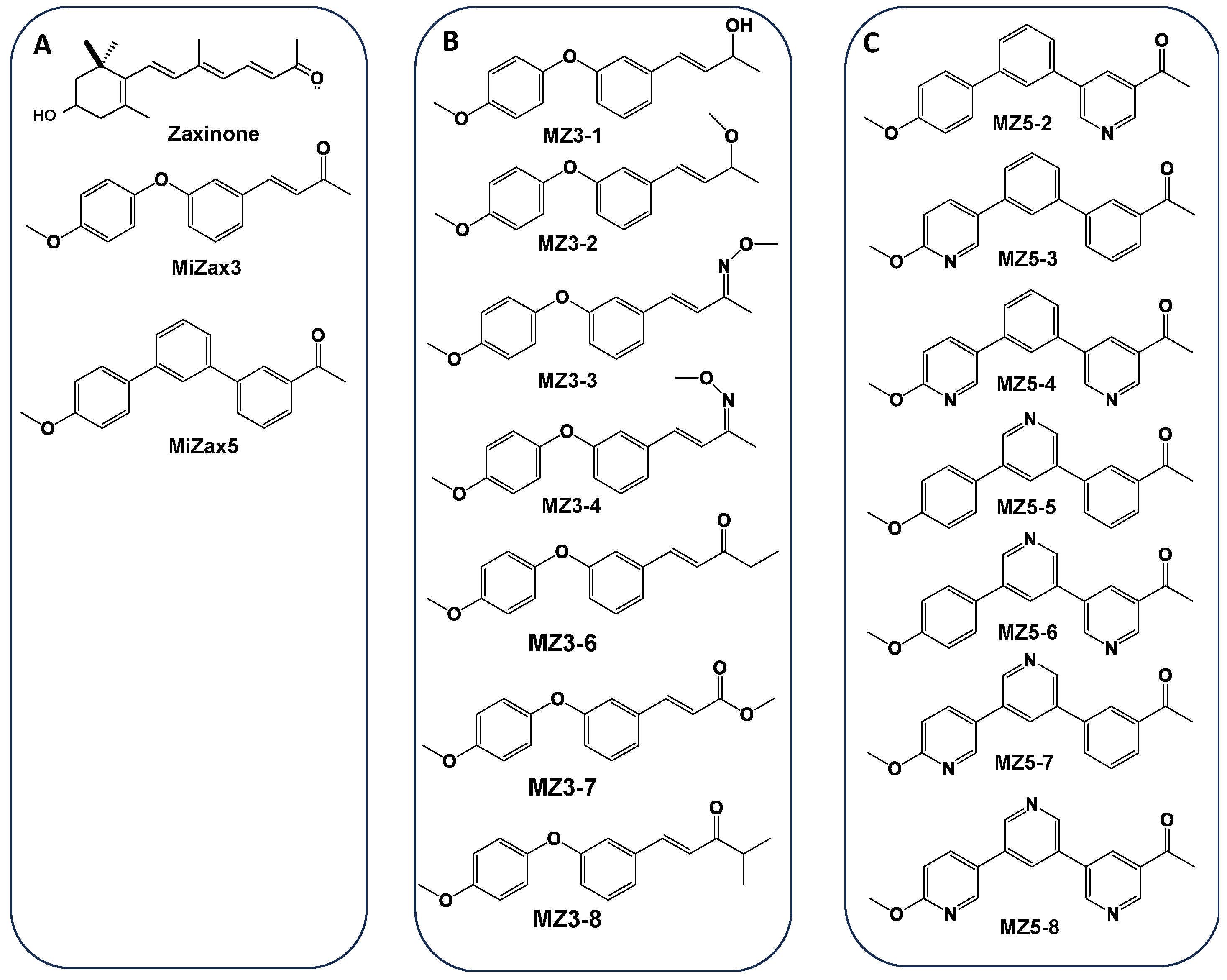
New Series of Zaxinone Mimics (MiZax) for Fundamental and Applied Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants Material and Chemicals
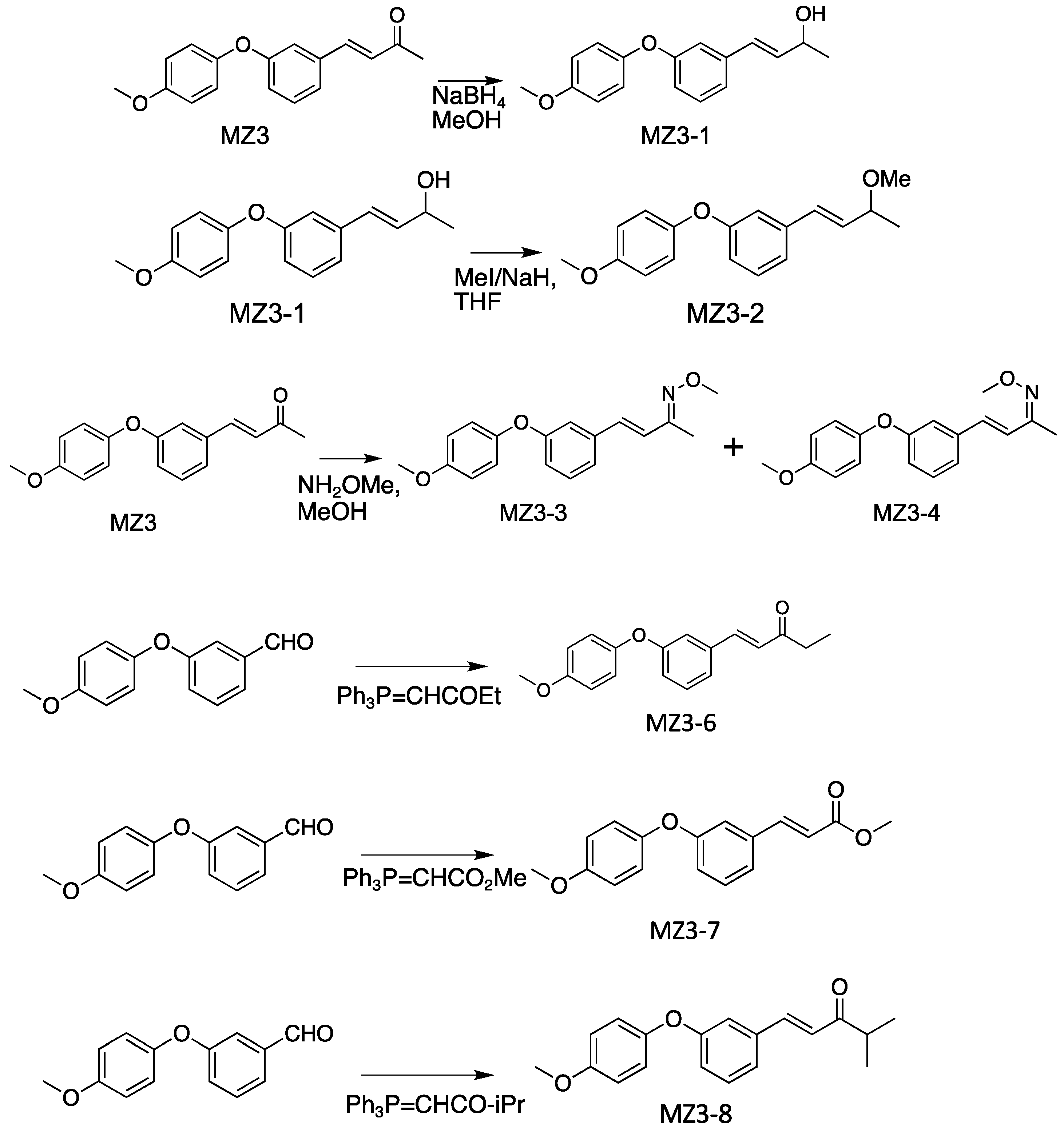
2.2. General Procedure for the Synthesis of MiZax3 Derivatives
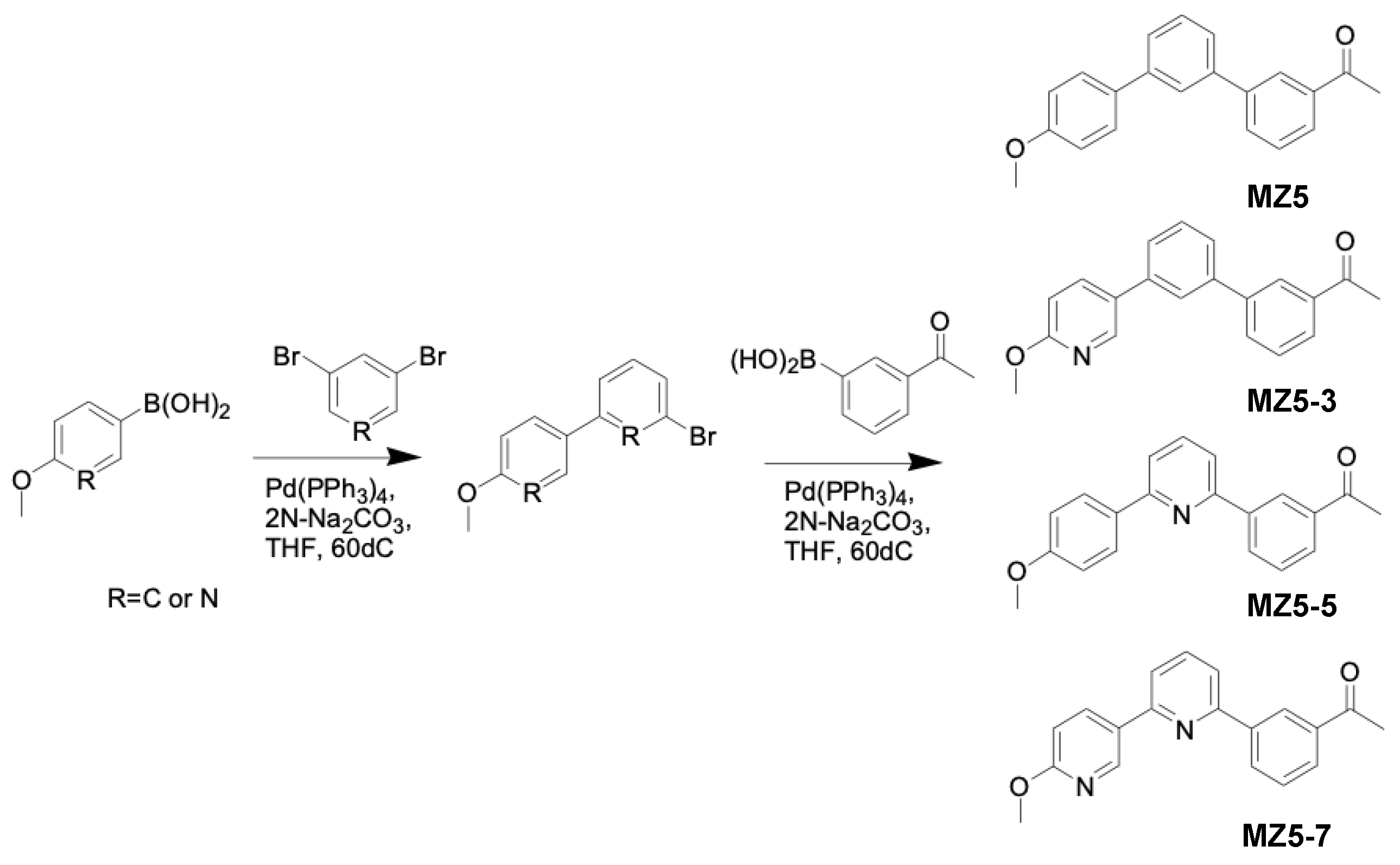
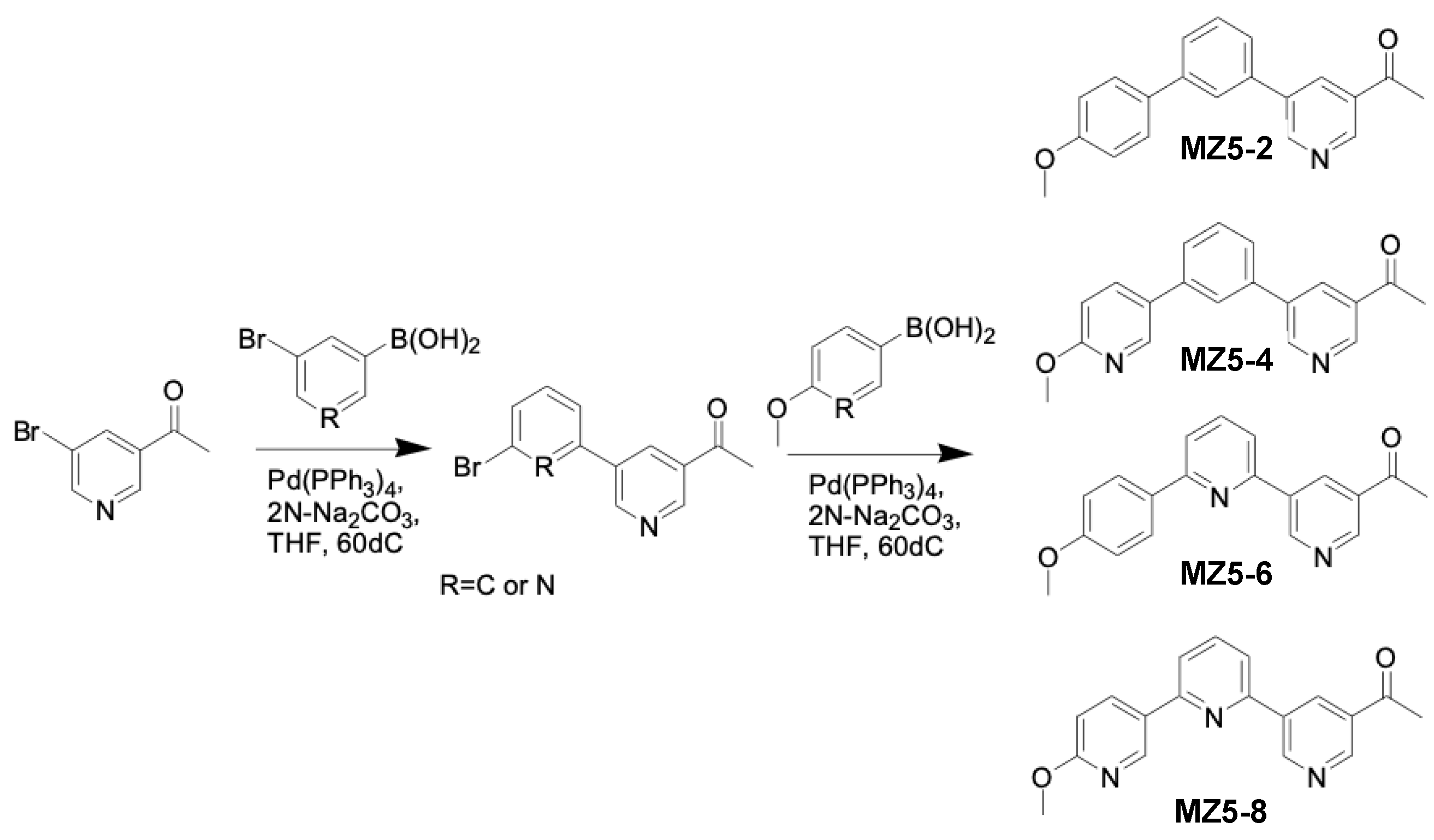
2.3. General Procedure for the Synthesis of MiZax5 Derivatives
2.4. Rice Cultivation Conditions
2.5. Striga Germination Bioassays
2.6. Strigolactone Quantification in Root Exudates
2.7. Striga Emergence in Pots under Greenhouse Conditions
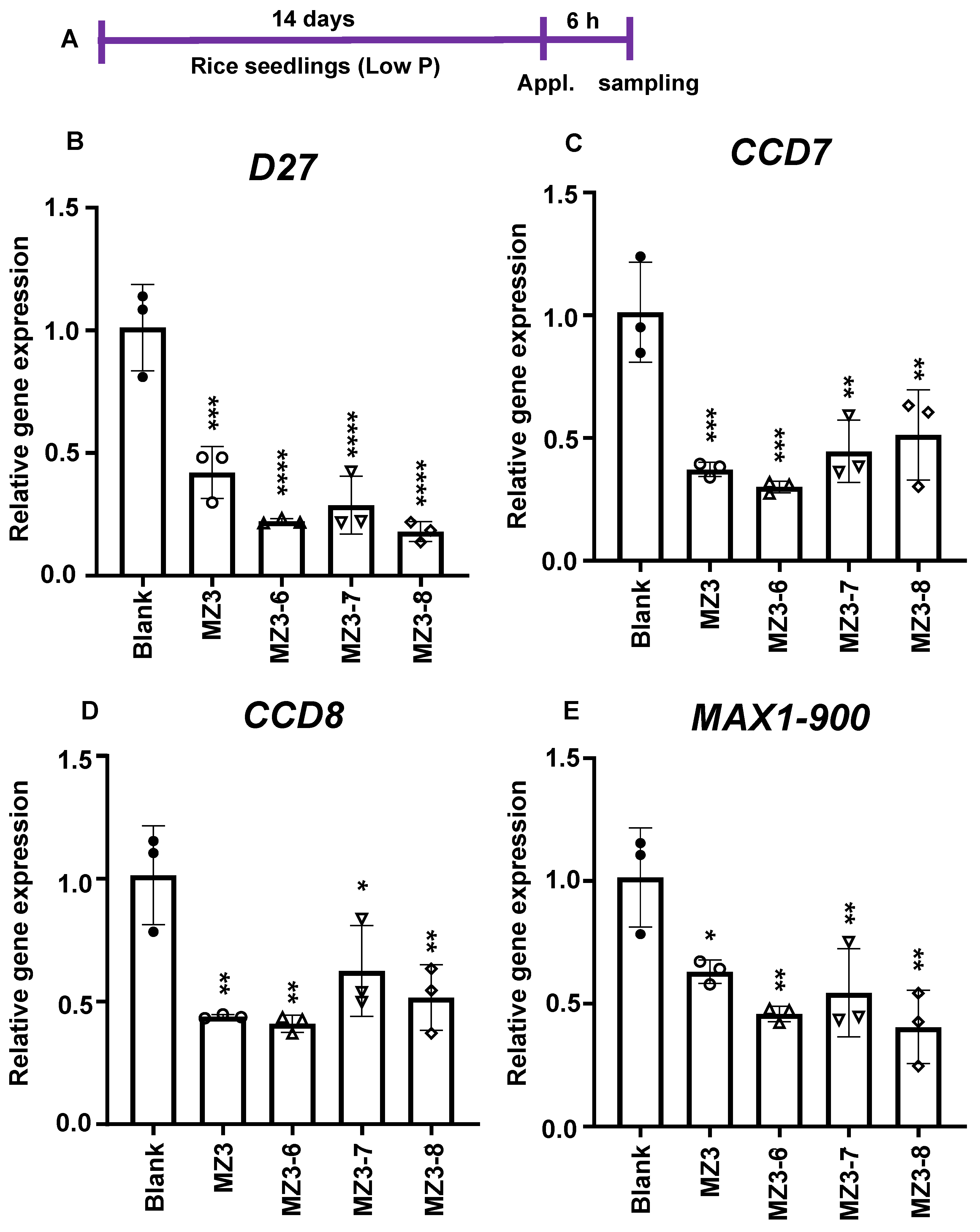
2.8. Gene Expression Analysis
2.9. Statistical Analysis
3. Results
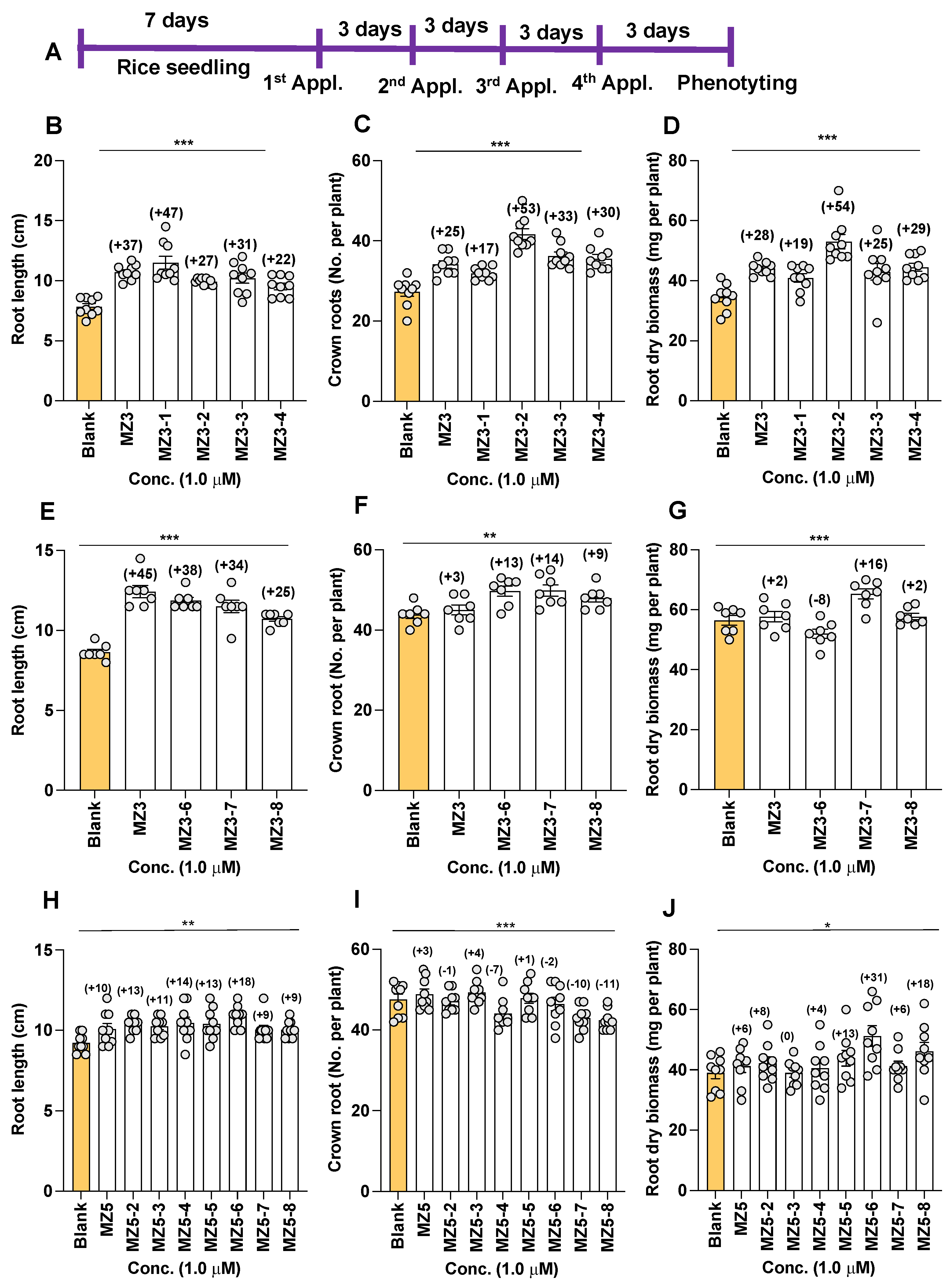
3.1. Effect of MZ3 and MZ5 Derivatives on Rice Growth and Development
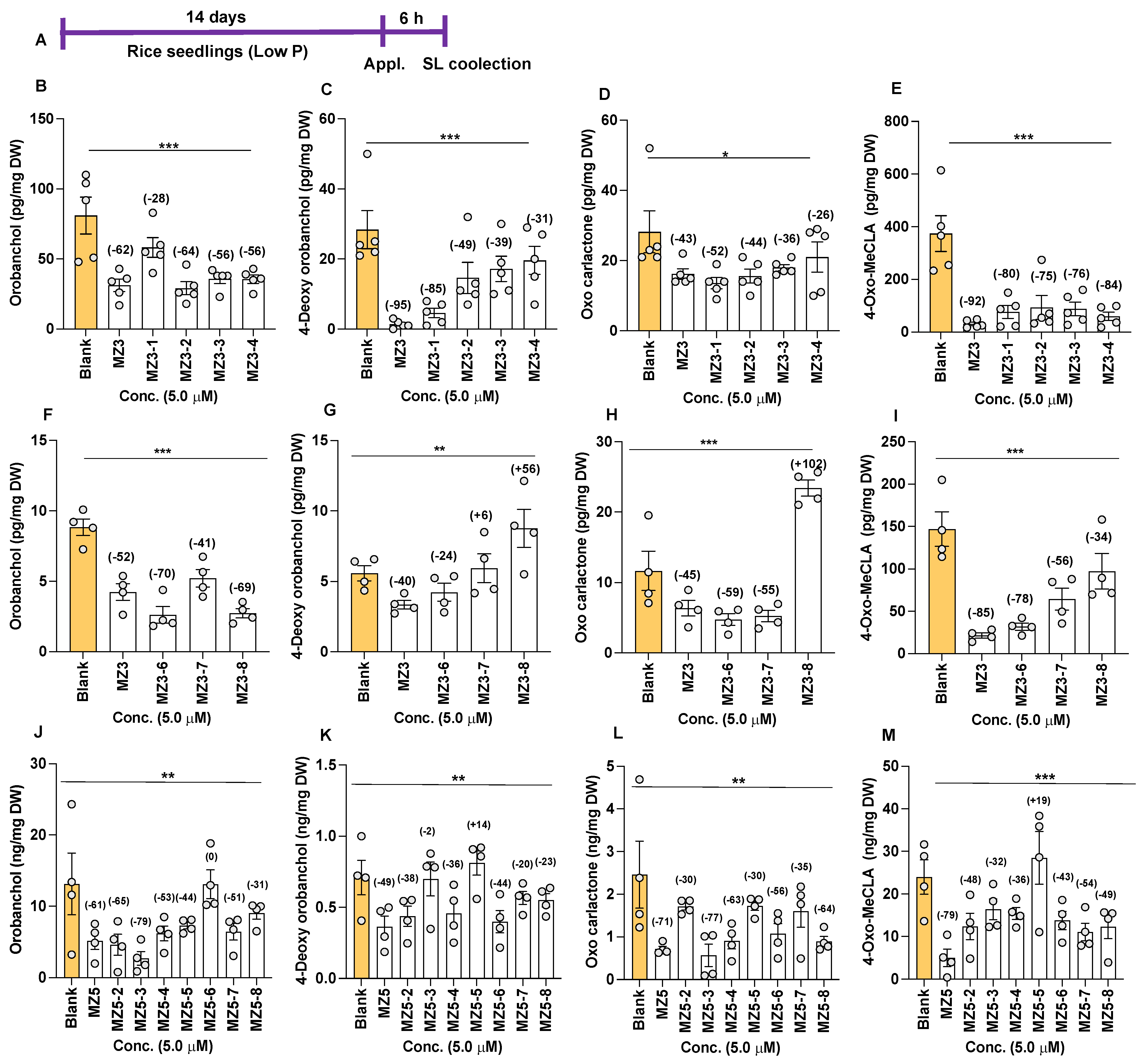
3.2. Effect of MZ3 and MZ5 Derivatives on SL Biosynthesis
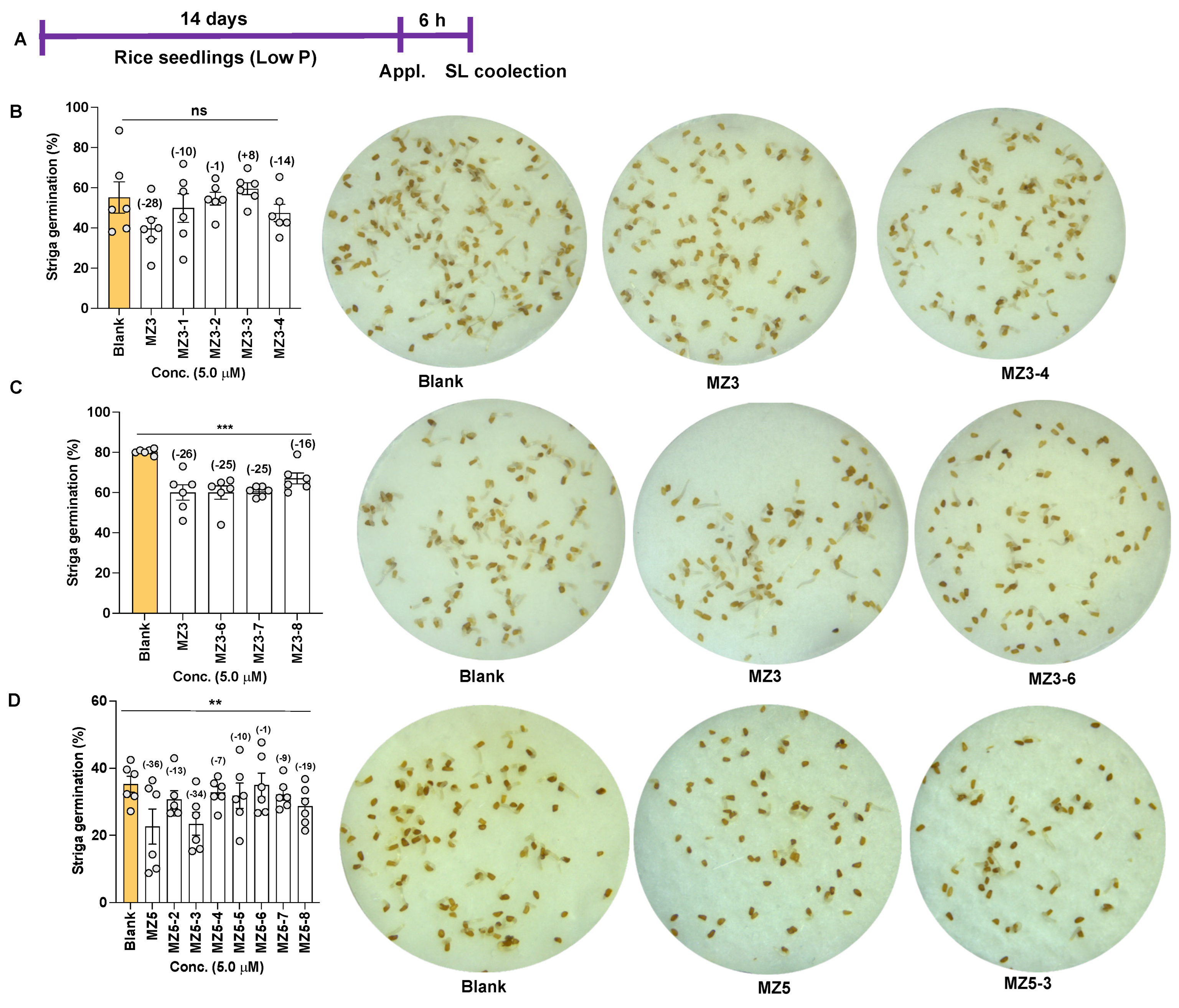
3.3. Effect of MZ3 and MZ5 Derivatives on Striga Germination
3.4. Effect of Selected MiZax on Striga Emergence and Host Growth in Pots under Greenhouse Conditions
4. Discussion
4.1. Rice Growth Improves with the Application of the New MiZax
4.2. Strigolactone Biosynthesis Is Negatively Regulated by the New Series of MiZax
4.3. Reduction of Striga Infestation by the Application of the New Series of MiZax
4.4. Possible Reasons for Differences Observed in the Bioactivity of the New MiZax
5. Perspectives, Future Outlook and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/home/en (accessed on 20 June 2023).
- Kanampiu, F.; Makumbi, D.; Mageto, E.; Omanya, G.; Waruingi, S.; Musyoka, P.; Ransom, J. Assessment of Management Options on Striga Infestation and Maize Grain Yield in Kenya. Weed Sci. 2018, 66, 516–524. [Google Scholar] [CrossRef]
- Kim, S.; Adetimirin, V.; The, C.; Dossou, R. Yield Losses in Maize due to Striga hermonthica in West and Central Africa. Int. J. Pest Manag. 2002, 48, 211–217. [Google Scholar] [CrossRef]
- Rodenburg, J.; Demont, M.; Zwart, S.J.; Bastiaans, L. Parasitic Weed Incidence and Related Economic Losses in Rice in Africa. Agric. Ecosyst. Environ. 2016, 235, 306–317. [Google Scholar] [CrossRef]
- M’Boob, S. A Regional Program for West and Central Africa. In Proceedings of the FAO/OAU All African Government Consultation on Striga Control, Maroua, Cameroun, 20–24 October1986; pp. 190–194. [Google Scholar]
- Kanampiu, F.K.; Kabambe, V.; Massawe, C.; Jasi, L.; Friesen, D.; Ransom, J.K.; Gressel, J. Multi-site, Multi-season Field Tests Demonstrate that Herbicide Seed-coating Herbicide-Resistance Maize Controls Striga spp. and Increases Yields in Several African Countries. Crop Prot. 2003, 22, 697–706. [Google Scholar] [CrossRef]
- Parker, C. Observations on the Current Status of Orobanche and Striga Problems Worldwide. Pest Manag. Sci. 2009, 65, 453–459. [Google Scholar] [CrossRef]
- Rodenburg, J.; Johnson, D.E. Weed Management in Rice-based Cropping Systems in Africa. Adv. Agron. 2009, 103, 149–218. [Google Scholar]
- Rodenburg, J.; Riches, C.R.; Kayeke, J.M. Addressing Current and Future Problems of Parasitic Weeds in Rice. Crop Prot. 2010, 29, 210–221. [Google Scholar] [CrossRef]
- Teka, H.B. Advance Research on Striga Control: A Review. Afr. J. Plant Sci. 2014, 8, 492–506. [Google Scholar]
- Ejeta, G. The Striga Scourge in Africa: A Growing Pandemic. In Integrating New Technologies for Striga Control: Towards Ending the Witch-Hunt; World Scientific: Singapore, 2007; pp. 3–16. [Google Scholar]
- Bàrberi, P. Ecological Weed Management in Sub-Saharan Africa: Prospects and Implications on Other Agroecosystem Services. Adv. Agron. 2019, 156, 219–264. [Google Scholar]
- Runo, S.; Kuria, E.K. Habits of a Highly Successful Cereal Killer, Striga. PLoS Pathog. 2018, 14, e1006731. [Google Scholar] [CrossRef]
- Bebawi, F.F.; Eplee, R.E.; Harris, C.E.; Norris, R.S. Longevity of Witchweed (Striga asiatica) Seed. Weed Sci. 1984, 32, 494–497. [Google Scholar] [CrossRef]
- Hearne, S.J. Control—The Striga Conundrum. Pest Manag. Sci. 2009, 65, 603–614. [Google Scholar] [CrossRef]
- Jamil, M.; Rodenburg, J.; Charnikhova, T.; Bouwmeester, H.J. Pre-Attachment Striga hermonthica Resistance of New Rice for Africa (NERICA) Cultivars Based on Low Strigolactone Production. New Phytol. 2011, 192, 964–975. [Google Scholar] [CrossRef]
- Jamil, M.; Kountche, B.A.; Al-Babili, S. Current Progress in Striga Management. Plant Physiol. 2021, 185, 1339–1352. [Google Scholar] [CrossRef]
- Ito, S.; Umehara, M.; Hanada, A.; Kitahata, N.; Hayase, H.; Yamaguchi, S.; Asami, T. Effects of Triazole Derivatives on Strigolactone Levels and Growth Retardation in Rice. PLoS ONE 2011, 6, 5–15. [Google Scholar] [CrossRef]
- Cook, C.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.I.; Hayashi, H. Plant Sesquiterpenes Induce Hyphal Branching in Arbuscular Mycorrhizal Fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef]
- Fiorilli, V.; Wang, J.Y.; Bonfante, P.; Lanfranco, L.; Al-Babili, S. Apocarotenoids: Old and New Mediators of the Arbuscular Mycorrhizal Symbiosis. Front. Plant Sci. 2019, 10, 1186–1192. [Google Scholar] [CrossRef]
- Decker, E.L.; Alder, A.; Hunn, S.; Ferguson, J.; Lehtonen, M.T.; Scheler, B.; Kerres, K.L.; Wiedemann, G.; Safavi-Rizi, V.; Nordzieke, S. Strigolactone Biosynthesis is Evolutionarily Conserved, Regulated by Phosphate Starvation and Contributes to Resistance against Phytopathogenic Fungi in a Moss, Physcomitrella patens. New Phytol. 2017, 216, 455–468. [Google Scholar] [CrossRef]
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a Novel Carotenoid-derived Plant Hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186. [Google Scholar] [CrossRef]
- Kaniganti, S.; Bhattacharya, J.; Petla, B.P.; Reddy, P.S. Strigolactone, a Neglected Plant Hormone, with a Great Potential for Crop Improvement: Crosstalk with Other Plant Hormones. Environ. Exp. Bot. 2022, 204, 105072. [Google Scholar] [CrossRef]
- Rehman, N.U.; Li, X.; Zeng, P.; Guo, S.; Jan, S.; Liu, Y.; Huang, Y.; Xie, Q. Harmony but not Uniformity: Role of Strigolactone in Plants. Biomolecules 2021, 11, 1616. [Google Scholar] [CrossRef]
- Wang, J.Y.; Lin, P.-Y.; Al-Babili, S. On the Biosynthesis and Evolution of Apocarotenoid Plant Growth Regulators. Semin. Cell Dev. Biol. 2021, 109, 3–11. [Google Scholar] [CrossRef]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The Path from β-carotene to Carlactone, a strigolactone-like Plant Hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef]
- Seto, Y.; Yamaguchi, S. Strigolactone Biosynthesis and Perception. Curr. Opin. Plant Biol. 2014, 21, 1–6. [Google Scholar] [CrossRef]
- Chen, G.-T.E.; Wang, J.Y.; Jamil, M.; Braguy, J.; Al-Babili, S. 9-cis-β-Apo-10′-carotenal is the Precursor of Strigolactones in planta. Planta 2022, 256, 88. [Google Scholar] [CrossRef]
- Ito, S.; Braguy, J.; Wang, J.Y.; Yoda, A.; Fiorilli, V.; Takahashi, I.; Jamil, M.; Felemban, A.; Miyazaki, S.; Mazzarella, T. Canonical Strigolactones are not the Major Determinant of Tillering but Important Rhizospheric Signals in Rice. Sci. Adv. 2022, 8, eadd1278. [Google Scholar] [CrossRef]
- Yoneyama, K.; Mori, N.; Sato, T.; Yoda, A.; Xie, X.; Okamoto, M.; Iwanaga, M.; Ohnishi, T.; Nishiwaki, H.; Asami, T. Conversion of Carlactone to Carlactonoic Acid is a Conserved Function of MAX 1 Homologs in Strigolactone Biosynthesis. New Phytol. 2018, 218, 1522–1533. [Google Scholar] [CrossRef]
- Haider, I.; Yunmeng, Z.; White, F.; Li, C.; Incitti, R.; Alam, I.; Gojobori, T.; Ruyter-Spira, C.; Al-Babili, S.; Bouwmeester, H.J. Transcriptome Analysis of the Phosphate Starvation Response Sheds Light on Strigolactone Biosynthesis in Rice. Plant J. 2023, 114, 355–370. [Google Scholar] [CrossRef]
- Zhang, Y.; Van Dijk, A.D.; Scaffidi, A.; Flematti, G.R.; Hofmann, M.; Charnikhova, T.; Verstappen, F.; Hepworth, J.; Van Der Krol, S.; Leyser, O. Rice Cytochrome P450 MAX1 Homologs Catalyze Distinct Steps in Strigolactone Biosynthesis. Nat. Chem. Biol. 2014, 10, 1028–1033. [Google Scholar] [CrossRef]
- Chen, G.-T.E.; Wang, J.Y.; Votta, C.; Braguy, J.; Jamil, M.; Kirschner, G.; Fiorilli, V.; Berqdar, L.; Balakrishna, A.; Blilou, I. Disruption of the Rice 4-Deoxyorobanchol Hydroxylase unravels specific functions of canonical strigolactones. bioRxiv 2023, 535333. [Google Scholar] [CrossRef]
- Wang, J.Y.; Haider, I.; Jamil, M.; Fiorilli, V.; Saito, Y.; Mi, J.; Baz, L.; Kountche, B.A.; Jia, K.-P.; Guo, X. The Apocarotenoid Metabolite Zaxinone Regulates Growth and Strigolactone Biosynthesis in Rice. Nat. Commun. 2019, 10, 810. [Google Scholar] [CrossRef]
- Wang, J.Y.; Alseekh, S.; Xiao, T.; Ablazov, A.; Perez de Souza, L.; Fiorilli, V.; Anggarani, M.; Lin, P.-Y.; Votta, C.; Novero, M. Multi-omics Approaches Explain the Growth-Promoting Effect of the Apocarotenoid Growth Regulator Zaxinone in Rice. Commun. Biol. 2021, 4, 1–11. [Google Scholar] [CrossRef]
- Wang, J.Y.; Jamil, M.; Lin, P.-Y.; Ota, T.; Fiorilli, V.; Novero, M.; Zarban, R.A.; Kountche, B.A.; Takahashi, I.; Martínez, C. Efficient Mimics for Elucidating Zaxinone Biology and Promoting Agricultura Applications. Mol. Plant 2020, 13, 1654–1661. [Google Scholar] [CrossRef]
- Wang, J.Y.; Jamil, M.; Hossain, M.G.; Chen, G.-T.E.; Berqdar, L.; Ota, T.; Blilou, I.; Asami, T.; Al-Solimani, S.J.; Mousa, M.A.A. Evaluation of the biostimulant activity of zaxinone mimics (MiZax) in crop plants. Front. Plant Sci. 2022, 13, 874858. [Google Scholar] [CrossRef]
- Wang, J.Y.; Jamil, M.; AlOtaibi, T.S.; Abdelaziz, M.E.; Ota, T.; Ibrahim, O.H.; Berqdar, L.; Asami, T.; Mousa, M.A.A.; Al-Babili, S. Zaxinone Mimics (MiZax) Efficiently Promote Growth and Production of Potato and Strawberry Plants under Desert Climate Conditions. Res. Sq. 2023. preprint. [Google Scholar] [CrossRef]
- Jamil, M.; Wang, J.Y.; Yonli, D.; Ota, T.; Berqdar, L.; Traore, H.; Margueritte, O.; Zwanenburg, B.; Asami, T.; Al-Babili, S. Striga hermonthica Suicidal Germination Activity of Potent Strigolactone Analogs: Evaluation from Laboratory Bioassays to Field Trials. Plants 2022, 11, 1045. [Google Scholar] [CrossRef]
- Braguy, J.; Ramazanova, M.; Giancola, S.; Jamil, M.; Kountche, B.A.; Zarban, R.A.Y.; Felemban, A.; Wang, J.Y.; Lin, P.-Y.; Haider, I.; et al. SeedQuant: A Deep Learning-based Tool for Assessing Stimulant and Inhibitor Activity on Root Parasitic Seeds. Plant Physiol. 2021, 186, 1632–1644. [Google Scholar] [CrossRef]
- Wang, J.Y.; Chen, G.-T.E.; Jamil, M.; Braguy, J.; Sioud, S.; Liew, K.X.; Balakrishna, A.; Al-Babili, S. Protocol for Characterizing Strigolactones Released by Plant Roots. STAR Protoc. 2022, 3, 101352. [Google Scholar] [CrossRef]
- Jamil, M.; Wang, J.Y.; Yonli, D.; Patil, R.H.; Riyazaddin, M.; Gangashetty, P.; Berqdar, L.; Chen, G.-T.E.; Traore, H.; Margueritte, O. A New Formulation for Strigolactone Suicidal Germination Agents, Towards Successful Striga Management. Plants 2022, 11, 808. [Google Scholar] [CrossRef]
- Wang, J.Y.; Braguy, J.; Chen, G.T.E.; Jamil, M.; Balakrishna, A.; Berqdar, L.; Al-Babili, S. Perspectives on the Metabolism of Strigolactone Rhizospheric Signals. Front. Plant Sci. 2022, 13, 1062107. [Google Scholar] [CrossRef]
- Xie, X.N.; Yoneyama, K.; Yoneyama, K. The Strigolactone Story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C. Strigolactone Inhibition of Shoot Branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Yoneyama, K.; Awad, A.A.; Xie, X.; Yoneyama, K.; Takeuchi, Y. Strigolactones as Germination Stimulants for Root Parasitic Plants. Plant Cell Physiol. 2010, 51, 1095–1103. [Google Scholar] [CrossRef]
- Jamil, M.; Charnikhova, T.; Cardoso, C.; Jamil, T.; Ueno, K.; Verstappen, F.; Asami, T.; Bouwmeester, H.J. Quantification of the Relationship Between Strigolactones and Striga hermonthica Infection in Rice under Varying Levels of Nitrogen and Phosphorus. Weed Res. 2011, 51, 373–385. [Google Scholar] [CrossRef]
- Jamil, M.; Charnikhova, T.; Houshyani, B.; van Ast, A.; Bouwmeester, H.J. Genetic Variation in Strigolactone Production and Tillering in Rice and its Effect on Striga hermonthica Infection. Planta 2012, 235, 473–484. [Google Scholar] [CrossRef]
- Jamil, M.; Van Mourik, T.A.; Charnikhova, T.; Bouwmeester, H.J. Effect of Diammonium Phosphate Application on Strigolactone Production and Striga hermonthica Infection in Three Sorghum Cultivars. Weed Res. 2013, 53, 121–130. [Google Scholar] [CrossRef]
- Jamil, M.; Charnikhova, T.; Verstappen, F.; Bouwmeester, H. Carotenoid Inhibitors Reduce Strigolactone Production and Striga hermonthica Infection in Rice. Arch. Biochem. Biophys. 2010, 504, 123–131. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Plant Biostimulants: Rationale, State of the Art and Evolution. Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 1–7. [Google Scholar]
- Rady, M.M.; Rehman, H. Supplementing Organic Biostimulants into Growing Media Enhances Growth and Nutrient Uptake of Tomato Transplants. Sci. Hortic. 2016, 203, 192–198. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, M.; Lin, P.-Y.; Berqdar, L.; Wang, J.Y.; Takahashi, I.; Ota, T.; Alhammad, N.; Chen, G.-T.E.; Asami, T.; Al-Babili, S. New Series of Zaxinone Mimics (MiZax) for Fundamental and Applied Research. Biomolecules 2023, 13, 1206. https://doi.org/10.3390/biom13081206
Jamil M, Lin P-Y, Berqdar L, Wang JY, Takahashi I, Ota T, Alhammad N, Chen G-TE, Asami T, Al-Babili S. New Series of Zaxinone Mimics (MiZax) for Fundamental and Applied Research. Biomolecules. 2023; 13(8):1206. https://doi.org/10.3390/biom13081206
Chicago/Turabian StyleJamil, Muhammad, Pei-Yu Lin, Lamis Berqdar, Jian You Wang, Ikuo Takahashi, Tsuyoshi Ota, Noor Alhammad, Guan-Ting Erica Chen, Tadao Asami, and Salim Al-Babili. 2023. "New Series of Zaxinone Mimics (MiZax) for Fundamental and Applied Research" Biomolecules 13, no. 8: 1206. https://doi.org/10.3390/biom13081206
APA StyleJamil, M., Lin, P.-Y., Berqdar, L., Wang, J. Y., Takahashi, I., Ota, T., Alhammad, N., Chen, G.-T. E., Asami, T., & Al-Babili, S. (2023). New Series of Zaxinone Mimics (MiZax) for Fundamental and Applied Research. Biomolecules, 13(8), 1206. https://doi.org/10.3390/biom13081206








