A Zebrafish Seizure Model of cblX Syndrome Reveals a Dose-Dependent Response to mTor Inhibition
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish and Genotyping
2.2. Reagents
2.3. Zebrabox Behavioral Analysis
2.4. Torin1 and PTZ Inhibition
2.5. Statistical Analysis
3. Results
3.1. Larval Response to Low-Dose PTZ
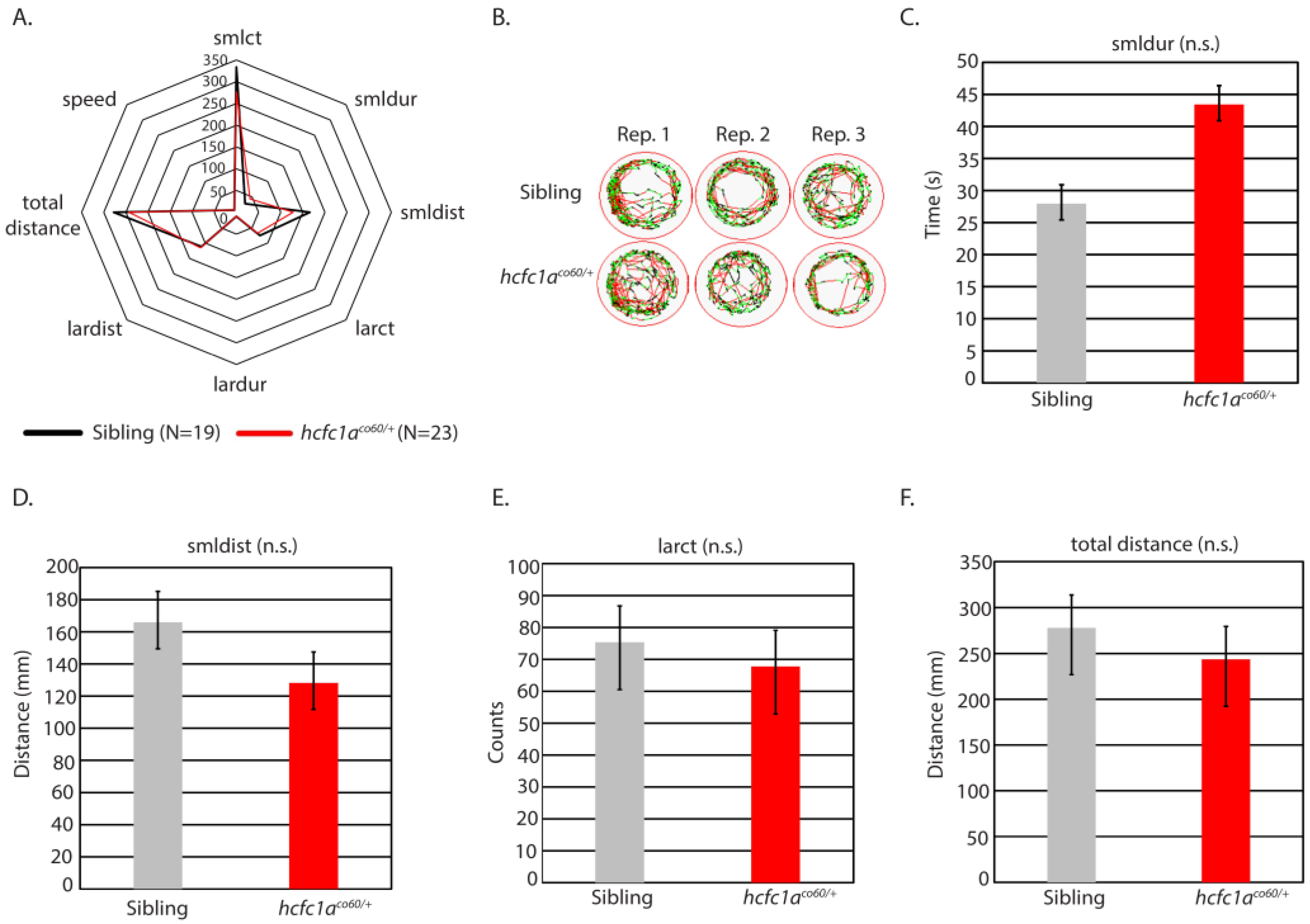
3.2. Mutation of hcfc1a Does Not Increase Seizure Susceptibility to Low-Dose PTZ
3.3. Inhibition of mTor Reduces Seizure-like Behavior in Wildtype Animals
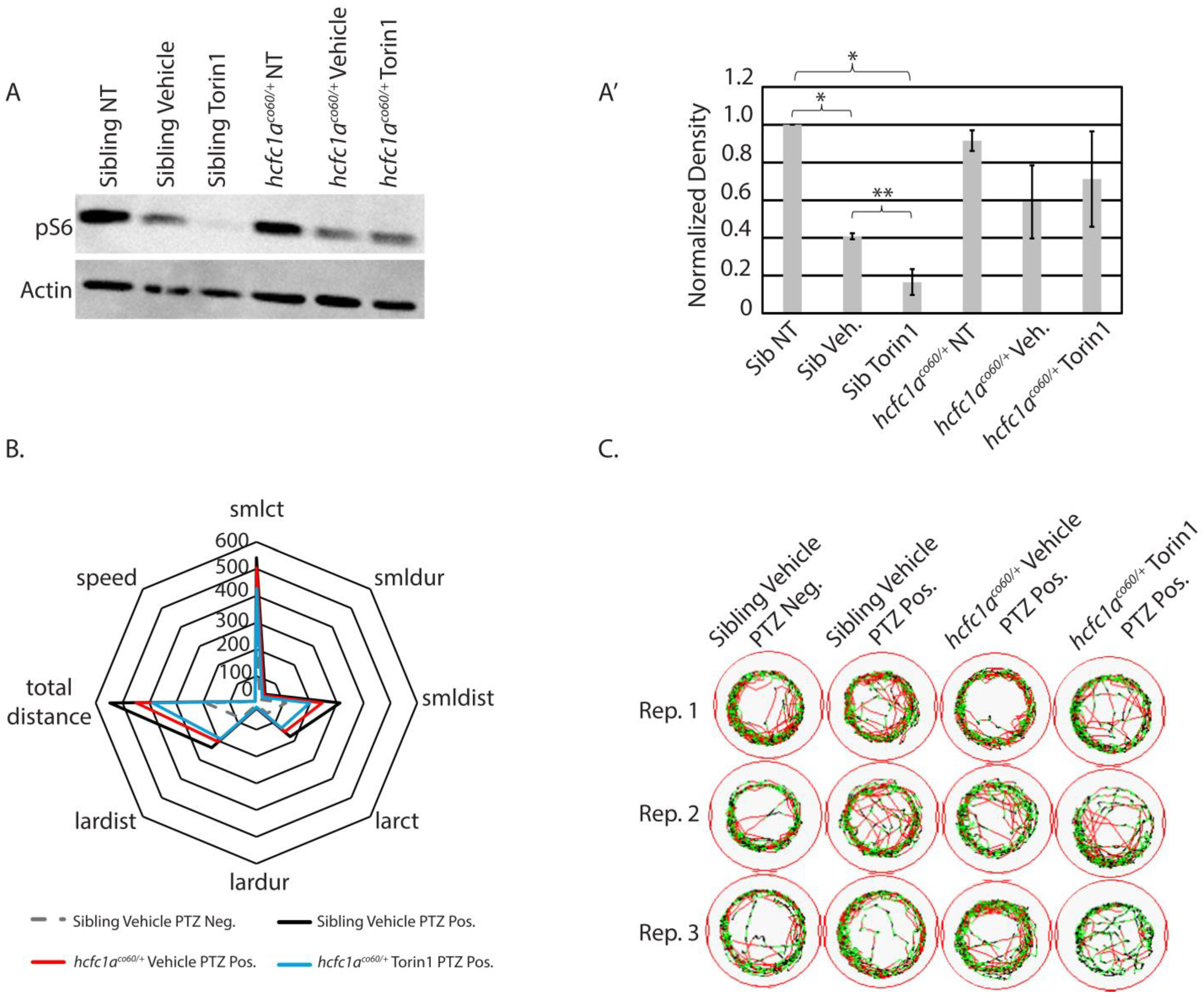
3.4. The Effects of mTor Inhibition on S6 Phosphorylation
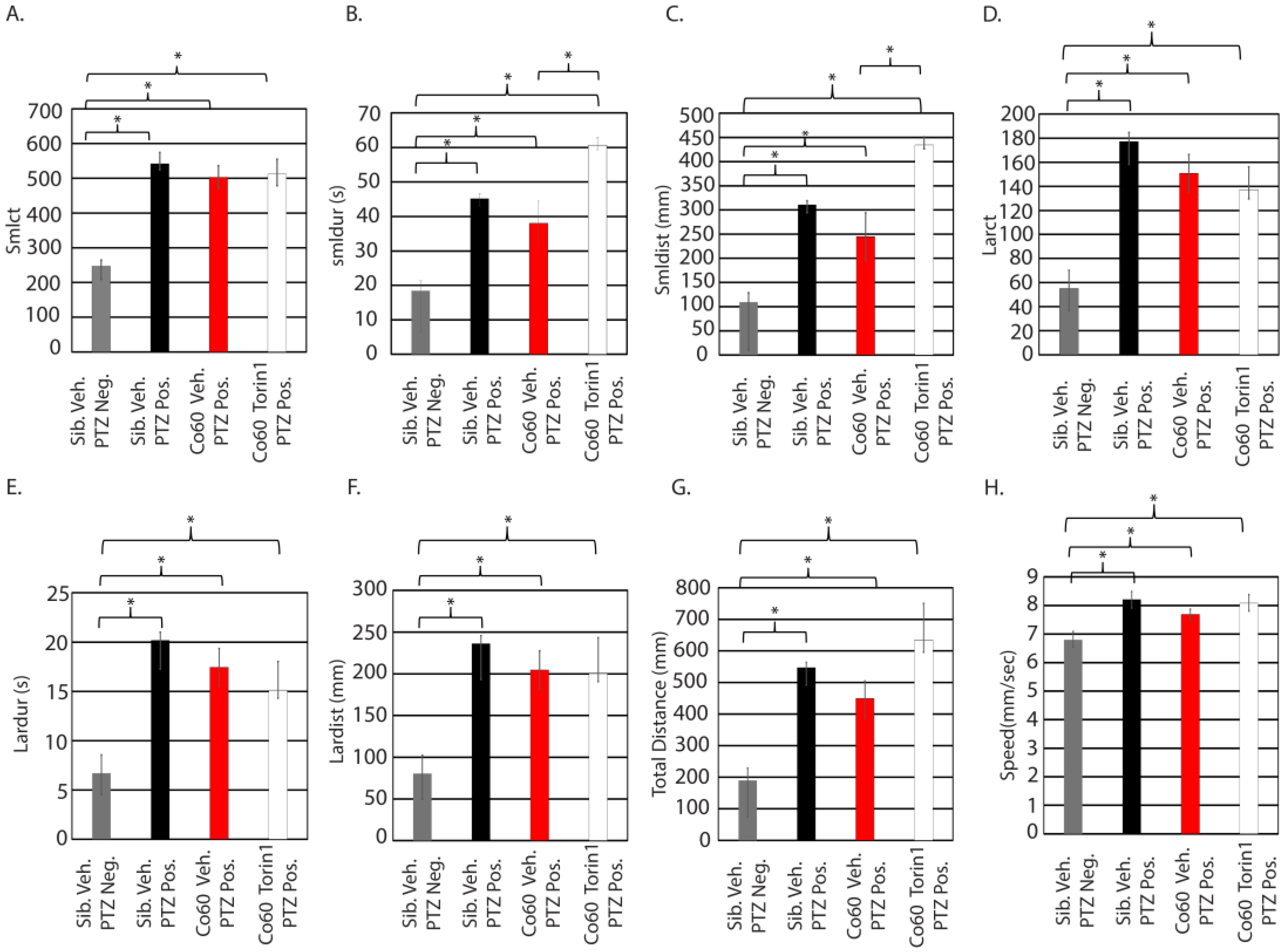
3.5. Inhibition of mTor at 250 nM Reduces Small/Short Behavioral Parameters in Mutant Animals Exposed to Seizure-Inducing Doses of PTZ
3.6. Excessive Inhibition of mTor in the Context of hcfc1a Mutation Results in Refractory Epilepsy
4. Discussion
4.1. Integration with Existing Knowledge
4.2. Mechanistic Implications
4.3. Limitations and Technical Considerations
4.4. Therapeutic Relevance
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michaud, J.; Praz, V.; Faresse, N.J.; Jnbaptiste, C.K.; Tyagi, S.; Schütz, F.; Herr, W. HCFC1 Is a Common Component of Active Human CpG-Island Promoters and Coincides with ZNF143, THAP11, YY1, and GABP Transcription Factor Occupancy. Genome Res. 2013, 23, 907–916. [Google Scholar] [CrossRef]
- Yu, H.-C.; Sloan, J.L.; Scharer, G.; Brebner, A.; Quintana, A.M.; Achilly, N.P.; Manoli, I.; Coughlin, C.R., 2nd; Geiger, E.A.; Schneck, U.; et al. An X-Linked Cobalamin Disorder Caused by Mutations in Transcriptional Coregulator HCFC1. Am. J. Hum. Genet. 2013, 93, 506–514. [Google Scholar] [CrossRef]
- Huang, L.; Jolly, L.A.; Willis-Owen, S.; Gardner, A.; Kumar, R.; Douglas, E.; Shoubridge, C.; Wieczorek, D.; Tzschach, A.; Cohen, M.; et al. A Noncoding, Regulatory Mutation Implicates HCFC1 in Nonsyndromic Intellectual Disability. Am. J. Hum. Genet. 2012, 91, 694–702. [Google Scholar] [CrossRef]
- Jolly, L.A.; Nguyen, L.S.; Domingo, D.; Sun, Y.; Barry, S.; Hancarova, M.; Plevova, P.; Vlckova, M.; Havlovicova, M.; Kalscheuer, V.M.; et al. HCFC1 Loss-of-Function Mutations Disrupt Neuronal and Neural Progenitor Cells of the Developing Brain. Hum. Mol. Genet. 2015, 24, 3335–3347. [Google Scholar] [CrossRef]
- Koufaris, C.; Alexandrou, A.; Tanteles, G.A.; Anastasiadou, V.; Sismani, C. A Novel HCFC1 Variant in Male Siblings with Intellectual Disability and Microcephaly in the Absence of Cobalamin Disorder. Biomed. Rep. 2016, 4, 215–218. [Google Scholar] [CrossRef]
- Wongkittichote, P.; Wegner, D.J.; Shinawi, M.S. Novel Exon-Skipping Variant Disrupting the Basic Domain of HCFC1 Causes Intellectual Disability without Metabolic Abnormalities in Both Male and Female Patients. J. Hum. Genet. 2021, 66, 717–724. [Google Scholar] [CrossRef]
- He, N.; Guan, B.-Z.; Wang, J.; Liu, H.-K.; Mao, Y.; Liu, Z.-G.; Yin, F.; Peng, J.; Xiao, B.; Tang, B.-S.; et al. HCFC1 Variants in the Proteolysis Domain Are Associated with X-Linked Idiopathic Partial Epilepsy: Exploring the Underlying Mechanism. Clin. Transl. Med. 2023, 13, e1289. [Google Scholar] [CrossRef]
- Quintana, A.M.; Yu, H.-C.; Brebner, A.; Pupavac, M.; Geiger, E.A.; Watson, A.; Castro, V.L.; Cheung, W.; Chen, S.-H.; Watkins, D.; et al. Mutations in THAP11 Cause an Inborn Error of Cobalamin Metabolism and Developmental Abnormalities. Hum. Mol. Genet. 2017, 26, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.L.; Reyes, J.F.; Reyes-Nava, N.G.; Paz, D.; Quintana, A.M. Hcfc1a Regulates Neural Precursor Proliferation and Asxl1 Expression in the Developing Brain. BMC Neurosci. 2020, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Minocha, S.; Herr, W. Cortical and Commissural Defects Upon HCF-1 Loss in Nkx2.1-Derived Embryonic Neurons and Glia. Dev. Neurobiol. 2019, 79, 578–595. [Google Scholar] [CrossRef] [PubMed]
- Chern, T.; Achilleos, A.; Tong, X.; Hill, M.C.; Saltzman, A.B.; Reineke, L.C.; Chaudhury, A.; Dasgupta, S.K.; Redhead, Y.; Watkins, D.; et al. Mutations in Hcfc1 and Ronin Result in an Inborn Error of Cobalamin Metabolism and Ribosomopathy. Nat. Commun. 2022, 13, 134. [Google Scholar] [CrossRef]
- Youn, H.S.; Kim, T.-Y.; Park, U.-H.; Moon, S.-T.; An, S.-J.; Lee, Y.-K.; Hwang, J.-T.; Kim, E.-J.; Um, S.-J. Asxl1 Deficiency in Embryonic Fibroblasts Leads to Cellular Senescence via Impairment of the AKT-E2F Pathway and Ezh2 Inactivation. Sci. Rep. 2017, 7, 5198. [Google Scholar] [CrossRef]
- Jansen, L.A.; Mirzaa, G.M.; Ishak, G.E.; O’Roak, B.J.; Hiatt, J.B.; Roden, W.H.; Gunter, S.A.; Christian, S.L.; Collins, S.; Adams, C.; et al. PI3K/AKT Pathway Mutations Cause a Spectrum of Brain Malformations from Megalencephaly to Focal Cortical Dysplasia. Brain 2015, 138, 1613–1628. [Google Scholar] [CrossRef]
- Castro, V.L.; Paz, D.; Virrueta, V.; Estevao, I.L.; Grajeda, B.I.; Ellis, C.C.; Quintana, A.M. Missense and Nonsense Mutations of the Zebrafish Hcfc1a Gene Result in Contrasting mTor and Radial Glial Phenotypes. Gene 2023, 864, 147290. [Google Scholar] [CrossRef]
- Okoh, J.; Mays, J.; Bacq, A.; Oses-Prieto, J.A.; Tyanova, S.; Chen, C.-J.; Imanbeyev, K.; Doladilhe, M.; Zhou, H.; Jafar-Nejad, P.; et al. Targeted Suppression of mTORC2 Reduces Seizures across Models of Epilepsy. Nat. Commun. 2023, 14, 7364. [Google Scholar] [CrossRef] [PubMed]
- Moavero, R.; Mühlebner, A.; Luinenburg, M.J.; Craiu, D.; Aronica, E.; Curatolo, P. Genetic Pathogenesis of the Epileptogenic Lesions in Tuberous Sclerosis Complex: Therapeutic Targeting of the mTOR Pathway. Epilepsy Behav. 2022, 131, 107713. [Google Scholar] [CrossRef] [PubMed]
- Giannantoni, N.M.; Restuccia, D.; Della Marca, G.; Alfano, R.M.; Vollono, C. A Novel TSC2 Mutation Causing Tuberless Tuberous Sclerosis. Seizure 2014, 23, 580–582. [Google Scholar] [CrossRef]
- Dhamne, S.C.; Modi, M.E.; Gray, A.; Bonazzi, S.; Craig, L.; Bainbridge, E.; Lalani, L.; Super, C.E.; Schaeffer, S.; Capre, K.; et al. Seizure Reduction in TSC2-Mutant Mouse Model by an mTOR Catalytic Inhibitor. Ann. Clin. Transl. Neurol. 2023, 10, 1790–1801. [Google Scholar] [CrossRef] [PubMed]
- Gérard, M.; Morin, G.; Bourillon, A.; Colson, C.; Mathieu, S.; Rabier, D.; Billette de Villemeur, T.; Ogier de Baulny, H.; Benoist, J.F. Multiple Congenital Anomalies in Two Boys with Mutation in HCFC1 and Cobalamin Disorder. Eur. J. Med. Genet. 2015, 58, 148–153. [Google Scholar] [CrossRef]
- Singh, T.; Mishra, A.; Goel, R.K. PTZ Kindling Model for Epileptogenesis, Refractory Epilepsy, and Associated Comorbidities: Relevance and Reliability. Metab. Brain Dis. 2021, 36, 1573–1590. [Google Scholar] [CrossRef]
- Hansen, S.L.; Sperling, B.B.; Sánchez, C. Anticonvulsant and Antiepileptogenic Effects of GABAA Receptor Ligands in Pentylenetetrazole-Kindled Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Baraban, S.C.; Taylor, M.R.; Castro, P.A.; Baier, H. Pentylenetetrazole Induced Changes in Zebrafish Behavior, Neural Activity and c-Fos Expression. Neuroscience 2005, 131, 759–768. [Google Scholar] [CrossRef]
- Afrikanova, T.; Serruys, A.-S.K.; Buenafe, O.E.M.; Clinckers, R.; Smolders, I.; de Witte, P.A.M.; Crawford, A.D.; Esguerra, C.V. Validation of the Zebrafish Pentylenetetrazol Seizure Model: Locomotor versus Electrographic Responses to Antiepileptic Drugs. PLoS ONE 2013, 8, e54166. [Google Scholar] [CrossRef]
- Milder, P.C.; Zybura, A.S.; Cummins, T.R.; Marrs, J.A. Neural Activity Correlates with Behavior Effects of Anti-Seizure Drugs Efficacy Using the Zebrafish Pentylenetetrazol Seizure Model. Front. Pharmacol. 2022, 13, 836573. [Google Scholar] [CrossRef]
- Shaw, P.A.G.; Panda, S.K.; Stanca, A.; Luyten, W. Optimization of a Locomotion-Based Zebrafish Seizure Model. J. Neurosci. Methods 2022, 375, 109594. [Google Scholar] [CrossRef] [PubMed]
- Szep, D.; Dittrich, B.; Gorbe, A.; Szentpeteri, J.L.; Aly, N.; Jin, M.; Budan, F.; Sik, A. A Comparative Study to Optimize Experimental Conditions of Pentylenetetrazol and Pilocarpine-Induced Epilepsy in Zebrafish Larvae. PLoS ONE 2023, 18, e0288904. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.Y.; Renquist, B.J. High Throughput Danio Rerio Energy Expenditure Assay. J. Vis. Exp. 2016, 107, e53297. [Google Scholar] [CrossRef]
- MacPhail, R.C.; Brooks, J.; Hunter, D.L.; Padnos, B.; Irons, T.D.; Padilla, S. Locomotion in Larval Zebrafish: Influence of Time of Day, Lighting and Ethanol. Neurotoxicology 2009, 30, 52–58. [Google Scholar] [CrossRef]
- Fraser, T.W.K.; Khezri, A.; Jusdado, J.G.H.; Lewandowska-Sabat, A.M.; Henry, T.; Ropstad, E. Toxicant Induced Behavioural Aberrations in Larval Zebrafish Are Dependent on Minor Methodological Alterations. Toxicol. Lett. 2017, 276, 62–68. [Google Scholar] [CrossRef]
- Reyes-Nava, N.; Yu, H.-C.; Coughlin, C.R., 2nd; Shaikh, T.H.; Quintana, A.M. Abnormal Expression of GABAA Receptor Sub-Units and Hypomotility upon Loss of Gabra1 in Zebrafish. Biol. Open 2020, 9, bio051367. [Google Scholar] [CrossRef]
- Reyes-Nava, N.G.; Paz, D.; Pinales, B.E.; Perez, I.; Gil, C.B.; Gonzales, A.V.; Grajeda, B.I.; Estevao, I.L.; Ellis, C.C.; Castro, V.L.; et al. Characterization of the Zebrafish Gabra1sa43718/Sa43718 Germline Loss of Function Allele Confirms a Function for Gabra1 in Motility and Nervous System Development. Differentiation 2024, 138, 100790. [Google Scholar] [CrossRef]
- Hoyberghs, J.; Bars, C.; Ayuso, M.; Van Ginneken, C.; Foubert, K.; Van Cruchten, S. DMSO Concentrations up to 1% Are Safe to Be Used in the Zebrafish Embryo Developmental Toxicity Assay. Front. Toxicol. 2021, 3, 804033. [Google Scholar] [CrossRef]
- Christou, M.; Kavaliauskis, A.; Ropstad, E.; Fraser, T.W.K. DMSO Effects Larval Zebrafish (Danio Rerio) Behavior, with Additive and Interaction Effects When Combined with Positive Controls. Sci. Total Env. 2020, 709, 134490. [Google Scholar] [CrossRef]
- Hagen, E.V.; Harper, M.M.M.; Zhang, Y.; Hamilton, T.J. Exploring the Impact of Acute Solvent Exposure on Larval Zebrafish Behaviour. Front. Behav. Neurosci. 2025, 19, 1717998. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a Comprehensive Catalog of Zebrafish Behavior 1.0 and Beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Thoreen, C.C.; Kang, S.A.; Chang, J.W.; Liu, Q.; Zhang, J.; Gao, Y.; Reichling, L.J.; Sim, T.; Sabatini, D.M.; Gray, N.S. An ATP-Competitive Mammalian Target of Rapamycin Inhibitor Reveals Rapamycin-Resistant Functions of mTORC1. J. Biol. Chem. 2009, 284, 8023–8032. [Google Scholar] [CrossRef] [PubMed]
- Baraban, S.C. A Zebrafish-Centric Approach to Antiepileptic Drug Development. Dis. Model. Mech. 2021, 14, dmm049080. [Google Scholar] [CrossRef] [PubMed]
- Gawel, K.; Langlois, M.; Martins, T.; van der Ent, W.; Tiraboschi, E.; Jacmin, M.; Crawford, A.D.; Esguerra, C.V. Seizing the Moment: Zebrafish Epilepsy Models. Neurosci. Biobehav. Rev. 2020, 116, 1–20. [Google Scholar] [CrossRef]
- Ellis, L.D.; Seibert, J.; Soanes, K.H. Distinct Models of Induced Hyperactivity in Zebrafish Larvae. Brain Res. 2012, 1449, 46–59. [Google Scholar] [CrossRef]
- Gauvin, D.V.; Dormer, K.N.; Holloway, F.A. Pentylenetetrazole Can Induce a Conditioned Place Preference. Pharmacol. Biochem. Behav. 1991, 40, 987–990. [Google Scholar] [CrossRef]
- Aniol, V.A.; Stepanichev, M.Y.; Yakovlev, A.A.; Lazareva, N.A.; Gulyaeva, N.V. Anxiogenic Effect of Pentylenetetrazole at a Subconvulsive Dose Is Accompanied by Decreased Cellular Proliferation and Neuronal NO-Synthase Expression in the Posterior Part of the Hippocampus. Neurochem. J. 2024, 18, 734–741. [Google Scholar] [CrossRef]
- Ravizza, T.; Scheper, M.; Di Sapia, R.; Gorter, J.; Aronica, E.; Vezzani, A. mTOR and Neuroinflammation in Epilepsy: Implications for Disease Progression and Treatment. Nat. Rev. Neurosci. 2024, 25, 334–350. [Google Scholar] [CrossRef]
- Boff, M.O.; Xavier, F.A.C.; Diz, F.M.; Gonçalves, J.B.; Ferreira, L.M.; Zambeli, J.; Pazzin, D.B.; Previato, T.T.R.; Erwig, H.S.; Gonçalves, J.I.B.; et al. mTORopathies in Epilepsy and Neurodevelopmental Disorders: The Future of Therapeutics and the Role of Gene Editing. Cells 2025, 14, 662. [Google Scholar] [CrossRef]
- Teng, L.-Y.; Chang, M.J.; Kim, S.H. mTOR Inhibition in Epilepsy: A Literature Review. Adv. Neurol. 2024, 3, 3568. [Google Scholar] [CrossRef]
- Swiech, L.; Perycz, M.; Malik, A.; Jaworski, J. Role of mTOR in Physiology and Pathology of the Nervous System. Biochim. Biophys. Acta 2008, 1784, 116–132. [Google Scholar] [CrossRef]
- Griffith, J.L.; Wong, M. The mTOR Pathway in Treatment of Epilepsy: A Clinical Update. Future Neurol. 2018, 13, 49–58. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Park, U.-H.; Moon, S.; Kang, M.; Youn, H.; Hwang, J.-T.; Kim, E.-J.; Um, S.-J. Asxl1 Ablation in Mouse Embryonic Stem Cells Impairs Neural Differentiation without Affecting Self-Renewal. Biochem. Biophys. Res. Commun. 2019, 508, 907–913. [Google Scholar] [CrossRef]
- Inoue, D.; Fujino, T.; Sheridan, P.; Zhang, Y.-Z.; Nagase, R.; Horikawa, S.; Li, Z.; Matsui, H.; Kanai, A.; Saika, M.; et al. A Novel ASXL1-OGT Axis Plays Roles in H3K4 Methylation and Tumor Suppression in Myeloid Malignancies. Leukemia 2018, 32, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.G.; Subramanian, L.; Kriegstein, A.R. mTOR Signaling Regulates the Morphology and Migration of Outer Radial Glia in Developing Human Cortex. eLife 2020, 9, e58737. [Google Scholar] [CrossRef] [PubMed]
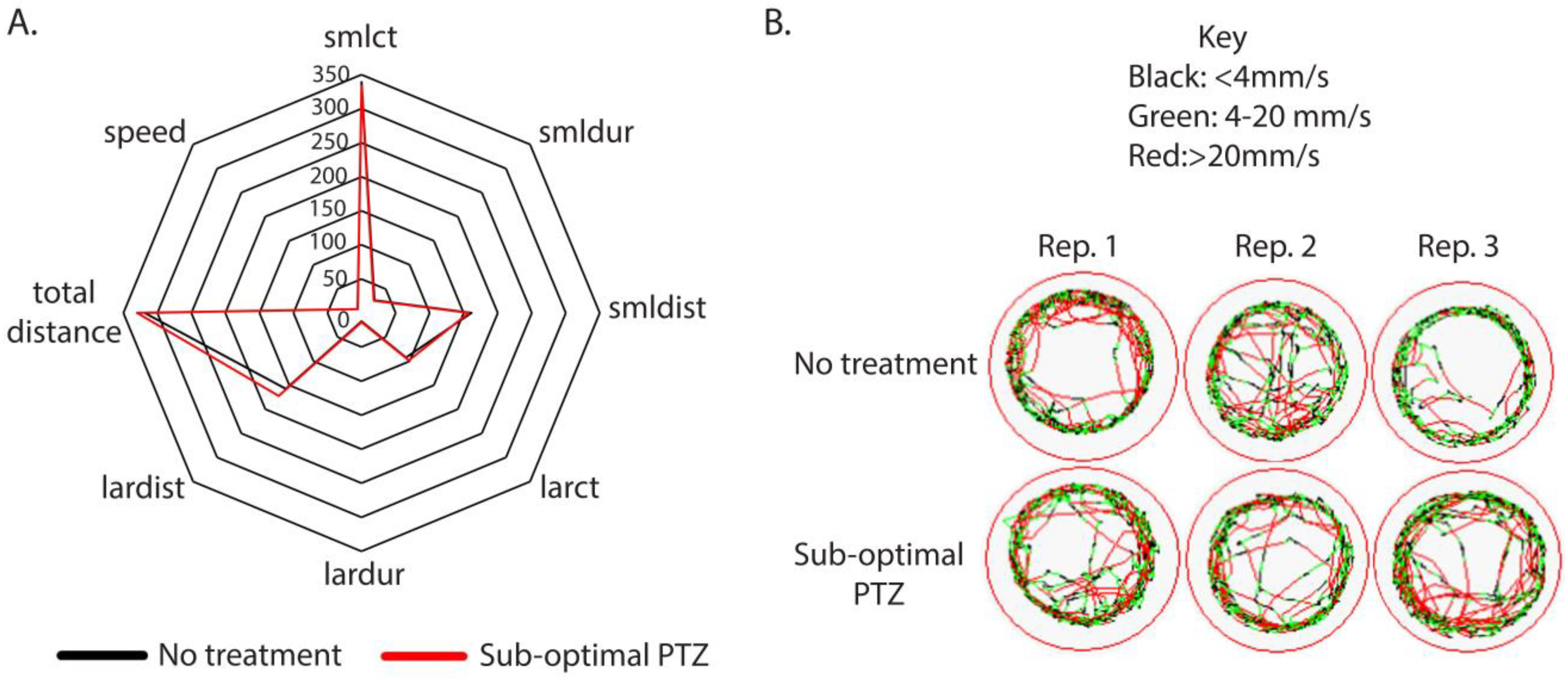


| PTZ | Small Count | Small Duration | Small Distance | Large Count | Large Duration | Large Distance | Total Distance (mm) | Speed | # of Parameters Affected |
|---|---|---|---|---|---|---|---|---|---|
| 1.0 µM | +59.17 | NC | +33.32 | +39.7 | +5.42 | +66.32 | +99.62 | +0.67 | 7 |
| 10 pM | NC | NC | NC | NC | NC | NC | NC | +0.83 | 1 |
| 1 pM | +194.93 | +18.73 | +116.85 | NC | NC | NC | +108.63 | −1.56 | 5 |
| 0.1 pM | +51.26 | NC | NC | +19.70 | +2.55 | NC | NC | NC | 3 |
| 0.01 pM | −49.65 | −4.92 | −32.97 | −22.48 | −2.62 | NC | −59.34 | NC | 6 |
| 0.001 pM | NC | NC | NC | NC | NC | NC | NC | NC | NC |
| Torin1 | Small Count | Small Duration | Small Distance | Large Count | Large Duration | Large Distance | Total Distance (mm) | Speed | # of Parameters Affected |
|---|---|---|---|---|---|---|---|---|---|
| 250 nM | NC | NC | NC | −48.41 | NC | NC | −163.30 | NC | 2 |
| 350 nM | −127.81 | NC | NC | −67.52 | −7.35 | NC | NC | NC | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Gil, C.B.; Paz, D.; Pinales, B.E.; Castro, V.L.; Perucho, C.E.; Gonzales, A.; Francia, G.; Masenga, S.K.; Hinton, A., Jr.; Quintana, A.M. A Zebrafish Seizure Model of cblX Syndrome Reveals a Dose-Dependent Response to mTor Inhibition. J. Dev. Biol. 2026, 14, 2. https://doi.org/10.3390/jdb14010002
Gil CB, Paz D, Pinales BE, Castro VL, Perucho CE, Gonzales A, Francia G, Masenga SK, Hinton A Jr., Quintana AM. A Zebrafish Seizure Model of cblX Syndrome Reveals a Dose-Dependent Response to mTor Inhibition. Journal of Developmental Biology. 2026; 14(1):2. https://doi.org/10.3390/jdb14010002
Chicago/Turabian StyleGil, Claudia B., David Paz, Briana E. Pinales, Victoria L. Castro, Claire E. Perucho, Annalise Gonzales, Giulio Francia, Sepiso K. Masenga, Antentor Hinton, Jr., and Anita M. Quintana. 2026. "A Zebrafish Seizure Model of cblX Syndrome Reveals a Dose-Dependent Response to mTor Inhibition" Journal of Developmental Biology 14, no. 1: 2. https://doi.org/10.3390/jdb14010002
APA StyleGil, C. B., Paz, D., Pinales, B. E., Castro, V. L., Perucho, C. E., Gonzales, A., Francia, G., Masenga, S. K., Hinton, A., Jr., & Quintana, A. M. (2026). A Zebrafish Seizure Model of cblX Syndrome Reveals a Dose-Dependent Response to mTor Inhibition. Journal of Developmental Biology, 14(1), 2. https://doi.org/10.3390/jdb14010002








