Various Expressions of PIK3C2A and TXNIP Genes and Their Potential Role as Independent Risk Factors for Chronic Stable Angina and Acute Coronary Syndrome
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Subjects
2.2. Inclusion and Exclusion Criteria
2.3. Blood Sample Collection and Processing
2.4. Biochemical Investigations
2.5. RNA Isolation and Real-Time Quantitative PCR (qRT-PCR)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, S.; Jiang, J.; Zhang, Y.; Chen, T.; Zhu, M.; Fang, C.; Mi, Y. Discovery of potential plasma protein biomarkers for acute myocardial infarction via proteomics. J. Thorac. Dis. 2019, 11, 3962–3972. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Bays, H.; Agarwala, A.; German, C.; Satish, P.; Iluyomade, A.; Dudum, R.; Thakkar, A.; Al Rifai, M.; Mehta, A.; Thobani, A.; et al. Ten things to know about ten cardiovascular disease risk factors—2022. Am. J. Prev. Cardiol. 2022, 10, 100342. [Google Scholar] [CrossRef]
- Turi, V.; Iurciuc, S.; Crețu, O.M.; Tit, D.M.; Bungau, S.; Apostol, A.; Moleriu, R.D.; Bustea, C.; Behl, T.; Diaconu, C.C.; et al. Arterial function in hypertensive pregnant women. Is arterial stiffness a marker for the outcomes in pregnancy? Life Sci. 2021, 264, 118723. [Google Scholar] [CrossRef]
- Shah, N.; Kelly, A.M.; Cox, N.; Wong, C.; Soon, K. Myocardial infarction in the “young”: Risk factors, presentation, management, and prognosis. Heart Lung Circ. 2016, 25, 955–960. [Google Scholar] [CrossRef]
- Arenberg, M.E.; Risch, N.; Berkman, L.F.; Floderus, B.; de Faire, U. Genetic susceptibility to death from coronary heart disease in a study of twins. N. Engl. J. Med. 1994, 330, 1041–1046. [Google Scholar] [CrossRef]
- Zdravkovic, S.; Wienke, A.; Pedersen, N.L.; Marenberg, M.E.; Yashin, A.I.; De Faire, U. Heritability of death from coronary heart disease: A 36-year follow-up of 20 966 Swedish twins. J. Intern. Med. 2002, 252, 247–254. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Wang, S.; Cheng, H.; Xu, L.; Pei, G.; Wang, Y.; Fu, C.; Jiang, Y.; He, C.; et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct. Target. Ther. 2022, 7, 78. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, J.M.; Posadas-Sánchez, R.; Blachman-Braun, R.; Vargas-Alarcón, G.; Posadas-Romero, C.; Rodríguez-Cortés, A.A.; López-Bautista, F.; Tovilla-Zárate, C.A.; Rojas-Toledo, E.X.; Borgonio-Cuadra, V.M.; et al. HHIPL-1 (rs2895811) gene polymorphism is associated with cardiovascular risk factors and cardiometabolic parameters in Mexicans patients with myocardial infarction. Gene 2018, 663, 34–40. [Google Scholar] [CrossRef]
- Catapano, A.L.; Lautsch, D.; Tokgözoglu, L.; Ferrieres, J.; Horack, M.; Farnier, M.; Toth, P.P.; Brudi, P.; Tomassini, J.E.; Ambegaonkar, B.; et al. Prevalence of potential familial hypercholesterolemia (FH) in 54,811 statin-treated patients in clinical practice. Atherosclerosis 2016, 252, 1–8. [Google Scholar] [CrossRef]
- Karakas, B.; Bachman, K.E.; Park, B.H. Mutation of the PIK3CA oncogene in human cancers. Br. J. Cancer 2006, 94, 455–459. [Google Scholar] [CrossRef]
- Cantley, L.C. The Phosphoinositide 3-Kinase Pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, Y.; Chen, Y.; Xiang, Y.; Xie, Y. Role of the microRNA, miR-206, and its target PIK3C2a in endothelial progenitor cell function—Potential link with coronary artery disease. FEBS J. 2015, 282, 3758–3772. [Google Scholar] [CrossRef] [PubMed]
- Mountford, J.K.; Petitjean, C.; Putra, H.W.K.; McCafferty, J.A.; Setiabakti, N.M.; Lee, H.; Tønnesen, L.L.; McFadyen, J.D.; Schoenwaelder, S.M.; Eckly, A.; et al. The class II PI 3-kinase, PI3KC2α, links platelet internal membrane structure to shear-dependent adhesive function. Nat. Commun. 2015, 6, 6535. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Yoshida, K.; Cui, H.; Wakayama, T.; Takuwa, N.; Okamoto, Y.; Du, W.; Qi, X.; Asanuma, K.; Sugihara, K.; et al. Endothelial PI3K-C2α, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat. Med. 2012, 18, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Margaria, J.P.; Ratto, E.; Gozzelino, L.; Li, H.; Hirsch, E. Class II PI3Ks at the Intersection between Signal Transduction and Membrane Trafficking. Biomolecules 2019, 9, 104. [Google Scholar] [CrossRef]
- Mohamed, I.; Hafez, S.S.; Fairaq, A.; Ergul, A.; Imig, J.; El-Remessy, A.B. Thioredoxin-interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high-fat diet. Diabetologia 2013, 57, 413–423. [Google Scholar] [CrossRef]
- Patwari, P.; Higgins, L.J.; Chutkow, W.A.; Yoshioka, J.; Lee, R.T. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006, 281, 21884–21891. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.-H.; Chen, X.-Y.; Jian-Hua, Z.; Wang, M.-X.; Jin-Sheng, L.; Zhang, Q.-Y.; Wang, W.; Wang, R.; Kang, L.-L.; et al. Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid. Redox Signal. 2015, 22, 848–870. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar]
- Steg, P.G.; James, S.K.; Atar, D.; Badano, L.P.; Blömstrom-Lundqvist, C.; Borger, M.A.; Di Mario, C.; Dickstein, K.; Ducrocq, G.; Fernandez-Aviles, F.; et al. ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur. Heart J. 2012, 33, 2569–2619. [Google Scholar] [PubMed]
- Gibbons, R.J.; Abrams, J.; Chatterjee, K.; Daley, J.; Deedwania, P.C.; Douglas, J.S.; Ferguson, T.B., Jr.; Fihn, S.D.; Fraker, T.D., Jr.; Gardin, J.M.; et al. ACC/AHA 2002 Guideline Update for the Management of Patients with Chronic Stable Angina: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for the Management of Patients with Chronic Stable Angina). Available online: http://www.acc.org/qualityandscience/clinical/guidelines/stable/stable_clean.pdf (accessed on 14 November 2007).
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Xu, X.; Xiang, Y.; Yang, G.; Ming, X.; He, S.; Liang, B.; Zhang, X.; Zheng, F. Thioredoxin-interacting protein promotes activation and inflammation of monocytes with DNA demethylation in coronary artery disease. J. Cell. Mol. Med. 2020, 24, 3560–3571. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Prevalence of coronary heart disease–United States, 2006–2010. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 1377–1381. [Google Scholar]
- Prassl, R.; Laggner, P. Molecular structure of low density lipoprotein: Current status and future challenges. Eur. Biophys. J. 2008, 38, 145–158. [Google Scholar] [CrossRef]
- Tan, B.; Liu, M.; Yang, Y.; Liu, L.; Meng, F. Low expression of PIK3C2A gene. Medicine 2019, 98, e15061. [Google Scholar] [CrossRef]
- Xiaoyu, L.; Wei, Z.; Ming, Z.; Jia, G. Anti-apoptotic Effect of MiR-223-3p Suppressing PIK3C2A in Cardiomyocytes from Myocardial Infarction Rat Through Regulating PI3K/Akt Signaling Pathway. Cardiovasc. Toxicol. 2021, 21, 669–682. [Google Scholar] [CrossRef]
- Wang, B.F.; Yoshioka, J. The Emerging Role of Thioredoxin-Interacting Protein in Myocardial Ischemia/Reperfusion Injury. J. Cardiovasc. Pharmacol. Ther. 2016, 22, 219–229. [Google Scholar] [CrossRef]
- Bedarida, T.; Domingues, A.; Baron, S.; Ferreira, C.; Vibert, F.; Cottart, C.H.; Paul, J.L.; Escriou, V.; Bigey, P.; Gaussem, P.; et al. Reduced endothelial thioredoxin-interacting protein protects arteries from damage induced by metabolic stress in vivo. FASEB J. 2018, 32, 3108–3118. [Google Scholar] [CrossRef]
- Neidhardt, S.; Garbade, J.; Emrich, F.; Klaeske, K.; Borger, M.A.; Lehmann, S.; Jawad, K.; Dieterlen, M.-T. Ischemic Cardiomyopathy Affects the Thioredoxin System in the Human Myocardium. J. Card. Fail. 2019, 25, 204–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, L.-S.; Cai, Y.-M.; He, J.-X.; Zhang, Z.-N.; Wu, G.; Zheng, J. TXNIP knockdown alleviates hepatocyte ischemia reperfusion injury through preventing p38/JNK pathway activation. Biochem. Biophys. Res. Commun. 2018, 502, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, J.; Yang, X.; Sun, X.; Xu, Q.; Wang, B.; Zhong, P.; Wei, Z. Altered Expression of TXNIP in the peripheral leukocytes of patients with coronary atherosclerotic heart disease. Medicine 2017, 96, e9108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lian, K.; Zhang, L.; Wang, R.; Yi, F.; Gao, C.; Xin, C.; Zhu, D.; Li, Y.; Yan, W.; et al. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res. Cardiol. 2014, 109, 415. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, S.; Chang, S.; Ren, D.; Shali, S.; Li, C.; Yang, H.; Huang, Z.; Ge, J. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-κB/NLRP3 inflammasome signaling pathway. J. Mol. Cell. Cardiol. 2020, 142, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhong, P.; Xu, Y.; Wang, B.; Zhu, T.; Zhang, W.; Wang, H.; Wei, Z.; Huang, J. Differential Expression of TXNIP Isoforms in the Peripheral Leukocytes of Patients with Acute Myocardial Infarction. Dis. Markers 2018, 2018, 9051481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Tang, S. Retrospective analysis of the relationship between elevated plasma levels of TXNIP and carotid intima-media thickness in subjects with impaired glucose tolerance and early Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2015, 109, 372–377. [Google Scholar] [CrossRef]
- Domingues, A.; Jolibois, J.; de Rougé, P.M.; Nivet-Antoine, V. The Emerging Role of TXNIP in Ischemic and Cardiovascular Diseases; A Novel Marker and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 1693. [Google Scholar] [CrossRef]
- Cubukcu, A.; Murray, I.; Anderson, S. What’s the risk? Assessment of patients with stable chest pain. Echo Res. Pract. 2015, 2, 41–48. [Google Scholar] [CrossRef]
- Saeed, M.; Stene, L.C.; Ariansen, I.; Tell, G.S.; Tapia, G.; Joner, G.; Skrivarhaug, T. Nine-fold higher risk of acute myocardial infarction in subjects with type 1 diabetes compared to controls in Norway 1973–2017. Cardiovasc. Diabetol. 2022, 21, 59. [Google Scholar] [CrossRef]
- Yao, H.; Farnier, M.; Tribouillard, L.; Chague, F.; Brunel, P.; Maza, M.; Brunet, D.; Rochette, L.; Bichat, F.; Cottin, Y.; et al. Coronary lesion complexity in patients with heterozygous familial hypercholesterolemia hospitalized for acute myocardial infarction: Data from the RICO survey. Lipids Health Dis. 2021, 20, 45. [Google Scholar] [CrossRef]
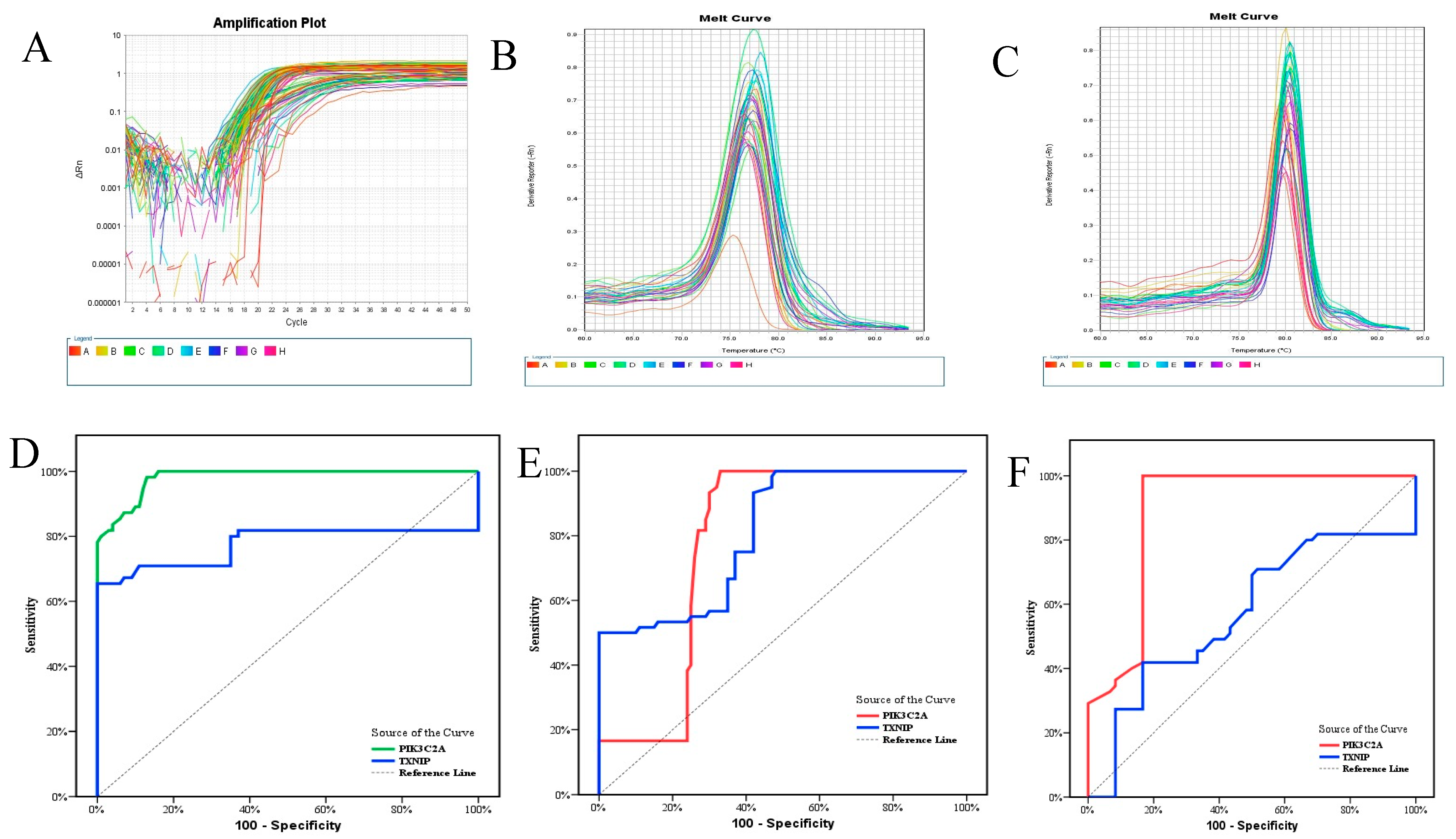
| Group I (n = 100) | Group II (n = 60) | Group III (n = 55) | Test of Sig. | p | Significance between Groups | |||
|---|---|---|---|---|---|---|---|---|
| I vs. II | I vs. III | II vs. III | ||||||
| Gender | ||||||||
| Male | 76 (76%) | 45 (75%) | 47 (85.5%) | χ2 = 2.337 | 0.3 | NS | NS | NS |
| Female | 24 (24%) | 15 (25%) | 8 (14.5%) | |||||
| Age (years) | ||||||||
| Mean ± SD | 54.6 ± 6 | 53.7 ± 7.7 | 56.7 ± 9.4 | F = 2.494 | 0.9 | NS | NS | NS |
| BMI | ||||||||
| Mean ± SD | 28.6 ± 3.6 | 29.3 ± 4.5 | 30.4 ± 3.5 | F = 3.785 * | 0.02 * | 0.488 | 0.018 * | 0.308 |
| Smoking No Smoker Ex-smoker | 69 (69%) 21 (21%) 10 (10%) | 20 (33.3%) 20 (33.3%) 20 (33.3%) | 13 (23.6%) 25 (45.5%) 17 (30.9%) | χ2 = 38.092 | <0.001 * | <0.001 * | <0.001 * | 0.355 |
| DM | 10 (10%) | 20 (33.3%) | 18 (32.7%) | χ2 = 16.385 * | <0.001 * | <0.001 * | <0.001 * | 0.945 |
| HTN | 10 (10%) | 25 (41.7%) | 30 (54.5%) | χ2 = 38.540 * | <0.001 * | <0.001 * | <0.001 * | 0.167 |
| Previous MI | 0 (0%) | 40 (66.7%) | 5 (9.1%) | χ2 = 106.968 * | <0.001 * | <0.001 * | FEp = 0.005 * | <0.001 * |
| CA | ||||||||
| Normal | 100 (100%) | 0 (0%) | 0 (0%) | χ2 = 339.658 * | MCp < 0.001 * | MCp < 0.001 * | <0.001 * | MCp < 0.001 * |
| 1VD | 0 (0%) | 10 (16.7%) | 22 (40%) | |||||
| 2VD | 0 (0%) | 10 (16.7%) | 23 (41.8%) | |||||
| 3VD | 0 (0%) | 25 (41.7%) | 10 (18.2%) | |||||
| Presentation | ||||||||
| CSA | 0 (0%) | 60 (100%) | 0 (0%) | χ2 = 430.0 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| NSTEMI | 0 (0%) | 0 (0%) | 15 (27.3%) | |||||
| STEMI | 0 (0%) | 0 (0%) | 40 (72.7%) | |||||
| EF | ||||||||
| Mean ± SD | 61.2 ± 1 | 56.5 ± 11.4 | 50.9 ± 7.2 | F = 36.2 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| WMAS | ||||||||
| No | 100 (100%) | 10 (16.7%) | 0 (0%) | χ2 = 181.65 * | <0.001 * | <0.001 * | <0.001 * | FEp = 0.001 * |
| Yes | 0 (0%) | 50 (83.3%) | 55 (100%) | |||||
| Systolic BP | ||||||||
| Mean ± SD | 127 ± 7 | 127.5 ± 20.2 | 126.5 ± 14 | F = 0.07 | 0.9 | NS | NS | NS |
| Diastolic BP | ||||||||
| Mean ± SD | 85.8 ± 4.8 | 84.2 ± 12.1 | 86.5 ± 7.6 | F = 1.2 | 0.3 | NS | NS | NS |
| Pulse | ||||||||
| Mean ± SD | 91.1 ± 3.3 | 76.4 ± 10.3 | 86.5 ± 3.3 | F = 108.5 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| Group I (n = 100) | Group II (n = 60) | Group III (n = 55) | Test of Sig. | p | Significance between Groups | |||
|---|---|---|---|---|---|---|---|---|
| I vs. II | I vs. III | II vs. III | ||||||
| T. cholesterol (mg/dL) | ||||||||
| Mean ± SD | 160.7 ± 6.4 | 185.9 ± 10.7 | 181.5 ± 8.6 | F = 207.7 * | <0.001 * | <0.001 * | <0.001 * | 0.013 * |
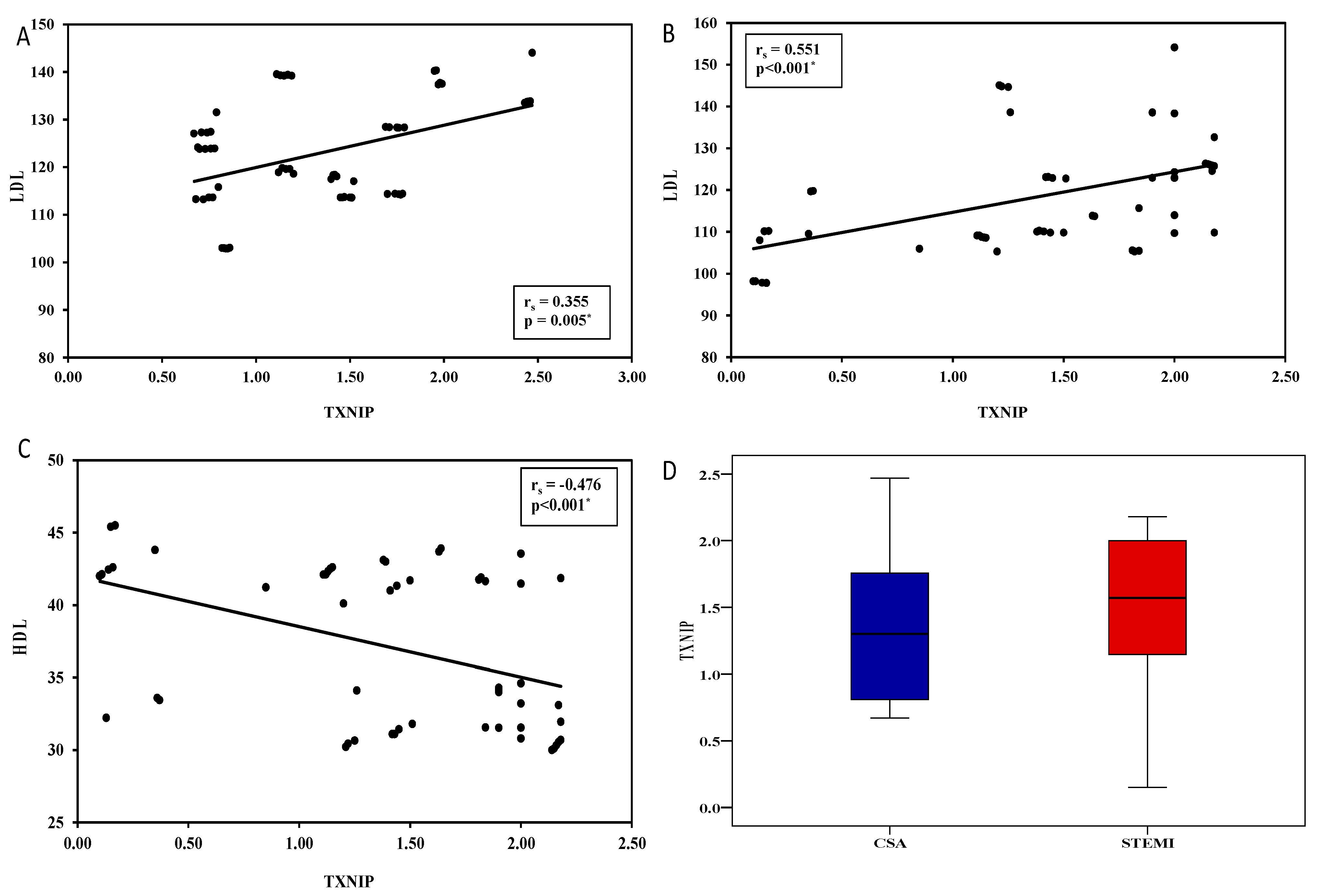
| LDLc (mg/dL) | ||||||||
| Mean ± SD | 89.2 ± 6.4 | 123 ± 11 | 118.6 ± 13.6 | F = 272.2 * | <0.001 * | <0.001 * | <0.001 * | 0.048 * |
| HDLc (mg/dL) | ||||||||
| Mean ± SD | 51.1 ± 1.9 | 39.9 ± 5.2 | 37.1 ± 5.5 | F = 256.3 * | <0.001 * | <0.001 * | <0.001 * | 0.001 * |
| TGS (mg/dL) | ||||||||
| Mean ± SD | 102 ± 7.3 | 114.9 ± 15.4 | 129 ± 18.8 | F = 72.2 | <0.001 * | <0.001 * | <0.001 * | 0.001 * |
| VLDLc (mg/dL) | ||||||||
| Mean ± SD | 20.4 ± 1.5 | 23 ±3.1 | 25.8 ± 3.8 | F = 72.2 | <0.001 * | <0.001 * | <0.001 * | 0.001 * |
| Hypercholesterolemia Yes No | 0 (0%) 100 (100%) | 35 (58.3%) 25 (41.7%) | 28 (50.9%) 27 (49.1%) | χ2 = 78.3 | <0.001 * | <0.001 * | <0.001 * | 0.424 |
| Troponin Negative Positive | 100 (100%) 0 (0%) | 60 (100%) 0 (0%) | 0 (0%) 55 (100%) | χ2 = 215.0 * | <0.001 * | – | <0.001 * | <0.001 * |
| HB (gm/dL) | ||||||||
| Mean ± SD | 14.7 ± 0.8 | 13.8 ± 1 | 12.9 ± 2.2 | F = 29.99 * | <0.001 * | <0.001 * | <0.001 * | 0.003 * |
| PIK3C2A | ||||||||
| Mean ± SD | 0.793 ± 0.6 | 0.218 ± 0.1 | 0.054 ± 0.03 | H = 122.4 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * |
| Median (Min.–Max.) | 0.675 (0.08–1.77) | 0.26 (0.04–0.38) | 0.056 (0.01–0.093) | |||||
| TXNIP | ||||||||
| Mean ± SD | 0.8 ± 0.3 | 1.3 ± 0.5 | 1.4 ± 0.7 | H = 55.6 * | <0.001 * | <0.001 * | <0.001 * | 0.700 |
| Median (Min.–Max.) | 0.6 (0.4–1.2) | 1.3 (0.7–2.5) | 1.5 (0.1–2.2) | |||||
| CSA | ACS | |||||
|---|---|---|---|---|---|---|
| N | PIK3C2A | TXNIP | N | PIK3C2A | TXNIP | |
| Median (Min.–Max.) | Median (Min.–Max.) | Median (Min.–Max.) | Median (Min.–Max.) | |||
| Gender | ||||||
| Male | 45 | 0.26 (0.04–0.38) | 1.42 (0.67–2.47) | 47 | 0.05 (0.01–0.09) | 1.41 (0.10–2.18) |
| Female | 15 | 0.26 (0.05–0.38) | 0.77 (0.68–1.79) | 8 | 0.08 (0.07–0.09) | 1.71 (1.42–2.18) |
| U(p) | 320.50 (0.771) | 187.0 * (0.010 *) | 52.0 * (0.001 *) | 122.50 (0.119) | ||
| DM | ||||||
| No | 40 | 0.26 (0.04–0.38) | 1.30 (0.67–1.99) | 37 | 0.05 (0.01–0.09) | 1.81 (0.15–2.18) |
| Yes | 20 | 0.18 (0.04–0.37) | 1.45 (0.68–2.47) | 18 | 0.06 (0.01–0.09) | 1.39 (0.10–2.18) |
| U(p) | 316.0 (0.186) | 320.50 (0.213) | 310.50 (0.686) | 206.50 * (0.023 *) | ||
| HTN | ||||||
| No | 35 | 0.25 (0.04–0.38) | 1.15 (0.67–1.78) | 25 | 0.05 (0.01–0.09) | 1.25 (0.10–2.18) |
| Yes | 25 | 0.27 (0.04–0.38) | 1.75 (0.69–2.47) | 30 | 0.07 (0.01–0.09) | 1.90 (0.15–2.18) |
| U(p) | 434.0 (0.958) | 252.0 * (0.005 *) | 268.0 (0.070) | 208.0 *(0.005 *) | ||
| Smoking | ||||||
| No | 20 | 0.21 (0.04–0.38) | 1.24 (0.68–1.99) | 13 | 0.08 (0.05–0.09) | 1.43 (0.10–2.18) |
| Smoker | 20 | 0.21 (0.04–0.38) | 1.33 (0.67–1.78) | 25 | 0.05 (0.01–0.09) | 1.82 (0.85–2.18) |
| Ex-smoker | 20 | 0.28 (0.16–0.38) | 1.30 (0.82–2.47) | 17 | 0.03 (0.01–0.08) | 1.25 (0.15–2.00) |
| H(p) | 8.433 (0.015 *) | 1.049 (0.592) | 8.992 (0.011 *) | 6.647 (0.036 *) | ||
| Presentation | ||||||
| CSA | 60 | 0.26 (0.04–0.38) | 1.30 (0.67–2.47) | 0 | – | – |
| NSTEMI | 0 | – | – | 15 | 0.06 (0.02–0.08) | 1.22 (0.10–2.00) |
| STEMI | 0 | – | – | 40 | 0.06 (0.01–0.09) | 1.57 (0.15–2.18) |
| CHEST PAIN | 0 | – | – | 0 | – | – |
| H(p) | – | – | 259.0 (0.438) | 194.50 * (0.046 *) | ||
| WMAS | ||||||
| No | 10 | 0.28 (0.19–0.37) | 1.65 (0.82–2.47) | 0 | – | – |
| Yes | 50 | 0.24 (0.04–0.38) | 1.30 (0.67–1.99) | 55 | 0.06 (0.01–0.09) | 1.45 (0.10–2.18) |
| U(p) | 137.50 (0.025 *) | 175.0 (0.137) | – | – | ||
| Hypercholesterolemia | ||||||
| Hypercholesterolemia | 35 | 0.27 (0.04–0.38) | 1.71 (0.69–2.47) | 28 | 0.06 (0.01–0.09) | 1.90 (0.13–2.18) |
| No-hypercholesterolemia | 25 | 0.25 (0.04–0.28) | 0.85 (0.67–1.52) | 27 | 0.06 (0.01–0.09) | 1.20 (0.10–2.18) |
| U(p) | 351.50 (0.655) | 185.50 (0.001 *) | 351.50 (0.655) | 185.50 (0.001 *) | ||
| Troponin | ||||||
| Negative | 60 | 0.26 (0.04–0.38) | 1.30 (0.67–2.47) | 0 | – | – |
| Positive | – | – | – | 55 | 0.06 (0.01–0.09) | 1.45 (0.10–2.18) |
| U(p) | – | – | – | – | ||
| CA | ||||||
| MVD | 25 | 0.26 (0.04–0.38) | 1.50 (0.68–2.47) | 10 | 0.05 (0.02–0.08) | 1.58 (1.20–2.00) |
| 1VD | 25 | 0.24 (0.04–0.38) | 1.13 (0.67–1.99) | 22 | 0.06 (0.04–0.09) | 1.57 (0.15–2.18) |
| 2VD | 10 | 0.27 (0.05–0.38) | 1.24 (0.69–1.79) | 23 | 0.06 (0.01–0.09) | 1.43 (0.10–2.18) |
| H(p) | 2.010 (0.366) | 5.475 (0.065) | 2.055 (0.358) | 0.171 (0.918) | ||
| AUC | p | 95%CI | Cut off | Sensitivity | Specificity | PV | NPV | |
|---|---|---|---|---|---|---|---|---|
| Acute coronary syndrome from control | ||||||||
| PIK3C2A | 0.981 | <0.001 * | 0.966–0.996 | ≤0.091 | 98.18 | 87.0 | 80.6 | 98.9 |
| TXNIP | 0.775 | <0.001 * | 0.673–0.876 | >1.195 | 70.91 | 89.0 | 78.0 | 84.8 |
| Acute coronary syndrome from chronic stable angina | ||||||||
| PIK3C2A | 0.892 | <0.001 * | 0.828–0.957 | 0.081 | 81.82 | 83.33 | 81.8 | 83.3 |
| TXNIP | 0.572 | 0.184 | 0.464–0.680 | >1.19 | 70.91 | 48.33 | 55.7 | 64.4 |
| PIK3C2A + TXNIP | 0.893 | <0.001 * | 0.829–0.957 | 98.18 | 83.33 | 84.4 | 98.0 | |
| Chronic stable angina from control | ||||||||
| PIK3C2A | 0.782 | <0.001 * | 0.708–0.856 | ≤0.28 | 81.67 | 73.0 | 64.5 | 86.9 |
| TXNIP | 0.813 | <0.001 * | 0.747–0.879 | >0.7 | 93.33 | 58.0 | 57.1 | 93.5 |
| PIK3C2A + TXNIP | 0.963 | <0.001 * | 0.939–0.987 | 95.0 | 80.0 | 74.0 | 96.4 | |
| Chronic Stable Angina Patients (n = 60) from Controls (n = 100). | ||||
|---|---|---|---|---|
| Univariate | #Multivariate | |||
| p | OR (95%CI) | p | OR (95%CI) | |
| PIK3C2A @1 | <0.001 * | 0.958 (0.941–0.976) | <0.001 * | 0.994 (0.991–0.997) |
| TXNIP @2 | <0.001 * | 1.362 (1.228–1.510) | <0.001 * | 2.635 (1.729–4.016) |
| Body weight | <0.001 * | 1.146 (1.084–1.211) | <0.001 * | 1.187 (1.085–1.299) |
| acute coronary syndrome patients (n = 55) from chronic stable angina patients (n = 60). | ||||
| p | OR (95%CI) | p | OR (95%CI) | |
| PIK3C2A | <0.001 | 0.728 (0.639–0.829) | <0.001 * | 0.706 (0.614–0.812) |
| Hb | 0.012 * | 0.733 (0.575–0.935) | 0.013 * | 0.525 (0.317–0.871) |
| T. Cholesterol | 0.019 * | 0.954 (0.917–0.992) | 0.004 * | 0.865 (0.784–0.955) |
| acute coronary syndrome patients (n = 55) from controls (n = 100). | ||||
| p | OR (95%CI) | p | OR (95%CI) | |
| DM | 0.001 * | 4.378 (1.848–10.374) | 0.131 | 0.248 (0.041–1.515) |
| Smoking | <0.001 * | 7.191 (3.388–15.265 | 0.034 * | 6.623 (1.155–37.971) |
| PIK3C2A@1 | <0.001 * | 0.175 (0.056–0.462) | 0.002 * | 0.118 (0.031–0.445 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, S.E.; Abouelenin, M.A.H.; Samy, N.I.; Omar, M.M.; Alrefai, A.A. Various Expressions of PIK3C2A and TXNIP Genes and Their Potential Role as Independent Risk Factors for Chronic Stable Angina and Acute Coronary Syndrome. Biomolecules 2023, 13, 302. https://doi.org/10.3390/biom13020302
Soliman SE, Abouelenin MAH, Samy NI, Omar MM, Alrefai AA. Various Expressions of PIK3C2A and TXNIP Genes and Their Potential Role as Independent Risk Factors for Chronic Stable Angina and Acute Coronary Syndrome. Biomolecules. 2023; 13(2):302. https://doi.org/10.3390/biom13020302
Chicago/Turabian StyleSoliman, Shimaa E., Mai A. H. Abouelenin, Neven I. Samy, Marwa M. Omar, and Abeer A. Alrefai. 2023. "Various Expressions of PIK3C2A and TXNIP Genes and Their Potential Role as Independent Risk Factors for Chronic Stable Angina and Acute Coronary Syndrome" Biomolecules 13, no. 2: 302. https://doi.org/10.3390/biom13020302
APA StyleSoliman, S. E., Abouelenin, M. A. H., Samy, N. I., Omar, M. M., & Alrefai, A. A. (2023). Various Expressions of PIK3C2A and TXNIP Genes and Their Potential Role as Independent Risk Factors for Chronic Stable Angina and Acute Coronary Syndrome. Biomolecules, 13(2), 302. https://doi.org/10.3390/biom13020302






