Abstract
The wide variety of potential pathogeneses for alopecia and the wide variety of active pharmaceutical ingredients (APIs) to treat and manage those pathogeneses highlight the importance of the development of stable and effective topical treatments. Topical options for alopecia on the market remain limited and oral products may result in unwanted systemic adverse effects. This study is meant to fill the gap by determining compatibility in terms of beyond-use date (BUD) of APIs with theoretical or demonstrated benefits for topical use for alopecia. The compatibility of seven formulations was tested: F1 = clobetasol 0.05% in TrichoWashTM; F2 = ketoconazole 2% in TrichoWashTM; F3 = spironolactone 1% in TrichoWashTM; F4 = latanoprost 0.1% in TrichoCreamTM; F5 = pyridoxine HCl 0.5%, vitamin A acetate 1%, and vitamin E succinate 12.1 IU in TrichoCondTM; F6 = Caffeine 2%, menthol 1%, and pyridoxine HCl 0.5% in TrichoWashTM; F7 = Latanoprost 0.1%, minoxidil 5%, and finasteride 0.25% in TrichoSolTM. All formulations presented a BUD of 6 months, except for F4 and F7, which showed compatibility for 3 months. This validates the compatibility of the APIs with the TrichotechTM vehicles, and that they are highly convenient for compounding pharmacies.
1. Introduction
Alopecia is a pervasive issue around the world. One study of men between the ages of 18 to 49 years, found that 42% of men had moderate to extensive hair loss. Evidence suggests that androgenetic alopecia, the most common form of alopecia, affects over half of men over 50 years of age; and in women, the reported incidence of alopecia is 29 to 38% for patients over 70 years of age [1,2]. Though alopecia itself often does not cause significant adverse health effects, it can increase and worsen anxiety and depression and those with alopecia often have lower self-esteem and body image [3].
Given the high incidence and significant psychosocial impact of alopecia, options for the management and treatment of hair loss are essential. Commercially available topical options are limited, and oral options can be related to possible adverse effects. In this study, we explored the compatibility of several active pharmaceutical ingredients (APIs) in bases designed to be used for patients with alopecia, including TrichoWashTM (a ready-to-use vehicle for personalized non-irritant shampoos), TrichoCondTM (a ready-to-use vehicle for personalized conditioners and with moisturizing properties), TrichoCreamTM (a ready-to-use vehicle for personalized creams for eyelashes, eyebrows, and beards), and TrichoSolTM (a ready-to-use vehicle for personalized hair solutions; consisting of a highly spreadable hydrophilic solution containing mineral salts of vegetable origin and is free from alcohol and propylene glycol), each of which contains TrichoTechTM, a patented technology designed to promote scalp health. All vehicles are free from parabens, formaldehyde, PABA, boric acid, benzyl alcohol, benzyl benzoate, propylene glycol, lanolin, triclosan, 1,4-dioxane, petrolatum, and artificial colorants [4].
The chosen APIs were caffeine, clobetasol propionate, finasteride, ketoconazole, latanoprost, menthol, minoxidil, pyridoxine hydrochloride, spironolactone, vitamin A acetate, and vitamin E succinate. Those comprised FDA-approved APIs for androgenetic alopecia (finasteride, minoxidil, and dutasteride) and also APIs with scientific evidence of potential for such conditions, either in isolation or as add-on therapies. Studies evaluating caffeine have found that topical preparations can significantly increase the number of hairs in anagen phase (growth phase) [5]. Its safety and efficacy profile based on current information warrants studies to ensure compatibility in topical vehicles. Clobetasol propionate is a steroid that has demonstrated efficacy in adults as well as pediatric patients suffering from alopecia areata [6,7]. Alternatively, finasteride for topical use has been well studied at a variety of strengths for androgenetic alopecia; the topical use allows the successful treatment of alopecia while avoiding higher systemic levels associated with oral use [8,9,10]. Similar to finasteride, spironolactone is another anti-androgenic drug that has been studied for topical use to avoid unwanted adverse effects that may be associated with oral use, such as those associated with its mechanism of action as a diuretic [11]. Other options such as latanoprost or minoxidil have a history of topical use but may not be available at the appropriate strength for use on the scalp, such as in the case of latanoprost or, as is the case with minoxidil, are often available in hydroalcoholic vehicles or vehicles that contain propylene glycol, which may cause drying or irritation of the scalp or even contact dermatitis [12,13]. In addition to these APIs, other vitamin deficiencies have been linked to hair loss as well, and topical options for these vitamins, such as pyridoxine, vitamin A, or vitamin E may help to support a healthy scalp and correct deficiencies that may be contributing to hair loss or lack of new hair growth [14].
The wide variety of potential pathogeneses for alopecia and the wide variety of APIs to treat and manage those pathogeneses highlight the importance of the development of stable and effective topical treatments. Topical options for alopecia on the market remain limited and oral products may result in unwanted systemic adverse effects. This study is meant to fill the gap by determining the compatibility of active ingredients with theoretical or demonstrated benefits as a topical option for alopecia. In this study ketoconazole, clobetasol propionate, spironolactone, and latanoprost were evaluated as individual agents in vehicles designed for topical use on the scalp for alopecia. Combination products of pyridoxine HCl, vitamin E, and vitamin A, as well as caffeine, menthol, and pyridoxine, and lastly, a combination of latanoprost, minoxidil, and finasteride were evaluated as combination formulations to demonstrate compatibility in bases well-suited for use for alopecia.
2. Materials and Methods
2.1. Preparation of Active Pharmaceutical Ingredients Cream Samples
The composition of the seven tested samples is described in Table 1. Those formulations (qualitative composition and concentrations) were chosen within the context of personalized medicine, meaning that compounding pharmacies often receive those prescriptions with doses and combinations different from the products available in the market, so to offer unique treatments for some patients. All formulations were prepared as described below and all materials were provided by Fagron (Saint Paul, MN, USA).

Table 1.
Active Pharmaceutical Ingredients Studied and their Formulations.
- F1. Clobetasol 0.05% in TrichoWashTM
- Weigh clobetasol propionate in a hood;
- Measure approximately 50% of the required TrichoWashTM and incorporate clobetasol propionate until a paste is obtained, using glass mortar and pestle;
- Transfer to a light-resistant polyethylene dispensing container, adding the required total amount of TrichoWashTM, rinsing the mortar;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
- F2. Ketoconazole 2% in TrichoWashTM
- Weigh ketoconazole in a hood;
- Dissolve ketoconazole with DMSO, using glass mortar and pestle;
- Slowly add approximately 80% of the required TrichoWashTM, using diametric dilution;
- Transfer to a light-resistant polyethylene dispensing container and bring to volume;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
- F3. Spironolactone 1% in TrichoWashTM
- Weigh spironolactone in a hood;
- Dissolve spironolactone with ethoxy diglycol, using a magnetic stirrer;
- In an Unguator jar, weigh approximately 50% of the required TrichoWashTM. Add the spironolactone/ethoxy diglycol solution, and then the rest of the required TrichoWashTM;
- Spin on Unguator (FagronLab, Scheßlitz, Germany) slowly, on direct speed 2/2/2/.
- Transfer to a light-resistant polyethylene dispensing container and bring to volume;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
- F4. Latanoprost 0.1% in TrichoCreamTM
- Measure required amount of latanoprost;
- Weigh 50% of required TrichoCreamTM;
- Incorporate latanoprost into TrichoCreamTM;
- Add the remaining amount of required TrichoCreamTM to Unguator jar and spin at normal speed;
- Transfer to a light-resistant polyethylene dispensing jar;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
- F5. Pyridoxine HCl 0.5%, Vitamin A acetate 1%, and Vitamin E succinate 12.1 IU in TrichoCondTM
- Weigh pyridoxine HCl, vitamin A acetate, and Vitamin E succinate in a hood;
- Place vitamin A in purified water and heat to 50 °C and mix until homogeneously suspended;
- Measure approximately 50% of the required TrichoCondTM (which is an emulsion) and incorporate Vitamin A, Vitamin E, and pyridoxine HCl into a paste for the solubilization of the APIs, using a glass mortar and pestle;
- Once mixed, transfer to a light-resistant polyethylene dispensing container and bring to volume with TrichoCondTM;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
- F6. Caffeine 2%, menthol 1%, and pyridoxine HCl 0.5% in TrichoWashTM
- Weigh caffeine, menthol, and pyridoxine HCl in a hood;
- Triturate and dissolve menthol in DMSO, using a separate glass mortar and pestle;
- Heat purified water to 150 °C and dissolve caffeine, mixing;
- Dissolve pyridoxine HCl in heated caffeine/water mixture, then let it achieve room temperature;
- Tare a speed mixer jar and add the pyridoxine HCl/caffeine/water mixture and the menthol/DMSO mixture;
- Bring to volume with the heated TrichoWashTM and lightly stir with a glass stir rod;
- Transfer to a light-resistant polyethylene dispensing container;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
- F7. Latanoprost 0.1%, minoxidil 5%, and finasteride 0.25% in TrichoSolTM
- Weigh minoxidil and finasteride in a hood;
- Triturate and dissolve finasteride in ethoxy diglycol, using a magnetic stirrer;
- Measure required amount of latanoprost;
- Measure approximately 80% of the required TrichoSolTM. Add finasteride/ethoxy diglycol, then dissolve minoxidil, with mixing;
- Add latanoprost to the previous solution and mix well;
- Transfer to a light-resistant polyethylene dispensing container and bring to volume with TrichoSolTM;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
2.2. Compatibility Study
The API samples were assayed by high performance liquid chromatography (HPLC) at pre-determined time points to verify the compatibility of the API in the vehicles. Aliquots for quantification (variable for each API) were withdrawn and diluted to obtain work solutions in the concentration described in Table 2. Sampling times were initial (T = 0), 3 months (T = 3), and 6 months (T = 6), depending on the formulation. All products were stored at controlled room temperature (20–25 °C) throughout the whole study.

Table 2.
Chromatographic Conditions Used in the Compatibility Study.
All products were assayed three times, and the results were expressed as the mean from the independent measurements. The evaluation parameter was the percent recovery, using the HPLC method (results given as percentage ± standard deviation).
2.3. Chromatographic Conditions
All analyses were performed by stability-indicating HPLC methods using the conditions described in Table 2. The APIs contents were calculated by direct comparison of their area with the one of the standard. All samples were diluted to the work concentration with mobile phase, and then filtered through a 0.45 mm filter membrane and degassed using an ultrasonic apparatus for 30 min immediately before use. The standards were also diluted in the mobile phase. All columns were from Phenomenex (Torrance, CA, USA) and were connected with a pre-column with the same packing (4.0 × 3.0 mm, 5 mm) from the same manufacturer. All volumetric glassware and analytical balances used were calibrated.
3. Results
At each sampling time, the visual appearance of the products was also evaluated to verify their homogeneity and physical compatibility (data not shown). Throughout the whole study, none of the following phenomena were observed: caking, flocculation, macroscopically visible crystal growth, odor generation, phase separation, precipitation, or turbidity.
The chemical compatibility results are shown in Table 3 and Figure 1, expressed as the percent of recovery in relation to T = 0. For the products to be considered stable, the relative percentage recovery should lie within 90% to 110%.

Table 3.
Compatibility of the Active Pharmaceutical Ingredients in the Hair Vehicles.
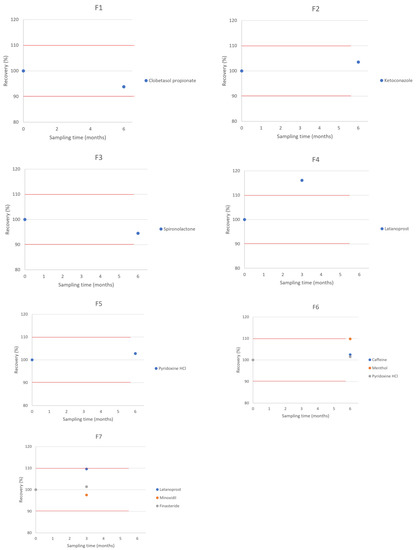
Figure 1.
Compatibility of the Selected APIs in the Hair Vehicles. Red lines represent the lower and upper limits, corresponding to 90% and 110% of labeled concentration.
In our study, a beyond-use date (BUD) of 6 months was observed for all products stored at room temperature, except for the ones containing latanoprost, which presented a BUD of 3 months.
In the absence of compatibility information for a specific formulation, USP <795> (Pharmaceutical compounding—nonsterile preparations) can be used as a guideline, which advises a beyond-use date of no more than 30 days [15]. The longer BUD of 3 or 6 months presented in this study offers increased convenience for both the compounding pharmacist and the patient.
4. Conclusions
We have tested seven formulations using TrichoTechTM hair vehicles, and found the following beyond-use dates (BUD):
- F1 = clobetasol 0.05% in TrichoWashTM. BUD = 6 months.
- F2 = ketoconazole 2% in TrichoWashTM. BUD = 6 months.
- F3 = spironolactone 1% in TrichoWashTM. BUD = 6 months.
- F4 = latanoprost 0.1% in TrichoCreamTM. BUD = 3 months.
- F5 = pyridoxine HCl 0.5%, vitamin A acetate 1% and vitamin E succinate 12.1 IU in TrichoCondTM. BUD = 6 months.
- F6 = caffeine 2%, menthol 1%, and pyridoxine HCl 0.5% in TrichoWashTM. BUD = 6 months.
- F7 = latanoprost 0.1%, minoxidil 5%, and finasteride 0.25% in TrichoSolTM. BUD = 3 months.
All formulations presented a beyond-use date of at least 3 or 6 months. Thus, this study showed that the APIs were all compatible with the TrichoTechTM vehicles in the conditions described, reinforcing that the vehicles are compatible with a wide range of APIs. These results validate that the TrichoTechTM hair vehicles are an adequate choice for use by compounders.
Author Contributions
Conceptualization, methodology, C.Z., S.T.; writing—original draft preparation, writing—review and editing, H.P., C.Z., S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors work for Fagron. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Rhodes, T.; Girman, C.J.; Savin, R.C.; Kaufman, K.D.; Guo, S.; Lilly, F.R.W.; Siervogel, R.M.; Chumlea, C.W. Prevalence of Male Pattern Hair Loss in 18–49 Year Old Men. Dermatol. Surg. 1998, 24, 1330–1332. [Google Scholar] [CrossRef]
- Emer, J.; Levy, L.L.L. Female pattern alopecia: Current perspectives. Int. J. Women’s Health 2013, 5, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.; McHale, S. The psychological impact of alopecia. BMJ 2005, 331, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.; Jardim, M.; Antunes, V.M.D.S.; Michelin, L.F.G.; dos Santos, B.A.R.; Barbosa, C.M.V.; Spindola, D.G.; Bincoletto, C.; Oliveira, C.R. In Vitro Effects of the Phytocomplex TrichoTechTM on Human Fibroblasts: Proliferative Potential and Effects on Gene Expression of FGF-7 and FGF-10. J. Cosmet. Dermatol. Sci. Appl. 2017, 7, 1–13. [Google Scholar] [CrossRef][Green Version]
- Dhurat, R.; Chitallia, J.; May, T.W.; Jayaraaman, A.M.; Madhukara, J.; Anandan, S.; Vaidya, P.; Klenk, A. An Open-Label Randomized Multicenter Study Assessing the Noninferiority of a Caffeine-Based Topical Liquid 0.2% versus Minoxidil 5% Solution in Male Androgenetic Alopecia. Ski. Pharmacol. Physiol. 2017, 30, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Lenane, P.; MacArthur, C.; Parkin, P.C.; Krafchik, B.; DeGroot, J.; Khambalia, A.; Pope, E. Clobetasol Propionate, 0.05%, vs Hydrocortisone, 1%, for Alopecia Areata in Children. JAMA Dermatol. 2014, 150, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Piraccini, B.M.; Pazzaglia, M.; Vincenzi, C. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J. Am. Acad. Dermatol. 2003, 49, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Loconsole, G.; Cammisa, G.; Mastrolonardo, G.; Vena, G. Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a 16-month therapy course. J. Dermatol. Treat. 1997, 8, 189–192. [Google Scholar] [CrossRef]
- Hajheydari, Z.; Akbari, J.; Saeedi, M.; Shokoohi, L. Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Srisuwanwattana, P.; Chalermroj, N.; Khunkhet, S. A randomized, double-blind controlled study of the efficacy and safety of topical solution of 0.25% finasteride admixed with 3% minoxidil vs. 3% minoxidil solution in the treatment of male androgenetic alopecia. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2257–2263. [Google Scholar] [CrossRef] [PubMed]
- Dill-Müller, D.; Zaun, H. Topical Treatment of Androgenetic Alopecia with Spironolactone. J. Eur. Acad. Dermatol. Venereol. 1997, 9, S31. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Lönnfors, S.; Hillmann, K.; Bartels, N.G. A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J. Am. Acad. Dermatol. 2012, 66, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Aleid, N.M.; Fertig, R.; Maddy, A.; Tosti, A. Common Allergens Identified Based on Patch Test Results in Patients with Suspected Contact Dermatitis of the Scalp. Ski. Appendage Disord. 2016, 3, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. 2018, 9, 51–70. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia. <795> Pharmaceutical Compounding—Nonsterile Preparations; United States Pharmacopeia: Rockville, MD, USA, 2020. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).