Abstract
Oral health is an essential element in maintaining general well-being. By preserving the complex equilibrium within the oral microbial community, commensal microorganisms can protect against extrinsic pathogenic threats. However, when an imbalance occurs, the organism is susceptible to a broad range of infections. Synthetic drugs can be administered to help the body fight against the fungal, bacterial, or viral burden. Nonetheless, they may produce undesirable consequences such as toxicity, adverse effects, and drug resistance. In this respect, research has focused on finding safer and more efficient alternatives. Particularly, increasing attention has been drawn towards developing novel formulations based on natural compounds. This paper reviews the plant-based, algae-based, and beehive products investigated for their antimicrobial properties, aiming to thoroughly present the state of the art on oral infection prevention in the ear, nose, and throat (ENT) field.
1. Introduction
The oral microbiome is an essential component of the human microbial community, playing a vital role in protecting against the colonization of extrinsic microbes, which can affect overall health. In addition, the oral microbiome is associated with systemic diseases, as the mouth represents an entry point to both the respiratory and digestive systems, which are also highly vascularized [1,2,3].
An imbalance in the complex equilibrium between the various microorganisms from the healthy oral cavity is intimately connected to the pathogenesis and development of numerous oral and systemic diseases [2,4,5]. Furthermore, factors such as poor oral hygiene, trauma, malnutrition, use of antibiotics, wear of dentures, underlying medical conditions, and radio- or chemotherapy also contribute to the occurrence of oral infection [4,6,7].
Specific pathogens can overgrow and spread in the oral mucosae, extend to surrounding tissues, and, if left untreated, produce systemic infections [6,8]. To avoid such a cascade of events, preventing oral infections has become an intense research topic. Preventive medicine is mostly focused on reducing oral infections and their associated complications via good oral hygiene [6,9]. In this respect, mouth rinses and toothpaste usually contain active ingredients like chlorhexidine, hyaluronic acid, and fluorides, which, although effective, may present some clinical disadvantages (e.g., taste alterations, mouth dryness, tooth discoloration, calculus accumulation, mucosal lesions) [9]. What is more, the use of synthetic chemical antimicrobials has been shown to produce drug-resistant microorganism strains which are no longer affected by conventional treatments. Hence, there emerged the need to develop novel treatment options [10,11].
To overcome the issues associated with chemical products, natural compounds have attracted interest in preventing and treating oral infections [5,10,11,12]. In this respect, a broad range of natural sources has been investigated for their pharmacological properties to find better solutions for bacterial, fungal, and viral oral infections [13,14,15].
This review comprehensively presents the oral microbiome composition, its potential imbalances, and conventional treatments for the most frequent oral infections. A special focus is further given to natural antimicrobial compounds that are systematically described from the points of view of their sources and potential applications.
2. Oral Microbiome Composition
The human oral cavity is colonized by diverse microbial flora, mainly comprised of bacteria, fungi, and viruses [16] (Figure 1).
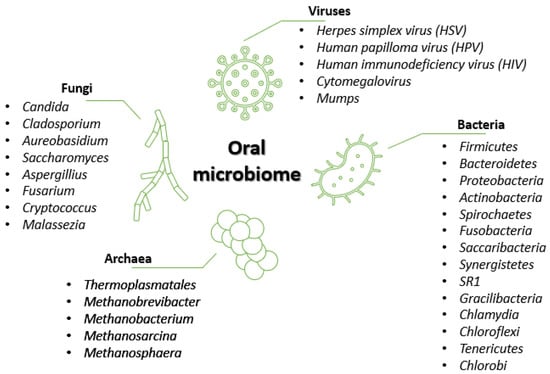
Figure 1.
Oral microbiome composition. Created based on information from references in the literature [2,4,5,16,17].
Bacteria represent the predominant microorganism type, with the human oral cavity containing more than 500 different species [2,18]. According to the literature [2,4,18], 95% of the oral bacterial community belongs to 6 major phyla, namely, Firmicutes, Proteobacteria, Actinobacteria, Bacteroidetes, Fusobacteria, and Spirochaetes. The remaining 5% of the taxa comprise microorganisms from phyla like Saccharibacteria, Synergistetes, SR1, Gracilibacteria, Chlamydia, Chloroflexi, Tenericutes, and Chlorobi [2]. Some genera, such as Streptococcus, Gemella, Granulicatella, Veillonella, and Fusobacterium, inhabit almost all oral sub-niches, whereas other genera, such as Prevotella, Bacteroides, Corynebacterium, Pasteurella, and Neisseria, have been found in selected sites [4].
Despite being studied to a lesser degree as compared to bacteria, fungi are widely present in the oral cavity. They are usually reported as opportunistic pathogens in immunocompromised individuals, but fungal organisms also belong to the healthy oral microbiota, which includes up to 101 fungal species [2]. The most common genus is Candida (with C. albicans as the predominant species), followed by Cladosporium, Aureobasidium, Saccharomyces, Aspergillus, Fusarium, Cryptococcus, and Malassezia [2,4].
Archaea represents a minor part of the oral microflora, restricted to limited species such as Thermoplasmatales, Methanobrevibacter, Methanobacterium, Methanosarcina, and Methanosphaera. The prevalence and numbers of these methanogens are increased in periodontitis patients, but archaea can be found in healthy individuals as well [2].
Several viruses can also be present in the oral cavity, and can be involved in oral ulcers, oral tumors, oral infections, and periodontitis [16]. Except for herpes simplex virus (HSV) and cytomegalovirus, viruses appear to be oral transients, primarily affecting other body structures [19]. Unlike the other components of the oral microbiota, most viruses in the mouth are associated with diseases [2]. Viruses like HSV, human papillomavirus (HPV), human immunodeficiency virus (HIV), cytomegalovirus, and mumps are responsible for lesions inside and around the mouth, salivary gland infection, gingivostomatitis, papilloma, condylomas, focal epithelial hyperplasia, and more [2,16].
3. Oral Infections: Causative Pathogens and Aggravating Potential
As oral health implies the maintenance of a complex microbiotic equilibrium, fluctuations in the availability of oxygen, nutrients, and the pH-mediating effect of saliva can result in the growth of some microorganisms and further cause opportunistic infections [3,4,7,20]. Such changes may occur in immunosuppressed individuals, patients undergoing radiotherapy, chemotherapy, prolonged antimicrobial therapy, and steroid administration, and people suffering from xerostomia, diabetes, or cancer [3,7,20,21,22,23,24,25,26,27,28]. Nonetheless, otherwise healthy individuals may develop oral infections due to a series of risk factors such as smoking, alcohol consumption, poor nutrition, ill-fitting prosthesis, infancy, old age, or pregnancy [12,20,24,26,27,29].
The persistence of pathogens inside the mouth leads to focal infection points, which may further cause secondary health problems such as biofilm formation, difficulty in chewing and swallowing, altered taste sensation, halitosis, systemic malnutrition, and weight loss [28,30].
To particularize the discussion and better understand how such infections may aggravate, several of the most common ENT-related oral infections are further described.
3.1. Candidiasis
One of the most common oral infections, especially amongst HIV-positive individuals and the elderly population, is oral candidiasis [5,20,24,31,32,33]. Also known as “thrush” or “candidosis”, this fungal infection is characterized by Candida spp. overgrowth and invasion into superficial tissue layers, subsequently damaging the oral mucosal surface [4].
Amongst Candida species, Candida albicans is considered the primary causative pathogen of oral candidiasis [5,27,34,35]. This is due to its high capability for adherence to oral tissues and denture surfaces, resulting in biofilm formation [35,36]. Nonetheless, this oral infection may also be caused by non-albicans Candida species such as C. glabrata, C. guillermondii, C. krusei, C. parapsilosis, C. pseudotropicalis, C. stellatoidea, C. tropicalis, C. keyfr, and C. dubliniensis, which are prevalent and important opportunistic pathogens in immunocompromised patients [5,20,35,37,38,39,40,41].
Candidiasis can occur at the level of oropharynx, hypopharynx, and larynx, usually producing severe odynophagia and swallowing difficulties [31]. However, 60% of oral candidiasis has been reported in the oral and pharyngeal region in a population of healthy, ambulatory, and immunocompetent individuals [20], while fungal laryngeal infection is rather uncommon, being more frequently observed in immunocompromised patients or individuals with mechanical, chemical, or thermal injuries to the mucosal barrier [25]. Candidiasis may appear in four different forms [32], as described in Figure 2.
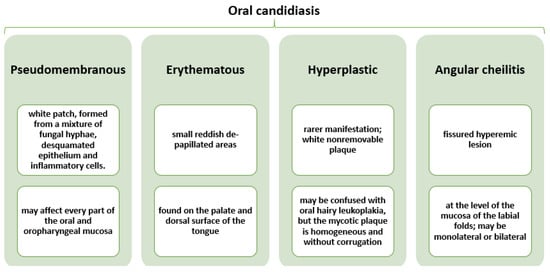
Figure 2.
Characterization of different forms of oral candidiasis. Created based on information from reference [32].
The accumulation of pathogens on the host’s mucous membranes, acrylic surfaces of removable orthodontic devices, and denture prostheses leads to the production of proteolytic enzymes that damage mucosal cells [12]. Thus, a dangerous focus of inflammation is created that increases the risk of cerebral strokes, decompensated glycemia, and focal and autoimmune diseases [27].
To prevent the occurrence of systemic Candida infections, prophylaxis treatment against this fungus may be provided to patients at risk. Nonetheless, this must proceed with care, as in stem cell transplant recipients and hematological malignancies a microbiota imbalance may be shifted towards the overgrowth of Aspergillus and other molds that produce dangerous fungal infections instead [42].
3.2. Aspergillosis
Aspergillosis represents the second most common type of opportunistic fungal infection after candidiasis [20,43,44,45]. As the name suggests, this oral mycosis is caused by Aspergillus spp., with the most frequently identified species being Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, and Aspergillus terreus [20,45].
The main exposure route to this pathogen is through the inhalation of spores that commonly colonize the upper and lower respiratory tracts. Hence, Aspergillus spp. first causes rhinosinusitis and broncho-pulmonary infections that may further spread to the skin, orbits, nose, larynx, and palate [44,45,46]. Tissue invasion is uncommon in immunocompetent individuals, but life-threatening complications can occur in patients with HIV infection, hematological malignancies, diabetes mellitus, or drug-induced immunosuppressive states [20,43,44,46]. This happens because in healthy people acquiring this infection the inhaled fungus is destroyed by macrophages and neutrophils, whereas in immunocompromised patients microorganisms may pass through without being intercepted due to neutropenia or neutrophil dysfunction [44,45].
Invasive aspergillosis remains a highly lethal form of opportunistic mycosis despite available antifungal therapies and improvements in underlying disease management [43]. Therefore, early detection and treatment are crucial in avoiding severe complications [44].
3.3. Herpes
The oral herpes viruses HSV-1 and HSV-2 are very common and infectious DNA viruses that persist in the host organism, reactivating periodically due to stress or immunosuppression [24,47,48]. The viral activity is manifested through the appearance of small, painful ulcerations that are most often located in the mouth, hard palate, gums, and on the lips and skin around the mouth, which can coalesce to form giant herpetic lesions [16,31].
Generally, HSV-1 is associated with orofacial infections, swollen lymph nodes, fever, muscle aches, and encephalitis, while HSV-2 is primarily connected to genital infections [16,24,49]. However, HSV-2 infection may spread to the mouth through oral sex, thus producing oral herpes [16].
One of the most frequently reported clinical manifestations of primary HSV infection is primary herpetic gingivostomatitis (PHGS), which occurs in 25–30% of affected children. The pain associated with PHGS disturbs food and water intake, sleep, physical well-being, and the psychological status of both patients and family [50].
HSV can form persistent, long-term, latent infections in sensory neurons and produce lesions at the entry point of the human body [49]. Oral herpes viruses debilitate patients and affect oral health, also having an important psychological impact [47,50]. These viruses have been reported in connection with diseases like diabetes, cancer, myocardial infarction, and Alzheimer’s disease [24,51]. With increasing seroprevalence rates, HSV infection treatment is challenging, irrespective of the various available drugs. Specifically, long-term treatment with antiviral formulations is associated with toxicities and drug resistance. Therefore, new antiviral therapies must be developed [49].
3.4. Cytomegalovirus
Cytomegalovirus is a member of the Herpesviridae family that can infect many tissues, including salivary glands and deep periodontal pockets [19,52]. Like HPV, cytomegalovirus establishes lifelong latency after the primary infection, manifested through periodical lytic reactivation and viral shedding [53].
This DNA virus can be acquired in two main ways: at mucosal sites through community exposure or by blood-borne transmission [52]. In children, it can cause various conditions such as jaundice, enteritis, central nervous system disturbances, or congenital defects, depending on the age of acquiring the virus. In contrast, adults may present mononucleosis-like symptoms or be completely asymptomatic. Hence, because of the persistent asymptomatic infections it creates, cytomegalovirus can easily be transmitted through the saliva of healthy asymptomatic adult carriers [19].
In immunosuppressed individuals and HIV-positive patients, human cytomegalovirus is a cause of high morbidity and mortality rates. This is due to the small number of available drugs, low potency, poor oral bioavailability, and emergence of drug resistance, which impede the proper treatment of such viral infections [54].
3.5. HPV
HPV is a DNA virus belonging to the Papillomaviridae family, and infects the skin and mucous membranes [55]. It is one of the most common causes of sexually transmitted infections [56]. Due to the changes in sexual habits in recent decades (e.g., a reduction in the age of onset of sexual activity, an increase in the number of partners, and changes in oro-genital sexual habits), the epidemiological features of HPV infections have also changed, leading to the emergence of an otorhinolaryngological pathology that was rarely seen previously [57,58]. Consequently, many epidemiologists have defined HPV as an endemic infection [58]. Most oral HPV infections are latent or subclinical, spontaneously regressing between 1 and 2 years after the virus is acquired [59].
HPV infections are generally asymptomatic, but they can induce benign tumor formation in some people and cause premalignant lesions that may further develop into cancer [55,60,61]. In the oral mucosa, they have been associated with warts, papilloma, focal epithelial hyperplasia, leucoplakia, oral neoplasia, and condyloma [32,55,58] (Figure 3).
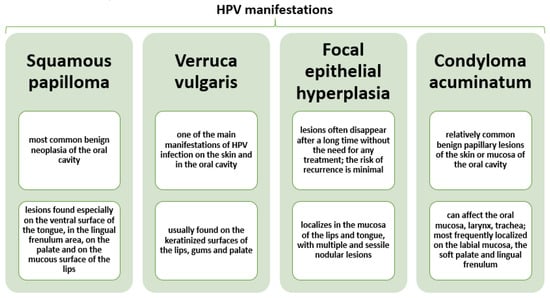
Figure 3.
Characterization of most frequent HPV oral clinical manifestations. Created based on information from reference [32].
A dramatic increase in the incidence of HPV-induced carcinoma [57,62] has been reported. This virus causes around 70% of all oropharyngeal squamous cell cancer in the United States, with an increased incidence among men which has more than doubled in the past 20 years [63]. Hence there is a growing need to focus on HPV oral diseases and develop efficient prevention and treatment methods [58].
3.6. Bacterial Infections
Some of the most common oral pathologies are of bacterial origins, caused by the overgrowth of microorganisms like Streptococcus mutans, Streptococcus salivarius, Streptococcus sanguinis, Streptococcus aureus, Porfiromonas gingivalis, Prevotella intermedia, Actinobacilus actinomycetemcomitans, Enterococcus faecalis, Escherichia coli, Enterobacter spp., Klebsiella spp., and Pseudomonas spp., among others [9,64]. Streptococcus mitis is another type of bacteria in the human mouth that is commonly found in the throat and nasopharynx as a colonized organism. It can cause infection in immunocompromised patients with moderate or severe clinical diseases [33]. The risk for oral infections by opportunistic bacteria is also increased in individuals taking chemotherapeutic drugs, as this may alter the receptor interaction between pathogens and epithelial cells and increase bacterial adhesion, while reducing the salivary secretion rate and oral pH. Local factors such as dentures, implants, piercings, wounds, mucositis, and xerostomia have also been reported to contribute to bacterial infection development [64].
Oral mucosal infections can appear either as localized lesions or as generalized stomatitis, with symptoms ranging from almost unnoticeable discomfort to severe pain. In addition, the treatment of bacterial infections in the oral cavity is difficult due to impaired host defense and antibiotic multi-resistance possessed by these pathogens. Hence, prevention is essential and can only be achieved through strict oral hygiene measures [64].
4. Synthetic Antimicrobial Drugs for the Treatment of Oral Infections
Depending on the type of oral infection, localization, and aggravating status, several treatment options can be employed.
The most conventional and efficient currently available drugs for treating oral candidiasis are polyenes (e.g., amphotericin B, nystatin), azoles (e.g., miconazole, clotrimazole, fluconazole, itraconazole, voriconazole, posaconazole, ketoconazole), and echinocandins (e.g., anidulafungin, caspofungin, micafungin), which can be administered either locally or systemically. Nonetheless, toxicity, adverse effects, and acquired resistance hinder the use of these antifungals [37,65,66,67].
Similar antifungal agents (i.e., amphotericin B, itraconazole, voriconazole, echinocandins) are also considered for treating aspergillosis in patients with normal immune systems. However, their effect in immunocompromised individuals is not very clear. In addition to antifungal therapy, surgical debridement may be involved [43,46].
Concerning HSV infections, the most accepted therapies imply the use of viral DNA replication inhibitors [68]. The drug of choice for this purpose is acyclovir [16,48,68]. Related nucleoside analogs such as valacyclovir, famciclovir, and ganciclovir may also be involved in the prophylaxis and treatment of HSV infections as they have a similar anti-HSV mechanism [48]. Particularly, valaciclovir has been noted to bring several advantages over acyclovir usage, namely greater oral bioavailability, high plasma levels of the parent compound, greater efficacy, and decreased dosing frequency [16]. Prior to acyclovir’s introduction, the first antiviral for systemic administration was vidarabine. This substance lacks specificity and is more toxic and less metabolically stable than acyclovir, but it is still applied for treating acyclovir-resistant HSV strains [16].
The treatment options are also limited against cytomegalovirus infections, for which the currently used antiviral drugs are ganciclovir, cidofovir, and foscarnet. The best results are obtained when administering the antiviral agents as preemptive treatment (when an asymptomatic cytomegalovirus infection is detected by laboratory analysis) because it helps avoid unnecessary drug toxicity and resistance [54].
Regarding bacterial infections, antibiotics represent the main traditional therapy for microbial control [69,70]. However, the efficiency of antibiotics is hindered by the resistance of slime-like biofilms [69]. Other possibilities for combating bacterial biofilms are bacteriophages and quorum-sensing inhibitors [70].
Table 1 summarizes the most used conventional treatments against oral infections as an overview of the above-presented synthetic antimicrobial drugs.

Table 1.
The most commonly used synthetic antimicrobial drugs for treating oral infections.
5. Natural Sources of Antimicrobial Compounds
Therapeutic challenges such as adverse effects, low efficiency, and drug resistance developed by numerous pathogens towards conventional treatments have created a need for the development of novel products [71,72,73]. As an alternative to synthetic drugs, antimicrobial compounds from natural sources have gained increasing attention. Whether used to treat the symptoms, subsequential conditions, or the infection itself, natural compounds are of great importance in dealing with oral infections [5,8,10,11,12,54,68,74,75,76].
There are various mechanisms through which bioactive compounds can exert their antimicrobial activity. These include, but are not limited to, destruction of the cell wall or membrane, hindering microbial DNA replication/repair, inhibiting ribosomal protein synthesis, inducing reactive oxygen species production, inhibiting energy synthesis, inhibiting bacterial toxins to the host, inhibiting biofilm formation, reversing antimicrobial resistance, and synergetic effects with antibiotics [77,78].
In this respect, essential oils, extracts, juices, and pure compounds from various natural sources (Figure 4) have been investigated for their antifungal, antibacterial, antiviral, and antibiofilm activities.

Figure 4.
Examples of natural sources of antimicrobial compounds.
5.1. Plant-Derived Natural Compounds
Cinnamon (Cinnamomum zeylanicum), a widely used culinary ingredient, has also found applications in medicine, being studied during pregnancy, for diabetes control, and for gynecological problems. Features of interest for oral infections, such as anti-inflammatory, antioxidative, and antimicrobial properties, have also been investigated. It was reported that cinnamon essential oils, extracts, and pure compounds have antibacterial and antifungal properties that can be exploited in the development of mouth rinses, toothpaste, or root canal irrigating solutions. However, as the antifungal activity was observed to be more pronounced than the antibacterial potential, cinnamon could serve as the main or complementary agent in treating candidiasis [9]. Particularly, the effects of mouthwash and spray containing cinnamon essential oil on Candida spp have been analyzed. A reduction of 61% and 33% of fungi isolates from oral mucosa and dentures, respectively, was noted, whereas the participants of the study reported a pleasant taste and only a few product-related complaints [79].
Turmeric (Curcuma longa) is an evergreen herb endowed with many pharmacological properties of interest for oral infection therapies. Its chloroform extract contains sesquiterpenes, turpentine, and fatty acids that are linked to overall antibiofilm activity. Notably, sesquiterpenes have the ability to destroy bacterial cell membranes due to their lipophilicity, which affects the growth and metabolism of bacteria [70]. Curcumin, the major constituent of turmeric, is rather investigated for its antifungal properties, as it displays potent activity against C. albicans, Aspergillus spp., Paracoccidioides brasiliensis, and Cryptococcus neoformans. Nonetheless, curcumin was reported to also be effective against bacteria such as Streptococcus pyogenes (at a median MIC of 31.25 µg/mL), methicillin-sensitive S. aureus (250 µg/mL), Acinetobacter lwoffii (250 µg/mL), and individual strains of Enterococcus faecalis (62.5 µg/mL) and Pseudomonas aeruginosa (62.5 µg/mL). Furthermore, curcumin can attain antibiofilm activity by inhibiting bacterial quorum-sensing (QS) systems and removing already existing biofilms. The mechanisms of action against microbial strains include induction of the apoptosis pathways and photodynamic action via production of cytotoxic reactive oxygen species against both planktonic and biofilm forms. Hence, these bioactive compounds are promising constituents of new medications with superior performance and fewer adverse effects [80,81].
Green tea (Camelia sinensis) is an important natural source of multi-purpose antimicrobial phytochemicals [5]. The aqueous extract of green tea can decrease the number of viable fungal cells in biofilms formed on acrylic resin [82]. Specifically, C. sinensis has shown remarkable antifungal activity against Candida spp., for example C. albicans (at an MIC of 0.125 µg/mL), C. parapsilosis (0.125 µg/mL), C. tropicalis (0.125–0.250 µg/mL), and C. glabrata (0.125–0.250 µg/mL) [83]. Anti-infectious properties have also been demonstrated for tannins isolated from C. sinensis extract [84]. In addition, green tea is a valuable source of polyphenols that endow this plant with antioxidant and antiviral properties. Polyphenols can inhibit enzymes that damage cell membranes and prevent the binding and penetration of viruses to cells [85]. Moreover, tea polyphenols have the ability to modify odorant sulfur components, thus abolishing bad breath (halitosis) [5].
Citrus fruits represent a rich source of phytochemicals with many benefits for human health. Possessing numerous therapeutic properties such as anticancer, antiviral, antitumor, antioxidant, and anti-inflammatory activities, citrus fruits have also attracted interest for preventing and treating oral infections [86]. Particularly, limonene, a monocyclic monoterpene found in the rind of citrus fruits, has been shown to have strong anti-biofilm activity against S. mutans (~75% biofilm inhibition at a concentration of 400 µg/mL), when used as a coating on oral implants [87]. Additionally, limonene interferes with the growth of yeast cells, being able to inhibit pathogens such as C. albicans, C. krusei, C. glabrata, and C. parapsilosis [88].
Peppermint (Mentha piperita) is another herbal remedy that finds applications for diverse symptoms and diseases. It has been recognized to have antiseptic, antibacterial, and antifungal properties [89]. Peppermint essential oil was shown to inhibit C. albicans and C. dubliniensis biofilm formation at a concentration of a maximum of 2 μL/mL in a dose-dependent manner. The antifungal effect is induced by the high concentration of menthol, which can enter the fungal cell membrane and disrupt it. Through this action mechanism, peppermint is also efficient against azole-resistant strains [90].
Castor oil plant (Ricinus communis) is an alternative for the creation of antifungal root canal irrigating solutions, mouthwashes, sanitizers, and toothbrushes for complete dentures. Studies compared the effectiveness of castor oil with that of conventional drugs, with the results showing similar potency to miconazole [91]. Another study evaluated and compared the antimicrobial activity of leaf, stem, and root extracts. It was reported that, at a 500 μg/mL concentration, the ethanol extract of the leaves presented antibacterial activity against P. aeruginosa and antifungal activity against C. albicans. The ethanol extract of the roots was effective against P. aeruginosa and C. glabrata, while the ethanol extract of the stems only inhibited P. aeruginosa. The ethyl acetate extract of the leaves had bacteriostatic activity against S. aureus, whereas the hexane extract of the roots exhibited antibacterial effects against B. subtilis [92]. Hence, R. communis may be used for denture stomatitis treatment, improving the clinical status of elderly patients [91].
Pomegranate (Punica granatum) bark extract has an antifungal activity that can also be exploited for treating denture stomatitis. P. granatum also presents antiviral, antioxidant, anti-inflammatory, and anti-carcinogenic properties, which are attractive features in creating pharmaceutical formulations against oral infections [91]. Moreover, pomegranate peel is rich in polyphenols responsible for its broad antimicrobial activity against both Gram-positive and Gram-negative bacteria, including methicillin-resistant S. aureus. Specifically, for the latter-mentioned pathogen, methanol peel extract was seen effective at a concentration as low as 12 μg/mL, with an inhibition zone of 12.5 mm [93].
Basil (Ocimum basilicum) extracts were investigated against Candida spp. adhesion on acrylic surfaces of removable orthodontic appliances. It was reported that two extracts (i.e., ethyl acetate and n-hexane fraction) were able to inhibit the growth, adherence, and formation of C. albicans and C. dubliniensis biofilms in a proportion of 73% and 78%, respectively, in the vicinity of ethyl acetate fractions, and 65% and 78%, respectively, in the vicinity of the n-hexane fraction. Therefore, they can be included in antifungal solutions or mouthwashes that can prevent and treat oral Candida infections [12].
Another natural anti-Candida treatment may be based on coriander (Coriandrum sativum) essential oil. This essential oil showed similar inhibitory activity to nystatin against Candida spp. planktonic cells and C. albicans biofilm (0.125 mg/mL for C. albicans CBS 562 and 1 mg/mL for C. albicans clinical isolate 13A5). Hence, this plant has a promising potential for oral candidiasis prevention and treatment [90,94].
Horsetail (Equisetum giganteum) also exhibits antifungal properties. When added to denture fixative powder, this plant’s hydroethanolic extracts influenced C. albicans biofilm formation on acrylic surfaces, minimizing its colonization and reducing its metabolism [91]. At a concentration of 16 mg/mL, an up to 79% reduction in biofilm cell viability was reported 24 h after treatment [95]. Moreover, horsetail antimicrobial effects were also reported against other pathogens such as Streptococcus pyogenes, Bacillus cereus, Bacillus subtilis, Enterococcus faecalis, Staphylococcus aureus, and Staphylococcus epidermidis [91].
Cranberry (Vaccinium macrocarpon) represents another natural source of interest against oral bacterial species. Cranberry juice can inhibit acid production, attachment, and biofilm formation, and even reverse microbial co-aggregation in the form of high molecular weight non-dialysable material (NDM) [96]. Thus, it is considered a promising preventive measure to include an NDM fraction in toothpastes and mouthwashes to better control oral diseases [97,98]. The most abundant flavonoids extracted from these fruits, proanthocyanidins (PACs), have been shown particular antimicrobial, antiadhesion, anti-inflammatory, and antioxidative properties [99]. A recent study specifically investigated PAC activity against P. aeruginosa. It was reported that PACs extracted from cranberry inhibit biofilm formation by 40.9% and 55.7%, at concentrations of 1 μg/mL and 10 μg/mL, respectively. These flavonoids were also shown to reduce preformed biofilm by 54.1% (p < 0.05) at 10 μg/mL concentration, and by 39.6% at (p < 0.01) at a concentration of 100 μg/mL [100].
Resveratrol is another important compound from cranberry. This polyphenolic antioxidant can be also found in peanuts (Arachis hypogea), blueberries (Vaccinium spp.), Japanese knotweed (Polygonum cuspidatum), and grapevines (Vitis vinifera). Resveratrol demonstrated antimicrobial activity against bacteria and fungi such as B. cereus (at an MIC of 50 μg/mL), S. aureus (100–512 μg/mL), E. faecalis (100–342 μg/mL), M. smegmatis (64 μg/mL), S. pneumoniae (100 μg/mL), S. pyogenes (>200 μg/mL), E. coli (250—512 μg/mL), K. pneumoniae (250–512 μg/mL), P. aeruginosa (200–512 μg/mL), and C. albicans (20–300 μg/mL). Its mechanisms of action against microbial strains include inhibition of ATP hydrolysis and synthesis, DNA fragmentation, and membrane damage due to increased potassium leakage and increased propidium iodide uptake [101].
Antibacterial activity was reported for garlic (Allium sativum) extract as well. Specifically, the active component allicin has been shown to permeate the bacterial membrane, destroy the cell structure, change the gene expression of microorganisms, and react with thiol enzymes to induce oxidative stress [70,102]. As allicin is a volatile compound, researchers had the idea to test its efficiency in the gas phase. It was reported that most of Pseudomonas, Streptococcus, and Staphylococcus isolates were completely inhibited by allicin at a 64 μg/mL concentration [103]. Moreover, antifungal properties were also noted, as a crude extract of 49 μg/mL concentration inhibited the growth of C. albicans [77].
Summer savory (Satureja hortensis) is also of interest for developing pharmaceuticals and natural therapies for infectious diseases. Its essential oil has been tested against 23 bacteria and 15 fungi species, showing great antimicrobial potential. Contrastingly, methanol extracts were not as efficient; only the nonpolar subfraction was reported to have antibacterial activity against five bacterial species, namely Bacillus subtilis (250 μg/mL), Enterococcus faecalis (500 μg/mL), Pseudomonas aeruginosa (250 μg/mL), Salmonella enteritidis (500 μg/mL), and Streptococcus pyogenes (500 μg/mL) [104].
The lavender tree (Heteropyxis natalensis) is traditionally used for oral care. The ethanolic extract of its leaves and twigs was investigated for antimicrobial activity against several oral microorganisms, of which Actinomyces israelii was found to be the most sensitive (at an MIC of 0.88 mg/mL). H. natalensis can also reduce the acid produced by S. mutans and L. paracasei, diminishing the metabolic effects of cavity-causing bacteria while only moderately influencing commensal microorganisms. Hence, this plant’s extract may be used for preventing excessive tissue damage in oral diseases by reducing pro-inflammation [15].
Tasmanian blue gum (Eucalyptus globulus) leaves were also reported to have antibacterial activity against oral bacteria (e.g., P. gingivalis, S. mutans). Including 0.4–0.6% eucalyptus extract in chewing gum significantly contributed to the inhibition of plaque formation, inflammation, and gingiva bleeding [105,106].
Gum Arabic tree (Acacia nilotica) has been used in ancient medicine for treating a broad range of diseases (e.g., abdominal aches, sore throat, dysentery, asthma, diabetes, hypertension). The plant’s twig has also gained attention for dental care due to its phytochemical content; fractions of A. nilotica twig methanol extract presented inhibitory properties against selected oral pathogens (zones of inhibition in the range 14–40 mm). The most potent effect was obtained for E. faecalis, with an MIC of 80 μg/mL. In addition, the identified bioactive compounds (e.g., catechins, catechol, gallic acid, sitosterol, kaempferol, etc.) can be included in herbal toothpaste, endodontic irrigating solutions, mouth fresheners, mouthwashes, and dental gels for maintaining healthy oral microflora [30].
Baikal skullcap (Scutellaria baicalensis) has attracted interest for various therapeutic purposes. Baicalein, the most important compound from this plant’s root extract, has antimicrobial, antioxidant, anticancer, and anti-inflammatory activities, which can be applied for treating several diseases. In addition, these naturally occurring anti-biofilm compounds are considered promising for novel strategies in combating pathogenic bacteria and treating biofilm-associated infections [11]. For instance, over 70% inhibition of C. albicans biofilms was registered for concentrations between 4 and 32 μg/mL [107].
Another natural source of antimicrobial, antiviral, and anti-inflammatory compounds is almond (Prunus dulcis) skin. Researchers have created a mix of polyphenols present in natural almond skin which displays anti-herpetic pharmacological properties that can be exploited for designing topical formulations. Nonetheless, further studies must be performed in order to establish possible synergistic effects with currently approved antibiotics and antivirals [68].
A similar anti-herpetic action was indicated for polyphenols extracted from natural shelled pistachios (Pistacia vera) kernels. Thus, pistachio extracts could serve as a novel treatment for HSV-1 infections, either alone or combined with standard antiviral therapies. Moreover, the antiviral and anti-inflammatory properties suggest possible further interest in using pistachio product waste as a source of bioactive compounds for pharmaceutical formulations [48].
One polyphenol of particular importance is tannic acid. Its unique antiviral and antibacterial properties have attracted interest in developing new strategies for preventing and treating oral infections. Tannic acid presents significant antimicrobial activity against influenza A virus, papillomaviruses, noroviruses, HSV-1, HSV-2, and HIV, as well as activity against both Gram-positive and Gram-negative bacteria without the toxicity associated with classic drugs [84].
The collateral effects of oral infections can also be diminished using plant-derived natural compounds. For instance, Echinacea purpurea extract has been investigated for sore throat therapy [105]. It was noticed that the effects on the sore throat of a sage/echinacea spray were comparable with those of chlorhexidine/lidocaine, with 60% of the patients in each group becoming symptom-free after 3 days [108].
Coconut oil is an alternative therapeutic option, especially in irradiated head and neck cancer patients. Coconut oil can “coat” the mouth and form a barrier that maintains the moisture of mucosal surfaces. Thus, it represents a feasible, low-cost, and safe strategy for managing xerostomia, which is a common complication of many diseases and represents a burden on patients’ quality of life [28].
5.2. Honey and Beehive Products
The medicinal properties of honey and beehive products have attracted interest for use in otorhinolaryngology. In addition, the immunomodulatory and antimicrobial properties of honey, propolis, royal jelly, and bee pollen are useful for diverse applications [109].
Honey has antibacterial, antiviral, and anti-inflammatory impacts, a low toxicity profile, and wound healing-enhancing properties [50,110]. Its mechanisms of action include hyperosmolarity, low pH, production of hydrogen peroxide, and a unique composition containing antioxidant compounds [110]. Particularly, in patients who underwent radio- and chemotherapy of the oropharyngeal region, honey was shown to reduce the intensity of oral mucositis, Candida infection, and pathogenic bacteria, while allowing faster healing [50,109]. Furthermore, the antiviral activity of honey was studied as an alternative to synthetic drugs for treating herpes lesions. A study compared honey versus acyclovir topical application. By analyzing factors such as healing time, pain relief, resolution of local signs, and duration of acute attacks, it was concluded that honey is superior to synthetic products by 43%, 39%, 28%, and 35%, respectively. These findings were attributed to the copper, ascorbic acid, and hydrogen peroxide contents of honey, which can inactivate HSV [111,112]. A particularly appealing type of honey for antimicrobial applications is Manuka honey, which has been shown effective in preventing biofilm growth and reducing acid production. Its antimicrobial potency is related to the Unique Manuka Factor (UMF) rating, which depends on the methylglycoxal and total phenol content [113,114,115]. The effects of several types of honey are compared in Table 2.

Table 2.
Comparison of the effects of different types and concentrations of honey on S. aureus. Reprinted with permission from ref. [115]. Copyright 2017 Elsevier B.V.
Propolis is a non-toxic, antimicrobial, anticancer, antibiotic, antifungal, antiviral, and anti-inflammatory natural product [5,116]. These biological and curative properties drew attention for inhibiting biofilm formation and treatment of denture stomatitis [91]. In particular, propolis extracts can be included in medicinal products, mouthwashes, toothpaste, and dental varnishes to control the growth of Candida spp. [8]. Research has shown that red propolis alcoholic extract exerts fungistatic and fungicidal activity on C. albicans (at 32–64 μg/mL and 64–512 μg/mL, respectively), C. tropicalis (32–64 μg/mL and 64 μg/mL), and C. glabrata (64 μg/mL and 64–256 μg/mL) strains isolated from chronic periodontitis cases [117]. An in vivo study on patients with full dentures demonstrated the anti-Candida activity of a mouthrinse based on a hydroalcoholic extract of propolis. The yeast strains showed antifungal activity in the following order of decreasing sensitivity: C. albicans, C. tropicalis, C. krusei, and C. guilliermondii [118].
Royal jelly, the yellow-white creamy and acidic secretion produced by worker honeybees to feed the queen honeybee, has attracted interest due to its composition rich in minerals, vitamins, fatty acids, sugars, proteins, and free amino acids. It has been observed to have immunomodulatory, wound-healing, bacteriostatic, antioxidant, and antimicrobial properties against yeasts and Gram-negative and Gram-positive bacteria [119,120,121]. Royal jelly administration showed promising results in patients undergoing radio- and chemotherapy, improving the signs and symptoms of oral mucositis and shortening the healing time [122]. Royal jelly has also been tested for the treatment of herpetic lesions as a natural alternative to acyclovir, showing inhibitory effects on HSV-1 at 250 μg/mL concentration [123].
Bee pollen is another bee product presenting useful pharmacological properties such as antifungal, antimicrobial, antiviral, anti-inflammatory, and immunostimulating activity. Its ethanol extract has been shown to be effective against S. aureus, E. coli, K. pneumoniae, P. aeruginosa, and C. albicans, becoming an interesting alternative for preventing and managing oral infections [124].
5.3. Other Natural Sources and Compounds
Mushrooms do not fall into any of the above-presented categories, but their health benefits are important to be mentioned in the context of alternative antimicrobial therapies. Mushrooms have bioactive compounds that have been shown to present antiviral properties. They contain polysaccharides, carbohydrate-binding proteins (i.e., polysaccharopeptide, peptidomannan), proteins (i.e., ubiquitin-like protein, nebrodeolysin, lectin, lentin), peptides, enzymes (i.e., laccase, tyrosinase), polyphenols, triterpenes, triterpenoids, and several other compounds that can inhibit viral entry, replication, viral enzymes, and the expression of viral proteins and cellular proteins. Antiviral compounds from mushrooms can enhance the immune system, helping the organism fight against HSV-1, HSV-2, HIV, and the influenza A virus, among others. Hence, mushroom-derived bioactive metabolites could serve as antiviral candidates against DNA and RNA viruses [125].
Various compounds possessing important antibacterial and antibiofilm activities have also been obtained from microalgae. Ethanolic extracts of Chorella vulgaris and Dunaliella salina are promising for inhibiting bacterial biofilm formation. Specifically, the compounds responsible for the antimicrobial properties may be flavonoids, tannins, and terpenoids from C. vulgaris extract and 3,3,5-trimethylheptane, n-hexadecane, polyunsaturated fatty acids, β-ionone, and neophytadiene from D. salina extract [126].
Natural polysaccharides extracted from cyanobacteria and macroalgae have been established as potent antiviral compounds that can disturb virus–cell interactions and inhibit virus adsorption or penetration into the host cells [49,54]. From Nostoc flagelliform nostoflan can be isolated, which is an antiviral polysaccharide that can act against cytomegalovirus infections. With an even higher potency against human cytomegalovirus than nostoflan, calcium spirulan can be used in alternative natural therapies. This sulfated polysaccharide isolated from Spirulina platensis owes its antiviral properties to the presence of sulfated groups, but further investigations are needed to clarify its structural formula [54]. Algal polysaccharides also have promising activity against influenza B virus and mumps, as is the case of compounds obtained from Gelidium cartilagineum (Linnaeus) Gaillon. Sulfated polysaccharides present in algae can also be employed in the development of novel HSV infection therapies; however, so far, only a small number of species have been investigated for anti-HSV properties [49]. The rich content in bioactive compounds of seaweeds has attracted interest in their antimicrobial potential as well. Hence, algae can be used for developing cost-effective therapies with only minor toxicity and fewer secondary effects than synthetic antibiotics [10].
A group of natural compounds that have attracted research interest towards infection treatment is represented by antimicrobial peptides (AMPs) [127]. Particularly, AMPs have been investigated for the control of bacterial biofilms [8]. AMPs are part of the line of defense against pathogens in higher organisms, but in microorganisms they compete for nutrients. Thus, natural AMPs are relatively safe and well-tolerated by humans, also being highly effective. However, in antifungal therapy only a few peptides were employed. Their use is hindered by hemolytic activity, low bioavailability, a poor ability to cross physiological barriers, and loss of activity in high salt concentrations [128].
To determine a clear correlation between the natural sources, therapeutic properties, inhibited pathogens, responsible bioactive compounds, and potential applications in treating oral diseases, Table 3 was created.

Table 3.
Correlation between natural antimicrobial compounds, microorganisms, oral diseases, and potential applications.
6. Hybrid Treatment Options
Some oral pathogens have also developed resistance to single-plant extracts [5], thus creating the need for the development of hybrid treatments.
One option is to create novel formulations that combine several plants [5]. An example of such mixture is made from Azadirachta indica, Mangifera indica L., Hemidesmus indicus (L.) R.Br., Caryophyllus aromaticus L., Cinnamomum zeylanicum Blume, Quercus infectoria Oliv., Emblica officinalis Gaertn., Terminalia belerica Roxb., and Terminalia chebula Retz. The chewable poly-herbal tablet was shown to inhibit the growth of dental bacteria, demonstrating good antimicrobial activity [143]. Another medicinal plant mixture is Sho-Saiko-to. Each of its seven herbal components (i.e., Bupleureum falcatum, Glycyrrhiza uralensis, Panax ginseng, Pinelliae ternatae, Scutellaria baicalens, Zingiber officinale, and Ziziphus jujube) contain several active biochemical constituents which work in synergy to treat oral infections [5,144].
Another possibility is the design of antimicrobial drugs by combining the benefits of natural compounds with the advantages of nanotechnology [145]. One successful association is the nanostructure made of polylactic acid-based composite films embedded with magnetite nanoparticles conjugated in situ with Eucalyptus essential oil. The inorganic material has a role in stabilizing and potentiating the essential oil, while the polymer modulates the biocompatibility and stability of magnetite. Such coatings do not affect the viability of eukaryote cells, but they significantly interfere with the formation and maturation of bacterial biofilms. Hence, effective anti-infective therapeutic nanosystems are obtained that can offer targeted and controlled treatment [146].
Studies combining silver nanoparticles with algae extracts have also shown promising results [126]. For instance, silver nanoparticles containing Oscillatoria spp. green algae methanol extract exhibit strong antibiofilm and antibacterial activity against pathogens such as S. aureus, E. coli, P. aeruginosa, and B. cereus. Their enhanced performance, coupled with their low cytotoxicity, are important criteria for their potential use in pharmacological applications [147].
Hydrogels embedded with tannic acid-modified silver nanoparticles were tested against herpes virus infection and were shown to be effective. These nanostructures affect viral attachment, impede penetration, and reduce post-infection spread [84,148].
The synergic action of natural compounds and conventional drugs represents another hybrid treatment option. It was reported that the combination between curcumin and antibiotics could restore the sensitivity of bacteria to synthetic drugs. Hence, it diminishes bacterial toxicity while promoting the influx of antibiotics [70]. Regarding viral infections, the combined use of acyclovir and honey resulted in better outcomes than the antifungal drug used alone in treating PHGS; specifically, children presented a significant earlier disappearance of oral lesions (3 days vs. 6 days), drooling (2 days vs. 4 days), and eating difficulty (3 days vs. 8 days). Moreover, honey improved inflammation and decreased the associated pain, allowing the patients to maintain a normal diet and recover from the infection [50].
To provide an overview of the status of hybrid treatment options, Table 4 summarizes several examples of patents in the field of oral care products.

Table 4.
Examples of patented applications of oral care products based on natural compounds.
7. The Role of Diet and Nutrition in Preventing Oral Infections
Like any form of life, microorganisms need nutrients to live and grow. The host’s diet can also be their source of nourishment, influencing the number and types of microbes in the oral cavity [19]. Therefore, by controlling the diet, some oral infections can be prevented.
A deficiency in micronutrients (e.g., B vitamins) is associated with oral manifestations like glossitis, cheilitis, and angular stomatitis. It has also been noticed that undernutrition aggravates oral infections, contributing to life-threatening conditions such as noma, a dehumanizing oro-facial type of gangrene [160].
On the other hand, the ingestion of high levels of carbohydrates is not beneficial either, as microorganisms use them as their primary energy source. Hence, a large preponderance of microbes is seen in individuals consuming large amounts of refined sugar and those drinking beer [5,19].
A healthy diet should be rich in fruits, vegetables, and wholegrain starchy foods, while the intake levels of free sugars and fat should be as low as possible [160]. The ingestion of bioactive natural compounds promotes oral health through their negative immunoregulatory and anti-inflammatory activities [161]. Everyday widely available products like edible mushrooms, honey, green tea, cranberries, grapes, milk, coffee, and alcohol-free red wine are natural foods and beverages proven to inhibit bacterial adhesion in the oral cavity [5].
Therefore, maintaining a balanced diet is an important factor in preventing oral diseases and infection invasion.
8. Conclusions and Future Perspectives
To conclude, oral infections are a hot topic of research, especially due to the emerging drug resistance to conventional treatments, contamination and invasion potential, and psychological impact. The toxicity, adverse effects, and low efficacy of existing synthetic drugs have driven the exploration of natural alternatives. Many compounds and products derived from plants, algae, fungi, and other natural sources have been investigated for their antimicrobial properties, leading to promising results against oral-related pathogens. Hence, it can be expected that novel natural pharmaceutical formulations and oral hygiene products will soon emerge on the market.
However, the tested species represent only a small part of the thousands of sources available in nature. Hence, research efforts should be directed towards the other useful plants that remained unexplored. Moreover, the mechanisms of action of some of the discussed natural antimicrobial compounds have not yet been fully elucidated, requiring additional investigations. Particularly, the selective targeting ability towards pathogens instead of probiotics should be evaluated for the discussed natural-based treatment alternatives. Another problem to be solved in the near future is assessment of the toxicological safety of the extracts and pure compounds that have been only tested in vitro so far. Ultimately, close attention should be given to match the treatment with the causative pathogen and the customized needs of each individual for the development of personalized anti-infective therapies.
Author Contributions
A.-G.N. and A.M.G. participated in the review, writing, and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI—UEFISCDI, project number 271PED/2020, within PNCDI III.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arweiler, N.B.; Netuschil, L. The oral microbiota. In Microbiota of the Human Body: Implications in Health and Disease; Schwiertz, A., Ed.; Springer International Publishing: Cham, Germany, 2016; pp. 45–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.R.; Gabaldón, T. The human oral microbiome in health and disease: From sequences to ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Krüger, W.; Vielreicher, S.; Kapitan, M.; Jacobsen, I.D.; Niemiec, M.J. Fungal-bacterial interactions in health and disease. Pathogens 2019, 8, 70. [Google Scholar] [CrossRef]
- Chinsembu, K.C. Plants and other natural products used in the management of oral infections and improvement of oral health. Acta Trop. 2016, 154, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Coll, P.P.; Lindsay, A.; Meng, J.; Gopalakrishna, A.; Raghavendra, S.; Bysani, P.; O’Brien, D. The prevention of infections in older adults: Oral health. J. Am. Geriatr. Soc. 2020, 68, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Back-Brito, G.N.; El Ackhar, V.N.R.; Querido, S.M.R.; dos Santos, S.S.F.; Jorge, A.O.C.; de Macedo Reisc, A.d.S.; Koga-Ito, C.Y. Staphylococcus spp., Enterobacteriaceae and Pseudomonadaceae oral isolates from Brazilian HIV-positive patients. Correlation with CD4 cell counts and viral load. Arch. Oral Biol. 2011, 56, 1041–1046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salehi, B.; Kregiel, D.; Mahady, G.; Sharifi-Rad, J.; Martins, N.; Rodrigues, C.F. Management of Streptococcus mutans-Candida spp. oral biofilms’ infections: Paving the way for effective clinical interventions. J. Clin. Med. 2020, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Yanakiev, S. Effects of Cinnamon (Cinnamomum spp.) in dentistry: A review. Molecules 2020, 25, 4184. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a Source of valuable antimicrobial compounds: Extraction and applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef]
- Ozma, M.A.; Khodadadi, E.; Pakdel, F.; Kamounah, F.S.; Yousefi, M.; Yousefi, B.; Asgharzadeh, M.; Ganbarov, K.; Kafil, H.S. Baicalin, a natural antimicrobial and anti-biofilm agent. J. Herb. Med. 2021, 27, 100432. [Google Scholar] [CrossRef]
- Roozbehani, N.; Golfeshan, F.; Pakshir, K.; Doorandishan, M.; Jassbi, A.R.; Mosaddad, S.A. Chemical composition and effectiveness of Ocimum basilicum L. extracts on the adhesion of Candida albicans and C. dubliniensis on acrylic surfaces of removable orthodontic appliances. Biointerface Res. Appl. Chem. 2021, 11, 9477–9489. [Google Scholar] [CrossRef]
- Shi, Y.; Gu, R.; Liu, C.; Ni, J.; Wu, T. Chinese medicinal herbs for sore throat. Cochrane Database Syst. Rev. 2007, 3, CD004877. [Google Scholar] [CrossRef]
- Mihai, A.D.; Chircov, C.; Grumezescu, A.M.; Holban, A.M. Magnetite nanoparticles and essential oils systems for advanced antibacterial therapies. Int. J. Mol. Sci. 2020, 21, 7355. [Google Scholar] [CrossRef]
- Henley-Smith, C.J.; Botha, F.S.; Hussein, A.A.; Nkomo, M.; Meyer, D.; Lall, N. Biological activities of heteropyxis natalensis against micro-organisms involved in oral infections. Front. Pharmacol. 2018, 9, 291. [Google Scholar] [CrossRef]
- Asai, D.; Nakashima, H. Pathogenic viruses commonly present in the oral cavity and relevant antiviral compounds derived from natural products. Medicines 2018, 5, 120. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, X.; Zhou, X.; Ren, B.; Xiao, L.; Li, Y.; Li, M.; Guo, Q. Basic biology of oral microbes. In Atlas of Oral Microbiology; Zhou, X., Li, Y., Eds.; Academic Press: Oxford, UK, 2015; pp. 1–14. [Google Scholar] [CrossRef]
- Reynolds-Campbell, G.; Nicholson, A.; Thoms-Rodriguez, C.-A. Oral bacterial infections: Diagnosis and management. Dent. Clin. North Am. 2017, 61, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Schuster, G.S. Oral flora and pathogenic organisms. Infect. Dis. Clin. North Am. 1999, 13, 757–774. [Google Scholar] [CrossRef]
- Santosh, A.B.R.; Muddana, K.; Bakki, S.R. Fungal infections of oral cavity: Diagnosis, management, and association with COVID-19. SN Compr. Clin. Med. 2021, 3, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Schuurhuis, J.M.; Stokman, M.A.; Witjes, M.J.H.; Langendijk, J.A.; van Winkelhoff, A.J.; Vissink, A.; Spijkervet, F.K.L. Head and neck intensity modulated radiation therapy leads to an increase of opportunistic oral pathogens. Oral Oncol. 2016, 58, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Medeiros, P.; Cardoso, T.; Campello, G. Immunossupressed patients admitted into intensive care with infection: Risk factors for infection by multidrug resistant pathogens and hospital mortality. Intensive Care Med. Exp. 2015, 3, A125. [Google Scholar] [CrossRef]
- Palmieri, M.; Sarmento, D.J.S.; Falcão, A.P.; Martins, V.A.O.; Brandão, T.B.; Morais-Faria, K.; Ribeiro, A.C.P.; Hasséus, B.; Giglio, D.; Braz-Silva, P.H. Frequency and evolution of acute oral complications in patients undergoing radiochemotherapy treatment for head and neck squamous cell carcinoma. Ear Nose Throat J. 2019, 100, 5. [Google Scholar] [CrossRef] [PubMed]
- Meurman, J.H.; Hämäläinen, P. Oral health and morbidity—Implications of oral infections on the elderly. Gerodontology 2006, 23, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Chong, A.W.; Mun, K.S. Fungal laryngitis causing airway compromise in post irradiated patient. Acta Oto-Laryngol. Case Rep. 2016, 1, 123–125. [Google Scholar] [CrossRef]
- Sharma, V.K.; Tailor, M.; Chaudhary, V.K.; Rawat, D.S.; Verma, P.C.; Singh, B.K. Fungal infections in otorhinolaryngology: A descriptive study. Int. Multispeciality J. Health 2017, 3, 117–124. [Google Scholar]
- Wiench, R.; Skaba, D.; Matys, J.; Grzech-Leśniak, K. Efficacy of toluidine blue—Mediated antimicrobial photodynamic therapy on Candida spp. A Syst. Rev. Antibiot. 2021, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Quimby, A.E.; Hogan, D.; Khalil, D.; Hearn, M.; Nault, C.; Johnson-Obaseki, S. Coconut oil as a novel approach to managing radiation-induced xerostomia: A primary feasibility study. Int. J. Otolaryngol. 2020, 2020, 8537643. [Google Scholar] [CrossRef] [PubMed]
- Quindós, G.; Gil-Alonso, S.; Marcos-Arias, C.; Sevillano, E.; Mateo, E.; Jauregizar, N.; Eraso, E. Therapeutic tools for oral candidiasis: Current and new antifungal drugs. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e172–e180. [Google Scholar] [CrossRef]
- Kumari, R.; Mishra, R.C.; Sheoran, R.; Yadav, J.P. Fractionation of antimicrobial compounds from acacia nilotica twig extract against oral pathogens. Biointerface Res. Appl. Chem. 2020, 10, 7097–7105. [Google Scholar]
- Iacovou, E.; Vlastarakos, P.V.; Papacharalampous, G.; Kampessis, G.; Nikolopoulos, T.P. Diagnosis and treatment of HIV-associated manifestations in otolaryngology. Infect. Dis. Rep. 2012, 4, 22–29. [Google Scholar] [CrossRef]
- Scasso, F.; Ferrari, G.; De Vincentiis, G.C.; Arosio, A.; Bottero, S.; Carretti, M.; Ciardo, A.; Cocuzza, S.; Colombo, A.; Conti, B.; et al. Emerging and re-emerging infectious disease in otorhinolaryngology. Acta Otorhinolaryngol. Ital. 2018, 38, S1–S106. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, P.; Khorshidi, H.; Raoofi, S.; Dehghani Nazhvani, A.; Badiee, P. Comparative evaluation of conventional and nanosilver-containing leucocyte and platelet-rich fibrin/biomaterial in the anti-biofilm formation of standard species of Candida and Streptococcus. Jundishapur J. Microbiol. 2018, 11, e68423. [Google Scholar] [CrossRef]
- Okonogi, S.; Phumat, P.; Khongkhunthian, S.; Suttiat, K.; Chaijareenont, P. Denture-soaking solution containing piper betle extract-loaded polymeric micelles; inhibition of candida albicans, clinical study, and effects on denture base resin. Antibiotics 2021, 10, 440. [Google Scholar] [CrossRef]
- Muadcheingka, T.; Tantivitayakul, P. Distribution of Candida albicans and non-albicans Candida species in oral candidiasis patients: Correlation between cell surface hydrophobicity and biofilm forming activities. Arch. Oral Biol. 2015, 60, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Lamfon, H.A. Denture biofilm and dentureassociated stomatitis, A literature review. Egypt. Dent. J. 2021, 67, 775–787. [Google Scholar] [CrossRef]
- Namangkalakul, W.; Benjavongkulchai, S.; Pochana, T.; Promchai, A.; Satitviboon, W.; Howattanapanich, S.; Phuprasong, R.; Ungvijanpunya, N.; Supakanjanakanti, D.; Chaitrakoonthong, T.; et al. Activity of chitosan antifungal denture adhesive against common Candida species and Candida albicans adherence on denture base acrylic resin. J. Prosthet. Dent. 2020, 123, 181.e181–181.e187. [Google Scholar] [CrossRef]
- Tejada, G.; Barrera, M.G.; García, P.; Sortino, M.; Lamas, M.C.; Lassalle, V.; Alvarez, V.; Leonardi, D. Nanoparticulated systems based on natural polymers loaded with miconazole nitrate and lidocaine for the treatment of topical candidiasis. AAPS Pharm. Sci. Tech. 2020, 21, 278. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Cruz, M.; Vega-González, A.; Mendoza-Novelo, B.; López-Romero, E.; Ruiz-Baca, E.; Quintanar-Escorza, M.A.; Villagómez-Castro, J.C. The effect of biomaterials and antifungals on biofilm formation by Candida species: A review. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2513–2527. [Google Scholar] [CrossRef] [PubMed]
- Muhvić-Urek, M.; Saltović, E.; Braut, A.; Kovačević Pavičić, D. Association between Vitamin D and Candida-Associated Denture Stomatitis. Dent. J. 2020, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Verma, R.; Murari, A.; Agrawal, A. Oral candidiasis: An overview. J. Oral Maxillofac. Pathol. 2014, 18, S81–S85. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; de Almeida Júnior, J.N.; Slavin, M.A.; Chen, S.C.A.; Sorrell, T.C. Candida and invasive mould diseases in non-neutropenic critically ill patients and patients with haematological cancer. Lancet Infect. Dis. 2017, 17, e344–e356. [Google Scholar] [CrossRef]
- Fuqua, T.H.; Sittitavornwong, S.; Knoll, M.; Said-Al-Naief, N. Primary invasive oral aspergillosis: An updated literature review. J. Oral Maxillofac. Surg. 2010, 68, 2557–2563. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Lee, K.H.; Colquhoun, A.N.; Evans, S.A. Invasive oral aspergillosis in a patient with acute myeloid leukaemia. Aust. Dent. J. 2010, 55, 214–218. [Google Scholar] [CrossRef]
- Vinay, B.H.; Mohan, A.; Haritha, P.; Lakshmi, K.R. A rare coexistence of aspergillosis with actinomycosis. J. Oral Maxillofac. Pathol. 2017, 21, 277–281. [Google Scholar] [CrossRef]
- Biswas, K.D.; Choudhary, A.; Ghosh, S.K.; Biswas, S. Primary laryngeal aspergillosis in an immunocompetent host. Bengal J. Otolaryngol. Head Neck Surg. 2018, 26, 131–133. [Google Scholar] [CrossRef]
- Fukuchi, K.; Sakagami, H.; Sugita, Y.; Takao, K.; Asai, D.; Terakubo, S.; Takemura, H.; Ohno, H.; Horiuchi, M.; Suguro, M.; et al. Quantification of the ability of natural products to prevent herpes virus infection. Medicines 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Smeriglio, A.; Mandalari, G.; Sciortino, M.T. In Vitro Anti-HSV-1 activity of polyphenol-rich extracts and pure polyphenol compounds derived from pistachios kernels (Pistacia vera L.). Plants 2020, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Lobine, D.; Rengasamy, K.R.R.; Gowrishankar, S.; Tewari, D.; Zengin, G.; Kim, D.H.; Sivanesan, I. Marine Algae: A potential resource of anti-HSV molecules. Processes 2019, 7, 887. [Google Scholar] [CrossRef]
- Awad, O.G.A.; Hamad, A.-M.H. Honey can help in herpes simplex gingivostomatitis in children: Prospective randomized double blind placebo controlled clinical trial. Am. J. Otolaryngol. 2018, 39, 759–763. [Google Scholar] [CrossRef]
- Brigandi, L.A.; Lanfranchi, P.V.; Scheiner, E.D.; Busch, S.L. Herpes simplex virus infection presenting as a piriform sinus mass. Ear Nose Throat J. 2006, 85, 450–456. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Foulon, I.; Pass, R.; Ville, Y. Cytomegalovirus infection during pregnancy: State of the science. Am. J. Obstet. Gynecol. 2020, 223, 330–349. [Google Scholar] [CrossRef]
- Mayer, B.T.; Krantz, E.M.; Swan, D.; Ferrenberg, J.; Simmons, K.; Selke, S.; Huang, M.-L.; Casper, C.; Corey, L.; Wald, A.; et al. Transient oral human cytomegalovirus infections indicate inefficient viral spread from very few initially infected cells. J. Virol. 2017, 91, e00380-17. [Google Scholar] [CrossRef]
- Stoeva, S.; Efferth, T. Human cytomegalovirus: Drug resistance and new treatment options using natural products. Mol. Med. Rep. 2008, 1, 781–785. [Google Scholar] [CrossRef]
- Esquenazi, D.; Filho, I.B.; da Costa Carvalho, M.d.G.; de Barros, F.S. The frequency of human papillomavirus findings in normal oral mucosa of healthy people by PCR. Braz. J. Otorhinolaryngol. 2010, 76, 78–84. [Google Scholar] [CrossRef]
- Matos, L.L.d.; Miranda, G.A.; Cernea, C.R. Prevalence of oral and oropharyngeal human papillomavirus infection in Brazilian population studies: A systematic review. Braz. J. Otorhinolaryngol. 2015, 81, 554–567. [Google Scholar] [CrossRef]
- Fernández-López, C.; Morales-Angulo, C. Otorhinolaryngology manifestations secondary to oral sex. Acta Otorrinolaringol. 2017, 68, 169–180. [Google Scholar] [CrossRef]
- Campisi, G.; Mauceri, R.; Tozzo, P.; Panzarella, V. Commonalities between ENT specialists and oral medicine experts: Old HPV diseases and new oral HPV-cancer along the borders. Oral 2021, 1, 11. [Google Scholar] [CrossRef]
- Palaia, G.; Ciolfi, C.; Del Vecchio, A.; Ciolfi, A.; Tenore, G.; Romeo, U. Prevention of recurrence of oral HPV-related lesions: Systematic review of the literature and meta-analysis. Appl. Sci. 2021, 11, 4194. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y. Human papillomavirus infection in oral potentially malignant disorders and cancer. Arch. Oral Biol. 2017, 83, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Di Cosola, M.; Bottalico, L.; Topi, S.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Cazzolla, A.P.; Dipalma, G. Focus on HPV infection and the molecular mechanisms of oral carcinogenesis. Viruses 2021, 13, 559. [Google Scholar] [CrossRef]
- Jeruzal-Świątecka, J.; Pietruszewska, W. Awareness of human papillomavirus and its oncogenic potential in head and neck cancer among students: Still more questions than answers. Int. J. Environ. Res. Public Health 2020, 17, 8667. [Google Scholar] [CrossRef]
- D’Souza, G.; McNeel, T.S.; Fakhry, C. Understanding personal risk of oropharyngeal cancer: Risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann. Oncol. 2017, 28, 3065–3069. [Google Scholar] [CrossRef]
- Dahlén, G. Bacterial infections of the oral mucosa. Periodontology 2009, 49, 13–38. [Google Scholar] [CrossRef]
- Garcia-Cuesta, C.; Sarrion-Pérez, M.-G.; Bagán, J.V. Current treatment of oral candidiasis: A literature review. J. Clin. Exp. Dent. 2014, 6, e576–e582. [Google Scholar] [CrossRef]
- Abraham, C.M. Advances and emerging techniques in the identification, diagnosis and treatment of oral candidiasis. Open Pathol. J. 2011, 5, 8–12. [Google Scholar] [CrossRef][Green Version]
- Gheorghe, D.C.; Niculescu, A.-G.; Bîrcă, A.C.; Grumezescu, A.M. Biomaterials for the prevention of oral candidiasis development. Pharmaceutics 2021, 13, 803. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The antimicrobial and antiviral activity of polyphenols from almond (Prunus dulcis L.) skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef]
- Kanwar, I.; Sah, A.K.; Suresh, P.K. Biofilm-mediated antibiotic-resistant oral bacterial infections: Mechanism and combat strategies. Curr. Pharm. Des. 2017, 23, 2084–2095. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, E.; Cheng, Y.; Mahmood, T.; Ge, F.; Zhou, K.; Bao, M.; Lv, L.; Li, L.; Yi, J.; et al. Is combined medication with natural medicine a promising therapy for bacterial biofilm infection? Biomed. Pharmacother. 2020, 128, 110184. [Google Scholar] [CrossRef]
- Nazarparvar, M.; Shako, A.; Ranjbariyan, A. Chemical composition and antimicrobial activity against food poisoning of alcoholic extract of Nigella Sativa L. Biointerface Res. Appl. Chem. 2020, 10, 6991–7001. [Google Scholar] [CrossRef]
- Selamoglu, Z.; Sevindik, M.; Bal, C.; Ozaltun, B.; Sen, I.; Pasdaran, A. Antioxidant, antimicrobial and DNA protection activities of phenolic content of Tricholoma virgatum (Fr.) P. Kumm. Biointerface Res. Appl. Chem. 2020, 10, 5500–5506. [Google Scholar] [CrossRef]
- Soto-Chilaca, G.A.; Mejia-Garibay, B.; Navarro-Amador, R.; Ramirez-Corona, N.; Palou, E.; Lopez-Malo, A. Cinnamaldehyde-loaded chitosan nanoparticles: Characterization and antimicrobial activity. Biointerface Res. Appl. Chem. 2019, 9, 4060–4065. [Google Scholar] [CrossRef]
- Aghazadeh, Z.; Aghazadeh, M.; Kafil, H.S.; Falsafi, P.; Rahbar, M. Gingerly effervescent tablets: Investigating the effect of cytotoxicity on gingival fibroblasts and antimicrobial properties under laboratory conditions. Biointerface Res. Appl. Chem. 2019, 9, 4534–4538. [Google Scholar] [CrossRef]
- Abusrewil, S.; Alshanta, O.A.; Albashaireh, K.; Alqahtani, S.; Nile, C.J.; Scott, J.A.; McLean, W. Detection, treatment and prevention of endodontic biofilm infections: What’s new in 2020? Critical Reviews in Microbiology 2020, 46, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Podgoreanu, P.; Negrea, S.M.; Buia, R.; Delcaru, C.; Trusca, S.B.; Lazar, V.; Chifiriuc, M.C. Alternative strategies for fighting multidrug resistant bacterial infections. Biointerface Res. Appl. Chem. 2019, 9, 3834–3841. [Google Scholar] [CrossRef]
- Mickymaray, S. efficacy and mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 118. [Google Scholar] [CrossRef]
- de Araújo, M.R.C.; Maciel, P.P.; Castellano, L.R.C.; Bonan, P.R.F.; da Nóbrega Alves, D.; de Medeiros, A.C.D.; de Castro, R.D. Efficacy of essential oil of cinnamon for the treatment of oral candidiasis: A randomized trial. Spec. Care Dent. 2021, 41, 349–357. [Google Scholar] [CrossRef]
- Cheraghipour, K.; Ezatpour, B.; Masoori, L.; Marzban, A.; Sepahvand, A.; Rouzbahani, A.K.; Moridnia, A.; Khanizadeh, S.; Mahmoudvand, H. Anti-candida activity of curcumin: A review. Curr. Drug Discov. Technol. 2020, 16. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a natural antimicrobial agent with strain-specific activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Antunes, D.P.; Salvia, A.C.R.D.; de Araújo, R.M.; Di Nicoló, R.; Koga Ito, C.Y.; de Araujo, M.A.M. Effect of green tea extract and mouthwash without alcohol on Candida albicans biofilm on acrylic resin. Gerodontology 2015, 32, 291–295. [Google Scholar] [CrossRef]
- Kumar, D.; Ayesha, M.J.; Gautam, P.; Joshi, H.; Kumar, N. A Recent report on ‘plants with anti-Candida properties’. Int. J. Cur. Res. Rev. 2020, 12, 25. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef] [PubMed]
- Narotzki, B.; Reznick, A.Z.; Aizenbud, D.; Levy, Y. Green tea: A promising natural product in oral health. Arch. Oral Biol. 2012, 57, 429–435. [Google Scholar] [CrossRef]
- Okwu, D.E. Citrus fruits: A rich source of phytochemicals and their roles in human health. Int. J. Chem. Sci. 2008, 6, 451–471. [Google Scholar]
- Subramenium, G.A.; Vijayakumar, K.; Pandian, S.K. Limonene inhibits streptococcal biofilm formation by targeting surface-associated virulence factors. J. Med. Microbiol. 2015, 64, 879–890. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Rossi, D.C.P.; Jabes, D.L.; Barbosa, D.A.; Cunha, F.F.M.; Nunes, L.R.; Arruda, D.C.; Pelleschi Taborda, C. In Vitro and In Vivo inhibitory activity of limonene against different isolates of Candida spp. J. Fungi 2020, 6, 183. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Chifiriuc, M.C.; Marinas, I.; Saviuc, C.; Mihaiescu, D.; Lazar, V. Ocimum basilicum and Mentha piperita essential oils influence the antimicrobial susceptibility of Staphylococcus aureus strains. Lett. Appl. Nano. Bio. Sci. 2012, 1, 14–17. [Google Scholar]
- Nuță, D.C.; Limban, C.; Chiriță, C.; Chifiriuc, M.C.; Costea, T.; Ioniță, P.; Nicolau, I.; Zarafu, I. Contribution of essential oils to the fight against microbial biofilms—A review. Processes 2021, 9, 537. [Google Scholar] [CrossRef]
- Sugio, C.Y.C.; Mengoa, M.G.R.; Gomes, A.C.G.; Garcia, A.A.M.N.; de Oliveira, T.M.; Hermana, K. Use of natural products in the prevention and treatment of denture stomatitis. Open Access J. Biomed. Sci. 2020, 1, 201–206. [Google Scholar]
- Santos, P.M.; Batista, D.L.J.; Ribeiro, L.A.F.; Boffo, E.F.; de Cerqueira, M.D.; Martins, D.; de Castro, R.D.; de Souza-Neta, L.C.; Pinto, E.; Zambotti-Villela, L.; et al. Identification of antioxidant and antimicrobial compounds from the oilseed crop Ricinus communis using a multiplatform metabolite profiling approach. Ind. Crop. Prod. 2018, 124, 834–844. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Antimicrobial potential of pomegranate peel: A review. Int. J. Food Sci. Technol. 2019, 54, 959–965. [Google Scholar] [CrossRef]
- Furletti, V.F.; Teixeira, I.P.; Obando-Pereda, G.; Mardegan, R.C.; Sartoratto, A.; Figueira, G.M.; Duarte, R.M.T.; Rehder, V.L.G.; Duarte, M.C.T.; Höfling, J.F. Action of Coriandrum sativum L. Essential Oil upon Oral Candida albicans Biofilm Formation. Evid. -Based Complementary Altern. Med. 2011, 2011, 985832. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.A.; Bernardo, L.P.; Moreno, J.M.L.; Lara, V.S.; Porto, V.C. Equisetum giganteum influences the ability of Candida albicans in forming biofilms over the denture acrylic resin surface. Pharm. Biol. 2017, 55, 1698–1702. [Google Scholar] [CrossRef]
- Alexander, B.; John, S. Oral health benefits of cranberry: A review. J. Dent. Med. Sci 2018, 18, 41–44. [Google Scholar]
- Gundidza, M.; Deans, S.G.; Kennedy, A.I.; Mavi, S.; Waterman, P.G.; Gray, A.I. The essential oil from Heteropyxis natalensis harv: Its antimicrobial activities and phytoconstituents. J. Sci. Food Agric. 1993, 63, 361–364. [Google Scholar] [CrossRef]
- Philip, N.; Leishman, S.J.; Bandara, H.M.H.N.; Healey, D.L.; Walsh, L.J. Randomized controlled study to evaluate microbial ecological effects of CPP-ACP and cranberry on dental plaque. Clin. Transl. Res. 2019, 5, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Feghali, K.; Feldman, M.; La, V.D.; Santos, J.; Grenier, D. Cranberry proanthocyanidins: Natural weapons against periodontal diseases. J. Agric. Food Chem. 2012, 60, 5728–5735. [Google Scholar] [CrossRef]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; van Hoek, M.L. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2014, 14, 499. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Harris, J.C.; Cottrell, S.; Plummer, S.; Lloyd, D. Antimicrobial properties of Allium sativum (garlic). Appl. Microbiol. Biotechnol. 2001, 57, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Levina, N.; Van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef] [PubMed]
- Güllüce, M.; Sökmen, M.; Daferera, D.; Aǧar, G.; Özkan, H.; Kartal, N.; Polissiou, M.; Sökmen, A.; Şahin, F. In Vitro antibacterial, antifungal, and antioxidant activities of the essential oil and methanol extracts of herbal parts and callus cultures of Satureja hortensis L. J. Agric. Food Chem. 2003, 51, 3958–3965. [Google Scholar] [CrossRef]
- Ghazi-Moghadam, K.; Inançlı, H.M.; Bazazy, N.; Plinkert, P.K.; Efferth, T.; Sertel, S. Phytomedicine in otorhinolaryngology and pulmonology: Clinical trials with herbal remedies. Pharmaceuticals 2012, 5, 853. [Google Scholar] [CrossRef]
- Nagata, H.; Inagaki, Y.; Tanaka, M.; Ojima, M.; Kataoka, K.; Kuboniwa, M.; Nishida, N.; Shimizu, K.; Osawa, K.; Shizukuishi, S. Effect of eucalyptus extract chewing gum on periodontal health: A double-masked, randomized trial. J. Periodontol. 2008, 79, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Dai, B.; Wang, Y.; Huang, S.; Xu, Y.; Cao, Y.; Gao, P.; Zhu, Z.; Jiang, Y. In Vitro activity of baicalein against Candida albicans biofilms. Int. J. Antimicrob. Agents 2008, 32, 73–77. [Google Scholar] [CrossRef]
- Schapowal, A.; Berger, D.; Klein, P.; Suter, A. Echinacea/sage or chlorhexidine/lidocaine for treating acute sore throats: A randomized double-blind trial. Eur. J. Med. Res. 2009, 14, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Henatsch, D.; Wesseling, F.; Kross, K.W.; Stokroos, R.J. Honey and beehive products in otorhinolaryngology: A narrative review. Clin. Otolaryngol. 2016, 41, 519–531. [Google Scholar] [CrossRef]
- Tharakan, T.; Bent, J.; Tavaluc, R. Honey as a treatment in otorhinolaryngology: A review by subspecialty. Ann. Otol. Rhinol. Laryngol. 2018, 128, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Laccourreye, O. Honey in otorhinolaryngology: When, why and how? Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 133–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Waili, N.S. Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med. Sci. Monit. 2004, 10, MT94–MT98. [Google Scholar] [PubMed]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Nigam, P.S. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655. [Google Scholar] [CrossRef]
- Ramsay, E.I.; Rao, S.; Madathil, L.; Hegde, S.K.; Baliga-Rao, M.P.; George, T.; Baliga, M.S. Honey in oral health and care: A mini review. J. Oral Biosci. 2019, 61, 32–36. [Google Scholar] [CrossRef]
- Almasaudi, S.B.; Al-Nahari, A.A.M.; Abd El-Ghany, E.S.M.; Barbour, E.; Al Muhayawi, S.M.; Al-Jaouni, S.; Azhar, E.; Qari, M.; Qari, Y.A.; Harakeh, S. Antimicrobial effect of different types of honey on Staphylococcus aureus. Saudi. J. Biol. Sci. 2017, 24, 1255–1261. [Google Scholar] [CrossRef]
- Khurshid, Z.; Naseem, M.; Zafar, M.S.; Najeeb, S.; Zohaib, S. Propolis: A natural biomaterial for dental and oral healthcare. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 265–274. [Google Scholar] [CrossRef]
- Siqueira, A.B.; Rodriguez, L.R.; Santos, R.K.; Marinho, R.R.; Abreu, S.; Peixoto, R.F.; Gurgel, B.C. Antifungal activity of propolis against Candida species isolated from cases of chronic periodontitis. Braz. Oral Res. 2015, 29, 73–77. [Google Scholar] [CrossRef]
- Ota, C.; Unterkircher, C.; Fantinato, V.; Shimizu, M.T. Antifungal activity of propolis on different species of Candida. Mycoses 2001, 44, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, S.; Akyol, S. The consumption of propolis and royal jelly in preventing upper respiratory tract infections and as dietary supplementation in children. J. Intercult. Ethnopharmacol. 2016, 5, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New insights into the biological and pharmaceutical properties of royal jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [PubMed]
- Alreshoodi, F.M.; Sultanbawa, Y. Antimicrobial activity of royal jelly. Anti-Infect. Agents 2015, 13, 50–59. [Google Scholar] [CrossRef]
- Erdem, Ö.; Güngörmüs, Z. The Effect of royal jelly on oral mucositis in patients undergoing radiotherapy and chemotherapy. Holist. Nurs. Pract. 2014, 28, 242–246. [Google Scholar] [CrossRef]
- Hashemipour, M.A.; Tavakolineghad, Z.; Arabzadeh, S.A.M.; Iranmanesh, Z.; Nassab, S.A.H.G. Antiviral activities of honey, royal jelly, and acyclovir against HSV-1. Wounds 2014, 26, 47–54. [Google Scholar]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in dermatology and skin care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef]
- Seo, D.J.; Choi, C. Antiviral bioactive compounds of mushrooms and their antiviral mechanisms: A review. Viruses 2021, 13, 350. [Google Scholar] [CrossRef]
- López, Y.; Soto, S.M. The usefulness of microalgae compounds for preventing biofilm infections. Antibiotics 2020, 9, 9. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Boyce, H.; Ibrahim, A.; Hoag, S.W.; Karlsson, A.J.; Meiller, T.F.; Jabra-Rizk, M.A. Development and In Vivo Evaluation of a Novel Histatin-5 Bioadhesive Hydrogel Formulation against Oral Candidiasis. Antimicrob. Agents Chemother. 2016, 60, 881. [Google Scholar] [CrossRef]
- Bondaryk, M.; Staniszewska, M.; Zielińska, P.; Urbańczyk-Lipkowska, Z. Natural antimicrobial peptides as inspiration for design of a new generation antifungal compounds. J. Fungi 2017, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Dhanavade, M.J.; Jalkute, C.B.; Ghosh, J.S.; Sonawane, K.D. Study antimicrobial activity of lemon (Citrus lemon L.) peel extract. Br. J. Pharmacol. Toxicol. 2011, 2, 119–122. [Google Scholar]
- Vieira, C.; Evangelista, S.; Cirillo, R.; Lippi, A.; Maggi, C.A.; Manzini, S. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediat. Inflamm. 2000, 9, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Valera, M.C.; Maekawa, L.E.; de Oliveira, L.D.; Jorge, A.O.; Shygei, É.; Carvalho, C.A. In Vitro antimicrobial activity of auxiliary chemical substances and natural extracts on Candida albicans and Enterococcus faecalis in root canals. J. Appl. Oral Sci. Rev. FOB 2013, 21, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Amor, G.; Sabbah, M.; Caputo, L.; Idbella, M.; De Feo, V.; Porta, R.; Fechtali, T.; Mauriello, G. Basil essential oil: Composition, antimicrobial properties, and microencapsulation to produce active chitosan films for food packaging. Foods 2021, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Alavarce, R.A.S.; Saldanha, L.L.; Almeida, N.L.M.; Porto, V.C.; Dokkedal, A.L.; Lara, V.S. The beneficial effect of Equisetum giganteum L. against Candida biofilm formation: New approaches to denture stomatitis. Evid. -Based Complementary Altern. Med. 2015, 2015, 939625. [Google Scholar] [CrossRef] [PubMed]
- Harkat-Madouri, L.; Asma, B.; Madani, K.; Bey-Ould Si Said, Z.; Rigou, P.; Grenier, D.; Allalou, H.; Remini, H.; Adjaoud, A.; Boulekbache-Makhlouf, L. Chemical composition, antibacterial and antioxidant activities of essential oil of Eucalyptus globulus from Algeria. Ind. Crop. Prod. 2015, 78, 148–153. [Google Scholar] [CrossRef]
- Damjanović-Vratnica, B.; Đakov, T.; Šuković, D.; Damjanović, J. Antimicrobial effect of essential oil isolated from Eucalyptus globulus Labill. from Montenegro. Czech J. Food Sci. 2011, 29, 277–284. [Google Scholar] [CrossRef]
- Revathi, S.; Govindarajan, R.K.; Rameshkumar, N.; Hakkim, F.L.; Mohammed, A.-B.; Krishnan, M.; Kayalvizhi, N. Anti-cancer, anti-microbial and anti-oxidant properties of Acacia nilotica and their chemical profiling. Biocatal. Agric. Biotechnol. 2017, 11, 322–329. [Google Scholar] [CrossRef]
- Luo, J.; Dong, B.; Wang, K.; Cai, S.; Liu, T.; Cheng, X.; Lei, D.; Chen, Y.; Li, Y.; Kong, J.; et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE 2017, 12, e0176883. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiao, H.; Meng, J.; Qiao, M.; Du, H.; He, M.; Ming, K.; Liu, J.; Wang, D.; Wu, Y. Baicalin Inhibits biofilm formation and the quorum-sensing system by regulating the MsrA drug efflux pump in Staphylococcus Saprophyticus. Front. Microbiol. 2019, 10, 2800. [Google Scholar] [CrossRef]
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharm. Rev. 2015, 9, 63–72. [Google Scholar] [CrossRef]
- Nasir, N.A.M.M.; Abllah, Z.; Jalaludin, A.A.; Shahdan, I.A.; Abd Manan, W.N.H.W. Virgin coconut oil and its antimicrobial properties against pathogenic microorganisms: A review. In Proceedings of the International Dental Conference of SUMATERA Utara 2017 (IDCSU 2017), Kota Medan, Indonesia, 7–9 December 2017; pp. 192–199. [Google Scholar]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee Pollen: Chemical composition and therapeutic application. Evid. -Based Complementary Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef]
- Thombre, R.; Khadpekar, A.; Phatak, A. Anti-bacterial activity of various medicinal plants against mixed dental flora. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 179. [Google Scholar]
- Weiskirchen, R. Hepatoprotective and Anti-fibrotic Agents: It’s time to take the next step. Front. Pharmacol. 2016, 6, 303. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.B.; Rayyif, S.M.I.; Curutiu, C.; Birca, A.C.; Oprea, O.-C.; Grumezescu, A.M.; Ditu, L.-M.; Gheorghe, I.; Chifiriuc, M.C.; Mihaescu, G.; et al. Eugenol-functionalized magnetite nanoparticles modulate virulence and persistence in pseudomonas aeruginosa clinical strains. Molecules 2021, 26, 2189. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, O.; Popescu, R.C.; Grumezescu, V.; Mogoșanu, G.D.; Mogoantă, L.; Iordache, F.; Holban, A.M.; Vasile, B.Ș.; Bîrcă, A.C.; Oprea, O.-C.; et al. MAPLE coatings embedded with essential oil-conjugated magnetite for anti-biofilm applications. Materials 2021, 14, 1612. [Google Scholar] [CrossRef] [PubMed]
- Adebayo-Tayo, B.; Salaam, A.; Ajibade, A. Green synthesis of silver nanoparticle using Oscillatoria sp. extract, its antibacterial, antibiofilm potential and cytotoxicity activity. Heliyon 2019, 5, e02502. [Google Scholar] [CrossRef]
- Szymańska, E.; Orłowski, P.; Winnicka, K.; Tomaszewska, E.; Bąska, P.; Celichowski, G.; Grobelny, J.; Basa, A.; Krzyżowska, M. Multifunctional tannic Acid/Silver nanoparticle-based mucoadhesive hydrogel for improved local treatment of HSV infection: In Vitro and In Vivo studies. Int. J. Mol. Sci. 2018, 19, 387. [Google Scholar] [CrossRef]
- Rokitowski, K.L.; Hart, A.T.; Lutrario, C.A. Natural Oral Care Compositions. U.S. Patent Application No. 8715625B1, 6 May 2014. [Google Scholar]
- Trivedi, H.M.; Xu, T.; Herles, S. Oral Care Compositions Containing Compounds from Magnolia and Hops Extracts. U.S. Patent Application No. 20060134024A1, 12 February 2014. [Google Scholar]
- Bogdan, C.; Pop, A.; Iurian, S.M.; Benedec, D.; Moldovan, M.L. Research advances in the use of bioactive compounds from vitis vinifera by-products in oral care. Antioxidants 2020, 9, 502. [Google Scholar] [CrossRef]
- Lawlor, T.M. Oral Care Compositions. U.S. Patent Application No. 20050281758A1, 15 September 2004. [Google Scholar]
- Vezin, J.-C. Use of an Aqueous Grape Seed Extract Combined with at Least One Fluorine Salt to Combat the Formation or Accumulation of Dental Biofilm and Compositions Comprising Said Combination. CA Patent Application No. 2683880A1, 12 September 2017. [Google Scholar]
- Shine, L.J. Oral Rinse Composition and Method. U.S. Patent Application No. 8273385B1, 25 September 2012. [Google Scholar]
- Hurwitz, M.M. Oral Hygiene Tablets and Capsules for Direct Oral Delivery of Active Ingredients. U.S. Patent Application No. 8728446B2, 20 May 2014. [Google Scholar]
- Shuanghua, L. Gargle Tablet. CN Patent Application No. 1306814A, 25 January 2000. [Google Scholar]
- Choubey, S.; Varughese, L.R.; Kumar, V.; Beniwal, V. Medicinal importance of gallic acid and its ester derivatives: A patent review. Pharm. Pat. Anal. 2015, 4, 305–315. [Google Scholar] [CrossRef]
- White, T.F.A. Medicine for Treating Oral Diseases and Preparation Method Thereof. CN Patent Application No. 103202901A, 17 July 2013. [Google Scholar]
- Dolezal, D. Oral Care Products and Fromulations. WO Patent Application No. 2019018659, 21 January 2019. [Google Scholar]
- Moynihan, P.J. The role of diet and nutrition in the etiology and prevention of oral diseases. Bull. World Health Organ. 2005, 83, 694–699. [Google Scholar]
- Yoo, S.; Yang, H.C.; Lee, S.; Shin, J.; Min, S.; Lee, E.; Song, M.; Lee, D. A deep learning-based approach for identifying the medicinal uses of plant-derived natural compounds. Front. Pharmacol. 2020, 11, 584875. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).