Abstract
Chloroplast genomes are considered to be highly conserved. Nevertheless, differences in their sequences are an important source of phylogenetically informative data. Chloroplast genomes are increasingly applied in evolutionary studies of angiosperms, including Magnoliaceae. Recent studies have focused on resolving the previously debated classification of the family using a phylogenomic approach and chloroplast genome data. However, most Neotropical clades and recently described species have not yet been included in molecular studies. We performed sequencing, assembly, and annotation of 15 chloroplast genomes from Neotropical Magnoliaceae species. We compared the newly assembled chloroplast genomes with 22 chloroplast genomes from across the family, including representatives from each genus and section. Family-wide, the chloroplast genomes presented a length of about 160 kb. The gene content in all species was constant, with 145 genes. The intergenic regions showed a higher level of nucleotide diversity than the coding regions. Differences were higher among genera than within genera. The phylogenetic analysis in Magnolia showed two main clades and corroborated that the current infrageneric classification does not represent natural groups. Although chloroplast genomes are highly conserved in Magnoliaceae, the high level of diversity of the intergenic regions still resulted in an important source of phylogenetically informative data, even for closely related taxa.
1. Introduction
Chloroplasts in plant cells evolved by endosymbiosis and contain their own genetic systems [1,2]. The typical angiosperm chloroplast is a circular sequence that consists of a structure divided into four main regions: two inverted repeats regions (IRa and IRb) and two single-copy regions (SC), called the small single-copy (SSC) and large single-copy (LSC) regions [3,4]. Usually, angiosperm chloroplasts have lengths between 72 and 217 kb and contain between 110 and 130 genes [5]. The chloroplast genome (plastome) is usually very conserved regarding gene content, intron content, and gene organization [6,7]. This has been related to the organization of plastid genes on partitions, the usually uniparental inheritance, as well as some highly effective repair mechanisms [8]. However, structural rearrangements, gene loss, IR expansions, and inversions occur in certain lineages [9,10]. The plastome has proven to be a valuable source of information for phylogenetics, population genetics, and evolutionary studies [11,12,13,14,15].
In the last decade, plastome-based molecular phylogenetic studies have increased, mainly due to new sequencing techniques that expanded the quantity of data obtained and reduced costs [16,17]. Next-generation sequencing, also known as high-throughput sequencing (HTS) or massively parallel sequencing, refers to different sequencing techniques that process DNA in genomic libraries and have the capability of obtaining DNA sequences from hundreds to thousands of different loci and from one to several individuals in just one run [18,19,20]. The high quantity of data achievable with these techniques makes them effective, even for degraded DNA, such as the DNA present in herbarium specimens [21,22,23]. Several strategies have been developed to optimize the quality and quantity of the HTS data, and many of these were reviewed by [19,24,25,26]. One of the most commonly used strategies is whole genome sequencing (WGS) or the genome skimming technique [27], which consists of the preparation and sequencing of libraries from the complete cellular DNA. This results in DNA reads from different origins: the nuclear, mitochondrial, and plastome genomes. In most plants, this strategy generally results in a much higher proportion of plastome reads compared to other regions of the genome because of the high molarity of these organelles in each cell. This strategy was effective in several plant groups [5,15,28,29,30] and has been applied in studies that use chloroplast genomes to solve evolutionary questions in different angiosperm clades [13,31,32,33]. Most attention has focused on sequencing crop genomes, and there is little information on wild groups. In an evolutionary context, it is of vital importance to focus on early divergent or "basal" angiosperm groups, i.e., the family Magnoliaceae [34,35,36,37,38]. For the Magnoliaceae, to date, 86 complete plastomes have been published and are available in the NCBI GenBank [39]; however, most of these correspond to Asian species [40,41,42,43,44,45].
Magnoliaceae is one of the earliest divergent lineages of angiosperms [46]. It comprises more than 300 species distributed in the temperate and tropical forests of Southeast Asia and the Americas [43,47]. The classification within the family has had a turbulent history, with up to 16 genera having been recognized. However, the "multi-generic" view is increasingly in disuse, and recent classifications prefer to maintain two genera with the species-rich genus Magnolia, which is subdivided into subgenera, sections, and subsections [48,49,50,51,52,53], although many of the morphology-based infrageneric taxa are not monophyletic [43,54,55]. The most recent morphology-based classification of the family, which has been widely accepted, only includes two subfamilies (Magnolioideae and Liriodendroideae) and two genera: Liriodendron L. [56] and Magnolia L. [56]. The latter is, in turn, divided into three subgenera, 12 sections, and 13 subsections: subgen. Gynopodium (2 sections), subgen. Magnolia (8 sections and 7 subsections), and subgen. Yulania (2 sections and 6 subsections), of which only subgen. Magnolia is found in the Neotropics [49]. Nevertheless, the most recent classification, which is based on WGS via genome skimming, divides Magnolia only into 15 sections [43].
Previous phylogenetic analyses have mainly focused on Asian Magnolia species and, the relationships between the magnolias from the Americas have been poorly investigated [43,47,57]. This bias results in incomplete knowledge of the phylogenetic relationships of the Neotropical species. Moreover, around 60 Neotropical species have been newly published in the last decade [58,59,60,61,62,63]. Many of these recently described species do not have clear morphological boundaries, casting doubt on their correct delimitation [43,64]. Indeed, although databases, such as POWO [65], record 339 accepted species, the largest phylogenetic studies published to date include only between 86 and 99 of them [43,54,57]. Some authors [54,55,66,67,68,69] suggested that a study including a broader taxon sampling is necessary to address questions that have not been resolved by studies based on Sanger sequencing (i.e., chloroplast and nuclear DNA markers). Such is the case of the relationships between the Neotropical Magnolia taxa or the deep relationships within the subgenus Magnolia, which, based on several molecular studies, has been shown not to be monophyletic [43,54,55,69].
In this study, we compared the structure and attributes of the Magnoliaceae plastome, with an emphasis on the Neotropical Magnolia species. This investigation will serve as a starting point towards resolving questions about the phylogenetic relationships, species delimitations, and character evolution, in this family. We used WGS and chloroplast assembly and included all sections of Neotropical Magnolia. Our research questions were the following: (1) Do the plastome gene composition and length vary within the Magnoliaceae? (2) Does the plastome gene order vary within the Magnoliaceae? (3) What are the most divergent regions of the Magnoliaceae plastome? (4) What are the positions within the infrageneric classification of Magnolia of the Neotropical species newly included in the present study? (5) What is the evidence-based infrageneric classification of Magnolia obtained from WGS presented here and in previous studies?
2. Results
2.1. Chloroplast Assembly and the Annotation of Neotropical Magnolia
The 15 newly generated circular genome maps are depicted in Figure A1 and information for all 37 plastomes is presented in Table 1. The mean assembly coverage varied from 19.8× in M. cubensis to 195× in M. argyrothricha, with a mean of 78.2×. All plastome sequences showed a typical quadripartite structure containing an LSC and an SSC separated by two IR regions (IRa and IRb). The annotations generated by GeSeq reported a total of 145 genes for all species, of which 92 corresponded to protein-coding genes, 45 to transfer RNAs (tRNAs), and 8 to ribosomal RNAs (rRNAs). Of the 145 genes, 94 were unique genes, 21 appear to be duplicated, and 3 appear to be triplicated. The duplicated genes were the dehydrogenase gene ndhB; ribosomal proteins rpl23, rps7, and rps12; open reading frames ycf1, ycf2, and ycf15; tRNAs trnA-UGC, trnI-CAU, trnI-GAU, trnL-CAA, trnN-GUU, trnR-ACG, and trnV-GAC; and rRNAs rrn4.5, rrn5, rrn16, and rrn23. The triplicated genes were rpl2, trnE-UUC, and trnM-CAU.

Table 1.
Plastome sequence length, assembly coverage, GC content, and gene content of the 37 Magnoliaceae plastomes included in the analyses; newly assembled plastomes are highlighted in gray. The classification is according to [51,60]. NA = Not applicable, LSC = large single copy region, SSC = small single copy region, IR = inverted repeat region, CDS = coding DNA sequence, tRNA = transfer RNA, rRNA = ribosomal RNA. 1: Synonym of Magnolia conifera var. chingii, treated as M. glaucifolia in [43]. 2: Synonym of M. vrieseana, treated as M. ovalis in [43].
2.2. The Magnoliaceae Plastome
In general, the plastomes of all studied Magnoliaceae species were conserved for their GC content, gene content, and plastome length (Table 1). The latter ranged from 159,121 bp in M. chimantensis to 160,232 bp in M. wilsonii, with a mean of 159,787 bp. At the same time, the LSC regions varied from 87,541 bp in M. chimantensis to 88,294 bp in M. sinica, with a mean of 88,018 bp. Moreover, the SSC regions were very stable in size, with a range from 18,690 bp in M. liliifera to 18,998 bp in Liriodendron chinense and a mean length of 18,772 bp. Finally, the IR regions ranged from 26,333 bp in L. chinense to 26,603 bp in M. fraseri, with a mean of 26,544 bp. The GC content for the family was 0.39 for all the species included in the analysis.
Within Magnoliaceae, there was a difference of only 1111 base pairs between the smallest (M. chimantensis) and the largest (M. wilsonii) plastome included in the analyses. With the caveat that only a small number of species were included for each section, section Talauma, with ten species sampled, showed the smallest plastomes within the genus (mean 159,704 bp), especially subsection Dugandiodendron (mean 159,185 bp). Section Oyama, represented in this study by two species, showed the largest plastomes in the family, with a mean of 160,204 bp. Subsection Dugandiodendron presented the smallest LSC and SSC regions in the family, with means of 87,590 and 18,717, respectively. Section Oyama showed the largest LSC (mean 88,267) and the two species of genus Liriodendron showed the largest SSC regions (mean 18,982). When comparing the IR sizes, the smallest was found in Liriodendron, with a mean of 26,357, while the largest was observed in section Auriculata (26,603 bp).
Concerning gene content, all the plastomes included in the analysis shared the same content; all species showed the same 92 CDS, 45 mRNAs, and 8 tRNAs (Table 2). The 21 genes duplicated in the Neotropical species were also duplicated family-wide. The same goes for the genes rpl2, trnE-UUC, and trnM-CAU, which appeared to be triplicated in the 37 analyzed species.

Table 2.
Genes found in the 37 Magnoliaceae plastomes. Duplicated genes are underlined. Triplicated genes are in bold.
Gene Organization and Nucleotide Diversity of the Magnoliaceae plastomes
The Shuffle-LAGAN alignment in mVista resulted in the similarity plot shown in Figure A2. In general, the Magnoliaceae plastomes presented high similarity values across the whole genome, with small regions of lower similarity, especially in intergenic regions, although the similarity values went below 50% in only a few sites. The IR regions resulted in the most conserved partitions, with values of 100% for similarity at most of their lengths. When comparing between Magnolia and Liriodendron, differences were more common; however, these remained confined to the intergenic regions and the similarities were still above 50%. The Mauve alignment results are presented in Figure 1. Four colinear blocks were identified: the first one corresponded to the LSC region and the first IR region; for the SSC we identified two colinear blocks, and the fourth block corresponded to the second IR. All regions of the 37 Magnoliaceae plastomes are in the same order and orientation.

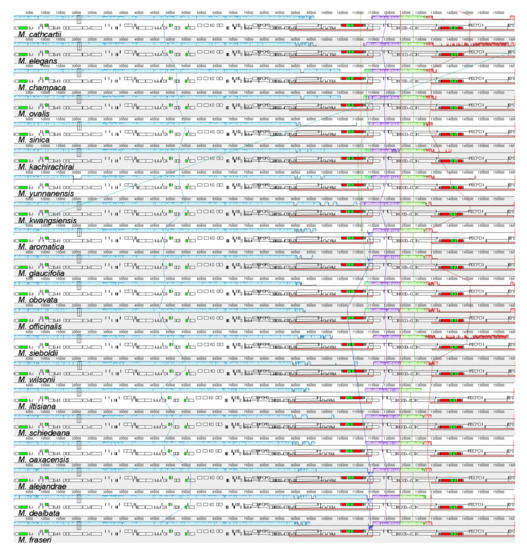
Figure 1.
Mauve progressive alignment, including all 37 Magnoliaceae plastomes. Blocks of the same color connected by a line represent colinear regions. Blocks below the graphs represent coding regions. Colinear regions appear in the same order in all species, which suggests that no significant rearrangement has been found.
From the sliding window analysis performed in DNAsp, we obtained the nucleotide diversity values (Pi) from all samples (Figure 2). These ranged from 0 to 0.0236 and presented a mean of 0.0042. The most diverse sites corresponded to genes, such as PetL, ccsA, and ndhD, as well as intergenic regions in the LSC and SSC regions, while the IR regions presented the lowest diversity.
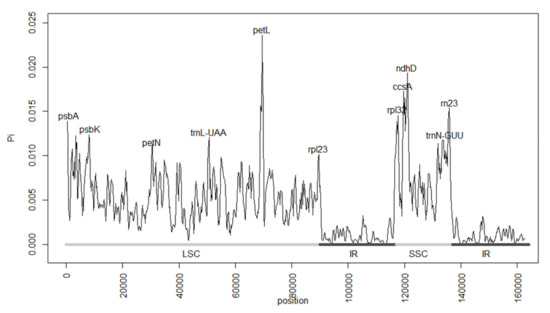
Figure 2.
Nucleotide diversity (Pi) values resulting from the sliding window analysis of the 37 included Magnoliaceae plastomes. The Pi values ranged from 0 to 0.0236. The most diverse sites corresponded to genes such as PetL, ccsA, and ndhD, while the IR regions presented the lowest diversity. LSC = large single copy region; IR = inverted repeat regions; SSC = small single copy region.
A comparison of the inverted repeat regions of the 37 species is shown in Figure 3. In the junction between the LSC and the IRb regions, the gene rpl12 was completely found in the IR region, while rps19 occurred mainly in the LSC, presenting a small overlap (1–21 base pairs) with the IRb region. In the junctions IRb/SSC and SSC/IRa we found the gene ycf1 overlapping with both pairs of regions, although mostly with the IR regions (IRa and IRb). The junction between the IRa and LSC regions presented the gene trnH on the LSC but slightly overlapping with the IRa, while the gene rpl12 was found in the IRa region.
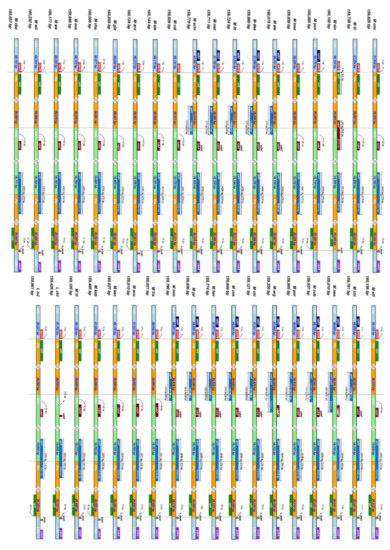
Figure 3.
Expansion and contraction of 37 Magnoliaceae IR regions, analyzed with IRscope. All four junctions (LSC/IRb, IRb/SSC, SSC/IRa, and IRa/LSC) are shown, as well as their flanking genes.
From the positive selection analysis performed in EasyCodeML [70], 13 genes with sites under positive selection were identified (likelihood ratio test p < 0.05): atpB, cemA, ndhD, ndhF, ndhH, pbf1, psaA, psaB, rbcL, rplI14, rpoA, rps3, and ycf1.
2.3. Neotropical Magnolia Phylogeny
The phylogenetic tree shows the position of the newly assembled plastomes (Figure 4). The relationships obtained for the Magnolia sections report two clades: the first one is formed by the sections Talauma and Gwillimia, and the second one includes the remaining 10. This second clade is divided into three subclades: clade A contains sections Gynopodium, Kmeria, Michelia, and Yulania; clade B contains sections Magnolia, Manglietia, and Rhytidospermum; and clade C contains Auriculata and Macrophylla; the latter clade being sister to the first two. Comparing the two clades obtained against the three subgenera that are recognized based on morphologic traits, those are not monophyletic. The subgenus Gynopodium partly includes subclade A, being paraphyletic; subgen. Magnolia entirely comprises clade I and subclades B and C, resulting in a polyphyletic subgenus, and subgen. Yulania contains the remaining part of subclade A and is paraphyletic.
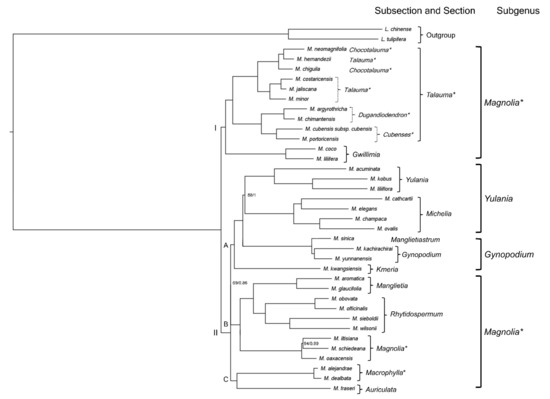
Figure 4.
Phylogenetic relationships obtained from the Bayesian inference analysis of the newly assembled plastomes from 15 Neotropical species plus 22 plastomes downloaded from the NCBI; Liriodendron was used as an outgroup. The classification was according to [51,60]; The Neotropical subsections are shown in dotted brackets. The numbers at the nodes represent the ML bootstrap and BI posterior probability support values, respectively; nodes without numbers correspond to 100/1 support values. * = Neotropical groups.
Particularly within the section Talauma, two clades can be distinguished: the first one groups the subsections Chocotalauma and Talauma, and the second one includes Cubenses and Dugandiodendron. In the remaining Neotropical sections (Macrophylla and Magnolia), only one clade is recognized for each.
Both the Bayesian inference analysis (BI) and the maximum likelihood analysis (ML) showed nearly the same results. The only difference was observed in the subgenus Gynopodium. In the ML tree, M. kachirachirai was a sister to the clade that includes M. sinica and M. yunnanensis. In contrast, in the BI tree, both species of section Gynopodium form a clade and M. sinica is a sister to it (Figure 4).
3. Discussion
3.1. Chloroplast Assembly and the Annotation of Neotropical Magnolia
WGS, followed by the use of assembly tools, has resulted in an effective manner to obtain chloroplast genomes for different families of angiosperms [13,31,32,33], as well as of different Magnoliaceae species [40,41,42,44]. In this study, the plastomes of 15 Neotropical Magnolia species were assembled through the use of short Illumina reads and the GetOrganelle assembler. GetOrganelle is a pipeline that has been proposed as the default option for plastome assemblies, due to the good performance shown compared to other tools [71]. In our study, this performance was corroborated by the obtention of highly consistent results and the assembly of complete circular plastomes of all the species.
The newly assembled plastomes had lengths of about 160 kb (Table 1), which is consistent with the lengths observed in previously assembled Magnolia plastomes [40,41,42,43,44,45]; partition lengths (LSC, IR, and SSC regions) were also consistent and similar to those observed in previous studies. For the GC content, other studies have found similar values [40], while for gene content, the previously annotated Magnoliaceae plastomes presented between 113 and 131 genes [41,42]. This difference in the number of annotated genes could be related to the tool used for the annotation. Other studies have used software, such as DOGMA [72] or cpGAVAS [73], to annotate other Magnoliaceae plastomes [41,42]. Comparisons of these two software programs against GeSeq have shown discrepancies in the number of identified genes, usually with the latter showing better results [74,75].
3.2. The Magnoliaceae Plastome
Many angiosperm lineages have shown some degree of variation in their plastomes [10]. This ranges from the an expansion or contraction of their IR regions [76,77] to rearrangements in the gene content and order [78], to even the complete deletion of an entire partition of the plastid [79]. However, plastomes often tend to be very stable and conserved [4,80]. This is the case for the Magnoliaceae plastome, where little variability was observed.
Angiosperm plastome length ranges from 72 kb to 217 kb, although in most species it is between 120 and 160 kb [5,7,9]. The Magnoliaceae plastome falls within that range with a length of about 160 kb. This size is comparable to those observed in other “basal angiosperms” [81], such as Lauraceae [82], Chloranthaceae [83], and Nymphaeaceae [84].
Family-wide, plastome size varied only slightly (i.e., c. 1000 base pairs at most). Several studies have indicated that factors such as gene loss, IR variation, and intergenic variation are three of the main drivers of genome length variation [85,86]. In the case of Magnoliaceae, gene content was constant across the family and only a small variation in size was found in the partitions (Table 1); however, intergenic variability was relatively high in several parts of the genome (Figure 2). In this manner, intergenic variation could be one of the main factors affecting chloroplast genome size. This often is the case in closely related species [81].
Although our present study only included a small fraction of Magnoliaceae species, there is some indication that plastome size is conserved among lineages (Table 1). Similar plastome lengths were found in species of the same section and subsection. Noteworthy cases are the plastomes found in subsection Dugandiodendron, which are the smallest plastomes assembled for the family. Liriodendron plastomes showed a particularly large SSC region and the smallest IR region in the family. This could be related to the expansions and contractions of the IR region that were observed when comparing this genus with Magnolia (Figure 3).
The Magnoliaceae plastome structure was highly conserved. The 37 plastomes presented the typical angiosperm quadripartite structure, which includes the LSC, IRb, SSC, and IRa regions. These four partitions are usually conserved in most angiosperms, although in some rare cases one of these could be lost [79,87,88].
Our results showed that Magnoliaceae plastomes are highly conserved in both gene content and gene order. The annotated genomes showed that all species included in the analysis presented the same CDSs, rRNAs, and tRNAs (Table 2). Gene loss in plastomes has been reported in several families of angiosperms [79,81,88,89], although it is most common among parasitic species [78]. The Shuffle-LAGAN alignment in mVista and the progressive alignment in Mauve (Figure 1 and Figure A2) confirmed that the genomes in all species are colinear and no major rearrangements have occurred, not even between Liriodendron and Magnolia. Contrary to Magnoliaceae, gene rearrangements have independently appeared in different plant families [78,79,90].
Mutations in the IR regions involve important changes to the chloroplast genome, due to either the duplication of genes from the SC regions or the deletion of one copy of a duplicated gene from the IR region [89,91]. The IR regions in the Magnoliaceae plastomes were also highly conserved among lineages, with lower molecular diversity values than those observed in the SC regions (Figure 2). No important expansions or contractions were observed within Magnolia (Figure 3), although a small expansion of the IR involving the ycf1 gene was observed in comparison to the IR region in Liriodendron. Previous studies have shown that IR and IR expansion/contractions are often similar between closely related species [91,92,93,94], which is also the case within Magnoliaceae.
Usually, diversity is smaller within species and it increases at higher taxonomic levels [30,79]. However, some pantropical genera, such as Aristolochia, have shown a higher nucleotide diversity, especially in intergenic regions [94]. The little genetic variation presented by Magnoliaceae has been previously observed [47,55]. Compared to other groups, the nucleotide diversity in Magnoliaceae is similar to that observed in some genera such as Blumea [91] and Fragaria [95] but smaller than expected for a widely distributed family, such as Myrtaceae [96].
In the case of positive selected sites, Magnoliaceae species showed 13 genes with sites under selection; most of these are related to the photosynthetic process (atpB, ndhD, ndhF, pbf1, psaA, psaB, and rbcL). Other studies have argued that positive selection in those genes could be related to differences in the photosynthetic pathways or in the habitat of each species [97,98].
3.3. Neotropical Magnolia Phylogeny
Similar relationships were obtained as those reported by [43], who distinguished two clades and three subclades based on WGS evidence, although the same sections were included, but not the same species. The Neotropical taxa incorporated in the present study had never been sampled before.
Within our clade I (Talauma-Gwillimia), it is possible to separate the subsections Cubenses and Dugandiodendron as a distinct section: Splendentes, as also reported by [43]. In this way, Splendentes would be a fourth section in the Neotropical region (besides Macrophylla, Magnolia, and Talauma) and the classification in the subsections for this region would need to be reconsidered, given that in our hypothesis there are genetic synapomorphies that join the Cubenses and Dugandiodendron species into a clade. As for the Asian section Gwillimia, it is maintained here as a sister group to the clade of Splendentes and Talauma, a position that was first revealed by [47,99] but altered in studies conducted afterward [54,69]. Our study, thus, confirms this sister relationship, as in other detailed analyses [43,69]. In contrast, subsection Chocotalauma does not form a separate clade. This result is preliminary because (1) the classification of M. chiguila to Chocotalauma has been questioned, given a stipular scar was observed (pers. comm. Emily Veltjen); and (2) the four other Chocotalauma species: M. calimaensis, M. calophylla, M. mashpi, and M. striatifolia are not yet considered. The four species should be included to further corroborate whether Chocotalauma really could be considered as a separate subsection [60].
Similarly, the relationships obtained in clade II correspond with those of [43], in which the division of this clade into three subclades is maintained, which shows the same relationships among the sampled sections. However, the relationships of the three subclades are solved here, with subclade C (section Macrophylla) as a sister to clades A and B, while [43] reported that there was a trichotomy between them, when taking into account the coding sequences (CDSs) dataset of the chloroplast genome.
As for the three subgenera identified by Figlar [50,51], it is once again proven that a new infrageneric classification reflecting natural groups supported by synapomorphies is needed (especially within polyphyletic subgenus Magnolia, considering our Neotropical framework), since Figlar’s morphology-based proposal [50,51] does not correspond with the results obtained in this study, reflecting the same pattern as in previous works using molecular evidence [43,47,54,57,69].
4. Materials and Methods
4.1. Sampling, DNA Extraction, and Sequencing
DNA was extracted from Magnolia leaf tissue, either freshly collected and dried in silica gel or from herbarium collections. The 15 sampled species included members of all Neotropical sections and covered a representative Neotropical distribution; species names and authors are according to [100]. Table 3 presents the complete sampling list, indicating the classification according to Figlar [50,51] and Pérez [60], the country of origin, and the herbarium voucher. The DNA extractions were carried out through a modified CTAB protocol [101,102]. The DNA quality was checked using a spectrophotometer (Nanodrop 2000 UV-Vis).

Table 3.
Sampled Neotropical Magnolia species. The classification is according to [51,60]. The collection is either a herbarium, in which case the acronyms are according to [103], or living specimens from the natural reserve “El Refugio” in Dagua, Colombia [104]. NA = Not applicable.
The 15 selected samples are part of an ongoing WGS study on Neotropical Magnolia phylogeny and evolution for which a total of 192 newly sequenced Neotropical Magnolia samples were generated. Sequencing was executed by Rapid Genomics (Gainesville, FL, USA) following a HiSeq protocol defined by Rapid Genomics using an Illumina platform. Demultiplexing was carried out using BCLtofastq. The sequencing results are shown in Table A1.
4.2. Chloroplast Assembly and Annotation
All analyses were carried out on a Unix platform, either on the Huitzilin HPC of the Instituto de Ecología, A.C., running CentOS 8, or on a Windows 10 desktop running Ubuntu 20.04.01 over the Linux Subsystem for Windows environment. Complete command-line examples of the code used for the assembly are shown in Algorithm A1. A first quality check of the demultiplexed samples was performed using the software FastQC v. 0.11.7 [105], and quality reports were performed with multiQC [106] to identify the quality of the reads and if adapters were present. Trimmomatic v. 0.38 [107] was used to filter low-quality reads and perform the adapter trimming, applying a sliding-window of 5:20 and removing all the reads shorter than 30 bases. This was followed by a second quality check with FastQC and multiQC to ensure the correct removal of the adapters.
GetOrganelle v. 1.7.0 [108] was used to assemble the plastome; this is a complete Python pipeline that uses Illumina reads to perform de novo plastome assemblies. This software consists of a pipeline that starts with the use of Bowtie2 [109] to align reads to a seed sequence; i.e., a sequence from a related species. Then, new reads are recruited through extending iterations. Later, GetOrganelle uses SPAdes [110] to begin the de novo assembly of the recruited reads. Finally, BLAST [111] was used to compare the assembled sequences and identify target contigs. As seeds for the assembly, complete chloroplast sequences from three Neotropical Magnolia species (M. pacifica subsp. tarahumara A. Vázquez/MN990636.1, M. dealbata Zucc./NC_023235.1, and M. ovata (A. St. Hil.) Spreng./NC_048993.1) were selected and downloaded from the NCBI GenBank database [39]. The script “Get_organelle_from_reads.py” was used with the “embplant_pt” option, as well as 15 extension rounds and kmer values between 21 and 105. The results were visualized with Bandage v. 0.8.1 [112] to ensure that a correct assembly graph was produced.
To accomplish a complete sampling that included all genera and sections in the family, 22 complete plastomes were downloaded from the NCBI (Table 4). These included 20 accessions from Magnolia sections with Asian distributions and two from Liriodendron. Both the 15 newly assembled sequences and the 22 NCBI accessions were annotated following the same protocol to ensure a correct comparison of gene content. The plastome annotation was performed using the online software GeSeq v. 1.55 (https://chlorobox.mpimp-golm.mpg.de/geseq.html accessed on 20 November 2021) [113]. Chloë v. 0.1.0 (https://chloe.plantenergy.edu.au/; unpublished, accessed on 20 November 2021) was used as a support annotator, and ARAGORN v. 1.2.38 [114] and tRNAscan-SE v. 2.0.7 [115] were used as tRNA annotators, keeping the best annotation only. As a reference for the annotation, we utilized the base MPI-MP reference set, as well as the Magnoliaceae plastomes available at the NCBI RefSeq [116]. Finally, we used OGDRAW v. 1.3.1 [117] to generate the circular genome map of each plastome.

Table 4.
Magnoliaceae plastomes downloaded from [39]; the classification is according to [51,60]. NA = Not applicable. 1: Synonym of Magnolia conifera var. chingii (Dandy) V.S.Kumar. 2: Synonym of M. vrieseana (Miq.) Baill. ex Pierre.
4.3. Genome Comparison
All 37 assembled plastomes and their annotations were submitted to mVista [118]. We used the Shuffle-LAGAN mode [119] to align the sequences and perform pairwise comparisons. Next, the 37 plastomes were aligned using Mauve v. 2.4.0 [120], with the progressive alignment option and the gene orders were compared.
DNAsp v. 6.12.03 [121] was used to calculate the nucleotide diversity among the aligned genomes using a sliding window approach with a window length of 600 bp and a step size of 200 bp. All the complete annotated plastomes were submitted to IRscope [122] to analyze the expansion and contraction of the IR regions.
EasyCodeML 1.4 [70] was used to identify positive selection sites. All the coding sequences of the unique CDSes were extracted and concatenated in a supermatrix for each species. The supermatrix was aligned using MAFFT v. 7.475 [123], and IQ-Tree v. 2.0.3 [124] was used to create a phylogenetic tree of the supermatrix. Each of the individual CDSes were aligned and used as inputs for EasyCodeML. The branch site model was selected, and a likelihood ratio test was performed, as implemented in EasyCodeML, to test the significance of the results.
4.4. Phylogenomics of the Magnoliacceae Plastome
The plastomes of the 37 species included in the analysis were aligned using MAFFT. The MAFFT alignment was used to build species trees using maximum likelihood (ML) and Bayesian inference (BI). The ML approach was performed in IQ-Tree v. 2.0.3 [124] with an ultrafast bootstrap to estimate the branch support values. The software MrBayes V. 3.2.7 was used for the BI analysis, using a GTR invgamma model with 10,000,000 generations and a burn-in of 25%.
5. Conclusions
Chloroplast genomes have proven to be a reliable source of information for addressing phylogenetic questions in angiosperms, even for closely related taxa. This is also the case for Magnoliaceae, where the chloroplast genome has previously been used to address evolutionary questions, such as the Magnoliaceae divergence time and the evolutionary relationships between the main Magnoliaceae clades. However, this has always occurred with a sampling bias towards Asian species, leaving a gap in the knowledge for the Magnoliaceae from the Americas (especially in the Neotropics). To fill this gap, we generated 15 new complete annotated Neotropical Magnolia plastomes; those were compared to 22 plastomes from other species of the family, representing all currently accepted clades. We found that the Magnoliaceae plastome is highly conserved in gene content and organization, regardless of its high species diversity and wide geographic distribution. Chloroplast genomes in Magnoliaceae only present subtle differences in size and nucleotide diversity, mainly in the intergenic regions. However, these differences are sufficient to yield phylogenetically informative data, as shown by previous studies, and will provide information to resolve relationships between the Neotropical magnolias in future studies. Further research is needed to elucidate whether the low genetic variation and highly conserved structure of Magnolia plastomes are reflected in an equally conserved morphology.
Author Contributions
Conceptualization, M.-S.S. and E.V.; fieldwork, F.A.A.N., E.V. and M.-S.S.; lab work, E.V., P.A. and F.A.A.N.; data generation and analyses, S.G.-D. and F.A.A.N.; writing—original draft preparation, S.G.-D.; writing—review and editing, S.G.-D., F.A.A.N., E.V., P.A., I.L. and M.-S.S.; supervision, M.-S.S.; project administration, E.V.; funding acquisition, M.-S.S. and E.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation Franklinia, project number 2019-03; the Instituto de Ecología, A.C., the Consejo Nacional de Ciencia y Tecnología (CONACYT) through a Ph.D. grant, CVU 848616; the Ghent University Special Research Fund (BOF: Bijzonder Onderzoeksfonds); and the Research Foundation Flanders.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study will be openly available at the NCBI GeneBank after publishing.
Acknowledgments
We are grateful to the following colleagues who supported us in the field and/or provided us with samples: Eduardo Calderón, Ricardo Callejas Posada, Alex Dahua Machoa, Luis Fernando Giraldo Gallego, Luis Roberto González Torres, Francisco Hernández Najarro, Majela Hernández Rodríguez, José Esteban Jiménez, Esteban Manuel Martínez Salas, Martín Mata Rosas, David Neill, Alejandro Palmarola Bejerano, Ernesto Testé Lozano, Christian Torres Santana, José Antonio Vázquez García, and Susana Vega Betancur. We also thank the herbarium K for allowing us to study and destructively sample the collections of M. chimantensis and M. hernandezii. We are grateful to the authorities of the following countries for granting us collection permits: Cuba (CICA-CITMA 15/16), Puerto Rico (2016 EPE-011), and Mexico (SGPA/DGGFS/712/1063/18). We thank Eduardo Cires Rodríguez and Enrique Ibarra Laclette for their comments and suggestions for this manuscript. We are also grateful for the technical support of Emanuel Villafán and the resources for high-performance computing that the Instituto de Ecología, A.C. (INECOL) made available for conducting the research reported in this paper. We thank José Trinidad Soto Rodríguez and Marisol Alicia Zurita Solís for their help in the programming and discussion. Finally, we also thank the three anonymous reviewers for their helpful comments and remarks to improve the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
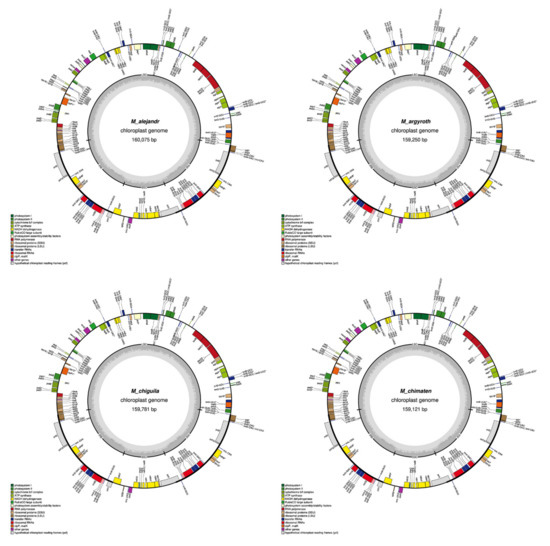
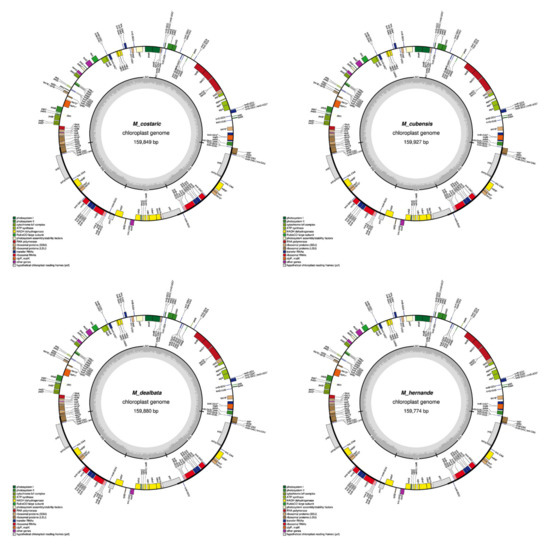
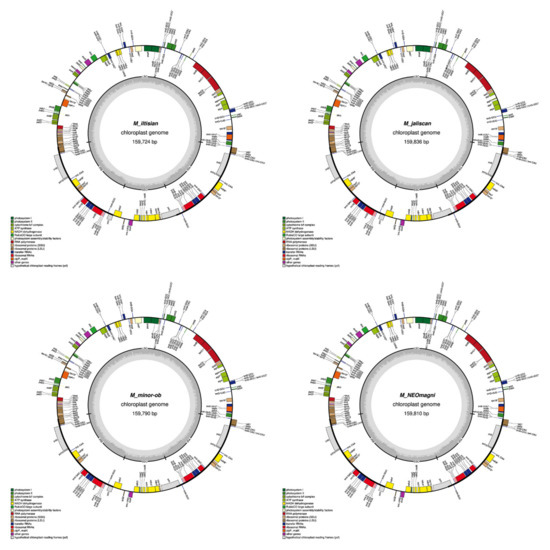
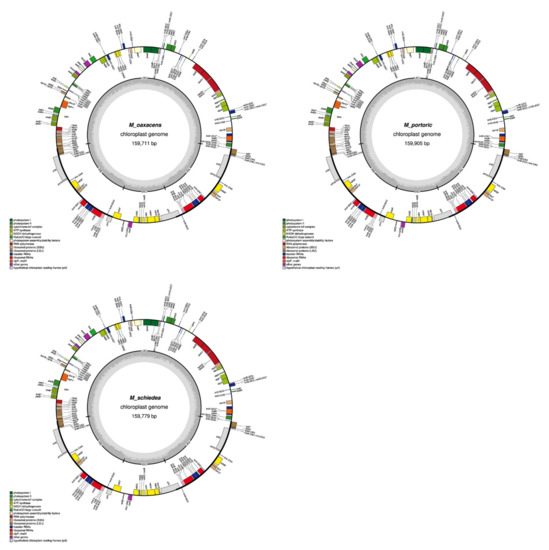
Figure A1.
Graphical maps of the 15 newly annotated plastomes generated by OGDRAW.
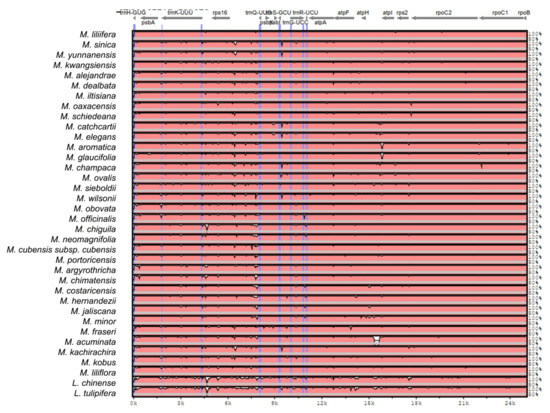
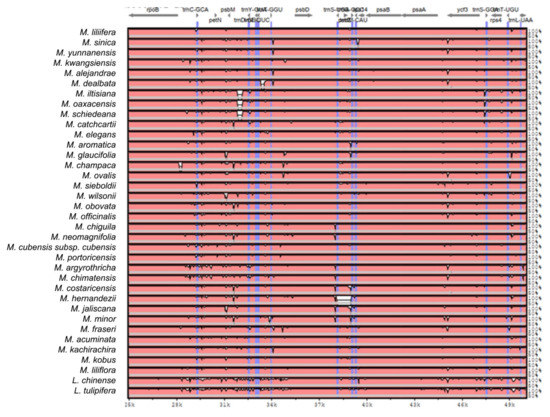
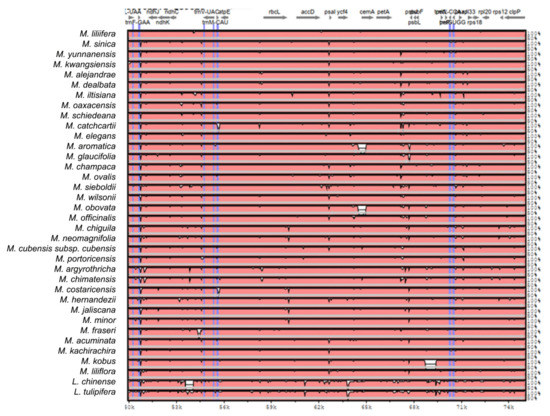
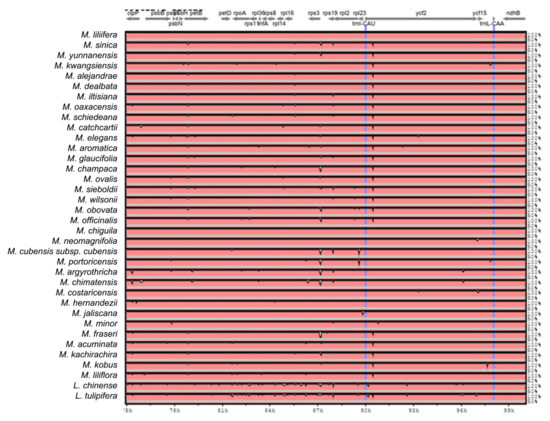
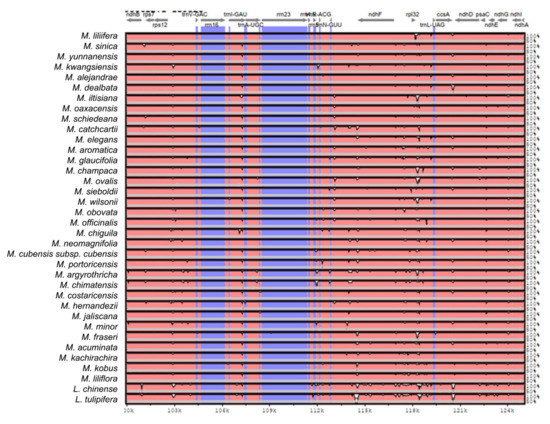
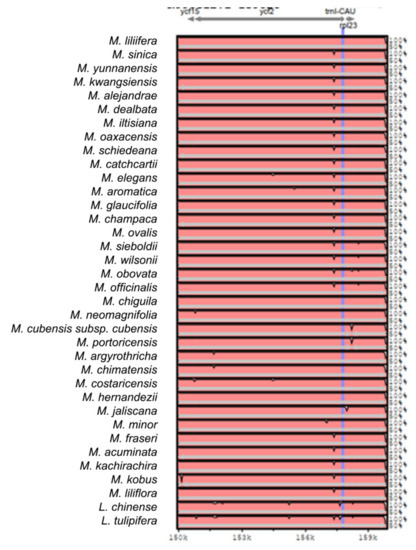
Figure A2.
Sequence identity plot produced by Shuffle-LAGAN alignment in mVista comparing 37 Magnolia species; Magnolia coco was used as reference. Grey arrows represent genes with their orientation. Pink areas are conserved non-coding sequences (CNS). Blue areas are exons. The Y-axis represents the percentage of conservation of each species against the reference.

Table A1.
Sequencing results of the newly assembled Magnolia species. Columns show the number of paired raw reads, the number of paired reads after quality trimming with Trimmomatic, and the NCBI reference number. NA = Not applicable.
Table A1.
Sequencing results of the newly assembled Magnolia species. Columns show the number of paired raw reads, the number of paired reads after quality trimming with Trimmomatic, and the NCBI reference number. NA = Not applicable.
| Section (Subsection) | Species | Voucher | Raw Reads | Trimmed Reads | NCBI Reference |
|---|---|---|---|---|---|
| Macrophylla | M. alejandrae García-Mor. and Iamonico | M. Mata 1188b | 1,583,270 | 1,369,706 | TBD |
| M. dealbata Zucc. | M. Mata 0866a | 1,576,433 | 1,435,083 | TBD | |
| Magnolia | M. iltisiana A. Vázquez | S. Cházaro B. and M. Rodríguez 8590 | 2,654,727 | 2,564,393 | TBD |
| M. oaxacensis A. Vázquez | M.S. Samain and E. Martínez 2019-019 | 1,857,563 | 1,792,016 | TBD | |
| M. schiedeana Schltdl. | F. Aldaba 187 | 1,713,367 | 1,672,698 | TBD | |
| Talauma (Chocotalauma) | M. chiguila F. Arroyo, Á.J. Pérez, and A. Vázquez | E. Veltjen and A. Dahua 2018-005 | 1,678,118 | 1,582,603 | TBD |
| M. neomagnifolia I.M.Turner | NA | 1,772,721 | 1,731,278 | TBD | |
| Talauma (Cubenses) | M. cubensis Urb. subsp. cubensis | A. Palmarola et al. HFC-89195 | 638,499 | 586,472 | TBD |
| M. portoricensis Bello | E. Veltjen et al. 2016-033 | 1,613,856 | 1,549,640 | TDB | |
| Talauma (Dugandiodendron) | M. argyrothricha (G. Lozano C.) Govaerts | NA | 2,008,282 | 1,910,887 | TBD |
| M. chimantensis Steyerm. and Maguire | J. Steyermark 1191 | 1,603,135 | 1,514,091 | TBD | |
| Talauma (Talauma) | M. costaricensis A. Vázquez | J.E. Jiménez 4622 | 1,889,242 | 1,806,280 | TBD |
| M. hernandezii (G. Lozano C.) Govaerts | Hernández 1001 | 1,089,114 | 716,041 | TBD | |
| M. jaliscana A. Vázquez and R. Guzmán | J.A. Vázquez García et al. 9335 | 1,800,168 | 1,671,463 | TBD | |
| M. minor (Urb.) Govaerts | B. Falcón HFC88953 | 1,750,979 | 1,611,208 | TBD |
| Algorithm A1. Unix commands used for the assembly of the WGS reads. |
| ### run fastQC mkdir raw_wgs/out_fastQC fastqc raw_wgs/*.fastq.gz --outdir=raw_wgs/out_fastQC ### run multiQC mkdir raw_wgs/out_fastQC/multiQC multiqc raw_wgs/out_fastQC --outdir raw_wgs/out_fastQC/multiqc #################### ##### trimming ##### mkdir trimmed while read p; do echo "#######################################"; echo "$p"; echo "#######################################"; trimmomatic PE -phred33 -trimlog trimmed/$p’.log’ raw_wgs/$p’_R1.fastq.gz’ raw_wgs/$p’_R2.fastq.gz’ trimmed/$p’_R1_paired.fastq’ trimmed/$p’_R1_unpaired.fastq’ trimmed/$p’_R2_paired.fastq’ trimmed/$p’_R2_unpaired.fastq’ ILLUMINACLIP:Trimmomatic-0.39/adapters/TruSeq3-PE-2.fa:2:30:10:1:TRUE SLIDINGWINDOW:5:20 ; done < raw_wgs/samples.txt ################################ ##### Quality check of ##### ##### trimmed reads ##### ### run fastQC mkdir trimmed/out_fastQC fastqc trimmed/*.fastq.gz --outdir=trimmed/out_fastQC ### run multiQC mkdir trimmed/out_fastQC/multiQC multiqc trimmed/out_fastQC --outdir trimmed/out_fastQC/multiqc ################################################### ##### chloroplast assembly using getOrganelle ##### ### run getOrganelle while read f; do get_organelle_from_reads.py --max-reads 3E7 -1 trimmed/$f’_R1_paired.fastq’ -2 trimmed/$f’_R2_paired.fastq.gz’ -o getOrganelle/$f -s targets/*_complete.fasta -R 25 -k 21,45,65,85,105 -F embplant_pt -t 8 ; echo ’###############################################################’; echo sample $f finished; echo ’###############################################################’; done < trimmed/samples.txt |
References
- Cooper, G. Chloroplast and Other Plastids. In The Cell: A Molecular Approach; ASM Press: New York, NY, USA, 2007; p. 820. ISBN 978-0-87893-219-1. [Google Scholar]
- Jensen, P.E.; Leister, D. Chloroplast evolution, structure and functions. Prime Rep. 2014, 6, 40. [Google Scholar] [CrossRef]
- Grivet, D.; Heinze, B.; Vendramin, G.G.; Petit, R.J. Genome walking with consensus primers: Application to the large single copy region of chloroplast DNA. Mol. Ecol. Notes 2001, 1, 345–349. [Google Scholar] [CrossRef]
- Henriquez, C.L.; Abdullah; Ahmed, I.; Carlsen, M.M.; Zuluaga, A.; Croat, T.B.; McKain, M.R. Molecular evolution of chloroplast genomes in Monsteroideae (Araceae). Planta 2020, 251, 72. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Su, T.; An, M.-T.; Hu, G.-X. The Complete Chloroplast Genome of the Vulnerable Oreocharis esquirolii (Gesneriaceae): Structural Features, Comparative and Phylogenetic Analysis. Plants 2020, 9, 1692. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D. Comparative organization of chloroplast genomes. Annu. Rev. Genet. 1985, 19, 325–354. [Google Scholar] [CrossRef]
- Daniell, H.; Lin, C.-S.; Yu, M.; Chang, W.-J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicke, S.; Schneeweiss, G.M.; dePamphilis, C.W.; Müller, K.F.; Quandt, D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Zheng, Y. Dynamic evolution and phylogenomic analysis of the chloroplast genome in Schisandraceae. Sci. Rep. 2018, 8, 9285. [Google Scholar] [CrossRef]
- Gonçalves, D.J.P.; Jansen, R.K.; Ruhlman, T.A.; Mandel, J.R. Under the rug: Abandoning persistent misconceptions that obfuscate organelle evolution. Mol. Phylogenet. Evol. 2020, 151, 106903. [Google Scholar] [CrossRef]
- Jansen, R.K.; Kaittanis, C.; Saski, C.; Lee, S.-B.; Tomkins, J.; Alverson, A.J.; Daniell, H. Phylogenetic analyses of Vitis (Vitaceae) based on complete chloroplast genome sequences: Effects of taxon sampling and phylogenetic methods on resolving relationships among rosids. BMC Evol. Biol. 2006, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.-Q.; Ge, S. The phylogeny of the BEP clade in grasses revisited: Evidence from the whole-genome sequences of chloroplasts. Mol. Phylogenet. Evol. 2012, 62, 573–578. [Google Scholar] [CrossRef]
- Duan, L.; Harris, A.; Mao, L.-Y.; Zhang, Z.-R.; Arslan, E.; Ertuğrul, K.; Loc, P.K.; Hayashi, H.; Wen, J.; Chen, H.-F. Chloroplast Phylogenomics and Biogeography of Liquorice (Leguminosae: Glycyrrhiza). In Prime Archives in Plant Sciences; Vide Leaf: Hyderabad, India, 2020; ISBN 978-81-944664-9-9. [Google Scholar]
- Li, H.; Liu, B.; Davis, C.C.; Yang, Y. Plastome phylogenomics, systematics, and divergence time estimation of the Beilschmiedia group (Lauraceae). Mol. Phylogenet. Evol. 2020, 10, 6901. [Google Scholar] [CrossRef]
- Su, C.; Duan, L.; Liu, P.; Liu, J.; Chang, Z.; Wen, J. Chloroplast phylogenomics and character evolution of eastern Asian Astragalus (Leguminosae): Tackling the phylogenetic structure of the largest genus of flowering plants in Asia. Mol. Phylogenet. Evol. 2020, 10, 7025. [Google Scholar] [CrossRef]
- Cronn, R.; Liston, A.; Parks, M.; Gernandt, D.S.; Shen, R.; Mockler, T. Multiplex sequencing of plant chloroplast genomes using Solexa sequencing-by-synthesis technology. Nucleic Acids Res. 2008, 36, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Parks, M.; Cronn, R.; Liston, A. Increasing phylogenetic resolution at low taxonomic levels using massively parallel sequencing of chloroplast genomes. BMC Biol. 2009, 7, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardis, E.R. Next-Generation Sequencing Platforms. Annu. Rev. Anal. Chem. 2013, 6, 287–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, C.F.; Bacon, C.D.; Antonelli, A.; Cano, Á.; Hofmann, T. An introduction to plant phylogenomics with a focus on palms. Bot. J. Linn. Soc. 2016, 182, 234–255. [Google Scholar] [CrossRef] [Green Version]
- Bleidorn, C. Phylogenomics: An Introduction; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-319-54062-7. [Google Scholar]
- Bakker, F.T.; Bieker, V.C.; Martin, M.D. Editorial: Herbarium Collection-Based Plant Evolutionary Genetics and Genomics. Front. Ecol. Evol. 2020, 8, 603948. [Google Scholar] [CrossRef]
- Zeng, C.-X.; Hollingsworth, P.M.; Yang, J.; He, Z.-S.; Zhang, Z.-R.; Li, D.-Z.; Yang, J.-B. Genome skimming herbarium specimens for DNA barcoding and phylogenomics. Plant Methods 2018, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Brewer, G.E.; Clarkson, J.J.; Maurin, O.; Zuntini, A.R.; Barber, V.; Bellot, S.; Biggs, N.; Cowan, R.S.; Davies, N.M.J.; Dodsworth, S.; et al. Factors Affecting Targeted Sequencing of 353 Nuclear Genes From Herbarium Specimens Spanning the Diversity of Angiosperms. Front. Plant Sci. 2019, 10, 1102. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, E.A.; Wen, J. Using nuclear gene data for plant phylogenetics: Progress and prospects II. Next-gen approaches. J. Syst. Evol. 2015, 53, 371–379. [Google Scholar] [CrossRef]
- McKain, M.R.; Johnson, M.G.; Uribe-Convers, S.; Eaton, D.; Yang, Y. Practical considerations for plant phylogenomics. Appl. Plant Sci. 2018, 6, e1038. [Google Scholar] [CrossRef]
- Dodsworth, S.; Pokorny, L.; Johnson, M.G.; Kim, J.T.; Maurin, O.; Wickett, N.J.; Forest, F.; Baker, W.J. Hyb-Seq for Flowering Plant Systematics. Trends Plant Sci. 2019, 24, 887–891. [Google Scholar] [CrossRef]
- Straub, S.C.K.; Parks, M.; Weitemier, K.; Fishbein, M.; Cronn, R.C.; Liston, A. Navigating the tip of the genomic iceberg: Next-generation sequencing for plant systematics. Am. J. Bot. 2012, 99, 349–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.; Tang, Y.; Xu, X. The complete chloroplast genome of Sedum emarginatum (Crassulaceae). Mitochondrial DNA Part B 2020, 5, 3082–3083. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Liu, Y.; Xu, C.; Gao, Y.; Yuan, Q.; Suo, Z.; Zhang, Z.; Sun, J. Chloroplast phylogenomic insights into the evolution of Distylium (Hamamelidaceae). BMC Genom. 2021, 22, 293. [Google Scholar] [CrossRef]
- Li, L.; Hu, Y.; He, M.; Zhang, B.; Wu, W.; Cai, P.; Huo, D.; Hong, Y. Comparative chloroplast genomes: Insights into the evolution of the chloroplast genome of Camellia sinensis and the phylogeny of Camellia. BMC Genom. 2021, 22, 138. [Google Scholar] [CrossRef]
- Azarin, K.; Usatov, A.; Makarenko, M.; Khachumov, V.; Gavrilova, V. Comparative analysis of chloroplast genomes of seven perennial Helianthus species. Gene 2021, 774, 145418. [Google Scholar] [CrossRef]
- Liu, K.; Wang, R.; Guo, X.-X.; Zhang, X.-J.; Qu, X.-J.; Fan, S.-J. Comparative and Phylogenetic Analysis of Complete Chloroplast Genomes in Eragrostideae (Chloridoideae, Poaceae). Plants 2021, 10, 109. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Wang, J.; Shen, H.; Ai, B.; Gao, W.; Zhang, C.; Fei, Q.; Yuan, D.; Wu, Z.; et al. Chloroplast genomes in Populus (Salicaceae): Comparisons from an intensively sampled genus reveal dynamic patterns of evolution. Sci. Rep. 2021, 11, 9471. [Google Scholar] [CrossRef]
- Chen, F.; Dong, W.; Zhang, J.; Guo, X.; Chen, J.; Wang, Z.; Lin, Z.; Tang, H.; Zhang, L. The Sequenced Angiosperm Genomes and Genome Databases. Front. Plant Sci. 2018, 9, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Song, Y.; Li, X.; Chen, J.; Mo, L.; Zhang, X.; Lin, Z.; Zhang, L. Genome sequences of horticultural plants: Past, present, and future. Hortic. Res. 2019, 6, 112. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization–diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.D.; Kang, Y.; Kim, C. Application of Genomic Big Data in Plant Breeding: Past, Present, and Future. Plants 2020, 9, 1454. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.F. Angiosperm Phylogeny Website. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 29 October 2021).
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, E.; Park, S.; Park, J.; Kim, S. The chloroplast genome sequence of Magnolia kobus DC. (Magnoliaceae). Mitochondrial DNA Part B 2018, 3, 342–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Wu, T.; Fu, Y.; Hao, J.; Ma, H.; Zhu, Y.; Sima, Y. Complete chloroplast genome sequence of Michelia champaca var. champaca Linnaeus, an ornamental tree species of Magnoliaceae. Mitochondrial DNA Part B 2020, 5, 2839–2841. [Google Scholar] [CrossRef] [PubMed]
- Sima, Y.; Wu, T.; Fu, Y.; Hao, J.; Chen, S. Complete chloroplast genome sequence of Lirianthe coco (Loureiro) N. H. Xia & C. Y. Wu (Magnoliaceae), a popular ornamental species. Mitochondrial DNA Part B 2020, 5, 2410–2412. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Nie, Z.; Chen, H.; Chen, F.; Figlar, R.B.; Wen, J. Major clades and a revised classification of Magnolia and Magnoliaceae based on whole plastid genome sequences via genome skimming. J. Syst. Evol. 2020, 58, 673–695. [Google Scholar] [CrossRef]
- Xu, X.; Wu, S.; Xia, J.; Yan, B. The complete chloroplast genome of Magnolia delavayi, a threatened species endemic to Southwest China. Mitochondrial DNA Part B 2020, 5, 2734–2735. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, S.; Yanpeng, Y.; Zhou, L.; Bo, R.; Li, W.; Shi, X.; Feixia, H.; Cheng, P.; Xianmei, Y.; et al. Chloroplast genome and historical biogeography of the three Magnolias. Res. Square 2021, 13, 21. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Azuma, H.; García-Franco, J.G.; Rico-Gray, V.; Thien, L.B. Molecular phylogeny of the Magnoliaceae: The biogeography of tropical and temperate disjunctions. Am. J. Bot. 2001, 88, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Dandy, J.E. The Genera of Magnolieae. Bull. Misc. Inform. Kew 1927, 1927, 257. [Google Scholar] [CrossRef]
- Figlar, R.B.; Nooteboom, H.P. Notes on Magnoliaceae IV. Blum.-J. Plant. Tax. Plant. Geog. 2004, 49, 87–100. [Google Scholar] [CrossRef]
- Figlar, R.B. A New Classification for Magnolia. In Rhododendrons with Camellias and Magnolias; The Royal Horticulture Society: London, UK, 2006; pp. 69–82. [Google Scholar]
- Figlar, R.B. A Brief Taxonomic History of Magnolia. Available online: https://www.magnoliasociety.org/ClassificationArticle (accessed on 4 September 2021).
- Magnoliaceae in Flora of China @ Efloras.Org. Available online: http://efloras.org/florataxon.aspx?flora_id=2&taxon_id=10530 (accessed on 29 October 2021).
- Sima, Y.-K.; Lu, S.-G. A New System for the Family Magnoliaceae. In Proceedings of the Second International Symposium on the Family Magnoliaceae, Guangzhou, China, 5–9 May 2009; Huazhong University Science & Technology Press: Wuhan, China, 2012; pp. 55–71. [Google Scholar]
- Nie, Z.-L.; Wen, J.; Azuma, H.; Qiu, Y.-L.; Sun, H.; Meng, Y.; Sun, W.-B.; Zimmer, E.A. Phylogenetic and biogeographic complexity of Magnoliaceae in the Northern Hemisphere inferred from three nuclear data sets. Mol. Phylogenet. Evol. 2008, 48, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Veltjen, E.; Testé, E.; Palmarola-Bejarano, A.; Asselman, P.; Hernández-Rodríguez, M.; González-Torres, L.R.; Chatrou, L.W.; Goetghebeur, P.; Larridon, I.; Samain, M.-S. The evolutionary history of the caribbean magnolias (Magnoliaceae): Testing species delimitations and biogeographical hypotheses using molecular data. Mol. Phylogenet. Evol. 2022, in press. [Google Scholar] [CrossRef]
- Linnaeus, C. Species Plantarum; Salvius: Stockholm, Sweden, 1753. [Google Scholar]
- Kim, S.; Park, C.-W.; Kim, Y.-D.; Suh, Y. Phylogenetic relationships in family Magnoliaceae inferred from ndhF sequences. Am. J. Bot. 2001, 88, 717–728. [Google Scholar] [CrossRef]
- Arroyo, F.; Serna-González, M. Two new species of Magnolia (Magnoliaceae) from Peru. Phytotaxa 2020, 471, 54–60. [Google Scholar] [CrossRef]
- De Azevedo, C.O.; Marinho, L.C.; Machado, A.F.P.; Arroyo, F.; Vázquez-García, J.A. Magnolia brasiliensis (Magnoliaceae), a new species and new record for the Northeastern region of Brazil. Brittonia 2018, 70, 306–311. [Google Scholar] [CrossRef]
- Pérez, Á.J.; Arroyo, F.; Neill, D.A.; Vázquez-García, J.A. Magnolia chiguila and M. mashpi (Magnoliaceae): Two new species and a new subsection (Chocotalauma, sect. Talauma) from the Chocó biogeographic region of Colombia and Ecuador. Phytotaxa 2016, 286, 267. [Google Scholar] [CrossRef]
- Serrano, M.J.; Grajeda-Estrada, R.; Villalobos, A.; Álvarez-Ruano, M.R.; Vázquez-García, J.A. Magnolia poqomchi, a new species of subsection Magnolia (Magnoliaceae) from San Cristóbal Verapaz, Alta Verapaz, Guatemala. Phytotaxa 2020, 454, 231–243. [Google Scholar] [CrossRef]
- Vázquez-García, J.A.; Pérez-Farrera, M.Á.; Martínez-Camilo, R.; Muñiz-Castro, M.Á.; Martínez-Meléndez, N. Magnolia lacandonica (subsection Talauma, Magnoliaceae), a new rainforest species from Chiapas, Mexico. Phytotaxa 2013, 79, 30–36. [Google Scholar] [CrossRef]
- Vázquez-García, J.A.; Gómez-Domínguez, H.; López-Cruz, A. Magnolia perezfarrerae, una especie nueva y una clave para las especies mexicanas de Magnolia (sección Talauma, subsección Talauma, Magnoliaceae). Bot. Sci. 2013, 91, 417–425. [Google Scholar] [CrossRef]
- Muñiz-Castro, M.Á.; Castro-Félix, P.; Carranza-Aranda, A.S.; Vázquez-García, J.A.; Santerre, A. Population genetics, species boundaries, and conservation in the Magnolia pacifica species complex along a continentality and moisture gradient in western Mexico. Bot. Sci. 2020, 25, 51. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 29 October 2021).
- Qiu, Y.-L.; Chase, M.W.; Les, D.H.; Parks, C.R. Molecular Phylogenetics of the Magnoliidae: Cladistic Analyses of Nucleotide Sequences of the Plastid Gene rbcL. Ann. Mo. Bot. Gard. 1993, 80, 587–606. [Google Scholar] [CrossRef]
- Qiu, Y.-L.; Parks, C.R.; Chase, M.W. Molecular divergence in the eastern Asia-eastern North America disjunct section Rytidospermum of Magnolia (Magnoliaceae). Am. J. Bot. 1995, 82, 1589–1598. [Google Scholar] [CrossRef]
- Qiu, Y.-L.; Chase, M.W.; Parks, C.R. A chloroplast DNA Phylogenetic study of the eastern Asia-eastern North America disjunct section Rytidospermum of Magnolia (Magnoliaceae). Am. J. Bot. 1995, 82, 1582–1588. [Google Scholar] [CrossRef]
- Kim, S.; Suh, Y. Phylogeny of Magnoliaceae based on ten chloroplast DNA regions. J. Plant Biol. 2013, 56, 290–305. [Google Scholar] [CrossRef]
- Gao, F.; Chen, C.; Arab, D.A.; Du, Z.; He, Y.; Ho, S.Y.W. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 2019, 9, 3891–3898. [Google Scholar] [CrossRef] [Green Version]
- Freudenthal, J.A.; Pfaff, S.; Terhoeven, N.; Korte, A.; Ankenbrand, M.J.; Förster, F. A systematic comparison of chloroplast genome assembly tools. Genome Biol. 2020, 21, 254. [Google Scholar] [CrossRef]
- Wyman, S.K.; Jansen, R.K.; Boore, J.L. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 2004, 20, 3252–3255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Shi, L.; Zhu, Y.; Chen, H.; Zhang, J.; Lin, X.; Guan, X. CpGAVAS, an integrated web server for the annotation, visualization, analysis, and GenBank submission of completely sequenced chloroplast genome sequences. BMC Genom. 2012, 13, 715. [Google Scholar] [CrossRef] [Green Version]
- Guyeux, C.; Charr, J.-C.; Tran, H.T.M.; Furtado, A.; Henry, R.J.; Crouzillat, D.; Guyot, R.; Hamon, P. Evaluation of chloroplast genome annotation tools and application to analysis of the evolution of coffee species. PLoS ONE 2019, 14, e0216347. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, K.; Lucas, S.J. Comparison of different annotation tools for characterization of the complete chloroplast genome of Corylus avellana cv Tombul. BMC Genom. 2019, 20, 874. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shao, J.; Zhang, H.; Jiang, M.; Huang, L.; Zhang, Z.; Yang, D.; He, M.; Ronaghi, M.; Luo, X.; et al. Sequencing and Analysis of Strobilanthes cusia (Nees) Kuntze Chloroplast Genome Revealed the Rare Simultaneous Contraction and Expansion of the Inverted Repeat Region in Angiosperm. Front. Plant Sci. 2018, 9, 324. [Google Scholar] [CrossRef] [Green Version]
- Abdullah; Henriquez, C.L.; Mehmood, F.; Carlsen, M.M.; Islam, M.; Waheed, M.T.; Poczai, P.; Croat, T.B.; Ahmed, I. Complete Chloroplast Genomes of Anthurium huixtlense and Pothos scandens (Pothoideae, Araceae): Unique Inverted Repeat Expansion and Contraction Affect Rate of Evolution. J. Mol. Evol. 2020, 88, 562–574. [Google Scholar] [CrossRef]
- Frailey, D.C.; Chaluvadi, S.R.; Vaughn, J.N.; Coatney, C.G.; Bennetzen, J.L. Gene loss and genome rearrangement in the plastids of five Hemiparasites in the family Orobanchaceae. BMC Plant Biol. 2018, 18, 30. [Google Scholar] [CrossRef] [Green Version]
- Cauz-Santos, L.A.; da Costa, Z.P.; Callot, C.; Cauet, S.; Zucchi, M.I.; Bergès, H.; van den Berg, C.; Vieira, M.L.C. A Repertory of Rearrangements and the Loss of an Inverted Repeat Region in Passiflora Chloroplast Genomes. Genome Biol. Evol. 2020, 12, 1841–1857. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Yang, J.; Lv, G. Complete chloroplast genome of seven Fritillaria species, variable DNA markers identification and phylogenetic relationships within the genus. PLoS ONE 2018, 13, e0194613. [Google Scholar] [CrossRef] [Green Version]
- Xiao-Ming, Z.; Junrui, W.; Li, F.; Sha, L.; Hongbo, P.; Lan, Q.; Jing, L.; Yan, S.; Weihua, Q.; Lifang, Z.; et al. Inferring the evolutionary mechanism of the chloroplast genome size by comparing whole-chloroplast genome sequences in seed plants. Sci. Rep. 2017, 7, 1555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-L.; Song, Y.; Ni, J.; Yao, X.; Tan, Y.-H.; Xu, Z.-F. Comparative chloroplast genomics and phylogenetics of nine Lindera species (Lauraceae). Sci. Rep. 2018, 8, 8844. [Google Scholar] [CrossRef] [Green Version]
- Han, E.-K.; Cho, W.-B.; Choi, G.; Lee, J.-H. The complete chloroplast genome of Sarcandra glabra (Chloranthaceae): A perianthless basal angiosperm. Mitochondrial DNA Part B 2018, 3, 661–662. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Gichira, A.W.; Li, Z.; Nzei, J.M.; Guo, Y.; Wang, Q.; Chen, J. Intergeneric Relationships within the Early-Diverging Angiosperm Family Nymphaeaceae Based on Chloroplast Phylogenomics. Int. J. Mol. Sci. 2018, 19, 3780. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, K.H.; Morden, C.W.; Palmer, J.D. Function and evolution of a minimal plastid genome from a nonphotosynthetic parasitic plant. Proc. Natl. Acad. Sci. USA 1992, 89, 10648–10652. [Google Scholar] [CrossRef] [Green Version]
- Greiner, S.; Wang, X.; Rauwolf, U.; Silber, M.V.; Mayer, K.; Meurer, J.; Haberer, G.; Herrmann, R.G. The complete nucleotide sequences of the five genetically distinct plastid genomes of Oenothera, subsection Oenothera: I. Sequence evaluation and plastome evolution. Nucleic Acids Res. 2008, 36, 2366–2378. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-P.; Huang, J.-P.; Wu, C.-S.; Hsu, C.-Y.; Chaw, S.-M. Comparative Chloroplast Genomics Reveals the Evolution of Pinaceae Genera and Subfamilies. Genome Biol. Evol. 2010, 2, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Chris Blazier, J.; Guisinger, M.M.; Jansen, R.K. Recent loss of plastid-encoded ndh genes within Erodium (Geraniaceae). Plant Mol. Biol. 2011, 76, 263–272. [Google Scholar] [CrossRef]
- Zhu, A.; Guo, W.; Gupta, S.; Fan, W.; Mower, J.P. Evolutionary dynamics of the plastid inverted repeat: The effects of expansion, contraction, and loss on substitution rates. New Phytol. 2016, 209, 1747–1756. [Google Scholar] [CrossRef] [Green Version]
- Guisinger, M.M.; Kuehl, J.V.; Boore, J.L.; Jansen, R.K. Extreme Reconfiguration of Plastid Genomes in the Angiosperm Family Geraniaceae: Rearrangements, Repeats, and Codon Usage. Mol. Biol. Evol. 2011, 28, 583–600. [Google Scholar] [CrossRef] [Green Version]
- Abdullah; Mehmood, F.; Rahim, A.; Heidari, P.; Ahmed, I.; Poczai, P. Comparative plastome analysis of Blumea, with implications for genome evolution and phylogeny of Asteroideae. Ecol. Evol. 2021, 3, 7614. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. The chloroplast genome sequence of bittersweet (Solanum dulcamara): Plastid genome structure evolution in Solanaceae. PLoS ONE 2018, 13, e0196069. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; He, J.; Ding, C.; Lyu, R.; Pei, L.; Cheng, J.; Xie, L. Comparative Analysis of Complete Chloroplast Genomes of Anemoclema, Anemone, Pulsatilla, and Hepatica Revealing Structural Variations Among Genera in Tribe Anemoneae (Ranunculaceae). Front. Plant Sci. 2018, 1, 97. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zuo, Y.; Zhu, X.; Liao, S.; Ma, J. Complete Chloroplast Genomes and Comparative Analysis of Sequences Evolution among Seven Aristolochia (Aristolochiaceae) Medicinal Species. Int. J. Mol. Sci. 2019, 20, 1045. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Sun, R.; Liu, H.; Chang, L.; Li, S.; Zhao, M.; Shennan, C.; Lei, J.; Dong, J.; Zhong, C.; et al. Complete chloroplast genome sequencing of ten wild Fragaria species in China provides evidence for phylogenetic evolution of Fragaria. Genomics 2021, 113, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Landis, J.B.; Wang, H.-X.; Zhu, Z.-X.; Wang, H.-F. Comparative analysis of chloroplast genome structure and molecular dating in Myrtales. BMC Plant Biol. 2021, 21, 219. [Google Scholar] [CrossRef]
- Tian, X.; Guo, J.; Zhou, X.; Ma, K.; Ma, Y.; Shi, T.; Shi, Y. Comparative and Evolutionary Analyses on the Complete Plastomes of Five Kalanchoe Horticultural Plants. Front. Plant Sci. 2021, 12, 651. [Google Scholar] [CrossRef]
- Raman, G.; Park, K.T.; Kim, J.-H.; Park, S. Characteristics of the completed chloroplast genome sequence of Xanthium spinosum: Comparative analyses, identification of mutational hotspots and phylogenetic implications. BMC Genom. 2020, 21, 855. [Google Scholar] [CrossRef]
- Azuma, H.; Thien, L.B.; Kawano, S. Molecular Phylogeny of Magnolia (Magnoliaceae) Inferred from cpDNA Sequences and Evolutionary Divergence of the Floral Scents. J. Plant. Res. 1999, 112, 291–306. [Google Scholar] [CrossRef]
- Missouri Botanical Garden Tropicos. Available online: https://www.tropicos.org/home (accessed on 10 November 2021).
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Larridon, I.; Walter, H.E.; Guerrero, P.C.; Duarte, M.; Cisternas, M.A.; Hernández, C.P.; Bauters, K.; Asselman, P.; Goetghebeur, P.; Samain, M.-S. An integrative approach to understanding the evolution and diversity of Copiapoa (Cactaceae), a threatened endemic Chilean genus from the Atacama Desert. Am. J. Bot. 2015, 102, 1506–1520. [Google Scholar] [CrossRef] [Green Version]
- Thiers, B. Index Herbariorum: A global directory of public herbaria and associated staff; New York Botanical Garden: New York, NY, USA, 2019. [Google Scholar]
- Reserva Natural “El Refugio”. Available online: http://elrefugionatura.jimdofree.com/about-1/ (accessed on 7 December 2021).
- Andrews, S. FastQC. A Quality Control Tool for High Throughput Sequence Data. 2019. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 20 November 2021).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.-J.; Yu, W.-B.; Yang, J.-B.; Song, Y.; dePamphilis, C.W.; Yi, T.-S.; Li, D.-Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wick, R.R.; Schultz, M.B.; Zobel, J.; Holt, K.E. Bandage: Interactive visualization of de novo genome assemblies. Bioinformatics 2015, 31, 3350–3352. [Google Scholar] [CrossRef] [Green Version]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq—Versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef]
- Laslett, D.; Canback, B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. Methods Mol. Biol. 2019, 1962, 1–14. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [Green Version]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef]
- Brudno, M.; Malde, S.; Poliakov, A.; Do, C.B.; Couronne, O.; Dubchak, I.; Batzoglou, S. Glocal alignment: Finding rearrangements during alignment. Bioinformatics 2003, 19, i54–i62. [Google Scholar] [CrossRef] [Green Version]
- Darling, A.C.E.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple Alignment of Conserved Genomic Sequence with Rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [Green Version]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. IRscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics 2018, 34, 3030–3031. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).