Dry Matter Gains in Maize Kernels Are Dependent on Their Nitrogen Accumulation Rates and Duration during Grain Filling
Abstract
1. Introduction
2. Results
2.1. Grain Yield, Kernel Number per Plant, Kernel Weight and Final Kernel N Content
2.2. Plant Growth and N Uptake during the Effective Grain-Filling Period
2.3. Nitrogen Nutrition Index at Silking and at the Onset of the Linear Phase
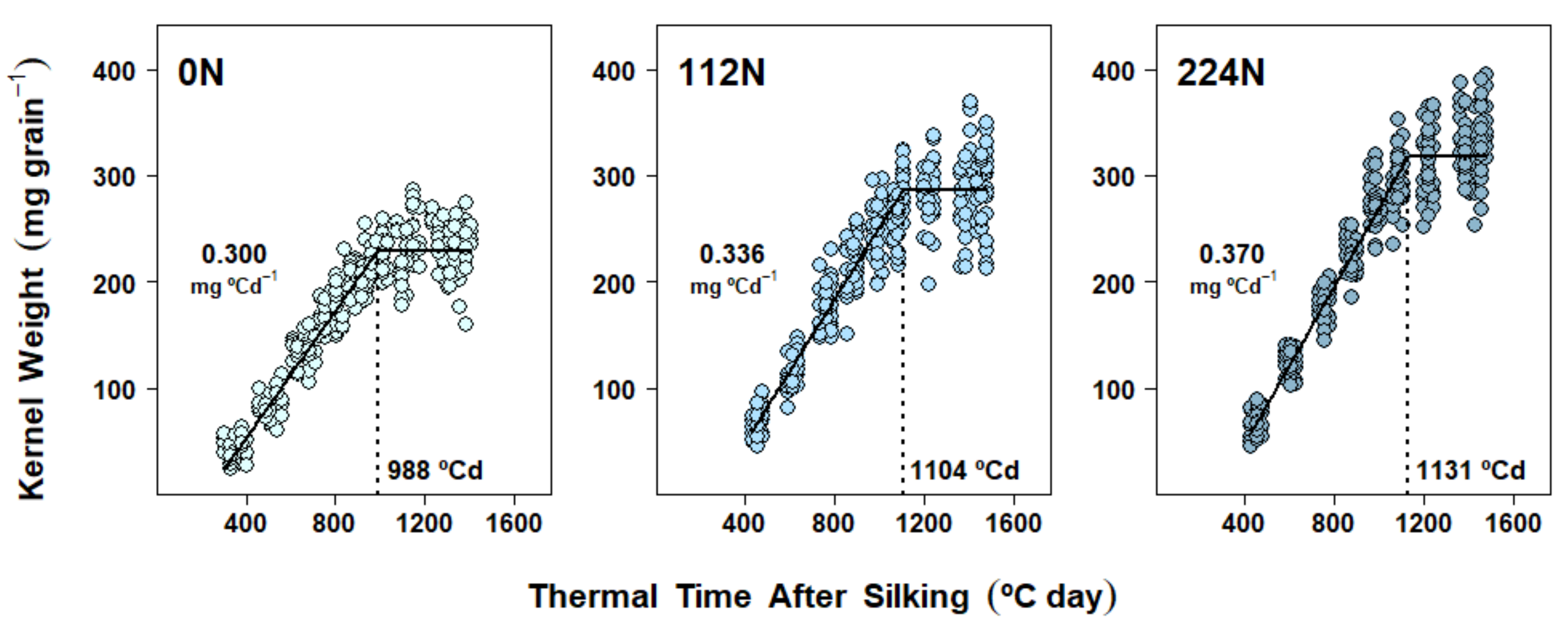
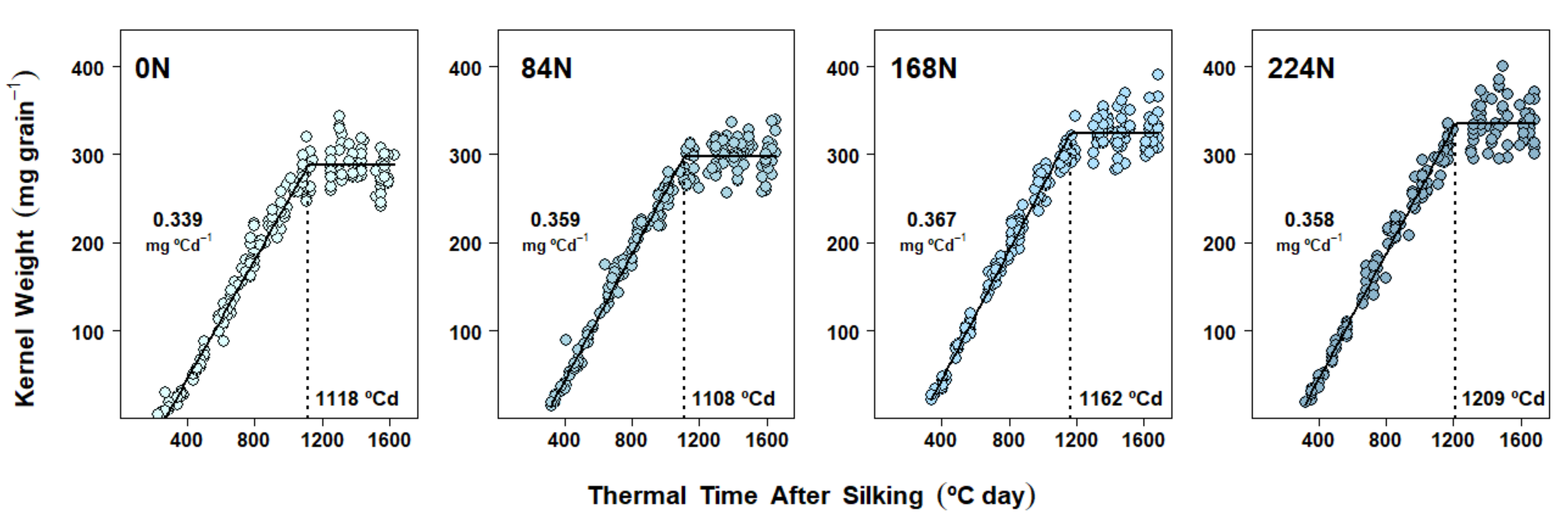
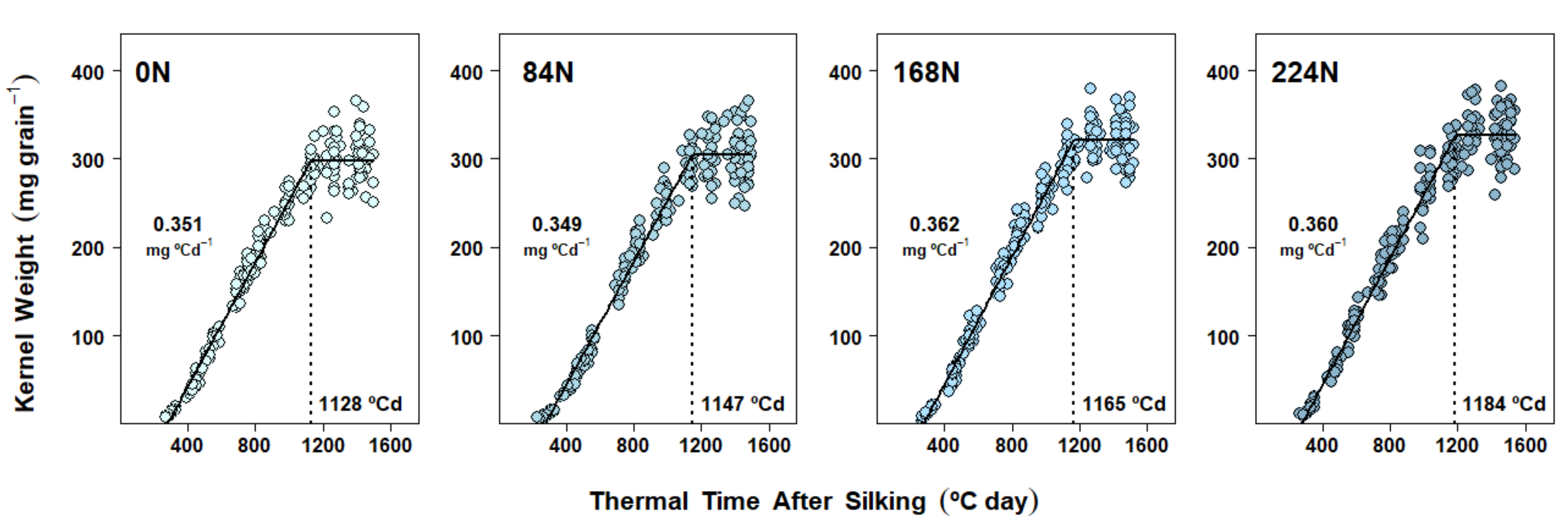
2.4. Dry Matter Accumulation Dynamics in Kernels
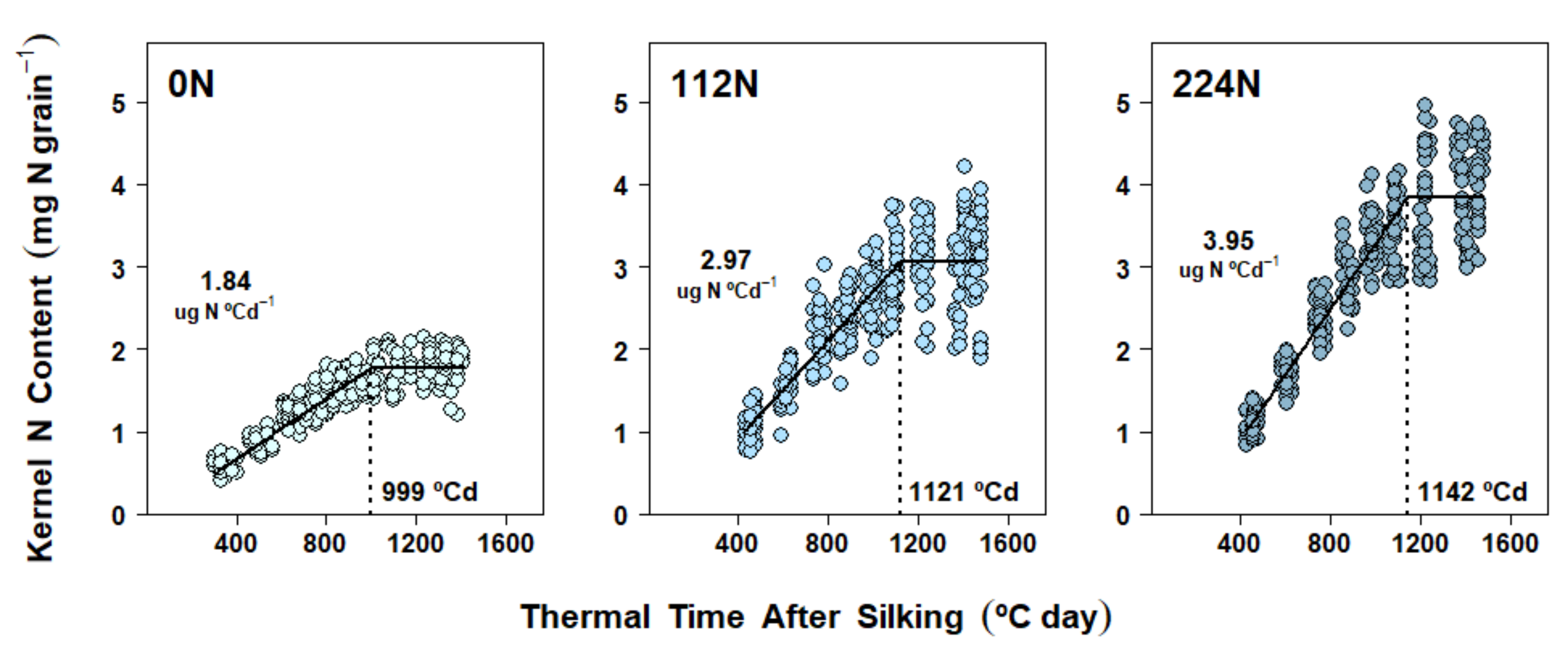
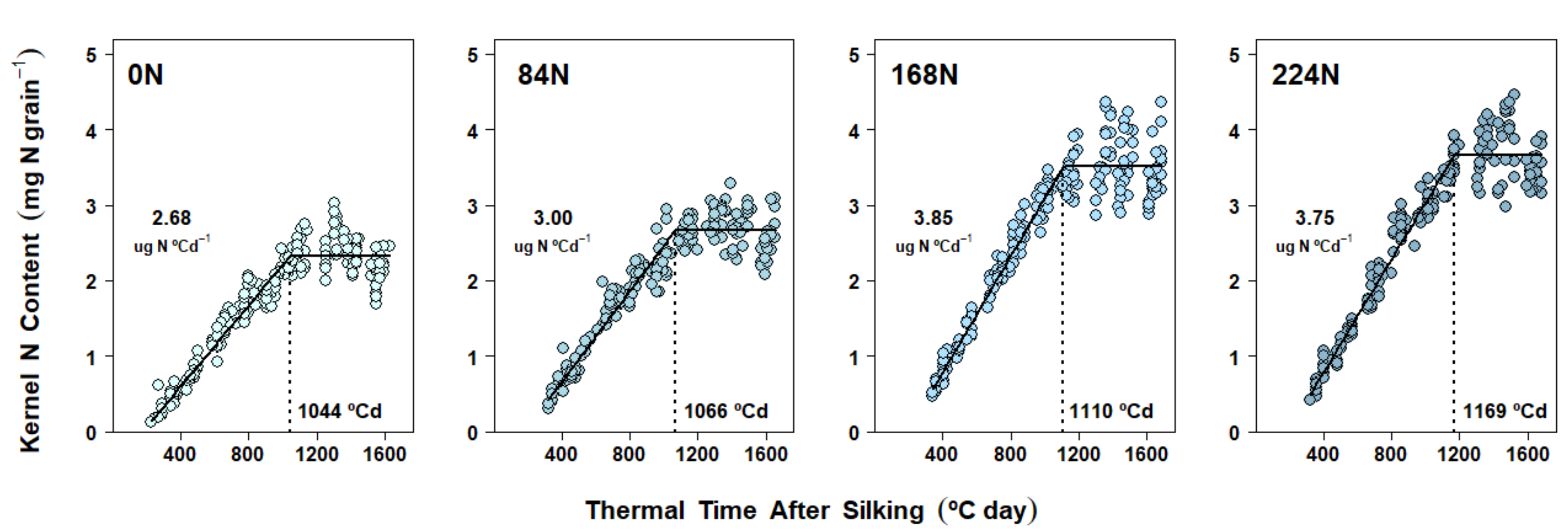
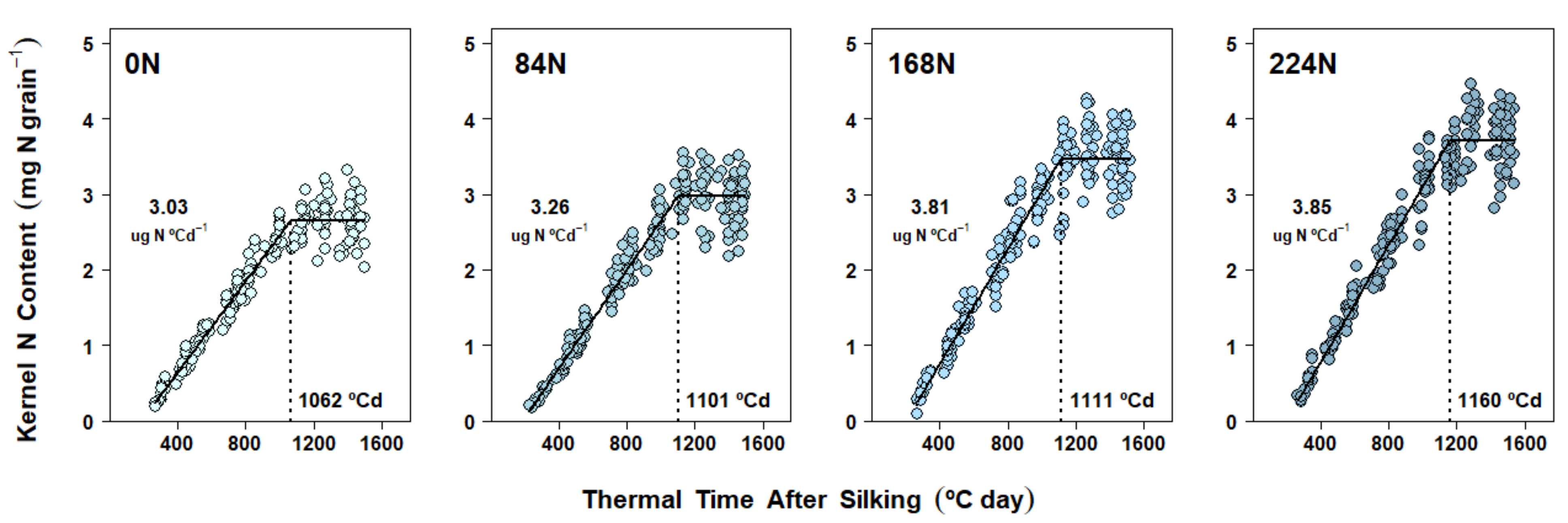
2.5. Nitrogen Accumulation Dynamics in Kernels
2.6. Relationships between Parameters
3. Discussion
3.1. Overview of Responses to Nitrogen Timing and Plant Density Treatments
3.2. Nitrogen Availability Effects on Kernel Dry Matter Accumulation Dynamics
3.3. Nitrogen Availability Effects on Kernel Nitrogen Accumulation Dynamics
3.4. Strong Relationships of Kernel Nitrogen and Dry Matter Gains during Linear Grain Fill
4. Materials and Methods
4.1. Field Experiments
4.2. Measurements and Calculations
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otegui, M.E. Prolificacy and grain yield components in modern Argentinian maize hybrids. Maydica 1995, 40, 371–376. [Google Scholar]
- Chapman, S.C.; Edmeades, G.O. Selection improves drought tolerance in tropical maize populations: II. Direct and correlated responses among secondary traits. Crop Sci. 1999, 39, 1315–1324. [Google Scholar] [CrossRef]
- Borrás, L.; Slafer, G.A.; Otegui, M.E. Seed dry weight response to source-sink manipulations in wheat, maize and soybean: A quantitative reappraisal. Field Crop. Res. 2004, 86, 131–146. [Google Scholar] [CrossRef]
- Andrade, F.H.; Ferreiro, M.A. Reproductive growth of maize, sunflower and soybean at different source levels during grain filling. Field Crop. Res. 1996, 48, 155–165. [Google Scholar] [CrossRef]
- Tollenaar, M. Sink-source relationships during reproductive development in maize. A review. Maydica 1977, 22, 49–75. [Google Scholar]
- Westgate, M.E.; Boyer, J.S. Reproduction at low silk and pollen water potentials in maize. Crop Sci. 1986, 26, 951–956. [Google Scholar] [CrossRef]
- Sadras, V.O. Evolutionary aspects of the trade-off between seed size and number in crops. Field Crop. Res. 2007, 100, 125–138. [Google Scholar] [CrossRef]
- Borrás, L.; Gambín, B.L. Trait dissection of maize kernel weight: Towards integrating hierarchical scales using a plant growth approach. Field Crop. Res. 2010, 118, 1–12. [Google Scholar] [CrossRef]
- Echarte, L.; Andrade, F.H.; Sadras, V.O.; Abbate, P. Kernel weight and its response to source manipulations during grain filling in Argentinean maize hybrids released in different decades. Field Crop. Res. 2006, 96, 307–312. [Google Scholar] [CrossRef]
- Chen, K.; Camberato, J.J.; Tuinstra, M.R.; Kumudini, S.V.; Tollenaar, M.; Vyn, T.J. Genetic improvement in density and nitrogen stress tolerance traits over 38 years of commercial maize hybrid release. Field Crop. Res. 2016, 196, 438–451. [Google Scholar] [CrossRef]
- Bonelli, L.E.; Monzon, J.P.; Cerrudo, A.; Rizzalli, R.H.; Andrade, F.H. Maize grain yield components and source-sink relationship as affected by the delay in sowing date. Field Crop. Res. 2016, 198, 215–225. [Google Scholar] [CrossRef]
- Zhou, B.; Yue, Y.; Sun, X.; Wang, X.; Wang, Z.; Ma, W.; Zhao, M. Maize grain yield and dry matter production responses to variations in weather conditions. Agron. J. 2016, 108, 196–204. [Google Scholar] [CrossRef]
- Jones, R.J.; Schreiber, B.M.N.; Roessler, J.A. Kernel sink capacity in maize: Genotypic and maternal regulation. Crop Sci. 1996, 36, 301–306. [Google Scholar] [CrossRef]
- Jones, R.J.; Roessler, J.; Ouattar, S. Thermal environment during endosperm cell division in maize: Effects on number of endosperm cells and starch granules. Crop Sci. 1985, 25, 830–834. [Google Scholar] [CrossRef]
- Johnson, D.R.; Tanner, J.W. Calculation of the rate and duration of grain filling in corn (Zea mays L.). Crop Sci. 1972, 12, 485–486. [Google Scholar] [CrossRef]
- Poneleit, C.G.; Egli, D.B. Kernel growth rate and duration in maize as affected by plant density and genotype. Crop Sci. 1979, 19, 385–388. [Google Scholar] [CrossRef]
- Borrás, L.; Westgate, M.E.; Otegui, M.E. Control of kernel weight and kernel water relations by post-flowering source-sink ratio in maize. Ann. Bot. 2003, 91, 857–867. [Google Scholar] [CrossRef]
- Westgate, M.E.; Boyer, J.S. Water status of the developing grain of maize. Agron. J. 1986, 78, 714–719. [Google Scholar] [CrossRef]
- Borrás, L.; Westgate, M.E. Predicting maize kernel sink capacity early in development. Field Crop. Res. 2006, 95, 223–233. [Google Scholar] [CrossRef]
- Rajcan, I.; Tollenaar, M. Source:sink ratio and leaf senescence in maize: I. Dry matter accumulation and partitioning during grain filling. Field Crop. Res. 1999, 60, 245–253. [Google Scholar] [CrossRef]
- Uhart, S.A.; Andrade, F.H. Nitrogen and carbon accumulation and remobilization during grain filling in maize under different source/sink ratios. Crop Sci. 1995, 35, 183–190. [Google Scholar] [CrossRef]
- D’Andrea, K.E.; Piedra, C.V.; Mandolino, C.I.; Bender, R.; Cerri, A.M.; Cirilo, A.G.; Otegui, M.E. Contribution of reserves to kernel weight and grain yield determination in maize: Phenotypic and genotypic variation. Crop Sci. 2016, 56, 697–706. [Google Scholar] [CrossRef]
- Reddy, V.; Daynard, T. Endosperm characteristics associated with rate of grain filling and kernel size in corn. Maydica 1983, 28, 339–355. [Google Scholar] [CrossRef]
- Gambín, B.L.; Borrás, L.; Otegui, M.E. Kernel water relations and duration of grain filling in maize temperate hybrids. Field Crop. Res. 2007, 101, 1–9. [Google Scholar] [CrossRef]
- Borrás, L.; Zinselmeier, C.; Senior, M.L.; Westgate, M.E.; Muszynski, M.G. Characterization of grain-filling patterns in diverse maize germplasm. Crop Sci. 2009, 49, 999–1009. [Google Scholar] [CrossRef]
- Rattalino Edreira, J.I.; Mayer, L.I.; Otegui, M.E. Heat stress in temperate and tropical maize hybrids: Kernel growth, water relations and assimilate availability for grain filling. Field Crop. Res. 2014, 166, 162–172. [Google Scholar] [CrossRef]
- Wang, J.; Kang, S.; Du, T.; Tong, L.; Ding, R.; Li, S. Estimating the upper and lower limits of kernel weight under different water regimes in hybrid maize seed production. Agric. Water Manag. 2019, 213, 128–134. [Google Scholar] [CrossRef]
- Hisse, I.R.; D’Andrea, K.E.; Otegui, M.E. Source-sink relations and kernel weight in maize inbred lines and hybrids: Responses to contrasting nitrogen supply levels. Field Crop. Res. 2019, 230, 151–159. [Google Scholar] [CrossRef]
- Wei, S.; Wang, X.; Li, G.; Qin, Y.; Jiang, D.; Dong, S. Plant density and nitrogen supply affect the grain-filling parameters of maize kernels located in different ear positions. Front. Plant Sci. 2019, 10, 180. [Google Scholar] [CrossRef]
- Liu, K.; Ma, B.L.; Luan, L.; Li, C. Nitrogen, phosphorus, and potassium nutrient effects on grain filling and yield of high-yielding summer corn. J. Plant Nutr. 2011, 34, 1516–1531. [Google Scholar] [CrossRef]
- Melchiori, R.J.M.; Caviglia, O.P. Maize kernel growth and kernel water relations as affected by nitrogen supply. Field Crop. Res. 2008, 108, 198–205. [Google Scholar] [CrossRef]
- Crawford, T.W.; Rendig, V.V.; Broadbent, F.E. Sources, fluxes, and sinks of nitrogen during early reproductive growth of maize (Zea mays L.). Plant Physiol. 1982, 70, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.L.; Camberato, J.J.; Jackson, W.A.; Moll, R.H. Utilization of previously accumulated and concurrently absorbed nitrogen during reproductive growth in maize. Plant Physiol. 1986, 82, 247–253. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. Grain nitrogen source changes over time in maize: A review. Crop Sci. 2013, 53, 366–377. [Google Scholar] [CrossRef]
- Singletary, G.W.; Below, F.E. Nitrogen-induced changes in the growth and metabolism of developing maize kernels grown in vitro. Plant Physiol. 1990, 92, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Cazetta, J.O.; Seebauer, J.R.; Below, F.E. Sucrose and nitrogen supplies regulate growth of maize kernels. Ann. Bot. 1999, 84, 747–754. [Google Scholar] [CrossRef]
- Chen, Q.; Mu, X.; Chen, F.; Yuan, L.; Mi, G. Dynamic change of mineral nutrient content in different plant organs during the grain filling stage in maize grown under contrasting nitrogen supply. Eur. J. Agron. 2016, 80, 137–153. [Google Scholar] [CrossRef]
- Ning, P.; Maw, M.J.W.; Yang, L.; Fritschi, F.B. Carbon accumulation in kernels of low-nitrogen maize is not limited by carbon availability but by an imbalance of carbon and nitrogen assimilates. J. Plant Nutr. Soil Sci. 2021, 1–10. [Google Scholar] [CrossRef]
- Lemaire, G.; Gastal, F. N uptake and distribution in plant canopies. In Diagnosis of the Nitrogen Status in Crops; Springer: Berlin/Heidelberg, Germany, 1997; pp. 3–43. [Google Scholar]
- Paul, M.J.; Foyer, C.H. Sink regulation of photosynthesis. J. Exp. Bot. 2001, 52, 1383–1400. [Google Scholar] [CrossRef]
- Fernie, A.R.; Bachem, C.W.B.; Helariutta, Y.; Neuhaus, H.E.; Prat, S.; Ruan, Y.L.; Stitt, M.; Sweetlove, L.J.; Tegeder, M.; Wahl, V.; et al. Synchronization of developmental, molecular and metabolic aspects of source–sink interactions. Nat. Plants 2020, 6, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Cerrudo, A.; Di Matteo, J.; Fernandez, E.; Robles, M.; Olmedo Pico, L.; Andrade, F.H. Yield components of maize as affected by short shading periods and thinning. Crop Pasture Sci. 2013, 64, 580–587. [Google Scholar] [CrossRef]
- Mueller, S.M.; Messina, C.D.; Vyn, T.J. Simultaneous gains in grain yield and nitrogen efficiency over 70 years of maize genetic improvement. Sci. Rep. 2019, 9, 9095. [Google Scholar] [CrossRef]
- Frey, N.M. Dry matter accumulation in kernels of maize. Crop Sci. 1981, 21, 118–122. [Google Scholar] [CrossRef]
- Below, F.E.; Cazetta, J.O.; Seebauer, J.R. Carbon/nitrogen interactions during ear and kernel development of maize. Crop Sci. 2000, 15–24. [Google Scholar] [CrossRef]
- Mueller, S.M.; Camberato, J.J.; Messina, C.; Shanahan, J.; Zhang, H.; Vyn, T.J. Late-split nitrogen applications increased maize plant nitrogen recovery but not yield under moderate to high nitrogen rates. Agron. J. 2017, 109, 2689. [Google Scholar] [CrossRef]
- Fernandez, J.A.; DeBruin, J.; Messina, C.D.; Ciampitti, I.A. Late-season nitrogen fertilization on maize yield: A meta-analysis. Field Crop. Res. 2019, 247, 107586. [Google Scholar] [CrossRef]
- Nasielski, J.; Deen, B. Nitrogen applications made close to silking: Implications for yield formation in maize. Field Crop. Res. 2019, 243, 107621. [Google Scholar] [CrossRef]
- Sala, R.G.; Westgate, M.E.; Andrade, F.H. Source/sink ratio and the relationship between maximum water content, maximum volume, and final dry weight of maize kernels. Field Crop. Res. 2007, 101, 19–25. [Google Scholar] [CrossRef]
- Chen, Y.; Hoogenboom, G.; Ma, Y.; Li, B.; Guo, Y. Maize kernel growth at different floret positions of the ear. Field Crop. Res. 2013, 149, 177–186. [Google Scholar] [CrossRef]
- DeBruin, J.L.; Schussler, J.R.; Mo, H.; Cooper, M. Grain yield and nitrogen accumulation in maize hybrids released during 1934 to 2013 in the US Midwest. Crop Sci. 2017, 57, 1431–1446. [Google Scholar] [CrossRef]
- Fernandez, J.A.; Messina, C.D.; Rotundo, J.; Ciampitti, I.A. Integrating nitrogen and water-soluble carbohydrates dynamics in maize: A comparison between hybrids from different decades. Crop Sci. 2020, 1–14. [Google Scholar] [CrossRef]
- Gallais, A.; Coque, M. Genetic variation and selection for nitrogen use efficiency in maize: A synthesis. Maydica 2005, 50, 531–547. [Google Scholar]
- Plénet, D.; Lemaire, G. Relationships between dynamics of nitrogen uptake and dry matter accumulation in maize crops. Determination of critical N concentration. Plant Soil 1999, 216, 65–82. [Google Scholar] [CrossRef]
- Pommel, B.; Gallais, A.; Coque, M.; Quilleré, I.; Hirel, B.; Prioul, J.L.; Andrieu, B.; Floriot, M. Carbon and nitrogen allocation and grain filling in three maize hybrids differing in leaf senescence. Eur. J. Agron. 2006, 24, 203–211. [Google Scholar] [CrossRef]
- Rajcan, I.; Tollenaar, M. Source: Sink ratio and leaf senescence in maize: II. Nitrogen metabolism during grain filling. Field Crop. Res. 1999, 60, 255–265. [Google Scholar] [CrossRef]
- Yang, L.; Guo, S.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Use of the stable nitrogen isotope to reveal the source-sink regulation of nitrogen uptake and remobilization during grain filling phase in maize. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef]
- Ning, P.; Fritschi, F.B.; Li, C. Temporal dynamics of post-silking nitrogen fluxes and their effects on grain yield in maize under low to high nitrogen inputs. Field Crop. Res. 2017, 204, 249–259. [Google Scholar] [CrossRef]
- Seebauer, J.R.; Singletary, G.W.; Krumpelman, P.M.; Ruffo, M.L.; Below, F.E. Relationship of source and sink in determining kernel composition of maize. J. Exp. Bot. 2010, 61, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.M.; Messina, C.D.; Vyn, T.J. The role of the exponential and linear phases of maize (Zea mays L.) ear growth for determination of kernel number and kernel weight. Eur. J. Agron. 2019, 111, 125939. [Google Scholar] [CrossRef]
- Borrás, L.; Otegui, M.E. Maize kernel weight response to postflowering source-sink ratio. Crop Sci. 2001, 41, 1816–1822. [Google Scholar] [CrossRef]
- Ritchie, S.W.; Hanway, J.J. How a Corn Plant Develops; Special Report N°48; Iowa State University of Science and Technology; Cooperative Extension Service, Iowa, EEUU: Ames, IO, USA, 1982. [Google Scholar]
- NOAA National Centers for Environmental Information Climate Data Online (CDO). Available online: https://www.ncdc.noaa.gov/cdo-web/ (accessed on 11 January 2021).
- INClimate—The Indiana State Climate Office Purdue Mesonet. Available online: https://ag.purdue.edu/indiana-state-climate/ (accessed on 11 January 2021).
- Etheridge, R.D.; Pesti, G.M.; Foster, E.H. A comparison of nitrogen values obtained utilizing the Kjeldahl nitrogen and Dumas combustion methodologies (Leco CNS 2000) on samples typical of an animal nutrition analytical laboratory. Anim. Feed Sci. Technol. 1998, 73, 21–28. [Google Scholar] [CrossRef]
- Muchow, R.C. Effect of high temperature on grain-growth in field-grown maize. Field Crop. Res. 1990, 23, 145–158. [Google Scholar] [CrossRef]
- R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2019. Available online: https://www.R-project.org/ (accessed on 5 February 2021).
- Pinheiro, J.; Bates, D.; DebRoy, S.; R Core Team. nlme: Nonlinear Mixed-Effects Models. 2021. Available online: https://cran.r-project.org/web/package=nlme (accessed on 19 March 2021).
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Lenth, R. emmeans: Estimated marginal means (Least-squares means). 2021. Available online: https://cran.r-project.org/package=emmeans (accessed on 26 March 2021).
- De Mendiburu, F. agricolae: Statistical Procedures for Agricultural Research. 2021. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 12 February 2021).
| GY | KNP | KW | KNc | KNC | PGR3 | PGR3.R6 | PNUR3 | PNUR3.R6 | NNIR1 | NNIR3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N Timing Application | |||||||||||
| Planting | 10.5 | 483 | 261.1 | 1.05 | 2.82 | 170 | 61 | 1.58 | 0.47 | 0.94 | 0.69 |
| Planting_V6 | 11.3 | 468 | 274.4 | 1.08 | 3.05 | 168 | 71 | 1.64 | 0.56 | 0.94 | 0.73 |
| Planting_V12 | 11.2 | 492 | 263.4 | 1.08 | 2.92 | 166 | 65 | 1.60 | 0.50 | 0.99 | 0.72 |
| N Rate (kg N ha−1) | |||||||||||
| 0 N | 5.3 c | 314 b | 213.3 c | 0.80 c | 1.71 c | 124 b | 15 c | 0.72 c | 0.15 c | 0.57 c | 0.40 c |
| 112 N | 11.9 b | 560 a | 271.3 b | 1.11 b | 3.02 b | 186 a | 78 b | 1.85 b | 0.43 b | 1.05 b | 0.80 b |
| 224 N | 15.7 a | 569 a | 314.3 a | 1.29 a | 4.07 a | 193 a | 103 a | 2.24 a | 0.96 a | 1.24 a | 0.94 a |
| F-test | |||||||||||
| N Timing (T) | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| N Rate (N) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| T × N | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| GY | KNP | KW | KNc | KNC | PGR3 | PGR3.R6 | PNUR3 | PNUR3.R6 | NNIR1 | NNIR3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N Rate (kg N ha−1) | |||||||||||
| 0 N | 7.4 d | 255 c | 267.7 b | 0.86 c | 2.29 c | 119 c | 29 b | 0.77 c | 0.13 b | 0.43 c | 0.44 c |
| 84 N | 11.4 c | 356 b | 283.9 b | 0.95 b | 2.70 b | 143 b | 54 b | 1.20 b | 0.20 b | 0.70 b | 0.63 b |
| 168 N | 15.3 b | 475 a | 309.6 a | 1.13 a | 3.50 a | 164 ab | 87 a | 1.94 a | 0.28 b | 1.01 a | 0.97 a |
| 224 N | 16.6 a | 505 a | 318.3 a | 1.15 a | 3.65 a | 165 a | 110 a | 2.03 a | 0.64 a | 1.08 a | 0.95 a |
| Plant Density (plant m−2) | |||||||||||
| 7.9 D | 12.8 | 455 a | 301.7 a | 1.03 | 3.13 | 163 a | 86 a | 1.70 a | 0.37 | 0.82 | 0.77 |
| 10.4 D | 12.5 | 340 b | 288.0 b | 1.01 | 2.95 | 131 b | 54 b | 1.26 b | 0.25 | 0.79 | 0.73 |
| F-test | |||||||||||
| N Rate (N) | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | 0.005 | 0.004 | <0.001 | 0.026 | <0.001 | <0.001 |
| Density (D) | ns | <0.001 | 0.010 | ns | ns | <0.001 | 0.001 | 0.002 | ns | ns | ns |
| N × D | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| GY | KNP | KW | KNc | KNC | PGR3 | PGR3.R6 | PNUR3 | PNUR3.R6 | NNIR1 | NNIR3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N Rate (kg N ha−1) | |||||||||||
| 0 N | 7.5 c | 288 c | 264.9 c | 0.88 c | 2.34 c | 127 b | 26 b | 0.88 b | 0.10 | 0.57 c | 0.50 b |
| 84 N | 9.5 b | 342 b | 282.3 b | 0.95 bc | 2.68 bc | 131 b | 47 a | 1.06 b | 0.20 | 0.74 b | 0.60 b |
| 168 N | 11.0 ab | 406 a | 295.3 ab | 1.06 ab | 3.12 ab | 169 a | 47 a | 1.66 a | 0.19 | 0.94 a | 0.77 a |
| 224 N | 12.2 a | 424 a | 299.2 a | 1.16 a | 3.48 a | 167 a | 60 a | 1.84 a | 0.20 | 0.98 a | 0.87 a |
| Plant Density (plant m−2) | |||||||||||
| 7.9 D | 10.0 | 405 a | 287.6 | 1.03 | 2.97 a | 162 a | 50 | 1.51 a | 0.20 | 0.82 | 0.69 |
| 10.4 D | 10.1 | 325 b | 283.2 | 1.00 | 2.84 b | 135 b | 39 | 1.21 b | 0.14 | 0.80 | 0.68 |
| F-test | |||||||||||
| N Rate (N) | 0.004 | <0.001 | 0.008 | 0.008 | 0.004 | <0.001 | 0.011 | 0.002 | ns | 0.002 | 0.006 |
| Density (D) | ns | <0.001 | ns | ns | 0.012 | <0.001 | ns | 0.012 | ns | ns | ns |
| N × D | 0.024 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| GY | KNP | KW | KNC | EGFR | GFD | KNAD | KNAR | PGR3 | PGR3.R6 | PNUR3 | PNUR3.R6 | NNIR1 | NNIR3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GY | 0.84 | 0.90 | 0.95 | 0.74 | 0.69 | 0.86 | 0.83 | 0.80 | 0.97 | 0.93 | 0.74 | 0.89 | 0.96 | |
| KNP | ** | 0.61 | 0.83 | 0.42 | 0.36 | 0.77 | 0.60 | 0.95 | 0.90 | 0.94 | 0.81 | 0.96 | 0.88 | |
| KW | *** | * | 0.92 | 0.93 | 0.89 | 0.87 | 0.95 | 0.65 | 0.82 | 0.81 | 0.56 | 0.73 | 0.86 | |
| KNC | *** | ** | *** | 0.80 | 0.73 | 0.92 | 0.92 | 0.86 | 0.91 | 0.96 | 0.75 | 0.92 | 0.96 | |
| EGFR | ** | ns | *** | ** | 0.84 | 0.71 | 0.92 | 0.52 | 0.61 | 0.64 | 0.38 | 0.57 | 0.71 | |
| GFD | * | ns | *** | * | ** | 0.83 | 0.88 | 0.42 | 0.59 | 0.60 | 0.25 | 0.51 | 0.69 | |
| KNAD | *** | ** | *** | *** | * | ** | 0.88 | 0.79 | 0.83 | 0.89 | 0.60 | 0.86 | 0.90 | |
| KNAR | ** | * | *** | *** | *** | *** | *** | 0.69 | 0.72 | 0.82 | 0.48 | 0.77 | 0.87 | |
| PGR3 | ** | *** | * | *** | ns | ns | ** | * | 0.81 | 0.95 | 0.73 | 0.96 | 0.88 | |
| PGR3.R6 | *** | *** | ** | *** | * | . | ** | * | ** | 0.92 | 0.83 | 0.89 | 0.92 | |
| PNUR3 | *** | *** | ** | *** | * | . | *** | ** | *** | *** | 0.75 | 0.98 | 0.98 | |
| PNUR3.R6 | ** | ** | . | ** | ns | ns | * | ns | * | ** | ** | 0.78 | 0.66 | |
| NNIR1 | *** | *** | * | *** | . | ns | *** | ** | *** | *** | *** | ** | 0.94 | |
| NNIR3 | *** | *** | *** | *** | * | * | *** | *** | *** | *** | *** | * | *** |
| Study | Location | Planting Date | Plant Density | N Rate | N Timing Application | Plot Size | Reps |
|---|---|---|---|---|---|---|---|
| Exp. 1 | La Crosse, IN | 16 May 2017 | 8.3 plants m−2 | Sub-plot : 0 112 kg N ha−1224 kg N ha−1 | Whole plot: At planting Split between planting and V6 Last 56 kg N ha−1 at V12 | 12 rows 0.76 m row width228 m row length | 3 |
| Exp. 2 | West Lafayette, IN | 8 May 2018 | Sub-plot: 7.9 plants m−2 10.4 plants m−2 | Whole plot: 0 84 kg N ha−1 168 kg N ha−1 224 kg N ha−1 | At planting | 4 rows 0.76 m row width 14.5 m row length | 3 |
| Exp. 3 | West Lafayette, IN | 3 June 2019 | Sub-plot: 7.9 plants m−2 10.4 plants m−2 | Whole plot: 0 84 kg N ha−1 168 kg N ha−1 224 kg N ha−1 | At planting | 4 rows 0.76 m row width 14.5 m row length | 3 |
| Climate Parameter | Growth Stage Interval | Experiment 1 | Experiment 2 | Experiment 3 |
|---|---|---|---|---|
| Mean Minimum Temperature (°C) | P-V12 | 13.3 | 16.2 | 17.5 |
| V12-R1 | 16.1 | 18.8 | 15.0 | |
| R1-R3 | 15.5 | 16.9 | 16.4 | |
| R3-R6 | 11.7 | 17.0 | 13.8 | |
| Mean Maximum Temperature (°C) | P-V12 | 26.6 | 27.6 | 28.3 |
| V12-R1 | 26.8 | 29.2 | 27.6 | |
| R1-R3 | 27.2 | 28.2 | 28.8 | |
| R3-R6 | 25.1 | 27.3 | 26.1 | |
| Cumulative Rainfall (mm) | P-V12 | 201 | 201 | 127 |
| V12-R1 | 79 | 48 | 4 | |
| R1-R3 | 96 | 14 | 29 | |
| R3-R6 | 84 * | 164 | 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedo Pico, L.B.; Vyn, T.J. Dry Matter Gains in Maize Kernels Are Dependent on Their Nitrogen Accumulation Rates and Duration during Grain Filling. Plants 2021, 10, 1222. https://doi.org/10.3390/plants10061222
Olmedo Pico LB, Vyn TJ. Dry Matter Gains in Maize Kernels Are Dependent on Their Nitrogen Accumulation Rates and Duration during Grain Filling. Plants. 2021; 10(6):1222. https://doi.org/10.3390/plants10061222
Chicago/Turabian StyleOlmedo Pico, Lía B., and Tony J. Vyn. 2021. "Dry Matter Gains in Maize Kernels Are Dependent on Their Nitrogen Accumulation Rates and Duration during Grain Filling" Plants 10, no. 6: 1222. https://doi.org/10.3390/plants10061222
APA StyleOlmedo Pico, L. B., & Vyn, T. J. (2021). Dry Matter Gains in Maize Kernels Are Dependent on Their Nitrogen Accumulation Rates and Duration during Grain Filling. Plants, 10(6), 1222. https://doi.org/10.3390/plants10061222






