Abstract
Komagataella phaffii (formerly known as Pichia pastoris) has become an increasingly important microorganism for recombinant protein production. This yeast species has gained high interest in an industrial setting for the production of a wide range of proteins, including enzymes and biopharmaceuticals. During the last decades, relevant bioprocess progress has been achieved in order to increase recombinant protein productivity and to reduce production costs. More recently, the improvement of cell features and performance has also been considered for this aim, and promising strategies with a direct and substantial impact on protein productivity have been reported. In this review, cell engineering approaches including metabolic engineering and energy supply, transcription factor modulation, and manipulation of routes involved in folding and secretion of recombinant protein are discussed. A lack of studies performed at the higher-scale bioreactor involving optimisation of cultivation parameters is also evidenced, which highlights new research aims to be considered.
1. Introduction
The methylotrophic yeast Komagataella phaffii, formerly known as Pichia pastoris [1], is one of the most prominent recombinant protein (rProt) production platforms [2,3]. As a methylotrophic yeast, it can oxidise methanol for energy production and biomass formation [4]. Given its ability to grow readily on relatively inexpensive culture media, it was initially used as a cellular protein source (SCP, single-cell protein). Over the years, it has become an attractive host system for the production of rProt from bacterial, fungal, plant, and mammalian/human origins [2,5,6,7].
K. phaffii stands out among other yeasts and microorganisms due to several beneficial features (for more information, see an excellent comprehensive review from Ata et al. [8]). In addition to its ability to metabolise methanol through the methanol utilisation (MUT) pathway, K. phaffii can grow on inexpensive media at high cell density, reaching a biomass concentration exceeding 100 g/L of dry cell weight (DCW) [9,10,11]. Genetic manipulation tools for transgene expression in this species are well-established, resulting in the targeted and stable integration of rProt genes. Proteins carrying a suitable secretion signal, such as the widely utilised alpha mating factor (MATα) secretion signal, can be accumulated in the culture supernatant due to its efficient secretory machinery in an environment relatively free from other proteins and contaminants, as less than 10% of the endogenous proteins are secreted [2,12]. In addition, K. phaffii can perform diverse protein processing and post-translational modifications typical of higher eukaryotes, such as glycosylation and disulphide bond formation [3,13], and it lacks other known disadvantages that are present in bacterial systems (formation of inclusion bodies and presence of endotoxins) or mammalian cell systems (high cultivation and handling costs) [14]. Using this host system, rProt can be produced in either a constitutive or induced manner, depending on the type of promoter used to drive recombinant gene expression.
The success of K. phaffii for rProt synthesis has been facilitated by strong methanol-inducible promoters from the alcohol oxidase genes (alcohol oxidase 1, AOX1, and to a lesser extent, alcohol oxidase 2, AOX2), and also from the glyceraldehyde 3-phosphate dehydrogenase (GAP) promoter PGAP, which exhibits strong constitutive expression in the presence of glucose and glycerol [15]. Notwithstanding the efficiency, tight control and rProt productivity obtained when using PAOX1 to drive transgene expression, this production system presents some drawbacks associated with methanol utilisation. Indeed, methanol is toxic to cells, inducing cell oxidative stress, and its use comes with a subsequent high oxygen demand for catabolism [16]. Additionally, as methanol is highly flammable, its use can imply safety issues, especially at an industrial scale. Taking these drawbacks into consideration, current research is being undertaken to evaluate alternatives in order to reduce or discard methanol use.
Important efforts have been carried out in order to improve the understanding of the physiology and cell response of K. phaffii under various genetic backgrounds (engineered strains) and bioprocess operations [17,18,19] with the goal of increasing rProt productivity, cell capabilities and fitness, and metabolic performance. These advancements have been reported in several reviews that focus on bioreactor processes [20,21], genetic manipulation techniques [22,23,24], and metabolic engineering [25]. However, there is a need to highlight and summarise the latest cell engineering approaches and strategies regarding manipulation of the methanol pathway, co-factor metabolism, transcription modulation, protein folding and secretion, as well as catabolism of alternative carbon sources, as illustrated in Figure 1, and their contribution to K. phaffii’s promising future as a robust and highly efficient host for the production of a huge variety of recombinant proteins.
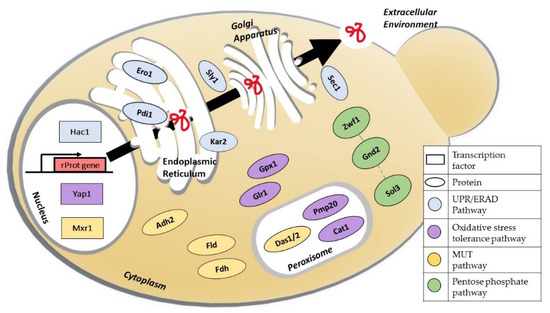
Figure 1.
Schematic overview of the main pathways, proteins, and transcription factors involved in cellular engineering strategies for the improvement of recombinant protein production in Komagataella phaffii. Abbreviations: Hac1: UPR-regulating transcription factor; Yap1: oxidative stress response transcription factor; Mxr1: methanol expression regulator 1; Pdi1: protein disulphide isomerase; Kar2: immunoglobulin-binding protein; Ero1: endoplasmic reticulum oxidoreductase; Sly1: hydrophilic protein involved in ER/Golgi vesicles trafficking; Sec1: Sm-like protein involved in docking and fusion of exocytic vesicles; Gpx1: glutathione peroxidase; Glr1: glutathione reductase; Pmp20: peroxisome-membrane-associated protein 20; Cat1: catalase; Das1/2: dihydroxyacetone synthase 1 and 2; Fld: formaldehyde dehydrogenase; Fdh: formate dehydrogenase; Zwf1: glucose-6-phosphate dehydrogenase; Sol3: 6-gluconolactonase; GND2: 6-phosphogluconate dehydrogenase.
2. Metabolic Engineering for Improved Metabolism and Energy Supply
rProt production and secretion generate a high metabolic burden, as protein synthesis leads to increased nutrient and energy demands [26]. In addition, production of rProt can often trigger endoplasmic reticulum (ER) stress and oxidative stress, causing (NAD(P)H) co-factor unbalance [27]. As stated above, K. phaffii can metabolise methanol as the sole carbon source; however, it can also metabolise other non-frequently used alkylated nitrogen sources, such as methylamine and choline. The main metabolic drawback of methanol catabolism is the production of toxic metabolites such as formaldehyde and hydrogen peroxide [28]. At high methanol concentration (above 5% vol/vol), disruption of the peroxisome can occur, thus impairing methanol catabolism [29,30]. Furthermore, methanol catabolism requires high oxygen consumption that can limit the productivity of the bioreactor process, especially at large scale where the oxygen transfer capacity is lower [31]. Therefore, the methanol concentration control in the culture medium is crucial to obtain high productivity of rProt.
In processes based on the PAOX1 expression system, co-substrates such as sorbitol can be used with the goal of dedicating methanol mainly as the inducer for the expression system, while sorbitol is used for biomass and energy formation [32,33,34]. This has been evidenced by metabolic flux analysis of a simplified metabolic network describing cell growth, methanol and sorbitol catabolism, and energy formation, which was subsequently confirmed in a bioreactor culture [35]. In this case, it was observed that an appropriate methanol/sorbitol mixture ratio (methanol fraction 0.60 C-mol/C-mol) could increase the PAOX1 induction level (β-galactosidase activity of 8.6 ± 0.8 × 103 Miller unit), compared to cultures with 100% methanol supplementation (7.8 ± 0.7 × 103 Miller unit) [35]. Glycerol has also been widely used as a co-substrate, and different culture strategies have been implemented. Recently, a combined μ-stat (constant exponential feeding rate) and m-stat (constant methanol concentration) feeding process was developed for β-glucosidase FBG1 production in a 5 L bioreactor. This co-stat feeding strategy allowed reaching a productivity of 403 mg/L of β-glucosidase, which was 2.6- and 4.4-fold higher than the titre obtained in μ-stat and m-stat modes, respectively [36].
Metabolic engineering of the host cell has also been considered, aiming at improving rProt productivity. Recent published work on engineering of the catabolic pathway of methanol and alternative carbon sources, along with co-factor engineering, is described in the following sections and summarised in Table 1.

Table 1.
Improving heterologous protein production by modifying metabolism and energy supply.
2.1. Engineering of the Methanol Catabolic Pathway
The MUT pathway can be divided into two main stages. First, methanol is oxidised by two alcohol oxidases (Aox1, Aox2) into formaldehyde in the peroxisome, before being further metabolised in two distinct metabolic routes. In the assimilatory branch, formaldehyde is converted into dihydroxyacetone (DHA) and GAP by the peroxisomal dihydroxyacetone synthase (Das). This pathway ends in the cytosol with the generation of fructose 1,6-biphosphate. In the dissimilatory branch, formaldehyde is oxidised to carbon dioxide in three steps by the enzymatic route formed by formaldehyde dehydrogenase (Fld), formyl glutathione hydrolase (Fgh), and formate dehydrogenase (Fdh), with the release of NADH [45], as illustrated in Figure 2.
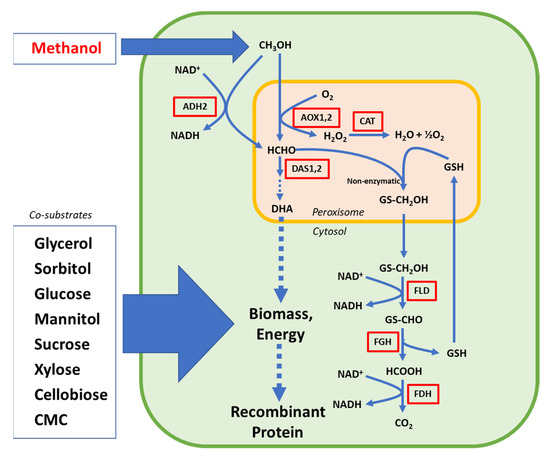
Figure 2.
Diagram illustrating the main steps in the MUT pathway and co-substrate alternatives for enhancement of biomass and energy supply in Komagataella phaffii. AOX: alcohol oxidase; CAT: catalase; DAS: dihydroxyacetone synthase; FLD: formaldehyde dehydrogenase; FGH: S-formylglutathione hydrolase; FDH; formate dehydrogenase; ADH: alcohol dehydrogenase; GS(H): glutathione; CMC: carboxymethyl cellulose.
Engineering of the MUT pathway has led to higher substrate-product conversion yield (YS/P) and rProt productivity. In a MutS strain (strain with disrupted AOX1), the overexpression of DAS resulted in a 2- to 3-fold increase in YS/P from methanol to produce horseradish peroxidase (Hrp) and Candida antarctica lipase B (CalB), with reported values of 3.06 U/mmol and 2.05 U/mmol, respectively, in comparison with the reference strains for each case (~1 U/mmol). Meanwhile, the overexpression of FLD with the same reporter proteins exhibited a 2-fold increase in YS/P from methanol (1.65 UHRP/mmol and 2.13 UCalB/mmol) [37]. On the other hand, the deletion of both DAS isoforms (DAS1 and DAS2) has increased green fluorescent protein (GFP) expression in single and double knockout strains. The highest rProt production was observed in the DAS1 deletion strain, which produced 30% more GFP than the control strain. This yield was followed by the DAS2 deletion strain and finally the double mutant DAS1-DAS2, with GFP expression levels of ~22% and ~15% in each case, both higher than the wild-type strain [38].
An alternative methanol metabolism implemented for recombinant protein production has been presented by Zavec et al. [46] with the recent reassessment of the Mut- strain (strain with AOX1 and AOX2 disruption), which, contrary to its generally recognised inability for methanol metabolization [47], showed that it is indeed able to metabolise methanol with a similar rProt yield when compared to a MutS strain, due to the promiscuous activity of the alcohol dehydrogenase enzyme Adh2. Based on these results and using metabolic engineering, the overexpression of ADH2 revealed a significant increase in the productivity (qP) of the camelid antibody fragment vHH (237 μg/g∙h) in the Mut-PAOX1vHHpFLD1Adh2 strain compared to the Mut-PAOX1vHH parent strain (88 μg/g∙h), and a slightly higher increase with the strain MutSPAOX1vHH (205 μg/g∙h). It is worth noting that despite the similarity in productivity between the Mut- improved strain and the MutS strain, the latter showed an additional advantage by having lower rates of oxygen uptake and heat production [39].
As far as the main mechanism for induction of the PAOX1/2 promoters is concerned, few studies have ventured to question whether methanol could be the sole inducer of the system. Tyurin and Kozlov [40] discussed the exclusive inducing activity of methanol and proposed the use of formate as a possible inducer of the PAOX1/2 system. Thus, to rule out the possibility of intracellular methanol reduction using potassium formate salt as an inducer, FDH was deleted. With this modification, the expression levels of β-galactosidase were increased by 2-fold (~4500 Miller units) compared to the wild-type strain (~2200 Miller units) [40].
Modifying the MUT pathway and the redistribution of metabolic flux for rProt expression in key metabolic steps directed to a target product has also been successful [37,38,39,40]. However, little attention has been paid in recent years to modifying this pathway to improve the production of different recombinant proteins than those abovementioned, and some high-value compounds, such as malic acid and S-adenosylmethionine, have been produced when a knockout of FDL and DAS was introduced [48,49]. Thus, the work presented by Zavec et al. [39] and Tyurin and Kozlov [40] highlights the need to question the previously acknowledged and accepted premise of the MUT pathway behaviour and re-evaluate methanol metabolism from different perspectives.
2.2. Engineering of Co-Substrate Catabolic Pathways
Due to the already described problems with the use of methanol in rProt production, co-feeding strategies of methanol with auxiliary carbon sources have been studied to mitigate this problem. Based on the results of Inan and Meagher [50], it was identified that substrates such as sorbitol and mannitol could be used as co-substrates in combination with methanol, as these do not repress the PAOX1 promoter, while glycerol and glucose do exert repression of this inducible promoter. Nevertheless, glycerol is mainly used in co-feeding strategies with methanol because it generates higher biomass yield, higher growth rate, and improved productivity [33]. The use of glucose has been favoured due to the deletion of the hexose transporter Hxt1, which made it possible to obtain a strain for the expression of rProt in a methanol-free medium [51]. On the other hand, the co-feeding with sorbitol positively affects cell growth and energy supply for heterologous protein production, which makes it one of the most widely used co-substrates to produce rProt in methanol-inducing media [31,33,52]. In order to improve xylanase expression under sorbitol-methanol co-feeding conditions, the gene identified in K. phaffii as the initial acceptor of the pexophagy process (ATG30) was deleted to favour peroxisome retention in cells under the influence of the addition of carbon sources that trigger this cellular response. This modification improved xylanase production, reporting an activity of ~1140.7 U/mL in cultures supplemented with 2% sorbitol, representing an increase of ~11.4% compared to the control culture containing 0.5% sorbitol [41]. This demonstrates that by employing cell engineering strategies, the production of a highly in-demand enzyme in the food and paper industry can be successfully increased [53].
K. phaffii does not normally metabolise sucrose because of the absence of an invertase enzyme [54]. However, a study evaluating the influence of the carbon source on cell size and production of an anti-LDL-single-chain variable fragment (scFV) [55], expressed in K. phaffii the Suc2 invertase enzyme from Saccharomyces cerevisiae [56] under the control of PAOX1. When this strain was grown in sucrose-supplemented medium, both the antibody fragment concentration (93.7 mg/L) and the specific yield on biomass (3.96 mg/g DCW) showed comparable values to glycerol-supplemented cultures (72.7 mg/L and 3.04 mg/g DCW, respectively). Additionally, when evaluating the use of glucose, this condition showed the lowest productivity (63.3 mg/L), even though using this medium generated a higher cell volume compared to sucrose or glycerol (0.766 μm3 in glucose, 0.214 μm3 in sucrose, 0.202 μm3 in glycerol) [55,57]. This suggests that high production of this heterologous protein in K. phaffii in sucrose-based cultures may be mainly related to cell number rather than cell concentration, in addition to the repressive effect on the PAOX1 promoter from both glycerol and glucose [55,57].
The study of these auxiliary carbon sources in recent years has mainly focused on managing the conditions and operating parameters in culture systems [20,28,35,58]; therefore, an interesting and complementary scope of study could be carried out looking into the modification in their respective metabolic pathways, in conjunction with the manipulation and optimisation of these cultivation parameters.
2.3. Engineering of Turnover Co-Factor Metabolism
The metabolic burden that arises from processing rProt folding, posttranslational modifications, and secretion has shown that the regeneration of the oxidised co-factor significantly affects energy metabolism, becoming a bottleneck [42,59,60]. The overexpression of genes encoding enzymes catalysing NADPH-producing reactions has proven its usefulness for overcoming redox unbalance. An example of this is the overexpression of the S. cerevisiae POS5-encoded NADH kinase, which increased 2-fold the specific productivity of a Fab antibody fragment [27].
Constitutive overexpression of the genes encoding the glucose-6-phosphate dehydrogenase (ZWF1) and 6-gluconolactonase (SOL3) that catalyse NADPH-generating reactions led to a 3.8-fold increase in the productivity of human superoxide dismutase (hSod) [42]. Besides this, overexpression of SOL3 and the gene encoding 6-phosphogluconate dehydrogenase (GND2) resulted in a 2.2-fold increase (~6.5 mg/L) in recombinant human interferon gamma (hIFN-γ) in comparison with the control strain GS115/hIFN-γ (2.5 mg/L) [43].
2.4. Engineering for Alternative Carbon Source Catabolism
In the context of circular economy, the trend has been the valorisation or increased added value of renewable raw materials, such as agricultural, forestry, food and industry bioproducts and/or waste streams. This goal would require K. phaffii to incorporate additional metabolic skills in order to metabolise substrates such as xylose, cellobiose, and cellulose [44,61].
The heterologous expression of genes involved in xylose catabolism, namely the xylose isomerase (XI) from Orpinomyces spp. and an endogenous xylulokinase, allowed xylose conversion resulting in a yield (Y x/s) of 0.378 g/g, nearly 2-fold higher than that of the parental strain GS115, reaching values comparable to those achieved with glucose or glycerol (0.310 g/g and 0.435~0.490 g/g, respectively) [44]. Using the same engineered strain, the β-mannanase production titre reached approximately 80 U/mL.
In a different research study, the expression of three heterologous cellulases, namely Aspergillus niger β-glucosidase (AnBGL1), A. niger endoglucanase (AnEG-A), and a Trichoderma reesei exoglucanase (TrCBH2), allowed the resulting strain to grow on cellobiose and carboxymethyl cellulose [61].
Another metabolic engineering strategy in the search for alternative carbon source metabolism consisted of increasing the acetate tolerance in K. phaffii. Indeed, acetate, which can be obtained from syngas by the hydrolysis of cellulosic biomass or by anaerobic fermentation of different agroindustrial wastes [62], is a cheap and largely available carbon source. This increased tolerance was conferred by the PGAP-driven overexpression of the native gene PAS_Chr3_1091 encoding the putative serine/threonine protein kinase Hrk1, together with the S. cerevisiae gene ScAcs1* encoding acetyl-CoA [63].
Recently, K. phaffii was engineered for CO metabolization through a reorganisation of the MUT pathway as well as the xylulose monophosphate (XuMP) pathway, in order to generate a Calvin–Benson–Bassham resembling cycle. This modification was obtained by the expression of eight recombinant proteins from five different organisms: from Ogataea polymorpha (glyceraldehyde-3-phosphate dehydrogenase Tdh3, phosphoglycerate kinase Pgk1), Ogataea parapolymorpha (transketolase Tkl1, triosephosphate isomerase Tpi1), Spinacia oleracea (phosphoribulokinase Prk), Thiobacillus denitrificans (ribulose-1,5-bisphosphate carboxylase-oxygenase RuBisCO), and Escherichia coli (chaperones GroEL and GroES); and the deletion of three native genes (AOX1, DAS1, DAS2) [64]. This strategy opens up new opportunities for rProt synthesis from alternative carbon sources.
3. Transcription Factor Engineering in K. phaffii for Recombinant Protein Production
Numerous investigations have focused on how to modify gene expression patterns and regulatory networks during rProt production [65,66,67]. The growing understanding of cellular transcriptome modulation during highly stressful processes, such as rProt synthesis in high-density cultures [68,69,70], has allowed identifying regulatory elements of interest for the improvement of production processes [71,72,73]. These regulatory elements may regulate not only the promoter used for the recombinant gene expression, but also key steps of rProt synthesis, such as posttranslational modifications and secretion [74]. Recent research on this regard is summarised in Table 2.

Table 2.
Improving heterologous protein production by transcription factor modification.
For instance, Yap1 has been identified as a transcription factor associated with the control of the oxidative stress response by modulating the expression level of more than 150 genes [87,88]. Overexpression of the Yap1 encoding gene led to a 2-fold increase in the expression of the recombinant gene encoding trypsin, reaching at least 80 µg/g WCW (wet cell weight) of trypsin under the regulation of PGAP [79].
Several groups have also investigated transcription factors associated with protein maturation and secretion. The protein Hac1 is a transcription regulator involved in the unfolded protein response (UPR), which increases the synthesis of endoplasmic reticulum resident proteins required for protein folding, as well as components of the secretory pathway [74,82]. Overexpression of this transcription factor has proven to be a very important tool to increase the production yield of different recombinant proteins [81,85,86,89,90,91,92,93,94]. Huang et al. [93] evaluated the effect of HAC1p on a raw starch hydrolysing α-amylase (Gs4j-amyA) to improve heterologous production of the enzyme in K. phaffii, further evaluating the variation in copy number and promoter used in the overexpression of HAC1 [93]. In this case, a strain with 12 copies of the GS4J-AMYA gene driven by PAOX1 and a basal expression of 305 U/mL was used, and upon incorporation of six copies of PAOX1-driven HAC1, amylase activity increased to 2200 U/mL. However, the excessive number of genes under the control of the AOX1 promoter seemed to interfere, limiting the transcription of GS4J-AMYA; therefore, by using the PGAP constitutive promoter to regulate the expression of HAC1, the amylase activity increased to 3700 U/mL. This illustrates the importance of the strategy followed with each auxiliary gene (in this case HAC1), this being as important as the choice of the auxiliary gene itself.
In some cases, an improvement can be achieved by decreasing or eliminating the expression of an auxiliary gene. One example of this comes from the transcriptional factor associated with galactose metabolism ATT1, a homologue of the GAL4 gene in S. cerevisiae, which specifically recognises sequences called galactose upstream activating sequence (GALUAS) and is mainly associated with genes related to sugar metabolism [71]. In relation to this, glycol-engineered K. phaffii strains are very valuable tools to produce more complex recombinant glycoproteins [95]. However, in addition to enabling the production of rProt with human-like glycosylation patterns, glycoengineered strains also change the glycan structures of all endogenous glycoproteins [78]. Although the exact physiological consequences of such widespread glycan remodelling are not well-understood, it is evident that modifying the glycosylation pathway can affect the overall fitness of the host cell [96,97]. In this context, Jiang and colleagues reported that silencing ATT1 increased the rProt yield with a 1.5-fold change in comparison to the control strain, reaching 1.98 g/L of human epidermal growth factor receptor 2 (anti-HER2) mAb. In addition, this increased the cell temperature tolerance by enduring 35 °C for up to 150 h with a low cell lysis rate in a 15 L bioreactor [78].
Additionally, Ata et al. [98] constructed a synthetic library based on PGAP, where they found 41 putative transcription factor binding sites (TFBS) in K. phaffii. As PGAP is a carbon-source-related promoter [15], TFs related to carbon source utilisation and their corresponding binding sites were considered as potential targets. Based on this, 10 strain variants were generated with deletion or duplication of TFBS, and the yield and effect of each modification was evaluated with the intracellular production of eGFP and/or extracellular production of recombinant human growth hormone (rhGH) as reporter proteins. The best results were obtained with the duplication of TFBS associated with the overexpression of the Gal4-like transcription factor; combining these two approaches resulted in a 2.2-fold increase in specific rhGH yield compared to PGAP in fed-batch bioreactor cultivation [98]. The specific glucose consumption and ethanol production rates were increased by 1.7- and 8-fold, respectively, in the K. phaffii Gal4-like transcription factor overexpressing mutants when compared to the wild-type, and it was noticed that the overexpression of the Gal4-like transcription factor resulted in a switch from Crabtree negative to Crabtree positive behaviour [71].
A similar approach was carried out for PAOX1 to convert it into a methanol-free expression system [99]. The individual overexpression of two out of the three known transcription factor genes involved in regulation of the MUT pathway, namely MRX1 and MIT1, activated PAOX1 when the glycerol as repressing carbon source was depleted (derepressed expression) [77]. In parallel, Wang et al. [100] reported that the knockout of the three transcription factors MIG1, MIG2, and NRG1 alongside MIT1 overexpression also resulted in induction of PAOX1 [100]. Although these two studies obtained lower gene expression levels than for methanol-induced PAOX1, the goal here resides in the development of a methanol-free bioprocess that offers a safer alternative, hugely relevant to industry.
Yu et al. [101] reported the separate effects of the co-overexpression of nine proteins under PAOX1 regulation, on PGAP-driven k-carrageenase production, including seven chaperones (Pdi: protein disulphide isomerase; Ire1: endoplasmic reticulum stress transducer; ero1: endoplasmic reticulum oxidoreductase; Kar2: immunoglobulin-binding protein; Aha1: activator of Hsp90 ATPase; Ypt6; GTPase; Prx1: thioredoxin-linked peroxidase) and two transcription factors Yap1 and Rpn4 (proteasome subunit transcription factors) [101,102]. Overexpression of Rpn4 yielded a 1.36-fold (7.07 U/mL) increase in the production of active k-carrageenase in the medium, and Yap1 yielded a 1.72-fold increase (7.42 U/mL), in contrast with the 2.73 U/mL obtained without methanol induction of auxiliary genes (transcription factors or chaperones). The cell engineering approach used in this work is noteworthy given the ability to compare the effect of co-expression of auxiliary genes in the same strain, using the methanol-inducible promoter PAOX1 for these and a constitutively expressed promoter such as PGAP for the rProt of interest.
Consequently, the modulation of expression of genes encoding transcriptional regulators offers a variety of alternatives enabling an increment in the rProt production yield. However, the high number of genes affected by the modulated expression of these factors should be considered [76,79,103]. Given that, in general, the comparisons are performed under optimised process conditions for the wild-type strain, the results obtained by adapting the bioprocess to other transcriptional variants of K. phaffii are unknown [23].
4. Improving Protein Folding and Secretion
The main hindrances in the production and secretion of proteins are the folding and processing of complex proteins in the ER. When proteins fold inappropriately or exceed the capacity of the ER, unfolded proteins can accumulate and tend to form aggregates. The cellular response generally involves synthesis and induction of folding-assisting proteins such as chaperones or foldases [13,104,105]. To overcome this bottleneck, different researchers have proposed the modification of some genetic factors such as codon sequence optimisation, promoter selection [106], gene dosage [107], and co-expression of folding helper proteins to improve rProt expression [108]. Some examples of these approaches are summarised in Table 3.
Regarding codon sequence optimisation, the codon usage bias can be adapted to the host [106], and this strategy is widely used with the assistance of software tools focused on replacing rare codons with frequently used codons in K. phaffii [109]. Karaoğlan and Erden-Karaoğlan [106] described a model for the expression of the protein endo-poly-galacturonase (Pgl) of A. niger, whose sequence was subjected to codon optimisation, evaluating its performance under the regulation of two promoters (PAOX and PADH2). The highest production level was achieved with the codon-optimised PGL using the pADH2 obtaining a productivity of 42.33 U/mL (4-fold increase) in shake flasks.
The co-expression of chaperones can promote the correct folding of rProts and reduce the intracellular aggregation of proteins [101]. The protein disulphide isomerase (Pdi1) present in the ER plays a crucial role in restoration and isomerisation of disulphide bonds in nascent proteins [7,101]. Lan et al. [110] showed that the expression of the marine Streptomyces sp. lipase Mas1 increased 1.7-fold when it was co-expressed with the chaperone Pdi1, compared to the control strain harbouring only MAS1 [111].
Another chaperone protein frequently co-expressed with rProt is Kar2, a homologue of the mammalian immunoglobulin-binding protein (BiP) [108,111] that acts as a sensor for misfolded proteins, participating in protein folding or directing recalcitrant proteins to the ER-associated degradation pathway (ERAD) [112]. An example of co-expression was reported by Sellada et al., expressing a hydrophobin class II (Hfbi) which, when co-expressed with Kar2, resulted in a 22-fold increase in productivity with respect to the strain without the chaperone [108].
Another strategy that also improves protein folding and secretion is the expression of transcription factors such as Yap1 and Hac1, as it was presented in the previous section. However, when these are expressed together with other folding facilitator proteins such as Pdi1 and Kar2, although it was expected to result in an increase in the production of the rProt of interest, the work of Sun et al. [81] and Duan et al. [113] showed that the rProt productivity was either maintained or decreased.
For this reason, many researchers have implemented more than one modification simultaneously, integrating the co-expression of chaperones and/or foldases, in conjunction with other genetic manipulation tools such as optimisation of codon usage, gene copy number, co-expression/modulation of transcription factors, and variation in culture conditions with the purpose of improving rProt productivity [108,111,114,115]. A clear summary example is reported by Ben Azoun et al. [116], with the expression of the rabies virus glycoprotein (RABV-G), where the molecular factors addressed were gene optimisation, secretion signal sequence, gene copy number, and the co-expression of different proteins. Gene optimisation increased productivity at approximately 2.1-fold, and the overexpression of the two folding factors PDI1 or ERO1 remarkably increased the expression level of RABV-G by 9.5-fold and 3.3-fold, respectively, in the high copy strains of RABV-G (more information available in Table 3).
These findings demonstrate that there is no combinatorial strategy that provides equal benefit for the secretion of all recombinant proteins, and further research is necessary to find the most suitable strategy to obtain the desired recombinant product.

Table 3.
Improving heterologous protein production by protein folding and secretion.
Table 3.
Improving heterologous protein production by protein folding and secretion.
| Auxiliary Gene | Modification | Pathways Involved | Heterologous Product | Production (Fold Change) | Operation Mode | Scale | Strain | Ref. |
|---|---|---|---|---|---|---|---|---|
| PDI1 | OE | Folding | Fab (S) | +1.9 | Batch | Flask | X-33 | [117] |
| PDI1 PDI1 w/ CN | OE | Folding | Na-ASP1 | +3.2 + 7.9 | Batch | Flask | X-33 | [118] |
| KAR2 PDI1 PDI1/KAR2 | OE | Folding | A33scFv (S) | +3 No effect No effect | Batch | 2.5 L Bioreactor | GS200 | [119] |
| PDI1 KAR2 ERO1 SEC1 SLY1 | OE | Folding and Trafficking | IL2-HSA (S) | +2.2 +1.9 +2.3 +2.5 +1.9 | Batch | Flask | GS115 | [111] |
| PDI1 + CN KAR2 + CN ERO1 CN | OE | Folding | Hydrophobin (S) | +7.8 +22 +30 No effect | Batch | Flask | GS115 | [108] |
| PDI1 ERO1 CN | OE | Folding | hLYZ (S) | +2.43 +2.30 +1.57 | Batch | 5 L Bioreactor | GS115 | [7] |
| YDJ1 SSA1 SEC63 KAR2 | OE | Folding and Trafficking | CalB (S) | +1.6 +1.4 +1.4 −0.7 | Batch | Flask | GS115 | [115] |
| PDI1 ERO1 GPX1 GLR1 YAP1 | OE | Folding | RABV-G (S) | +9.5 +3.3 +8.2 +1.2 No effect | Batch | Flask | KM71H/GS115 | [114,116] |
Abbreviations: PDI1: protein disulphide isomerase; KAR2: immunoglobulin-binding protein; ERO1: endoplasmic reticulum oxidoreductase; SEC1: Sm-like protein involved in docking and fusion of exocytic vesicles; SLY1: hydrophilic protein involved in ER/Golgi vesicles trafficking; GPX1: glutathione peroxidase; GLR1: glutathione reductase; YDJ1: type I HSP40 co-chaperone; SSA1: Hsp70 family ATPase involved in protein folding; SEC63: protein-transporting protein; Fab: antibody fragment; Na-ASP1: Necator americanus secretory protein; A33scFv: A33 single-chain antibody fragment; IL2-HSA: human albumin fusion protein; hLYZ: human lysozyme; CalB: Candida antarctica lipase B; RABV-G: rabies virus glycoprotein; CN: gen copy number.
5. Conclusions
K. phaffii has reached a well-recognised position as a successful platform to produce biotechnologically and commercially attractive products (as shown in Table 1, Table 2 and Table 3), mainly due to its ability to produce a diversity of functionally active heterologous proteins, ranging from proteins of microbial origin to complex eukaryotic proteins with several applications [3,120,121].
Metabolic modifications aided by gene codon optimisation, promoter engineering, and genomic engineering with different synthetic biology tools have made it possible to implement new metabolic routes with the objective of developing more robust strains, with improved capabilities and fitness, which can provide higher rProt productivity levels and mitigate issues related to methanol metabolism, the catabolism of cheap raw carbon sources (e.g., xylose, cellulose), and also the potential conversion of K. phaffii into an autotrophic microorganism [64].
Further study of metabolic modifications directed to assimilation routes of carbon and energy sources in response to the culture media will have a repressive or derepressive response to the highly used PAOX1/2 induction system and to the metabolic system in general.
On the other hand, modifications to increase/improve protein folding and secretion, such as codon usage optimisation, increase in gene copy number, co-expression/modulation of transcription factors, co-expression of chaperones, and modification of culture conditions have allowed the expression of a wide range of proteins with diverse application fields. However, the use of one or many of these modifications does not ensure an increase in rProt expression, especially in the case of proteins that are difficult to express, such as antibodies, membrane proteins, toxins, and proteins with non-standard amino acids, among others [107,109,114,116]. Each protein appears to be as unique as the combinatorial strategies that can be incorporated to enhance its expression. So far, there is no combinatorial strategy that enhances all recombinant proteins equally, and further research is needed in order to find the right strategy for the specific desired product, turning the optimisation into a product-based approach [13].
Furthermore, due to the variety of available strains of K. phaffii used to produce recombinant proteins, it is necessary to have a model that integrates information related to cell behaviour in a way that is capable of developing hypotheses focused on optimisation of production processes [122]. The genome-wide metabolic model characterises cell physiology, which allows obtaining valuable information on metabolism and designing possible strategies to improve a strain through in silico simulations [123]. This computational approach, combined with synthetic biology techniques, potentially forms a basis for the rational analysis and design of the K. phaffii metabolic network to improve recombinant protein production [124,125].
Finally, the latest advances and improvements addressed in this review have been reported mainly at laboratory scale (flask) with only a few examples at bioreactor level or high cell density. This highlights the need to expand the research scope on the scale-up of these processes, in order to determine cultivation parameters suited for higher-scale and for industrially relevant cultivation modalities (fed-batch and, increasingly, continuous mode) and strain performance in controlled conditions. In this regard, engineering tools such as response surface methodology can be used, as they have been successfully applied to improve K.-phaffii-based bioprocesses [126,127,128,129].
Author Contributions
Conceptualization, C.B., J.Q., R.V., P.F. and J.B.; Investigation, C.B., J.Q., R.V., S.B.-G., P.F. and J.B.; Writing—original draft preparation, C.B., J.Q., R.V., S.B.-G., P.F. and J.B.; Writing—review and editing, C.B., J.Q., R.V., S.B.-G., C.A. and J.B.; Funding acquisition: C.A., S.B.-G., P.F. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDECYT Regular grant number 1191196, FONDECYT Iniciación grant number 11200933, ANILLO Regular de Ciencia y Tecnología grant number ACT210068, and Becas Doctorado Nacional grant numbers 21191422, 21211138, 21211233—Agencia Nacional de Investigación y Desarrollo (ANID), Chile; and Wallonie-Bruxelles International through the Cooperation bilateral Belgique-Chili project SUB/2019/435787.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kurtzman, C.P. Biotechnological strains of Komagataella (Pichia) pastoris are Komagataella phaffii as determined from multigene sequence analysis. J. Ind. Microbiol. Biotechnol. 2009, 36, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Sun, H.; Wang, M.; Li, Y. Pichia pastoris as a Versatile Cell Factory for the Production of Industrial Enzymes and Chemicals: Current Status and Future Perspectives. Biotechnol. J. 2019, 14, e1800694. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Hirz, M.; Pichler, H.; Schwab, H. Protein expression in Pichia pastoris: Recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol. 2014, 98, 5301–5317. [Google Scholar] [CrossRef] [PubMed]
- Peña, D.A.; Gasser, B.; Zanghellini, J.; Steiger, M.; Mattanovich, D. Metabolic engineering of Pichia pastoris. Metab. Eng. 2018, 50, 2–15. [Google Scholar] [CrossRef]
- Maccani, A.; Landes, N.; Stadlmayr, G.; Maresch, D.; Leitner, C.; Maurer, M.; Gasser, B.; Ernst, W.; Kunert, R.; Mattanovich, D. Pichia pastoris secretes recombinant proteins less efficiently than Chinese hamster ovary cells but allows higher space-time yields for less complex proteins. Biotechnol. J. 2014, 9, 526–537. [Google Scholar] [CrossRef]
- Raza, A.; Pothula, R.; Abdelgaffar, H.; Bashir, S.; Jurat-Fuentes, J.L. Supplemental Information 3: Gene sequence for beta-glucosidase from Bacillus tequelensis. PeerJ 2020, 8, e8792. [Google Scholar] [CrossRef]
- He, H.; Wu, S.; Mei, M.; Ning, J.; Li, C.; Ma, L.; Zhang, G.; Yi, L. A Combinational Strategy for Effective Heterologous Production of Functional Human Lysozyme in Pichia pastoris. Front. Bioeng. Biotechnol. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Ata, Ö.; Ergün, B.G.; Fickers, P.; Heistinger, L.; Mattanovich, D.; Rebnegger, C.; Gasser, B. What makes Komagataella phaffii non-conventional? FEMS Yeast Res. 2021, 21, 21. [Google Scholar] [CrossRef]
- Henríquez, M.; Braun-Galleani, S.; Nesbeth, D.N. Whole cell biosynthetic activity of Komagataella phaffii (Pichia pastoris) GS115 strains engineered with transgenes encoding Chromobacterium violaceum ω-transaminase alone or combined with native transketolase. Biotechnol. Prog. 2020, 36, e2893. [Google Scholar] [CrossRef]
- Braun-Galleani, S.; Henríquez, M.-J.; Nesbeth, D.N. Whole cell biosynthesis of 1-methyl-3-phenylpropylamine and 2-amino-1,3,4-butanetriol using Komagataella phaffii (Pichia pastoris) strain BG-10 engineered with a transgene encoding Chromobacterium violaceum ω-transaminase. Heliyon 2019, 5, e02338. [Google Scholar] [CrossRef]
- Heistinger, L.; Gasser, B.; Mattanovich, D. Microbe Profile: Komagataella phaffii: A methanol devouring biotech yeast formerly known as Pichia pastoris. Microbiol. 2020, 166, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Burgard, J.; Grünwald-Gruber, C.; Altmann, F.; Zanghellini, J.; Valli, M.; Mattanovich, D.; Gasser, B.; Gruber-Grünwald, C. The secretome of Pichia pastoris in fed-batch cultivations is largely independent of the carbon source but changes quantitatively over cultivation time. Microb. Biotechnol. 2019, 13, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Raschmanová, H.; Weninger, A.; Knejzlík, Z.; Melzoch, K.; Kovar, K. Engineering of the unfolded protein response pathway in Pichia pastoris: Enhancing production of secreted recombinant proteins. Appl. Microbiol. Biotechnol. 2021, 105, 4397–4414. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.M.; Obaidi, I.; Quigley, A.; Xie, Y.; Butler, M. Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Biotechnol. Adv. 2020, 43, 107552. [Google Scholar] [CrossRef]
- Özçelik, A.T.; Yılmaz, S.; Inan, M. Pichia pastoris Promoters. Methods Pharmacol. Toxicol. 2019, 1923, 97–112. [Google Scholar] [CrossRef]
- Jia, L.; Li, T.; Wu, Y.; Wu, C.; Li, H.; Huang, A. Enhanced human lysozyme production by Pichia pastoris via periodic glycerol and dissolved oxygen concentrations control. Appl. Microbiol. Biotechnol. 2021, 105, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.; Haridas, S.; Wolfe, K.H.; Lopes, M.R.; Hittinger, C.T.; Göker, M.; Salamov, A.A.; Wisecaver, J.H.; Long, T.M.; Calvey, C.H.; et al. Comparative genomics of biotechnologically important yeasts. Proc. Natl. Acad. Sci. USA 2016, 113, 9882–9887. [Google Scholar] [CrossRef]
- Heistinger, L.; Gasser, B.; Mattanovich, D. Creation of Stable Heterothallic Strains of Komagataella phaffii Enables Dissection of Mating Gene Regulation. Mol. Cell. Biol. 2018, 38, e00398-17. [Google Scholar] [CrossRef]
- Braun-Galleani, S.; Dias, J.A.; Coughlan, A.Y.; Ryan, A.P.; Byrne, K.P.; Wolfe, K.H. Genomic diversity and meiotic recombination among isolates of the biotech yeast Komagataella phaffii (Pichia pastoris). Microb. Cell Factories 2019, 18, 1–13. [Google Scholar] [CrossRef]
- Nieto-Taype, M.A.; Garcia-Ortega, X.; Albiol, J.; Seguí, J.L.M.; Valero, F. Continuous Cultivation as a Tool Toward the Rational Bioprocess Development With Pichia Pastoris Cell Factory. Front. Bioeng. Biotechnol. 2020, 8, 632. [Google Scholar] [CrossRef]
- Liu, W.-C.; Inwood, S.; Gong, T.; Sharma, A.; Yu, L.-Y.; Zhu, P. Fed-batch high-cell-density fermentation strategies for Pichia pastoris growth and production. Crit. Rev. Biotechnol. 2019, 39, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Bernauer, L.; Radkohl, A.; Lehmayer, L.G.K.; Emmerstorfer-Augustin, A. Komagataella phaffii as Emerging Model Organism in Fundamental Research. Front. Microbiol. 2021, 11, 11. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z. Engineering strategies for enhanced production of protein and bio-products in Pichia pastoris: A review. Biotechnol. Adv. 2018, 36, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Jiang, L.; Lian, J. Development of synthetic biology tools to engineer Pichia pastoris as a chassis for the production of natural products. Synth. Syst. Biotechnol. 2021, 6, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Schwarzhans, J.-P.; Luttermann, T.; Geier, M.; Kalinowski, J.; Friehs, K. Towards systems metabolic engineering in Pichia pastoris. Biotechnol. Adv. 2017, 35, 681–710. [Google Scholar] [CrossRef]
- Kafri, M.; Metzl-Raz, E.; Jona, G.; Barkai, N. The Cost of Protein Production. Cell Rep. 2015, 14, 22–31. [Google Scholar] [CrossRef]
- Tomàs-Gamisans, M.; Andrade, C.C.P.; Maresca, F.; Monforte, S.; Ferrer, P.; Albiol, J. Redox Engineering by Ectopic Overexpression of NADH Kinase in Recombinant Pichia pastoris (Komagataella phaffii): Impact on Cell Physiology and Recombinant Production of Secreted Proteins. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Jia, L.; Mpofu, E.; Tu, T.; Huai, Q.; Sun, J.; Chen, S.; Ding, J.; Shi, Z. Transcriptional analysis for carbon metabolism and kinetic modeling for heterologous proteins productions by Pichia pastoris in induction process with methanol/sorbitol co-feeding. Process Biochem. 2017, 59, 159–166. [Google Scholar] [CrossRef]
- Cai, H.; Doi, R.; Shimada, M.; Hayakawa, T.; Nakagawa, T. Metabolic regulation adapting to high methanol environment in the methylotrophic yeast Ogataea methanolica. Microb. Biotechnol. 2021, 14, 1512–1524. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Zhao, F.; Zhang, Y.; Yang, X.; Lin, Y.; Han, S. Methanol oxidase from Hansenula polymorpha shows activity in peroxisome-deficient Pichia pastoris. Biochem. Eng. J. 2022, 180, 108369. [Google Scholar] [CrossRef]
- Carly, F.; Niu, H.; Delvigne, F.; Fickers, P. Influence of methanol/sorbitol co-feeding rate on pAOX1 induction in a Pichia pastoris Mut+ strain in bioreactor with limited oxygen transfer rate. J. Ind. Microbiol. Biotechnol. 2016, 43, 517–523. [Google Scholar] [CrossRef]
- Azadi, S.; Mahboubi, A.; Naghdi, N.; Solaimanian, R.; Mortazavi, S.A. Evaluation of Sorbitol-Methanol Co-Feeding Strategy on Production of Recombinant Human Growth Hormone in Pichia Pastoris. Iran. J. Pharm. Res. 2017, 16, 1555–1564. [Google Scholar] [PubMed]
- Berrios, J.; Flores, M.-O.; Díaz-Barrera, A.; Altamirano, C.; Martínez, I.; Cabrera, Z. A comparative study of glycerol and sorbitol as co-substrates in methanol-induced cultures of Pichia pastoris: Temperature effect and scale-up simulation. J. Ind. Microbiol. Biotechnol. 2017, 44, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Zepeda, A.B.; Pessoa, A.; Farías, J.G. Carbon metabolism influenced for promoters and temperature used in the heterologous protein production using Pichia pastoris yeast. Braz. J. Microbiol. 2018, 49, 119–127. [Google Scholar] [CrossRef]
- Niu, H.; Jost, L.; Pirlot, N.; Sassi, H.; Daukandt, M.; Rodriguez, C.; Fickers, P. A quantitative study of methanol/sorbitol co-feeding process of a Pichia pastoris Mut+/pAOX1-lacZ strain. Microb. Cell Factories 2013, 12, 33. [Google Scholar] [CrossRef]
- Liu, W.; Xiang, H.; Zhang, T.; Pang, X.; Su, J.; Liu, H.; Ma, B.; Yu, L. Development of a New High-Cell Density Fermentation Strategy for Enhanced Production of a Fungus β-Glucosidase in Pichia pastoris. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Krainer, F.W.; Dietzsch, C.; Hajek, T.; Herwig, C.; Spadiut, O.; Glieder, A. Recombinant protein expression in Pichia pastoris strains with an engineered methanol utilization pathway. Microb. Cell Factories 2012, 11, 22. [Google Scholar] [CrossRef]
- Geier, M.; Brandner, C.; Strohmeier, G.A.; Hall, M.; Hartner, F.S.; Glieder, A. Engineering Pichia pastoris for improved NADH regeneration: A novel chassis strain for whole-cell catalysis. Beilstein J. Org. Chem. 2015, 11, 1741–1748. [Google Scholar] [CrossRef]
- Zavec, D.; Troyer, C.; Maresch, D.; Altmann, F.; Hann, S.; Gasser, B.; Mattanovich, D. Beyond alcohol oxidase: The methylotrophic yeast Komagataella phaffii utilizes methanol also with its native alcohol dehydrogenase Adh2. FEMS Yeast Res. 2021, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Tyurin, O.V.; Kozlov, D.G. Deletion of the FLD gene in methylotrophic yeasts Komagataella phaffii and Komagataella kurtzmanii results in enhanced induction of the AOX1 promoter in response to either methanol or formate. Microbiol. 2015, 84, 408–411. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Y.; Zhang, J.; Zhang, J. Enhancement of xylanase expression by Komagataella phaffii through pexophagy inhibition. Biotechnol. Biotechnol. Equip. 2019, 33, 855–862. [Google Scholar] [CrossRef]
- Nocon, J.; Steiger, M.; Mairinger, T.; Hohlweg, J.; Rußmayer, H.; Hann, S.; Gasser, B.; Mattanovich, D. Increasing pentose phosphate pathway flux enhances recombinant protein production in Pichia pastoris. Appl. Microbiol. Biotechnol. 2016, 100, 5955–5963. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.A.; Veeranki, V.D. Metabolic Engineering of Pichia pastoris GS115 for Enhanced Pentose Phosphate Pathway (PPP) Flux toward Recombinant Human Interferon Gamma (HIFN-γ) Production. Mol. Biol. Rep. 2018, 45, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Sun, H.; Chen, Z.; Li, Y.; Zhu, T. Construction of efficient xylose utilizing Pichia pastoris for industrial enzyme production. Microb. Cell Factories 2015, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Cereghino, J.L.; Cregg, J.M. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 2000, 24, 45–66. [Google Scholar] [CrossRef]
- Zavec, D.; Gasser, B.; Mattanovich, D. Characterization of methanol utilization negative Pichia pastoris for secreted protein production: New cultivation strategies for current and future applications. Biotechnol. Bioeng. 2020, 117, 1394–1405. [Google Scholar] [CrossRef]
- Chiruvolu, V.; Cregg, J.M.; Meagher, M.M. Recombinant protein production in an alcohol oxidase-defective strain of Pichia pastoris in fedbatch fermentations. Enzym. Microb. Technol. 1997, 21, 277–283. [Google Scholar] [CrossRef]
- Guo, F.; Dai, Z.; Peng, W.; Zhang, S.; Zhou, J.; Ma, J.; Dong, W.; Xin, F.; Zhang, W.; Jiang, M. Metabolic engineering of Pichia pastoris for malic acid production from methanol. Biotechnol. Bioeng. 2021, 118, 357–371. [Google Scholar] [CrossRef]
- Liu, T.; Liu, B.; Zhou, H.; Zhang, J. Knockout of the DAS gene increases S-adenosylmethionine production in Komagataella phaffii. Biotechnol. Biotechnol. Equip. 2021, 35, 29–36. [Google Scholar] [CrossRef]
- Inan, M.; Meagher, M.M. Non-repressing carbon sources for alcohol oxidase (AOX1) promoter of Pichia pastoris. J. Biosci. Bioeng. 2001, 92, 585–589. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, W.; Zhou, X.; Bai, P.; Cregg, J.M.; Zhang, Y. Catabolite Repression of Aox in Pichia pastoris Is Dependent on Hexose Transporter PpHxt1 and Pexophagy. Appl. Environ. Microbiol. 2010, 76, 6108–6118. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mohsin, A.; Chu, J.; Zhuang, Y.; Liu, Y.; Guo, M. Enhanced protein production by sorbitol co-feeding with methanol in recombinant Pichia pastoris strains. Biotechnol. Bioprocess Eng. 2017, 22, 767–773. [Google Scholar] [CrossRef]
- Moreira, L.R.S.; Filho, E.X.F. Insights into the mechanism of enzymatic hydrolysis of xylan. Appl. Microbiol. Biotechnol. 2016, 100, 5205–5214. [Google Scholar] [CrossRef] [PubMed]
- Çalık, P.; Ata, O.; Güneş, H.; Massahi, A.; Boy, E.; Keskin, A.; Öztürk, S.; Zerze, G.H.; Özdamar, T.H. Recombinant protein production in Pichia pastoris under glyceraldehyde-3-phosphate dehydrogenase promoter: From carbon source metabolism to bioreactor operation parameters. Biochem. Eng. J. 2015, 95, 20–36. [Google Scholar] [CrossRef]
- Arias, C.A.D.; Molino, J.V.D.; Marques, D.D.A.V.; Maranhão, A.Q.; Parra, D.A.S.; Junior, A.P.; Converti, A. Influence of carbon source on cell size and production of anti LDL (-) single-chain variable fragment by a recombinant Pichia pastoris strain. Mol. Biol. Rep. 2019, 46, 3257–3264. [Google Scholar] [CrossRef]
- Boehm, T.; Pirie-Shepherd, S.; Trinh, L.-B.; Shiloach, J.; Folkman, J. Disruption of the KEX1 gene in Pichia pastoris allows expression of full-length murine and human endostatin. Yeast 1999, 15, 563–572. [Google Scholar] [CrossRef]
- Arias, C.A.D.; Marques, D.D.A.V.; Malpiedi, L.P.; Maranhão, A.Q.; Parra, D.A.S.; Converti, A.; Junior, A.P. Cultivation of Pichia pastoris carrying the scFv anti LDL (-) antibody fragment. Effect of preculture carbon source. Braz. J. Microbiol. 2017, 48, 419–426. [Google Scholar] [CrossRef]
- Canales, C.; Altamirano, C.; Berrios, J. The growth of Pichia pastoris Mut+ on methanol-glycerol mixtures fits to interactive dual-limited kinetics: Model development and application to optimised fed-batch operation for heterologous protein production. Bioprocess Biosyst. Eng. 2018, 41, 1827–1838. [Google Scholar] [CrossRef]
- Geertman, J.-M.A.; van Maris, A.J.; van Dijken, J.P.; Pronk, J.T. Physiological and genetic engineering of cytosolic redox metabolism in Saccharomyces cerevisiae for improved glycerol production. Metab. Eng. 2006, 8, 532–542. [Google Scholar] [CrossRef]
- Kim, S.; Hahn, J.-S. Efficient production of 2,3-butanediol in Saccharomyces cerevisiae by eliminating ethanol and glycerol production and redox rebalancing. Metab. Eng. 2015, 31, 94–101. [Google Scholar] [CrossRef]
- Kickenweiz, T.; Glieder, A.; Wu, J.C. Construction of a cellulose-metabolizing Komagataella phaffii (Pichia pastoris) by co-expressing glucanases and β-glucosidase. Appl. Microbiol. Biotechnol. 2017, 102, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.R.; Su, X.; Shrestha, S.K.; Yang, S.Y.; Soh, Y. 2E-Decene-4,6-diyn-1-ol-acetate inhibits osteoclastogenesis through mitogen-activated protein kinase-c-Fos-NFATc1 signalling pathways. Clin. Exp. Pharmacol. Physiol. 2021, 49, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Bai, C.; Liu, Y.; Song, L.; Tian, L.; Yan, Y.; Zhou, J.; Zhou, X.; Zhang, Y.; Cai, M. Modulation of acetate utilization in Komagataella phaffii by metabolic engineering of tolerance and metabolism. Biotechnol. Biofuels 2019, 12, 61. [Google Scholar] [CrossRef]
- Gassler, T.; Sauer, M.; Gasser, B.; Egermeier, M.; Troyer, C.; Causon, T.; Hann, S.; Mattanovich, D.; Steiger, M.G. The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2. Nat. Biotechnol. 2020, 38, 210–216. [Google Scholar] [CrossRef]
- Resina, D.; Bollók, M.; Khatri, N.K.; Valero, F.; Neubauer, P.; Ferrer, P. Transcriptional response of P. pastoris in fed-batch cultivations to Rhizopus oryzae lipase production reveals UPR induction. Microb. Cell Factories 2007, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Glieder, A. Regulation of Pichia pastoris promoters and its consequences for protein production. New Biotechnol. 2012, 30, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Ergün, B.G.; Demir, I.; Özdamar, T.H.; Gasser, B.; Mattanovich, D.; Çalık, P. Engineered Deregulation of Expression in Yeast with Designed Hybrid-Promoter Architectures in Coordination with Discovered Master Regulator Transcription Factor. Adv. Biosyst. 2020, 4, e1900172. [Google Scholar] [CrossRef]
- Gasser, B.; Sauer, M.; Maurer, M.; Stadlmayr, G.; Mattanovich, D. Transcriptomics-Based Identification of Novel Factors Enhancing Heterologous Protein Secretion in Yeasts. Appl. Environ. Microbiol. 2007, 73, 6499–6507. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Miao, H.; Tang, X.; Xu, B.; Wu, Q.; Mu, Y.; Huang, Z. Transcriptomic Analysis of Pichia pastoris (Komagataella phaffii) GS115 During Heterologous Protein Production Using a High-Cell-Density Fed-Batch Cultivation Strategy. Front. Microbiol. 2020, 11, 463. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhan, C.; Zhang, Z.; Liu, X.; Liu, H.; Bai, Z. Transcriptional analysis of impacts of glycerol transporter 1 on methanol and glycerol metabolism in Pichia pastoris. FEMS Yeast Res. 2017, 18, 18. [Google Scholar] [CrossRef]
- Ata, Ö.; Rebnegger, C.; Tatto, N.E.; Valli, M.; Mairinger, T.; Hann, S.; Steiger, M.G.; Çalık, P.; Mattanovich, D. A single Gal4-like transcription factor activates the Crabtree effect in Komagataella phaffii. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kalender, Ö.; Çalık, P. Transcriptional regulatory proteins in central carbon metabolism of Pichia pastoris and Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2020, 104, 7273–7311. [Google Scholar] [CrossRef] [PubMed]
- Mitsis, T.; Efthimiadou, A.; Bacopoulou, F.; Vlachakis, D.; Chrousos, G.; Eliopoulos, E. Transcription factors and evolution: An integral part of gene expression (Review). World Acad. Sci. J. 2020. [Google Scholar] [CrossRef]
- Bankefa, O.E.; Wang, M.; Zhu, T.; Li, Y. Hac1p homologues from higher eukaryotes can improve the secretion of heterologous proteins in the yeast Pichia pastoris. Biotechnol. Lett. 2018, 40, 1149–1156. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, Y.; Wang, P.; Li, X.; Zhan, C.; Linhardt, R.J.; Zhang, F.; Liu, X.; Zhan, J.; Bai, Z. Characterization and application of a putative transcription factor (SUT2) in Pichia pastoris. Mol. Genet. Genom. 2020, 295, 1295–1304. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Zhang, X.; Li, C.; Liu, X.; Lin, Y.; Liang, S. Fhl1p protein, a positive transcription factor in Pichia pastoris, enhances the expression of recombinant proteins. Microb. Cell Factories 2019, 18, 1–12. [Google Scholar] [CrossRef]
- Chang, C.-H.; Hsiung, H.-A.; Hong, K.-L.; Huang, C.-T. Enhancing the efficiency of the Pichia pastoris AOX1 promoter via the synthetic positive feedback circuit of transcription factor Mxr1. BMC Biotechnol. 2018, 18, 81. [Google Scholar] [CrossRef]
- Jiang, B.; Argyros, R.; Bukowski, J.; Nelson, S.; Sharkey, N.; Kim, S.; Copeland, V.; Davidson, R.C.; Chen, R.; Zhuang, J.; et al. Inactivation of a GAL4 -Like Transcription Factor Improves Cell Fitness and Product Yield in Glycoengineered Pichia pastoris Strains. Appl. Environ. Microbiol. 2015, 81, 260–271. [Google Scholar] [CrossRef][Green Version]
- Delic, M.; Graf, A.B.; Koellensperger, G.; Troyer, C.; Hann, S.; Mattanovich, D.; Gasser, B. Overexpression of the transcription factor Yap1 modifies intracellular redox conditions and enhances recombinant protein secretion. Microb. Cell 2014, 1, 376–386. [Google Scholar] [CrossRef]
- Ruth, C.; Buchetics, M.; Vidimce, V.; Kotz, D.; Naschberger, S.; Mattanovich, D.; Pichler, H.; Gasser, B. Pichia pastoris Aft1—A novel transcription factor, enhancing recombinant protein secretion. Microb. Cell Factories 2014, 13, 1–15. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, J.; Zhai, X.; Zhu, S.; Qu, Z.; Yuan, W.; Wang, Z.; Wei, C. Coexpression of Kex2 Endoproteinase and Hac1 Transcription Factor to Improve the Secretory Expression of Bovine Lactoferrin in Pichia pastoris. Biotechnol. Bioprocess Eng. 2019, 24, 934–941. [Google Scholar] [CrossRef]
- Guerfal, M.; Ryckaert, S.; Jacobs, P.P.; Ameloot, P.; Van Craenenbroeck, K.; Derycke, R.; Callewaert, N. The HAC1 gene from Pichia pastoris: Characterization and effect of its overexpression on the production of secreted, surface displayed and membrane proteins. Microb. Cell Factories 2010, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Thallinger, G.G.; Zellnig, G.; Drew, D.; Cregg, J.M.; Glieder, A.; Freigassner, M. Towards improved membrane protein production in Pichia pastoris: General and specific transcriptional response to membrane protein overexpression. New Biotechnol. 2014, 31, 538–552. [Google Scholar] [CrossRef]
- Liu, J.; Han, Q.; Cheng, Q.; Chen, Y.; Wang, R.; Li, X.; Liu, Y.; Yan, D. Efficient Expression of Human Lysozyme Through the Increased Gene Dosage and Co-expression of Transcription Factor Hac1p in Pichia pastoris. Curr. Microbiol. 2020, 77, 846–854. [Google Scholar] [CrossRef] [PubMed]
- De Waele, S.; Vandenberghe, I.; Laukens, B.; Planckaert, S.; Verweire, S.; Van Bogaert, I.; Soetaert, W.; Devreese, B.; Ciesielska, K. Optimized expression of the Starmerella bombicola lactone esterase in Pichia pastoris through temperature adaptation, codon-optimization and co-expression with HAC1. Protein Expr. Purif. 2018, 143, 62–70. [Google Scholar] [CrossRef]
- Han, M.; Wang, W.; Zhou, J.; Gong, X.; Xu, C.; Li, Y.; Li, Q. Activation of the Unfolded Protein Response via Co-expression of the HAC1i Gene Enhances Expression of Recombinant Elastase in Pichia pastoris. Biotechnol. Bioprocess Eng. 2020, 25, 302–307. [Google Scholar] [CrossRef]
- Yano, T.; Yurimoto, H.; Sakai, Y. Activation of the Oxidative Stress Regulator PpYap1 through Conserved Cysteine Residues during Methanol Metabolism in the Yeast Pichia pastoris. Biosci. Biotechnol. Biochem. 2009, 73, 1404–1411. [Google Scholar] [CrossRef]
- Yano, T.; Takigami, E.; Yurimoto, H.; Sakai, Y. Yap1-Regulated Glutathione Redox System Curtails Accumulation of Formaldehyde and Reactive Oxygen Species in Methanol Metabolism of Pichia pastoris. Eukaryot. Cell 2009, 8, 540–549. [Google Scholar] [CrossRef]
- Lin, X.-Q.; Liang, S.-L.; Han, S.-Y.; Zheng, S.-P.; Ye, Y.-R.; Lin, Y. Quantitative iTRAQ LC–MS/MS proteomics reveals the cellular response to heterologous protein overexpression and the regulation of HAC1 in Pichia pastoris. J. Proteom. 2013, 91, 58–72. [Google Scholar] [CrossRef]
- Li, C.; Lin, Y.; Zheng, X.; Pang, N.; Liao, X.; Liu, X.; Huang, Y.; Liang, S. Combined strategies for improving expression of Citrobacter amalonaticus phytase in Pichia pastoris. BMC Biotechnol. 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Hohenblum, H.; Gasser, B.; Maurer, M.; Borth, N.; Mattanovich, D. Effects of gene dosage, promoters, and substrates on unfolded protein stress of recombinant Pichia pastoris. Biotechnol. Bioeng. 2004, 85, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Miao, L.; Huang, H.; Li, Y.; Zhu, T. High-level production of glucose oxidase in Pichia pastoris: Effects of Hac1p overexpression on cell physiology and enzyme expression. Enzym. Microb. Technol. 2020, 141, 109671. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Gao, Y.; Zhou, X.; Zhang, Y.; Cai, M. Regulating unfolded protein response activator HAC1p for production of thermostable raw-starch hydrolyzing α-amylase in Pichia pastoris. Bioprocess Biosyst. Eng. 2016, 40, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Haberhauer-Troyer, C.; Delic, M.; Gasser, B.; Mattanovich, D.; Hann, S.; Koellensperger, G. Accurate quantification of the redox-sensitive GSH/GSSG ratios in the yeast Pichia pastoris by HILIC–MS/MS. Anal. Bioanal. Chem. 2013, 405, 2031–2039. [Google Scholar] [CrossRef]
- Pekarsky, A.; Veiter, L.; Rajamanickam, V.; Herwig, C.; Grünwald-Gruber, C.; Altmann, F.; Spadiut, O. Production of a recombinant peroxidase in different glyco-engineered Pichia pastoris strains: A morphological and physiological comparison. Microb. Cell Factories 2018, 17, 1–15. [Google Scholar] [CrossRef]
- Barnard, G.C.; Kull, A.R.; Sharkey, N.S.; Shaikh, S.S.; Rittenhour, A.M.; Burnina, I.; Jiang, Y.; Li, F.; Lynaugh, H.; Mitchell, T.; et al. High-throughput screening and selection of yeast cell lines expressing monoclonal antibodies. J. Ind. Microbiol. Biotechnol. 2010, 37, 961–971. [Google Scholar] [CrossRef]
- Zha, D. Glycoengineered Pichia-Based Expression of Monoclonal Antibodies. Methods Mol. Biol. 2013, 988, 31–43. [Google Scholar] [CrossRef]
- Ata, Ö.; Prielhofer, R.; Gasser, B.; Mattanovich, D.; Çalık, P. Transcriptional engineering of the glyceraldehyde-3-phosphate dehydrogenase promoter for improved heterologous protein production in Pichia pastoris. Biotechnol. Bioeng. 2017, 114, 2319–2327. [Google Scholar] [CrossRef]
- Vogl, T.; Sturmberger, L.; Fauland, P.C.; Hyden, P.; Fischer, J.E.; Schmid, C.; Thallinger, G.G.; Geier, M.; Glieder, A. Methanol independent induction in Pichia pastoris by simple derepressed overexpression of single transcription factors. Biotechnol. Bioeng. 2018, 115, 1037–1050. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Shi, L.; Qi, F.; Zhang, P.; Zhang, Y.; Zhou, X.; Song, Z.; Cai, M. Methanol-Independent Protein Expression by AOX1 Promoter with trans-Acting Elements Engineering and Glucose-Glycerol-Shift Induction in Pichia pastoris. Sci. Rep. 2017, 7, srep41850. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Z.; Chen, M.; Yang, M.; Li, L.; Mou, H. Enhancing the expression of recombinant κ-carrageenase in Pichia pastoris using dual promoters, co-expressing chaperones and transcription factors. Biocatal. Biotransformation 2019, 38, 104–113. [Google Scholar] [CrossRef]
- Shirozu, R.; Yashiroda, H.; Murata, S. Identification of minimum Rpn4-responsive elements in genes related to proteasome functions. FEBS Lett. 2015, 589, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Barbay, D.; Mačáková, M.; Sützl, L.; De, S.; Mattanovich, D.; Gasser, B. Two homologs of the Cat8 transcription factor are involved in the regulation of ethanol utilization in Komagataella phaffii. Curr. Genet. 2021, 67, 641–661. [Google Scholar] [CrossRef]
- Delic, M.; Valli, M.; Graf, A.B.; Pfeffer, M.; Mattanovich, D.; Gasser, B. The secretory pathway: Exploring yeast diversity. FEMS Microbiol. Rev. 2013, 37, 872–914. [Google Scholar] [CrossRef] [PubMed]
- Delic, M.; Göngrich, R.; Mattanovich, D.; Gasser, B. Engineering of Protein Folding and Secretion—Strategies to Overcome Bottlenecks for Efficient Production of Recombinant Proteins. Antioxidants Redox Signal. 2014, 21, 414–437. [Google Scholar] [CrossRef] [PubMed]
- Karaoğlan, M.; Erden-Karaoğlan, F. Effect of codon optimization and promoter choice on recombinant endo-polygalacturonase production in Pichia pastoris. Enzym. Microb. Technol. 2020, 139, 109589. [Google Scholar] [CrossRef]
- Che, Z.; Cao, X.; Chen, G.; Liang, Z. An effective combination of codon optimization, gene dosage, and process optimization for high-level production of fibrinolytic enzyme in Komagataella phaffii (Pichia pastoris). BMC Biotechnol. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Sallada, N.D.; Harkins, L.E.; Berger, B.W. Effect of gene copy number and chaperone coexpression on recombinant hydrophobin HFBI biosurfactant production in Pichia pastoris. Biotechnol. Bioeng. 2019, 116, 2029–2040. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, T.; Lu, L.; Cai, F.; Lin, J.; Jiang, Y.; Lin, Y. Codon pair optimization (CPO): A software tool for synthetic gene design based on codon pair bias to improve the expression of recombinant proteins in Pichia pastoris. Microb. Cell Factories 2021, 20, 1–10. [Google Scholar] [CrossRef]
- Lan, D.; Qu, M.; Yang, B.; Wang, Y. Enhancing production of lipase MAS1 from marine Streptomyces sp. strain in Pichia pastoris by chaperones co-expression. Electron. J. Biotechnol. 2016, 22, 62–67. [Google Scholar] [CrossRef]
- Guan, B.; Chen, F.; Su, S.; Duan, Z.; Chen, Y.; Li, H.; Jin, J. Effects of co-overexpression of secretion helper factors on the secretion of a HSA fusion protein (IL2-HSA) inpichia pastoris. Yeast 2016, 33, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.; Vanz, A.L.; Lünsdorf, H.; Nimtz, M.; Rinas, U. Fate of the UPR marker protein Kar2/Bip and autophagic processes in fed-batch cultures of secretory insulin precursor producing Pichia pastoris. Microb. Cell Factories 2018, 17, 123. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Ding, L.; Wei, D.; Zhou, H.; Chu, J.; Zhang, S.; Qian, J. Screening endogenous signal peptides and protein folding factors to promote the secretory expression of heterologous proteins in Pichia pastoris. J. Biotechnol. 2019, 306, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ben Azoun, S.; Ben Zakour, M.; Sghaier, S.; Kallel, H. Expression of rabies virus glycoprotein in the methylotrophic yeast Pichia pastoris. Biotechnol. Appl. Biochem. 2017, 64, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Samuel, P.; Vadhana, A.K.P.; Kamatchi, R.; Antony, A.; Meenakshisundaram, S. Effect of molecular chaperones on the expression of Candida antarctica lipase B in Pichia pastoris. Microbiol. Res. 2013, 168, 615–620. [Google Scholar] [CrossRef]
- Ben Azoun, S.; Belhaj, A.E.; Göngrich, R.; Gasser, B.; Kallel, H. Molecular optimization of rabies virus glycoprotein expression in Pichia pastoris. Microb. Biotechnol. 2016, 9, 355–368. [Google Scholar] [CrossRef]
- Gasser, B.; Maurer, M.; Gach, J.; Kunert, R.; Mattanovich, D. Engineering of Pichia pastoris for improved production of antibody fragments. Biotechnol. Bioeng. 2006, 94, 353–361. [Google Scholar] [CrossRef]
- Inan, M.; Aryasomayajula, D.; Sinha, J.; Meagher, M.M. Enhancement of protein secretion in Pichia pastoris by overexpression of protein disulfide isomerase. Biotechnol. Bioeng. 2006, 93, 771–778. [Google Scholar] [CrossRef]
- Damasceno, L.M.; Anderson, K.A.; Ritter, G.; Cregg, J.M.; Old, L.J.; Batt, C.A. Cooverexpression of chaperones for enhanced secretion of a single-chain antibody fragment in Pichia pastoris. Appl. Microbiol. Biotechnol. 2007, 74, 381–389. [Google Scholar] [CrossRef]
- Gasser, B.; Prielhofer, R.; Marx, H.; Maurer, M.; Nocon, J.; Steiger, M.; Puxbaum, V.; Sauer, M.; Mattanovich, D. Pichia pastoris: Protein production host and model organism for biomedical research. Futur. Microbiol. 2013, 8, 191–208. [Google Scholar] [CrossRef]
- Spohner, S.; Müller, H.; Quitmann, H.; Czermak, P. Expression of enzymes for the usage in food and feed industry with Pichia pastoris. J. Biotechnol. 2015, 202, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Saitua, F.; Torres, P.; Pérez-Correa, J.R.; Agosin, E. Dynamic genome-scale metabolic modeling of the yeast Pichia pastoris. BMC Syst. Biol. 2017, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.K.; Selvarasu, S.; Camattari, A.; Ryu, J.; Lee, H.; Ahn, J.; Lee, H.; Lee, D.-Y. Genome-scale metabolic reconstruction and in silico analysis of methylotrophic yeast Pichia pastoris for strain improvement. Microb. Cell Factories 2010, 9, 50. [Google Scholar] [CrossRef]
- Tomàs-Gamisans, M.; Ferrer, P.; Albiol, J. Integration and Validation of the Genome-Scale Metabolic Models of Pichia pastoris: A Comprehensive Update of Protein Glycosylation Pathways, Lipid and Energy Metabolism. PLoS ONE 2016, 11, e0148031. [Google Scholar] [CrossRef]
- Tomàs-Gamisans, M.; Ferrer, P.; Albiol, J. Fine-tuning the P. pastoris iMT1026 genome-scale metabolic model for improved prediction of growth on methanol or glycerol as sole carbon sources. Microb. Biotechnol. 2018, 11, 224–237. [Google Scholar] [CrossRef]
- Canales, C.; Altamirano, C.; Berrios, J. Effect of dilution rate and methanol-glycerol mixed feeding on heterologous Rhizopus oryzae lipase production with Pichia pastoris Mut+ phenotype in continuous culture. Biotechnol. Prog. 2015, 31, 707–714. [Google Scholar] [CrossRef]
- Kastberg, L.L.B.; Ard, R.; Jensen, M.K.; Workman, C.T. Burden Imposed by Heterologous Protein Production in Two Major Industrial Yeast Cell Factories: Identifying Sources and Mitigation Strategies. Front. Fungal Biol. 2022, 3, 1. [Google Scholar] [CrossRef]
- Torres, P.; Saa, P.A.; Albiol, J.; Ferrer, P.; Agosin, E. Contextualized genome-scale model unveils high-order metabolic effects of the specific growth rate and oxygenation level in recombinant Pichia pastoris. Metab. Eng. Commun. 2019, 9, e00103. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Huang, Z.; Qian, M.; Zhang, Q.; Feng, J. Process development of recombinant Aspergillus flavus urate oxidase production in Pichia pastoris intracellularly and its characterization as a potential biosimilar. Process Biochem. 2021, 102, 376–385. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).