites was used (Table 2). The study was conducted on mature seeds. The seeds of I. maackii were taken from one of the locules in the only mature fruit of LE01010783. For I. laevigata and I. pseudacorus, seeds were collected from different individuals for each site. The seeds of I. laevigata were from three wild localities in Russia, one of which is located near Lake Baikal, from where the species was described [43], and from two localities in Primorsky Krai. The seeds of I. pseudacorus were from the living collection of the BGI, Vladivostok, and also from two localities in Sakhalin Island (originally identified as I. maackii), one of which is indicated in references [16,44], and from a native population in the Don River delta.
The terminology used in the descriptions follows reference [45]. For the taxonomy, the Shenzhen Code (hereafter, ICN [46]) was consulted. Relevant literature, including the protologue of I. maackii [3], was also analyzed. The herbarium codes follow Index Herbariorum [47].

Table 2.
Collection site data for seeds of the Iris species studied.
Table 2.
Collection site data for seeds of the Iris species studied.
| No. | Species | Origin | Voucher (*) |
|---|---|---|---|
| 1 | I. maackii | China, Ussuri River | R. Maack (LE01010783, holotype) |
| 2 | I. laevigata | Buryatia, Lake Baikal, near the Vydrinaya River estuary, 51°29′29.6″ N 104°50′39.3″ E | Yu.N. Pochinchik (VBGI) |
| 3 | Primorsky Krai, Bolshaya Ussurka River, Roshchino Village, 45°53′12.6″ N 134°50′48.3″ E | L.M. Pshennikova (VBGI) | |
| 4 | Primorsky Krai, Khasansky District, Cape L’va, 42°41′60.0″ N 131°14′12.3″ E | E.A. Chubar (VBGI) | |
| 5 | I. pseudacorus | Primorsky Krai, Vladivostok, BGI FEB RAS, 43°13′27″ N 131°59′38″ E | E.V. Boltenkov (VBGI, cult.) |
| 6 | Sakhalin Island, 3 km south of Shebunino Village | s. coll. (No. 2086, sub I. maackii) ** | |
| 7 | Sakhalin Island, Dolinsk | s. coll. (No. 2066, sub I. maackii) ** | |
| 8 | Rostov Oblast, Don River delta, 47°07′49.3″ N 39°28′07.7″ E | A.N. Shmaraeva (RWBG) |
The collection site No. 6 was mentioned in references [16,44]; all sites of I. laevigata and I. pseudacorus are from Russia. * Herbarium codes follow Index Herbariorum [47]. ** Seed laboratory, Botanical Gardens of Peter the Great, Komarov Botanical Institute RAS (St. Petersburg, Russia).
2.2. Morphometric Analysis
The morphometric analysis was based on four seed characters (see Table 1), and used 50 seeds from each collection site (Table 2 and Table S2). All the statistical analyses were performed in the R software [48], version 4.1.2 [49]. The data were evaluated by one-way analysis of variance (ANOVA). After multiple statistical testing, the calculated p-values were adjusted using the procedure proposed by Benjamini and Hochberg [50]. To test the ANOVA assumptions, the Shapiro–Wilk test for the normality of the distribution [49] and Levene’s test for the homogeneity of the variance [51] were performed. The effect size (η2) for ANOVA and Cohen’s d for the difference in the means were calculated using the respective functions of the R add-on package “lsr”, version 0.6.1 [52]. If the ANOVA showed a statistically significant difference among species, then subsequent pairwise comparisons were made using Dunnett’s many-to-one test [53]. The data for I. maackii were used as a control. The inequality of variance was taken into account by using the heteroscedastic consistent covariance estimation provided in the R add-on package “sandwich”, version 2.3.0 [54,55]. The differences between the mean values of each collection site (Table 2) and the control were considered statistically significant at a p-value < 0.05.
Finally, a principal component analysis (PCA) [56] was performed on the morphometric parameters of the seeds, i.e., the L, W, T, and L/W ratio (see Table 1), to visualize the distribution of species over the space of the quantitative multivariate data and to assess their delimitation. For the PCA analysis, the built-in function prcomp was used, and the results of the analysis were extracted and visualized using the respective functions of the factoextra R package [57]. In the PCA scatter plot, only the first (PC1) and second (PC2) principal components were considered to represent the data.
3. Results
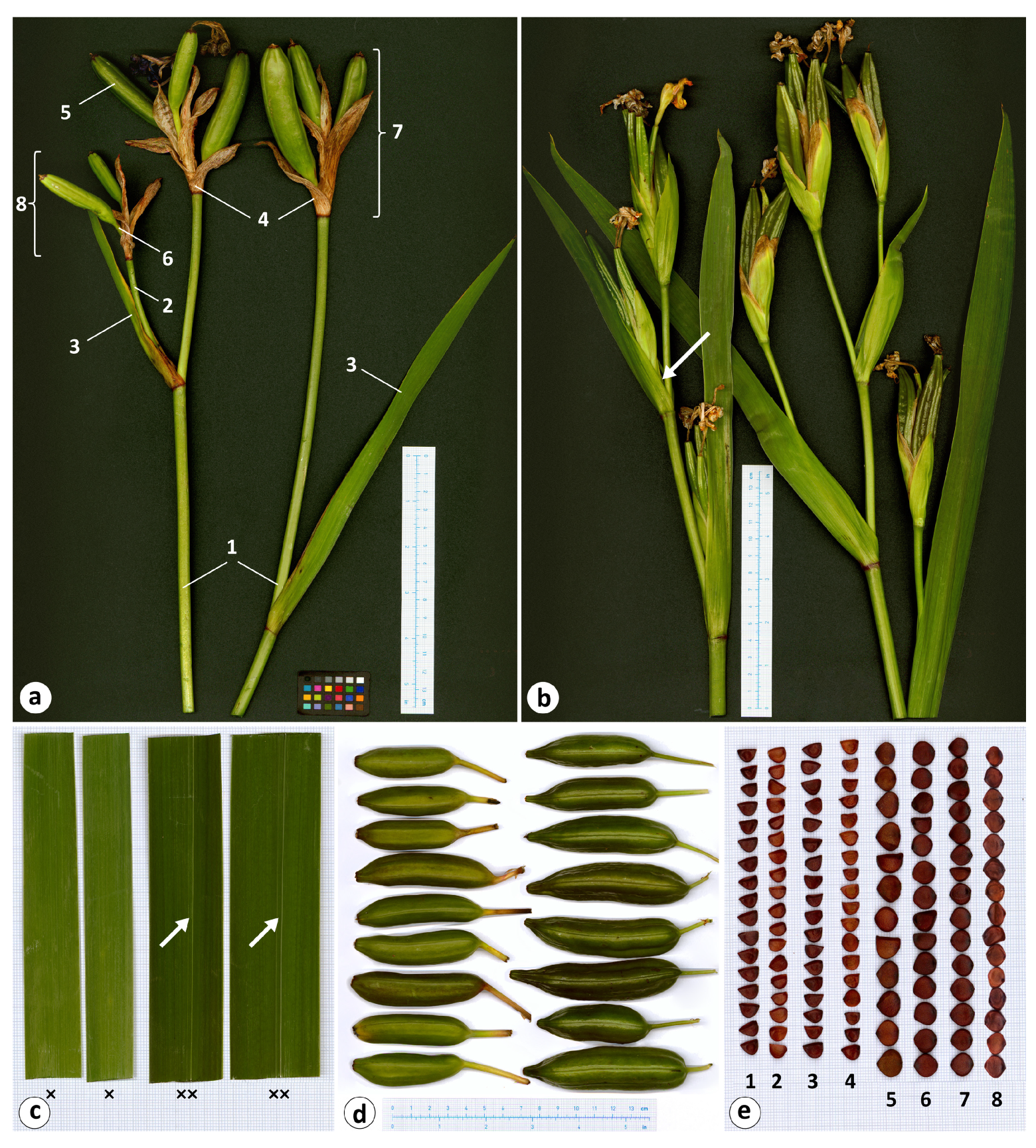
The selected characters of the species under study are shown in Figure 3 and Table 3 (also see Table S1). The holotype of I. maackii and the I. laevigata individuals were found to have no more than one lateral cluster per flowering stem, while in I. pseudacorus, there were usually more than one, and up to four, lateral clusters (Figure 1 and Figure 3a,b). Iris maackii and I. laevigata had smoothed rosette leaves that lacked the prominent midrib, a taxonomic feature of I. laevigata, with an average width of 1.7 cm; in I. pseudacorus, these leaves were prominently ribbed and broader, on average, by 60%, to 4.4 cm wide (Figure 1 and Figure 3c). The shoot of the upper lateral cluster was conspicuous and comparatively long in I. maackii and I. laevigata; in I. pseudacorus, it was usually inconspicuous or shorter than in I. laevigata (Figure 3a,b and Table 3). In I. maackii and I. laevigata, the upper cauline leaf was usually longer than in I. pseudacorus (Figure 3a,b). The bracts of I. laevigata were dry during fruiting; in I. pseudacorus, they were green. The number of fruit per stem was no greater than seven in I. maackii and I. laevigata, and they had smoothed surfaces and were obtuse at the apex; in I. pseudacorus, they were numerous (on average, 10), three-angled, and conspicuously beaked at the apex (Figure 3d). All three species shared the following characters: the bract and pedicel length, the number of fruit per terminal cluster and per upper lateral cluster, and the length and width of the fruit, which were oblong, cylindrical, and obtuse at the base (Figure 1 and Figure 3, Table 3).

Figure 3.
Morphological characters of Iris laevigata and I. pseudacorus: (a) flowering stems of I. laevigata (marks are as follows: 1, stem; 2, lateral shoot; 3, upper cauline leaf; 4, outer bract; 5, fruit; 6, pedicel; 7, terminal cluster; 8, lateral cluster); (b) flowering stems of I. pseudacorus (arrow indicates the inconspicuous lateral shoot); (c) a middle part of the rosette leaves (×, I. laevigata; ××, I. pseudacorus; arrows indicate the prominent midrib); (d) fruit (left row, I. laevigata; right row, I. pseudacorus); and (e) seeds (1–8 are collection site numbers; see Table 1). Photos by the author. Images were taken using an ObjectScan 1600 scanner (Microtek International Inc., Taiwan).

Table 3.
Comparative morphology of the Iris species studied.
The seed characters of the species under study are presented in Table 4 (also see Table S2). Morphologically, the seeds from all collection sites (Table 2) were brown, glossy, and flattened with a smooth surface and a more or less fragile testa (Figure 3e). The seed shape was oblong (L/W ratio: 1.4) or D-shaped in I. maackii; oblong (L/W ratio: 1.3–1.4), D-shaped, or, rarely, almost round in I. laevigata; in I. pseudacorus, the seed shape was almost round (L/W ratio: 0.9–1.1), predominantly subacute, or, much rarer, rounded at the chalaza. The seeds of I. maackii were similar in size to those of I. laevigata (Table 4). The seed length in I. laevigata ranged from 4.5 to 7.5 mm, the width from 3.8 to 6.6 mm, and the thickness from 1.4 to 3.6 mm. In I. pseudacorus, the values of these characters were greater: the seed length ranged from 5.6 to 9.6 mm, the width from 5.2 to 10.3 mm, and the thickness from 1.7 to 4.7 mm.

Table 4.
Morphological characters of seeds from the Iris species studied.
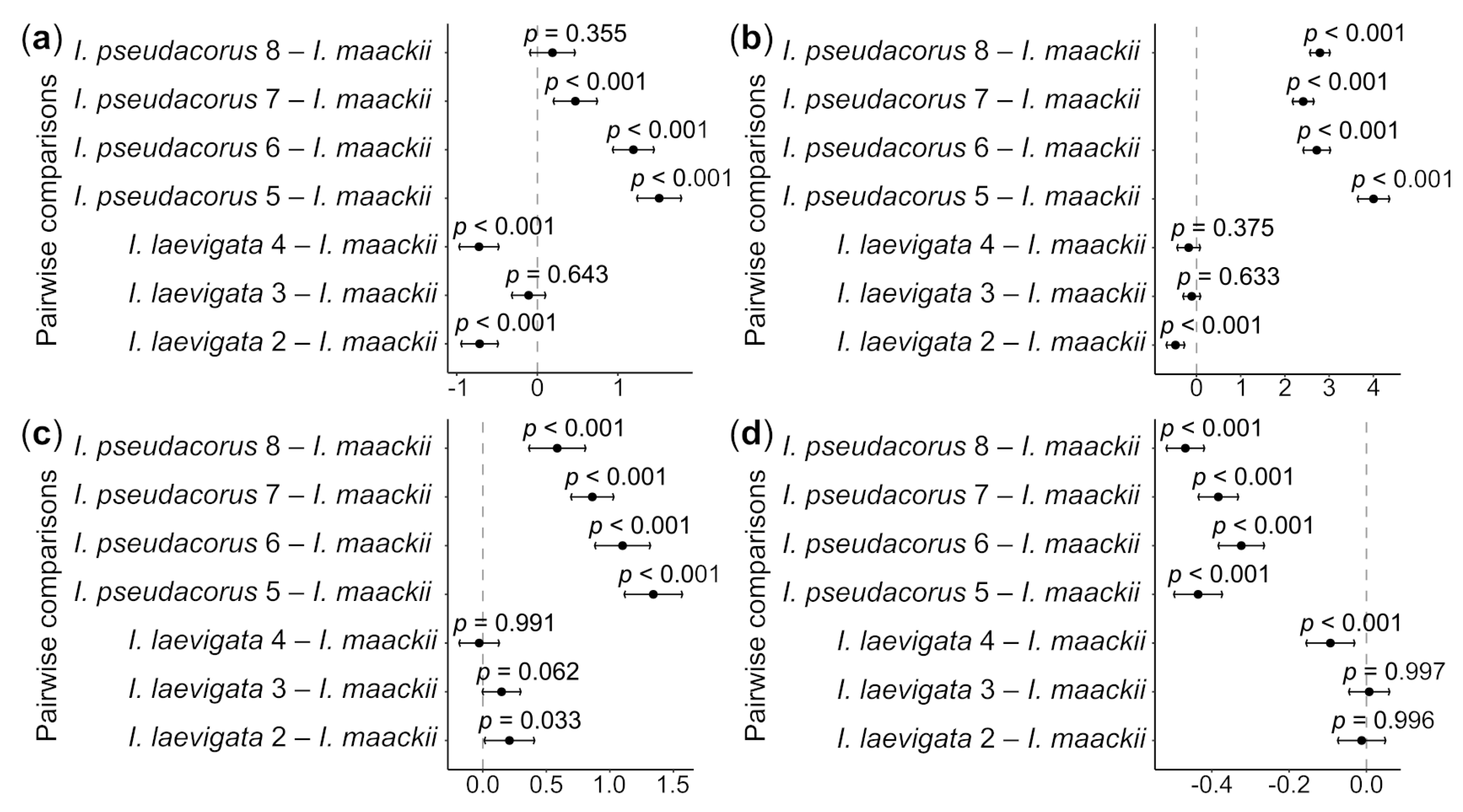
A statistically significant difference in all the morphometric parameters of the seeds was observed between the collection sites of I. laevigata and I. pseudacorus (see Table 1) and I. maackii (Figure 4; for ANOVA results, see Table S3). In particular, the seeds from sites 2 and 4 were approximately 10% shorter than those of I. maackii, with this difference being statistically significant (p < 0.001); the difference in the mean seed length between site 3 and I. maackii was 1.5%, being statistically non-significant (p > 0.05). The seeds from sites 5–7 were approximately 6.8–21.9% longer than the seeds of I. maackii, with this difference being statistically significant (p < 0.001). The difference in the seed length between site 8 and I. maackii was no greater than 2.7% and was statistically non-significant (p > 0.05).
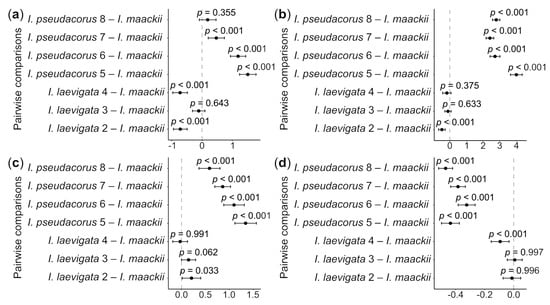
Figure 4.
Whisker plots showing the results of Dunnett’s test of differences in the mean values of length (a), width (b), thickness (c), and L/W ratio (d) between the Iris laevigata and I. pseudacorus collection sites (see Table 2) and I. maackii, selected as a control. The results of the test are presented as p-values, differences between the mean values of each experimental group (black dots), and 95% confidence intervals of these differences (whiskers) for each pairwise comparison. X-axis is differences in means. Dash-dotted line indicates zero difference. See Table S3 for more details.
No statistically significant differences in the seed width and thickness were found between collection sites 3 and 4 and I. maackii (p > 0.05), while there was a small, approximately 9.4%, but statistically significant (p < 0.001), difference between site 2 and I. maackii. There was no difference in the L/W ratio between sites 2 and 3 and I. maackii (p > 0.05). However, a small, approximately 6.8%, but statistically significant (p < 0.001), difference was found in the L/W ratio between site 4 and I. maackii. For I. pseudacorus, the mean seed width was approximately 37% greater, the seed thickness approximately 33.5% greater, and the L/W ratio approximately 41% smaller than for I. maackii and these differences were statistically significantly different (p < 0.001).
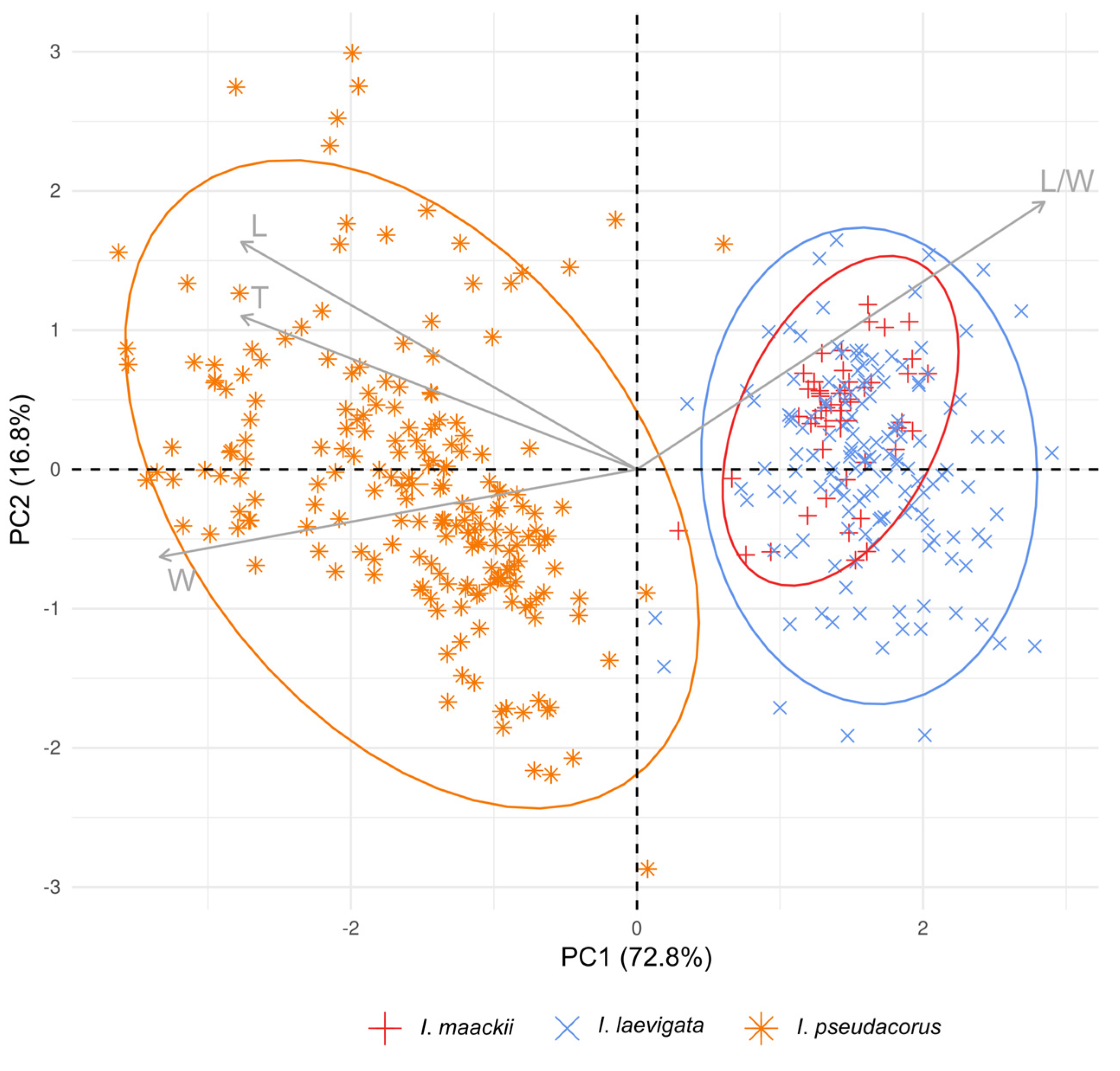
In a PCA scatter plot (Figure 5), the first two principal components explained 89.6% of the total variance and revealed two distinct groups, corresponding to I. laevigata and I. pseudacorus. The first principal component (PC1) explained 72.8% of the total variance and contributed to discriminating the species (Figure 5; also see Table S4). Based on their correlation with the PC1 axis, the L, W, and T morphometric parameters were related to I. pseudacorus on the left side. In contrast, the L/W ratio was more significant for I. maackii and I. laevigata, which completely overlapped on the right side.

Figure 5.
Principal component analysis of the morphometric parameters of seeds from Iris maackii (red), I. laevigata (blue), and I. pseudacorus (orange). Ellipses show 95% high-density regions for normal distributions representing two groups. Arrows indicate contribution of each morphometric parameter. The codes of the morphological characters of seeds are as follows: L, length; W, width; T, thickness; and L/W, length-to-width ratio. See Table S4 for more details.
4. Discussion
4.1. What Is Iris maackii from Northeast Asia According to the Literature?
The taxonomic history of I. maackii began with a single fruiting specimen (Figure 1) collected by Maack from the middle reaches of the Ussuri River, in an area of present-day China, which was originally identified as I. pseudacorus by Regel [2]. Maximowicz came to the conclusion that Maack’s specimen was not I. pseudacorus in the terms of Linnaeus [58] (p. 38), because the leaves lacked the prominent midrib and, thus, the new species I. maackii Maxim. was described [3]. In addition, the mention of I. pseudacorus from Siberia by Regel [2] was indirectly related to references [59,60], according to which this species occurred in the vicinity of Selenginsk, Republic of Buryatia, and near Lake Baikal, Russia. It was rightly noted [4,61] that I. pseudacorus did not occur in Siberia or Manchuria, and its mention by Gmelin [59] (p. 31, No. 29) referred to I. laevigata, which had been described based on plants from the Baikal region and Dahuria [43].
Komarov noted that I. maackii had never been found in the type locality after Maack [62]. Fedtschenko reported that the bract texture and fruit shape of I. maackii were the same as those of I. laevigata, and regarded I. maackii as a synonym of I. laevigata [4]. Ivan Shishkin, a florist of the Far Eastern Branch of the USSR Academy of Sciences, made six expeditions in 1927–1929 with the aim to extend the floristic knowledge of the Iman River and surroundings [7]. In particular, he came to the conclusion that only I. laevigata, including I. pseudacorus in the terms of Regel [2], and I. maackii in the terms of Komarov [62] could be found in this area. Thus, the name I. maackii was synonymized with I. laevigata [5,8,9,10].
On the other hand, I. maackii was indicated for Sakhalin Island and the Kuril Islands and characterized as a plant with a prominently veined mid-rib and yellow flowers [44,63,64,65], although these are the diagnostic features of I. pseudacorus. However, most recent authors state that I. maackii from the Russian Far East is I. pseudacorus, which was introduced there and naturalized [11,66,67,68]. Apparently, Russian settlers in this region introduced I. pseudacorus as a bactericidal rather than an ornamental plant. The essential oils from the rhizomes of I. pseudacorus were shown to have antimicrobial activity [69,70]. Moreover, this plant can reduce the number of coliform bacteria by 50% and Salmonella by 70% in wastewater [27]. In addition, the Far Eastern and European plants of I. pseudacorus have the same chromosome number, 2n = 34 [66] (p. 127), and are not different in the noncoding regions of plastid DNA [26].
In addition, I. maackii was indicated to grow in the Liaoning, Heilongjiang, and Jilin provinces of northeastern China [18,25,71,72,73,74,75]. Zhao noted that the characteristics of I. maackii from Liaoning Province are similar to I. pseudacorus, and, therefore, he doubted that I. maackii was a true species [76]. The authors of the Flora of China noted that further study was needed to determine whether or not I. maackii is separable from I. pseudacorus [25]. Rodionenko suggested that I. maackii from China was the adventive I. pseudacorus [28]. Recently, it has been confirmed that the plants from Liaoning Province cited as I. maackii do, in fact, belong to I. pseudacorus, while I. laevigata was not found in the province [77].
Thus, authors in the beginning of the 21th century treat I. maackii from Northeast Asia as I. pseudacorus. Notwithstanding the above, I. maackii is currently considered an accepted species native to northeastern China and the Russian Far East [15,16,17,18,19,20,21,22,23,24,34,36,37,38,39].
4.2. What Is Iris maackii vs. I. laevigata and I. pseudacorus According to Morphology?
An examination of the holotype of I. maackii (Figure 1) showed it to be identical to I. laevigata in the rosette leaf width and texture, flowering stem branching, length of the upper lateral shoot, bract texture during fruiting, number of fruit per flowering stem, fruit shape, and size and shape of the seeds (Table 3 and Table 4, Figure 1 and Figure 3). In addition, after the characterization of 153 individuals of I. laevigata and I. pseudacorus for 15 morphological characters, it was found that these species could be differentiated from each other, especially in the rosette leaf texture, the flowering stem branching, and the fruit shape (Figure 3 and Table 3).
Among the species studied, the seeds varied in size and shape. By using Dunnett’s test, differences in the morphometric parameters of seeds were found between I. maackii and some of the collection sites of I. laevigata (Figure 4). However, these differences were no greater than 10% and were related to the origin of the seeds: the seeds of I. maackii were from a single available locule of the same fruit and, therefore, had similar sizes (Figure 3e), while the seeds of I. laevigata were from different individuals. In the PCA scatter plot, the characters of I. maackii completely overlapped with those of I. laevigata (Figure 5); the characters were taxonomically useful when the overlap was equal to or lower than a threshold of 25% [78].
Previously, it was reported that the seed characters of I. laevigata were almost identical to those of I. pseudacorus [79,80,81]. However, that finding is not in concurrence with the present study. An analysis of the morphometric parameters of seeds based on Dunnett’s test showed that I. laevigata and I. pseudacorus are distinct (Figure 4). In view of the results of the PCA analysis, the three taxa could unambiguously be separated into two distinct groups with clearly different features in their seed characters (Figure 5). In particular, it was confirmed that the plants listed in references [16,44] as I. maackii from the neighborhood of Shebunino Village, Sakhalin Island, belonged to I. pseudacorus (Figure 4 and Figure 5). Thus, the morphological differences between I. laevigata and I. pseudacorus also include seed characters such as the size and shape.
4.3. Taxonomic Treatment
Based on detailed morphological and morphometric comparisons among I. maackii, I. laevigata, and I. pseudacorus, two species are recognized in the present study, I. laevigata and I. pseudacorus. With regards to I. maackii, the author postulates that this name is a taxonomic synonym of I. laevigata. Information on the accepted species (highlighted in bold italics) is provided below.
Iris laevigata Fisch., Index Seminum (St.Petersburg (Petropolitanus)) 5: 36, 1839.—Lectotype (designated by Alexeeva [82] (p. 417)): In paludibus ad Baicalem, [fl.], 1829, Turcz[aninow] s.n. Herb. C.F. Ledebour (LE01010777!).—http://rr.herbariumle.ru/01010777 (accessed on 13 August 2023).
=Iris maackii Maxim., Bull. Acad. Imp. Sci. Saint-Pétersbourg 26(3): 541, 1880.—Holotype: (China, Heilongjiang Province) (note handwritten by E. Regel): Iris Pseud-Acorus L. teste Rgl. Legit Maack; (note handwritten by C.J. Maximowicz): Iris maackii Maxim. Gegenüber d. [der] Ima Mündung linkes Ussuri uter, [fr.], (15 (27) July 1859); (note handwritten by V.L. Komarov): Уссури, левый берег прoтив устья Имана (Ussuri River, left bank opposite the mouth of the Iman River) (LE01010783!).—Figure 1.
=Iris pseudacorus auct. non L. [2,60].
Iris pseudacorus L., Sp. Pl. 1: 38, 1753.—“I. pseudacorus var. mandshurica L.H.Bailey”, Man. Cult. Pl., ed. 2: 273, 1949, nom. inval. (Art. 38.1 of the ICN).—Lectotype (designated by Crespo [83] (p. 56)): (Specimen from a cultivated plant). PseudoAcorus 7, [fl.], s.d., s. coll. s.n. Herb. Linnaeus (LINN No. 61.7!).—https://www.linnean-online.org/805/ (accessed on 13 August 2023).
=Iris maackii auct. non Maxim. [15,16,25,44,63,64,65,71,72,73,74,75].
5. Conclusions
Since the early 20th century, the taxonomic identity of Iris maackii (Iridaceae) has been unclear, and there have been various speculations as to whether it is an independent species or not. Currently, in most databases, it is regarded as a distinct species native to northeastern China and the Russian Far East [17,18,19,20,21,22,23,24,34,36,37,38,39]. The present report provides a re-evaluation of the taxonomic identity of I. maackii based on a morphological study. In addition, an overview of the taxonomic history of I. maackii, based on numerous publications of scientists from 1861 to the present time, was conducted to establish its true identity. Since I. maackii is known on the basis of a single specimen (Figure 1), there was difficulty with the availability of material for the morphological comparison of this species with I. laevigata and I. pseudacorus, to which it is associated. However, as a result of a careful examination of the holotype of I. maackii, a total of 20 morphological characters were selected.
As argued in the present contribution, I. maackii is a taxonomic synonym of I. laevigata on the basis of a set of characters, including smoothed rosette leaves, one-branched flowering stems, an elongated shoot of the upper lateral cluster, dry bracts during fruiting, fruit that is smoothed and obtuse at the apex, and mostly oblong, D-shaped seeds. In addition, it is of equal importance that the species-specificity of the seed size and shape can be useful in the taxonomic differentiation of I. laevigata and I. pseudacorus. In order to avoid further confusion, it is here stated that the names I. maackii and I. pseudacorus must never be conflated. The present results confirm that the plants from northeastern China and the Russian Far East (viz. Sakhalin Island, Kuril Islands, and Primorsky Krai) indicated in the literature (e.g., [15,16,44]) and databases [17,18,19,20,21,22,23,24,34,36,37,38,39] as I. maackii should be considered I. pseudacorus. An important point is that I. pseudacorus is non-native in Northeast Asia and has become highly invasive in natural and artificial waterbodies in the Neotropics, Afrotropics, Neartic, and Australasia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12193349/s1, Table S1: Raw data of the morphological analysis of the Iris species studied (the numbers of the morphological characters correspond to those in Table 1); Table S2: Raw data of the morphological analysis of seeds from the Iris species (the codes of the morphological characters are provided in Table 1; for the collection site numbers, i.e., 1–8, see Table 2); Table S3: Results of ANOVA and pairwise comparisons between the mean values for each collection site (see Table 1) and the control (I. maackii) using Dunnett’s many-to-one test for the morphometric parameters of seeds; Table S4: Results of the principal component analysis of the morphometric parameters of seeds.
Funding
This research received no external funding.
Data Availability Statement
All data supporting reported results are presented as Supplementary Materials.
Acknowledgments
The author is grateful to Marina Yarichina and Kirill Tkachenko (Komarov Botanical Institute, St. Petersburg, Russia), to Elena Chubar (A.V. Zhirmunsky National Scientific Center of Marine Biology, Vladivostok, Russia (NSCMB FEB RAS)), and to Antonina Shmarayeva (Southern Federal University, Rostov-on-Don, Russia) for providing the seeds of the irises. Special thanks are due to Alexander Kalachev (NSCMB FEB RAS) for his help with the PCA analyses. This study was carried out in the framework of the institutional research project No. 122040800085-4.
Conflicts of Interest
The author declares no conflict of interest.
References
- Maack, R. Journey Through the Valley of the Ussuri River; V. Bezobrazov & Co.: St. Petersburg, Russia, 1861; Volume 1. [Google Scholar]
- Regel, E. Tentamen Florae Ussuriensis Oder Versuch Einer Flora des Ussuri-Gebietes. Mémoires De L’académie Impériale Des Sci. De St. Pétersbourg Septième Série 1861, 4, 1–228. [Google Scholar]
- Maximowicz, C.J. Diagnoses plantarum novarum asiaticarum, III. Bull. Acad. Imp. Sci. Saint-Pétersbourg 1880, 26, 420–542. [Google Scholar]
- Fedtschenko, O. Irideen-Studien; Was ist Iris Maacki Maxim? Allg. Bot. Z. Syst. 1906, 12, 89–90. [Google Scholar]
- Komarov, V.L. Flora Manshuriae, III. Trudy Imp. S. Peterburgsk. Bot. Sada 1907, 25, 335–853. [Google Scholar]
- Koidzumi, G. Contributiones ad cognitionem florae Asiae orientalis. Bot. Mag. 1925, 39, 299–318. [Google Scholar] [CrossRef]
- Shishkin, I.K. Materialy k flore bassejna r. Imana (DVK, Khabarovskij okrug) [Materials to the flora of the Iman river basin (Khabarovsk Okrug)]. Zap. Vladivostoksk. Otd. Gosud. Russk. Geogr. Obsc. 1930, 5, 5–173. [Google Scholar]
- Fedtschenko, B.A. Iris. In Flora of the USSR; Komarov, V.L., Ed.; Izdatel’stvo Akademii nauk SSSR: Leningrad, Russia, 1935; Volume 4, pp. 511–557. [Google Scholar]
- Peckham, E.A.S. Alphabetical Iris Check List; Waverly Press: Baltimore, MD, USA, 1939. [Google Scholar]
- Kitagawa, M. Neo-Lineamenta Florae Manshuricae; J. Cramer: Vaduz, Liechtenstein, 1979. [Google Scholar]
- Pavlova, N.S. Iridaceae Juss. In Flora of the Russian Far East; Kozhevnikov, A.E., Probatova, N.S., Eds.; Dalnauka: Vladivostok, Russia, 2006; pp. 277–279. [Google Scholar]
- Linnegar, S. Series Laevigatae (Diels) Lawrence. In A Guide to Species Irises: Their Identification and Cultivation; The Species Group of the British Iris Society, Ed.; Cambridge University Press: Cambridge, UK, 2012; pp. 160–167. [Google Scholar]
- Hortipedia. Available online: https://en.hortipedia.com/Iris_laevigata (accessed on 13 August 2023).
- Lim, K.Y.; Matyasek, R.; Kovarik, A.; Leitch, A. Parental origin and genome evolution in the allopolyploid Iris versicolor. Ann. Bot. 2007, 100, 219–224. [Google Scholar] [CrossRef]
- Alekseeva, N.B. Genus Iris L. (Iridaceae) in Russia. Turczaninowia 2008, 11, 5–68. [Google Scholar]
- Alexeeva, N.B. Seed morphology in the genus Iris (Iridaceae) from Russia. Vavilovia 2020, 3, 5–28. [Google Scholar] [CrossRef]
- Catalogue of Life. Available online: https://www.catalogueoflife.org/data/taxon/3PZV6 (accessed on 13 August 2023).
- Catalogue of Life China 2023 Annual Checklist. Available online: http://www.sp2000.org.cn/species/show_species_details/T20171000043881 (accessed on 13 August 2023).
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=381594 (accessed on 13 August 2023).
- Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:438832-1 (accessed on 13 August 2023).
- The Global Biodiversity Information Facility. Available online: https://www.gbif.org/ (accessed on 13 August 2023).
- The Plant List. Available online: http://www.theplantlist.org/tpl1.1/record/kew-322090 (accessed on 13 August 2023).
- World Flora Online. Available online: http://www.worldfloraonline.org/taxon/wfo-0000783584 (accessed on 13 August 2023).
- World Plants. Available online: https://www.worldplants.de/world-plants-complete-list/complete-plant-list/#plantUid-74714 (accessed on 13 August 2023).
- Zhao, Y.-T.; Noltie, H.J.; Mathew, B. Iridaceae. In Flora of China; Wu, Z.-Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2000; Volume 24, pp. 297–313. [Google Scholar]
- Boltenkov, E.; Artyukova, E.; Kozyrenko, M.; Erst, A.; Trias-Blasi, A. Iris sanguinea is conspecific with I. sibirica (Iridaceae) according to morphology and plastid DNA sequence data. PeerJ 2020, 8, e10088. [Google Scholar] [CrossRef]
- Sutherland, W.J. Iris pseudacorus L. J. Ecol. 1990, 78, 833–848. [Google Scholar] [CrossRef]
- Rodionenko, G.I. On the taxonomic structure of Iris pseudacorus s.l. (Iridaceae). Bot. Zhurn. 2003, 88, 133–138. [Google Scholar]
- Yousefi, Z.; Mohseni-Bandpei, A. Nitrogen and phosphorus removal from wastewater by subsurface wetlands planted with Iris pseudacorus. Ecol. Eng. 2010, 36, 777–782. [Google Scholar] [CrossRef]
- Machado, A.I.; Fragoso, R.; Dordio, A.V.; Duarte, E. Performance of Iris pseudacorus and Typha domingensis for furosemide removal in a hydroponic system. Int. J. Phytoremediation 2020, 22, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Nawrot, N.; Wojciechowska, E.; Kuittinen, S.; Szczepańska, K.; Dembska, G.; Pappinen, A. Cadmium accumulation by Phragmites australis and Iris pseudacorus from stormwater in floating treatment wetlands microcosms: Insights into plant tolerance and utility for phytoremediation. J. Environ. Manag. 2023, 331, 117339. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Cheng, L.-J.; Zhou, Q.-X. Phytoremediation of petroleum contaminated soils with Iris pseudacorus L. and the metabolic analysis in roots. J. Environ. Sci. 2016, 37, 1531–1538. [Google Scholar]
- Gervazoni, P.; Sosa, A.; Franceschini, C.; Coetzee, J.; Faltlhauser, A.; Fuentes-Rodriguez, D.; Martínez, A.; Hill, M. The alien invasive yellow flag (Iris pseudacorus L.) in Argentinian wetlands: Assessing geographical distribution through different data sources. Biol. Inv. 2020, 22, 3183–3193. [Google Scholar] [CrossRef]
- Asianflora. Available online: http://www.asianflora.com/Iridaceae/Iris-maackii.htm (accessed on 13 August 2023).
- Leipzig Catalogue of Vascular Plants. Available online: https://lifegate.idiv.de (accessed on 13 August 2023).
- Missouri Botanical Garden. Available online: http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=281340&isprofile=0& (accessed on 13 August 2023).
- SIGNA. Available online: http://www.signa.org/index.pl?Iris-maackii (accessed on 13 August 2023).
- The American Iris Society Iris Encyclopedia. Available online: https://wiki.irises.org/Spec/SpecMaackii (accessed on 13 August 2023).
- Wikipedia. Available online: https://en.wikipedia.org/wiki/Iris_maackii (accessed on 13 August 2023).
- Gallego-Tévar, B.; Grewell, B.J.; Whitcraft, C.R.; Futrell, J.C.; Bárcenas-Moreno, G.; Castillo, J.M. Contrasted impacts of yellow flag iris (Iris pseudacorus) on plant diversity in tidal wetlands within its native and invaded distribution ranges. Diversity 2022, 14, 326. [Google Scholar] [CrossRef]
- Minuti, G.; Stiers, I.; Coetzee, J.A. Climatic suitability and compatibility of the invasive Iris pseudacorus L. (Iridaceae) in the Southern Hemisphere: Considerations for biocontrol. Biol. Control 2022, 169, 104886. [Google Scholar] [CrossRef]
- Castillo, J.M.; Gallego-Tévar, B.; Grewell, B.J. Wrack burial limits germination and establishment of yellow flag iris (Iris pseudacorus L.). Plants 2023, 12, 1510. [Google Scholar] [CrossRef]
- Fischer, F.E.L.; Meyer, C.A. Index Quintus Seminum, Quae Hortus Botanicus Imperialis Petropolitanus Pro Mutua Commutatione Offert: Accedunt Animadversiones Botanicae Nonnullae; Academiae Caesareae Petropolitanae: Petropoli, Russia, 1839; Issue 5. [Google Scholar]
- Ponomarchuk, G.I.; Lyubitskaya, E.A. Floristic findings in south of Far East. Byull. Glavn. Bot. Sada 1983, 128, 51–52. [Google Scholar]
- Beentje, H. The Kew Plant Clossary; Kew Publishing: Kew, UK, 2012. [Google Scholar]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. (Eds.) International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017 [Regnum Vegetabile Volume 159]; Koeltz Botanical Books: Glashütten, Germany, 2018. [Google Scholar] [CrossRef]
- Index Herbariorum. Available online: https://sweetgum.nybg.org/ih/ (accessed on 13 August 2023).
- Ihaka, R.; Gentleman, R. R: A language for data analysis and graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 13 August 2023).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Navarro, D. Learning Statistics with R: A Tutorial for Psychology Students and Other Beginners. (Version 0.6.1). Available online: https://kpu.pressbooks.pub/learningstatistics/ (accessed on 13 August 2023).
- Dunnett, C.W. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 1955, 50, 1096–1121. [Google Scholar] [CrossRef]
- Zeileis, A. Econometric computing with HC and HAC covariance matrix estimators. J. Stat. Soft. 2004, 11, 1–17. [Google Scholar] [CrossRef]
- Zeileis, A.; Köll, S.; Graham, N. Various versatile variances: An object-oriented implementation of clustered covariances in R. J. Stat. Softw. 2020, 95, 1–36. [Google Scholar] [CrossRef]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy: The Principles and Practices of Numerical Classification; Freeman: San Francisco, CA, USA, 1973. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, Version 1.0.7. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 13 August 2023).
- Linnaeus, C. Species Plantarum; Laurentii Salvii: Stockholm, Sweden, 1753; Volume 1. [Google Scholar] [CrossRef]
- Gmelin, J.G. Flora Sibirica sive Historia Plantarum Sibiriae; Typographia Academiae Scientiarum: St. Petersburg, Russia, 1747; Volume 1. [Google Scholar]
- Georgi, J.G. Bemerkungen einer Reise im Russischen Reich in den Jahren 1773 und 1774; Kaiserlichen Academie der Wissenschaften: St. Petersburg, Russia, 1775. [Google Scholar]
- Fedtschenko, B.A. Iridaceae. In Flora Transbaicalica; Fedtschenko, B.A., Ed.; Sumptibus Societatis Respublicanae Geographicae Troitzkosavsk–Kiachta: Leningrad, Russia, 1931; Volume 2, pp. 155–157. [Google Scholar]
- Komarov, V.L. Flora Manshuriae, I. Trudy Imp. S.-Peterburgsk. Bot. Sada 1901, 20, 1–559. [Google Scholar]
- Miyabe, K.; Kudô, Y. Flora of Hokkaido and Saghalien III: Monocotyledoneae Araceae to Orchidaceae. J. Fac. Agric. Hokkaido Imp. Univ. 1932, 26, 279–387. [Google Scholar]
- Voroschilov, V.N. Flora Sovetskogo Dal’nego Vostoka [Flora of the Soviet Far East]; Nauka: Moscow, Russia, 1966. [Google Scholar]
- Neczaeva, T.I. Iridaceae Juss. In Key for the Vascular Plants of Sakhalin and Kurile Islands; Tolmatchev, A.I., Ed.; Nauka: Leningrad, Russia, 1974; p. 121. [Google Scholar]
- Probatova, N.S.; Barkalov, V.Y.; Rudyka, E.G. Caryology of the Flora of Sakhalin and the Kurile Islands; Dalnauka: Vladivostok, Russia, 2007. [Google Scholar]
- Barkalov, V.Y. Flora of the Kuril Islands; Dalnauka: Vladivostok, Russia, 2009. [Google Scholar]
- Takahashi, H. Plants of the Kuril Islands; Hokkaido University Press: Sapporo, Japan, 2015. [Google Scholar]
- Ramtin, M.; Massiha, A.; Khoshkholgh-Pahlaviani, M.R.M.; Issazadeh, K.; Assmar, M.; Zarrabi, S. Evaluation of the antibacterial activities of essential oils of Iris pseudacorus and Urtica dioica. Zahedan J. Res. Med. Sci. 2014, 16, 35–39. [Google Scholar]
- Michalak, A.; Krauze-Baranowska, M.; Migas, P.; Kawiak, A.; Kokotkiewicz, A.; Królicka, A. Iris pseudacorus as an easily accessible source of antibacterial and cytotoxic compounds. J. Pharm. Biomed. Anal. 2021, 195, 113863. [Google Scholar] [CrossRef]
- Zhao, Y.-T. Some notes on the genus Iris of China. Acta Phytotax. Sin. 1980, 18, 53–62. Available online: https://www.jse.ac.cn/EN/Y1980/V18/I1/53 (accessed on 13 August 2023).
- Waddick, J.W.; Zhao, Y.-T. Iris of China; Timber Press: Portland, OR, USA, 1992. [Google Scholar]
- Fu, P.Y. Clavis Plantarum Chinae Boreali-Orientalis, 2nd ed.; Science Press: Beijing, China, 1995. [Google Scholar]
- Fu, L.; Hong, T. Higher Plants of China; Qingdao Publishing House: Qingdao, China, 2002; Volume 13, p. 286. [Google Scholar]
- Wu, Z.-Y.; Raven, P.H. Flora of China Illustrations; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2002; Volume 24, p. 344. [Google Scholar]
- Zhao, Y.-T. The wild irises of China. In Proceedings of the International Symposium on Iris, Celebrating the 50th Anniversary of the New Zealand Iris Society, Tauranga, New Zealand, 2–6 November 2000; pp. 39–43. [Google Scholar]
- Zheng, Y.; Meng, T.; Bi, X.; Lei, J. Investigation and evaluation of wild Iris resources in Liaoning Province, China. Genet. Resour. Crop Evol. 2017, 64, 967–978. [Google Scholar] [CrossRef]
- Jiménez-Mejías, P.; Luceño, M.; Martín-Bravo, S. Species boundaries within the southwest old world populations of the Carex flava group (Cyperaceae). Syst. Bot. 2014, 39, 117–131. [Google Scholar] [CrossRef]
- Dykes, W.R. Some garden irises. J. Roy. Hort. Soc. New Ser. 1914, 40, 226–233. [Google Scholar]
- Dykes, W.R. Iris laevigata, Fischer. Gard. Chron. Ser. 3 1920, 67, 274. [Google Scholar]
- Dykes, W.R. A Handbook of Garden Irises; M. Hopkinson & Co.: London, UK, 1924. [Google Scholar] [CrossRef]
- Alexeeva, N.B. Iridaceae Juss. In Catalogue of the Type Specimens of the Vascular Plants from Siberia and the Russian Far East kept in the Herbarium of the Komarov Botanical Institute (LE); Sokolova, I.V., Ed.; KMK Scientific Press: Moscow, Russia, 2012; pp. 415–419. [Google Scholar]
- Crespo, M.B. Nomenclatural types of Iberian irises (Iris and related genera, Iridaceae). Fl. Montiber. 2012, 53, 49–62. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).