Ceramide as a Promising Tool for Diagnosis and Treatment of Clinical Diseases: A Review of Recent Advances
Abstract
1. Introduction
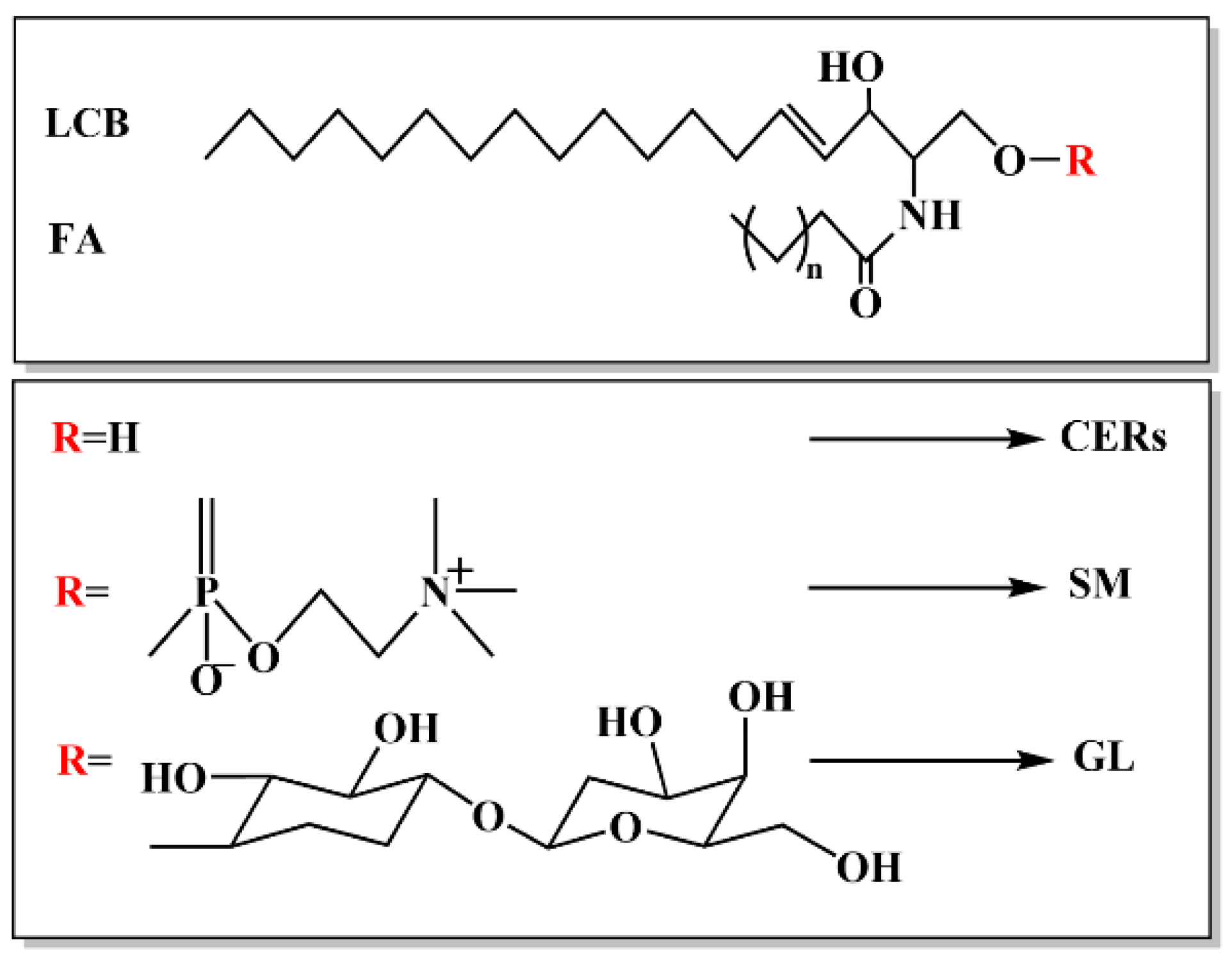
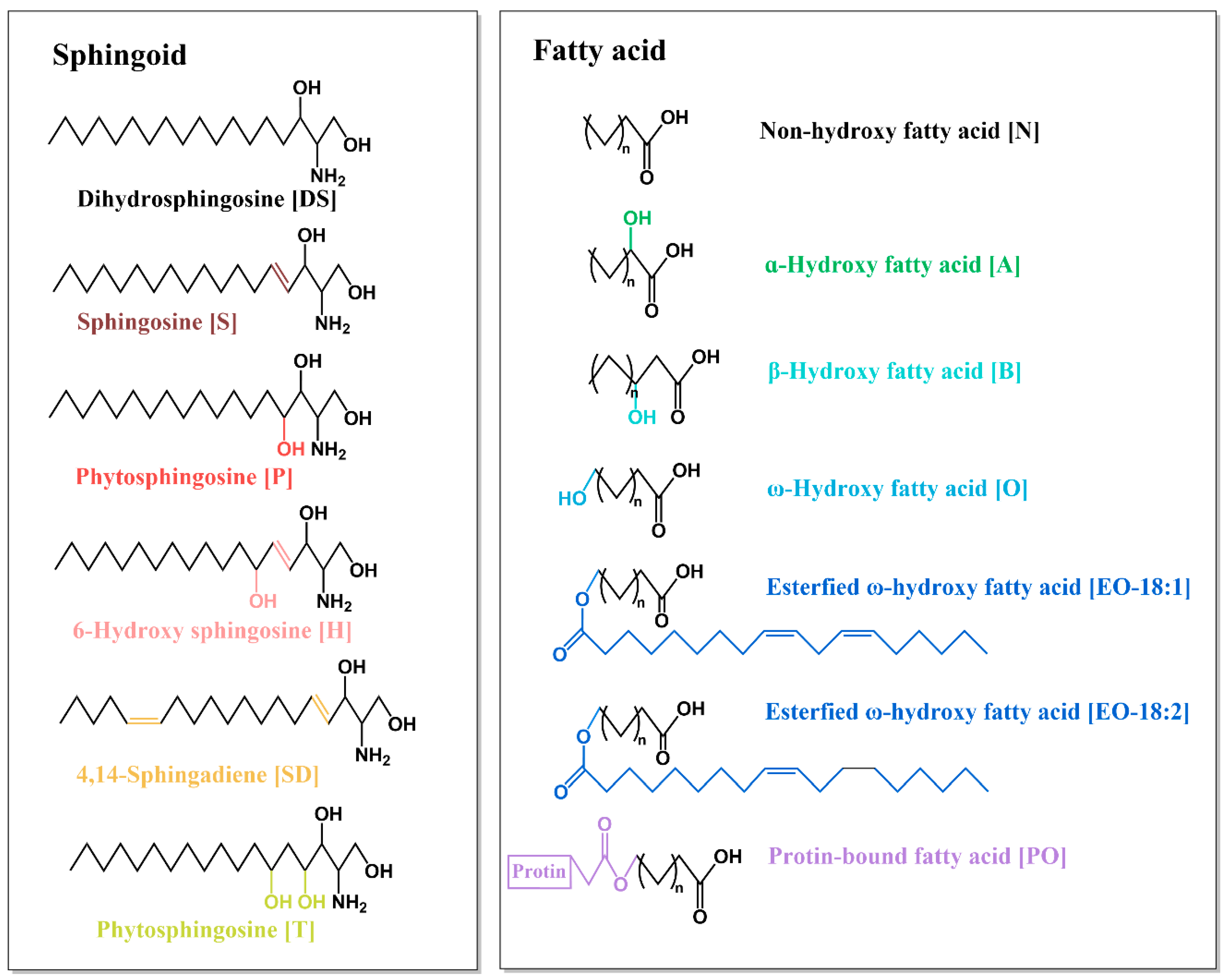
1.1. Structure of Ceramide
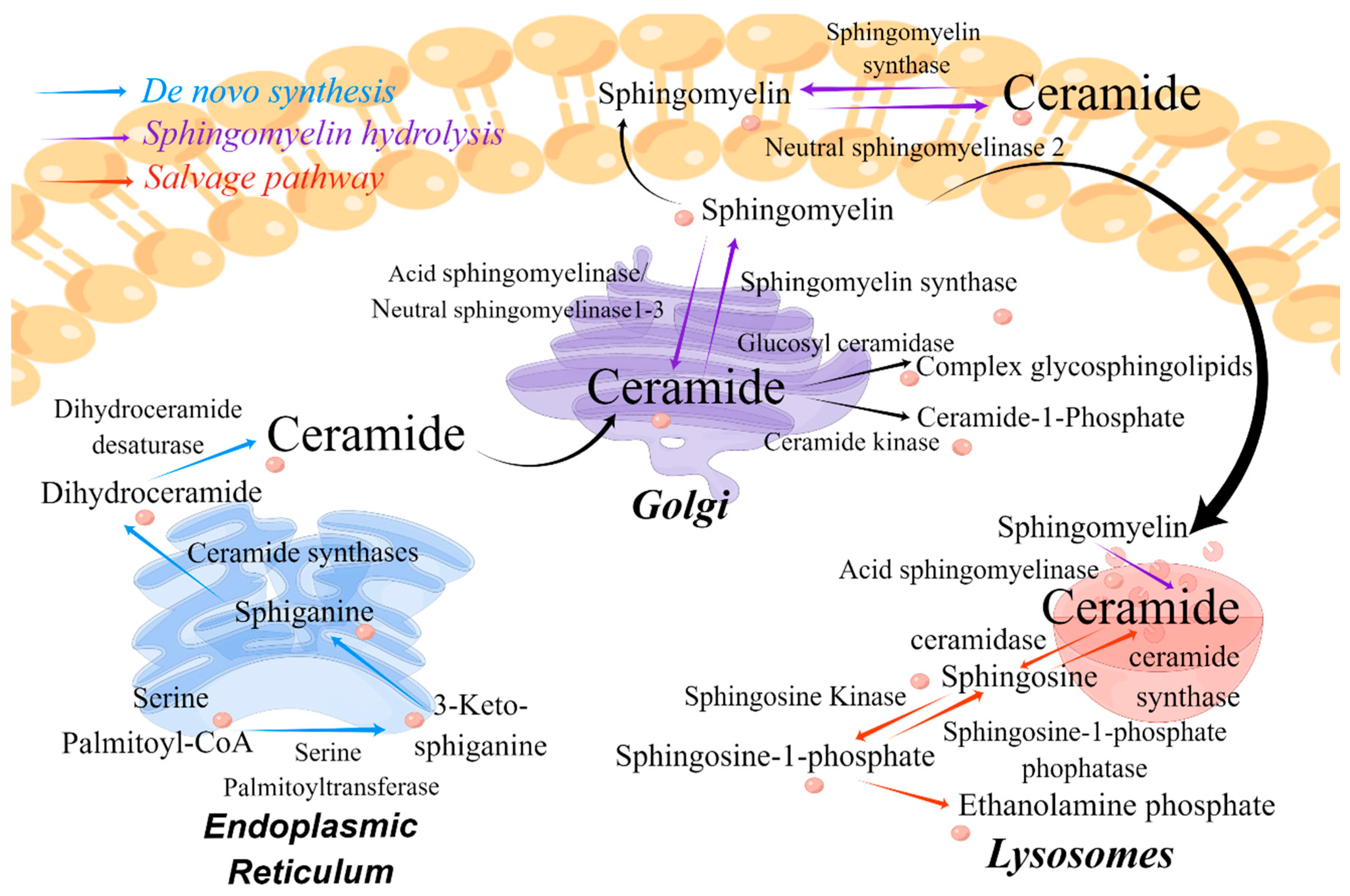
1.2. Biosynthesis and Metabolism of Ceramide
1.3. Functions of Ceramide in Cell Signaling and Apoptosis
2. Application of Ceramide Measurement
2.1. Ceramide and CVD: Ceramide Scoring Criteria Have Been Established
2.2. Ceramide and Cancer: Insights into Application of Sphingolipid-Based Drugs
2.3. Ceramide and Dermatosis: A Biomarker and Key Component in Skin Permeability Barrier
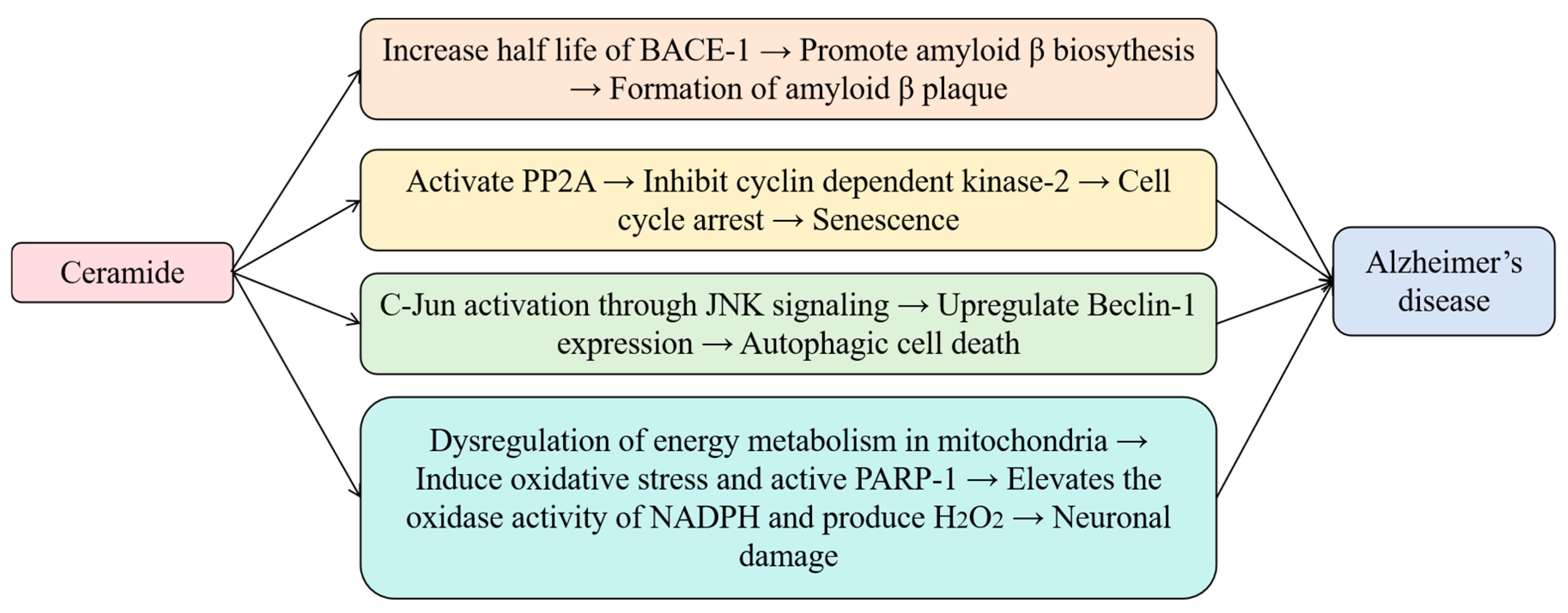
2.4. Ceramide and Alzheimer’s Disease: Exploring Relationships Between Lipid Dysregulation and Neurodegenerative Diseases’ Mechanism
2.5. Ceramide and Metabolic Syndromes: Promote Better Understanding of Disease and Treatment Mechanism
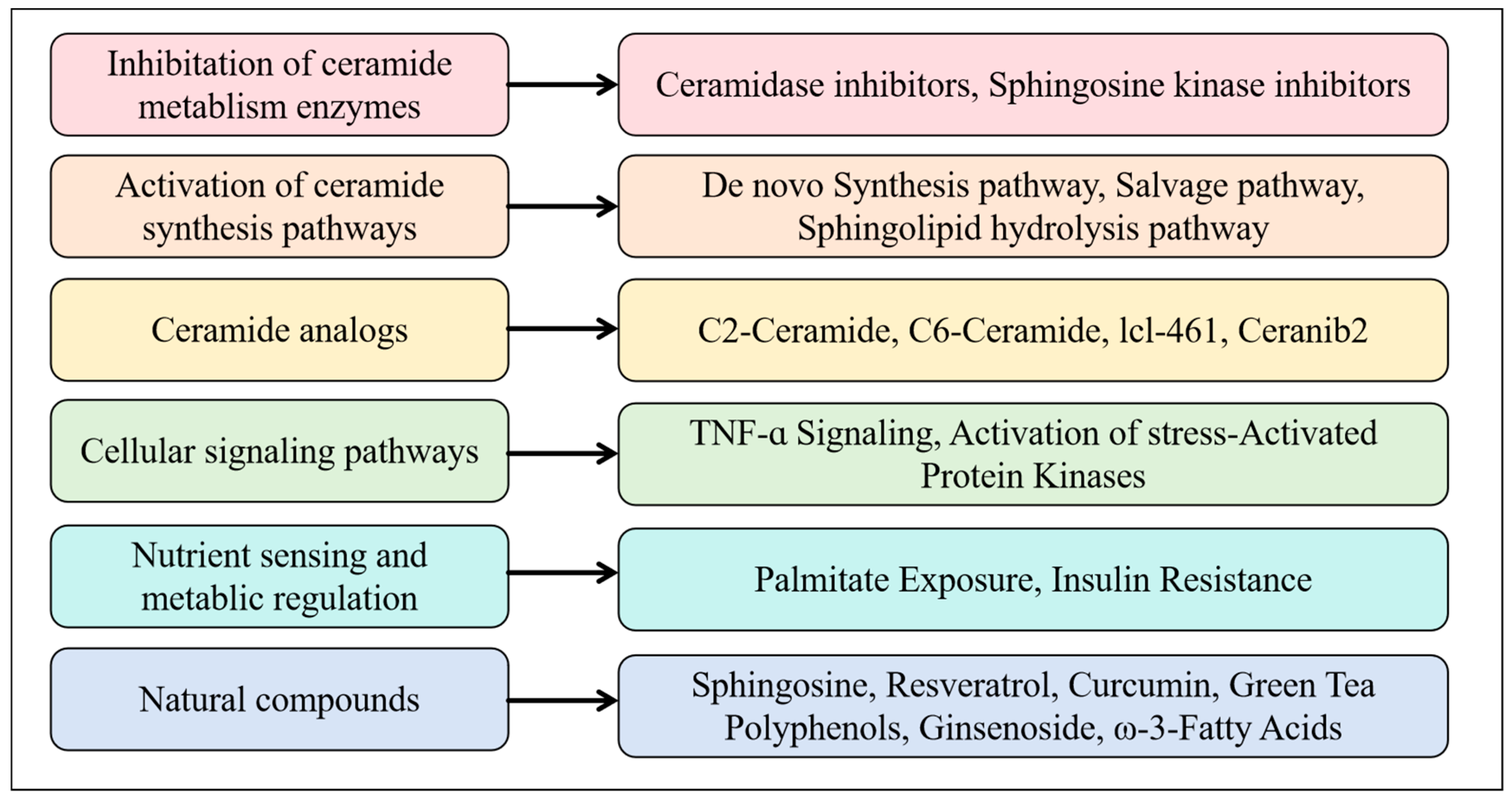
3. Challenges and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, B.Y.; Yang, H.Q.; Pan, Q.C.; Liu, Y.; Cong, L.; Tang, J.W.; Li, X.Z. Evaluation of moisturizing and repairing effects of ceramide complexes in formulations. China Clean. Ind. 2022, 8, 70–77. [Google Scholar] [CrossRef]
- Chang, Y.L.; Gao, J.P.; Zhang, X.J. Research progress on the correlation between ceramide and atopic dermatitis. Chin. J. Dermatol. 2018, 51, 79–80. [Google Scholar] [CrossRef]
- Zhang, M.; Shen, T. Research progress on the relationship between ceramide and the prevention and treatment of obesity related diseases. Acta Univ. Med. Anhui 2020, 55, 146–149. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.C.; Liu, Y.M. Research progress on ceramide and related heart failure. Chin. J. Evid. Based Cardiovasc. Med. 2023, 15, 1015–1017. [Google Scholar] [CrossRef]
- Guo, Y. Study on the Correlation BETWEEN ceramide and Large Artery Atherosclerotic Stroke. Master’s Thesis, Jilin University, Changchun, China, 2022. Volume 1. pp. 1–70. [Google Scholar] [CrossRef]
- Canals, D.; Salamone, S.; Hannun, Y.A. Visualizing bioactive ceramides. Chem. Phys. Lipids 2018, 216, 142–151. [Google Scholar] [CrossRef]
- Wajapeyee, N.; Beamon, T.C.; Gupta, R. Roles and Therapeutic Targeting of Ceramide Metabolism in Cancer. J. Mol. Metab. 2024, 83, 101936. [Google Scholar] [CrossRef]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Duan, L.; Shang, S.; Guo, H.; Zhao, C.; Li, Y.; Dai, H. Research progress on the role of ceramides in pancreatic beta cell apoptosis. J. Mod. Clin. Med. 2018, 44, 321–323+326. Available online: https://link.cnki.net/urlid/51.1688.R.20180921.1441.002 (accessed on 7 March 2025).
- Canals, D.; Hannun, Y.A. Biological function, topology, and quantification of plasma membrane Ceramide. Adv. Biol. Regul. 2024, 91, 101009–101015. [Google Scholar] [CrossRef]
- SenthilKumar, G.; Zirgibel, Z.; Cohen, K.E.; Katunaric, B.; Jobe, A.M.; Shult, C.G.; Limpert, R.H.; Freed, J.K. Ying and Yang of Ceramide in the Vascular Endothelium. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1725–1736. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Y.; Cheng, X.; Song, Q.; Wang, Y.; He, L.; Chen, T. The relevance between abnormally elevated serum ceramide and cognitive impairment in Alzheimer’s disease model mice and its mechanism. Psychopharmacology 2024, 241, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Ohya, Y.; Ogiso, Y.; Matsuda, M.; Sakae, H.; Nishida, K.; Miki, Y.; Fox, T.E.; Kester, M.; Sakamoto, W.; Nabe, T.; et al. Pronecroptotic Therapy Using Ceramide Nanoliposomes Is Effective for Triple-Negative Breast Cancer Cells. Cells 2024, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Tao, S.; Yang, L.; Chong, S.; Zhang, Z.; Zhong, S.; Wu, Y. Efficacy and Safety of Moisturizers Containing Ceramide in Improving Dry Skin. Chin. J. Aesthetic Med. 2022, 31, 62–66. [Google Scholar] [CrossRef]
- Adam, W.; Izabela, D.; Szymon, S.; Wojciech, Ł.; Ewa, O.; Elżbieta, S. Cannabidiol and Cannabigerol Modify the Composition and Physicochemical Properties of Keratinocyte Membranes Exposed to UVA. Int. J. Mol. Sci. 2023, 24, 12424. [Google Scholar] [CrossRef]
- Liu, Y.; Bing, L.; Li, S.Q.; Li, H.S.; Xu, L.S.; Liu, Y.X. Correlation between ceramide content in plasma and atherosclerosis. Chin. J. Clin. Healthc 2022, 25, 315–318. [Google Scholar] [CrossRef]
- Georgios, G.; Niklas, S.; Nerea, F.; Dimitra, B.; Eva, H.; Harald, F.; Verena, K.; Albrecht, P.; Stefan, Z.; Bernd, K.; et al. Serum sphingolipidomic analyses reveal an upregulation of C16-ceramide and sphingosine-1-phosphate in hepatocellular carcinoma. Oncotarget 2016, 7, 18095–18105. [Google Scholar] [CrossRef]
- Kauhanen, D.; Sysi-Aho, M.; Koistinen, K.M.; Laaksonen, R.; Sinisalo, J.; Ekroos, K. Development and validation of a high-throughput LC-MS/MS assay for routine measurement of molecular ceramides. Anal. Bioanal. Chem. 2016, 408, 3475–3483. [Google Scholar] [CrossRef]
- Zhang, R. Establishment of LC-MS/MS Based Ceramide Quantification Platform and Its Application in Patients with Coronary Heart Disease 2020; Beijing University of Chemical Technology: Beijing, China, 2020; pp. 1–69. [Google Scholar]
- Yuan, S. Study on Association Between Obesity Complicated with Diabetes with Bisphenol a Exposure and Ceramides in adipose Tissue. Master’s Thesis, Anhui Medical University, Hefei, China, 2021; pp. 1–53. [Google Scholar]
- Qiu, L.; Sun, W. Simultaneous quantitative analysis of different ceramide species in cells by high-performance liquid chromatography tandem mass spectrometry. J. Zhejiang Univ. (Med. Sci.) 2015, 44, 429–434. [Google Scholar]
- Shao, Q.; Shui, D.; Ye, W.; Zheng, L.; Lv, J.; Tang, C. Determination of Ceramide NP in Cosmetics by HPLC-MS/MS. Mod. Chem. Res. 2024, 22, 92–94. [Google Scholar] [CrossRef]
- Zhang, X. Inhibition of Ceramide De Novo Improves Insulin Sensitivity in Bisphenol A-Exposed Mice. Master Thesis, Anhui Medical University, Hefei, China, 2020. [Google Scholar]
- Blachnio-Zabielska, A.U.; Persson, X.M.; Koutsari, C.; Zabielski, P.; Jensen, M.D. A liquid chromatography/tandem mass spectrometry method for measuring the in vivo incorporation of plasma free fatty acids into intramyocellular ceramides in humans. Rapid Commun. Mass Spectrom. RCM 2012, 26, 1134–1140. [Google Scholar] [CrossRef]
- Fillet, M.; Van Heugen, J.C.; Servais, A.C.; De Graeve, J.; Crommen, J. Separation, identification and quantitation of ceramides in human cancer cells by liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2002, 949, 225–233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- An, Y.; Huang, N.; Yang, R.; Chen, X. Deep learning-based model for risk prediction of cardiovascular diseases. Chin. J. Med. Phys. 2019, 36, 1103–1112. [Google Scholar]
- Shao, Q.; Qian, Y.; Wang, T.; Han, J. Application of Ceramide-Based Cardiovascular Risk Prediction. Adv. Cardiovasc. Dis. 2023, 44, 814–818+840. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, X.; Wang, H.; Liu, H. Research advances in predictive value of ceramide in cardiovascular disease. Chin. J. Cardiovasc. Med. 2020, 25, 189–192. [Google Scholar] [CrossRef]
- Hilvo, M.; Meikle, P.J.; Pedersen, E.R.; Tell, G.S.; Dhar, I.; Brenner, H.; Schöttker, B.; Lääperi, M.; Kauhanen, D.; Koistinen, K.M.; et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 2020, 41, 371–380. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, H.; Yang, X.; Gao, H.; Lin, T.; Yang, R. Predictive value of serum sST2, NT-proBNP and CERT score combined with Fried frailty scale for prognosis of elderly patients with chronic heart failure. Chin. J. Mult. Organ. Dis. Elder. 2023, 22, 29–34. [Google Scholar] [CrossRef]
- Yao, K.; Yan, Z.W.; Xu, D.M.; Liu, X.B.; Shen, C.X.; Hu, W.; Wang, Z.; Wu, R.D.; Tang, X.L.; Sun, A.J.; et al. Effect of combined testing of ceramides with high-sensitive troponin T on the detection of acute coronary syndrome in patients with chest pain in China: A prospective observational study. BMJ Open 2019, 9, e028211. [Google Scholar] [CrossRef]
- Cheng, J.M.; Suoniemi, M.; Kardys, I.; Vihervaara, T.; de Boer, S.P.M.; Akkerhuis, K.M.; Sysi-Aho, M.; Ekroos, K.; Garcia-Garcia, H.M.; Oemrawsingh, R.M.; et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Atherosclerosis 2015, 243, 560–566. [Google Scholar] [CrossRef]
- Poss, A.M.; Maschek, J.A.; Cox, J.E.; Hauner, B.J.; Hopkins, P.N.; Hunt, S.C.; Holland, W.L.; Summers, S.A.; Playdon, M.C. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J. Clin. Investig. 2020, 130, 1363–1376. [Google Scholar] [CrossRef]
- Beska, B.; Mills, G.B.; Ratcovich, H.; Wilkinson, C.; Damluji, A.A.; Kunadian, V. Impact of multimorbidity on long-term outcomes in older adults with non-ST elevation acute coronary syndrome in the North East of England: A multi-centre cohort study of patients undergoing invasive care. BMJ Open 2022, 12, e061830. [Google Scholar] [CrossRef]
- Shi, S.; Zh, L.; Ye, L.; Luo, T. Disease Predict of Cardiovascular Based on Random Forest. Intell. Comput. Appl. 2021, 11, 176–178+181. [Google Scholar]
- Yoshikazu, U.; Kyungho, P. Ceramides in Skin Health and Disease: An Update. Am. J. Clin. Dermatol. 2021, 22, 853–866. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, H.; Jin, G.; Du, i.; Cui, Y.; Fan, H. Ceramide signaling pathways in parasitic diseases. Chin. J. Zoonoses 2021, 37, 268–277. [Google Scholar]
- Dubois, N.; Rio, E.; Ripoche, N.; Ferchaud-Roucher, V.; Gaugler, M.-H.; Campion, L.; Krempf, M.; Carrie, C.; Mahé, M.; Mirabel, X.; et al. Plasma ceramide, a real-time predictive marker of pulmonary and hepatic metastases response to stereotactic body radiation therapy combined with irinotecan. Radiother. Oncol. 2016, 119, 229–235. [Google Scholar] [CrossRef]
- Kazuki, M.; Tsutomu, K.; Junko, T.; Emmanuel, G.; Qianya, Q.; Li, Y.; Toshifumi, W.; Kazuaki, T.; Masayuki, N. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget 2018, 9, 19874–19890. [Google Scholar] [CrossRef]
- Jiang, Y.; Tie, C.; Wang, Y.; Cheng, X.; Bian, D.; Wang, T.; Liu, M.; Duan, Z.; Zheng, S.; Zhang, J. The diagnostic value of serum sphingolipids for HBV-related AFP negative hepato cellular carcinoma. Chin. J. Gastroenterol. Hepatol. 2018, 27, 563–568. [Google Scholar] [CrossRef]
- Markowski, A.R.; Błachnio-Zabielska, A.U.; Guzińska-Ustymowicz, K.; Markowska, A.; Pogodzińska, K.; Roszczyc, K.; Zińczuk, J.; Zabielski, P. Ceramides Profile Identifies Patients with More Advanced Stages of Colorectal Cancer. Biomolecules 2020, 10, 632. [Google Scholar] [CrossRef]
- Zhang, X.; Sakamoto, W.; Canals, D.; Ishibashi, M.; Matsuda, M.; Nishida, K.; Toyoshima, M.; Shigeta, S.; Taniguchi, M.; Senkal, C.E.; et al. Ceramide synthase 2-C24:1-ceramide axis limits the metastatic potential of ovarian cancer cells. FASEB J. 2021, 35, e21287. [Google Scholar] [CrossRef]
- Claudiu, R.; Tudor, E.D.; Emil, M.; Florin, G.; Carmen, S.; Adrian, S.M.; Al, H.N. Lipidomic Signatures for Colorectal Cancer Diagnosis and Progression Using UPLC-QTOF-ESI+MS. Biomolecules 2021, 11, 417. [Google Scholar] [CrossRef]
- Paul, B.; Lewinska, M.; Andersen, J.B. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022, 4, 100479. [Google Scholar] [CrossRef]
- Jiawei, Z.; Honglin, T.; Bin, H.; Qian, Z.; Qi, K.; Yan, D.; Jie, T.; Bei, X.; Jiafu, F.; Lin, Y. Lipid metabolism characterization in gastric cancer identifies signatures to predict prognostic and therapeutic responses. Front. Genet. 2022, 13, 959170. [Google Scholar] [CrossRef]
- Liina, S.; Ioana, B.E.; Mitja, L.; Antti, J.; Sinikka, O.; Sakari, H.; Jalid, S.; Hagen, K.; du, B.A.; Sven, M.; et al. A Novel Two-Lipid Signature Is a Strong and Independent Prognostic Factor in Ovarian Cancer. Cancers 2021, 13, 1764. [Google Scholar] [CrossRef] [PubMed]
- Shashank, D.; Svenja, B.; Mina, J.G.; Hassan, D.G.; Sergei, K.; Guilherme, R.; Helene, J.; Patrick, N.; Dagmar, M.; Markus, S.; et al. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat. Commun. 2019, 10, 1832–1843. [Google Scholar] [CrossRef]
- Yura, Y.; Masui, A.; Hamada, M. Inhibitors of Ceramide- and Sphingosine-Metabolizing Enzymes as Sensitizers in Radiotherapy and Chemotherapy for Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 2062. [Google Scholar] [CrossRef]
- Lee, H.; Rotolo, J.A.; Mesicek, J.; Penate-Medina, T.; Rimner, A.; Liao, W.-C.; Yin, X.; Ragupathi, G.; Ehleiter, D.; Gulbins, E.; et al. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS ONE 2017, 6, e19783. [Google Scholar] [CrossRef]
- Li, F.; Zhang, N. Ceramide: Therapeutic Potential in Combination Therapy for Cancer Treatment. Curr. Drug Metab. 2016, 17, 37–51. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Y.-Z.; Peng, Y.; Yi, N.; Gu, X.; Jin, Y.; Bai, X. Ceramide production mediates cinobufotalin-induced growth inhibition and apoptosis in cultured hepatocellular carcinoma cells. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 5763–5771. [Google Scholar] [CrossRef]
- Sophie, K.; O’Boyle, N.M. Skin Lipids in Health and Disease: A Review. Chem. Phys. Lipids 2021, 236, 105055–105068. [Google Scholar] [CrossRef]
- Jia, Y.; Gan, Y.; He, C.F.; Chen, Z.; Zhou, C. The mechanism of skin lipids influencing skin status. J. Dermatol. Sci. 2018, 89, 112–119. [Google Scholar] [CrossRef]
- Jungersted, J.M.; Hellgren, L.I.; Jemec, G.B.; Agner, T. Lipids and skin barrier function—A clinical perspective. Contact Dermat. 2008, 58, 255–262. [Google Scholar] [CrossRef]
- Momoko, K.; Masatoshi, M.; Yusuke, O.; Akio, K. Comparative profiling and comprehensive quantification of stratum corneum ceramides in humans and mice by LC/MS/MS. J. Lipid Res. 2020, 61, 884–895. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Liu, Z.; Wang, X.; Liu, Y.; Jiang, P.; Xu, Y. Update of the ceramide and related dermatosis. Chin. J. Lepr. Skin Dis. 2020, 36, 626–630. [Google Scholar] [CrossRef]
- Lin, M.H.; Hsu, F.F.; Crumrine, D.; Meyer, J.; Elias, P.M.; Miner, J.H. Fatty acid transport protein 4 is required for incorporation of saturated ultralong-chain fatty acids into epidermal ceramides and monoacylglycerols. Sci. Rep. 2019, 9, 13254–13271. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Danielle, B.J.L.; Elie, S.; Audrey, S.; Arlette, B.-G.; Rime, M.-J.; Ali, T. Comprehensive characterization and simultaneous analysis of overall lipids in reconstructed human epidermis using NPLC/HR-MSn: 1-O-E (EO) Cer, a new ceramide subclass. Anal. Bioanal. Chem. 2020, 412, 777–793. [Google Scholar] [CrossRef]
- BoKyung, K.; Cheol, S.J.; Seok, S.H.; KwangHyeon, L.; Won, L.J.; Ku, A.S.; Phil, H.S. Decrease of Ceramides with Long-Chain Fatty Acids in Psoriasis: Possible Inhibitory Effect of Interferon Gamma on Chain Elongation. Exp. Dermatol. 2021, 31, 122–132. [Google Scholar] [CrossRef]
- Fölster-Holst, R.; Naß, C.; Dähnhardt-Pfeiffer, S.; Freitag-Wolf, S. Analysis of the structure and function of the epidermal barrier in patients with ichthyoses—Clinical and electron microscopical investigations. J. Eur. Acad. Dermatol. Venereol. JEADV 2022, 36, 726–738. [Google Scholar] [CrossRef]
- Rinnov, M.R.; Halling, A.S.; Gerner, T.; Ravn, N.H.; Knudgaard, M.H.; Trautner, S.; Goorden, S.M.I.; Ghauharali-van der Vlugt, K.J.M.; Stet, F.S.; Skov, L.; et al. Skin biomarkers predict development of atopic dermatitis in infancy. Allergy 2022, 78, 791–802. [Google Scholar] [CrossRef]
- Dan, D.; Chunyan, H.; Shuo, W.; Mei, W.; Na, G.; Ping, S. Toward Personalized Interventions for Psoriasis Vulgaris: Molecular Subtyping of Patients by Using a Metabolomics Approach. Front. Mol. Biosci. 2022, 9, 945917. [Google Scholar] [CrossRef]
- Jihyun, K.; Eui, K.B.; Elena, G.; Evgeny, B.; Jaewoong, B.; Seokjin, K.; HyeYoung, K.; Ha, L.U.; Shin, K.M.; Minyoung, J.; et al. Alterations of Epidermal Lipid Profiles and Skin Microbiome in Children With Atopic Dermatitis. Allergy Asthma Immunol. Res. 2023, 15, 186–200. [Google Scholar] [CrossRef]
- Evgeny, B.; Jihyun, K.; Eui, K.B.; Elena, G.; Taras, L.; Irina, B.; Sofia, B.A.; Olivia, X.; Jiwon, K.; Sukyung, K.; et al. Stratum Corneum Lipid and Cytokine Biomarkers at Two Months of Age Predict the Future Onset of Atopic Dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1307–1316. [Google Scholar] [CrossRef]
- Matwiejuk, M.; Myśliwiec, H.; Lukaszuk, B.; Lewoc, M.; Malla, H.; Myśliwiec, P.; Dadan, J.; Chabowski, A.; Flisiak, I. Crosstalk between Serum and Skin Sphingolipids in Psoriasis. Int. J. Mol. Sci. 2023, 24, 14872. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.; Min, K.S.; KeLun, Z.; Zhexue, W.; Hemin, L.; Hye, K.J.; Li, K.H.; Ri, K.Y.; Hyeong, K.S.; Jin, K.W.; et al. Head and neck dermatitis is exacerbated by Malassezia furfur colonization, skin barrier disruption, and immune dysregulation. Front. Immunol. 2023, 14, 1114321. [Google Scholar] [CrossRef]
- Su, Q.; Hu, X.; Yang, M.; He, H.; Jia, Y. Lipidomic analysis of facial skin surface lipids in acne in young women. Int. J. Cosmet. Sci. 2024, 46, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Fei, G.; Keyan, S.; Zhenlin, H.; Xin, D. Role of Omega-Hydroxy Ceramides in Epidermis: Biosynthesis, Barrier Integrity and Analyzing Method. Int. J. Mol. Sci. 2023, 24, 5035. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem. Pharmacol. 2023, 211, 115522–115535. [Google Scholar] [CrossRef]
- Zelnik, I.D.; Rozman, B.; Rosenfeld-Gur, E.; Ben-Dor, S.; Futerman, A.H. A Stroll Down the CerS Lane. Adv. Exp. Med. Biol. 2019, 1159, 49–63. [Google Scholar] [CrossRef]
- Sastry, P.S. Lipids of nervous tissue: Composition and metabolism. Prog. Lipid Res. 1985, 24, 69–176. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; Jin, H.K.; Bae, J.S. Diverse Roles of Ceramide in the Progression and Pathogenesis of Alzheimer’s Disease. Biomedicines 2022, 10, 1956. [Google Scholar] [CrossRef]
- van Kruining, D.; Losen, M.; Crivelli, S.M.; de Jong, J.J.; Jansen, J.F.; Backes, W.H.; Monereo-Sánchez, J.; van Boxtel, M.P.J.; Köhler, S.; Linden, D.E.J.; et al. Plasma ceramides relate to mild cognitive impairment in middle-aged men: The Maastricht Study. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2023, 15, e12459. [Google Scholar] [CrossRef]
- Menzel, R.; Rybak, J. Insights from the past: The work of Hans von Alten on the evolution of brain structure, ecological adaptation, and cognition in hymenopteran species. Learn. Mem. 2024, 31, a053922. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Tong, M.; Nguyen, V.; Setshedi, M.; Longato, L.; Wands, J.R. Ceramide-mediated insulin resistance and impairment of cognitive-motor functions. J. Alzheimer’s Dis. JAD 2010, 21, 967–984. [Google Scholar] [CrossRef] [PubMed]
- Carmen, P.; Lourdes, Á.; Marta, R.; Lorena, G.; Miguel, B.; Consuelo, C. Plasma Lipidomics Approach in Early and Specific Alzheimer’s Disease Diagnosis. J. Clin. Med. 2022, 11, 5030. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Haughey, N.J.; Bandaru, V.V.R.; Schech, S.; Carrick, R.; Carlson, M.C.; Mori, S.; Miller, M.I.; Ceritoglu, C.; Brown, T.; et al. Plasma ceramides are altered in mild cognitive impairment and predict cognitive decline and hippocampal volume loss. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2010, 6, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Dakterzada, F.; Benítez, I.D.; Targa, A.; Carnes, A.; Pujol, M.; Jové, M.; Mínguez, O.; Vaca, R.; Sánchez-de-la-Torre, M.; Barbé, F.; et al. Cerebrospinal fluid lipidomic fingerprint of obstructive sleep apnoea in Alzheimer’s disease. Alzheimer’s Res. Ther. 2023, 15, 134–146. [Google Scholar] [CrossRef]
- Marrano, N.; Biondi, G.; Borrelli, A.; Rella, M.; Zambetta, T.; Di Gioia, L.; Caporusso, M.; Logroscino, G.; Perrini, S.; Giorgino, F.; et al. Type 2 Diabetes and Alzheimer’s Disease: The Emerging Role of Cellular Lipotoxicity. Biomolecules 2023, 13, 183. [Google Scholar] [CrossRef]
- Kinga, C.; Robert, S. Ceramide in the molecular mechanisms of neuronal cell death. The role of sphingosine-1-phosphate. Mol. Neurobiol. 2014, 50, 26–37. [Google Scholar] [CrossRef]
- Czubowicz, K.; Jęśko, H.; Wencel, P.; Lukiw, W.J.; Strosznajder, R.P. The Role of Ceramide and Sphingosine-1-Phosphate in Alzheimer’s Disease and Other Neurodegenerative Disorders. Mol. Neurobiol. 2019, 56, 5436–5455. [Google Scholar] [CrossRef]
- Yoon, J.H.; Seo, Y.; Jo, Y.S.; Lee, S.; Cho, E.; Cazenave-Gassiot, A.; Shin, Y.-S.; Moon, M.H.; An, H.J.; Wenk, M.R.; et al. Brain lipidomics: From functional landscape to clinical significance. Sci. Adv. 2022, 8, eadc9317. [Google Scholar] [CrossRef]
- Xing, Y.; Tang, Y.; Zhao, L.; Wang, Q.; Qin, W.; Ji, X.; Zhang, J.; Jia, J. Associations between plasma ceramides and cognitive and neuropsychiatric manifestations in Parkinson’s disease dementia. J. Neurol. Sci. 2016, 370, 82–87. [Google Scholar] [CrossRef]
- McGrath, E.R.; Himali, J.J.; Xanthakis, V.; Duncan, M.S.; Schaffer, J.E.; Ory, D.S.; Peterson, L.R.; DeCarli, C.; Pase, M.P.; Satizabal, C.L.; et al. Circulating ceramide ratios and risk of vascular brain aging and dementia. Ann. Clin. Transl. Neurol. 2020, 7, 160–168. [Google Scholar] [CrossRef]
- Oizumi, H.; Sugimura, Y.; Totsune, T.; Kawasaki, I.; Ohshiro, S.; Baba, T.; Kimpara, T.; Sakuma, H.; Hasegawa, T.; Kawahata, I.; et al. Plasma sphingolipid abnormalities in neurodegenerative diseases. PLoS ONE 2022, 17, e0279315. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, H.M.; Xie, L.J.; Zou, D.D.; Liu, P.; Hu, Z.F.; Ma, R.M.; Shi, Y.J.; Zheng, G.H.; Zhang, G.J. Serum ceramide concentrations are associated with depression in patients after ischemic stroke-A two-center case-controlled study. Clin. Chim. Acta; Int. J. Clin. Chem. 2021, 518, 110–115. [Google Scholar] [CrossRef]
- Mondal, K.; Del Mar, N.A.; Gary, A.A.; Grambergs, R.C.; Yousuf, M.; Tahia, F.; Stephenson, B.; Stephenson, D.J.; Chalfant, C.E.; Reiner, A.; et al. Sphingolipid changes in mouse brain and plasma after mild traumatic brain injury at the acute phases. Lipids Health Dis. 2024, 23, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, G.; Liu, J.; Lv, H.; Zhang, G. Clinica lvalue of change of serum ceramide C16, C24 levels and their ratio in diagnosis for depression after intracerebralhemorrhage. Int. J. Lab. Med. 2021, 42, 2958–2962. [Google Scholar] [CrossRef]
- Fernández-Irigoyen, J.; Cartas-Cejudo, P.; Iruarrizaga-Lejarreta, M.; Santamaría, E. Alteration in the Cerebrospinal Fluid Lipidome in Parkinson’s Disease: A Post-Mortem Pilot Study. Biomedicines 2021, 9, 491. [Google Scholar] [CrossRef]
- Tomasik, J.; Harrison, S.J.; Rustogi, N.; Olmert, T.; Barton-Owen, G.; Han, S.Y.S.; Cooper, J.D.; Eljasz, P.; Farrag, L.P.; Friend, L.V.; et al. Metabolomic Biomarker Signatures for Bipolar and Unipolar Depression. JAMA Psychiatry 2024, 81, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Tringali, C.; Giussani, P. Ceramide and Sphingosine-1-Phosphate in Neurodegenerative Disorders and Their Potential Involvement in Therapy. Int. J. Mol. Sci. 2022, 23, 7806. [Google Scholar] [CrossRef]
- Crivelli, S.M.; Luo, Q.; Kruining, D.V.; Giovagnoni, C.; Mané-Damas, M.; den Hoedt, S.; Berkes, D.; De Vries, H.E.; Mulder, M.T.; Walter, J.; et al. FTY720 decreases ceramides levels in the brain and prevents memory impairments in a mouse model of familial Alzheimer’s disease expressing APOE4. Biomed. Pharmacother. 2022, 152, 113240–113251. [Google Scholar] [CrossRef]
- Uranbileg, B.; Isago, H.; Sakai, E.; Kubota, M.; Saito, Y.; Kurano, M. Alzheimer’s disease manifests abnormal sphingolipid metabolism. Front. Aging Neurosci. 2024, 16, 1368839–1368853. [Google Scholar] [CrossRef]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Hajduch, E.; Le Stunff, H. Serum ceramides could predict durable diabetes remission following gastric bypass surgery. Med 2022, 3, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Błachnio-Zabielska, A.; Zabielski, P.; Baranowski, M.; Gorski, J. Effects of streptozotocin-induced diabetes and elevation of plasma FFA on ceramide metabolism in rat skeletal muscle. Horm. Metab. Res. = Horm. Stoffwechselforschung = Horm. Metab. 2010, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, L.; Ubhayasekera, S.J.; Bergquist, J.; Sargsyan, E.; Bergsten, P. Palmitate-induced impairments of β-cell function are linked with generation of specific ceramide species via acylation of sphingosine. Endocrinology 2015, 156, 802–812. [Google Scholar] [CrossRef]
- Samad, F.; Hester, K.D.; Yang, G.; Hannun, Y.A.; Bielawski, J. Altered adipose and plasma sphingolipid metabolism in obesity: A potential mechanism for cardiovascular and metabolic risk. Diabetes 2006, 55, 2579–2587. [Google Scholar] [CrossRef]
- Kim, J.I.; Huh, J.Y.; Sohn, J.H.; Choe, S.S.; Lee, Y.S.; Lim, C.Y.; Jo, A.; Park, S.B.; Han, W.; Kim, J.B. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol. Cell. Biol. 2015, 35, 1686–1699. [Google Scholar] [CrossRef]
- Aburasayn, H.; Al Batran, R.; Ussher, J.R. Targeting ceramide metabolism in obesity. Am. J. Physiology. Endocrinol. Metab. 2016, 311, E423–E435. [Google Scholar] [CrossRef]
- Haus, J.M.; Kashyap, S.R.; Kasumov, T.; Zhang, R.; Kelly, K.R.; Defronzo, R.A.; Kirwan, J.P. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009, 58, 337–343. [Google Scholar] [CrossRef]
- Lopez, X.; Goldfine, A.B.; Holland, W.L.; Gordillo, R.; Scherer, P.E. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J. Pediatr. Endocrinol. Metab. JPEM 2013, 26, 995–998. [Google Scholar] [CrossRef]
- Warshauer, J.T.; Lopez, X.; Gordillo, R.; Hicks, J.; Holland, W.L.; Anuwe, E.; Blankfard, M.B.; Scherer, P.E.; Lingvay, I. Effect of pioglitazone on plasma ceramides in adults with metabolic syndrome. Diabetes/Metab. Res. Rev. 2015, 31, 734–744. [Google Scholar] [CrossRef]
- Chung, J.O.; Koutsari, C.; Blachnio-Zabielska, A.U.; Hames, K.C.; Jensen, M.D. Intramyocellular Ceramides: Subcellular Concentrations and Fractional De Novo Synthesis in Postabsorptive Humans. Diabetes 2017, 66, 2082–2091. [Google Scholar] [CrossRef]
- Razak Hady, H.; Błachnio-Zabielska, A.U.; Szczerbiński, Ł.; Zabielski, P.; Imierska, M.; Dadan, J.; Krętowski, A.J. Ceramide Content in Liver Increases Along with Insulin Resistance in Obese Patients. J. Clin. Med. 2019, 8, 2197. [Google Scholar] [CrossRef] [PubMed]
- León-Aguilar, L.F.; Croyal, M.; Ferchaud-Roucher, V.; Huang, F.; Marchat, L.A.; Barraza-Villarreal, A.; Romieu, I.; Ramakrishnan, U.; Krempf, M.; Ouguerram, K.; et al. Maternal obesity leads to long-term altered levels of plasma ceramides in the offspring as revealed by a longitudinal lipidomic study in children. Int. J. Obes. 2019, 43, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Tong, S.; Yong, W.; Ke, K.; Yi, Z.; Xu, H.; Yao, Z. Correlation between adipose tissue ceramides levels and obesity complicated with diabetes mellitus. Anhui Med. J. 2021, 42, 835–839. [Google Scholar] [CrossRef]
- Morze, J.; Wittenbecher, C.; Schwingshackl, L.; Danielewicz, A.; Rynkiewicz, A.; Hu, F.B.; Guasch-Ferré, M. Metabolomics and Type 2 Diabetes Risk: An Updated Systematic Review and Meta-analysis of Prospective Cohort Studies. Diabetes Care 2022, 45, 1013–1024. [Google Scholar] [CrossRef]
- Lytle, K.A.; Chung, J.O.; Bush, N.C.; Triay, J.M.; Jensen, M.D. Ceramide concentrations in liver, plasma, and very low-density lipoproteins of humans with severe obesity. Lipids 2023, 58, 107–115. [Google Scholar] [CrossRef]
- Passaro, A.P.; Marzuillo, P.; Guarino, S.; Scaglione, F.; Miraglia Del Giudice, E.; Di Sessa, A. Omics era in type 2 diabetes: From childhood to adulthood. World J. Diabetes 2021, 12, 2027–2035. [Google Scholar] [CrossRef]
- Guo, K.; Savelieff, M.G.; Rumora, A.E.; Alakwaa, F.M.; Callaghan, B.C.; Hur, J.; Feldman, E.L. Plasma Metabolomics and Lipidomics Differentiate Obese Individuals by Peripheral Neuropathy Status. J. Clin. Endocrinol. Metab. 2022, 107, 1091–1109. [Google Scholar] [CrossRef]
- Peterson, L.R.; Jiang, X.; Chen, L.; Goldberg, A.C.; Farmer, M.S.; Ory, D.S.; Schaffer, J.E. Alterations in plasma triglycerides and ceramides: Links with cardiac function in humans with type 2 diabetes. Lipid Res. 2020, 61, 1065–1074. [Google Scholar] [CrossRef]
- Wang, H.; He, C. Research and application status of ceramide in skin. China Surfactant Deterg. Cosmet. 2019, 49, 51–57. [Google Scholar] [CrossRef]
- Wang, S.; Jin, Z.; Wu, B.; Morris, A.J.; Deng, P. Role of dietary and nutritional interventions in ceramide-associated diseases. J. Lipid Res. 2024, 66, 100726. [Google Scholar] [CrossRef]

| Detective Method | Sample Matrix | Pretreatment Method | Chromatographic COLUMN | Mobile Phase | Gradient Elution | Linear Range | LOD (fmol) on Column |
|---|---|---|---|---|---|---|---|
| LC-ESI-MS/MS [17] | Serum | Liquid–liquid extraction | Luna C18column (150 mm × 2 mm ID, 5 µm particle size, 100 Å pore size) | Phase A: water: formic acid (100:0.1, v/v); Phase B: acetonitrile: tetrahydrofuran: formic acid (50:50:0.1, v/v/v) | 0~0.6 min: A/B (60%/40%); 0.6~3.9 min: A/B (60%/40%~0/100%); 3.9~10.4 min: A/B (0/100%); 10.4~10.9 min: A/B (60%/40%). | Cer(18:0): 0.18–300 ng/mL; Cer(20:0): 0.24–400 ng/mL; Cer(24:1): 0.24–400 ng/mL; dhCer(24:0): 0.6–1000 ng/mL; dhCer(18:0): 0.3–500 ng/mL; | Not Mentioned |
| LC-ESI-MS/MS [18] | Plasma | Protein precipitation technology | Acquity BEH C18 (2.1 × 50 mm, 1.7 μm) | Phase A: 10 mM ammonium acetate + 0.1% formic acid in water; Phase B: 10 mM ammonium acetate + 0.1% formic acid in acetonitrile:2-propanol (4:3) | 0~0.5 min: A/B (15%/85%); 0.5~1.5 min: A/B (15%/85%~0/100%); 1.5~4 min: A/B (0/100%); 4.5~5.0 min: A/B (15%/85%). | Cer(d18:1/16:0): 0.008~2 pmol/μL; Cer(d18:1/18:0): 0.008~2 pmol/μL; Cer(d18:1/24:0): 0.08~20 pmol/μL; Cer(d18:1/24:1): 0.08~20 pmol/μL. | Not Mentioned |
| LC-ESI-MS/MS [19] | Plasma | Protein precipitation technology | Water ACQUITY UPLC BEH Phenyl (3 mm × 100 mm, 1.7 nm) | Phase A: 0.2% formic acid, 1 mM ammonium formate aqueous solution Phase B: 0.2% formic acid, 1 mM ammonium formate methanol solution | 0~2 min: A/B (20%/80%); 2~8 min: A/B (5%/95%); 8~9 min: A/B (2%/98%); 9~10 min: A/B (20%/80%). | Cer(d18:1/12:0): 1.953~8000 nM Cer(d18:1/14:0): 1.953~8000 nM Cer(d18:1/14:0): 1.953~8000 nM Cer(d18:1/18:0): 1.953~8000 nM Cer(d18:1/20:0): 1.953~8000 nM Cer(d18:1/22:0): 1.953~8000 nM Cer(d18:1/24:1): 1.953~8000 nM Cer(d18:1/24:0): 1.953~8000 nM | Not Mentioned |
| LC-APCI-MS/MS [20] | Fat Tissue | Liquid–liquid extraction and protein precipitation technology | Shimadzu Shim-pack C18 (50 mm × 2.1 mm, 2.0 µm) | Phase A: acetonitrile/water (6:4, ν/ν) + 0.1% formic acid + 0.1% ammonium formate; Phase B: acetonitrile/isopropanol (1:9, ν/ν) + 0.1% formic acid + 0.1% ammonium formate. | 0–3 min: A/B (50%/50%); 4–12.5 min: A/B (30%/70%); 12.5–14 min: A/B (50%/50%). | Cer (d18:1/16:0): 125~5000 ng/mL Cer (d18:1/17:0): 1 µg/mL Cer (d18:1/18:0): 125~5000 ng/mL Cer (d18:1/20:0): 125~5000 ng/mL Cer (d18:1/24:0): 125~5000 ng/mL Cer [d18:1/24:1(15Z)]: 125~5000 ng/mL | Not Mentioned |
| LC-ESI-MS/MS [21] | Cell | Liquid–liquid extraction | ZORBAX Eclipse XDB-C8 (150.0 mm × 2.1 mm, 3.5 μm) | Phase A: 1 mmol/L ammonium formate and 0.2% formic acid in methanol solution, Phase B: 100% methanol | 0~20 min: A/B (20%/80%~1/99%); 20~35 min: A/B (1%/99%~0/100%). | Cer16: 0.02~1.0 μg/mL; Cer17: 0.02~1.0 μg/mL; Cer24: 0.02~1.0 μg/mL; Cer18: 0.001~1.0 μg/mL. | Not Mentioned |
| LC-ESI-MS/MS [22] | Cosmetics | Ultrasound-assisted extraction | Waters XBridge Protein BEH C4 (2.5 μm, 2.1 mm × 50 mm) | Phase A: acetonitrile; Phase B: water. | 0~2 min: A/B (40%/60%); 8~12 min: A/B (100/0%); 12.1~15 min: A/B (60%/40%). | Cer(t18:0/18:1): 5~100 ng/mL | Cer(t18:0/18:1): 0.125 µg/g |
| LC-APCI-MS/MS [23] | Fat Tissue | Liquid–liquid extraction | Shimadzu shim-pack GIST C18 (2.0 μm, 2.1 × 50 mm) | Phase A: acetonitrile/water [60:40, v/v] + 0.1%, formic acid + 0.1% formic acid, amine. Phase B: acetonitrile/iso-propanol [1:9, v/v] + 0.1% formic acid + 0.1% formic acid, amine. | 0~1.0 min: A/B (50%/50%); 1.0~7.0 min: A/B (30%/80%); 7.1 ~8.5 min: A/B (50%/50%). | Not Mentioned | Not Mentioned |
| LC-ESI-MS/MS [24] | Tissues and Plasma | Liquid–liquid extraction | Zorbax SB-C8 (2.1 ×150 mm, 1.8 μm) | Phase A: 1 mM ammonium formate, 0.1% formic acid in water. Phase B: 2 mM ammonium formate, 0.1% formic acid in methanol. | 0 min: A/B (80%/20%); 0–1.5 min: A/B (80%/20%~90%/10%); 1.5–2.3 min isocratic at A/B (90%/10%); 2.3–9.3 min: A/B (90%/10%~99/1%); 9.3–11 min isocratic at A/B (99/1%); 11–11.3 min:A/B (99%/1%~80%/20%); 11.3–13 min isocratic at A/B (80%/20%). | Not Mentioned | C14:0-Cer: 3.1 μg/mL. C16:0-Cer: 2.9 μg/mL C17:0-Cer: 2.8 μg/mL. C18:1-Cer:2.8 μg/mL. C18:0-Cer: 2.8 μg/mL. C20:0-Cer: 2.6 μg/mL. C24:1-Cer: 2.4 μg/mL. C24:0-Cer: 2.4 μg/mL. |
| LC-ESI-MS/MS [25] | Cells | Liquid–liquid extraction | RP C18 Nuecleosil AB column (5 μm, 70 × 2 mm I.D.) | Phase A: water-acetonitrilie-2-propanol (8:1:1, v/v/v); Phase B: acetonitrile-2-propanol (9:1, v/v) | 0~2 min: A/B (35%/65%); 7~13 min: A/B (10%/90%); 15 min: A/B (0/100%); 16~18 min: A/B (35%/65%). | Not Mentioned | C16-Cer:0.253 ng/500 μg proteins. C18-Cer:0.253 ng/500 μg proteins. C20-Cer:0.253 ng/500 μg proteins |
| (a) | |||||||
| Risk Score Table | Biomarkers | Score | Total Score | ||||
| Q1 | Q2 | Q3 | Q4 | ||||
| CERT1 | Cer(d18:1/16:0) | 0 | 0 | 1 | 2 | 0~12 | |
| Cer(d18:1/18:0) | 0 | 0 | 1 | 2 | |||
| Cer(d18:1/24:1) | 0 | 0 | 1 | 2 | |||
| Cer(d18:1/16:0)/Cer(d18:1/24:0) | 0 | 0 | 1 | 2 | |||
| Cer(d18:1/18:0)/Cer(d18:1/24:0) | 0 | 0 | 1 | 2 | |||
| Cer(d18:1/24:1)/Cer(d18:1/24:0) | 0 | 0 | 1 | 2 | |||
| CERT2 | Cer(d18:1/24:1)/Cer(d18:1/24:0) | 0 | 1 | 2 | 3 | 0~12 | |
| Cer(d18:1/16:0)/PC(16:0/22:5) | 0 | 1 | 2 | 3 | |||
| Cer(d18:1/16:0)/PC(14:0/22:6) | 0 | 1 | 2 | 3 | |||
| PC(16:0/16:0) | 0 | 1 | 2 | 3 | |||
| CERT2-TnT | Cer(d18:1/24:1)/Cer(d18:1/24:0) | 0 | 1 | 2 | 3 | 0~15 | |
| Cer(d18:1/16:0)/PC(16:0/22:5) | 0 | 1 | 2 | 3 | |||
| Cer(d18:1/16:0)/PC(14:0/22:6) | 0 | 1 | 2 | 3 | |||
| PC(16:0/16:0) | 0 | 1 | 2 | 3 | |||
| hs-TnT | 0 | 1 | 2 | 3 | |||
| (b) | |||||||
| Risk Score Table | Risk Rating | ||||||
| Low Risk | Medium Risk | Medium-high Risk | High Risk | ||||
| CERT1 | 0~2 | 3~6 | 7~9 | 10~12 | |||
| CERT2 | 0~3 | 4~6 | 7~9 | 9~12 | |||
| CERT2-TnT | 0~4 | 5~7 | 8~10 | 11~15 | |||
| Study | Method | Participants | Sample | Conclusions | Future Perspectives | |
|---|---|---|---|---|---|---|
| Test | Control | |||||
| Grammatikos, 2016 [17] | LC-APCI-MS/MS | n = 122 HCC | n = 127 cirrhosis | Serum | The levels of long-chain and ultra-long-chain ceramides (C16–C24) were significantly higher in patients with HCC than in patients with cirrhosis (p < 0.001). | The C16 ceramide and its metabolite S1P might serve as new diagnostic markers of HCC in patients with liver disease. |
| Dubois N, 2016 [38] | LC-ESI-MS/MS | n = 35 colorectal CA before treatment | n = 35 colorectal CA after treatment | Plasma | Patients with controlled tumors within 1 year had higher ceramide levels, whereas 50% of patients with decreased ceramide levels experienced an increase in tumor volume. | Total plasma ceramide may serve as a biomarker of liver and lung oligometastases of colorectal cancer, enabling the classification of high-risk patients. |
| Kazuki Moro, 2017 [39] | LC-ESI-MS/MS | n = 44 breast CA | n = 36 peri-tumor and n = 44 normal breast tissues | Breast tissue | Cer(14:0), Cer(16:0), Cer(18:1), Cer(18:0), Cer(20:0), Cer(22:0), Cer(24:1), Cer(24:0), Cer(26:1), and Cer(26:0) were higher in breast cancer than in peritumor or normal breast tissue. Ceramide levels in cancer tissue were significantly negatively correlated with the nuclear grade (p = 0.04) and Ki-67 index (p = 0.09). The Area Under the Curve (AUC) scores of Receiver Operating Characteristic Curve (ROC) were 0.7226 for normal tissue and 0.7228 for peritumor tissue, respectively, showing that breast cancer tissue may be distinguished from normal breast tissue by ceramide levels. | Ceramide levels were higher in breast cancer tissue than in other tissues and were negatively correlated with aggressive phenotypes. Higher gene expressions of ceramide-related enzymes had a worse prognosis in breast cancer. |
| Jiang, 2018 [40] | LC-ESI-MS/MS | n = 15 hepatitis B related-AFP-negative HCC | n = 49 patients with hepatitis B cirrhosis | Serum | The expression level of Cer(d18:1/8:0)-1-P in patients with AFP-negative HCC was 2.177 nmol/mL, which was significantly higher than that in patients with hepatitis B cirrhosis (p < 0.05). The level of Cer(d18:1/8:0)-1-P can be used to identify hepatitis B-related AFP-negative HCC, with a sensitivity of 81.6% and a specificity of 86.7%. | Upregulated peripheral serum Cer(d18:1/8:0)-1-P might serve as a diagnostic marker of hepatitis B-related AFP-negative HCC. |
| Adam R. Markowski, 2020 [41] | LC-ESI-MS/MS | Colorectal tumor | Normal colorectal tissue | Colorectal tissue | The levels of sphingolipids in colorectal cancer tissues differed from those in surrounding healthy tissues, with increased levels of SPA, S1P, and Cer(14:0) and significantly lower levels of Cer(18:0) and Cer(20:0) in tumors. The levels of specific ceramides in colorectal cancer tissues and plasma depended on the stage of colorectal cancer. In ROC, sensitivities of plasma Cer(16:0), Cer(18:1), Cer(20:0), Cer(24:1) were 0.085, 0.092, 0.088, 0.088, respectively, in CRC(TNM III+IV). | Combined measurement of the plasma concentrations of several ceramides facilitates the differentiation between early and advanced lesions of colorectal cancer and is useful as a screening test for the detection of early colorectal cancer. |
| Xuewei Zhang, 2021 [42] | LC-ESI-MS/MS | CerS2-knockout cells | Control cells | Cell lipid | The CerS2-Cer(24:1) ceramide pathway limits ovarian cancer metastasis by restricting lamellipod formation in ovarian cancer cells. CerS2-Cer(24:1) | Provides insights for the development of ceramide-based therapies and the identification of biomarkers of metastatic ovarian cancer. |
| Fatty Acid | Non-Hydroxy Fatty Acid [N] | α-HYDROXY Fatty Acid [A] | β-HYDROXY Fatty Acid [B] | ω-Hydroxy Fatty Acid [O] | Esterified ω-Hydroxy Fatty Acid [EO] | Protein-Bound Fatty Acid [PO] | |
|---|---|---|---|---|---|---|---|
| Sphingoid | |||||||
| [DS] | CER[NDS] | CER[ADS] | CER[BDS] | CER[ODS] | CER[EODS] | CER[PODS] | |
| [S] | CER[NS] | CER[AS] | CER[BS] | CER[OS] | CER[EOS] | CER[POS] | |
| [P] | CER[NP] | CER[AP] | CER[BP] | CER[OP] | CER[EOP] | CER[POP] | |
| [H] | CER[NH] | CER[AH] | CER[BH] | CER[OH] | CER[EOH] | CER[POH] | |
| [SD] | CER[NSD] | CER[ASD] | CER[BSD] | CER[OSD] | CER[EOSD] | CER[POSD] | |
| Study | Method | Skin Diseases | Ceramides and Derivatives | Future Perspectives | |
|---|---|---|---|---|---|
| Test | Control | ||||
| Bo-Kyung Kim, 2021 [59] | LC-ESI-MS/MS | Psoriasis-like murine epidermis and human psoriatic stratum corneum | Healthy controls | Long-chain-ceramide ↓ Short-chain-ceramide ↑ | IFN-γ may regulate ELOVL and CerS levels by downregulating transcription factors. Transcription factors, such as PPARs and liver X receptor agonists in the ceramide elongation process, may serve as potential therapeutic agents for lengthening the ceramide FA chain in psoriasis. |
| Fölster Holst, 2022 [60] | HPTLC | Ichthyoses lesional skin | Ichthyoses nonlesional skin | CER[EOS] ↓ | Analysis of intercellular lipid lamellae organization and corneocyte membrane undulation may improve the understanding of the epidermal barrier in ichthyoses and assist in evaluating the effects of topical skin preparations. |
| Maria Rasmussen Rinnov, 2022 [61] | LS-ESI-MS/MS | Pediatric AD | Healthy controls | CER[DS] ↑ DHS ↑ CER[P] ↓ | CER[P] had the highest prediction accuracy among the biomarkers (accuracy = 75.6%, sensitivity = 84%). The combination of five lipid ratios (CER[DS]-(d17:0)/CER[DS]-(d18:0), CER[DS]-(d18:0)/CER[S]-(d18:1), CER[P]- (t18:0)/CER[S]- (d18:1), CER[DS]- (d17:0)/CER[DS]- (d18:0), CER[S]- (d17:1)/CER[S]- (d18:1)) gave an accuracy of 89.4% to the prediction of AD within the first 12 months. |
| Dan Dai, 2022 [62] | LC-ESI-MS/MS | Patients with PVM | Patients with PV | Cer (d18:1/18:0) were positively correlated with the PASI in severe PV | Patients with PV at different severity levels have distinct metabolic profiles, aiding in understanding disease progression and establishing precision treatment strategies for PV. |
| Jihyun Kim, 2023 [63] | LC-ESI-MS/MS | AD lesional skin | AD nonlesional skin | C18-CER[NS] N-acylated with C16, C18, and C22 Fas ↑ C24-32 -CER [NS]/C14-22-CER [NS] and total CER[EO]/total CER[NS] were negatively correlated with transepidermal water loss. | Pediatric AD skin showed aberrant lipid profiles associated with microbial dysbiosis and cutaneous barrier dysfunction. |
| Evgeny Berdyshev, 2023 [64] | LC-ESI-MS/MS | Children with AD family history | Children without AD family history | CER[PO] ↓ Short-chain CER[N] and CER[A] ↑ | Noninvasive skin tape strip analysis of ceramides can identify asymptomatic children at risk of future AD with high probability. A combination of lipids and cytokines serves as a powerful biomarker for predicting AD development, paving the way for precision medicine in AD. |
| Mateusz Matwiejuk, 2023 [65] | LC-ESI-MS/MS | Patients with psoriasis | Healthy controls | Positive associations between CER_t and CER_s, SFA_t and CER_s, and SFO_t and CER_s | Sphingolipid metabolism is impaired in both the affected skin and serum in patients with psoriasis. Skin and serum lipids show interrelationship, suggesting systemic involvement and correlations between specific sphingolipids. |
| Howard Chu, 2023 [66] | LC-ESI-MS/MS | AD with HND | AD without HND | CER[EOS] ↓ CER[EOP] ↓ | |
| Qianqian Su, 2024 [67] | LC-ESI-MS/MS | Acne in women | Healthy controls | Ceramide chain length ↓ | Skin surface lipids are closely associated with acne development. Lipidomics is a useful tool for analyzing skin surface lipids in different types of acne. |
| Stage | Characteristics/Symptoms |
|---|---|
| Preclinical AD | 1. Measurable biomarkers and detectable changes in the brain, CSF, and blood. |
| 2. Absence of symptoms such as memory loss. | |
| MCI due to AD | 1. Measurable biomarkers and detectable changes in the brain related to AD pathology |
| 2. Moderate cognitive decline, mainly affecting the performance of small daily tasks (such as paying bills or preparing meals). | |
| Dementia | 1. Measurable biomarkers and detectable changes in the brain, related to AD pathology. |
| 2. Substantial memory loss. | |
| 3. Behavioral and personality changes. | |
| 4. Severe impairments in completing daily tasks. |
| Study | Method | Skin Diseases/Condition | Sample | Ceramides and Derivatives | Future Perspectives | |
|---|---|---|---|---|---|---|
| Test | Control | |||||
| Michelle M. Mielke, 2010 [78] | LC-ESI-MS/MS | Patients with MCI | Healthy controls | Plasma | Cer(22:0) ↓ Cer(24:0) ↓ | Ultra-long-chain ceramides in the plasma predict memory loss and right hippocampal volume loss in patients with MCI and may be early indicators of AD progression. |
| PeñaBautista Carmen, 2022 [76] | LC-ESI-MS/MS | Preclinical AD | Healthy controls | Plasma | Cer↑ | The study of lipid profiles in plasma samples can help identify early stages of AD and potential new biomarkers. |
| MCI-AD | Healthy controls | |||||
| Daan van Kruining, 2023 [73] | LC-ESI-MS/MS | Men with MCI | Healthy controls | Plasma | Cer(18:0) ↑ Cer(24:1) ↑ Ceramide chain lengths ↑ (Cer(20:0), Cer(22:0), and Cer(24:1) ↑ are associated with larger volume of the hippocampus) | The study highlights the importance of considering sex and age-related factors when examining sphingolipid and CERT metabolism related to cognitive function. No associations of plasma sphingolipids with MCI or brain volumes were found in women. Further analyses of plasma ceramides as potential markers of MCI in middle-aged men are warranted. |
| Study | Method | Nervous System Diseases/Condition | Sample | Ceramides and Derivatives | Future Perspectives | |
|---|---|---|---|---|---|---|
| Test | Controls | |||||
| Xing Y, 2016 [83] | LC-ESI-MS/MS | PDD | PD-NC | Plasma | Cer(14:0) ↑ Cer(24:1) ↑ Cer(22:0), Cer(20:0), and Cer(18:0) were associated with hallucinations, anxiety and sleep behavior disturbances, respectively | In PDD, increased ceramide levels were correlated with decreased memory function and a higher odd of multiple neuropsychiatric symptoms. |
| Emer R McGrath, 2020 [84] | LC-ESI-MS/MS | Dementia-free Framingham Offspring Study cohort | Plasma | Cer22:0/Cer16:0 ↓ Cer24:0/Cer16:0 ↓ | Circulating ceramide ratios may serve as biomarkers for predicting dementia risk in cognitively healthy adults. | |
| Hideki Oizumi, 2022 [85] | LC-ESI-MS/MS | ND groups (including IPD, DLB, MSA, AD, and PSP) | Healthy controls | Plasma | S1P ↓ | The study indicates the important role of abnormal sphingolipid metabolism in neurodegeneration. |
| Lv Hong, 2022 [86] | LC-ESI-MS/MS | Patients with PSD | Patients without PSD | Plasma | Cer(16:0) ↑ Cer(18:0) ↑ Cer(24:0) ↑ Cer(24:1) ↑ | Serum ceramides may become an essential candidate biomarker for PSD diagnosis and may aid in monitoring the other biomarkers in the pathway. |
| Patients with PSD | Patients with MD | Cer(16:0) ↑ Cer(18:0) ↑ Cer(24:0) ↑ | ||||
| Koushik Mondal, 2024 [87] | LC-ESI-MS/MS | TBI mouse model | Healthy controls | Brain tissue | Sphingosine ↑ C1P ↑ | Alterations in sphingolipid metabolite composition, particularly sphingomyelinases and short chain ceramides, may contribute to the induction and regulation of neuroinflammatory events in the early stages of TBI, suggesting targets for novel diagnostic, prognostic, and therapeutic strategies in the future. |
| Plasma | Cer(22:0)) ↓ (at 1 day) Cer (22:0) ↑ (at 7 days) Cer (24:1) ↑ (at 3 days) Cer (24:1) ↓ (at 7 days) Cer (24:0) ↓ SM (22:0) ↓ (at 3 days) SM (22:0) ↑ (at 7 days) | |||||
| Study | Method | Participants | Sample | Main Findings | Conclusions | |
|---|---|---|---|---|---|---|
| Test | Controls | |||||
| Haus Jacob, 2009 [101] | LC-ESI-MS/MS | n = 13 T2D | n = 14 healthy control | Plasma | Patients with T2D had higher levels of Cer(d18:1/18:0), Cer(d18:1/20:0), Cer(d18:1/24:1), and total ceramides. Insulin sensitivity was inversely correlated with Cer(d18:1/18:0), Cer(d18:1/20:0), Cer(d18:1/24:1), Cer(d18:1/24:0), and total ceramides. | Plasma ceramide levels were increased in patients with obesity and T2D and were positively correlated with insulin resistance. |
| Ximena Lopez, 2013 [102] | LC-ESI-MS/MS | n = 14 women with obesity and T2D | n = 14 women with obesity and T2D | Fasting plasma | Cer(d18:1/22:0) and Cer(d18:1/20:0) were elevated, and Cer(d18:1/18:0) and Cer(d18:0/24:1) were twice that in healthy individuals (p < 0.05). | Plasma ceramides were elevated in T2D, reflecting tissue insulin resistance, potentially due to low adiponectin levels. |
| Jeremy Warshauer, 2015 [103] | LC-ESI-MS/MS | n = 19 pioglitazone | n = 18 placebo | Plasma | Cer(d18:1/18:0), Cer(d18:1/20:0), Cer(d18:1/24:1), Cer(d18:0/18:0), Cer(d18:0/24:1), lactosylceramides Cer(d18:1/16:0), hexosylceramides Cer(d18:1/16:0), Cer(d18:1/16:0), and Cer(d18:1/22:0) were markedly reduced after 6 months of pioglitazone treatment (all p < 0.01). | Plasma ceramide levels were markedly decreased in patients with MetS who received pioglitazone for 6 months. Some changes were correlated with insulin resistance and adiponectin levels. |
| Jacob Haus, 2017 [104] | LC-ESI-MS/MS | n = 76 SS | n = 76 IMF | Skeletal muscle lipids | SS ceramides, especially those whose chain length was C16 to C18 (Cer(d18:1/16:0) and Cer(d18:1/18:1)) or generated under the stimulation of plasma palmitate, were associated with biomarkers of insulin resistance. However, the IMF level was not correlated with any metabolic parameters. | Skeletal muscle SS ceramides, especially C16~18 chain lengths, and the de novo synthesis of intramyocellular ceramide from plasma palmitate are associated with insulin resistance markers. |
| Hady Razak Hady, 2019 [105] | LC-ESI-MS/MS | n = 31 IGT group (women = 12, men = 19), and n = 33 T2D (women = 19, men = 14) | Normal glucose tolerance group (NGT, women = 30, men = 36) | Liver | Hepatic ceramides were higher in women with T2D than in women with NGT (p < 0.05). Glycemic parameters, such as FBG, OGTT at 120 min, and HbA1c, were correlated with ceramides. Hepatic ceramides were higher in men with IGT than in men with NGT, but only Cer(d18:1/22:0) was correlated with all glycemic parameters | Ceramide contributed to the induction of hepatic insulin resistance, and it may differ between men and women. |
| Luis Felipe León-Aguilar, 2019 [106] | LC-ESI-MS/MS | n = 91 docosahexaenoic acid | n = 92 placebo | Plasma | The total abundance of plasma ceramides in overweight and obese mothers, especially Cer(d18:1/20:0), Cer(d18:1/22:0), Cer(d18:1/23:0), and Cer(d18:1/24:0), was significantly decreased. Compared with children of normal weight mothers, the levels of Cer(d18:1/22:0), Cer(d18:1/23:0), and Cer(d18:1/24:0) were similar in the 4-year-old children of overweight or obese mothers. | Maternal obesity led to long-term changes in plasma ceramide levels of their offspring, and lipids may serve as early predictors of metabolic disease risk that result from maternal obesity. |
| Yuan, 2021 [107] | LC-APCI-MS/MS | n = 56 OD | n = 144 OND | Abdominal adipose tissue | Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/24:0), Cer(d18:1/24:0), and total ceramides in the fat tissue of OD group were higher than those in the OND group (p < 0.05), whereas the difference in Cer(d18:1/20:0) was not statistically significant (p > 0.05). IL-1 and IL-18 in serum and fat tissue of OD group were higher than those in the OND group (p < 0.05). | Ceramide level in the fat tissue of patients with obesity was associated with the inflammation of fat tissue and increased diabetes risk. |
| Jakub Morze, 2022 [108] | Meta-analysis | n = 11,771 T2D | n = 59,425 healthy controls | Plasma, serum, and urine | Higher plasma and serum levels of phosphatidylethanolamines and ceramides included in the meta-analysis were associated with a higher risk of type 2 diabetes. | Several plasma and serum metabolites, including amino acids, lipids, and carbohydrates, were associated with the risk of type 2 diabetes. |
| Kelli Lytle, 2023 [109] | LC-ESI-MS/MS | n = 25 obesity | Liver, plasma, and VLDL particles | (i) The proportion of Cer(14:0), Cer(18:0), Cer(20:0), and Cer(24:1) in the liver and whole plasma were positively correlated. (ii) Hepatic fat was positively correlated with the proportion of hepatic Cer18:1, Cer18:0, and Cer20:0 but not with total hepatic ceramide concentration. (iii) The proportions of whole plasma ceramide subspecies, especially Cer(14:0), Cer(18:0), Cer(20:0), and C(24:1) chain length, are reflective of those of hepatic ceramide subspecies in individuals with obesity. | A correlation was observed between the levels of ceramides in the liver and plasma of patients with obesity. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Feng, R.; Zhou, R.; Zhang, Z.; Liu, K.; Wang, S. Ceramide as a Promising Tool for Diagnosis and Treatment of Clinical Diseases: A Review of Recent Advances. Metabolites 2025, 15, 195. https://doi.org/10.3390/metabo15030195
Shen X, Feng R, Zhou R, Zhang Z, Liu K, Wang S. Ceramide as a Promising Tool for Diagnosis and Treatment of Clinical Diseases: A Review of Recent Advances. Metabolites. 2025; 15(3):195. https://doi.org/10.3390/metabo15030195
Chicago/Turabian StyleShen, Xueping, Rui Feng, Rui Zhou, Zhaoyang Zhang, Kaiyong Liu, and Sheng Wang. 2025. "Ceramide as a Promising Tool for Diagnosis and Treatment of Clinical Diseases: A Review of Recent Advances" Metabolites 15, no. 3: 195. https://doi.org/10.3390/metabo15030195
APA StyleShen, X., Feng, R., Zhou, R., Zhang, Z., Liu, K., & Wang, S. (2025). Ceramide as a Promising Tool for Diagnosis and Treatment of Clinical Diseases: A Review of Recent Advances. Metabolites, 15(3), 195. https://doi.org/10.3390/metabo15030195





