Small Molecule Inhibitors of Nicotinamide N-Methyltransferase Enzyme for the Treatment of Osteosarcoma and Merkel Cell Carcinoma: Potential for the Development of a Targeted Therapeutic Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Inhibitors
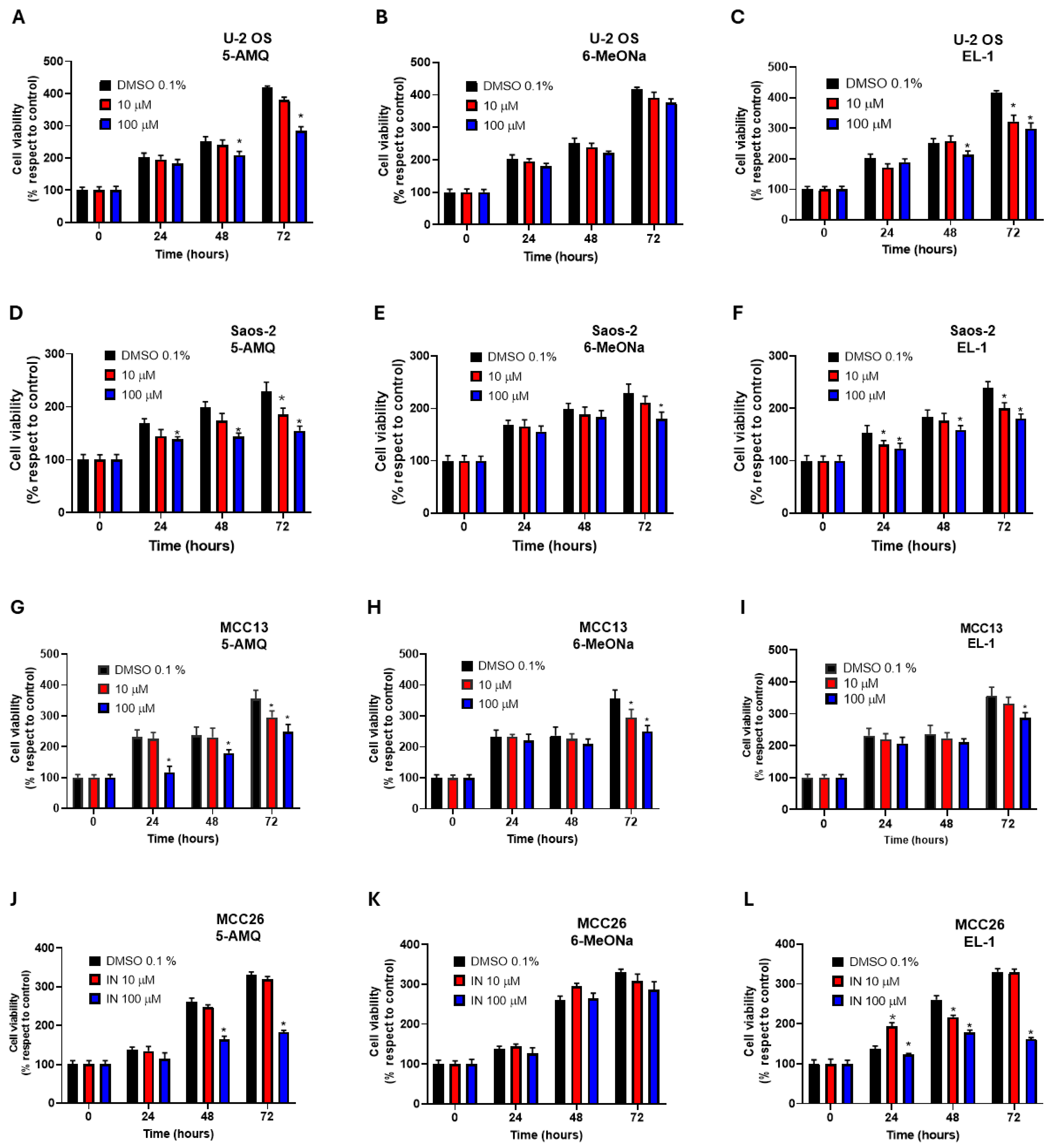
2.2. MTT Assay
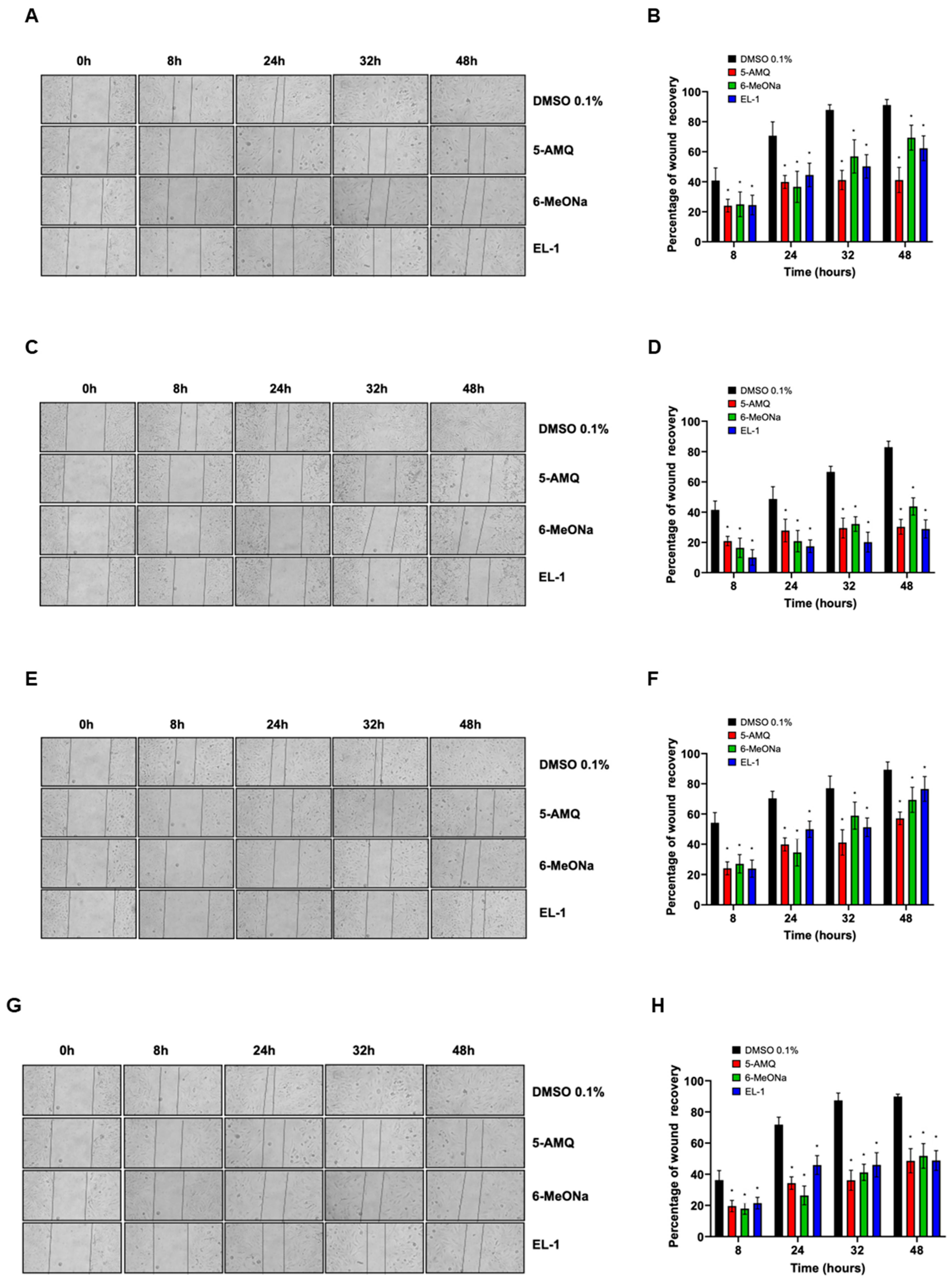
2.3. Monolayer Wound Healing Assay
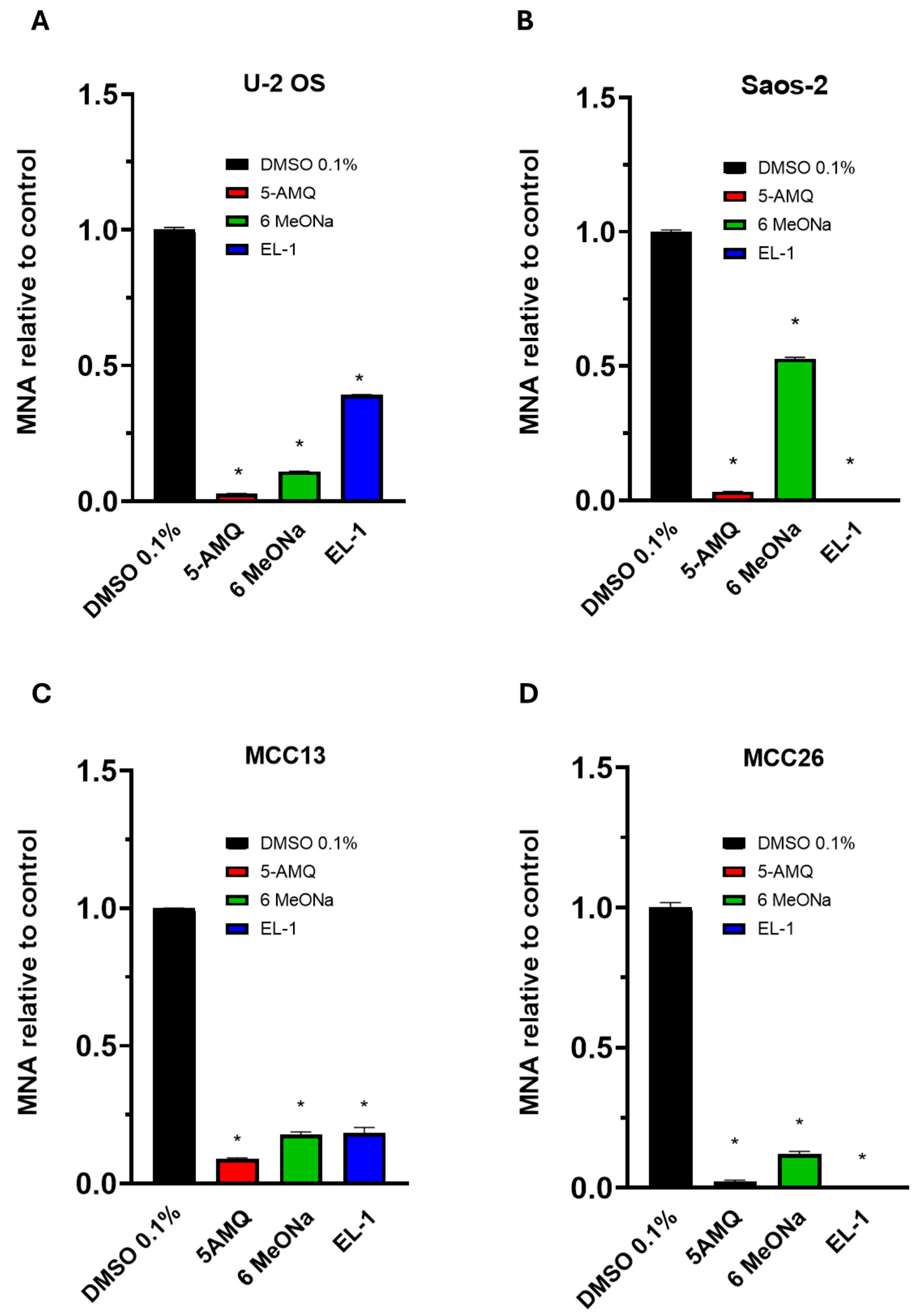
2.4. Quantitative Measurements of MNA Levels in Cultured Cells
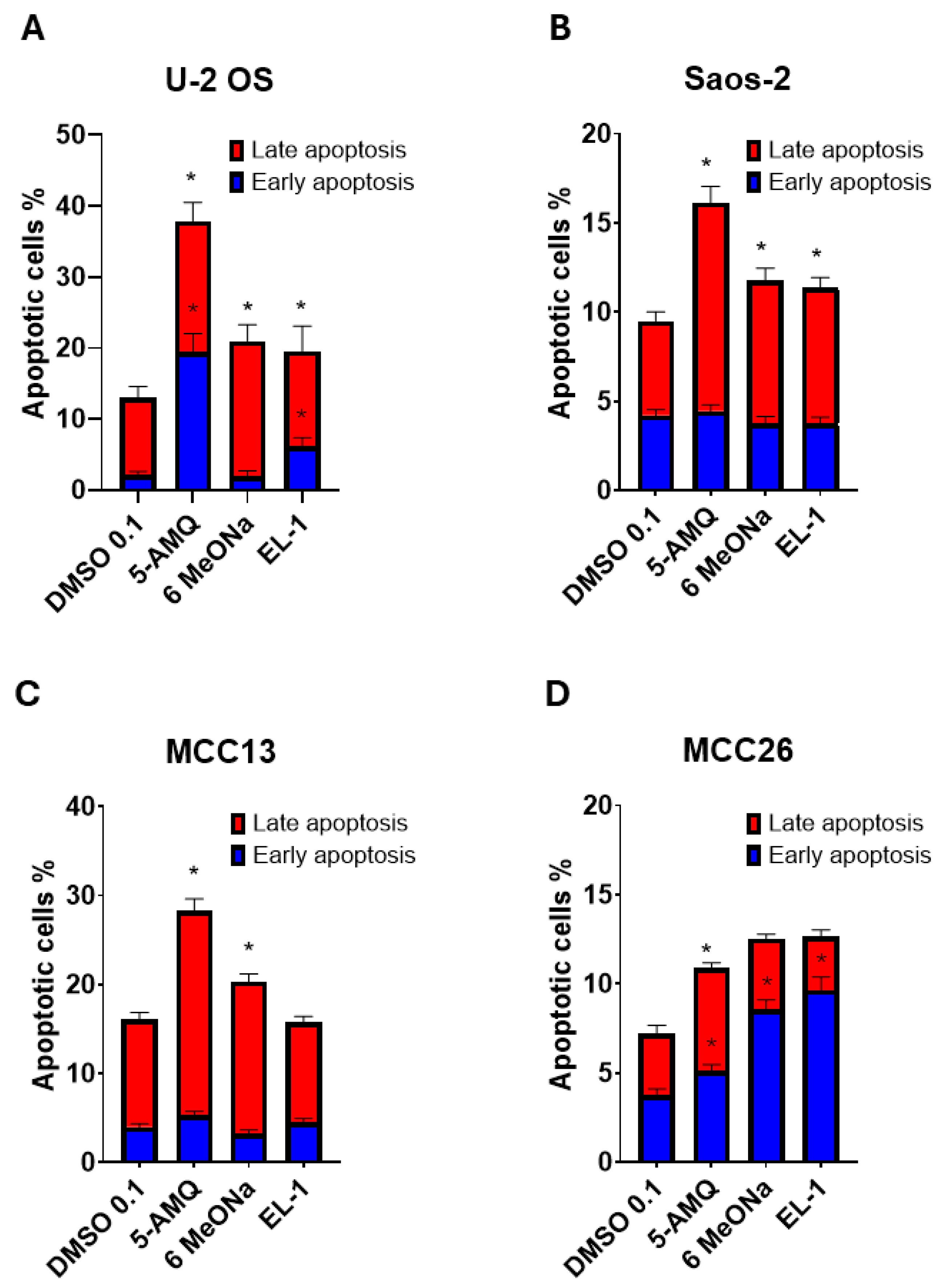
2.5. Apoptosis Assay
2.6. Expression Levels of Caspases Through Real-Time PCR and Western Blot Analysis
2.7. Intracellular ROS Production
2.8. Sensitivity to Chemotherapeutic Treatment
2.9. Statistical Analyses
3. Results
3.1. Effect of NNMT Inhibitors on Cell Viability and Migration Capacity
3.2. Intracellular MNA Levels Following Treatment with Small Molecule Compounds
3.3. Apoptosis Activation Induced by Treatment with NNMT Inhibitors
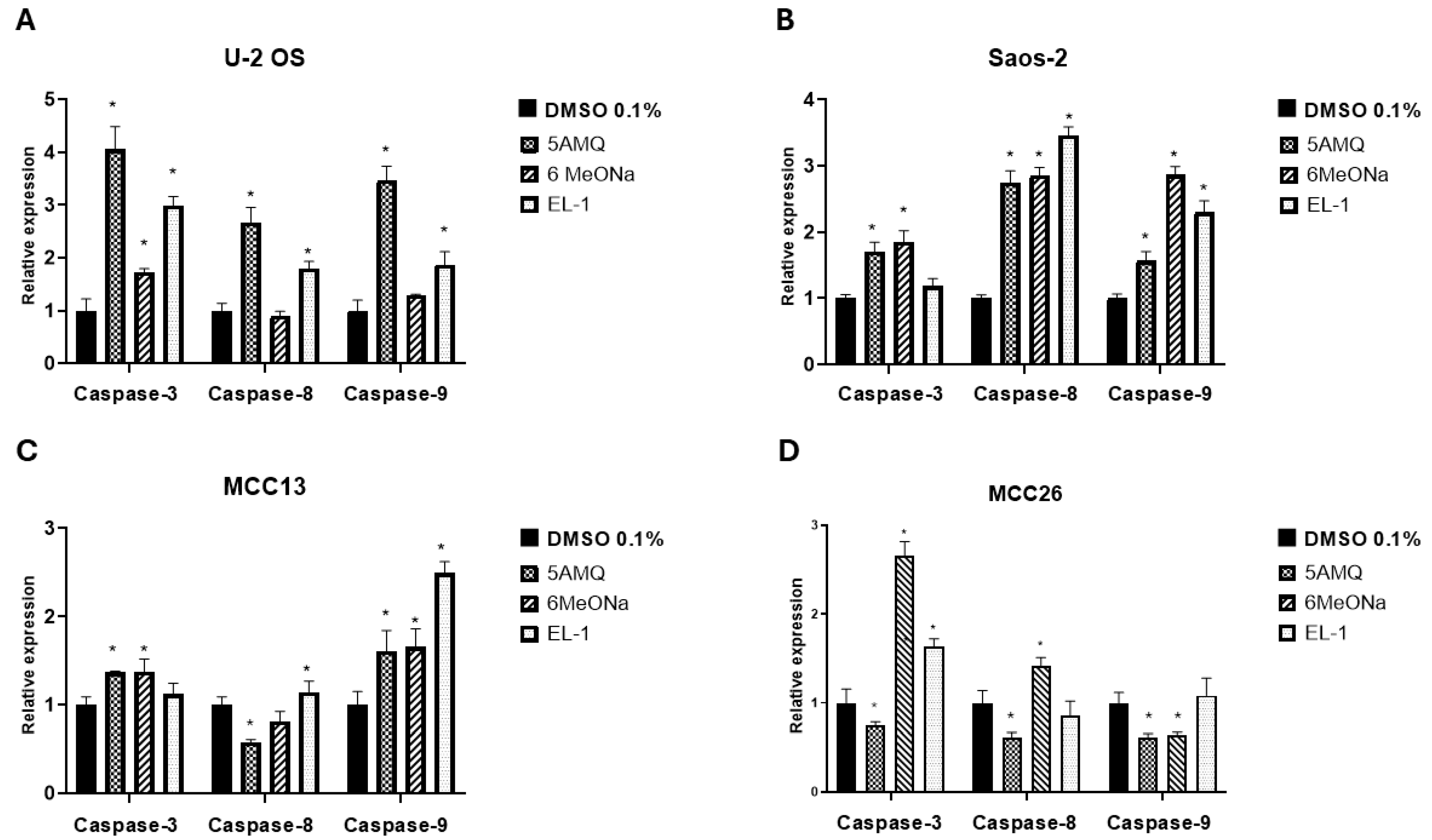
3.4. Effect of Treatment with NNMT Inhibitors on Expression Levels of Apoptotic Caspases
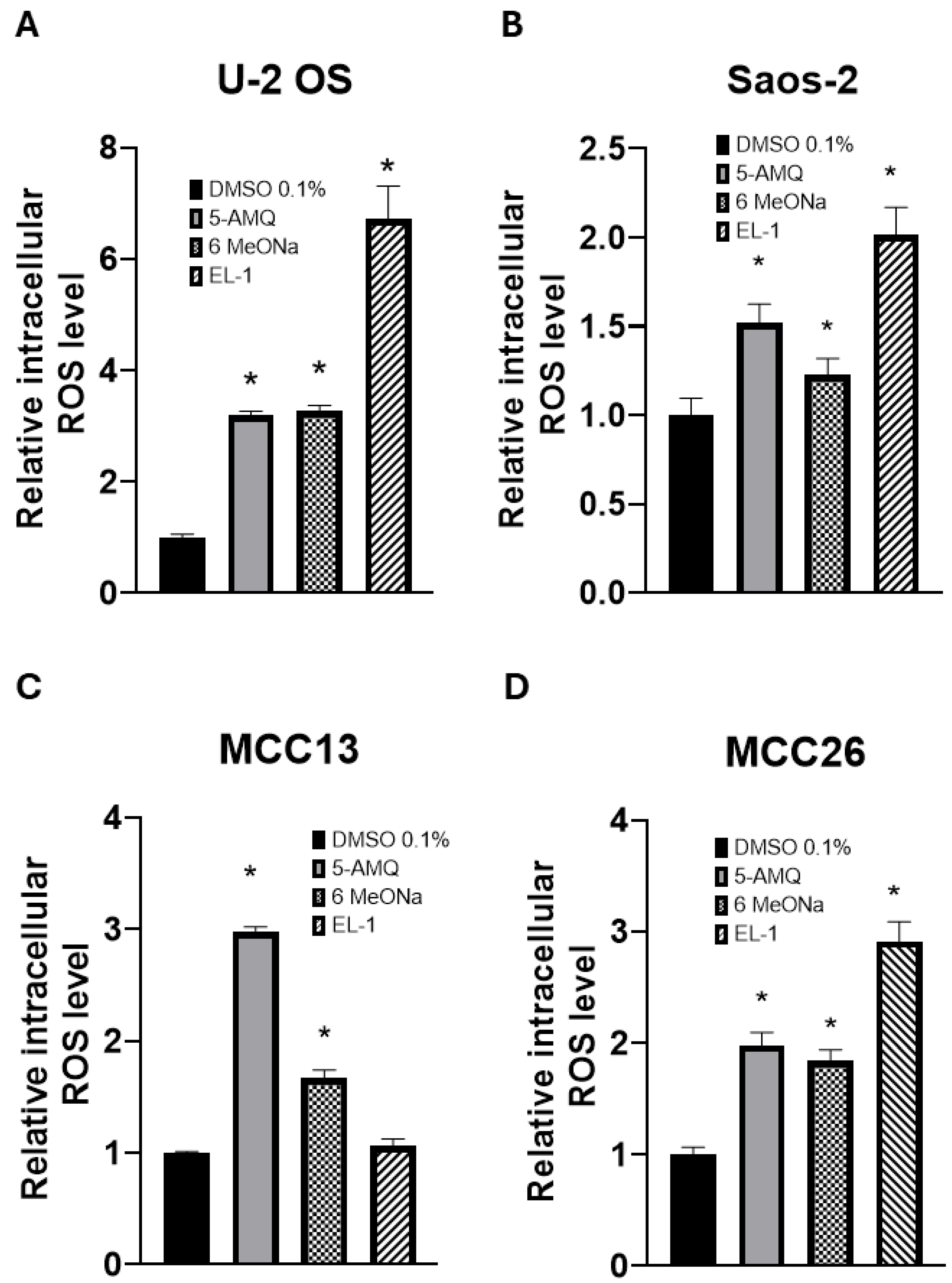
3.5. Modulation of Intracellular Oxidative Stress upon NNMT Inhibitor Treatment
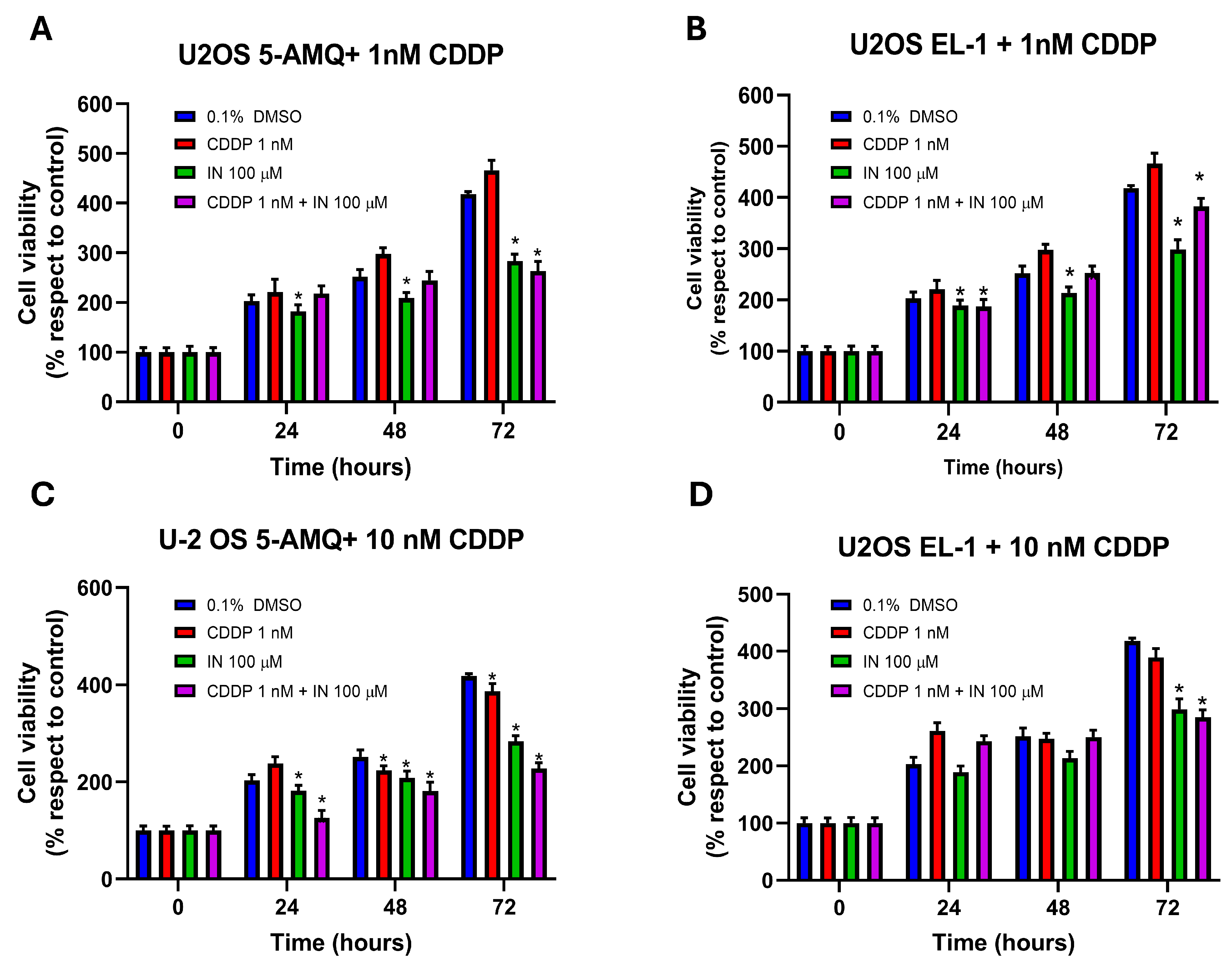
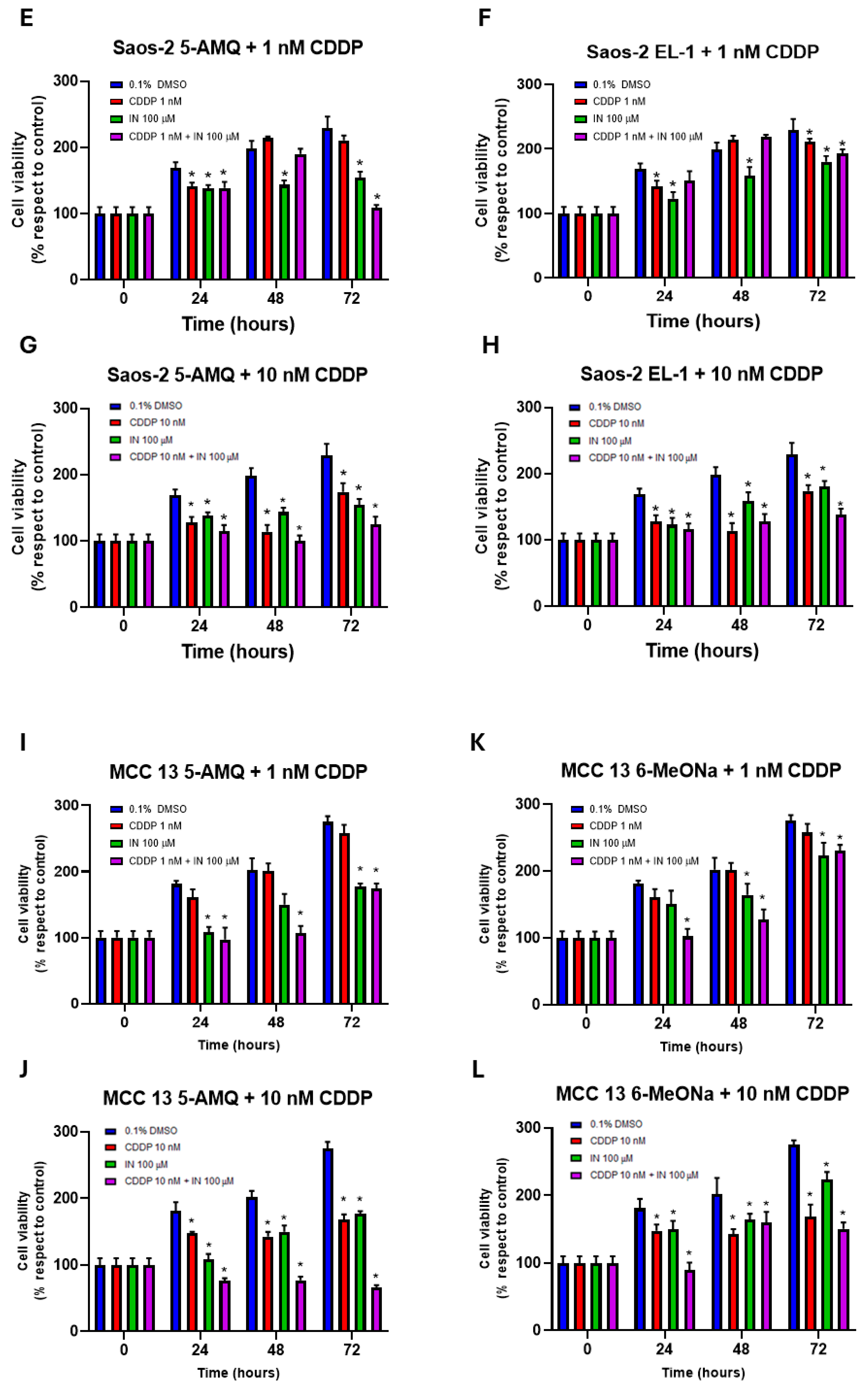
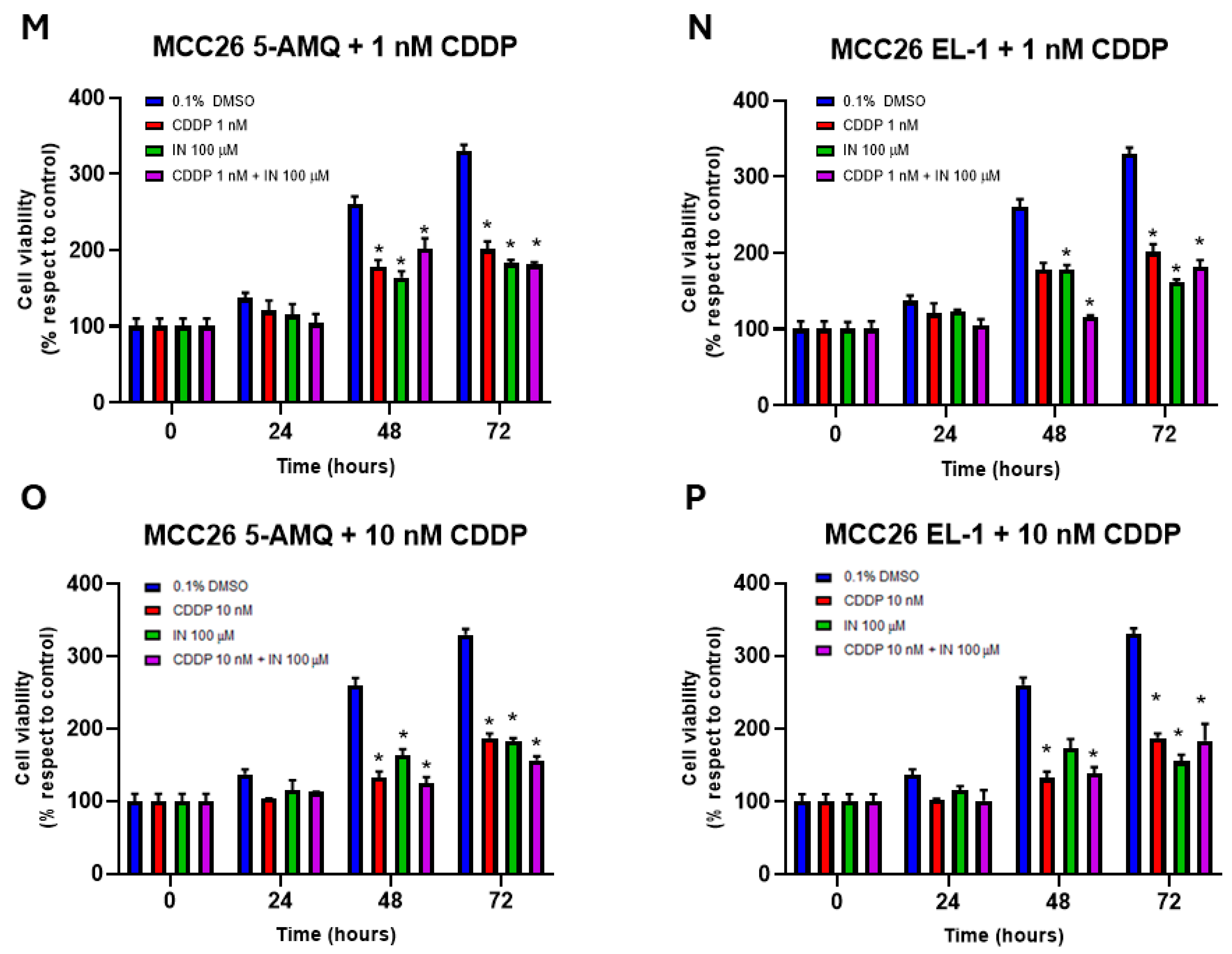
3.6. Effect of Treatment with NNMT Inhibitors on Chemosensitivity
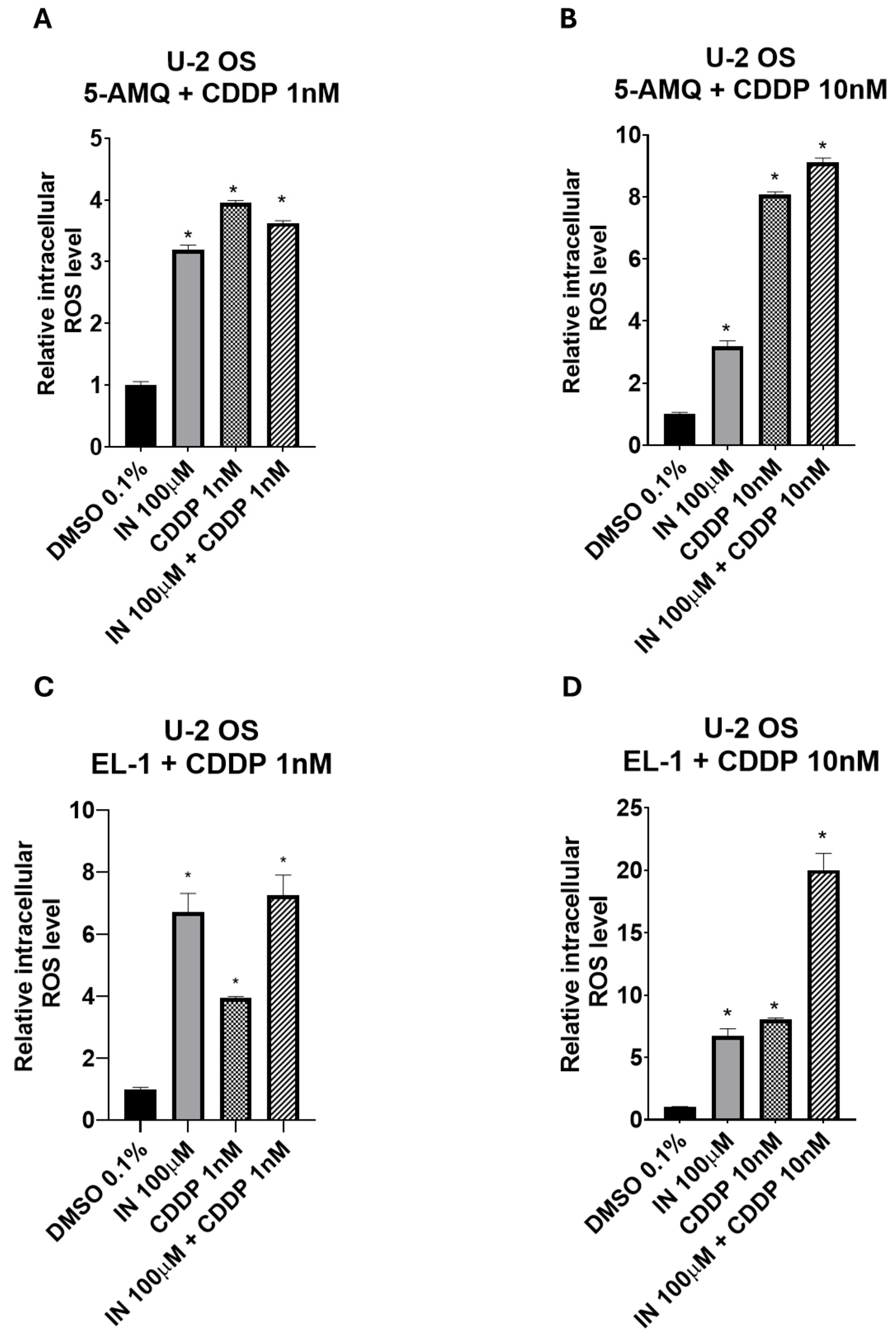
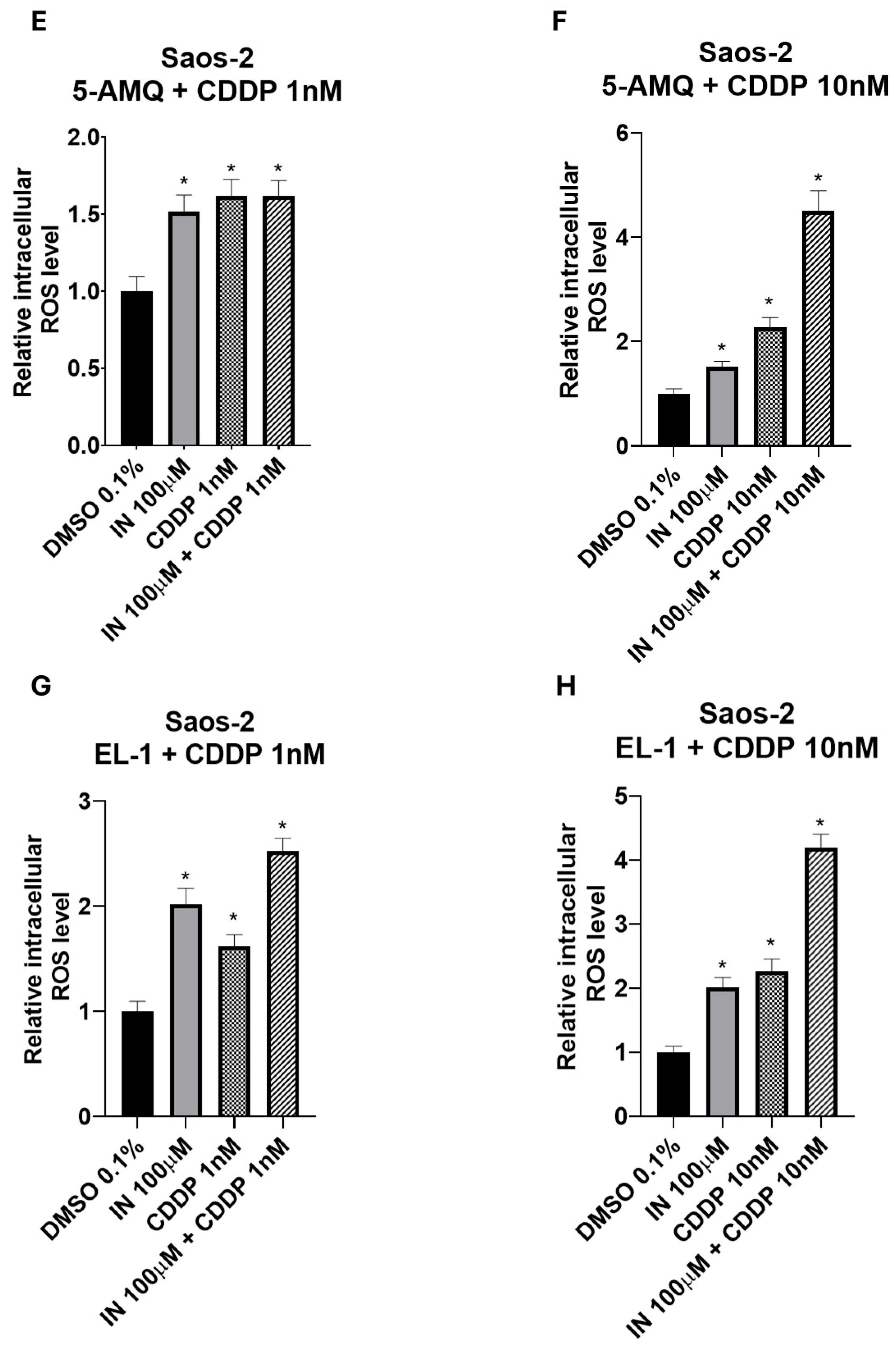
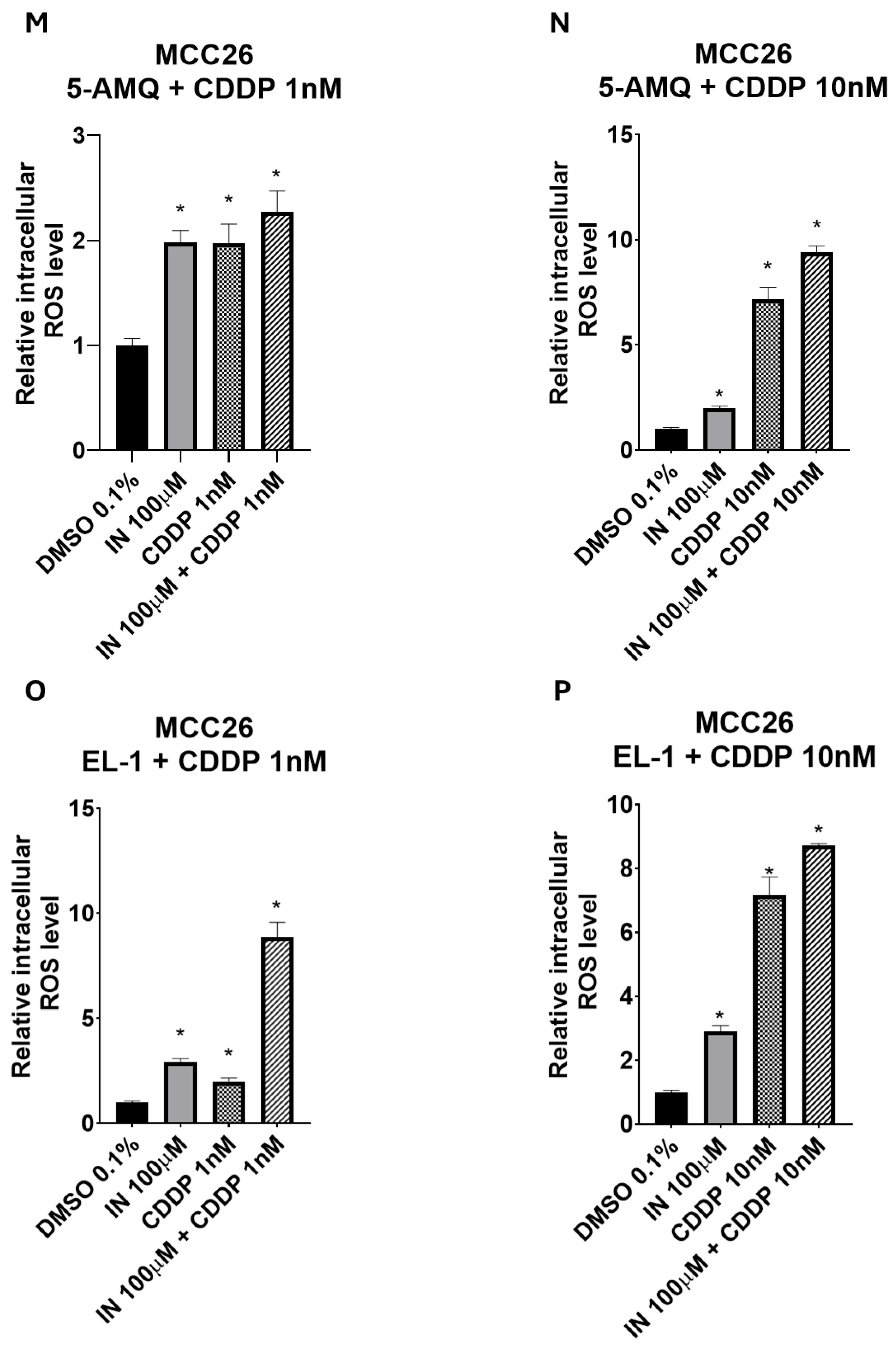
3.7. Impact of Treatment with NNMT Inhibitors Combined with Chemotherapy on Intracellular Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Breast Cancer |
| CDDP | Cisplatin |
| DCFH2-DA | 2′,7′-Dichlorodihydrofluorescein Diacetate |
| DMSO | Dimethyl Sulfoxide |
| EL-1 | Eli Lilly’s Pyrimidine 5-Carboxamide |
| 5-AMQ | 5-Amino-1-Methyl Quinolinium |
| GSH | Reduced Glutathione |
| GSSG | Oxidized Glutathione |
| MCC | Merkel Cell Carcinoma |
| MNA | N1-Methylnicotinamide |
| MTT | (4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| muSCs | Muscle Stem Cells |
| NA | Nicotinamide |
| NAD+ | Oxidized Nicotinamide Adenine Dinucleotide |
| NNMT | Nicotinamide N-Methyltransferase |
| OS | Osteosarcoma |
| ROS | Reactive Oxygen Species |
| SAH | S-Adenosyl-L-Homocysteine |
| SAM | S-Adenosyl-L-Methionine |
| 6MeONa | 6-Methoxynicotinamide |
References
- Aksoy, S.; Szumlanski, C.L.; Weinshilboum, R.M. Human liver nicotinamide N-methyltransferase. cDNA cloning, expression, and biochemical characterization. J. Biol. Chem. 1994, 269, 14835–14840. [Google Scholar] [CrossRef]
- Felsted, R.L.; Chaykin, S. N1-methylnicotinamide oxidation in a number of mammals. J. Biol. Chem. 1967, 242, 1274–1279. [Google Scholar] [CrossRef]
- Okamoto, H.; Ishikawa, A.; Yoshitake, Y.; Kodama, N.; Nishimuta, M.; Fukuwatari, T.; Shibata, K. Diurnal variations in human urinary excretion of nicotinamide catabolites: Effects of stress on the metabolism of nicotinamide. Am. J. Clin. Nutr. 2003, 77, 406–410. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.; Wang, T.; Deng, H. Complex roles of nicotinamide N-methyltransferase in cancer progression. Cell Death Dis. 2022, 13, 267. [Google Scholar] [CrossRef] [PubMed]
- Makarov, M.V.; Trammell, S.A.J.; Migaud, M.E. The chemistry of the vitamin B3 metabolome. Biochem. Soc. Trans. 2019, 47, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Are poly(ADP-ribosyl)ation by PARP-1 and deacetylation by Sir2 linked? Bioessays 2003, 25, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Sartini, D.; Pozzi, V.; Wilk, D.; Emanuelli, M.; Yee, V.C. Structural basis of substrate recognition in human nicotinamide N-methyltransferase. Biochemistry 2011, 50, 7800–7808. [Google Scholar] [CrossRef]
- Loring, H.S.; Thompson, P.R. Kinetic Mechanism of Nicotinamide N-Methyltransferase. Biochemistry 2018, 57, 5524–5532. [Google Scholar] [CrossRef]
- van Haren, M.J.; Taig, R.; Kuppens, J.; Sastre Torano, J.; Moret, E.E.; Parsons, R.B.; Sartini, D.; Emanuelli, M.; Martin, N.I. Inhibitors of nicotinamide N-methyltransferase designed to mimic the methylation reaction transition state. Org. Biomol. Chem. 2017, 15, 6656–6667. [Google Scholar] [CrossRef]
- Gao, Y.; van Haren, M.J.; Moret, E.E.; Rood, J.J.M.; Sartini, D.; Salvucci, A.; Emanuelli, M.; Craveur, P.; Babault, N.; Jin, J.; et al. Bisubstrate Inhibitors of Nicotinamide N-Methyltransferase (NNMT) with Enhanced Activity. J. Med. Chem. 2019, 62, 6597–6614. [Google Scholar] [CrossRef]
- Gao, Y.; van Haren, M.J.; Buijs, N.; Innocenti, P.; Zhang, Y.; Sartini, D.; Campagna, R.; Emanuelli, M.; Parsons, R.B.; Jespers, W.; et al. Potent Inhibition of Nicotinamide N-Methyltransferase by Alkene-Linked Bisubstrate Mimics Bearing Electron Deficient Aromatics. J. Med. Chem. 2021, 64, 12938–12963. [Google Scholar] [CrossRef]
- Markert, J.M.; Fuller, C.M.; Gillespie, G.Y.; Bubien, J.K.; McLean, L.A.; Hong, R.L.; Lee, K.; Gullans, S.R.; Mapstone, T.B.; Benos, D.J. Differential gene expression profiling in human brain tumors. Physiol. Genomics 2001, 5, 21–33. [Google Scholar] [CrossRef]
- Xu, J.; Moatamed, F.; Caldwell, J.S.; Walker, J.R.; Kraiem, Z.; Taki, K.; Brent, G.A.; Hershman, J.M. Enhanced expression of nicotinamide N-methyltransferase in human papillary thyroid carcinoma cells. J. Clin. Endocrinol. Metab. 2003, 88, 4990–4996. [Google Scholar] [CrossRef][Green Version]
- Lim, B.H.; Cho, B.I.; Kim, Y.N.; Kim, J.W.; Park, S.T.; Lee, C.W. Overexpression of nicotinamide N-methyltransferase in gastric cancer tissues and its potential post-translational modification. Exp. Mol. Med. 2006, 38, 455–465. [Google Scholar] [CrossRef]
- Roessler, M.; Rollinger, W.; Palme, S.; Hagmann, M.L.; Berndt, P.; Engel, A.M.; Schneidinger, B.; Pfeffer, M.; Andres, H.; Karl, J.; et al. Identification of nicotinamide N-methyltransferase as a novel serum tumor marker for colorectal cancer. Clin. Cancer Res. 2005, 11, 6550–6557. [Google Scholar] [CrossRef]
- Zhou, W.; Gui, M.; Zhu, M.; Long, Z.; Huang, L.; Zhou, J.; He, L.; Zhong, K. Nicotinamide N-methyltransferase is overexpressed in prostate cancer and correlates with prolonged progression-free and overall survival times. Oncol. Lett. 2014, 8, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Siadaty, M.S.; Berens, M.E.; Hampton, G.M.; Theodorescu, D. Overlapping gene expression profiles of cell migration and tumor invasion in human bladder cancer identify metallothionein 1E and nicotinamide N-methyltransferase as novel regulators of cell migration. Oncogene 2008, 27, 6679–6689. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.W.; Yang, T.C.; Lin, W.C.; Chang, W.H.; Wang, C.C.; Lai, M.K.; Lin, J.Y. Nicotinamide N-methyltransferase induces cellular invasion through activating matrix metalloproteinase-2 expression in clear cell renal cell carcinoma cells. Carcinogenesis 2011, 32, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Li, G.; Yu, H.; Xie, X. Down-regulation of nicotinamide N-methyltransferase induces apoptosis in human breast cancer cells via the mitochondria-mediated pathway. PLoS ONE 2014, 9, e89202. [Google Scholar] [CrossRef]
- Xie, X.; Yu, H.; Wang, Y.; Zhou, Y.; Li, G.; Ruan, Z.; Li, F.; Wang, X.; Liu, H.; Zhang, J. Nicotinamide N-methyltransferase enhances the capacity of tumorigenesis associated with the promotion of cell cycle progression in human colorectal cancer cells. Arch. Biochem. Biophys. 2014, 564, 52–66. [Google Scholar] [CrossRef]
- Xie, X.; Liu, H.; Wang, Y.; Zhou, Y.; Yu, H.; Li, G.; Ruan, Z.; Li, F.; Wang, X.; Zhang, J. Nicotinamide N-methyltransferase enhances resistance to 5-fluorouracil in colorectal cancer cells through inhibition of the ASK1-p38 MAPK pathway. Oncotarget 2016, 7, 45837–45848. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, J.; Wu, W.; Xie, S.; Yu, H.; Li, G.; Zhu, T.; Li, F.; Lu, J.; Wang, G.Y.; et al. Nicotinamide N-methyltransferase enhances chemoresistance in breast cancer through SIRT1 protein stabilization. Breast Cancer Res. 2019, 21, 64. [Google Scholar] [CrossRef]
- Kassem, H.; Sangar, V.; Cowan, R.; Clarke, N.; Margison, G.P. A potential role of heat shock proteins and nicotinamide N-methyl transferase in predicting response to radiation in bladder cancer. Int. J. Cancer 2002, 101, 454–460. [Google Scholar] [CrossRef]
- Pozzi, V.; Molinelli, E.; Campagna, R.; Serritelli, E.N.; Cecati, M.; De Simoni, E.; Sartini, D.; Goteri, G.; Martin, N.I.; van Haren, M.J.; et al. Knockdown of nicotinamide N-methyltransferase suppresses proliferation, migration, and chemoresistance of Merkel cell carcinoma cells in vitro. Hum. Cell 2024, 37, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Serritelli, E.N.; Sartini, D.; Campagna, R.; Pozzi, V.; Martin, N.I.; van Haren, M.J.; Salvolini, E.; Cecati, M.; Rubini, C.; Emanuelli, M. Targeting nicotinamide N-methyltransferase decreased aggressiveness of osteosarcoma cells. Eur. J. Clin. Investig. 2024, 54, e14185. [Google Scholar] [CrossRef]
- Neelakantan, H.; Wang, H.Y.; Vance, V.; Hommel, J.D.; McHardy, S.F.; Watowich, S.J. Structure-Activity Relationship for Small Molecule Inhibitors of Nicotinamide N-Methyltransferase. J. Med. Chem. 2017, 60, 5015–5028. [Google Scholar] [CrossRef]
- Ruf, S.; Hallur, M.S.; Anchan, N.K.; Swamy, I.N.; Murugesan, K.R.; Sarkar, S.; Narasimhulu, L.K.; Putta, V.; Shaik, S.; Chandrasekar, D.V.; et al. Novel nicotinamide analog as inhibitor of nicotinamide N-methyltransferase. Bioorg. Med. Chem. Lett. 2018, 28, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, R.W. Novel Pyrimidine-5-carboxamide Compounds as NNMT Inhibitors for Treating Diabetes. ACS Med. Chem. Lett. 2021, 12, 538–539. [Google Scholar] [CrossRef] [PubMed]
- Kansara, M.; Teng, M.W.; Smyth, M.J.; Thomas, D.M. Translational biology of osteosarcoma. Nat. Rev. Cancer 2014, 14, 722–735. [Google Scholar] [CrossRef]
- He, H.; Ni, J.; Huang, J. Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol. Lett. 2014, 7, 1352–1362. [Google Scholar] [CrossRef]
- Strong, J.; Hallaert, P.; Brownell, I. Merkel Cell Carcinoma. Hematol. Oncol. Clin. N. Am. 2024, 38, 1133–1147. [Google Scholar] [CrossRef]
- Hernandez, L.E.; Mohsin, N.; Yaghi, M.; Frech, F.S.; Dreyfuss, I.; Nouri, K. Merkel cell carcinoma: An updated review of pathogenesis, diagnosis, and treatment options. Dermatol. Ther. 2022, 35, e15292. [Google Scholar] [CrossRef]
- Bedard, P.L.; Hyman, D.M.; Davids, M.S.; Siu, L.L. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 2020, 395, 1078–1088. [Google Scholar] [CrossRef]
- Neelakantan, H.; Vance, V.; Wetzel, M.D.; Wang, H.L.; McHardy, S.F.; Finnerty, C.C.; Hommel, J.D.; Watowich, S.J. Selective and membrane-permeable small molecule inhibitors of nicotinamide N-methyltransferase reverse high fat diet-induced obesity in mice. Biochem. Pharmacol. 2018, 147, 141–152. [Google Scholar] [CrossRef]
- Babula, J.J.; Bui, D.; Stevenson, H.L.; Watowich, S.J.; Neelakantan, H. Nicotinamide N-methyltransferase inhibition mitigates obesity-related metabolic dysfunction. Diabetes Obes. Metab. 2024, 26, 5272–5282. [Google Scholar] [CrossRef]
- Neelakantan, H.; Brightwell, C.R.; Graber, T.G.; Maroto, R.; Wang, H.L.; McHardy, S.F.; Papaconstantinou, J.; Fry, C.S.; Watowich, S.J. Small molecule nicotinamide N-methyltransferase inhibitor activates senescent muscle stem cells and improves regenerative capacity of aged skeletal muscle. Biochem. Pharmacol. 2019, 163, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Kannt, A.; Rajagopal, S.; Kadnur, S.V.; Suresh, J.; Bhamidipati, R.K.; Swaminathan, S.; Hallur, M.S.; Kristam, R.; Elvert, R.; Czech, J.; et al. A small molecule inhibitor of Nicotinamide N-methyltransferase for the treatment of metabolic disorders. Sci. Rep. 2018, 8, 3660. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Huang, Y.; Li, P.; Yang, M.; Zeng, S.; Chen, D.; Wang, Q.; Liu, H.; Luo, K.; et al. Targeting nicotinamide N-methyltransferase overcomes resistance to EGFR-TKI in non-small cell lung cancer cells. Cell Death Discov. 2022, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, B.; Miao, H.; Liu, L.; Wang, Z.; Jiang, C.; Yang, Y.; Qiu, S.; Li, X.; Geng, Y.; et al. Nicotinamide N -methyltransferase promotes M2 macrophage polarization by IL6 and MDSC conversion by GM-CSF in gallbladder carcinoma. Hepatology 2023, 78, 1352–1367. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 1, a008656, Erratum in Cold Spring Harb. Perspect. Biol. 2015, 7, a026716. [Google Scholar] [CrossRef] [PubMed]
- Mistry, R.J.; Klamt, F.; Ramsden, D.B.; Parsons, R.B. Nicotinamide N-methyltransferase expression in SH-SY5Y human neuroblastoma cells decreases oxidative stress. J. Biochem. Mol. Toxicol. 2020, 34, e22439. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhou, X.; Wang, Y.; Huang, X.; Yang, J.; Zeng, J.; Li, G.; Xie, X.; Zhang, J. Nicotinamide N-methyltransferase inhibits autophagy induced by oxidative stress through suppressing the AMPK pathway in breast cancer cells. Cancer Cell Int. 2020, 20, 191. [Google Scholar] [CrossRef]
- Wang, T.; Pulkkinen, O.I.; Aittokallio, T. Target-specific compound selectivity for multi-target drug discovery and repurposing. Front. Pharmacol. 2022, 13, 1003480. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pompei, V.; Cecati, M.; Serritelli, E.N.; Gerini, E.; Campagna, R.; Pozzi, V.; Van Haren, M.J.; Martin, N.I.; Emanuelli, M.; Sartini, D. Small Molecule Inhibitors of Nicotinamide N-Methyltransferase Enzyme for the Treatment of Osteosarcoma and Merkel Cell Carcinoma: Potential for the Development of a Targeted Therapeutic Strategy. Biomolecules 2025, 15, 1553. https://doi.org/10.3390/biom15111553
Pompei V, Cecati M, Serritelli EN, Gerini E, Campagna R, Pozzi V, Van Haren MJ, Martin NI, Emanuelli M, Sartini D. Small Molecule Inhibitors of Nicotinamide N-Methyltransferase Enzyme for the Treatment of Osteosarcoma and Merkel Cell Carcinoma: Potential for the Development of a Targeted Therapeutic Strategy. Biomolecules. 2025; 15(11):1553. https://doi.org/10.3390/biom15111553
Chicago/Turabian StylePompei, Veronica, Monia Cecati, Emma Nicol Serritelli, Eleonora Gerini, Roberto Campagna, Valentina Pozzi, Matthijs J. Van Haren, Nathaniel I. Martin, Monica Emanuelli, and Davide Sartini. 2025. "Small Molecule Inhibitors of Nicotinamide N-Methyltransferase Enzyme for the Treatment of Osteosarcoma and Merkel Cell Carcinoma: Potential for the Development of a Targeted Therapeutic Strategy" Biomolecules 15, no. 11: 1553. https://doi.org/10.3390/biom15111553
APA StylePompei, V., Cecati, M., Serritelli, E. N., Gerini, E., Campagna, R., Pozzi, V., Van Haren, M. J., Martin, N. I., Emanuelli, M., & Sartini, D. (2025). Small Molecule Inhibitors of Nicotinamide N-Methyltransferase Enzyme for the Treatment of Osteosarcoma and Merkel Cell Carcinoma: Potential for the Development of a Targeted Therapeutic Strategy. Biomolecules, 15(11), 1553. https://doi.org/10.3390/biom15111553










