Abstract
Our aim is to investigate the recent liver biopsy findings of autoimmune liver diseases at a university hospital located in an urban area of Japan. The study included 259 patients (mean age 56.8 ± 12.5; male/female, 46/213) who underwent a liver biopsy for primary biliary cirrhosis (PBC) or autoimmune hepatitis (AIH). We analyzed their liver biopsy findings according to age and gender. Among 127 PBC patients, Scheuer stages 1, 2, 3, and 4 were 42, 54, 18, and 13, respectively. Among 101 AIH patients, fibrosis stages F1, F2, F3, and F4 were 37, 32, 19, and 13, respectively, and inflammatory activity grades A1, A2, and A3 were 22, 25, and 54, respectively. Among PBC aged ≥65 years, Scheuer stages 1–3 and 4 patients were 27 and 6, respectively. The proportion of Scheuer stage 4 patients in PBC aged ≥65 years tended to be higher than that in PBC aged <65 years (p = 0.0659). Of interest, the proportion of AIH patients with moderate or severe activity (A2 or A3) in males was higher than in females (p = 0.0311). From the point of view of fibrosis stage or inflammatory activity grade of the liver, the proportion of AIH patients aged ≥65 years was similar to that aged <65 years. Although we identified six older cirrhotic patients with AIH, three of them were male. The progression of fibrosis and inflammatory activity of the liver should be noted when we treat older patients suffering from autoimmune liver diseases. Liver biopsy plays an important role in obtaining accurate information on autoimmune liver diseases in older patients.
1. Introduction
Primary biliary cirrhosis (PBC) is a progressive chronic cholestatic liver disease, characterized by non-suppurative cholangitis, that mainly affects middle-aged women [1,2]. The reported number of patients with PBC has increased dramatically in the United States [2], United Kingdom [3], and Japan [4,5]. Understanding of the pathophysiology and earlier diagnosis of PBC has increased its prevalence worldwide [6]. The natural history of PBC has improved greatly during the past two decades because of its diagnosis at earlier stages and the widespread use of ursodeoxycholic acid (UDCA) [7,8]. As a result, far fewer patients require liver transplantation [7].
Autoimmune hepatitis (AIH) is characterized by chronic inflammatory liver disease, which, when not treated with prednisolone alone or in combination with azathioprine, leads to liver cirrhosis. It also affects mainly women [9]. AIH can occasionally have clinical, laboratory, histological, and genetic characteristics identical to those of PBC [10]. Some cases of AIH are difficult to diagnose [10,11,12,13,14]. The incidence of AIH differs in various geographic regions. The incidence of type 1 AIH among Caucasoid populations in Europe and North America ranges from 0.1 to 1.9/100,000/year [15]. A population-based epidemiological study of AIH in New Zealand demonstrated that age-standardized incidence and prevalence were 1.7 and 18.9 per 100,000, respectively [16]. In Japan, the prevalence is as high as 1.0 per 10,000 [17]. Except for atypical presentations of AIH [12,18,19], early administration of corticosteroid usually leads to a relatively better prognosis for this disease.
Histological assessment of the liver is valuable for evaluation and management of patients with liver diseases even if sensitive and relatively accurate blood testing is available [20]. Liver biopsy also plays an important role in the diagnosis, staging, prognosis and management of PBC and AIH [20]. In Japan, recent opportunities to manage patients with autoimmune liver diseases such as AIH and PBC have been increasing. This tendency is especially apparent in the older population, as many more people live to an advanced age in Japan. For older patients with liver diseases, the risk of cirrhosis and hepatocellular carcinoma (HCC) is more critical than for the young [21], although there is a contrary opinion [22]. In the present study, to clarify the features of older patients with autoimmune liver diseases at a university hospital located in urban Japan, we analyzed the histological features of these diseases by reviewing the recent findings of liver biopsies.
2. Experimental Section
2.1. Research Subjects
Between 2000 and 2011, 259 patients were diagnosed with AIH and PBC through liver biopsy specimens; we reviewed and re-analyzed these liver biopsy findings at the Department of Gastroenterology and Nephrology, Chiba University, Graduate School of Medicine, Japan. All subjects gave their informed consent for inclusion before participating in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Chiba University School of Medicine (No. 1841).
2.2. Liver Biopsy
We conducted liver biopsies as follows: a patient lay face-up on a bed, right hand resting above the head. The patient was given a local anesthetic to the body surface area where the biopsy needle would be inserted. The patient was administered 15 mg of pentazocine hydrochloride, 0.25 mg of atropine sulfate, and 25 mg of hydroxyzine pamoate as premedication. We made a small incision in the right side of the patient’s abdomen, just below the rib cage. Liver biopsy was performed with 14–20 gauge automatic or manual Tru-cut biopsy needles under ultrasound guidance. Biopsy samples were immediately placed into 10% formalin solution (Wako Pure Chemical, Osaka, Japan).
2.3. Evaluation of Biopsy Samples
Three hepatologists blindly evaluated the histopathological findings of liver biopsy samples with hematoxylin and eosin (HE) stain. Scheuer’s classification was used for evaluation of histopathological findings of PBC: a lesion characteristic of bile ducts was classified as Stage I; florid bile duct lesions were judged as Stage II; and hepatic fibrosis and cirrhosis were categorized as Stages III and IV, respectively [23]. The liver tissue samples from AIH patients were evaluated with fibrosis staging and inflammatory activity grading of the liver in chronic liver diseases [24]. We classified samples from AIH patients into no fibrosis (F0), mild fibrosis (F1), moderate fibrosis (F2), severe fibrosis (F3), and cirrhosis (F4); or minimal activity (A0), mild activity (A1), moderate activity (A2), and severe activity (A3) for staging the extent of fibrosis or grading the activity of inflammation, respectively.
2.4. Statistical Analysis
For all tests, two-sided P-values were calculated and the results were considered statistically significant at p < 0.05. Statistical analysis was performed using the Excel statistics program for Windows, version 7 (SSRI, Tokyo, Japan) and DA Stats software (O. Nagata, Nifty Serve: PAF01644).
3. Results
3.1. Patient Characteristics
A total of 259 patients with autoimmune liver diseases were enrolled into the present study (Table 1). Female patients dominated (82.2%) the patient groups: 81.7% and 82.9% of PBC and AIH patients, respectively. According to our records, age was unknown in nine patients with PBC and 35 patients with AIH, although liver biopsy was still performed on these patients. The number of older patients, ≥65 years, was at least 27.4%: 25.2% and 29.7% of PBC and AIH patients, respectively. Liver biopsy findings from four PBC and 27 AIH patients were not available.

Table 1.
Characteristics of patients with autoimmune liver diseases in the present study.
| Total Patients (n = 259) | PBC (n = 131) | AIH (n = 128) | p-values | |
|---|---|---|---|---|
| Age (years) | 56.8 ± 12.5 | 57.2 ± 10.7 | 56.5 ± 14.2 | N.S. |
| Older Patients (≥65 years) | 71 | 33 | 38 | N.S. |
| Gender (male/female) | 46/213 | 24/107 | 22/106 | N.S. |
| Histopathological findings | ||||
| Staging of Fibrosis (F1/F2/F3/F4) | - | - | 37/32/19/13 | - |
| Grading of Activity (A1/A2/A3) | - | - | 22/25/54 | - |
| Scheuer Stage (1/2/3/4) | - | 42/54/18/13 | - | - |
Note: PBC, primary biliary cirrhosis; AIH, autoimmune hepatitis.
3.2. Annual Changes in the Number of PBC Patients with Liver Biopsy
In the present study, the total number of PBC was 131. Liver biopsy was performed in 127 of 131 patients in the years shown in Figure 1A. Between three and 15 PBC cases, including 13 with Scheuer stage 4, were biopsied in each year. Among them, males and females were 24 and 103, respectively (Figure 1B,C). Among the 13 patients with Scheuer stage 4, four were males and nine were females.
3.3. Older PBC Patients Tended to Have Advanced Fibrosis
PBC patients aged <65 years and ≥65 years numbered 91 and 33, respectively (Figure 2A,B). Among PBC patients aged <65 years, Scheuer stages 1–3 and stage 4 patients numbered 86 and five, respectively. Among PBC patients aged ≥65 years, Scheuer stages 1–3 and stage 4 patients numbered 27 and 6 respectively. The proportion of Scheuer stage 4 patients aged ≥65 years tended to be higher than that in those aged <65 years (p = 0.0659). The 11 PBC patients with Scheuer stage 4 are shown in Table 2A. All six patients aged ≥65 years with Scheuer stage 4 were female.
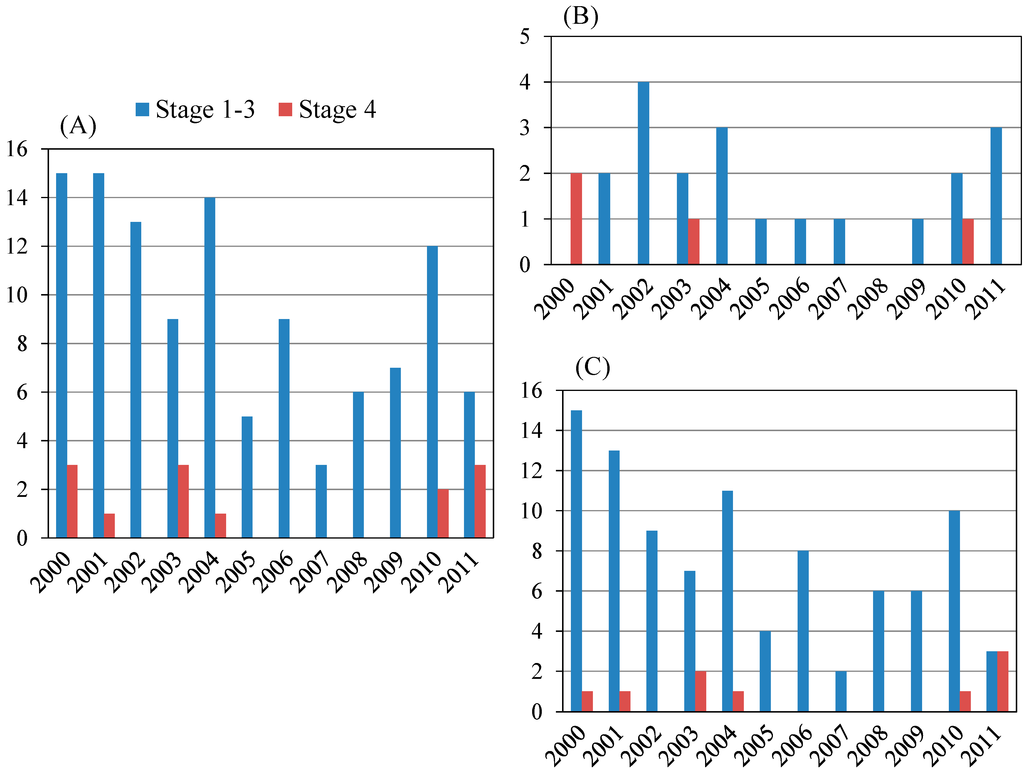
Figure 1.
Scheuer stage of primary biliary cirrhosis (PBC) patients in the present study. (A) Total patients (n = 127), Stage 1–3: Stage 4 = 114:13; (B) Male patients (n = 24), Stage 1–3: Stage 4 = 20:4; (C) Female patients (n = 103), Stage 1–3: Stage 4 = 94:9.
Figure 1.
Scheuer stage of primary biliary cirrhosis (PBC) patients in the present study. (A) Total patients (n = 127), Stage 1–3: Stage 4 = 114:13; (B) Male patients (n = 24), Stage 1–3: Stage 4 = 20:4; (C) Female patients (n = 103), Stage 1–3: Stage 4 = 94:9.
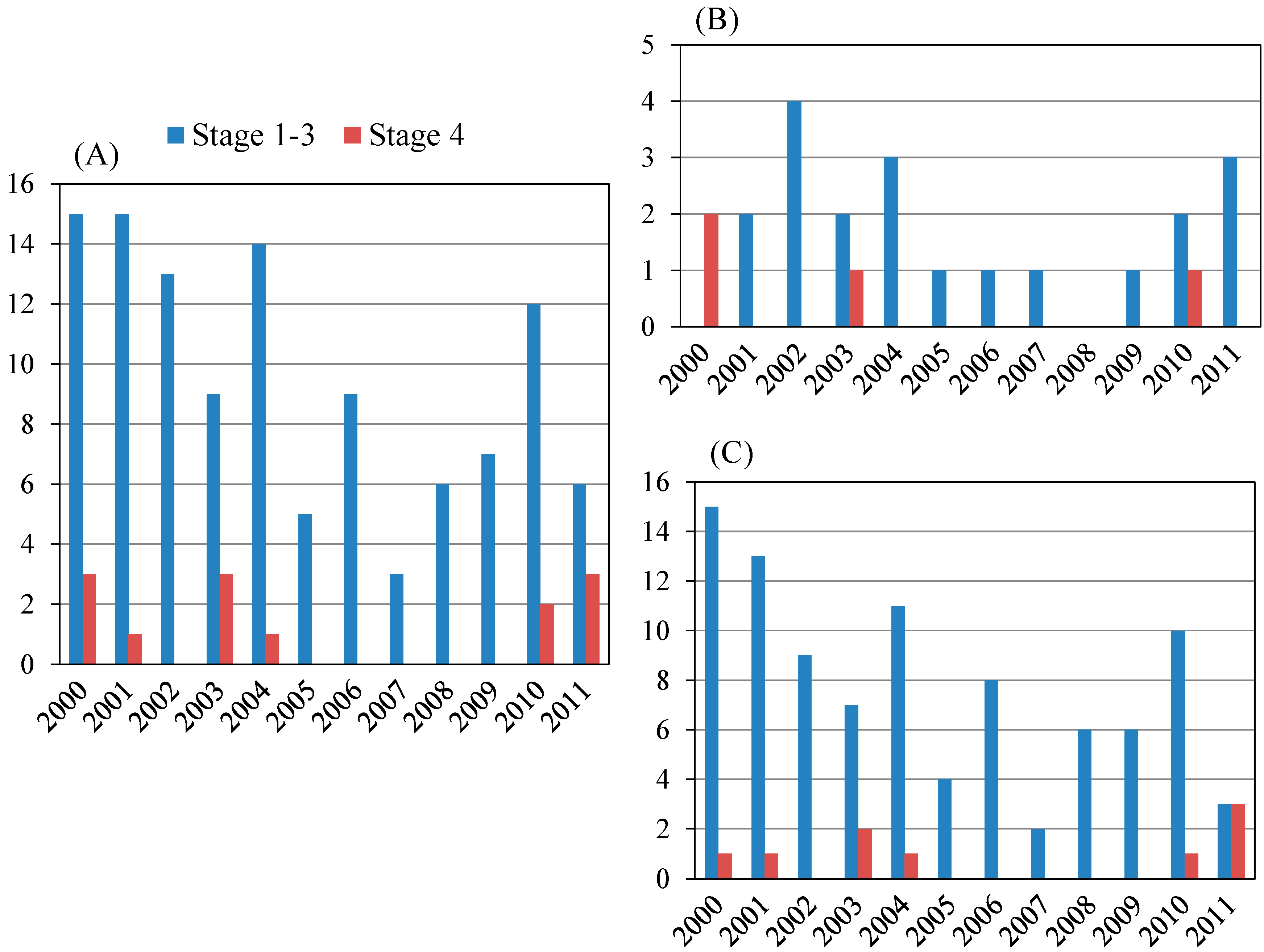
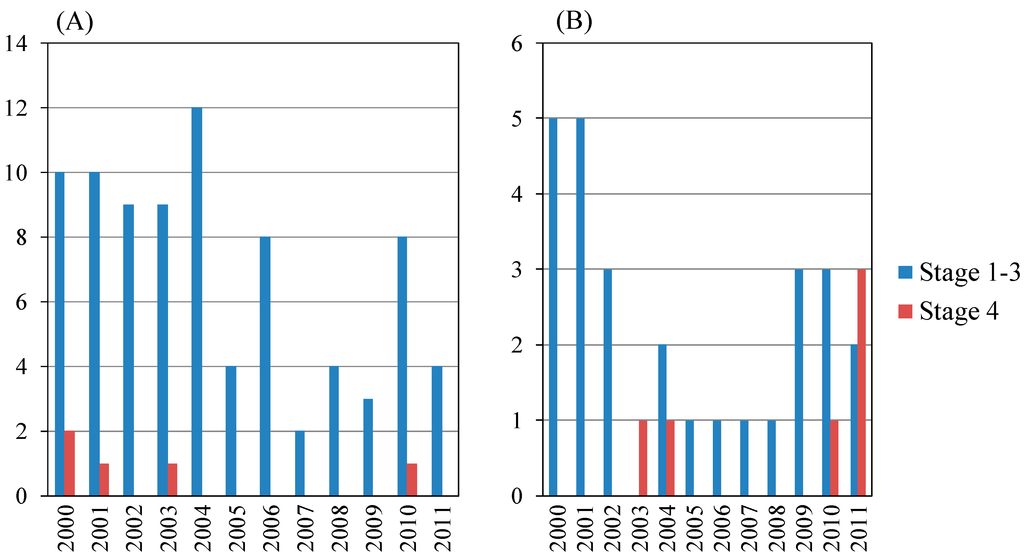
Figure 2.
Scheuer stage of primary biliary cirrhosis (PBC) patients according to age. (A) Age <65 (years) (n = 91); (B) Age ≥65 (years) (n = 33).
Figure 2.
Scheuer stage of primary biliary cirrhosis (PBC) patients according to age. (A) Age <65 (years) (n = 91); (B) Age ≥65 (years) (n = 33).
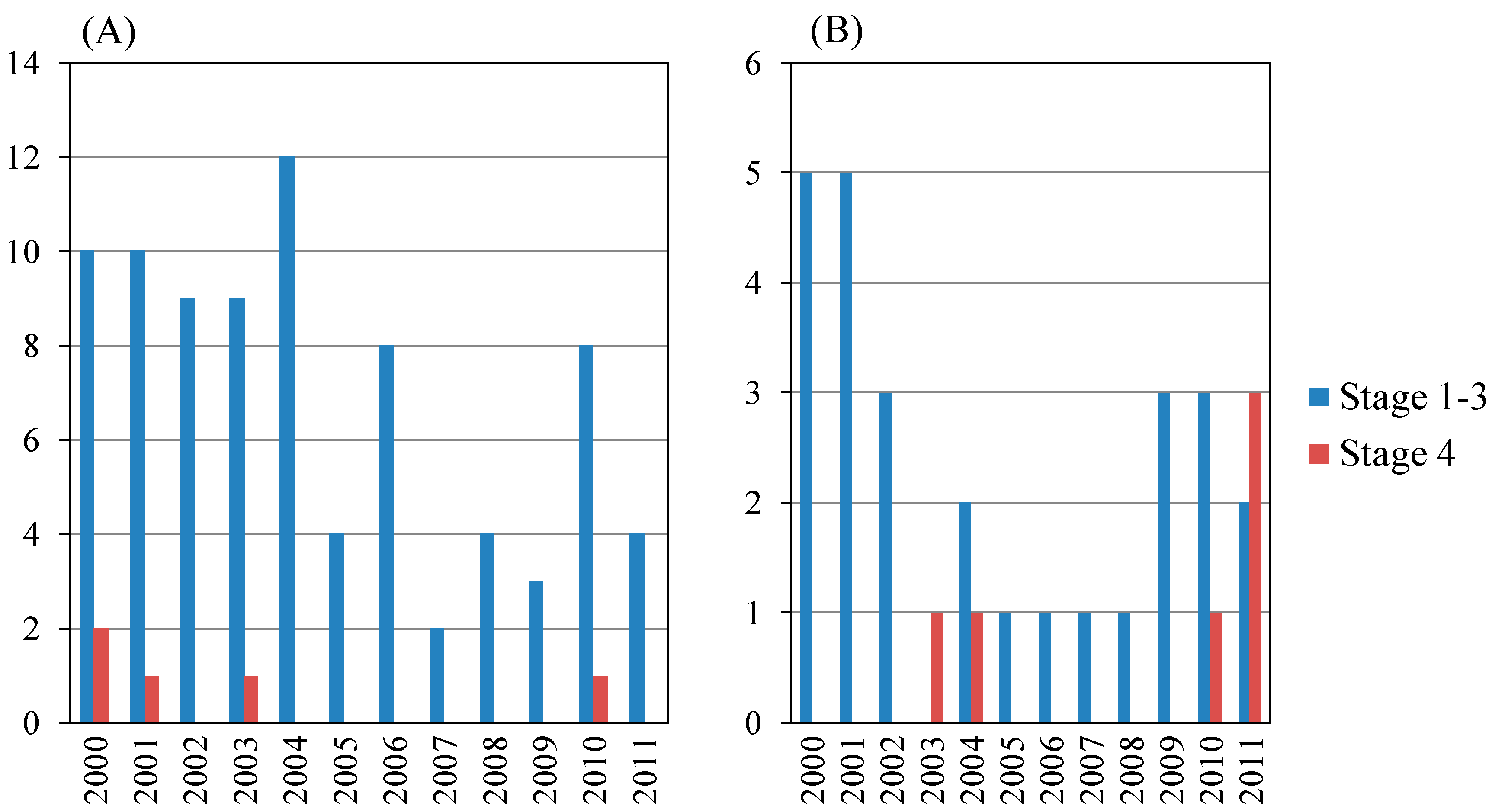

Table 2.
Cirrhotic patients of primary biliary cirrhosis and autoimmune hepatitis in the present study. (A) Patients with Scheuer stage 4 of primary biliary cirrhosis; (B) Cirrhotic patients with autoimmune hepatitis.
| (A) | ||
| Year of Diagnosis | Age at Diagnosis (years) | Gender |
| 2000 | 49 | Male |
| 2000 | 57 | Female |
| 2001 | 42 | Female |
| 2003 | 60 | Female |
| 2010 | 59 | Male |
| 2003 | 80 | Female |
| 2004 | 68 | Female |
| 2010 | 70 | Female |
| 2011 | 73 | Female |
| 2011 | 78 | Female |
| 2011 | 80 | Female |
| (B) | ||
| Year of diagnosis | Age at diagnosis (years) | Gender |
| 2001 | 58 | Female |
| 2002 | 45 | Female |
| 2004 | 62 | Female |
| 2004 | 61 | Female |
| 2006 | 36 | Male |
| 2011 | 58 | Female |
| 2000 | 67 | Male |
| 2004 | 68 | Female |
| 2005 | 72 | Male |
| 2005 | 74 | Male |
| 2009 | 70 | Female |
| 2009 | 70 | Female |
3.4. Annual Changes in the Number of AIH Patients with Liver Biopsy
In the present study, the total number of AIH was 128. Table 3A shows the yearly schedule of liver biopsy of 101 of the 128 patients. Among them, males and females numbered 18 and 83, respectively (Table 3A,B). Between 2000 and 2011, the annual number of AIH was between six and 16 cases, including 13 cirrhotic patients (four males and nine females). The proportion of cirrhotic patients with AIH did not differ statistically between males and females (Table 3A). The proportion of AIH patients with moderate or severe activity (A2 or A3) was higher for males than for females (p = 0.0311) (Table 3B). Of interest, among the AIH patients, all male patients had A2 or A3 inflammatory activity grade of the liver.

Table 3.
Histopathological findings of the liver in autoimmune hepatitis according to gender in the present study. (A) Staging of fibrosis; (B) Grading of inflammatory activity.
| (A) | ||||||
| Total Patients (n = 101) | Male (n = 18) | Female (n = 83) | ||||
| Year | F1-F3 | F4 | F1-F3 | F4 | F1-F3 | F4 |
| 2000 | 13 | 1 | 3 | 1 | 10 | 0 |
| 2001 | 11 | 1 | 2 | 0 | 9 | 1 |
| 2002 | 8 | 1 | 1 | 0 | 7 | 1 |
| 2003 | 7 | 0 | 2 | 0 | 5 | 0 |
| 2004 | 9 | 4 | 1 | 0 | 8 | 4 |
| 2005 | 7 | 2 | 2 | 2 | 5 | 0 |
| 2006 | 7 | 1 | 0 | 1 | 7 | 0 |
| 2007 | 7 | 0 | 0 | 0 | 7 | 0 |
| 2008 | 5 | 0 | 1 | 0 | 4 | 0 |
| 2009 | 5 | 2 | 1 | 0 | 4 | 2 |
| 2010 | 4 | 0 | 0 | 0 | 4 | 0 |
| 2011 | 5 | 1 | 1 | 0 | 4 | 1 |
| Total | 88 | 13 | 14 | 4 | 74 | 9 |
| (B) | ||||||
| Total patients (n = 101) | Male (n = 18) | Female (n = 83) | ||||
| Year | A1 | A2 or A3 | A1 | A2 or A3 | A1 | A2 or A3 |
| 2000 | 3 | 11 | 0 | 4 | 3 | 7 |
| 2001 | 3 | 9 | 0 | 2 | 3 | 7 |
| 2002 | 1 | 8 | 0 | 1 | 1 | 7 |
| 2003 | 1 | 6 | 0 | 2 | 1 | 4 |
| 2004 | 3 | 10 | 0 | 1 | 3 | 9 |
| 2005 | 1 | 8 | 0 | 4 | 1 | 4 |
| 2006 | 1 | 7 | 0 | 1 | 1 | 6 |
| 2007 | 1 | 6 | 0 | 0 | 1 | 6 |
| 2008 | 3 | 2 | 0 | 1 | 3 | 1 |
| 2009 | 2 | 5 | 0 | 1 | 2 | 4 |
| 2010 | 2 | 2 | 0 | 0 | 2 | 2 |
| 2011 | 1 | 5 | 0 | 1 | 1 | 4 |
| Total | 22 | 79 | 0 | 18 | 22 | 61 |
3.5. Clinical Features of Older Patients with AIH
AIH patients aged <65 years and ≥65 years numbered 57 and 36, respectively (Figure 3 and Table 4). Among those aged <65 years, fibrosis stage 1–3 and 4 patients numbered 51 and six, respectively, and among those aged ≥65 years, fibrosis stage 1–3 and 4 patients numbered 30 and six, respectively. The proportion of cirrhotic (F4) patients with AIH aged ≥65 years was the same in those aged <65 years (not statistically significant) (Table 4A). The 12 cirrhotic patients with AIH are shown in Table 2B. We identified six older cirrhotic patients with AIH, but three of them were male (Table 2B).
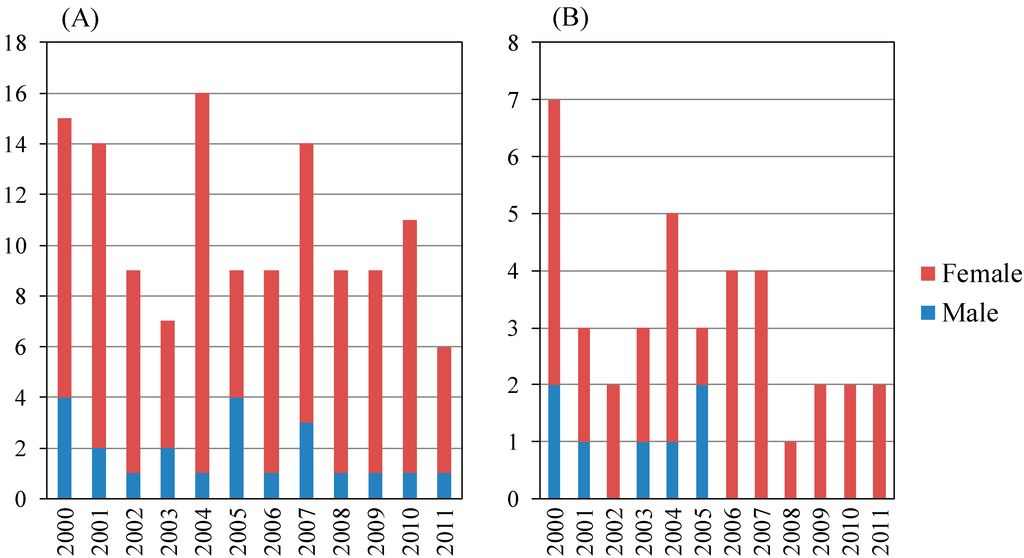
Figure 3.
Annual numbers of autoimmune hepatitis patients with liver biopsy in the present study. (A) Total patients (n = 128); male: female = 22:106; (B) Age ≥65 (years) (n = 38), male: female = 7:31.
Figure 3.
Annual numbers of autoimmune hepatitis patients with liver biopsy in the present study. (A) Total patients (n = 128); male: female = 22:106; (B) Age ≥65 (years) (n = 38), male: female = 7:31.
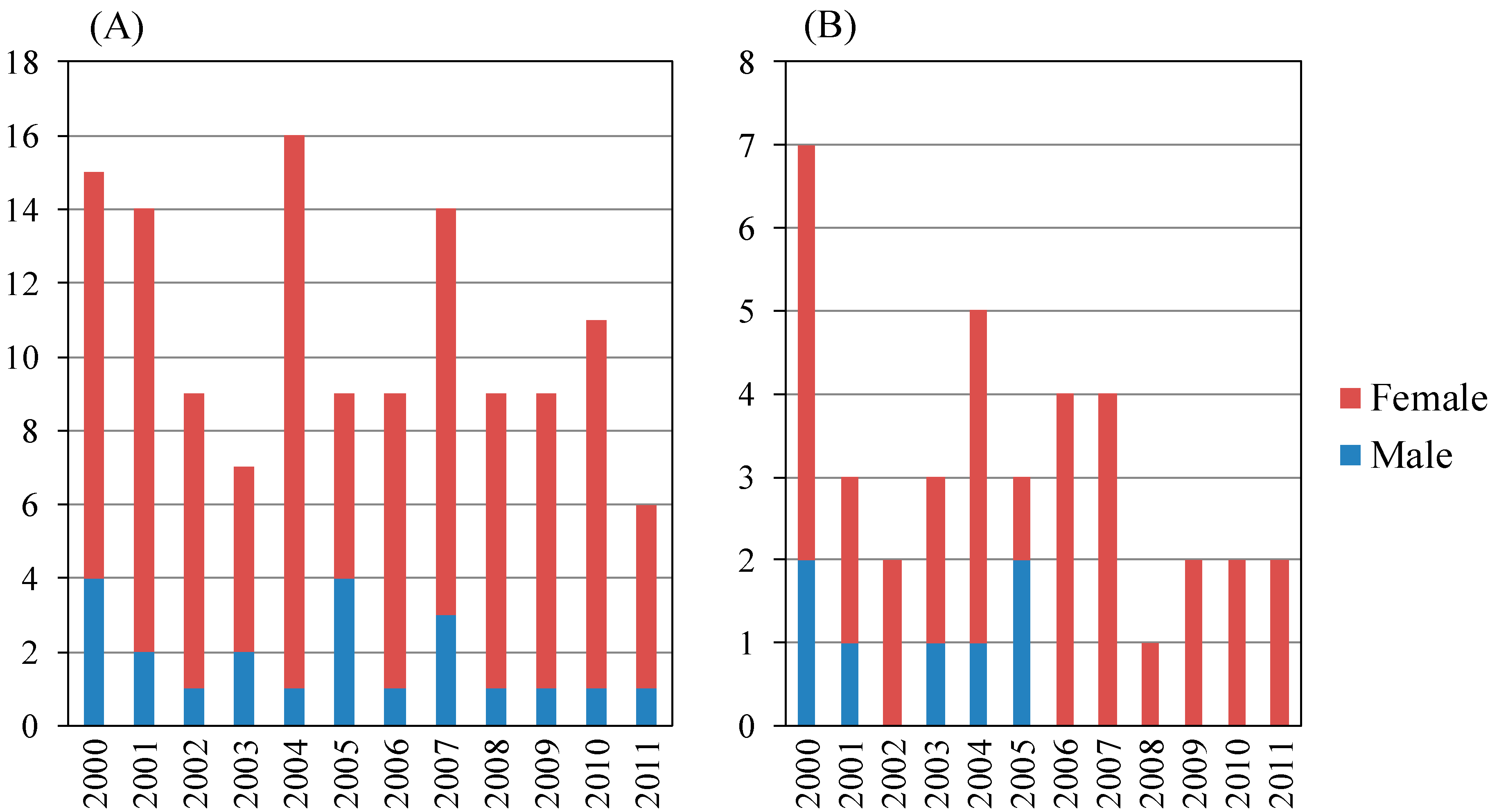

Table 4.
Histopathological findings of the liver in autoimmune hepatitis according to age in the present study. (A) Staging of fibrosis; (B) Grading of inflammatory activity.
| (A) | ||||
| Age < 65 (years) | Age ≥ 65 (years) | |||
| Year | F1-F3 | F4 | F1-F3 | F4 |
| 2000 | 7 | 0 | 6 | 1 |
| 2001 | 8 | 1 | 3 | 0 |
| 2002 | 6 | 1 | 2 | 0 |
| 2003 | 4 | 0 | 3 | 0 |
| 2004 | 5 | 2 | 4 | 1 |
| 2005 | 6 | 0 | 1 | 2 |
| 2006 | 3 | 1 | 4 | 0 |
| 2007 | 2 | 0 | 3 | 0 |
| 2008 | 2 | 0 | 1 | 0 |
| 2009 | 2 | 0 | 0 | 2 |
| 2010 | 3 | 0 | 1 | 0 |
| 2011 | 3 | 1 | 2 | 0 |
| Total | 51 | 6 | 30 | 6 |
| (B) | ||||
| Age < 65 (years) | Age ≥ 65 (years) | |||
| Year | A1 | A2 or A3 | A1 | A2 or A3 |
| 2000 | 3 | 4 | 0 | 7 |
| 2001 | 2 | 7 | 1 | 2 |
| 2002 | 1 | 6 | 0 | 2 |
| 2003 | 0 | 4 | 1 | 2 |
| 2004 | 3 | 4 | 0 | 5 |
| 2005 | 1 | 5 | 0 | 3 |
| 2006 | 1 | 3 | 0 | 4 |
| 2007 | 0 | 2 | 1 | 2 |
| 2008 | 2 | 0 | 1 | 0 |
| 2009 | 1 | 1 | 1 | 1 |
| 2010 | 1 | 2 | 1 | 0 |
| 2011 | 1 | 3 | 0 | 2 |
| Total | 16 | 41 | 6 | 30 |
Among AIH patients aged < 65 years, patients with inflammatory activity grades A1 or A2 and A3 numbered 16 and 41, respectively. Among AIH patients aged ≥65 years, patients with inflammatory activity grades A1 or A2, and A3 numbered six and 30, respectively. The proportions of A2 and A3 patients with AIH aged ≥65 years were similar to those aged <65 years (not statistically significant) (Table 4B).
4. Discussion
In the present study, recent trends for patients with autoimmune liver diseases and liver biopsies performed at a university hospital located in an urban area of Japan were investigated. Among the patients with PBC or AIH, at least 25.2% or 29.7%, respectively, were ≥65 years old. This can likely be attributed to the fact that the opportunity to diagnose and treat older people suffering from autoimmune liver diseases has increased in line with the aging of society in Japan. As a result of the development of non-invasive serum markers of hepatic fibrosis and transient elastography [25,26,27], with a concomitant decrease in occasions for liver biopsy, these numbers might in fact be underestimated. Kim et al. [2] reported that, in the United States, the peak incidence of patient age with PBC was 60–69 years for men and 70–79 years for women. More recently, Japanese national surveillance [28] reported that the peak incidence of patient age with PBC was 60–69 years for men and 50–59 years for women, regardless of asymptomatic or symptomatic PBC.
In the present study, we demonstrated that the proportion of PBC Scheuer stage 4 patients aged ≥65 years tended to be higher, and that all our six patients with stage 4 were female (Table 2A). Harada et al. [29] reported that the histological stage at the time of HCC diagnosis was an independent risk factor for HCC in females and that the time between the diagnosis of PBC and that of HCC was significantly shorter in males than in females. Attention should be paid to older PBC patients with advanced fibrosis.
For AIH patients, it seems that aging has no effect on stage, indicating that this disease can appear at any age, and could be classified into three different types of AIH [30]. Miyake et al. [31] reported that there are no apparent differences in the characteristics of AIH between older and younger patients, supporting the results of the present study. Although we identified six older cirrhotic patients with AIH, three of them were males. Thus, AIH is not just a severe female disease. Among older patients with AIH, confirming the accuracy of information about the disease seems important. Interestingly, we discovered that the proportion of AIH patients with moderate or severe activity (A2 or A3) is higher for males than for females (Table 3), indicating that among male patients with liver dysfunction of unknown etiology, AIH should be considered a possible etiology of their liver diseases. Liver biopsies could be useful in male patients with liver dysfunction of unknown etiology. It was reported that an androgenic/anabolic steroid could induce toxic hepatitis [32]. Gender differences in the proportion of AIH patients with moderate or severe activity (A2 or A3) might be caused by the effects of sex steroid hormone receptors such as androgen receptors [33,34]. Further studies will be needed. The number of female patients with PBC or AIH was greater than male patients, and this may reflect the population of these autoimmune liver diseases. In spite of this tendency, the number of older cirrhotic patients with AIH was equally split between males and females (Table 2B). This may be due to the fact that male patients with AIH tend to have a more severe stage than females, or that it might be difficult to diagnose male patients with AIH using the present criteria [35,36]. Special attention should also be paid to the diagnosis and/or treatment of older male patients with AIH. In such cases, liver biopsies could be useful, and older patients with autoimmune liver diseases may have more severe cases than expected.
In Japan, HCC developed in 5.1% of patients with AIH, and cirrhosis was commonly found in elderly individuals [37]. Ohira et al. [37] reported that the mean age at diagnosis of HCC was 69 years, and that the male-to-female ratio was 1:5.7. AIH patients with liver cirrhosis are at high risk for HCC. Although diagnosis of AIH has recently been performed using the AIH scoring system [35,36] in consideration of the possibility of other liver diseases, other fibrogenetic factors such as non-alcoholic fatty liver diseases and alcohol intake should also be considered [36,38]. In the present study, we found 12 cirrhotic patients with AIH (Table 2); only one patient revealed alcohol intake, but four patients were overweight or obese. In Japanese cirrhotic AIH patients, these fibrogenetic factors might have partly contributed to this condition.
Previous studies have established that PBC is associated with HLA-DR8 [39] and six non-HLA loci: IL12A, IL12RB2, IRF/TNPO3, ORMDL3/IKZF3, MMEL1, and SP1B [40]. Mells et al. [40] reported that STAT4, DENND1B, CD80, IL7R, CXCR5, TNFRSF16F, and NFKB1 were new candidate genes associated with the genetic risk factors for PBC. A recent genome-wide association study revealed TNFSF15 and POU2AF1 as susceptibility loci for PBC in the Japanese population [41]. Another genome-wide association study revealed that AIH type 1 was associated with variants in the major histocompatibility complex region, and identified the variants SH2B3 and CARD10 as likely risk factors [42].
In PBC patients, the aspartate aminotransferase-to-platelet ratio index (APRI) was significantly correlated with the histological degree of liver fibrosis, with a limited value of scores between 1.0 and 1.5 [43]. Although it was reported that APRI appeared to be of no value in AIH patients [44], serum surrogate markers such as hyaluronic acid level, type 4 collagen level, and APRI, as well as liver stiffness measurement, are helpful in predicting significant fibrosis of the liver in non-viral liver diseases [25]. In the near future, we will not have to perform a liver biopsy to diagnose PBC and AIH. Further studies of the differences between AIH pathology and PBC pathology might shed some new light on better management of these diseases.
In Japan, opportunities to manage patients with autoimmune liver diseases such as AIH and PBC have been on the increase. In conclusion, we should pay particular attention to the progression of fibrosis and inflammatory activity of the liver when we treat older patients suffering from autoimmune liver diseases. For this reason, at the present time, liver biopsy is recommended for obtaining accurate information.
Acknowledgments
This work was conducted by the Scholarship Program, Chiba University, School of Medicine, Chiba, Japan (Tatsuo Kanda, Katsuhiro Hagiwara, and Osamu Yokosuka). We would like to thank the medical staff of Chiba University Hospital for their assistance and support during this study.
Author Contributions
Yuki Haga and Tatsuo Kanda collected and analyzed data, saw the patients, and contributed to the writing and editing of the manuscript. Katsuhiro Hagiwara collected and helped analyze data. Reina Sasaki, Masato Nakamura., Shin Yasui, Makoto Arai, and Osamu Yokosuka saw the patients. Xia Jiang, Shuang Wu, and Shingo Nakamoto collected and analyzed data and helped edit the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Imam, M.H.; Lindor, K.D. The natural history of primary biliary cirrhosis. Semin. Liver Dis. 2014, 34, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Lindor, K.D.; Locke, G.R., 3rd; Therneau, T.M.; Homburger, H.A.; Batts, K.P.; Yawn, B.P.; Petz, J.L.; Melton, L.J., 3rd; Dickson, E.R. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology 2000, 119, 1631–1636. [Google Scholar] [PubMed]
- James, O.F.; Bhopal, R.; Howel, D.; Gray, J.; Burt, A.D.; Metcalf, J.V. Primary biliary cirrhosis once rare, now common in the United Kingdom? Hepatology 1999, 30, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Inoue, K.; Hirohara, J.; Arita, S.; Higuchi, K.; Omata, M.; Toda, G. Long-term prognosis of primary biliary cirrhosis (PBC) in Japan and analysis of the factors of stage progression in asymptomatic PBC (a-PBC). Hepatol. Res. 2002, 22, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Imazeki, F.; Hirasawa, Y.; Ikeuchi, T.; Mikata, R.; Zhang, K.Y.; Kurihara, T.; Arai, M.; Fukai, K.; et al. Body mass index in Japanese patients with autoimmune liver disease: Overweight patients with primary biliary cirrhosis tend to be asymptomatic. Hepatogastroenterology 2007, 54, 1758–1760. [Google Scholar] [PubMed]
- Nakamura, M. Clinical significance of autoantibodies in primary biliary cirrhosis. Semin. Liver Dis. 2014, 34, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Poupon, R. Evidence-based treatment of primary biliary cirrhosis. Dig. Dis. 2014, 32, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Imazeki, F.; Saisho, H. Bezafibrate treatment: A new medical approach for PBC patients? J. Gastroenterol. 2003, 38, 573–578. [Google Scholar]
- Strassburg, C.P.; Manns, M.P. Treatment of autoimmune hepatitis. Semin. Liver Dis. 2009, 29, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; Czaja, A.J.; Gorham, J.D.; Krawitt, E.L.; Mieli-Vergani, G.; Vergani, D.; Vierling, J.M. Diagnosis and management of autoimmune hepatitis. Hepatology 2010, 51, 2193–2213. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Hirasawa, Y.; Imazeki, F.; Nagao, K.; Saisho, H. Occurrence of autoimmune hepatitis during the course of primary biliary cirrhosis: Report of two cases. Dig. Dis. Sci. 2006, 51, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Hirasawa, Y.; Imazeki, F.; Nagao, K.; Suzuki, Y.; Saisho, H. Acute-onset autoimmune hepatitis resembling acute hepatitis: A case report and review of reported cases. Hepatogastroenterology 2005, 52, 1233–1235. [Google Scholar] [PubMed]
- Abe, M.; Onji, M.; Kawai-Ninomiya, K.; Michikata, K.; Matsuura, B.; Hiasa, Y.; Horiike, N. Clinicopathologic features of the severe form of acute type 1 autoimmune hepatitis. Clin. Gastroenterol. Hepatol. 2007, 5, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Yamamoto, K. Current status of autoimmune hepatitis in Japan. Acta Med. Okayama 2008, 62, 217–226. [Google Scholar] [PubMed]
- Boberg, K.M. Prevalence and epidemiology of autoimmune hepatitis. Clin. Liver Dis. 2002, 6, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Ngu, J.H.; Bechly, K.; Chapman, B.A.; Burt, M.J.; Barclay, M.L.; Gearry, R.B.; Stedman, C.A. Population-based epidemiology study of autoimmune hepatitis: A disease of older women? J. Gastroenterol. Hepatol. 2010, 25, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Toda, G.; Zeniya, M.; Watanabe, F.; Imawari, M.; Kiyosawa, K.; Nishioka, M.; Tsuji, T.; Omata, M. Present status of autoimmune hepatitis in Japan—Correlating the characteristics with international criteria in an area with a high rate of HCV infection. Japanese National Study Group of Autoimmune Hepatitis. J. Hepatol. 1997, 26, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Yasui, S.; Fujiwara, K.; Yonemitsu, Y.; Oda, S.; Nakano, M.; Yokosuka, O. Clinicopathological features of severe and fulminant forms of autoimmune hepatitis. J. Gastroenterol. 2011, 46, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Yasui, S.; Tawada, A.; Okitsu, K.; Yonemitsu, Y.; Chiba, T.; Arai, M.; Kanda, T.; Imazeki, F.; Nakano, M.; et al. Autoimmune fulminant liver failure in adults: Experience in a Japanese center. Hepatol. Res. 2011, 41, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Caldwell, S.H.; Goodman, Z.D.; Nelson, R.C.; Smith, A.D. American Association for Study of Liver Diseases. Liver biopsy. Hepatology 2009, 49, 1017–1044. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Nakamoto, S.; Yasui, S.; Nakamura, M.; Miyamura, T.; Wu, S.; Jiang, X.; Arai, M.; Imazeki, F.; Yokosuka, O. Occurrence and Recurrence of Hepatocellular Carcinoma Were Not Rare Events during Phlebotomy in Older Hepatitis C Virus-Infected Patients. Case Rep. Oncol. 2014, 7, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Kita, R.; Kimura, T.; Ohara, Y.; Takeda, H.; Sakamoto, A.; Saito, S.; Nishijima, N.; Nasu, A.; Komekado, H.; et al. Transcatheter arterial chemoembolization for intermediate-stage hepatocellular carcinoma: Clinical outcome and safety in elderly patients. J. Cancer 2014, 5, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, P. Primary biliary cirrhosis. Proc. R. Soc. Med. 1967, 60, 1257–1260. [Google Scholar] [PubMed]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Obara, N.; Ueno, Y.; Fukushima, K.; Nakagome, Y.; Kakazu, E.; Kimura, O.; Wakui, Y.; Kido, O.; Ninomiya, M.; Kogure, T.; et al. Transient elastography for measurement of liver stiffness measurement can detect early significant hepatic fibrosis in Japanese patients with viral and nonviral liver diseases. J. Gastroenterol. 2008, 43, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Rust, M.; Rosenberg, W.; Parkes, J.; Herrmann, E.; Zeuzem, S.; Sarrazin, C. Comparison of ELF, FibroTest and FibroScan for the non-invasive assessment of liver fibrosis. BMC Gastroenterol. 2010, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Tawada, A.; Maruyama, H.; Kamezaki, H.; Shimada, T.; Ishibashi, H.; Takahashi, M.; Kanda, T.; Fujiwara, K.; Imazeki, F.; Yokosuka, O. Magnitude of contrast-enhanced ultrasonography as a noninvasive predictor for hepatic fibrosis: Comparison with liver stiffness measurement and serum-based models. Hepatol. Int. 2013, 7, 749–757. [Google Scholar] [CrossRef]
- Japan Intractable Diseases Information Center. Available online: http://www.nanbyou.or.jp/entry/93 (accessed on 17 August 2014).
- Harada, K.; Hirohara, J.; Ueno, Y.; Nakano, T.; Kakuda, Y.; Tsubouchi, H.; Ichida, T.; Nakanuma, Y. Incidence of and risk factors for hepatocellular carcinoma in primary biliary cirrhosis: National data from Japan. Hepatology 2013, 57, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Floreani, A.; Liberal, R.; Vergani, D.; Mieli-Vergani, G. Autoimmune hepatitis: Contrasts and comparisons in children and adults—A comprehensive review. J. Autoimmun. 2013, 46, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Miyaoka, H.; Abe, M.; Furukawa, S.; Shigematsu, S.; Furukawa, E.; Ikeda, R.; Okita, S.; Okada, T.; Yoshida, O.; et al. Clinical characteristics of autoimmune hepatitis in older aged patients. Hepatol. Res. 2006, 36, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Stimac, D.; Milić, S.; Dintinjana, R.D.; Kovac, D.; Ristić, S. Androgenic/Anabolic steroid-induced toxic hepatitis. J. Clin. Gastroenterol. 2002, 35, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Kanda, T.; Nakamoto, S.; Miyamura, T.; Wu, S.; Yokosuka, O. Involvement of androgen receptor and glucose-regulated protein 78 kDa in human hepatocarcinogenesis. Exp. Cell Res. 2014, 323, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Jiang, X.; Yokosuka, O. Androgen receptor signaling in hepatocellular carcinoma and pancreatic cancers. World J. Gastroenterol. 2014, 20, 9229–9236. [Google Scholar] [PubMed]
- Alvarez, F.; Berg, P.A.; Bianchi, F.B.; Bianchi, L.; Burroughs, A.K.; Cancado, E.L.; Chapman, R.W.; Cooksley, W.G.; Czaja, A.J.; Desmet, V.J.; et al. International Autoimmune Hepatitis Group Report: Review of criteria for diagnosis of autoimmune hepatitis. J. Hepatol. 1999, 31, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Hennes, E.M.; Zeniya, M.; Czaja, A.J.; Parés, A.; Dalekos, G.N.; Krawitt, E.L.; Bittencourt, P.L.; Porta, G.; Boberg, K.M.; Hofer, H.; et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008, 48, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Abe, K.; Takahashi, A.; Zeniya, M.; Ichida, T. Clinical features of hepatocellular carcinoma in patients with autoimmune hepatitis in Japan. J. Gastroenterol. 2013, 48, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Kanda, T.; Nakamura, M.; Miyamura, T.; Yasui, S.; Nakamoto, S.; Wu, S.; Arai, M.; Imazeki, F.; Yokosuka, O. Acute liver injury in a patient with alcohol dependence: A case resembling autoimmune hepatitis or drug-induced liver injury. Case Rep. Gastroenterol. 2014, 8, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, P.T.; Baragiotta, A.; Heneghan, M.A.; Floreani, A.; Venturi, C.; Underhill, J.A.; Jones, D.E.; James, O.F.; Bassendine, M.F. HLA class II alleles, genotypes, haplotypes, and amino acids in primary biliary cirrhosis: A large-scale study. Hepatology 2006, 44, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Mells, G.F.; Floyd, J.A.; Morley, K.I.; Cordell, H.J.; Franklin, C.S.; Shin, S.Y.; Heneghan, M.A.; Neuberger, J.M.; Donaldson, P.T.; Day, D.B.; et al. Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat. Genet. 2011, 43, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Nishida, N.; Kawashima, M.; Aiba, Y.; Tanaka, A.; Yasunami, M.; Nakamura, H.; Komori, A.; Nakamuta, M.; Zeniya, M.; et al. Genome-wide association study identifies TNFSF15 and POU2AF1 as susceptibility loci for primary biliary cirrhosis in the Japanese population. Am. J. Hum. Genet. 2012, 91, 721–728. [Google Scholar] [CrossRef] [PubMed]
- De Boer, Y.S.; van Gerven, N.M.; Zwiers, A.; Verwer, B.J.; van Hoek, B.; van Erpecum, K.J.; Beuers, U.; van Buuren, H.R.; Drenth, J.P.; den Ouden, J.W.; et al. Genome-wide association study identifies variants associated with autoimmune hepatitis type 1. Gastroenterology 2014, 147, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, A.; Sattler, T.; Unold, H.P.; Grambihler, A.; Teufel, A.; Koch, S.; Schuchmann, M.; Biesterfeld, S.; Wörns, M.A.; Galle, P.R.; et al. Predictive Scores in Primary Biliary Cirrhosis: A Retrospective Single Center Analysis of 204 Patients. J. Clin. Gastroenterol. 2014. [Google Scholar] [CrossRef]
- Loaeza-del-Castillo, A.; Paz-Pineda, F.; Oviedo-Cárdenas, E.; Sánchez-Avila, F.; Vargas-Vorácková, F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann. Hepatol. 2008, 7, 350–357. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
