Identifying Metabolomic Profiles of Insulinemic Dietary Patterns
Abstract
1. Introduction
2. Results
2.1. EDIH Validation Study
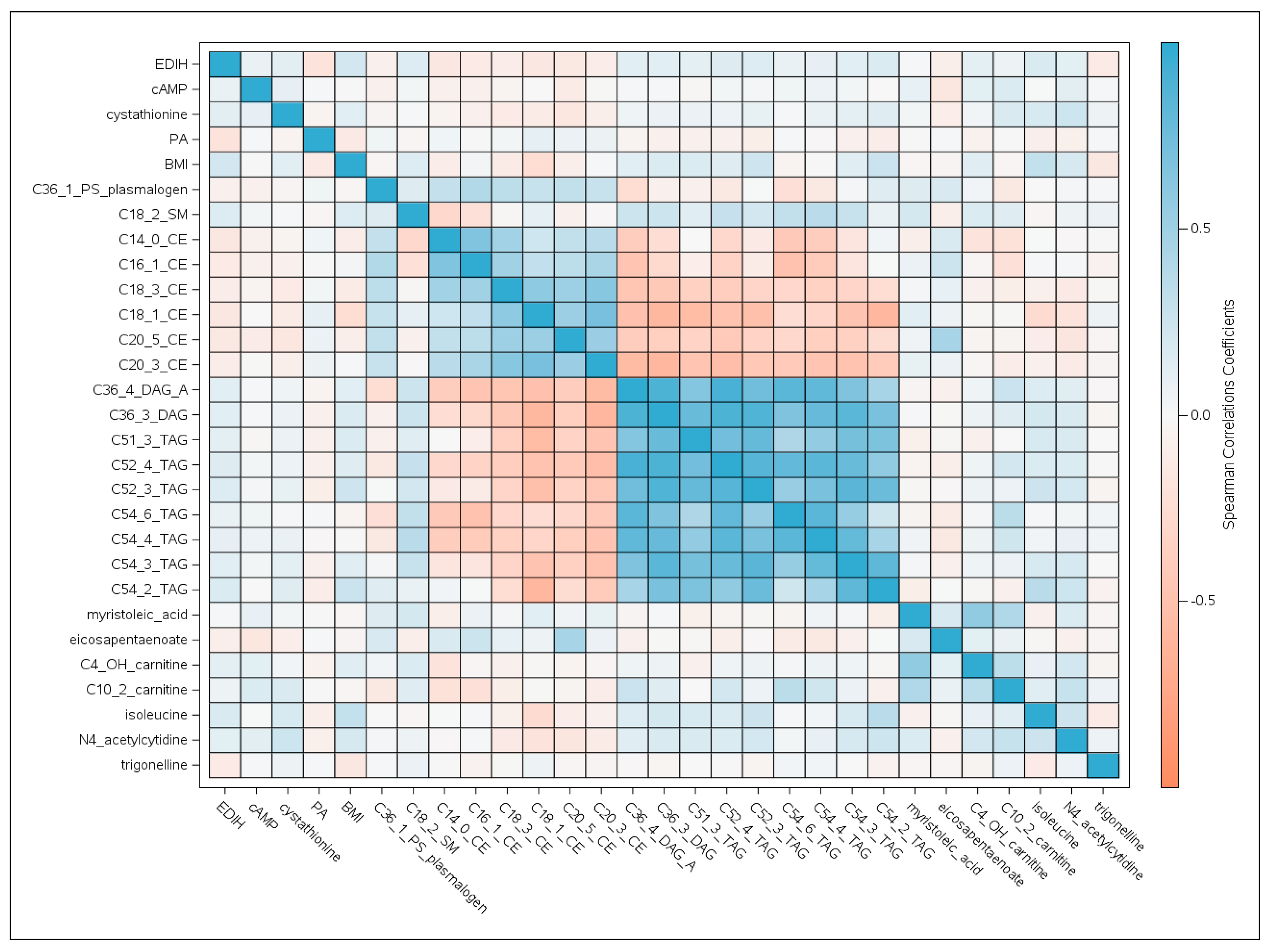
2.2. Metabolomic Profiles of Insulinemic Diets
2.2.1. Characteristics of the EDIH Metabolomics Study Population
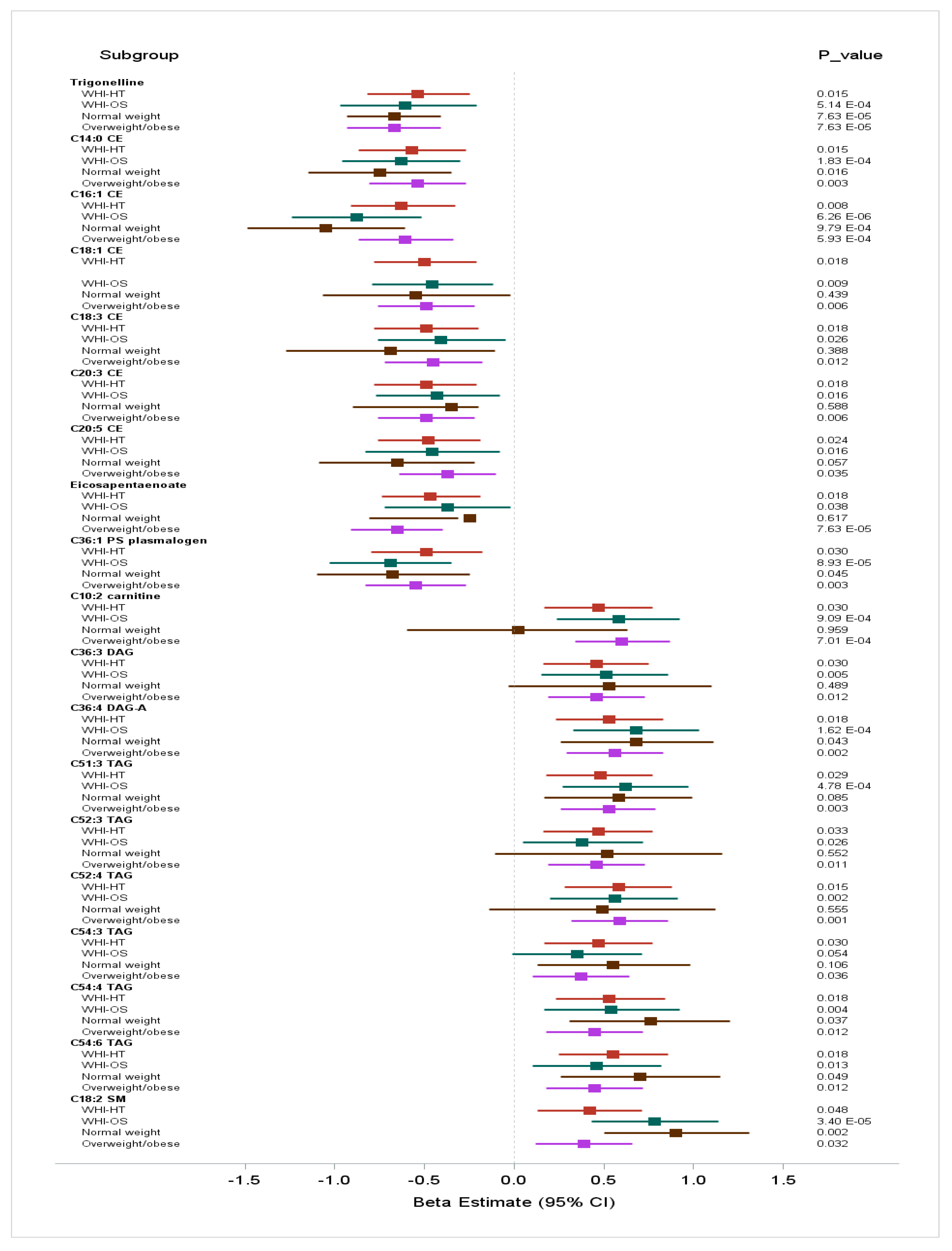
2.2.2. Two-stage Discovery and Replication of Metabolites Associated with Insulinemic Diets
2.2.3. Among Underweight and Normal Weight Women (BMI: 15 to <25 kg/m2, n = 630)
2.2.4. Among Overweight and Obese Women (BMI: 25 to 50 kg/m2, n = 1289)
3. Discussion
4. Methods
4.1. Study Population
4.2. Dietary Assessment and Calculation of the Empirical Dietary Index for Hyperinsulinemia (EDIH) Score
4.3. C-peptide Measurement
4.4. Assessment of Metabolites
4.5. Covariates
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tabung, F.K.; Wang, W.; Fung, T.T.; Hu, F.B.; Smith-Warner, S.A.; Chavarro, J.E.; Charles, S.F.; Willett, W.C.; Giovannucci, E.L. Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. Br. J. Nutr. 2016, 116, 1787–1798. [Google Scholar] [CrossRef]
- Tabung, F.K.; Nimptsch, K.; Giovannucci, E.L. Postprandial duration influences the association of insulin-related dietary indices and plasma C-peptide concentrations in adult men and women. J. Nutr. 2018, 149, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Wang, W.; Fung, T.T.; Smith-Warner, S.A.; Keum, N.N.; Wu, K.; Fuchs, C.S.; Hu, F.B.; Giovannucci, E.L. Association of dietary insulinemic potential and colorectal cancer risk in men and women. Am. J. Clin. Nutr. 2018, 108, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fung, T.T.; Wang, M.; Smith-Warner, S.A.; Giovannucci, E.L.; Tabung, F.K. Association of the insulinemic potential of diet and lifestyle with risk of digestive system cancers in men and women. JNCI Cancer Spectr. 2018, 2, pky080. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Crosslin, D.R.; Haynes, C.; Dungan, J.; Newby, L.K.; Hauser, E.R.; Ginsburg, G.S.; et al. Association of a Peripheral Blood Metabolic Profile with Coronary Artery Disease and Risk of Subsequent Cardiovascular Events. Circ. Cardiovasc. Genet. 2010, 3, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Paynter, N.P.; Balasubramanian, R.; Giulianini, F.; Wang, D.D.; Tinker, L.F.; Gopal, S.; Deik, A.A.; Bullock, K.; Pierce, K.A.; Scott, J.; et al. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation 2018, 137, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hu, F.B.; Ruiz-Canela, M.; Bulló, M.; Toledo, E.; Wang, D.D.; Corella, D.; Gómez-Gracia, E.; Fiol, M.; Estruch, R.; et al. Plasma Metabolites from Choline Pathway and Risk of Cardiovascular Disease in the PREDIMED (Prevention with Mediterranean Diet) Study. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2017, 6, e006524. [Google Scholar] [CrossRef] [PubMed]
- Mayers, J.R.; Wu, C.; Clish, C.B.; Kraft, P.; Torrence, M.E.; Fiske, B.P.; Yuan, C.; Bao, Y.; Townsend, M.K.; Tworoger, S.S.; et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med. 2014, 20, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.S.; Vander Heiden, M.G.; Giovannucci, E.L.; Mucci, L.A. Metabolomic Biomarkers of Prostate Cancer: Prediction, Diagnosis, Progression, Prognosis, and Recurrence. Cancer Epidemiol. Biomark. Prev. 2016, 25, 887–906. [Google Scholar] [CrossRef]
- Playdon, M.C.; Ziegler, R.G.; Sampson, J.N.; Stolzenberg-Solomon, R.; Thompson, H.J.; Irwin, M.L.; Mayne, S.T.; Hoover, R.N.; Moore, S.C. Nutritional metabolomics and breast cancer risk in a prospective study. Am. J. Clin. Nutr. 2017, 106, 637–649. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite Profiles and the Risk of Developing Diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Almanza-Aguilera, E.; Llorach, R.; Vázquez-Fresno, R.; Estruch, R.; Corella, D.; Sorli, J.V.; Carmona, F.; Sanchez-Pla, A.; Salas-Salvado, J.; et al. Non-targeted metabolomic biomarkers and metabotypes of type 2 diabetes: A cross-sectional study of PREDIMED trial participants. Diabetes Metab. 2019, 45, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Kettunen, J.; Würtz, P.; Haller, T.; Havulinna, A.S.; Kangas, A.J.; Soininen, P.; Esko, T.; Tammesoo, M.L.; Mägi, R.; et al. Biomarker Profiling by Nuclear Magnetic Resonance Spectroscopy for the Prediction of All-Cause Mortality: An Observational Study of 17,345 Persons. PLoS Med. 2014, 11, e1001606. [Google Scholar] [CrossRef] [PubMed]
- Playdon, M.C.; Lampe, J.W.; Tinker, L.F.; Prentice, R.; Hayden, K.M.; Van Horn, L.; Sampson, J.; Stolzenberg-Solomon, R.; Moore, S.C. Objective biomarkers of usual diet: A metabolomics analysis of weighed food intake. Am. J. Clin. Nutr. 2016, 104, 776–789. [Google Scholar] [CrossRef]
- Playdon, M.C.; Moore, S.C.; Derkach, A.; Reedy, J.; Subar, A.F.; Sampson, J.N.; Albanes, D.; Gu, F.; Kontto, J.; Lassale, C.; et al. Identifying biomarkers of dietary patterns by using metabolomics. Am. J. Clin. Nutr. 2017, 105, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Satija, A.; Fung, T.T.; Clinton, S.K.; Giovannucci, E.L. Long-term change in both dietary insulinemic and inflammatory potential is associated with weight gain in adult women and men. J. Nutr. 2019, 149, 804–815. [Google Scholar] [CrossRef]
- Ferrannini, E.; Natali, A.; Bell, P.; Cavallo-Perin, P.; Lalic, N.; Mingrone, G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J. Clin. Investig. 1997, 100, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Erion, K.A.; Corkey, B.E. Hyperinsulinemia: A Cause of Obesity? Curr. Obes. Rep. 2017, 6, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.D.; White, E.; Lewis, C.E.; Kotchen, J.M.; Hendrix, S.L.; Trevisan, M. The women’s health initiative observational study: Baseline characteristics of participants and reliability of baseline measures. Ann. Epidemiol. 2003, 13, S107–S121. [Google Scholar] [CrossRef]
- Ritenbaugh, C.; Patterson, R.E.; Chlebowski, R.T.; Caan, B.; Fels-Tinker, L.; Howard, B.; Ockene, J. The women’s health initiative dietary modification trial: Overview and baseline characteristics of participants. Ann. Epidemiol. 2003, 13, S87–S97. [Google Scholar] [CrossRef]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Dietary α-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007, 85, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, J.C.; Vinagre, C.G.; Pozzi, F.S.; Slywitch, E.; Maranhão, R.C. Metabolism of triglyceride-rich lipoproteins and transfer of lipids to high-density lipoproteins (HDL) in vegan and omnivore subjects. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.D.; Hueb, W.; Oliveira, A.A.; Ramires, J.A.F.; Maranhão, R.C. Plasma kinetics of a cholesterol-rich emulsion in subjects with or without coronary artery disease. J. Lipid Res. 2003, 44, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.M.; César, T.B.; Mesquita, C.H.; Maranhão, R.C. High Cholesterol Intake Modifies Chylomicron Metabolism in Normolipidemic Young Men. J. Nutr. 2006, 136, 971–976. [Google Scholar]
- Li, J.; Ren, S.; Piao, H.-L.; Wang, F.; Yin, P.; Xu, C.; Lu, X.; Ye, G.; Shao, Y.; Yan, M.; et al. Integration of lipidomics and transcriptomics unravels aberrant lipid metabolism and defines cholesteryl oleate as potential biomarker of prostate cancer. Sci. Rep. 2016, 6, 20984. [Google Scholar] [CrossRef] [PubMed]
- Klein-Platat, C.; Schlienger, J.-L.; Drai, J.; Oujaa, M.; Simon, C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am. J. Clin. Nutr. 2005, 82, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Paillard, F.; Catheline, D.; Duff, F.L.; Bouriel, M.; Deugnier, Y.; Pouchard, M.; Daubert, J.-C.; Legrand, P. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 436–440. [Google Scholar] [CrossRef]
- Jansen, E.; Khaw, K.-T.; Wareham, N.J.; Patel, P.S.; Luben, R.N.; Sharp, S.J.; Forouhi, M.G. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: A pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Norfolk cohort. Am. J. Clin. Nutr. 2010, 92, 1214–1222. [Google Scholar]
- Chavarro, J.E.; Kenfield, S.A.; Stampfer, M.J.; Loda, M.; Campos, H.; Sesso, H.D.; Ma, J. Blood levels of saturated and monounsaturated fatty acids as markers of de novo lipogenesis and risk of prostate cancer. Am. J. Epidemiol. 2013, 178, 1246–1255. [Google Scholar] [CrossRef]
- Silva Figueiredo, P.; Carla Inada, A.; Marcelino, G.; Maiara Lopes Cardozo, C.; de Cássia Freitas, K.; de Cássia Avellaneda Guimarães, R.; de Castro, A.P.; Aragão do Nascimento, V.; Aiko Hiane, P. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients 2017, 9, 1158. [Google Scholar] [CrossRef]
- Oscarsson, J.; Hurt-Camejo, E. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and their mechanisms of action on apolipoprotein B-containing lipoproteins in humans: A review. Lipids Health Dis. 2017, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kong, D.; Wang, Q.; Wu, W.; Tang, Y.; Bai, T.; Guo, L.; Wei, L.; Zhang, Q.; Yu, Y.; et al. Niacin ameliorates ulcerative colitis via prostaglandin D (2)-mediated D prostanoid receptor 1 activation. EMBO Mol. Med. 2017, 9, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of the receptor (Gpr109a) for niacin and the commensal metabolite butyrate suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, A.E.; Olthof, M.R.; Meeuse, J.C.; Seebus, E.; Heine, R.J.; van Dam, R.M. Acute Effects of Decaffeinated Coffee and the Major Coffee Components Chlorogenic Acid and Trigonelline on Glucose Tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Flock, M.R.; Kris-Etherton, P.M. Diverse physiological effects of long-chain saturated fatty acids: Implications for cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Goñi, F.M.; Alonso, A. Structure and functional properties of diacylglycerols in membranes. This work is dedicated to Professor Vittorio Luzzati on occasion of his 75th birthday. Prog. Lipid Res. 1999, 38, 1–48. [Google Scholar] [CrossRef]
- Yuan, C.; AlEssa, H.B.; Malik, V.S.; Hu, F.B.; Willett, W.C.; Tobias, D.K.; Huang, T. Dietary patterns and cardiometabolic and endocrine plasma biomarkers in US women. Am. J. Clin. Nutr. 2016, 105, 432–441. [Google Scholar]
- Blumenthal, J.A.; Babyak, M.A.; Sherwood, A.; Craighead, L.; Lin, P.-H.; Johnson, J.; Lana, L.W.; Jenny, T.W.; Cynthia, K.; Mark, F.; et al. Effects of the Dietary Approaches to Stop Hypertension Diet Alone and in Combination with Exercise and Caloric Restriction on Insulin Sensitivity and Lipids. Hypertension 2010, 55, 1199–1205. [Google Scholar] [CrossRef]
- Hannon, B.A. Dietary Fiber Is Independently Related to Blood Triglycerides among Adults with Overweight and Obesity. Curr. Dev. Nutr. 2018, 3, nzy094. [Google Scholar] [CrossRef]
- Slattery, M.J.; Bredella, M.A.; Thakur, H.; Torriani, M.; Misra, M. Insulin resistance and impaired mitochondrial function in obese adolescent girls. Metab. Syndr. Relat. Disord. 2014, 12, 56–61. [Google Scholar] [CrossRef]
- Fleischman, A.; Kron, M.; Systrom, D.M.; Hrovat, M.; Grinspoon, S.K. Mitochondrial function and insulin resistance in overweight and normal-weight children. J. Clin. Endocrinol. Metab. 2009, 94, 4923–4930. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.G.; Carpenter, K.; Hammond, J.; Christodoulou, J.; Wilcken, B. Quantitative fibroblast acylcarnitine profiles in mitochondrial fatty acid β-oxidation defects: Phenotype/metabolite correlations. Mol. Genet. Metab. 2002, 76, 327–334. [Google Scholar]
- Osorio, J.H.; Pourfarzam, M. Determination of normal acylcarnitine levels in a healthy pediatric population as a diagnostic tool in inherited errors of mitochondrial fatty acid β-oxidation. An. Pediatría 2007, 67, 548–552. [Google Scholar]
- Schmidt, J.A.; Rinaldi, S.; Ferrari, P.; Carayol, M.; Achaintre, D.; Scalbert, A.; Cross, A.J.; Gunter, M.J.; Fensom, G.K.; Appleby, P.N.; et al. Metabolic profiles of male meat eaters, fish eaters, vegetarians, and vegans from the EPIC-Oxford cohort. Am. J. Clin. Nutr. 2015, 102, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Guasch-Ferré, M.; Bhupathiraju, S.N.; Kanaya, A.M.; Gadgil, M.D.; Newgard, C.B.; Bain, J.R.; Muehlbauer, M.J.; Ilkayeva, O.R.; Scholtens, D.M.; et al. Dietary Patterns among Asian Indians Living in the United States Have Distinct Metabolomic Profiles That Are Associated with Cardiometabolic Risk. J. Nutr. 2018, 148, 1150–1159. [Google Scholar]
- Grzelczyk, A.; Gendaszewska-Darmach, E. Novel bioactive glycerol-based lysophospholipids: New data—New insight into their function. Biochimie 2013, 95, 667–679. [Google Scholar] [CrossRef]
- Xu, Y. Sphingosylphosphorylcholine and lysophosphatidylcholine: G protein-coupled receptors and receptor-mediated signal transduction. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2002, 1582, 81–88. [Google Scholar] [CrossRef]
- del Bas, J.M.; Caimari, A.; Rodriguez-Naranjo, M.I.; Childs, C.E.; Paras Chavez, C.; West, A.L.; Miles, E.A.; Arola, L.; Calder, P.C. Impairment of lysophospholipid metabolism in obesity: Altered plasma profile and desensitization to the modulatory properties of n–3 polyunsaturated fatty acids in a randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 266–279. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Tinker, L.; Shaw, P.A.; Schoeller, D.; Bingham, S.A.; Horn, L.V.; Beresford, S.A.; Caan, B.; Thomson, C.; Satterfield, S.; et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women’s Health Initiative. Am. J. Epidemiol. 2008, 167, 1247–1259. [Google Scholar] [CrossRef]
- Prentice, R.L.; Mossavar-Rahmani, Y.; Huang, Y.; Van Horn, L.; Beresford, S.A.; Caan, B.; Tinker, L.; Schoeller, D.; Bingham, S.; Eaton, C.B.; et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am. J. Epidemiol. 2011, 174, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Women’s Health Initiative Study Group. Design of the Women’s Health Initiative Clinical Trial and Observational Study. Control. Clin. Trials 1998, 19, 61–109. [Google Scholar] [CrossRef]
- Patterson, R.E.; Neuhouser, M.L.; Hedderson, M.M.; Schwartz, S.M.; Standish, L.J.; Bowen, D.J. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J. Am. Diet. Assoc. 2003, 103, 323–328. [Google Scholar] [PubMed]
- Patterson, R.E.; Kristal, A.R.; Tinker, L.F.; Carter, R.A.; Bolton, M.P.; Agurs-Collins, T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann. Epidemiol. 1999, 9, 178–187. [Google Scholar] [CrossRef]
- Hu, F.B.; Rimm, E.; Smith-Warner, S.A.; Feskanich, D.; Stampfer, M.J.; Ascherio, A.; Sampson, L.; Willett, W.C. Reproducibility and validity of dietary patterns assessed with a food frequency questionnaire. Am. J. Clin. Nutr. 1999, 69, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.L.; Manson, J.; Wallace, R.; Lund, B.; Hall, D.; Davis, S.; Shumaker, S.; Wang, C.-Y.; Stein, E.; Prentice, R.L. Implementation of the women’s health initiative study design. Ann. Epidemiol. 2003, 13, S5–S17. [Google Scholar] [CrossRef]
- Kabat, G.C.; Kim, M.Y.; Strickler, H.D.; Shikany, J.M.; Lane, D.; Luo, J.; Ning, Y.; Gunter, M.J.; Rohan, T.E. A longitudinal study of serum insulin and glucose levels in relation to colorectal cancer risk among postmenopausal women. Br. J. Cancer 2012, 106, 227–232. [Google Scholar] [CrossRef]
- Bajad, S.U.; Lu, W.; Kimball, E.H.; Yuan, J.; Peterson, C.; Rabinowitz, J.D. Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography-tandem mass spectrometry. J. Chromatogr. A 2006, 1125, 76–88. [Google Scholar] [CrossRef]
- Rhee, E.P.; Cheng, S.; Larson, M.G.; Walford, G.A.; Lewis, G.D.; McCabe, E.; Yang, E.; Farrell, L.; Fox, C.S.; O’Donnell, C.J.; et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J. Clin. Investig. 2011, 121, 1402–1411. [Google Scholar] [CrossRef]
- Townsend, M.; Clish, C.B.; Kraft, P.; Wu, C.; Souza, A.L.; Deik, A.A.; Tworoger, S.S.; Wolpin, B.M. Reproducibility of Metabolomic Profiles among Men and Women in 2 Large Cohort Studies. Clin. Chem. 2013, 59, 1657–1667. [Google Scholar] [CrossRef]
- Irwin, M.L.; McTiernan, A.; Manson, J.E.; Thomson, C.A.; Sternfeld, B.; Stefanick, M.L.; Wactawski-Wende, J.; Craft, L.; Lane, D.; Martin, L.W.; et al. Physical Activity and Survival in Postmenopausal Women with Breast Cancer: Results from the Women’s Health Initiative. Cancer Prev. Res. 2011, 4, 522–529. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Quintile 1 (−4.44 to <−0.81), n = 183 | Quintile 2 (–0.81 to <–0.31), n = 184 | Quintile 3 (–0.31 to <0.07), n = 184 | Quintile 4 (0.07 to <0.66), n = 184 | Quintile 5 (0.66 to 4.93), n = 184 |
|---|---|---|---|---|---|
| C-peptide, ng/mL | 1.14 ± 0.80 | 1.20 ± 0.77 | 1.32 ± 0.76 | 1.36 ± 0.72 | 1.54 ± 0.78 |
| Age at screening, years | 66.7 ± 6.9 | 66.9 ± 6.7 | 67.1 ± 6.5 | 67.1 ± 6.6 | 65.3 ± 6.6 |
| Body mass index, kg/m2 | 26.1 ± 4.5 | 26.6 ± 4.9 | 27.2 ± 5.5 | 28.0 ± 5.4 | 29.0 ± 5.7 |
| Body mass index categories, % | |||||
| 15–<18.5 (thin) | 1.1 | 1.1 | 1.1 | 1.1 | 0 |
| 18.5–<25 (normal weight) | 46.4 | 39.7 | 40.2 | 29.3 | 25.0 |
| 25–<30 (overweight) | 36.6 | 39.7 | 34.2 | 44.6 | 38.0 |
| 30–50 (obese) | 15.9 | 19.5 | 24.5 | 25.0 | 37.0 |
| Physical activity, MET-hour/week | 10.0 ± 11.7 | 9.4 ± 12.3 | 9.1 ± 11.0 | 7.3 ± 10.1 | 5.3 ± 7.7 |
| Aspirin/NSAID user, % | 53 | 56 | 57.1 | 53.3 | 52.3 |
| Educational level, % | |||||
| Some high school or lower educational level | 2.7 | 3.8 | 3.3 | 5.4 | 6.5 |
| High school graduate/some college or associate degree | 45.9 | 57.1 | 48.4 | 65.2 | 69.6 |
| ≥4y of college | 51.4 | 39.1 | 48.4 | 29.4 | 23.9 |
| Race/ethnicity, % | |||||
| African American | 6.0 | 6.5 | 8.7 | 9.2 | 13.0 |
| European American | 89.6 | 86.4 | 84.2 | 89.1 | 79.4 |
| Other | 4.4 | 7.1 | 7.1 | 1.7 | 7.6 |
| Smoking status, % | |||||
| Never | 42.6 | 50.5 | 51.1 | 56.0 | 48.4 |
| Former | 51.9 | 43.5 | 44.0 | 37.5 | 42.4 |
| Current | 5.5 | 6.0 | 4.9 | 6.5 | 9.2 |
| Menopausal hormone use, % | |||||
| Unopposed estrogen use, ever | 32.8 | 39.7 | 37.0 | 42.9 | 34.8 |
| Estrogen plus progestin use, ever | 29.0 | 26.6 | 18.5 | 19.0 | 21.7 |
| Statistical Models | EDIH Quintiles | P-Trend 4 | ||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Absolute concentrations (ng/mL) | ||||||
| Model 1 | 1.14 (1.07, 1.22) | 1.20 (1.12, 1.28) | 1.33 (1.24, 1.42) | 1.37 (1.28, 1.46) | 1.54 (1.44, 1.64) | <0.0001 |
| Model 2 | 1.21 (0.94, 1.56) | 1.26 (0.99, 1.61) | 1.40 (1.10, 1.80) | 1.37 (1.08, 1.76) | 1.53 (1.19, 1.96) | <0.0001 |
| Model 3 | 1.19 (0.95, 1.50) | 1.22 (0.98, 1.53) | 1.30 (1.04, 1.63) | 1.41 (1.04, 1.63) | 1.41 (1.13, 1.77) | <0.0001 |
| Relative concentrations (percent change) | ||||||
| Model 1 | 0 (ref) | 5 (−7, 18) | 16 (3, 31) | 20 (6, 35) | 34 (19, 51) | <0.0001 |
| Model 2 | 0 (ref) | 4 (−7, 17) | 16 (3, 30) | 13 (1, 28) | 26 (12, 42) | <0.0001 |
| Model 3 | 0 (ref) | 3 (−8, 14) | 12 (1, 25) | 9 (−2, 22) | 18 (6, 32) | <0.0001 |
| Normal weight (BMI: 15 to <25 kg/m2, n = 340): absolute concentrations (ng/mL) | ||||||
| Model 1 + BMI | 0.98 (0.89, 1.05) | 0.94 (0.87, 1.03) | 1.14 (1.04, 1.24) | 1.07 (0.97, 1.18) | 1.09 (0.97, 1.22) | 0.02 |
| Model 3 | 0.90 (0.67, 1.21) | 0.87 (0.65, 1.17) | 1.09 (0.73, 1.32) | 0.98 (0.73, 1.32) | 0.97 (0.71, 1.32) | 0.09 |
| Normal weight (BMI: 15 to <25 kg/m2, n = 340): relative concentrations (percent change) | ||||||
| Model 1 + BMI | 0 (ref) | −2 (−16, 13) | 18 (1, 37) | 10 (−6, 30) | 12 (−5, 34) | 0.02 |
| Model 3 | 0 (ref) | −3 (−17, 14) | 21 (3, 42) | 9 (−8, 30) | 8 (−11, 30) | 0.09 |
| Overweight/obese (BMI: 25 to 50 kg/m2, n = 579): absolute concentrations (ng/mL) | ||||||
| Model 1 + BMI | 1.37 (1.26, 1.50) | 1.44 (1.33, 1.57) | 1.47 (1.36, 1.60) | 1.51 (1.40, 1.63) | 1.68 (1.56, 1.80) | 0.008 |
| Model 3 | 1.48 (1.10, 1.99) | 1.55 (1.16, 2.08) | 1.59 (1.19, 2.13) | 1.60 (1.20, 2.13) | 1.82 (1.36, 2.42) | 0.0005 |
| Overweight/obese (BMI: 25 to 50 kg/m2, n = 579): relative concentrations (percent change) | ||||||
| Model 1 + BMI | 0 (ref) | 5 (−9, 22) | 7 (−7, 25) | 10 (−6, 27) | 22 (6, 41) | 0.008 |
| Model 3 | 0 (ref) | 5 (−10, 22) | 8 (−7, 25) | 8 (−7, 25) | 23 (6, 42) | 0.0005 |
| - | Quintile 1 (–5.36 to <–0.72) n = 383 | Quintile 2 (–0.72 to <–0.21) n = 384 | Quintile 3 (–0.21 to <0.20) n = 384 | Quintile 4 (0.20 to <0.74) n = 384 | Quintile 5 (0.74 to 6.64) n = 384 |
|---|---|---|---|---|---|
| Food/food groups, servings/week | |||||
| Red meat | 3.3 ± 3.2 | 3.0 ± 2.8 | 3.2 ± 3.1 | 3.4 ± 2.7 | 4.8 ± 4.2 |
| Sugar-sweetened beverages | 0.4 ± 1.0 | 0.5 ± 1.4 | 0.5 ± 1.3 | 1.3 ± 2.8 | 4.3 ± 9.0 |
| Cream soup | 0.2 ± 0.4 | 0.2 ± 0.3 | 0.3 ± 0.4 | 0.3 ± 0.4 | 0.5 ± 0.8 |
| Processed meat | 1.2 ± 1.5 | 1.3 ± 1.5 | 1.7 ± 1.7 | 1.9 ± 2.1 | 3.7 ± 3.5 |
| Butter and margarine | 3.0 ± 3.6 | 3.3 ± 3.8 | 4.5 ± 4.3 | 6.0 ± 4.8 | 10.5 ± 9.0 |
| Poultry | 2.3 ± 1.7 | 2.3 ± 1.7 | 2.4 ± 1.8 | 2.5 ± 1.8 | 3.2 ± 2.4 |
| White/non-oily fish | 1.6 ± 1.5 | 1.4 ± 1.3 | 1.4 ± 1.2 | 1.5 ± 1.6 | 1.7 ± 1.8 |
| French fries | 0.2 ± 0.3 | 0.2 ± 0.3 | 0.2 ± 0.4 | 0.3 ± 0.5 | 0.7 ± 1.1 |
| Tomatoes | 3.6 ± 3.2 | 3.7 ± 3.6 | 3.2 ± 3.0 | 3.7 ± 3.6 | 4.4 ± 4.8 |
| Low-fat dairy | 14.8 ± 12.5 | 13.4 ± 11.8 | 14.2 ± 13.2 | 12.3 ± 11.9 | 13.5 ± 13.8 |
| Eggs | 0.7 ± 1.0 | 0.8 ± 1.4 | 0.9 ± 1.1 | 1.0 ± 1.1 | 1.6 ± 2.3 |
| Refined grains | 25.2 ± 14.5 | 21.4 ± 12.2 | 20.2 ± 12.7 | 19.8 ± 11.8 | 24.4 ± 14.4 |
| Whole grains | 9.9 ± 6.5 | 8.3 ± 5.3 | 7.6 ± 5.1 | 6.7 ± 4.6 | 7.3 ± 5.6 |
| Wine | 3.9 ± 6.0 | 1.1 ± 2.1 | 0.6 ± 1.3 | 0.5 ± 1.4 | 0.3 ± 1.0 |
| Tea/coffee | 21.1 ± 15.2 | 16.4 ± 12.0 | 14.0 ± 12.4 | 12.9 ± 11.7 | 13.0 ± 12.5 |
| Whole fruit | 18.3 ± 10.4 | 16 ± 8.8 | 13.0 ± 7.6 | 10.0 ± 7.0 | 9.4 ± 7.3 |
| High-fat dairy | 3.2 ± 4.1 | 2.3 ± 3.4 | 2.3 ± 3.1 | 2.1 ± 2.4 | 2.8 ± 3.3 |
| Green-leafy vegetables | 7.8 ± 6.1 | 6.3 ±4.5 | 5.7 ± 4. | 5.0 ± 4.5 | 4.7 ± 4.0 |
| Nutrient intakes | |||||
| Fiber, g/d | 19.8 ± 7.6 | 16.8 ± 6.2 | 14.7 ± 5.6 | 12.8 ± 5.5 | 13.6 ± 6.3 |
| Carbohydrate, g/d | 235 ± 83 | 202 ± 66 | 185 ± 70 | 170 ± 65 | 204 ± 93 |
| Protein, g/d | 72.9 ± 29.0 | 64.1 ± 26.3 | 62.5 ± 28.5 | 59.7 ± 24.7 | 72.0 ± 32.9 |
| Total fat, g/d | 58.5 ± 29.0 | 50.8 ± 28.4 | 53.7 ± 30.2 | 56.1 ± 28.0 | 75.6 ± 41.7 |
| Saturated fat, g/d | 19.8 ± 10.7 | 17.0 ± 9.9 | 18.0 ± 10.9 | 18.6 ± 10.0 | 25.3 ± 15.0 |
| Cholesterol, g/d | 201 ± 119 | 191 ± 130 | 198 ± 119 | 207 ± 109 | 286 ± 191 |
| Calcium, mg/d | 978 ± 496 | 817 ± 413 | 780 ± 469 | 671 ± 380 | 737 ± 425 |
| Lycopene, mcg/d | 5539 ± 3657 | 5125 ± 3246 | 4164 ± 2557 | 4389 ± 3466 | 4651 ± 3465 |
| - | - | - | Associations in WHI-HT (Discovery, n = 1109) | Associations in WHI-OS (Replication, n = 810) | ||
|---|---|---|---|---|---|---|
| Metabolite | HMDB ID | Category | Beta Estimate (95% CI) | FDR-Adjusted P-value | Beta Estimate (95% CI) | FDR-Adjusted P-value |
| C14:0 CE | HMDB0006725 | Cholesterol esters | −0.57 (−0.87, −0.27) | 0.015 | −0.63 (−0.96, −0.30) | 1.83 × 104 |
| C16:1 CE | HMDB0000658 | Cholesterol esters | −0.63 (−0.91, −0.33) | 0.008 | −0.88 (−1.24, −0.52) | 6.26 × 106 |
| C18:1 CE | HMDB0000918 | Cholesterol esters | −0.50 (−0.78, −0.21) | 0.018 | −0.46 (−0.79, −0.12) | 0.009 |
| C18:3 CE | HMDB0010370 | Cholesterol esters | −0.49 (−0.78, −0.20) | 0.018 | −0.41 (−0.76, −0.05) | 0.026 |
| C20:3 CE | HMDB0006736 | Cholesterol esters | −0.49 (−0.78, −0.21) | 0.018 | −0.43 (−0.77, −0.08) | 0.016 |
| C20:5 CE | HMDB0006731 | Cholesterol esters | −0.48 (−0.76, −0.19) | 0.024 | −0.46 (−0.83, −0.08) | 0.016 |
| Trigonelline | HMDB0000875 | Alkaloid and derivatives | −0.54 (−0.82, −0.25) | 0.015 | −0.61 (−0.97, −0.27) | 5.14 × 104 |
| C36:1 PS plasmalogen | Unknown | Other | −0.49 (−0.80, −0.18) | 0.030 | −0.69 (−1.03, −0.35) | 8.93 × 105 |
| Eicosapentaenoate | HMDB0001999 | Fatty acids | −0.47 (−0.74, −0.19) | 0.018 | −0.37 (−0.72, −0.02) | 0.038 |
| Myristoleic acid | HMDB0002000 | Fatty acids | 0.43 (0.14, 0.73) | 0.047 | 0.16 (−0.16, 0.49) | 0.325 |
| C4−OH carnitine | HMDB0013127 | Acylcarnitines | 0.40 (0.12, 0.68) | 0.048 | 0.25 (−0.12, 0.61) | 0.179 |
| C10:2 carnitine | HMDB0013325 | Acylcarnitines | 0.47 (0.17, 0.77) | 0.030 | 0.58 (0.24, 0.92) | 9.09 × 104 |
| C18:2 SM | HMDB0012101 | Sphingomyelins | 0.42 (0.13, 0.71) | 0.048 | 0.78 (0.43, 1.14) | 3.40 × 105 |
| C36:3 DAG | HMDB0007219 | Diacylglycerols | 0.46 (0.16, 0.75) | 0.030 | 0.51 (0.15, 0.86) | 0.005 |
| C36:4 DAG−A | HMDB0007248 | Diacylglycerols | 0.53 (0.23, 0.83) | 0.018 | 0.68 (0.33, 1.03) | 1.62 × 104 |
| C51:3 TAG | Unknown | Triacylglycerols | 0.48 (0.18, 0.77) | 0.030 | 0.62 (0.27, 0.97) | 4.78 × 104 |
| C52:3 TAG | HMDB0005384 | Triacylglycerols | 0.47 (0.16, 0.77) | 0.033 | 0.38 (0.05, 0.72) | 0.026 |
| C52:4 TAG | HMDB0005363 | Triacylglycerols | 0.58 (0.28, 0.88) | 0.015 | 0.56 (0.20, 0.91) | 0.002 |
| C54:2 TAG | HMDB0005403 | Triacylglycerols | 0.44 (0.15, 0.73) | 0.035 | 0.20 (−0.15, 0.55) | 0.269 |
| C54:3 TAG | HMDB0005405 | Triacylglycerols | 0.47 (0.17, 0.77) | 0.030 | 0.35 (−0.01, 0.71) | 0.054 |
| C54:4 TAG | HMDB0005370 | Triacylglycerols | 0.53 (0.23, 0.84) | 0.018 | 0.54 (0.17, 0.92) | 0.004 |
| C54:6 TAG | HMDB0005391 | Triacylglycerols | 0.55 (0.25, 0.86) | 0.018 | 0.46 (0.10, 0.82) | 0.013 |
| cAMP | HMDB0000058 | Purines and Pyrimidines | 0.37 (0.12, 0.62) | 0.047 | 0.20 (−0.68, 0.27) | 0.401 |
| N4-acetylcytidine | HMDB0005923 | Purines and Pyrimidines | 0.43 (0.16, 0.71) | 0.030 | 0.10 (−0.24, 0.44) | 0.563 |
| Isoleucine | HMDB0000172 | Amino acids | 0.47 (0.20, 0.74) | 0.018 | 0.13 (−0.23, 0.49) | 0.472 |
| Cystathionine | HMDB0000099 | Amino Acids | 0.51 (0.23, 0.79) | 0.018 | 0.07 (−0.28, 0.42) | 0.689 |
| Metabolite | HMDB ID | Category | Beta Estimate (95% CI) | FDR-Adjusted P-value |
|---|---|---|---|---|
| C14:0 CE | HMDB0006725 | Cholesteryl esters | −0.75 (−1.15, −0.35) | 0.016 |
| C16:1 CE | HMDB0000658 | Cholesteryl esters | −1.05 (−1.49, −0.61) | 0.001 |
| C20:5 CE | HMDB0006731 | Cholesteryl esters | −0.65 (−1.09, −0.22) | 0.057 |
| N-acetylornithine | HMDB0003357 | Other | −0.82 (−1.23, −0.42) | 0.006 |
| C22:6 LPE | HMDB0011526 | Lysophosphatidylethanolamine | −0.56 (−0.98, −0.14) | 0.097 |
| C34:0 PS | HMDB0012356 | Other | −0.68 (−1.12, −0.24) | 0.053 |
| C30:0 PC | HMDB0007869 | Phosphatidylcholines | −0.60 (−1.02, −0.18) | 0.079 |
| C30:1 PC | HMDB0007870 | Phosphatidylcholines | −0.53 (−0.93, −0.14) | 0.097 |
| C32:1 PC | HMDB0007873 | Phosphatidylcholines | −0.85 (−1.27, −0.42) | 0.008 |
| C32:1 PC plasmalogen-A | HMDB0013404 | Phosphatidylcholine plasmalogens | −0.53 (−0.92, −0.15) | 0.095 |
| C34:1 PC | HMDB0007972 | Phosphatidylcholines | −0.77 (−1.19, −0.35) | 0.019 |
| C36:1 PS plasmalogen | Unavailable | Phosphatidylethanolamine plasmalogens | −0.68 (−1.10, −0.25) | 0.045 |
| C36:4 PE | HMDB0008937 | Phosphatidylethanolamine | −0.58 (−1.01, −0.15) | 0.097 |
| C36:5 PC | HMDB0007890 | Phosphatidylcholines | −0.76 (−1.20, −0.32) | 0.031 |
| 1-methylguanosine | HMDB0001563 | Purines and Pyrimidines | −0.61 (−1.02, −0.21) | 0.057 |
| Urate | HMDB0000289 | Purines and Pyrimidines | −0.54 (−0.93, −0.15) | 0.095 |
| Palmitoleic acid | HMDB0003229 | Fatty acids | −0.61 (−1.03, −0.19) | 0.079 |
| Myristoleic acid | HMDB0002000 | Fatty acids | 0.69 (0.27, 1.12) | 0.043 |
| C18:0 LPC plasmalogen | HMDB0011149 | Lysophosphatidylcholine plasmalogens | 0.55 (0.14, 0.97) | 0.097 |
| C18:1 LPC plasmalogen | HMDB0011149 | Lysophosphatidylcholine plasmalogens | 0.56 (0.14, 0.98) | 0.097 |
| C18:2 SM | HMDB0012101 | Sphingomyelins | 0.90 (0.50, 1.31) | 0.002 |
| C22:1 MAG | HMDB0011582 | Monoacylglycerols | −0.60 (−1.01, −0.19) | 0.076 |
| C36:4 DAG-A | HMDB0007248 | Diacylglycerols | 0.68 (0.26, 1.11) | 0.043 |
| C51:3 TAG | Unavailable | Triacylglycerols | 0.58 (0.17, 0.99) | 0.085 |
| C54:3 TAG | HMDB0005405 | Triacylglycerols | 0.55 (0.13, 0.98) | 0.106 |
| C54:4 TAG | HMDB0005370 | Triacylglycerols | 0.76 (0.31, 1.20) | 0.037 |
| C54:6 TAG | HMDB0005391 | Triacylglycerols | 0.70 (0.26, 1.15) | 0.050 |
| Trimethylamine-N-oxide | HMDB0000925 | Other | 0.56 (0.15, 0.98) | 0.096 |
| Glycoursodeoxycholate | HMDB0000708 | Bile acids | 0.58 (0.15, 1.02) | 0.097 |
| Metabolite | HMDB ID | Category | Beta Estimate (95% CI) | FDR-Adjusted P-value |
|---|---|---|---|---|
| Eicosapentaenoate | HMDB0001999 | Fatty acids | −0.65 (−0.91, −0.40) | 7.63 × 105 |
| Palmitoleic acid | HMDB0003229 | Fatty acids | −0.39 (−0.67, −0.12) | 0.032 |
| Myristoleic acid | HMDB0002000 | Fatty acids | 0.38 (−0.11, 0.64) | 0.035 |
| 2−hydroxyhexadecanoate | HMDB0031057 | Fatty acids | 0.39 (0.12, 0.66) | 0.032 |
| C14:0 CE | HMDB0006725 | Cholesterol esters | −0.54 (−0.81, −0.27) | 0.003 |
| C16:1 CE | HMDB0000658 | Cholesterol esters | −0.61 (−0.87, −0.34) | 5.93 × 104 |
| C18:1 CE | HMDB0000918 | Cholesterol esters | −0.49 (−0.76, −0.22) | 0.006 |
| C18:3 CE | HMDB0010370 | Cholesterol esters | −0.45 (−0.72, −0.18) | 0.012 |
| C20:3 CE | HMDB0006736 | Cholesterol esters | −0.49 (−0.76, −0.22) | 0.006 |
| C20:5 CE | HMDB0006731 | Cholesterol esters | −0.37 (−0.64, −0.10) | 0.035 |
| Trigonelline | HMDB0000875 | Alkaloid and derivatives | −0.67 (−0.93, −0.41) | 7.63 × 105 |
| C16:1 LPC | HMDB0010383 | Phosphatidylcholines | −0.54 (−0.81, −0.26) | 0.003 |
| C20:1 LPC | HMDB0010391 | Phosphatidylcholines | −0.54 (−0.82, −0.27) | 0.003 |
| C24:0 LPC | HMDB0008038 | Phosphatidylcholines | −0.49 (−0.76, −0.23) | 0.005 |
| C28:0 PC | HMDB0007866 | Phosphatidylcholines | −0.37 (−0.65, −0.10) | 0.040 |
| C30:0 PC | HMDB0007869 | Phosphatidylcholines | −0.40 (−0.67, −0.13) | 0.026 |
| C30:1 PC | HMDB0007870 | Phosphatidylcholines | −0.43 (−0.70, −0.17) | 0.014 |
| C32:1 PC | HMDB0007873 | Phosphatidylcholines | −0.40 (−0.66, −0.13) | 0.026 |
| C34:1 PC | HMDB0007972 | Phosphatidylcholines | −0.35 (−0.62, −0.08) | 0.046 |
| C40:10 PC | HMDB0008511 | Phosphatidylcholines | −0.34 (−0.61, −0.08) | 0.050 |
| C32:1 PC plasmalogen-A | HMDB0013404 | Phosphatidylcholine plasmalogens | −0.40 (−0.67, −0.13) | 0.024 |
| C34:2 PC plasmalogen-B | HMDB0011210 | Phosphatidylcholine plasmalogens | −0.44 (−0.70, −0.18) | 0.012 |
| C14:0 LPC | HMDB0010379 | Lysophosphatidylcholines | −0.39 (−0.66, −0.12) | 0.032 |
| C14:0 LPC-A | HMDB0010379 | Lysophosphatidylcholines | −0.44 (−0.71, −0.17) | 0.014 |
| C18:1 LPC | HMDB0002815 | Lysophosphatidylcholines | −0.36 (−0.63, −0.09) | 0.042 |
| C18:3 LPC | HMDB0010387 | Lysophosphatidylcholines | −0.38 (−0.66, −0.10) | 0.040 |
| C20:3 LPC | HMDB0010393 | Lysophosphatidylcholines | −0.37 (−0.64, −0.09) | 0.040 |
| C16:0 LPE | HMDB0011503 | Lysophosphatidylethanolamines | −0.45 (−0.72, −0.18) | 0.012 |
| C18:1 LPE | HMDB0011506 | Lysophosphatidylethanolamines | −0.38 (−0.66, −0.11) | 0.036 |
| C22:6 LPE-B | HMDB0011526 | Lysophosphatidylethanolamines | −0.36 (−0.63, −0.09) | 0.040 |
| C14:0 SM | HMDB0012097 | Sphingomyelins | −0.45 (−0.71, −0.18) | 0.012 |
| C18:2 SM | HMDB0012101 | Sphingomyelins | 0.39 (0.12, 0.66) | 0.032 |
| C24:1 SM | HMDB0012107 | Sphingomyelins | −0.43 (−0.70, −0.16) | 0.017 |
| C4-OH carnitine | HMDB0013127 | Acylcarnitines | 0.43 (0.17, 0.68) | 0.012 |
| C6 carnitine | HMDB0000705 | Acylcarnitines | 0.47 (0.20, 0.74) | 0.011 |
| C7 carnitine | HMDB0013238 | Acylcarnitines | 0.47 (0.21, 0.72) | 0.006 |
| C9 carnitine | HMDB0013288 | Acylcarnitines | 0.43 (0.17, 0.70) | 0.014 |
| C10:2 carnitine | HMDB0013325 | Acylcarnitines | 0.60 (0.34, 0.87) | 7.01 × 104 |
| C14:2 carnitine | HMDB0013331 | Acylcarnitines | 0.38 (0.11, 0.65) | 0.032 |
| C36:3 DAG | HMDB0007219 | Diacylglycerols | 0.46 (0.19, 0.73) | 0.012 |
| C36:4 DAG-A | HMDB0007248 | Diacylglycerols | 0.56 (0.29, 0.83) | 0.002 |
| C51:3 TAG | Unknown | Triacylglycerols | 0.53 (0.26, 0.79) | 0.003 |
| C52:2 TAG | HMDB0005369 | Triacylglycerols | 0.34 (0.08, 0.60) | 0.047 |
| C52:3 TAG | HMDB0005384 | Triacylglycerols | 0.46 (0.19, 0.73) | 0.011 |
| C52:4 TAG | HMDB0005363 | Triacylglycerols | 0.59 (0.32, 0.86) | 0.001 |
| C54:2 TAG | HMDB0005403 | Triacylglycerols | 0.38 (0.12, 0.65) | 0.032 |
| C54:3 TAG | HMDB0005405 | Triacylglycerols | 0.37 (0.10, 0.64) | 0.036 |
| C54:4 TAG | HMDB0005370 | Triacylglycerols | 0.45 (0.18, 0.72) | 0.012 |
| C54:6 TAG | HMDB0005391 | Triacylglycerols | 0.45 (0.18, 0.72) | 0.012 |
| Isoleucine | HMDB0000172 | Amino acids | 0.47 (0.22, 0.72) | 0.005 |
| Dimethylglycine | HMDB0000092 | Amino Acids | 0.40 (0.12, 0.66) | 0.032 |
| Cystathionine | HMDB0000099 | Amino Acids | 0.33 (0.08, 0.58) | 0.046 |
| 2-aminooctanoate | HMDB0000991 | Amino Acids | 0.38 (0.10, 0.66) | 0.038 |
| Pantothenate | HMDB0000210 | Amino Acids | 0.34 (0.61, 0.08) | 0.047 |
| N-methylproline | HMDB0094696 | Amino Acids | 0.44 (0.70, 0.17) | 0.012 |
| C36:1 PS plasmalogen | Unknown | Other | 0.55 (0.83, 0.27) | 0.003 |
| X4-pyridoxate | Unknown | Other | 0.42 (0.67, 0.16) | 0.014 |
| Proline betaine | HMDB0004827 | Other | 0.40 (0.66, 0.14) | 0.024 |
| Indole-3-propionate | HMDB0002302 | Other | 0.35 (0.61, 0.09) | 0.040 |
| Cortisol | HMDB0000063 | Steroids | 0.37 (0.64, 0.10) | 0.040 |
| C23:0 Ceramide (d18:1) | HMDB0000950 | Ceramides | 0.39 (0.12, 0.66) | 0.032 |
| N4-acetylcytidine | HMDB0005923 | Purines and Pyrimidines | 0.37 (0.11, 0.62) | 0.032 |
| Cytidine | HMDB0000089 | Purines and Pyrimidines | 0.37 (0.10, 0.64) | 0.040 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabung, F.K.; Balasubramanian, R.; Liang, L.; Clinton, S.K.; Cespedes Feliciano, E.M.; Manson, J.E.; Van Horn, L.; Wactawski-Wende, J.; Clish, C.B.; Giovannucci, E.L.; et al. Identifying Metabolomic Profiles of Insulinemic Dietary Patterns. Metabolites 2019, 9, 120. https://doi.org/10.3390/metabo9060120
Tabung FK, Balasubramanian R, Liang L, Clinton SK, Cespedes Feliciano EM, Manson JE, Van Horn L, Wactawski-Wende J, Clish CB, Giovannucci EL, et al. Identifying Metabolomic Profiles of Insulinemic Dietary Patterns. Metabolites. 2019; 9(6):120. https://doi.org/10.3390/metabo9060120
Chicago/Turabian StyleTabung, Fred K., Raji Balasubramanian, Liming Liang, Steven K. Clinton, Elizabeth M. Cespedes Feliciano, JoAnn E. Manson, Linda Van Horn, Jean Wactawski-Wende, Clary B. Clish, Edward L. Giovannucci, and et al. 2019. "Identifying Metabolomic Profiles of Insulinemic Dietary Patterns" Metabolites 9, no. 6: 120. https://doi.org/10.3390/metabo9060120
APA StyleTabung, F. K., Balasubramanian, R., Liang, L., Clinton, S. K., Cespedes Feliciano, E. M., Manson, J. E., Van Horn, L., Wactawski-Wende, J., Clish, C. B., Giovannucci, E. L., & Rexrode, K. M. (2019). Identifying Metabolomic Profiles of Insulinemic Dietary Patterns. Metabolites, 9(6), 120. https://doi.org/10.3390/metabo9060120





