An Overview of Contrasting Experimental Results on Dynamics of Kinesin-1 Molecular Motors: Insight into the Underlying Mechanism
Abstract
1. Introduction
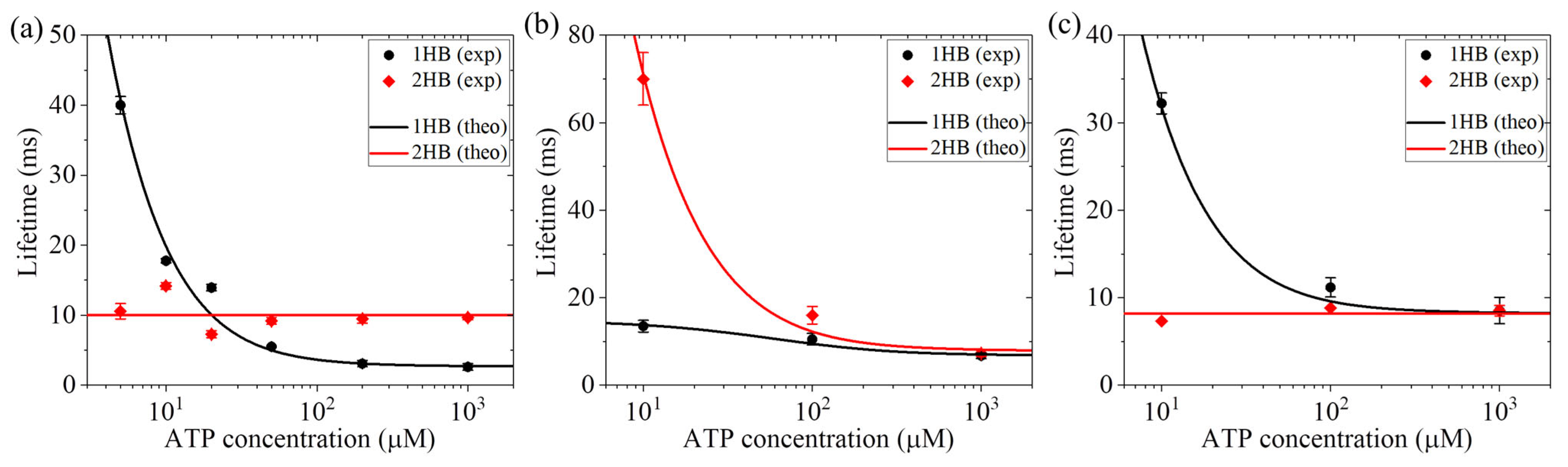
2. ATP Binding Occurs in One-Head-Bound or Two-Heads-Bound State
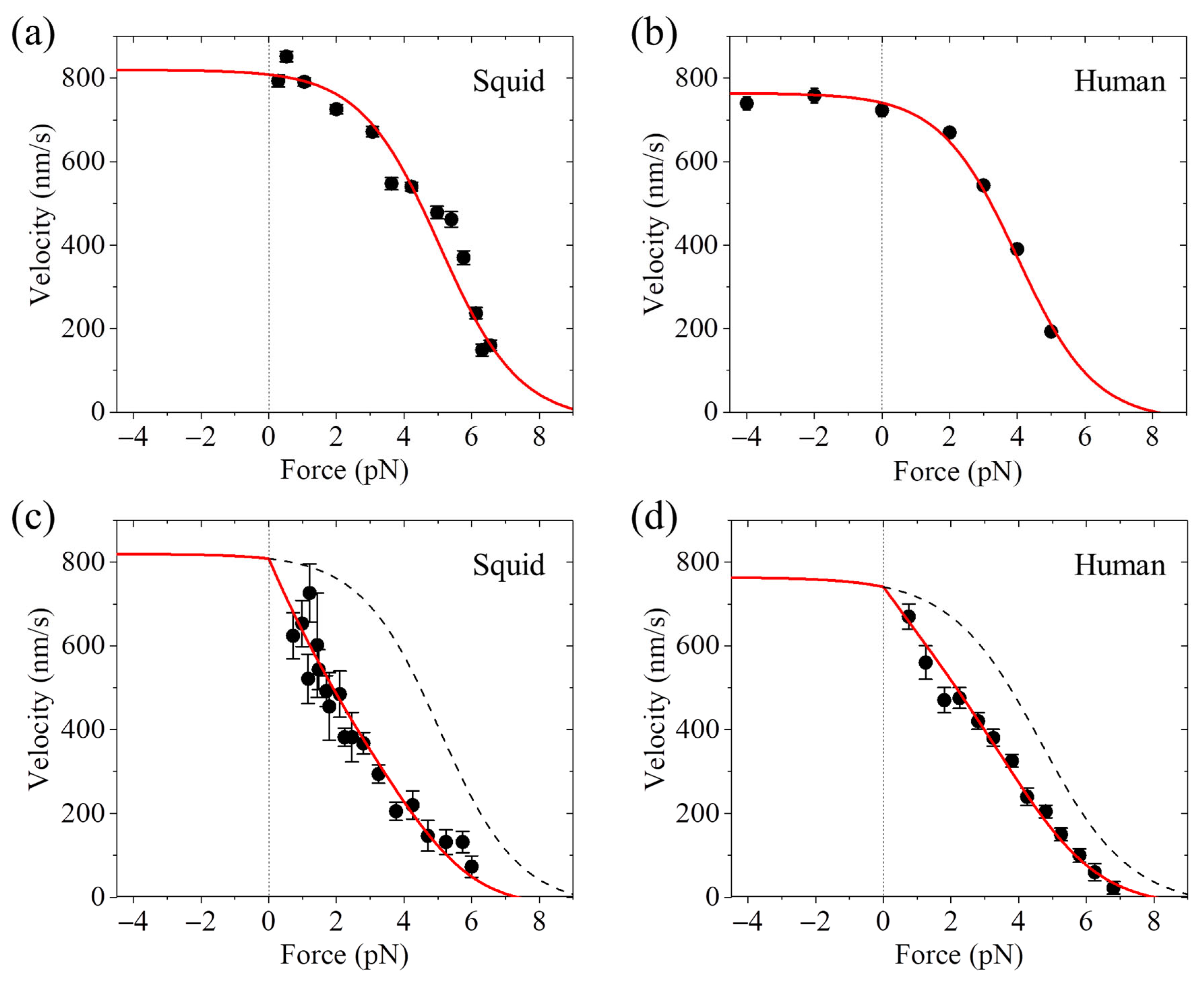
3. Velocity Versus Backward Load Has a Sigmoid or Linear Form
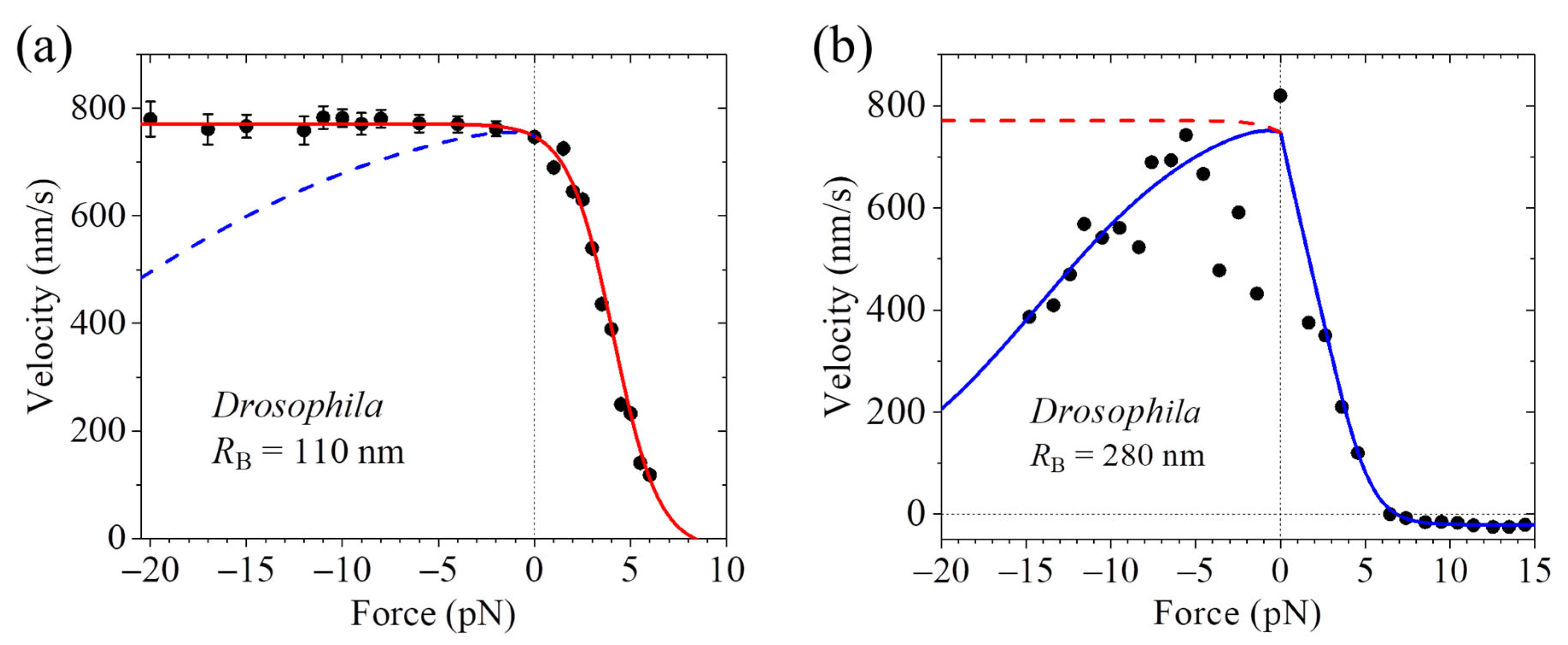
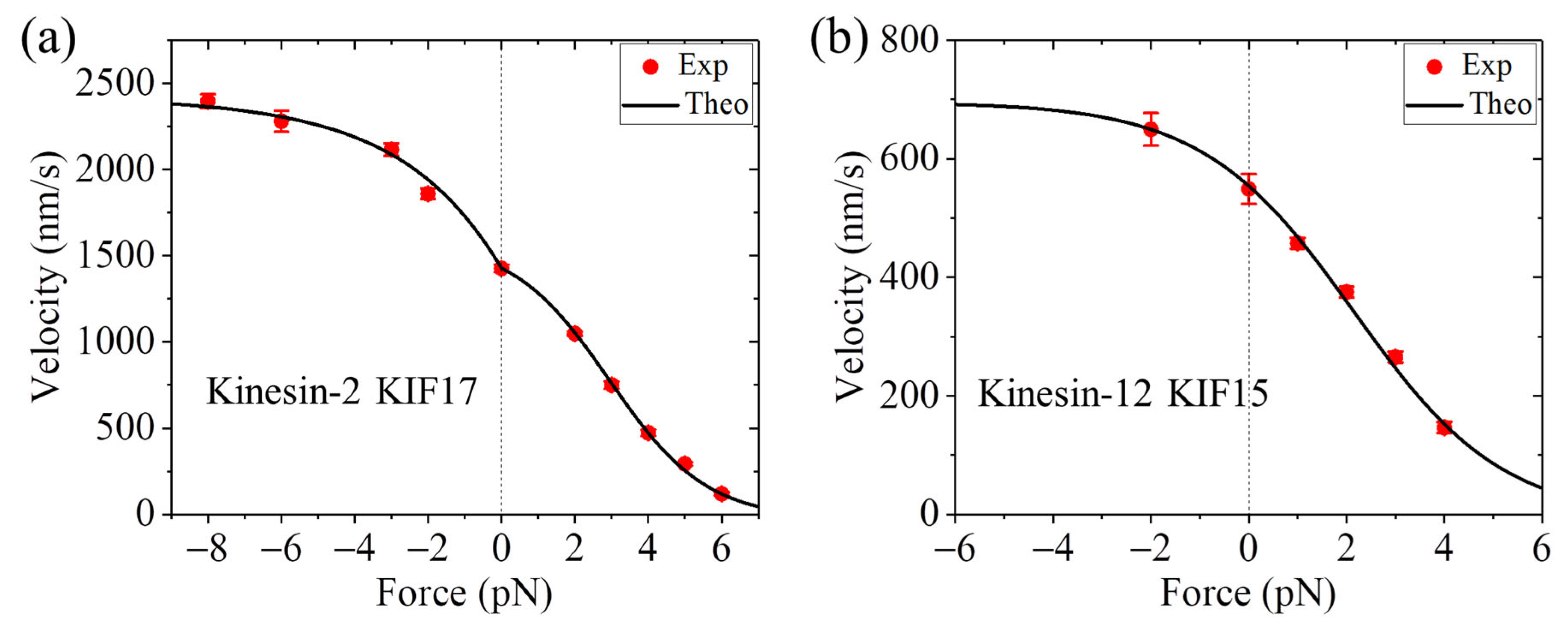
4. Velocity Is Independent of or Decreases with or Increases with Forward Load
5. Kinesin Pauses for a Short or Long Time upon Reaching Roadblocks
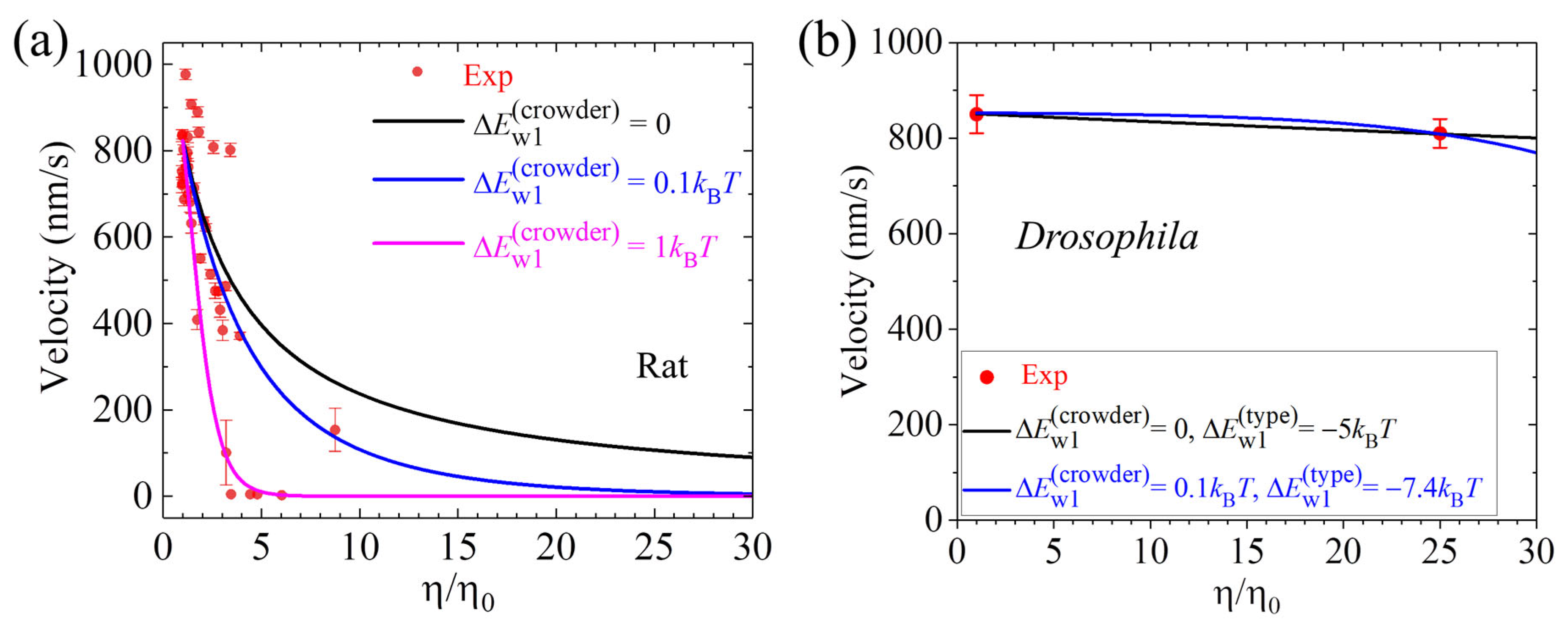
6. Velocity Decreases Sensitively with or Depends Insensitively on the Solution Viscosity
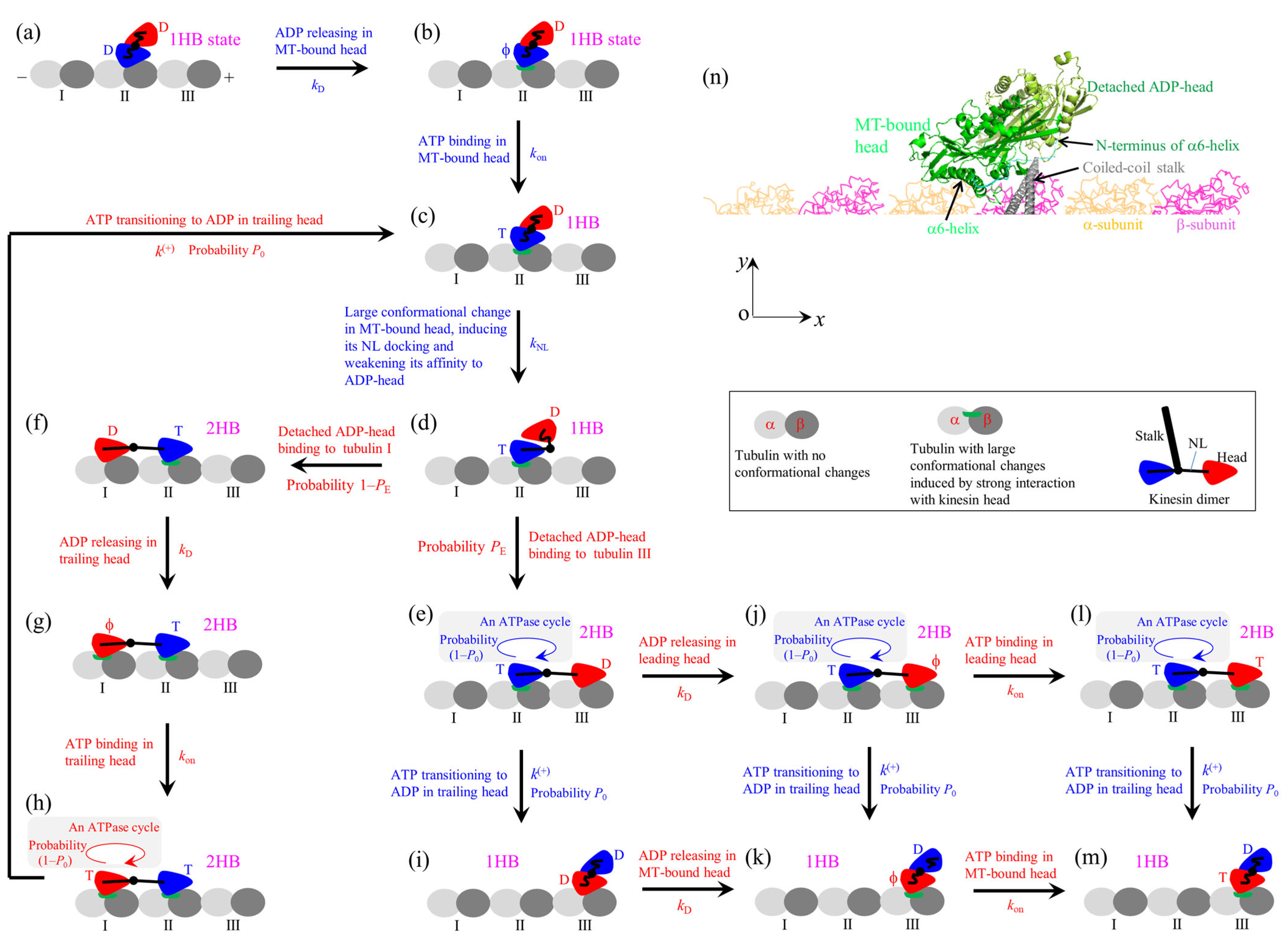
7. A Model Can Consistently Explain the Contrasting Experimental Results
7.1. Explanation of the Conflicting Experimental Results on ATP Binding in 1HB or 2HB State
7.2. Explanation of the Contrasting Experimental Results on Velocity Versus Backward Load
7.3. Explanation of the Contrasting Experimental Results on Velocity Versus Forward Load
7.4. Explanation of the Conflicting Experimental Results on Pausing Time upon Reaching Roadblocks
7.5. Explanation of the Conflicting Experimental Results on Dependence of Velocity Upon Solution Viscosity
7.6. Explanation of Other Experimental Results
8. Concluding Remarks and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | adenosine diphosphate |
| AMD | atomistic molecular dynamics |
| ATP | adenosine triphosphate |
| ATPase | adenosine triphosphatase |
| BSA | bovine serum albumin |
| ϕ | nucleotide free |
| INT | intermediate |
| MT | microtubule |
| NBP | nucleotide-binding pocket |
| NL | neck linker |
| 1HB | one-head-bound |
| 2HB | two-heads-bound |
| WT | wild type |
Appendix A. Main Elements in the Model of Figure 7
References
- Lawrence, C.J.; Dawe, R.K.; Christie, K.R.; Cleveland, D.W.; Dawson, S.C.; Endow, S.A.; Goldstein, L.S.B.; Goodson, H.V.; Hirokawa, N.; Howard, J.; et al. A standardized kinesin nomenclature. J. Cell Biol. 2004, 167, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Vale, R.D. The molecular motor toolbox for intracellular transport. Cell 2003, 112, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Okada, Y.; Hirokawa, N. Analysis of the kinesin superfamily: Insights into structure and function. Trends Cell Biol. 2005, 15, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Noda, Y.; Tanaka, Y.; Niwa, S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 2009, 10, 682. [Google Scholar] [CrossRef]
- Iino, R.; Kinbara, K.; Bryant, Z. Introduction: Molecular motors. Chem. Rev. 2020, 120, 1–4. [Google Scholar] [CrossRef]
- Vale, R.D.; Reese, T.S.; Sheetz, M.P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 1985, 42, 39–50. [Google Scholar] [CrossRef]
- Brady, S.T. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature 1985, 317, 73–75. [Google Scholar] [CrossRef]
- Howard, J. The movement of kinesin along microtubules. Annu. Rev. Physiol. 1996, 58, 703–729. [Google Scholar] [CrossRef]
- Cross, R.A. The kinetic mechanism of kinesin. Trends Biochem. Sci. 2004, 29, 301–309. [Google Scholar] [CrossRef]
- Asbury, C.L. Kinesin: World’s tiniest biped. Curr. Opin. Cell Biol. 2005, 17, 89–97. [Google Scholar]
- Block, S.M. Kinesin motor mechanics: Binding, stepping, tracking, gating, and limping. Biophys. J. 2007, 92, 2986–2995. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.A. Mechanochemistry of the kinesin-1 ATPase. Biopolymers 2017, 105, 476–482. [Google Scholar] [CrossRef]
- Kozielski, F.; Sack, S.; Marx, A.; Thormählen, M.; Schönbrunn, E.; Biou, V.; Thompson, A.; Mandelkow, E.M.; Mandelkow, E. The crystal structure of dimeric kinesin and implications for microtubule-dependent motility. Cell 1997, 91, 985–994. [Google Scholar] [CrossRef]
- Block, S.M.; Goldstein, L.S.; Schnapp, B.J. Bead movement by single kinesin molecules studied with optical tweezers. Nature 1990, 348, 348–352. [Google Scholar] [CrossRef]
- Svoboda, K.; Schmidt, C.; Schnapp, B.; Block, S. Direct observation of kinesin stepping by optical trapping interferometry. Nature 1993, 365, 721–727. [Google Scholar] [CrossRef]
- Kull, F.J.; Sablin, E.P.; Lau, R.; Fletterick, R.J.; Vale, R.D. Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature 1996, 380, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Sindelar, C.V.; Downing, K.H. An atomic-level mechanism for activation of the kinesin molecular motors. Proc. Natl. Acad. Sci. USA 2010, 107, 4111–4116. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Zhou, K.; Xu, C.; Csencsits, R.; Cochran, J.C.; Sindelar, C.V. High-resolution structures of kinesin on microtubules provide a basis for nucleotide-gated force-generation. eLife 2014, 3, e04686. [Google Scholar] [CrossRef][Green Version]
- Gigant, B.; Wang, W.; Dreier, B.; Jiang, Q.; Pecqueur, L.; Plückthun, A.; Wang, C.; Knossow, M. Structure of a kinesin-tubulin complex and implications for kinesin motility. Nat. Struct. Mol. Biol. 2013, 20, 1001–1007. [Google Scholar] [CrossRef]
- Cao, L.; Wang, W.; Jiang, Q.; Wang, C.; Knossow, M.; Gigant, B. The structure of apo-kinesin bound to tubulin links the nucleotide cycle to movement. Nat. Comm. 2014, 5, 5364. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Yajima, H.; Nitta, R.; Inoue, S.; Ogura, T.; Sato, C.; Hirokawa, N. X-ray and Cryo-EM structures reveal mutual conformational changes of kinesin and GTP-state microtubules upon binding. EMBO J. 2015, 34, 1270–1286. [Google Scholar] [CrossRef]
- Hackney, D.D. Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc. Natl. Acad. Sci. USA 1994, 91, 6865–6869. [Google Scholar] [CrossRef]
- Hackney, D.D. Kinesin ATPase: Rate-limiting ADP release. Proc. Natl. Acad. Sci. USA 1998, 37, 800–813. [Google Scholar] [CrossRef]
- Moyer, M.L.; Gilbert, S.P.; Johnson, K.A. Pathway of ATP hydrolysis by monomeric and dimeric kinesin. Biochemistry 1998, 37, 800–813. [Google Scholar] [CrossRef]
- Rosenfeld, S.S.; Jefferson, G.M.; King, P.H. ATP reorients the neck linker of kinesin in two sequential steps. J. Biol. Chem. 2001, 276, 40167–40174. [Google Scholar] [CrossRef]
- Mickolajczyk, K.J.; Deffenbaugh, N.C.; Arroyo, J.O.; Andrecka, J.; Kukura, P.; Hancock, W.O. Kinetics of nucleotide-dependent structural transitions in the kinesin-1 hydrolysis cycle. Proc. Natl. Acad. Sci. USA 2015, 112, E7186–E7193. [Google Scholar] [CrossRef] [PubMed]
- Isojima, H.; Iino, R.; Niitani, Y.; Noji, H.; Tomishige, M. Direct observation of intermediate states during the stepping motion of kinesin-1. Nat. Chem. Biol. 2016, 12, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.O.; Scheiderer, L.; Engelhardt, T.; Engelhardt, J.; Matthias, J.; Hell, S.W. MINFLUX dissects the unimpeded walking of kinesin-1. Science 2023, 379, 1004–1010. [Google Scholar] [CrossRef]
- Deguchi, T.; Iwanski, M.K.; Schentarra, E.-M.; Heidebrecht, C.; Schmidt, L.; Heck, J.; Weihs, T.; Schnorrenberg, S.; Hoess, P.; Liu, S.; et al. Direct observation of motor protein stepping in living cells using MINFLUX. Science 2023, 379, 1010–1015. [Google Scholar] [CrossRef]
- Schnitzer, M.J.; Block, S.M. Kinesin hydrolyses one ATP per 8-nm step. Nature 1997, 388, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Coppin, C.M.; Pierce, D.W.; Hsu, L.; Vale, R.D. The load dependence of kinesin’s mechanical cycle. Proc. Natl. Acad. Sci. USA. 1997, 94, 8539–8544. [Google Scholar] [CrossRef]
- Kojima, H.; Muto, E.; Higuchi, H.; Yanagida, T. Mechanics of single kinesin molecules measured by optical trapping nanometry. Biophys. J. 1997, 73, 2012–2022. [Google Scholar] [CrossRef]
- Visscher, K.; Schnitzer, M.J.; Block, S.M. Single kinesin molecules studied with a molecular force clamp. Nature 1999, 400, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, M.; Higuchi, H.; Yanagida, T. Chemomechanical coupling of the forward and backward steps of single kinesin molecules. Nat. Cell Biol. 2002, 4, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.S.; Appleyard, D.C.; Labno, A.K.; Georges, A.; Karplus, M.; Belcher, A.M.; Hwang, W.; Lang, M.J. Kinesin’s cover-neck bundle folds forward to generate force. Proc. Natl. Acad. Sci. USA 2008, 105, 19247–19252. [Google Scholar] [CrossRef]
- Carter, N.J.; Cross, R.A. Mechanics of the kinesin step. Nature 2005, 435, 308–312. [Google Scholar] [CrossRef]
- Andreasson, J.O.L.; Milic, B.; Chen, G.-Y.; Guydosh, N.R.; Hancock, W.O.; Block, S.M. Examining kinesin processivity within a general gating framework. eLife 2015, 4, e07403. [Google Scholar] [CrossRef] [PubMed]
- Milic, B.; Andreasson, J.O.L.; Hancock, W.O.; Block, S.M. Kinesin processivity is gated by phosphate release. Proc. Natl. Acad. Sci. USA 2014, 111, 14136–14140. [Google Scholar] [CrossRef]
- Sudhakar, S.; Abdosamadi, M.K.; Jachowski, T.J.; Bugiel, M.; Jannasch, A.; Schäffer, E. Germanium nanospheres for ultraresolution picotensiometry of kinesin motors. Science 2021, 71, eabd9944. [Google Scholar] [CrossRef]
- Hyeon, C.; Onuchic, J.N. Mechanical control of the directional stepping dynamics of the kinesin motor. Proc. Natl. Acad. Sci. USA 2007, 104, 17382–17387. [Google Scholar] [CrossRef]
- Zhang, Z.; Thirumalai, D. Dissecting the kinematics of the kinesin step. Structure 2012, 20, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Kanada, R.; Kuwata, T.; Kenzaki, H.; Takada, S. Structure-based molecular simulations reveal the enhancement of biased Brownian motions in single-headed kinesin. PLoS Comput. Biol. 2013, 9, e1002907. [Google Scholar] [CrossRef]
- Hwang, W.; Lang, M.J.; Karplus, M. Force generation in Kinesin hinges on cover-neck bundle formation. Structure 2008, 16, 62–71. [Google Scholar] [CrossRef]
- Geng, Y.-Z.; Ji, Q.; Liu, S.-X.; Yan, S.-W. Initial conformation of kinesin’s neck linker. Chin. Phys. B 2014, 23, 108701. [Google Scholar] [CrossRef]
- Chakraborty, S.; Zheng, W. Decrypting the structural, dynamic, and energetic basis of a monomeric Kinesin interacting with a tubulin dimer in three ATPase states by all-atom molecular dynamics simulation. Biochemistry 2015, 54, 859–869. [Google Scholar] [CrossRef]
- Wang, J.-G.; Shi, X.-X.; Liu, Y.-R.; Wang, P.-Y.; Chen, H.; Xie, P. Investigation of the structural and dynamic basis of kinesin dissociation from microtubule by atomistic molecular dynamics simulations. Chin. Phys. B 2022, 31, 058702. [Google Scholar] [CrossRef]
- Geng, Y.-Z.; Lu, L.-A.; Jia, N.; Zhang, B.-B.; Ji, Q. Kinesin-microtubule interaction reveals the mechanism of kinesin-1 for discriminating the binding site on microtubule. Chin. Phys. B 2023, 32, 108701. [Google Scholar] [CrossRef]
- Schnitzer, M.J.; Visscher, K.; Block, S.M. Force production by single kinesin motors. Nat. Cell Biol. 2000, 2, 718–723. [Google Scholar] [CrossRef]
- Fisher, M.E.; Kolomeisky, A.B. Simple mechanochemistry describes the dynamics of kinesin molecules. Proc. Natl. Acad. Sci. USA 2001, 98, 7748–7753. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, C.; Onuchic, J.N. Internal strain regulates the nucleotide binding site of the kinesin leading head. Proc. Natl. Acad. Sci. USA 2007, 104, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Liepelt, S.; Lipowsky, R. Kinesin’s network of chemomechanical motor cycles. Phys. Rev. Lett. 2007, 98, 258102. [Google Scholar] [CrossRef]
- Khataee, H.; Liew, A.W.C. A mathematical model describing the mechanical kinetics of kinesin stepping. Bioinformatics 2014, 30, 353–359. [Google Scholar]
- Sumi, T. Design principles governing chemomechanical coupling of kinesin. Sci. Rep. 2017, 7, 1163. [Google Scholar] [CrossRef]
- Takaki, R.; Mugnai, M.L.; Goldtzvik, Y.; Thirumalai, D. How kinesin waits for ATP affects the nucleotide and load dependence of the stepping kinetics. Proc. Natl. Acad. Sci. USA 2019, 116, 23091–23099. [Google Scholar] [CrossRef]
- Guo, S.-K.; Wang, P.-Y.; Xie, P. A model of processive movement of dimeric kinesin. J. Theor. Biol. 2017, 414, 62–75. [Google Scholar] [CrossRef]
- Xie, P. Insight into the chemomechanical coupling mechanism of kinesin molecular motors. Commun. Theor. Phys. 2021, 73, 057601. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z. Origin of tradeoff between movement velocity and attachment duration of kinesin motor on microtubule. Chin. Phys. B 2024, 33, 028708. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Liu, Y.; Xie, P. On load dependence of detachment rate of kinesin motor. Chin. Phys. B 2025, 34, 018702. [Google Scholar] [CrossRef]
- Asbury, C.L.; Fehr, A.N.; Block, S.M. Kinesin moves by an asymmetric hand-over-hand mechanism. Science 2003, 302, 2130–2134. [Google Scholar] [PubMed]
- Yildiz, A.; Tomishige, M.; Vale, R.D.; Selvin, P.R. Kinesin walks hand-over-hand. Science 2004, 303, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Toleikis, A.; Carter, N.J.; Cross, R.A. Backstepping mechanism of kinesin-1. Biophys J. 2020, 119, 1984–1994. [Google Scholar] [CrossRef]
- Kondo, Y.; Kazuo Sasaki, K.; Higuchi, H. Fast backward steps and detachment of single kinesin molecules measured under a wide range of loads. Traffic 2023, 24, 463–474. [Google Scholar] [CrossRef]
- Mori, T.; Vale, R.D.; Tomishige, M. How kinesin waits between steps. Nature 2007, 450, 750–754. [Google Scholar] [CrossRef]
- Alonso, M.C.; Drummond, D.R.; Kain, S.; Hoeng, J.; Amos, L.; Cross, R.A. An ATP gate controls tubulin binding by the tethered head of kinesin-1. Science 2007, 316, 120–123. [Google Scholar] [CrossRef][Green Version]
- Chen, G.-Y.; Mickolajczyk, K.J.; Hancock, W.O. The kinesin-5 chemomechanical cycle is dominated by a two-heads-bound state. J. Biol. Chem. 2016, 291, 20283–20294. [Google Scholar] [CrossRef]
- Xie, P. ATP-concentration-dependent fractions of one-head-bound and two-heads-bound states of kinesin motor during its chemomechanical coupling cycle. J. Phys. Chem. Lett. 2024, 15, 3893–3899. [Google Scholar] [CrossRef]
- Shi, X.-X.; Liu, Y.-R.; Xie, P. Factors determining kinesin motor in a predominant one-head-bound or two-heads-bound state during its stepping cycle. Biomolecules 2025, 15, 717. [Google Scholar] [CrossRef]
- Higuchi, H.; Muto, E.; Inoue, Y.; Yanagida, T. Kinetics of force generation by single kinesin molecules activated by laser photolysis of caged ATP. Proc. Natl. Acad. Sci. USA 1997, 94, 4395–4400. [Google Scholar] [CrossRef] [PubMed]
- Visscher, K.; Block, S.M. Versatile optical traps with feedback control. Methods Enzymol. 1998, 298, 460–489. [Google Scholar] [PubMed]
- Block, S.M.; Asbury, C.L.; Shaevitz, J.W.; Lang, M.J. Probing the kinesin reaction cycle with a 2D optical force clamp. Proc. Natl. Acad. Sci. USA 2003, 100, 2351–2356. [Google Scholar] [CrossRef] [PubMed]
- Kaseda, K.; Higuchi, H.; Horose, K. Coordination of kinesin’s two heads studied with mutant heterodimers. Proc. Natl. Acad. Sci. USA 2002, 99, 16058–16063. [Google Scholar] [CrossRef]
- Svoboda, K.; Block, S.M. Force and velocity measured for single kinesin molecules. Cell 1994, 77, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Ishiwata, S. Temperature dependence of force, velocity, and processivity of single kinesin molecules. Biochem. Biophys. Res. Commun. 2000, 272, 895–899. [Google Scholar] [CrossRef]
- Xie, P. Effects of stalk orientation and size of trapped bead on force–velocity relation of kinesin motor determined using single molecule optical trapping methods. J. Biol. Phys. 2025, 51, 7. [Google Scholar] [CrossRef]
- Milic, B.; Andreasson, J.O.L.; Hogan, D.W.; Block, S.M. Intraflagellar transport velocity is governed by the number of active KIF17 and KIF3AB motors and their motility properties under load. Proc. Natl. Acad. Sci. USA 2017, 114, E6830–E6838. [Google Scholar] [CrossRef]
- Milic, B.; Chakraborty, A.; Han, K.; Bassik, M.C.; Block, S.M. KIF15 nanomechanics and kinesin inhibitors, with implications for cancer chemotherapeutics. Proc. Natl. Acad. Sci. USA 2018, 115, E4613–E4622. [Google Scholar] [CrossRef] [PubMed]
- Xie, P. Effect of the neck linker on processive stepping of kinesin motor. Biophysica 2023, 3, 46–68. [Google Scholar] [CrossRef]
- Xie, P. Dynamics of kinesin motor proteins under longitudinal and sideways loads. J. Theor. Biol. 2021, 530, 110879. [Google Scholar] [CrossRef]
- Crevel, I.M.-T.; Nyitrai, M.; Alonso, M.C.; Weiss, S.; Geeves, M.A.; Cross, R.A. What kinesin does at roadblocks: The coordination mechanism for molecular walking. EMBO J. 2004, 23, 23–32. [Google Scholar] [CrossRef]
- Telley, I.A.; Bieling, P.; Surrey, T. Obstacles on the microtubule reduce the processivity of kinesin-1 in a minimal in vitro system and in cell extract. Biophys. J. 2009, 96, 3341–3353. [Google Scholar] [CrossRef]
- Schneider, R.; Korten, T.; Walter, W.J.; Diez, S. Kinesin-1 motors can circumvent permanent roadblocks by side-shifting to neighboring protofilaments. Biophys. J. 2015, 108, 2249–2257. [Google Scholar] [CrossRef]
- Korten, T.; Diez, S. Setting up roadblocks for kinesin-1: Mechanism for the selective speed control of cargo carrying microtubules. Lab Chip 2008, 8, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Kim, B.; Grabner, H.; Ries, J.; Kulomaa, M.; Vogel, V. Tuning the “roadblock” effect in kinesin-based transport. Nano Lett. 2012, 12, 3466–3471. [Google Scholar] [CrossRef]
- Tjioe, M.; Shukla, S.; Vaidya, R.; Troitskaia, A.; Bookwalter, C.S.; Trybus, K.M.; Chemla, Y.R.; Selvin, P.R. Multiple kinesins induce tension for smooth cargo transport. eLife 2019, 8, e50974. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Troitskaia, A.; Swarna, N.; Maity, B.K.; Tjioe, M.; Bookwalter, C.S.; Trybus, K.M.; Chemla, Y.R.; Selvin, P.R. High-throughput force measurement of individual kinesin-1 motors during multi-motor transport. Nanoscale 2022, 14, 12463. [Google Scholar] [CrossRef] [PubMed]
- Siahaan, V.; Krattenmacher, J.; Hyman, A.A.; Diez, S.; Hernández-Vega, A.; Lansky, Z.; Braun, M. Kinetically distinct phases of tau on microtubules regulate kinesin motors and severing enzymes. Nat. Cell Biol. 2019, 21, 1086–1092. [Google Scholar] [CrossRef]
- Sozański, K.; Ruhnow, F.; Wisniewska, A.; Tabaka, M.; Diez, S.; Holyst, R. Small Crowders Slow Down Kinesin-1 Stepping by Hindering Motor Domain Diffusion. Phys. Rev. Lett. 2015, 115, 218102. [Google Scholar] [CrossRef]
- Nettesheim, G.; Nabti, I.; Murade, G.U.; Jaffe, G.R.; King, S.J.; Shubeita, G.T. Macromolecular crowding acts as a physical regulator of intracellular transport. Nat. Phys. 2020, 16, 1144–1151. [Google Scholar] [CrossRef]
- Xie, P. Effect of small molecular crowders on dynamics of kinesin molecular motors. J. Theor. Biol. 2024, 578, 111685. [Google Scholar] [CrossRef]
- Shi, X.-X.; Guo, S.-K.; Wang, P.-Y.; Chen, H.; Xie, P. All-atom molecular dynamics simulations reveal how kinesin transits from one-head-bound to two-heads-bound state. Proteins 2020, 88, 545–557. [Google Scholar] [CrossRef]
- Xie, P. Modeling study of effects of tubulin carboxy-terminal tails on dynamics of kinesin and dynein motors. Protein J. 2025, 44, 399–408. [Google Scholar] [CrossRef]
- Kikkawa, M.; Hirokawa, N. High-resolution cryo-EM maps show the nucleotide binding pocket of KIF1A in open and closed conformations. EMBO J. 2006, 25, 4187–4194. [Google Scholar] [CrossRef]
- Yildiz, A.; Tomishige, M.; Gennerich, A.; Vale, R.D. Intramolecular strain coordinates kinesin stepping behavior along microtubules. Cell 2008, 134, 1030–1041. [Google Scholar] [CrossRef]
- Clancy, B.E.; Behnke-Parks, W.M.; Andreasson, J.O.L.; Rosenfeld, S.S.; Block, S.M. A universal pathway for kinesin stepping. Nat. Struct. Mol. Biol. 2011, 18, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Xie, P. Molecular mechanism of interaction between kinesin motors affecting their residence times on microtubule lattice and end. J. Theor. Biol. 2023, 571, 111556. [Google Scholar] [CrossRef]
- Xie, P.; Guo, S.-K.; Chen, H. A generalized kinetic model for coupling between stepping and ATP hydrolysis of kinesin molecular motors. Int. J. Mol. Sci. 2019, 20, 4911. [Google Scholar] [CrossRef]
- Roostalu, J.; Hentrich, C.; Bieling, P.; Telley, I.A.; Schiebel, E.; Surrey, T. Directional switching of the kinesin cin8 through motor coupling. Science 2011, 332, 94–99. [Google Scholar] [CrossRef]
- Gerson-Gurwitz, A.; Thiede, C.; Movshovich, N.; Fridman, V.; Podolskaya, M.; Danieli, T.; Lakamper, S.; Klopfenstein, D.R.; Schmidt, C.F.; Gheber, L. Directionality of individual kinesin-5 Cin8 motors is modulated by loop 8, ionic strength and microtubule geometry. EMBO J. 2011, 30, 4942–4954. [Google Scholar] [CrossRef]
- Fridman, V.; Gerson-Gurwitz, A.; Shapira, O.; Movshovich, N.; Lakamper, S.; Schmidt, C.F.; Gheber, L. Kinesin-5 Kip1 is a bi-directional motor that stabilizes microtubules and tracks their plus-ends in vivo. J. Cell Sci. 2013, 126, 4147–4159. [Google Scholar] [CrossRef] [PubMed]
- Edamatsu, M. Bidirectional motility of the fission yeast kinesin-5, Cut7. Biochem. Biophys. Res. Commun. 2014, 446, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Pandey, H.; Al--Bassam, J.; Gheber, L. Bidirectional motility of kinesin-5 motor proteins: Structural determinants, cumulative functions and physiological roles. Cell. Mol. Life Sci. 2018, 75, 1757–1771. [Google Scholar] [CrossRef]
- Xie, P. A common ATP-dependent stepping model for kinesin-5 and kinesin-1: Mechanism of bi-directionality of kinesin-5. Biophys. Chem. 2021, 271, 106548. [Google Scholar] [CrossRef]
- Gicking, A.M.; Wang, P.; Liu, C.; Mickolajczyk, K.J.; Guo, L.; Hancock, W.O.; Qiu, W. The Orphan kinesin PAKRP2 achieves processive motility via a noncanonical stepping mechanism. Biophys. J. 2019, 116, 1270–1281. [Google Scholar] [CrossRef]
- Guo, S.-K.; Xie, P. A common chemomechanical coupling model for orphan and conventional kinesin molecular motors. Biophys. Chem. 2020, 264, 106427. [Google Scholar] [CrossRef]
- Mickolajczyk, K.J.; Hancock, W.O. Kinesin processivity is determined by a kinetic race from a vulnerable one-head-bound state. Biophys. J. 2017, 112, 2615–2623. [Google Scholar] [CrossRef]
- Zaniewski, T.M.; Gicking, A.M.; Fricks, J.; Hancock, W.O. A kinetic dissection of the fast and superprocessive kinesin-3 KIF1A reveals a predominant one-head-bound state during its chemomechanical cycle. J. Biol. Chem. 2020, 295, 17889–17903. [Google Scholar] [CrossRef] [PubMed]
- Helenius, J.; Brouhard, G.; Kalaidzidis, Y.; Diez, S.; Howard, J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature 2006, 441, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.R.; Wagenbach, M.; Asbury, C.L.; Wordeman, L. Catalysis of the microtubule on-rate is the major parameter regulating the depolymerase activity of MCAK. Nat. Struct. Mol. Biol. 2010, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Xie, P. A model for chemomechanical coupling of kinesin—3 motor. Cell. Mol. Bioeng. 2024, 17, 137–151. [Google Scholar] [CrossRef]
- Case, R.B.; Rice, S.; Hart, C.L.; Ly, B.; Vale, R.D. Role of the kinesin neck linker and catalytic core in microtubule--based motility. Curr. Biol. 2000, 10, 157–160. [Google Scholar] [CrossRef]
- Andreasson, J.O.L.; Shastry, S.; Hancock, W.O.; Block, S.M. The mechanochemical cycle of mammalian kinesin-2 KIF3A/B under load. Curr. Biol. 2015, 25, 1166–1175. [Google Scholar] [CrossRef]
- Endow, S.A.; Waligora, K.W. Determinants of kinesin motor polarity. Science 1998, 281, 1200–1202. [Google Scholar] [CrossRef]
- Case, R.B.; Pierce, D.W.; Hom-Booher, N.; Hart, C.L.; Vale, R.D. The directional preference of kinesin motors is specified by an element outside of the motor catalytic domain. Cell 1997, 90, 959–966. [Google Scholar] [CrossRef]
- Diehl, M.R.; Zhang, K.; Lee, H.J.; Tirrell, D.A. Engineering cooperativity in biomotor-protein assemblies. Science 2006, 311, 1468–1471. [Google Scholar] [CrossRef]
- Schimert, K.I.; Budaitis, B.G.; Reinemann, D.N.; Lang, M.J.; Verhey, K.J. Intracellular cargo transport by single-headed kinesin motors. Proc. Natl. Acad. Sci. USA 2019, 116, 6152–6161. [Google Scholar] [CrossRef]
- Xie, P. Mechanochemical coupling of two coupled kinesin monomers: Comparison with that of the single dimer. RSC Mechanochem 2025, 2, 127–141. [Google Scholar] [CrossRef]
- Crevel, I.M.T.C.; Lockhart, A.; Cross, R.A. Weak and strong states of kinesin and Ncd. J. Mol. Biol. 1996, 257, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Sosa, H.; Peterman, E.J.G.; Moerner, W.E.; Goldstein, L.S.B. ADP-induced rocking of the kinesin motor domain revealed by single-molecule fluorescence polarization microscopy. Nat. Struct. Biol. 2001, 8, 540–544. [Google Scholar] [CrossRef]
- Verhey, K.J.; Ohi, R. Causes, costs and consequences of kinesin motors communicating through the microtubule lattice. J. Cell Sci. 2023, 136, jcs260735. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-X.; Wang, P.-Y.; Chen, H.; Xie, P. Studies of conformational changes of tubulin induced by interaction with kinesin using atomistic molecular dynamics simulations. Int. J. Mol. Sci. 2021, 22, 6709. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-X.; Fu, Y.-B.; Guo, S.-K.; Wang, P.-Y.; Chen, H.; Xie, P. Investigating role of conformational changes of microtubule in regulating its binding affinity to kinesin by all-atom molecular dynamics simulation. Proteins 2018, 86, 1127–1139. [Google Scholar] [CrossRef]
- Vilfan, A.; Frey, E.; Schwabl, F.; Thormahlen, M.; Song, Y.H.; Mandelkow, E. Dynamics and cooperativity of microtubule decoration by the motor protein kinesin. J. Mol. Biol. 2001, 312, 1011–1026. [Google Scholar] [CrossRef]
- Muto, E.; Sakai, H.; Kaseda, K. Long-range cooperative binding of kinesin to a microtubule in the presence of ATP. J. Cell Biol. 2005, 168, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.H.; Campas, O.; Montel, F.; Woehlke, G.; Spatz, J.P.; Bassereau, P.; Cappello, G. Dynamic kinesin-1 clustering on microtubules due to mutually attractive interactions. Phys. Biol. 2008, 5, 046004. [Google Scholar] [CrossRef][Green Version]
- Wijeratne, S.S.; Fiorenza, S.A.; Neary, A.E.; Subramanian, R.; Betterton, M.D. Motor guidance by long-range communication on the microtubule highway. Proc. Natl. Acad. Sci. USA 2022, 119, e2120193119. [Google Scholar] [CrossRef]
- Xie, P. A model for cooperativity of kinesin-4 motors by communicating through the microtubule track. Chem. Phys. 2024, 581, 112274. [Google Scholar] [CrossRef]
- Rice, S.; Lin, A.W.; Safer, D.; Hart, C.L.; Naber, N.; Carragher, B.O.; Cain, S.M.; Pechatnikova, E.; Wilson-Kubalek, E.M.; Whittaker, M.; et al. A structural change in the kinesin motor protein that drives motility. Nature 1999, 402, 778–784. [Google Scholar] [CrossRef]
- Asenjo, A.B.; Weinberg, Y.; Sosa, H. Nucleotide binding and hydrolysis induces a disorder-order transition in the kinesin neck-linker region. Nat. Struc. Mol. Biol. 2006, 13, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, M. The role of microtubules in processive kinesin movement. Trends Cell Biol. 2008, 18, 128–135. [Google Scholar] [CrossRef]
- Benoit, M.P.M.H.; Asenjo, A.B.; Paydar, M.; Dhakal, S.; Kwok, B.H.; Sosa, H. Structural basis of mechano-chemical coupling by the mitotic kinesin KIF14. Nat. Commun. 2021, 12, 3637. [Google Scholar] [CrossRef]
- Benoit, M.P.M.H.; Rao, L.; Asenjo, A.B.; Gennerich, A.; Sosa, H. Cryo-EM unveils kinesin KIF1A’s processivity mechanism and the impact of its pathogenic variant P305L. Nat. Commun. 2024, 15, 5530. [Google Scholar] [CrossRef] [PubMed]
- Nitta, R.; Okada, Y.; Hirokawa, N. Structural model for strain-dependent microtubule activation of Mg-ADP release from kinesin. Nat. Struct. Mol. Biol. 2008, 15, 1067–1075. [Google Scholar] [CrossRef]
- Okada, Y.; Hirokawa, N. A processive single-headed motor: Kinesin superfamily protein KIF1A. Science 1999, 283, 1152–1157. [Google Scholar] [CrossRef]
- Rosenfeld, S.S.; Fordyce, P.M.; Jefferson, G.M.; King, P.H.; Block, S.M. Stepping and stretching: How kinesin uses internal strain to walk processively. J. Biol. Chem. 2003, 278, 18550–18556. [Google Scholar] [PubMed]
- Hariharan, V.; Hancock, W.O. Insights into the mechanical properties of the kinesin neck linker domain from sequence analysis and molecular dynamics simulations. Cell Mol. Bioeng. 2009, 2, 177–189. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, P. An Overview of Contrasting Experimental Results on Dynamics of Kinesin-1 Molecular Motors: Insight into the Underlying Mechanism. Biomolecules 2025, 15, 1453. https://doi.org/10.3390/biom15101453
Xie P. An Overview of Contrasting Experimental Results on Dynamics of Kinesin-1 Molecular Motors: Insight into the Underlying Mechanism. Biomolecules. 2025; 15(10):1453. https://doi.org/10.3390/biom15101453
Chicago/Turabian StyleXie, Ping. 2025. "An Overview of Contrasting Experimental Results on Dynamics of Kinesin-1 Molecular Motors: Insight into the Underlying Mechanism" Biomolecules 15, no. 10: 1453. https://doi.org/10.3390/biom15101453
APA StyleXie, P. (2025). An Overview of Contrasting Experimental Results on Dynamics of Kinesin-1 Molecular Motors: Insight into the Underlying Mechanism. Biomolecules, 15(10), 1453. https://doi.org/10.3390/biom15101453





