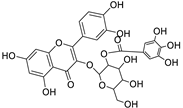
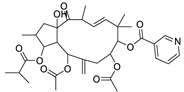
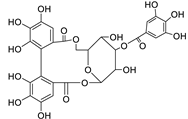
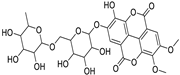
Abstract
Euphorbia is a large genus of flowering plants with a great diversity in metabolic pattern. Testing the cytotoxic potential of fifteen Euphorbia species revealed highest activity of E. officinarum L. against human colon adenocarcinoma (CACO2) cell line (IC50 7.2 µM) and of E. lactea Haw. against human hepatoma (HepG2) and human breast adenocarcinoma (MCF-7) cell lines (IC50 5.2 and 5.1 µM, respectively). Additionally, metabolic profiling of the fifteen tested species, using LC-HRMS, for dereplication purposes, led to the annotation of 44 natural compounds. Among the annotated compounds, diterpenoids represent the major class. Dereplication approach and multivariate data analysis are adopted in order to annotate the compounds responsible for the detected cytotoxic activity. Results of Principle component analysis (PCA) come in a great accordance with results of biological testing, which emphasized the cytotoxic properties of E. lactea Haw. A similarity correlation network showed that the two compounds with the molecular formula C16H18O8 and C20H30O10, are responsible for cytotoxic activity against MCF-7 and HepG2 cell lines. Similarly, the compound with molecular formula C18H35NO correlates with cytotoxic activity against CACO2.
1. Introduction
Cancer represents one of the most lethal diseases worldwide. Cancer treatments have adverse effects. Moreover, not all tumors react in the same way to the treatment. Natural products are considered as a promising approach to cancer control and management [1]. Several studies investigated the cytotoxic potential of phytoconstituents against variable cancer cell lines [2].
Genus Euphorbia belongs to family Euphorbiaceae, spurge family, which is composed of about 50 tribes, 300 genera, and 8000 species [3]. Euphorbia is the third largest genus of flowering plants, only after Astragalus (Fabaceae) and Psychotria (Rubiaceae) with approximately 2160 species [4]. Members of this genus are characterized by the production of a milky irritant latex which is exuded when they are injured. Euphorbia L. are widely distributed throughout both tropical and temperate regions and range in morphology from small, annual or perennial herbaceous plants to woody shrubs, trees and even large desert succulents [3,5].
Different Euphorbia species are used traditionally for the treatment of digestive system disorders as diarrhea, jaundice, constipation, colic and indigestion [4]. Furthermore, they are also used for the treatment of skin diseases, gonorrhea, migraines, intestinal parasites, inhibition of HIV-1 viral infection, warts and for mediating pain due to their antipyretic and analgesic activity [5].
Euphorbia exhibited a wide variety of compounds with diverse pharmaceutical activities. Diterpenes, triterpenes, steroids, phenolics and flavonoids are among secondary metabolites isolated from genus Euphorbia [5]. In the past few years, many studies have been performed on the cytotoxic activity of Euphorbia diterpenes as they proved to have moderate or strong anti-proliferative activity due to the lactone structures. Euphorbia diterpenes also reported to own potent antineoplastic activity towards various cancer cell lines (e.g., chronic myeloid leukemia and nasopharyngeal, pancreatic, lung, ovarian, and colon carcinomas) [3].
Plants have a great challenge in metabolomics due to the high chemical and physical diversity of their metabolites [6]. Furthermore, Metabolomics is being applied to identify and biotechnologically optimize the production of pharmacologically active secondary metabolites [7]. In this framework, liquid-chromatography coupled to high resolution mass spectrometry (LC-HRMS) is performed and, by untargeted data-dependent MS/MS experiments, much information on the chemical composition of crude extracts can be created [8]. This interesting task cannot be achieved by a single analytical technique rather several analytical platforms are needed [9]. The dereplication approach and multivariate data analysis are used in order to identify compounds in a mixture responsible for the anti-proliferative effects of plant extracts, and provide a better understanding of the mechanisms of action of medicinal plants [10]. Principle component analysis (PCA) is an unsupervised clustering method requiring no knowledge of the dataset and acts to reduce the dimensionality of multivariate data while preserving most of the variance within [11].
Consequently, this study is designed to investigate the cytotoxic potential of fifteen Euphorbia species against three cancer cell lines, i.e., HepG2 (human hepatoma), MCF-7 (Human breast adenocarcinoma), and CACO2 (human colon adenocarcinoma) cells. Moreover, metabolic profiling tools and dereplication processes are adopted to investigate the differences in secondary metabolite pattern between the 15 species and annotate the compounds responsible for the tested anti-proliferative activity of the tested Euphorbia species
2. Results
2.1. Cytotoxic Activity
The cytotoxic activity of fifteen Euphorbia species against three cancer cell lines was evaluated (Table 1). Results reveal that five Euphorbia species display activity against HepG2 where E. lactea Haw. and E. obesa Hook. are the most active (IC50 5.2 and 6.3 µg/mL, respectively). Moreover, five species are active against MCF-7 where E. lactea Haw. and E. grandialata R.A. Dyer exhibit highest activity (IC50 5.1 and 7.5 µg/mL, respectively). On the other hand, eight Euphorbia species show cytotoxic activity against CACO2 where E. officinarum L. and E. royleana Boiss. are the most active (IC50 7.2 and 9.1 µg/mL, respectively). Among fifteen Euphorbia, three species, E. tirucalli L., E. horrida Boiss., and E. ingens E. Mey. are inactive against the tested cell lines.

Table 1.
IC50 µM values of methanolic Euphorbia extracts in different cancer cell lines.
2.2. LC-HR/MS Analysis
Metabolic profiling of 15 Euphorbia species by LC-HR-MS for dereplication purposes has resulted in the characterization of a variety of metabolites, of which diterpenes are predominant. The dereplication study of the metabolites (Table 2) using the Dictionary of Natural Products (DNP) database followed by chemotaxonomic filtration resulted to the characterization of 44 natural compounds from the 15 studied Euphorbia species. The annotated compounds can be classified into: diterpenoids (21 compounds), sterols (seven compounds), triterpenoids (four compounds), flavone glycosides (four compounds), tannins (three compounds), sesquiterpenoids (two compounds), alkaloid (one compound), acetophenone glycosides (one compound), and isoquinoline-carboxylic acid (one compound). As illustrated in (Figure 1), diterpenoids represent the most predominant chemical class in the tested species.

Table 2.
List of secondary metabolites isolated from fifteen Euphorbia species.
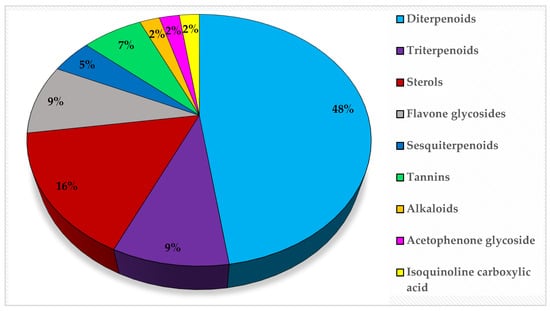
Figure 1.
Percentage of different classes of metabolites distributed in the tested 15 Euphorbia species.
2.3. Metabolic and Molecular Correlations Analysis
To provide a holistic coverage of Euphorbia’s metabolic profile, the crude extracts of the tested species were analyzed in both positive and negative ion electrospray ionization (ESI) MS modes as changes in ESI polarity can often hinder competitive ionization and suppression effects, revealing otherwise suppressed metabolite signals. This resulted in approximately 3000 molecular ions of both polarities. Principle component analysis (PCA) was applied to the dataset to explore the relative variability and/or similarity of the chemical profiles among the tested species. PCA score plot (Figure 2A) exhibited a respective total variance of 48% and 16% for PC1 and PC2, and highlighted the outlying of EU2, EU9, and EU13 corresponding to the crude extracts of E. caput-medusae L., E. horrida Boiss. and E. lactea Haw., respectively. The dispersal of the former extracts revealed for their unique chemical profiles which led to probe the metabolites contributed to such segregation. PCA loading plot (Figure 2B) demonstrated the discriminatory molecules at m/z (retention time in minutes) 592.268 [M+] (tR 29.13) characteristic for E. caput-medusae L. and E. horrida Boiss., while E. lactea Haw. was characterized by 402.225 [M+] (tR 24.58). The molecular correlation network uses Pearson correlation coefficient to detect molecules highly correlated with bioactivities (represented by percentage of inhibition against cancer cells). Features (molecules) are connected by edges (correlation values) where an edge’s width is corresponding to the strength of this correlation [12]. The threshold of Pearson correlation coefficient was set to 0.8 and a network of metabolites linked to the bioactivity is depicted in (Figure 3A). The network is mapped so nodes are colored in pie chart according to their concentration in the tested species and labeled with feature’s molecular weight. An extracted network of the molecules directly linked to the bioactivity and their neighbor ions (Figure 3B), showed metabolites at m/z [M+] (retention time in minutes) 338.100 (9.40) and 430.184 (10.94) are highly correlated with MCF-7 activity. In addition, the other molecule directly linked to HPEG2 activity at m/z 503.506 [M+] (tR = 29.66), was not reported before, that refers to a new chemical structure still to be discovered. The network did not detect any molecules correlated with CACO2 cytotoxic activity, even though the threshold was decreased. To highlight the molecules could be contributed to the cytotoxic activity against CACO2 cancer cell line, an OPLS-DA (orthogonal partial least square discrimination analysis) module was created. OPLS-DA was validated by permutation test. The test showed the original R2 and Q2 values were more than the permuted values and the cumulative value of Q2 is −0.08 (less than zero) which is indicative for the good prediction ability of the model. Discriminatory elements were confirmed by descriptive statistics, i.e., p-value<0.05, coefficient of variation (95% confidence limits do not cross zero), and variable importance (VIP values >1). The score plot (Figure 4A) demonstrated a clear separation between the active and inactive extracts with a strong goodness of fit R2 = 0.96 and a goodness of prediction Q2 = 0.46. The S-loading plot (Figure 4B) is a very useful tool to compare the variable magnitude against its reliability, where axes plotted from the predictive components are the covariance p against the correlation p(cor). The molecules highly correlated with the CACO2 activity were checked and only those with high coefficient of variation, whose 95% confidence level limits not crossing zero, were chosen. The significant metabolite highly correlated with the CACO2 cytotoxicity was at m/z [M]+ 281.272 (tR = 29.16).
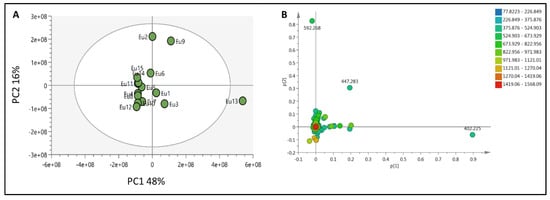
Figure 2.
(A): PCA score plot for all crude extracts of the tested Euphorbia species, (B): PCA loading plot showing the distinctive metabolites.
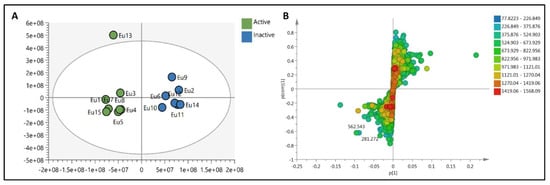
Figure 3.
(A): The whole molecular network showing the clusters of different features. (B): extracted network showing the metabolites highly correlated to the cytotoxic activity against MCF-7 and HPEG2. Nodes are colored in pie chart and molecules grouped according to their concentration in the tested species. Edges are correlation values and the edge’s width is proportional with this value.
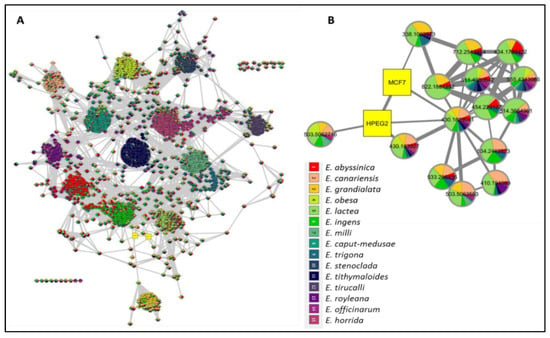
Figure 4.
(A): OPLS-DA score plot showing a clear separation between (●) CACO2 cytotoxic extracts and (●) inactive ones, (B): S-loading plot showing the metabolites highly correlated with CACO2 cytotoxicity.
3. Discussion
3.1. Cytotoxic Activity
However, the anticancer activity of E. lactea Haw. remains largely unexplored, its methanolic extract exhibits high activity against both HepG2 and MCF-7 cell lines (IC50 5.2 and 5.1 µM, respectively). Previous studies tested the ethanolic extract of E. lactea Haw. against another hepatic cancer cell line HEp-2 and the IC50 was found to be 89 µg/mL [13]. In addition, it was reported that the hydro-alcoholic extract of E. lactea Haw. exhibited cytotoxic and anti-migratory activities toward HN22 cells [14]. Additionally, E. officinarum L. and E. royleana Boiss. are the most active against CACO2. Interestingly, the cytotoxicity of E. officinarum L. hasn’t tested before on any cancer cell lines, and the cytotoxic activity of the hexane extract of E. royleana Boiss. was only studied on a potato disc and found to be 61.66% at 800 µg/mL [15]. Current results also show mild cytotoxic activity of E. trigona Mill. extract against both MCF-7 and CACO2. The latex of E. trigona Mill. was previously tested on HT-29 (colon cancer cell line) and found to be inactive [16]. Notably, among the fifteen tested extracts, three species, namely E. tirucalli L., E. horrida Boiss., and E. ingens E. Mey., are inactive against the three tested cell lines. However, previous studies reported high cytotoxic activity of the butanol extract of E. tirucalli L. against MCF-7 [17] as well as moderate activity of high concentration of aqueous extract of the same species (100–150 µg/mL) against human leukocytes [18].
3.2. LC-HR/MS Analysis
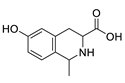
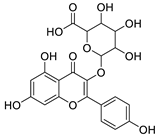
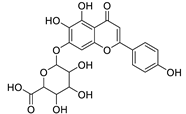
The distribution of the compounds in the 15 Euphorbia species reveals the presence of different chemical classes such as terpenoids, sterols, flavonoids and tannins and all of these classes were reported previously in Euphorbia species [5,19]. Also, Milliamine J alkaloid (44) is detected only in E. milli Des Moul. and this was reported before [20]. Additionally, l-methyl-6-hydroxy-l,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (1) and the acetophenone ebractelatinoside C (19) record the highest concentrations in the same species.
Basically, diterpenoids, such as jatrophanes, lathyranes, tiglianes, ingenanes, myrsinols nucleus, and those with oxygen-containing functionalities, are the majority among secondary metabolites of the genus Euphorbia. About 400 diterpenoids have been isolated from different Euphorbia species [21]. This comes in great accordance with our results that detect diterpenes as the main class of constituents in all tested Euphorbia species, where four different nuclei, i.e., lathyrane (10 compounds), tigliane (5 compounds), inginane (4 compounds), and jatrophane (2 compounds), are detected. Furthermore, inginane nucleus represents the highest concentration compared to other nuclei where compound (21) ingenol-3-angelate-5,20-diacetate is detected in high concentration especially in E. abyssinica J.F. Gmel., followed by compound (25), 17-hydroxyingenol-17-benzoate-20-angelate, that is highly represented in E. horrida Boiss. Similarly, a study of the diterpenoid ester content of E. cupanii, using liquid chromatography coupled to tandem mass spectrometry and molecular networking coupled to unsupervised substructure annotation (MS2LDA) was recently published and showed the presence of premyrsinane/myrsinane diterpene esters [22].
Herein, the sesquiterpene supinaionoside A (3), is the leading among other annonated sesquiterpenes and is detected in E. abyssinica J.F. Gmel. for the first time. On the other hand, triterpenes have been frequently reported in Euphorbia species [5]. Among the detected triterpenoids, euphorbiane (6) and canaric acid (9) exist in high concentration in E. milli Des Moul.
Considering sterol content, 24-hydroperoxytirucalla-8,25-dien-3β-ol-7-one (18) is recorded in the highest concentration in E. lactea Haw. and E. ingens E. Mey. Also, many sterols were identified in E. ingens [23]. Regarding flavonoids, rhamnetin-3-α-arabinofuranoside (12) is the main flavonoid detected and recorded in high concentration in almost all the fifteen tested species, a result that agrees with previous report about the prevalence of rhamnetin glycosides within the Euphorbiaceae [24]. In the same context, recent LC-DAD-MSn fingerprint of the phenolics of E. hirta, E. heterophylla and E. convolvuloides concluded the presence of flavonoids, coumarins and phenolic acids [25]. Tannins, specifically; helioscopinin B (38) and 3,3’,4-tri-O-methyl-4’-O-rutinosyl-ellagic acid (42) are detected in high concentration in E. stenoclada Baill. and E. abyssinica J.F. Gmel., respectively.
3.3. Metabolic and Molecular Correlations Analysis
The dereplication study revealed that molecule at m/z [M+] 592.268 is corresponding to molecular formula (C35H36N4O5) which may be phaeophorbide A that is a chlorophyll degradation product formed by enzymic hydrolysis of phaeophytin A by chlorophyllase. More interestingly, signal at m/z 402.225 is equivalent to molecular formula C20H34O8 that may correspond to either taxane or grayanane type of diterpenoid molecule i.e., pierisformosoid which was reported in the literature for its cytotoxic, analgesic and antifeedant properties [71,72]. The PCA and dereplication results matched with the investigated biological activities of the Euphorbia’s crude extracts which emphasized the cytotoxic properties of E. lactea Haw. The biological investigations revealed the cytotoxic activities of E. lactea Haw. against HPEG2 and MCF-7 cancer cell lines and E. officinarum L. against CACO2 cell line. To pinpoint the molecules mediated for such bioactivities, a similarity correlation network was implemented. The molecules 338.100 (9.40) and 430.184 (10.94) that correlated with MCF-7 were equivalent to molecular formula C16H18O8 and C20H30O10 respectively. In addition, the molecule at 430.184 was further correlated to the cytotoxic activity against HPEG2. Moreover, the correlated metabolite with the CACO2 cytotoxicity 281.272 (tR = 29.16) that was equivalent to C18H35NO. More amazingly, all these molecular formulae not reported to be isolated previously from genus Euphorbia and need further investigation. In summary, metabolomics was a powerful tool that gave a shorter access to the bioactive metabolites could mediate for the demonstrated cytotoxicity of Euphorbia’s extracts against MCF-7, HPEG2 and CACO2 cancer cell line. Moreover, the dereplication study relied on a precise molecular formula prediction along with 1HNMR to minimize the number of hits per each molecular formula, leading to the tentative identification of the top hits [73].
4. Materials and Methods
4.1. Plant Material
The aerial non-flowering parts of fifteen Euphorbia species were collected during October 2018 from Helal Cactus farm, Abdel Samad village, El-Mansuriya, Giza, Egypt. All the collected species consisted mainly of stems and leaves. The species were identified and authenticated by Prof. Dr. Abdel-Halim Mohammed; Professor of Agriculture, Flora department, Agricultural museum, Dokki, Giza, Egypt. Fresh plants were cut into small pieces and directly macerated in methanol 80%.
4.2. Methanolic Extracts for Metabolic and Cytotoxic Studies
Methanolic extracts were prepared by maceration of 50 g fresh samples in (3 × 200 mL) 80% methanol (Sigma-Aldrich, Darmstadt, Germany). The extracts were separately filtered and evaporated to dryness using rotatory evaporator. Dried extracts were stored at 4 °C.
4.3. Cytotoxic Activity
Cytotoxicity of the methanolic extracts was evaluated in cell lines using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [74,75]. HepG2 (human hepatoma), MCF-7 (Human breast adenocarcinoma), and CACO2 (human colon adenocarcinoma) cells were maintained in RPMI medium (Merck, Darmstadt, Germany), supplemented with 10% fetal bovine serum (FBS). Cancer cells were cultured at 37 °C, 5% (v/v) CO2 in RPMI1640 medium, supplemented with 5% (v/v) fetal bovine serum (FBS), 1% (w/v) L-glutamine, 1% sodium pyruvate and 0.4% (w/v) antibiotics (50 U/mL penicillin, 50 mg/mL streptomycin). Cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA; HPACC, Salisbury, UK) and routinely sub-cultured twice per week. Alcoholic extracts were dissolved in DMSO at a concentration of 0.05 g/0.5 mL as a stock solution and filtered to remove any particulate matter. Further dilutions were made in culture medium. DMSO used for the assay was of ACS reagent grade from Sigma Aldrich (Darmstadt, Germany). The water used was reverse osmosis water purified using a Millipore cartridge filter. The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide) substance, and all the other reagents and substances were obtained commercially (Sigma Aldrich, USA). The glass vials (2 mL) utilized were Fisher-brand with Titeseal closure (Fisher Scientific). To normalize cell viability values, each plate included a triplicate of cells treated with the various methanolic extracts carrier on DMSO to define 100% viable cells as well as a triplicate of cells incubated with a cytotoxic mixture (200 ng/mL Tumor Necrosis Factor TNF, 200 ng/mL CD95L (Fas ligand), 200 ng/mL TRAIL (TNF-related apoptosis-inducing ligand), 25 g/mL CHX (Cycloheximide), 1% (w/v) sodium azide) to define maximal cell death and thus 0% viability. The viable cells produced a dark blue formazan product, whereas no such staining was formed in the dead cells. All samples were transferred to a 96-well plate and absorbance was measured at 570 nm using a SpectraMax plus Microplate Reader (Molecular Devices, CA, USA). The cell viability was expressed relative to the untreated control cells. All other viability values were normalized according to the averages of these triplicates and analyzed by the Graph Pad Prism 5 software (La Jolla, CA, USA), 50%. 5-Flurouracil was used as a positive control.
4.4. LC-HR/MS Analysis
One mg of each extract (of the fifteen species) was weighted using sensitive electric balance (Sartorius, type 1712, Germany) and dissolved in one mL HPLC grade methanol then it was analyzed on an Acquity Ultra Performance Liquid Chromatography system coupled to a Synapt G2 HDMS quadrupole time-of-flight hybrid mass spectrometer (Waters, Milford, CT, USA). The HPLC column was an ACE (ACE, Mainz, Germany) C18, 75 mm × 3.0 mm, 5 µm column. The mobile phase consisted of HPLC grade water (A) that was obtained in-house from a direct Q-3 water purification system (Millipore, Watford, UK) and acetonitrile (B) with 0.1% formic acid in each solvent. All reagents were of analytical grade and were purchased (Fisher Scientific, Hemel Hempstead, UK). The gradient program started with 10% B linearly increased to 100% B at a flow rate of 300 µL/min for 30 min and remained isocratic for 5 min before linearly decreasing back to 10% B in 1 min. The column was then re-equilibrated with 10% B for 9 min before the next injection. The total analysis time for each sample was 45 min. The injection volume was 10 µL, and the tray temperature was maintained at 12 °C. High resolution mass spectrometry was carried out in both positive and negative ESI ionization modes with a spray voltage at 4.5 kV and capillary temperature at 320 °C. The mass range was set from m/z 150–1500. Both negative and positive ionization switch modes were used to include the highest number of metabolites from the investigated methanol extracts subjected to LC–HR-ESIMS analysis. The dereplication was achieved for each m/z ion peak with metabolites recorded in the customized databases based on established parameters (m/z threshold of ±3 ppm and retention time) [76], which provided a high level of confidence in metabolites identity. Consequently, the number of the remaining unknown metabolites in each species was refine. The raw data were processed, aligned, and merged into one dataset according to the method previously developed in our lab [77,78].
5. Conclusions
The present study highlighted the cytotoxic activity and metabolic profiling of fifteen Euphorbia species where E. lactea Haw. shows the highest cytotoxic activity against HepG2 and MCF-7 (IC50 5.2 and 5.1, respectively). On the other hand, E. officinarum L. is the most active against CACO2 IC50 7.2. The molecular interaction network is implemented in order to correlate the chemical and biological profiles. Interestingly, molecule detected at m/z 503.506 [M+] (tR = 29.66), directly linked to HepG2 activity, was not reported before, suggesting that a new chemical structure still to be discovered. Also, the molecular correlations analysis reveals for the unique chemical profiles of E. caput-medusae L., E. horrida Boiss., and E. lactea Haw. where means of 592.268 [M+] (tR 29.13) is characteristic for E. caput-medusae L. and E. horrida Boiss. while 402.225 [M+] (tR 24.58) is characterized for E. lactea Haw.
Author Contributions
Conceptualization, S.S.E.-H.; data curation, N.M.L. and E.A.; formal analysis, N.M.L. and M.N.A.; funding acquisition, N.M.L. and E.A.; investigation, N.M.L.; methodology, data processing, metabolomics and chemometric studies, A.F.T. and U.R.A.; project administration, R.M. and E.A.; resources, N.M.L. and E.A.; Software, U.R.A.; supervision, S.S.E.-H. and S.F.A.; validation, A.F.T. and U.R.A.; visualization, R.M. and S.F.A.; writing—original draft, N.M.L. and A.F.T.; writing—review & editing, E.A., U.R.A. and A.F.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We would like to thank Nahda University for support. We thank M. Müller and M. Krischscke for LC-MS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karikas, G.A. Anticancer and chemopreventing natural products: Some biochemical and therapeutic aspects. J. BUON 2010, 15, 627–638. [Google Scholar] [PubMed]
- Hücre, B.T.; Aktiviteleri, H.S. Cytotoxic Activities of Certain Medicinal Plants on Different Cancer Cell Lines. Turk. J. Pharm. Sci. 2017, 14, 222–230. [Google Scholar]
- Vasas, A.; Hohmann, J. Euphorbia Diterpenes: Isolation, Structure, Biological Activity, and Synthesis (2008−2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Grace, O.M.; Saslis-Lagoudakis, C.H.; Nilsson, N.; Simonsen, H.T.; Rønsted, N. Global medicinal (Euphorbiaceae) uses of Euphorbia L. J. Ethnopharmacol. 2015, 176, 90–101. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, Y.; Ding, A.; You, F.; Zhang, L.; Duan, J. 13C-NMR Data of Three Important Diterpenes Isolated from Euphorbia Species. Molecules 2009, 14, 4454–4475. [Google Scholar] [CrossRef]
- Gomaa, A.A.R.; Samy, M.N.; Abdelmohsen, U.R.; Krischke, M.; Mueller, M.J.; Wanas, A.S.; Desoukey, S.Y.; Kamel, M.S. Metabolomic profiling and anti-infective potential of Zinnia elegans and Gazania rigens (Family Asteraceae). Nat. Prod. Res. 2018, 34, 1–4. [Google Scholar] [CrossRef]
- Tawfike, A.F.; Viegelmann, C.; Edrada-Ebel, R. Metabolomics and dereplication strategies in natural products. In Metabolomics Tools for Natural Product Discovery; Humana Press: Totowa, NJ, USA, 2013; pp. 227–244. [Google Scholar]
- Allard, P.M.; Péresse, T.; Bisson, J.; Gindro, K.; Marcourt, L.; Pham, V.C.; Roussi, F.; Litaudon, M.; Wolfender, J.L. Integration of molecular networking and in-silico MS/MS fragmentation for natural products dereplication. Anal. Chem. 2016, 88, 3317–3323. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrum. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Chassagne, F.; Haddad, M.; Amiel, A.; Phakeovilay, C.; Manithip, C.; Bourdy, G.; Deharo, E.; Marti, G. A metabolomic approach to identify anti-hepatocarcinogenic compounds from plants used traditionally in the treatment of liver diseases. Fitoterapia 2018, 127, 226–236. [Google Scholar] [CrossRef]
- Farag, M.A.; Tawfike, A.F.; Donia, M.S.; Ehrlich, A.; Wessjohann, L.A. Influence of Pickling Process on Allium cepa and Citrus limon Metabolome as Determined via Mass Spectrometry-Based Metabolomics. Molecules 2019, 24, 928. [Google Scholar] [CrossRef]
- Tawfike, A.F.; Romli, M.; Clements, C.; Abbott, G.; Young, L.; Schumacher, M.; Diederich, M.; Farag, M.; Edrada-Ebel, R. Isolation of anticancer and anti-trypanosome secondary metabolites from the endophytic fungus Aspergillus flocculus via bioactivity guided isolation and MS based metabolomics. J. Chromatogr. B 2019, 1106, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Whelan, L.C.; Ryan, M.F. Ethanolic extracts of Euphorbia and other ethnobotanical species as inhibitors of human tumour cell growth. Phytomedicine 2003, 10, 53–58. [Google Scholar] [CrossRef]
- Wongprayoon, P.; Charoensuksai, P. Cytotoxic and anti-migratory activities from hydroalcoholic extract of Euphorbia lactea Haw. Against HN22 cell line. Thai J. Pharm. Sci. 2018, 13, 69–77. [Google Scholar]
- Ashraf, A.; Sarfraz, R.A.; Rashid, M.A.; Shahid, M. Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an important medicinal plant (Euphorbia royleana) from Pakistan. J. Food Drug Anal. 2015, 23, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.; Quirós, L.M.; Castañón, S. Purification and partial characterization of a ribosome-inactivating protein from the latex of Euphorbia trigona Miller with cytotoxic activity toward human cancer cell lines. Phytomedicine 2015, 22, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Choene, M.; Motadi, L. Validation of the antiproliferative effects of Euphorbia tirucalli extracts in breast cancer cell lines. Mol. Biol. 2016, 50, 98–110. [Google Scholar] [CrossRef]
- Waczuk, E.P.; Kamdem, J.P.; Abolaji, A.O.; Meinerz, D.F.; Bueno, D.C.; do Nascimento Gonzaga, T.K.; do Canto Dorow, T.S.; Boligon, A.A.; Athayde, M.L.; da Rocha, J.B.T.; et al. Euphorbia tirucalli aqueous extract induces cytotoxicity, genotoxicity and changes in antioxidant gene expression in human leukocytes. Toxicol. Res. 2015, 4, 739–748. [Google Scholar] [CrossRef]
- Vasas, A. Isolation and Structure Elucidation of Diterpenes from Hungarian Euphorbia Species. Ph.D. Thesis, University of Szeged, Szeged, Hungary, 2006. [Google Scholar]
- Zani, C.L.; Marston, A.; Hamburger, M.; Hostettmann, K. Molluscicidal milliamines from Euphorbia milii var. hislopii. Phytochemistry 1993, 34, 89–95. [Google Scholar] [CrossRef]
- Avila, L.; Perez, M.; Sanchez-Duffhues, G.; Hernández-Galán, R.; Muñoz, E.; Cabezas, F.; Echeverri, F. Effects of diterpenes from latex of Euphorbia lactea Haw. and Euphorbia laurifolia on human immunodeficiency virus type 1 reactivation. Phytochemistry 2010, 71, 243–248. [Google Scholar] [CrossRef]
- Nothias-Esposito, M.; Nothias, L.F.; Da Silva, R.R.; Retailleau, P.; Zhang, Z.; Leyssen, P.; Roussi, F.; Touboul, D.; Paolini, J.; Dorrestein, P.C. Investigation of Premyrsinane and Myrsinane Esters in Euphorbia cupanii and Euphobia pithyusa with MS2LDA and Combinatorial Molecular Network Annotation Propagation. J. Nat. Prod. 2019, 82, 1459–1470. [Google Scholar] [CrossRef]
- Nielsen, P.E.; Nishimura, H.; Liang, Y.; Calvin, M. Steroids from Euphorbia and other latex-bearing plants. Phytochemistry 1979, 18, 103–104. [Google Scholar] [CrossRef]
- Seigler, D.S. Phytochemistry and systematics of the Euphorbiaceae. Ann. Missouri Bot. Gard. 1994, 81, 380–401. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Dall’Acqua, S.; Sinan, K.I.; Sut, S.; Ferrarese, I.; Etienne, O.K.; Sadeer, N.B.; Ak, G.; Zengin, G. Phenolic compounds analysis of three Euphorbia species by LC-DAD-MSn and their biological properties. J. Pharm. Biomed. Anal. 2020, 189, 113477. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Schütte, H.R. 1-Methyl-6-hydroxy-1.2.3.4-tetrahydroisochinolin-3-carbonsäure im Milchsaft von Euphorbia myrsinites L. Z. Naturforsch. B 1968, 23, 491–493. [Google Scholar] [CrossRef]
- Fürstenberger, G.; Hecker, E. On the Active Principles of the Spurge Family (Euphorbiaceae) XI. [1] the Skin Irritant and Tumor Promoting Diterpene Esters of Euphorbia tirucalli L. Originating from South Africa. Z. Naturforsch. C 1985, 40, 631–646. [Google Scholar] [CrossRef]
- Cai, W.H.; Matsunami, K.; Otsuka, H. Supinaionosides A and B: Megastigmane Glucosides and Supinanitrilosides A—F: Hydroxynitrile Glucosides from the Whole Plants of Euphorbia supina. Chem. Pharm. Bull. 2009, 57, 840–845. [Google Scholar] [CrossRef]
- Fattorusso, E.; Lanzotti, V.; Taglialatela-Scafati, O.; Tron, G.C.; Appendino, G. Bisnorsesquiterpenoids from Euphorbia resinifera Berg. and an expeditious procedure to obtain resiniferatoxin from its fresh latex. Eur. J. Org. Chem. 2002, 1, 71–78. [Google Scholar] [CrossRef]
- Khan, A.Q.; Rasheed, T.; Kazmi, S.N.U.H.; Ahmed, Z.; Malik, A. Cycloeuphordenol, a new triterpene from Euphorbia tirucalli. Phytochemistry 1988, 27, 2279–2281. [Google Scholar] [CrossRef]
- Yu, H.J.; Shen, C.C.; Yi, H.M.; Chen, T.H.; Hsueh, M.L.; Lin, C.C.; Don, M.J. Euphorbiane: A novel triterpenoid with an unprecedented skeleton from Euphorbia tirucalli. J. Chin. Chem. Soc. 2013, 60, 191–194. [Google Scholar] [CrossRef]
- Afza, N.; Malik, A.; Siddiqui, S. Isolation and structure of cycloeuphornol, a new triterpene from Euphorbia tirucalli. Pak. J. Sci. Ind. Res. 1979, 22, 173–176. [Google Scholar]
- De, P.T.; Urones, J.G.; Marcos, I.S.; Basabe, P.; Cuadrado, M.S.; Moro, R.F. Triterpenes from Euphorbia broteri. Phytochemistry 1987, 26, 1767–1776. [Google Scholar] [CrossRef]
- Daoubi, M.; Benharref, A.; Hernandez-Galan, R.; Macías-Sánchez, A.J.; Collado, I.G. Two novel steroids from Euphorbia officinarum latex. Nat. Prod. Res. 2004, 18, 177–181. [Google Scholar] [CrossRef]
- Mueller, R.; Pohl, R. Flavonol glycosides of Euphorbia amygdaloides and their quantitative determination at various stages of plant development. 5. Flavonoids of native Euphorbiaceae. Planta Med. 1970, 18, 114–129. [Google Scholar]
- Tanaka, R.; Matsunaga, S. Supinenolones A, B and C, fernane type triterpenoids from Euphorbia supina. Phytochemistry 1989, 28, 3149–3154. [Google Scholar] [CrossRef]
- Toume, K.; Nakazawa, T.; Hoque, T.; Ohtsuki, T.; Arai, M.A.; Koyano, T.; Ishibashi, M. Cycloartane triterpenes and ingol diterpenes isolated from Euphorbia neriifolia in a screening program for death-receptor expression-enhancing activity. Planta Med. 2012, 78, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Öksüz, S.; Gil, H.; Chai, R.R.; Pezzuto, J.M.; Cordell, G.A.; Ulubelen, A. Biologically active compounds from the Euphorbiaceae; 2. Two triterpenoids of Euphorbia cyparissias. Planta Med. 1994, 60, 594–596. [Google Scholar] [CrossRef]
- Kato, T.; Frei, B.; Heinrich, M.; Sticher, O. Antibacterial hydroperoxysterols from Xanthosoma robustum. Phytochemistry 1996, 41, 1191–1195. [Google Scholar] [CrossRef]
- Cabrera, G.M.; Seldes, A.M. Hydroperoxycycloartanes from Tillandsia recurvate. J. Nat. Prod. 1995, 58, 1920–1924. [Google Scholar] [CrossRef]
- Dumkow, K. Kaempferol-3-glucuronide and quercetin-3-glucuronide, principal flavonoids of Euphorbia lathyris L. and their separation on acetylated polyamide. Z. Naturforsch. B 1969, 24, 358. [Google Scholar] [CrossRef]
- Hu, Q.; Dai, L.L.; Wang, L.; Xiao, Y.H.; Pan, Z.Q. Study on optimization of extraction and separation processes of breviscapine. Chem. Bioeng. 2009, 26, 58–60. [Google Scholar]
- Zhang, W.; Di, D.; Wen, B.; Liu, X.; Jiang, S. Determination of Scutellarin in Scutellaria barbata Extract by Liquid Chromatography–Electrochemical Detection. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 2133–2140. [Google Scholar] [CrossRef]
- Miyaichi, Y.; Kizu, H.; Tomimori, T.; Lin, C.C. Studies on the Constituents of Scutellaria Species. XI: On the Flavonoid Constituents of the Aerial Parts of Scutellaria indica L. Chem. Pharm. Bull. 1989, 37, 794–797. [Google Scholar] [CrossRef]
- Zayed, S.M.; Farghaly, M.; Taha, H.; Gotta, H.; Hecker, E. Dietary cancer risk conditional cancerogens in produce of livestock fed on species of spurge (Euphorbiaceae). J. Cancer Res. Clin. Oncol. 1998, 124, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhu, C.; Cheng, W.; Fan, X.; Chen, X.; Yang, S.; Guo, Y.; Ye, F.; Shi, J. Chemical constituents of the roots of Euphorbia micractina. J. Nat. Prod. 2009, 72, 1620–1626. [Google Scholar] [CrossRef]
- Wenxiang, W.; Xingbao, D. Acetophenone Derivatives From Euphorbia Ebracteolata Hayata. Acta Pharm. Sin. 1999, 7, 514–517. [Google Scholar]
- Khan, A.Q.; Rasheed, T.; Malik, A. Tirucalicine: A new macrocyclic diterpene from Euphorbia tirucalli. Heterocycles 1988, 27, 2851–2856. [Google Scholar]
- Marco, J.A.; Sanz-Cervera, J.F.; Yuste, A. Ingenane and lathyrane diterpenes from the latex of Euphorbia canariensis. Phytochemistry 1997, 45, 563–570. [Google Scholar] [CrossRef]
- Yamamura, S.; Shizuri, Y.; Kosemura, S.; Ohtsuka, J.; Tayama, T.; Ohba, S.; Terada, Y. Diterpenes from Euphorbia helioscopia. Phytochemistry 1989, 28, 3421–3436. [Google Scholar] [CrossRef]
- Forgo, P.; Rédei, D.; Hajdu, Z.; Szabó, P.; Szabó, L.; Hohmann, J. Unusual tigliane diterpenes from Euphorbia grandicornis. J. Nat. Prod. 2011, 74, 639–643. [Google Scholar] [CrossRef]
- Gschwendt, M.; Hecker, E. Über die Wirkstoffe der Euphorbiaceen. Z. Krebsforsch. Klin. Onkol. 1973, 80, 335–350. [Google Scholar] [CrossRef]
- Fürstenberger, G.; Hecker, E. New highly irritant euphorbia factors from latex of Euphorbia tirucalli L. Experientia 1977, 33, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Li, Y.; Wang, S.F.; Zhao, Y.L.; Liu, K.C.; Wang, X.M.; Yang, Y.P. Ingol and ingenol diterpenes from the aerial parts of Euphorbia royleana and their antiangiogenic activities. J. Nat. Prod. 2009, 72, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Malik, A. A new macrocyclic diterpene ester from the latex of Euphorbia tirucalli. J. Nat. Prod. 1990, 53, 728–731. [Google Scholar] [CrossRef]
- Adolf, W.; Köhler, I.; Hecker, E. Lathyrane type diterpene esters from Euphorbia lathyris. Phytochemistry 1984, 23, 1461–1463. [Google Scholar] [CrossRef]
- Daoubi, M.; Marquez, N.; Mazoir, N.; Benharref, A.; Hernández-Galán, R.; Munoz, E.; Collado, I.G. Isolation of new phenylacetylingol derivatives that reactivate HIV-1 latency and a novel spirotriterpenoid from Euphorbia officinarum latex. Bioorg. Med. Chem. 2007, 15, 4577–4584. [Google Scholar] [CrossRef]
- Opferkuch, H.J.; Hecker, E. New diterpenoid irritants from Euphorbia ingens. Tetrahedron Lett. 1974, 15, 261–264. [Google Scholar] [CrossRef]
- Noori, M.; Chehreghani, A.; Kaveh, M. Flavonoids of 17 species of Euphorbia (Euphorbiaceae) in Iran. Toxicol. Environ. Chem. 2009, 91, 631–641. [Google Scholar] [CrossRef]
- Singh, H.; Mishra, A.; Mishra, A.K. The chemistry and pharmacology of Cleome genus: A review. Biomed. Pharmacother. 2018, 101, 37–48. [Google Scholar] [CrossRef]
- Phan, N.M.; Nguyen, T.P.; Le, T.D.; Mai, T.C.; Phong, M.T.; Mai, D.T. Two new flavonol glycosides from the leaves of Cleome viscosa L. Phytochem. Lett. 2016, 18, 10–13. [Google Scholar] [CrossRef]
- Iwashina, T.; Kokubugata, G. Flavone and flavonol glycosides from the leaves of Triumfetta procumbens in Ryukyu Islands. Bull. Natl. Mus. Nat. Sci. Ser. B 2012, 38, 63–67. [Google Scholar]
- Hamad, M.N. Isolation of rutin from Ruta graveolens (Rutaceae) cultivated in Iraq by precipitation and fractional solubilization. Pharm. Glob. 2012, 3, 1. [Google Scholar]
- Williams, C.A.; Harborne, J.B.; Eagles, J. Leaf flavonoid diversity in the Australian genus Patersonia. Phytochemistry 1989, 28, 1891–1896. [Google Scholar] [CrossRef]
- Yang, Z.G.; Jia, L.N.; Shen, Y.; Ohmura, A.; Kitanaka, S. Inhibitory effects of constituents from Euphorbia lunulata on differentiation of 3T3-L1 cells and nitric oxide production in RAW264. 7 cells. Molecules 2011, 16, 8305–8318. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.A. Flavonol glycosides of Euphorbia retusa and E. sanctae-catharinae. Phytochemistry 1985, 24, 371–372. [Google Scholar] [CrossRef]
- Corea, G.; Fattorusso, E.; Lanzotti, V.; Motti, R.; Simon, P.N.; Dumontet, C.; Di Pietro, A. Structure-activity relationships for euphocharacins A-L, a new series of jatrophane diterpenes, as inhibitors of cancer cell P-glycoprotein. Planta Med. 2004, 70, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Tanaka, T.; Nonaka, G.I.; Nishioka, I. Tannins and Related Compounds. XCV.: Isolation and Characterization of Helioscopinins and Helioscopins, Four New Hydrolyzable Tannins from Euphorbia helioscopia L.(1). Chem. Pharm. Bull. 1990, 38, 1518–1523. [Google Scholar] [CrossRef]
- Lin, S.J.; Yeh, C.H.; Yang, L.M.; Liu, P.C.; Hsu, F.L. Phenolic compounds from Formosan Euphorbia tirucalli. J. Chin. Chem. Soc. 2001, 48, 105–108. [Google Scholar] [CrossRef]
- Bindra, R.S.; Satti, N.K.; Suri, O.P. Isolation and structures of ellagic acid derivatives from Euphorbia acaulis. Phytochemistry 1988, 27, 2313–2315. [Google Scholar] [CrossRef]
- Niu, C.S.; Li, Y.; Liu, Y.B.; Ma, S.G.; Liu, F.; Cui, L.; Yu, H.B.; Wang, X.J.; Qu, J.; Yu, S.S. Grayanane diterpenoids with diverse bioactivities from the roots of Pieris Formosa. Tetrahedron 2018, 74, 375–382. [Google Scholar] [CrossRef]
- Shen, Y.C.; Prakash, C.V.; Chen, Y.J.; Hwang, J.F.; Kuo, Y.H.; Chen, C.Y. Taxane Diterpenoids from the Stem Bark of Taxus mairei. J. Nat. Prod. 2001, 64, 950–952. [Google Scholar] [CrossRef]
- Tawfike, A.F.; Tate, R.; Abbott, G.; Young, L.; Viegelmann, C.; Schumacher, M.; Diederich, M.; Edrada-Ebel, R. Metabolomic tools to assess the chemistry and bioactivity of endophytic Aspergillus strain. Chem. Biodiverse. 2017, 14, e1700040. [Google Scholar] [CrossRef]
- Cheng, C.; Othman, E.; Stopper, H.; Edrada-Ebel, R.; Hentschel, U.; Abdelmohsen, U. Isolation of petrocidin A, a new cytotoxic cyclic dipeptide from the marine sponge-derived bacterium Streptomyces sp. SBT348. Mar. Drugs 2017, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; Attia, E.Z.; Hajjar, D.; Anany, M.A.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S.; Wajant, H.; Gulder, T.A.; Abdelmohsen, U.R. New cytotoxic cyclic peptide from the marine sponge-associated Nocardiopsis sp. UR67. Mar. Drugs 2018, 16, 290. [Google Scholar] [CrossRef] [PubMed]
- Tawfike, A.F.; Attia, E.Z.; Desoukey, S.Y.; Hajjar, D.; Makki, A.A.; Schupp, P.J.; Edrada-Ebel, R.; Abdelmohsen, U.R. New bioactive metabolites from the elicited marine sponge-derived bacterium Actinokineospora spheciospongiae sp. nov. AMB Express 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, L.; Zhang, T.; Viegelmann, C.; Martinez, I.; Cheng, C.; Dowdells, C.; Abdelmohsen, U.R.; Gernert, C.; Hentschel, U.; Edrada-Ebel, R. Metabolomic tools for secondary metabolite discovery from marine microbial symbionts. Mar. Drugs 2014, 12, 3416–3448. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).