Abstract
This study aimed to determine blood and milk metabolic parameters and their correlations for the purpose of evaluating metabolic status in dairy cows. Blood and milk samples were collected from 100 Holstein dairy cows during morning milking. The cows were allocated to four groups according to the production period, including cows in early (n = 18), full (n = 26), mid (n = 25) and late (n = 31) lactation. The value of non-esterified fatty acids (NEFA), β-hydroxybutyrate (BHB), glucose, triglycerides (TG), total cholesterol (TChol), total protein (TP), albumin, globulin, urea, total bilirubin (TBil), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), and lactate dexydrogenase (LDH) in the blood were determined. The following milk parameters were measured: fat, protein, lactose, urea, AST, ALT, ALP, GGT, LDH and BHB. Blood serum NEFA, BHB, TBil, AST, ALT, ALP and LDH were higher in early lactation cows, whereas glucose, TP, globulin and urea levels were significantly lower in early lactation cows. Milk fat and lactose levels were lower in early lactation cows, whereas milk protein and the activities of AST, ALT, ALP and LDH in milk were highly greater in early lactation cows. Milk fat was positively correlated with glucose, TP and TG, and negatively correlated with BHB, NEFA, TBil, ALT, LDH and ALP levels in the blood. Enzyme activities in milk were positively correlated with those in blood and with blood NEFA, BHB and TBil levels, and negatively correlated with blood glucose, TChol and TG. A significant positive correlation existed between blood and milk BHB values. Many correlations showed the same slope during all lactation periods. In conclusion, similar changes in blood and milk metabolite concentration during lactation and milk to blood correlations confirm that milk has great potential in predicting of blood metabolites and metabolic status of cows.
1. Introduction
Dairy cows are commonly affected by production diseases, i.e., diseases related to poor nutrition or management [1,2]. Metabolic profile testing is routinely used to reveal metabolic disorders in dairy cattle. During lactation, blood and milk biochemical parameters are usually checked to evaluate animal health and milk yield, especially when the herd is at high risk of developing metabolic diseases [3,4,5,6]. Major health disorders in high-yielding cows occur around parturition and during lactation. States of negative energy balance (fasting, parturition and lactation) cause excessive fat mobilization and accumulation in liver cells, causing ketogenesis and disturbance of liver physiology and morphology [7,8,9,10].
Clinical and laboratory monitoring during the transition period and early lactation play an important role in detecting subclinical nutritional and metabolic diseases. The main blood markers of lipid mobilization in dairy cows are BHB, the most important ketone body, and NEFA [11,12,13]. Lipid metabolites are important in pathogenesis of many metabolic disease and stress adaptation in cows [14]. NEFA are accumulated as TG in the liver, primarily due to the decreased hepatic synthesis of very low-density lipoproteins (VLDL). However, in liver lipidosis, endogenous synthesis decreases, leading to a reduction in blood glucose, TP, albumin, globulin, TChol, TG and urea concentrations. Moreover, as the excretory capacity of liver cells is reduced, the blood levels of some metabolites such as TBil, ammonia and bile acids are generally elevated [15,16]. Fatty liver and diffuse infiltration of hepatocytes are characterized by cell membrane damage, hepatocyte destruction, and the release of cytoplasmic enzymes, whose blood activities are considerably increased. Therefore, blood serum ALT, aspartate aminotransferase (AST), ALP, LDH and GGT activities are useful indicators of postpartum liver function [8,17,18]. As there is little information about changes in ALT, AST, LDH, GGT and ALP activities in milk, practical attention has been focused on the assessment of enzyme activities in milk, with many enzymes being proposed and listed as reliable markers for early diagnosis of subclinical disease [19,20,21]. The activities of these enzymes in milk and blood sera of cows were evaluated and exhibited close relationships, as shown by the results of correlation analysis and regression models [22,23].
Milk parameters originate from blood and food components, and elucidating relationships among these parameters individually in food, blood and milk helps to understand the health and production status of animals [21,22,23,24,25]. Milk may be a preferred matrix whenever possible because it is non-invasive and easy to collect, and it is often used to identify ketosis and other production disease [26,27]. Variations in milk fat and milk BHB levels indicate lipomobilization and ketogenesis [28,29]. Milk, the outcome of various biochemical activities in mammary secretory cells, contains fat, protein, lactose, enzymes, vitamins and various minerals. Its composition is dependent on various factors, i.e., stage of lactation, lactation number, breed, feeding pattern, environmental conditions and udder health [30].
However, modern management practices involve more frequent or even daily monitoring of the health status of high-yielding dairy cows to control milk yield and quality and address potential health problems. This study aimed to determine blood and milk metabolic parameters and their relationships for the purpose of evaluating the metabolic status of dairy cows for early diagnosis of subclinical metabolic disease at different stages of lactation.
2. Materials and Methods
2.1. Animals and Study Design
A total of 100 dairy cows were randomly selected from the same Holstein herd containing almost 1000 cows (Vrbas, Vojvodina, Serbia). Clinically healthy cows were allocated to four experimental groups: Group 1—early lactation cows (n = 18), from 1 to 49 days of lactation; Group 2—full lactation cows (n = 26), from 50 to 109 days of lactation; Group 3—mid-lactation cows (n = 25), between 110 and 209 days of lactation; and Group 4—late lactation cows (n = 31), from 210 to 305 and more days of lactation. The cows were high-yielding, aged 4 years on average (with an average of 2.7 lactations), with a preceding lactation of about 8500L (average weekly yield was 26.5 L/cow/day). The average body condition score (BCS) was 3.36 ± 0.55 for all experimental cows. The experimental cows were housed in free-stall barns. Diet and housing conditions were adapted to the purposes of the experiment, with diet tailored to the cows’ energy requirements during different periods of lactation using National Research Council (NRC) standards [31].
On the farm, the experimental dairy cows were fed a total mixed ration (TMR) twice daily and had ad libitum access to water. The ration for early lactation cows contained: dry matter (DM) 21.5 kg; net energy of lactation 153.2 MJ; crude protein (CP) 18.3% DM; rumen undegradable protein 39.69% CP; fat 4.92% DM; fiber 17.2% DM; acid detergent fiber (ADF) 22.6% DM, and neutral detergent fiber (NDF) 37.16% DM. Generally, diets were designed to provide the consumption of about 23 kg DM, i.e., 43 kg of feed. At different stages of lactation, diets were balanced to increase the percentage of corn silage from 30% in the early lactation period to 50% in the dry period, whereas the grain proportion was reduced from 5–8% to 3–4% in respective periods. Balancing did not change the overall average chemical composition of the diet.
2.2. Blood Analysis
Blood samples were taken 4 to 6 h after milking and feeding from the coccygeal vein into evacuated serum separator tubes. After clotting for 3 h at 4 °C and centrifugation (1500 G, 10 min), blood sera were analyzed for the following biochemical parameters: glucose, non-esterified fatty acids (NEFA), β-hydroxybutyrate (BHB), total cholesterol (TChol), triglycerides (TG), total bilirubin (TBil), total protein (TP), urea, albumin, globulin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT) and lactate dexydrogenase (LDH), which were determined by colorimetric kits (Biosystem, Spain and Randox, Carlisle, UK) and a Chemray spectrophotometer (Rayto, Shenzhen, China). All analyses were performed at the Laboratory of Pathophysiology, Department of Veterinary Medicine, University of Novi Sad.
2.3. Milk Analysis
Milk samples were collected during morning milking into tubes with and without additives on the same day blood was sampled. The chemical composition of milk was determined at the Central Laboratory for Milk Quality Control at Agriculture faculty of Novi Sad. Milk samples were analyzed by a FOSS milk analyzer, and their chemical composition was assessed by a MILKOSCANFT analyzer (Milko-Scan 133 B, Foss Electric, Denmark) using Fourier-transform infrared spectroscopy. Milk fat, protein and lactose contents were determined. Before analysis, samples were heated in a water bath at 40 + 2 °C. After homogenization, about 5 mL of milk was taken by the apparatus. Upon serum separation, milk was subjected to biochemical tests for the determination of the enzymes (AST, ALT, ALP, GGT, LDH), urea and BHB. Milk serum was separated after centrifugation at 10,000× g for 30 min and was transferred to new tubes for analysis. The biochemical reagents and apparatus used for milk serum analysis were the same as for blood serum.
2.4. Statistical Analysis
All blood and milk metabolic parameters were included in PCA analysis and unit variance scaling was applied to rows. Singular value decomposition with imputation was used to calculate principal components. The X and Y axes showed principal component 1 and principal component 2. Principal components analysis (PCA) was used to obtain a graphical representation of the four groups of cows to estimate which stage of lactation was expected to show the largest deviation in the parameters tested. Then, the effect of lactation period on blood and milk biochemical parameters was examined by ANOVA analysis coupled with an LSD post hoc test. Associations between milk and blood biochemical parameters were determined by Pearson’s coefficient of correlation. Importantly, estimating lipolysis and ketogenesis in dairy cows through milk parameters allowed continuous monitoring of energy balance. Regression lines between blood NEFA and BHB values and the parameters with which they were significantly correlated at the entire lactation level were derived. Then, the homogeneity of slopes of the regression lines between NEFA and BHB levels and milk parameters was tested as a function of lactation period to determine which parameters can be used throughout lactation, and which milk parameters should be considered relative to lactation period for the estimation of lipolysis and ketogenesis, i.e., the energy balance (EB). SPSS statistics software (IBM, USA) was used.
3. Results
The PCA score plots showed that cows from different period of lactation were clustered differently so that early lactation cows were clustered separately from cows in other three period of lactation (Figure 1). Considering that all metabolic parameters from blood and milk were included in the PCA analysis, the obtained result requires further examination of statistically significant differences between lactation periods for each metabolic parameter.
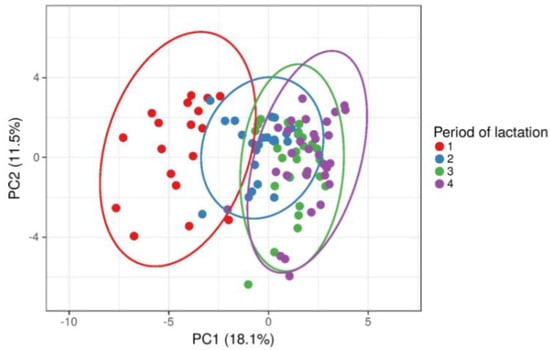
Figure 1.
Principal components analysis (PCA) and clustering of cows based on lactation period (early—1, mid—2, full—3 and late—4) after including all blood and milk metabolic parameters.
Blood biochemical parameters in cows during the four lactation periods are summarized in Table 1. Serum NEFA and BHB concentrations were highly significantly greater in early lactation cows than in cows during the other lactation periods (p < 0.001), whereas mean TG and TChol concentrations were significantly lower (p < 0.01) in early lactation cows. In addition, glucose, TP, globulin and urea levels were significantly lower in Group 1 (early lactation) cows (p < 0.01), which also exhibited a slight (but not significant) decrease in albumin concentration. By contrast, TBil and serum AST, ALT, ALP and LDH activities were significantly increased in early lactation cows compared with cows in the other periods of lactation (p < 0.01).

Table 1.
Blood metabolic parameter in early (Group 1), mid (Group 2), full (Group 3) and late (Group 4) lactation dairy cows.
Table 2 shows significant changes in most milk metabolic parameters between the experimental groups of cows in this study. Milk fat levels were significantly lower (p < 0.001) in early lactation cows than in the other periods of lactation, whereas the mean values of milk protein were highly significantly greater (p < 0.001) in early and late lactation than in the other periods of lactation. Milk lactose levels were significantly lower (p < 0.001) in early lactation cows than during mid and late lactation. The activities of AST, ALT, ALP and LDH in milk were also significantly higher in early lactation (p < 0.01) than in the other stages of lactation. There was no significant difference (p > 0.05) in milk serum BHB and urea values between the experimental groups of cows.

Table 2.
Milk metabolic parameters in early (Group 1), mid (Group 2), full (Group 3) and late (Group 4) lactation dairy cows.
Table 3 shows the coefficients of correlation between blood and milk biochemical parameters calculated for all cows in this experiment. Milk fat was positively correlated (p < 0.05) with glucose, TP and TG, and negatively correlated (p < 0.05) with BHB, NEFA, TBil, ALT, LDH and ALP levels in the blood. There was a significantly positive correlation (p < 0.05) between milk protein and blood GGT. Enzyme activities in milk were positively correlated (p < 0.05) with those in blood (except for LDH) and with blood NEFA, BHB and TBil levels, and negatively correlated (p < 0.05) with blood glucose, TChol and TG. A significant positive correlation (p < 0.01) existed between blood and milk BHB values.

Table 3.
Correlation between milk composition and diagnostic blood metabolic parameters.
The regression lines for the statistically significant correlations between blood NEFA and BHB levels and selected milk parameters are presented in Figure 2. The regression lines for milk fat to blood BHB, milk fat to blood NEFA, and milk BHB to blood BHB relationships showed good homogeneity with the similar slopes through all four lactation periods. However, in other relationships, the regression lines changed their slope, indicating that lactation period should be taken into consideration when evaluating lipolysis and ketogenesis using milk parameters (Figure 2). The change in the value of NEFA and BHB in the function of enzymes in milk was much greater in the later periods of lactation. This requires additional research.
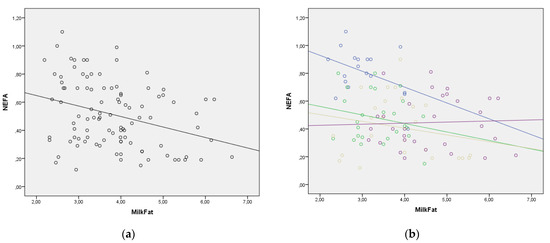
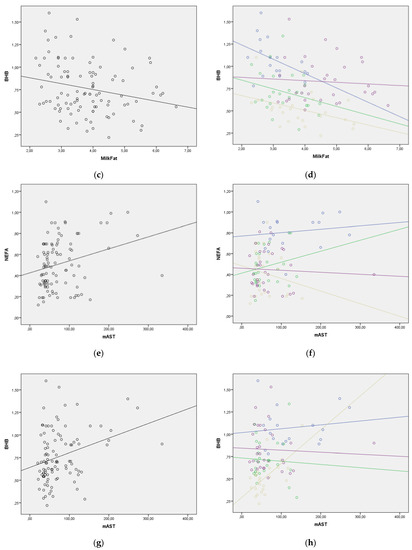
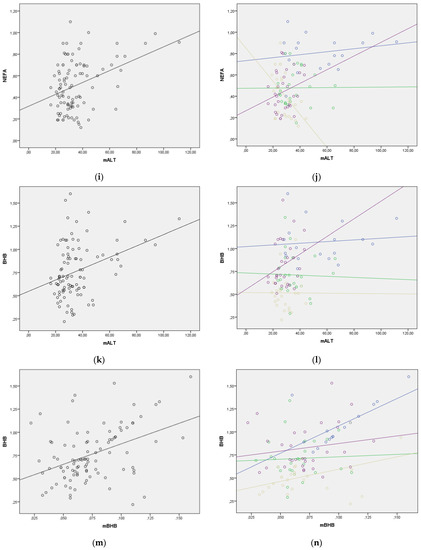
Figure 2.
(a–n) Correlation between blood NEFA and BHB values and milk parameters with lactation period added as a covariate (lactation period and the color of both the line and the figures: early lactation-Group 1—blue; full lactation-Group 2—green; mid lactation-Group 3—golden; late lactation-Group 4—violet). (a) Correlation between milk fat and blood NEFA in whole lactation; (b) Correlation between milk fat and blood NEFA with lactation period as covariate; (c) Correlation between milk fat and blood BHB in whole lactation; (d) Correlation between milk fat and blood BHB with lactation period as covariate; (e) Correlation between milk AST (mAST) and blood NEFA in whole lactation; (f) Correlation between milk AST (mAST) and blood NEFA with lactation period as covariate; (g) Correlation between milk AST (mAST) and blood BHB in whole lactation; (h) Correlation between milk AST (mAST) and blood BHB with lactation period as covariate; (i) Correlation between milk ALT (mALT) and blood NEFA in whole lactation; (j) Correlation between milk ALT (mALT) and blood NEFA with lactation period as covariate; (k) Correlation between milk ALT (mALT) and blood BHB in whole lactation; (l) Correlation between milk ALT (mALT) and blood BHB with lactation period as covariate; (m) Correlation between milk BHB (mBHB) and blood BHB in whole lactation; (n) Correlation between milk BHB (mBHB) and blood BHB with lactation period as covariate.
4. Discussion
Modern dairy farming often results in forced milk production, giving rise to metabolic disorders in cows. To predict such disorders and related subclinical diseases, it is necessary to establish the physiological ranges of biochemical parameters in a clinically healthy herd [26,32,33].
This study examined the associations between different blood and milk metabolic biomarkers in dairy cows at various stages of lactation by correlation analyses, focusing on the relationship between blood NEFA and BHB levels and milk metabolic parameters using single linear regressions. Blood NEFA as the best indicator of negative energy balance (NEB) and lipomobilization during lactation [12,25,34,35] was significantly elevated (p < 0.01) in early lactation cows compared to mid, full and late lactation cows. Blood and milk serum concentrations of BHB, another indicator of energy metabolism in early lactation cows, were also significantly higher (p < 0.01) than in the other groups of lactation cows, indicating intense fat reserve mobilization. Subclinical ketosis and clinical ketosis involve blood serum BHB levels above 1.2 mmol/L and above 2.9 mmol/L, respectively [2,11,28,36]. Early lactation cows had indicative BHB values (1.07 ± 0.22 mmol/L), without the presence of clinical signs. Blood and milk concentrations of the lipomobilization and ketogenesis marker, BHB, were positively correlated (p < 0.01) in this study, which is in agreement with a previous study [37]. Serum BHB and NEFA levels in puerperal cows clearly indicated the presence of some degree of ketogenesis and hepatic fatty infiltration due to intense lipomobilization in the post-partum period [38,39,40,41,42].
In the present study, glycemia values in mid, full and late lactation cows were within the physiological range of 2.5 to 4.2 mmol/L [32]. Nevertheless, glucose levels decreased in early lactation cows compared to the other lactation groups of cows (p < 0.01). This hypoglycemia in early lactation cows previously reported in various studies [7,10,42,43] may be associated with a lower liver gluconeogenesis process and with the sudden activity of the mammary gland and increased lactose synthesis. During puerperium, decreased values (p < 0.01) were also found for the other blood biochemical parameters, at least partially synthesized in the liver, such as TG, TChol, albumin, globulin, and urea. This indicated an increased accumulation of TG and TChol in hepatocytes in puerperal cows, most likely due to the depleted synthesis of VLDLs in the liver [44].
In cows with liver cell damage, nitrogen metabolism parameters, including uremia, proteinemia and albuminemia, decreased [8,33,45]. Although the levels of these three parameters in cows during the lactation period in the present study were within the physiological range, i.e., 60–80 g/L for proteinemia, 30–40 g/L for albuminemia and 1.66–6.66 mmol/L for uremia [32], they declined in puerperal cows compared to lactation females, which confirmed that the synthesis of these parameters in the liver was reduced due to the development of fatty infiltration of the liver [8,17,18,25,45]. This statement was confirmed by the significantly higher (p < 0.01) blood concentration of TBil in early lactation cows, which experienced a decline in the excretory capacity of the liver during fatty liver development. During the first month of lactation, 5–10% of high-yielding dairy cows suffer from severe hepatic lipidosis, and 30–40% of cows develop mild hepatic lipidosis [8], which indicates that almost 50% of these cows are at risk for metabolic disorders. Fatty infiltration of the liver causes lesions in the hepatic tissue and a general increase in the levels of the enzymes indicating hepatocyte injury, i.e., AST, GGT, and GLDH [6,17,18,46].
In this experiment, the activities of blood and milk serum AST, ALT, ALP and LDH were significantly higher (p < 0.01) in early and full lactation cows than in the other two groups of cows, suggesting mild fat infiltration of liver cells and a release of these enzymes in circulation, as induced by lipomobilization. Changes in blood and milk AST, ALT, ALP, LDH and GGT activities at different lactation stages indicated a mild degree of hepatic lesions in early lactation cows, probably due to fat infiltration. There were significant positive correlations between blood and milk serum AST (r = 0.450; p < 0.01), ALT (r = 0.649; p < 0.01), ALP (r = 0.344; p < 0.01) and GGT (r = 0.211; p < 0.05) activities in this study (Table 3). These results are supported by the reports of other authors [21,22,23,28,47], who showed that milk enzyme activities can be good indicators of lipid mobilization and ketogenesis in cows during lactation for early detection of subclinical disease. The high correlation coefficient of the work agrees with the finding of Liu et al. [21], and the high significance arises due to the large number of samples examined in this experiment. The activities of some milk enzymes were strongly positively correlated (p < 0.05) with negative energy balance (NEB) and lipomobilization biomarkers (NEFA, BHB), as well as with the blood biomarkers of the excretory capacity of the liver (TBil), and negatively correlated (p < 0.05) with the parameters of the functional state of the liver (glucose, TChol and TG).
The stage of lactation has a significant effect on raw milk composition in dairy cows [30,33]. Risk factors for developing general or mammary gland diseases should be examined in combination with fat and protein as major milk components by determining milk urea as an indicator of a balanced diet [47]. In this research, milk fat content was lower (p < 0.05) in early lactation cows (Group 1) than in the other three groups of lactating cows. Milk protein and lactose levels significantly declined (p < 0.01) from early to late lactation. There was no significant difference (p > 0.05) in milk urea levels between the experimental groups of cows. Milk fat levels are dependent on ration composition. Early lactation cows tend to mobilize body reserves while ingesting rations that are low in effective fiber; accordingly, milk fat levels decrease [48,49,50]. Milk fat can also be produced from volatile fatty acids and, inter alia, from the acetic acid formed in the rumen of cows [51]. Low milk fat levels are induced by a lack of the major precursor, acetic acid, in rumen [26,33,52]. Lactation performance and milk fat synthesis increased with branched-chain volatile fatty acid supplementation by improving ruminal fermentation, nutrient digestibility and mRNA expressions of genes related to milk fat synthesis [52].
It is well known that milk composition of dairy cows is affected by their energy balance (EB), especially during early lactation [42]. Cows in NEB mobilize their adipose tissue, which elevates blood NEFA levels [9,41]. This increase in NEFA supply for milk fat synthesis causes increases in milk fat content and milk fat:protein ratio (FPR) during the lactation period. Moreover, lipomobilization leads to changes in milk fatty acids composition [29,42,53]. The composition of milk fatty acids and its changes are promising EB predictors because they can be measured from routinely collected test-day milk samples [29,53,54]. Milk fatty acids are related to ruminal pH and subacute ruminal acidosis [55]. The authors found that milk fatty acids, alone, are better EB predictors than milk yield, milk FPR, and body traits combined.
In this study, milk fat content during lactation was positively correlated (p < 0.05) with total protein, glucose and TG levels, and negatively correlated with TBil, ALT, LDH, ALP, BHB and NEFA in the blood serum. These results were also confirmed by the regression lines for the relationships milk fat: blood BHB, milk fat: blood NEFA, milk BHB: blood BHB, which showed good homogeneity with similar slopes through the four stages of lactation. These correlation and regression relationships clearly indicate that milk fat was directly correlated with blood glucose, protein and triglycerides over the entire course of lactation, and negatively correlated with the lipomobilization and NEB parameters (NEFA, BHB), i.e., indicators of the functional and morphological state of the liver (enzymes, bilirubin). These results are consistent with studies showing that milk fat content in dairy cows during the first months of lactation can be monitored with moderately high accuracy using routine milk measurements [26,29,42,53,54]. Low milk fat content is commonly used in farms to indicate subacute ruminal acidosis and predict the effectiveness of diet structure for chewing [48,49,50]. Milk fat can be a very interesting indicator for assessing metabolic stress in the form of lipolysis and ketogenesis, because it correlates with NEFA and BHB in the blood, so that the slope of the regression curve is similar in all periods of lactation, which gives additional value to this parameter. In earlier research, it was found that in cows with metabolic disease in early lactation there is a change in the value of milk fat and the milk fat to protein ratio [27]. Milk fat is an indicator to which special attention must be paid in the future, especially because this parameter is measured routinely and daily. Energy balance of cows could be predicted by milk traits obtained by herd testing, and milk BHB concentration and blood NEFA predictions are potentially useful tools for management purposes [56,57].
An increase in milk protein content and a decrease in milk fat content cause subclinical acidosis [58], as shown in this experiment in early lactation cows. On the other hand, considering the high sensitivity of the fat:protein ratio (FPR) > 1.42 or lower (>1.35 or >1.25), these cut-offs could be used as a screening test to avoid testing all the cows if a herd is systematically monitored for subclinical ketosis (SCK) [59]. Milk urea and protein levels are indicators of metabolic nitrogen balance, which characterizes the health and reproductive ability of cows [60]. The negative regression dependency reported for the milk protein to milk urea ratio was stronger in early lactation but decreased in mid and late lactation [61]. In this study, there was a significantly positive correlation (p < 0.05) between milk protein and blood GGT activity. The energy to protein ratio is the most important nutritional factor in cow rations. Urea level increases with increasing intake of rumen degradable protein, but also when energy in rations is lacking, since no optimal amount of protein can be utilized from the ration due to decreased activity of rumen bacteria. As the feed energy supply increases, the concentration of urea in milk decreases [26,62]. In early lactation (Group 1), milk urea levels were non-significantly lower (p > 0.05) than in the other three lactation periods due to the decreased energy supply (NEB) through diet in the puerperal period, as well as due to increased lipomobilization from body reserves. Blood urea tended to have a significant positive relationship with milk fat and a significant negative relationship with milk protein, and a mainly positive relationship with milk lactose [63]. Milk lactose content was significantly lower (p < 0.01) in early and late lactations than in full and mid lactation. The confirmed decrease in milk lactose content and yield during lactation is in agreement with the results of Henao-Velásquez et al. [64] who explained this relationship as a decrease in milk yield in continuous lactation when milk secretion is regulated by lactose synthesis in the mammary gland.
This study emphasizes that milk is the most promising matrix for the purpose of diagnosing subclinical metabolic disease because it is easy to sample and allows whole herd testing during routine recording. The latest results, published several weeks ago, confirm the possibility of predicting blood metabolic parameters from milk samples obtained during routine milking [65]. The author found moderate correlations between the observed and predicted parameters, with which our result agrees. New research should further investigate metabolic stress using milk samples as a function of lactation stage to assess the health and productivity characteristics of cows.
5. Conclusions
Dynamic changes in metabolite values in blood and milk during lactation are identical, which confirms the possibility of using metabolic parameters from milk for the purposes of assessing metabolic status in cows. Correlation, regression and covariance analysis between blood and milk metabolic parameters confirms that milk parameters can be indicators in the evaluation of the metabolic status of cows. Parameters from milk are a significant indicator of the metabolic stress of cows because they correlate with the parameters of lipolysis and ketogenesis in the blood of cows. It is necessary to know the lactation period in order to correctly interpret the mutual relationships of metabolic parameters. Milk samples are obtained non-invasively, which additionally makes it suitable for evaluating the metabolic status in everyday practice, when the milk comes from a healthy udder.
Author Contributions
Conceptualization, B.A. and R.D.; methodology, B.A. and M.C.; validation, S.B.-B. and M.C.; resources, B.A., M.P. and A.Č.; writing-original draft preparation, B.A, A.Č. and R.D.; writing, review and editing, M.P., J.M. and S.B.-B.; supervision, R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of education, science and technological development, grant number 451-03-9/2021-14/200117.
Institutional Review Board Statement
The study protocol was approved by the Institutional Ethics Committee of the University of Novi Sad (protocol code IV/2017/02).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Associations of elevated nonesterified fatty acids and β-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J. Dairy Sci. 2010, 93, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Brunner, N.; Groeger, S.; Raposo, J.C.; Bruckmaier, R.M.; Gross, J.J. Prevalence of subclinical ketosis and production diseases in dairy cows in Central and South America, Africa, Asia, Australia, New Zealand, and Eastern Europe. Trans. Anim. Sci. 2019, 3, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Staufenbiel, R.; Gelfert, C.C. Metabolic profile test as a management tool in dairy herds. In Proceedings of the 5th Middle-European Buiatrics Congress, Hajduszoboszlo, Hungary, 2–5 June 2004; p. 721. [Google Scholar]
- Whitaker, D.A.; Macrae, A.I.; Burrough, E. Nutrition, fertility and dairy herd productivity. Cattle Pract. 2005, 13, 27–32. [Google Scholar]
- Cook, N.; Oetzelg, G.; Nordlund, K. Modern techniques for monitoring high-producing dairy cows 1. Practical applications. Practice 2006, 28, 598–603. [Google Scholar] [CrossRef]
- Le Blanc, S.J. Monitoring metabolic health of dairy cattle in transition period. J. Reprod. Dev. 2010, 56, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Overton, T.R.; Waldron, M.R. Nutritional management of transition dairy cows: Strategies to optimize metabolic health. J. Dairy Sci. 2004, 87, E105–E119. [Google Scholar] [CrossRef]
- Bobe, G.; Young, J.W.; Beitz, D.C. Pathology, etiology, prevention, treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef]
- Dann, H.M.; Morin, D.E.; Murphy, M.R.; Bollerog, A.; Drackely, J.K. Prepartum intake, postpartum induction of ketosis, and periparturient disorders affect the metabolic status of dairy cows. J. Dairy Sci. 2005, 88, 3249–3264. [Google Scholar] [CrossRef]
- Djokovic, R.; Cincovic, M.; Belic, B.; Toholj, B.; Davidov, I.; Hristovska, T. Relationship between blood metabolic hormones, metabolites and energy balance in Simmental dairy cows during peripartum period and lactation. Pak. Vet. J. 2015, 35, 163–167. [Google Scholar]
- Oetzel, G.R. Monitoring and testing dairy herds for metabolic disease. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 651–674. [Google Scholar] [CrossRef]
- Gonzalez, F.D.; Murino, R.; Pereira, V.; Campos, R.; Benedito, J.L. Relationship among blood indicators of lipomobilization and hepatic function during early lactation in high yielding dairy cows. J. Vet. Sci. 2011, 12, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Belić, B.; Cincović, M.; Lakić, I.; Đoković, R.; Petrović, M.; Ježek, J.; Starič, J. Metabolic status of dairy cows grouped by anabolic and catabolic indicators of metabolic stress in early lactation. Acta Sci. Vet. 2018, 46, 9. [Google Scholar] [CrossRef]
- Petrović, M.Ž.; Cincović, M.; Starič, J.; Djoković, R.; Belić, B.; Radinović, M.; Majkić, M.; Ilić, Z.Ž. The Correlation between Extracellular Heat Shock Protein 70 and Lipid Metabolism in a Ruminant Model. Metabolites 2022, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Giannuzzi, D.; Tessari, R.; Pegolo, S.; Fiore, E.; Gianesella, M.; Trevisi, E.; Ajmone MArsan, P.; Premi, M.; Piccioli-Cappelli, F.; Tagliapietra, F.; et al. Associations between ultrasound measurements and hematochemical parameters for the assessment of liver metabolic status in Holstein–Friesian cows. Sci. Rep. 2021, 11, 16314. [Google Scholar] [CrossRef] [PubMed]
- Schären, M.; Riefke, B.; Slopianka, M.; Keck, M.; Gruendemann, S.; Wichard, J.; Brunner, N.; Klein, S.; Snedec, T.; Theinert, K.B.; et al. Aspects of transition cow metabolomics—Part III: Alterations in the metabolome of liver and blood throughout the transition period in cows with different liver metabotypes. J. Dairy Sci. 2021, 104, 9245–9262. [Google Scholar] [CrossRef]
- Lubojacka, V.; Pechova, A.; Dvorak, R.; Drastich, P.; Kummer, V.; Poul, J. Liver steatosis following supplementation with fat in dairy cows diets. Acta Vet. Brno 2005, 74, 217–224. [Google Scholar] [CrossRef][Green Version]
- Stojevi, Z.; Piršljin, J.; Milinković-Tur, S.; Zdelar-Tuk, M.; Ljubić, B.B. Activities of AST, ALT and GGT in clinically healthy dairy cows during lactation and in the dry period. Vet. Arhiv 2005, 75, 67–73. [Google Scholar]
- Babaei, H.; Mansuori-Najand, L.; Molaei, M.M.; Kheradmand, A.; Sharifan, M. Assessment of lactate dehydrogenase, alkaline phosphatase and aspartate aminotransferase activities in cow’s milk as an indicator of subclinical mastitis. Vet. Res. Commun. 2007, 31, 419–425. [Google Scholar] [CrossRef]
- Katsoulos, P.D.; Christodoulopoulos, G.; Minas, A.; Karatzia, M.A.; Pourliotis, K.; Kritas, S.K. The role of lactate dehydrogenase, alkaline phosphatase and aspartate aminotransferase in the diagnosis of subclinical intramammary infections in dairy sheep and goats. J. Dairy Res. 2010, 77, 107–111. [Google Scholar] [CrossRef]
- Liu, P.; He, B.X.; Yang, X.L.; Hou, X.L.; Han, J.B.; Han, Y.H.; Nie, P.; Deng, H.F.; Du, X.H. Bioactivity evaluation of certain hepatic enzymes in blood plasma and milk of Holstein cows. Pak. Vet. J. 2012, 32, 601–604. [Google Scholar]
- Liu, P.; Hou, L.X.; Nie, P.; Aahan, H.Y.; Hoang, F.Y.; Zoun, X.Z.; Deng, F.H.; Song, P.; Li, M.; Xiang, H.B. Dynamic Monitoring of ALT and correlation analysis in blood plasma and milk of Holstein cows. Agric. J. 2013, 8, 51–55. [Google Scholar] [CrossRef]
- Ghadaa, E.M. Investigation of some enzymes level in blood and milk serum in two stages of milk yield dairy cows at Assiut city. Assiut Vet. Med. J. 2014, 60, 110–120. [Google Scholar] [CrossRef]
- Jozwik, A.; Strzalkowska, N.; Bagnicka, E.; Grzybek, W.; Krzyzewski, J.; Polowska, E.; Kolataj, A.; Horbanczuk, J.O. Relationship between milk yield, stage of lactation, and some blood serum metabolic parameters of dairy cows. Czech. J. Anim. Sci. 2012, 57, 353–360. [Google Scholar] [CrossRef]
- Djoković, R.; Cincović, M.; Ilić, Z.; Kurćubić, V.; Andjelić, B.; Petrović, M.; Lalić, N.; Jašović, B. Relationships between contents of biochemical metabolites in blood and milk in dairy cows during transition and mid lactation. Int. J. Appl. Res. Vet. Med. 2019, 17, 1–9. [Google Scholar]
- Pires, J.A.A.; Larsen, T.; Leroux, C. Milk metabolites and fatty acids as noninvasive biomarkers of metabolic status and energy balance in early-lactation cows. J. Dairy Sci. 2022, 105, 201–220. [Google Scholar] [CrossRef]
- Bondan, C.; Folchini, J.A.; Guimarães, L.; Noro, M.; Zanella, R.; Alves, L.P.; Fontaneli, R.S.; Gonzalez, F. Milk yield and composition in dairy cows with post-partum disorders. Arq. Bras. Med. Vet. Zootec. 2021, 73, 639–646. [Google Scholar] [CrossRef]
- Benedet, A.; Manuelian, C.L.; Zidi, A.; Penasa, M.; De Marchi, M. Invited review: β-hydroxybutyrate concentration in blood and milk and its associations with cow performance. Animal 2019, 13, 1676–1689. [Google Scholar] [CrossRef]
- Churakov, M.; Karlsson, J.; Edvarsson-Rasmussen, A.; Holtenius, K. Milk fatty acids as indicators of negative energy balance of dairy cows in early lactation. Animal 2012, 15, 100253. [Google Scholar] [CrossRef] [PubMed]
- Brinez, W.J.; Valbuena, E.; Castro, G.; Tovar, A.; Ruiz, R.J.; Roman, R. Effects of breed, season, lactation stage and parity number on composition of raw milk of crossbreed cows. Rev. Cietifica 2003, 13, 490–498. [Google Scholar]
- NRC (National Research Council). Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Radostits, O.M.; Blood, D.C.; Gay, C.C.; Hinchcliff, K.W. Veterinary Medicine, A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses, 9th ed.; W.B. Saunders Company Ltd.: London, UK; New York, NY, USA; Philadelphia, PA, USA; San Francisco, CA, USA; St. Louis, MO, USA; Sydney, Australia, 2007. [Google Scholar]
- Reist, M.; Erdin, D.; Von Euw, D.; Tschuemperlin, K.; Leunberger, H.; Chiliard, Y.; Hammon, H.M.; Morel, C.; Philipona, C.; Zbinden, Y.; et al. Estimation of energy balance at the individual and herd level using blood and milk traits in high-yielding dairy cows. J. Dairy Sci. 2002, 85, 3314–3327. [Google Scholar] [CrossRef]
- Cincović, M.R.; Belić, B.; Radojičić, B.; Hristov, S.; Đoković, R. Influence of lipolysis and ketogenesis to metabolic and hematological parameters in dairy cows during periparturient period. Acta Vet. 2012, 62, 429–444. [Google Scholar] [CrossRef]
- Lakić, I.; Cincović, M.R.; Belić, B.; Đoković, R.; Majkić, M.; Petrović, M.Ž.; Nikolić, S. Lipolysis and ketogenesis in cows in early lactation. Acta Agric. Serb. 2018, 23, 265–276. [Google Scholar] [CrossRef]
- Duffield, T. Subclinical ketosis in lactating dairy cattle. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 231–253. [Google Scholar] [CrossRef]
- Ježek, J.; Cincović, M.R.; Nemec, M.; Belić, B.; Djoković, R.; Klinkon, M.; Starič, J. Beta-hydroxybutyrate in milk as screening test for subclinical ketosis in dairy cows. Pol. J. Vet. Sci. 2017, 20, 507–512. [Google Scholar] [CrossRef]
- McFadden, J.W. Lipid biology in the periparturient dairy cow: Contemporary perspectives. Animal 2020, 14, s165–s175. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, M.; Bionaz, M.; Trevisi, E. Interaction between inflammation and metabolism in periparturient dairy cows. J. Anim. Sci. 2020, 98, S155–S174. [Google Scholar] [CrossRef]
- Civelek, T.; Aydin, I.; Cingi, C.C.; Yilmaz, O.; Kabu, M. Serum non-esterified fatty acids and beta-hydroxybutyrate in dairy cows with retained placenta. Pak. Vet. J. 2011, 31, 341–344. [Google Scholar]
- Drackley, J.K.; Dann, H.M.; Douglas, G.N.; Janovick-Gurtzky, N.A.; Lutherland, N.B.; Underwood, J.P.; Loor, J. Physiological and pathological adaptations in dairy cows that may increase susceptibility to periparturient diseases and disorders. Ital. J. Anim. Sci. 2005, 4, 323–344. [Google Scholar] [CrossRef]
- Gross, J.; Van Dorland, H.A.; Bruckmaiker, R.M.; Schwar, F.J. Performance and metabolic profile of dairy cows during a lactation and deliberately induced negative energy balance with subsequent realimentation. J. Dairy Sci. 2011, 94, 1820–1830. [Google Scholar] [CrossRef]
- Stang, B.D.; Bertics, S.J.; Grummer, R.R.; Armentanol, E. Effect of long chain fatty acids on triglycerides accumulation, gluconeogenesis and ureogenesis in bovine hepatocytes. J. Dairy Sci. 1998, 81, 728–739. [Google Scholar] [CrossRef]
- Sevinc, M.; Basoglu, A.; Guzulbekta, H. Lipid and lipoprotein levels in dairy cows with fatty liver. Turk. J. Vet. Anim. Sci. 2003, 27, 295–299. [Google Scholar]
- Djoković, R.; Ilić, Z.; Kurćubić, V.; Petrović, M.; Dosković, D. Functional and morphological state of the liver in Simmental dairy cows during transitional period. Rev. Méd. Vét. 2011, 162, 574–579. [Google Scholar]
- Pechova, A.; Llek, J.; Halouzka, R. Diagnosis and control of the development of hepatic lipidosis in dairy cows in the peri-parturient period. Acta Vet. Brno 1997, 66, 235–243. [Google Scholar] [CrossRef]
- Djoković, R.; Cincović, M.; Ilić, Z.; Kurćubić, V.; Fratrić, N.; Petrović, M.; Andjelić, B. The correlations between serum enzyme activities in blood and milk in the different stage of lactation in Holstein dairy cows. In Proceedings of the 30th World Buiatrics Congress, Sapporo, Japan, 28 August–1 September 2018; p. 305. [Google Scholar]
- Nozad, S.; Ramin, A.G.; Moghadam, G. Diurnal variations in milk, urea, protein and lactose concentrations in Holstein dairy cows. Acta Vet. 2011, 61, 3–12. [Google Scholar] [CrossRef]
- Nozad, S.; Ramin, A.G.; Moghadam, G.; Rezaei, S.A.; Babapour, A.; Ramin, S. Relationship between blood urea, protein, creatinine, triglycerides and macro-mineral concentrations with the quality and quantity of milk in dairy Holstein cows. Vet. Res. Forum 2007, 3, 55–59. [Google Scholar]
- Hamann, J.; Kromker, V. Potential of specific milk composition variables for cow health management. Livest. Prod. Sci. 1997, 48, 201–208. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Guo, G.; Huo, W.J.; Zhang, S.L.; Pei, C.X.; Zhang, Y.L.; Wang, H. Effects of branched-chain volatile fatty acids on lactation performance and mRNA expression of genes related to fatty acid synthesis in mammary gland of dairy cows. Animal 2018, 12, 2071–2079. [Google Scholar] [CrossRef]
- Mordak, R.; Kupczyński, R.; Kuczaj, M.; Niżański, W. Analysis of correlations between selected blood markers of liver function and milk composition in cows during late lactation period. Ann. Anim. Sci. 2020, 20, 871–886. [Google Scholar] [CrossRef]
- Mäntysaari, P.; Mäntysaari, E.A.; Kokkonen, T.; Mehtiö, T.S.; Kajava, S.; Grelet, C.; Lidauer, P.; Lidauer, M.H. Body and milk traits as indicators of dairy cow energy status in early lactation. J. Dairy Sci. 2019, 102, 7904–7916. [Google Scholar] [CrossRef]
- Woolpert, M.E.; Dann, H.M.; Cotanch, K.W.; Melilli, C.; Chase, L.E.; Grant, R.J.; Barbano, D.M. Management, nutrition, and lactation performance are related to bulk tank milk de novo fatty acid concentration on northeastern US dairy farms. J. Dairy Sci. 2016, 99, 8486–8497. [Google Scholar] [CrossRef]
- Komisarek, J.; Stefańska, B.; Nowak, W. The effect of ruminal fluid pH on milk fatty acids composition in cattle. Ann. Anim. Sci. 2022, 22, 625–631. [Google Scholar] [CrossRef]
- Mäntysaari, P.; Juga, J.; Lidauer, M.H.; Häggman, J.; Mehtiö, T.; Christensen, J.M.; Mäntysaari, E.A. The relationships between early lactation energy status indicators and endocrine fertility traits in dairy cows. J. Dairy Sci. 2022, 105, 6833–6844. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, A.; Sasaki, O.; Tanigawa, T.; Kubota, A.; Takeda, H.; Saito, Y. Prediction of energy balance from milk traits of Holsteins in Japan. Anim. Sci. J. 2022, 93, e13757. [Google Scholar] [CrossRef]
- Eicher, R. Evaluation of the metabolic and nutritional situation in dairy herds: Diagnostic use of milk components. In Proceedings of the World Buiatrics Congress, Quebec City, QC, Canada, 11–16 July 2004. [Google Scholar]
- Jenkins, T.N.; Gustavo Peña, G.; Carlos Risco, C. Utility of in line milk fat and protein ratio to diagnose subclinical ketosis and to assign propylene glycol treatment in lactating dairy cows. Can. Vet. J. 2015, 56, 850–854. [Google Scholar] [PubMed]
- Sammad, A.; Khan, M.Z.; Abbas, Z.; Hu, L.; Ullah, Q.; Wang, Y.; Zhu, H.; Wang, Y. Major Nutritional Metabolic Alterations Influencing the Reproductive System of Postpartum Dairy Cows. Metabolites 2022, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Tinkovicova-Lackova, P.; Maskalova, M.; Vajda, V. Evaluation of the milk urea content in relation to milk production and composition in dairy cows. Acta Vet. Brno 2019, 88, 277–285. [Google Scholar] [CrossRef]
- Jilek, F.; Rehak, D.; Volek, J.; Stipkova, M.; Nemcova, E.; Fiedlerova, M.; Rajmon, A.R.; Svetskova, D. Effect of herd, parity stage of lactation and milk yield on urea concentration in milk. Czech. J. Anim. Sci. 2006, 51, 510–517. [Google Scholar] [CrossRef]
- Chladek, G.; Machal, L. Blood plasma urea concentration and its relationship with milk production parameters in Czech pied cows. J. Cent. Euro. Agric. 2004, 5, 337–346. [Google Scholar]
- Henao-Velasquez, A.P.; Munera-Bedoya, O.D.; Herrera, A.C.; Aqudelo-Trujillo, J.H.; Cenon–Munoz, M.F. Lactose and milk urea nitrogen, fluctuations during lactation in Holstein cows. R. Bras. Zootec. 2014, 43, 479–484. [Google Scholar] [CrossRef]
- Giannuzzi, D.; Mota, L.F.M.; Pegolo, S.; Gallo, L.; Schiavon, S.; Tagliapietra, F.; Katz, G.; Fainboym, D.; Minuti, A.; Trevisi, E.; et al. In-line near-infrared analysis of milk coupled with machine learning methods for the daily prediction of blood metabolic profile in dairy cattle. Sci. Rep. 2022, 12, 8058. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).