Kaempferol Regulates Lipid Homeostasis, Endocannabinoid System, and PPARα in Rat Cerebral Cortex Following BCCAO/R
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Keeping
2.2. Surgery
2.3. Sampling
2.4. Measurement of Fatty Acid in Brain Tissue and Plasma
2.5. Endocannabinoid and Congener Quantification in Tissue and Plasma
2.6. Western Blot Assays for CB1R, CB2R, COX-2, PPARα, GFAP, and Iba1
2.7. Immunohistochemistry for GFAP and Iba1
2.8. Statistical Analysis
3. Results
3.1. Analysis of Fatty Acid Profiles, Endocannabinoids, and Congeners in Frontal Cortex
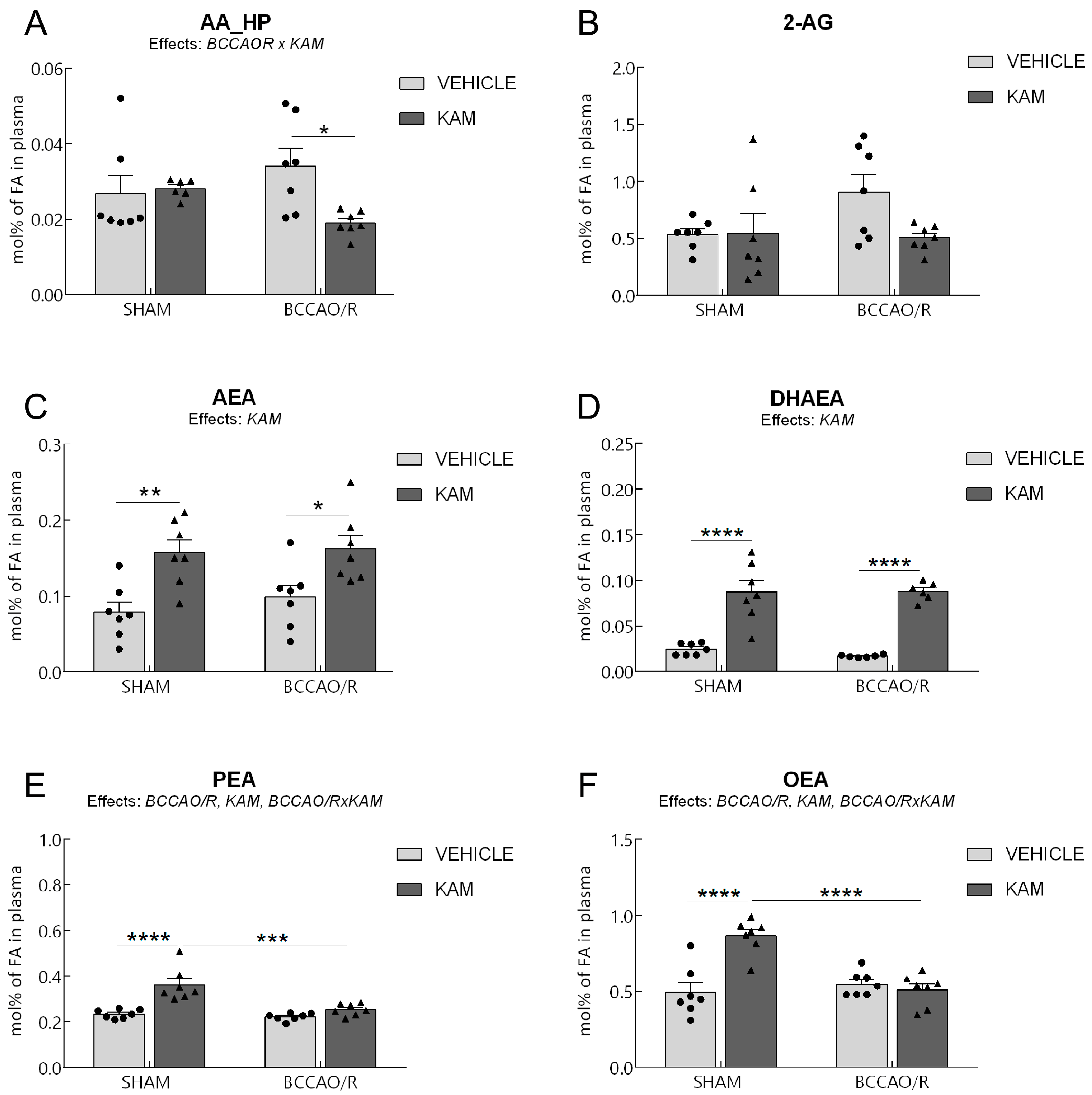
3.2. Analysis of Fatty Acid Profiles, Endocannabinoids, and Congeners in Plasma
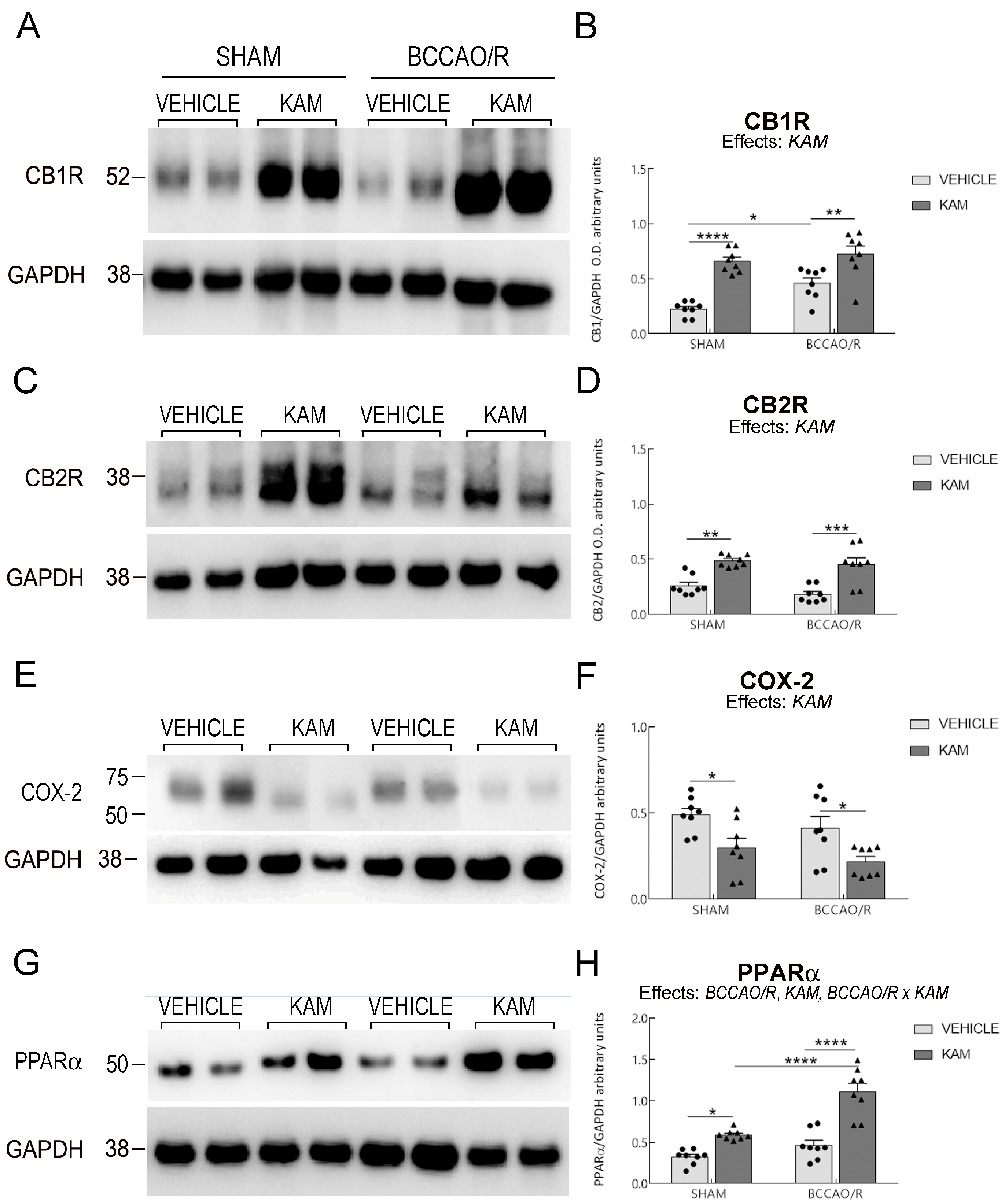
3.3. Analysis of CB1R, CB2R, COX-2, PPARα, GFAP, and Iba1 in Frontal Cortex
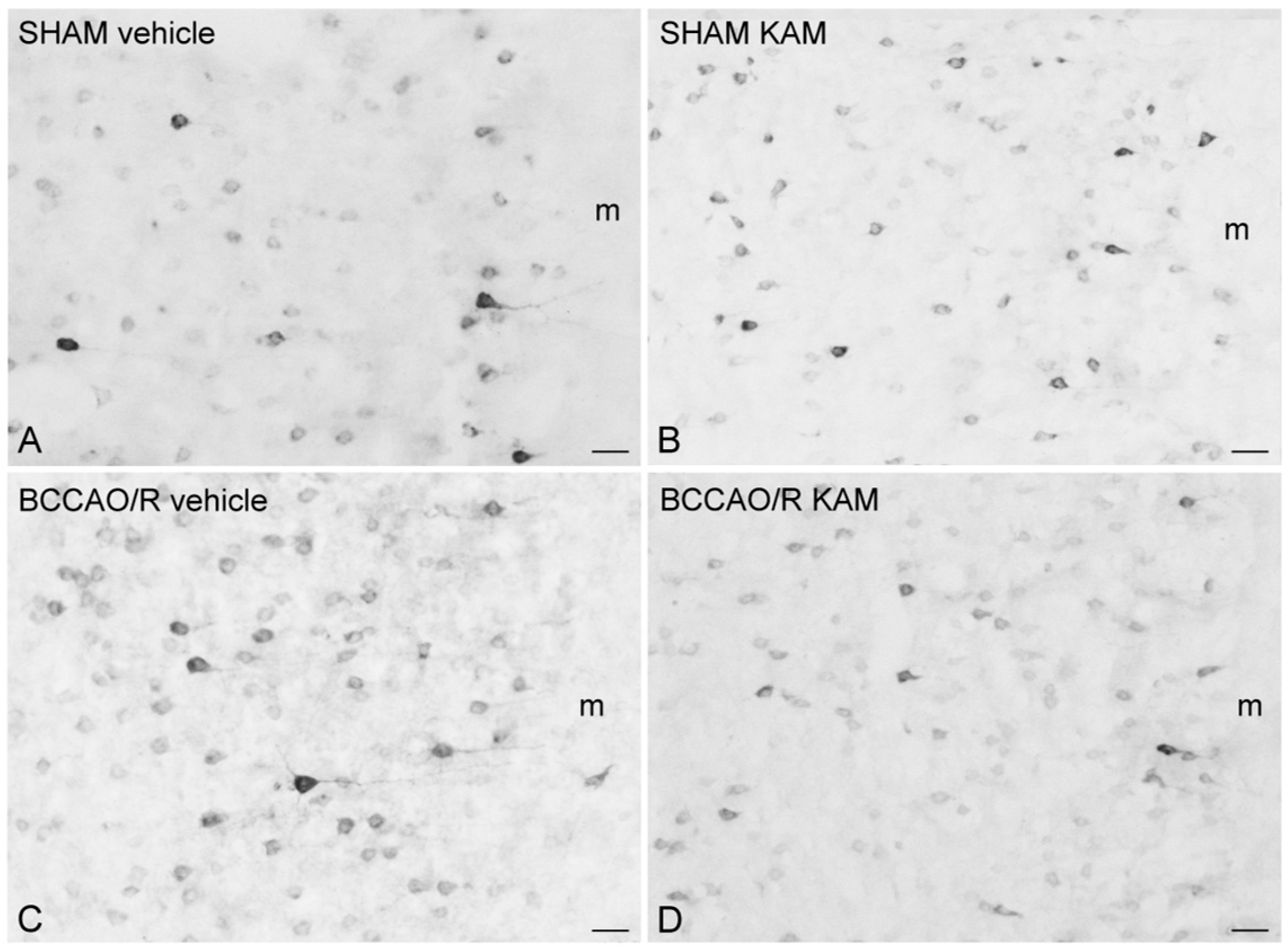
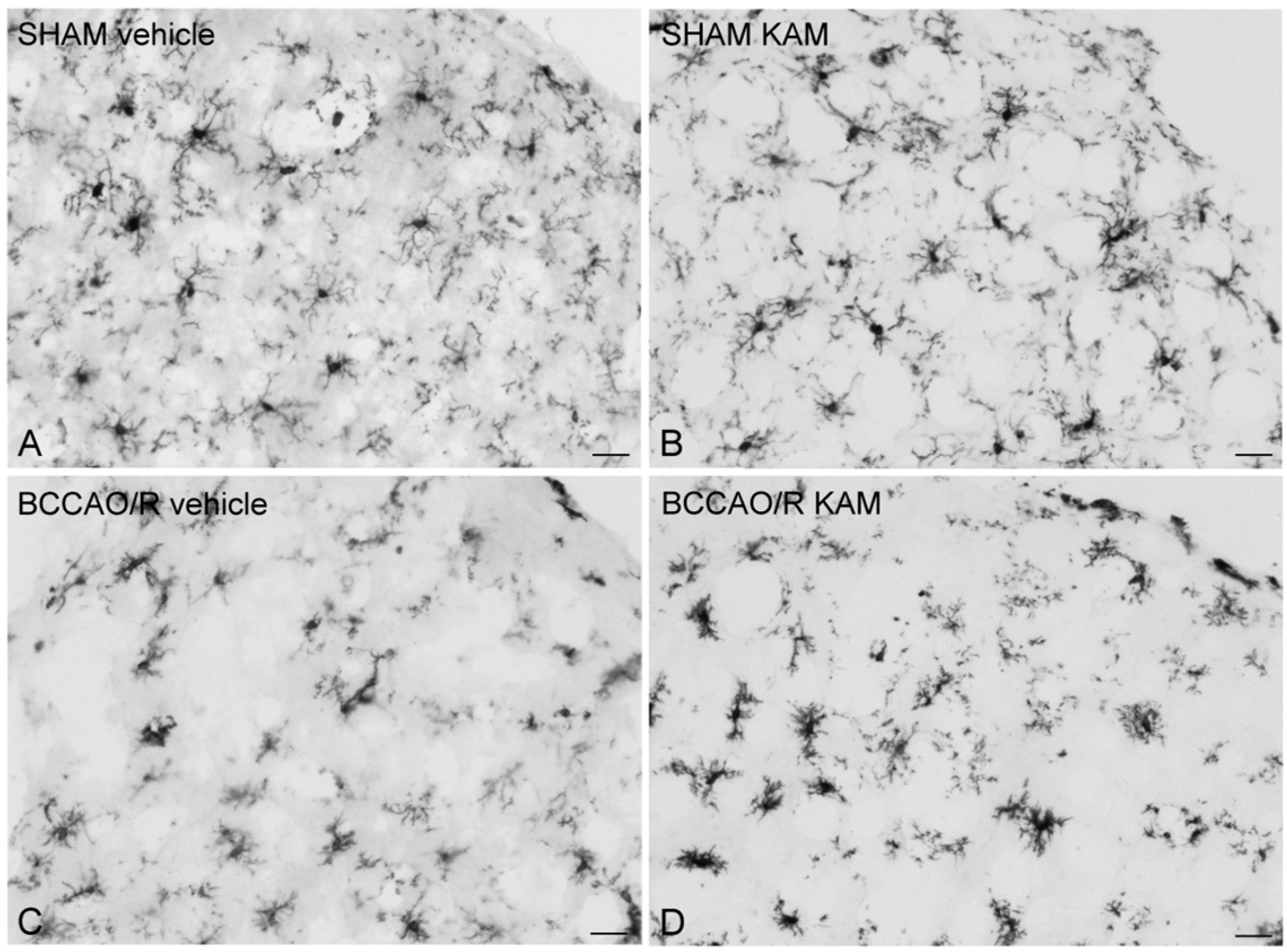
3.4. Immunohistochemistry for COX-2 and Iba1
4. Discussion
4.1. Key Effects of KAM Administration
4.2. KAM in Preclinical and Clinical Research
Bioavailability of KAM and Translational Relevance in Human Studies
4.3. Molecular Markers and Mechanisms
4.3.1. COX-2
4.3.2. PPARα
4.4. Effects of KAM on Glial Markers
4.5. Systemic Effects Observed in Plasma
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| AA_HP | arachidonic acid_hydroperoxides |
| AEA | arachidonoylethanolamide |
| 2-AG | 2-arachidonoylglycerol |
| BCCAO | Bilateral Common Carotid Artery Occlusion |
| CBR | cannabinoid receptor |
| CB1R | cannabinoid receptor type 1 |
| CB2R | cannabinoid receptor type 2 |
| COX-2 | cyclooxygenase-2 |
| DHA | docosahexaenoic acid |
| DHAEA | docosahexaenoylethanolamide |
| eCBs | endocannabinoids |
| ECS | endocannabinoid system |
| FA | fatty acids |
| FAAH | fatty acid amide hydrolase |
| GFAP | glial fibrillary acidic protein |
| Iba1 | ionized calcium-binding adapter molecule 1 |
| KAM | kaempferol |
| NAEs | N-acylethanolamines |
| OEA | oleoylethanolamide |
| PEA | palmitoylethanolamide |
| PUFA | polyunsaturated fatty acids |
| PPARα | peroxisome proliferator-activated receptor alpha |
| PVDF | polyvinylidene fluoride |
| SFA | saturated fatty acids |
| TBS-T | (Tris base, sodium chloride, Tween 20) |
| UFA | unsaturated fatty acids |
References
- Petrovic-Djergovic, D.; Goonewardena, S.N.; Pinsky, D.J. Inflammatory Disequilibrium in Stroke. Circ. Res. 2016, 119, 142–158. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kawabori, M.; Yenari, M.A. Innate Inflammatory Responses in Stroke: Mechanisms and Potential Therapeutic Targets. Curr. Med. Chem. 2014, 21, 2076–2097. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and Foe for Ischemic Stroke. J Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef]
- Liebeskind, D.S. Collateral Circulation. Stroke 2003, 34, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, M.; Sako, K.; Yura, S.; Yonemasu, Y. Local Cerebral Glucose Utilisation Following Acute and Chronic Bilateral Carotid Artery Ligation in Wistar Rats: Relation to Changes in Local Cerebral Blood Flow. Exp. Brain Res. 1993, 95, 1–7. [Google Scholar] [CrossRef]
- Yanpallewar, S. Nimodipine Attenuates Biochemical, Behavioral and Histopathological Alterations Induced by Acute Transient and Long-Term Bilateral Common Carotid Occlusion in Rats. Pharmacol. Res. 2004, 49, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Quartu, M.; Poddighe, L.; Melis, T.; Serra, M.P.; Boi, M.; Lisai, S.; Carta, G.; Murru, E.; Muredda, L.; Collu, M.; et al. Involvement of the Endocannabinoid System in the Physiological Response to Transient Common Carotid Artery Occlusion and Reperfusion. Lipids Health Dis. 2017, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Ganesana, M.; Venton, B.J. Early Changes in Transient Adenosine during Cerebral Ischemia and Reperfusion Injury. PLoS ONE 2018, 13, e0196932. [Google Scholar] [CrossRef]
- Poddighe, L.; Carta, G.; Serra, M.P.; Melis, T.; Boi, M.; Lisai, S.; Murru, E.; Muredda, L.; Collu, M.; Banni, S.; et al. Acute Administration of Beta-Caryophyllene Prevents Endocannabinoid System Activation during Transient Common Carotid Artery Occlusion and Reperfusion. Lipids Health Dis. 2018, 17, 23. [Google Scholar] [CrossRef]
- Serra, M.P.; Boi, M.; Carta, A.; Murru, E.; Carta, G.; Banni, S.; Quartu, M. Anti-Inflammatory Effect of Beta-Caryophyllene Mediated by the Involvement of TRPV1, BDNF and trkB in the Rat Cerebral Cortex after Hypoperfusion/Reperfusion. Int. J. Mol. Sci. 2022, 23, 3633. [Google Scholar] [CrossRef]
- De Ley, G.; Nshimyumuremyi, J.B.; Leusen, I. Hemispheric Blood Flow in the Rat after Unilateral Common Carotid Occlusion: Evolution with Time. Stroke 1985, 16, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.; Panzenbeck, M.J. Collateral Development after Carotid Artery Occlusion in Fischer 344 Rats. Stroke 1990, 21, 316–321. [Google Scholar] [CrossRef]
- Plaschke, K.; Bardenheuer, H.J.; Martin, E.; Sartor, K.; Heiland, S. Evolution of Apparent Diffusion Coefficient and Transverse Relaxation Time (T2) in the Subchronic Stage of Global Cerebral Oligemia in Different Rat Models. Exp. Brain Res. 2006, 169, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Banni, S.; Montisci, R.; Sanfilippo, R.; Finco, G.; Sanna, D.; Giordano, E.; Murru, E.; Cordeddu, L.; Carta, G.; Banni, D.; et al. Physiological Response to Lipid Peroxidation in Ischemia and Reperfusion during Carotid Endarterectomy. Lipids Health Dis. 2010, 9, 41. [Google Scholar] [CrossRef]
- Bakar, B.; Kose, E.A.; Kupana Ayva, S.; Sarkarati, B.; Kasimcan, M.O.; Kilinc, K. Effects of Low-Dose Methotrexate in Spinal Cord Injury in Rats. Ulus. Travma Acil Cerrahi Derg. 2013, 19, 285–293. [Google Scholar] [CrossRef][Green Version]
- Quartu, M.; Serra, M.P.; Boi, M.; Pillolla, G.; Melis, T.; Poddighe, L.; Del Fiacco, M.; Falconieri, D.; Carta, G.; Murru, E.; et al. Effect of Acute Administration of Pistacia lentiscus L. Essential Oil on Rat Cerebral Cortex Following Transient Bilateral Common Carotid Artery Occlusion. Lipids Health Dis. 2012, 11, 8. [Google Scholar] [CrossRef]
- Kirisci, M.; Gunes, H.; Kocarslan, A.; Ozcan Metin, T.; Altintas Aykan, D.; Seyithanoglu, M.; Doganer, A.; Bayrak, G.; Aksu, E. Protective Effects of Adrenomedullin on Rat Cerebral Tissue After Transient Bilateral Common Carotid Artery Occlusion and Reperfusion. Braz. J. Cardiovasc. Surg. 2020, 35, 314–322. [Google Scholar] [CrossRef]
- Zoechling, A.; Reiter, E.; Eder, R.; Wendelin, S.; Liebner, F.; Jungbauer, A. The Flavonoid Kaempferol Is Responsible for the Majority of Estrogenic Activity in Red Wine. Am. J. Enol. Vitic. 2009, 60, 223–232. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Aaqil Khan, M.; Asaf, S.; Lubna; Park, J.-R.; Lee, I.-J.; Kim, K.-M. Flavonone 3-Hydroxylase Relieves Bacterial Leaf Blight Stress in Rice via Overaccumulation of Antioxidant Flavonoids and Induction of Defense Genes and Hormones. Int. J. Mol. Sci. 2021, 22, 6152. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.P.; Grotewold, E. Flavonoids as Developmental Regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.S.; Altamimi, A.S.A.; Afzal, M.; Almalki, W.H.; Kazmi, I.; Alzarea, S.I.; Gupta, G.; Shahwan, M.; Kukreti, N.; Wong, L.S.; et al. Kaempferol: Paving the Path for Advanced Treatments in Aging-Related Diseases. Exp. Gerontol. 2024, 188, 112389. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A Review of the Dietary Flavonoid, Kaempferol on Human Health and Cancer Chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Herrera, T.E.S.; Tello, I.P.S.; Mustafa, M.A.; Jamil, N.Y.; Alaraj, M.; Atiyah Altameem, K.K.; Alasheqi, M.Q.; Hamoody, A.-H.M.; Alkhafaji, A.T.; Shakir, M.N.; et al. Kaempferol: Unveiling Its Anti-Inflammatory Properties for Therapeutic Innovation. Cytokine 2025, 186, 156846. [Google Scholar] [CrossRef]
- Yang, L.; Gao, Y.; Bajpai, V.K.; El-Kammar, H.A.; Simal-Gandara, J.; Cao, H.; Cheng, K.-W.; Wang, M.; Arroo, R.R.J.; Zou, L.; et al. Advance toward Isolation, Extraction, Metabolism and Health Benefits of Kaempferol, a Major Dietary Flavonoid with Future Perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 2773–2789. [Google Scholar] [CrossRef]
- Huang, J.; Qi, Z. MiR-21 Mediates the Protection of Kaempferol against Hypoxia/Reoxygenation-Induced Cardiomyocyte Injury via Promoting Notch1/PTEN/AKT Signaling Pathway. PLoS ONE 2020, 15, e0241007. [Google Scholar] [CrossRef]
- Silva Dos Santos, J.; Gonçalves Cirino, J.P.; De Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef]
- Yu, L.; Chen, C.; Wang, L.-F.; Kuang, X.; Liu, K.; Zhang, H.; Du, J.-R. Neuroprotective Effect of Kaempferol Glycosides against Brain Injury and Neuroinflammation by Inhibiting the Activation of NF-κB and STAT3 in Transient Focal Stroke. PLoS ONE 2013, 8, e55839. [Google Scholar] [CrossRef]
- Ballav, S.; Lokhande, K.B.; Yadav, R.S.; Ghosh, P.; Swamy, K.V.; Basu, S. Exploring Binding Mode Assessment of Novel Kaempferol, Resveratrol, and Quercetin Derivatives with PPAR-α as Potent Drug Candidates against Cancer. Mol. Divers. 2023, 27, 2867–2885. [Google Scholar] [CrossRef]
- Heneka, M.; Landreth, G. PPARs in the Brain. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2007, 1771, 1031–1045. [Google Scholar] [CrossRef]
- Reddy, J.K.; Hashimoto, T. PEROXISOMALβ-OXIDATION ANDPEROXISOMEPROLIFERATOR–ACTIVATEDRECEPTORα: An Adaptive Metabolic System. Annu. Rev. Nutr. 2001, 21, 193–230. [Google Scholar] [CrossRef]
- Yu, S.; Reddy, J. Transcription Coactivators for Peroxisome Proliferator-Activated Receptors. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2007, 1771, 936–951. [Google Scholar] [CrossRef]
- Collino, M.; Aragno, M.; Mastrocola, R.; Benetti, E.; Gallicchio, M.; Dianzani, C.; Danni, O.; Thiemermann, C.; Fantozzi, R. Oxidative Stress and Inflammatory Response Evoked by Transient Cerebral Ischemia/Reperfusion: Effects of the PPAR-α Agonist WY14643. Free. Radic. Biol. Med. 2006, 41, 579–589. [Google Scholar] [CrossRef]
- Collino, M.; Patel, N.S.A.; Thiemermann, C. Review: PPARs as New Therapeutic Targets for the Treatment of Cerebral Ischemia/Reperfusion Injury. Ther. Adv. Cardiovasc. Dis. 2008, 2, 179–197. [Google Scholar] [CrossRef]
- Mandrekar-Colucci, S.; Sauerbeck, A.; Popovich, P.G.; McTigue, D.M. PPAR Agonists as Therapeutics for CNS Trauma and Neurological Diseases. ASN Neuro 2013, 5, AN20130030. [Google Scholar] [CrossRef]
- D’Agostino, G.; La Rana, G.; Russo, R.; Sasso, O.; Iacono, A.; Esposito, E.; Raso, G.M.; Cuzzocrea, S.; Lo Verme, J.; Piomelli, D.; et al. Acute Intracerebroventricular Administration of Palmitoylethanolamide, an Endogenous Peroxisome Proliferator-Activated Receptor-α Agonist, Modulates Carrageenan-Induced Paw Edema in Mice. J. Pharmacol. Exp. Ther. 2007, 322, 1137–1143. [Google Scholar] [CrossRef]
- Ricote, M.; Glass, C.K. PPARs and Molecular Mechanisms of Transrepression. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2007, 1771, 926–935. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The Mechanisms of Action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef]
- Agarwal, S.; Yadav, A.; Chaturvedi, R.K. Peroxisome Proliferator-Activated Receptors (PPARs) as Therapeutic Target in Neurodegenerative Disorders. Biochem. Biophys. Res. Commun. 2017, 483, 1166–1177. [Google Scholar] [CrossRef]
- Diep, T.A.; Madsen, A.N.; Holst, B.; Kristiansen, M.M.; Wellner, N.; Hansen, S.H.; Hansen, H.S. Dietary Fat Decreases Intestinal Levels of the Anorectic Lipids through a Fat Sensor. FASEB J. 2011, 25, 765–774. [Google Scholar] [CrossRef]
- Hansen, H.S. Palmitoylethanolamide and Other Anandamide Congeners. Proposed Role in the Diseased Brain. Exp. Neurol. 2010, 224, 48–55. [Google Scholar] [CrossRef]
- Lin, Q.; Ruuska, S.E.; Shaw, N.S.; Dong, D.; Noy, N. Ligand Selectivity of the Peroxisome Proliferator-Activated Receptor α. Biochemistry 1999, 38, 185–190. [Google Scholar] [CrossRef]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The Nuclear Receptor Peroxisome Proliferator-Activated Receptor-α Mediates the Anti-Inflammatory Actions of Palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef]
- Carta, G.; Poddighe, L.; Serra, M.P.; Boi, M.; Melis, T.; Lisai, S.; Murru, E.; Muredda, L.; Collu, M.; Banni, S.; et al. Preventive Effects of Resveratrol on Endocannabinoid System and Synaptic Protein Modifications in Rat Cerebral Cortex Challenged by Bilateral Common Carotid Artery Occlusion and Reperfusion. Int. J. Mol. Sci. 2018, 19, 426. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ito, S.; Suzuki, M.; Nagahori, T.; Yamamoto, T.; Konno, H. Forebrain Ischemia Induced by Temporary Bilateral Common Carotid Occlusion in Normotensive Rats. J. Neurol. Sci. 1989, 90, 155–165. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Amsterdam, The Netherlands; Elsevier: Boston, MA, USA, 2007; ISBN 978-0-12-547612-6. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Banni, S.; Carta, G.; Contini, M.S.; Angioni, E.; Deiana, M.; Dessì, M.A.; Melis, M.P.; Corongiu, F.P. Characterization of Conjugated Diene Fatty Acids in Milk, Dairy Products, and Lamb Tissues. J. Nutr. Biochem. 1996, 7, 150–155. [Google Scholar] [CrossRef]
- Melis, M.P.; Angioni, E.; Carta, G.; Murru, E.; Scanu, P.; Spada, S.; Banni, S. Characterization of Conjugated Linoleic Acid and Its Metabolites by RP-HPLC with Diode Array Detector. Eur. J. Lipid Sci. Technol. 2001, 103, 617–621. [Google Scholar] [CrossRef]
- Piscitelli, F.; Carta, G.; Bisogno, T.; Murru, E.; Cordeddu, L.; Berge, K.; Tandy, S.; Cohn, J.S.; Griinari, M.; Banni, S.; et al. Effect of Dietary Krill Oil Supplementation on the Endocannabinoidome of Metabolically Relevant Tissues from High-Fat-Fed Mice. Nutr. Metab. 2011, 8, 51. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Lapi, D. Protective Effects of Quercetin on Rat Pial Microvascular Changes during Transient Bilateral Common Carotid Artery Occlusion and Reperfusion. Front. Physio. 2012, 3, 32. [Google Scholar] [CrossRef]
- Lapi, D.; Vagnani, S.; Pignataro, G.; Esposito, E.; Paterni, M.; Colantuoni, A. Rat Pial Microvascular Responses to Transient Bilateral Common Carotid Artery Occlusion and Reperfusion: Quercetin’s Mechanism of Action. Front. Physio. 2012, 3, 99. [Google Scholar] [CrossRef]
- López-Sánchez, C.; Martín-Romero, F.J.; Sun, F.; Luis, L.; Samhan-Arias, A.K.; García-Martínez, V.; Gutiérrez-Merino, C. Blood Micromolar Concentrations of Kaempferol Afford Protection against Ischemia/Reperfusion-Induced Damage in Rat Brain. Brain Res. 2007, 1182, 123–137. [Google Scholar] [CrossRef]
- Hu, K.; Nordbeck, P. Preclinical and Clinical Effects of the Flavanol Kaempferol: Oxidative Stress, Myocardial Inflammation, and the Impact of Human Metabolism. J. Cardiovasc. Pharmacol. 2019, 74, 324–325. [Google Scholar] [CrossRef]
- Chaubey, S.; Singh, L. Deciphering the Mechanisms Underlying the Neuroprotective Potential of Kaempferol: A Comprehensive Investigation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 2275–2292. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, Y.; Xu, Y.; Han, X.; Peng, J.; Liu, K.; Sun, C.K. Protective Effect of Flavonoid-Rich Extract from Rosa Laevigata Michx on Cerebral Ischemia–Reperfusion Injury through Suppression of Apoptosis and Inflammation. Neurochem. Int. 2013, 63, 522–532. [Google Scholar] [CrossRef]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderio, J.; Saw, C.L.; Kong, A. Metabolism, Oral Bioavailability and Pharmacokinetics of Chemopreventive Kaempferol in Rats. Biopharm. Drug Dispos. 2009, 30, 356–365. [Google Scholar] [CrossRef]
- De Vries, J.H.; Hollman, P.C.; Meyboom, S.; Buysman, M.N.; Zock, P.L.; Van Staveren, W.A.; Katan, M.B. Plasma Concentrations and Urinary Excretion of the Antioxidant Flavonols Quercetin and Kaempferol as Biomarkers for Dietary Intake. Am. J. Clin. Nutr. 1998, 68, 60–65. [Google Scholar] [CrossRef]
- DuPont, M.S.; Day, A.J.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A. Absorption of Kaempferol from Endive, a Source of Kaempferol-3-Glucuronide, in Humans. Eur. J. Clin. Nutr. 2004, 58, 947–954. [Google Scholar] [CrossRef]
- Williams, J.J.; Mayurasakorn, K.; Vannucci, S.J.; Mastropietro, C.; Bazan, N.G.; Ten, V.S.; Deckelbaum, R.J. N-3 Fatty Acid Rich Triglyceride Emulsions Are Neuroprotective after Cerebral Hypoxic-Ischemic Injury in Neonatal Mice. PLoS ONE 2013, 8, e56233. [Google Scholar] [CrossRef]
- Costa, B.; Conti, S.; Giagnoni, G.; Colleoni, M. Therapeutic Effect of the Endogenous Fatty Acid Amide, Palmitoylethanolamide, in Rat Acute Inflammation: Inhibition of Nitric Oxide and Cyclo-oxygenase Systems. Br. J. Pharmacol. 2002, 137, 413–420. [Google Scholar] [CrossRef]
- Jhaveri, M.D.; Richardson, D.; Robinson, I.; Garle, M.J.; Patel, A.; Sun, Y.; Sagar, D.R.; Bennett, A.J.; Alexander, S.P.H.; Kendall, D.A.; et al. Inhibition of Fatty Acid Amide Hydrolase and Cyclooxygenase-2 Increases Levels of Endocannabinoid Related Molecules and Produces Analgesia via Peroxisome Proliferator-Activated Receptor-Alpha in a Model of Inflammatory Pain. Neuropharmacology 2008, 55, 85–93. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C. Endocannabinoid 2-Arachidonoylglycerol Protects Neurons by Limiting COX-2 Elevation. J. Biol. Chem. 2008, 283, 22601–22611. [Google Scholar] [CrossRef]
- Sahoo, S.; Bhattacharya, R.; Yadav, M.; Panda, D.S.; Guria, T.; Mishra, K. An Overview of Arachidonic Acid Metabolic Pathway and Recent Updates on a Few Heterocyclic Derivatives Showing COX-2 Inhibition. J. Drug Deliv. Technol. 2023, 13, 1078–1091. [Google Scholar] [CrossRef]
- Thors, L.; Belghiti, M.; Fowler, C.J. Inhibition of Fatty Acid Amide Hydrolase by Kaempferol and Related Naturally Occurring Flavonoids. Br. J. Pharmacol. 2008, 155, 244–252. [Google Scholar] [CrossRef]
- Ahmad, H.; Rauf, K.; Zada, W.; McCarthy, M.; Abbas, G.; Anwar, F.; Shah, A.J. Kaempferol Facilitated Extinction Learning in Contextual Fear Conditioned Rats via Inhibition of Fatty-Acid Amide Hydrolase. Molecules 2020, 25, 4683. [Google Scholar] [CrossRef]
- Melis, M.; Carta, G.; Pistis, M.; Banni, S. Physiological Role of Peroxisome Proliferator-Activated Receptors Type Alpha on Dopamine Systems. CNS Neurol. Disord.-Drug Targets 2013, 12, 70–77. [Google Scholar] [CrossRef]
- Murru, E.; Muntoni, A.L.; Manca, C.; Aroni, S.; Pistis, M.; Banni, S.; Carta, G. Profound Modification of Fatty Acid Profile and Endocannabinoid-Related Mediators in PPARα Agonist Fenofibrate-Treated Mice. Int. J. Mol. Sci. 2022, 24, 709. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Spector, A.A. N-Docosahexaenoylethanolamine: A Neurotrophic and Neuroprotective Metabolite of Docosahexaenoic Acid. Mol. Asp. Med. 2018, 64, 34–44. [Google Scholar] [CrossRef]
- Porcedda, C.; Manca, C.; Carta, G.; Piras, F.; Banni, S.; Sogos, V.; Murru, E. Anti-Neuroinflammatory Effects of Conjugated Linoleic Acid Isomers, C9,T11 and T10,C12, on Activated BV-2 Microglial Cells. Front. Cell. Neurosci. 2024, 18, 1442786. [Google Scholar] [CrossRef]
- Tóth, A.; Boczán, J.; Kedei, N.; Lizanecz, E.; Bagi, Z.; Papp, Z.; Édes, I.; Csiba, L.; Blumberg, P.M. Expression and Distribution of Vanilloid Receptor 1 (TRPV1) in the Adult Rat Brain. Mol. Brain Res. 2005, 135, 162–168. [Google Scholar] [CrossRef]
- Viader, A.; Blankman, J.L.; Zhong, P.; Liu, X.; Schlosburg, J.E.; Joslyn, C.M.; Liu, Q.-S.; Tomarchio, A.J.; Lichtman, A.H.; Selley, D.E.; et al. Metabolic Interplay between Astrocytes and Neurons Regulates Endocannabinoid Action. Cell Rep. 2015, 12, 798–808. [Google Scholar] [CrossRef]
- Ábrahám, H.; Lázár, G. Early Microglial Reaction Following Mild Forebrain Ischemia Induced by Common Carotid Artery Occlusion in Rats. Brain Res. 2000, 862, 63–73. [Google Scholar] [CrossRef]
- Artamonov, M.; Zhukov, O.; Shuba, I.; Storozhuk, L.; Khmel, T.; Klimashevsky, V.; Mikosha, A.; Gul, M. Incorporation of Labelled N-Acylethanolamine (NAE) into Rat Brain Regions in Vivo and Adaptive Properties of Saturated NAE under x-Ray Irradiation. Ukr. Biochem. J. 2005, 77, 51–62. [Google Scholar]
- Orio, L.; Alen, F.; Pavón, F.J.; Serrano, A.; García-Bueno, B. Oleoylethanolamide, Neuroinflammation, and Alcohol Abuse. Front. Mol. Neurosci. 2019, 11, 490. [Google Scholar] [CrossRef]
- Ioghen, O.; Chițoiu, L.; Gherghiceanu, M.; Ceafalan, L.C.; Hinescu, M.E. CD36—A Novel Molecular Target in the Neurovascular Unit. Eur. J. Neurosci. 2021, 53, 2500–2510. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carta, G.; Serra, M.P.; Murru, E.; Boi, M.; Manca, C.; Lai, Y.; Cabboi, M.; Carta, A.; Banni, S.; Quartu, M. Kaempferol Regulates Lipid Homeostasis, Endocannabinoid System, and PPARα in Rat Cerebral Cortex Following BCCAO/R. Biomolecules 2025, 15, 1440. https://doi.org/10.3390/biom15101440
Carta G, Serra MP, Murru E, Boi M, Manca C, Lai Y, Cabboi M, Carta A, Banni S, Quartu M. Kaempferol Regulates Lipid Homeostasis, Endocannabinoid System, and PPARα in Rat Cerebral Cortex Following BCCAO/R. Biomolecules. 2025; 15(10):1440. https://doi.org/10.3390/biom15101440
Chicago/Turabian StyleCarta, Gianfranca, Maria Pina Serra, Elisabetta Murru, Marianna Boi, Claudia Manca, Ylenia Lai, Monica Cabboi, Antonella Carta, Sebastiano Banni, and Marina Quartu. 2025. "Kaempferol Regulates Lipid Homeostasis, Endocannabinoid System, and PPARα in Rat Cerebral Cortex Following BCCAO/R" Biomolecules 15, no. 10: 1440. https://doi.org/10.3390/biom15101440
APA StyleCarta, G., Serra, M. P., Murru, E., Boi, M., Manca, C., Lai, Y., Cabboi, M., Carta, A., Banni, S., & Quartu, M. (2025). Kaempferol Regulates Lipid Homeostasis, Endocannabinoid System, and PPARα in Rat Cerebral Cortex Following BCCAO/R. Biomolecules, 15(10), 1440. https://doi.org/10.3390/biom15101440








