An Anti-Inflammatory Poly(PhosphorHydrazone) Dendrimer Capped with AzaBisPhosphonate Groups to Treat Psoriasis
Abstract
1. Introduction
2. Materials and Methods
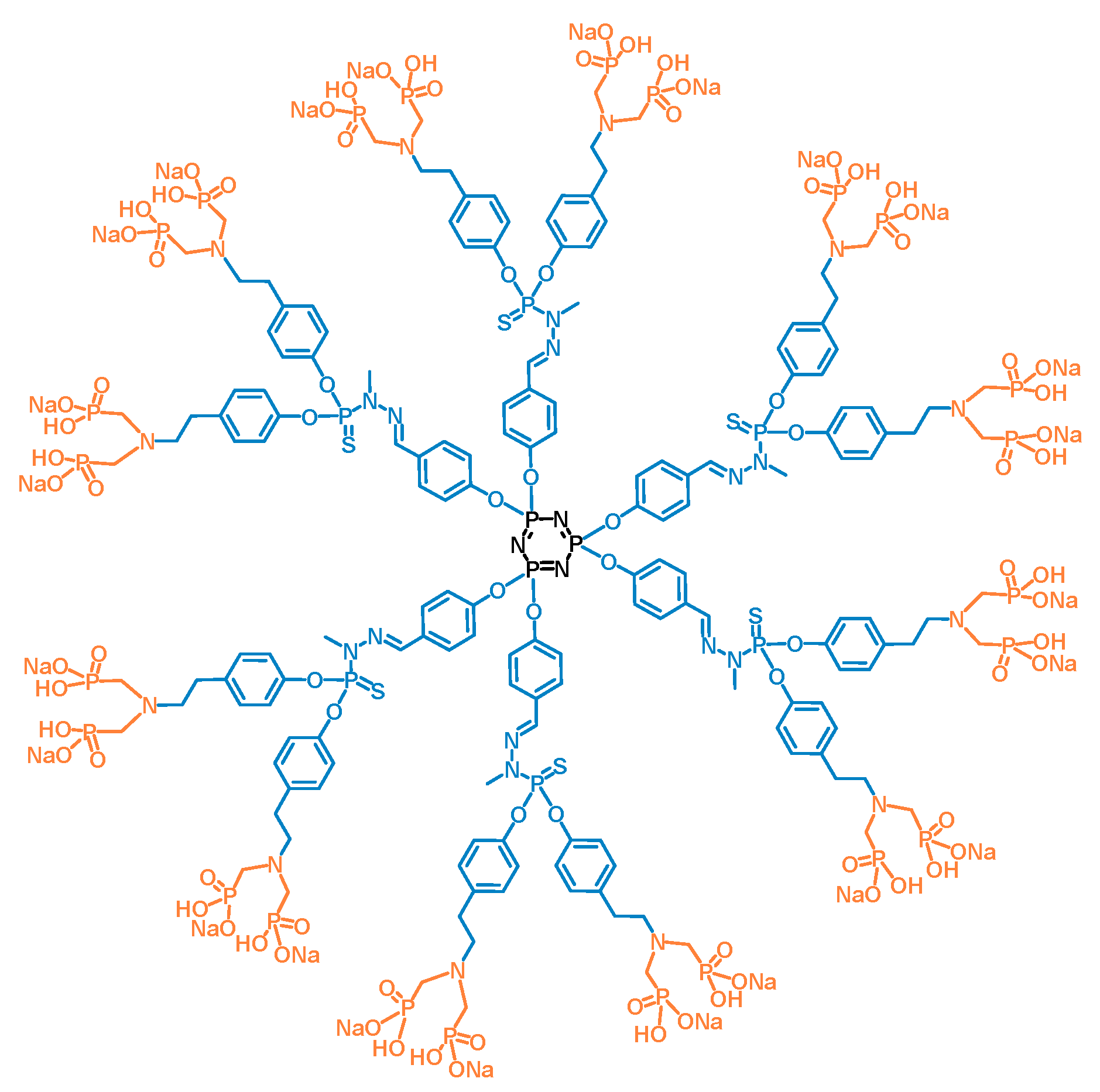
2.1. Synthesis of the ABP Dendrimer
2.2. Animals
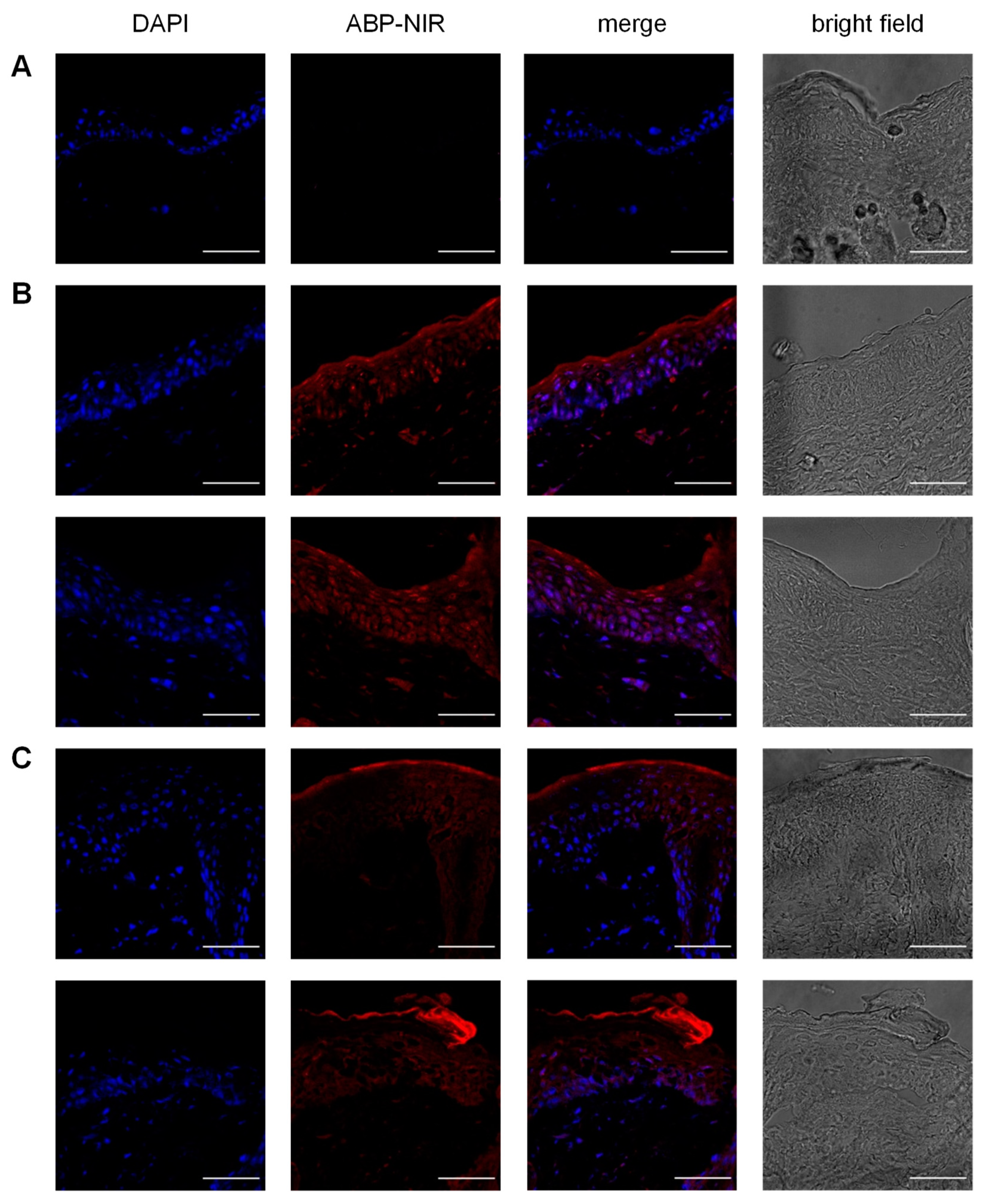
2.3. Skin Penetration Study
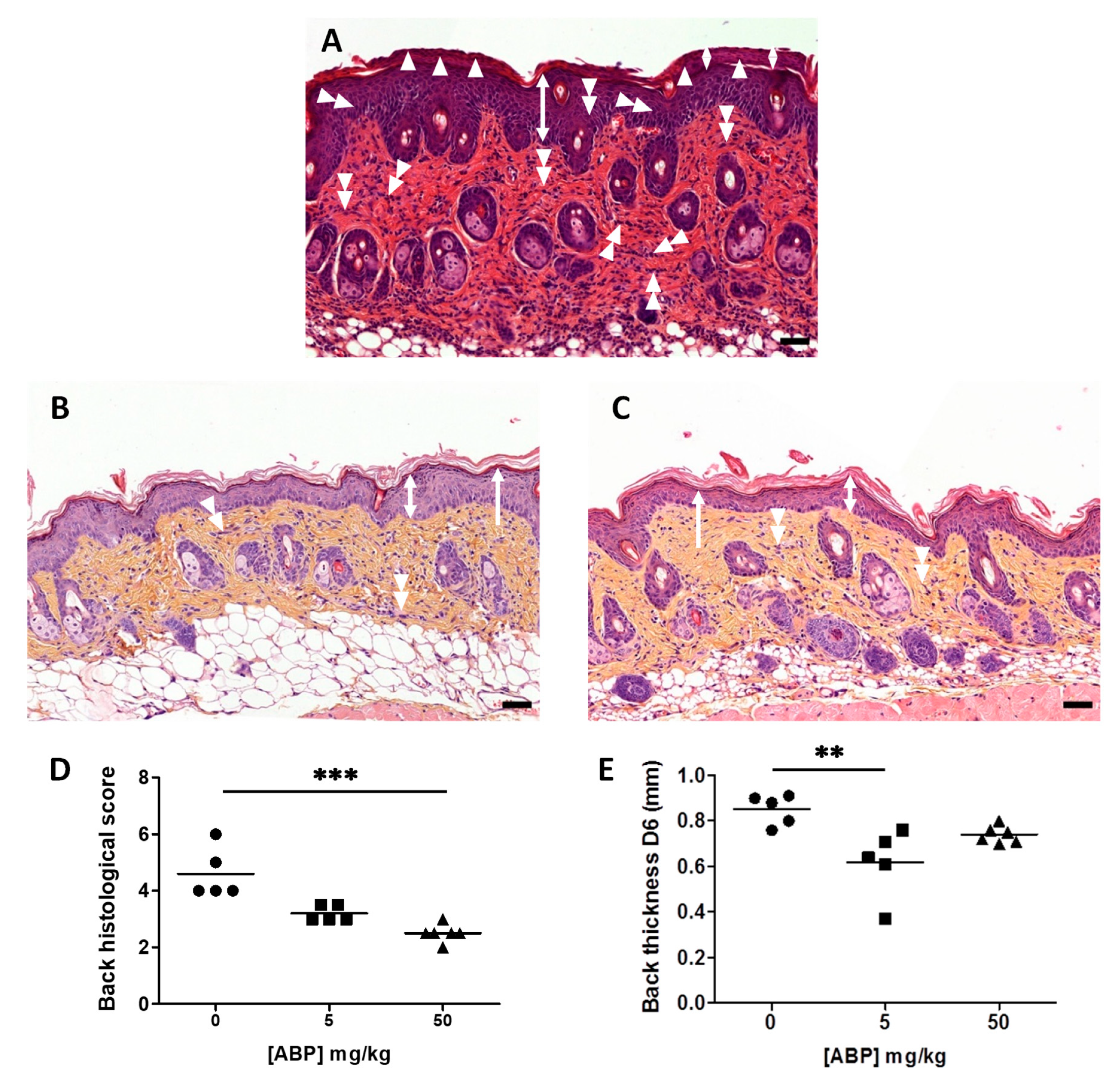
2.4. Histopathology
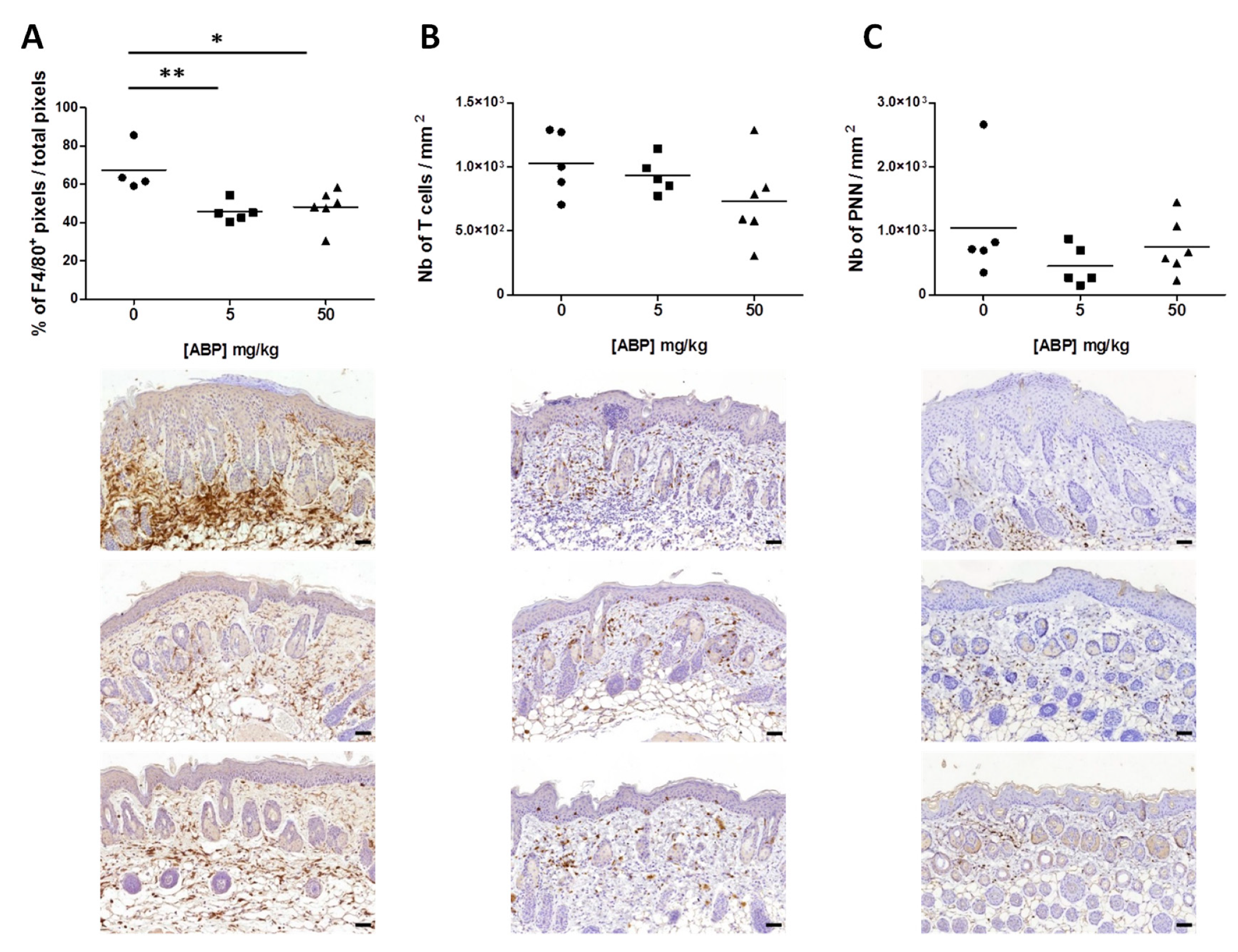
2.5. Immunohistochemistry
2.6. Statistical Analyses
3. Results
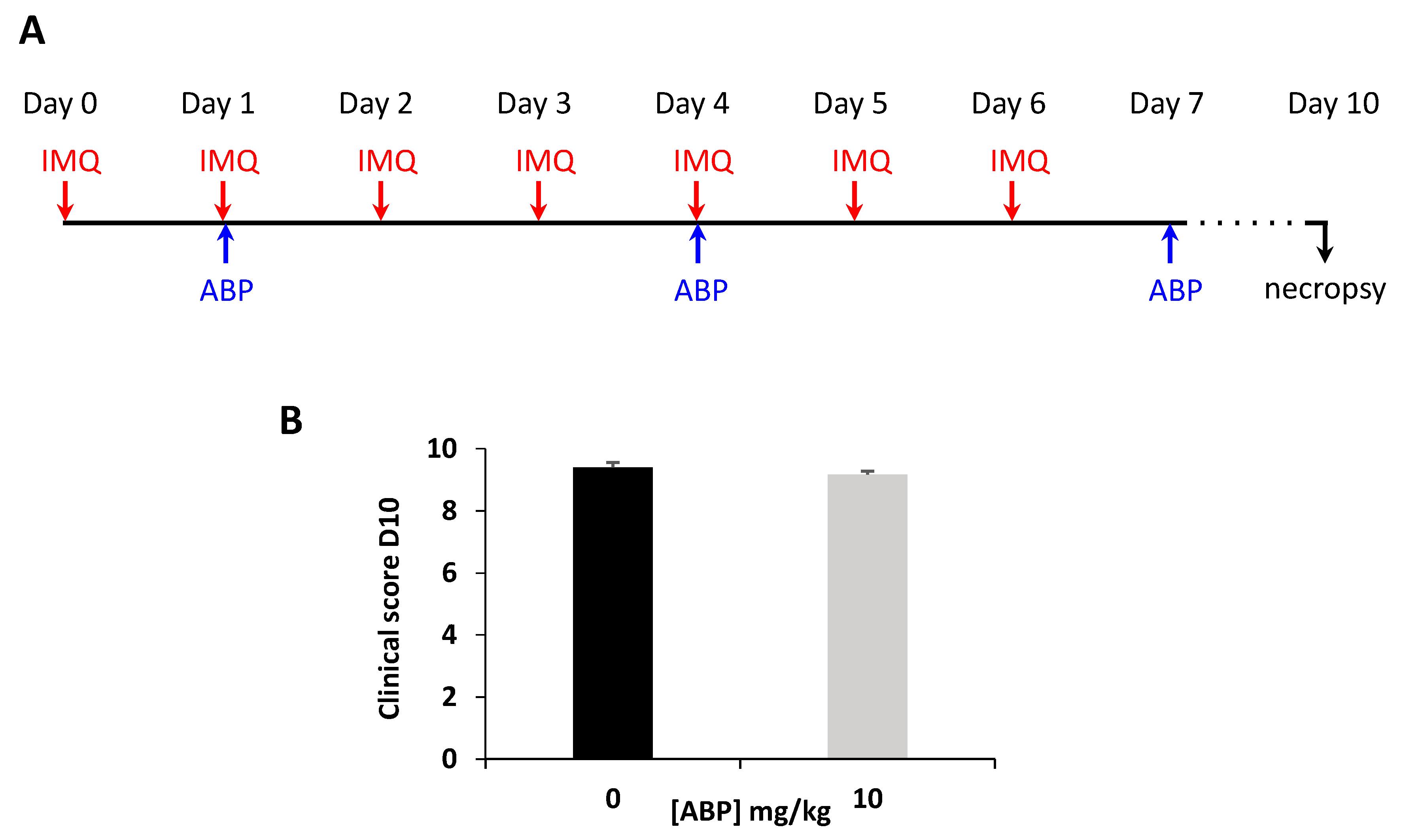
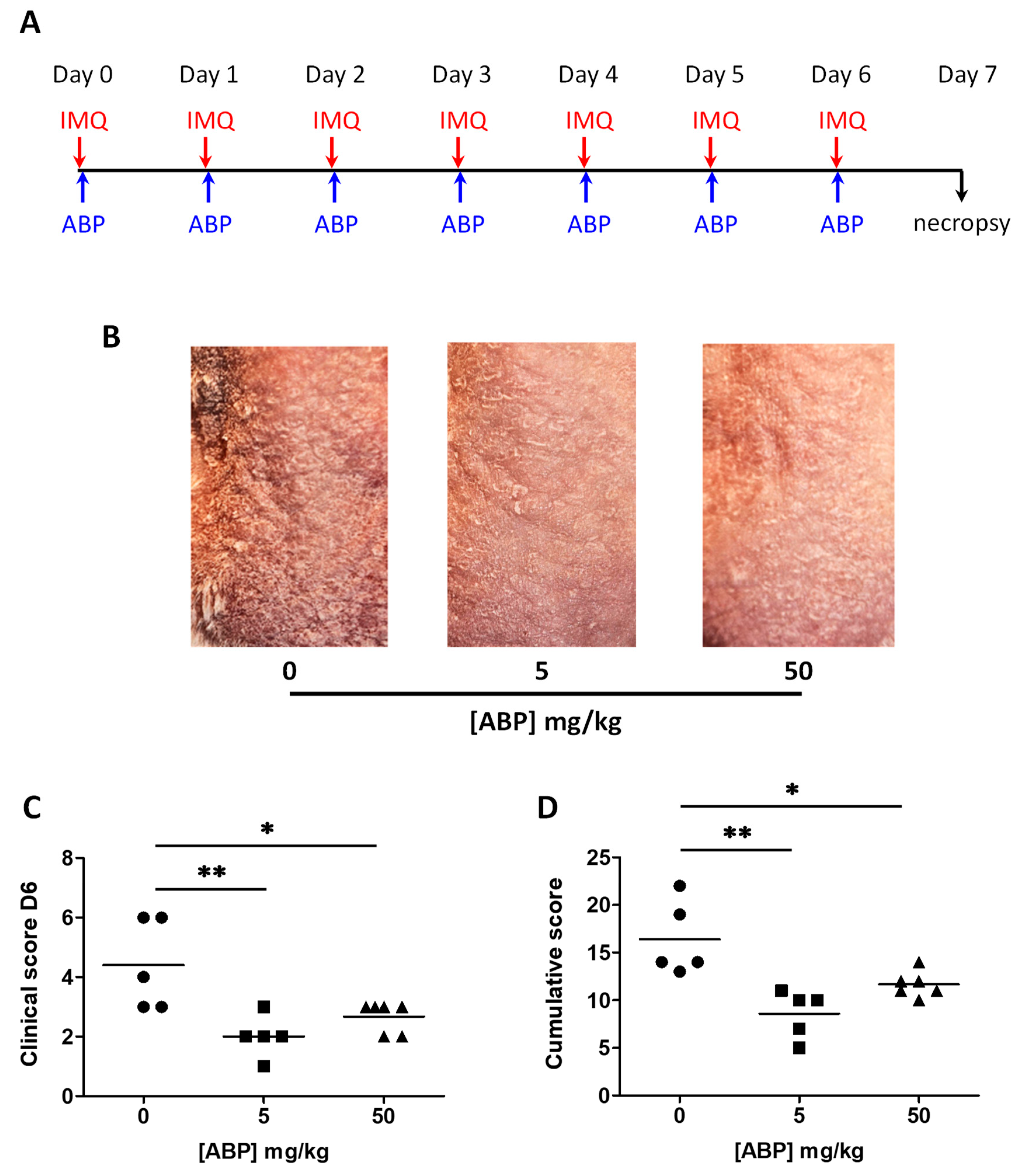
3.1. Clinical Assessment of the Efficacy of the ABP Dendrimer
3.2. Histological Assessment of the Efficacy of the ABP Dendrimer
3.3. Immunological Assessment of the Efficacy of the ABP Dendrimer
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The World Health Organization. Global Report on Psoriasis; WHO Press: Geneva, Switzerland , 2016. [Google Scholar]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 326, 983–994. [Google Scholar] [CrossRef]
- Hedrich, C.M. Shaping the spectrum—From auto-inflammation to auto-immunity. Clin. Immunol. 2016, 165, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Guérard, S.; Fortin, M.M.; Rusu, D.; Soucy, J.; Poubelle, P.E.; Pouliot, R. Pathological crosstalk in vitro between T lymphocytes and lesional keratinocytes in psoriasis: Necessity of direct cell-to-cell contact. Lab. Invest. 2012, 92, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Cline, A.; Hill, D.; Lewallen, R.; Feldman, S.R. Current status and future prospects for biologic treatment of psoriasis. Expert Rev. Clin. Immunol. 2016, 12, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Papp, K.A.; Gooderham, M.; Pariser, D.M.; Augustin, M.; Kerdel, F.A.; Fakharzadeh, S.; Goyal, K.; Calabro, S.; Langholff, W.; et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: Results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Bergers, L.I.J.C.; Reijnders, C.M.A.; van den Broeck, L.J.; Spiekstra, S.W.; de Gruijl, T.D.; Weijers, E.M.; Gibbs, S. Immune-competent human skin disease models. Drug Discov. Today 2016, 21, 1479–1488. [Google Scholar] [CrossRef]
- Wagner, E.F.; Schonthaler, H.B.; Guinea-Viniegra, J.; Tschachler, E. Psoriasis: What have we learned from mouse models. Nat. Rev. Rheumatol. 2010, 6, 704–714. [Google Scholar] [CrossRef]
- van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Buttler, B.; Nestle, F.O. TLRs to cytokines: Mechanistic insights from the imiquimod mouse model of psoriasis. Eur. J. Immunol. 2013, 43, 3138–3146. [Google Scholar] [CrossRef]
- Baek, J.O.; Byamba, D.; Wu, W.H.; Kim, T.G.; Lee, M.G. Assessment of an imiquimod-induced psoriatic mouse model in relation to oxidative stress. Arch. Dermatol. Res. 2012, 304, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Arif, T.; Adil, M. Nanotechnology - Dermatological perspective. Int. J. Nanomed. Nanosurg. 2016, 2. [Google Scholar] [CrossRef]
- Rahman, M.; Akhter, S.; Ahmad, J.; Ahmad, M.Z.; Beg, S.; Ahmad, F.J. Nanomedicine-based drug targeting for psoriasis: Potentials and emerging trends in nanoscale pharmacotherapy. Expert Opin. Drug Deliv. 2014, 12, 635–652. [Google Scholar] [CrossRef]
- Agrawal, U.; Mehra, N.K.; Gupta, U.; Jain, N.K. Hyperbranched dendritic nano-carrier for topical delivery of dithranol. J. Drug Target. 2013, 21, 497–506. [Google Scholar] [CrossRef]
- Fruchon, S.; Poupot, R. Pro-inflammatory versus anti-inflammatory effects of dendrimers: The two faces of immuno-modulatory nanoparticles. Nanomaterials 2017, 7, 251. [Google Scholar] [CrossRef]
- Hayder, M.; Fruchon, S.; Fournié, J.J.; Poupot, M.; Poupot, R. Anti-inflammatory properties of dendrimers per se. Sci. World J. 2011, 11, 1367–1382. [Google Scholar] [CrossRef]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournié, J.J.; Cantagrel, A.; et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci. Transl. Med. 2011, 3, 81ra35. [Google Scholar] [CrossRef]
- Hayder, M.; Varilh, M.; Turrin, C.O.; Saoudi, A.; Caminade, A.M.; Poupot, R.; Liblau, R.S. Phosphorus-based dendrimer ABP treats neuroinflammation by promoting IL-10-producing CD4+ T cells. Biomacromolecules 2015, 16, 3425–3433. [Google Scholar] [CrossRef]
- Fruchon, S.; Caminade, A.M.; Abadie, C.; Davignon, J.L.; Combette, J.M.; Turrin, C.O.; Poupot, R. An azabisphosphonate-capped poly(phosphorhydrazone) dendrimer for the treatment of endotoxin-induced uveitis. Molecules 2013, 18, 9305–9316. [Google Scholar] [CrossRef]
- Fruchon, S.; Poupot, R. The ABP dendrimer, a drug-candidate against inflammatory diseases that triggers the activation of interleukin-10 producing immune cells. Molecules 2018, 23, E1272. [Google Scholar] [CrossRef]
- Fruchon, S.; Poupot, M.; Martinet, L.; Turrin, C.O.; Majoral, J.P.; Fournié, J.J.; Caminade, A.M.; Poupot, R. Anti-inflammatory and immuno-suppressive activation of human monocytes by a bio-active dendrimer. J. Leukoc. Biol. 2009, 85, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Degboé, Y.; Fruchon, S.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Poupot, R.; Cantagrel, A.; Davignon, J.L. Modulation of pro-inflammatory activation of monocytes and dendritic cells by aza-bis-phosphonate dendrimer as an experimental therapeutic agent. Arthritis Res. Ther. 2014, 16, R98. [Google Scholar] [CrossRef] [PubMed]
- Portevin, D.; Poupot, M.; Rolland, O.; Turrin, C.O.; Fournié, J.J.; Majoral, J.P.; Caminade, A.M.; Poupot, R. Regulatory activity of azabisphosphonate-capped dendrimers on human CD4+ T cell proliferation enhances ex-vivo expansion of NK cells from PBMCs for immunotherapy. J. Transl. Med. 2009, 7, 82. [Google Scholar] [CrossRef]
- Poupot, M.; Turrin, C.O.; Caminade, A.M.; Fournié, J.J.; Attal, M.; Poupot, R.; Fruchon, S. Poly(phosphorhydrazone) dendrimers: Yin and yang of monocyte activation for human NK cell amplification applied to immunotherapy against Multiple Myeloma. Nanomedicine 2016, 12, 2321–2330. [Google Scholar] [CrossRef]
- Rolland, O.; Turrin, C.O.; Bacquet, G.; Poupot, R.; Poupot, M.; Caminade, A.M.; Majoral, J.P. Efficient synthesis of phosphorus-containing dendrimers capped with isosteric functions of amino-bis(methylene) phosphonic acids. Tetrahedron Lett. 2009, 50, 2078–2082. [Google Scholar] [CrossRef]
- Ledall, J.; Fruchon, S.; Garzoni, M.; Pavan, G.M.; Caminade, A.M.; Turrin, C.O.; Blanzat, M.; Poupot, R. Interaction studies reveal specific recognition of an anti-inflammatory polyphosphorhydrazone dendrimer by human monocytes. Nanoscale 2015, 7, 17672–17684. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Fruchon, S.; Turrin, C.O.; Poupot, M.; Ouali, A.; Maraval, A.; Garzoni, M.; Maly, M.; Furer, V.; Kovalenko, V.; et al. The key role of the scaffold on the efficiency of dendrimer nanodrugs. Nat. Commun. 2015, 6, 7722. [Google Scholar] [CrossRef]
- Hayder, M.; Garzoni, M.; Bochicchio, D.; Caminade, A.M.; Couderc, F.; Ong-Meang, V.; Davignon, J.L.; Turrin, C.O.; Pavan, G.M.; Poupot, R. Three-dimensional directionality is a pivotal structural feature for the bioactivity of azabisphosphonate-capped poly(phosphorhydrazone) nanodrug dendrimers. Biomacromolecules 2018, 19, 712–720. [Google Scholar] [CrossRef]
- Poupot, M.; Griffe, L.; Marchand, P.; Maraval, A.; Rolland, O.; Martinet, L.; L’Faqihi-Olive, F.E.; Turrin, C.O.; Caminade, A.M.; Fournié, J.J.; et al. Design of phosphorylated dendritic architectures to promote human monocyte activation. FASEB J. 2006, 20, 2339–2351. [Google Scholar] [CrossRef]
- Fruchon, S.; Bellard, E.; Beton, N.; Goursat, C.; Oukhrib, A.; Caminade, A.M.; Blanzat, M.; Turrin, C.O.; Golzio, M.; Poupot, R. Biodistribution and biosafety of a poly(phosphorhydrazone) dendrimer, an anti-inflammatory drug candidate. Biomolecules 2019, 9, E475. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Faunce, D.E.; Stacey, M.; Terajewicz, A.; Nakamura, T.; Zhang-Hoover, J.; Kerley, M.; Mucenski, M.L.; Gordon, S.; Stein-Streilein, J. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in periphery tolerance. J. Exp. Med. 2005, 201, 1615–1625. [Google Scholar] [CrossRef]
- Shaunak, S. Perspective: Dendrimer drugs for infection and inflammation. Biochem. Biophys. Res. Commun. 2015, 468, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S.; Sridevi, S.; Chalasani, K.B.; Jain, A.K.; Jain, S.K.; Jain, N.K.; Diwan, P.V. Dendrimer-mediated transdermal delivery: Enhanced bioavailability of indomethacin. J. Control. Release 2003, 90, 335–343. [Google Scholar] [CrossRef]
- Borowska, K.; Wolowiec, S.; Rubaj, A.; Glowniak, K.; Sieniawska, E.; Radej, S. Effect of polyamidoamine dendrimer G3 and G4 on skin permeation of 8-methoxypsoralene – In vivo study. Int. J. Pharm. 2012, 426, 280–283. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Gorain, B.; Choudhury, H.; Srivastava, A.; Kesharwani, P. Dendrimer entrapped microsponge gel of dithranol for effective topical treatment. Heliyon 2019, 5, e01343. [Google Scholar] [CrossRef]
- Tsakovska, I.; Pajeva, I.; Al Sharif, M.; Alov, P.; Fioravanzo, E.; Kovarich, S.; Worth, A.P.; Richarz, A.N.; Yang, C.; Mostrag-Szlichtyng, A.; et al. Quantitative structure-skin permeability relationships. Toxicology 2017, 387, 27–42. [Google Scholar] [CrossRef]
- Venuganti, V.V.; Perumal, O.P. Poly(amidoamine) dendrimers as skin penetration enhancers: Influence of charge, generation, and concentration. J. Pharm. Sci. 2009, 98, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, Y.; Sun, M.; Wang, Z.; Fan, A.; Zhao, Y. Modulating topical drug delivery via skin pre-treatment with low-generation poly(amidoamine) dendrimers. J. Drug Del. Sci. Tech. 2014, 24, 555–557. [Google Scholar] [CrossRef]
- Huber, C.; Christophers, E. “Keratolytic” effect of salicylic acid. Arch. Dermatol. Res. 1977, 257, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Venuganti, V.V.; Sahdev, P.; Hildreth, M.; Guan, X.; Perumal, O. Structure-skin permeability relationship of dendrimers. Pharm. Res. 2011, 28, 2246–2260. [Google Scholar] [CrossRef] [PubMed]
- Fruchon, S.; Mouriot, S.; Thiollier, T.; Grandin, C.; Caminade, A.M.; Turrin, C.O.; Contamin, H.; Poupot, R. Repeated intravenous injections in non-human primates demonstrate preclinical safety of an anti-inflammatory phosphorus-based dendrimer. Nanotoxicology 2015, 9, 933–941. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jebbawi, R.; Oukhrib, A.; Clement, E.; Blanzat, M.; Turrin, C.-O.; Caminade, A.-M.; Lacoste, E.; Fruchon, S.; Poupot, R. An Anti-Inflammatory Poly(PhosphorHydrazone) Dendrimer Capped with AzaBisPhosphonate Groups to Treat Psoriasis. Biomolecules 2020, 10, 949. https://doi.org/10.3390/biom10060949
Jebbawi R, Oukhrib A, Clement E, Blanzat M, Turrin C-O, Caminade A-M, Lacoste E, Fruchon S, Poupot R. An Anti-Inflammatory Poly(PhosphorHydrazone) Dendrimer Capped with AzaBisPhosphonate Groups to Treat Psoriasis. Biomolecules. 2020; 10(6):949. https://doi.org/10.3390/biom10060949
Chicago/Turabian StyleJebbawi, Ranime, Abdelouahd Oukhrib, Emily Clement, Muriel Blanzat, Cédric-Olivier Turrin, Anne-Marie Caminade, Eric Lacoste, Séverine Fruchon, and Rémy Poupot. 2020. "An Anti-Inflammatory Poly(PhosphorHydrazone) Dendrimer Capped with AzaBisPhosphonate Groups to Treat Psoriasis" Biomolecules 10, no. 6: 949. https://doi.org/10.3390/biom10060949
APA StyleJebbawi, R., Oukhrib, A., Clement, E., Blanzat, M., Turrin, C.-O., Caminade, A.-M., Lacoste, E., Fruchon, S., & Poupot, R. (2020). An Anti-Inflammatory Poly(PhosphorHydrazone) Dendrimer Capped with AzaBisPhosphonate Groups to Treat Psoriasis. Biomolecules, 10(6), 949. https://doi.org/10.3390/biom10060949






