Abstract
cis,cis-Muconic acid (MA) is a valuable C6 dicarboxylic acid platform chemical that is used as a starting material for the production of various valuable polymers and drugs, including adipic acid and terephthalic acid. As an alternative to traditional chemical processes, bio-based MA production has progressed to the establishment of de novo MA pathways in several microorganisms, such as Escherichia coli, Corynebacterium glutamicum, Pseudomonas putida, and Saccharomyces cerevisiae. Redesign of the metabolic pathway, intermediate flux control, and culture process optimization were all pursued to maximize the microbial MA production yield. Recently, MA production from biomass, such as the aromatic polymer lignin, has also attracted attention from researchers focusing on microbes that are tolerant to aromatic compounds. This paper summarizes recent microbial MA production strategies that involve engineering the metabolic pathway genes as well as the heterologous expression of some foreign genes involved in MA biosynthesis. Microbial MA production will continue to play a vital role in the field of bio-refineries and a feasible way to complement various petrochemical-based chemical processes.
1. Introduction
cis, cis-Muconic acid (MA) is a six-carbon (C6) compound with two carboxylic functional groups at both ends and two double bonds in the middle [Figure 1]. A report published by Research and Market in 2019 showed that the turnover of the MA market was US$ 79.6 million in 2018, which is expected to be US$ 119.4 million in 2024 [1]. MA in the presence of catalysts can be converted to valuable industrial chemicals, such as adipic acid or caprolactam. In particular, adipic acid is a high-demand bulk intermediate chemical that can produce nylon-6,6, terephthalic acid (TPA), and polyurethanes. MA-driven intermediates, including adipic acid, are widely used in food additives, medicines, and cosmetics as well as in the textile industry [2]. On the other hand, the production of most of these polymers is petroleum-based. Specifically, the production of adipic acid produces numerous carcinogenic substances, such as cyclohexane, cyclohexanol, and cyclohexanone, via benzene, and causes environmental contamination problems. Recently, the production of benzene-free bio adipic acid through microbes has attracted attention to avoid these issues and meet the increasing demand for adipic acid. A key objective of related research is to secure economic feasibility by the production of high MA-producing microbial strains [2].
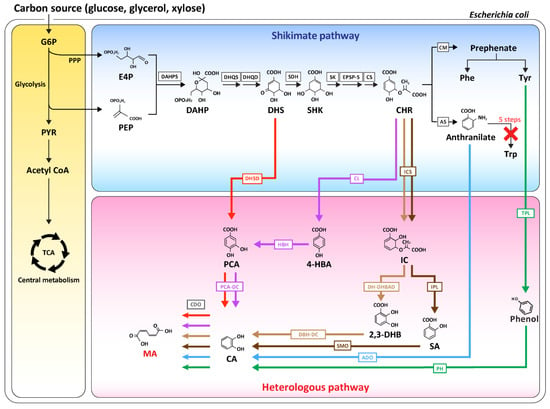
Figure 1.
Schematic overview of the metabolic pathway for MA biosynthesis in E. coli. The red arrow (pathway 1), purple arrow (pathway 2), tan arrow (pathway 3), brown arrow (pathway 4), blue arrow (pathway 5), and green arrow (pathway 6) represent MA biosynthetic pathways 1, 2, 3, 4, 5, and 6, respectively. G6P, glucose-6- phosphate; PPP, pentose phosphate pathway; PYR, pyruvate; PEP, phosphoenolpyruvate; E4P, erythrose4-phosphate; DAHP, 3-deoxy-D-arabinoheptulosonate-7-phosphate; DHS, 3-dehydroshikimate; SHK, shikimic acid; Phe, phenylalanine; Tyr, tyrosine; Trp, tryptophan; CHR, chorismate; 4-HBA, 4-hydroxybenzoate; IC, isochorismate; 2,3-DHB, 2,3-dihydroxybenzoate; SA, salicylate; PCA, protocatechuate; CA, catechol; MA, muconic acid. DAHPS, DAHP synthase; DHQS, DHQ synthase; DHQD, DHQ dehydratase; SDH, shikimate dehydrogenase; SK, shikimate kinase; EPSP-S, 5-enolpyruvylshikimate-3-phosphate synthase; CS, chorismate synthase; CM, chorismate mutase; AS, anthranilate synthase; DHSD, DHS dehydratase; PCA-DC, protocatechuate decarboxylase; CDO, catechol 1,2-dioxygenase; TPL, tyrosine phenol lyase; PH, phenol hydroxylase; CL, chorismate pyruvate-lyase; ICS, isochorismate synthase; IM, isochorismatase; DH-DHBAD, 2,3-dihydro-2,3-DHBA dehydrogenase; IPL, isochorismate pyruvate lyase; DBH-DC, 2,3-dihydroxybenzoate decarboxylase; SMO, salicylate 1-monoxygenase; ADO, anthranilate 1,2-dioxygenase.
Draths and Frost (1994) first reported bio-based MA production. Moreover, the stable production of MA could be achieved in an environment-friendly manner by the establishment of a de novo biosynthetic pathway from 3-dehydroshikimate (DHS) in the shikimate pathway using glucose as a carbon source in Escherichia coli [2,3]. Since then, MA biosynthesis has been conducted by many researchers via re-engineering the shikimate pathway [2,3,4,5]. The shikimate pathway can be applied using three routes: (i) the pathway that synthesizes MA by the utilization of DHS as a precursor, (ii) the pathway with chorismate as a starting intermediate, and (iii) the biosynthesis of MA from aromatic amino acids (tyrosine, phenylalanine, and tryptophan). In addition, high MA productivity was induced through additional pathway engineering, such as glycolysis or the pentose phosphate pathway to send the carbon flux inside the cell to the shikimate pathway [2,3,4,5].
In research on microbial MA production, E. coli has been used representatively for many decades, including most research results and industrial feasibility studies. On the other hand, with the accumulation of various genetic and culture technologies, MA production utilizing various microbial strains with several merits has become possible, and remarkable progress has been achieved. Therefore, along with studies focusing on E. coli, this review paper also summarizes the production of bio-based MA with Corynebacterium glutamicum, Klebsiella pneumoniae, and Pseudomonas putida.
2. MA Production from Escherichia coli
2.1. MA Biosynthesis Through Redesign of the Shikimate Pathway
Previous studies used MA production via microbial strains with a simple one- or two-step process by the addition of compounds containing phenol rings, such as benzoate or catechol [4,5,6]. On the other hand, E. coli allowed the production of a real bio-based target MA through total biosynthesis using various pathways with glucose as a starter. Because the entire biosynthetic pathways that produce MA are not available in E. coli, it was necessary to develop a new pathway through the insertion of heterogeneous genes. Niu et al. reported a benzene-free microbial MA synthesis process that did not use carcinogenic benzene or benzene-derived chemicals as the problematic feedstock in the production of adipic acid [3]. In addition, this process did not cause any environmental problems, such as the generation of nitrous oxide that was a byproduct during MA synthesis. They used the E. coli AB2834 strain, in which aroE (encodes shikimate dehydrogenase) was replaced with leaky aroE to use DHS, which accumulates in the shikimate pathway. The MA biosynthesis pathway was then established in E. coli AB2834 through the heterologous expression of three genes. First, the K. pneumoniae-derived aroZ gene (encodes DHS dehydratase) was integrated into the chromosome; the aroY gene (encodes protocatechuate decarboxylase) derived from K. pneumoniae, and the catA gene (encodes Catechol 1,2-dioxygenase) derived from Acinetobacter calcoaceticus were expressed as the plasmids to generate E. coli WNl/pWN2.248. This engineered E. coli strain produced 36.8 g/L of MA from glucose [3]. Additional fermentation process optimization (Pathway 1 in Figure 1) resulted in an increase in the production yield to 59.2 g/L of MA [7].
Several studies have examined MA production via chorismate, which is another intermediate in the shikimate pathway. A pathway for the production of 2,3-dihydroxybenzoate (2,3-DHB) from chorismate was established by the expression of the genes that code EntC (encodes isochorismate synthase), EntB (encodes isochorismatase A), and EntA (encodes 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase). For two heterogeneous genes required to proceed from 2.3-DHB to MA, 2,3-DHB decarboxylase (EntX) from K. pneumonia and CatA from P. putida KT2440 were overexpressed using a high copy number plasmid. This enabled the production of 605 mg/L of MA from E. coli, in which the aroG (encodes DAHP synthase) and aroL genes (encoding shikimate kinase) were overexpressed to strengthen the shikimate pathway (Pathway 3 in Figure 1) [8,9].
In addition to DHS and chorismate, MA can also be produced by a pathway through catechol from anthranilate, which is the first intermediate of the tryptophan biosynthetic branch. Gram-per-liter levels of MA could be produced through the additional expression of the enzymes anthranilate 1,2-dioxygenase (ADO), which converts anthranilate to catechol and catechol 1,2-dioxygenase (CDO), which is engaged in the conversion of catechol to MA. In addition, 389.96 mg/L of MA was produced in a flask by the establishment of an anthranilate overproducer MA-4 strain. At the same time, tryptophan biosynthesis was blocked, and the key enzyme of the shikimate pathway was overexpressed (Pathway 5 in Figure 1) [10].
In addition, the MA biosynthesis pathway was divided into three in the presence of the starting intermediate of DHS and chorismate in E. coli. Each of these synthetic pathways were introduced to identify the best pathway to produce MA effectively [11]. Pathway 4 produced MA via chorismate, isochorismate, salicylate, and catechol in sequence, in which salicylate was converted to catechol by salicylate 1-monoxygenase and catechol was converted to MA in the presence of catechol 1,2-dioxygenase (Pathway 4 in Figure 1). Pathway 4 was designed based on the research showing that E. coli LS-8 produced 1.5 g/L of MA through the module engineering of these enzymes [12]. Pathway 2 was designed to produce MA from protocatechuate (PCA) through chorismate and 4-hydroxybenzoate. This was based on reports that 170 mg/L of MA was produced in minimal media prepared through engineered strains via the expression of three non-native genes (pobA, aroY, and catA, which code 4-hydroxybenzoate hydroxylase, protocatechuate decarboxylase, and catechol 1,2-dioxygenase, respectively) [13]. Pathway 1 was designed so that MA could be synthesized by PCA and catechol with the starting intermediate of traditional DHA. When the above three pathways were tested using an E. coli ATCC31882 derivative, which is an L-phenylalanine-overproducing strain, the traditional MA-producing Pathway 1 produced the highest yield of MA. The method involved fusing the protein that was engaged in the effective conversion of the intermediate. In addition, the fusion proteins of AroC (chorismate synthase) and MenF (isochorismate synthase) (Pathway 4 in Figure 1), and AroD (3-dehydroquinate dehydratase) and AroZ (DHS dehydratase) (Pathway 1 in Figure 1) were overexpressed to increase the carbon flux from chorismate to isochorismate. The level of MA production in the batch with pH control (with CaCO3) was 4.45 g/L. This proved that the traditional pathway to produce MA from DHS was still effective [11].
As another example of producing MA through the establishment of various pathways, several biosynthesis pathways were established in E. coli, and MA was produced via a biosynthesis route that was executed in the order of the DHS-derived route, chorismite-derived route, and tyrosine-phenol route (Pathway 6 in Figure 1). E. coli NST74 ΔpheA ΔpykA ΔpykF Δcrr (3.1 g/L minimal media) produced the highest MA production yield through the “metabolic funnel” (combination of Pathway 1 and 2), which is a parallel route to the combination of DHS and chorismite [14]. Choi et al. reported the highest production yield of MA in E. coli, which produced 64.5 g/L of MA by engineering the E. coli AB2834 strain pathway. This strain could produce up to 117 g/L (0.39 g/g) of the intermediate DHS. After establishing the heterogeneous aroZ, aroY, and catA (Pathway 1 in Figure 1) in the strain as the operon forms, a search was conducted for an efficient promoter based on the RNA-seq to express the heterogeneous genes. Finally, MA production of 64.5 g/L in 7-L fed-batch fermentation could be achieved, without accumulating intermediates in the E. coli strain [15].
2.2. MA Production via Various Carbon Sources and Co-Cultivation
Recently, the utilization of agricultural and industrial biomass as a feedstock in a bio-refinery has attracted increasing interest [16]. Of such materials, renewable lignocellulose materials promise an economically sustainable supply that is inexpensive and abundant. Lignin is a natural polymer in nature and a key component in woody plants. Recently, many studies have reported the use of lignin monomers and hemicellulose components rather than glucose or glycerol as carbon sources for the production of MA.
Lignocellulose, when pretreated, is degraded into glucose and xylose. Several studies have reported the use of these intermediates for MA production in E. coli. The Dahms pathway, which is the xylose degradation pathway, was introduced into E. coli. Two metabolically parallel pathways were then designed so that xylose was input directly into the TCA cycle as a carbon source through a catabolic pathway for growth. At the same time, glucose was input only into the shikimate pathway to produce MA. With the simultaneous input of the carbon sources of glucose and xylose, 4.09 g/L of MA was produced in minimal media [17].
A study on novel MA production was published in 2015 and involved two engineered E. coli-E. coli cocultures with glycerol as the carbon source. Two types of strains were established: (i) one strain produced high levels of DHS effectively by the deletion of ydiB (encoding quinate/shikimate dehydrogenase) and aroE (shikimate dehydrogenase) in a tyrosine overproducer E. coli, and (ii) in another strain, heterologous genes, aroZ, aroY, and catA, were inserted so that DHS could be converted to MA. The system was established to move the DHS effectively between the two E. coli strains, and ShiA (Shikimate transporter) permease, which is engaged in DHS assimilation, was overexpressed so that the secreted DHS could be absorbed efficiently into the cells. The two strains were cocultured in a bioreactor and produced 2 g/L of MA [18]. Moreover, a coculture of the two E. coli strains that used glucose or xylose with similar strategies provided an MA production of 4.7 g/L in the bioreactor [19].
These results suggest that MA could be biosynthesized through the engineering of numerous pathways in a single strain. On the other hand, it was also proven that coculture of engineered strains in accordance with the objective of each strain was equally effective.
3. MA Production from C. Glutamicum
Kinoshita et al. isolated a Gram-positive bacterium from the soil that produced large amounts of L-glutamate and called it Micrococcus glutamicus. Later, that strain was re-named C. glutamicum, and it has been used for the mass production of various amino acids, organic acids, polymer precursors, and biofuels. The strain is generally recognized as safe (GRAS) because of its characteristic of being a non-endotoxin. C. glutamicum is a representative industrial strain that produces L-glutamate and L-lysine, which are used as food additives with a production of more than 1.5 × 106 tons/year and 0.9 × 106 tons/year, respectively [20,21,22,23,24,25].
Because of the excellent characteristics of the strain for industry, many researchers have conducted studies on the production of useful materials from C. glutamicum through pathway engineering, and research on the strain to produce good quantities of MA have progressed, centering on the research by Becher et al. and Lee et al. [26,27]. Both groups removed the catB gene (encoding muconate cycloisomerase), which degraded MA from C. glutamicum, to accumulate MA as the final product in C. glutamicum (Figure 2).
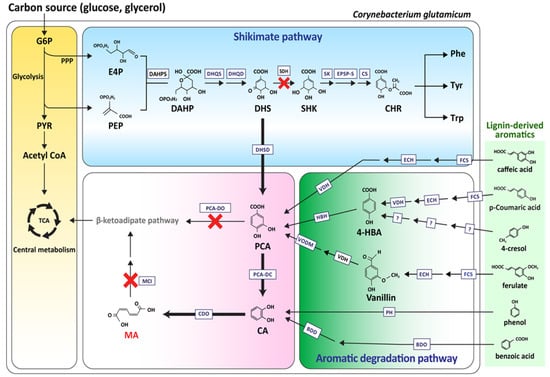
Figure 2.
Schematic overview of the aromatic compound degradation metabolic pathway in C. glutamicum for MA biosynthesis. G6P, glucose-6- phosphate; PPP, pentose phosphate pathway; PYR, pyruvate; PEP, phosphoenolpyruvate; E4P, erythrose4-phosphate; DAHP, 3-deoxy-D-arabinoheptulosonate-7-phosphate; DHS, 3-dehydroshikimate; SHK, shikimic acid; Phe, phenylalanine; Tyr, tyrosine; Trp, tryptophan; CHR, chorismate; 4-HBA, 4-hydroxybenzoate; IC, isochorismate; 2,3-DHB, 2,3 dihydroxybenzoate; SA, salicylate; PCA, protocatechuate; CA, catechol; MA, muconic acid. DAHPS, DAHP synthase; DHQS, DHQ synthase; DHQD, DHQ dehydratase; SDH, shikimate dehydrogenase; SK, shikimate kinase; EPSP-S, 5-enolpyruvylshikimate-3-phosphate synthase; CS, chorismate synthase; DHSD, DHS dehydratase; PCA-DC, protocatechuate decarboxylase; CDO, catechol 1,2-dioxygenase; MCI, muconate cycloisomerase; PCA-DO, protocatechuate 3,4-dioxygenase; FCS, feruloyl–CoA synthetase; ECH, enoyl–CoA hydratase/aldolase; VDH, vanillin dehydrogenase; HBH, p-hydroxybenzoate hydroxylase; VODM, vanillate O-demethylase: PH, phenol hydroxylase; BDO, benzoate dioxygenase; BDD, benzoate diol dehydrogenase.
Becher et al. produced MA from lignin-derived aromatic compounds, instead of glucose. When the lignin was treated hydrothermally in supercritical water, it hydrolyzed to aromatic compounds. The aromatic compounds entered the TCA cycle in the form of acetyl-CoA and succinyl-CoA, via catechol and the MA branch in the β-ketoadipate pathway inside C. glutamicum [28,29]. C. glutamicum devoid of catB in the β-ketoadipate pathway was prepared to accumulate MA in this pathway. Benzoic acid, phenol, and catechol were then added, which resulted in the accumulation of MA. In addition, the catA gene that coded catechol-1,2-dioxygenase, the final catalyst in MA biosynthesis by C. glutamicum, was replaced with a strong promotor. A fed-batch culture was then conducted by feeding the catechol on an hourly basis. The culture yielded 85 g/L of MA from catechol. The results showed that 1.8 g/L of MA was produced in the presence of aromatics when the hydrothermally depolymerized softwood lignin was applied in the engineered C. glutamicum strain [28].
Lee et al. engineered the shikimate pathway in C. glutamicum to produce MA from glucose. The catB, PCA dioxygenase alpha/beta subunit genes (pcaG/H), and shikimate dehydrogenase gene (aroE) were removed from the β-ketoadipate pathway to accumulate DHS and PCA, which were the precursors for MA production. Subsequently, to connect the missing conversion from PCA to catechol, the encoding PCA decarboxylase (aroY) gene derived from K. pneumoniae and the kpdBD (encoding PCA decarboxylase subunit) gene, which was a subunit of AroY, were codon-optimized in C. glutamicum so MA would accumulate. This resulted in MA production (340 mg/L) from glucose. The established strain could produce 53.8 g/L of MA through 50-L scale fed-batch fermentation and media optimization [29]. The uptake of glucose by C. glutamicum through the PTS system results in the consumption of phosphoenolpyruvate (PEP), an important starter of the shikimate pathway. The aim was to reduce PEP consumption by removing phosphotransferase (ptsl) from the PTS system. On the other hand, although the glucose uptake rate was reduced five-fold compared to the parental strain, cell growth was retarded. This problem was resolved by strengthening the inositol permease transporter, which is another glucose uptake system, by removing the iolR gene, which encoded the IolR repressor that repressed the inositol permease transporter. In addition, the qsuB gene (encoding 3-dehydroshikimate dehydratase), which converted DHS (one of the essential pathways for MA production) to PCA, was overexpressed along with YBD (aroY and kpdBD), after which 4.5 g/L of MA was produced, showing a 12.2% increase [30,31].
From the above results, a possible MA production pathway from glucose using a C. glutamicum strain with a well-established amino acid production system was proposed as an industrial-scale pilot. In addition, the strain tolerates aromatic compounds, including the precursors (PCA, catechol) contained in C. glutamicum, proving that it could be a beneficial strain for high-level MA production.
4. MA Production from Other Microorganisms
Some microorganisms belonging to the genera Pseudomonas, Arthrobacter, Corynebacterium, Brevibacterium, Microbacterium, and Sphingobacterium were reported to metabolize benzoate via the catechol branch of the β-ketoadipate pathway to produce MA.
Benzoate is first converted to benzoate diol catalyzed by benzoate 1,2-dioxygenase encoded by benABC. The oxidative decarboxylation of benzoate diol to catechol is then performed by benzoate diol dehydrogenase encoded by benD. Ring fission of catechol between the hydroxyl groups is catalyzed by CatA encoded by catA to form MA. The latter metabolite is then converted to muconolactone by muconate cycloisomerase encoded by catB. Muconolactone is finally converted to tricarboxylic acid cycle intermediates after several metabolic steps.
4.1. MA Production from Saccharomyces Cerevisiae
E. coli strains that can only grow under neutral pH conditions are not beneficial in cost-competitive industrial production processes because, even if they are improved to high MA producing strains, the MA is purified under low pH conditions. In contrast, Saccharomyces cerevisiae is beneficial in industrial production because it can be fermented at a low pH, has high robustness, is resistant to toxic inhibitors and fermentation products, has microbial contamination resistance, and has a high level of public acceptance [32].
Weber C et al. reported the results for MA production by introducing the heterologous biosynthetic pathway from DHS using S. cerevisiae. As with the other strains, a three-step synthetic pathway (dehydroshikimate dehydratase from Podospora anserina, protocatechuic acid decarboxylase from Enterobacter cloacae, and catechol 1,2-dioxygenase from Candida albicans) was introduced into the yeast, and further genetic modification and feedback inhibition mitigation were applied. The S. cerevisiae MuA12 strain, where the precursor availability was enhanced, could produce 141 mg/L of MA in a flask culture [33].
Similarly, three enzymes, AroZ from Podospora anserina, AroY from K. pneumoniae, and HQD2 from Candida albicans, were introduced to a multicopy plasmid, and the transketolase gene (TKL1) was overexpressed for carbon flux. The resulting system produced 559.5 mg/L of MA from glucose through the engineered S. cerevisiae MuA12 [34].
In addition to rational engineering for MA production, a combined adaptive laboratory evolution (ALE) strategy and rational metabolic engineering were also employed. An S. cerevisiae strain that produced more aromatic amino acid was secured using exogenous amino acid supplementation (particularly, tryptophan) and anti-metabolite selection [4-fluorophenylalanine and G418 (antibiotic)] methods, and 2.1 g/L of MA was produced from the MuA-5.01.1.02+aro1t+scPAD1 strain in fed-batch fermentation [35].
In addition to an engineering strategy at the metabolic site, such as gene deletion or overexpression of the structural genes, another study produced MA and shikimate from S. cerevisiae by removing Ric1, which is a transcriptional repressor. In silico modeling and pathway analysis confirmed the production of 2705 mg/L of MA and its precursor using the BY47471-MA4 strain [36].
Recently, it was reported that S. cerevisiae could produce MA from lignocellulosic biomass hydrolysate using recombinant xylose-fermenting yeast. In addition to the exogenous MA biosynthetic pathway, the xylose isomerase gene from Bacteroides vulgatus and pentose phosphate pathway genes from S. cerevisiae were overexpressed in the yeast strain, and the overexpression of the Aro1 gene (with a stop codon of AroE) and a feedback-resistant Aro4opt mutant gene from S. cerevisiae were also applied. Under aerobic conditions, the MA titer reached 424 mg/L, and 1286 mg/L MA was produced with the supplement of 1 g/L catechol. Fermentation of an oil palm empty fruit bunch hydrolysate resulted in 31.3 g/L ethanol and 53.4 mg/L MA [37].
4.2. MA Production from Amycolatopsis Species
Amycolatopsis spp. also tolerate lignin-based aromatics, such as catechol, guaiacol, phenol, toluene, p-coumarate, and benzoate, and even favor these aromatics as carbon sources over sugar. The aqueous phase, obtained through hydrothermal conversion as a lignin treatment, contained a large quantity of guaiacol of 7 g/L. Research on the metabolically engineered Amycolatopsis sp. ATCC 39,116 revealed 3.1 g/L of MA production from the guaiacol, while 1.8 mM of MA was produced with the lignin hydrolysate [38].
4.3. MA Production from Pseudomonas Species
P. putida has excellent tolerance towards organic solvents and was the first soil microbe among Gram-negative bacteria to be recognized as a safety strain from the Recombinant DNA Advisory Committee [39,40,41]. Therefore, P. putida has attracted attention as a metabolic engineering chassis for applications in the industrial bioengineering field. Moreover, P. putida can be used as a carbon source for the growth and energy production of lignin-related aromatics, such as vanillin [42,43,44,45].
Sonoki et al. conducted an experiment on P. putida to produce MA from lignin without glucose through metabolic engineering. The precursor was induced to accumulate by the removal of pcaG/H and catB from the β-ketoadipate pathway using the same strategy as with C. glutamicum. The PCA decarboxylase gene was overexpressed to complete the MA pathway. A medium containing 25 nM each of aromatic compound 4-hydroxybenzoic acid (4-HBA) and vanillin produced MA with a yield of 19.0%, whereas 0.11 mM MA was biosynthesized through softwood lignin [46,47].
Vardon et al. substituted protocatechuate 3,4 dioxygenase (PcaG/H) with protocatechuate decarboxylase (AroY) to convert aromatics from the catechol and protocatechuate branches to MA with P. putida KT2440. To block the synthesized MA, P. putida KT2440-CJ103 was established, in which a catBCA operon (metabolism operon of MA through β-ketoadipate pathway) was substituted with Ptac_catA_dmpKLMNOP (encoding phenol monooxygenase), and 13.5 g/L of MA was produced from the p-coumarate through fed-batch fermentation conducted for 78.5 h [48].
Two subunit genes, which play important roles in PCA decarboxylase and decarboxylase activity, were expressed as a genome-integrated gene to reduce PCA accumulation, which is a bottleneck in MA production, resolve the conversion of PCA to MA, and increase MA production. The KT2440-CJ184 (P. putida KT2440 ΔcatRBC::Ptac:catA ΔpcaHG::Ptac:aroY:ecdB:ecdD) strain expressed a codon-optimized AroY, EcdB (subunit of decarboxylase AroY) and EcdD (subunit of decarboxylase AroY) from E. cloacae, and produced 15.59 g/L of MA in bioreactor cultivation that contained p-coumarate, which is an aromatic lignin monomer, and glucose [49]. This was followed by the accumulation of 4-hydroxybenzoate (4-HBA) and vanillin, which are intermediates of PCA in the same group. The catabolite repression control (Crc) protein, which is a regulator of carbon catabolite repression, was located and deleted. This increased the MA conversion rate from p-coumarate by 12% [50].
Furthermore, a pathway was engineered that could catabolize a range of aromatic compounds based on P. putida KT2440 and could convert them to 16 catabolic intermediates, which exhibited a substantive chemical diversity. Enzymes derived from Sphingobacterium sp., Paenibacillus sp., and P. putida were introduced to redesign the aromatic catabolic pathway so that these catabolic-intermediate molecules could be produced from an aromatic compound or glucose. The 16 target molecules were then produced and analyzed based on a bioreactor. Among the numerous mutants produced in this study, the P. putida KT2440 ΔcatRBC::Ptac:catA ΔpcaHG::Ptac:aroY:ecdB:asbF ΔpykA::aroGD146N:aroY:ecdB:asbF ΔpykF Δppc Δpgi-1 Δpgi-2 Δgcd (CJ522) strain was used to produce MA; 12 g/L of MA was produced through a fed-batch mode bioreactor cultivation from glucose. This is a practical example of the use of microbial strains for the production of chemically diverse molecules as building blocks [51].
Although the β-ketoadipate pathway is not present in E. coli or S. cerevisiae, it is endogenous to aromatic degrading organisms, such as P. putida. This is because P. putida has been engineered extensively to produce MA directly from pretreated biomass as well as from lignin-derived monomers. Table 1 shows the list of engineered microbial strains for MA biosynthesis.

Table 1.
List of engineered microbial strains for MA biosynthesis.
5. Conclusions and Perspectives
Recently, information on the metabolic pathways of numerous microbes has become readily available, owing to the development of extensive genomic analysis, well-established molecular tools, system biology techniques, and fermentation techniques. Therefore, with such technologies, it has become possible to build new metabolisms that did not exist in nature or to reinforce existing ones. MA synthesis via PCA and catechol as starters in the shikimate pathway has been applied to various microbes, starting from primary research with E. coli.
In addition, MA production with E. coli has been diversified with microbes, such as C. glutamicum and P. putida, which have resistance to the toxicity of aromatic compounds. These aromatic-tolerant microbes possess abundant pathways and enzymes that can produce MA with aromatic compounds that are being discarded. This paper summarizes the use of microbes with strong potential as microbial cell factories at an industrial level for MA production. Finally, various other aromatics should be applied to obtain higher MA titers considering the economic and industrial aspects in the mass production of MA and the metabolic engineering approaches and optimized process operations using a range of microbes.
Funding
This study was supported by the Agricultural Microbiome R&D Program, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea (as part of the (multi-ministerial) Genome Technology to Business Translation Program). No. 918008-04. This work was also funded by Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01318701) Rural Development Administration, Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Muconic Acid Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2019–2024. Available online: https://www.researchandmarkets.com/reports/4775781/muconic-acid-market-global-industry-trends (accessed on 1 June 2019).
- Draths, K.M.; Frost, J.W. Environmentally compatible synthesis of adipic acid from D-glucose. J. Am. Chem. Soc. 1994, 116, 399–400. [Google Scholar] [CrossRef]
- Niu, W.; Draths, K.M.; Frost, J.W. Benzene-free synthesis of adipic acid. Biotechnol. Prog. 2002, 18, 201–211. [Google Scholar] [CrossRef]
- Parke, D. Positive regulation of phenolic catabolism in Agrobacterium tumefaciens by the pcaQ gene in response to beta-carboxy-cis,cis-muconate. J. Bacteriol. 1993, 175, 3529–3535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Neidle, E.L.; Ornston, L.N. Benzoate and muconate, structurally dissimilar metabolites, induce expression of catA in Acinetobacter calcoaceticus. J. Bacteriol. 1987, 169, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.W.; Hsieh, J.H. Oxidative bioconversion of toluene to 1,3-butadiene-1,4-dicarboxylic acid (cis,cis-muconic acid). World J. Microbiol. Biotechnol. 1990, 6, 127–143. [Google Scholar] [CrossRef]
- Bui, V.; Lau, M.K.; MacRae, D.; Schweitzer, D. Methods for Producing Isomers of Muconic Acid and Muconate Salts. U.S. Patent 20130030215A1, 31 January 2013. [Google Scholar]
- Sun, X.; Lin, Y.; Yuan, Q.; Yan, Y. Biological production of muconic acid via a prokaryotic 2,3-dihydroxybenzoic acid decarboxylase. ChemSusChem 2014, 7, 2478–2481. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, P. Muconic acid production from glucose using enterobactin precursors in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2015, 42, 701–709. [Google Scholar] [CrossRef]
- Sun, X.; Lin, Y.; Huang, Q.; Yuan, Q.; Yan, Y. A novel muconic acid biosynthesis approach by shunting tryptophan biosynthesis via anthranilate. Appl. Environ. Microbiol. 2013, 79, 4024–4030. [Google Scholar] [CrossRef]
- Fujiwara, R.; Noda, S.; Tanaka, T.; Kondo, A. Muconic Acid Production Using Gene-Level Fusion Proteins in Escherichia coli. ACS Synth. Biol. 2018, 7, 2698–2705. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, X.; Yuan, Q.; Yan, Y. Extending shikimate pathway for the production of muconic acid and its precursor salicylic acid in Escherichia coli. Metab. Eng. 2014, 23, 62–69. [Google Scholar] [CrossRef]
- Sengupta, S.; Jonnalagadda, S.; Goonewardena, L.; Juturu, V. Metabolic engineering of a novel muconic acid biosynthesis pathway via 4-hydroxybenzoic acid in Escherichia Coli. Appl. Environ. Microbiol. 2015, 81, 8037–8043. [Google Scholar] [CrossRef][Green Version]
- Thompson, B.; Pugh, S.; Machas, M.; Nielsen, D.R. Muconic Acid Production via Alternative Pathways and a Synthetic “Metabolic Funnel”. ACS Synth. Biol. 2018, 7, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Seo, S.Y.; Park, S.O.; Lee, H.N.; Song, J.S.; Kim, J.Y.; Park, J.H.; Kim, S.; Lee, S.J.; Chun, G.T.; et al. Cell Factory Design and Culture Process Optimization for Dehydroshikimate Biosynthesis in Escherichia coli. Front. Bioeng. Biotechnol. 2019, 7, 241. [Google Scholar] [CrossRef] [PubMed]
- Hazeena, S.H.; Sindhu, R.; Pandey, A.; Binod, P. Lignocellulosic bio-refinery approach for microbial 2,3-Butanediol production. Bioresour. Technol. 2020, 302, 122873. [Google Scholar] [CrossRef]
- Fujiwara, R.; Noda, S.; Tanaka, T.; Kondo, A. Metabolic engineering of Escherichia coli for shikimate pathway derivative production from glucose-xylose co-substrate. Nat. Commun. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.; Pereira, B.; Stephanopoulos, G. Engineering, E. coli–E. coli cocultures for production of muconic acid from glycerol. Microb. Cell Fact. 2015, 14, 134. [Google Scholar] [CrossRef]
- Zhang, H.; Pereira, B.; Li, Z.; Stephanopoulos, G. Engineering Escherichia coli coculture systems for the production of biochemical products. Proc. Natl. Acad. Sci. USA 2015, 112, 8266–8271. [Google Scholar] [CrossRef]
- Kinoshita, S.; Udaka, S.; Shimono, M. Studies on the amino acid fermentation. Part 1. Production of L-glutamic acid by various microorganisms. J. Gen. Appl. Microbiol. 2004, 50, 331–343. [Google Scholar]
- Lee, J.Y.; Na, Y.A.; Kim, E.; Lee, H.S.; Kim, P. The Actinobacterium Corynebacterium glutamicum, an Industrial Workhorse. J. Microbiol. Biotechnol. 2016, 26, 807–822. [Google Scholar] [CrossRef]
- Leuchtenberger, W.; Huthmacher, K.; Drauz, K. Biotechnological production of amino acids and derivatives: Current status and prospects. Appl. Microbiol. Biotechnol. 2005, 69, 1–8. [Google Scholar] [CrossRef]
- Takors, R.; Bathe, B.; Rieping, M.; Hans, S.; Kelle, R.; Huthmacher, K. Systems biology for industrial strains and fermentation processes--example: Amino acids. J. Biotechnol. 2007, 129, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, A.; Katsumata, R.; Oka, T.; Furuya, A. Functional expression of the genes of Escherichia coli in gram-positive Corynebacterium glutamicum. Mol. Gen. Genet. 1984, 196, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Nešvera, J.; Pátek, M. Tools for genetic manipulations in Corynebacterium glutamicum and their applications. Appl. Microbiol. Biotechnol. 2011, 90, 1641–1654. [Google Scholar] [CrossRef]
- Becker, J.; Kuhl, M.; Kohlstedt, M.; Starck, S.; Wittmann, C. Metabolic engineering of Corynebacterium glutamicum for the production of cis, cis-muconic acid from lignin. Microb. Cell Fact. 2018, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Shin, W.S.; Seo, S.Y.; Choi, S.S.; Song, J.S.; Kim, J.Y.; Park, J.H.; Lee, D.; Kim, S.Y.; Lee, S.J.; et al. Corynebacterium Cell Factory Design and Culture Process Optimization for Muconic Acid Biosynthesis. Sci. Rep. 2018, 8, 18041. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.H.; Zhou, N.Y.; Liu, S.J. Degradation and assimilation of aromatic compounds by Corynebacterium glutamicum: Another potential for applications for this bacterium? Appl. Microbiol. Biotechnol. 2012, 95, 77–89. [Google Scholar] [CrossRef]
- Shen, X.H.; Huang, Y.; Liu, S.J. Genomic analysis and identification of catabolic pathways for aromatic compounds in Corynebacterium glutamicum. Microbes Environ. 2005, 20, 160–167. [Google Scholar] [CrossRef][Green Version]
- Lindner, S.N.; Seibold, G.M.; Henrich, A.; Krämer, R.; Wendisch, V.F. Phosphotransferase system-independent glucose utilization in Corynebacterium glutamicum by inositol permeases and glucokinases. Appl. Environ. Microbiol. 2011, 77, 3571–3581. [Google Scholar] [CrossRef]
- Shin, W.S.; Lee, D.; Lee, S.J.; Chun, G.T.; Choi, S.S.; Kim, E.S.; Kim, S. Characterization of a non-phosphotransferase system for cis,cis-muconic acid production in Corynebacterium glutamicum. Biochem. Biophys. Res. Commun. 2018, 499, 279–284. [Google Scholar] [CrossRef]
- Weber, C.; Brückner, C.; Weinreb, S.; Lehr, C.; Essl, C.; Boles, E. Biosynthesis of cis,cis-muconic acid and its aromatic precursors, catechol and protocatechuic acid, from renewable feedstocks by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2012, 78, 8421–8430. [Google Scholar] [CrossRef]
- Curran, K.A.; Leavitt, J.M.; Karim, A.S.; Alper, H.S. Metabolic engineering of muconic acid production in Saccharomyces cerevisiae. Metab. Eng. 2013, 15, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Suastegui, M.; Matthiesen, J.E.; Carraher, J.M.; Hernandez, N.; Rodriguez Quiroz, N.; Okerlund, A.; Cochran, E.W.; Shao, Z.; Tessonnier, J.P. Combining Metabolic Engineering and Electrocatalysis: Application to the Production of Polyamides from Sugar. Angew. Chem. Int. Ed. Engl. 2016, 55, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, J.M.; Wagner, J.M.; Tu, C.C.; Tong, A.; Liu, Y.; Alper, H.S. Biosensor-Enabled Directed Evolution to Improve Muconic Acid Production in Saccharomyces cerevisiae. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Suástegui, M.; Yu Ng, C.; Chowdhury, A.; Sun, W.; Cao, M.; House, E.; Maranas, C.D.; Shao, Z. Multilevel engineering of the upstream module of aromatic amino acid biosynthesis in Saccharomyces cerevisiae for high production of polymer and drug precursors. Metab. Eng. 2017, 42, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Peng, B.; Huang, S.; Geng, A. Recombinant xylose-fermenting yeast construction for the co-production of ethanol and cis,cis-muconic acid from lignocellulosic biomass. Bioresour. Technol. Rep. 2020, 9, 100395. [Google Scholar] [CrossRef]
- Barton, N.; Horbal, L.; Starck, S.; Kohlstedt, M.; Luzhetskyy, A.; Wittmann, C. Enabling the valorization of guaiacol-based lignin: Integrated chemical and biochemical production of cis,cis-muconic acid using metabolically engineered Amycolatopsis sp ATCC 39116. Metab. Eng. 2018, 45, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.E.; Weinel, C.; Paulsen, I.T.; Dodson, R.J.; Hilbert, H.; Martins dos Santos, V.A.; Fouts, D.E.; Gill, S.R.; Pop, M.; Holmes, M.; et al. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 2002, 4, 799–808. [Google Scholar] [CrossRef]
- Nikel, P.I.; Chavarría, M.; Danchin, A.; de Lorenzo, V. From dirt to industrial applications: Pseudomonas putida as a Synthetic Biology chassis for hosting harsh biochemical reactions. Curr. Opin. Chem. Biol. 2016, 34, 20–29. [Google Scholar] [CrossRef]
- Aparicio, T.; de Lorenzo, V.; Martínez-García, E. CRISPR/Cas9-Based Counterselection Boosts Recombineering Efficiency in Pseudomonas putida. Biotechnol. J. 2018, 13, e1700161. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Ogino, C.; Kondo, A. Microbial conversion of biomass into bio-based polymers. Bioresour. Technol. 2017, 245, 1664–1673. [Google Scholar] [CrossRef]
- Xie, N.Z.; Liang, H.; Huang, R.B.; Xu, P. Biotechnological production of muconic acid: Current status and future prospects. Biotechnol. Adv. 2014, 32, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Vardon, D.R.; Rorrer, N.A.; Salvachúa, D.; Settle, A.E.; Johnson, C.W.; Menart, M.J.; Cleveland, N.S.; Ciesielski, P.N.; Steirer, K.X.; Dorgan, J.R.; et al. cis,cis-Muconic acid: Separation and catalysis to bio-adipic acid for nylon-6,6 polymerization. Green Chem. 2016, 18, 3397–3413. [Google Scholar] [CrossRef]
- Wackett, L.P. Pseudomonas putida―a versatile biocatalyst. Nat. Biotechnol. 2003, 21, 136–138. [Google Scholar] [CrossRef]
- Sonoki, T.; Morooka, M.; Sakamoto, K.; Otsuka, Y.; Nakamura, M.; Jellison, J.; Goodell, B. Enhancement of protocatechuate decarboxylase activity for the effective production of muconate from lignin-related aromatic compounds. J. Biotechnol. 2014, 192, 71–77. [Google Scholar] [CrossRef]
- Sonoki, T.; Takahashi, K.; Sugita, H.; Hatamura, M.; Azuma, Y.; Sato, T.; Suzuki, S.; Kamimura, N.; Masai, E. Glucose-Free cis,cis-Muconic Acid Production via New Metabolic Designs Corresponding to the Heterogeneity of Lignin. ACS Sustain. Chem. Eng. 2018, 6, 1256–1264. [Google Scholar] [CrossRef]
- Vardon, D.R.; Franden, M.A.; Johnson, C.W.; Karp, E.M.; Guarnieri, M.T.; Linger, J.G.; Salm, M.J.; Strathmann, T.J.; Beckham, G.T. Adipic acid production from lignin. Energy Environ. Sci. 2015, 8, 617–628. [Google Scholar] [CrossRef]
- Johnson, C.W.; Salvachúa, D.; Khanna, P.; Smith, H.; Peterson, D.J.; Beckham, G.T. Enhancing muconic acid production from glucose and lignin-derived aromatic compounds via increased protocatechuate decarboxylase activity. Metab. Eng. Commun. 2016, 3, 111–119. [Google Scholar] [CrossRef]
- Johnson, C.W.; Abraham, P.E.; Linger, J.G.; Khanna, P.; Hettich, R.L.; Beckham, G.T. Eliminating a global regulator of carbon catabolite repression enhances the conversion of aromatic lignin monomers to muconate in Pseudomonas putida KT2440. Metab. Eng. Commun. 2017, 5, 19–25. [Google Scholar] [CrossRef]
- Johnson, C.W.; Salvachúa, D.; Rorrer, N.A.; Black, B.A.; Vardon, D.R.; St. John, P.C.; Cleveland, N.S.; Dominick, G.; Elmore, J.R.; Grundl, N.; et al. Innovative Chemicals and Materials from Bacterial Aromatic Catabolic Pathways. Joule 2019, 3, 1523–1537. [Google Scholar] [CrossRef]
- Horwitz, A.A.; Walter, J.M.; Schubert, M.G.; Kung, S.H.; Hawkins, K.; Platt, D.M.; Hernday, A.D.; Mahatdejkul-Meadows, T.; Szeto, W.; Chandran, S.S.; et al. Efficient Multiplexed Integration of Synergistic Alleles and Metabolic Pathways in Yeasts via CRISPR-Cas. Cell Syst. 2015, 1, 88–96. [Google Scholar] [CrossRef]
- Skjoedt, M.L.; Snoek, T.; Kildegaard, K.R.; Arsovska, D.; Eichenberger, M.; Goedecke, T.J.; Rajkumar, A.S.; Zhang, J.; Kristensen, M.; Lehka, B.J.; et al. Engineering prokaryotic transcriptional activators as metabolite biosensors in yeast. Nat. Chem. Biol. 2016, 12, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Pyne, M.E.; Narcross, L.; Melgar, M.; Kevvai, K.; Mookerjee, S.; Leite, G.B.; Martin, V.J.J. An Engineered Aro1 Protein Degradation Approach for Increased cis,cis-Muconic Acid Biosynthesis in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2018, 84, e01095-18. [Google Scholar] [CrossRef] [PubMed]
- Brückner, C.; Oreb, M.; Kunze, G.; Boles, E.; Tripp, J. An expanded enzyme toolbox for production of cis, cis-muconic acid and other shikimate pathway derivatives in Saccharomyces cerevisiae. FEMS Yeast Res. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Kildegaard, K.R.; Tramontin, L.R.R.; Chekina, K.; Li, M.; Goedecke, T.J.; Kristensen, M.; Borodina, I. CRISPR/Cas9-RNA interference system for combinatorial metabolic engineering of Saccharomyces cerevisiae. Yeast 2019, 36, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Øzmerih, S.; Guerreiro, R.; Meireles, A.C.; Carolas, A.; Milne, N.; Jensen, M.K.; Ferreira, B.S.; Borodina, I. Improvement of cis,cis-Muconic Acid Production in Saccharomyces cerevisiae through Biosensor-Aided Genome Engineering. ACS Synth. Biol. 2020, 9, 634–646. [Google Scholar] [CrossRef]
- Bang, S.; Choi, C.Y. DO-stat fed-batch production of cis, cis-muconic acid from benzoic acid by Pseudomonas putida BM014. J. Ferment. Bioeng. 1995, 79, 381–383. [Google Scholar] [CrossRef]
- van Duuren, J.B.; Wijte, D.; Karge, B.; dos Santos, V.A.; Yang, Y.; Mars, A.E.; Eggink, G. pH-stat fed-batch process to enhance the production of cis, cis-muconate from benzoate by Pseudomonas putida KT2440-JD1. Biotechnol. Prog. 2012, 28, 85–92. [Google Scholar] [CrossRef]
- Xie, N.Z.; Wang, Q.Y.; Zhu, Q.X.; Qin, Y.; Tao, F.; Huang, R.B.; Xu, P. Optimization of medium composition for cis,cis-muconic acid production by a Pseudomonas sp. mutant using statistical methods. Prep. Biochem. Biotechnol. 2014, 44, 342–354. [Google Scholar] [CrossRef]
- Salvachúa, D.; Johnson, C.W.; Singer, C.A.; Rohrer, H.; Peterson, D.J.; Black, B.A.; Knapp, A.; Beckham, G.T. Bioprocess development for muconic acid production from aromatic compounds and lignin. Green Chem. 2018, 20, 5007–5019. [Google Scholar] [CrossRef]
- Kohlstedt, M.; Starck, S.; Barton, N.; Stolzenberger, J.; Selzer, M.; Mehlmann, K.; Schneider, R.; Pleissner, D.; Rinkel, J.; Dickschat, J.S.; et al. From lignin to nylon: Cascaded chemical and biochemical conversion using metabolically engineered Pseudomonas putida. Metab. Eng. 2018, 47, 279–293. [Google Scholar] [CrossRef]
- Shinoda, E.; Takahashi, K.; Abe, N.; Kamimura, N.; Sonoki, T.; Masai, E. Isolation of a novel platform bacterium for lignin valorization and its application in glucose-free cis,cis-muconate production. J. Ind. Microbiol. Biotechnol. 2019, 46, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Bentley, G.J.; Narayanan, N.; Jha, R.K.; Salvachúa, D.; Elmore, J.R.; Peabody, G.L.; Black, B.A.; Ramirez, K.; De Capite, A.; Michener, W.E.; et al. Engineering glucose metabolism for enhanced muconic acid production in Pseudomonas putida KT2440. Metab. Eng. 2020, 59, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Yoshikawa, N.; Seki, M.; Mikawa, T.; Imada, Y. Microbial production of cis, cis-muconic acid from benzoic acid. Appl. Microbiol. Biotechnol. 1988, 28, 20–25. [Google Scholar] [CrossRef]
- Kaneko, A.; Ishii, Y.; Kirimura, K. High-yield production of cis,cis-muconic acid from catechol in aqueous solution by biocatalyst. Chem. Lett. 2011, 40, 381–383. [Google Scholar] [CrossRef]
- Han, L.; Liu, P.; Sun, J.; Wu, Y.; Zhang, Y.; Chen, W.; Lin, J.; Wang, Q.; Ma, Y. Engineering catechol 1, 2-dioxygenase by design for improving the performance of the cis, cis-muconic acid synthetic pathway in Escherichia coli. Sci. Rep. 2015, 5, 13435. [Google Scholar] [CrossRef]
- Jung, H.M.; Jung, M.Y.; Oh, M.K. Metabolic engineering of Klebsiella pneumoniae for the production of cis,cis-muconic acid. Appl. Microbiol. Biotechnol. 2015, 99, 5217–5225. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).