Metabolomic Insights into Smoking-Induced Metabolic Dysfunctions: A Comprehensive Analysis of Lipid and Amino Acid Metabolomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Biochemical Analysis
2.2.1. Determination of Serum Glucose
2.2.2. Determination of Serum Insulin
2.2.3. Determination of Insulin Resistance
2.2.4. Determination of HbA1c Levels
2.2.5. Lipid Biochemical Parameters
2.2.6. Estimation of Kidney Function Biomarkers
2.2.7. Assessment of Liver Biomarkers
2.2.8. Estimation of Inflammatory Biomarkers
2.3. Estimation of Cadmium and Zinc in Serum by ICP-OES
2.3.1. Reagents and Standard Solutions
2.3.2. Conventional Digestion of Samples for ICP-OES Analysis
2.4. Qualitative Analysis of Metabolomes with LC-MS/MS
2.4.1. Sample Preparation for LC–MS/MS Analysis
2.4.2. Conditions of the Instrument
2.4.3. Annotation and Identification
2.5. Statistical Analysis
3. Results
3.1. Biochemical Analysis
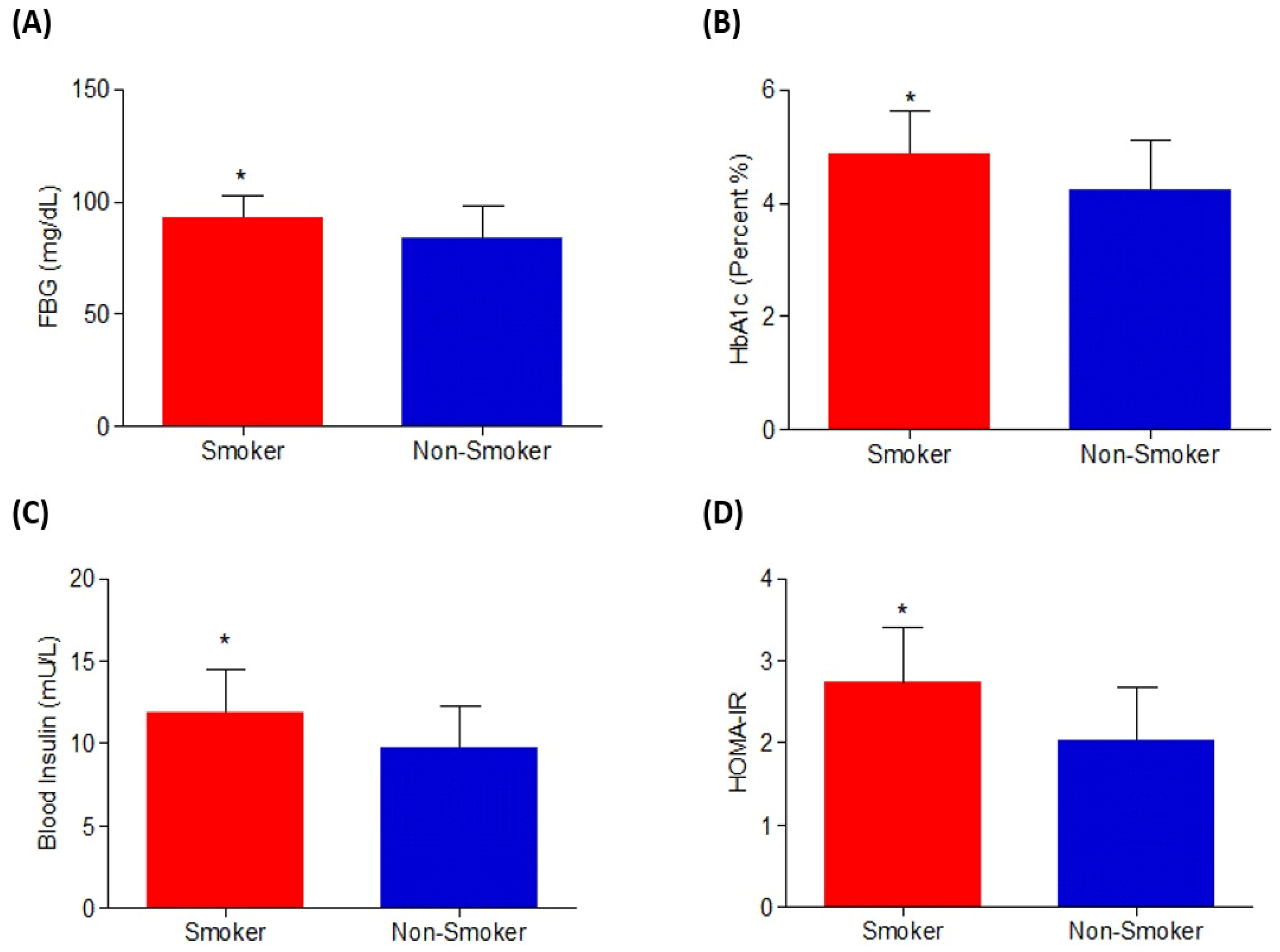
3.1.1. Effect on Glycemic Index Parameters
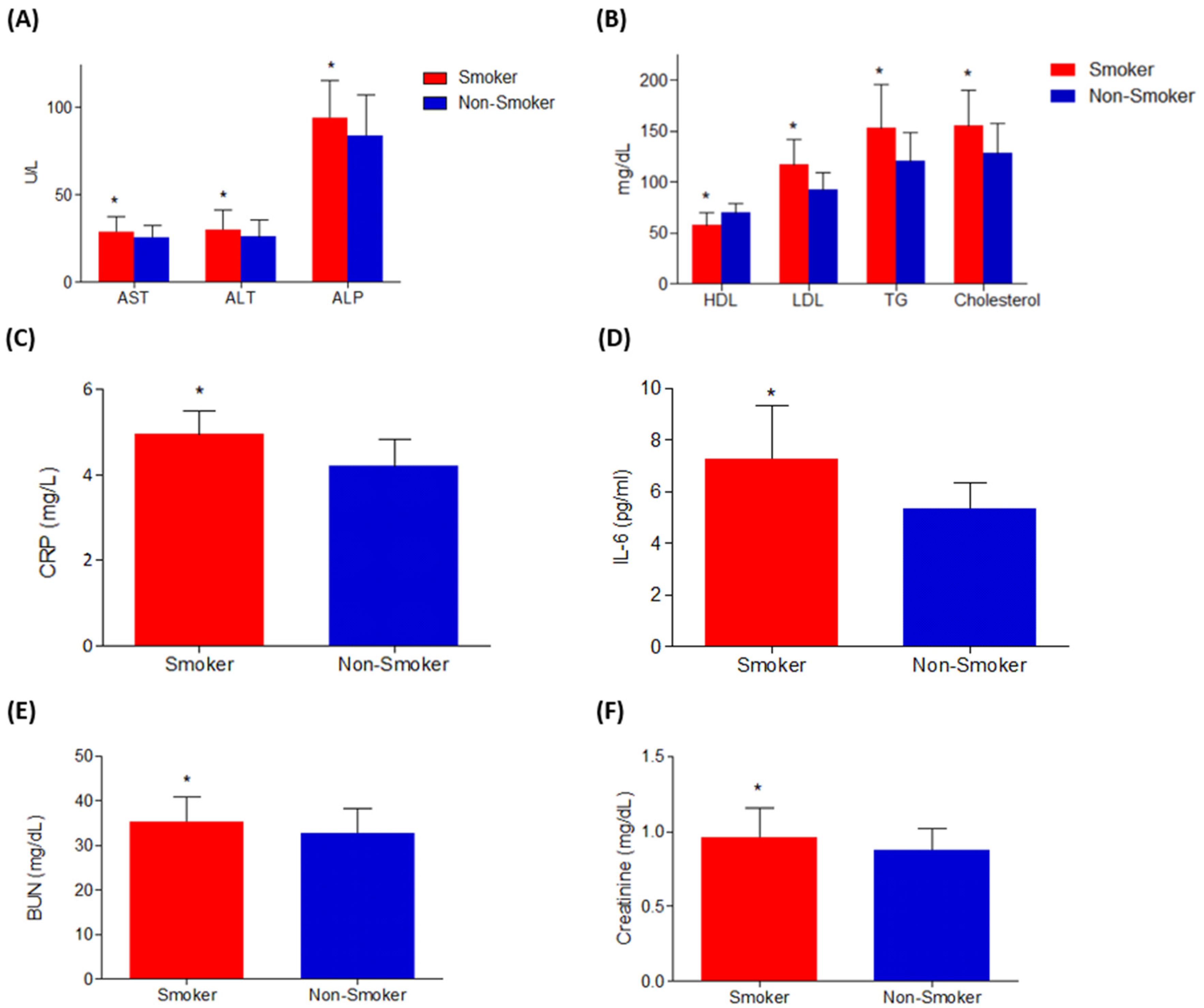
3.1.2. Effects on Liver, Inflammatory, Kidney Biomarkers, and Lipid Profile
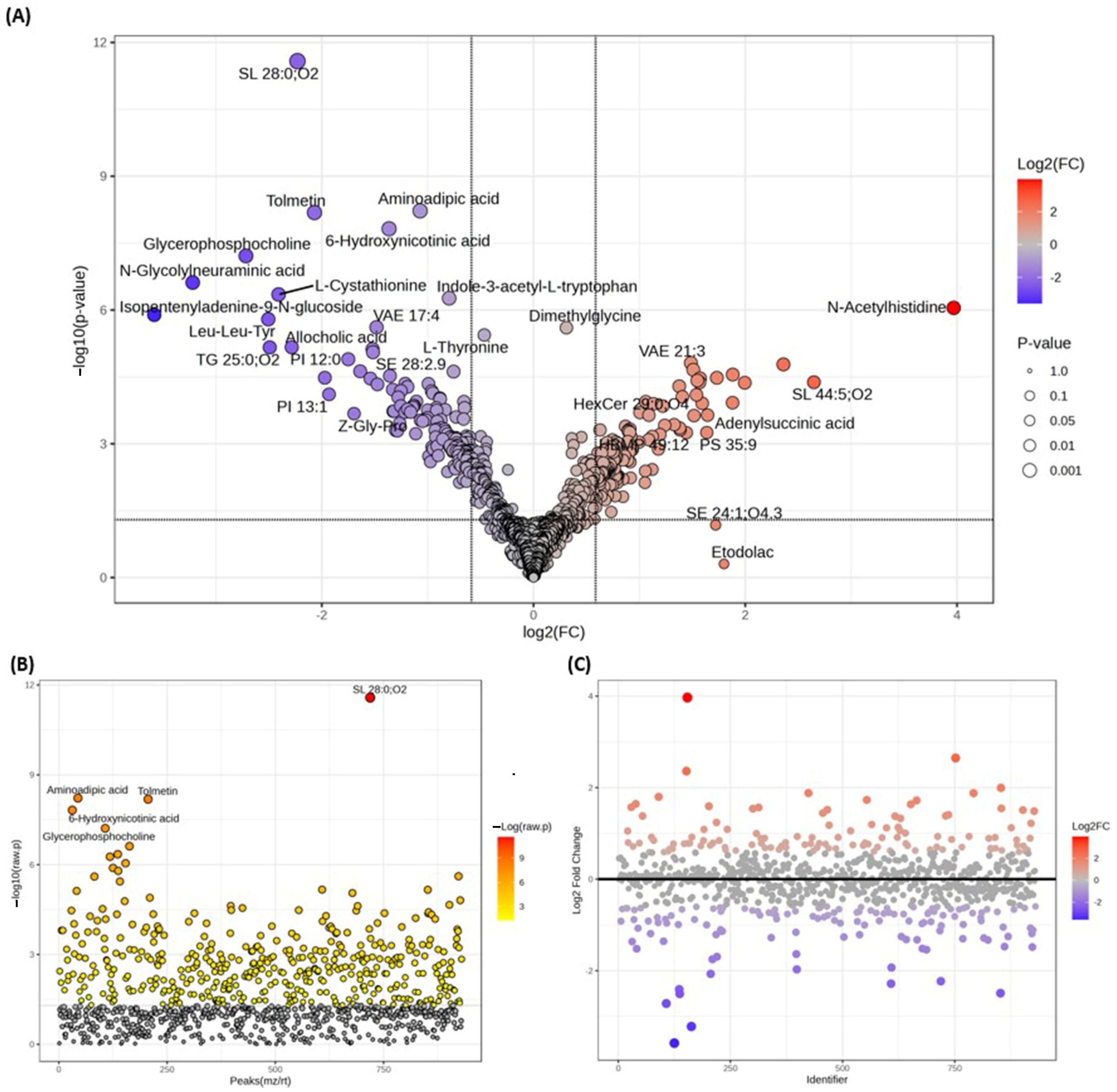
3.2. Analysis of Metabolomes by LC-MS/MS
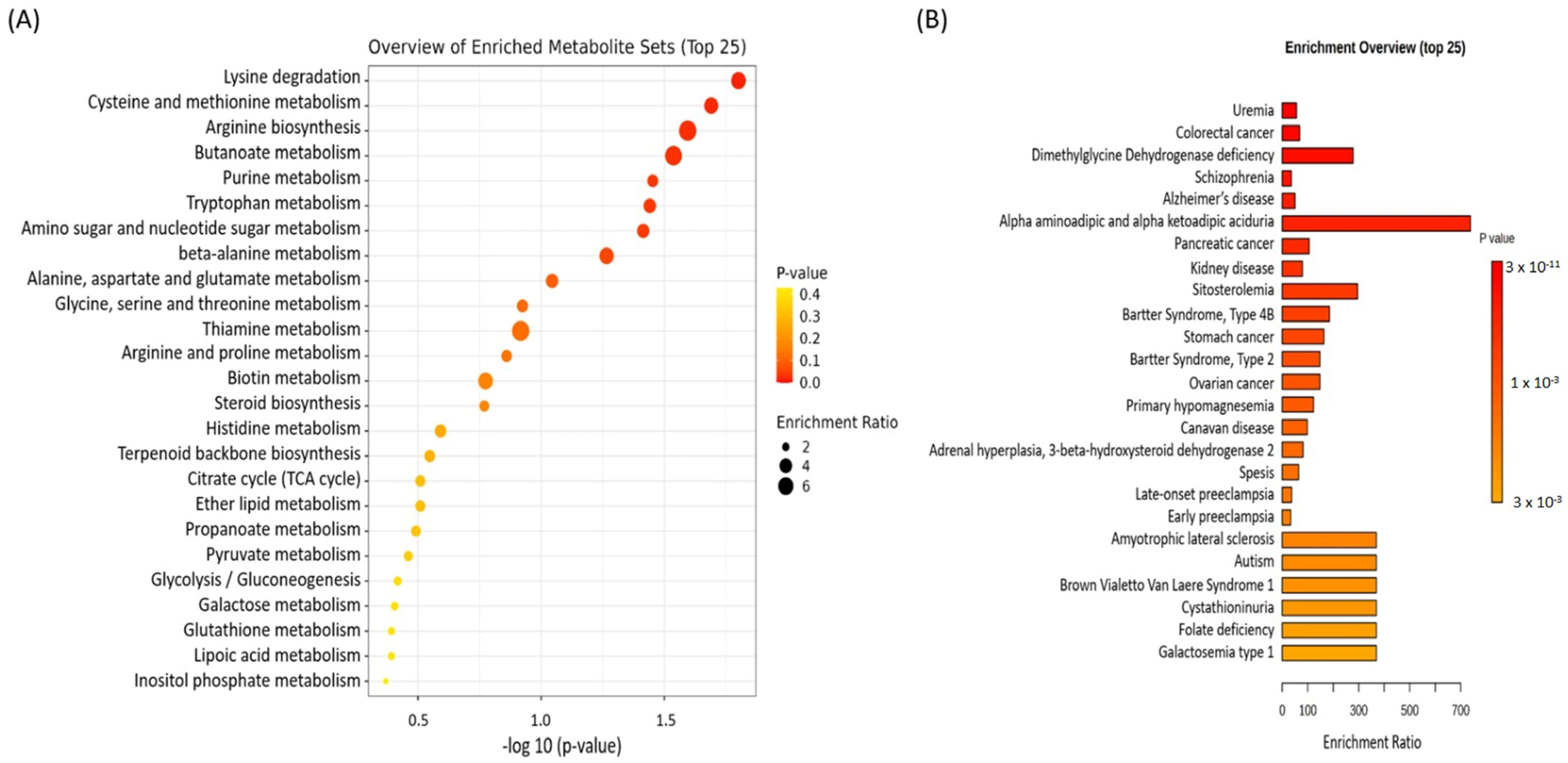
3.2.1. Metabolomic Enrichment Analysis
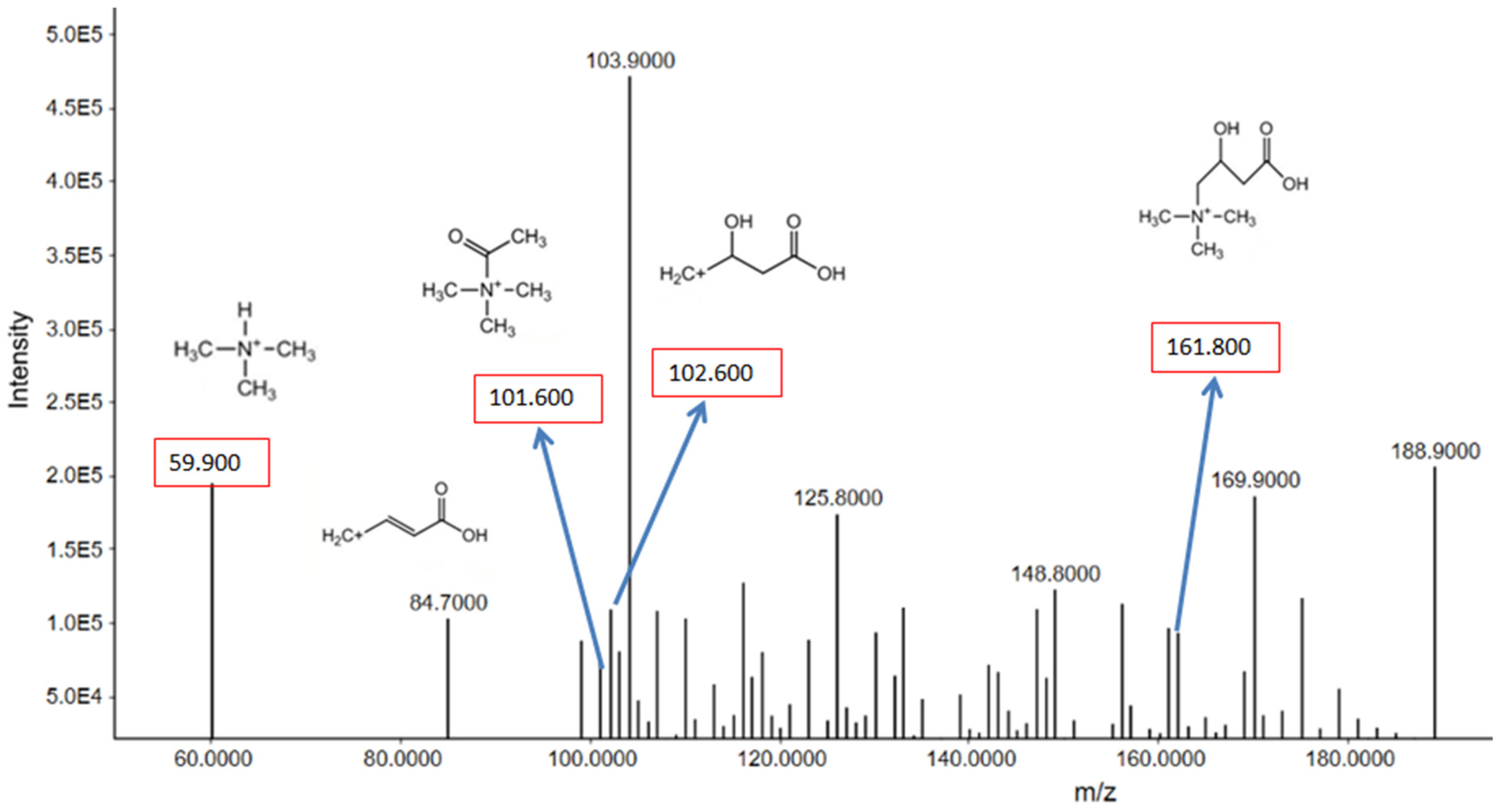
3.2.2. Metabolomes Profiling Using Product Ion Fragmentation Method
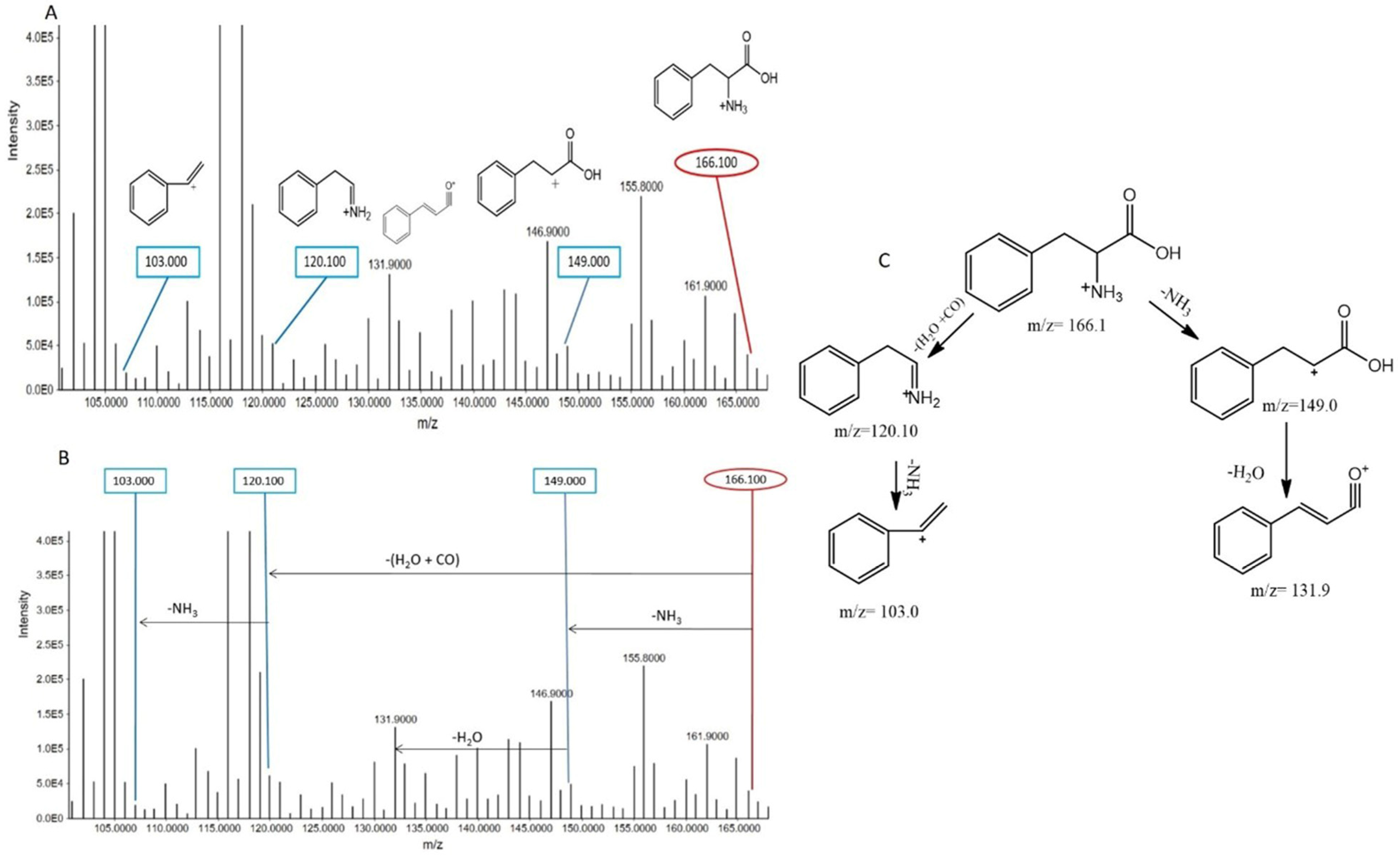
Phenylalanine
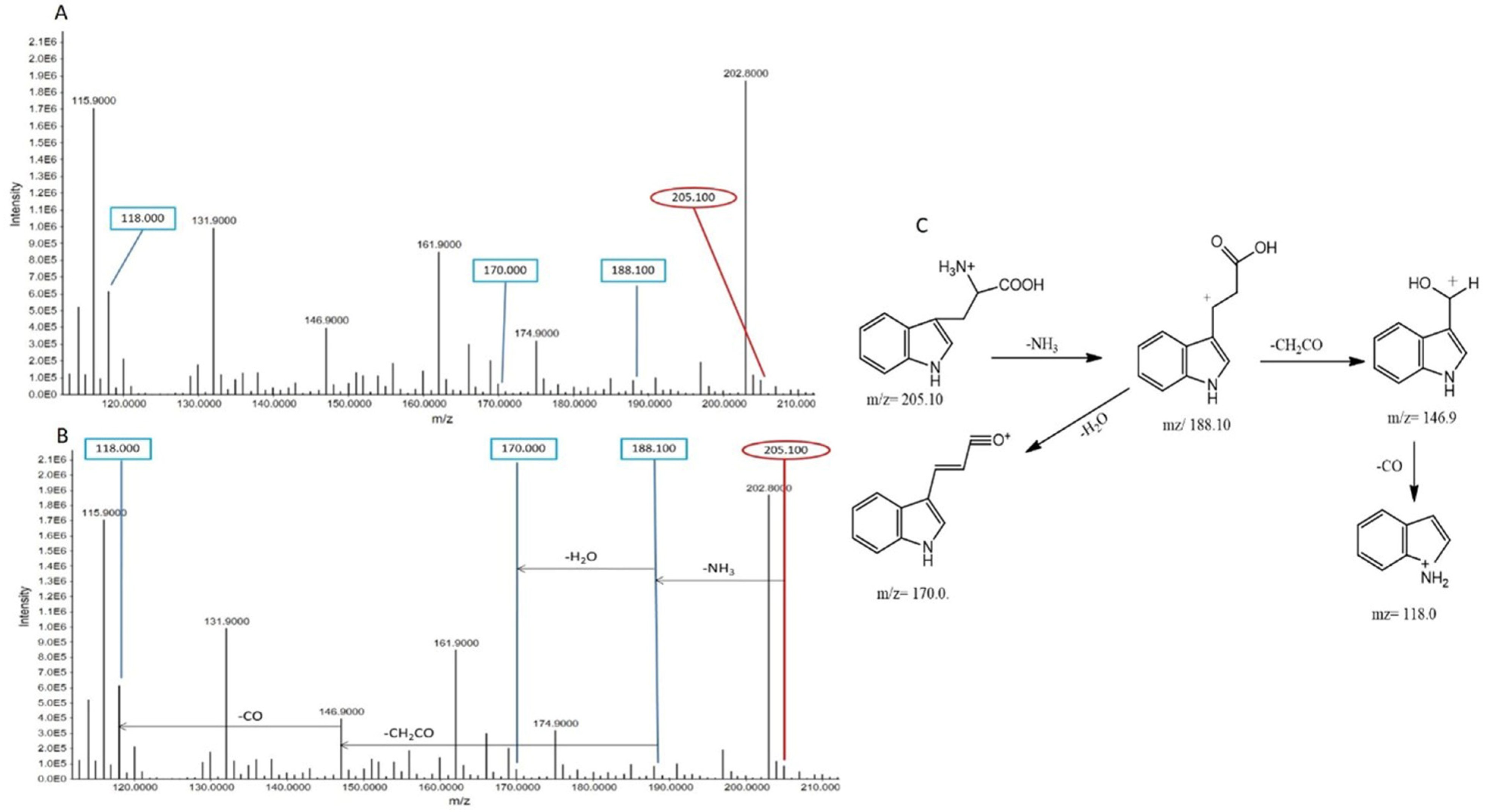
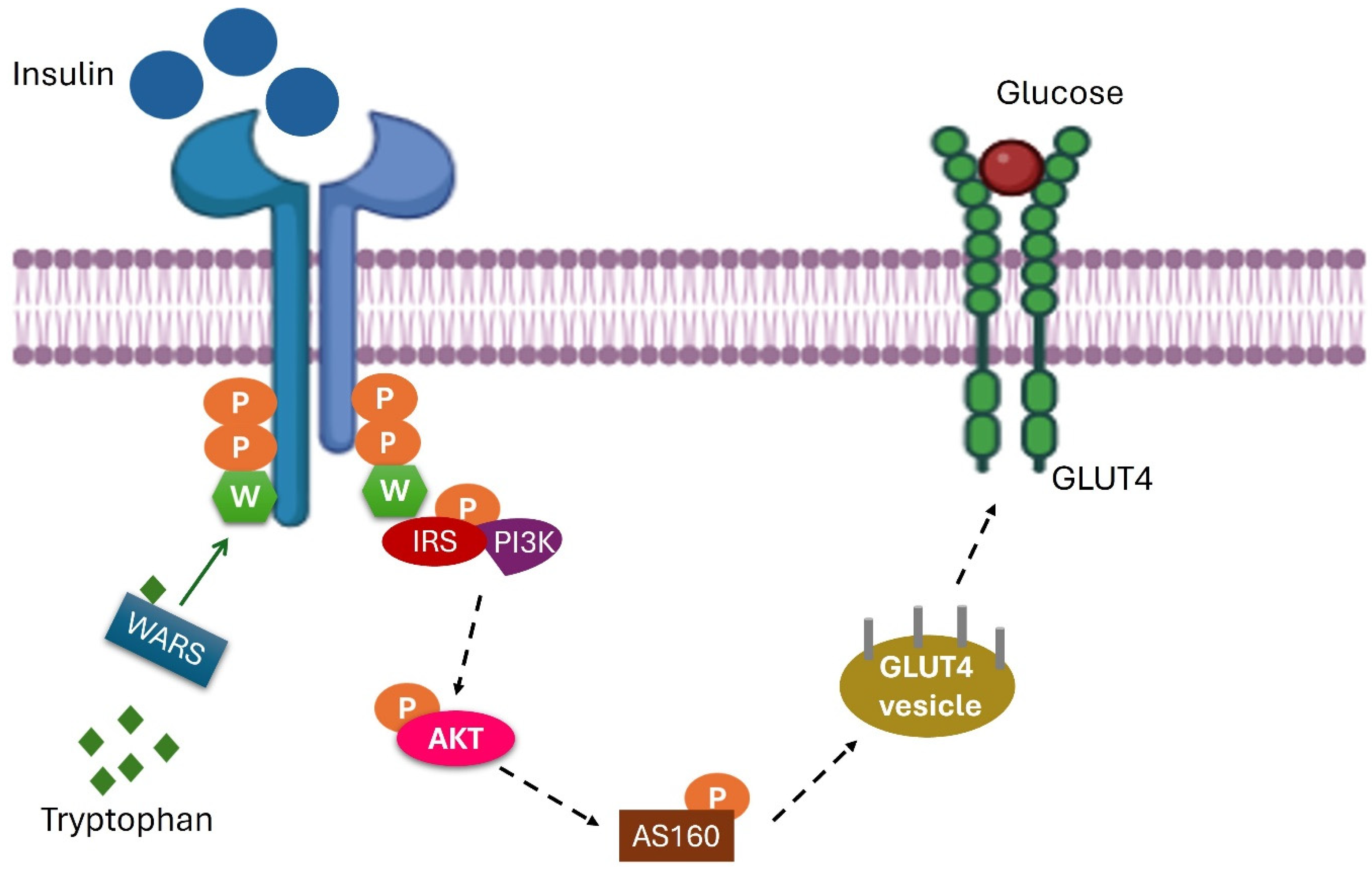
Tryptophan
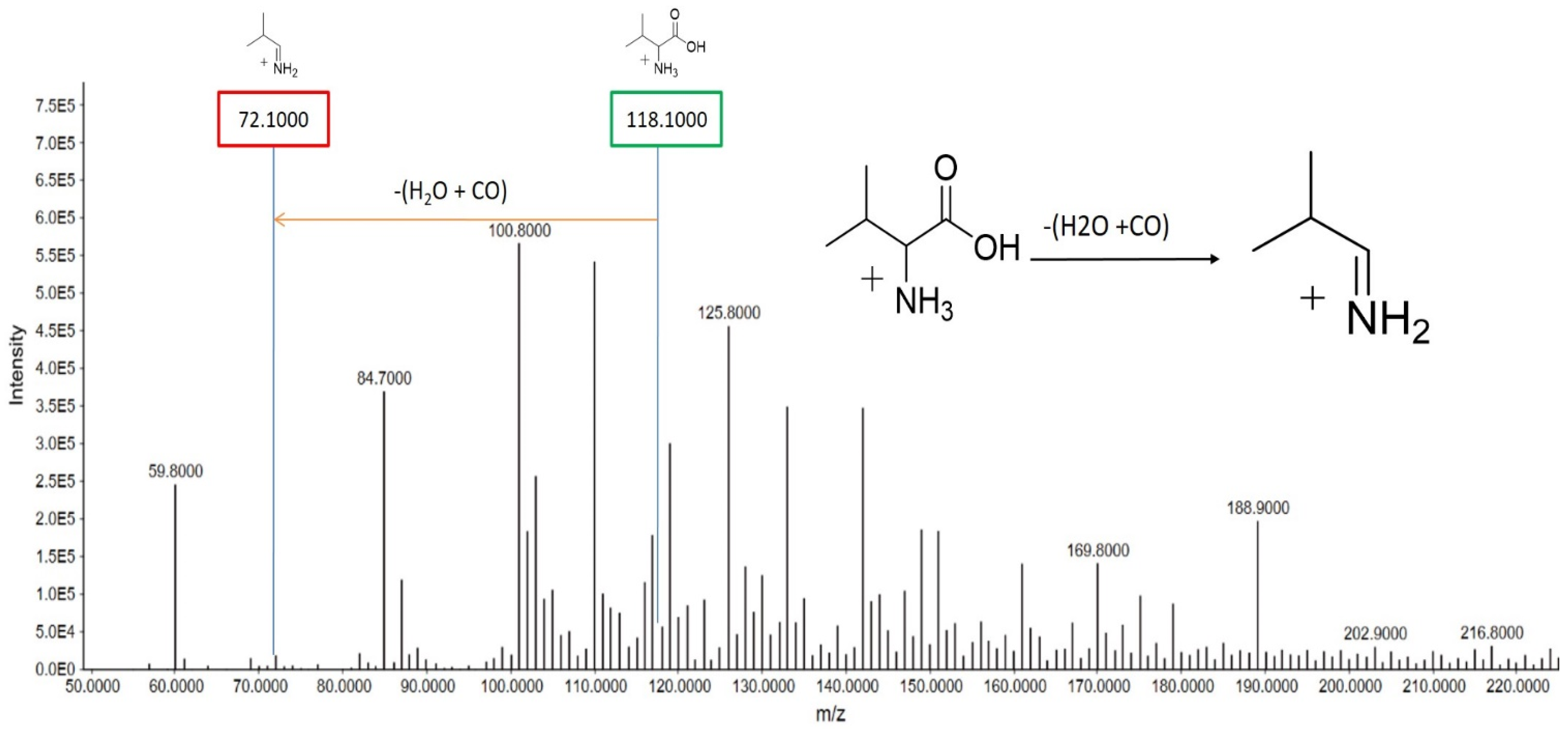
Valine
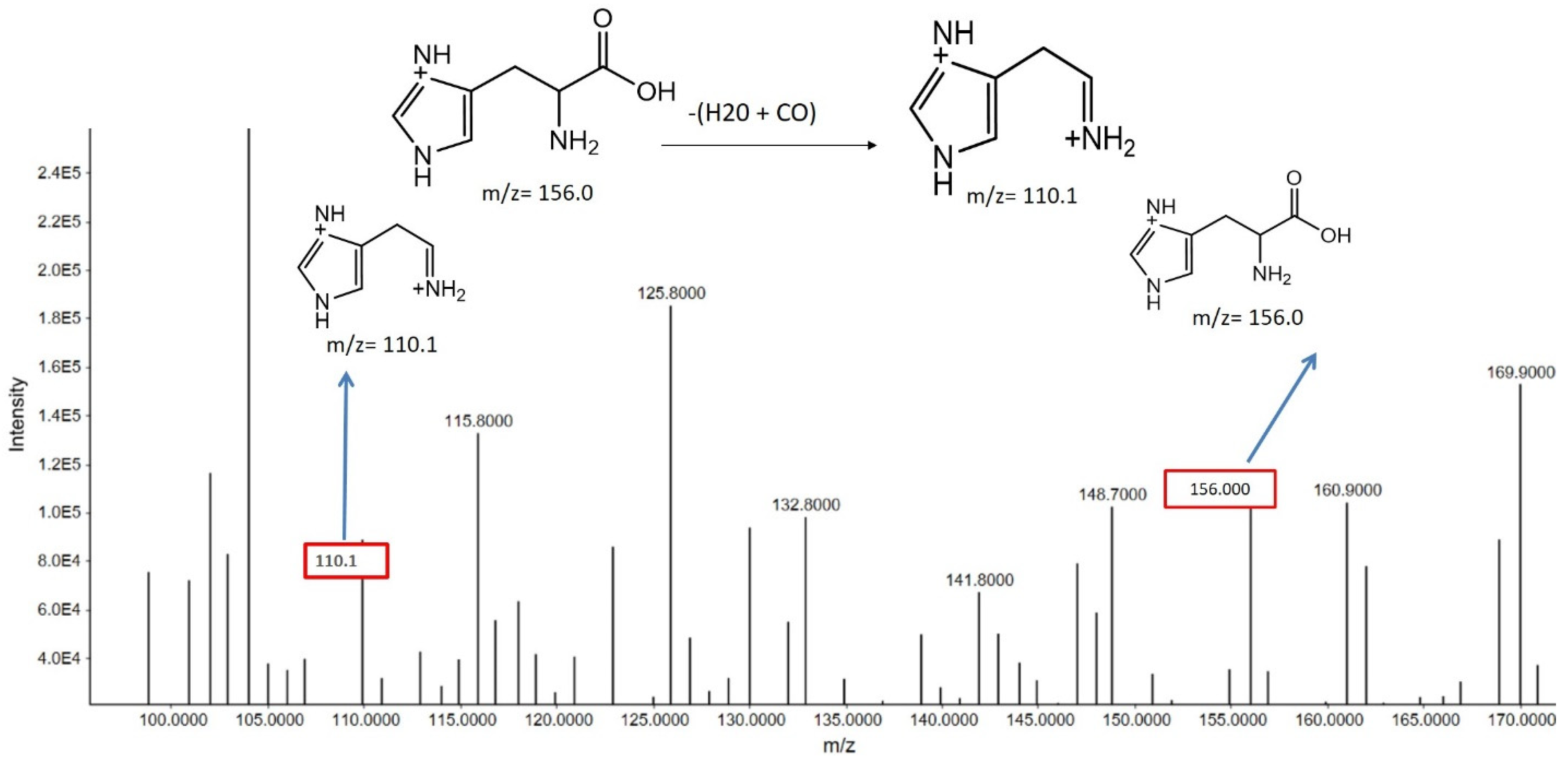
Histidine
Carnitine
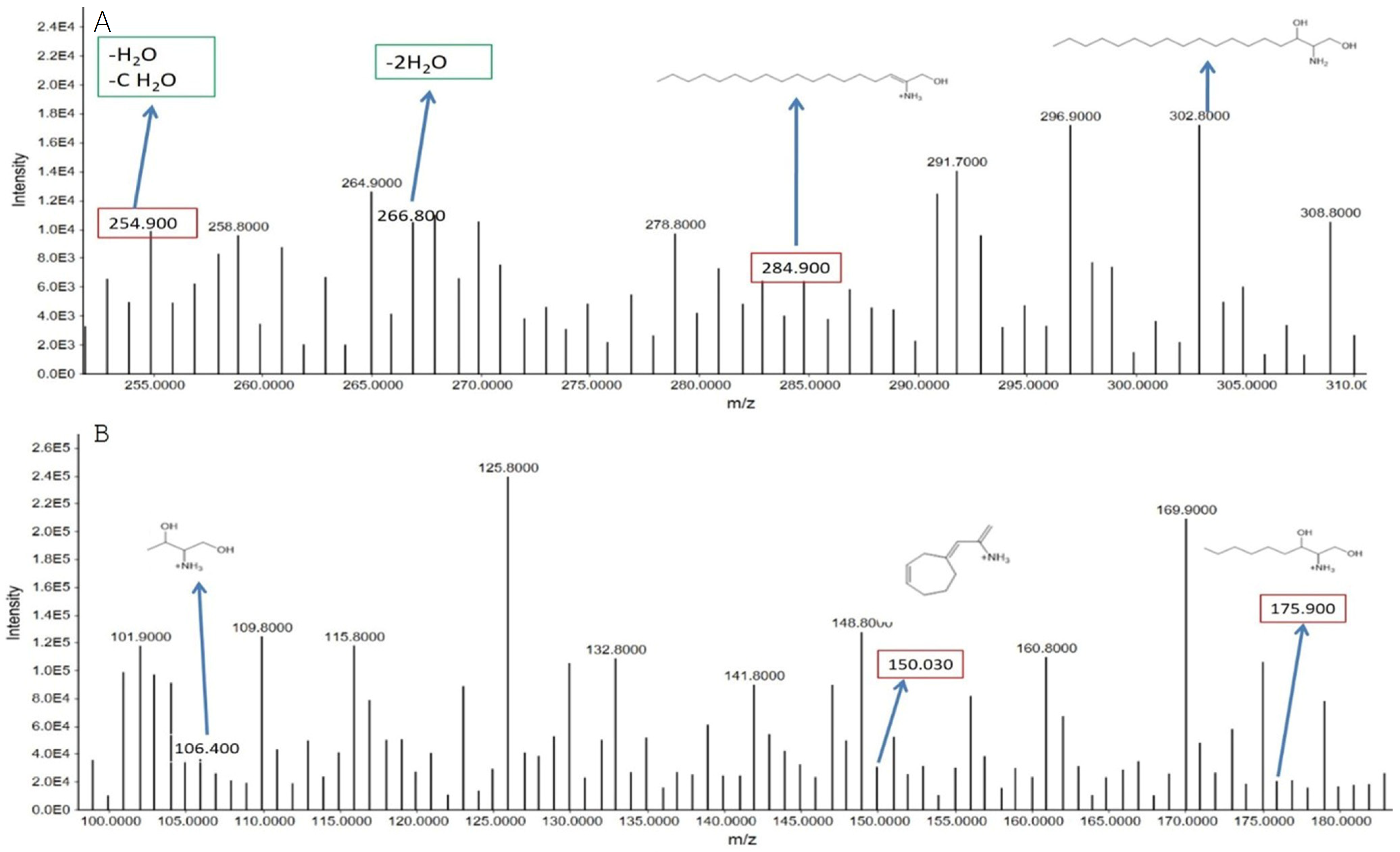
Sphinganine
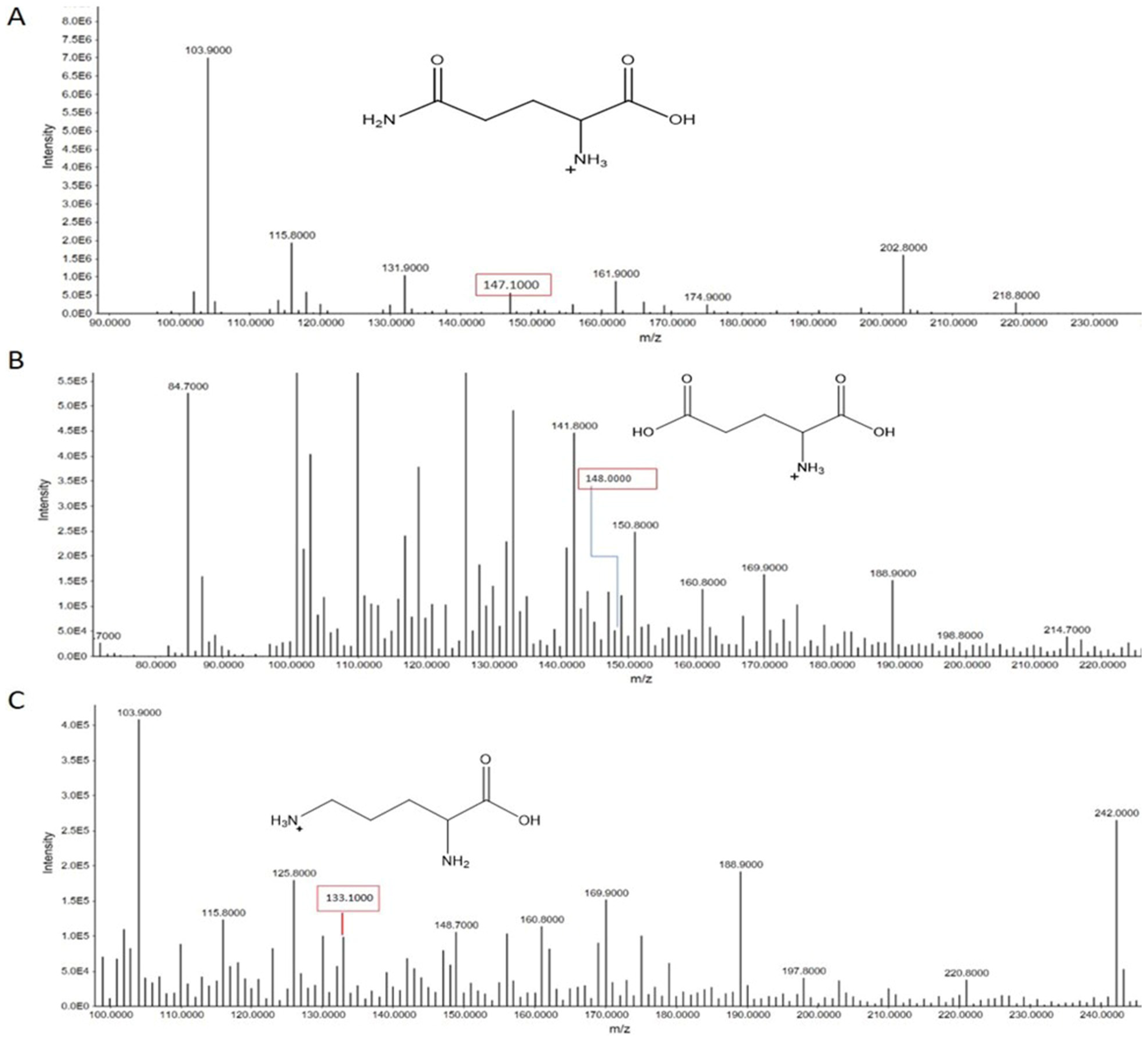
3.2.3. Miscellaneous Amino Acids
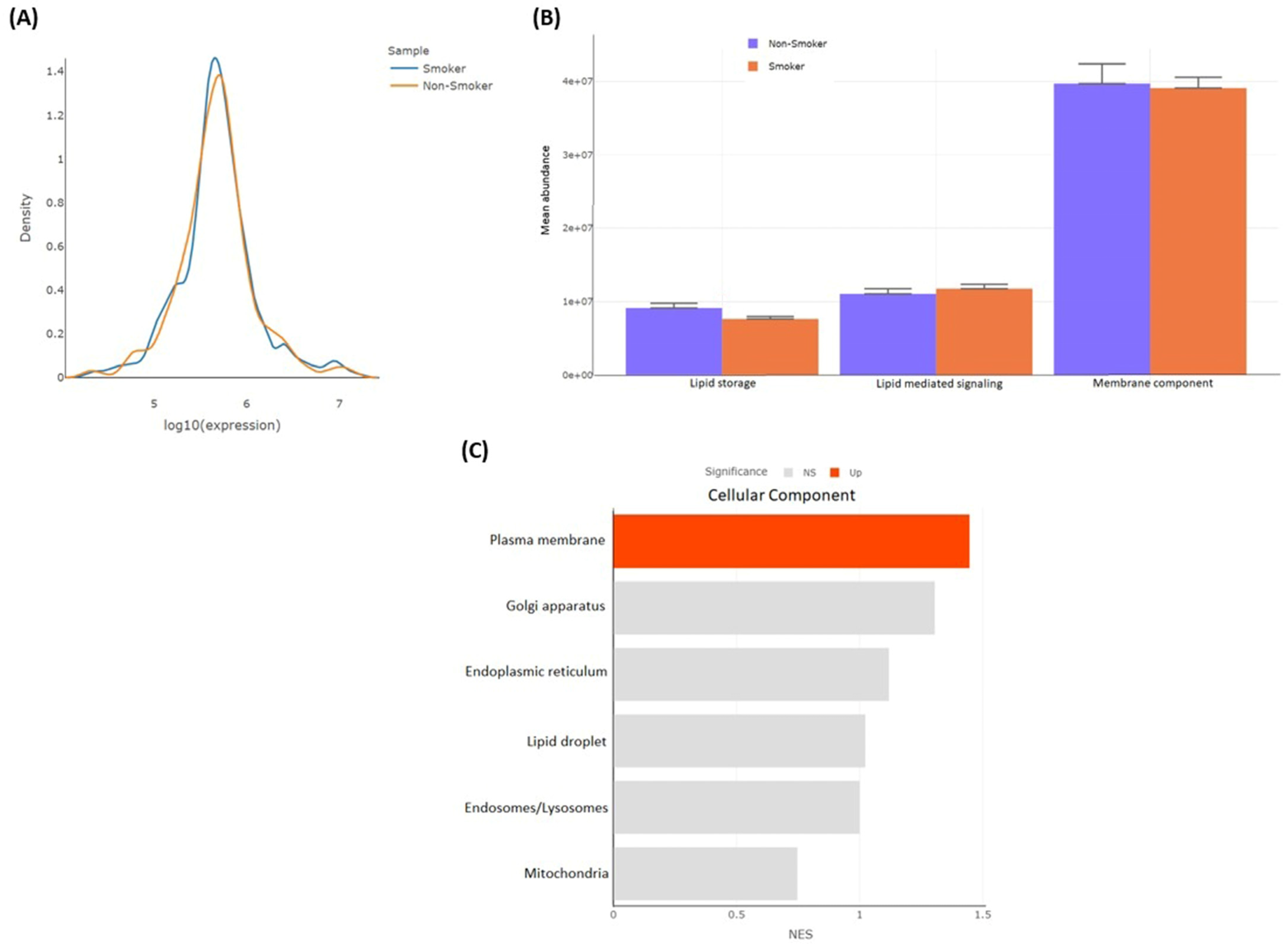
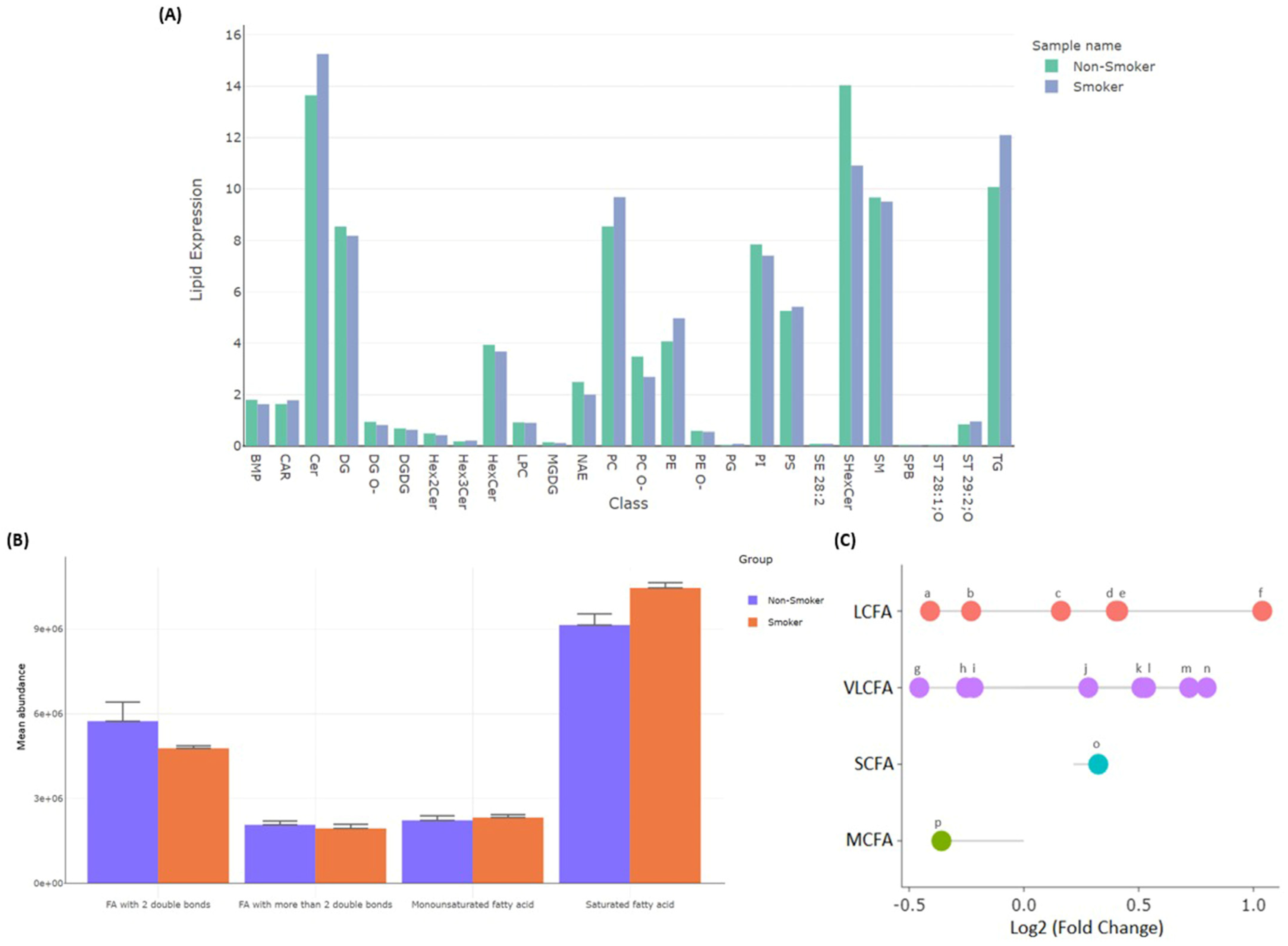
3.2.4. Differentially Detected Lipidomes
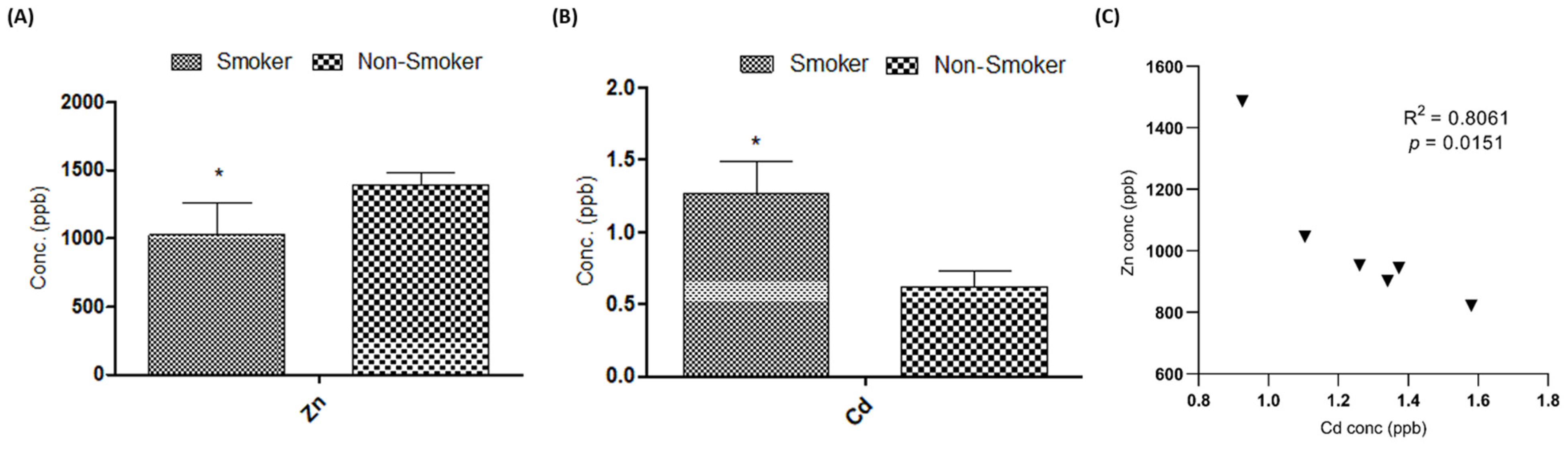
3.3. Effect on Serum Cd and Zn Levels
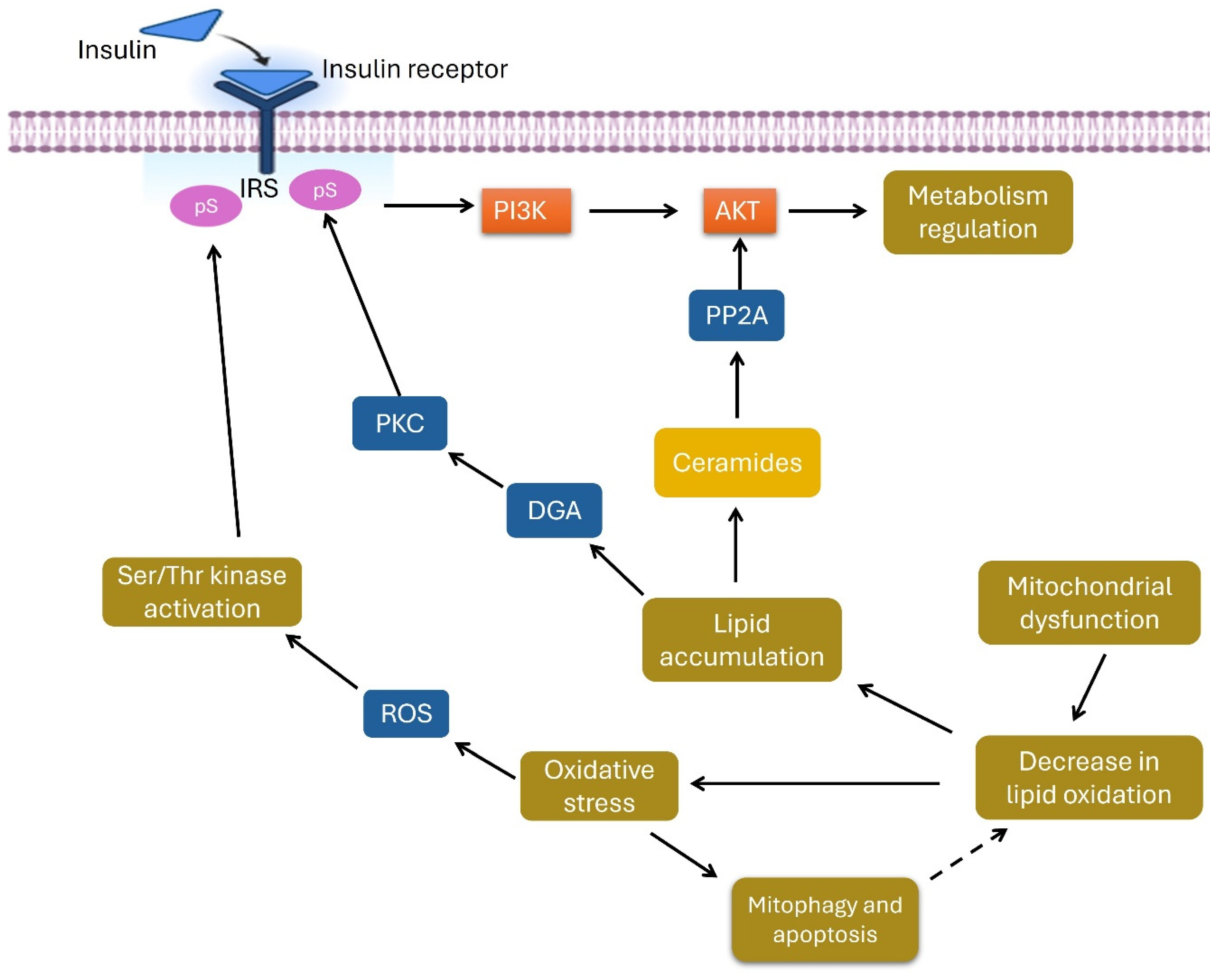
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benowitz, N.L. Nicotine Addiction. N. Engl. J. Med. 2010, 362, 2295–2303. [Google Scholar] [CrossRef]
- Fiore, M.C.; Jaén, C.; Baker, T.B.; Bailey, W.C.; Benowitz, N.L.; Curry, S.J.; Dorfman, S.F.; Froelicher, E.; Goldstein, M. A Clinical Practice Guideline for Treating Tobacco Use and Dependence: 2008 Update: A US public health service report. Am. J. Prev. Med. 2008, 35, 158–176. [Google Scholar]
- Lerman, C.; Tyndale, R.; Patterson, F.; Wileyto, E.P.; Shields, P.G.; Pinto, A.; Benowitz, N. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin. Pharmacol. Ther. 2006, 79, 600–608. [Google Scholar] [CrossRef]
- Tan, X.; Vrana, K.; Ding, Z.-M. Cotinine: Pharmacologically active metabolite of nicotine and neural mechanisms for its actions. Front. Behav. Neurosci. 2021, 15, 758252. [Google Scholar] [CrossRef]
- Shoaib, S.M.; Afzal, S.; Feezan, A.; Akash, M.S.H.; Nadeem, A.; Mir, T.M. Metabolomics Analysis and Biochemical Profiling of Arsenic-Induced Metabolic Impairment and Disease Susceptibility. Biomolecules 2023, 13, 1424. [Google Scholar] [CrossRef]
- Fan, J.; Zhou, Y.; Meng, R.; Tang, J.; Zhu, J.; Aldrich, M.C.; Cox, N.J.; Zhu, Y.; Li, Y.; Zhou, D. Cross-talks between gut microbiota and tobacco smoking: A two-sample Mendelian randomization study. BMC Med. 2023, 21, 163. [Google Scholar] [CrossRef]
- Bajaj, M. Nicotine and Insulin Resistance: When the Smoke Clears. Diabetes 2012, 61, 3078–3080. [Google Scholar] [CrossRef]
- Ahmadkhaniha, R.; Yousefian, F.; Rastkari, N. Impact of smoking on oxidant/antioxidant status and oxidative stress index levels in serum of the university students. J. Environ. Health Sci. Eng. 2021, 19, 1043–1046. [Google Scholar] [CrossRef]
- Chen, B.; Zeng, G.; Sun, L.; Jiang, C. When smoke meets gut: Deciphering the interactions between tobacco smoking and gut microbiota in disease development. Sci. China Life Sci. 2024, 67, 854–864. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, P.; Yang, X.; Chen, M.; Dong, Y.; Li, J. Untargeted metabolomics unravel serum metabolic alterations in smokers with hypertension. Front. Physiol. 2023, 14, 1127294. [Google Scholar] [CrossRef]
- Harada, S.; Ohmomo, H.; Matsumoto, M.; Sata, M.; Iida, M.; Hirata, A.; Miyagawa, N.; Kuwabara, K.; Kato, S.; Toki, R.; et al. Metabolomics Profiles Alterations in Cigarette Smokers and Heated Tobacco Product Users. J. Epidemiol. 2023, 34, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, K.; Levänen, B.; Palmberg, L.; Åkesson, A.; Lindén, A. Cadmium in tobacco smokers: A neglected link to lung disease? Eur. Respir. Rev. 2018, 27, 170122. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, K. Multiplatform Metabolome Profiling to Identify Specific Signatures and Biomarkers in Blood Samples: Untargeted Approach. Ph.D. Thesis, Universidad de La Rioja, Logroño, Spain, 2023. [Google Scholar]
- Dator, R.; Villalta, P.W.; Thomson, N.; Jensen, J.; Hatsukami, D.K.; Stepanov, I.; Warth, B.; Balbo, S. Metabolomics profiles of smokers from two ethnic groups with differing lung cancer risk. Chem. Res. Toxicol. 2020, 33, 2087–2098. [Google Scholar] [CrossRef]
- Novelle, M.G.; Wahl, D.; Diéguez, C.; Bernier, M.; de Cabo, R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res. Rev. 2015, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Saba, S.; Akash, M.S.H.; Rehman, K.; Saleem, U.; Fiayyaz, F.; Ahmad, T. Assessment of heavy metals by ICP-OES and their impact on insulin stimulating hormone and carbohydrate metabolizing enzymes. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1682–1691. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, X.; Huang, Z.; Pan, Y.; Westbrook, A.; Li, S.; Bazzano, L.; Chen, W.; He, J.; Kelly, T.; et al. Examination of serum metabolome altered by cigarette smoking identifies novel metabolites mediating smoking-BMI association. Obesity 2022, 30, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ji, X.; Rahman, I. Dysregulated Metabolites Serve as Novel Biomarkers for Metabolic Diseases Caused by E-Cigarette Vaping and Cigarette Smoking. Metabolites 2021, 11, 345. [Google Scholar] [CrossRef]
- Soulimane, S.; Simon, D.; Herman, W.H.; Lange, C.; Lee, C.M.; Colagiuri, S.; Shaw, J.E.; Zimmet, P.Z.; Magliano, D.; Ferreira, S.R.; et al. HbA1c, fasting and 2 h plasma glucose in current, ex- and never-smokers: A meta-analysis. Diabetologia 2014, 57, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Vlassopoulos, A.; Lean, M.E.; Combet, E. Influence of smoking and diet on glycated haemoglobin and ‘pre-diabetes’ categorisation: A cross-sectional analysis. BMC Public Health 2013, 13, 1013. [Google Scholar] [CrossRef]
- Halimi, J.M.; Giraudeau, B.; Vol, S.; Cacès, E.; Nivet, H.; Lebranchu, Y.; Tichet, J. Effects of current smoking and smoking discontinuation on renal function and proteinuria in the general population. Kidney Int. 2000, 58, 1285–1292. [Google Scholar] [CrossRef]
- Larregle, E.V.; Varas, S.M.; Oliveros, L.B.; Martinez, L.D.; Antón, R.; Marchevsky, E.; Giménez, M.S. Lipid metabolism in liver of rat exposed to cadmium. Food Chem. Toxicol. 2008, 46, 1786–1792. [Google Scholar] [CrossRef]
- Cheng, Y.-Y.; Huang, N.-C.; Chang, Y.-T.; Sung, J.-M.; Shen, K.-H.; Tsai, C.-C.; Guo, H.-R. Associations between arsenic in drinking water and the progression of chronic kidney disease: A nationwide study in Taiwan. J. Hazard. Mater. 2017, 321, 432–439. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Li, T.; Xian, H.; Al-Aly, Z. Blood urea nitrogen and risk of insulin use among people with diabetes. Diabetes Vasc. Dis. Res. 2018, 15, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, J.; Liu, X.; Han, F.; Cai, Q.; Peng, G.; Zhang, K.; Chen, W.; Wang, J.; Huang, H. Cigarette smoking reduced renal function deterioration in hypertensive patients may be mediated by elevated homocysteine. Oncotarget 2016, 7, 86000–86010. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.C.; Xu, Z.L.; Zhao, M.Y.; Xu, K. The association between smoking and renal function in people over 20 years old. Front. Med. 2022, 9, 870278. [Google Scholar] [CrossRef]
- Hyder, O.; Chung, M.; Cosgrove, D.; Herman, J.M.; Li, Z.; Firoozmand, A.; Gurakar, A.; Koteish, A.; Pawlik, T.M. Cadmium exposure and liver disease among US adults. J. Gastrointest. Surg. 2013, 17, 1265–1273. [Google Scholar] [CrossRef]
- Hong, D.; Min, J.Y.; Min, K.B. Association Between Cadmium Exposure and Liver Function in Adults in the United States: A Cross-sectional Study. J. Prev. Med. Public Health 2021, 54, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Aldaham, S.; Foote, J.A.; Chow, H.H.; Hakim, I.A. Smoking Status Effect on Inflammatory Markers in a Randomized Trial of Current and Former Heavy Smokers. Int. J. Inflam. 2015, 2015, 439396. [Google Scholar] [CrossRef] [PubMed]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free. Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxidative Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S. Is Chronic Kidney Disease Due to Cadmium Exposure Inevitable and Can It Be Reversed? Biomedicines 2024, 12, 718. [Google Scholar] [CrossRef]
- Mosna, K.; Jurczak, K.; Krężel, A. Differentiated Zn (II) binding affinities in animal, plant, and bacterial metallothioneins define their zinc buffering capacity at physiological pZn. Metallomics 2023, 15, mfad061. [Google Scholar] [CrossRef] [PubMed]
- Grzybowska, E.A. Calcium-binding proteins with disordered structure and their role in secretion, storage, and cellular signaling. Biomolecules 2018, 8, 42. [Google Scholar] [CrossRef]
- Yu, H.-t.; Zhen, J.; Leng, J.-y.; Cai, L.; Ji, H.-l.; Keller, B.B. Zinc as a countermeasure for cadmium toxicity. Acta Pharmacol. Sin. 2021, 42, 340–346. [Google Scholar] [CrossRef]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem.-Biol. Interact. 2014, 211, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hao, W.; Shi, H.; Hou, Y.; Xu, Q. Calcium homeostasis disruption-a bridge connecting cadmium-induced apoptosis, autophagy and tumorigenesis. Oncol. Res. Treat. 2015, 38, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, X.; Liu, X.; Xie, C.; Shi, J. The role of the kynurenine pathway in cardiovascular disease. Front. Cardiovasc. Med. 2024, 11, 1406856. [Google Scholar] [CrossRef] [PubMed]
- Gherghina, M.E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress-Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- Prokopieva, V.D.; Yarygina, E.G.; Bokhan, N.A.; Ivanova, S.A. Use of Carnosine for Oxidative Stress Reduction in Different Pathologies. Oxid. Med. Cell. Longev. 2016, 2016, 2939087. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Yang, S.; Seng, S. Mechanisms of Cancer Induction by Tobacco-Specific NNK and NNN. Cancers 2014, 6, 1138–1156. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Burns, K.; Manolikos, C.; Fatovich, D.; Bell, D.A. Hyperammonaemia: Review of the pathophysiology, aetiology and investigation. Pathology 2024, 56, 763–772. [Google Scholar] [CrossRef]
- Balkhi, H.M.; Gul, T.; Banday, M.Z.; Haq, E. Glutamate excitotoxicity: An insight into the mechanism. Int. J. Adv. Res. 2014, 2, 361–373. [Google Scholar]
- D’Ambrosio, V.; Capolongo, G.; Goldfarb, D.; Gambaro, G.; Ferraro, P.M. Cystinuria: An update on pathophysiology, genetics, and clinical management. Pediatr. Nephrol. 2022, 37, 1705–1711. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Liang, X.; Zou, L.; Ong, C.N.; Yuan, J.-M.; Koh, W.-P.; Pan, A. Serum amino acids in association with prevalent and incident type 2 diabetes in a Chinese population. Metabolites 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Onmaz, M.; Eryavuz Onmaz, D.; Demirbas, N.; Kutlu, R.; Ünlü, A.; Hatir, A. Tobacco induces abnormal metabolism of tryptophan via the kynurenine pathway. Turk. J. Biochem. 2025. [Google Scholar] [CrossRef]
- Okafor, I.; Uzoma, G.; Nvani, L. Cobalamin, Folate and Pyridoxal 5- Phosphate Level of Smokers in Calabar Cross River State. J. Glob. Oncol. 2018, 4, 15s. [Google Scholar] [CrossRef]
- Kozieł, K.; Urbanska, E.M. Kynurenine pathway in diabetes mellitus—Novel pharmacological target? Cells 2023, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-X.; Zhang, K.-H.; Zhou, Q.; Hu, S.-H.; Lin, Y.; Xu, W.; Zhao, S.-M.; Yuan, Y.-Y. Tryptophanylation of insulin receptor by WARS attenuates insulin signaling. Cell. Mol. Life Sci. 2024, 81, 25. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Quinville, B.M.; Deschenes, N.M.; Ryckman, A.E.; Walia, J.S. A comprehensive review: Sphingolipid metabolism and implications of disruption in sphingolipid homeostasis. Int. J. Mol. Sci. 2021, 22, 5793. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Hu, X.; Li, J.; Bai, B.; Tian, L. Lipidomics analysis of impaired glucose tolerance and type 2 diabetes mellitus in overweight or obese elderly adults. Endocr. Connect. 2023, 12, e230212. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S. Oxidative Stress and ROS Link Diabetes and Cancer. J. Mol. Pathol. 2024, 5, 96–119. [Google Scholar] [CrossRef]
- Zardini Buzatto, A.; Tatlay, J.; Bajwa, B.; Mung, D.; Camicioli, R.; Dixon, R.A.; Li, L. Comprehensive serum lipidomics for detecting incipient dementia in Parkinson’s disease. J. Proteome Res. 2021, 20, 4053–4067. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Particulars |
|---|---|
| Instrument | The triple quadrupole LC/MS 6495C from Agilent accompanied by electrospray ionization source |
| Mode of ion | Positive ion scan mode |
| Sheath gas temperature | 300 °C |
| Sheath gas flow rate | 8 L/min |
| Range of scanning mass | 50 to 1000 m/z |
| Flow rate of auxiliary gas | 14/min |
| Capillary voltage | 3000 V |
| Nozzle voltage | 1500 V |
| Biochemical Parameters | Non-Smoker | Smoker | p-Value |
|---|---|---|---|
| Glycemic index markers | |||
| FBG (mg/dL) | 84.000 ± 14.004 | 93.000 ± 9.785 | <0.05 a |
| HbA1c (%) | 4.235 ± 0.885 | 4.876 ± 0.762 | <0.05 a |
| Insulin (U/mL) | 9.788 ± 2.501 | 11.9 ± 2.635 | <0.05 a |
| Lipid profile biomarkers | |||
| Cholesterol (mg/dL) | 128.666 ± 28.854 | 154.888 ± 35.565 | <0.05 a |
| LDL (mg/dL) | 92.363 ± 16.842 | 117.545 ± 24.138 | <0.05 a |
| HDL (mg/dL) | 70.363 ± 9.08 | 57.181 ± 12.440 | <0.05 a |
| Triglycerides (mg/dL) | 120.478 ± 28.348 | 153.043 ± 43.347 | <0.05 a |
| Inflammatory biomarkers | |||
| CRP (mg/L) | 4.2 ± 0.618 | 4.937 ± 0.568 | <0.05 a |
| IL-6 (pg/mL) | 5.21 ± 0.769 | 6.137± 0.979 | <0.05 a |
| Liver function biomarkers | |||
| ALT (U/L) | 25.919 ± 10.011 | 30.112 ± 11.512 | <0.05 a |
| AST (U/L) | 25.758 ± 7.049 | 28.93 ± 8.853 | <0.05 a |
| ALP (U/L) | 83.677 ± 23.327 | 93.806 ± 21.350 | <0.05 a |
| Kidney function biomarkers | |||
| Creatinine (mg/dL) | 0.873 ± 0.143 | 0.959 ± 0.197 | <0.05 a |
| BUN (mg/dL) | 32.642 ± 5.534 | 35.285 ± 5.645 | <0.05 a |
| Pathway Name | Total | Expected | Hits | p-Value |
|---|---|---|---|---|
| Lysine degradation | 30 | 0.546 | 3 | 0.0158 a |
| Cysteine and methionine metabolism | 33 | 0.6 | 3 | 0.0204 a |
| Arginine biosynthesis | 14 | 0.255 | 2 | 0.0254 a |
| Butanoate metabolism | 15 | 0.273 | 2 | 0.029 a |
| Purine metabolism | 70 | 1.27 | 4 | 0.0352 a |
| Tryptophan metabolism | 41 | 0.746 | 3 | 0.0362 a |
| Amino sugar and nucleotide sugar metabolism | 42 | 0.764 | 3 | 0.0385 a |
| beta-Alanine metabolism | 21 | 0.382 | 2 | 0.0542 a |
| Alanine, aspartate and glutamate metabolism | 28 | 0.509 | 2 | 0.0902 b |
| Glycine, serine and threonine metabolism | 33 | 0.6 | 2 | 0.119 b |
| Thiamine metabolism | 7 | 0.127 | 1 | 0.121 b |
| Arginine and proline metabolism | 36 | 0.655 | 2 | 0.138 b |
| Biotin metabolism | 10 | 0.182 | 1 | 0.168 b |
| Steroid biosynthesis | 41 | 0.746 | 2 | 0.17 b |
| Histidine metabolism | 16 | 0.291 | 1 | 0.256 b |
| Terpenoid backbone biosynthesis | 18 | 0.327 | 1 | 0.283 b |
| Citrate cycle (TCA cycle) | 20 | 0.364 | 1 | 0.309 b |
| Ether lipid metabolism | 20 | 0.364 | 1 | 0.309 b |
| Propanoate metabolism | 21 | 0.382 | 1 | 0.322 b |
| Pyruvate metabolism | 23 | 0.418 | 1 | 0.346 b |
| Glycolysis/Gluconeogenesis | 26 | 0.473 | 1 | 0.382 b |
| Galactose metabolism | 27 | 0.491 | 1 | 0.393 b |
| Glutathione metabolism | 28 | 0.509 | 1 | 0.405 b |
| Lipoic acid metabolism | 28 | 0.509 | 1 | 0.405 b |
| Inositol phosphate metabolism | 30 | 0.546 | 1 | 0.427 b |
| Differentially Detected Metabolites in MS/MS Spectra of Non-Smokers | ||||
| Sr. No | R.T. | m/z | Metabolite | Area |
| 1 | 8.736 | 259.2 | NAOrn 8:0 | 11,496 |
| 2 | 8.091 | 270.2 | NAGly 13:1 | 12,542 |
| 3 | 4.065 | 275.2 | NAOrn 8:0;O | 423,486 |
| 4 | 5.347 | 278.2 | DG 10:0|DG 5:0_5:0 | 12,878 |
| 5 | 6.456 | 325.2 | DG 13:0 | 14,618 |
| 6 | 14.555 | 758.7 | Cer49:3;O4|Cer,13:1;O3/36:2(2OH) | 1,840,580 |
| 7 | 2.304 | 948.9 | TG 57:1|TG 8:0_11:0_38:1 | 105,025 |
| Differentially Detected Metabolites in MS/MS Spectra of Smokers | ||||
| Sr. No | R.T | m/z | Metabolite | Area |
| 1 | 3.334 | 330.3 | SPB 19:1;O3 | 24,725 |
| 2 | 3.9 | 355.3 | NAGly 18:2 | 7024 |
| 3 | 9.286 | 358.3 | CAR 13:0 | 15,329 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, M.A.; Iqbal, H.; Ilyas, K.; Rehman, K.; Hussain, A.; Akash, M.S.H.; Shahid, M.; Chen, S. Metabolomic Insights into Smoking-Induced Metabolic Dysfunctions: A Comprehensive Analysis of Lipid and Amino Acid Metabolomes. Metabolites 2025, 15, 96. https://doi.org/10.3390/metabo15020096
Aslam MA, Iqbal H, Ilyas K, Rehman K, Hussain A, Akash MSH, Shahid M, Chen S. Metabolomic Insights into Smoking-Induced Metabolic Dysfunctions: A Comprehensive Analysis of Lipid and Amino Acid Metabolomes. Metabolites. 2025; 15(2):96. https://doi.org/10.3390/metabo15020096
Chicago/Turabian StyleAslam, Muhammad Amtiaz, Hajra Iqbal, Kainat Ilyas, Kanwal Rehman, Amjad Hussain, Muhammad Sajid Hamid Akash, Mudassar Shahid, and Shuqing Chen. 2025. "Metabolomic Insights into Smoking-Induced Metabolic Dysfunctions: A Comprehensive Analysis of Lipid and Amino Acid Metabolomes" Metabolites 15, no. 2: 96. https://doi.org/10.3390/metabo15020096
APA StyleAslam, M. A., Iqbal, H., Ilyas, K., Rehman, K., Hussain, A., Akash, M. S. H., Shahid, M., & Chen, S. (2025). Metabolomic Insights into Smoking-Induced Metabolic Dysfunctions: A Comprehensive Analysis of Lipid and Amino Acid Metabolomes. Metabolites, 15(2), 96. https://doi.org/10.3390/metabo15020096











