Formulation Optimization of Extemporaneous Oral Liquids Containing Naloxone and Propranolol for Pediatric Use
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
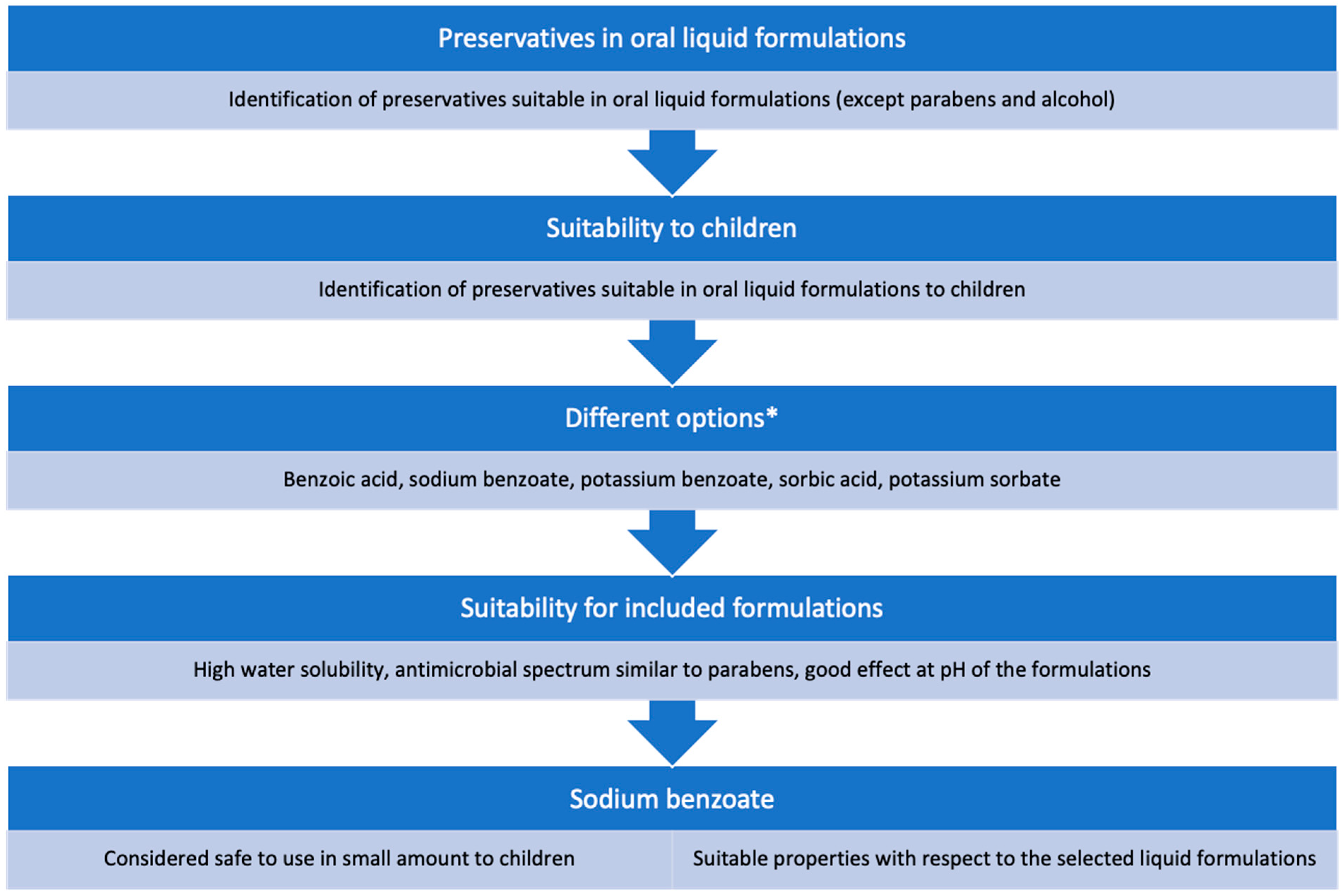
2.2. Choice of Preservative and Solubilizer
2.3. Manufacture of the Liquid Formulations
2.4. Experimental Plan
2.5. Optimization and Evaluation Using MODDE Pro
2.6. Evaluation of Oral Liquids
2.6.1. Solubility
2.6.2. pH
2.6.3. Viscosity
2.6.4. Osmolality
2.6.5. Dispensed Dose and Dosing Accuracy
2.6.6. Preservative Effect
3. Results and Discussion
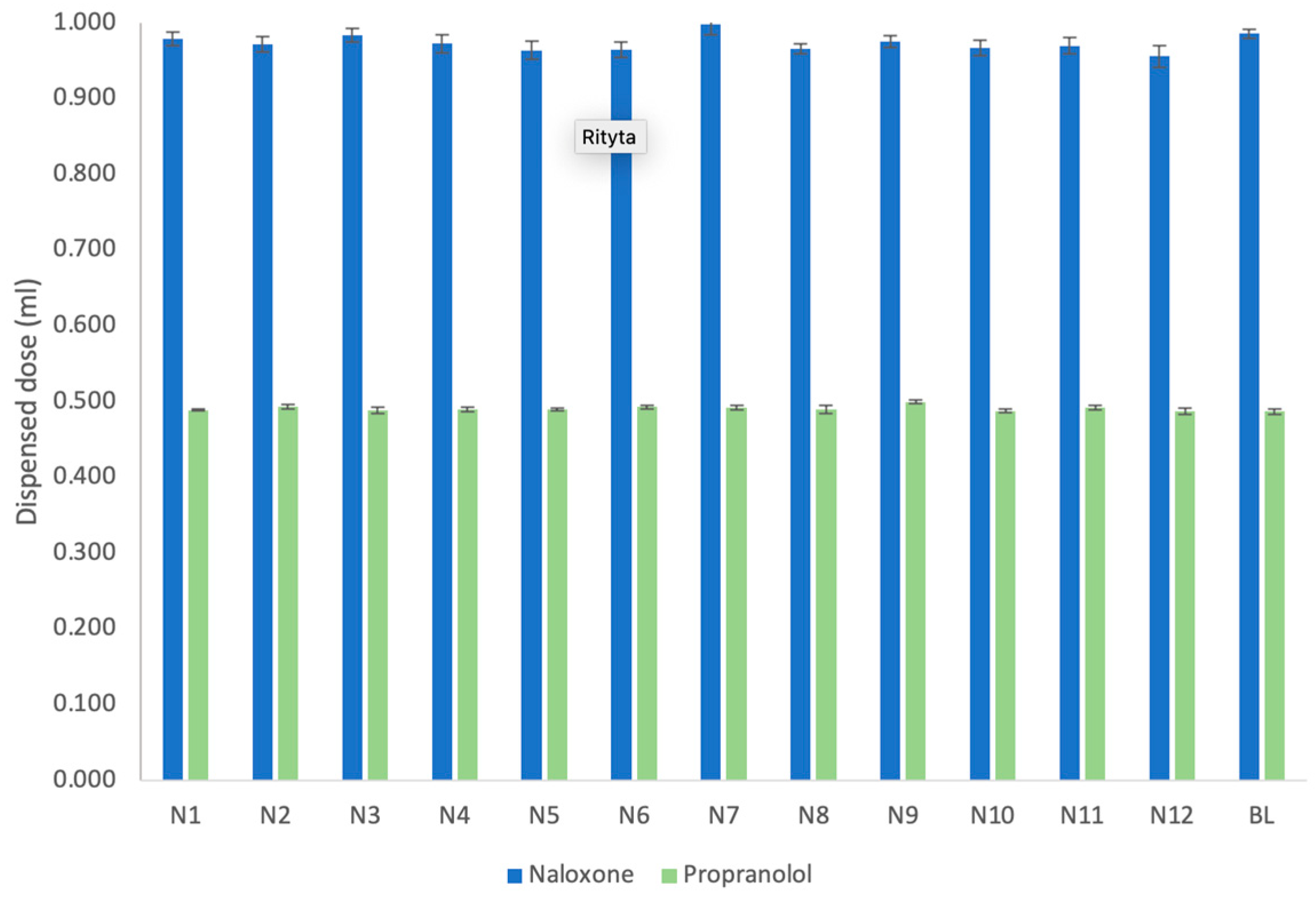
3.1. Dispensed Dose and Dosing Accuracy
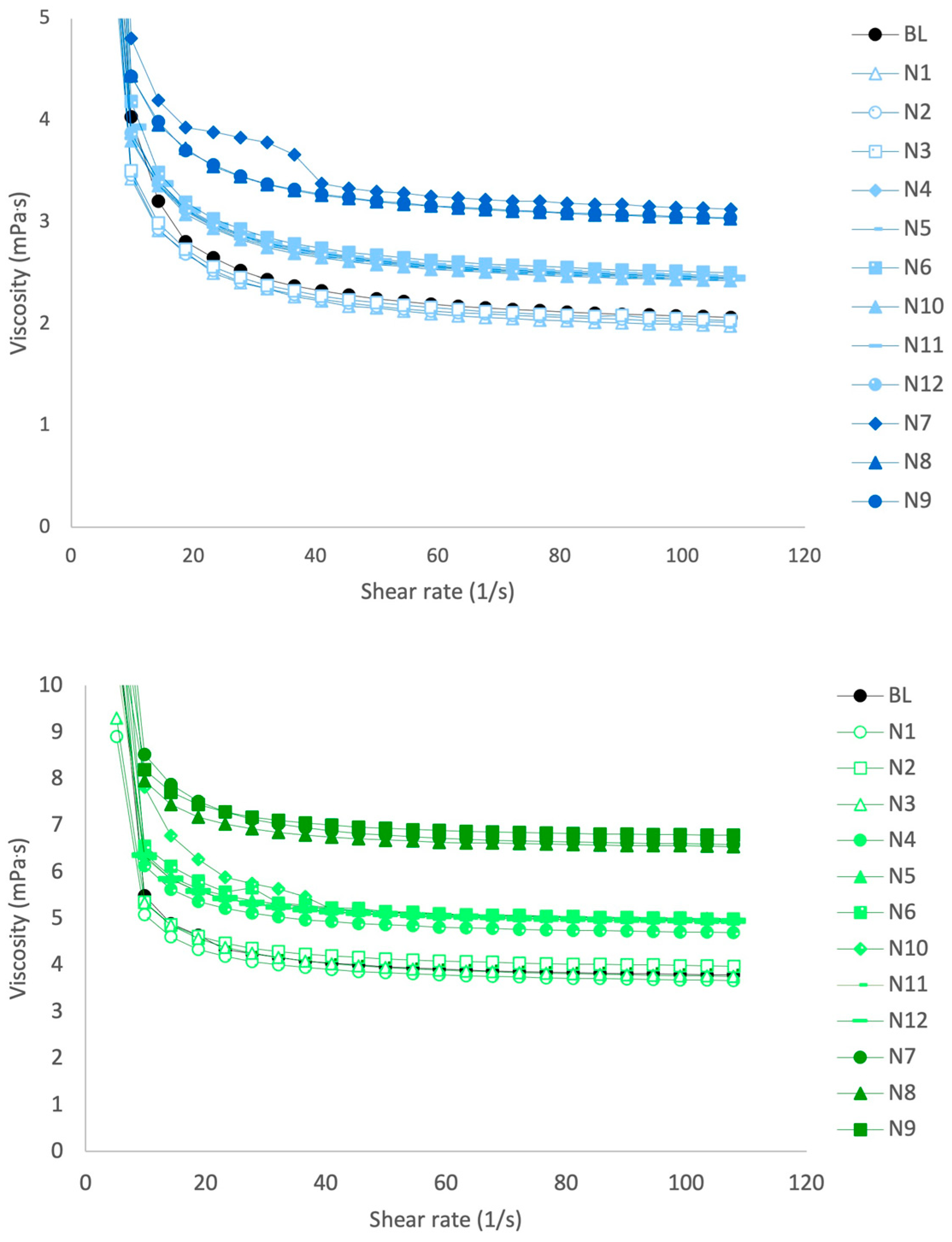
3.2. Viscosity
3.3. Solubility
3.4. Osmolality
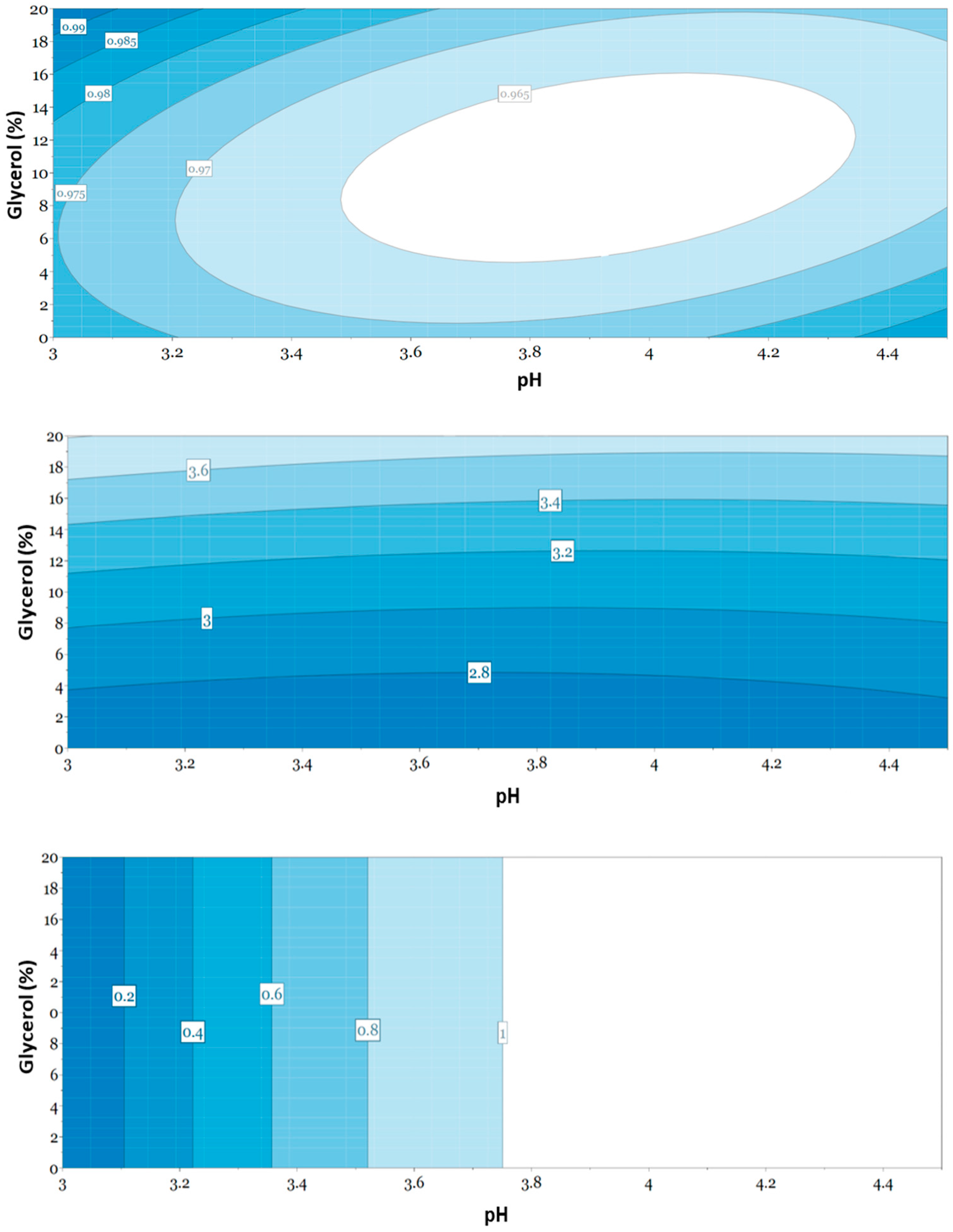
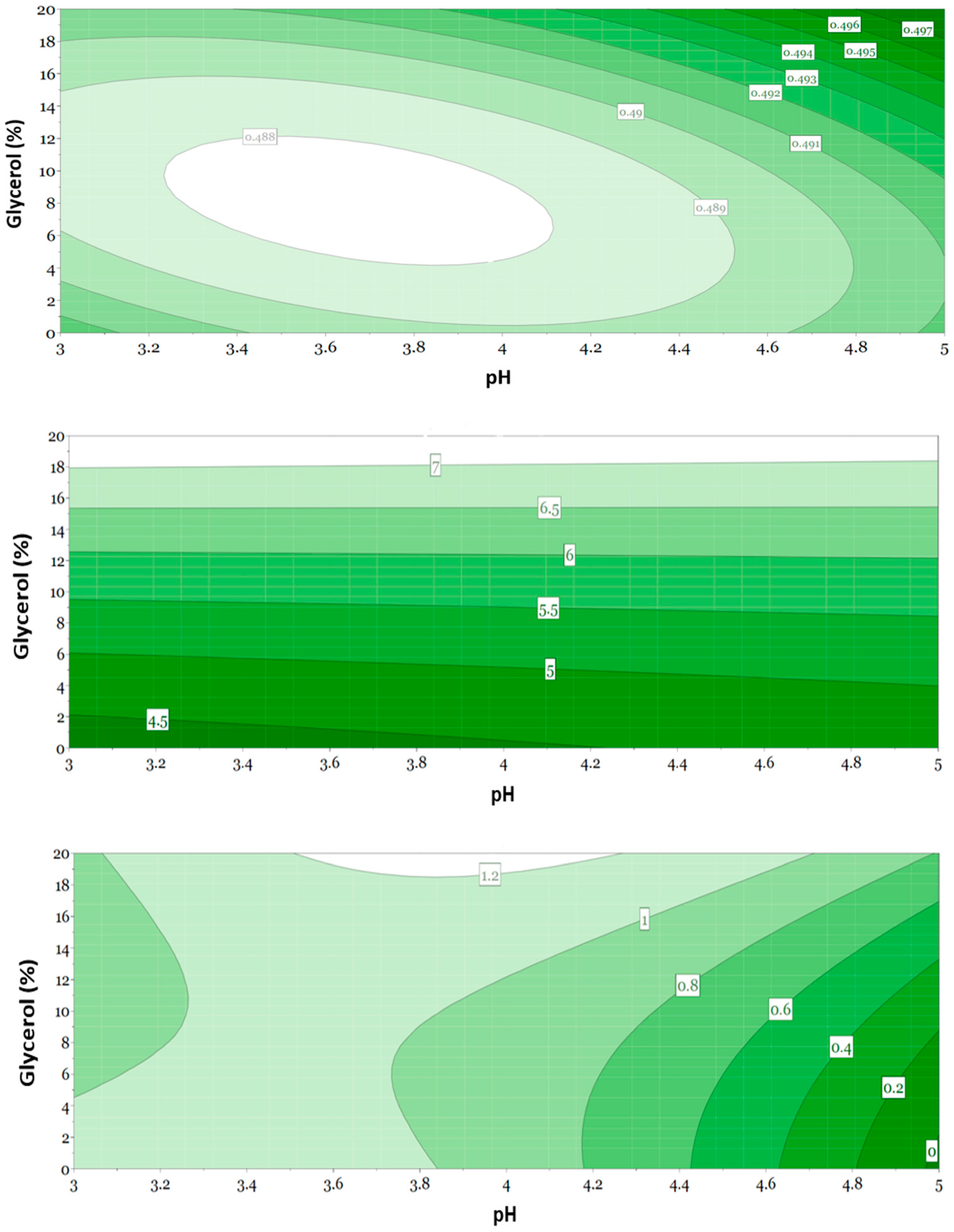
3.5. Evaluation of Responses Using Multivariate Data Analysis
3.6. Evaluation of Selected Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, H.C.; Garbe, M.C.; Lees, J.; Aziz, N.; Chaaban, H.; Miller, J.L.; Johnson, P.; DeLeon, S. Off-label medication use in children, more common than we think: A Systematic review of the literature. J. Okla State Med. Assoc. 2018, 111, 776–783. [Google Scholar] [PubMed]
- Madathilethu, J.; Roberts, M.; Peak, M.; Blair, J.; Prescott, R.; Ford, J.L. Content uniformity of quartered hydrocortisone tablets in comparison with mini-tablets for paediatric dosing. BMJ Paediatr. Open 2018, 2, e000198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zidan, A.; Tawakkul, M.; Sayeed, V.A.; Khan, M. Tablet splitting: Product quality assessment of metoprolol succinate extended-release tablets. Int. J. Pharm. 2010, 401, 25–31. [Google Scholar] [CrossRef]
- Freeman, M.K.; White, W.; Iranikhah, M. Tablet splitting: A review of weight and content uniformity. Consult. Pharm. 2012, 27, 341–352. [Google Scholar] [CrossRef]
- Nidanapu, R.P.; Rajan, S.; Mahadevan, S.; Gitanjali, B. Tablet splitting of antiepileptic drugs in pediatric epilepsy: Potential effect on plasma drug concentrations. Paediatr. Drugs 2016, 18, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Schier, J.G.; Howland, M.A.; Hoffman, R.S.; Nelosn, L.S. Fatality from administration of labetalol and crushed extended-release nifedipine. Ann. Pharmacother. 2003, 37, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Guideline on Pharmaceutical Development of Medicines for Paediatric Use. European Medicines Agency. 2014. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmaceutical-development-medicines-paediatric-use_en.pdf (accessed on 7 October 2021).
- Reflection Paper: Formulations of Choice for the Paediatric Population. European Medicines Agency. 2006. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-formulations-choice-paediatric-population_en.pdf (accessed on 7 October 2021).
- Development of Paediatric Medicines: Points to Consider in Formulation. World Health Organization. 2012. WHO Technical Report Series; No 970, Annex 5. Available online: https://www.who.int/medicines/areas/quality_safety/quality_assurance/Annex5TRS-970.pdf?ua=1 (accessed on 7 October 2021).
- Ernest, T.B.; Elder, D.P.; Martini, L.G.; Roberts, M.; Ford, J.L. Developing paediatric medicines: Identifying the needs and recognizing the challenges. J. Pharm. Pharmacol. 2007, 59, 1043–1055. [Google Scholar] [CrossRef] [Green Version]
- Van Riet-Nales, D.A.; Kozarewicz, P.; Aylward, B.; de Vries, R.; Egberts, T.C.G.; Rademaker, C.M.A.; Schobben, A.F.A.M. Paediatric drug development and formulation design—A European perspective. AAPS PharmSciTech 2017, 18, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Krause, J.; Breitkreutz, J. Improving drug delivery in paediatric medicine. Pharm. Med. 2008, 22, 41–50. [Google Scholar] [CrossRef]
- Lopez, F.L.; Ernest, T.B.; Tuleu, C.; Gul, M.O. Formulation approaches to pediatric oral drug delivery: Benefits and limitations of current platforms. Expert Opin. Drug Deliv. 2015, 12, 1727–1740. [Google Scholar] [CrossRef]
- Batchelor, H.K.; Marriott, J.F. Formulations for children: Problems and solutions. Br. J. Clin. Pharmacol. 2015, 79, 405–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuleu, C.; Breitkreutz, J. Educational paper: Formulation-related issues in pediatric clinical pharmacology. Eur. J. Pediatr. 2013, 172, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Haywood, A.; Glass, B.D. Liquid dosage forms extemporaneously prepared from commercially available products—Considering new evidence on stability. J. Pharm. Pharm. Sci. 2013, 16, 441–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistry, P.; Batchelor, H. Evidence of acceptability of oral paediatric medicines: A review. J. Pharm. Pharmacol. 2017, 69, 361–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klang, M.; McLymont, V.; Ng, N. Osmolality, pH, and compatibility of selected oral liquid medications with an enteral nutrition product. J. Parenter. Enter Nutr. 2013, 37, 689–694. [Google Scholar] [CrossRef]
- Salunke, S.; Brandys, B.; Giacoia, G.; Tuleu, C. The STEP (Safety and Toxicity of Excipients for Paediatrics) database: Part 2-the pilot version. Int. J. Pharm. 2013, 457, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Rouaz, K.; Chiclana-Rodríguez, B.; Nardi-Ricart, A.; Suñé-Pou, M.; Mercadé-Frutos, D.; Suñé-Negre, J.M.; Pérez-Lozano, P.; García-Montoya, E. Excipients in the Paediatric Population: A Review. Pharmaceutics 2021, 13, 387. [Google Scholar] [CrossRef]
- Swarbrick, J.; Boylan, J.C. Encyclopedia of Pharmaceutical Technology; Marcel Dekker: New York, NY, USA, 1996; Volume 13, pp. 2983–2992. [Google Scholar]
- Aulton, M.E.; Taylor, K.M. Aulton’s Pharmaceutics—The Design and Manufacture of Medicine, 5th ed.; Churchill Livingstone Elsevier: Edinburgh, UK, 2013. [Google Scholar]
- Zupanets, K.O.; Shebeko, S.K.; Ratushna, K.L.; Katilov, O.V. Cumulative Risks of Excipients in Pediatric Phytomucolytic Syrups: The Implications for Pharmacy Practice. Sci. Pharm. 2021, 89, 32. [Google Scholar] [CrossRef]
- Batchelor, H.K.; Fotaki, N.; Klein, S. Paediatric oral biopharmaceutics: Key considerations and current challenges. Adv. Drug Deliv. Rev. 2014, 73, 102–126. [Google Scholar] [CrossRef] [Green Version]
- Generally Recognized as Safe (GRAS). FDA. Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 11 December 2021).
- Nowak, K.; Ratajczak-Wrona, W.; Górska, M.; Jabłońska, E. Parabens and their effects on the endocrine system. Mol. Cell Endocrinol. 2018, 474, 238–251. [Google Scholar] [CrossRef]
- Reflection Paper on the Use of Methyl-and Propylparaben as Excipients in Human Medicinal Products for Oral Use. European Medicines Agency. 2015. Available online: www.ema.europa.eu (accessed on 15 October 2021).
- Safety & Toxicity of Excipients for Paediatrics database (STEP) London. European Pediatric Formulation Initiative. 2015. Available online: https://step-db.ucl.ac.uk/eupfi/appDirectLink.do?appFlag=login (accessed on 17 October 2021).
- Mulla, H.; Yakkundi, S.; McElnay, J.; Lutsar, I.; Metsvaht, T.; Varendi, H.; Nellis, G.; Nunn, A.; Duncan, J.; Pandya, H.; et al. An observational study of blood concentrations and kinetics of methyl- and propyl- parabens in neonates. Pharm. Res. 2015, 32, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.F.; Ahlfors, C.E.; Wennberg, R.P. The effect of paraben preservatives on albumin binding of bilirubin. J. Pediatr. 1976, 89, 475–478. [Google Scholar] [CrossRef]
- Contreras, L.; Cram, A.; English, C.; Heimlich, J. Pharmaceutical Excipients in Pediatric Formulations. In Handbook of Pharmaceutical Excipients; Pharmaceutical Press: London, UK, 2016. [Google Scholar]
- Whittaker, A.; Currie, A.E.; Turner, M.A.; Field, D.J.; Mulla, H.; Pandya, H.C. Toxic additives in medication for preterm infants. Arch. Dis. Child Fetal Neonatal. Ed. 2009, 94, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Valeur, K.S.; Hertel, S.A.; Lundstrøm, K.E.; Holst, H. The cumulative daily tolerance levels of potentially toxic excipients ethanol and propylene glycol are commonly exceeded in neonates and infants. Basic Clin. Pharmacol. Toxicol. 2018, 122, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Tissel, L.A. Handbook on Injectable Drugs, 13th ed.; Publications Production Center of the American Society of Health-System Pharmacists: Bethesda, MD, USA, 2005. [Google Scholar]
- Drug Bank. The Governors of the University of Alberta. 2016. Available online: https://www.drugbank.ca/ (accessed on 13 December 2021).
- Product Information Naloxone Hydrochloride Dihydrate. Sigma Aldrich. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Sheet/2/n7758pis.pdf (accessed on 4 December 2019).
- Product Information Propranolol Hydrochloride. Sigma Aldrich. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Sheet/1/p5544pis.pdf (accessed on 4 December 2021).
- Neuberger, J.M.; Schweitzer, S.; Rolland, M.O.; Burghard, R. Effect of sodium benzoate in the treatment of atypical nonketotic hyperglycinaemia. J. Inherit Metab Dis. 2000, 23, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Bateman, B.; Warner, J.O.; Hutchinson, E.; Dean, T.; Rowlandson, P.; Gant, C.; Grundy, J.; Fitzgerald, C.; Stevenson, J. The effects of a double blind, placebo controlled, artificial food colourings and benzoate preservative challenge on hyperactivity in a general population sample of preschool children. Arch. Dis. Child 2004, 89, 506–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.Y.; Chang, R.C.; Sowa, M.E.; Chang, A.C.; Abdenur, J.E. Prevention of metabolic decompensation in an infant with mutase deficient methylmalonic aciduria undergoing cardiopulmonary bypass. World J. Pediatr. 2014, 10, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Peltola, H.; Roine, I.; Fernandez, J.; Zavala, I.; Ayala, S.G.; Mata, A.G.; Arbo, A.; Bologna, R.; Mino, G.; Goyo, J.; et al. Adjuvant Glycerol and/or Dexamethasone to Improve the Outcomes of Childhood Bacterial Meningitis: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect Dis. 2007, 45, 1277–1286. [Google Scholar] [CrossRef] [Green Version]
- Singhi, S.; Järvinen, A.; Peltola, H. Increase in serum osmolality is possible mechanism for the beneficial effects of glycerol in childhood bacterial meningitis. Pediatr. Infect Dis. J. 2008, 27, 892–896. [Google Scholar] [CrossRef]
- Sankar, J.; Singhi, P.; Bansal, A.; Ray, P.; Singhi, S. Role of dexamethasone and oral glycerol in reducing hearing and neurological sequelae in children with bacterial meningitis. Ind. Pediatr. 2007, 44, 649–656. [Google Scholar]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients; The Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019.
- Tofil, N.M.; Benner, K.W.; Faro, S.J.; Winkler, M.K. The use of enteral naloxone to treat opioid-induced constipation in a pediatric intensive care unit. Pediatr. Crit. Care Med. 2006, 7, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Padden Elliott, J.; McConaha, J.; Cornish, N.; Bunk, E.; Hilton, L.; Modany, A.; Bucker, I. Influence of viscosity and consumer use on accuracy of oral medication dosing devices. J. Pharm. Technol. 2014, 30, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, P.; Christopherson, J.; Ambrose, P.J.; Corelli, R.L. Accuracy of oral liquid measuring devices: Comparison of dosing cup and oral dosing syringe. Ann. Pharmacother. 2008, 42, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Peacock, G.; Parnapy, S.; Raynor, S.; Wetmore, S. Accuracy and precision of manufacturer-supplied liquid medication administration devices before and after patient education. J. Am. Pharm. Assoc. 2010, 50, 84–86. [Google Scholar] [CrossRef]
- Neves, I.; Auxtero, M.D. Dosing Accuracy of Oral Extemporaneous Suspensions of Antibiotics: Measuring Procedures and Administration Devices. Pharmaceutics 2021, 13, 528. [Google Scholar] [CrossRef]
- Berthe-Aucejo, A.; Girard, D.; Lorrot, M.; Bellettre, X.; Faye, A.; Mercier, J.C.; Brion, F.; Bourdon, O.; Prot-Labarthe, S. Evaluation of frequency of paediatric oral liquid medication dosing errors by caregivers: Amoxicillin and josamycin. Arch. Dis. Child 2016, 101, 359–364. [Google Scholar] [CrossRef]
- Walsh, J.; Bickmann, D.; Breitkreutz, J.; Chariot-Goulet, M.; European Paediatric Formulation Initiative (EuPFI). Delivery devices for the administration of paediatric formulations: Overview of current practice, challenges and recent developments. Int. J. Pharm. 2011, 30, 221–231. [Google Scholar] [CrossRef]
- Fernández, P.A.; Cabañas, P.M.J.; Clemente, B.S.; Oliveras, A.M.; Castillo, S.F.; Hidalgo, A.E. Osmolality of oral liquid dosage forms to be administered to newborns in a hospital. Farm. Hosp. 2007, 31, 311–314. [Google Scholar] [CrossRef]
- Barness, L.A.; Mauer, A.M.; Holliday, M.A.; Anderson, A.S.; Dallman, P.R.; Forbes, G.B.; Goldbloom, R.B.; Haworth, J.C.; Jesse, M.J.; Scriver, C.R.; et al. American Academy of Pediatrics. Commentary on breastfeeding and infant formulas, including proposed standards for formulas. Pediatrics 1976, 57, 278–285. [Google Scholar] [CrossRef]
- Ellis, Z.M.; Tan, H.S.G.; Embleton, N.D.; Sangild, P.T.; van Elburg, R.M. Milk feed osmolality and adverse events in newborn infants and animals: A systematic review. Arch. Dis. Child Fetal Neonatal. Ed. 2019, 104, F333–F340. [Google Scholar] [CrossRef] [Green Version]
- Shah, D.D.; Kuzmov, A.; Clausen, D.; Siu, A.; Robinson, C.A.; Kimler, K.; Meyers, R.; Shah, P. Osmolality of Commonly Used Oral Medications in the Neonatal Intensive Care Unit. J. Pediatr. Pharmacol. Ther. 2021, 26, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tsoi, J.K.; Wong, H.M.; Chu, C.H.; Matinlinna, J.P. Paediatric Over-the-Counter (OTC) Oral Liquids Can Soften and Erode Enamel. Dent. J. 2017, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passos, I.A.; Sampaio, F.C.; Martínez, C.R.; Freitas, C.H. Sucrose concentration and pH in liquid oral pediatric medicines of long-term use for children. Rev. Panam. Salud Publica 2010, 27, 132–137. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Questions and Answers on Benzoic Acid and Benzoates Used as Excipients in Medicinal Products for Human Use. Committee for Medicinal Products for Human Use (CHMP). 2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-benzoic-acid-benzoates-used-excipients-medicinal-products-human-use_en.pdf (accessed on 25 November 2021).
- LeBel, M.; Ferron, L.; Masson, M.; Pichette, J.; Carrier, C. Benzyl alcohol metabolism and elimination in neonates. Dev. Pharmacol. Ther. 1988, 11, 347–356. [Google Scholar] [CrossRef]
- Evaluation of Certain Food Additives and Contaminants, Forty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives 1996, WHO Technical Report Series 868, World Health Organization, Geneva. Available online: https://apps.who.int/iris/handle/10665/41962/ (accessed on 21 January 2022).
- Opinion of the Scientific Committee on Food on Benzoic acid and Its Salts, SCF/CS/ADD/CONS/48. European Commission, 2002. Available online: https://ec.europa.eu/food/system/files/2020-12/sci-com_scf_out137_en.pdf (accessed on 21 January 2022).
| Property | Naloxone | Propranolol |
|---|---|---|
| pH stability (drug) | 2.5–5.0 | 2.8–4.0 |
| pH stability (product) | 3.0–4.0 | ≤3 |
| pKa | 7.84–10.07 | 9.42 |
| Water solubility (drug) (mg/mL) | 5.64 | 0.079 |
| Water solubility (hydrochloride salt) (mg/mL) | 50 | 10 |
| Material | Function | Manufacturer | Country |
|---|---|---|---|
| Naloxone hydrochloride dihydrate | API | Sanofi Chimie | France |
| Propranolol hydrochloride | API | Cosma S.p.A. | Italy |
| Stock solution of methyl parahydroxybenzoate | Preservative | ||
| Ethanol 96% | Univar | Sweden | |
| Methyl parahydroxybenzoate | Merck KGaA | Germany | |
| Water, purified | APL 1 | Sweden | |
| Sodium benzoate | Preservative | Emerald Performance Materials | Netherlands |
| Sodium citrate | Buffering agent | Jungbunzlauer AG | Austria |
| Citric acid monohydrate | Buffering agent | Jungbunzlauer AG | Austria |
| Sugar syrup 63% | Sweetening agent, viscosity-increasing agent | ||
| Sucrose | Nordic Sugar AB | Sweden | |
| Methyl parahydroxybenzoate | Merck KGaA | Germany | |
| Water, purified | APL | Sweden | |
| Glycerol 85% | Solvent, sweetening agent, viscosity-increasing agent | Oleon NV | France |
| Glycerol | |||
| Water, purified | |||
| Hydrochloric acid 1 M and 5 M | pH adjustment | Merck KGaA | Germany |
| Water, purified | Solvent | APL | Sweden |
| Preparation | Material | Amount |
|---|---|---|
| Naloxone 1 mg/mL | Naloxone hydrochloride dihydrate | 1 mg |
| Methyl parahydroxybenzoate 1 | 1 mg | |
| (+6 mg Ethanol 96%) | ||
| Hydrochloric acid 1 M | q.s. pH 3–3.5 | |
| Water, purified | ad 1 mL | |
| Propranolol 10 mg/mL | Propranolol hydrochloride | 10 mg |
| pH 3.9 | Sodium citrate | 2.1 mg |
| Citric acid monohydrate | 2.8 mg | |
| Sugar syrup | 320 mg | |
| Methyl parahydroxybenzoate 1 | 0.68 mg | |
| (+4.08 mg Ethanol 96%) | ||
| Water, purified | ad 1 mL |
| Order of Manufacturing | Experiment Number | Experiment Name | pH | Glycerol (%) |
|---|---|---|---|---|
| 1 | 7 | N7 | 3 | 20 |
| 2 | 4 | N4 | 3 | 10 |
| 3 | 3 | N3 | 4.5 | 0 |
| 4 | 6 | N6 | 4.5 | 10 |
| 5 | 2 | N2 | 3.75 | 0 |
| 6 | 1 | N1 | 3 | 0 |
| 7 | 9 | N9 | 4.5 | 20 |
| 8 | 12 | N12 | 3.75 | 10 |
| 9 | 11 | N11 | 3.75 | 10 |
| 10 | 8 | N8 | 3.75 | 20 |
| 11 | 5 | N5 | 3.75 | 10 |
| 12 | 10 | N10 | 3.75 | 10 |
| Order of Manufacturing | Experiment Number | Experiment Name | pH | Glycerol (%) |
|---|---|---|---|---|
| 9 | 9 | N9 | 5 | 20 |
| 3 | 3 | N3 | 5 | 0 |
| 10 | 10 | N10 | 4 | 10 |
| 5 | 5 | N5 | 4 | 10 |
| 12 | 12 | N12 | 4 | 10 |
| 8 | 8 | N8 | 4 | 20 |
| 2 | 2 | N2 | 4 | 0 |
| 7 | 7 | N7 | 3 | 20 |
| 11 | 11 | N11 | 4 | 10 |
| 1 | 1 | N1 | 3 | 0 |
| 6 | 6 | N6 | 5 | 10 |
| 4 | 4 | N4 | 3 | 10 |
| Naloxone 1 mg/mL | Osmolality (mOsm/kg) | Propranolol 10 mg/mL | Osmolality (mOsm/kg) |
|---|---|---|---|
| Baseline | 136 ± 0.007 | Baseline | 983 ± 0.005 |
| N1 | 97 ± 0.000 | N1 | 1121 ± 0.011 |
| N2 | 96 ± 0.000 | N2 | 941 ± 0.011 |
| N3 | 81 ± 0.000 | N3 | 918 ± 0.003 |
| Preparation | Material | Amount |
|---|---|---|
| Naloxone 1 mg/mL | Naloxone hydrochloride dihydrate | 1 mg |
| Sodium benzoate 1 | 0.5 or 1 mg | |
| Hydrochloric acid 1 M | q.s. pH 3.9–4.1 | |
| Water, purified | ad 1 mL | |
| Propranolol 10 mg/mL | Propranolol hydrochloride | 10 mg |
| Sodium citrate | 2.1 mg | |
| Citric acid monohydrate | 2.8 mg | |
| Sugar syrup | 320 mg | |
| Sodium benzoate 1 | 0.5 or 1 mg | |
| Hydrochloric acid 1 M | q.s. pH 3.2–3.6 | |
| Water, purified | ad 1 mL |
| Preparation | Dispensed Dose (mL) (% Error) | Viscosity 1 (mPa·s) | Osmolality (mOsm/kg) |
|---|---|---|---|
| Naloxone 1 mg/mL | |||
| Baseline | 0.986 ± 0.006 (1.4) | 2.72 ± 0.017 | 136 ± 0.007 |
| SB 0.05% | 0.959 ± 0.008 (4.1) | 2.60 ± 0.008 | 13 ± 0.000 |
| SB 0.1% | 0.972 ± 0.006 (2.8) | 2.61 ± 0.005 | 22 ± 0.000 |
| Propranolol 10 mg/mL | |||
| Baseline | 0.487 ± 0.004 (2.6) | 4.48 ±0.016 | 983 ± 0.005 |
| SB 0.05% | 0.491 ± 0.001 (1.8) | 4.22 ± 0.012 | 933 ± 0.002 |
| SB 0.1% | 0.491 ± 0.001 (1.8) | 4.31 ± 0.015 | 952 ± 0.003 |
| Microorganism | Amount Sodium Benzoate (%) | Start CFU/mL | Log10 Reduction 14 Days | Log10 Reduction 28 Days |
|---|---|---|---|---|
| Staphylococcus aureus | 0.05 | 2.4 × 105 | 5 | NI |
| 0.1 | 2.4 × 105 | 5 | NI | |
| Pseudomonas aeruginosa | 0.05 | 2.8 × 105 | 5 | NI |
| 0.1 | 2.8 × 105 | 5 | NI | |
| Escherichia coli | 0.05 | 3.5 × 105 | 5 | NI |
| 0.1 | 3.5 × 105 | 5 | NI | |
| Candida albicans | 0.05 | 6.5 × 105 | 5 | NI |
| 0.1 | 6.5 × 105 | 5 | NI | |
| Aspergillus brasiliensis | 0.05 | 5.3 × 105 | 2 | NI |
| 0.1 | 5.3 × 105 | 5 | NI |
| Microorganism | Amount Sodium Benzoate (%) | Start CFU/mL | Log10 Reduction 14 Days | Log10 Reduction 28 Days |
|---|---|---|---|---|
| Staphylococcus aureus | 0.05 | 2.4 × 105 | 5 | NI |
| 0.1 | 2.4 × 105 | 5 | NI | |
| Pseudomonas aeruginosa | 0.05 | 2.8 × 105 | 5 | NI |
| 0.1 | 2.8 × 105 | 5 | NI | |
| Escherichia coli | 0.05 | 3.5 × 105 | 5 | NI |
| 0.1 | 3.5 × 105 | 5 | NI | |
| Candida albicans | 0.05 | 6.5 × 105 | 5 | NI |
| 0.1 | 6.5 × 105 | 5 | NI | |
| Aspergillus brasiliensis | 0.05 | 5.3 × 105 | 5 | NI |
| 0.1 | 5.3 × 105 | 5 | NI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attebäck, M.; Hedin, B.; Mattsson, S. Formulation Optimization of Extemporaneous Oral Liquids Containing Naloxone and Propranolol for Pediatric Use. Sci. Pharm. 2022, 90, 15. https://doi.org/10.3390/scipharm90010015
Attebäck M, Hedin B, Mattsson S. Formulation Optimization of Extemporaneous Oral Liquids Containing Naloxone and Propranolol for Pediatric Use. Scientia Pharmaceutica. 2022; 90(1):15. https://doi.org/10.3390/scipharm90010015
Chicago/Turabian StyleAttebäck, Maria, Bengt Hedin, and Sofia Mattsson. 2022. "Formulation Optimization of Extemporaneous Oral Liquids Containing Naloxone and Propranolol for Pediatric Use" Scientia Pharmaceutica 90, no. 1: 15. https://doi.org/10.3390/scipharm90010015
APA StyleAttebäck, M., Hedin, B., & Mattsson, S. (2022). Formulation Optimization of Extemporaneous Oral Liquids Containing Naloxone and Propranolol for Pediatric Use. Scientia Pharmaceutica, 90(1), 15. https://doi.org/10.3390/scipharm90010015






