Abstract
The analysis of urinary volatile organic compounds (VOCs) is a promising field of research with the potential to discover new biomarkers for cancer early detection. This systematic review aims to summarise the published literature concerning cancer-associated urinary VOCs. A systematic online literature search was conducted to identify studies reporting urinary VOC biomarkers of cancers in accordance with the recommendations of the Cochrane Library and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines. Thirteen studies comprising 1266 participants in total were included in the review. Studies reported urinary VOC profiles of five cancer subtypes: prostate cancer, gastrointestinal cancer, leukaemia/lymphoma, lung cancer, and bladder cancer. Forty-eight urinary VOCs belonging to eleven chemical classes were identified with high diagnostic performance. VOC profiles were distinctive for each cancer type with limited cross-over. The metabolic analysis suggested distinctive phenotypes for prostate and gastrointestinal cancers. The heterogenicity of study design, methodological and reporting quality may have contributed to inconsistencies between studies. Urinary VOC analysis has shown promising performance for non-invasive diagnosis of cancer. However, limitations in study design have resulted in inconsistencies between studies. These limitations are summarised and discussed in order to support future studies.
1. Introduction
There remains an important unmet clinical need to improve the earlier detection of cancer. Early symptoms of many forms of cancer are often vague and may be mistaken for common benign conditions. Without access to acceptable and affordable methods of assessing patients who present with such symptoms, diagnosis is often delayed until the cancer is at an advanced, sometimes incurable, stage.
There is growing evidence linking different cancers to the increased/decreased production of volatile organic compounds (VOCs) [1,2,3,4,5,6]. As end products of metabolism, cancer-related VOCs are potentially produced by oxidative stress and peroxidation of cell membranes or as a consequence of gene or protein alterations in cancer cells. VOCs levels can reflect pathophysiological processes including inflammation, necrosis, and cancer development [7,8]. Owing to their volatility at ambient temperature VOCs produced within cancer tissues travel in the systemic circulation before being freely excreted. In humans, VOCs have been detected in a wide range of samples, including breath, urine, blood, faeces, tissue, and skin.
Compared to other biological samples such as breath, blood, and tissue, urine has the advantage of being easy and inexpensive to collect and handle. Furthermore, urine has the potential to provide insight not only to local environment of the urogenital tract but also systemic metabolism as it contains the effluent of renal filtration. Studies that have analysed urinary VOCs in cancer patients have yielded promising results [9,10,11]. Hanai et al. reported nine VOCs that were present at higher concentrations in urine of lung cancer patients [12]. Arasaradnam et al. demonstrated for the first time the utility of urine specimens to discriminate healthy individuals from pancreatic ductal adenocarcinoma (PDAC) patients through the detection of VOCs. Their findings were also able to distinguish between early and late stage PDAC [10].
Despite promising findings, there remains uncertainty as to the role of urinary VOC analysis in cancer early detection. A major perceived challenge is the lack of standardisation of methods that may hinder efforts to achieve reproducibility of findings and in turn wider adoption in clinical practice.
The purpose of this systematic review is to summarise the published literature concerning cancer-associated urinary VOCs. Specific objectives were to identify published urinary VOC markers of cancer; explore emerging metabolic pathways of cancer specific VOCs; and evaluate the methodological quality of published studies.
2. Results
The systematic literature search identified 886 studies. After screening and assessment for eligibility, a total of 13 studies were included (Figure 1) [9,11,13,14,15,16,17,18,19,20,21,22,23]. Details of included studies are presented in Table 1. Included studies were from Europe (n = 10), North America (n = 2), and Asia (n = 1) reporting the outcomes of 1266 patients of which 700 had been diagnosed with cancer. Studies reported the urinary VOC profiles of prostate cancer (n = 5); gastrointestinal cancer (n = 7); lung cancer (n = 1); haematological malignancies (n = 1); and bladder cancer (n = 1).
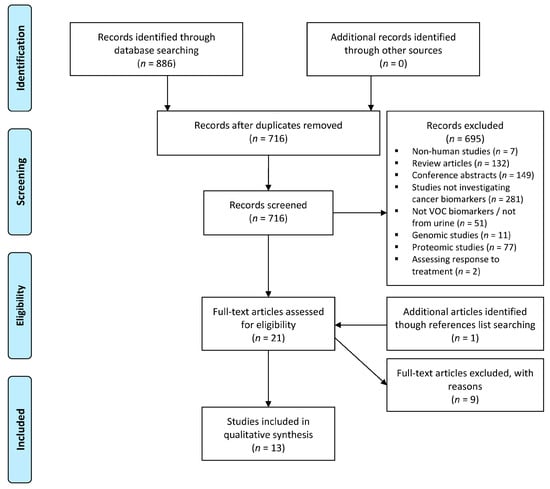
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. VOC: volatile organic compound. The systematic literature search identified 886 studies, with 13 studies included after screening and assessment for eligibility.

Table 1.
Study characteristics. Urinary volatile organic compound analysis for cancer diagnosis: analytical and biostatistical techniques for biomarker discovery.
Gas chromatography mass spectrometry (GC-MS) was the most commonly used analytical technique (10 studies). Selected ion flow tube mass spectrometry (SIFT-MS) was used in three studies. Field asymmetric ion mobility spectrometry (FAIMS) was used in a single study. Two studies used more than one analytical technique [14,21]. In the majority of studies (n = 9), VOCs were analysed within urine headspace, as opposed to the fluid phase. Other techniques used for the extraction of urinary VOCs included solid phase microextraction (SPME) and stir bar sorptive extraction with or without derivatisation.
2.1. Quality Assessment
Outcomes of quality assessments are summarised in Table 2. Bias and applicability of outcomes were analysed with QUADAS-2 (Table 2). Of the 13 included studies, there was considered to be an overall low risk of bias and high applicability of these studies to the review question.

Table 2.
Quality assessment with Standards for Reporting of Diagnostic Accuracy studies (STARD), Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2), and Chemical Analysis Working Group Metabolomics Standard Initiative (CAWG-MSI) score. There was an overall low risk of bias and high applicability of the 13 studies to the review question. The completeness and transparency of reporting was inadequate. There was an overall inadequate reporting of metadata of the studies.
General reporting quality of the studies was assessed by the STARD checklist (Table 2). STARD scores ranged from 11 to 27 with a mean of 19.9 ± 4.6 where the maximum score is 41, indicating that reporting standards were often inadequate.
Reporting of metadata in metabolomics datasets was assessed using CAWG-MSI (Table S1) [24]. Only three studies reported greater than 50% of the CAWG-MSI criteria [13,14,18]. Eight of the 13 included studies used a relative quantification of compounds [11,13,14,15,16,20,21,23], whilst five studies provided an absolute quantification of compounds [9,17,18,19,22]. In general, studies provided an adequate description of sample preparation, experimental analysis, and instrumental performance. No study provided an acceptable description of method validation. Three of the eight studies that analysed the relative quantification of metabolites were identified used internal standards [13,14,15], and five studies described methods used for assessing instrument variation [11,13,14,20,21]. Of the five studies that used absolute quantification, four did not report accuracy or precision validation data for their method on the instrument [9,17,19,22], while one study reported the limits of quantification and detection of the method [18]. Six studies provided a detailed description of data pre-processing [13,14,15,19,20,21]. Level one metabolite identification was reported in two studies [17,18]. Level two metabolite identification was reported in nine studies [9,11,13,14,15,16,19,20,23].
2.2. Urinary VOCs
A total of 48 cancer-associated urinary VOCs were reported within the 13 identified studies, with significant variation observed between different cancer types. VOCs belonged to 11 chemical classes (Figure 2). Five of the VOCs, 2,6-dimethyl-7-octen-2-ol, p-cresol, phenol, acetic acid, and dimethyl disulphide, were reported in more than one study as being associated with cancer (Table 3).
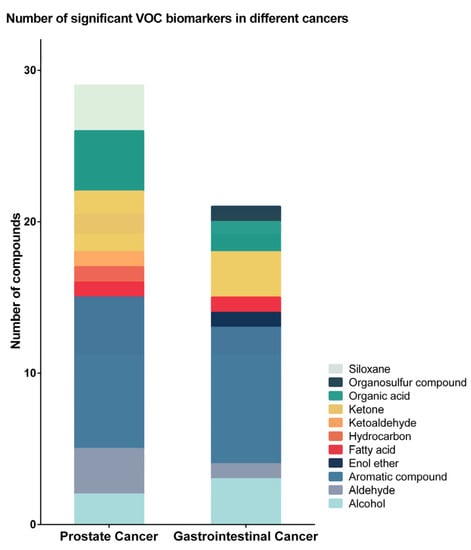
Figure 2.
Number of identified volatile organic compounds (VOCs) in different cancers. For prostate cancer, 29 urinary VOCs from nine chemical classes were identified, and the majority of them were aromatic compounds, ketones, and organic acids. For gastrointestinal cancer, 21 VOCs from eight chemical classes were identified, 19 of which were not identified in prostate cancer urine. The majority were aromatic compounds, alcohols, and ketones.

Table 3.
List of all volatile organic compounds (VOCs), their chemical class, and studies that identified them to be increased or decreased in cancers.
For prostate cancer, 29 urinary VOCs from nine chemical classes were identified (Figure 2 and Table 3). The majority of identified VOCs were aromatic compounds, ketones, and organic acids (Figure 2). Most VOCs showed decreased concentrations in the urine of prostate cancer patients compared to the urine of non-cancer patients. Alcohols, ketones, and organic acids were reported to have the largest decrease in concentration in prostate cancer. In comparison, aldehydes and siloxanes were identified to be increased in the urine of prostate cancer patients (Figure 3).
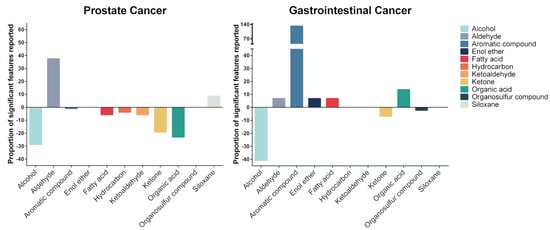
Figure 3.
Proportion of identified compound chemical classes in different cancers. Most prostate cancer-related VOCs showed decreased concentrations in the urine of prostate cancer patients compared to the urine of non-cancer patients. The majority of the gastrointestinal cancer-related VOCs showed increased concentrations, particularly of aromatic VOCs.
For gastrointestinal cancers (gastroesophageal, colorectal, and hepato-biliary), 21 urinary VOCs from eight chemical classes were identified, 19 of which were not identified in prostate cancer urine (Figure 2 and Table 3). The majority of the gastrointestinal cancer-related VOCs were aromatic compounds, alcohols, and ketones (Figure 2). Enol ether and organosulfur compounds were unique to the urine of gastrointestinal cancer patients. Compare to prostate cancer, the majority of the gastrointestinal cancer-related VOCs showed increased concentrations, particularly aromatic VOCs. Alcohols, ketones and organosulfur compounds were identified to be decreased in the urine of gastrointestinal cancer patients (Figure 3).
Six leukaemia and lymphoma-related urinary VOC biomarkers were reported by a single study [23]. These VOCs were identified to be increased in the urine of patients with these haematological malignancies, except for anisole, which was decreased in the urine of lymphoma patients. Formaldehyde were reported to be associated with bladder cancer by a single study [17]. No urinary VOC biomarker for lung cancer was reported. (Table 3).
2.3. Metabolic Analysis
Metabolic pathway analysis showed different levels of VOCs related to KEGG metabolic pathways that belonged to carbohydrate, lipid, amino acid, and energy metabolism. Glycolysis and the gluconeogenesis pathway had the most significant impact among metabolic pathways related to cancer specific urinary VOCs. Prostate cancer was associated with seven metabolic pathways categorised into carbohydrate, lipid, and amino acid metabolism. Gastrointestinal cancer was associated with six metabolic pathways belonging to the three pathway categories as well as the energy metabolism. Gastrointestinal cancer had a greater association with carbohydrate metabolism and energy metabolism compared to prostate cancer, suggesting different underlying metabolic profiles of these cancers. (Figure 4 and Table S2).
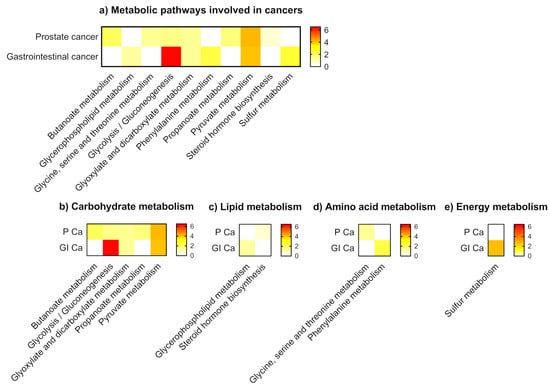
Figure 4.
Metabolic pathways involved in cancers. (a) All metabolic pathways (b) Carbohydrate metabolism (c) Lipid metabolism (d) Amino acid metabolism (e) Energy metabolism. NOTE: P Ca: prostate cancer; GI Ca: gastrointestinal cancer. Gastrointestinal cancer had a greater association with carbohydrate metabolism and energy metabolism compared to prostate cancer, suggesting different underlying metabolic profiles of these cancers.
3. Discussion
This systematic review provides an overview of the use of urinary VOCs for cancer diagnosis. The principal findings were a description of characteristic cancer associated urinary VOC biomarkers; promising diagnostic performance of urinary VOCs for the detection of prostate and gastrointestinal cancers; and a lack of standardisation in reported practices for urinary VOCs analysis.
Early detection is one of the most important factors influencing cancer survival. Many cancers, including those identified in this review, present with vague symptoms leading to a delay in their investigation and detection. Late diagnosis is associated with worse overall survival. For patients diagnosed with advanced (stage IV) prostate cancer, five-year survival is 49% compared to almost 100% for patients with early (stage I/II) disease [25]. Therefore, it is crucial to develop accurate, acceptable, and affordable methods for cancer early detection.
GC-MS was the most common analytical platform used for urinary VOC analysis, with multiple sampling techniques aiding pre-analysis VOC extraction. However, the majority of the studies failed to report adequate information concerning patient recruitment and study design, including strategies for mitigation of bias. No study performed an adequate validation of results. Three of the 13 studies validated their initial discoveries in independent cohorts [13,15,18]. Four studies applied internal standard normalisation to data [13,14,15,18], and two reported a use of quality control measures [13,14]. Therefore, future studies are needed to establish an “optimal” method for urinary VOC analysis. These studies should acknowledge the importance of standardisation and adoption of quality control measures to ensure accuracy and reproducibility.
The majority of studies identified by this review investigated urinary VOCs of prostate and gastrointestinal cancers. The wide variation in reported cancer-associated VOCs likely reflects diversity in the underlying tumour metabolic profiles and/or methodological variability secondary to the lack of standardised practices for urinary VOCs analysis. Protocols for the analysis of urinary metabolites have been published previously [26,27,28]. Whilst the current review was not intended to establish similar guidance, important considerations for the specific analysis of urinary VOCs are summarised in Table 4.

Table 4.
Considerations for analysis of urinary volatile organic compounds.
In general, both prostate and gastrointestinal cancer-specific VOCs had high sensitivity and specificity for cancer detection. As previously mentioned, a limited number of studies validated their findings in an independent dataset. In prostate cancer, the majority of cancer-specific VOCs were decreased compared to controls. In comparison, VOCs that were associated with gastrointestinal cancers tended to be found at elevated urinary levels. Identified VOCs originated from a wide variety of metabolic pathways, including carbohydrate metabolism, lipid metabolism, amino acid metabolism, and energy metabolism. Metabolic analysis derived provisional evidence that the metabolic pathways of both prostate and gastrointestinal cancer specific VOCs were different, with the latter being association more with carbohydrate metabolism and energy metabolism.
The origin of VOCs within the body and the mechanism by which they enter the urine remains incompletely understood. It is presumed that cancer-specific VOCs are of endogenous origin and produced as a result of abnormal metabolism either within the tumour itself or related tissues. VOCs released by tissues may travel in the systemic circulation from where they may be excreted via the lungs, skin, or renal tract. In the case of prostate cancer, there may also be local release directly from the prostate gland into urine. It has been hypothesised that the tumour-associated intestinal microbiome may also contribute to VOC production in gastrointestinal cancers. Further studies are needed to determine the underlying mechanisms of VOC production in cancer and the kinetics of their release.
This systematic review suffers from a number of limitations that principally concern the relatively small number of published studies within this field. A wide variation in the methodologies used by individual studies was observed, making it difficult to draw strong conclusions. Studies rarely utilised robust quality control strategies, and few studies validate findings within an independent patient cohort. Inadequate reporting of clinical parameters, including cancer stage, made it difficult to evaluate the performance of urinary VOC analysis on diagnosing early-stage cancer. For those studies that did report cancer stage, it was evident that the majority of enrolled patients had locally advanced disease. Therefore, observed metabolic differences can not been seen to truly represent “early” disease that is the ultimate target of the test. It should also be noted that the majority of the articles in this review originated from Europe and may therefore not be representative of other populations.
This systematic review summarises the progress of urinary VOC analysis for the diagnosis of cancer. Although there were variations in study quality, urinary VOC analysis exhibited promising performance for developing non-invasive diagnostic tools for cancer diagnosis and demonstrating metabolic profiling of different types of cancer. In order to develop future studies and translate their results to large clinical practice, the methodological weakness and limitations summarised in this review must be overcome.
4. Materials and Methods
4.1. Literature Search
A systematic online literature search was conducted to identify all studies that measured differences in urinary VOCs between cancer patients and relevant controls in accordance with the recommendations of the Cochrane library and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [29]. Databases that were searched included Medline (1946–13th December 2019) via OvidSP, Ovid Embase (1947–13th December 2019), and Cochrane Library. The following terms were used in the search strategy: urine, volatile organic compounds, biomarkers, metabolomics, metabolic profiling, magnetic resonance spectroscopy; mass spectrometry, and carcinoma. All variations in spelling including truncated search term using wild card characters and the “related articles” function were used in combination with the Boolean operators AND and OR. Full information of search strategy is provided as an online supplementary file. Reference lists of qualified articles were screened to include potentially relevant studies.
Two independent reviewers, Q.W. and P.B., screened the titles and abstracts of all studies identified through database searching. The full text of potentially relevant articles was reviewed for eligibility. Only original research articles published in the English language were considered. Included studies were those that identified potential VOC biomarkers of cancers by profiling the urine of patients and relevant controls (patients without cancer) using mass spectrometry based technologies. Studies were excluded if they did not report named VOC biomarkers or if they reported results from mixed cancer cohorts where the results of each subtype could not be clearly separated. Review articles, conference abstracts, articles not written in the English language, and animal and cell studies were excluded. A third reviewer (G.B.H.) was consulted when disagreement in study inclusion arose.
4.2. Outcome Measures
The following information from included articles was extracted and summarised: year of publication, country of origin, study design, recruitment period, number of participants, cancer type, analytical platform used, sampling technique methodology, quality control method(s), normalisation method(s), number of VOCs identified, identity of VOCs increased/decreased in cancer, method of statistical analysis (including prediction model used), sensitivity and specificity, and area under the receiver operating characteristic (AU-ROC) curve.
4.3. Quality Assessment
Quality of all the studies was assessed using 3 tools. The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool was used to assess the risk of bias and applicability of the study [30]. The QUADAS-2 was divided into risk of bias of patient selection, diagnostic test, reference standard and patient flow and timing. This test also investigated the applicability of patient selection, diagnostic test, and reference standard to the systematic review. The Standards for Reporting of Diagnostic Accuracy studies (STARD) tool was used to assess reporting quality of the study [31]. This tool evaluated all the sections including title, abstract, introduction, methods, results and discussion to provide a comprehensive figure of the completeness and transparency of reporting of diagnostic accuracy studies. The Chemical Analysis Working Group (CAWG)-Metabolomics Standard Initiative (MSI) criteria were used to assess the quality of metadata of the study [24]. CAWG-MSI proposed minimum reporting standards related to the chemical analysis aspects of metabolomics experiments including sample preparation, experimental analysis, quality control, metabolite identification, and data pre-processing.
4.4. Metabolic Analysis
All VOCs identified were checked and classed according to the Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway and the Human Metabolome Database (HMDB). Statistical analysis was performed using the pathway analysis module in MetaboAnalyst 4.0, which is based on the R programming language (version 3.5.3, The R Project for Statistical Computing, www.r-project.org). VOCs that were significantly increased or decreased in each study were selected. In the pathway analysis module, compound names were standardised against HMDB, KEGG, and PubChem to match well-annotated compounds in KEGG pathway libraries. Based on KEGG pathway libraries, parameters used to analyse data were a hypergeometric test for over representation analysis and a relative-betweeness centrality test for pathway topology analysis [32,33,34]. Normalisation was performed following an equation for weighted means of each identified VOC: the proportion of the total number of VOCs identified per study, divided by the total number of VOCs identified in each cancer type, then multiplied by the total number of studies in which this VOC was identified (Figure S1).
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-1989/11/1/17/s1, Search strategy, Prisma checklist, Figure S1: Equation for weighted means of each identified volatile organic compound (VOC), Table S1: Quality assessment of metabolic metadata based on CAWG-MSI guidelines, Table S2: List of biomarkers noted to be significantly increased and/or decreased in cancers and associated metabolic pathway.
Author Contributions
Q.W. and P.B. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualisation, Q.W. and P.B.; Acquisition, analysis, or interpretation of data: Q.W. and P.B.; Drafting of the manuscript: Q.W. and P.B.; Critical revision of the manuscript for important intellectual content: P.B., A.M., I.B. and G.B.H.; Study supervision: G.B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, Y.; Liu, Y.; Li, W.; Jin, Y.; Tang, Z.; Duan, Y. Investigation of potential breath biomarkers for the early diagnosis of breast cancer using gas chromatography-mass spectrometry. Clin. Chim. Acta 2014, 436, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Saalberg, Y.; Wolff, M. VOC breath biomarkers in lung cancer. Clin. Chim. Acta 2016, 459, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.F.; Di Lena, M.; Porcelli, F.; Trizio, L.; Travaglio, E.; Tutino, M.; Dragonieri, S.; Memeo, V.; de Gennaro, G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br. J. Surg. 2013, 100, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Huang, J.; Abbassi-Ghadi, N.; Mackenzie, H.A.; Veselkov, K.A.; Hoare, J.M.; Lovat, L.B.; Španěl, P.; Smith, D.; Hanna, G.B. Mass Spectrometric Analysis of Exhaled Breath for the Identification of Volatile Organic Compound Biomarkers in Esophageal and Gastric Adenocarcinoma. Ann. Surg. 2015, 262, 981–990. [Google Scholar] [CrossRef]
- Markar, S.R.; Brodie, B.; Chin, S.T.; Romano, A.; Spalding, D.; Hanna, G.B. Profile of exhaled-breath volatile organic compounds to diagnose pancreatic cancer. Br. J. Surg. 2018, 105, 1493–1500. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720. [Google Scholar] [CrossRef]
- Frank Kneepkens, C.M.; Lepage, G.; Roy, C.C. The potential of the hydrocarbon breath test as a measure of lipid peroxidation. Free Radic. Biol. Med. 1994, 17, 127–160. [Google Scholar] [CrossRef]
- Huang, J.; Kumar, S.; Abbassi-Ghadi, N.; Span?l, P.; Smith, D.; Hanna, G.B. Selected ion flow tube mass spectrometry analysis of volatile metabolites in urine headspace for the profiling of gastro-esophageal cancer. Anal. Chem. 2013, 85, 3409–3416. [Google Scholar] [CrossRef]
- Arasaradnam, R.; Wicaksono, A.; O’Brien, H.; Kocher, H.M.; Covington, J.A.; Crnogorac-Jurcevic, T. Non-invasive Diagnosis of Pancreatic Cancer Through Detection of Volatile Organic Compounds in Urine. Gastroenterology 2017, 154. [Google Scholar] [CrossRef]
- Khalid, T.; Aggio, R.; White, P.; De Lacy Costello, B.; Persad, R.; Al-Kateb, H.; Jones, P.; Probert, C.S.; Ratcliffe, N. Urinary Volatile Organic Compounds for the Detection of Prostate Cancer. PLoS ONE 2015, 10, e0143283. [Google Scholar] [CrossRef]
- Hanai, Y.; Shimono, K.; Matsumura, K.; Vachani, A.; Albelda, S.; Yamazaki, K.; Beauchamp, G.K.; Oka, H. Urinary volatile compounds as biomarkers for lung cancer. Biosci. Biotechnol. Biochem. 2012, 76, 679–684. [Google Scholar] [CrossRef]
- Lima, A.R.; Pinto, J.; Azevedo, A.I.; Barros-Silva, D.; Jerónimo, C.; Henrique, R.; de Lourdes Bastos, M.; Guedes de Pinho, P.; Carvalho, M. Identification of a biomarker panel for improvement of prostate cancer diagnosis by volatile metabolic profiling of urine. Br. J. Cancer 2019, 121, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Struck-Lewicka, W.; Kordalewska, M.; Bujak, R.; Yumba Mpanga, A.; Markuszewski, M.; Jacyna, J.; Matuszewski, M.; Kaliszan, R.; Markuszewski, M.J. Urine metabolic fingerprinting using LC–MS and GC–MS reveals metabolite changes in prostate cancer: A pilot study. J. Pharm. Biomed. Anal. 2015, 111, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Su, X.; Annabi, M.H.; Schreiter, B.R.; Prince, T.; Ackerman, A.; Morgas, S.; Mata, V.; Williams, H.; Lee, W.-Y. Application of Urinary Volatile Organic Compounds (VOCs) for the Diagnosis of Prostate Cancer. Clin. Genitourin. Cancer 2019. [Google Scholar] [CrossRef]
- Jiménez-Pacheco, A.; Salinero-Bachiller, M.; Iribar, M.C.; López-Luque, A.; Miján-Ortiz, J.L.; Peinado, J.M. Furan and p-xylene as candidate biomarkers for prostate cancer. Urol. Oncol. 2018, 36, e221–e243. [Google Scholar]
- Spanel, P.; Smith, D.; Holland, T.A.; Al Singary, W.; Elder, J.B. Analysis of formaldehyde in the headspace of urine from bladder and prostate cancer patients using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 1999, 13, 1354–1359. [Google Scholar]
- Chen, Y.; Zhang, J.; Guo, L.; Liu, L.; Wen, J.; Xu, L.; Yan, M.; Li, Z.; Zhang, X.; Nan, P.; et al. A characteristic biosignature for discrimination of gastric cancer from healthy population by high throughput GC-MS analysis. Oncotarget 2016, 7, 87496–87510. [Google Scholar] [CrossRef]
- Navaneethan, U.; Parsi, M.A.; Lourdusamy, D.; Grove, D.; Sanaka, M.R.; Hammel, J.P.; Vargo, J.J.; Dweik, R.A. Volatile Organic Compounds in Urine for Noninvasive Diagnosis of Malignant Biliary Strictures: A Pilot Study. Dig. Dis. Sci. 2015, 60, 2150–2157. [Google Scholar]
- Panebianco, C.; Kelman, E.; Vene, K.; Gioffreda, D.; Tavano, F.; Vilu, R.; Terracciano, F.; Pata, I.; Adamberg, K.; Andriulli, A.; et al. Cancer sniffer dogs: How can we translate this peculiarity in laboratory medicine? Results of a pilot study on gastrointestinal cancers. Clin. Chem. Lab. Med. 2017, 56. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; McFarlane, M.J.; Ryan-Fisher, C.; Westenbrink, E.; Hodges, P.; Hodges, P.; Thomas, M.G.; Chambers, S.; O’Connell, N.; Bailey, C.; et al. Detection of colorectal cancer (CRC) by urinary volatile organic compound analysis. PLoS ONE 2014, 9, e108750. [Google Scholar] [CrossRef] [PubMed]
- Rozhentsov, A.; Koptina, A.; Mitrakov, A.A.; Sharipova, T.; Tsapaev, I.; Ryzhkov, V.L.; Lychagin, K.A.; Furina, R.R.; Mitrakova, N.N. A New Method to Diagnose Cancer Based on Image Analysis of Mass Chromatograms of Volatile Organic Compounds in Urine. Sovrem. Tehnol. Med. 2014, 6, 151–157. [Google Scholar]
- Silva, C.L.; Passos, M.; Câmara, J.S. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br. J. Cancer 2011, 105, 1894–1904. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metab. Off. J. Metab. Soc. 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Cancer Research UK prostate cancer survival statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/survival#heading-Three (accessed on 28 September 2020).
- Want, E.J.; Wilson, I.D.; Gika, H.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Holmes, E.; Nicholson, J.K. Global metabolic profiling procedures for urine using UPLC–MS. Nat. Protoc. 2010, 5, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Chan, E.C.Y.; Pasikanti, K.K.; Nicholson, J.K. Global urinary metabolic profiling procedures using gas chromatography–mass spectrometry. Nat. Protoc. 2011, 6, 1483. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shojaie, A.; Michailidis, G. A comparative study of topology-based pathway enrichment analysis methods. BMC Bioinform. 2019, 20, 546. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).