Talking Different Languages: The Role of Plant–Plant Communication When an Invader Beats up a Strange Neighborhood
Abstract
:1. Introduction
2. Results
2.1. Exposure Experiment
2.1.1. Aboveground Dry Mass (AGDM)
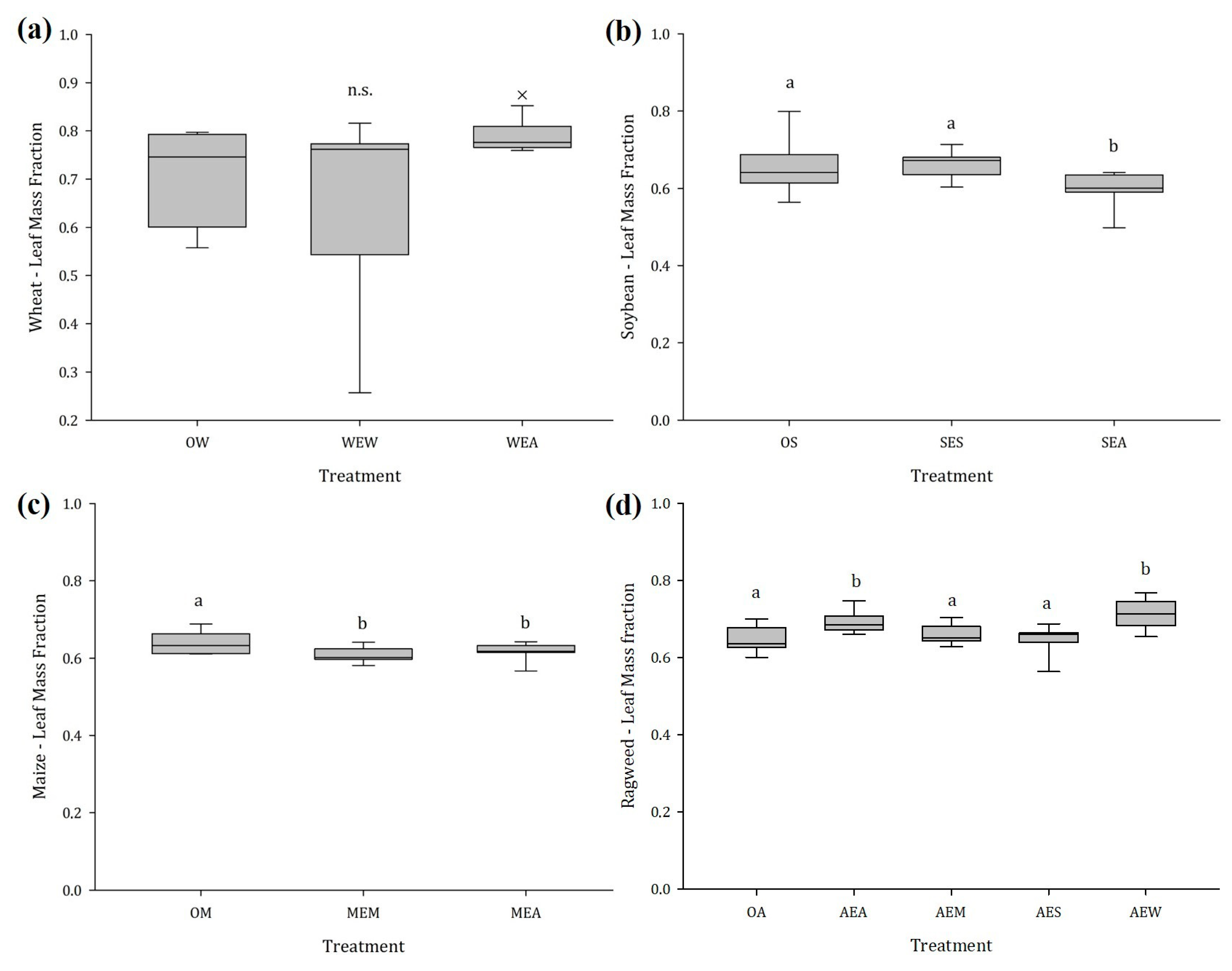
2.1.2. Stem and Leaf Mass Fraction
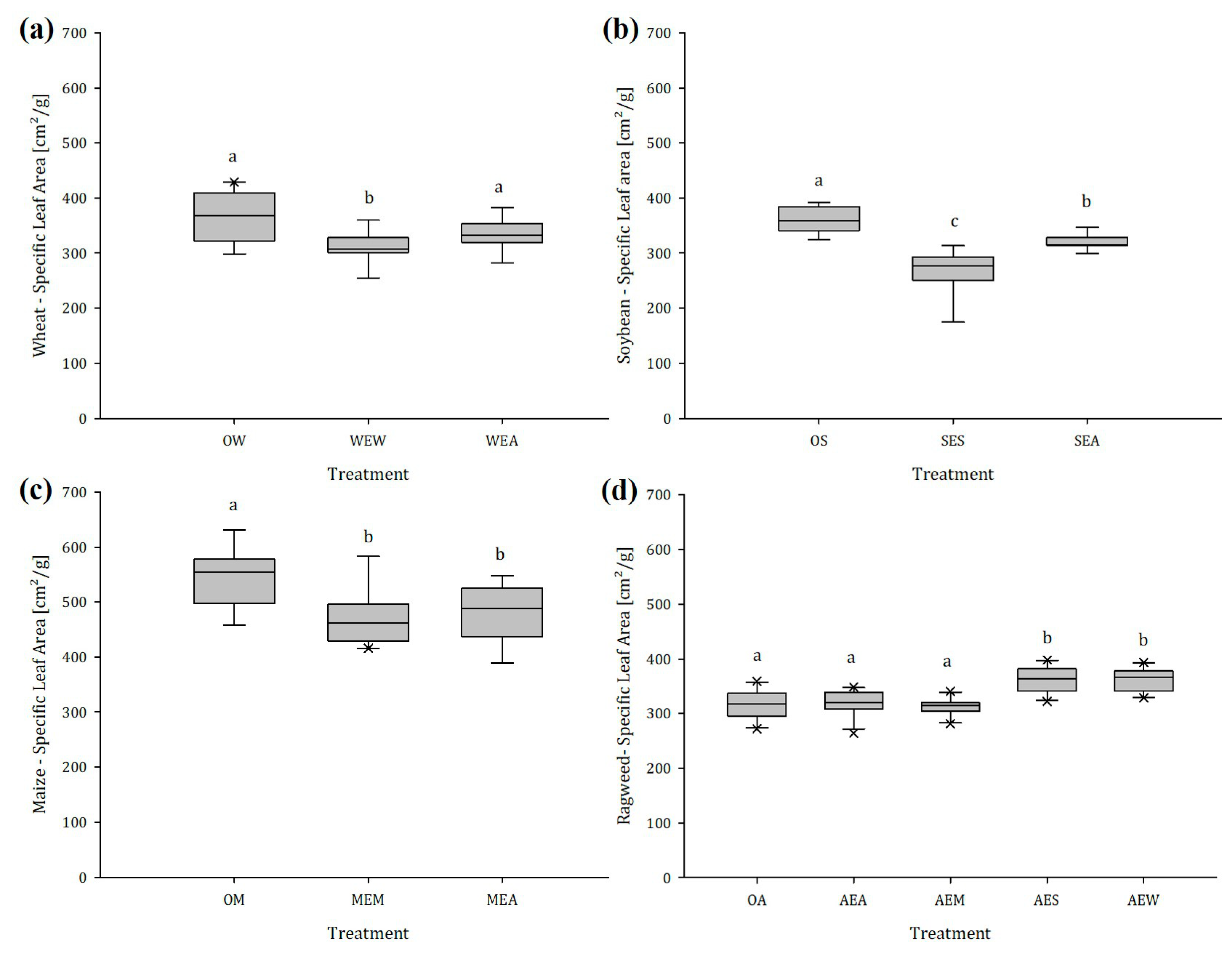
2.1.3. Specific Leaf Area (SLA)
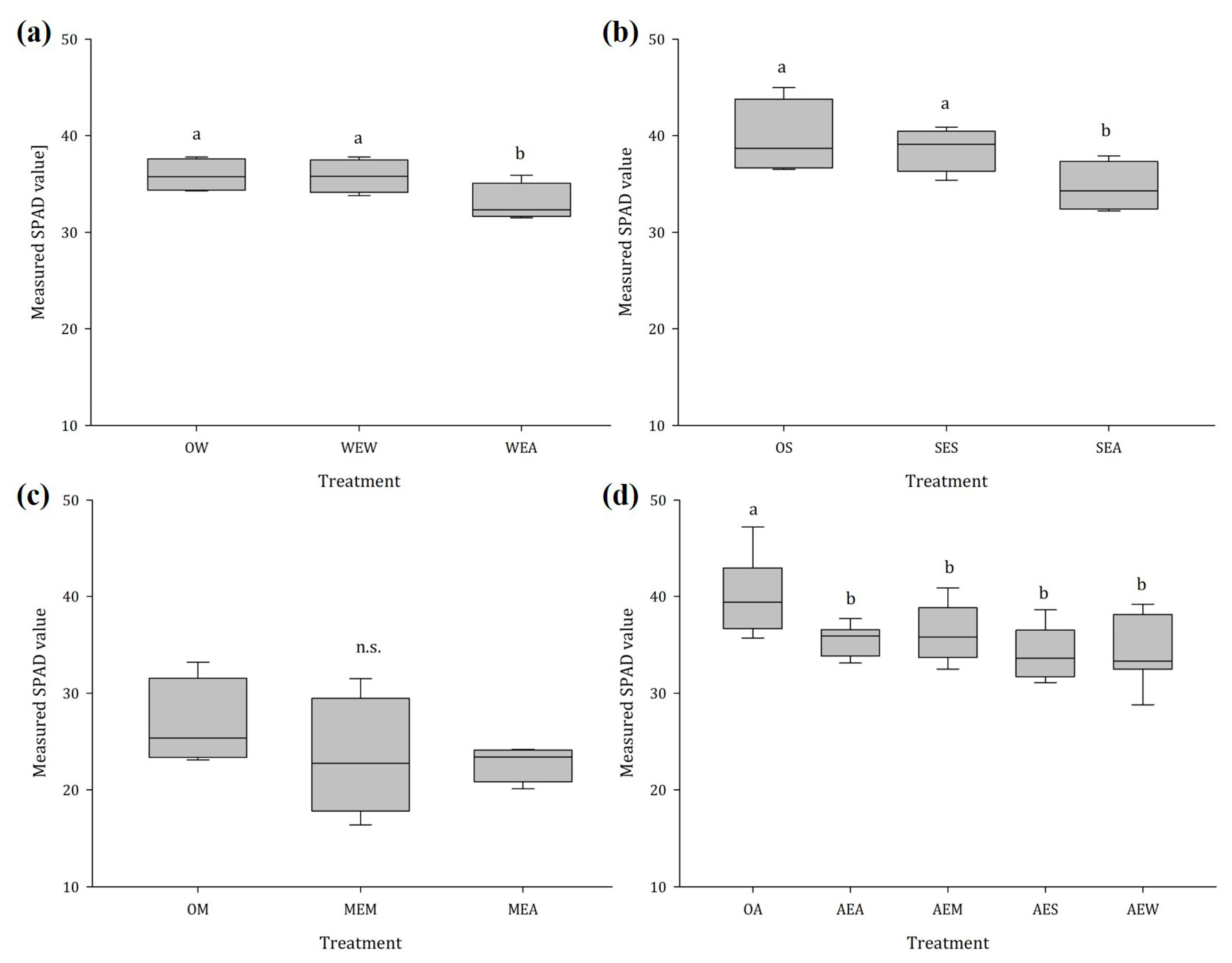
2.1.4. Chlorophyll Content
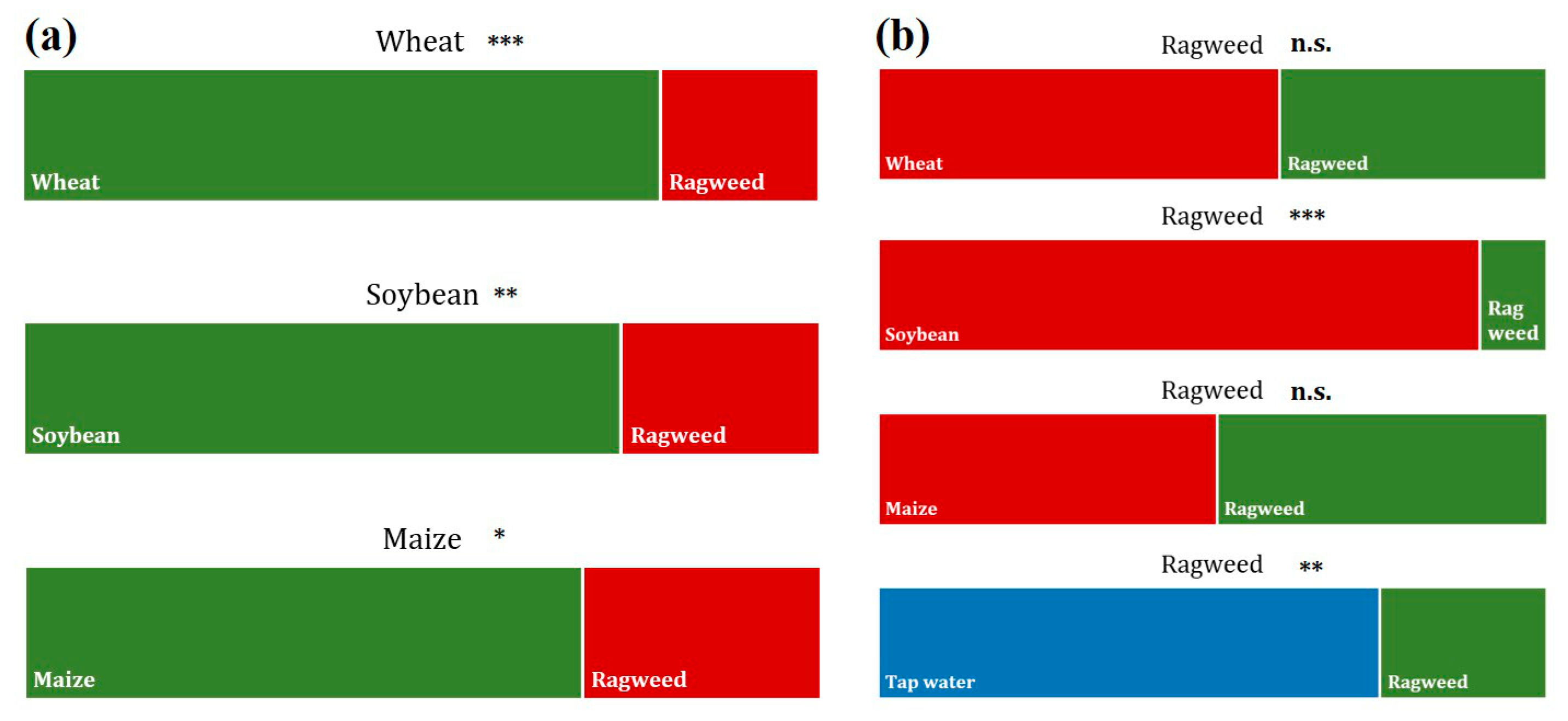
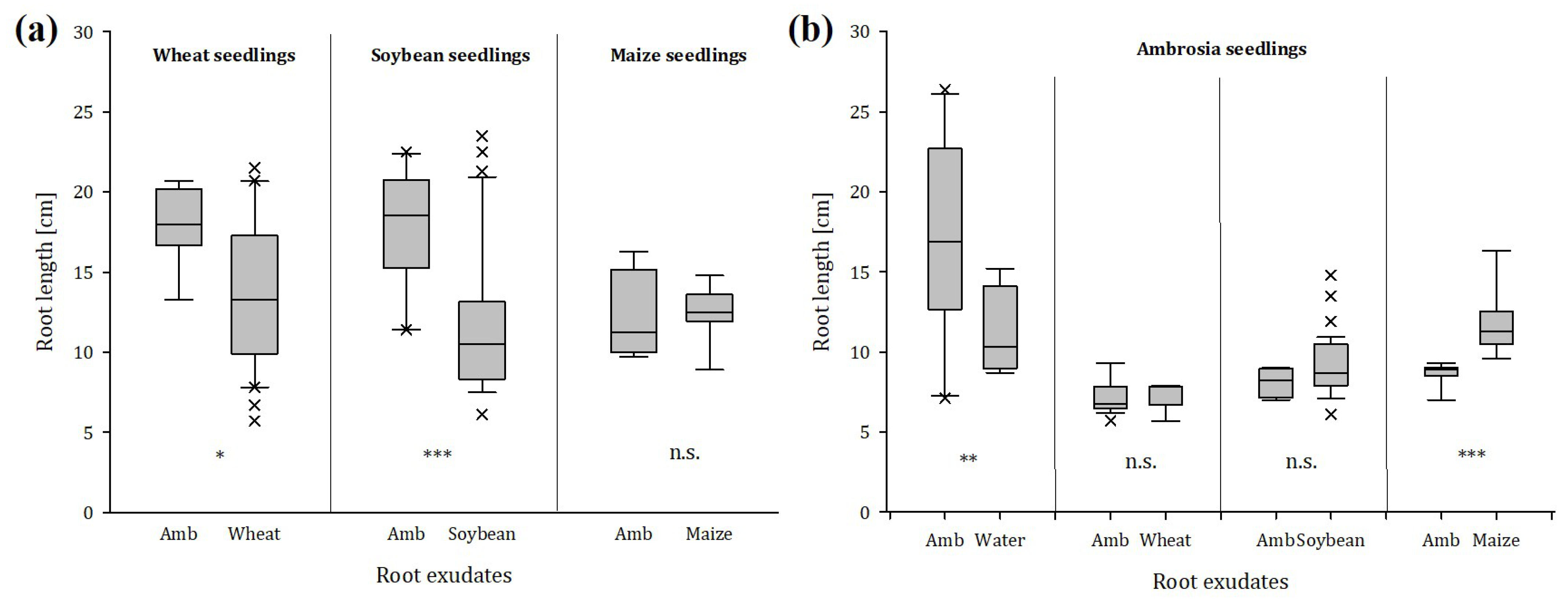
2.1.5. Root Choice Experiment
3. Discussion
3.1. Crop Growth Response after Exposure to VOCs from Ambrosia and Their Kin (Self-Exposure)
3.2. Ragweed Growth Response after Exposure to VOCs from Crops and Its Kin (Self-Exposure)
3.3. Changes in Crop Chlorophyll Content after Exposure to VOCs from Conspecifics and Ragweed
3.4. Root Seedlings Preference/Avoidance Behavior in Response to Ragweed or Conspecifics
4. Material and Methods
4.1. Plant Material
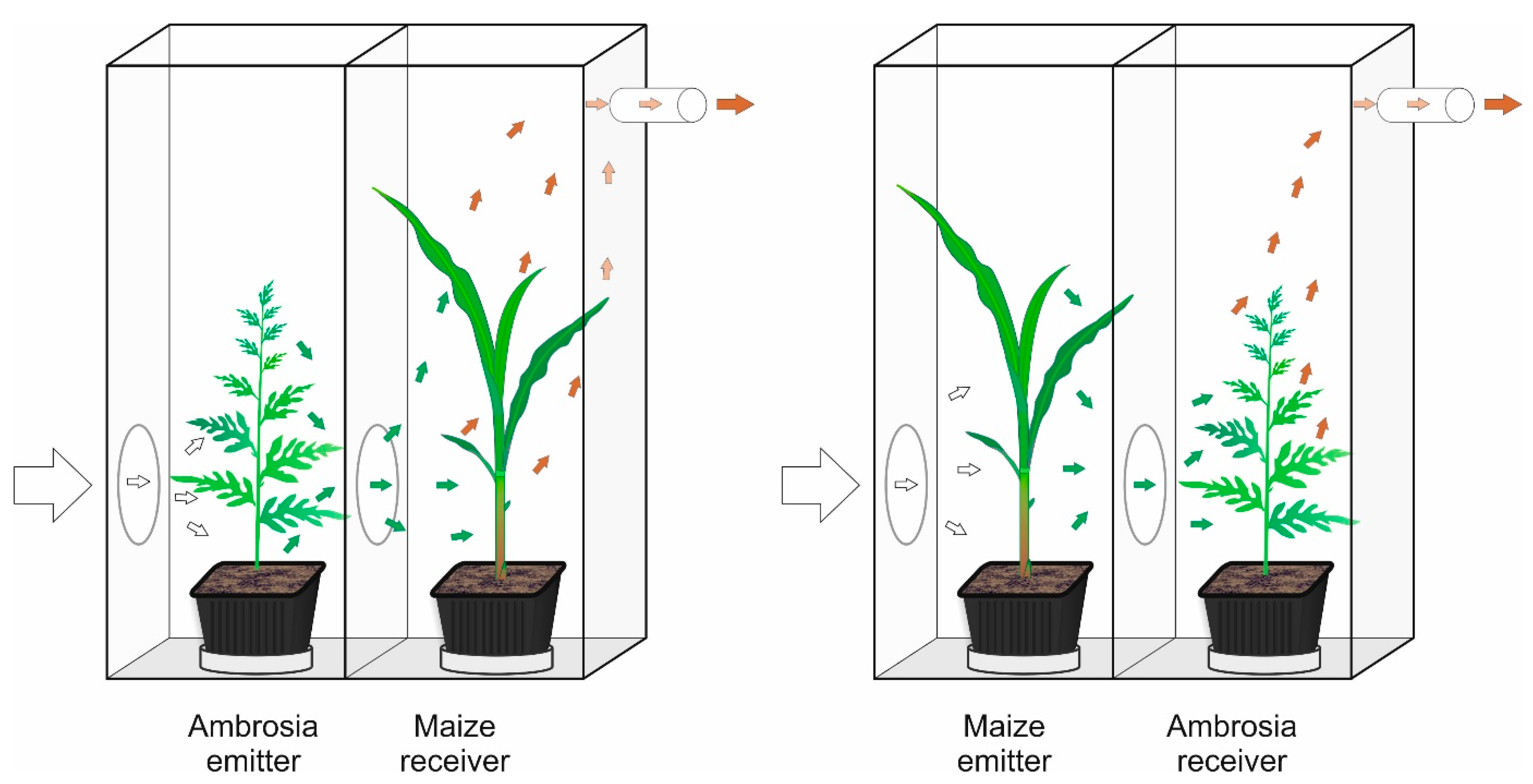
4.2. Exposure System
4.3. Measurements
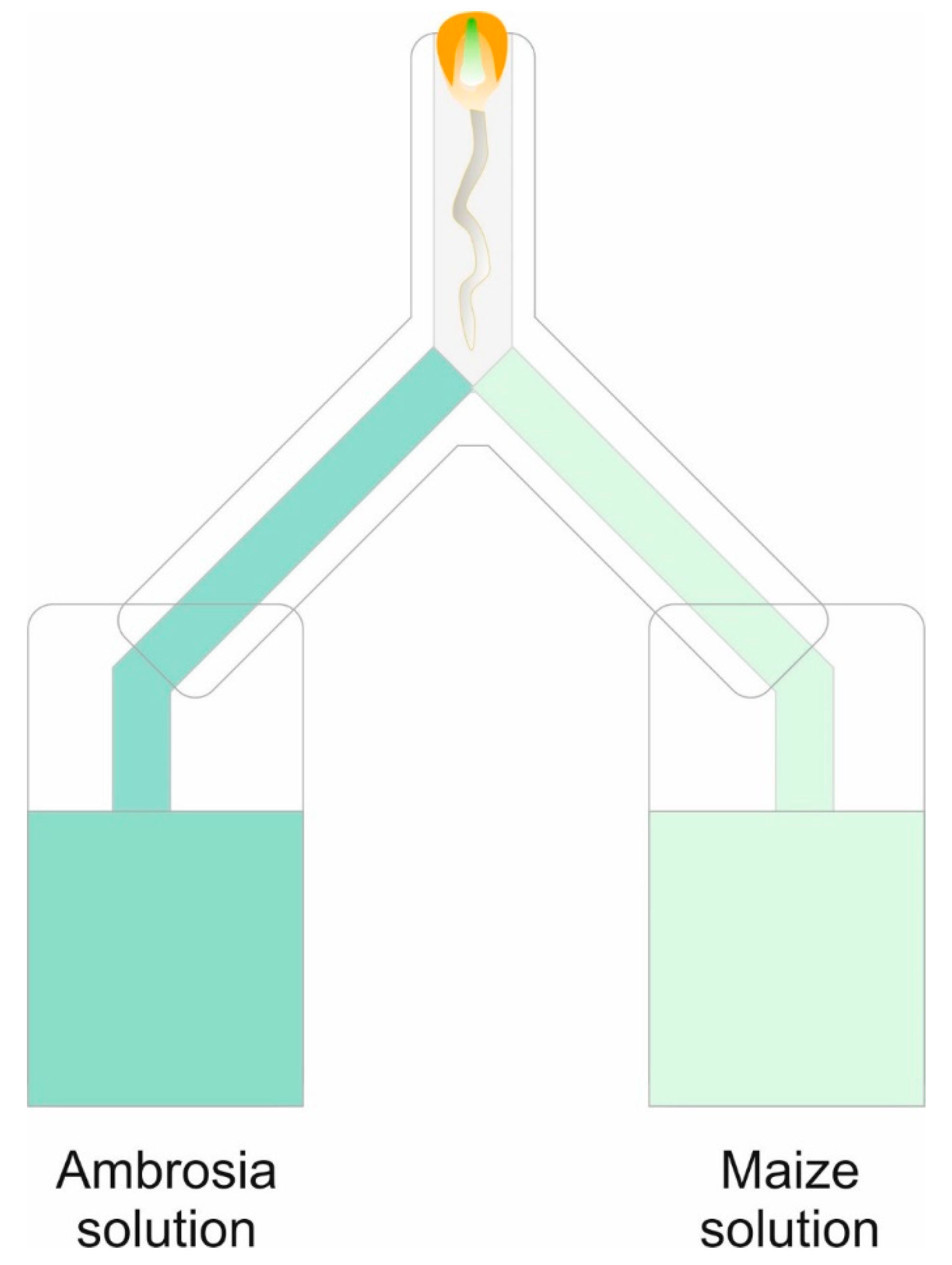
4.4. Root Choice Experiment
4.5. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williamson, M. Biological Invasions; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Thorpe, A.S.; Thelen, G.C.; Diaconu, A.; Callaway, R.M. Root exudate is allelopathic in invaded community but not in native community: Field evidence for the novel weapons hypothesis. J. Ecol. 2009, 97, 641–645. [Google Scholar] [CrossRef]
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and economic costs of nonindigenous species in the United States. Bioscience 2000, 50, 53–65. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P. Plant invasions: Merging the concepts of species invasiveness and community invasibility. Prog. Phys. Geog. 2006, 30, 409–431. [Google Scholar] [CrossRef]
- Walker, L.R.; Smith, S.D. Impacts of Invasive Plants on Community and Ecosystem Properties. In Assessment and Management of Plant Invasions; Luken, J.O., Thieret, J.W., Eds.; Springer: New York, NY, USA, 1997; pp. 69–86. [Google Scholar]
- Rejmánek, M.; Richardson, D.M.; Pyšek, P. Plant Invasions and Invasibility of Plant Communities. In Vegetation Ecology; van der Maarel, E., Franklin, J., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 387–424. [Google Scholar]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Pauchard, A.; Traveset, A.; Vilà, M. Linking the impacts of plant invasion on community functional structure and ecosystem properties. J. Veg. Sci. 2016, 27, 1233–1242. [Google Scholar] [CrossRef]
- Karban, R. Plant behaviour and communication. Ecol. Lett. 2008, 11, 727–739. [Google Scholar] [CrossRef]
- Elhakeem, A.; Markovic, D.; Broberg, A.; Anten, N.P.R.; Ninkovic, V. Aboveground mechanical stimuli affect belowground plant-plant communication. PLoS ONE 2018, 13, e0195646. [Google Scholar] [CrossRef]
- Ninkovic, V. Volatile communication between barley plants affects biomass allocation. J. Exp. Bot. 2003, 54, 1931–1939. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Baldwin, I.T. Plant volatiles. Curr. Biol. 2010, 20, R392–R397. [Google Scholar] [CrossRef]
- Ninkovic, V.; Rensing, M.; Dahlin, I.; Markovic, D. Who is my neighbor? Volatile cues in plant interactions. Plant Signal Behav. 2019, 14, 1634993. [Google Scholar] [CrossRef] [PubMed]
- Pierik, R.; Mommer, L.; Voesenek, L.A. Molecular mechanisms of plant competition: Neighbour detection and response strategies. Funct. Ecol. 2013, 27, 841–853. [Google Scholar] [CrossRef]
- Yamawo, A.; Tagawa, J.; Hada, Y.; Suzuki, N. Different combinations of multiple defence traits in an extrafloral nectary-bearing plant growing under various habitat conditions. J. Ecol. 2014, 102, 238–247. [Google Scholar] [CrossRef]
- Hamilton, W.D. Genetical evolution of social behaviour. I. J. Theor. Biol. 1964, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.P.; Swanton, C.J.; Van Acker, R.C.; Dudley, S.A. Kin recognition, multilevel selection and altruism in crop sustainability. J. Ecol. 2017, 105, 930–934. [Google Scholar] [CrossRef]
- Ehlers, B.K.; Bilde, T. Inclusive fitness, asymmetric competition and kin selection in plants. Oikos 2019, 128, 765–774. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Chen, B.J.W. Detect thy family: Mechanisms, ecology and agricultural aspects of kin recognition in plants. Plant Cell Environ. 2021, 44, 1059–1071. [Google Scholar] [CrossRef]
- Dudley, S.A.; Murphy, G.P.; File, A.L. Kin recognition and competition in plants. Funct. Ecol. 2013, 27, 898–906. [Google Scholar] [CrossRef]
- Yang, X.-F.; Li, L.-L.; Xu, Y.; Kong, C.-H. Kin recognition in rice (Oryza sativa) lines. New Phytol. 2018, 220, 567–578. [Google Scholar] [CrossRef]
- Takigahira, H.; Yamawo, A. Competitive responses based on kin-discrimination underlie variations in leaf functional traits in Japanese beech (Fagus crenata) seedlings. Evol. Ecol. 2019, 33, 521–531. [Google Scholar] [CrossRef]
- Dudley, S.A.; File, A.L. Kin recognition in an annual plant. Biol. Lett. 2007, 3, 435–438. [Google Scholar] [CrossRef]
- Semchenko, M.; Saar, S.; Lepik, A. Plant root exudates mediate neighbour recognition and trigger complex behavioural changes. New Phytol. 2014, 204, 631–637. [Google Scholar] [CrossRef]
- Karban, R.; Shiojiri, K.; Ishizaki, S.; Wetzel, W.C.; Evans, R.Y. Kin recognition affects plant communication and defence. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123062. [Google Scholar] [CrossRef]
- Ninkovic, V. Volatile Interaction Between Undamaged Plants: A Short Cut to Coexistence. In Plant Communication from an Ecological Perspective; Baluška, F., Ninkovic, V., Eds.; Signaling and Communication in Plants; Springer: Berlin, Germany, 2010; pp. 75–86. [Google Scholar]
- Depuydt, S. Arguments for and against self and non-self root recognition in plants. Front. Plant Sci. 2014, 5, 614. [Google Scholar] [CrossRef]
- Thelen, G.C.; Vivanco, J.M.; Newingham, B.; Good, W.; Bais, H.P.; Landres, P.; Caesar, A.; Callaway, R.M. Insect herbivory stimulates allelopathic exudation by an invasive plant and the suppression of natives. Ecol. Lett. 2005, 8, 209–217. [Google Scholar] [CrossRef]
- Toole, E.H.; Brown, E. Final results of the duvel buried seed experiment. J. Agric. Res. 1946, 72, 201–210. [Google Scholar]
- Hall, R.M.; Urban, B.; Skalova, H.; Moravcová, L.; Sölter, U.; Starfinger, U.; Kazinczi, G.; van Valkenburg, J.; Fenesi, A.; Konstantinovic, B.; et al. Seed viability of common ragweed (Ambrosia artemisiifolia L.) is affected by seed origin and age, but also by testing method and laboratory. NeoBiota 2021, 70, 193–221. [Google Scholar] [CrossRef]
- Kazinczi, G.; Novak, R.; Pathy, Z.; Beres, I. Common ragweed (Ambrosia artemisiifolia L.): A review with special regards to the results in Hungary. III. Resistant biotypes, control methods and authority arrangements. Herbologia 2008, 9, 119–144. [Google Scholar]
- Kazinczi, G.; Beres, I.; Novak, R.; Karaman, J. Focusing again on common ragweed (Ambrosia artemisiifolia L.). Novenyvdelem 2009, 45, 389–403. [Google Scholar]
- Hall, R.M.; Urban, B.; Wagentristl, H.; Karrer, G.; Winter, A.; Czerny, R.; Kaul, H.-P. Common ragweed (Ambrosia artemisiifolia L.) causes severe yield losses in soybean and impairs Bradyrhizobium japonicum infection. Agronomy 2021, 11, 1616. [Google Scholar] [CrossRef]
- Brückner, D.J.; Lepossa, A.; Herpai, Z. Inhibitory effect of ragweed (Ambrosia artemisiifolia L.)-inflorescence extract on the germination of Amaranthus hypochondriacus L. and growth of two soil algae. Chemosphere 2003, 51, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Pajević, S.; Borišev, M.; Orčić, D.; Boža, P.; Nikolić, N. Photosynthetic and biochemical characteristics of invasive species (Ambrosia artemisiifolia L., Ambrosia trifida L. and Iva xanthifolia Nutt.) depending on soil humidity and phenological phase. Russ. J. Ecol. 2010, 41, 498–505. [Google Scholar] [CrossRef]
- Bonea, D.; Bonciu, E.; Niculescu, M.; Olaru, A.L. The allelopathic, cytotoxic and genotoxic effect of Ambrosia artemisiifolia on the germination and root meristems of Zea mays. Caryologia 2017, 71, 24–28. [Google Scholar] [CrossRef]
- Mutch, D.R.; Martin, T.E.; Kosola, K.R. Red clover (Trifolium pratense) suppression of common ragweed (Ambrosia artemisiifolia) in winter wheat (Triticum aestivum). Weed Technol. 2003, 17, 181–185. [Google Scholar] [CrossRef]
- Vidotto, F.; Tesio, F.; Ferrero, A. Allelopathic effects of Ambrosia artemisiifolia L. in the invasive process. Crop Protect. 2013, 54, 161–167. [Google Scholar] [CrossRef]
- Molinaro, F.; Monterumici, C.M.; Ferrero, A.; Tabasso, S.; Negre, M. Bioherbicidal activity of a germacranolide sesquiterpene dilactone from Ambrosia artemisiifolia L. J. Environ. Sci. Health B 2016, 51, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.D. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 1965, 13, 115–155. [Google Scholar] [CrossRef]
- Chen, B.J.; During, H.J.; Anten, N.P. Detect thy neighbor: Identity recognition at the root level in plants. Plant Sci. 2012, 195, 157–167. [Google Scholar] [CrossRef]
- Goodnight, C.J.; Scheartz, J.M.; Stevens, L. Contextual analysis of models of group selection, soft selection, hard selection, and the evolution of altruism. Am. Nat. 1992, 140, 743–761. [Google Scholar] [CrossRef]
- Cahill, J.F.; McNickle, G.G. The behavioral ecology of nutrient foraging by plants. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 289–311. [Google Scholar] [CrossRef]
- Faria, G.S.; Gardner, A. Does kin discrimination promote cooperation? Biol. Lett. 2020, 16, 20190742. [Google Scholar] [CrossRef]
- Grime, J.P. Vegetation classification by reference to strategies. Nature 1974, 250, 26–31. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant Ecological Strategies: Some Leading Dimensions of Variation between Species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Louault, F.; Pillar, V.D.; Aufrère, J.; Garnier, E.; Soussana, J.-F. Plant traits and functional types in response to reduced disturbance in a semi-natural grassland. J. Veg. Sci. 2005, 16, 151–160. [Google Scholar] [CrossRef]
- Grime, J.P. Plant strategies and vegetation processes. Plant Biol. 1979, 23, 245–254. [Google Scholar] [CrossRef]
- Essl, F.; Biró, K.; Brandes, D.; Broennimann, O.; Bullock, J.M.; Chapman, D.S.; Chauvel, B.; Dullinger, S.; Fumanal, B.; Guisan, A.; et al. Biological flora of the British isles: Ambrosia artemisiifolia. J. Ecol. 2015, 103, 1069–1098. [Google Scholar] [CrossRef]
- Lepik, A.; Abakumova, M.; Zobel, K.; Semchenko, M. Kin recognition is density-dependent and uncommon among temperate grassland plants. Funct. Ecol. 2012, 26, 1214–1220. [Google Scholar] [CrossRef]
- Masclaux, F.; Hammond, R.L.; Meunier, J.; Gouhier-Darimont, C.; Keller, L.; Reymond, P. Competitive ability not kinship affects growth of Arabidopsis thaliana accessions. New Phytol. 2010, 185, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Milla, R.; Iriondo, A.E.M. Congruence between geographic range distribution and local competitive ability of two Lupinus species. Am. J. Bot. 2011, 98, 1456–1464. [Google Scholar] [CrossRef]
- Oyerinde, R.O.; Otusanya, O.O.; Akpor, O.B. Allelopathic effect of Tithonia diversifolia on the germination, growth and chlorophyll contents of maize (Zea mays L.). J. Sci. Res. Essay 2009, 4, 1553–1558. [Google Scholar]
- Elisante, F.; Tarimo, M.T.; Ndakidemi, P.A. Allelopathic effect of seed and leaf aqueous extracts of Datura stramonium on leaf chlorophyll content, shoot and root elongation of Cenchrus ciliaris and Neonotonia wightii. Am. J. Plant Sci. 2013, 4, 2332–2339. [Google Scholar] [CrossRef]
- Han, C.; Shao, H.; Zhou, S.; Mei, Y.; Cheng, Z.; Huang, L.; Lv, G. Chemical composition and phytotoxicity of essential oil from invasive plant, Ambrosia artemisiifolia L. Ecotoxicol. Environ. Saf. 2021, 211, 111879. [Google Scholar] [CrossRef]
- Benyas, E.; Hassanpouraghdam, M.B.; Zehtab Salmasi, S.; Khatamian Oskooei, O.S. Allelopathic effects of Xanthium strumarium L. shoot aqueous extract on germination, seedling growth and chlorophyll content of lentil (Lens culinaris Medic.). Rom. Biotechnol. Lett. 2010, 15, 5223–5228. [Google Scholar]
- Ibrahim, D.; Kenanoglu, B.B.; Jalink, H.; Mavi, K. Chlorphyll Fluorescence sorting method to improve seedling emergence potential and vigour of commercial tomato and cucumber seed lots. Int. J. Res. Agric. For. 2013, 3, 14465–14477. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, H.; Wang, J.; Sui, X.; Xu, N. Adaptive changes in chlorophyll content and photosynthetic features to low light in Physocarpus amurensis Maxim and Physocarpus opulifolius “Diabolo”. PeerJ 2016, 4, e2125. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Y.; Lu, Y.; Liao, Y.; Nie, J.; Yuan, X.; Chen, F. Use of a leaf chlorophyll content index to improve the prediction of above-ground biomass and productivity. PeerJ 2019, 6, e6240. [Google Scholar] [CrossRef] [PubMed]
- Fredeen, A.L.; Raab, T.K.; Rao, I.M.; Terry, N. Effects of phosphorus nutrition on photosynthesis in Glycine max (L.) Merr. Planta 1990, 181, 399–405. [Google Scholar] [CrossRef]
- Nagata, N.; Tanaka, R.; Satoh, S.; Tanaka, A. Identification of a vinyl reductase gene for chlorophyll synthesis in Arabidopsis thaliana and implications for the evolution of Prochlorococcus species. Plant Cell 2005, 17, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Biedrzycki, M.L.; Jilany, T.A.; Dudley, S.A.; Bais, H.P. Root exudates mediate kin recognition in plants. Commun. Integr. Biol. 2010, 3, 28–35. [Google Scholar] [CrossRef]
- Semchenko, M.; John, E.A.; Hutchings, M.J. Effects of physical connection and genetic identity of neighbouring ramets on root-placement patterns in two clonal species. New Phytol. 2007, 176, 644–654. [Google Scholar] [CrossRef]
- Markovic, D.; Nikolic, N.; Glinwood, R.; Seisenbaeva, G.; Ninkovic, V. Plant responses to brief touching: A mechanism for early neighbour detection? PLoS ONE 2016, 11, e0165742. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.E.; Brown, J.S.; Moll, J.D. Roots in space: A spatially explicit model for below-ground competition in plants. Proc. R. Soc. B Biol. Sci. 2007, 274, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J.; Ninkovic, V.; Ahmed, E. Volatiles from different barley cultivars affect aphid acceptance of neighbouring plants. Acta Agric. Scand. B Soil Plant Sci. 1999, 49, 152–157. [Google Scholar] [CrossRef]
- Yamamoto, A.; Nakamura, T.; Adu-Gyamfi, J.J.; Saigusa, M. Relationship between chlorophyll content in leaves of sorghum and pigeonpea determined by extraction method and by chlorophyll meter (Spad-502). J. Plant Nutr. 2006, 25, 2295–2301. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, R.M.; Markovic, D.; Kaul, H.-P.; Wagentristl, H.; Urban, B.; Durec, N.; Renner-Martin, K.; Ninkovic, V. Talking Different Languages: The Role of Plant–Plant Communication When an Invader Beats up a Strange Neighborhood. Plants 2023, 12, 3298. https://doi.org/10.3390/plants12183298
Hall RM, Markovic D, Kaul H-P, Wagentristl H, Urban B, Durec N, Renner-Martin K, Ninkovic V. Talking Different Languages: The Role of Plant–Plant Communication When an Invader Beats up a Strange Neighborhood. Plants. 2023; 12(18):3298. https://doi.org/10.3390/plants12183298
Chicago/Turabian StyleHall, Rea Maria, Dimitrije Markovic, Hans-Peter Kaul, Helmut Wagentristl, Bernhard Urban, Nora Durec, Katharina Renner-Martin, and Velemir Ninkovic. 2023. "Talking Different Languages: The Role of Plant–Plant Communication When an Invader Beats up a Strange Neighborhood" Plants 12, no. 18: 3298. https://doi.org/10.3390/plants12183298
APA StyleHall, R. M., Markovic, D., Kaul, H.-P., Wagentristl, H., Urban, B., Durec, N., Renner-Martin, K., & Ninkovic, V. (2023). Talking Different Languages: The Role of Plant–Plant Communication When an Invader Beats up a Strange Neighborhood. Plants, 12(18), 3298. https://doi.org/10.3390/plants12183298









