Abstract
The traditional Ecuadorian spice Ishpingo, characterized by a strong cinnamon-like aroma, is constituted by the dry cupules of Amazonian species Ocotea quixos. Nevertheless, bark and leaves also present aromatic properties and are sometimes used as substitutes. In the present study, the essential oils, distilled from these morphological structures, are comparatively analyzed for their chemical and enantiomeric compositions. A total of 88 components were identified with 2 orthogonal GC columns, whereas 79, corresponding to more than 94%, were also quantified with at least 1 column. Major compounds were (E)-methyl cinnamate in cupules (35.9–34.2%), (E)-cinnamaldehyde in bark (44.7–47.0%), and (E)-cinnamyl acetate (46.0–50.4%) in leaves. For what concerns the enantioselective analysis, 10 chiral terpenes and terpenoids were detected, of which 6 were present as enantiomeric pairs in at least 1 essential oil, the others being enantiomerically pure. Both quantitative and enantioselective analyses were submitted to Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA), where their results confirmed significative difference among the three products.
1. Introduction
Despite that many countries are characterized by an important biodiversity all around the world, only 17 of them are known as “megadiverse”, as they account together for “two thirds of all non-fish vertebrate species and three quarters of all higher plant species” []. According to the UN Environment Programme, there are 17 megadiverse countries, including Ecuador. This is the reason why Ecuador is an invaluable reservoir of native and endemic botanical species, most of them unprecedented as sources of new bioactive natural products []. In this context, the authors have been studying for a long time the secondary metabolites of the Ecuadorian flora, as a contribution to the knowledge in its phytochemistry and phytopharmacology [,,,,,,,]. The reasons of our interest in new EOs reside in the simplicity of their obtention, the potential commercial interest, the range of biological activities (most of them unexplored), and the fact that, so far, gas chromatography-olfactometry (GC-O) and enantioselective analysis are not frequently applied to EO investigation [,,,,,,,].
However, despite new natural products and unexplored species undoubtedly arousing the highest interest from an academic point of view, many already studied plants may also need deeper investigation. That is why the present study focused on the EO of a major traditional species within the Ecuadorian flora: Ocotea quixos.
Ocotea quixos (Lam.) Kosterm. belongs to the family Lauraceae, which is also known with the accepted name Mespilodaphne quixos (Lam.) Rohwer and the synonyms Laurus quixos Lam. and Nectandra cinnamomoides (Kunth) Nees. Ocotea quixos Kosterm. ex O.C. Schmidt is a homonym []. Despite that some specimens have been identified also in Colombia and Peru, the very main area of dispersion corresponds to Ecuador, where it is one of the 48 species of this genus known in the country. Ocotea quixos is a tree, both native and cultivated, growing in the Amazonian region between 0 and 1000 m above sea level []. This plant is quite popular in Ecuador, where it is traditionally used as a food aroma, due to its strong similarity with cinnamon (Cinnamomum verum J. Presl) and to whose botanical family it belongs. The main spice, i.e., the plant derivative assuming the highest commercial importance, is constituted by the dry cupules. Other parts of the plant, such as bark and leaves, also characterized by a cinnamon-like aroma, are commonly used. Furthermore, O. quixos is a very slow-growing species, whose cupules can be collected only biyearly, from plants 15–20 years old. All these conditions make the production quite limited and, consequently, the spice is very expensive [].
For what concerns the previous studies about O. quixos EOs, some important articles must be cited. On one hand, the chemical composition of volatile fractions, distilled from cupules, leaves and bark, were separately described [,,,]. For cupules, Bruni et al.’s study shows that the principal components detected were (E)-cinnamaldehyde, methylcinnamate and 1-8-cineol []. Sacchetti et al. found that the main components on O. quixos leaves were (E)-β-caryophyllene, (E)-cinnamyl acetate, sabinene, geranial and (E)-cinnamaldehyde []; whereas Valarezo et al. showed that the major components in leaves’ samples were (E)-cinnamyl acetate, (E)-methyl cinnamate and (E)-β-caryophyllene []. Finally, bark EO was studied by Noriega et al., showing that the main components were (E)-cinnamaldehyde, (E)-o-methoxy cinnamaldehyde, (E)-cinnamyl acetate and (E)-methyl cinnamate [].
On the other hand, many biological activities, such as antimicrobial, antioxidant, antiplatelet, anti-inflammatory and larvicidal against Aedes aegypti, were demonstrated for some of these EOs [,,,]. More recently, we described the termiticidal and repellent activity of the leaves’ EO against Nasutitermes corniger [].
The aim of the present study was to investigate the similarity among the EOs obtained from the three main morphological structures (cupules, bark and leaves). Additionally, the enantioselective analysis of the chiral components was carried out for the first time. This investigation was conducted as a component of a great governmental programme, dedicated to forest conservation and sustainable productions, through the application of non-timber forest products []. To the best of the authors’ knowledge, this is the first investigation of O. quixos EOs about these items.
2. Results
2.1. Qualitative and Quantitative Chemical Analyses
Three essential oils were obtained from dry cupules, bark and leaves of O. quixos, with a distillation yield by weight of 1.79%, 1.04% and 1.52%, respectively. Both qualitative and quantitative analyses were carried out by capillary gas chromatography (GC), over non-polar (polydimethylsiloxane and 5% phenyl groups) and polar (polyethylene glycol) stationary phases. In the qualitative analysis, the GC instrument was coupled with a mass spectrometer (GC-MS), whereas a flame ionization detector was applied to the quantitative one (GC-FID). A total of 88 components were identified, of which 79 were also quantified in at last one EO, with at least one column. All the quantified metabolites corresponded to more than 94% of the whole EO mass in every case. The main fraction was constituted by shikimic acid derivatives (74.5–89.1%) for all the morphological structures. They could be identified as derivatives of cinnamic acid, which is perfectly consistent with the strong cinnamon-like odor of all these EOs. In particular, (E)-methyl cinnamate was the major constituent of cupules’ EO (35.9–34.2%), (E)-cinnamaldehyde was most abundant in bark volatile fraction (44.7–47.0%), whereas leaves were dominated by (E)-cinnamyl acetate (46.0–50.4%). After phenylpropanoids, the second main fraction was constituted by monoterpenes. In this case, they altogether ranged from about 3% in leaves, to about 19% in cupules. The complete chemical analyses are reported in Table 1, whereas the comparative chromatograms are represented in Figure 1.

Table 1.
Chemical analysis of the EOs from the three morphological structures, with both DB-5ms and HP-INNOWax columns.
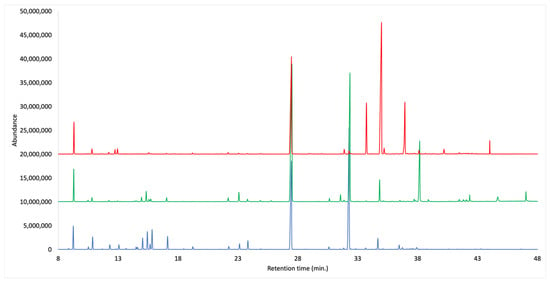
Figure 1.
GC-MS chromatograms with DB-5ms column of the EOs from cupules, bark and leaves of O. quixos.
2.2. Enantioselective Analysis
Since the monoterpene fraction is not negligible in these EOs, an enantioselective analysis was carried out through a cyclodextrin-based capillary column. A total of 10 chiral terpenes and terpenoids were detected, of which 6 were present as enantiomeric pairs in at least one essential oil, the others being enantiomerically pure. This was the case of (1R,4S)-(-)-camphene, (R)-(-)-linalool and (1R,2S,6S,7S,8S)-(-)-α-copaene; whereas, in many other cases, the compound being enantiomerically pure is a morphological structure but presented an enantiomeric excess in the others (α-pinene, β-pinene, α-phellandrene, limonene and terpinen-4-ol). The complete enantioselective analysis is detailed in Table 2.

Table 2.
Enantioselective analysis of the EOs with a 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin-based column.
2.3. Statistical Analysis
To treat the problem more objectively, the data obtained was submitted to a PCA (Principal Component Analysis) and HCA (Hierarchical Clustering Analysis) approach. PCA based on 76 essential oil compounds resulted in two principal components: F1 (36.08%) and F2 (21.85%) with 57.9% of the total variance. The PCA showed three different groups corresponding to cupules, leaves and bark essential oils (Figure 2). These results allow to establish that, from the point of view of their EO chemical composition, the three morphological parts can be considered as different among them.
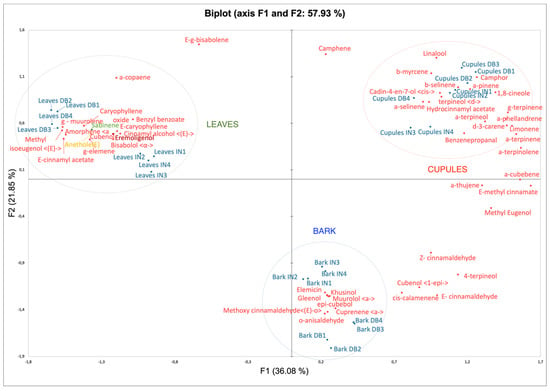
Figure 2.
Principal Component Analysis (PCA) of leaves, bark and cupules and observations and variables Biplot Analysis.
On one hand, the main contributors in leaves’ PCA were (E)-cinnamyl acetate, (E)-methyl isoeugenol, γ-muurolene, amorphene, caryophyllene oxide and cinnamyl alcohol, among others. In the case of barks, they were (E)-o-methoxy cinnamaldehyde, o-anisaldehyde, cuprenene, epicubebol, muurolol, elemicin, khusinol and gleenol. In cupules, they were camphor, β-selinene, α-pinene, 1,8-cineole and cis-cadin-4-en-7-ol. (E)-cinnamaldehyde and (E)-methyl cinnamate are principal contributors between cupules and bark (Figure 2). On the other hand, through HCA, it is possible to consider that barks and cupules’ essential oils are more similar among them than leaves’ essential oils.
3. Discussion
The present study demonstrated that the essential oils, distilled from the three main morphological structures of O. quixos, are significantly different from both the chemical and enantiomerical point of view. For this reason, instead of simply concluding that they cannot be used indifferently, it would be better to indicate the different properties and possible applications, according to what is known in literature about the respective main components. For what concerns the chemical composition, the EOs from cupules and bark are dominated by (E)-methyl cinnamate and (E)-cinnamaldehyde (although in different relative abundance), whereas the one from leaves is characterized by (E)-cinnamyl acetate and (E)-cinnamaldehyde. Since (E)-cinnamaldehyde is common to all these EOs, we could suppose that it exerts its properties in all cases, with the highest activity in bark and the lowest activity in leaves. According to literature, (E)-cinnamaldehyde presented important antifungal, antibacterial, larvicidal, repellent and anti-diabetic activities [,,,,]. The antifungal activity was widely investigated, obtaining excellent in vitro results against Aspergillus flavus, Coriolus versicolor, Laetiporus sulphureus, Saccharomyces cerevisiae, Aspergillus parasiticus, Aspergillus niger, Candida albicans, Collectotrichum gloeosporioides, Rhizoctonia solani, Fusarium solani and Ganoderma austral, most of which are pathogenic. Furthermore, this molecule resulted remarkably active against fluconazole-resistant strains of C. albicans. The (E)-cinnamaldehyde antifungal mechanism is supposed to be varied, probably interfering with the cell wall biosynthesis, the membrane functions and inhibiting important enzymes []. On the other hand, the antibacterial activity has been mainly described for (E)-cinnamaldehyde-dominated EOs, where our molecule was confirmed to be the active component. Both Gram-positive and Gram-negative bacteria resulted to be sensitive to (E)-cinnamaldehyde, which is quite interesting since usually Gram-negative bacteria are more resistant to antibiotics. The main reason for this property is the ability of (E)-cinnamaldehyde to inhibit porins (membrane proteins), affecting the osmotic equilibrium of the cell and increasing its vulnerability. However, many mechanisms other than osmotic interference have been confirmed, such as ATPase inhibition, cell division inhibition, mobility inhibition, biofilm formation and anti-quorum sensing effect. However, the most important property is probably the synergic antibacterial effect, resulting in the increased efficacy of classical antibiotics when administrated in addition to (E)-cinnamaldehyde []. Another important biological activity of this metabolite is its toxicity against Culex pipiens pallens, a subspecies of common mosquito, and Aedes aegypti larvae. These activities, that can be exerted respectively by fumigation and irroration, open the way to possible applications in the Amazonian Forest, where O. quixos is cultivated and biting pests are responsible for spreading serious diseases [,]. Furthermore, we must consider the anti-diabetic properties of (E)-cinnamaldehyde that was widely assayed in animal models, showing a variety of physiological effects on different tissues and organs, all converging toward the control of glycemia []. About (E)-methyl cinnamate, many biological activities such as antibacterial, antifungal, antispasmodic, myorelaxant and anti-inflammatory have been described. However, the most recent and interesting property is probably its activity against pre-osteoblast survival, migration and differentiation, which suggests possible applications to the treatment of many bone diseases []. Finally, to speculate over possible activities for our leaves’ EO, the properties of (E)-cinnamyl acetate must be considered. Despite that this compound is quite less investigated in respect to the other cinnamic derivatives, the main biological activity is an excellent anti-inflammatory capacity. The pharmacological mechanism is the suppression of nitric oxide production []. All these observations, together with the statistical results previously described, can be considered quite representative of the different EOs obtained from O. quixos. In fact, their quali-quantitative chemical compositions are very consistent with those presented in literature by other authors.
The chemical analysis of the three essential oils substantially confirms the analytical results present in literature for the volatile fractions of O. quixos [,,,]. Furthermore, an enantioselective evaluation of the terpene components was carried out here for the first time. For what concerns the chemical composition, a simplified representation is shown in Figure 3.
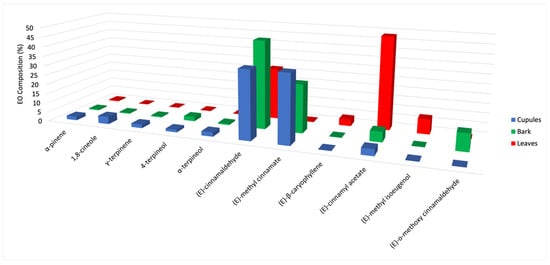
Figure 3.
Simplified comparative histogram of the mean quantitative analyses for major components (>2% for at least one EO).
In this graph, only those constituents, whose abundance is >2% by at least one column, are represented. From a qualitative point of view, a fact immediately stood up: the relative abundance of the three main components appeared dramatically different within the samples. On one hand, the main component changed in each EO, being (E)-methyl cinnamate dominant in cupules, (E)-cinnamaldehyde in bark and (E)-cinnamyl acetate in leaves. On the other hand, (E)-methyl cinnamate is quite absent where (E)-cinnamyl acetate is overwhelming (leaves), whereas the opposite is observed in cupules and bark. These observations could induce to think that the three EOs are basically different and, subsequently, the three morphological structures are not so similar in their EO composition to be used as spices or equivalent sources of a cinnamon-like aroma.
For what concerns the enantioselective analysis, a quite interesting phenomenon aroused. In fact, where a chiral compound was detected in more than one EO, the enantiomeric distribution was not respected everywhere. This is the case of α-phellandrene, limonene, terpinene-4-ol and α-terpineol, whose major enantiomer is levorotatory in a certain morphological structure and dextrorotatory in another. In this respect, the enantiomer composition of limonene is the most peculiar, since it appears to be enantiomerically pure in cupules (dextrorotatory), undetected in barks and almost racemic in leaves (with the levorotatory isomer as the major one). Anyway, the sum of analyzed chiral components corresponded to 8.3%, 4.3% and 3.2% of the total EO for cupules, bark and leaves, respectively. These amounts are quite small to imagine a great influence of the enantiomeric distribution on the total aroma profile. Actually, none of these chiral terpenes is a major constituent of any EO. These results are represented in Figure 4.
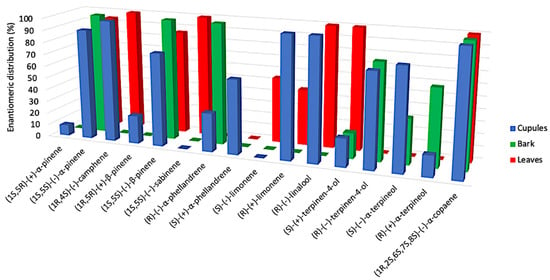
Figure 4.
Comparative histogram of the enantioselective analysis for the three EOs.
4. Materials and Methods
4.1. Instruments and Chemicals
Essential oil qualitative and enantioselective analyses were performed using an Agilent Technologies GC-MS system (GC 6890N, Autoinjector model 7683). The device was coupled to a mass spectrometry detector (MSD) model 5973 INERT from Agilent Technologies (Santa Clara, CA, USA). The MSD was programmed in SCAN mode (range 40–350 m/z) through an electron ionization (EI) source at 70 eV. The transfer line temperature was set at 280 °C, whereas the MS ion source was kept at 200 °C. For quantitative analyses, a common flame ionization detector (FID) was used instead of MSD. The FID worked with a mixture of hydrogen and air at the flow of 30 mL/min and 300 mL/min, respectively. The detector was operated at 250 °C. The non-polar column was composed by a 5% phenyl methylpolysiloxane stationary phase (DB-5ms from Agilent Technologies, 30 m long, 0.25 mm internal diameter and 0.25 μm film thickness), while the polar column was fit with a polyethylene glycol phase (HP-INNOWax from Agilent Technologies, 30 m × 0.25 mm × 0.25 μm). The enantioselective analysis was achieved into 30% diethyl-tert-butyldimethylsilyl-β-cyclodextrin capillary column on PS-086 as the chiral selector. The column was 25 m × 250 μm internal diameter × 0.25 μm phase thickness and was purchased from Mega, MI, Italy. Helium purity grade (Indura, Guayaquil, Ecuador) was used as gas for GC analyses at a 1 mL/min flow rate. The C10–C25 n-alkanes mixture, the internal standard for GC analysis (n-nonane) and all analytical grade solvents (purity >99%) were acquired from Sigma-Aldrich. Isopropyl caproate, as calibration standard for GC-FID, was produced and purified in the authors’ laboratory at 98.8% purity.
Statistical analyses were carried out with XLSTAT (Version 2021.3.1.1155, Addinsoft, Paris, France) and MS Excel (Version 16.51, Redmond, WA, USA).
4.2. Plant Material
Dry cupules, bark and leaves of O. quixos were purchased in 2019 from the Chankuap Foundation (Soasti, 10 de Agosto y Tarqui, Morona Santiago, Macas, Ecuador). All vegetal materials were obtained from cultivated plants, grown by the foundation’s rural providers in Morona-Santiago province (Amazonia) of Ecuador.
4.3. EO Distillation and Sample Preparation
An amount of 100 g of plant material from each morphological structure was distilled in duplicate, through a laboratory-scale glassy Dean-Stark apparatus, obtaining six EO samples. Each distillation was carried out for 2 h, affording an organic phase that spontaneously separated from water. After separation, the EOs were dried over anhydrous sodium sulphate and kept in darkness, at −15 °C, until use.
For all chemical and enantioselective analyses, the samples were prepared as previously described in literature []. In this process, two samples were prepared from each EO, finally counting on a total of 12 analytical samples, that were directly injectable in GC.
4.4. Chemical Qualitative Analysis
In qualitative analyses, a representative analytical sample of each EO was submitted to GC-MS over both polar and non-polar stationary phases. With each column, the n-alkanes homologous series was also injected. The GC conditions with DB-5ms were as follows: 50 °C as initial constant temperature for 5 min, subsequently, a gradient of 3 °C/min until 155 °C, followed by an additional gradient of 15 °C/min until 250 °C. The last temperature was maintained constant for 2 min. With the INNOWax column, the thermal program was identical, except for the last temperature that was fixed at 230 °C. The injector was set at 250 °C and operated in split mode (40:1) with both columns. As usual, all the analytes were identified, with both columns, by comparing each EI-MS spectra and the respective linear retention index (LRI) with data from literature (see Table 1). The LRIs were calculated according to Van Den Dool and Kratz [].
4.5. Chemical Quantitative Analysis
The quantitative analyses were carried out injecting the 12 analytical samples in both columns and expressing the percent results as mean values and standard deviation. The GC configuration and analytical method were the same as the qualitative analysis. The quantification was conducted by means of a relative response factor (RRF) that was calculated based on each analyte’s combustion enthalpy [,]. A calibration curve was built for each column as previously described in literature [], using isopropyl caproate as calibration standard and n-nonane as internal standard.
4.6. Enantioselective Analysis
The enantiomeric composition was evaluated by GC-MS, injecting a representative sample for each EO. The MSD configuration and injection condition were the same as the qualitative analysis, whereas the thermal program was as follows: 50 °C for 5 min, then a gradient of 2 °C/min until 220 °C, finally, 220 °C for 5 min. The enantiomers were determined by injecting enantiomerically pure standards in the same column and applying identical instrumental conditions.
4.7. Statistical Analysis
The different observations (chemical quantitative and enantioselective) were analyzed through Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA) to assess the differences in chemical composition of essential oil among different parts of the plant and the different GC-FID analysis performed (DB-5ms and HP-INNOWax). PCA used correlation matrix (Pearson matrix). HCA was performed with Euclidean distance and Ward’s minimum variance method. Standard deviation (σ) was calculated using MS Excel.
5. Conclusions
In conclusion, each one of the three main dry morphological structures of O. quixos produced, by hydrodistillation, an EO in similar and very good yields. On one hand, the statistical analyses demonstrated that the three EOs must be considered different from both the chemical and enantiomeric points of view. On the other hand, despite the analyzed fraction of chiral constituents which did not exceed 8% of the whole EO, some monoterpenes (α-pinene, β-pinene and limonene) are known to be characterized by a quite low threshold odor concentration and a different aroma for the two enantiomers. Consequently, the three morphological structures can certainly be used as spices or sources of EOs, however, these three products cannot be commercially considered as equivalent. Since, currently, the three morphological structures are almost indifferently used as cinnamon-like aromas, these results could open the way to a more rational and economically sustainable production of cinnamon-like aromas in the Amazonian region of Ecuador.
Author Contributions
Conceptualization, G.G.; supervision, O.M.; investigation, M.M. and M.V.; data curation, G.G. and O.M.; writing—original draft preparation, G.G. and O.M.; writing—review and editing, G.G. and O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PROAmazonia-UTPL project, agreement number 555-2018, Ministerio de Agricultura y Ganadería and Ministerio del Ambiente, Agua y Transición Ecológica of Ecuador. Founded by Green Climate Fund (GCF), Global Environment Facility Trust Fund (GEF) and UTPL, managed by United Nations Development Program (UNDP).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data are available from the authors.
Acknowledgments
We are grateful to the Universidad Técnica Particular de Loja (UTPL) for supporting open access publication. We are also very grateful to Carlo Bicchi (University of Turin, Turin, Italy) for providing enantiomerically pure standards and Aminael Sánchez (Universidad Técnica Particular de Loja) for his support in statistical analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Megadiverse Countries, UNEP-WCMC. Available online: https://www.biodiversitya-z.org/content/megadiverse-countries (accessed on 21 July 2021).
- Malagón, O.; Ramírez, J.; Andrade, J.; Morocho, V.; Armijos, C.; Gilardoni, G. Phytochemistry and ethnopharmacology of the Ecuadorian flora. A review. Nat. Prod. Commun. 2016, 11, 1934578X1601100307. [Google Scholar] [CrossRef] [Green Version]
- Chiriboga, X.; Gilardoni, G.; Magnaghi, I.; Vita Finzi, P.; Zanoni, G.; Vidari, G. New anthracene derivatives from Coussarea macrophylla. J. Nat. Prod. 2003, 66, 905–909. [Google Scholar] [CrossRef]
- Gilardoni, G.; Chiriboga, X.; Finzi, P.V.; Vidari, G. New 3,4-secocycloartane and 3,4-secodammarane triterpenes from the Ecuadorian plant Coussarea macrophylla. Chem. Biodivers. 2015, 12, 946–954. [Google Scholar] [CrossRef]
- Herrera, C.; Morocho, V.; Vidari, G.; Bicchi, C.; Gilardoni, G. Phytochemical investigation of male and female Hedyosmum scabrum (Ruiz & Pav.) Solms leaves from Ecuador. Chem. Biodivers. 2018, 15, e1700423. [Google Scholar]
- Torres-Naranjo, M.; Suárez, A.I.; Gilardoni, G.; Cartuche, L.; Flores, P.; Morocho, V. Chemical constituents of Muehlenbeckia tamnifolia (Kunth) Meisn (Polygonaceae) and its in vitro α-amilase and α-glucosidase inhibitory activities. Molecules 2016, 21, 1461. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; Suarez, A.I.; Bec, N.; Armijos, C.; Gilardoni, G.; Larroque, C.; Vidari, G. Carnosol from Lepechinia mutica and tiliroside from Vallea stipularis: Two promising inhibitors of BuChE. Rev. Bras. Farmacogn. 2018, 28, 559–563. [Google Scholar] [CrossRef]
- Vidari, G.; Abdo, S.; Gilardoni, G.; Ciapessoni, A.; Gusmeroli, M.; Zanoni, G. Fungitoxic metabolites from Erigeron apiculatus. Fitoterapia 2006, 77, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Quílez, A.; Berenguer, B.; Gilardoni, G.; Souccar, C.; De Mendonça, S.; Oliveira, L.F.S.; Martin-Calero, M.J.; Vidari, G. Anti- secretory, anti-inflammatory, and anti-Helicobacter pylori activities of several fractions isolated from Piper carpunya Ruiz & Pav. J. Ethnopharmacol. 2010, 128, 583–589. [Google Scholar]
- Morocho, V.; Valarezo, L.P.; Tapia, D.A.; Cartuche, L.; Cumbicus, N.; Gilardoni, G. A rare dirhamnosyl flavonoid and other radical-scavenging metabolites from Cynophalla mollis (Kunth) J. Presl and Colicodendron scabridum (Kunt) Seem. (Capparaceae) of Ecuador. Chem. Biodivers. 2021, 16, e2100260. [Google Scholar]
- Ramírez, J.; Andrade, M.D.; Vidari, G.; Gilardoni, G. Essential oil and major non-volatile secondary metabolites from the leaves of Amazonian Piper subscutatum. Plants 2021, 10, 1168. [Google Scholar] [CrossRef]
- Gilardoni, G.; Matute, Y.; Ramírez, J. Chemical and enantioselective analysis of the leaf essential oil from Piper coruscans Kunth (Piperaceae), a costal and Amazonian native species of Ecuador. Plants 2020, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; Gilardoni, G.; Jácome, M.; Montesinos, J.; Rodolfi, M.; Guglielminetti, M.; Cagliero, C.; Bicchi, C.; Vidari, G. Chemical composition, enantiomeric analysis, AEDA sensorial evaluation and antifungal activity of the essential oil from the Ecuadorian plant Lepechinia mutica Benth (Lamiaceae). Chem. Biodivers. 2017, 14, e1700292. [Google Scholar] [CrossRef] [PubMed]
- Gilardoni, G.; Montalván, M.; Ortiz, M.; Vinueza, D.; Montesinos, J.V. The flower essential oil of Dalea mutisii Kunth (Fabaceae) from Ecuador: Chemical, enantioselective, and olfactometric analyses. Plants 2020, 9, 1403. [Google Scholar] [CrossRef] [PubMed]
- García, J.; Gilardoni, G.; Cumbicus, N.; Morocho, V. Chemical analysis of the essential oil from Siparuna echinata (Kunth) A. DC. (Siparunaceae) of Ecuador and isolation of the rare terpenoid Sipaucin A. Plants 2020, 9, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa, S.; Bec, N.; Larroque, C.; Ramirez, J.; Sgorbini, B.; Bicchi, C.; Gilardoni, G. Chemical, enantioselective, and sensory analysis of a cholinesterase inhibitor essential oil from Coreopsis triloba S.F. Blake (Asteraceae). Plants 2019, 8, 448. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, S.; Bec, N.; Larroque, C.; Ramírez, J.; Sgorbini, B.; Bicchi, C.; Cumbicus, N.; Gilardoni, G. A novel chemical profile of a selective in vitro cholinergic essential oil from Clinopodium taxifolium (Kunth) Govaerts (Lamiaceae), a native Andean species of Ecuador. Molecules 2021, 26, 45. [Google Scholar] [CrossRef]
- Calva, J.; Bec, N.; Gilardoni, G.; Larroque, C.; Cartuche, L.; Bicchi, C.; Montesinos, J. Acorenone B: AChE and BChE inhibitor as a major compound of the essential oil distilled from the Ecuadorian species Niphogeton dissecta (Benth.) JF Macbr. Pharmaceuticals 2017, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Tropicos.org. Missouri Botanical Garden. Available online: https://www.tropicos.org/name/17805788 (accessed on 21 July 2021).
- Jorgensen, P.; Leon-Yanez, S. Catalogue of the Vascular Plants of Ecuador; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; pp. 1–1182. [Google Scholar]
- Naranjo, P.; Kijjoa, A.; Giesbrecht, A.M.; Gottlieb, O.R. Ocotea quixos, American cinnamon. J. Ethnopharmacol. 1981, 4, 233–236. [Google Scholar] [CrossRef]
- Bruni, R.; Medici, A.; Andreotti, E.; Fantin, C.; Muzzoli, M.; Dehesa, M.; Romagnoli, C.; Sacchetti, G. Chemical composition and biological activities of Ishpingo essential oil, a traditional Ecuadorian spice from Ocotea quixos (Lam.) Kosterm. (Lauraceae) flower calices. Food Chem. 2004, 85, 415–421. [Google Scholar] [CrossRef]
- Sacchetti, G.; Guerrini, A.; Noriega, P.; Bianchi, A.; Bruni, R. Essential oil of wild Ocotea quixos (Lam.) Kosterm. (Lauraceae) leaves from Amazonian Ecuador. Flavour Fragr. J. 2006, 21, 674–676. [Google Scholar] [CrossRef]
- Valarezo, E.; Vullien, A.; Conde-Rojas, D. Variability of the chemical composition of the essential oil from the Amazonian Ishpingo species (Ocotea quixos). Molecules 2021, 26, 3961. [Google Scholar] [CrossRef]
- Noriega, P.; Mosquera, T.; Paredes, E.; Parra, M.; Zappia, M.; Herrera, M.; Villegas, A.; Osorio, E. Antimicrobial and antioxidant bioautography activity of bark essential oil from Ocotea quixos (Lam.) Kosterm. J. Planar. Chromat. 2018, 31, 163–168. [Google Scholar] [CrossRef]
- Tognolini, M.; Barocelli, E.; Ballabeni, V.; Bruni, R.; Bianchi, A.; Chiavarini, M.; Impicciatore, M. Comparative screening of plant essential oils: Phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 2006, 78, 1419–1432. [Google Scholar] [CrossRef]
- Ballabeni, V.; Tognolini, M.; Giorgio, C.; Bertoni, S.; Bruni, R.; Barocelli, E. Ocotea quixos Lam. essential oil: In vitro and in vivo investigation on its anti-inflammatory properties. Fitoterapia 2010, 81, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Scalvenzi, L.; Radice, M.; Toma, L.; Severini, F.; Boccolini, D.; Bella, A.; Guerrini, A.; Tacchini, M.; Sacchetti, G.; Chiurato, M.; et al. Larvicidal activity of Ocimum campechianum, Ocotea quixos and Piper aduncum essential oils against Aedes aegypti. Parasite 2019, 26, 23. [Google Scholar] [CrossRef] [Green Version]
- Ballabeni, V.; Tognolini, M.; Bertoni, S.; Bruni, R.; Guerrini, A.; Moreno-Rueda, G.; Barocelli, E. Antiplatelet and antithrombotic activities of essential oil from wild Ocotea quixos (Lam.) Kosterm. (Lauraceae) calices from Amazonian Ecuador. Pharmacol. Res. 2007, 55, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Crespo, Y.; Ureta-Leones, D.; García-Quintana, Y.; Montalván, M.; Gilardoni, G.; Malagón, O. Preliminary predictive model of termiticidal and repellent activities of essential oil extracted from Ocotea quixos leaves against Nasutitermes corniger (Isoptera: Termitidae) using one-factor response surface methodology design. Agronomy 2021, 11, 1249. [Google Scholar] [CrossRef]
- PROAmazonia Program. Available online: https://www.proamazonia.org (accessed on 21 July 2021).
- Adams, R.P. Identification of Essential Oil Components by Gas. Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 10-1932633219. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- Kundakovic, T.; Fokialakis, N.; Kovacevic, N.; Chinou, I. Essential oil composition of Achillea lingulata and A. umbellate. Flavour Fragr. J. 2007, 22, 184–187. [Google Scholar] [CrossRef]
- Cho, I.H.; Namgung, H.-J.; Choi, H.-K.; Kim, Y.-S. Volatiles and key odorants in the pileus and stipe of pine-mushroom (Tricholoma matsutake Sing.). Food Chem. 2008, 106, 71–76. [Google Scholar] [CrossRef]
- Saroglou, V.; Marin, P.D.; Rancic, A.; Veljic, M.; Skaltsa, H. Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochem. Syst. Ecol. 2007, 35, 146–152. [Google Scholar] [CrossRef]
- Adamiec, J.; Rossner, J.; Velisek, J.; Cejpek, K.; Savel, J. Minor Strecker degradation products of phenylalanine and phenylglycine. Eur. Food Res. Technol. 2001, 212, 135–140. [Google Scholar] [CrossRef]
- Mondello, L.; Dugo, P.; Basile, A.; Dugo, G. Interactive use of linear retention indices, on polar and apolar columns, with a MS-library for reliable identification of complex mixtures. J. Microcolumn Sep. 1995, 7, 581–591. [Google Scholar] [CrossRef]
- Quijano, C.E.; Linares, D.; Pino, J.A. Changes in volatile compounds of fermented cereza agria [Phyllanthus acidus (L.) Skeels] fruit. Flavour Fragr. J. 2007, 22, 392–394. [Google Scholar] [CrossRef]
- Aubert, C.; Pitrat, M. Volatile compounds in the skin and pulp of Queen Anne’s pocket melon. J. Agric. Food Chem. 2006, 54, 8177–8182. [Google Scholar] [CrossRef] [PubMed]
- Bisio, A.; Ciarallo, G.; Romussi, G.; Fontana, N.; Mascolo, N.; Capasso, R.; Biscardi, D. Chemical Composition of Essential Oils from some Salvia species. Phytother. Res. 1998, 12, s117–s120. [Google Scholar] [CrossRef]
- Raina, V.K.; Kumar, A.; Srivastava, S.K.; Syamsundar, K.V.; Kahol, A.P. Essential oil composition of ‘kewda’ (Pandanus odoratissimus) from India. Flavour Fragr. J. 2004, 19, 434–436. [Google Scholar] [CrossRef]
- Shreaz, S.; Wani, W.A.; Behbehani, J.M.; Raja, V.; Irshad, M.; Karched, M.; Ali, I.; Siddiqi, W.A.; Hun, L.T. Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 2016, 112, 116–131. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Wei-Bin, M.; Jun-Tao, F.; Zhi-Li, J.; Xing, Z. Fumigant activity of 6 selected essential oil compounds and combined effect of methyl salicylate and trans-cinnamaldehyde against Culex pipiens pallens. J. Am. Mosq. Control. Assoc. 2014, 30, 199–203. [Google Scholar]
- Sen-Sung, C.; Ju-Yun, L.; Kun-Hsien, T.; Wei-June, C.; Shang-Tzen, C. Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J. Agric. Food Chem. 2004, 52, 4395–4400. [Google Scholar]
- Zhu, R.; Liu, H.; Liu, C.; Wang, L.; Ma, R.; Chen, B.; Li, L.; Niu, J.; Fu, M.; Zhang, D.; et al. Cinnamaldehyde in diabetes: A review of pharmacology, pharmacokinetics and safety. Pharmacol. Res. 2017, 122, 78–89. [Google Scholar] [CrossRef]
- Park, K.-R.; Lee, H.; Cho, M.; Yun, H.-M. A phytochemical constituent, (E)-methyl-cinnamate isolated from Alpinia katsumadai Hayata suppresses cell survival, migration, and differentiation in pre-osteoblasts. Int. J. Mol. Sci. 2020, 21, 3700. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Chua, M.T.; Wang, S.Y.; Chang, S.T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463. [Google Scholar] [CrossRef]
- De Saint Laumer, J.Y.; Cicchetti, E.; Merle, P.; Egger, J.; Chaintreau, A. Quantification in gas chromatography: Prediction of flame ionization detector response factors from combustion enthalpies and molecular structures. Anal. Chem. 2010, 82, 6457–6462. [Google Scholar] [CrossRef] [PubMed]
- Tissot, E.; Rochat, S.; Debonneville, C.; Chaintreau, A. Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour Fragr. J. 2012, 27, 290–296. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).