Foliar Selenium Application to Reduce the Induced-Drought Stress Effects in Coffee Seedlings: Induced Priming or Alleviation Effect?
Abstract
:1. Introduction
2. Results
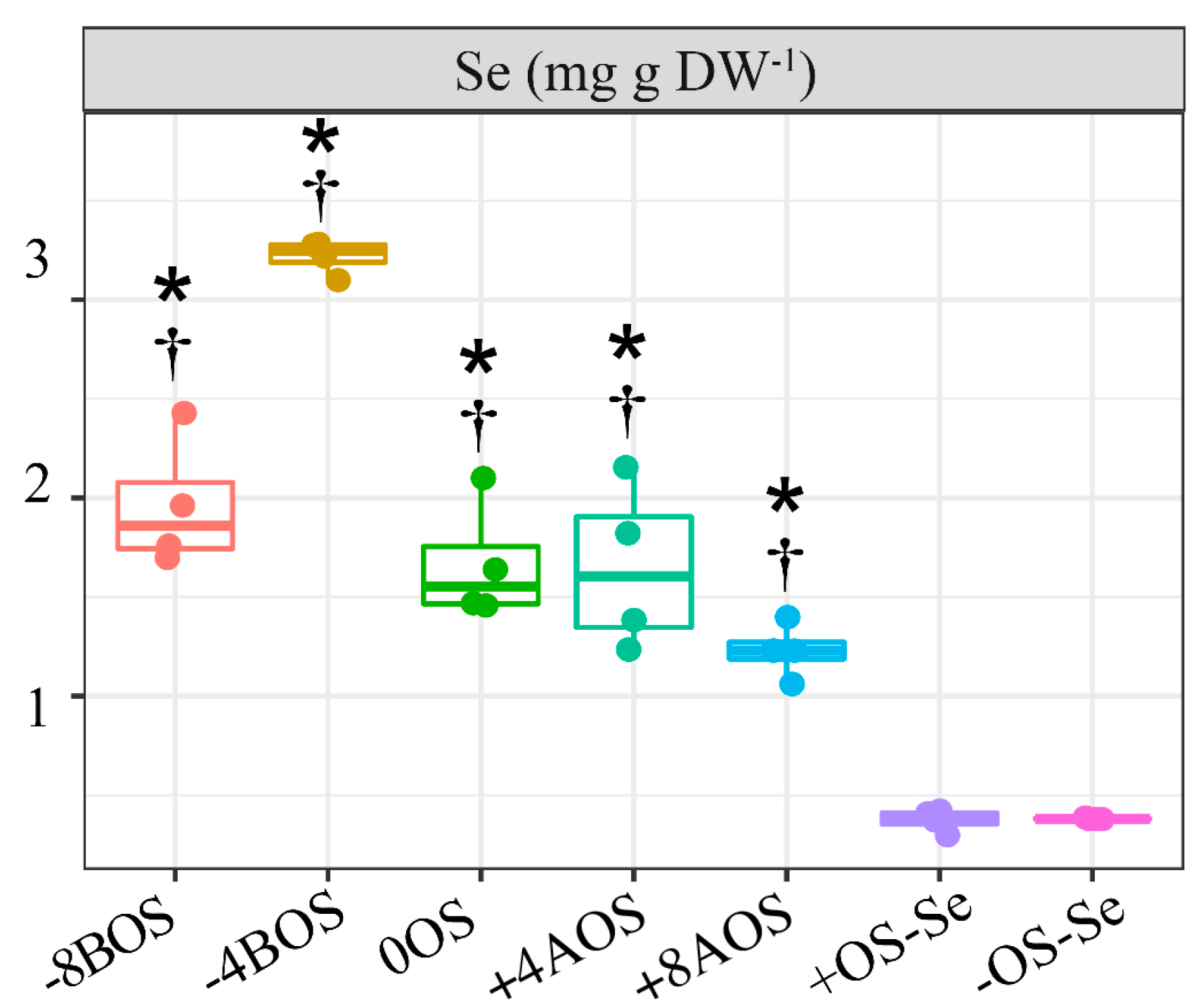
2.1. Analysis of Se Content
2.2. Antioxidant Enzymes (APX, CAT, GR, and SOD), H2O2, and MDA
2.3. Carbohydrates, Protein, Amino Acids, and Proline
2.4. Chlorophyll Fluorescence Parameters (MultispeQ®)
2.5. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Study Site
4.2. Experimental Design and Treatments
4.3. Application of Foliar Treatments
4.4. Osmotic Stress Imposition and Leaf Water Status
4.5. Leaf Sample Collection and Preparation
4.6. Determination of Examined Parameters
4.6.1. Selenium Content in Leaves and Detection Limit (LOD and LOQ)
4.6.2. Carbohydrates, Total Protein, and Total Free Amino Acids
4.6.3. Proline
4.6.4. Antioxidant Enzymes (SOD, CAT, APX, and GR)
4.6.5. Hydrogen Peroxide and Lipid Peroxidation (Malondialdehyde)
4.6.6. Chlorophyll Fluorescence Parameters (MultispeQ®)
4.7. Statistical Analysis and PCA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In IPCC Climate Change 2014: Synthesis Report; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Ortiz-Bobea, A.; Ault, T.R.; Carrillo, C.M.; Chambers, R.G.; Lobell, D.B. Anthropogenic Climate Change Has Slowed Global Agricultural Productivity Growth. Nat. Clim. Chang. 2021, 11, 306–312. [Google Scholar] [CrossRef]
- Kotz, M.; Levermann, A.; Wenz, L. The Effect of Rainfall Changes on Economic Production. Nature 2022, 601, 223–227. [Google Scholar] [CrossRef]
- Cerdán, C.R.; Rebolledo, M.C.; Soto, G.; Rapidel, B.; Sinclair, F.L. Local Knowledge of Impacts of Tree Cover on Ecosystem Services in Smallholder Coffee Production Systems. Agric. Syst. 2012, 110, 119–130. [Google Scholar] [CrossRef]
- Chain-Guadarrama, A.; Martínez-Salinas, A.; Aristizábal, N.; Ricketts, T.H. Ecosystem Services by Birds and Bees to Coffee in a Changing Climate: A Review of Coffee Berry Borer Control and Pollination. Agric. Ecosyst. Environ. 2019, 280, 53–67. [Google Scholar] [CrossRef]
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Stoffelen, P. An Annotated Taxonomic Conspectus of the Genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006, 152, 465–512. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ramalho, J.D.C. Impacts of Drought and Temperature Stress on Coffee Physiology and Production: A Review. Braz. J. Plant Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought Stress in Plants: A Review on Morphological Characteristics and Pigments Composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Ahmad, Z.; Waraich, E.A.; Akhtar, S.; Anjum, S.; Ahmad, T.; Mahboob, W.; Hafeez, O.B.A.; Tapera, T.; Labuschagne, M.; Rizwan, M. Physiological Responses of Wheat to Drought Stress and Its Mitigation Approaches. Acta Physiol. Plant. 2018, 40, 80. [Google Scholar] [CrossRef]
- Nawaz, F.; Ahmad, R.; Ashraf, M.Y.Y.; Waraich, E.A.A.; Khan, S.Z.Z. Effect of Selenium Foliar Spray on Physiological and Biochemical Processes and Chemical Constituents of Wheat under Drought Stress. Ecotoxicol. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of Drought Stress on Photosynthesis and Photosynthetic Electron Transport Chain in Young Apple Tree Leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef] [PubMed]
- Semedo, J.N.; Rodrigues, W.P.; Dubberstein, D.; Martins, M.Q.; Martins, L.D.; Pais, I.P.; Rodrigues, A.P.; Leitão, A.E.; Partelli, F.L.; Campostrini, E.; et al. Coffee Responses to Drought, Warming and High [CO2] in a Context of Future Climate Change Scenarios. In Climate Change Management; Alves, F., Leal Filho, W., Azeiteiro, U., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 465–477. ISBN 978-3-319-72873-5. [Google Scholar]
- Feng, R.; Wei, C.; Tu, S. The Roles of Selenium in Protecting Plants against Abiotic Stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Bhadwal, S.; Sharma, S. Selenium Alleviates Carbohydrate Metabolism and Nutrient Composition in Arsenic Stressed Rice Plants. Rice Sci. 2022, 29, 385–396. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S. Agronomic Biofortification of Plant Foods with Minerals, Vitamins and Metabolites with Chemical Fertilizers and Liming. J. Plant Nutr. 2020, 43, 1534–1554. [Google Scholar] [CrossRef]
- Abul-Soud, M.; Abd-Elrahman, S. Foliar Selenium Application to Improve the Tolerance of Eggplant Grown under Salt Stress Conditions. Int. J. Plant Soil. Sci. 2016, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ravello, R.A.V.; de Oliveira, C.; Lessa, J.; Boas, L.V.V.; de Castro, E.M.; Guilherme, L.R.G.; Lopes, G. Selenium Application Influenced Selenium Biofortification and Physiological Traits in Water-Deficit Common Bean Plants. Crop Pasture Sci. 2021, 73, 44–55. [Google Scholar] [CrossRef]
- De Sousa, G.F.; Silva, M.A.; de Morais, E.G.; Van Opbergen, G.A.Z.; Van Opbergen, G.G.A.Z.; de Oliveira, R.R.; Amaral, D.; Brown, P.; Chalfun-Junior, A.; Guilherme, L.R.G. Selenium Enhances Chilling Stress Tolerance in Coffee Species by Modulating Nutrient, Carbohydrates, and Amino Acids Content. Front. Plant Sci. 2022, 13, 1000430. [Google Scholar] [CrossRef]
- Lanza, M.G.D.B.; Reis, A.R. dos Roles of Selenium in Mineral Plant Nutrition: ROS Scavenging Responses against Abiotic Stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium Application in Two Methods Promotes Drought Tolerance in Solanum Lycopersicum Plant by Inducing the Antioxidant Defense System. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Silva, M.A.; De Sousa, G.F.; Van Opbergen, G.A.Z.; Van Opbergen, G.G.A.Z.; Corguinha, A.P.B.; Bueno, J.M.M.; Brunetto, G.; Leite, J.M.; dos Santos, A.A.; Lopes, G.; et al. Foliar Application of Selenium Associated with a Multi-Nutrient Fertilizer in Soybean: Yield, Grain Quality, and Critical Se Threshold. Plants 2023, 12, 2028. [Google Scholar] [CrossRef]
- Lončarić, Z.; Ivezić, V.; Kerovec, D.; Rebekić, A. Foliar Zinc-Selenium and Nitrogen Fertilization Affects Content of Zn, Fe, Se, p, and Cd in Wheat Grain. Plants 2021, 10, 1549. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Yang, Z.; Shui, Y.; Liu, X.; Chen, J.; Khan, S.; Wang, J.; Gao, Z. Methods of Selenium Application Differentially Modulate Plant Growth, Selenium Accumulation and Speciation, Protein, Anthocyanins and Concentrations of Mineral Elements in Purple-Grained Wheat. Front. Plant Sci. 2020, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M. Application of Selenium A Useful Way to Mitigate Drought Stress: A Review. Open Access J. Biog. Sci. Res. 2020, 3, 39. [Google Scholar] [CrossRef]
- De Mateus, M.P.B.; Tavanti, R.F.R.; Tavanti, T.R.; Santos, E.F.; Jalal, A.; Reis, A.R. dos Selenium Biofortification Enhances ROS Scavenge System Increasing Yield of Coffee Plants. Ecotoxicol. Environ. Saf. 2021, 209, 111772. [Google Scholar] [CrossRef]
- Luo, H.; Xing, P.; Liu, J.; Pan, S.; Tang, X.; Duan, M. Selenium Improved Antioxidant Response and Photosynthesis in Fragrant Rice (Oryza sativa L.) Seedlings during Drought Stress. Physiol. Mol. Biol. Plants 2021, 27, 2849–2858. [Google Scholar] [CrossRef]
- Lopes, G.; Ávila, F.W.; Guilherme, L.R.G. Selenium Behavior in the Soil Environment and Its Implication for Human Health. Ciênc. Agrotec. 2017, 41, 605–615. [Google Scholar] [CrossRef]
- Mirlean, N.; Seus-Arrache, E.R.; Vlasova, O. Selenium Deficiency in Subtropical Littoral Pampas: Environmental and Dietary Aspects. Environ. Geochem. Health 2018, 40, 543–556. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants: Fourth Edition; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9780429192036. [Google Scholar]
- Gabos, M.B.; Alleoni, L.R.F.; Abreu, C.A. Background Levels of Selenium in Some Selected Brazilian Tropical Soils. J. Geochem. Explor. 2014, 145, 35–39. [Google Scholar] [CrossRef]
- Thuc, L.V.; Sakagami, J.I.; Hung, L.T.; Huu, T.N.; Khuong, N.Q.; Vi, L.L.V. Foliar Selenium Application for Improving Drought Tolerance of Sesame (Sesamum indicum L.). Open Agric. 2021, 6, 93–101. [Google Scholar] [CrossRef]
- Becerra-Vázquez, Á.G.; Coates, R.; Sánchez-Nieto, S.; Reyes-Chilpa, R.; Orozco-Segovia, A. Effects of Seed Priming on Germination and Seedling Growth of Desiccation-Sensitive Seeds from Mexican Tropical Rainforest. J. Plant Res. 2020, 133, 855–872. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, F.; Jiang, D. Priming: A Promising Strategy for Crop Production in Response to Future Climate. J. Integr. Agric. 2017, 16, 2709–2716. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on Crops: Their Impact under Abiotic Stress Conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for Plant Growth and Mitigation of Abiotic Stresses: A Metabolomics Perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef]
- Jolayemi, O.L.; Malik, A.H.; Ekblad, T.; Fredlund, K.; Olsson, M.E.; Johansson, E. Protein-Based Biostimulants to Enhance Plant Growth—State-of-the-Art and Future Direction with Sugar Beet as an Example. Agronomy 2022, 12, 3211. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Sahoo, L.; Panda, S.K. Molecular Physiology of Osmotic Stress in Plants. In Molecular Stress Physiology of Plants; Springer India: Delhi, India, 2013; pp. 179–192. ISBN 9788132208075. [Google Scholar]
- Reddy, Y.A.N.; Reddy, Y.N.P.; Ramya, V.; Suma, L.S.; Reddy, A.B.N.; Krishna, S.S. Drought Adaptation: Approaches for Crop Improvement; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128200896. [Google Scholar]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early Drought Stress Warning in Plants: Color Pictures of Photosystem II Photochemistry. Climate 2022, 10, 179. [Google Scholar] [CrossRef]
- Suma, L.S. Characterization of Selected Germplasm Accessions for Drought Tolerance in Finger Millet (Eleusine coracana (L.) Gaertn. Doctoral Dissertation, University of Agricultural Sciences, GKVK., Bengaluru, India, 2014. [Google Scholar]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The Determinants of Leaf Turgor Loss Point and Prediction of Drought Tolerance of Species and Biomes: A Global Meta-Analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef]
- Read, J.; Sanson, G.D.; Garine-Wichatitsky, M.d.; Jaffre, T. Sclerophylly in Two Contrasting Tropical Environments: Low Nutrients vs. Low Rainfall. Am. J. Bot. 2006, 93, 1601–1614. [Google Scholar] [CrossRef]
- Kostopoulou, P.; Barbayiannis, N.; Noitsakis, B. Water Relations of Yellow Sweetclover under the Synergy of Drought and Selenium Addition. Plant Soil. 2010, 330, 65–71. [Google Scholar] [CrossRef]
- Ribeiro, C.; Stitt, M.; Hotta, C.T. How Stress Affects Your Budget—Stress Impacts on Starch Metabolism. Front. Plant Sci. 2022, 13, 65–71. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a Determinant of Plant Fitness under Abiotic Stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of Leaf Starch Degradation by Abscisic Acid Is Important for Osmotic Stress Tolerance in Plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef] [PubMed]
- Kempa, S.; Krasensky, J.; Dal Santo, S.; Kopka, J.; Jonak, C. A Central Role of Abscisic Acid in Stress-Regulated Carbohydrate Metabolism. PLoS ONE 2008, 3, e3935. [Google Scholar] [CrossRef] [PubMed]
- Danso, O.P.; Asante-badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X. Selenium Biofortification: Strategies, Progress and Challenges. Agriculture 2023, 13, 416. [Google Scholar] [CrossRef]
- Lee, B.-R.; Jin, Y.-L.; Jung, W.-J.; Avice, J.-C.; Morvan-Bertrand, A.; Ourry, A.; Park, C.-W.; Kim, T.-H. Water-Deficit Accumulates Sugars by Starch Degradation-Not by de Novo Synthesis-in White Clover Leaves (Trifolium repens). Physiol. Plant 2008, 134, 403–411. [Google Scholar] [CrossRef]
- Sitnicka, D.; Orzechowski, S. Cold-Induced Starch Degradation in Potato Leaves—Intercultivar Differences in the Gene Expression and Activity of Key Enzymes. Biol. Plant 2014, 58, 659–666. [Google Scholar] [CrossRef]
- Malik, J.A.; Kumar, S.; Thakur, P.; Sharma, S.; Kaur, N.; Kaur, R.; Pathania, D.; Bhandhari, K.; Kaushal, N.; Singh, K.; et al. Promotion of Growth in Mungbean (Phaseolus aureus Roxb.) by Selenium Is Associated with Stimulation of Carbohydrate Metabolism. Biol. Trace Elem. Res. 2011, 143, 530–539. [Google Scholar] [CrossRef]
- Silva, M.A.; De Sousa, G.F.; Bañuelos, G.; Amaral, D.; Brown, P.H. Selenium Speciation in Se-Enriched Soybean Grains from Bio- Fortified Plants Grown under Different Methods of Selenium Application. Foods 2023, 12, 1214. [Google Scholar] [CrossRef]
- Silva, M.A.; de Sousa, G.F.; Corguinha, A.P.B.; de Lima Lessa, J.H.; Dinali, G.S.; Oliveira, C.; Lopes, G.; Amaral, D.; Brown, P.; Guilherme, L.R.G. Selenium Biofortification of Soybean Genotypes in a Tropical Soil via Se-Enriched Phosphate Fertilizers. Front. Plant Sci. 2022, 13, 988140. [Google Scholar] [CrossRef]
- Deng, X.; Liu, K.; Li, M.; Zhang, W.; Zhao, X.; Zhao, Z.; Liu, X. Difference of Selenium Uptake and Distribution in the Plant and Selenium Form in the Grains of Rice with Foliar Spray of Selenite or Selenate at Different Stages. Field Crops Res. 2017, 211, 165–171. [Google Scholar] [CrossRef]
- Manojlović, M.S.; Lončarić, Z.; Cabilovski, R.R.; Popović, B.; Karalić, K.; Ivezić, V.; Ademi, A.; Singh, B.R. Biofortification of Wheat Cultivars with Selenium. Acta Agric. Scand. B Soil. Plant Sci. 2019, 69, 715–724. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Winkel, L.H.E.; Lin, Z.-Q. (Eds.) Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects. In Plant Ecophysiology, 1st ed.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-56248-3. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chu, C. Selenium Uptake, Transport, Metabolism, Reutilization, and Biofortification in Rice. Rice 2022, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Z.; Luo, L.Y.; Fu, P.N.; Wang, Q.; Li, H.F. Selenium Uptake and Biotransformation in Brassica Rapa Supplied with Selenite and Selenate: A Hydroponic Work with HPLC Speciation and RNA-Sequencing. J. Agric. Food Chem. 2019, 67, 12408–12418. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lin, W.; Jiao, H.; Liu, J.; Chan, L.; Liu, X.; Wang, R.; Chen, T. Uptake, Transport, and Metabolism of Selenium and Its Protective Effects against Toxic Metals in Plants: A Review. Metallomics 2021, 13, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium Uptake, Translocation, Assimilation and Metabolic Fate in Plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef]
- Lima, L.W.; Pilon-Smits, E.A.H.; Schiavon, M. Mechanisms of Selenium Hyperaccumulation in Plants: A Survey of Molecular, Biochemical and Ecological Cues. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2343–2353. [Google Scholar] [CrossRef]
- Konze, J.R.; Schilling, N.; Kende, H. Enhancement of Ethylene Formation by Selenoamino Acids. Plant Physiol. 1978, 62, 397–401. [Google Scholar] [CrossRef]
- Tangjaidee, P.; Swedlund, P.; Xiang, J.; Yin, H.; Quek, S.Y. Selenium-Enriched Plant Foods: Selenium Accumulation, Speciation, and Health Functionality. Front. Nutr. 2023, 9, 962312. [Google Scholar] [CrossRef]
- Akladious, S.A. Influence of Different Soaking Times with Selenium on Growth, Metabolic Activities of Wheat Seedlings under Low Temperature Stress. Afr. J. Biotechnol. 2012, 11, 14792–14804. [Google Scholar]
- Schiavon, M.; Lima, L.W.; Jiang, Y.; Hawkesford, M.J. Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects. In Effects of Selenium on Plant Metabolism and Implications for Crops and Consumers; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 257–275. ISBN 978-3-319-56249-0. [Google Scholar]
- Trippe, R.C.; Pilon-Smits, E.A.H. Selenium Transport and Metabolism in Plants: Phytoremediation and Biofortification Implications. J. Hazard. Mater. 2021, 404, 124178. [Google Scholar] [CrossRef] [PubMed]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium Protects Wheat Seedlings against Salt Stress-Mediated Oxidative Damage by up-Regulating Antioxidants and Osmolytes Metabolism. Plant Physiol. Biochem. 2019, 137, 144–153. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Role of Glutathione in Plant Abiotic Stress Tolerance. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Wiley-Blackwell: Hoboken, NJ, USA, 2019; Volume 1, pp. 159–172. [Google Scholar] [CrossRef]
- Rahman, M.; Rahman, K.; Sathi, K.S.; Alam, M.M.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Supplemental Selenium and Boron Mitigate Salt-induced Oxidative Damages in Glycine max L. Plants 2021, 10, 2224. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akbar, A.; Parveen, A.; Rasheed, R.; Hussain, I.; Iqbal, M. Phenological Application of Selenium Differentially Improves Growth, Oxidative Defense and Ion Homeostasis in Maize under Salinity Stress. Plant Physiol. Biochem. 2018, 123, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; García-Caparrós, P.; Parvin, K.; Zulfiqar, F.; Ahmed, N.; Fujita, M. Selenium Supplementation and Crop Plant Tolerance to Metal/Metalloid Toxicity. Front. Plant Sci. 2022, 12, 792770. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.M.; Rimoldi Tavanti, R.F.; Gratão, P.L.; Alcock, T.D.; Reis, A.R. dos Selenate and Selenite Affect Photosynthetic Pigments and ROS Scavenging through Distinct Mechanisms in Cowpea (Vigna unguiculata (L.) Walp) Plants. Ecotoxicol. Environ. Saf. 2020, 201, 110777. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Mansoor, S.; Wani, O.A.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A Comprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants. Biomolecules 2022, 12, 43. [Google Scholar] [CrossRef]
- Malar, S.; Vikram, S.S.; Favas, P.J.C.; Perumal, V. Lead Heavy Metal Toxicity Induced Changes on Growth and Antioxidative Enzymes Level in Water Hyacinths [Eichhornia crassipes (Mart.)]. Bot. Stud. 2016, 55, 54. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, X.; Song, X.; Zhang, Y.; Jiang, J.; Han, Q.; Liu, M.; Qiao, G.; Zhuo, R. Overexpressing the Sedum Alfredii Cu/Zn Superoxide Dismutase Increased Resistance to Oxidative Stress in Transgenic Arabidopsis. Front. Plant Sci. 2017, 8, 1010. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, L.; Zhang, L.; Qiu, H.; Liu, C.; Wang, A.; Deng, F.; Zhu, J. A Cu/Zn Superoxide Dismutase Gene from Saussurea Involucrata Kar. & Kir., SiCSD, Enhances Drought, Cold, and Oxidative Stress in Transgenic Tobacco. Can. J. Plant Sci. 2017, 97, 816–826. [Google Scholar] [CrossRef]
- Liu, J.X.; Feng, K.; Duan, A.Q.; Li, H.; Yang, Q.Q.; Xu, Z.S.; Xiong, A.S. Isolation, Purification and Characterization of an Ascorbate Peroxidase from Celery and Overexpression of the AgAPX1 Gene Enhanced Ascorbate Content and Drought Tolerance in Arabidopsis. BMC Plant Biol. 2019, 19, 488. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Moustakas, M. Interaction of Proline, Sugars, and Anthocyanins during Photosynthetic Acclimation of Arabidopsis Thaliana to Drought Stress. J. Plant Physiol. 2012, 169, 577–585. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant Sugars Are Crucial Players in the Oxidative Challenge during Abiotic Stress: Extending the Traditional Concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Signorelli, S.; Dans, P.D.; Coitiño, E.L.; Borsani, O.; Monza, J. Connecting Proline and γ-Aminobutyric Acid in Stressed Plants through Non-Enzymatic Reactions. PLoS ONE 2015, 10, e0115349. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Borusiewicz, A.; Lozowicka, B. Fluazinam and Its Mixtures Induce Diversified Changes of Crucial Biochemical and Antioxidant Profile in Leafy Vegetable. Sci. Hortic. 2022, 298, 110988. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ronchi, C.P.; Maestri, M.; Barros, R.S. Ecophysiology of Coffee Growth and Production. Braz. J. Plant Physiol. 2007, 19, 485–510. [Google Scholar] [CrossRef]
- van der Vossen, H.; Bertrand, B.; Charrier, A. Next Generation Variety Development for Sustainable Production of Arabica Coffee (Coffea arabica L.): A Review. Euphytica 2015, 204, 243–256. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. In Circular: California Agricultural Experiment Station; University of Michigan Library: Ann Arbor, Michigan, 1950; Volume 347, pp. 1–32. [Google Scholar]
- Kane, C.D.; Jasoni, R.L.; Peffley, E.P.; Thompson, L.D.; Green, C.J.; Pare, P.; Tissue, D. Nutrient Solution and Solution PH Influences on Onion Growth and Mineral Content. J. Plant Nutr. 2006, 29, 375–390. [Google Scholar] [CrossRef]
- Salgado, O.G.G.; Teodoro, J.C.; Alvarenga, J.P.; de Oliveira, C.; de Carvalho, T.S.; Domiciano, D.; Marchiori, P.E.R.; Guilherme, L.R.G. Cerium Alleviates Drought-Induced Stress in Phaseolus Vulgaris. J. Rare Earths 2020, 38, 324–331. [Google Scholar] [CrossRef]
- Villela, F.A.; Doni Filho, L.; Sequeira, E.L. Tabela de Potencial Osmótico Em Função Da Concentração de Polietileno Glicol 6.000 e Da Temperatura. Pesqui. Agropecu. Bras. 1991, 26, 1957–1968. [Google Scholar]
- Fernandes, I.; Marques, I.; Paulo, O.S.; Batista, D.; Partelli, F.L.; Lidon, F.C.; Damatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. Understanding the Impact of Drought in Coffea Genotypes: Transcriptomic Analysis Supports a Common High Resilience to Moderate Water Deficit but a Genotype Dependent Sensitivity to Severe Water Deficit. Agronomy 2021, 11, 2255. [Google Scholar] [CrossRef]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap Pressure in Vascular Plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Tyree, M.T.; Hammel, H.T. The Measurement of the Turgor Pressure and the Water Relations of Plants by the Pressure-Bomb Technique. J. Exp. Bot 1972, 23, 267–282. [Google Scholar] [CrossRef]
- Kuhlgert, S.; Austic, G.; Zegarac, R.; Osei-Bonsu, I.; Hoh, D.; Chilvers, M.I.; Roth, M.G.; Bi, K.; TerAvest, D.; Weebadde, P.; et al. MultispeQ Beta: A Tool for Large-Scale Plant Phenotyping Connected to the Open PhotosynQ Network. R. Soc. Open Sci. 2016, 3, 160592. [Google Scholar] [CrossRef]
- U.S. EPA Method 3051A (SW-846); Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils. United States Environmental Protection Agency: Washington, DC, USA, 2007; p. 30.
- Silva Junior, E.C.; Wadt, L.H.O.; Silva, K.E.; Lima, R.M.B.; Batista, K.D.; Guedes, M.C.; Carvalho, G.S.; Carvalho, T.S.; Reis, A.R.; Lopes, G.; et al. Natural Variation of Selenium in Brazil Nuts and Soils from the Amazon Region. Chemosphere 2017, 188, 650–658. [Google Scholar] [CrossRef]
- Zanandrea, I.; Alves, J.D.; Deuner, S.; de Goulart, P.F.P.; Henrique, P.D.C.; Silveira, N.M. Tolerance of Sesbania Virgata Plants to Flooding. Aust. J. Bot. 2010, 57, 661. [Google Scholar] [CrossRef]
- Dische, Z. General Color Reactions. In Carbohydrate Chemistry; Whistler, R.L., Wolfram, M.L., Eds.; Academic Press: New York, NY, USA, 1962; pp. 477–512. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The Estimation of Carbohydrates in Plant Extracts by Anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Biemelt, S.; Keetman, U.; Albrecht, G. Re-Aeration Following Hypoxia or Anoxia Leads to Activation of the Antioxidative Defense System in Roots of Wheat Seedlings. Plant Physiol. 1998, 116, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases; Academic Press: New York, NY, USA, 1977; pp. 309–314. [Google Scholar]
- Havir, E.A.; McHale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Schaedle, M.; Bassham, J.A. Chloroplast Glutathione Reductase. Plant Physiol. 1977, 59, 1011–1012. [Google Scholar] [CrossRef]
- García-Limones, C.; Hervás, A.; Navas-Cortés, J.A.; Jiménez-Díaz, R.M.; Tena, M. Induction of an Antioxidant Enzyme System and Other Oxidative Stress Markers Associated with Compatible and Incompatible Interactions between Chickpea (Cicer arietinum L.) and Fusarium oxysporum f. Sp. ciceris. Physiol. Mol. Plant Pathol. 2002, 61, 325–337. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene Produced by Leaves Protects the Photosynthetic Apparatus against Ozone Damage, Quenches Ozone Products, and Reduces Lipid Peroxidation of Cellular Membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 302–310. ISBN 9780121819521. [Google Scholar]
- Chambers, J.M.; Hastie, T.J. Statistical Models in S; Wadsworth & Brooks: Pacific Grove, CA, USA, 1992; ISBN 9780534167646. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- R Foundation for Statistical Computing. R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Pinheiro, J.; Bates, D. Linear and Nonlinear Mixed Effects Models. Available online: https://cran.r-project.org/package=nlme (accessed on 10 July 2023).
- Kassambara, A.; Mundt, F. Extract and Visualize the Results of Multivariate Data Analyses. Available online: https://cran.r-project.org/package=factoextra (accessed on 10 July 2023).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, G.F.d.; Silva, M.A.; Carvalho, M.R.d.; Morais, E.G.d.; Benevenute, P.A.N.; Van Opbergen, G.A.Z.; Van Opbergen, G.G.A.Z.; Guilherme, L.R.G. Foliar Selenium Application to Reduce the Induced-Drought Stress Effects in Coffee Seedlings: Induced Priming or Alleviation Effect? Plants 2023, 12, 3026. https://doi.org/10.3390/plants12173026
Sousa GFd, Silva MA, Carvalho MRd, Morais EGd, Benevenute PAN, Van Opbergen GAZ, Van Opbergen GGAZ, Guilherme LRG. Foliar Selenium Application to Reduce the Induced-Drought Stress Effects in Coffee Seedlings: Induced Priming or Alleviation Effect? Plants. 2023; 12(17):3026. https://doi.org/10.3390/plants12173026
Chicago/Turabian StyleSousa, Gustavo Ferreira de, Maila Adriely Silva, Mariana Rocha de Carvalho, Everton Geraldo de Morais, Pedro Antônio Namorato Benevenute, Gustavo Avelar Zorgdrager Van Opbergen, Guilherme Gerrit Avelar Zorgdrager Van Opbergen, and Luiz Roberto Guimarães Guilherme. 2023. "Foliar Selenium Application to Reduce the Induced-Drought Stress Effects in Coffee Seedlings: Induced Priming or Alleviation Effect?" Plants 12, no. 17: 3026. https://doi.org/10.3390/plants12173026
APA StyleSousa, G. F. d., Silva, M. A., Carvalho, M. R. d., Morais, E. G. d., Benevenute, P. A. N., Van Opbergen, G. A. Z., Van Opbergen, G. G. A. Z., & Guilherme, L. R. G. (2023). Foliar Selenium Application to Reduce the Induced-Drought Stress Effects in Coffee Seedlings: Induced Priming or Alleviation Effect? Plants, 12(17), 3026. https://doi.org/10.3390/plants12173026







