Abstract
Polyploidy plays a crucial role in plant evolution and speciation. The development of male and female gametes is essential to the reproductive capacity of polyploids, but their gene expression pattern has not been fully explored in newly established polyploids. The present study aimed to reveal a detailed atlas of gene expression for gamete development in newly synthetic Brassica allohexaploids that are not naturally existing species. Comparative transcriptome profiling between developing anthers (staged from meiosis to mature pollen) and ovules (staged from meiosis to mature embryo sac) was performed using RNA-Seq analysis. A total of 8676, 9775 and 4553 upregulated differentially expressed genes (DEGs) were identified for the development of both gametes, for male-only, and for female-only gamete development, respectively, in the synthetic Brassica allohexaploids. By combining gene ontology (GO) biological process analysis and data from the published literature, we identified 37 candidate genes for DNA double-strand break formation, synapsis and the crossover of homologous recombination during male and female meiosis and 51 candidate genes for tapetum development, sporopollenin biosynthesis and pollen wall development in male gamete development. Furthermore, 23 candidate genes for mitotic progression, nuclear positioning and cell specification and development were enriched in female gamete development. This study lays a good foundation for revealing the molecular regulation of genes related to male and female gamete development in Brassica allohexaploids and provides more resourceful genetic information on the reproductive biology of Brassica polyploid breeding.
Keywords:
transcriptome; Brassica; allohexaploid; polyploid; male and female gamete development; meiosis; anther; ovule 1. Introduction
Polyploidy has long been recognized as an important evolutionary force in plants [1,2,3]. The formation of polyploids is attributed to genomic plasticity, and polyploid-induced changes can result in new genetic diversity and advantageous adaptations to the environment [4,5]. Polyploids generally have multiple phenotypes and greater growth vigor compared with their parents [6]. Polyploidy also leads to an increase in plant organs to improve outputs and adapt to various biological and abiotic stresses in plant breeding [7,8]. Allopolyploidy results concomitantly from the genome double after the hybridization of two or more species [9]. Brassica has long been considered as a model to explore polyploidization. Three basic diploid species in Brassica, Brassica rapa (AA, 2n = 20), Brassica nigra (BB, 2n = 16) and Brassica oleracea (CC, 2n = 18), have hybridized to give rise to three allotetraploid species, Brassica juncea (AABB, 2n = 36), Brassica napus (AACC, 2n = 38) and Brassica carinata (BBCC, 2n = 34). The combination of genetic variation from six species in Brassica could result in crops with increased adaptation and agronomic potential as well as improved heterosis from the contribution of alleles [10,11,12]. Brassica allohexaploids (2n = AABBCC) do not exist in nature but can be synthesized by hybridization between diploid and/or allotetraploid species of Brassica. The cross between B. carinata and B. rapa is the most commonly attempted and successful method in the five possible species combinations that can produce Brassica allohexaploids [13]. Hybridization and genomic doubling may lead to extensive transcriptomic changes in the synthesized trigenomic Brassica allohexaploids relative to their parents [14]. Brassica allohexaploids not only provide an effective way to improve the genetic diversity of Brassica and the intergenic hybridization of oilseed crops in the future, but they also offer excellent material for the study of polyploid plants.
So far, studies on Brassica allohexaploids have mainly focused on agronomic traits, meiotic behaviors and subgenome stabilities [15,16]. The development of male and female gametes is crucial to the reproductive capacity of sexually reproducing organisms, especially polyploids, and is strictly regulated by complex processes. However, the genes involved in male and female gamete development are not fully understood in Brassica allohexaploids. Therefore, it is very important to establish a reproductive transcriptome profiling of Brassica allohexaploids.
The male germline matures within the anther, whereas the female germline develops within the ovule [17]. The male and female gametophyte are the important reproductive units of angiosperms and are essential for sexual reproduction [18,19]. Both male and female germline development of angiosperms consist of two main stages: microsporogenesis and microgametogenesis, giving rise to male gametes, and megasporogenesis and megagametogenesis, leading to the formation of female gametes. Microsporogenesis and megasporogenesis are key to male and female reproduction and require the completion of meiosis to form microspores and functional megaspores (FM). Microgametogenesis includes an asymmetric division to form vegetative and generative cells, and the generative cells produce two male gametes after a mitotic division [20]. FM undergoes three rounds of mitosis, nuclear migration, cellularization and differentiation to form a mature seven-celled embryo sac, containing three antipodal cells, two synergid cells, one egg cell and one central cell that contains two polar nuclei, which complete megagametogenesis [19,21]. During gamete generation, male and female gametes have similar ploidy transition and cell cycle progressions, while they show many differences in gamete development and specialization.
RNA-Seq technology is used to analyze the structure and function of genes at the organismal level and to explore a range of biological pathways [22,23]. The establishment of Brassica transcriptome databases, such as the B. napus transcriptome database BnTIR, provide a lot of useful resources for the study of Brassica polyploidy at the transcriptional level [24]. In recent years, RNA-Seq has been successfully used in anther and ovule development in many species. In Brassica napus L., lipid metabolism genes involved in pollen extine formation, elaioplast and tapetosome biosynthesis were preferentially expressed in early anthers, and carbohydrate metabolism genes to form pollen intine and to accumulate starch in mature pollen grains were preferentially expressed in late anthers [25]. Comparative analysis of differential gene expression revealed multiple signal pathways during flowering of autotetraploid B. rapa [26]. The molecular processes involved in the development of female gametes in plants are much less understood than those involved in the development of male gametes due to the small number and difficult availability of female gametophytes. Using high-throughput sequencing analysis, female gamete development in Arabidopsis has been intensively studied, and a number of key genes regulating megasporogenesis and megagametogenesis have been found, offering fundamental knowledge of these developmental processes [21,27].
The aim of the study was to reveal a detailed atlas of gene expression for gamete development in synthetic Brassica allohexaploids. We performed differential expression analysis to identify common and preferential genes that regulate the developmental events of male and female gametes. This study provides rich genetic resources for the cloning and functional verification of genes related to male and female gamete development and lays a good foundation for revealing the molecular regulatory mechanism of reproductive development in Brassica allohexaploids.
2. Results
2.1. Transcriptome Sequencing and Sequence Alignment
RNA-Seq of anthers from meiosis to the mature pollen stage (Anther), ovules from meiosis to mature embryo sac stage (Ovule) and young leaves (Leaf) was performed by Illumina in synthetic Brassica allohexaploids. Leaf was used as vegetative tissue (organ) control, and each Brassica allohexaploid tissue (organ) received three biological replicates. RNA-Seq results are presented in Table 1 and Table 2. The number of clean reads from the nine RNA-Seq libraries ranged from 40,202,856 to 47,450,126 (Table 1). The Clean Q30 Bases Rate was greater than 94.29%, and the higher the value is, the better the sequencing quality (Table 1). All clean reads were then aligned to B. rapa, B. nigra and B. oleracea genome sequences using HISAT2 software. Mapped genome reads ranged from 21,502,559 to 39,794,184, and genome mapping rates ranged from 53.49% to 85.19% (Table 2). These results suggested that their quality met the requirements for transcriptome sequencing. Fragments per kilobase per million reads (FPKM) were determined for all genes of Anther, Ovule and Leaf in Brassica allohexaploids (Table S1). Correlation heat maps of the transcriptome from Anther, Ovule and Leaf showed that the three biological replicates of each tissue were grouped together with high correlation (Figure S1). These results indicated that the three biological replicates of per tissue had good repeatability in this study.

Table 1.
Statistics of the RNA-Seq data in the Brassica allohexaploids.

Table 2.
Statistics for clean reads mapped in the Brassica allohexaploids.
2.2. Anthers at Male Gamete Development Stage and Ovules at Female Gamete Development Stage Contained More Genes Than Leaves in Brassica Allohexaploids
A total of 100,804 genes were identified in the RNA-Seq data of Brassica allohexaploids, among which 96,286, 86,712 and 79,875 genes were expressed in Anther, Ovule and Leaf, respectively, providing sufficient data for studying male and female gamete development (Figure 1a). Among the 100,804 genes, 74,597 genes were expressed in all three tissues, and 9948, 1870 and 1514 genes were specifically expressed in Anther, Ovule and Leaf, respectively (Figure 1a). Comparative analysis showed that Anther and Ovule overlapped most, with 9111 genes commonly expressed in these two tissues; 2630 genes were commonly expressed in Anther and Leaf; and 1134 genes were commonly expressed in Ovule and Leaf (Figure 1a). Anther and Ovule contained more genes and more specific genes than vegetative tissue Leaf. In all three tissues, transcript abundance analysis indicated 74.1% to 78.6% genes with low expression (FPKM >0 to ≤10) and 19.6% to 24.0% genes with moderate expression (FPKM >10 to ≤100) but only 1.4% to 1.9% genes with high expression (FPKM > 100) (Figure 1b).

Figure 1.
Overview of gene expression in Anther, Ovule and Leaf of Brassica allohexaploids: (a) Venn diagram showing the overlap between expressed genes in Anther, Ovule and Leaf; (b) distribution of FPKM range in Anther, Ovule and Leaf; (c) the number of genes from A, B and C-genomes in Anther, Ovule and Leaf and the proportion of genes in the reference genomes; and (d) results of pairwise differential expression analysis in Anther, Ovule and Leaf.
In Anther, Ovule and Leaf, 33,837, 30,501 and 28,078 genes, respectively, were expressed from A-genome; 35,126, 31,413 and 28,842 genes, respectively, were expressed from B-genome; and 27,323, 24,798 and 22,955 genes, respectively, were expressed from C-genome (Figure 1c). The reference genomes of B. rapa, B. nigra and B. oleracea contained 41,020, 47,953 and 61,279 genes, respectively, thus the percentage of genes expressed in Anther, Ovule and Leaf from A-genome was 82.5%, 74.4% and 68.4%, respectively, while from B-genome the values were 73.3%, 65.5% and 60.1%, respectively, and from C-genome 44.6%, 40.5% and 37.5% (Figure 1c), respectively. These results indicated that the B-genome contained the largest number of genes in Anther, Ovule and Leaf in Brassica allohexaploids, while the A-genome was bias expressed. Three groups of differentially expressed genes (DEGs) were generated by pairing differential expression analysis of genes between all three tissues (Table S2). Volcano plots show DEGs of Anther vs. Ovule, Ovule vs. Leaf and Anther vs. Leaf (Figure S2). Among all comparisons, the number of DEGs in Anther and Ovule was the smallest, and 22,305 genes (14,366 upregulated and 7939 downregulated) were differentially expressed in Anther compared with Ovule (Figure 1d). Compared with Leaf, 30,893 genes (14,830 upregulated and 16,063 downregulated) were differentially expressed in Ovule (Figure 1d). However, 35,849 genes (19,513 upregulated and 16,336 downregulated) were differentially expressed in Anther compared with Leaf, which was the largest number of DEGs (Figure 1d). The findings showed that there were differences in gene expression between reproductive tissue and vegetative tissue and male gamete development had more genes expressed than female gamete development in Brassica allohexaploids.
2.3. Identification of Upregulated Genes Co-Expressed and Preferentially Expressed Genes in Male and Female Gamete Development of Brassica Allohexaploids
In order to study the common characteristics of male and female gamete development in Brassica allohexaploids, the 19,513 upregulated genes in Anther relative to Leaf and the 14,830 upregulated genes in Ovule relative to Leaf were overlapped. There were 25,667 upregulated genes co-expressed and 8676 preferentially expressed genes in male and female gamete development of Brassica allohexaploids (Figure 2a); 8605 of the 25,667 upregulated genes co-expressed in male and female gamete development were annotated using clusters of orthologous groups (COG) classification and functionally divided into 25 COG categories (Figure 2b). COG clusters were mainly showed in General function prediction only [1494~13.43%]; Transcription [1079~9.70%]; Posttranslational modification, protein turnover, chaperones [861~7.74%]; Replication, recombination and repair [778~6.99%]; Carbohydrate transport and metabolism [775~6.97%]; Signal transduction mechanisms [590~5.30%]; Translation, ribosomal structure and biogenesis [576~5.18%]; Cell cycle control, cell division, chromosome partitioning [559~5.03%]; Amino acid transport and metabolism [557~5.01%]; and Lipid transport and metabolism [470~4.23%] (Figure 2b). To identify the key biological process pathways of common genes in male and female gamete development of Brassica allohexaploids, 8676 preferentially expressed genes at male and female gamete development stages were analyzed by gene ontology (GO) biological process enrichment analysis. The significantly enriched GO terms had DNA repair (GO: 0006281), DNA replication (GO: 0006260), synapsis (GO: 0007129), cell cycle (GO: 0007049), microtubule-based movement (GO: 0007018), DNA recombination (GO: 0006310), double-strand break repair via homologous recombination (GO: 0000724), mismatch repair (GO: 0006298), resolution of meiotic recombination intermediates (GO: 0000712), double-strand break repair (GO: 0006302), chiasma assembly (GO: 0051026), double-strand break repair via synthesis-dependent strand annealing (GO: 0045003), homologous recombination (GO: 0035825), regulation of double-strand break repair (GO: 2000779) and regulation of double-strand break repair via homologous recombination (GO: 0010569) (Figure 2c). These results suggested that common genes in male and female gamete development may be related to the homologous recombination of meiosis in Brassica allohexaploids.
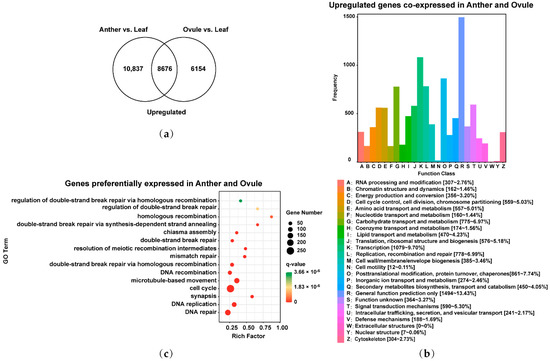
Figure 2.
Identification and functional analysis of upregulated genes co-expressed and preferentially expressed genes in Anther and Ovule of Brassica allohexaploids: (a) Venn diagram showing the overlap of upregulated genes between Anther vs. Leaf and Ovule vs. Leaf; (b) clusters of orthologous groups (COG) classification of upregulated genes co-expressed in Anther and Ovule; and (c) gene ontology (GO) biological process pathway enrichment analysis of preferentially expressed genes in Anther and Ovule.
Arabidopsis orthologs of seven genes, BniB034973 (orthologous to SPO11-1 (Sporulation 11-1)), BniB027699 and Bol025700 (orthologous to PRD1 (PUTATIVE RECOMBINATION INITIATION DEFECT 1)), Bra024921, Bra033241 and Bol007924 (orthologous to PRD3 (PUTATIVE RECOMBINATION INITIATION DEFECT 3)) and BniB044390 (orthologous to MTOPVIB (Meiotic topoisomerase VIB-like)) are necessary for meiotic double-strand breaks (DSBs) formation (Figure 3). SPO11-1 is essential for formation of DSBs in plants, and PRD1, PRD3 and MTOPVIB have already been shown to play a key role in DNA DSBs formation in Arabidopsis (Figure 3) [28,29,30,31]. In addition, some meiosis genes related to synapses are also enriched in the GO biological process during the development of male and female gametes. Bra004222 and BniB009412 are orthologous to ASY1 (Asynaptic 1), which encodes a protein essential for meiotic chromosomal synapsis and localizes to axis-associated chromatin [32]. In B. rapa, the axis-associated mutant plants of Bra004222 produce fewer crossovers (COs) due to abnormalities in meiosis (Figure 3) [33]. BniB041399 is a homolog of ASY3 (Asynaptic 3) (Figure 3). AYS3 and ASY1 have similar roles in Arabidopsis, and its deletion also disrupts synaptic complex (SC) formation [34]. Similarly, Bra003654, Bra035016 and Bol027500 are orthologs of ZYP1a encoding synaptonemal complex protein, which regulates chromosome synapsis and normal fidelity of crossing over (Figure 3) [35]. The orthologs of these candidate genes, Bra039674, Bra038126, BniB027427, BniB026849, BniB009174, Bol039109 and Bol035122 (orthologous to HEI10 (Homolog of human hei10)), Bra029127 (orthologous to SHOC1 (Shortage in chiasmata 1)) and Bra037488, BniB000031 and Bol043105 (orthologous to SPO22) are ZMM proteins, which are required for the formation of class I meiotic COs (Figure 3) [36,37,38]. The Arabidopsis orthologous gene MSH5 (Muts homolog 5) of Bra035777 and BniB043474 partners orthologous gene MSH4 (Muts homolog 4) of Bol032857 in class I meiotic CO pathway [39]. The Arabidopsis ortholog TOP3a (Topoisomerase 3alpha) of BniB047025 and RMI1 (RecQ mediated instability 1) of Bra035838, Bra031944 and BniB045608 maintain genome stability by limiting CO formation in favor of non-crossover (NCO) events (Figure 3) [40,41]. Bra034416, BniB042826 and Bol031970 are orthologous to FANCM (Fanconi anemia complementation group M), a highly conserved helicase, which functions as a major factor limiting meiotic CO formation (Figure 3) [42]. RECQ4A, an Arabidopsis ortholog of Bra018418, Bra031741 and Bol022020, has been found to play a key role in DNA repair and homologous recombination suppression (Figure 3) [43].

Figure 3.
The expression patterns of double-strand break formation, synapsis, class I crossover and non-crossover–related genes in Anther, Ovule and Leaf of Brassica allohexaploids.
2.4. Identification of Preferentially Expressed Genes in Male Gamete Development of Brassica Allohexaploids
To better understand the development of male gametes in Brassica allohexaploids, we analyzed the preferentially expressed genes in male gamete development. Venn diagrams showed that the overlap of 14,366 upregulated genes in Anther vs. Ovule and 19,513 upregulated genes in Anther vs. Leaf was 9775 preferentially expressed genes in male gamete development (Figure 4a). We used GO annotation to functionally classify these 9775 genes based on their biological process. We found that GO terms associated with the activation of protein kinase activity (GO: 0032147), protein ubiquitination (GO: 0016567), pollen wall assembly (GO: 0010208), anther wall tapetum development (GO: 0048658), pollen exine formation (GO: 0010584), sporopollenin biosynthetic process (GO: 0080110), pollen development (GO: 0009555), callose deposition in cell walls (GO: 0052543), anther development (GO: 0048653) and pollen sperm cell differentiation (GO: 0048235) were significantly overrepresented within the preferentially expressed genes in male gamete development (Figure 4b). These findings showed that preferentially expressed genes in male gamete development were concentrated in pollen wall formation and other related pathways in Brassica allohexaploids.
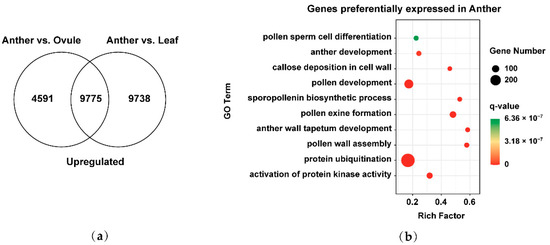
Figure 4.
Identification and functional analysis of preferentially expressed genes in male gamete development of Brassica allohexaploids: (a) Venn diagram showing the overlap of upregulated genes between Anther vs. Ovule and Anther vs. Leaf and (b) GO biological process pathway enrichment analysis of preferentially expressed genes in Anther.
The transcription factor AMS (ABORTED MICROSPORES), Arabidopsis ortholog of Bra002004, Bra013041, BniB011765, BniB025753, Bol004758 and Bol042692, plays a vital role in tapetum and pollen development (Figure 5) [44]. Arabidopsis ortholog MYB80 (MYB domain protein 80) also called MS188 (Male Sterile 188), a member of the R2R3 MYB transcription factor gene family, of Bra002847, Bra035604, BniB030464, BniB044215, Bol009875 and Bol035011, is required for tapetal and pollen development (Figure 5) [45]. Bra025337, BniB025201 and Bol042967 are orthologous to TDF1 (Tapetal Development and Function 1), regulating tapetal differentiation and function (Figure 5) [46]. Arabidopsis orthologs CYP703A2 of Bra032631, Bra033272, BniB042784, Bol018458 and Bol040704, as well as CYP704B1 of Bra004386, BniB031958 and Bol023932, are essential for sporopollenin synthesis in pollen development (Figure 5) [47,48]. Furthermore, Arabidopsis orthologs of some genes (Bra036646 and BniB001185 orthologous to ACOS5 (Acyl-CoA Synthetase 5), Bra034658, Bra011566, Bra017681, BniB036573, BniB040820, BniB048407, Bol013698 and Bol034656 orthologous to LAP5 (Less adhesive pollen 5) and Bra017147, BniB016901 and Bol025267 orthologous to LAP6 (Less adhesive pollen 6)) have been shown to perform important roles in pollen development and sporopollenin biosynthesis (Figure 5) [49,50]. Other important candidate genes Bra010535 and BniB015834 are orthologous to TKPR1 (TETRAKETIDE alpha-PYRONE REDUCTASE 1) (previously called DRL1 (Dihydroflavonol 4-reductase-like1)), regulating a metabolic pathway critical for sporopollenin monomer biosynthesis, pollen wall formation and male fertility (Figure 5) [51,52]. Bra004316, Bra008822, BniB031910 and Bol024018 are orthologous to TKPR2 (TETRAKETIDE alpha-PYRONE REDUCTASE 2), which regulates the biochemical pathway for sporopollenin monomer biosynthesis in Arabidopsis (Figure 5) [51]. Bra034793, Bra038691, BniB010680, BniB035126, Bol007277 and Bol010336 are orthologous to MS2 (Male Sterile 2), which encodes for a plastid-localized fatty acyl carrier protein reductase, is essential for pollen wall development in Arabidopsis (Figure 5) [53]. ABCG26 (ATP-Binding Cassette Transporter G26), Arabidopsis ortholog of Bra039378, BniB034970 and Bol015793, is important for male fertility and pollen exine formation (Figure 5) [54].

Figure 5.
The expression patterns of tapetum development, sporopollenin biosynthesis and pollen wall development related genes in Anther, Ovule and Leaf of Brassica allohexaploids.
2.5. Identification of Preferentially Expressed Genes in Female Gamete Development of Brassica Allohexaploids
We next analyzed the preferentially expressed genes involved in female gamete development to explore their key events in Brassica allohexaploids. 4553 genes preferentially expressed in female gamete development were identified by overlapping 7939 and 14,830 upregulated genes of Ovule vs. Anther and Ovule vs. Leaf (Figure 6a). These genes were analyzed by GO biological process pathway analysis. These GO terms related to cell differentiation (GO: 0030154), auxin biosynthetic process (GO: 0009851), integument development (GO: 0080060), cell division (GO: 0051301), glucosinolate biosynthetic process (GO: 0019761), auxin-activated signaling pathway (GO: 0009734), polarity specification of adaxial/abaxial axis (GO: 0009944), regulation of proanthocyanidin biosynthetic process (GO: 2000029), plant ovule development (GO: 0048481) and regulation of jasmonic acid mediated signaling pathway (GO: 2000022) were significantly enriched in female gamete development (Figure 6b). These results suggested that genes preferentially expressed in female gamete development may be related to cell division, nuclear positioning, cell differentiation and auxin-activated synthesis and signal transduction of Brassica allohexaploids.
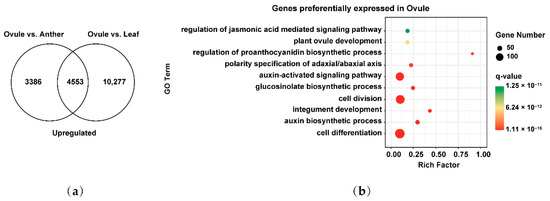
Figure 6.
Identification and functional analysis of preferentially expressed genes in female gamete development of Brassica allohexaploids: (a) Venn diagram showing the overlap of upregulated genes between Ovule vs. Anther and Ovule vs. Leaf; and (b) GO biological process pathway enrichment analysis of preferentially expressed genes in Ovule.
Megagametogenesis begins when FM passes through three rounds of nuclear division, producing an eight-nucleate syncytium. Many genes have been implicated in the mitosis entry and progression during female gametophyte development. Arabidopsis ortholog AUX1 (AUXIN RESISTANT 1) of Bra000160 and Bol020456, and PIN1 (PIN-FORMED 1) of Bra015983, BniB013785, BniB015990, Bol026255 and Bol039419 are essential in sporophytes for auxin import and local auxin biosynthesis to regulate mitosis (Figure 7) [55,56]. The anaphase-promoting complex/cyclosome (APC/C) is a multi-subunit E3 ligase, which plays a critical role in regulating cell-cycle progression [57]. Bra028755 and BniB038334 are orthologous to APC1 (Figure 7). The three mutants apc1-1, apc1-2, and apc1-3 had similar problems in female gametogenesis, such as degradation, aberrant nuclear number, and altered nuclei polarity in the embryo sac, as well as embryogenesis [57]. Arabidopsis ortholog NOP10 of Bra036475 affects megaspore mitosis and polar nuclear fusion in female gametophyte development (Figure 7) [58]. Bra007730, Bra040432, BniB007136 and Bol045717 are orthologous to RanGAP1 (Ran GTPase Activating Protein 1), which is essential in sporophytes and may regulate mitotic cell cycle progression of female gametophyte development (Figure 7) [59]. Arabidopsis ortholog TUBG1, γ-tubulin gene, of Bra007622 is involved in the position of the nuclei (Figure 7) [60]. Bra025912, Bra031027, BniB047288, Bol009730 and Bol030782 are Arabidopsis ortholog of NACK1 (NPK1-ACTIVATING KINESIN 1), which is necessary for cellularization and nuclear positioning during female gametophyte development (Figure 7) [61]. In addition, some genes play an important role in cell specification and development. BniB000251 is orthologous to NFD1 (Nuclear fusion defective1), encoding the Arabidopsis RPL21M protein, which regulates karyogamy in female gametophyte development (Figure 7) [62]. The Arabidopsis ortholog AMP1 (Altered meristem program 1) of Bra014794 and BniB007415 has been shown to play an essential role in synergid cell fate for megagametogenesis (Figure 7) [63].
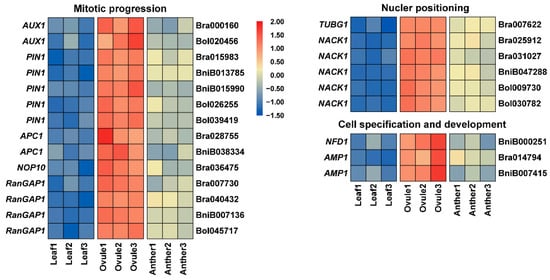
Figure 7.
The expression patterns of mitotic progression, nuclear positioning and cell specification and development related genes in Anther, Ovule and Leaf of Brassica allohexaploids.
2.6. Validation of Expression Profiling by Real-Time Quantitative PCR (RT-qPCR)
We further tested the reliability of RNA-Seq results by RT-qPCR. The randomly selected male and female gamete development-related genes BniB000031 (SPO22), Bra035838 (RMI1), Bol022020 (RECQ4A), Bra000160 (AUX1), Bol039419 (PIN1), BniB025753 (AMS), Bra025337 (TDF1), Bol034656 (LAP5) and BniB031910 (TKPR2) were verified by RT-qPCR. The results showed that the selected genes were positive by RT-qPCR (Figure 8). The expression patterns showed the same trend as that detected by RNA-Seq analysis (Figure 8).
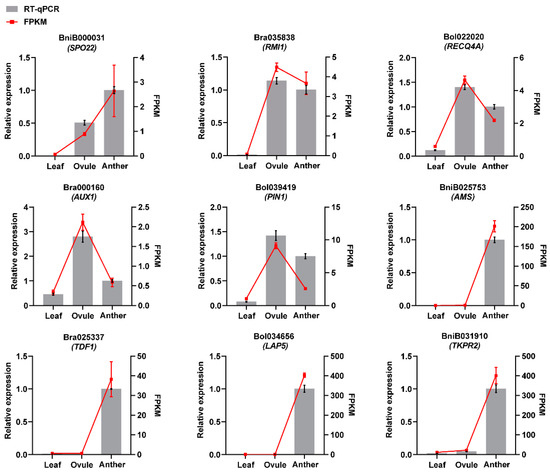
Figure 8.
RT-qPCR analysis of nine randomly selected genes. RT-qPCR results are shown by grey columns, and RNA-Seq findings are represented by red lines.
3. Discussion
3.1. Meiosis-Related Genes May Affect Homologous Recombination during Male and Female Gamete Development in Brassica Allohexaploids
The key events in both the male and female gamete development of Brassica allohexaploids mainly focused on meiosis-related genes and pathways. Polyploidy species may be used more as model organisms for meiosis in the future, and Brassica has attracted attention as model of allopolyploid meiotic regulation mechanisms [64]. Meiosis leads to the production of genetically unique haploid spores, which contribute to genome stability and genetic diversity. Crossover of homologous recombination ensures faithful segregation of homologous chromosomes in meiosis I [65]. Meiotic CO produces new combinations of alleles, increasing the genetic diversity of gametes [66]. Meiosis forms DNA DSB through programming, processes and repairs DSB by homologous recombination. In all eukaryotes, SPO11-1 is a strict requirement for meiosis DSB formation. In Arabidopsis and Brassica, meiotic DSB formation is required for synapsis and SC formation. Crossover or non-crossover recombination products can be isolated and genetically tested by recombination intermediates formed between homologous chromosomes. Consequently, we hypothesized that meiosis-related genes may affect homologous recombination during male and female gamete development in Brassica allohexaploids.
3.2. TDF1, AMS and MS188 May Influence Tapetum Development and Pollen Wall Formation in Brassica Allohexaploids
Male gamete development affects the effective pollination control system, which is the premise of utilizing heterosis of Brassica allohexaploids. The development of male gametes is accompanied by tapetal development, sporopollenin synthesis and pollen wall formation. Tapetum is the innermost layer of the four anther somatic cell layers, and provides material for pollen development. The main component of the exine is sporopollenin, which is produced by the tapetum. The tapetal development regulatory network during male gamete development has been studied in Arabidopsis, in which TDF1 may be involved in redox and cell degradation [67,68,69]. AMS is thought to control lipid transfer proteins in pollen wall building, repressing of upstream regulators and promoting of AMS protein degradation [44,67]. Most cell wall–related genes are regulated by transcription factor MS188, which is involved in both tapetum cell wall degradation and pollen wall synthesis [67]. TDF1 controls AMS directly through an AACCT cis-element, and TDF1 and AMS control downstream genes in a feed-forward loop [68]. In addition, MS188 can activate the expression of CYP704B1, ACOS5 and TKPR1, and form a feedforward loop with its direct upstream regulatory factor AMS to activate the sporopollenin biosynthesis pathway and rapidly form pollen wall in Arabidopsis [69]. Therefore, we speculated that these genes in Brassica allohexaploids may also potentially affect tapetum development and pollen wall formation.
3.3. AUX1 and PIN1 May Regulate the Formation of the Seven-Cell Embryo Sac in Brassica Allohexaploids
Female gamete development is rarely studied compared to male gamete development. To enable female gametophyte fertilization, hence plant reproduction, megagametogenesis comprises carefully controlled mitotic divisions, repositioning of nuclei along a polar axis and the acquisition of different identities by individual cells [70]. Auxin levels are known to be controlled by biosynthesis and transport, and it is critical for sporophytic developmental processes. During embryo sac development, localized auxin biosynthesis and import are essential for mitotic divisions, cell growth and patterning [55]. AUX1 and PIN1 influence mitosis progression at one-, two- and four-nucleate stages [55]. The final position of the nuclei foreshadows the cellularization pattern that divides the female gametophyte into seven cells. Furthermore, cellular identity is most likely determined by the location of the nuclei and related cells along the micropylar–chalazal axis. Therefore, these genes involved in mitosis, nuclear localization, cell differentiation and development may be related to the formation of mature seven-cell embryo sac in Brassica allohexaploids.
4. Materials and Methods
4.1. Plant Materials, Tissue Collection and RNA Extraction
A trigenomic Brassica allohexaploid (BBCCAA, 2n = 54) was generated by interspecific hybridization and chromosome doubling between maternal B. carinata (“VI047487”, BBCC, 2n = 34) and paternal B. rapa (“JK66-83”, AA, 2n = 20) in this study. These Brassica allohexaploid materials were grown in greenhouse with a 16-h light/8-h dark cycle at 22 °C. These plants were later chromosomally identified as allohexaploid (2n = 54). Anthers and ovules of these Brassica allohexaploids were collected according to the previous methods reported [25,27]. After flowering, anthers from meiosis to mature pollen stage (Anther) and ovules from meiosis to mature embryo sac stage (Ovule) were collected, and young leaves (Leaf) of main inflorescences were also collected as vegetative tissue (organ) control. Three biological replicates were taken from each tissue of Brassica allohexaploids. These samples were rapidly frozen in liquid nitrogen and stored at −80 °C to extract RNA. The purity of the samples was determined by NanoPhotometer® (IMPLEN, Westlake Village, CA, USA). The concentration and integrity of RNA samples were detected using Agilent 2100 RNA nano 6000 detection kit (Agilent Technology, Santa Clara, CA, USA).
4.2. cDNA Library Construction, Filter and Alignment
A total amount of 1–3 μg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using VAHTS Universal V6 RNA-Seq Library Prep Kit for Illumina ®. In order to guarantee the data quality used to analysis, the useful Perl script was used to filter the original data (Raw Data). The reference genomes and the annotation file were downloaded from B. rapa genome v1.5 sequence, B. nigra genome v1.1 sequence and B. oleracea genome v1.0 sequence (http://Brassicadb.cn, accessed on 1 May 2021). Bowtie2 v2.2.3 was used for building the genome index, and Clean Data was then aligned to the reference genomes using HISAT2 v2.1.0 [71]. The RNA-Seq data was uploaded to the NCBI Gene Expression Omnibus (GEO), and its accession number, GSE201456, may be used to retrieve it.
4.3. FPKM and DEGs
Reads count for each gene in each sample was counted by HTSeq v0.6.0 (Simon Anders, Heidelberg, Germany), and FPKM was then calculated to estimate the expression level of genes in each sample. DESeq2 estimated the expression level of each gene in per sample by the linear regression, then calculated the p-value with Wald test [72]. The p-value was corrected by the BH method. Genes with fold change ≥ 2 and q-value (adjusted p-value) ≤ 0.05 were identified as DEGs.
4.4. Function Enrichment Analysis
The DEGs aligned to COG were classified according to functions of genes (http://www.ncbi.nlm.nih.gov/COG/, accessed on 7 August 2021). The GO enrichment of DEGs was implemented by the hypergeometric test, in which p-value was calculated and adjusted as q-value, and data background was genes in the whole genome (http://geneontology.org/, accessed on 27 August 2021). GO terms with q-value < 0.05 were considered to be significantly enriched. GO enrichment analysis could exhibit the biological functions of the DEGs. The information about Arabidopsis ortholog of Brassica was obtained from Brassicaceae Database (BRAD) (http://Brassicadb.cn, accessed on 1 May 2021).
4.5. RT-qPCR
In order to verify the accuracy of RNA-Seq, nine candidate genes were randomly selected for RT-qPCR. The cDNA used the same RNA samples of the RNA-Seq. The RT-qPCR analysis was performed using the SYBR Green I. To standardize the results, BrGAPDH was employed as an internal reference control. Three biological replicates were used for each sample. Primer Premier 5.0 was used to design gene-specific primers for nine genes and these primer sequences were listed in Table S1. The CT values (the fractional cycle number at which the fluorescence crosses the specified threshold) were generated using CFX manager software and evaluated using the 2−ΔΔCt method [73].
5. Conclusions
The transcriptome profiling of anthers and ovules revealed the candidate genes of homologous recombination in male and female meiosis, the genes of tapetum development, sporopollenin biosynthesis and pollen wall formation in male gamete development and the genes of mitosis, nuclear localization, cell differentiation and development in female gamete development of Brassica allohexaploids. Our findings enhance the understanding of the genes involved in the male and female gamete development of Brassica allohexaploids and lay a foundation for the reproductive study of polyploids.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11121556/s1, Figure S1: Correlation heat map of the nine samples of Brassica allohexaploids using Pearson correlation coefficients; Figure S2: Volcano plots of differentially expressed genes (DEGs) for three comparisons in Brassica allohexaploids; Table S1: FPKM of all genes in Anther, Ovule and Leaf of Brassica allohexaploids; Table S2: Statistics of differentially expressed genes (DEGs) in Anther, Ovule and Leaf by pairwise comparison in Brassica allohexaploids; Table S3: List of primer sequences for quantitative fluorescence verification involving selected genes and internal reference.
Author Contributions
Conceptualization, C.J. and F.W.; data curation, C.J. and Z.T.; formal analysis, Y.L. and W.C.; funding acquisition, B.T.; investigation, C.J.; methodology, C.J.; project administration, F.W.; resources, X.W.; software, C.J. and G.S.; supervision, C.J.; validation, X.H.; visualization, C.J., Z.X. and N.L.; writing—original draft, C.J.; writing—review and editing, C.J., F.W. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Henan Provincial Natural Science Foundation of China (No. 202300410366) and the Program for Science and Technology Innovation Talents in Universities of Henan Province (No. 19HASTIT014).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The RNA-Seq data were deposited into NCBI GEO and can be accessed with the accession number GSE201456.
Acknowledgments
We would like to express our thanks to the anonymous reviewers for their useful comments.
Conflicts of Interest
The authors declared no conflict of interest.
References
- Jiao, Y.; Wickett, N.J.; Ayyampalayam, S.; Chanderbali, A.S.; Landherr, L.; Ralph, P.E.; Tomsho, L.P.; Hu, Y.; Liang, H.; Soltis, P.S.; et al. Ancestral polyploidy in seed plants and angiosperms. Nature 2011, 473, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Soltis, D.E.; Albert, V.A.; Leebens-Mack, J.; Bell, C.D.; Paterson, A.H.; Zheng, C.F.; Sankoff, D.; de Pamphilis, C.W.; Wall, P.K.; Soltis, P.S. Polyploidy and Angiosperm Diversification. Am. J. Bot. 2009, 96, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Soltis, P.S.; Soltis, D.E. Ancient WGD events as drivers of key innovations in angiosperms. Curr. Opin. Plant Biol. 2016, 30, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Leitch, A.R.; Leitch, I.J. Genomic plasticity and the diversity of polyploid plants. Science 2008, 320, 481–483. [Google Scholar] [CrossRef]
- Soltis, P.S.; Soltis, D.E. The role of genetic and genomic attributes in the success of polyploids. Proc. Natl. Acad. Sci. USA 2000, 97, 7051–7057. [Google Scholar] [CrossRef]
- Ni, Z.F.; Kim, E.D.; Ha, M.S.; Lackey, E.; Liu, J.X.; Zhang, Y.R.; Sun, Q.X.; Chen, Z.J. Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids. Nature 2009, 457, 327–332. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef]
- Chen, Z.J. Molecular mechanisms of polyploidy and hybrid vigor. Trends Plant Sci. 2010, 15, 57–71. [Google Scholar] [CrossRef]
- Chen, S.; Nelson, M.N.; Chevre, A.M.; Jenczewski, E.; Li, Z.Y.; Mason, A.S.; Meng, J.L.; Plummer, J.A.; Pradhan, A.; Siddique, K.H.M.; et al. Trigenomic Bridges for Brassica Improvement. Crit. Rev. Plant Sci. 2011, 30, 524–547. [Google Scholar] [CrossRef]
- Gaebelein, R.; Mason, A.S. Allohexaploids in the Genus Brassica. Crit. Rev. Plant Sci. 2018, 37, 422–437. [Google Scholar] [CrossRef]
- Zou, J.; Zhu, J.L.; Huang, S.M.; Tian, E.T.; Xiao, Y.; Fu, D.H.; Tu, J.X.; Fu, T.D.; Meng, J.L. Broadening the avenue of intersubgenomic heterosis in oilseed Brassica. Theor. Appl. Genet. 2010, 120, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.N.; Mason, A.S.; Farooq, M.A.; Islam, F.; Quezada-Martinez, D.; Hu, D.D.; Yang, S.; Zou, J.; Zhou, W.J. Challenges and prospects for a potential allohexaploid Brassica crop. Theor. Appl. Genet. 2021, 134, 2711–2726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zou, J.; Meng, J.; Mei, S.; Wang, J. Tracing the transcriptomic changes in synthetic Trigenomic allohexaploids of Brassica using an RNA-Seq approach. PLoS ONE 2013, 8, e68883. [Google Scholar] [CrossRef]
- Gaebelein, R.; Schiessl, S.V.; Samans, B.; Batley, J.; Mason, A.S. Inherited allelic variants and novel karyotype changes influence fertility and genome stability in Brassica allohexaploids. New Phytol. 2019, 223, 965–978. [Google Scholar] [CrossRef]
- Zhou, J.; Tan, C.; Cui, C.; Ge, X.; Li, Z. Distinct subgenome stabilities in synthesized Brassica allohexaploids. Theor. Appl. Genet. 2016, 129, 1257–1271. [Google Scholar] [CrossRef]
- Ma, H.; Sundaresan, V. Development of flowering plant gametophytes. Curr. Top. Dev. Biol. 2010, 91, 379–412. [Google Scholar] [CrossRef]
- Hafidh, S.; Fila, J.; Honys, D. Male gametophyte development and function in angiosperms: A general concept. Plant Reprod. 2016, 29, 31–51. [Google Scholar] [CrossRef]
- Yadegari, R.; Drews, G.N. Female gametophyte development. Plant Cell 2004, 16, S133–S141. [Google Scholar] [CrossRef]
- Mccormick, S. Male Gametophyte Development. Plant Cell 1993, 5, 1265–1275. [Google Scholar] [CrossRef]
- Yang, W.C.; Shi, D.Q.; Chen, Y.H. Female gametophyte development in flowering plants. Annu. Rev. Plant Biol. 2010, 61, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmi, U.; Waern, K.; Snyder, M. RNA-Seq: A method for comprehensive transcriptome analysis. Curr. Protoc. Mol. Biol. 2010, 4, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yu, L.; Wei, L.; Yu, P.; Wang, J.; Zhao, H.; Zhang, Y.; Zhang, S.; Yang, Z.; Chen, G.; et al. BnTIR: An online transcriptome platform for exploring RNA-seq libraries for oil crop Brassica napus. Plant Biotechnol. J. 2021, 19, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Zhang, P.P.; Lv, J.Y.; Cheng, Y.F.; Cui, J.M.; Zhao, H.X.; Hu, S.W. Global Dynamic Transcriptome Programming of Rapeseed (Brassica napus L.) Anther at Different Development Stages. PLoS ONE 2016, 11, e0154039. [Google Scholar] [CrossRef] [PubMed]
- Braynen, J.; Yang, Y.; Yuan, J.C.; Xie, Z.Q.; Cao, G.Q.; Wei, X.C.; Shi, G.Y.; Zhang, X.W.; Wei, F.; Tian, B.M. Comparative transcriptome analysis revealed differential gene expression in multiple signaling pathways at flowering in polyploid Brassica rapa. Cell Biosci. 2021, 11, 17. [Google Scholar] [CrossRef]
- Zhao, L.; He, J.; Cai, H.; Lin, H.; Li, Y.; Liu, R.; Yang, Z.; Qin, Y. Comparative expression profiling reveals gene functions in female meiosis and gametophyte development in Arabidopsis. Plant J. 2014, 80, 615–628. [Google Scholar] [CrossRef]
- Grelon, M.; Vezon, D.; Gendrot, G.; Pelletier, G. AtSPO11-1 is necessary for efficient meiotic recombination in plants. EMBO J. 2001, 20, 589–600. [Google Scholar] [CrossRef]
- De Muyt, A.; Vezon, D.; Gendrot, G.; Gallois, J.L.; Stevens, R.; Grelon, M. AtPRD1 is required for meiotic double strand break formation in Arabidopsis thaliana. EMBO J. 2007, 26, 4126–4137. [Google Scholar] [CrossRef]
- De Muyt, A.; Pereira, L.; Vezon, D.; Chelysheva, L.; Gendrot, G.; Chambon, A.; Laine-Choinard, S.; Pelletier, G.; Mercier, R.; Nogue, F.; et al. A high throughput genetic screen identifies new early meiotic recombination functions in Arabidopsis thaliana. PLoS Genet. 2009, 5, e1000654. [Google Scholar] [CrossRef]
- Vrielynck, N.; Chambon, A.; Vezon, D.; Pereira, L.; Chelysheva, L.; De Muyt, A.; Mezard, C.; Mayer, C.; Grelon, M. A DNA topoisomerase VI-like complex initiates meiotic recombination. Science 2016, 351, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.J.; Caryl, A.P.; Jones, G.H.; Franklin, F.C. Asy1, a protein required for meiotic chromosome synapsis, localizes to axis-associated chromatin in Arabidopsis and Brassica. J. Cell Sci. 2002, 115, 3645–3655. [Google Scholar] [CrossRef] [PubMed]
- Cuacos, M.; Lambing, C.; Pachon-Penalba, M.; Osman, K.; Armstrong, S.J.; Henderson, I.R.; Sanchez-Moran, E.; Franklin, F.C.H.; Heckmann, S. Meiotic chromosome axis remodelling is critical for meiotic recombination in Brassica rapa. J. Exp. Bot. 2021, 72, 3012–3027. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, M.; Higgins, J.D.; Osman, K.; Lambing, C.; Roitinger, E.; Mechtler, K.; Armstrong, S.J.; Perry, R.; Pradillo, M.; Cunado, N.; et al. Inter-Homolog Crossing-Over and Synapsis in Arabidopsis Meiosis Are Dependent on the Chromosome Axis Protein AtASY3. PLoS Genet. 2012, 8, e1002507. [Google Scholar] [CrossRef]
- Higgins, J.D.; Sanchez-Moran, E.; Armstrong, S.J.; Jones, G.H.; Franklin, F.C.H. The Arabidopsis synaptonemal complex protein ZYP1 is required for chromosome synapsis and normal fidelity of crossing over. Genes Dev. 2005, 19, 2488–2500. [Google Scholar] [CrossRef]
- Chelysheva, L.; Vezon, D.; Chambon, A.; Gendrot, G.; Pereira, L.; Lemhemdi, A.; Vrielynck, N.; Le Guin, S.; Novatchkova, M.; Grelon, M. The Arabidopsis HEI10 Is a New ZMM Protein Related to Zip3. PLoS Genet. 2012, 8, e1002799. [Google Scholar] [CrossRef]
- Macaisne, N.; Novatchkova, M.; Peirera, L.; Vezon, D.; Jolivet, S.; Froger, N.; Chelysheva, L.; Grelon, M.; Mercier, R. SHOC1, an XPF endonuclease-related protein, is essential for the formation of class I meiotic crossovers. Curr. Biol. 2008, 18, 1432–1437. [Google Scholar] [CrossRef]
- Chelysheva, L.; Gendrot, G.; Vezon, D.; Doutriaux, M.P.; Mercier, R.; Grelon, M. Zip4/Spo22 is required for class I CO formation but not for synapsis completion in Arabidopsis thaliana. PLoS Genet. 2007, 3, e83. [Google Scholar] [CrossRef]
- Higgins, J.D.; Vignard, J.; Mercier, R.; Pugh, A.G.; Franklin, F.C.; Jones, G.H. AtMSH5 partners AtMSH4 in the class I meiotic crossover pathway in Arabidopsis thaliana, but is not required for synapsis. Plant J. 2008, 55, 28–39. [Google Scholar] [CrossRef]
- Chelysheva, L.; Vezon, D.; Belcram, K.; Gendrot, G.; Grelon, M. The Arabidopsis BLAP75/Rmi1 Homologue Plays Crucial Roles in Meiotic Double-Strand Break Repair. PLoS Genet. 2008, 4, e1000309. [Google Scholar] [CrossRef][Green Version]
- Hartung, F.; Suer, S.; Knoll, A.; Wurz-Wildersinn, R.; Puchta, H. Topoisomerase 3alpha and RMI1 suppress somatic crossovers and are essential for resolution of meiotic recombination intermediates in Arabidopsis thaliana. PLoS Genet. 2008, 4, e1000285. [Google Scholar] [CrossRef] [PubMed]
- Knoll, A.; Higgins, J.D.; Seeliger, K.; Reha, S.J.; Dangel, N.J.; Bauknecht, M.; Schropfer, S.; Franklin, F.C.H.; Puchta, H. The Fanconi Anemia Ortholog FANCM Ensures Ordered Homologous Recombination in Both Somatic and Meiotic Cells in Arabidopsis. Plant Cell 2012, 24, 1448–1464. [Google Scholar] [CrossRef] [PubMed]
- Hartung, F.; Suer, S.; Puchta, H. Two closely related RecQ helicases have antagonistic roles in homologous recombination and DNA repair in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 18836–18841. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.C.; Pearce, S.; Band, L.R.; Yang, C.Y.; Ferjentsikova, I.; King, J.; Yuan, Z.; Zhang, D.B.; Wilson, Z.A. Biphasic regulation of the transcription factor ABORTED MICROSPORES (AMS) is essential for tapetum and pollen development in Arabidopsis. New Phytol. 2017, 213, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.A.; Li, S.F.; Parish, R.W. MYB80, a regulator of tapetal and pollen development, is functionally conserved in crops. Plant Mol. Biol. 2012, 78, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, H.; Li, H.; Gao, J.F.; Jiang, H.; Wang, C.; Guan, Y.F.; Yang, Z.N. Defective in Tapetal Development and Function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J. 2008, 55, 266–277. [Google Scholar] [CrossRef]
- Morant, M.; Jorgensen, K.; Schaller, H.; Pinot, F.; Moller, B.L.; Werck-Reichhart, D.; Bak, S. CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen. Plant Cell 2007, 19, 1473–1487. [Google Scholar] [CrossRef]
- Dobritsa, A.A.; Shrestha, J.; Morant, M.; Pinot, F.; Matsuno, M.; Swanson, R.; Moller, B.L.; Preuss, D. CYP704B1 Is a Long-Chain Fatty Acid omega-Hydroxylase Essential for Sporopollenin Synthesis in Pollen of Arabidopsis. Plant Physiol. 2009, 151, 574–589. [Google Scholar] [CrossRef]
- Souza, C.D.; Kim, S.S.; Koch, S.; Kienow, L.; Schneider, K.; McKim, S.M.; Haughn, G.; Kombrink, E.; Douglas, C.J. A Novel Fatty Acyl-CoA Synthetase Is Required for Pollen Development and Sporopollenin Biosynthesis in Arabidopsis. Plant Cell 2009, 21, 507–525. [Google Scholar] [CrossRef]
- Kim, S.S.; Grienenberger, E.; Lallemand, B.; Colpitts, C.C.; Kim, S.Y.; Souza, C.D.; Geoffroy, P.; Heintz, D.; Krahn, D.; Kaiser, M.; et al. LAP6/POLYKETIDE SYNTHASE A and LAP5/POLYKETIDE SYNTHASE B Encode Hydroxyalkyl alpha-Pyrone Synthases Required for Pollen Development and Sporopollenin Biosynthesis in Arabidopsis thaliana. Plant Cell 2010, 22, 4045–4066. [Google Scholar] [CrossRef]
- Grienenberger, E.; Kim, S.S.; Lallemand, B.; Geoffroy, P.; Heintz, D.; Souza, C.D.; Heitz, T.; Douglas, C.J.; Legrand, M. Analysis of TETRAKETIDE alpha-PYRONE REDUCTASE Function in Arabidopsis thaliana Reveals a Previously Unknown, but Conserved, Biochemical Pathway in Sporopollenin Monomer Biosynthesis. Plant Cell 2010, 22, 4067–4083. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.K.; Chu, H.; Yip, W.K.; Yeung, E.C.; Lo, C. An anther-specific dihydroflavonol 4-reductase-like gene (DRL1) is essential for male fertility in Arabidopsis. New Phytol. 2009, 181, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Yu, X.H.; Zhang, K.S.; Shi, J.X.; De Oliveira, S.; Schreiber, L.; Shanklin, J.; Zhang, D.B. Male Sterile2 Encodes a Plastid-Localized Fatty Acyl Carrier Protein Reductase Required for Pollen Exine Development in Arabidopsis. Plant Physiol. 2011, 157, 842–853. [Google Scholar] [CrossRef]
- Quilichini, T.D.; Friedmann, M.C.; Samuels, A.L.; Douglas, C.J. ATP-Binding Cassette Transporter G26 Is Required for Male Fertility and Pollen Exine Formation in Arabidopsis. Plant Physiol. 2010, 154, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Panoli, A.; Martin, M.V.; Alandete-Saez, M.; Simon, M.; Neff, C.; Swarup, R.; Bellido, A.; Yuan, L.; Pagnussat, G.C.; Sundaresan, V. Auxin Import and Local Auxin Biosynthesis Are Required for Mitotic Divisions, Cell Expansion and Cell Specification during Female Gametophyte Development in Arabidopsis thaliana. PLoS ONE 2015, 10, e0126164. [Google Scholar] [CrossRef] [PubMed]
- Ceccato, L.; Masiero, S.; Roy, D.S.; Bencivenga, S.; Roig-Villanova, I.; Ditengou, F.A.; Palme, K.; Simon, R.; Colombo, L. Maternal Control of PIN1 Is Required for Female Gametophyte Development in Arabidopsis. PLoS ONE 2013, 8, e66148. [Google Scholar] [CrossRef]
- Wang, Y.B.; Hou, Y.N.; Gu, H.Y.; Kang, D.M.; Chen, Z.L.; Liu, J.J.; Qu, L.J. The Arabidopsis Anaphase-Promoting Complex/Cyclosome Subunit 1 is Critical for Both Female Gametogenesis and Embryogenesis. J. Integr. Plant Biol. 2013, 55, 64–74. [Google Scholar] [CrossRef]
- Li, L.X.; Liao, H.Z.; Jiang, L.X.; Tan, Q.; Ye, D.; Zhang, X.Q. Arabidopsis thaliana NOP10 is required for gametophyte formation. J. Integr. Plant Biol. 2018, 60, 723–736. [Google Scholar] [CrossRef]
- Rodrigo-Peiris, T.; Xu, X.M.; Zhao, Q.; Wang, H.J.; Meier, I. RanGAP is required for post-meiotic mitosis in female gametophyte development in Arabidopsis thaliana. J. Exp. Bot. 2011, 62, 2705–2714. [Google Scholar] [CrossRef]
- Pastuglia, M.; Azimzadeh, J.; Goussot, M.; Camilleri, C.; Belcram, K.; Evrard, J.L.; Schmit, A.C.; Guerche, P.; Bouchez, D. gamma-tubulin is essential for microtubule organization and development in Arabidopsis. Plant Cell 2006, 18, 1412–1425. [Google Scholar] [CrossRef]
- Tanaka, H.; Ishikawa, M.; Kitamura, S.; Takahashi, Y.; Soyano, T.; Machida, C.; Machida, Y. The AtNACK1/HINKEL and STUD/TETRASPORE/AtNACK2 genes, which encode functionally redundant kinesins, are essential for cytokinesis in Arabidopsis. Genes Cells 2004, 9, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Portereiko, M.F.; Sandaklie-Nikolova, L.; Lloyd, A.; Dever, C.A.; Otsuga, D.; Drews, G.N. Nuclear fusion defective1 encodes the Arabidopsis RPL21M protein and is required for karyogamy during female gametophyte development and fertilization. Plant Physiol. 2006, 141, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Lau, S.; Jurgens, G. Twin plants from supernumerary egg cells in Arabidopsis. Curr. Biol. 2015, 25, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, M.; Eber, F.; Lucas, M.O.; Lode, M.; Chevre, A.M.; Jenczewski, E. Repeated Polyploidy Drove Different Levels of Crossover Suppression between Homoeologous Chromosomes in Brassica napus Allohaploids. Plant Cell 2010, 22, 2265–2276. [Google Scholar] [CrossRef]
- Page, S.L.; Hawley, R.S. Chromosome choreography: The meiotic ballet. Science 2003, 301, 785–789. [Google Scholar] [CrossRef]
- Youds, J.L.; Boulton, S.J. The choice in meiosis—Defining the factors that influence crossover or non-crossover formation. J. Cell Sci. 2011, 124, 501–513. [Google Scholar] [CrossRef]
- Li, D.D.; Xue, J.S.; Zhu, J.; Yang, Z.N. Gene Regulatory Network for Tapetum Development in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1559. [Google Scholar] [CrossRef]
- Lou, Y.; Zhou, H.S.; Han, Y.; Zeng, Q.Y.; Zhu, J.; Yang, Z.N. Positive regulation of AMS by TDF1 and the formation of a TDF1-AMS complex are required for anther development in Arabidopsis thaliana. New Phytol. 2018, 217, 378–391. [Google Scholar] [CrossRef]
- Wang, K.; Guo, Z.L.; Zhou, W.T.; Zhang, C.; Zhang, Z.Y.; Lou, Y.; Xiong, S.X.; Yao, X.Z.; Fan, J.J.; Zhu, J.; et al. The Regulation of Sporopollenin Biosynthesis Genes for Rapid Pollen Wall Formation. Plant Physiol. 2018, 178, 283–294. [Google Scholar] [CrossRef]
- Serbes, I.E.; Palovaara, J.; Gross-Hardt, R. Development and function of the flowering plant female gametophyte. Plant Dev. Evol. 2019, 131, 401. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).