Abstract
The order Crassvirales comprises dsDNA bacteriophages infecting bacteria in the phylum Bacteroidetes that are found in a variety of environments but are especially prevalent in the mammalian gut. This review summarises available information on the genomics, diversity, taxonomy, and ecology of this largely uncultured viral taxon. With experimental data available from a handful of cultured representatives, the review highlights key properties of virion morphology, infection, gene expression and replication processes, and phage-host dynamics.
1. Introduction
The crAss-like phages are currently thought to be the most abundant double-stranded DNA bacteriophages in the human gut. Yet, despite a wealth of metagenomic data available from the human gut, this highly abundant viral group escaped discovery until 2014 [1]. The near accidental discovery of crAssphage (later dubbed p-crAssphage, or prototypical crAssphage), a bacterial DNA virus (phage) that shared no DNA and very little protein homology with any of the known viruses, highlighted the very limited capacity that existed at the time to discover completely novel viruses, including very prominent ones. The discovery of p-crAssphage has invigorated microbiome researchers to examine the virome portion of the human gut and other environments more closely [2,3,4,5].
Once advanced tools for detection of putative viral genomes became available, a more rigorous examination of previously untraceable “viral dark matter” in metagenomic data revealed a whole plethora of novel viral clades, including “Flandersviridae”, “Quimbyviridae”, Gubaphage, and giant Lak phages [6,7,8,9]. The p-crAssphage itself turned out to be not an orphan but rather a member of an expansive and highly abundant clade of intestinal and environmental phages, initially known as crAss-like phages, now forming the order Crassvirales. While p-crAssphage and related phage strains (members of the recently created new species Carjivirus communis in the family Intestiviridae, subfamily Crudevirinae) are the most prominent members in the gut microbiomes of westernised populations [10,11], other genera, subfamilies, and families of Crassvirales appear to be dominant in human populations adhering to a pre-industrial lifestyle and diet and in non-human primates [10,11,12].
Current analyses estimate crAss-like phages to be present in 73% of global human gut metagenomes, where they can comprise up to 99% of the viral metagenomic reads [10,13]. Found even in remote populations, it is a ubiquitous global virus clade, and its detection in Old and New World primates suggests a possible ancient coevolutionary relationship with humans. Established crAssphage populations are highly stable in the human gut [10,13] with the same viral strain having been demonstrated to persist up to 4 years after original sampling [13,14]. As no detrimental associations between human health and the presence of crAssphage have yet been found, it has been deemed a benign entity in the gut microbiome [11,12]. A beneficial role in the human gut has been suggested by observations of depleted crAss-like phage populations in patients with inflammatory bowel disease, and a destabilisation of p-crAssphage is linked to obesity and metabolic syndrome [15,16].
Since their detection, the crAss-like phages have drawn great interest, yet their role in the gut microbial ecology remains obscure. Their known bacterial hosts, members of the phylum Bacteroidota, mainly family Bacteroidaceae, are essential mutualistic bacteria of the human gut, present at high densities, and are well documented to perform various functions beneficial to the human host, such as immunomodulation, colonisation resistance against invading pathogens, biosynthesis of vitamins, and cooperation with other commensal and mutualistic microbes through cross-feeding networks [17,18,19,20,21,22,23]. Phages can manipulate bacterial populations and communities through a variety of mechanisms: by lysing their bacterial targets (lytic infection) and affecting community structure and cross-feeding networks [24], by integrating prophages into the host genome (lysogeny) and altering host metabolism and fitness [25], and by transferring genes (phage transduction), which can confer advantages to the host (e.g., antibiotic resistance [26,27]). CrAss-like phages seem to be strictly lytic and incapable of lysogeny [28]. At the same time, longitudinal observations of the human gut microbiome, phage-host colonisation trials in germ-free animals, and in vitro phage-host co-cultures demonstrated that crAss-like phages can efficiently co-exist with their host bacteria without inflicting excessive damage at the population level [13,20,21,22,29].
What is the ecological role of this highly abundant virus infecting the family Bacteroidaceae, and what is the secret of their ecological success? Are they crucial for maintaining the stability, diversity, and functional versatility of their host populations, or are they just selfish parasites whose presence their bacterial partners have no choice but to tolerate? A macro view of the gut virome at the population and community levels would suggest there is either an ongoing active arms race between crAss-like phages and their hosts or an “uneasy truce” between the two in which some form of mutualistic interaction or collaboration between these entities has been established [30]. What aspects of their biology have enabled them to become extremely successful colonisers of the human gut? Several crAss-like representatives have been isolated in culture and have been the subjects of experiments designed to address these questions [18,19,20,21,22,31].
The discovery of crAss-like phages is exemplary in proving we have only begun to scratch the surface in uncovering the global viral diversity. Unique in the sense that it was discovered as an orphan genome before its isolation in the lab, crAssphage set a trend for virus discovery in silico, whereas historically phages would be isolated in the lab before their characterisation [32]. The ongoing metagenomics and metatranscriptomic revolution [33], facilitated by access to scalable computing resources and growing databases, is fuelling the pace of viral discovery in global human and mammalian gut samples as well as in any possible sampled environment [6,7,8,9,34,35].
In this review, we attempt to briefly summarise available data on the diversity, taxonomy, and ecology of cultured and uncultured Crassvirales species, as well as to briefly review the history of their discovery. We will also highlight recent progress in understanding their virion molecular structure, infection mechanisms, and phage-host dynamics, achieved with the help of a limited number of cultured species of Crassvirales.
3. Co-Evolution with Bacterial Hosts
Antagonistic interactions between phages and their hosts drive their co-evolution, encouraging both parties to develop novel strategies to increase their fitness and overcome any defence mechanisms developed by the opposite party [30,42]. Gene duplication is one such strategy found in crAss-like sequences that is speculated to bestow replicative advantages. For instance, p-crAssphage has two copies of repL, required for the initiation of DNA replication, which arose from an ancient gene duplication event [43]. CrAss-like phages assigned to candidate genus I (Crudevirinae subfamily of Intestiviridae) have been found to encode diversity-generating retroelements associated with tail protein genes (DGR) [44]. DGRs employ reverse transcriptase to selectively mutate a stretch of DNA coding sequence, giving rise to the creation of original hypervariable tail proteins [45]. This built-in DNA mutator cassette is a versatile protection mechanism that potentially enables phages to counteract bacterial defences (resistance) or exploit alternative bacterial hosts by altering their tail proteins involved in host recognition.
An analysis of nucleotide polymorphisms and the rate of non-synonymous to synonymous changes (dN/dS) in crAssphage genomes from a mother-child cohort with low interpersonal viral diversity showed rapid evolution and strong positive selection for mutations in genes coding for RNAP and tail proteins [46]. Considering the crucial role tail proteins have in bacterial host recognition and the overcoming of acquired phage resistance, it is fitting that their genes would be targets of active selection. One specific target of elevated variation is the BACON domain-containing protein, which is located proximal to the tail protein complex and is thought to have a role in binding to the cell surface of Bacteroides [47]. The rapid evolution of these genes could reflect crAss-like phage’s response to a Red Queen scenario where they are adapting to the host bacteria continuously cycling their cell-surface receptors.
5. Hallmark Genetic Features
An analysis of 596 diverse circular crAss-like phage genomes uncovered some unusual aspects of crAss-like phage architecture and biology [8]. In line with previous host assignments, CRISPR spacer searches of the prokaryotic genome database assigned the majority of crAss-like sequences to hosts in the phylum Bacteroidetes. Lower frequencies of spacer matches aligned to other bacterial phyla such as gut Firmicutes and Proteobacteria, and a minority proportion aligned to Archaeal genomes. The study also consolidated the genomic trademark of crAss-like phages as a syntenous block of eight highly conserved genes encoding the structural proteins MCP, TerL, portal protein, integration host factor (IHF), tail stabilization protein, tail tubular protein, and two uncharacterized proteins. Yutin et al. (2021) also identified two groups of environmental and gut phages appearing related to the core crAss-like phage phylogenetic assemblage, which were named as groups Zeta and Epsilon (continuing the original system of naming Crassvirales families as Alpha, Beta, Gamma, and Delta) [10]. These groups were characterised by larger genome sizes, ranging from 145 to 192 kb, almost twice as large as p-crAssphage’s 97 kb.
An interesting observation was the alternative choice of family A or B DNA polymerases by different clades. Families Intestiviridae, Crevaviridae, and Suoliviridae (Alpha, Gamma, and Delta groups) exclusively encode PolB, while Zeta and Beta groups (the latter being family Steigviridae) have no preference and can either carry PolA or PolB. When co-occurring within groups, PolA and PolB occupy the same position in the genome and are thought to be swapped in situ by homologous recombination. It is hypothesised that this phenomenon has arisen due to the selective pressure presented by an undefined Bacteroides defence mechanism. In addition to DNA polymerase switching, another notable finding was the use of alternative genetic codes, specifically in 243 crAss-like phages in the Beta, Delta (Steigviridae, Suoliviridae), and Zeta groups that reassign TAG stop codons as glutamine or TGA stop codons as tryptophan. Phages in the Zeta groups can switch genetic code at short phylogenetic distances, with closely related branches interspersed with phages reassigning TAG or TGA or using the standard code. An established method of phage codon reassignment is through the use of suppressor tRNAs, which encode an anticodon complementary to the standard stop codons charged with an amino acid [8]. Some crAss-like phages, especially in the Beta (Steigviridae) and Zeta groups, encode a plethora of tRNAs (1–34), with one Zeta phage (NCBI accession OJOH01000017) encoding 34 tRNAs. The encoding of suppressor tRNAs is not completely congruent with stop codon reassignment [8,50]. For example, in some Zeta clades, despite encoding suppressor tRNAs, no reassignment occurs, and in other cases where reassignment occurs, no suppressor tRNAs are present. This leaves the mechanism and motive behind genetic code switching in crAss-like phages ambiguous.
Another distinct feature of crAss-like phages that has not been observed in other phages is the presence of at least one nuclease of the PDDEXK family, with many encoding two or three of these. The exact function of these nucleases is unclear, but they are presumed to have a role in reproduction, with some phages having one variety located in the structural block (PDDEXK_a), another in the replication block (PDDEXK_b), or yet another inside a large protein associated with transcription. The blocks of short, unknown genes common in many crAss-like genomes that share no homologs outside the crAss-like cohort are probably the most intriguing. Postulated to be genes in their defence armoury, specifically anti-CRISPR proteins (Acr), their purpose is still up for debate as a machine learning Acr model failed to confirm their function.
Introns, a group of mobile genetic elements that act as self-splicing ribozymes (an RNA with enzymatic activity) [51], and inteins, proteinaceous parasites that perform self-splicing by cleaving peptide bonds [52], are typical of the genomic landscape in many bacteriophages [51,53]. The crAss-like phages of the Delta (Suoliviridae) and especially Zeta groups have a remarkable density of introns and inteins. In Delta phages, group I introns are inserted in the core phage genes—MCP, PolB, and primase—with TerL containing an intein. A stark example of this intron infestation is found in a Zeta phage’s (NCBI accession number: OHFV01000001) MCP gene, which contains three Group I self-splicing introns within its ORF fragments. In the Zeta group, their predicted RNAP ORFs are drastically fragmented by numerous stop codons and frame shifts, some occurring within the highly conserved DxDxD motif of RNAPB’. The disrupted nature of the genes highlights the complexity and nuance of annotating crAss-like phage genomes. Taking all of this into consideration, crAss-like phages likely employ unconventional encoding and expression mechanisms that have yet to be understood.
6. Alternative Genetic Coding Strategy: Experimentally Confirmed
LC-MS/MS metaproteomics of two metagenomic samples containing two different crAss-like phage genomes (one Suoliviridae and one Zeta group) identified phage peptides from MS/MS spectra. When mapped to the optimal gene predictions using both the standard genetic code (translation table 11) and table 15 (suppression of TAG stop codon with glutamine), around half of the peptides were found to only map to structural proteins (capsid, portal, and tail-associated) predicted using table 15. MS/MS peptide spectra supported the reassignment of the TAG codon to code for glutamine (Q). The contrast in mapping efficiency and correctness of annotation between the standard and non-standard genetic codes is stark and shows that consideration should be taken when predicting crAss-like phage genes [54].
Stop-codon recoding is widespread among different phage clades in the human gut microbiome, with crAss-like phages and Lak phages being found to recode the TAG or TGA stop codon (genetic codes 15 and 4) [8,55]. CrAss-like phages using an alternate code infect standard-code hosts (genetic code 11) [8]. It is uncertain why some phages have evolved genetic codes incompatible with host translation systems. Interestingly, only 35%/40% of TGA/TAG-recoded genomes contain a corresponding tRNA suppressor. Do the other 60% rely on non-standard code used by the host, or do they use an unknown mechanism to accommodate their alternate code? Importantly, CrAss-like phages, for the most part, use alternative coding for their “late” structural and lysis genes (which constitute a single cohesive transcriptional unit). Therefore, the use of recoded stop codons might be required for regulating the precise timing of structural protein expression and cell lysis. Specific gene families seem to be enriched with recoded stop codons: lysozyme-type amidase and spanin/holin (both proteins involved in the lysis-lysis cassette). In such scenarios, regulated genetic code switching might be helping to prevent premature lysis (abortive infection activated by some anti-phage defence systems). Recoding has also been suggested to enable phages to sense co-resident phages and coordinate their lysis timing. Temperate gut phages (non-crAss-like) also employ genetic code switching to control lysis-lysogeny decisions. Given that crAss-like phages are not thought to be lysogenic, it is still possible that recoding contributes to the regulation of lysis (productive vs. abortive infection) in order to fine-tune the steady state in phage-host pairs and ensure long-term persistence [50].
7. P-crAssphage as a Marker for Faecal Contamination
Given p-crAssphage’s ubiquity in human faecal samples, an industrial application of its prevalence has been its utilization as a microbial source tracker (MST) marker of human faecal contamination and pollution in environmental water, surfaces, and wastewater treatment [56,57]. One interest in using crAssphage as a marker for faecal pollution in environmental waters was borne out of the ability to distinguish human faecal waste from other animal faecal pollution [57]. That withstanding, crAss-like variants of animal gut origin are being researched to reliably assess animal faecal pollution of wastewater, with only 61% of wastewater confirmed positive for animal faecal matter showing positive for crAssphage [58].
8. Identification of Bacterial Hosts for crAss-like Phages
The classic approach to the isolation and identification of bacteriophages in environmental samples and linking them to their bacterial hosts is based on plaque assays. Subsequently, this bacteriophage can be further propagated from the plaque and studied. The success of the plaque assay approach in searching for phage-host pairs depends on several factors: the choice of the right bacterial host strain, the ability of the bacteriophage to form visible plaques, the suitability of the medium and other growth conditions for the bacterial growth and plaque formation, and the amount of infective phage particles in a lysate obtained from a sample. A successful combination of all these factors that would yield a plaque is not always possible, especially for such complex communities as the gut microbiota, which contains predominantly temperate phages [59,60,61] and phages infecting bacteria that remain mostly uncultured under laboratory conditions [1]. Despite all its weaknesses, the traditional plaque/spot assay approach was used for the isolation of several crAss-like phages, such as phi14:2, crAss001, DAC15, and DAC17 [19,31]. The first member of the now-extinct Crassvirales to be successfully isolated by this traditional method was in fact the Cellulophaga phage phi14:2. This marine virus was isolated and classified as a member of the family Podoviridae the year before the discovery of p-crAssphage and the sudden rise of interest in crAss-like phages as a novel taxonomic entity [1,31]. Phage enrichment in a liquid culture is a culture-based technique that does not rely on plaque formation. This technique, in combination with next-generation sequencing of the enriched virus-like particle (VLP)-associated DNA fraction, has been used for human gut phageome studies and resulted in the discovery of a significant amount of novel phage-host pairs, including those for crAss-like phages [23]. This plaque-independent, culture-assisted metagenomics approach was used for the identification and characterisation of crAss-like phages ΦcrAss001 [18] and ΦcrAss002 [20]. Additionally, the first crAss-like phages replicating in Bacteroides uniformis cells were detected with similar methods [21].
The paucity of cultured crAss-like phages has prompted the development of alternative approaches for identification of the phage-host pairs. The proximity ligation approach is based on chemically cross-linking DNA fragments that are in close proximity to each other in three-dimensional space. Cross-linking of the fragments results in them belonging to the same contigs after sequencing and data processing. This method allowed the assignment of several members of the crAss-like family to their bacterial hosts. A range of different hosts, all belonging to the Bacteroidetes phylum, was identified for 17 crAss-like phages using meta-HiC.
Microbe-seq, a new complex method of single-cell sequencing, is one of such approaches that uses single amplified genomes (SAG) [62]. This is a culture-independent method that allows for genomic data with strain resolution and is also capable of detecting host-phage interactions within complex populations. As phages associated with bacteria are encapsulated in the same single-cell droplets, the bacterial and viral genomes are linked in the output data by droplet-specific tags. It is thus possible to search for these phages in the resulting genomic data and track their connection with a specific bacterial strain. Using this approach, Bacteroides vulgatus from human gut samples was found to be significantly associated with the p-crAssphage and was therefore predicted to be its host. This result is consistent with other studies that propose the host of the crAssphage to be from the Bacteroides genus [63,64].
CRISPR spacers analysis resolved the crAss-like phage hosts at the order level (Bacteroidales), while more precise host identification by this method is problematic due to horizontal gene transfer leading to distant bacterial species sharing the same CRISPR direct repeats, which hinders taxonomic assignment of CRISPR regions themselves [64]. The correlation between bacterial and viral relative abundance pointed towards Bacteroides vulgatus and Ruminococcus spp. as possible hosts for crAss-like phages [65]. P-crAssphage was found to strongly co-occur and correlate quantitatively with Bacteroides dorei [66]. The credibility of this prediction remains questionable, as the results of the microbe-seq laboratory approach do not support it [62]. Other approaches that can potentially be applied to identify hosts for diverse uncultured crAss-like phages are listed in Box 1.
Box 1. Methods of host assignment for uncultured (difficult-to-culture) bacteriophages.
Many of the viruses discovered with metagenomics approaches have never been isolated in culture. The only methods that allow to link these uncultured phages to the bacterial hosts are bioinformatic predictions. The coevolution of phages and their hosts leaves specific signatures in the genomes of both. The signals for host-phage identification could be abundance profiles, codon usage similarity, genetic similarity, and CRISPR spacer matches [36,64]. Examples of the tools are HoPhage, HostFinder, PHIAF, and VirHostMatcher. Existing bioinformatics tools for in silico predictions of phage-host interactions were reviewed elsewhere [67].
One of the most widespread experimental, culture-independent methods of identifying and describing phage-host interactions is proximity ligation [68,69,70,71]. Recently, an improved protocol based on proximity ligation, called meta3C, was developed. Another recent modification of the proximity ligation method is the cross-linking of specifically bacterial ribosomal RNAs to the transcripts. rRNA-mRNA chimeras where mRNA belongs to a virus allow to identify the host of this virus—the organism that translates its transcripts.
Approaches that utilize single amplified genomes (SAG) are another powerful culture-independent tool for linking phages to hosts. In these approaches, DNA from individual cells is isolated and sequenced. The first step is separating individual cells from each other, which can be conducted with microfluidics or with flow-cytometry techniques. In the next step, the cells are lysed, their genomes are amplified, and they are subjected to next-generation sequencing. Applied to phage-bacteria interactions studies, SAG approaches reveal associations between viral DNA and bacterial DNA of different species and strains. These associations serve as the basis for predictions about which bacterial species serve as hosts for specific phages [72,73,74,75,76].
A virus adsorption-based approach to link bacteriophages from environmental samples to their bacterial hosts is adsorption sequencing (AdsorpSeq). It is based on the interactions between bacteriophages and receptors on the surface of their hosts. The phages that were able to bind to the cell envelopes isolated from a putative host are separated from free (unbound) phages with agarose electrophoresis and then identified by sequencing. This method was verified for several distinct species of Gram-negative bacteria, while its suitability for Gram-positive bacteria remains to be investigated [77]. One of the main drawbacks of all host identification methods that are based on the adsorption of phages to their bacterial hosts is the fact that not all phages that are able to bind to a bacterial surface are able to infect this specific bacterium. Therefore, the range of phages identified for a set of hosts with one of these methods is likely to be wider than the range of phages that successfully infect these hosts.
Viral tagging is another method based on phage adsorption. In this approach, viruses from environmental samples are labelled with fluorescent dye and mixed with supposed bacterial hosts [78,79]. Then the cells are separated by flow cytometry and activated by fluorescence. Infected cells are collected for viral DNA extraction and amplification that yields viral-tagged metagenomes. The single-cell viral tagging was implemented to understand the network of phages and their hosts in the human gut; the study yielded hundreds of predicted phage-host pairs.
Droplet digital PCR (ddPCR) [80,81,82] allows for the identification of the co-localisation of viruses and bacterial cells. Cells from an environmental sample are diluted and directly embedded in droplets or in microfluidic PCR chambers in such a manner that most droplets or chambers contain just one cell. qPCR is performed using two pairs of primers—to a viral marker gene and to a cell marker gene (the rRNA gene). Then the DNA from the droplets or chambers is retrieved and sequenced. The co-amplification of both viral and bacterial marker genes in one droplet or chamber indicates phage-bacteria association. Statistical analysis of these associations reveals the relations between phages and bacterial species inside the community. The method was verified on a microbial population of termite hindgut.
Emulsion-paired isolation-concatenation PCR (epicPCR) [83], initially used for profiling bacterial communities [84], was subsequently modified for linking uncultured phages to bacteria. In this approach, single cells from environmental samples are encapsulated in polyacrylamide beads in emulsion oil. Phusion PCR is performed inside each bead. One of the primers to the target gene (viral) carries an overhang matching the 16SrRNA bacterial gene. Co-localisation of the target gene with the host rRNA gene results in fused products that are later detected with next-generation sequencing. The applicability of the techniques above to crAss-like phages is yet to be tested.
9. Morphology and Molecular Structure of crAss-like Phages
An early in-depth analysis (combining sensitive PSI-BLAST and HHPred searches for protein homologies) of two representative Crassvirales phages, p-crAssphage (now Carjivirus communis, family Intestiviridae) and IAS virus (now Paundivirus hollandii, family Steigviridae), managed to assign functions to many of the hypothetical proteins encoded by both genomes [39]. Both viruses appear to have genes grouped into distinct functional modules: the replication module, the transcription module, and the structural protein module (the latter of which can be further split into capsid and tail protein sub-modules). This functional division agrees with other features of their global genome organisation:An inversion of GC-skew likely separates the genomes into two oppositely oriented replichores (one containing the replication module and the other containing the structural and transcription modules) [50]. These functional modules coincide with transcriptional modules of early (regulatory), middle (replication + optional transcription), and late genes (transcription + structural virion proteins), as discussed below (Figure 2).
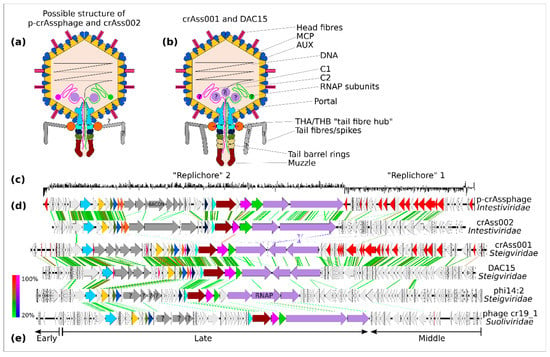
Figure 2.
Virion and genome structure of the typical representatives of the order Crassvirales. A schematic structure of Intestiviridae virions (a) is extrapolated from the cryoEM structural data available for Steigviridae (b). Colours of the protein subunits (a,b) are consistent with ORF colouring (d). GC-skew graph of the p-crAssphage genomes shows clear separation into two possible replichores (c). Despite significant divergence in the amino acid sequence of the encoded proteins, all families show clear genome collinearity (d). Three transcriptional modules can be identified: early (regulatory), middle (DNA replication), and late (structural proteins and other virion components), as exemplified by a Suoliviridae genome (e).
While the podovirus-like morphology of Crassvirales virions had been predicted based on genome analysis, it was not until the isolation of first-cultured Crassvirales that this morphology could be confirmed. Bacteroides phages crAss001 [18], DAC15, DAC17 [19], and Cellulophaga baltica phage phi14:2 [31] (all Steigviridae) all share a very similar morphology with isometric icosahedral capsids 77–88 nm in diameter (depending on the microscopy method) and short non-contractile tails of 36–44 nm decorated with appendages of different sizes. The Bacteroides xylanisolvens phage crAss002 (Jahgtovirus secundus, family Intestiviridae) has a capsid of 77 nm and a shorter tail of 18 nm [20]. A crude faecal filtrate rich in Crassvirales (mainly Intestiviridae and Crevaviridae) contained two types of virions with isometric heads (~76.5 nm) and shorter or longer tails [10]. Members of the family Suoliviridae, as well as additional proposed epsilon- and delta-groups of Crassvirales, have never been isolated in culture or observed microscopically.
A major advancement in understanding the virion structure and functions of individual structural proteins in Crassvirales came from a cryo-EM-based reconstruction of crAss001 virions, allowing for atomic-level modelling of many of the capsid and tail proteins [85]. This analysis revealed a T = 9 icosahedral capsid with an outer diameter of 88 nm. The capsid is mainly composed of the HK97-type fold major capsid protein (MCP) and an auxiliary capsid protein (AUX). The structure of the AUX protein is unique to the Crassvirales. In addition to these main subunit types, two types of head fibres, trimeric and dimeric, with recognisably Ig-like domains, are inserted into the central pockets formed by the MCP-AUX hexons. These fibres seem to be narrowly specific to particular taxa within Crassvirales, with only a fraction of viral diversity in this order having detectable homologues. At the same time, unrelated proteins may have analogous functions in other species of Crassvirales. Both types of fibres can potentially be involved in either host recognition, or perhaps binding to the intestinal mucus, increasing the persistence of phage in the gut and preventing its washout (BAM-model of gut phage persistence) [86]. Such a phenomenon has previously been reported, with hoc+T4 coliphage being capable of binding to the gut mucin.
A dodecameric portal assembly is inserted in one of the five-fold symmetrical capsid vertices, providing an entry point for DNA packaging and an interface for docking the tail barrel assembly. Together with the large terminase gene, primase, and the MCP gene, the portal protein gene forms a set of genes universally conserved across the order Crassvirales. Zones around the portal crown domains extending into the capsid matrix contain a lower density of packaged DNA and are believed to be occupied by cargo proteins, which eject from the capsid together with, or ahead of, the phage genome in the early stages of infection. These cargo proteins may include 1–3 large subunits of the vRNAP as well as the large proteins C1 and C2, however only the presence of C1 protein can be confirmed from cryo-EM reconstruction. All cargo proteins are likely to be present in a partially unfolded state, facilitating their passage through the portal and the tail barrel. Protein C1 appears to be stretched out, with parts of its sequence visible inside the capsid (around the portal crown) and the tail barrel. Such partially unfolded C1, as well as parts of other cargo proteins and the leading DNA end, may constitute the central body visible inside the portal channel and the tail barrel.
The tail barrel is composed of five stacked dodecameric rings. Rings 1–3 are composed of dedicated subunit types, whereas rings four and five use identical subunits. Interestingly, different clades of Crassvirales contain variable numbers of tail-barrel ring protein genes, implying a different number of rings and therefore tail length in different species. This agrees with EM observations of diverse Crassvirales. A hexameric tail muzzle protein forms the tail barrel assembly and represents a structural element highly conserved across the Crassvirales [10]. This multi-domain protein contains some elements shared with tail tip proteins from other phages, e.g., a ring-joining domain and a β-propeller domain containing the gate loop are similar to the coliphage T7 nozzle protein. At the same time, two Ig-like domains and, most notably, a domain with a novel type of fold, termed crAss fold, located at the very tip of the muzzle structure, are highly unique to the Crassvirales. It is believed that the crAss domain, together with Ig-like domains, might be responsible for the docking of phage particles on the cell surface. Conformational changes evoked by this docking event can trigger the opening of the gate located above, in the proximal part of the muzzle, ejecting the capsid contents.
Two types of protein subunits (THA and THB) are responsible for forming a collar structure around the tail neck. This structure forms a docking hub for the attachment of tail fibre/spike assemblies—flexible structures forming the outer and inner “cages” around the tail barrel. While five different proteins are likely to be involved in this assembly in crAss001, the exact structure could not be resolved using cryo-EM. Tail spike subunits in different Crassvirales have been annotated as containing carbohydrate binding/lectin-type domains (e.g., the BACON domain in p-crAssphage and related phages) as well as possible glycosyl-hydrolase domains, phosphatase domains, and CHAP domains [1,18,20,47]. Tail spike proteins with CPS-, EPS-, and LPS-depolymerising activity are a typical feature in phages with podovirus-like morphology [87]. It is therefore likely that the tail fibres/spikes of Crassvirales act as receptor binding proteins (RBPs) and are responsible for both the recognition of polysaccharide structures covering host cells, and the digestion of CPS/EPS/LPS polysaccharides in order to gain access to the outer membrane for docking. The genomic region occupied by the tail spike genes is characterised by a striking level of diversity, with different lineages of Crassvirales containing completely different numbers and types of genes, often with no detectable homology to their counterparts in crAss001 or p-crAssphage [10,18]. Even within narrower groups in Crassvirales (just p-crAssphage —Carjivirus communis), tail spike proteins are subjects to rapid evolution, e.g., DGR-dependent diversification [44] and evolution through duplication and HGT of BACON repeats [47]. Longitudinal analysis of p-crAssphage genomes persisting in the human gut revealed rapid evolution of tail spike proteins with evidence of positive selective pressure [44,88].
Overall, the genome and virion structure of Crassvirales resemble those of coliphage N4 (the latter being slightly smaller at 72 kb genome and 70 nm capsid). Interestingly, coliphage N4 also includes the rare feature of a high-molecular-weight (360 kDa) vRNAP [89,90].
11. Conclusions
The accidental discovery of p-crAssphage, the most abundant human gut bacteriophage, by cross-assembly of metagenomic reads in 2014 revealed how little we knew about the diversity and the role of bacterial viruses in the human gut microbiome and other complex microbial communities. The known viral diversity of dsDNA-tailed phages has bloomed in recent years due to the increasing availability of shotgun metagenomic and metaviromic data, improved virome analysis pipelines, sensitive protein homology search tools, and expanding collections of reference genomes [7,9,105]. The range of known crAss-like phages has expanded from a few “orphan” species into the expansive and highly diverse new viral order of Crassvirales [8,46]. Still, the phylogeny of the Crassvirales and their evolutionary relationships with other dsDNA viruses remain largely unexplored.
Metagenomics and metaviromics have proven themselves powerful technologies for virus discovery, host prediction, taxonomy, and abundance and distribution analysis [28]. However, in silico methods can be limited in their tangible revelations on the molecular biology, host range, and ecological impacts of crAss-like phages. Emerging engineering advances in single-cell sequencing techniques, viral tagging, and proximity ligation are set to provide new insights into the biology of diverse crAss-like phages.
The first in-depth characterisation of a crAss-like phage, crAss001, while providing a complete genome and data on virion structure and certain aspects of its life cycle [18,22,84], posed numerous new questions about the biology of this phage taxon. Being apparently a strictly lytic phage, crAss001 is capable of long-term persistence in the culture of its host Bacteroides intestinalis, which was shown to be associated with dynamic phase variation of capsular polysaccharide types. However, is that the only mechanism responsible? Could alternative mechanisms of persistence (lysogeny, pseudo-lysogeny, the carrier state, or chronic infection “hibernation” inside infected cells) also be part of it? Available evidence does not support the ability for true lysogeny in crAss-like phages, but other mechanisms cannot be ruled out.
Phage crAss002 infects its host Bacteroides xylanisolvens without forming plaques, despite being apparently a lytic phage [20]. Is that a result of phase variation greatly shifting towards the insensitive phase? Such phage-host interactions may be puzzling to the observer, but is it uniquely specific to the Bacteroides/crAss-like phage systems, or is it a widespread type of dynamic between phages and their bacterial hosts in natural settings, a better winning strategy for the phage? Perhaps the “classical” lytic process, favoured by many studies of phage biology, is a very artificial scenario that can only be achieved in perfect conditions (exactly the right growth phase, MOI, etc.).
As discussed in our other recent review [30], bacteriophages can be seen as an integral component of complex microbial communities, evolutionary partners rather than antagonists of bacteria, responsible for maintenance of taxonomic and functional diversity, stimulating resilience and adaptability of the microbiota. The tandem of Bacteroides bacteria and crAss-like phages in the gut might be an epitome of such relationships.
Author Contributions
L.S.: writing and original draft preparation; E.G.: writing and original draft preparation; B.G.: review and editing; A.N.S.: writing, original draft preparation, and review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the Wellcome Trust (grant number 220646/Z/20/Z), the European Research Council (ERC), under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 101001684), and Science Foundation Ireland (Grant number 12/RC/2273_P2).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dutilh, B.E.; Cassman, N.; McNair, K.; Sanchez, S.E.; Silva, G.G.Z.; Boling, L.; Barr, J.J.; Speth, D.R.; Seguritan, V.; Aziz, R.K.; et al. A Highly Abundant Bacteriophage Discovered in the Unknown Sequences of Human Faecal Metagenomes. Nat. Commun. 2014, 5, 4498. [Google Scholar] [CrossRef] [PubMed]
- Pratama, A.A.; van Elsas, J.D. The ‘Neglected’ Soil Virome—Potential Role and Impact. Trends Microbiol. 2018, 26, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, F.H.; Silveira, C.B.; Gregoracci, G.B.; Thompson, C.C.; Edwards, R.A.; Brussaard, C.P.D.; Dutilh, B.E.; Thompson, F.L. Marine Viruses Discovered via Metagenomics Shed Light on Viral Strategies throughout the Oceans. Nat. Commun. 2017, 8, 15955. [Google Scholar] [CrossRef] [PubMed]
- Garmaeva, S.; Sinha, T.; Kurilshikov, A.; Fu, J.; Wijmenga, C.; Zhernakova, A. Studying the Gut Virome in the Metagenomic Era: Challenges and Perspectives. BMC Biol. 2019, 17, 84. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.D.S.; Hill, C. Gut Bacteriophage: Current Understanding and Challenges. Front. Endocrinol. 2019, 10, 784. [Google Scholar] [CrossRef]
- Devoto, A.E.; Santini, J.M.; Olm, M.R.; Anantharaman, K.; Munk, P.; Tung, J.; Archie, E.A.; Turnbaugh, P.J.; Seed, K.D.; Blekhman, R.; et al. Megaphages Infect Prevotella and Variants Are Widespread in Gut Microbiomes. Nat. Microbiol. 2019, 4, 693–700. [Google Scholar] [CrossRef]
- Benler, S.; Yutin, N.; Antipov, D.; Rayko, M.; Shmakov, S.; Gussow, A.B.; Pevzner, P.; Koonin, E.V. Thousands of Previously Unknown Phages Discovered in Whole-Community Human Gut Metagenomes. Microbiome 2021, 9, 78. [Google Scholar] [CrossRef]
- Yutin, N.; Benler, S.; Shmakov, S.A.; Wolf, Y.I.; Tolstoy, I.; Rayko, M.; Antipov, D.; Pevzner, P.A.; Koonin, E.V. Analysis of Metagenome-Assembled Viral Genomes from the Human Gut Reveals Diverse Putative CrAss-like Phages with Unique Genomic Features. Nat. Commun. 2021, 12, 1044. [Google Scholar] [CrossRef]
- Camarillo-Guerrero, L.F.; Almeida, A.; Rangel-Pineros, G.; Finn, R.D.; Lawley, T.D. Massive Expansion of Human Gut Bacteriophage Diversity. Cell 2021, 184, 1098–1109.e9. [Google Scholar] [CrossRef]
- Guerin, E.; Shkoporov, A.; Stockdale, S.R.; Clooney, A.G.; Ryan, F.J.; Sutton, T.D.S.; Draper, L.A.; Gonzalez-Tortuero, E.; Ross, R.P.; Hill, C. Biology and Taxonomy of CrAss-like Bacteriophages, the Most Abundant Virus in the Human Gut. Cell Host Microbe 2018, 24, 653–664.e6. [Google Scholar] [CrossRef]
- Edwards, R.A.; Vega, A.A.; Norman, H.M.; Ohaeri, M.; Levi, K.; Dinsdale, E.A.; Cinek, O.; Aziz, R.K.; McNair, K.; Barr, J.J.; et al. Global Phylogeography and Ancient Evolution of the Widespread Human Gut Virus CrAssphage. Nat. Microbiol. 2019, 4, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Honap, T.P.; Sankaranarayanan, K.; Schnorr, S.L.; Ozga, A.T.; Warinner, C.; Lewis, C.M. Biogeographic Study of Human Gut-Associated CrAssphage Suggests Impacts from Industrialization and Recent Expansion. PLoS ONE 2020, 15, e0226930. [Google Scholar] [CrossRef] [PubMed]
- Shkoporov, A.N.; Clooney, A.G.; Sutton, T.D.S.; Ryan, F.J.; Daly, K.M.; Nolan, J.A.; McDonnell, S.A.; Khokhlova, E.V.; Draper, L.A.; Forde, A.; et al. The Human Gut Virome Is Highly Diverse, Stable, and Individual Specific. Cell Host Microbe 2019, 26, 527–541.e5. [Google Scholar] [CrossRef]
- Gulyaeva, A.; Garmaeva, S.; Ruigrok, R.A.A.A.; Wang, D.; Riksen, N.P.; Netea, M.G.; Wijmenga, C.; Weersma, R.K.; Fu, J.; Vila, A.V.; et al. Discovery, Diversity, and Functional Associations of CrAss-like Phages in Human Gut Metagenomes from Four Dutch Cohorts. Cell Rep. 2022, 38, 110204. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Echeverría, M.; Gallardo-Becerra, L.; Cornejo-Granados, F.; Leyva, A.O. A Loss of CrAssphage Stability in the Human Gut Virome Is Associated with Obesity and Metabolic Syndrome. bioRxiv 2022. [Google Scholar] [CrossRef]
- Clooney, A.G.; Sutton, T.D.S.; Shkoporov, A.N.; Holohan, R.K.; Daly, K.M.; O’Regan, O.; Ryan, F.J.; Draper, L.A.; Plevy, S.E.; Ross, R.P.; et al. Whole-Virome Analysis Sheds Light on Viral Dark Matter in Inflammatory Bowel Disease. Cell Host Microbe 2019, 26, 764–778.e5. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H. Gut Bacteroides Species in Health and Disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Khokhlova, E.V.; Fitzgerald, C.B.; Stockdale, S.R.; Draper, L.A.; Ross, R.P.; Hill, C. ΦCrAss001 Represents the Most Abundant Bacteriophage Family in the Human Gut and Infects Bacteroides intestinalis. Nat. Commun. 2018, 9, 4781. [Google Scholar] [CrossRef]
- Hryckowian, A.J.; Merrill, B.D.; Porter, N.T.; Van Treuren, W.; Nelson, E.J.; Garlena, R.A.; Russell, D.A.; Martens, E.C.; Sonnenburg, J.L. Bacteroides thetaiotaomicron-Infecting Bacteriophage Isolates Inform Sequence-Based Host Range Predictions. Cell Host Microbe 2020, 28, 371–379.e5. [Google Scholar] [CrossRef]
- Guerin, E.; Shkoporov, A.N.; Stockdale, S.R.; Comas, J.C.; Khokhlova, E.V.; Clooney, A.G.; Daly, K.M.; Draper, L.A.; Stephens, N.; Scholz, D.; et al. Isolation and Characterisation of ΦcrAss002, a CrAss-like Phage from the Human Gut That Infects Bacteroides Xylanisolvens. Microbiome 2021, 9, 89. [Google Scholar] [CrossRef]
- Hedžet, S.; Rupnik, M.; Accetto, T. Broad Host Range May Be a Key to Long-Term Persistence of Bacteriophages Infecting Intestinal Bacteroidaceae Species. Sci. Rep. 2022, 12, 21098. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Khokhlova, E.V.; Stephens, N.; Hueston, C.; Seymour, S.; Hryckowian, A.J.; Scholz, D.; Ross, R.P.; Hill, C. Long-Term Persistence of CrAss-like Phage CrAss001 Is Associated with Phase Variation in Bacteroides intestinalis. BMC Biol. 2021, 19, 163. [Google Scholar] [CrossRef]
- Fitzgerald, C.B.; Shkoporov, A.N.; Upadrasta, A.; Khokhlova, E.V.; Ross, R.P.; Hill, C. Probing the “Dark Matter” of the Human Gut Phageome: Culture Assisted Metagenomics Enables Rapid Discovery and Host-Linking for Novel Bacteriophages. Front. Cell. Infect. Microbiol. 2021, 11, 616918. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Bry, L.; Silver, P.A.; Gerber, G.K. Bacteriophages Dynamically Modulate the Gut Microbiota and Metabolome. bioRxiv 2018, 454579. [Google Scholar] [CrossRef]
- Campbell, D.E.; Ly, L.K.; Ridlon, J.M.; Hsiao, A.; Whitaker, R.J.; Degnan, P.H. Infection with Bacteroides Phage BV01 Alters the Host Transcriptome and Bile Acid Metabolism in a Common Human Gut Microbe. Cell Rep. 2020, 32, 108142. [Google Scholar] [CrossRef] [PubMed]
- Balcazar, J.L. Bacteriophages as Vehicles for Antibiotic Resistance Genes in the Environment. PLoS Pathog. 2014, 10, e1004219. [Google Scholar] [CrossRef]
- Penadés, J.R.; Chen, J.; Quiles-Puchalt, N.; Carpena, N.; Novick, R.P. Bacteriophage-Mediated Spread of Bacterial Virulence Genes. Curr. Opin. Microbiol. 2015, 23, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Yutin, N. The CrAss-like Phage Group: How Metagenomics Reshaped the Human Virome. Trends Microbiol. 2020, 28, 349–359. [Google Scholar] [CrossRef]
- Reyes, A.; Wu, M.; McNulty, N.P.; Rohwer, F.L.; Gordon, J.I. Gnotobiotic Mouse Model of Phage–Bacterial Host Dynamics in the Human Gut. Proc. Natl. Acad. Sci. USA 2013, 110, 20236–20241. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Turkington, C.J.; Hill, C. Mutualistic Interplay between Bacteriophages and Bacteria in the Human Gut. Nat. Rev. Microbiol. 2022, 20, 737–749. [Google Scholar] [CrossRef]
- Holmfeldt, K.; Solonenko, N.; Shah, M.; Corrier, K.; Riemann, L.; Verberkmoes, N.C.; Sullivan, M.B. Twelve Previously Unknown Phage Genera Are Ubiquitous in Global Oceans. Proc. Natl. Acad. Sci. USA 2013, 110, 12798–12803. [Google Scholar] [CrossRef]
- Rosario, K.; Breitbart, M. Exploring the Viral World through Metagenomics. Curr. Opin. Virol. 2011, 1, 289–297. [Google Scholar] [CrossRef]
- Koonin, E.V.; Krupovic, M.; Dolja, V.V. The Global Virome: How Much Diversity and How Many Independent Origins? Environ. Microbiol. 2022, 25, 40–44. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Stockdale, S.R.; Lavelle, A.; Kondova, I.; Heuston, C.; Upadrasta, A.; Khokhlova, E.V.; van der Kamp, I.; Ouwerling, B.; Draper, L.A.; et al. Viral Biogeography of the Mammalian Gut and Parenchymal Organs. Nat. Microbiol. 2022, 7, 1301–1311. [Google Scholar] [CrossRef]
- López-Bueno, A.; Tamames, J.; Velázquez, D.; Moya, A.; Quesada, A.; Alcamí, A. High Diversity of the Viral Community from an Antarctic Lake. Science 2009, 326, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.A.; McNair, K.; Faust, K.; Raes, J.; Dutilh, B.E. Computational Approaches to Predict Bacteriophage–Host Relationships. FEMS Microbiol. Rev. 2016, 40, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef]
- Draper, L.A.; Ryan, F.J.; Smith, M.K.; Jalanka, J.; Mattila, E.; Arkkila, P.A.; Ross, R.P.; Satokari, R.; Hill, C. Long-Term Colonisation with Donor Bacteriophages Following Successful Faecal Microbial Transplantation. Microbiome 2018, 6, 220. [Google Scholar] [CrossRef]
- Yutin, N.; Makarova, K.S.; Gussow, A.B.; Krupovic, M.; Segall, A.; Edwards, R.A.; Koonin, E.V. Discovery of an Expansive Bacteriophage Family That Includes the Most Abundant Viruses from the Human Gut. Nat. Microbiol. 2018, 3, 38–46. [Google Scholar] [CrossRef]
- Li, Y.; Gordon, E.; Shean, R.C.; Idle, A.; Deng, X.; Greninger, A.L.; Delwart, E. CrAssphage and Its Bacterial Host in Cat Feces. Sci. Rep. 2021, 11, 815. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, F.L.; Vlot, M.; de Jonge, P.A.; Dreesens, L.L.; Beaumont, H.J.E.; Lavigne, R.; Dutilh, B.E.; Brouns, S.J.J. Targeting Mechanisms of Tailed Bacteriophages. Nat. Rev. Microbiol. 2018, 16, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, P.D. Bacteria–Bacteriophage Coevolution in the Human Gut: Implications for Microbial Diversity and Functionality. Trends Microbiol. 2017, 25, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Treu, L.; Toppo, S.; Zschach, H.; Campanaro, S.; Dutilh, B.E. Evolutionary Study of the Crassphage Virus at Gene Level. Viruses 2020, 12, 1035. [Google Scholar] [CrossRef] [PubMed]
- Morozova, V.; Fofanov, M.; Tikunova, N.; Babkin, I.; Morozov, V.V.; Tikunov, A. First CrAss-Like Phage Genome Encoding the Diversity-Generating Retroelement (DGR). Viruses 2020, 12, 573. [Google Scholar] [CrossRef]
- Guo, H.; Arambula, D.; Ghosh, P.; Miller, J.F. Diversity-Generating Retroelements in Phage and Bacterial Genomes. Microbiol. Spectr. 2014, 2, 1237–1252. [Google Scholar] [CrossRef]
- Brown, B.P.; Chopera, D.; Havyarimana, E.; Wendoh, J.; Jaumdally, S.; Nyangahu, D.D.; Gray, C.M.; Martin, D.P.; Varsani, A.; Jaspan, H.B. CrAssphage Genomes Identified in Fecal Samples of an Adult and Infants with Evidence of Positive Genomic Selective Pressure within Tail Protein Genes. Virus Res. 2021, 292, 198219. [Google Scholar] [CrossRef]
- Jonge, P.A.; von Meijenfeldt, F.A.B.; van Rooijen, L.E.; Brouns, S.J.J.; Dutilh, B.E. Evolution of BACON Domain Tandem Repeats in CrAssphage and Novel Gut Bacteriophage Lineages. Viruses 2019, 11, 1085. [Google Scholar] [CrossRef]
- Shkoporov, A.; Stockdale, S.R.; Adriaenssens, E.M.; Yutin, N.; Koonin, E.V.; Dutilh, B.E.; Krupovic, M.; Edwards, R.A.; Tolstoy, I.; Hill, C. Taxonomic Proposal: Create One New Order (Crassvirales) Including Four New Families, Ten New Subfamilies, 42 New Genera and 73 New Species (Caudoviricetes). 2020. Available online: https://ictv.global/filebrowser/download/5740 (accessed on 22 March 2023).
- Roux, S.; Adriaenssens, E.M.; Dutilh, B.E.; Koonin, E.V.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Lavigne, R.; Brister, J.R.; Varsani, A.; et al. Minimum Information about an Uncultivated Virus Genome (MIUViG). Nat. Biotechnol. 2019, 37, 29–37. [Google Scholar] [CrossRef]
- Borges, A.L.; Lou, Y.C.; Sachdeva, R.; Al-Shayeb, B.; Penev, P.I.; Jaffe, A.L.; Lei, S.; Santini, J.M.; Banfield, J.F. Widespread Stop-Codon Recoding in Bacteriophages May Regulate Translation of Lytic Genes. Nat. Microbiol. 2022, 7, 918–927. [Google Scholar] [CrossRef]
- Edgell, D.R.; Chalamcharla, V.R.; Belfort, M. Learning to Live Together: Mutualism between Self-Splicing Introns and Their Hosts. BMC Biol. 2011, 9, 22. [Google Scholar] [CrossRef]
- Gogarten, J.P.; Senejani, A.G.; Zhaxybayeva, O.; Olendzenski, L.; Hilario, E. Inteins: Structure, Function, and Evolution. Annu. Rev. Microbiol. 2002, 56, 263–287. [Google Scholar] [CrossRef]
- Frost, L.S.; Leplae, R.; Summers, A.O.; Toussaint, A. Mobile Genetic Elements: The Agents of Open Source Evolution. Nat. Rev. Microbiol. 2005, 3, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.L.; Borges, A.L.; Giannone, R.J.; Morowitz, M.J.; Banfield, J.F.; Hettich, R.L. Experimental Validation That Human Microbiome Phages Use Alternative Genetic Coding. Nat. Commun. 2022, 13, 5710. [Google Scholar] [CrossRef] [PubMed]
- Crisci, M.A.; Chen, L.-X.; Devoto, A.E.; Borges, A.L.; Bordin, N.; Sachdeva, R.; Tett, A.; Sharrar, A.M.; Segata, N.; Debenedetti, F.; et al. Closely Related Lak Megaphages Replicate in the Microbiomes of Diverse Animals. iScience 2021, 24, 102875. [Google Scholar] [CrossRef] [PubMed]
- Stachler, E.; Kelty, C.; Sivaganesan, M.; Li, X.; Bibby, K.; Shanks, O.C. Quantitative CrAssphage PCR Assays for Human Fecal Pollution Measurement. Environ. Sci. Technol. 2017, 51, 9146–9154. [Google Scholar] [CrossRef]
- Park, G.W.; Ng, T.F.F.; Freeland, A.L.; Marconi, V.C.; Boom, J.A.; Staat, M.A.; Montmayeur, A.M.; Browne, H.; Narayanan, J.; Payne, D.C.; et al. CrAssphage as a Novel Tool to Detect Human Fecal Contamination on Environmental Surfaces and Hands. Emerg. Infect. Dis. 2020, 26, 1731. [Google Scholar] [CrossRef]
- García-Aljaro, C.; Ballesté, E.; Muniesa, M.; Jofre, J. Determination of CrAssphage in Water Samples and Applicability for Tracking Human Faecal Pollution. Microb. Biotechnol. 2017, 10, 1775–1780. [Google Scholar] [CrossRef]
- Reyes, A.; Haynes, M.; Hanson, N.; Angly, F.E.; Heath, A.C.; Rohwer, F.; Gordon, J.I. Viruses in the Faecal Microbiota of Monozygotic Twins and Their Mothers. Nature 2010, 466, 334–338. [Google Scholar] [CrossRef]
- Fitzgerald, C.B.; Shkoporov, A.N.; Sutton, T.D.S.; Chaplin, A.V.; Velayudhan, V.; Ross, R.P.; Hill, C. Comparative Analysis of Faecalibacterium Prausnitzii Genomes Shows a High Level of Genome Plasticity and Warrants Separation into New Species-Level Taxa. BMC Genom. 2018, 19, 931. [Google Scholar] [CrossRef]
- Cornuault, J.K.; Moncaut, E.; Loux, V.; Mathieu, A.; Sokol, H.; Petit, M.-A.; De Paepe, M. The Enemy from within: A Prophage of Roseburia intestinalis Systematically Turns Lytic in the Mouse Gut, Driving Bacterial Adaptation by CRISPR Spacer Acquisition. ISME J. 2020, 14, 771–787. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, S.; Yin, Y.; Zhang, H.; Needham, D.M.; Evans, E.D.; Dai, C.L.; Lu, P.J.; Alm, E.J.; Weitz, D.A. High-Throughput, Single-Microbe Genomics with Strain Resolution, Applied to a Human Gut Microbiome. Science 2022, 376, eabm1483. [Google Scholar] [CrossRef]
- Asnicar, F.; Manara, S.; Zolfo, M.; Truong, D.T.; Scholz, M.; Armanini, F.; Ferretti, P.; Gorfer, V.; Pedrotti, A.; Tett, A.; et al. Studying Vertical Microbiome Transmission from Mothers to Infants by Strain-Level Metagenomic Profiling. mSystems 2017, 2, e00164-16. [Google Scholar] [CrossRef]
- Sugimoto, R.; Nishimura, L.; Nguyen, P.T.; Ito, J.; Parrish, N.F.; Mori, H.; Kurokawa, K.; Nakaoka, H.; Inoue, I. Comprehensive Discovery of CRISPR-Targeted Terminally Redundant Sequences in the Human Gut Metagenome: Viruses, Plasmids, and More. PLoS Comput. Biol. 2021, 17, e1009428. [Google Scholar] [CrossRef] [PubMed]
- Tomofuji, Y.; Kishikawa, T.; Maeda, Y.; Ogawa, K.; Nii, T.; Okuno, T.; Oguro-Igashira, E.; Kinoshita, M.; Yamamoto, K.; Sonehara, K.; et al. Whole Gut Virome Analysis of 476 Japanese Revealed a Link between Phage and Autoimmune Disease. Ann. Rheum. Dis. 2022, 81, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Cinek, O.; Kramna, L.; Lin, J.; Oikarinen, S.; Kolarova, K.; Ilonen, J.; Simell, O.; Veijola, R.; Autio, R.; Hyöty, H. Imbalance of Bacteriome Profiles within the Finnish Diabetes Prediction and Prevention Study: Parallel Use of 16S Profiling and Virome Sequencing in Stool Samples from Children with Islet Autoimmunity and Matched Controls. Pediatr. Diabetes 2017, 18, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Coclet, C.; Roux, S. Global Overview and Major Challenges of Host Prediction Methods for Uncultivated Phages. Curr. Opin. Virol. 2021, 49, 117–126. [Google Scholar] [CrossRef]
- Bickhart, D.M.; Watson, M.; Koren, S.; Panke-Buisse, K.; Cersosimo, L.M.; Press, M.O.; Van Tassell, C.P.; Van Kessel, J.A.S.; Haley, B.J.; Kim, S.W.; et al. Assignment of Virus and Antimicrobial Resistance Genes to Microbial Hosts in a Complex Microbial Community by Combined Long-Read Assembly and Proximity Ligation. Genome Biol. 2019, 20, 153. [Google Scholar] [CrossRef]
- Marbouty, M.; Cournac, A.; Flot, J.-F.; Marie-Nelly, H.; Mozziconacci, J.; Koszul, R. Metagenomic Chromosome Conformation Capture (Meta3C) Unveils the Diversity of Chromosome Organization in Microorganisms. eLife 2014, 3, e03318. [Google Scholar] [CrossRef]
- Marbouty, M.; Baudry, L.; Cournac, A.; Koszul, R. Scaffolding Bacterial Genomes and Probing Host-Virus Interactions in Gut Microbiome by Proximity Ligation (Chromosome Capture) Assay. Sci. Adv. 2017, 3, e1602105. [Google Scholar] [CrossRef]
- Yaffe, E.; Relman, D.A. Tracking Microbial Evolution in the Human Gut Using Hi-C Reveals Extensive Horizontal Gene Transfer, Persistence and Adaptation. Nat. Microbiol. 2020, 5, 343–353. [Google Scholar] [CrossRef]
- Labonté, J.M.; Swan, B.K.; Poulos, B.; Luo, H.; Koren, S.; Hallam, S.J.; Sullivan, M.B.; Woyke, T.; Eric Wommack, K.; Stepanauskas, R. Single-Cell Genomics-Based Analysis of Virus–Host Interactions in Marine Surface Bacterioplankton. ISME J. 2015, 9, 2386–2399. [Google Scholar] [CrossRef] [PubMed]
- Swan, B.K.; Tupper, B.; Sczyrba, A.; Lauro, F.M.; Martinez-Garcia, M.; González, J.M.; Luo, H.; Wright, J.J.; Landry, Z.C.; Hanson, N.W.; et al. Prevalent Genome Streamlining and Latitudinal Divergence of Planktonic Bacteria in the Surface Ocean. Proc. Natl. Acad. Sci. USA 2013, 110, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Munson-McGee, J.H.; Peng, S.; Dewerff, S.; Stepanauskas, R.; Whitaker, R.J.; Weitz, J.S.; Young, M.J. A Virus or More in (Nearly) Every Cell: Ubiquitous Networks of Virus–Host Interactions in Extreme Environments. ISME J. 2018, 12, 1706–1714. [Google Scholar] [CrossRef]
- Martinez-Hernandez, F.; Fornas, O.; Lluesma Gomez, M.; Bolduc, B.; de la Cruz Peña, M.J.; Martínez, J.M.; Anton, J.; Gasol, J.M.; Rosselli, R.; Rodriguez-Valera, F.; et al. Single-Virus Genomics Reveals Hidden Cosmopolitan and Abundant Viruses. Nat. Commun. 2017, 8, 15892. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.Z.; Ishoey, T.; Novotny, M.A.; McLean, J.S.; Lasken, R.S.; Williamson, S.J. Single Virus Genomics: A New Tool for Virus Discovery. PLoS ONE 2011, 6, e17722. [Google Scholar] [CrossRef]
- de Jonge, P.A.; von Meijenfeldt, F.A.B.; Costa, A.R.; Nobrega, F.L.; Brouns, S.J.J.; Dutilh, B.E. Adsorption Sequencing as a Rapid Method to Link Environmental Bacteriophages to Hosts. iScience 2020, 23, 101439. [Google Scholar] [CrossRef]
- Deng, L.; Ignacio-Espinoza, J.C.; Gregory, A.C.; Poulos, B.T.; Weitz, J.S.; Hugenholtz, P.; Sullivan, M.B. Viral Tagging Reveals Discrete Populations in Synechococcus Viral Genome Sequence Space. Nature 2014, 513, 242–245. [Google Scholar] [CrossRef]
- Džunková, M.; Low, S.J.; Daly, J.N.; Deng, L.; Rinke, C.; Hugenholtz, P. Defining the Human Gut Host–Phage Network through Single-Cell Viral Tagging. Nat. Microbiol. 2019, 4, 2192–2203. [Google Scholar] [CrossRef]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef]
- Morella, N.M.; Yang, S.C.; Hernandez, C.A.; Koskella, B. Rapid Quantification of Bacteriophages and Their Bacterial Hosts in Vitro and in Vivo Using Droplet Digital PCR. J. Virol. Methods 2018, 259, 18–24. [Google Scholar] [CrossRef]
- Tadmor, A.D.; Ottesen, E.A.; Leadbetter, J.R.; Phillips, R. Probing Individual Environmental Bacteria for Viruses by Using Microfluidic Digital PCR. Science 2011, 333, 58–62. [Google Scholar] [CrossRef]
- Sakowski, E.G.; Arora-Williams, K.; Tian, F.; Zayed, A.A.; Zablocki, O.; Sullivan, M.B.; Preheim, S.P. Interaction Dynamics and Virus–Host Range for Estuarine Actinophages Captured by EpicPCR. Nat. Microbiol. 2021, 6, 630–642. [Google Scholar] [CrossRef]
- Spencer, S.J.; Tamminen, M.V.; Preheim, S.P.; Guo, M.T.; Briggs, A.W.; Brito, I.L.; Weitz, D.A.; Pitkänen, L.K.; Vigneault, F.; Virta, M.P.J.; et al. Massively Parallel Sequencing of Single Cells by EpicPCR Links Functional Genes with Phylogenetic Markers. ISME J. 2016, 10, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Bayfield, O.; Shkoporov, A.; Yutin, N.; Khokhlova, E.; Smith, J.; Hawkins, D.; Koonin, E.; Hill, C.; Antson, A. Structural Atlas of the Most Abundant Human Gut Virus. Reseqrch Square 2022. [Google Scholar] [CrossRef]
- Barr, J.J.; Auro, R.; Furlan, M.; Whiteson, K.L.; Erb, M.L.; Pogliano, J.; Stotland, A.; Wolkowicz, R.; Cutting, A.S.; Doran, K.S.; et al. Bacteriophage Adhering to Mucus Provide a Non–Host-Derived Immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 10771–10776. [Google Scholar] [CrossRef] [PubMed]
- Knecht, L.E.; Veljkovic, M.; Fieseler, L. Diversity and Function of Phage Encoded Depolymerases. Front. Microbiol. 2020, 10, 2949. [Google Scholar] [CrossRef] [PubMed]
- Siranosian, B.A.; Tamburini, F.B.; Sherlock, G.; Bhatt, A.S. Acquisition, Transmission and Strain Diversity of Human Gut-Colonizing CrAss-like Phages. Nat. Commun. 2020, 11, 280. [Google Scholar] [CrossRef]
- Wittmann, J.; Turner, D.; Millard, A.D.; Mahadevan, P.; Kropinski, A.M.; Adriaenssens, E.M. From Orphan Phage to a Proposed New Family–The Diversity of N4-Like Viruses. Antibiotics 2020, 9, 663. [Google Scholar] [CrossRef]
- Choi, K.; McPartland, J.; Kaganman, I.; Bowman, V.D.; Rothman-Denes, L.; Rossmann, M.G. Insight into DNA and Protein Transport in Double-Stranded DNA Viruses: The Structure of Bacteriophage N4. Microsc. Microanal. 2008, 14, 1574–1575. [Google Scholar] [CrossRef]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The Long-Term Stability of the Human Gut Microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Garmaeva, S.; Kurilshikov, A.; Vich Vila, A.; Gacesa, R.; Sinha, T.; Segal, E.; Weersma, R.K.; Wijmenga, C.; et al. The Long-Term Genetic Stability and Individual Specificity of the Human Gut Microbiome. Cell 2021, 184, 2302–2315.e12. [Google Scholar] [CrossRef]
- Minot, S.; Bryson, A.; Chehoud, C.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Rapid Evolution of the Human Gut Virome. Proc. Natl. Acad. Sci. USA 2013, 110, 12450–12455. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic Modulation of the Gut Microbiota and Metabolome by Bacteriophages in a Mouse Model. Cell Host Microbe 2019, 25, 803–814.e5. [Google Scholar] [CrossRef]
- Porter, N.T.; Hryckowian, A.J.; Merrill, B.D.; Fuentes, J.J.; Gardner, J.O.; Glowacki, R.W.P.; Singh, S.; Crawford, R.D.; Snitkin, E.S.; Sonnenburg, J.L.; et al. Phase-Variable Capsular Polysaccharides and Lipoproteins Modify Bacteriophage Susceptibility in Bacteroides thetaiotaomicron. Nat. Microbiol. 2020, 5, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.T.; Canales, P.; Peterson, D.A.; Martens, E.C. A Subset of Polysaccharide Capsules in the Human Symbiont Bacteroides thetaiotaomicron Promote Increased Competitive Fitness in the Mouse Gut. Cell Host Microbe 2017, 22, 494–506.e8. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xiao, H.; Wang, L.; Wang, X.; Tan, Z.; Han, Z.; Li, X.; Yang, F.; Liu, Z.; Song, J.; et al. Structural Changes in Bacteriophage T7 upon Receptor-Induced Genome Ejection. Proc. Natl. Acad. Sci. USA 2021, 118, e2102003118. [Google Scholar] [CrossRef]
- Drobysheva, A.V.; Panafidina, S.A.; Kolesnik, M.V.; Klimuk, E.I.; Minakhin, L.; Yakunina, M.V.; Borukhov, S.; Nilsson, E.; Holmfeldt, K.; Yutin, N.; et al. Structure and Function of Virion RNA Polymerase of a CrAss-like Phage. Nature 2021, 589, 306–309. [Google Scholar] [CrossRef]
- Lourenço, M.; Chaffringeon, L.; Lamy-Besnier, Q.; Pédron, T.; Campagne, P.; Eberl, C.; Bérard, M.; Stecher, B.; Debarbieux, L.; De Sordi, L. The Spatial Heterogeneity of the Gut Limits Predation and Fosters Coexistence of Bacteria and Bacteriophages. Cell Host Microbe 2020, 28, 390–401.e5. [Google Scholar] [CrossRef]
- Heilmann, S.; Sneppen, K.; Krishna, S. Coexistence of Phage and Bacteria on the Boundary of Self-Organized Refuges. Proc. Natl. Acad. Sci. USA 2012, 109, 12828–12833. [Google Scholar] [CrossRef]
- Maslov, S.; Sneppen, K. Population Cycles and Species Diversity in Dynamic Kill-the-Winner Model of Microbial Ecosystems. Sci. Rep. 2017, 7, 39642. [Google Scholar] [CrossRef] [PubMed]
- Voigt, E.; Rall, B.C.; Chatzinotas, A.; Brose, U.; Rosenbaum, B. Phage Strategies Facilitate Bacterial Coexistence under Environmental Variability. PeerJ 2021, 9, e12194. [Google Scholar] [CrossRef]
- Koskella, B.; Brockhurst, M.A. Bacteria-Phage Coevolution as a Driver of Ecological and Evolutionary Processes in Microbial Communities. FEMS Microbiol. Rev. 2014, 38, 916–931. [Google Scholar] [CrossRef] [PubMed]
- De Sordi, L.; Lourenço, M.; Debarbieux, L. “I Will Survive”: A Tale of Bacteriophage-Bacteria Coevolution in the Gut. Gut Microbes 2019, 10, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, J.; Krupovic, M.; Koonin, E.V. The Double-Stranded DNA Virosphere as a Modular Hierarchical Network of Gene Sharing. Mbio 2016, 7, e00978-16. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).