Glucocorticoid Receptor and Ovarian Cancer: From Biology to Therapeutic Intervention
Abstract
1. Introduction
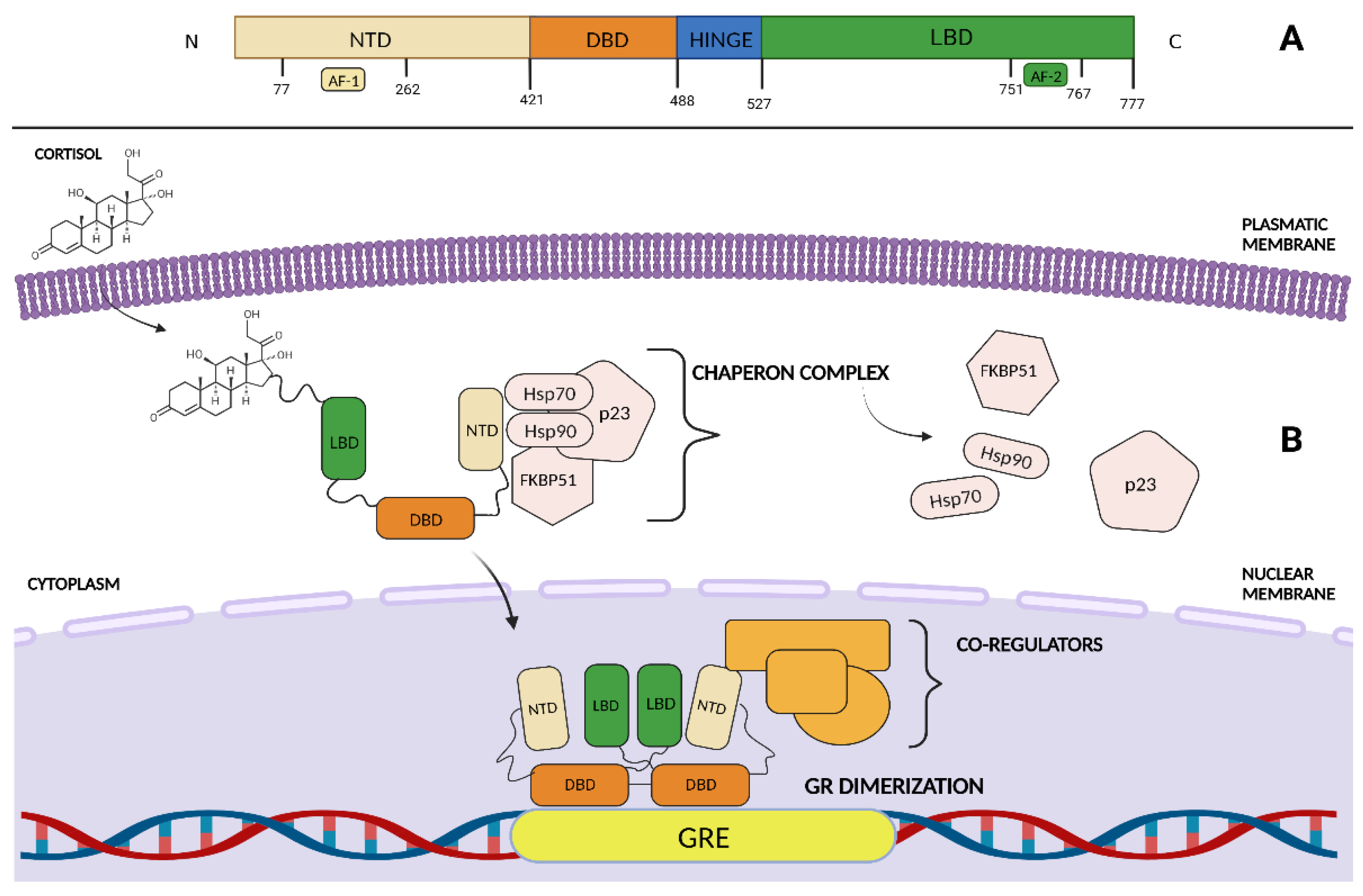
2. GR Structure and Function
3. GR Physiological and Pathological Functions
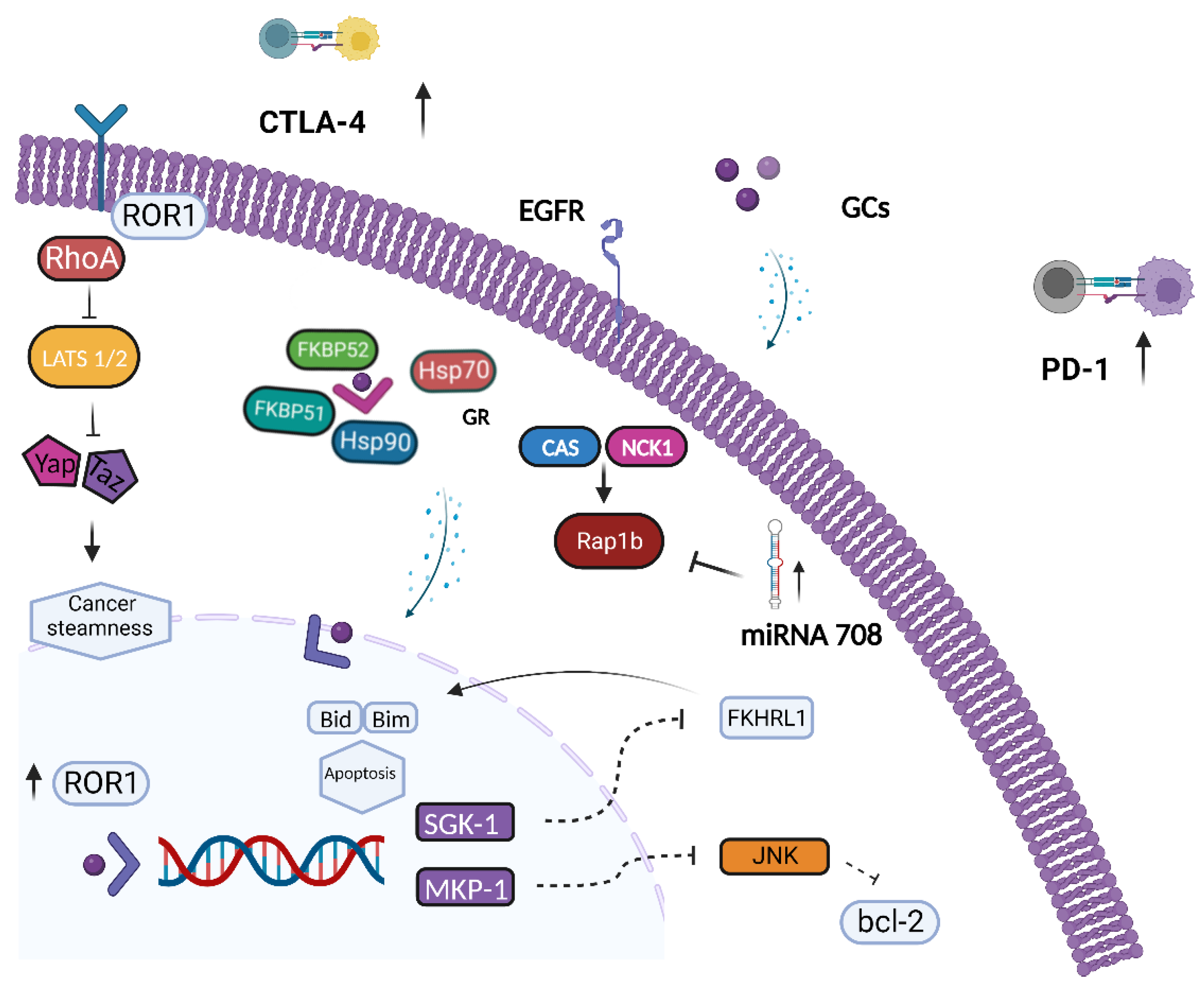
4. The Role of GR in Ovarian Cancer
5. GR and BRCA
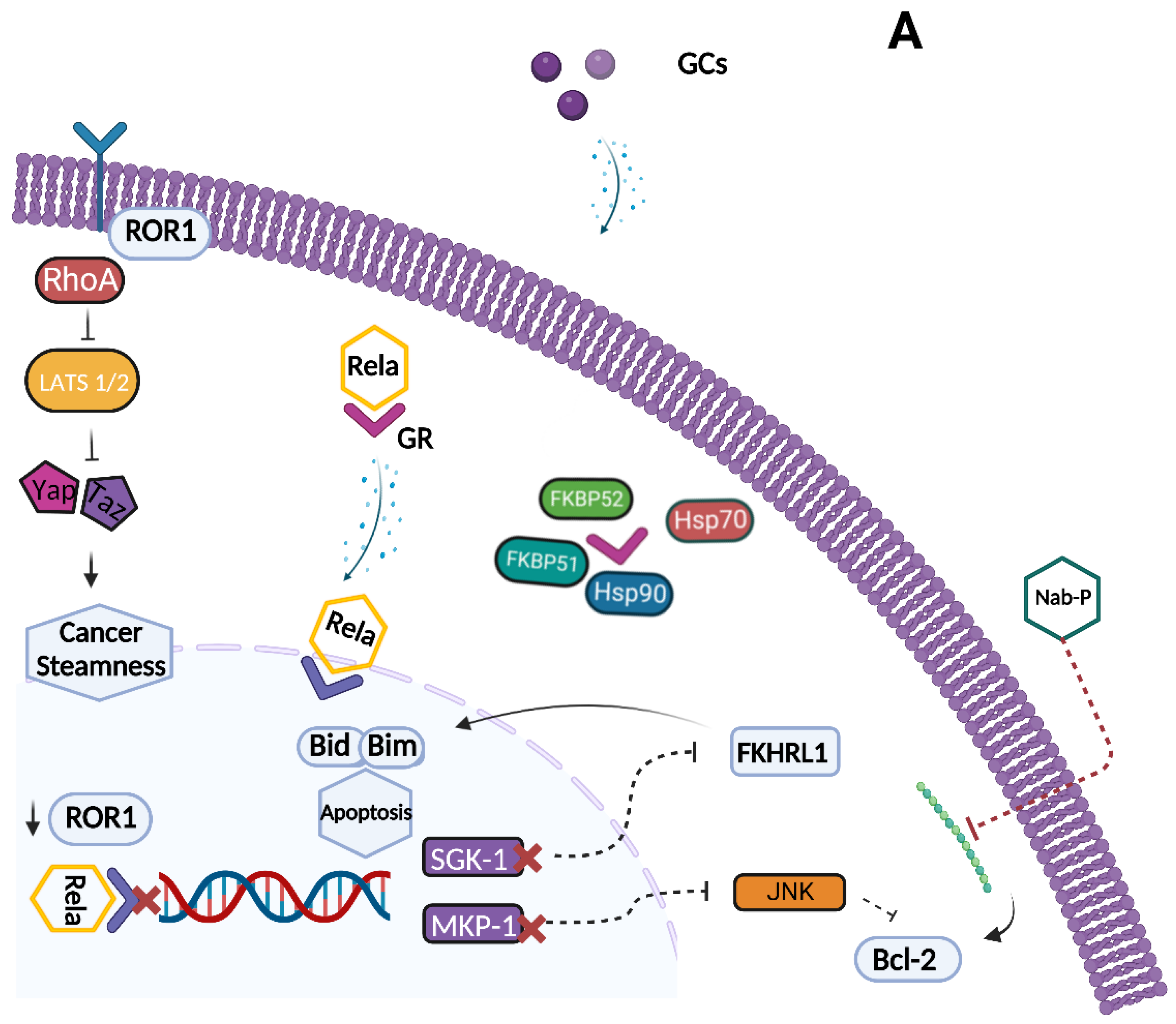
6. Glucocorticoid Receptor as a Potential Target for a Therapeutic Intervention in Ovarian Cancer
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Salani, R.; Backes, F.J.; Fung, M.F.K.; Holschneider, C.H.; Parker, L.P.; Bristow, R.E.; Goff, B.A. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am. J. Obstet. Gynecol. 2011, 204, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A. Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef]
- International Collaborative Ovarian Neoplasm 1 (ICON1); European Organisation for Research and Treatment of Cancer Collaborators–Adjuvant ChemoTherapy in Ovarian Neoplasm (EORTC–ACTION). International Collaborative Ovarian Neoplasm Trial 1 and Adjuvant ChemoTherapy in Ovarian Neoplasm Trial: Two Parallel Randomized Phase III Trials of Adjuvant Chemotherapy in Patients With Early-Stage Ovarian Carcinoma. Gynecol. Oncol. 2003, 95, 105–112. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Swisher, E.M.; Lin, K.K.; Oza, A.M.; Scott, C.L.; Giordano, H.; Sun, J.; Konecny, G.E.; Coleman, R.L.; Tinker, A.V.; O’Malley, D.M.; et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 75–87. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Mayayo-Peralta, I.; Zwart, W.; Prekovic, S. Duality of glucocorticoid action in cancer: Tumor-suppressor or oncogene? Endocrine-Related Cancer 2021, 28, R157–R171. [Google Scholar] [CrossRef]
- Lonard, D.M.; O’Malley, B.W. Nuclear Receptor Coregulators: Judges, Juries, and Executioners of Cellular Regulation. Mol. Cell 2007, 27, 691–700. [Google Scholar] [CrossRef]
- McKenna, N.J.; Lanz, R.B.; O’Malley, B.W. Nuclear Receptor Coregulators: Cellular and Molecular Biology. Endocr. Rev. 1999, 20, 321–344. [Google Scholar] [CrossRef] [PubMed]
- Vandevyver, S.; Dejager, L.; Libert, C. Comprehensive Overview of the Structure and Regulation of the Glucocorticoid Receptor. Endocr. Rev. 2014, 35, 671–693. [Google Scholar] [CrossRef]
- Oakley, R.H. Immunocytochemical analysis of the glucocorticoid receptor alpha isoform (GRα) using a GRα-specific antibody. Steroids 1999, 64, 742–751. [Google Scholar] [CrossRef]
- Vitellius, G.; Trabado, S.; Bouligand, J.; Delemer, B.; Lombès, M. Pathophysiology of Glucocorticoid Signaling. Ann. d'Endocrinologie 2018, 79, 98–106. [Google Scholar] [CrossRef]
- He, B.; Cruz-Topete, D.; Oakley, R.H.; Xiao, X.; Cidlowski, J.A. Human Glucocorticoid Receptor β Regulates Gluconeogenesis and Inflammation in Mouse Liver. Mol. Cell. Biol. 2016, 36, 714–730. [Google Scholar] [CrossRef]
- Thomas-Chollier, M.; Watson, L.C.; Cooper, S.B.; Pufall, M.A.; Liu, J.S.; Borzym, K.; Vingron, M.; Yamamoto, K.R.; Meijsing, S.H. A naturally occuring insertion of a single amino acid rewires transcriptional regulation by glucocorticoid receptor isoforms. Proc. Natl. Acad. Sci. USA 2013, 110, 17826–17831. [Google Scholar] [CrossRef] [PubMed]
- Moalli, P.A.; Pillay, S.; Krett, N.L.; Rosen, S.T. Alternatively spliced glucocorticoid receptor messenger RNAs in glucocorticoid-resistant human multiple myeloma cells. Cancer Res. 1993, 53, 3877–3879. [Google Scholar] [PubMed]
- de Lange, P.; Segeren, C.M.; Koper, J.W.; Wiemer, E.; Sonneveld, P.; Brinkmann, A.O.; White, A.; Brogan, I.J.; de Jong, F.H.; Lamberts, S.W.J. Expression in Hematological Malignancies of a Glucocorticoid Receptor Splice Variant That Augments Glucocorticoid Receptor-mediated Effects in Transfected Cells. Cancer Res. 2010, 61, 3937–3941. [Google Scholar] [CrossRef]
- Ramos-Ramírez, P.; Tliba, O. Glucocorticoid Receptor β (GRβ): Beyond Its Dominant-Negative Function. Int. J. Mol. Sci. 2021, 22, 3649. [Google Scholar] [CrossRef]
- Meijer, O.C.; Koorneef, L.L.; Kroon, J. Glucocorticoid receptor modulators. Ann. d'Endocrinologie 2018, 79, 107–111. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, K.; Berghe, W.V.; Haegeman, G. The Interplay between the Glucocorticoid Receptor and Nuclear Factor-κB or Activator Protein-1: Molecular Mechanisms for Gene Repression. Endocr. Rev. 2003, 24, 488–522. [Google Scholar] [CrossRef] [PubMed]
- Weikum, E.R.; Liu, X.; Ortlund, E.A. The nuclear receptor superfamily: A structural perspective. Protein Sci. 2018, 27, 1876–1892. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.-Y.; Lin, J.; Zhu, S.-Y.; Guo, J.-Y.; Cui, J.-G.; Li, J.-L. Atrazine-induced oxidative damage via modulating xenobiotic-sensing nuclear receptors and cytochrome P450 systems in cerebrum and antagonism of lycopene. Food Chem. Toxicol. 2022, 170, 113462. [Google Scholar] [CrossRef]
- Ding, S.; Yang, L.; Huang, L.; Kong, L.; Chen, M.; Su, Y.; Li, X.; Dong, X.; Han, Y.; Li, W.; et al. Chronic glucocorticoid exposure accelerates Aβ generation and neurotoxicity by activating calcium-mediated CN-NFAT1 signaling in hippocampal neurons in APP/PS1 mice. Food Chem. Toxicol. 2022, 168, 113407. [Google Scholar] [CrossRef]
- Bereshchenko, O.; Bruscoli, S.; Riccardi, C. Glucocorticoids, Sex Hormones, and Immunity. Front. Immunol. 2018, 9, 1332. [Google Scholar] [CrossRef]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef]
- Escoter-Torres, L.; Caratti, G.; Mechtidou, A.; Tuckermann, J.; Uhlenhaut, N.H.; Vettorazzi, S. Fighting the Fire: Mechanisms of Inflammatory Gene Regulation by the Glucocorticoid Receptor. Front. Immunol. 2019, 10, 1859. [Google Scholar] [CrossRef]
- Ronchetti, S.; Ricci, E.; Migliorati, G.; Gentili, M.; Riccardi, C. How Glucocorticoids Affect the Neutrophil Life. Int. J. Mol. Sci. 2018, 19, 4090. [Google Scholar] [CrossRef]
- Tuckermann, J.P.; Kleiman, A.; McPherson, K.; Reichardt, H.M. Molecular mechanisms of glucocorticoids in the control of inflammation and lymphocyte apoptosis. Crit. Rev. Clin. Lab. Sci. 2005, 42, 71–104. [Google Scholar] [CrossRef]
- Herold, M.J.; McPherson, K.G.; Reichardt, H.M. Glucocorticoids in T cell apoptosis and function. Cell. Mol. Life Sci. 2005, 63, 60–72. [Google Scholar] [CrossRef]
- Xing, K.; Gu, B.; Zhang, P.; Wu, X. Dexamethasone enhances programmed cell death 1 (PD-1) expression during T cell activation: An insight into the optimum application of glucocorticoids in anti-cancer therapy. BMC Immunol. 2015, 16, 39. [Google Scholar] [CrossRef]
- West, D.C.; Pan, D.; Tonsing-Carter, E.Y.; Hernandez, K.M.; Pierce, C.F.; Styke, S.C.; Bowie, K.R.; Garcia, T.I.; Kocherginsky, M.; Conzen, S.D. GR and ER Coactivation Alters the Expression of Differentiation Genes and Associates with Improved ER+ Breast Cancer Outcome. Mol. Cancer Res. 2016, 14, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Kocherginsky, M.; Conzen, S.D. Activation of the Glucocorticoid Receptor Is Associated with Poor Prognosis in Estrogen Receptor-Negative Breast Cancer. Cancer Res. 2011, 71, 6360–6370. [Google Scholar] [CrossRef] [PubMed]
- Abduljabbar, R.; Negm, O.H.; Lai, C.-F.; Jerjees, D.A.; Al-Kaabi, M.; Hamed, M.R.; Tighe, P.; Buluwela, L.; Mukherjee, A.; Green, A.; et al. Clinical and biological significance of glucocorticoid receptor (GR) expression in breast cancer. Breast Cancer Res. Treat. 2015, 150, 335–346. [Google Scholar] [CrossRef]
- Noureddine, L.; Trédan, O.; Hussein, N.; Badran, B.; Le Romancer, M.; Poulard, C. Glucocorticoid Receptor: A Multifaceted Actor in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 4446. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Jin, Y.; Nagaich, A.K. Interaction of Glucocorticoid Receptor (GR) with Estrogen Receptor (ER) α and Activator Protein 1 (AP1) in Dexamethasone-mediated Interference of ERα Activity. J. Biol. Chem. 2013, 288, 24020–24034. [Google Scholar] [CrossRef]
- Chen, Z.; Lan, X.; Wu, D.; Sunkel, B.; Ye, Z.; Huang, J.; Liu, Z.; Clinton, S.K.; Jin, V.X.; Wang, Q. Ligand-dependent genomic function of glucocorticoid receptor in triple-negative breast cancer. Nat. Commun. 2015, 6, 8323. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, G.; Zhang, H.; Xiong, Q.; Cheng, F.; Wang, H.; Luo, J.; Zhang, Y.; Shi, P.; Xu, J.; et al. Dexamethasone enhances the lung metastasis of breast cancer via a PI3K-SGK1-CTGF pathway. Oncogene 2021, 40, 5367–5378. [Google Scholar] [CrossRef]
- Regan Anderson, T.M.; Ma, S.H.; Raj, G.V.; Cidlowski, J.A.; Helle, T.M.; Knutson, T.P.; Krutilina, R.I.; Seagroves, T.N.; Lange, C.A. Breast Tumor Kinase (Brk/PTK6) Is Induced by HIF, Glucocorticoid Receptor, and PELP1-Mediated Stress Signaling in Triple-Negative Breast Cancer. Cancer Res. 2016, 76, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Obradović, M.M.S.; Hamelin, B.; Manevski, N.; Couto, J.P.; Sethi, A.; Coissieux, M.-M.; Münst, S.; Okamoto, R.; Kohler, H.; Schmidt, A.; et al. Glucocorticoids promote breast cancer metastasis. Nature 2019, 567, 540–544. [Google Scholar] [CrossRef]
- Yemelyanov, A.; Czwornog, J.; Chebotaev, D.; Karseladze, A.; Kulevitch, E.; Yang, X.; Budunova, I. Tumor suppressor activity of glucocorticoid receptor in the prostate. Oncogene 2007, 26, 1885–1896. [Google Scholar] [CrossRef]
- Sakellakis, M.; Flores, L.J. Is the glucocorticoid receptor a key player in prostate cancer?: A literature review. Medicine 2022, 101, e29716. [Google Scholar] [CrossRef]
- Song, C.; Kim, Y.; Min, G.E.; Ahn, H. Dihydrotestosterone enhances castration-resistant prostate cancer cell proliferation through STAT5 activation via glucocorticoid receptor pathway. Prostate 2014, 74, 1240–1248. [Google Scholar] [CrossRef]
- Yao, Y.; Yao, Q.-Y.; Xue, J.-S.; Tian, X.-Y.; An, Q.-M.; Cui, L.-X.; Xu, C.; Su, H.; Yang, L.; Feng, Y.-Y.; et al. Dexamethasone inhibits pancreatic tumor growth in preclinical models: Involvement of activating glucocorticoid receptor. Toxicol. Appl. Pharmacol. 2020, 401, 115118. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Menard, C.; Puig, P.E.; Ladoire, S.; Roux, S.; Martin, F.; Solary, E.; Le Cesne, A.; Zitvogel, L.; Chauffert, B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007, 56, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Veneris, J.T.; Darcy, K.M.; Mhawech-Fauceglia, P.; Tian, C.; Lengyel, E.; Lastra, R.R.; Pejovic, T.; Conzen, S.D.; Fleming, G.F. High glucocorticoid receptor expression predicts short progression-free survival in ovarian cancer. Gynecol. Oncol. 2017, 146, 153–160. [Google Scholar] [CrossRef]
- Roila, F.; Feyer, P.; Hesketh, P.J.; Jordan, K.; Olver, I.; Rapoport, B.L.; Roscoe, J.; Walsh, D.; Warr, D.; van der Wetering, M.; et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann. Oncol. 2016, 27, v119–v133. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, P.J.; Kris, M.G.; Basch, E.; Bohlke, K.; Barbour, S.Y.; Clark-Snow, R.A.; Danso, M.A.; Dennis, K.; Dupuis, L.L.; Dusetzina, S.B.; et al. Antiemetics: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 2782–2797. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, J.; Boursiquot, J.; Cournoyer, G.; Lemieux, J.; Masse, M.; Almanric, K.; Guay, M. Management of Hypersensitivity to Platinum- and Taxane-Based Chemotherapy: Cepo Review and Clinical Recommendations. Curr. Oncol. 2014, 21, 630–641. [Google Scholar] [CrossRef]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.; ESMO Guidelines Committee. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef]
- Fabi, A.; Bhargava, R.; Fatigoni, S.; Guglielmo, M.; Horneber, M.; Roila, F.; Weis, J.; Jordan, K.; Ripamonti, C. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann. Oncol. 2020, 31, 713–723. [Google Scholar] [CrossRef]
- Zhang, C.; Wenger, T.; Mattern, J.; Ilea, S.; Frey, C.; Gutwein, P.; Altevogt, P.; Bodenmüller, W.; Gassler, N.; Schnabel, P.A.; et al. Clinical and mechanistic aspects of glucocorticoid-induced chemotherapy resistance in the majority of solid tumors. Cancer Biol. Ther. 2007, 6, 278–287. [Google Scholar] [CrossRef]
- Zhang, C.; Marmé, A.; Wenger, T.; Gutwein, P.; Edler, L.; Rittgen, W.; Debatin, K.-M.; Altevogt, P.; Mattern, J.; Herr, I. Glucocorticoid-mediated inhibition of chemotherapy in ovarian carcinomas. Int. J. Oncol. 2006, 28, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Mikosz, C.A.; Brickley, D.R.; Sharkey, M.S.; Moran, T.W.; Conzen, S.D. Glucocorticoid Receptor-mediated Protection from Apoptosis Is Associated with Induction of the Serine/Threonine Survival Kinase Gene, sgk-1. J. Biol. Chem. 2001, 276, 16649–16654. [Google Scholar] [CrossRef]
- Brunet, A.; Park, J.; Tran, H.; Hu, L.S.; Hemmings, B.A.; Greenberg, M.E. Protein Kinase SGK Mediates Survival Signals by Phosphorylating the Forkhead Transcription Factor FKHRL1 (FOXO3a). Mol. Cell. Biol. 2001, 21, 952–965. [Google Scholar] [CrossRef]
- D’Antona, L.; Dattilo, V.; Catalogna, G.; Scumaci, D.; Fiumara, C.V.; Musumeci, F.; Perrotti, G.; Schenone, S.; Tallerico, R.; Spoleti, C.B.; et al. In Preclinical Model of Ovarian Cancer, the SGK1 Inhibitor SI113 Counteracts the Development of Paclitaxel Resistance and Restores Drug Sensitivity. Transl. Oncol. 2019, 12, 1045–1055. [Google Scholar] [CrossRef]
- Franklin, C.C.; Kraft, A.S. Conditional Expression of the Mitogen-activated Protein Kinase (MAPK) Phosphatase MKP-1 Preferentially Inhibits p38 MAPK and Stress-activated Protein Kinase in U937 Cells. J. Biol. Chem. 1997, 272, 16917–16923. [Google Scholar] [CrossRef]
- Hirsch, D.D.; Stork, P. Mitogen-activated Protein Kinase Phosphatases Inactivate Stress-activated Protein Kinase Pathways in Vivo. J. Biol. Chem. 1997, 272, 4568–4575. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Schmitt, W.; Berger, S.; Reles, A.; Siegert, A.; Lichtenegger, W.; Dietel, M.; Hauptmann, S. Expression of mitogen-activated protein kinase phosphatase-1 (MKP-1) in primary human ovarian carcinoma. Int. J. Cancer 2002, 102, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Melhem, A.; Yamada, S.D.; Fleming, G.F.; Delgado, B.; Brickley, D.R.; Wu, W.; Kocherginsky, M.; Conzen, S.D. Administration of Glucocorticoids to Ovarian Cancer Patients Is Associated with Expression of the Anti-apoptotic Genes SGK1 and MKP1/DUSP1 in Ovarian Tissues. Clin. Cancer Res. 2009, 15, 3196–3204. [Google Scholar] [CrossRef]
- Karvonen, H.; Arjama, M.; Kaleva, L.; Niininen, W.; Barker, H.; Koivisto-Korander, R.; Tapper, J.; Pakarinen, P.; Lassus, H.; Loukovaara, M.; et al. Glucocorticoids induce differentiation and chemoresistance in ovarian cancer by promoting ROR1-mediated stemness. Cell Death Dis. 2020, 11, 790. [Google Scholar] [CrossRef]
- Veskimäe, K.; Scaravilli, M.; Niininen, W.; Karvonen, H.; Jaatinen, S.; Nykter, M.; Visakorpi, T.; Mäenpää, J.; Ungureanu, D.; Staff, S. Expression Analysis of Platinum Sensitive and Resistant Epithelial Ovarian Cancer Patient Samples Reveals New Candidates for Targeted Therapies. Transl. Oncol. 2018, 11, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-X.; Wang, Y.; Fu, C.-C.; Diao, F.; Song, L.-N.; Li, Z.-B.; Yang, R.; Lu, J. Dexamethasone enhances cell resistance to chemotherapy by increasing adhesion to extracellular matrix in human ovarian cancer cells. Endocr.-Relat. Cancer 2010, 17, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, R.E.; Fegan, K.S.; Ren, X.; Hillier, S.G.; Duncan, W.C. Glucocorticoid Regulation of SLIT/ROBO Tumour Suppressor Genes in the Ovarian Surface Epithelium and Ovarian Cancer Cells. PLoS ONE 2011, 6, e27792. [Google Scholar] [CrossRef]
- Lin, K.-T.; Yeh, Y.-M.; Chuang, C.-M.; Yang, S.Y.; Chang, J.-W.; Sun, S.-P.; Wang, Y.-S.; Chao, K.-C.; Wang, L.-H. Glucocorticoids mediate induction of microRNA-708 to suppress ovarian cancer metastasis through targeting Rap1B. Nat. Commun. 2015, 6, 5917. [Google Scholar] [CrossRef]
- Huang, M.; Anand, S.; Murphy, E.A.; Desgrosellier, J.S.; Stupack, D.G.; Shattil, S.J.; Schlaepfer, D.D.; Cheresh, D.A. EGFR-dependent pancreatic carcinoma cell metastasis through Rap1 activation. Oncogene 2011, 31, 2783–2793. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.; Huck, B.; Keller, B.; Strotbek, M.; Schmid, S.; Boerries, M.; Busch, H.; Müller, D.; Olayioye, M.A. miR149 Functions as a Tumor Suppressor by Controlling Breast Epithelial Cell Migration and Invasion. Cancer Res. 2014, 74, 5256–5265. [Google Scholar] [CrossRef]
- Lin, K.-T.; Sun, S.-P.; Wu, J.-I.; Wang, L.-H. Low-dose glucocorticoids suppresses ovarian tumor growth and metastasis in an immunocompetent syngeneic mouse model. PLoS ONE 2017, 12, e0178937. [Google Scholar] [CrossRef]
- Qian, B.-Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Marigo, I.; Dolcetti, L.; Serafini, P.; Zanovello, P.; Bronte, V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 2008, 222, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Schweer, D.; McAtee, A.; Neupane, K.; Richards, C.; Ueland, F.; Kolesar, J. Tumor-Associated Macrophages and Ovarian Cancer: Implications for Therapy. Cancers 2022, 14, 2220. [Google Scholar] [CrossRef]
- Yin, M.; Shen, J.; Yu, S.; Fei, J.; Zhu, X.; Zhao, J.; Zhai, L.; Sadhukhan, A.; Zhou, J. Tumor-Associated Macrophages (TAMs): A Critical Activator In Ovarian Cancer Metastasis. OncoTargets Ther. 2019, ume 12, 8687–8699. [Google Scholar] [CrossRef]
- Cui, T.X.; Kryczek, I.; Zhao, L.; Zhao, E.; Kuick, R.; Roh, M.H.; Vatan, L.; Szeliga, W.; Mao, Y.; Thomas, D.G.; et al. Myeloid-Derived Suppressor Cells Enhance Stemness of Cancer Cells by Inducing MicroRNA101 and Suppressing the Corepressor CtBP2. Immunity 2013, 39, 611–621. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Wu, W.; Gao, H.; Liu, N.; Zhan, G.; Li, L.; Han, L.; Guo, X. Myeloid-derived suppressor cells promote epithelial ovarian cancer cell stemness by inducing the CSF2/p-STAT3 signalling pathway. FEBS J. 2020, 287, 5218–5235. [Google Scholar] [CrossRef] [PubMed]
- Veneris, J.T.; Huang, L.; Churpek, J.E.; Conzen, S.D.; Fleming, G.F. Glucocorticoid receptor expression is associated with inferior overall survival independent of BRCA mutation status in ovarian cancer. Int. J. Gynecol. Cancer 2019, 29, 357–364. [Google Scholar] [CrossRef]
- Pardo, B.; Gómez-González, B.; Aguilera, A. DNA Repair in Mammalian Cells. Cell. Mol. Life Sci. 2009, 66, 1039–1056. [Google Scholar] [CrossRef] [PubMed]
- Hartlerode, A.J.; Scully, R. Mechanisms of double-strand break repair in somatic mammalian cells. Biochem. J. 2009, 423, 157–168. [Google Scholar] [CrossRef]
- Manchana, T.; Phoolcharoen, N.; Tantbirojn, P. BRCA mutation in high grade epithelial ovarian cancers. Gynecol. Oncol. Rep. 2019, 29, 102–105. [Google Scholar] [CrossRef]
- Mylavarapu, S.; Das, A.; Roy, M. Role of BRCA Mutations in the Modulation of Response to Platinum Therapy. Front. Oncol. 2018, 8, 16. [Google Scholar] [CrossRef]
- Faraoni, I.; Graziani, G. Role of BRCA Mutations in Cancer Treatment with Poly(ADP-ribose) Polymerase (PARP) Inhibitors. Cancers 2018, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Ma, Y.X.; Wang, C.; Yuan, R.-Q.; Meng, Q.; Wang, J.-A.; Erdos, M.; Goldberg, I.D.; Webb, P.; Kushner, P.J.; et al. Role of direct interaction in BRCA1 inhibition of estrogen receptor activity. Oncogene 2001, 20, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ritter, H.D.; Antonova, L.; Mueller, C.R. The Unliganded Glucocorticoid Receptor Positively Regulates the Tumor Suppressor Gene BRCA1 through GABP Beta. Mol. Cancer Res. 2012, 10, 558–569. [Google Scholar] [CrossRef]
- Vilasco, M.; Communal, L.; Hugon-Rodin, J.; Penault-Llorca, F.; Mourra, N.; Wu, Z.; Forgez, P.; Gompel, A.; BRACAPS. Loss of glucocorticoid receptor activation is a hallmark of BRCA1-mutated breast tissue. Breast Cancer Res. Treat. 2013, 142, 283–296. [Google Scholar] [CrossRef]
- Fang, Y.-Y.; Li, D.; Cao, C.; Li, C.-Y.; Li, T.-T. Glucocorticoid receptor repression mediated by BRCA1 inactivation in ovarian cancer. BMC Cancer 2014, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Goyeneche, A.A.; Caroón, R.W.; Telleria, C.M. Mifepristone Inhibits Ovarian Cancer Cell Growth In vitro and In vivo. Clin. Cancer Res. 2007, 13, 3370–3379. [Google Scholar] [CrossRef]
- Tieszen, C.R.; A Goyeneche, A.; Brandhagen, B.N.; Ortbahn, C.T.; Telleria, C.M. Antiprogestin mifepristone inhibits the growth of cancer cells of reproductive and non-reproductive origin regardless of progesterone receptor expression. BMC Cancer 2011, 11, 207. [Google Scholar] [CrossRef]
- Ritch, S.J.; Brandhagen, B.N.; Goyeneche, A.A.; Telleria, C.M. Advanced assessment of migration and invasion of cancer cells in response to mifepristone therapy using double fluorescence cytochemical labeling. BMC Cancer 2019, 19, 376. [Google Scholar] [CrossRef]
- Ritch, S.J.; Noman, A.S.M.; Goyeneche, A.A.; Telleria, C.M. The metastatic capacity of high-grade serous ovarian cancer cells changes along disease progression: Inhibition by mifepristone. Cancer Cell Int. 2022, 22, 397. [Google Scholar] [CrossRef]
- Rocereto, T.F.; Saul, H.M.; Aikins, J.A.; Paulson, J. Phase II Study of Mifepristone (RU486) in Refractory Ovarian Cancer. Gynecol. Oncol. 2000, 77, 429–432. [Google Scholar] [CrossRef]
- Rocereto, T.F.; Brady, W.E.; Shahin, M.S.; Hoffman, J.S.; Small, L.; Rotmensch, J.; Mannel, R.S. A phase II evaluation of mifepristone in the treatment of recurrent or persistent epithelial ovarian, fallopian or primary peritoneal cancer: A gynecologic oncology group study. Gynecol. Oncol. 2010, 116, 332–334. [Google Scholar] [CrossRef]
- Ponikwicka-Tyszko, D.; Chrusciel, M.; Stelmaszewska, J.; Bernaczyk, P.; Chrusciel, P.; Sztachelska, M.; Scheinin, M.; Bidzinski, M.; Szamatowicz, J.; Huhtaniemi, I.T.; et al. Molecular mechanisms underlying mifepristone's agonistic action on ovarian cancer progression. Ebiomedicine 2019, 47, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V. Selective estrogen receptor modulation: Concept and consequences in cancer. Cancer Cell 2004, 5, 207–213. [Google Scholar] [CrossRef]
- Coghlan, M.J.; Jacobson, P.B.; Lane, B.; Nakane, M.; Lin, C.W.; Elmore, S.W.; Kym, P.R.; Luly, J.R.; Carter, G.W.; Turner, R.; et al. A Novel Antiinflammatory Maintains Glucocorticoid Efficacy with Reduced Side Effects. Mol. Endocrinol. 2003, 17, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Zalachoras, I.; Houtman, R.; Atucha, E.; Devos, R.; Tijssen, A.M.I.; Hu, P.; Lockey, P.M.; Datson, N.A.; Belanoff, J.K.; Lucassen, P.J.; et al. Differential targeting of brain stress circuits with a selective glucocorticoid receptor modulator. Proc. Natl. Acad. Sci. USA 2013, 110, 7910–7915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liang, Y.; Zhang, J. Natural and synthetic compounds as dissociated agonists of glucocorticoid receptor. Pharmacol. Res. 2020, 156, 104802. [Google Scholar] [CrossRef]
- Greenstein, A.E.; Hunt, H.J. Glucocorticoid receptor antagonism promotes apoptosis in solid tumor cells. Oncotarget 2021, 12, 1243–1255. [Google Scholar] [CrossRef]
- Colombo, N.; Nguyen, D.; Fleming, G.; Grisham, R.; Lorusso, D.; Van Gorp, T.; Oaknin, A.; Pashova, H.; Grauer, A. 721O Relacorilant, a selective glucocorticoid receptor modulator, in combination with nab-paclitaxel improves progression-free survival in patients with recurrent platinum-resistant ovarian cancer: A 3-arm, randomized, open-label, phase II study. Ann. Oncol. 2021, 32, S725. [Google Scholar] [CrossRef]
- Lorusso, D.; Greenstein, A.; Wadekar, S.; Tudor, I.; Hunt, H.; Guyer, B. 524MO Glucocorticoid receptor expression and activity in a phase II ovarian cancer trial of the glucocorticoid receptor modulator relacorilant in combination with nab-paclitaxel. Ann. Oncol. 2022, 33, S784–S785. [Google Scholar] [CrossRef]
- Olawaiye, A.; Monk, B.J.; Herzog, T.J.; Copeland, L.J.; Coleman, R.L.; Moore, K.N.; Randall, L.M.; Slomovitz, B.M.; O’Malley, D.M.; Eskander, R.N.; et al. ROSELLA: A phase 3 study of relacorilant in combination with nab-paclitaxel versus investigator’s choice in advanced, platinum-resistant, high-grade epithelial ovarian, primary peritoneal, or fallopian-tube cancer. J. Clin. Oncol. 2022, 40, TPS5620. [Google Scholar] [CrossRef]
- Li, B.-X.; Wang, H.-B.; Qiu, M.-Z.; Luo, Q.-Y.; Yi, H.-J.; Yan, X.-L.; Pan, W.-T.; Yuan, L.-P.; Zhang, Y.-X.; Xu, J.-H.; et al. Correction to: Novel smac mimetic APG-1387 elicits ovarian cancer cell killing through TNF-alpha, Ripoptosome and autophagy mediated cell death pathway. J. Exp. Clin. Cancer Res. 2018, 37, 108. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines☆. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef] [PubMed]

| Study Objective | Study Type | # of Pts | Results | References |
|---|---|---|---|---|
| Association between GR IHC expression and outcome of OC patients | Retrospective | 481 | mPFS = 20.4 months (GR IHC 2+, 3+) vs. 36 months (0 or 1+) HR = 1.66, 95% CI 1.29–2.14, p = 0.001 | [47] |
| Association between NR3C1 gene expression and overall survival of OC patients | Retrospective | 222 | Trend toward decreased OS in pts with high NR3C1 expression compared with low NR3C1 expression (p = 0.06) independently from BRCA mutational status | [76] |
| MKP1 expression in primary human ovarian carcinoma | Retrospective | 101 | mPFS = 18.3 (MPK1+) vs. 40.6 months (MKP1-) (95% CI 13.11–23.5, p = 0.019) | [60] |
| Association between MiRNA-708 expression and OC patients’ survival | Retrospective | 82 | Pts with high miR-708 expression had a significantly better OS (p = 0.04) and RFS (p = 0.026) than those with low miR-708 expression | [66] |
| Expression of the anti-apoptotic genes SGK1 and MKP1/DUSP1 in ovarian tissues upon DEX or NS administration | Prospective randomized | 10 | The average SKG1 and MKP1 mRNA expression was increased 6.1-fold vs. 1.5 and 8.2-fold vs. 1.1, in the DEX and NS arms respectively compared with baseline pretreatment levels | [61] |
| Study Objective | Phase | # of Patients | Results | References |
|---|---|---|---|---|
| Oral MF activity in refractory EOC | II | 44 | ORR = 26.5%, CR = 9%, PR = 17.5% | [90] |
| MF activity in recurrent or persistent HGSOC | II | 24 | Only 1 patient had a partial response (4.5%) | [91] |
| RELA efficacy and safety in combination with chemotherapy in Platinum-Resistant HGSOC | II | 60 (intermittent RELA plus Nab-P) vs. 58 (continuous RELA plus Nab-P) vs. 60 (Nab-P) | mPFS = 5.6 months vs. 3.8 months, HR 0.66, 95% CI 0.44–0.98, p = 0.038); ORR High GR expression = 40.4% vs. 18.8% χ p 0.037; ORR low GR expression= 32.0% vs. 47.1% χ p > 0.05, OS = 13.9 months vs. 12.2 months HR 0.63, p = 0.045. | [98,99] |
| Intermittent RELA plus nab-paclitaxel efficacy vs. TPC in in platinum resistant pre-treated HGSOC (ROSELLA, NCT05257408) | III | 360 (estimated) | Ongoing | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonaiuto, R.; Neola, G.; Cecere, S.C.; Caltavituro, A.; Cefaliello, A.; Pietroluongo, E.; De Placido, P.; Giuliano, M.; Arpino, G.; De Angelis, C. Glucocorticoid Receptor and Ovarian Cancer: From Biology to Therapeutic Intervention. Biomolecules 2023, 13, 653. https://doi.org/10.3390/biom13040653
Buonaiuto R, Neola G, Cecere SC, Caltavituro A, Cefaliello A, Pietroluongo E, De Placido P, Giuliano M, Arpino G, De Angelis C. Glucocorticoid Receptor and Ovarian Cancer: From Biology to Therapeutic Intervention. Biomolecules. 2023; 13(4):653. https://doi.org/10.3390/biom13040653
Chicago/Turabian StyleBuonaiuto, Roberto, Giuseppe Neola, Sabrina Chiara Cecere, Aldo Caltavituro, Amedeo Cefaliello, Erica Pietroluongo, Pietro De Placido, Mario Giuliano, Grazia Arpino, and Carmine De Angelis. 2023. "Glucocorticoid Receptor and Ovarian Cancer: From Biology to Therapeutic Intervention" Biomolecules 13, no. 4: 653. https://doi.org/10.3390/biom13040653
APA StyleBuonaiuto, R., Neola, G., Cecere, S. C., Caltavituro, A., Cefaliello, A., Pietroluongo, E., De Placido, P., Giuliano, M., Arpino, G., & De Angelis, C. (2023). Glucocorticoid Receptor and Ovarian Cancer: From Biology to Therapeutic Intervention. Biomolecules, 13(4), 653. https://doi.org/10.3390/biom13040653








