Abstract
The growing interest in polyphenols of natural origin and their plant sources encourages the study of their chemical composition and biological activity. Propolis is widely used as a source of phenolic compounds. The aim of this study is to evaluate and compare the chemical composition, antioxidant activity and sun protection factor (SPF) of the ethanolic extracts of the poplar buds, birch buds and pine buds of propolis plant precursors collected in Lithuania. The IC50 concentration of the extracts was evaluated using DPPH and ABTS methods. Extracts of poplar buds, birch buds and propolis showed a lower IC50 concentration by ABTS and DPPH methods compared with pine buds extracts. Poplar buds and propolis extracts showed the highest SPF value, while birch and pine buds extracts showed a lower SPF value. High-performance liquid chromatography (HPLC) analysis results showed that phenolic acids, such as p-coumaric acid and cinnamic acid, and flavonoids, such as pinobanksin and pinocembrin, were identified in all the tested extracts. Salicin has been identified only in poplar buds extracts. The results of antioxidant activity showed that propolis poplar and birch buds are a promising source of biologically active polyphenols.
1. Introduction
Various plant species and their extracts have been used for medicinal purposes since ancient times [1]. The most commonly studied groups of polyphenols are phenolic acids and flavonoids [2]. Scientists are increasingly focusing on naturally occurring polyphenols and their sources in order to investigate, evaluate and apply extracts of plant materials for therapeutic purposes [3]. Propolis is particularly valued for its various beneficial therapeutic properties, and antioxidant, anti-inflammatory, antibacterial and anti-tumor effects [4]. The therapeutic properties of propolis are due to the presence of various biologically active compounds, such as phenolic acids, flavonoids, sesquiterpenes, lignans, amino acids, vitamins, fatty acids and minerals [5,6]. The flavonoids in propolis are potent antioxidants that can bind free radicals and protect cells from lipid peroxidation [7]. Kim et al. found that propolis inhibited the UV-induced production of (MMP)-1 matrix metalloproteinase in human skin fibroblasts. Propolis reduced UV-induced MMP-1 expression and prevented collagen degradation in human skin tissues [8]. In vitro studies showed that the ethanolic extract of propolis had a protective effect against H2O2-induced cell death and inhibited the H2O2-induced decrease in collagen mRNA expression in L929 cells [9]. A study by Gastaldello et al. found that baccharin and coumaric acid isolated from green propolis have anticarcinogenic potential, which may be applicable for the development of new anticancer agents [10].
The process of propolis extraction is a complex one, the chemical composition of which depends on the vegetation of the region where the bees collect propolis. It is important to study in more detail the chemical composition of potential propolis precursors by geographical area and to compare it with the chemical composition of propolis. The chemical composition of propolis is closely related to plant sources, which vary from one geographical area to another [11]. In order to achieve a research-based quality assessment of propolis, it is relevant to investigate the chemical composition and bioactivity of propolis by looking for correlations with the plant precursors of propolis. For example, Pobiega et al. determined the composition of the Polish ethanolic extract of propolis was rich in phenolic acids, such as p-coumaric acid, ferulic acid and caffeic acid, and falvonoids, such as pinocembrin, galangin, pinobanksin and pinostrobin [12]. The plant precursors of North American and European propolis are thought to be resins from buds of poplar, aspen, birch, pine, alder, chestnut and oak [13,14,15,16]. In view of the widespread distribution of conifers and birch forests in Lithuania, it is of interest to investigate the chemical composition and biological activity of propolis extracts in comparison with those of birch, pine and poplar buds extracts.
It is known that phenolic compounds can exhibit various properties, such as antioxidant activity and anti-inflammatory activity [17], anti-ageing, antiproliferative, antibacterial and antiviral properties [18,19]. Oxidative stress is responsible for many degenerative diseases, including cancer and cardiovascular diseases [20,21]. Oxidative stress is associated with a high production of reactive oxygen species (ROS) in the cell, exceeding the cell’s ability to remove them efficiently, which can cause damage to DNA, proteins or lipids within the cell [22,23,24]. Research has shown that polyphenols can protect against UV radiation exposure in a variety of ways. Excessive exposure to UV radiation can cause adverse reactions and damage the cell’s DNA [25,26]. Natural polyphenols are often yellow, red or purple pigments that are able to absorb UV radiation [27]. Various clinical and in vitro studies have shown that exposure to UV radiation may be directly responsible for various skin diseases, skin ageing, dry skin, vasodilation, melanoma and skin tumors [27]. Phenolic compounds are characterized by their absorption spectrum, where UV radiation is filtered out, thereby reducing the penetration of harmful UV rays into the skin, oxidative stress and the damaging effects on DNA [26,28]. Protection against the adverse effects of UV radiation from the sun can be achieved by the use of natural polyphenol products, which not only have antioxidant and anti-inflammatory properties but also photoprotective properties [29,30].
Propolis and its plant precursors are potential candidates as active ingredients in skin care and pharmaceutical formulations for the protective effects of solar UV radiation. The photoprotective properties of Lithuanian propolis and propolis plant precursors have not been investigated so far. The results of this study are relevant for the application of propolis in UV-protective products. The aim of our planned study is to compare the chemical composition of ethanolic extracts of propolis collected in Lithuania with its plant precursors in balsam poplar buds (Populus balsamifera L.), birch buds (Betula pendula L.) and scots pine buds (Pinus sylvestris L.) to evaluate their antioxidant and photoprotective properties.
2. Results
2.1. Evaluation of the Total Content of Phenolic Compounds
The ethanolic extract of pine buds had the lowest content of phenolic compounds: 53.93 ± 3.24 mg CAE/g. The highest phenolic compounds content was observed in the balsam poplar buds ethanolic extract: 230.42 ± 14.83 mg CAE/g. A statistically significant difference (p < 0.05) was found between the pine buds extract and all the other extracts tested. There was no statistically significant difference (p > 0.05) in total phenolic compounds between the balsam poplar buds and propolis extracts. The results are presented in Table 1 and the appearance of the extracts presented in Figure 1.

Table 1.
Poplar buds, birch buds, pine buds and propolis total amount of phenolic compounds expressed as mg CAE/g dry weight (DW). Results are presented as mean and standard deviation of three measurements. Different subscript letters indicate a statistically significant difference between samples (p < 0.05).
Figure 1.
Physical appearance of poplar (1), birch (2), pine (3) buds and propolis (4) extracts.
2.2. Analysis of Active Compounds by HPLC Analysis
The chemical composition of the extracts produced was evaluated by HPLC (Table 2, Figure 2). The balsam poplar buds extracts were dominated by p-coumaric acid 12.696 ± 0.366 mg/g, cinnamic acid 8.866 ± 0.167 mg/g and galangin 6.396 ± 0.110 mg/g. These predominant compounds account for about 40%, 28% and 20% of the total identified compounds, respectively. Salicin was found in the poplar buds extract—0.556 ± 0.046 mg/g (Figure 2). In the birch buds extract, the predominant compound identified was pinocembrin—4.940 ± 0.125 mg/g, which accounts for about 70% of the total compounds identified. Additionally, identified in the birch buds extract was p-coumaric acid, which was found at 1.167 ± 0.078 mg/g, and apigenin, which was found at 1.030 ± 0.065 mg/g. In propolis, the predominant compounds identified were p-coumaric acid 15.776 ± 0.410 mg/g, ferulic acid 7.479 ± 0.227 mg/g and vanillin 4.980 ± 0.163 mg/g, which accounted for about 52%, 25% and 17% of the total identified compounds, respectively. Pinocembrin, ferulic acid and p-coumaric acid were detected in pine buds extract but in significantly lower amounts of compounds compared with poplar buds, birch buds and propolis extracts.

Table 2.
HPLC analysis of ethanolic poplar, birch, pine buds and propolis extracts (mg/g). Results expressed as mean and standard deviation (SD) of three measurements.
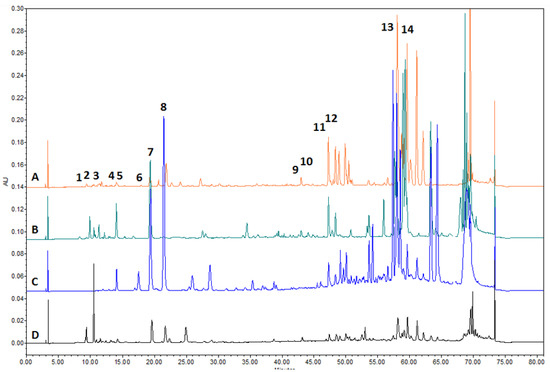
Figure 2.
Chromatogram of A—birch buds, B—poplar buds, C—propolis and D—pine buds extracts. 1—salicin, 2—neochlorogenic acid, 3—chlorogenic acid, 4—vanillic acid, 5—caffeic acid, 6—vanillin, 7—P-coumaric acid, 8—ferulic acid, 9—cinnamic acid, 10—quercetin, 11—pinobanksin, 12—apigenin, 13—kaempferol, 14—pinocembrin.
2.3. Antioxidant Activity of DPPH and ABTS In Vitro
To assess the antioxidant activity of the extracts, solutions of the extracts were made at different concentrations ranging from 100 µg CAE/mL to 1 µg CAE/mL (Figure 3). The IC50 of the extracts and standards (p-coumaric acid, quercetin) evaluated by ABTS showed a statistically significantly higher IC50 concentrations of the tested extracts compared with p-coumaric acid and quercetin (p < 0.05). The IC50ABTS of all tested extracts ranged from 49.92 ± 6.44 µg CAE/mL to 171.29 ± 10.01 µg CAE/mL. The lowest inhibitory concentrations were observed for the extracts of propolis, poplar buds and birch buds. There was no statistically significant difference in IC50ABTS concentrations between these extracts (p > 0.05). When antioxidant activity was evaluated by DPPH, the pine buds extract had the highest inhibitory concentration of 99.53 ± 9.07 µg CAE/mL. The lowest inhibitory concentration of the extracts tested was the poplar buds extract with 50.64 ± 6.49 µg CAE/mL. The IC50DPPH concentration of propolis was found to be 51.92 ± 4.95 µg CAE/mL. There was no statistically significant difference (p > 0.05) in IC50DPPH concentrations between the poplar buds and propolis extracts. A statistically significantly higher concentration of IC50DPPH was found in pine buds extracts compared with poplar buds, birch buds and propolis extracts (p < 0.05).

Figure 3.
Antioxidant activity of poplar, birch, pine buds and propolis extracts. Subgraph (A)—ABTS inhibition % of extracts (1 µg CAE/mL to 100 µg CAE/mL); subgraph (B)—DPPH inhibition % of extracts (1 µg CAE/mL to 100 µg CAE/mL); subgraph (C)—IC50ABTS concentration; subgraph (D)—IC50DPPH concentration. Subscripts of different letters indicate statistically significant differences between subjects (p < 0.05).
2.4. SPF Factor of Extracts
The sun protection factor (SPF) of the ethanolic extracts studied was assessed spectrophotometrically (Figure 4). The results showed that the SPF of 10 µg/mL extracts ranged from 2.010 to 4.851. The highest SPF was found in the propolis extract at 4.851. The lowest SPF was found in the pine buds extract at 2.010. The SPF values of the standard quercetin, p-coumaric acid and salicin were evaluated, and the highest SPF was found for p-coumaric acid at 8.921. The p-coumaric acid-dominated extracts of poplar buds and propolis showed almost twice the SPF compared with birch and pine buds extracts. P-coumaric acid had a higher SPF compared with quercetin.
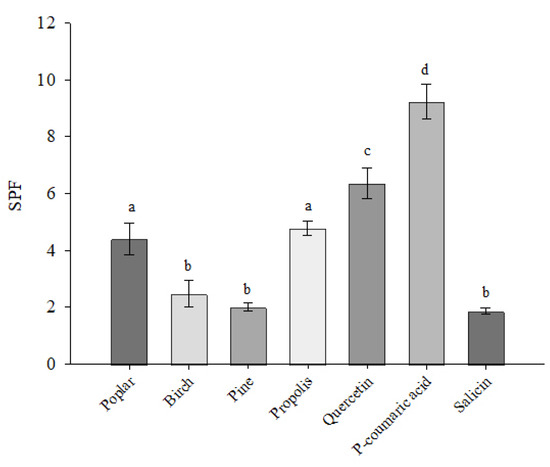
Figure 4.
SPF values of poplar, birch, pine buds and propolis extracts by spectrophotometric method. Results are presented at extract concentrations of 10 µg CAE/mL, standards of quercetin, p-coumaric acid and salicin at 10 µg/mL. Subscripts of different letters symbolize a statistically significant difference between samples (p < 0.05).
2.5. Correlation
A significant relation is observed between total phenolic compounds and antioxidant activity (Table 3). A strong negative correlation was observed between total phenolic compounds content and antioxidant activity (IC50ABTS r = −0.870; IC50DPPH r = −0.828). A strong correlation is observed between the total amount of phenolic compounds and SPF values (r = 0.845). Additionally, a strong correlation was found between the total amount of identified phenolic acids and SPF values (r = 0.962).

Table 3.
Correlation table comparing SPF factor (SPF), antioxidant activity (IC50ABTS and IC50DPPH), total phenolic compounds (TPC), total identified phenolic acids (sum_TPA), total identified flavonoids (sum_F) and total identified active compounds (Total_IAC).
3. Discussion
Propolis and its extracts are used for medicinal purposes. The results of our study confirmed that the chemical composition of propolis extracts depends on the plants from which the bees collect resins for propolis [4,31,32,33]. The p-coumaric acid was the predominant acid in the extracts of Lithuanian propolis [34,35]. Socha et al. studied propolis ethanolic extract from different regions of Poland and found p-coumaric acid to be the predominant phenolic acid, ranging from 37.54 to 116.95 mg/g, and high levels of ferulic acid [36]. The methanolic extract samples of Turkish propolis also contained p-coumaric acid and ferulic acid [37]. The results of this study show that p-coumaric acid is predominant in poplar buds extracts, with lower levels found in birch and pine buds extracts. Ferulic acid was also found in the propolis extracts studied and accounted for approximately 25% of the total compounds identified. Ferulic acid, which is one of the main acids in propolis, was not identified in the poplar and birch extracts, while small amounts were detected in the pine buds extracts. P-coumaric, chlorogenic and caffeic acids were the most abundant compounds identified in brown and green Brazilian propolis [38]. In our experimental studies, caffeic acid was not identified only in birch buds extracts. Chlorogenic acid was identified only in poplar buds extracts. Cinnamic acid was found in abundance in the poplar buds extracts, accounting for about 29% of the total compounds identified. Small amounts of cinnamic acid were found in extracts of propolis, birch and pine buds, whose amounts are 0.191 ± 0.009 mg/g, 0.097 ± 0.009 mg/g and 0.015 ± 0.001 mg/g, respectively.
The flavonoids identified and quantified in the extracts studied are important for their antioxidant and anti-inflammatory properties. A different range of flavonoids was found in the extracts studied. Quercetin and kaempferol were identified in birch buds extracts at 0.53 ± 0.06 mg/g and 1.53 ± 0.08 mg/g, respectively, and traces of these compounds were detected in pine buds extracts. Samples of methanolic extracts of Turkish propolis contained high levels of quercetin, a potent antioxidant [37]. Quercetin was not identified in the propolis and poplar buds extracts we analyzed, and small amounts were detected in birch and pine buds extracts. Galangin was identified only in the poplar buds extracts and accounted for about 21% of the total compounds identified in the extract. Szliszka et al. found that the flavonoids pinobansin, chrysin and methoxyflavonin were the predominant flavonoids in Polish propolis extract [39]. In Lithuanian propolis extracts, vanillin was the predominant phenolic aldehyde, and smaller amounts of flavonoids pinocembrin, pinobanksin and apigenin were also identified. Vanillin was identified only in propolis extracts. Pinocembrin and pinobanksin were identified in all extracts tested. The highest levels of pinocembrin were found in birch buds extracts. Lower amounts of pinocembrin were found in pine, poplar buds and propolis extracts (0.153 ± 0.005 mg/g, 1.263 ± 0.049 mg/g and 0.509 ± 0.030 mg/g, respectively). Salicin was found in poplar buds extracts and has important pharmacological effects in the treatment of fever, pain and inflammation [40]. Although poplar is one of the plant precursors of propolis, this compound has not been identified in propolis extracts. For most cases analyzed in the scientific literature, propolis is of mixed origin. Often, remnants from several plant precursors can be detected in its composition [16]. The results of the studies have confirmed that the chemical composition of propolis is closely linked to the dominant vegetation in the area of propolis collection [35,41].
The identified active compounds and their levels in the extracts of propolis and its plant precursors have important implications for their biological activity. P-coumaric acid, which is strongly predominant in propolis and poplar buds extracts, is known as a hydroxyl compound of cinnamic acid, which is able to reduce the peroxidation of low-density lipoproteins, has antimicrobial activity, contributes to the inhibition of cellular melanogenesis, and is known to have a positive effect on the regulation of the human immune system [42]. Reactive oxygen species (ROS) are produced by normal physiological stresses and cause a variety of cellular damages, which may lead to the accumulation of lipid peroxides in biological membranes [43]. ROS can damage biomolecules that are essential for the body, such as nucleic acids and carbohydrates, as well as affecting and damaging DNA, leading to mutations [44]. If ROS are not effectively removed from cellular components, they can lead to free radical chain reactions that damage biomolecules, resulting in various diseases and ailments. It has long been described in the scientific literature that ROS-induced oxidative stress is one of the main factors in the development of cataracts and the formation of hydrogen peroxide, which is a major intracellular ROS that can activate a wide range of signaling events, promote apoptosis of lens epithelial cells (HLE), and induce lens opacification, which can subsequently lead to the development of cataracts [45]. The adverse effects of UVB-activated ROS on DNA, proteins and lipids are also frequently addressed in the scientific literature [46,47]. In studies by An et al., a strong antimelanogenic effect of p-coumaric acid was observed in human epidermal melanocytes exposed to UVB. The study showed that p-coumaric acid is a potent and selective inhibitor of human TYR and may be useful as a hypopigmenting agent [48]. In an experimental study, it was found that all extracts exhibited antioxidant activity as assessed by DPPH and ABTS methods in vitro. Pine buds were found to have the highest IC50 concentration by both methods. Propolis and poplar buds extracts showed the lowest IC50 concentrations of 49.92 ± 5.71 µg CAE/mL, 50.89 ± 1.46 µg CAE/mL (ABTS) and 51.92 ± 4.95 µg CAE/mL, 50.64 ± 6.49 µg CAE/mL (DPPH), respectively. In the antioxidant activity assays performed by Wezgowiec et al., the IC50DPPH of the ethanolic propolis extract was found to range from 33.01 ± 2.73 µg/mL to 78.02 ± 4.86 µg/mL by different regions of the collected samples [49]. The results obtained correlate directly with the total phenolic compounds found in the extracts. In a study by Ahn et al., p-coumaric acid showed lower antiradical activity against DPPH and reducing power than the flavonoids quercetin or kaempferol [50].
Researchers are focusing on the ability of plant extracts to protect against the UV radiation that causes skin damage. Propolis has been shown to have anticancer [51,52,53,54], anti-inflammatory [55,56,57,58], antioxidant [59,60,61,62] and mainly antibacterial properties [63,64,65,66,67]. Quercetin, a flavonoid with well-known antioxidant activity [68,69], has been used as a reference antioxidant compound for the evaluation of the activity of different extracts. The results of our studies show that p-coumaric acid has a stronger SPF value compared with quercetin. The assessment of the SPF of the extracts showed that the extracts of propolis and poplar buds, which are dominated by p-coumaric acid, have the best effect. Our results show a correlation between the phenolic acids content and the SPF values of the extracts. The results show that the SPF increased accordingly with increasing concentrations of the identified active compounds in the extracts. The FDA (Food and Drug Administration of the United States and the European Union) recommends the use of ingredients with SPF values above 15 in pharmaceutical products [70].
4. Materials and Methods
4.1. Materials
Standard solvents and reagents used were of analytical grade. We produced 96% rectified ethanol (JSC “Vilniaus degtine”, Vilnius, Lithuania) and purified deionized water using water purification system Milli-Q® (Millipore, Burlington, MA, USA). Acetonitrile (Sigma-Aldrich, Steinheim, Germany), Folin-Ciocalteu’s reagent (Sigma-Aldrich, St. Louis, MO, USA) and sodium carbonate (Sigma-Aldrich, Saint-Quentin-Fallavier, France). Reference standards: p-coumaric acid, cinnamic acid, caffeic acid, vanillin, apigenin and chlorogenic acid (Sigma-Aldrich, Steinheim, Germany); salicin, pinobanksin, pinocembrin, galangin, ferulic acid and vanillic acid (Sigma-Aldrich, St. Louis, MO, USA). ABTS (2,20-azino-bis (3- ethylbenzothiazoline-6-sulfonic acid) (Sigma-Aldrich Chemie, Steinheim, Germany), DPPH (2,2-diphenyl-1-picrylhydrazyl) (Sigma-Aldrich, St. Louis, MO, USA). Ultrasonic bath (Bandelin electronic GmbH & Co.KG, Berlin, Germany).
4.2. Extraction
Dried balsam poplar buds, birch buds and pine buds were purchased commercially from Jadvyga Balvociute’s organic herb farm. The raw material was collected in the spring of 2022. Propolis was commercially purchased from R. Serksniene’s farm, in Lithuania, Raseiniai district. We used 70% ethanol (v/v) as extractant. The ratio of raw material and extractant was 1:10. The extraction was carried out using maceration. Macerates are stored in a dark glass bottle for 7 days at room temperature (21 ± 1 °C), mixing the contents of the macerate several times. The obtained extracts filtered through ashless filter paper.
4.3. Evaluation of Total Phenolic Compounds
The total phenolic content of balsam poplar buds, birch buds, pine buds and propolis extracts was evaluated according to Singleton et al. methodology with certain modifications [71]. The phenolic compounds content was determined using the Folin–Ciocalteu reagent. The reaction was carried out in 25 mL volumetric flasks: 1 mL of the test extract, 9 mL of purified water and 1 mL of Folin–Ciocalteu reagent were added and mixed, and after 2–3 min, 1.5 mL of (7.5%) Na2CO3 was added. The reaction mixtures were diluted with purified water to the mark of 25 mL. Samples were incubated for 40 min at room temperature (21 ± 1 °C) in the dark. The absorbance was measured using a spectrophotometer (Ag-ilent Technologies 8453 UV-Vis, Santa Clara, California, USA) at a wavelength of 760 nm. The results expressed as mg of p-coumaric acid equivalent/g of dry weight (mg CAE/g, DW).
4.4. HPLC Analysis
Analysis of the phenolic compounds of the extracts under study performed using high-performance liquid chromatography (HPLC). Chromatographic system “Waters 2695” with diode matrix detector “Waters 996” and chromatographic column ACE 5C18, 250 × 4.6 mm data are processed by Empower 2 Chromatography Data software. HPLC eluents consisted of acetonitrile and trifluoroacetic acid. Column temperature 25 °C, flow time 81 min, injection volume 10 µL, mobile phase flow rate 1 mL/min. The compounds present in the sample were identified by the retention time of analytes and reference materials and UV absorption (250 to 400 nm). Reference compounds: salicin (R2 = 0.9999), p-coumaric acid (R2 = 0.9999), cinnamic acid (R2 = 0.9999), caffeic acid (R2 = 0.9999), chlorogenic acid (R2 = 0.9999), pinocembrin (R2 = 0.9998), pinobanksin (R2 = 0.9999), vanillin (R2 = 0.9999), vanillic acid (R2 = 0.9999), ferulic acid (R2 = 0.9999), neichlorogenic acid (R2 = 0.9999), kaempferol (R2 = 0.9999), quercetin (R2 = 0.9999), apigenin (R2 = 0.9998) and galangin (R2 = 0.9999).
4.5. Antioxidant Activity by DPPH and ABTS Methods In Vitro
The antioxidant activity of extracts ABTS and DPPH in vitro was determined based on the methodology described by Rezzoug et.al with certain modifications [72].
ABTS primary solution was prepared (0.0548 g of ABTS is dissolved in 50 mL of purified water, 0.0095 g of K2S2O8). The prepared ABTS solution was kept in the dark for about 16 h. A working solution of ABTS•+ was prepared by diluting the original solution with purified water until the absorbance of 10 mm solution reaches 0.8 ± 0.03 at 734 nm wavelength. We mixed 50 µl of extract samples (from 1 µg CAE/mL to 100 µg CAE/mL) with 1450 µl of working ABTS•+ solution. The samples were incubated in the dark for 10 min and the absorbance of the samples was measured. Purified water was used as a blank.
For evaluation of DPPH antioxidant activity, a 60 µM DPPH solution in 96% ethanol (v/v) was prepared. WE mixed 1 mL of extracts samples (from 1 µg CAE/mL to 100 µg CAE/mL) with 2 mL of DPPH solution. The samples were incubated in the dark for 30 min and their absorbance was measured at 517 nm wavelength. We used 96% ethanol (v/v) as a blank.
The antiradical activity (%) was estimated based on the formula [73]:
Scavenging effect (%) =((A1 − A2)/A1) × 100
A1 represents absorption of DPPH or ABTS plus blank respectively; A2 represents absorbance of DPPH or ABTS radical with the test sample. The antioxidant activity of the test samples was expressed as an IC50 value.
4.6. SPF Factor of Extracts
The sun protection factor of balsam poplar buds, birch buds, pine buds and propolis extracts was evaluated according to Oliveira-Júnior et al. methodology with certain modifications [74]. During the study, the extracts were diluted with 96% ethanol (v/v) to a concentration of 10 µg CAE/mL. The absorption spectrum of the test samples was obtained in the range of 290–450 nm. A 1 cm quartz element was used for the study. Absorbance data were obtained from 290 to 320 nm in 5 nm increments. We used 96% ethanol (v/v) as a blank.
Sun protection factor (SPF) spectrophotometric results were calculated according to Mansur et al. equation [75]:
EE (λ)—erythemal effect spectrum; I (λ)—solar intensity spectrum; Abs (λ)—absorbance of extract; CF—correction factor (= 10).
The constants for the product of the erythemal effect and the intensity of the solar spectrum were estimated by Sayre et al. and are presented in Table 4 [76].

Table 4.
Normalized values of erythema effect and solar spectrum intensity [76].
4.7. Statistical Analysis
Results expressed as mean and standard deviation of three measurements. One-way ANOVA was used to determine the differences between the compared data that were statistically significant. Tukey’s multiple comparison test was applied. The differences evaluated as statistically significant at p < 0.05. Pearson correlation coefficient was determined to evaluate the data correlation (0.3 < |r| < 0.5, weak correlation; 0.5 < |r| < 0.7, medium correlation; 0.7 < |r| < 0.9, strong correlation; 0.9 < |r| ≤ 1, very strong correlation). Data processed and graphically presented using IBM SPSS Statistics 27 (SPSS Inc., Chicago, IL, USA) and SigmaPlot 13.0 (Systat Software, San Jose, CA, USA).
5. Conclusions
Buds of poplar, birch and pine growing in Lithuania are characterized by a different composition of active compounds and their quantities. The results of the research allow us to state that the range of active compounds prevailing in Lithuanian propolis extracts is close to birch and poplar buds extracts. Balsam poplar buds extracts and propolis extracts have a higher amount of tested compounds compared with birch and pine buds extracts. The results of this study provide new data on the SPF value of the extracts. The results of antioxidant activity and SPF values correlated with the determined amount of phenolic compounds in the extracts.
Author Contributions
Conceptualization, K.R. and M.S.; methodology, K.R., M.S., M.M. and L.R.; investigation, K.R., M.S., M.M. and L.R.; data curation, K.R., M.S.; writing—original draft preparation, K.R. and M.S.; writing—review and editing, K.R. and M.S.; visualization, K.R. and M.S.; supervision, K.R.; project administration, K.R.; funding acquisition, K.R. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beserra, F.P.; Gushiken, L.F.S.; Hussni, M.F.; Ribeiro, V.P.; Bonamin, F.; Jackson, C.J.; Pellizzon, C.H.; Bastos, J.K. Artepillin C as an Outstanding Phenolic Compound of Brazilian Green Propolis for Disease Treatment: A Review on Pharmacological Aspects. Phytother. Res. 2021, 35, 2274–2286. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, Food and Pharma. Current Knowledge and Directions for Future Research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and Their Benefits: A Review. Int. J. Food Prop. 2017, 20 (Suppl. S2), 1700–1741. [Google Scholar] [CrossRef]
- Bankova, V. Chemical Diversity of Propolis and the Problem of Standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Daleprane, J.B.; da Silva Freitas, V.; Pacheco, A.; Rudnicki, M.; Faine, L.A.; Dörr, F.A.; Ikegaki, M.; Salazar, L.A.; Ong, T.P.; Abdalla, D.S.P. Anti-Atherogenic and Anti-Angiogenic Activities of Polyphenols from Propolis. J. Nutr. Biochem. 2012, 23, 557–566. [Google Scholar] [CrossRef]
- Braakhuis, A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef]
- El Adham, E.K.; Hassan, A.I.; Dawoud, M.M.A. Evaluating the Role of Propolis and Bee Venom on the Oxidative Stress Induced by Gamma Rays in Rats. Sci. Rep. 2022, 12, 2656. [Google Scholar] [CrossRef]
- Kim, D.H.; Auh, J.-H.; Oh, J.; Hong, S.; Choi, S.; Shin, E.J.; Woo, S.O.; Lim, T.-G.; Byun, S. Propolis Suppresses UV-Induced Photoaging in Human Skin through Directly Targeting Phosphoinositide 3-Kinase. Nutrients 2020, 12, 3790. [Google Scholar] [CrossRef]
- Cao, X.-P.; Chen, Y.-F.; Zhang, J.-L.; You, M.-M.; Wang, K.; Hu, F.-L. Mechanisms Underlying the Wound Healing Potential of Propolis Based on Its in Vitro Antioxidant Activity. Phytomedicine 2017, 34, 76–84. [Google Scholar] [CrossRef]
- Gastaldello, G.H.; Cazeloto, A.C.V.; Ferreira, J.C.; Rodrigues, D.M.; Bastos, J.K.; Campo, V.L.; Zoccal, K.F.; Tefé-Silva, C. Green Propolis Compounds (Baccharin and p-Coumaric Acid) Show Beneficial Effects in Mice for Melanoma Induced by B16f10. Medicines 2021, 8, 20. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis Volatile Compounds: Chemical Diversity and Biological Activity: A Review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Pobiega, K.; Przybył, J.L.; Żubernik, J.; Gniewosz, M. Prolonging the Shelf Life of Cherry Tomatoes by Pullulan Coating with Ethanol Extract of Propolis During Refrigerated Storage. Food Bioprocess Technol. 2020, 13, 1447–1461. [Google Scholar] [CrossRef]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent Advances in Chemistry and Plant Origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Wilson, M.B.; Spivak, M.; Hegeman, A.D.; Rendahl, A.; Cohen, J.D. Metabolomics Reveals the Origins of Antimicrobial Plant Resins Collected by Honey Bees. PLoS ONE 2013, 8, e77512. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Bakier, S.; Pirożnikow, E.; Zambrzycka, M.; Swiecicka, I. Selective Behaviour of Honeybees in Acquiring European Propolis Plant Precursors. J. Chem. Ecol. 2016, 42, 475–485. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Szczepaniak, L.; Bakier, S. Rapid GC/MS Determination of Botanical Precursors of Eurasian Propolis. Food Chem. 2014, 142, 101–106. [Google Scholar] [CrossRef]
- Kim, Y.S.; Young, M.R.; Bobe, G.; Colburn, N.H.; Milner, J.A. Bioactive Food Components, Inflammatory Targets, and Cancer Prevention. Cancer Prev. Res. 2009, 2, 200–208. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic Compounds in Fruits—An Overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative Stress and Inflammation in Osteoarthritis Pathogenesis: Role of Polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, X.; Chen, F.; Wang, M. Dietary Polyphenols as Photoprotective Agents against UV Radiation. J. Funct. Foods 2017, 30, 108–118. [Google Scholar] [CrossRef]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-Induced Skin Damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Gregoris, E.; Fabris, S.; Bertelle, M.; Grassato, L.; Stevanato, R. Propolis as Potential Cosmeceutical Sunscreen Agent for Its Combined Photoprotective and Antioxidant Properties. Int. J. Pharm. 2011, 405, 97–101. [Google Scholar] [CrossRef]
- Lee, K.-H. Current Developments in the Discovery and Design of New Drug Candidates from Plant Natural Product Leads. J. Nat. Prod. 2004, 67, 273–283. [Google Scholar] [CrossRef]
- Karapetsas, A.; Voulgaridou, G.-P.; Konialis, M.; Tsochantaridis, I.; Kynigopoulos, S.; Lambropoulou, M.; Stavropoulou, M.-I.; Stathopoulou, K.; Aligiannis, N.; Bozidis, P.; et al. Propolis Extracts Inhibit UV-Induced Photodamage in Human Experimental In Vitro Skin Models. Antioxidants 2019, 8, 125. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Ferreres, F.; Custódio, A.R.; Ferreira, M.M.C.; Bankova, V.S.; García-Viguera, C.; Bretz, W.A. Evalution of Phenolic Compounds in Brazilian Propolis from Different Geographic Regions. Zeitschrift für Naturforschung C 2000, 55, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, É.W.; Negri, G.; Meira, R.M.S.A.; Message, D.; Salatino, A. Plant Origin of Green Propolis: Bee Behavior, Plant Anatomy and Chemistry. Evid.-Based Complement. Altern. Med. 2005, 2, 697212. [Google Scholar] [CrossRef] [PubMed]
- Uzel, A.; Sorkun, K.; Önçağ, Ö.; Çoğulu, D.; Gençay, Ö.; Sali˙h, B. Chemical Compositions and Antimicrobial Activities of Four Different Anatolian Propolis Samples. Microbiol. Res. 2005, 160, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskiene, K.; Savickas, A.; Ivanauskas, L.; Kalveniene, Z.; Kasparaviciene, G.; Banionyte, I.; Amsiejus, A.; Martirosyan, M.D. Analysis of Phenolic Acids in Propolis Using the High-Performance Liquid Chromatography Technique. Curr. Nutr. Food Sci. 2008, 4, 209–212. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Liaudanskas, M.; Ivanauskas, L.; Ivaskiene, M.; Ramanauskiene, K. Extracts of Poplar Buds (Populus balsamifera L., Populus nigra L.) and Lithuanian Propolis: Comparison of Their Composition and Biological Activities. Plants 2021, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic Composition and Antioxidant Activity of Propolis from Various Regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef]
- Aliyazıcıoglu, R.; Sahin, H.; Erturk, O.; Ulusoy, E.; Kolayli, S. Properties of Phenolic Composition and Biological Activity of Propolis from Turkey. Int. J. Food Prop. 2013, 16, 277–287. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of Bioactive Compounds Potential and Antioxidant Activity of Brown, Green and Red Propolis from Brazilian Northeast Region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef]
- Szliszka, E.; Sokół-Łętowska, A.; Kucharska, A.Z.; Jaworska, D.; Czuba, Z.P.; Król, W. Ethanolic Extract of Polish Propolis: Chemical Composition and TRAIL-R2 Death Receptor Targeting Apoptotic Activity against Prostate Cancer Cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 757628. [Google Scholar] [CrossRef]
- Kim, C.S.; Subedi, L.; Park, K.J.; Kim, S.Y.; Choi, S.U.; Kim, K.H.; Lee, K.R. Salicin Derivatives from Salix Glandulosa and Their Biological Activities. Fitoterapia 2015, 106, 147–152. [Google Scholar] [CrossRef]
- Popova, M.P.; Bankova, V.S.; Bogdanov, S.; Tsvetkova, I.; Naydenski, C.; Marcazzan, G.L.; Sabatini, A.-G. Chemical Characteristics of Poplar Type Propolis of Different Geographic Origin. Apidologie 2007, 38, 306–311. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic Studies on the Antioxidant Activity of P-Coumaric Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zheng, T.; Liang, Y.; Duan, L.; Zhang, Y.; Wang, L.-J.; He, G.; Xiao, H. P-Coumaric Acid Protects Human Lens Epithelial Cells against Oxidative Stress-Induced Apoptosis by MAPK Signaling. Oxidative Med. Cell. Longev. 2018, 2018, 8549052. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Lodovici, M.; Morbidelli, L.; Dolara, P. Hydrocaffeic and P-Coumaric Acids, Natural Phenolic Compounds, Inhibit UV-B Damage in WKD Human Conjunctival Cells in Vitro and Rabbit Eye in Vivo. Free Radic. Res. 2008, 42, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- An, S.M.; Koh, J.-S.; Boo, Y.C. P-Coumaric Acid Not Only Inhibits Human Tyrosinase Activity in Vitro but Also Melanogenesis in Cells Exposed to UVB. Phytother. Res. 2010, 24, 1175–1180. [Google Scholar] [CrossRef]
- Wezgowiec, J.; Wieczynska, A.; Wieckiewicz, W.; Kulbacka, J.; Saczko, J.; Pachura, N.; Wieckiewicz, M.; Gancarz, R.; Wilk, K.A. Polish Propolis—Chemical Composition and Biological Effects in Tongue Cancer Cells and Macrophages. Molecules 2020, 25, 2426. [Google Scholar] [CrossRef]
- Ahn, M.-R.; Kunimasa, K.; Kumazawa, S.; Nakayama, T.; Kaji, K.; Uto, Y.; Hori, H.; Nagasawa, H.; Ohta, T. Correlation between Antiangiogenic Activity and Antioxidant Activity of Various Components from Propolis. Mol. Nutr. Food Res. 2009, 53, 643–651. [Google Scholar] [CrossRef]
- Forma, E.; Bryś, M. Anticancer Activity of Propolis and Its Compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.F.; Dos Santos, H.F.; Bonamigo, T.; de Campos Domingues, N.L.; de Picoli Souza, K.; Dos Santos, E.L. Stingless Bee Propolis: New Insights for Anticancer Drugs. Oxidative Med. Cell. Longev. 2021, 2021, 2169017. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, P.; Muninathan, N.; Megalatha, S.T.; Suresh, A.; Kumar, K.S.; Jhansi, N.; Kalaivani, K.; Krishnamoorthy, G. An Insight into Anticancer Effect of Propolis and Its Constituents: A Review of Molecular Mechanisms. Evid.-Based Complement. Altern. Med. 2022, 2022, 5901191. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Ravaioli, S.; Santi, S.; Mariani, V.; Santarcangelo, C.; De Filippis, A.; Montanaro, L.; Arciola, C.R.; Daglia, M. Exploring the Anticancer Effects of Standardized Extracts of Poplar-Type Propolis: In Vitro Cytotoxicity toward Cancer and Normal Cell Lines. Biomed. Pharmacother. 2021, 141, 111895. [Google Scholar] [CrossRef]
- Machado, J.L.; Assunção, A.K.M.; da Silva, M.C.P.; dos Reis, A.S.; Costa, G.C.; de Sousa Arruda, D.; Rocha, B.A.; de Oliveira Lima Leite Vaz, M.M.; de Andrade Paes, A.M.; Guerra, R.N.M.; et al. Brazilian Green Propolis: Anti-Inflammatory Property by an Immunomodulatory Activity. Evid.-Based Complement. Altern. Med. 2012, 2012, 157652. [Google Scholar] [CrossRef]
- Szliszka, E.; Kucharska, A.Z.; Sokół-Łętowska, A.; Mertas, A.; Czuba, Z.P.; Król, W. Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages. Evid.-Based Complement. Altern. Med. 2013, 2013, 976415. [Google Scholar] [CrossRef]
- Shang, H.; Srikanth Bhagavathula, A.; Ali Aldhaleei, W.; Rahmani, J.; Karam, G.; Rinaldi, G.; Clark, C.; Salehisahlabadi, A.; Yuan, Q. Effect of Propolis Supplementation on C-Reactive Protein Levels and Other Inflammatory Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. King Saud Univ. Sci. 2020, 32, 1694–1701. [Google Scholar] [CrossRef]
- Xu, W.; Lu, H.; Yuan, Y.; Deng, Z.; Zheng, L.; Li, H. The Antioxidant and Anti-Inflammatory Effects of Flavonoids from Propolis via Nrf2 and NF-κB Pathways. Foods 2022, 11, 2439. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant Activity of Propolis of Various Geographic Origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Cavalaro, R.I.; da Cruz, R.G.; Dupont, S.; de Moura Bell, J.M.L.N.; de Souza Vieira, T.M.F. In Vitro and in Vivo Antioxidant Properties of Bioactive Compounds from Green Propolis Obtained by Ultrasound-Assisted Extraction. Food Chem. X 2019, 4, 100054. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Kwaśniewska-Sip, P.; Waśkiewicz, A.; Nowak, P.; Ratajczak, I. Effect of the Solvent on Propolis Phenolic Profile and its Antifungal, Antioxidant, and In Vitro Cytoprotective Activity in Human Erythrocytes Under Oxidative Stress. Molecules 2020, 25, 4266. [Google Scholar] [CrossRef]
- Mendonça, M.A.A.d.; Ribeiro, A.R.S.; Lima, A.K.d.; Bezerra, G.B.; Pinheiro, M.S.; Albuquerque-Júnior, R.L.C.d.; Gomes, M.Z.; Padilha, F.F.; Thomazzi, S.M.; Novellino, E.; et al. Red Propolis and Its Dyslipidemic Regulator Formononetin: Evaluation of Antioxidant Activity and Gastroprotective Effects in Rat Model of Gastric Ulcer. Nutrients 2020, 12, 2951. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.J.V.; Fonseca, Y.M.; Marquele-Oliveira, F.; Vicentini, F.T.M.C.; Furtado, N.A.J.C.; Sousa, J.P.B.; Lucisano-Valim, Y.M. Evaluation of the Potential of Brazilian Propolis against UV-Induced Oxidative Stress. Evid.-Based Complement. Altern. Med. 2011, 2011, 863917. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.S.; Cunha, A.; Oliveira, R.; Almeida-Aguiar, C. Propolis Antibacterial and Antioxidant Synergisms with Gentamicin and Honey. J. Appl. Microbiol. 2022, 132, 2733–2745. [Google Scholar] [CrossRef]
- Governa, P.; Cusi, M.G.; Borgonetti, V.; Sforcin, J.M.; Terrosi, C.; Baini, G.; Miraldi, E.; Biagi, M. Beyond the Biological Effect of a Chemically Characterized Poplar Propolis: Antibacterial and Antiviral Activity and Comparison with Flurbiprofen in Cytokines Release by LPS-Stimulated Human Mononuclear Cells. Biomedicines 2019, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Eskandarinia, A.; Kefayat, A.; Gharakhloo, M.; Agheb, M.; Khodabakhshi, D.; Khorshidi, M.; Sheikhmoradi, V.; Rafienia, M.; Salehi, H. A Propolis Enriched Polyurethane-Hyaluronic Acid Nanofibrous Wound Dressing with Remarkable Antibacterial and Wound Healing Activities. Int. J. Biol. Macromol. 2020, 149, 467–476. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Food and Drug Administration. Sunscreen: How to Help Protect Your Skin from the Sun. Available online: https://www.fda.gov/drugs/understanding-over-counter-medicines/sunscreen-how-help-protect-your-skin-sun#spf (accessed on 10 October 2022).
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A.; FlaminiGuido; Kilinçarslan, Ö.; Mammadov, R.; Bardaweel, S.K. Chemical Composition and Bioactivity of Essential Oils and Ethanolic Extracts of Ocimum Basilicum L. and Thymus Algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Altern. Med. 2019, 19, 146. [Google Scholar] [CrossRef] [PubMed]
- More, G.K.; Makola, R.T. In-Vitro Analysis of Free Radical Scavenging Activities and Suppression of LPS-Induced ROS Production in Macrophage Cells by Solanum Sisymbriifolium Extracts. Sci. Rep. 2020, 10, 6493. [Google Scholar] [CrossRef]
- de Oliveira-Júnior, R.G.; Ferraz, C.A.A.; Souza, G.R.; Guimarães, A.L.; de Oliveira, A.P.; Lima-Saraiva, S.R.G.D.; Rolim, L.A.; Rolim-Neto, P.J.; da Silva Almeida, J.R.G. Phytochemical Analysis and Evaluation of Antioxidant and Photoprotective Activities of Extracts from Flowers of Bromelia Laciniosa (Bromeliaceae). Biotechnol. Biotechnol. Equip. 2017, 31, 600–605. [Google Scholar] [CrossRef]
- Mansur, J.D.S.; Breder, M.N.R.; Mansur, M.C.D.A.; Azulay, R.D. Determinaçäo Do Fator de Proteçäo Solar Por Espectrofotometria. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A Comparison of In Vivo and In Vitro Testing of Sunscreening Formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).