Abstract
Clonazepam (CLZ), an antipsychotic drug reported for its efficiency in managing anxiety-related disorders, is being marketed only as conventional tablets. Some patients have abstention to swallow the conventional tablets; therefore, the proposed study was aimed at developing a buccal lozenge tablet by direct compression of two types of optimized granules. Conazepam’s water solubility was first enhanced by a solid dispersion technique for a fast and better dissolution of type 1 granules, while the impact of gelling polymers was investigated on controlled-release type 2 granules. The optimized formulae met the acceptable pharmacopeial limits for tablets’ evaluation. A differential scanning calorimetry study revealed the compatibility between the drug and used excipients. All formulae gave a burst release of CLZ in the first hour of investigation, followed by a sustained release over 24 h. The formula that showed the highest prolonged in vitro release (99.0 + 0.1%), following the Higuchi diffusion model (R2 = 0.99), was then selected for further study. The formula succeeded in controlling the induced stress in a rat model with a significant impact on the behavioral tests throughout the experiment. The results were further confirmed by a pharmacokinetic study that showed a significant increase in Cmax, Tmax, and AUC (1.5, 2, and 3.9 folds), respectively, compared to oral suspension. The newly proposed delivery system has proven a better efficacy with a reduced dosing frequency.
1. Introduction
Clonazepam (CLZ) is a benzodiazepine anticonvulsant drug, which is commonly indicated for seizure, panic, epilepsy, and anxiety disorders [1,2]. CLZ has a limited dissolution, as well as poor absorption and distribution profiles. It is rapidly absorbed after oral administration with an apparent volume of distribution of 3.0 L/kg and a clearance rate of 55.0 mL/min, and then is extensively metabolized by cytochrome P450 liver microsomal enzymes [3,4]. CLZ has a narrow therapeutic window of 0.02 to 0.08 µg/mL, therefore, symptoms of toxicity may develop rapidly. Based on the biopharmaceutics classification system (BSC), CLZ is classified as a class II drug [4]. CLZ is slightly soluble in methanol and ethanol, and is practically insoluble in water (Less than 0.1 mg/mL) [5], therefore, establishing a research method for the improvement of its aqueous solubility is valuable to enhance the drug’s absorption. It is a moderately basic drug with a pKa of less than 2.0 [6]. The use of hydrophilic polymers, such as hydroxypropyl methyl cellulose (HPMC) or different grades of polyethylene glycols (PEG), is valuable for improving CLZ solubility by the formulation of a solid dispersion and consequently positively affects its therapeutic efficacy [7]. Therefore, it is more reasonable to formulate a buccal tablet rather than oral ones in order to bypass the enterohepatic pathways.
Patients suffering from seizures, anxiety, depression, or some other psychotic-related disorders face difficulties in swallowing or are reluctant to ingest their tablets [8]. In such cases, buccal dosage forms, as candy lozenges, would be an effective solution appealing to those patients for an efficient medicine regimen.
The buccal route for drug delivery offers a promising route, not only for the treatment of local conditions in buccal mucosa, but also for systemic delivery by rapid and direct absorption of the drug through buccal mucosa into the systemic circulation. It constitutes a good alternative to the oral route for avoiding the accompanied problems, especially those drugs that degrade through the gastrointestinal pathway [9].
From a technical point of view, tablets are the most preferred dosage form for being easily manufactured with a high patient convenience and compliance, making them the first-choice dosage form. Conventional tablets require frequent dosing and consequently an unpredicted drug plasma level for the drugs of short half-lives [10]. In many cases, to manage the panic attack of a number of diseases, a pulse release of drug is required, and then a prolonged effect should be maintained by sustained drug release. On the basis of such a requirement, new approaches of tablet designs such as multilayered tablets have appeared in the field of the pharmaceutical industry [11]. Due to numerous obstacles concerning the compressibility and risks of bilayer separation, another approach for formulating two-in-one single-layer lozenge tablets based on different compositions is involved. Such tablets are prepared by the binary mixing of different types of fast- and controlled-release granules [12].
In the proposed study, optimized single-layer lozenge tablets, prepared by the direct compression of a binary mixture of two types of granules, was formulated for bimodal release of CLZ. Their efficacy in management of anxiety-related disorders was studied on an anxiety-induced rat model. Pharmacokinetic studies were then conducted to confirm the in vivo results.
2. Materials and Methods
2.1. Materials
Clonazepam was kindly supplied by the Egyptian International Pharmaceutical Industries Co. (EIPICo., Tenth of Ramadan City, Egypt). Sodium alginate, Lactose, HPMC K4000, HPMC K15m, and PEG 6000 were also kindly supplied by EIPICo., Egypt. Magnesium stearate and Avicel were purchased from Sigma Chemical, St. Louis, MO, USA. All other used reagents and chemicals were of analytical grades.
2.2. Preparation of Clonazepam Solid Dispersion
2.2.1. Preparation of Physical Mixtures (PMs) and Solid Dispersions (SDs) of CLZ
Hydrophilic polymers such as PEG 6000 and HPMC K4000 at different ratios to CLZ as per listed in Table 1 were used for preparation of six PMs and six SDs. The physical mixtures were prepared by simple trituration of CLZ and polymer for 5 min in a glass container, sieving, and storage in desiccators for further estimation.

Table 1.
Composition of Clonazepam, different physical mixtures, and solid dispersions.
For preparation of solid dispersion (SD), solvent evaporation method was used as previously described by Minhaz et al. [7]. Simply, an accurately weighed amount of polymer and CLZ were dissolved in a sufficient volume of ethanol. The solution was then transferred to a petri dish and the solvent was allowed to evaporate at room temperature for 1 h and then was kept in desiccators for 48 h for complete evaporation and dryness. The obtained SDs were crushed, pulverized, and subjected to sieving, then stored in a desiccator for further evaluation.
2.2.2. Dissolution Studies
A UV/VIS spectrophotometric method was used for determination of CLZ in formulated tablets dosage form [7,13], with slight modifications. The modified method was then re-validated according to ICH guidelines [14] for linearity, accuracy, and precision within a specified range of 1–30 µg/mL in water. The linearity calibration curve (Figure 1) was then used for calculating the concentration of CLZ in PM and SD samples. Accuracy results of the modified analytical method was 98.3 ± 1.2, in terms of %recovery relative standard deviations.
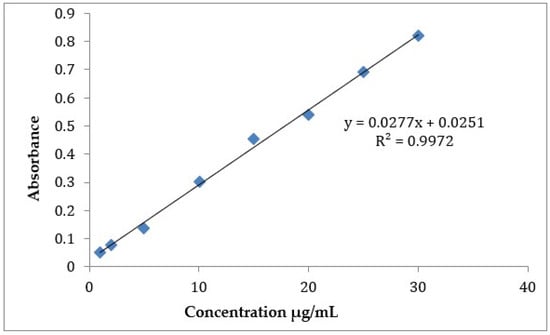
Figure 1.
Calibration curve of CLZ determination using UV/VIS spectrophotometry in distilled water.
CLZ’s PMs and SDs were subjected to dissolution studies using USP paddle apparatus (type II), in distilled water adjusted to pH 7, at a temperature of 37 °C ± 0.5 °C [13], and paddle speed set at 50 rpm. Samples of 1 mg of pure drug (the prescribed maintenance daily dose of CLZ) [8,15] was dispersed into 500 mL of dissolution medium for comparison. After equilibration, exactly 3 mL samples were withdrawn at specified time intervals, filtered and analyzed by UV-spectroscopy at 254 nm detection wavelength for quantification of CLZ [7,16]. Dissolution measurement studies were performed in triplicates (n = 3). The same procedures were conducted for PM and SD samples of an equivalent amount of CLZ.
2.3. Optimization of Formulation Excipient Type and Concentration
2.3.1. Optimization of Avicel: Lactose Ratio
Avicel, also named as microcrystalline cellulose, is widely used in tableting as a compression aid and filler during direct compression. Lactose on the other hand is reported as a diluent. To optimize the ratio of avicel concentration compared to lactose, six different formulations were prepared in order to investigate the impact of their ratio concentrations on the formulation characteristics (Table 2). Based on the results, the selected ratio was involved in the first type granules and used for optimization of the gelling polymer type and concentration for the second type of granules.

Table 2.
Formulation of different batches of bilayer tablets of Clonazepam for optimization.
2.3.2. Optimization of Gelling Polymer Type and Concentration
In total, six formulations were prepared to investigate the effect of the gelling agent type and concentration along with optimized concentration of binder on tablet formulation characteristics. Either HPMC k15m or Na alginate was employed as a drug release modifier at various concentrations (1, 2, 3% for HPMC k15m and 1, 2, 3% for Na Alginate) (Table 2).
The required quantity of each ingredient was taken for each specific formulation and the tablets were prepared by wet granulation technique for selection of optimized formula based on evaluation.
2.4. Preparation of Single-Layer Two-in-One Matrix Lozenge Tablets of CLZ
The developed method was a modification to a reported one by Morovati et al. [12]. Granules of different sizes and composition were prepared and mixed together prior to compression. CLZ-SD equivalent to 1 mg of the active drug as an initial dose and other additives such as lactose and avicel were firstly mixed via geometric dilution, then, the mixture was passed twice through a 20-mesh size sieve for uniform particle size. Small quantity of Cetyl alcohol was melted at a temperature below the melting point of CLZ and an appropriate amount of mixture 1 (A6) was added gradually and stirred with the melted mass (melt granulation). These granules were then passed through a 14-mesh size sieve and the first granule size portion was hence prepared.
Another mixture was prepared by mixing CLZ-SD equivalent to 1 mg of drug, Avicel PH102, lactose, and Na alginate via geometric dilution and mixing thoroughly, then the mixture was sieved. Appropriate amount of water was added to form a cohesive mass, which was then subsequently passed through a 16-mesh size sieve (the second granule size portion). Granules were dried in an electrical oven at 25 °C for 2 h and mixed together as per 500 mg of each portion. Sifted lubricating agent, Mg stearate (1% w/w), was added to the mixture just before the compression step. Compression was done at compression force of 80 kN for 15 s [17], using a single flat-faced punch tablet machine of 10 mm diameter (Korsch Frogerais, type AO, Berlin, Germany).
2.5. Characterization of Two-in-One Single-Layer CLZ Lozenge Tablets
For each batch, 20 tablets were used to calculate the average weight using analytical balance (Mettler-Toledo, CH9606, Greifensee, Switzerland). Lozenge tablets were evaluated for parameters of drug content, hardness, friability, weight variation, thickness, and content uniformity as per the method described in the official pharmacopeia [5].
2.5.1. Percentage Drug Content
The uniformity of drug content in tablets was determined according to the following described procedures [18]. Ten lozenge tablets of each batch were picked randomly, weighed, and crushed in a mortar. Powder amount equivalent to a dose of 2 mg of CLZ was obtained and dissolved in 10 mL of methanol. The volume was made up to 100 mL with phosphate buffer pH 6.8 and sonicated for 20 min. The absorbance was measured on a UV–Vis spectrophotometer at 254 nm, and the amount of CLZ was calculated. All the formulations should be within the standard limits (95–105% of drug) [19].
2.5.2. Hardness, Diameter, and Thickness
This test is used to check the crushing strength of tablets, to confirm whether they can withstand chipping, storage, transportation, and handling without breaking. Five lozenges were randomly selected from each batch and the hardness of each was measured using Pharma-Test Hardness Tester (Hamburg, Germany). The hardness is usually measured in terms of kg/cm2. The extent to which the thickness of each lozenge deviated from the standard was determined [20].
2.5.3. Friability
Ten lozenge tablets of each batch were first weighed (Winitial) and subjected to friability test using Roche friabilator. The instrument was run at 25 revolutions/min for 4 min. Lozenges were collected, dedusted, and reweighed (Wfinal). The percentage of weight lost, or friability (F), was calculated by the formula given below (Equation (1)), and results should not exceed 1% [20].
2.5.4. Disintegration Test
Disintegration time was determined for six lozenge tablets from each batch using Pharma-Test Disintegration Tester (Hamburg, Germany). Each tablet was placed singly in a tube of the basket. A disc was added to each tube and the apparatus was run using 900 mL of phosphate buffer solution (pH 6.8) maintained at 37 ± 0.5 °C. The basket assembly moved up and down at a constant frequency of 30 cycles/min. The time, in seconds, for complete disintegration of the tablet with no palpable mass remaining in the apparatus was measured and recorded.
2.5.5. In Vitro Drug Release
In vitro drug release was performed using USP dissolution test apparatus type II (paddle type) at 50 rpm and 37 ± 0.5 °C, using 900 mL of Sörensen’s phosphate buffer pH 6.8 as dissolution medium. Samples of 5 mL volume were withdrawn at predetermined time intervals and replaced by an equivalent volume of fresh medium. Samples were filtered through Whatman filters (0.45 μm) and analyzed by UV spectrophotometer at 254 nm using a suitable blank. Measurements were made in triplicates (n = 3).
2.5.6. Drug-Release Kinetics
CLZ release data were fitted to kinetics models, zero order, first order, and Higuchi, in order to investigate the kinetics and mechanism of drug release pattern from the prepared lozenge tablets.
2.5.7. Differential Scanning Calorimetry (DSC)
DSC studies were conducted to confirm the drug incorporation within the prepared lozenge tablets. The thermal behavior of pure drug, pure components, as well as physical mixture was recorded using a Model DT-60 DSC, Schimadzu, Japan. Accurately weighed samples were heated in thermally sealed aluminum pans over the temperature range of 0–400 °C at a constant rate of 10 °C/min under a nitrogen purge (30 mL/min) [21,22].
2.5.8. In Vivo Characterization of Optimized and Commercial Formulations
Ethical Approval
Adult albino male rats were obtained from animal breeding center, Zagazig University, Egypt (~250 g), and maintained at room temperature in 12 h light/dark cycles with free access to food and water. Rats were acclimatized at least one week before starting the experiments. The guidelines of Institutional Animal Care and Use Committee (IACUC) of Faculty of Pharmacy, Zagazig University were followed (Approval number: ZU-IACUC/2/F/46/2022).
Sucrose Preference Test
All rats underwent adaptive training from day 1 to day 4, with two bottles of pure water available on days 1 and 2, two bottles of 1% sucrose on day 3, and one bottle of pure water and one bottle of 1% sucrose on day 4. Rats were subjected to food and water deprivation for 12 h, then each rat was given 200 mL of pure water and 200 mL of 1% sucrose solution. The quantities of pure water and sucrose consumed were recorded after 1 h and again after 12 h [23].
Sucrose preference is defined as described in Equation (2). Induction of anxiety was performed for all rat groups except for the first one that served as a negative control group.
sucrose preference percentage (%) = sucrose solution consumption (g)/(sucrose solution consumption (g) + water consumption (g)) × 100%
Administration and Evaluation of Studied Formulations
After acclimatization, the animals were housed singly in metabolic cages. They were divided into four groups (n = 5):
- -
- Group1: had free access to water and food for 10 days and served as the negative control. This group was tested under the same conditions and was used to demonstrate the increase in anxiety behavior after induction in the other groups.
- -
- Group2: rats did not receive any treatment and served as positive control.
- -
- Group 3: rats received the marketed tablets of CLZ (Amotril®, containing 2 mg CLZ per tablet, manufactured by Amoun pharmaceuticals, Egypt) as an oral suspension, adjusted to contain 0.25 mg per kg of body weight of rats [24]. The suspension was stabilized with the help of 2% Tween 80 or 1% gum solution, immediately before administration [25].
- -
- Group 4: rats received the optimized formulation containing the same dose as group 3 (0.25 mg/kg) fixed to the buccal cavity, using urethane at a small dose of 2 mg/kg before drug administration only to ensure the retention of test formulations within the buccal cavity for a sufficient period of time.
Evaluation of Antianxiety Activity (Behavioral Assessment)
Sixty minutes after administration of treatment protocols to the animals, they were sequentially exposed to the following experimental models of anxiety. After testing each animal, all apparatuses were cleaned thoroughly with alcohol in order to mask the odor left by the animal for complete behavioral assessment.
Forced Swim Test
In a transparent glass cylinder (20 cm diameter × 50 cm high) filled with water (23–25 L) to a depth of 30 cm, rats were put individually and after 1 min of adaptable swimming, their immobility time was measured over a period of 5 min. The test was repeated after 1, 6, and 12 h. Rats that floated only to keep their heads above the water with no swimming were as immobile [23].
Open Field Test (OFT)
The method used in our experiment was that described by Belzung [26]. The OF test is a mild stressful task to be used for measuring the exploration performance such as latency time, number of squares crossed by animal, and rearing frequency. It can also help measure the emotionality changes by determining the grooming frequency. The test was carried out in a square box (100 × 100 × 30 cm) divided into 25 equal squares and the duration of each test was set for 5 min. The latency time after the initial movement was measured by recording the time in seconds required for the four paws of the rats to remain in the central square. The number of squares crossed was determined when all the four paws of rats were placed into another square. Moreover, rearing was described as the lifting of the upper body and forepaws of rats off the ground. Grooming frequency was determined by the friction movements by the rat forepaws to the face, head, nose, and ears in addition to body scratching using the hind paws. The test box was cleaned by 70% ethanol between each test to bypass the residual odor. The tests were videotaped and the blindness was preserved [27].
2.5.9. Pharmacokinetic Study
HPLC Analysis Method for CLZ Determination
Separation of CLZ was performed by HPLC (Thermo Scientific Surveyor Plus HPLC system, Thermo Scientific company, Waltham, MA, USA). The method was previously reported by Mura et al. [28], with little modification. The mobile phase was 40:60 (v/v) mixture of acetonitrile and 0.01 M sodium acetate buffer (pH = 7), flow rate of 1 mL/min, at room temperature (25 ± 1.0 °C), and measurements were run at wavelength 240 nm. Validation of the method was done in terms of linearity (5–750 ng/mL), and limit of quantification (5 ng/mL). Blood samples from rats’ plasma were quantified for the pharmacokinetic study. CLZ concentration was calculated according to the equation obtained from the calibration curve of validation study done for CLZ determination using HPLC (Figure 2).
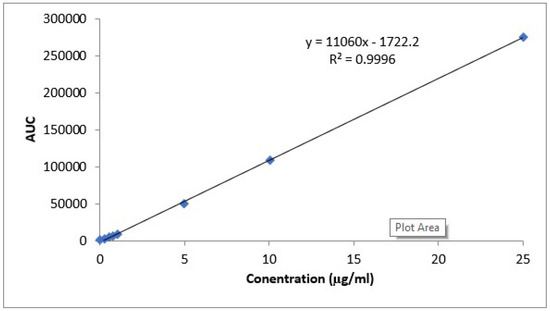
Figure 2.
Calibration curve of determination of CLZ in rat plasma using HPLC method.
In Vivo Kinetic Study
Three groups of six male albino rats, each weighing 200–250 g, were used for each formulation. All groups were fasted overnight before the experiment. The first group received 1 mL oral suspension of pure CLZ (0.25 mg/kg). The second group received the selected buccal lozenge formulation of CLZ administered to the oral cavity between the lower gum and bottom lip containing the same dose as the first group [29]. The third group was considered as negative control. Approximately 0.3 mL blood samples were withdrawn at 0, 1, 2, 3, 4, 5, 6, 7, 8, and 24 h. Blood samples were centrifuged at 4000 rpm for 10 min to separate the plasma. Plasma (100 µL) was transferred in a test tube to which 0.2 mL of 0.1 M NaOH aqueous solution was added and vortex-mixed using vortex mixer (Model 16,700 mixer, Thermolyne corporation, Dubuque, IA, USA). Then, 4 mL of the hexane–ethyl acetate (9:1, vol/vol) mixture was added and centrifuged at 12,000 rpm for 5 min. The organic phase was taken and the solvent was evaporated completely under nitrogen gas. The residue was dissolved in 40 µL of the HPLC mobile phase. Finally, the resultant solution (20 µL) was injected in the HPLC column [29].
Statistical Analysis
The data of pharmacokinetic and behavioral studies were expressed as mean values ± S.D (n = 5). Student’s t-test was employed to analyze the measured pharmacokinetic parameters. The behavioral parameters were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. The GraphPad Prism® program, version 5.00 was utilized.
3. Results and Discussion
CLZ is an oral anxiolytic agent that is commonly associated with serious variable bioavailability problems related to its poor water solubility [30]. Therefore, the prospective of the current study was aimed at developing a dosage form with an improved water solubility, and consequently, its therapeutic efficacy. The developed formulation was a bimodal two-in-one single-layer lozenge tablet based on the wet granulation technique. The optimization of the tablet formulations was an attempt to provide the bimodal release of CLZ from the compressed granules of two different sizes and compositions, to provide a first immediate and another sustained release granular type to achieve both a loading dose as a result of a burst release of CLZ to help the acute panic cases and a maintenance dose by the sustained granular ones. Tablets were assessed for various parameters such as friability, hardness, drug content uniformity, etc., for the best optimization.
3.1. Dissolution Enhancement of CLZ by Solid Dispersion
In vitro dissolution testing was performed for 60 min to ascertain the effect of the polymeric carrier (HPMC K4000, PEG 6000) on immediate drug release enhancement (Figure 3 and Figure 4). The impact of the incorporated polymer on the release of the drug from a solid dispersion was evaluated by comparing the dissolution of the drug in the prepared physical mixtures and the corresponding solid dispersions along with that of the pure drug. Theoretically, solid dispersions improve drug dissolution by decreasing the particle size, the formation of amorphous forms, and the improved wettability [7].
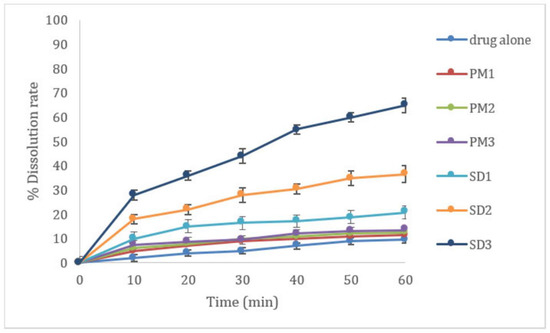
Figure 3.
Enhancement of dissolution profile of Clonazepam/HPMC K4000 PMs and SDs.
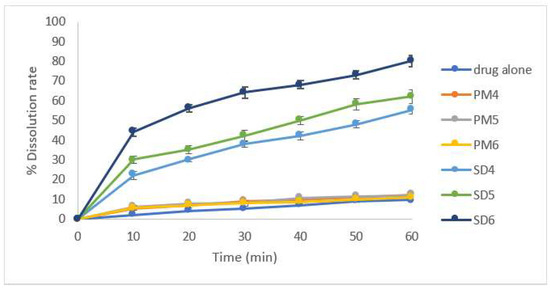
Figure 4.
Enhancement of dissolution profile of Clonazepam/PEG 6000 PMs and SDs.
Out of the different polymers examined, it was observed that the physical mixing of CLZ with both polymers at different concentrations had little impact on the dissolution rate of the drug. On the other hand, the formation of SD significantly enhanced the dissolution rate of CLZ. Moreover, when the PEG 6000 concentration was increased, the rate of drug release from solid dispersion was also increased significantly due to its wetting ability and conversion of CLZ from a crystalline to an amorphous state. The formulation containing a higher concentration of PEG 6000 recorded the highest drug release percent, so SD3 was selected for further study.
Similar results were obtained by Minhaz et al. [7], who stated that the drug release was highest from the formulation containing the highest ratio of PEG 6000, by facilitating wetting of the drug and improving its dissolution. Moreover, this formulation contained a small amount of crystal lattice compared to the pure drug alone, which indicates that a reduction of drug particle size may be responsible for improved dissolution. The delayed release in the HPMC-containing SDs may be due to the gelling-like properties of polymer that may be responsible for the delayed release of drug after dissolution in water.
3.2. Optimization of Avicel: Lactose Concentration
For an acceptable tablet hardness, friability, and enhanced dissolution of the contained drug, optimization of the incorporated polymers was a necessity. In the present study, the Avicel: lactose ratio was optimized to facilitate their compression into tablets with an acceptable friability and hardness. Six formulations were prepared using different Avicel: lactose ratios and results revealed that increasing the Avicel ratio to be equal to lactose facilitated the compression of ingredients and had a good impact on the hardness with least tablets friability.
3.3. Optimization of Polymer Type and Concentration
The incorporation of either HPMC or Na alginate at different concentrations was assessed. As shown in Table 3A,B, the results revealed that increasing the concentration from 1% to 3% for both HPMC and Na alginate, had a significant positive impact on the hardness due to the increased mechanical strength and/or resistance of the prepared tablets at the optimized binder concentration [31].

Table 3.
Characterization of different batches of formulations.
The results showed that all formulae complied with the pharmacopeial limits [5]. The average drug content of the examined tablets ranged from (95.1 + 2.5 to 99.6 + 0.5% of the label claim). The hardness values were satisfactory to make the lozenge tablets able to withstand the applied mechanical shocks during transport, and handling was noticed to increase by increasing the concentration of polymer. All the tablets exhibited friability values of less than 1% w/w as per stated in BP2017 [5]. All batches showed thickness, friability, weight variation, and content uniformity in acceptable limits.
3.4. In Vitro Drug Release
The in vitro drug release studies in Sörensen’s phosphate buffer of pH 6.8 was conducted to assess the success of formulations to provide the desired dual immediate and controlled drug delivery after buccal administration. It was obvious that a more significant burst release (p > 0.5) was observed in formulae containing Na alginate as the polymeric ingredient, which released approximately 30~40% of the incorporated dose compared to those containing HPMC, with a reduction in the releasing rate upon increasing the polymeric concentration. The B1 formulation showed a maximum release in 24 h (90%) due to the least Na alginate polymer concentration, and the release of the drug decreased by increasing the polymer concentration to 2 and 3% w/w to 80 and 75%, respectively, as shown in Figure 5. As per the HPMC-containing formulae, the release of CLZ decreased by increasing the concentration of HPMC. It was clear that the release of CLZ was much lower in tablets containing HPMC compared to those containing Na alginate. This may be attributed to the higher gelling properties of HPMC compared to Na alginate that hindered the release of the drug after dissolution of the tablets.
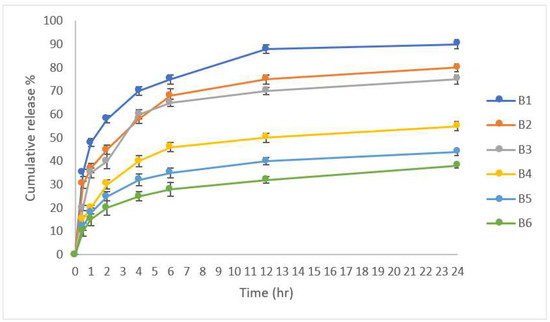
Figure 5.
In vitro drug release data of the prepared tablets (n = 3).
3.5. In Vitro Release Kinetic Study
The values of the correlation coefficient R2 indicate the appropriate kinetic model that describes the release of the drug, where the closer the R2 value is to one indicates the model fitting the release mechanism [32]. Based on the analysis of CLZ release data, it was clear that the Higuchi diffusion model elicited the highest R2 value. Therefore, it was selected to best describe the drug release from the studied formulae. The results are summarized in Table 4. The release of CLZ from the prepared formulae was governed by its permeability through the polymeric layer into dissolution medium as described by Patra et al. [33]. It was obvious that increasing the HPMC content not only led to a decrease in the burst effect, but also showed a more sustained effect. At a low polymer concentration, the hydrated matrix would be more porous with a low gel strength, which resulted in the rapid diffusion of the drug from the matrix. The slower release rate of CLZ may be due to hydration of HPMC with the formation of a gel layer that led to a longer diffusion path length with increasing the content of HPMC [34]. In brief, the key factor to control the mechanism and the rate of drug release is the concentration of the gelling polymer. As shown from the results in Table 4, it was found that the drug release from the formulations followed Higuchi’s kinetic model, and this explains that as the distance for diffusion was increased the drug diffused at a slower rate [35].

Table 4.
In vitro release kinetics study.
3.6. Differential Scanning Calorimetry (DSC)
The thermograms (DSC) of pure drug, excipients, and drug–polymer physical mixtures were studied to assess the drug–excipients compatibility and results are shown in Figure 6. The results indicated a sharp endothermic peak at 239.82 °C corresponding to the CLZ melting point and the same peak result was stated by Roni et al. [36]. The peak was more or less close to as documented in Merck index [37].
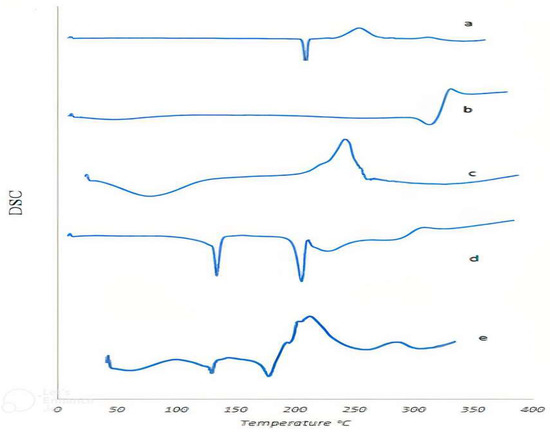
Figure 6.
Differential scanning colorimetry for (a) CLZ, (b) avecil, (c) sodium alginate, (d) lactose, and (e) physical mixture.
Sodium alginate showed a broad endotherm and exotherm at 78.55 °C (Figure 6c) due to the loss of moisture. The second exothermic peak at 248.43 °C is due to the loss of volatile components, chain rupture, and fragmentation of sodium alginate [38,39].
Two distinct endothermic peaks were clearly observed in the lactose thermogram (Figure 6d); the first was at 145 °C, corresponding to the point of dehydration, which was followed by a peak at 217 °C, for the melting point of the anhydrous crystal [31,40,41].
Figure 6e showed that the thermogram of the physical mixture of the tablet formulation containing CLZ had a shift lower than the melting point of the incorporated polymers and the drug itself. The DSC curve of pure Clonazepam showed a melting endothermic peak at 174.99 °C, while the physical mixture of drug and polymer exhibited an endothermic peak at 145.23 °C. The compatibility between the components of the tablets was confirmed by the observation of a distinct shift of their melting point peaks. This also ensured the homogenous dispersion of the tablet components in the amorphous form [42].
3.7. Evaluation of Anxiety-Related Symptoms
A behavioral assessment was performed, to confirm that the optimized single layered two-in-one lozenge tablets loaded with CLZ (B1) could achieve the burst release of the drug. In turn, the optimized lozenge tablets could exert its immediate anxiolytic activity in acute cases and also sustain its release to get a prolonged action. The resultant formula, hence, would minimize the need for frequent administration, compared to oral tablets, in an attempt to improve the patients’ compliance and efficacy of therapy by minimizing the fluctuation in the drug plasma concentration.
The sucrose preference test (SPT) was used to elicit anxiety-like symptoms in the laboratory animal models, and as reported, it increased the signs of anxiety behavior concomitant with increased indices of excitatory neurotransmitters. Rats subjected to the sucrose preference test had significantly lower sucrose preference percentages compared to the control group. The SPT was successful to verify the establishment of the anxiety-induced rat model sucrose consumption measured within 12 h (Figure 7). This was confirmed by the significant increase in the immobility time of the anxiety-induced animal groups in the forced swim test (FST) compared with the control group.
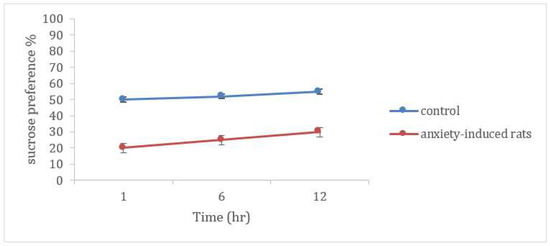
Figure 7.
Sucrose preference test in control and anxiety-induced rats.
The results of the open field test (OFT) showed a significantly lower latency time (p < 0.05) expressed as the shorter time spent by group 2 after the induction of anxiety compared to the control group 1 (Figure 8), a significant reduction in the number of squares crossed by the group of rats that received the studied formulation was also noticed (Figure 9). A significant increase in both the rearing and grooming frequencies elicited the extrapolation of emotional changes (Figure 10 and Figure 11).
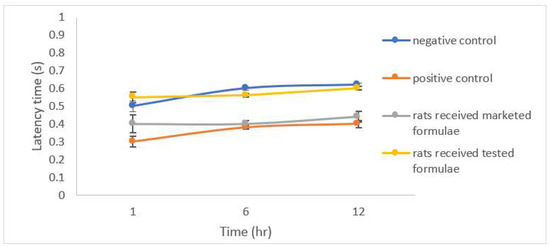
Figure 8.
Latency time in by different groups of rats.
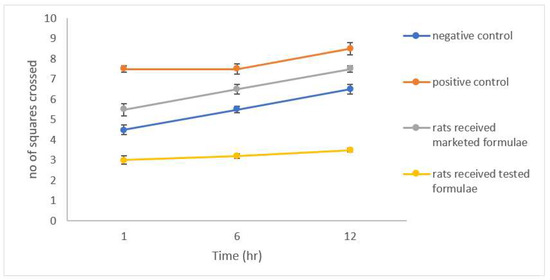
Figure 9.
Number of squares crossed by different groups of rats.
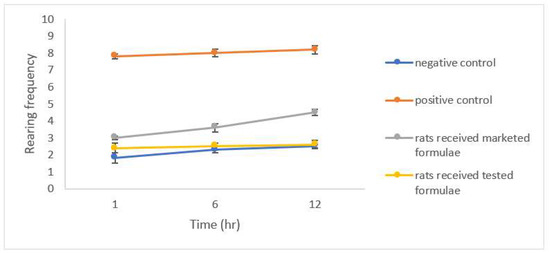
Figure 10.
Rearing frequency for different groups of rats.
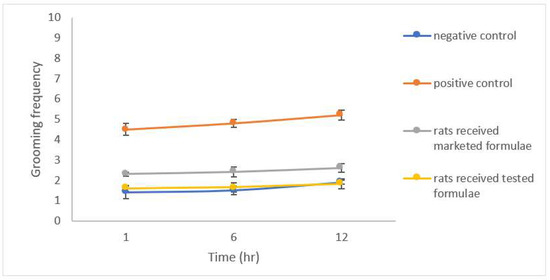
Figure 11.
Grooming frequency for different groups of rats.
The group of rats that received the studied formula showed a significant increase in latency time with a lower number of squares crossed by the rats, and lower rearing and grooming frequencies. The results were in accordance with Barros et al., who declared the same behavior for drugs belonging to gamma amino butyric acid (GABA) such as CLZ and explained their action by facilitating GABA-A action and the state of low firing potential produced by excitatory neurons [43].
3.8. Pharmacokinetics Study
The pharmacokinetic parameters were calculated using PK Solver and compared for the two groups (Table 5). The group that received the formula showed a higher plasma peak concentration compared to those that received the oral suspension. Therefore, the prepared lozenges are considered being the better formulation to obtain a rapid effect for anxiety. In addition to that, the Cmax showed a 1.5-fold increase for the lozenge group in comparison to the pure drug oral group at Tmax (4 h and 2 h, respectively), comprising a 2-fold increase for Tmax. The prolonged Tmax indicated the sustained drug release was followed by the rapid release of the prepared lozenges. Finally, AUC (Figure 12) was improved in the case of the lozenges group, comprising a 3.9-fold increase in comparison with the pure drug group. Thus, the in vivo kinetic study showed that the CLZ lozenges could achieve an effective plasma concentration quickly with a sustained effect for 24 h.

Table 5.
Pharmacokinetic parameters of CLZ after administration of oral lozenges compared with oral drug suspension in rats. (mean ± SD).
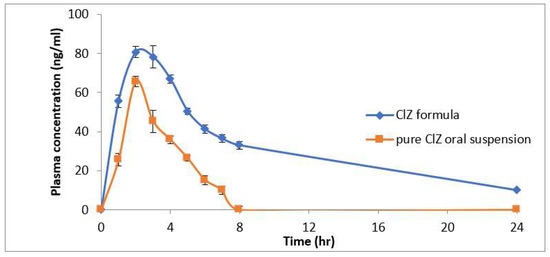
Figure 12.
Plasma conc. versus time profile of CLZ after administration of buccal lozenges compared with oral drug suspension in rats.
4. Conclusions
CLZ is an anxiolytic drug facing poor absorption based on its low water solubility. Its dissolution profile was first improved by a solid dispersion technique. Two types of granules were optimized for different release patterns of CLZ. The optimized single-layer two-in-one matrix lozenge tablets of CLZ were successfully prepared by the direct compression of two types of granules. A dual bimodal release of CLZ as a burst phase followed by an extended release one was exhibited. All formulae passed the pharmacopeial evaluation of tablets. In addition, the release pattern was best fitted to a diffusion mechanism. Behavioral studies had assured the impact of the optimized lozenge tablets to control the anxiety-related behavioral changes produced by sucrose preference tests in a rat model. A pharmacokinetic study was conducted for the optimized formula and compared to an oral solution and had confirmed the significant increase in the mean plasma concentrations as a function of time for the optimized formulations. Two-in-one matrix single layered tablets might be the appropriate choice to have an initial immediate release, followed by sustained release profile, especially when bilayer manufacturing is not possible.
Author Contributions
Conceptualization, E.G. and M.M.F.; methodology, E.G., M.M.F. and A.E.I.; software, E.G. and M.M.F.; validation, E.G., M.M.F. and A.E.I.; formal analysis E.G., M.M.F. and A.E.I.; investigation, E.G. and M.M.F.; resources, E.G., M.M.F. and S.E.D.; data curation, E.G. and M.M.F.; writing—original draft preparation, E.G., M.M.F. and A.E.I.; writing—review and editing, A.E.I. and S.E.D.; supervision, S.E.D.; project administration, E.G., M.M.F. and S.E.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Adult albino male rats were obtained from animal breeding center, Zagazig University, Egypt. The guidelines of Institutional Animal Care and Use Committee (IACUC) of Faculty of Pharmacy, Zagazig University were followed (Approval number: ZU-IACUC/2/F/46/2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sweetman, S. Martindale: The Complete Drug Reference; Pharmaceutical Press: London, UK, 2009; Volume 36. [Google Scholar]
- Koubeissi, M. Intravenous Clonazepam in Status Epilepticus. Epilepsy Curr. 2016, 16, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Maestrelli, F.; Nerli, G.; Mennini, N.; D’Ambrosio, M.; Luceri, C.; Mura, P.A. Development of a cyclodextrin-based mucoadhesive-thermosensitive in situ gel for clonazepam intranasal delivery. Pharmaceutics 2021, 13, 969. [Google Scholar] [CrossRef] [PubMed]
- Vyas, T.K.; Babbar, A.; Sharma, R.; Singh, S.; Misra, A. Intranasal mucoadhesive microemulsions of clonazepam: Preliminary studies on brain targeting. J. Pharm. Sci. 2006, 95, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Commission, B.P. British Pharmacopoeia 2017; Stationery Office: London, UK, 2017. [Google Scholar]
- Vårdal, L.; Øiestad, E.L.; Gjelstad, A.; Pedersen-Bjergaard, S. Electromembrane extraction of substances with weakly basic properties: A fundamental study with benzodiazepines. Bioanalysis 2018, 10, 769–781. [Google Scholar] [CrossRef]
- Minhaz, M.; Rahman, M.; Ahsan, M.; Chowdhury, M. Enhancement of solubility and dissolution properties of clonazepam by solid dispersions. Int. J. Pharm. Life Sci. 2012, 3, 1510–1518. [Google Scholar]
- Seshadri, V.C.; Manohari, P.J.; Kunchithapatham, J. Formulation and characterization of mouth dissolving tablets containing benzodiazepine. Indo Am. J. Pharm Res. 2013, 3, 1457–1474. [Google Scholar]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Pharm. Sci. 1998, 1, 15–30. [Google Scholar]
- Malonne, H.; Sonet, B.; Streel, B.; Lebrun, S.; De Niet, S.; Sereno, A.; Vanderbist, F. Pharmacokinetic evaluation of a new oral sustained release dosage form of tramadol. Br. J. Clin. Pharmacol. 2004, 57, 270–278. [Google Scholar] [CrossRef][Green Version]
- Chowdary, Y.A.; Raparla, R.; Madhuri, M. Formulation and evaluation of multilayered tablets of pioglitazone hydrochloride and metformin hydrochloride. J. Pharm. 2014, 2014, 848243. [Google Scholar] [CrossRef]
- Morovati, A.; Ghaffari, A. Single Layer Extended Release Two-in-One Guaifenesin Matrix Tablet: Formulation Method, Optimization, Release Kinetics Evaluation and Its Comparison with Mucinex® Using Box-Behnken Design. Iran. J. Pharm. Res. IJPR 2017, 16, 1349. [Google Scholar]
- Rashed, S.S.; Tushar, R.R.; Ahmed, F.; Vabna, N.J.; Jahan, L.; Billah, M.M. Comparative in vitro Dissolution Study of Clonazepam Tablets of Bangladesh by UV-Visible Spectrophotometry. ACTA Pharm. Sci. 2021, 59, 641–653. [Google Scholar] [CrossRef]
- ICHHT Guideline. ICH guidelines for Validation of analytical procedures: Text and methodology Q2 (R1). In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 2–4 March 2005; pp. 11–12. [Google Scholar]
- Bolla, S.; Segji, S.; Nallipogu, V.; Kumar, V.; Chetty, C. Formulation and evaluation of orodispersible tablets of clonazepam using natural superdisintegrants. J. Pharm. Biol. Sci. 2014, 9, 47–52. [Google Scholar] [CrossRef]
- Sharaf, Y.A.; El Deeb, S.; Ibrahim, A.E.; Al-Harrasi, A.; Sayed, R.A. Two Green Micellar HPLC and Mathematically Assisted UV Spectroscopic Methods for the Simultaneous Determination of Molnupiravir and Favipiravir as a Novel Combined COVID-19 Antiviral Regimen. Molecules 2022, 27, 2330. [Google Scholar] [CrossRef] [PubMed]
- Al Ashmawy, A.Z.G.; Eissa, N.G.; El Nahas, H.M.; Balata, G.F. Fast disintegrating tablet of Doxazosin Mesylate nanosuspension: Preparation and characterization. J. Drug Deliv. Sci. Technol. 2021, 61, 102210. [Google Scholar] [CrossRef]
- Abd El-Hay, S.S.; Elhenawee, M.; Maged, K.; Ibrahim, A.E. Cost-effective, green HPLC determination of losartan, valsartan and their nitrosodiethylamine impurity: Application to pharmaceutical dosage forms. R. Soc. Open Sci. 2022, 9, 220250. [Google Scholar] [CrossRef]
- Ashraf, Z.; Khurram, S.; Maqbool, U.; Khan, K.R.; Ajaz, U.; Fatima, S.F.; Naz, S.; Muneer, F.; Irfan, U.; Ayub, M. Assay of clopidogrel hydrogen sulphate in tablet form from different manufacturing sources by using UV spectroscopy. W. J. Pharm. Res. 2015, 4, 377–381. [Google Scholar]
- Ofori-Kwakye, K.; Osei-Yeboah, F.; Kipo, S.L. Formulation and quality evaluation of two conventional release tablet formulations. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 94–99. [Google Scholar]
- Mohire, N.; Yadav, A. Novel approach to formulate β-cyclodextrin complexed mouth dissolving tablet of metronidazole and its in-vitro evaluation. J. Pharm. Res. 2010, 3, 662–667. [Google Scholar]
- Sharma, D.; Singh, M.; Kumar, D.; Singh, G. Formulation development and evaluation of fast disintegrating tablet of cetirizine hydrochloride: A novel drug delivery for pediatrics and geriatrics. J. Pharm. 2014, 2014, 808167. [Google Scholar] [CrossRef]
- He, L.W.; Zeng, L.; Tian, N.; Li, Y.; He, T.; Tan, D.M.; Zhang, Q.; Tan, Y. Optimization of food deprivation and sucrose preference test in SD rat model undergoing chronic unpredictable mild stress. Anim. Models Exp. Med. 2020, 3, 69–78. [Google Scholar] [CrossRef]
- Nin, M.S.; Couto-Pereira, N.S.; Souza, M.F.; Azeredo, L.A.; Ferri, M.K.; Dalprá, W.L.; Gomez, R.; Barros, H.M. Anxiolytic effect of clonazepam in female rats: Grooming microstructure and elevated plus maze tests. Eur. J. Pharmacol. 2012, 684, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.; Barros, H.M. Clonazepam increases in vivo striatal extracellular glucose in diabetic rats after glucose overload. Pharmacol. Biochem. Behav. 2003, 76, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Crusio, W.E.; Gerlai, R.T. Handbook of Molecular-Genetic Techniques for Brain and Behavior Research; Elsevier: Amsterdam, The Netherland, 1999. [Google Scholar]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Mura, P.; Bragagni, M.; Mennini, N.; Cirri, M.; Maestrelli, F. Development of liposomal and microemulsion formulations for transdermal delivery of clonazepam: Effect of randomly methylated β-cyclodextrin. Int. J. Pharm. 2014, 475, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Sakata, O.; Onishi, H.; Machida, Y. Clonazepam oral droplets for the treatment of acute epileptic seizures. Drug Dev. Ind. Pharm. 2008, 34, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Al-Tahan, F.; Löscher, W.; Frey, H. Pharmacokinetics of clonazepam in the dog. Arch. Int. Pharmacodyn. Thérapie 1984, 268, 180–193. [Google Scholar]
- Gombas, A.; Szabó-Révész, P.; Kata, M.; Regdon, G.; Erős, I. Quantitative determination of crystallinity of α-lactose monohydrate by DSC. J. Therm. Anal. Calorim. 2002, 68, 503–510. [Google Scholar] [CrossRef]
- Wójcik-Pastuszka, D.; Krzak, J.; Macikowski, B.; Berkowski, R.; Osiński, B.; Musiał, W. Evaluation of the release kinetics of a pharmacologically active substance from model intra-articular implants replacing the cruciate ligaments of the knee. Materials 2019, 12, 1202. [Google Scholar] [CrossRef]
- Patra, C.N.; Kumar, A.B.; Pandit, H.K.; Singh, S.P.; MEDURI, V.D. Design and evaluation of sustained release bi-layer tablets of propranolol hydrochloride. Acta Pharm. 2007, 57, 479–489. [Google Scholar] [CrossRef]
- Barakat, N.S.; Elbagory, I.M.; Almurshedi, A.S. Controlled-release carbamazepine granules and tablets comprising lipophilic and hydrophilic matrix components. AAPS PharmSciTech 2008, 9, 1054–1062. [Google Scholar] [CrossRef]
- Merchant, H.A.; Shoaib, H.M.; Tazeen, J.; Yousuf, R.I. Once-daily tablet formulation and in vitro release evaluation of cefpodoxime using hydroxypropyl methylcellulose: A technical note. AAPS PharmSciTech 2006, 7, E178–E183. [Google Scholar] [CrossRef] [PubMed]
- Roni, M.A.; Islam, M.S.; Kibria, G.; Sadat, S.M.A.; Rony, R.; Rahman, H.; Jalil, R.U. Effects of poloxamer and HPMC on the dissolution of clonazepam-polyethylene glycol solid dispersions and tablets. Ind. J. Pharm. Educ. Res. 2011, 45, 139. [Google Scholar]
- O’Neil, M.J. The Merck Index: An Encyclopedia of Chemicals, Drugs and Biologicals; Royal Society of Chemistry: London, UK, 2013; Volume 15. [Google Scholar]
- Awasthi, R.; Kulkarni, G.T.; Ramana, M.V.; Pinto, T.d.J.A.; Kikuchi, I.S.; Ghisleni, D.D.M.; de Souza Braga, M.; De Bank, P.; Dua, K. Dual crosslinked pectin–alginate network as sustained release hydrophilic matrix for repaglinide. Int. J. Biol. Macromol. 2017, 97, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R. Floating in-situ raft forming liquid gastroretentive drug delivery system containing poorly water soluble drug. Bull. Pharm. Sci. 2021, 44, 301–312. [Google Scholar] [CrossRef]
- Larhrib, H.; Martin, G.P.; Prime, D.; Marriott, C. Characterisation and deposition studies of engineered lactose crystals with potential for use as a carrier for aerosolised salbutamol sulfate from dry powder inhalers. Eur. J. Pharm. Sci. 2003, 19, 211–221. [Google Scholar] [CrossRef]
- Kaialy, W.; Martin, G.P.; Ticehurst, M.D.; Royall, P.; Mohammad, M.A.; Murphy, J.; Nokhodchi, A. Characterisation and deposition studies of recrystallised lactose from binary mixtures of ethanol/butanol for improved drug delivery from dry powder inhalers. AAPS J. 2011, 13, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.M.; Mohammed, E.J. Preparation and In-Vitro Evaluation of Clopidogrel Bisulfate Liquisolid Compact. Iraqi J. Pharm. Sci. 2018, 135–149. [Google Scholar] [CrossRef]
- Barros, H.; Tannhauser, S.; Tannhauser, M.; Tannhauser, M. The effects of GABAergic drugs on grooming behaviour in the open field. Pharmacol. Toxicol. 1994, 74, 339–344. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).