Abstract
Background: Several studies have reported a reduced risk of COVID-19-related mortality in patients taking antidiabetic medications. This is an umbrella review, meta-analysis, and Bayesian sensitivity assessment of SGLT2 inhibitors (SGLT2is) in COVID-19 patients with type 2 diabetes mellitus (T2DM). Methods: A search was conducted on the MEDLINE (PubMed), EMBASE, Cochrane, and ClinicalTrials.gov databases on 5/12/2023. We performed an umbrella review of systematic reviews and meta-analyses on the effects of SGLT2is in T2DM patients with COVID-19 and critically appraised them using AMSTAR 2.0. Trials investigating SGLT2i use in COVID-19 patients post-hospitalisation and observational studies on prior SGLT2i use among COVID-19 patients were included in the meta-analysis, adhering to the PRISMA guidelines. Results: SGLT2is exhibited significantly lower odds of mortality (OR 0.67, 95% CI 0.53–0.84) and hospitalisation (OR 0.84, 0.75–0.94) in COVID-19 patients with T2DM. Bayesian sensitivity analyses corroborated most of the findings, with differences observed in hospitalisation and mortality outcomes. SGLT-2 inhibitors showed an OR of 1.20 (95% CI 0.64–2.27) for diabetic ketoacidosis. Publication bias was observed for hospitalisation, but not for mortality. The GRADE assessment indicated a low to very low quality of evidence because of the observational studies included. Conclusions: The prophylactic use of SGLT2is reduces mortality and hospitalisation among COVID-19 patients, particularly in patients with diabetes. The utility of SGLT2is after hospitalisation is uncertain and warrants further investigation. A limited efficacy has been observed under critical conditions. Individualised assessment is crucial before integration into COVID-19 management.
1. Introduction
COVID-19, the fast-spreading pandemic caused by SARS-CoV-2, had infected more than 772 million people globally by the end of November 2023, killing approximately 6.9 million [1]. Most of these deaths were attributed to comorbid conditions such as hypertension, diabetes, cardiovascular disease, and respiratory disorders [2]. It has also been seen that patients with COVID-19 have a high prevalence of diabetes [3]. The risk of severe infection in patients with diabetes and COVID-19 is associated with an increase in angiotensin-converting enzyme 2 (ACE 2), impaired T Cell function, and increased interleukin-6 (IL-6), which promotes COVID-19 infection due to increased viral entry and an impaired immune response [3]. Patients with type 2 diabetes mellitus (T2DM) are more prone to hospitalisation, respiratory dysfunction, and COVID-19-related death [4,5,6]. Studies have shown that poor glycaemic control is significantly associated with a poor clinical prognosis in patients with T2DM and COVID-19 [7,8]. Therefore, there is a keen interest in exploring antidiabetic therapy to improve the clinical outcomes of individuals with T2DM who are affected by COVID-19 [7].
Antidiabetic agents, such as Metformin, Dipeptidyl Peptidase-4 (DPP-4) inhibitors, Sulfonylurea, Glinides, Sodium–Glucose Co-Transporter 2 inhibitors (SGLT2is), glucagon-like peptide-1 (GLP-1) Receptor Agonists, α-glycosidase inhibitors, and thiazolidinediones (TZDs), are effective, safe, and widely used in the treatment of diabetes. Apart from their primary function in managing diabetes, there have been documented reports of anti-inflammatory and antiviral properties associated with antidiabetic agents [9]. Previous studies have shown that antidiabetic medications may significantly reduce COVID-19-related mortalities. At the same time, insulin treatment is associated with poor outcomes [10,11,12,13]. SGLT2is are glucose-lowering agents used in T2DM with other comorbidities, such as chronic kidney disease (CKD) and heart failure [14,15]. However, evidence regarding the benefits of SGLT2is in T2DM patients with COVID-19 is limited. This study aimed to assess the effectiveness and safety of SGLT2is in reducing mortality and morbidity in patients with COVID-19.
2. Methodology
2.1. Literature Searching
MEDLINE (PubMed), EMBASE, Cochrane, and the National Library of Medicine (ClinicalTrials.gov) were searched. The PICO elements for the three research questions are listed in Supplementary Table S1.
There were three primary objectives during the literature search.
- To identify studies investigating the effect of SGLT2is on COVID-19 patients and their outcomes after hospitalisation.
- To identify observational studies that have studied the effects of SGLT2is in patients who were already on the medication and later developed COVID-19.
- To identify systematic reviews and meta-analyses that have reviewed studies investigating the effects of SGLT-2 inhibitors and their outcomes in patients with diabetes diagnosed with COVID-19.
A search query was made for each database using a common framework. A protocol was pre-registered in PROSPERO (Registration: CRD42023493347).
2.2. Search Strategy
Search terms and their iterations were identified through Medical Subject Headings (MeSH) index terms and a text mining tool, PubMed PubReMiner [16]. Filters for observational study designs were adopted from the Harvard Countway Library guide [17], which is a modified version of the “fixed method B” proposed by Furlan et al. [18]. Filters for randomised trials in MEDLINE and EMBASE (sensitivity- and precision-maximising version) were adopted from the technical supplement to Chapter 4 of the Cochrane Handbook for Systematic Reviews of Interventions (version 6.2) [19]. A high-sensitivity and precision PubMed search filter developed by Salvador-Oliván et al. was used to identify systematic reviews and meta-analyses for an umbrella review [20]. This review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Table S2). The search queries are listed in Supplementary Table S3. The search date ranged from the earliest available date to 7 December 2023. The inclusion and exclusion criteria are presented in Supplementary Table S3.
2.3. Statistical Analysis
Relevant data were extracted from the studies as count data (numerator and denominator) or effect size (risk ratio or odds ratio with variability). Individual study estimates were pooled using the Mantel–Haenszel method [21] with a random-effects model, expecting clinical heterogeneity in the included studies. Heterogeneity was assessed using prediction intervals [22], I2 [23], and tau2 [24]. Subgroup analyses were performed based on the type of comparator used. Sensitivity analyses included a Bayesian meta-analysis with weakly informative priors (as these may provide better estimates when the number of studies is less [25]), leave-one-out meta-analysis, and subgrouping based on study quality. All analyses were performed using R version 4.3.0 [26] with a restricted maximum−likelihood estimator and Q−profile for tau2 and its CI.
The primary outcomes were as follows:
The odds ratio of mortality and hospitalisation among COVID-19 patients with a history of SGLT2i use compared to COVID-19 patients without a history of SGLT2i use.
2.4. Quality and Risk of Bias Assessment
The Revised Cochrane risk-of-bias tool for randomised trials (RoB 2) was used to assess the risk of bias analysis of randomised trials [27]. The Risk of Bias in Non-randomised Studies of Interventions (ROBINS-I) tool was used for observational studies [28]. The AMSTAR 2 tool was used to critically appraise the systematic reviews included in the umbrella review [29]. The class of evidence was rated based on the following criteria [30]:
- Convincing (class I): number of cases > 1000, p < 10−6, I2 < 50%, 95% prediction interval excluding the null, no small-study effects, and no excess significance bias;
- Highly suggestive (class II): when the number of cases was >1000, p < 10−6, the largest study with a statistically significant effect and class I criteria not met;
- Suggestive (class III): when the number of cases > 1000, p < 10−3, and class I–II criteria were not met;
- Weak (class IV) when p < 0.05, and class I–III criteria were not met.
- Non-significant when p > 0.05.
3. Results
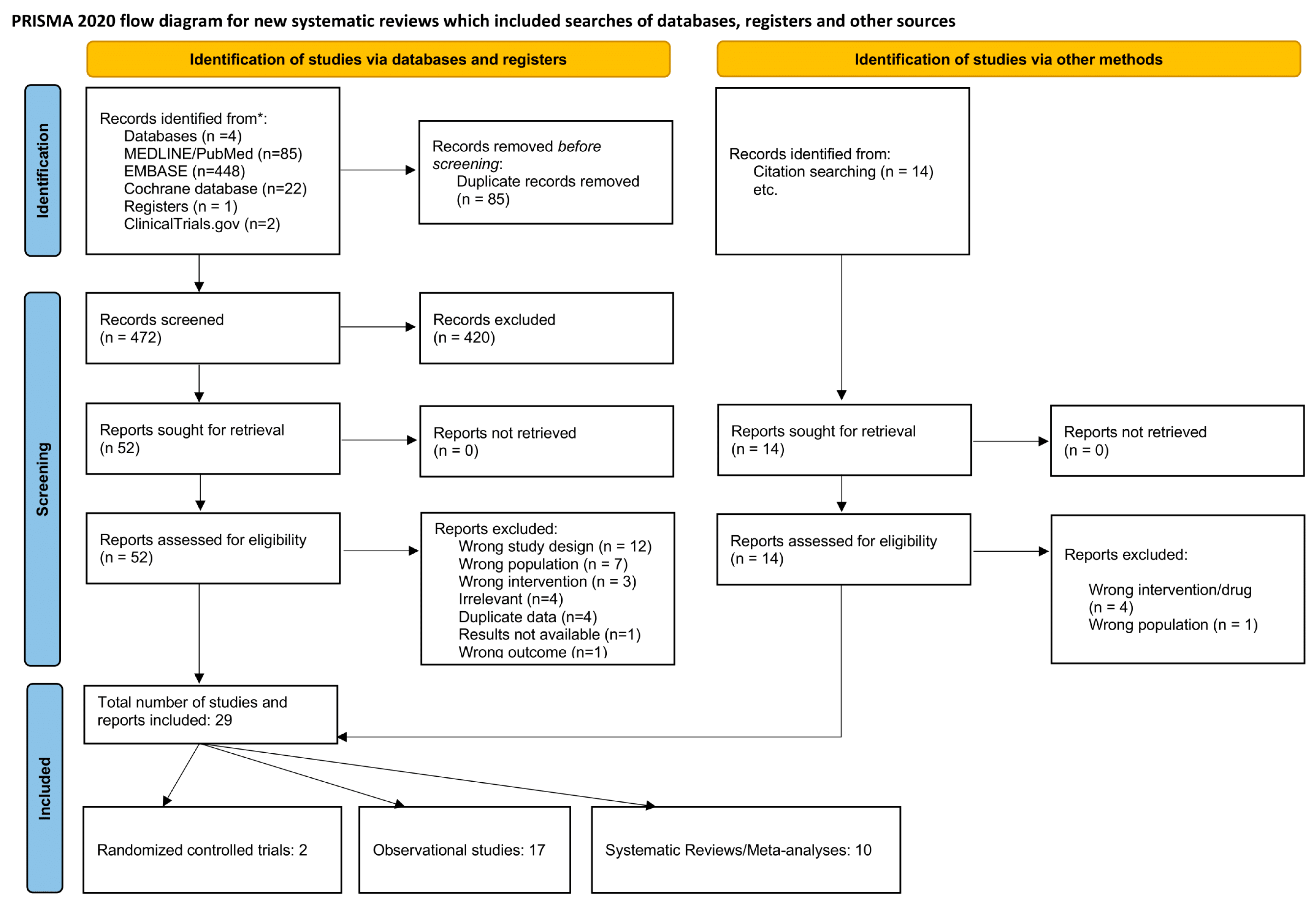
A total of 472 articles identified in the literature were screened after deduplication. Upon screening the titles and abstracts, 420 articles were deemed irrelevant. Subsequently, 52 articles underwent full-text assessment, with 32 exclusions for the following reasons: wrong study design (n = 12), wrong population (n = 7), wrong intervention (n = 3), irrelevant (n = 4), duplicate data (n = 4), unavailable results (n = 1), and wrong outcome (n = 1). Nine additional articles were incorporated through citation searching. The final review included 29 studies, comprising two randomised controlled trials, 17 observational studies, and 10 systematic reviews/meta-analyses. The screening process is illustrated in the PRISMA flowchart (Figure 1).
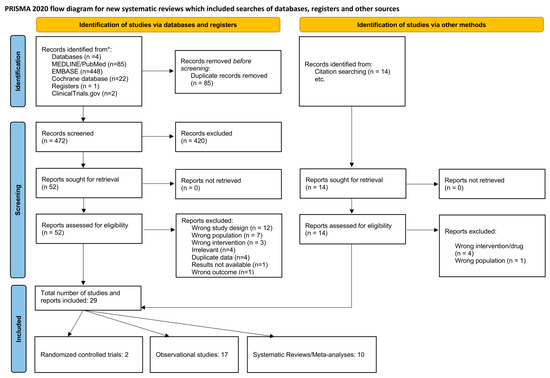
Figure 1.
PRISMA flowchart outlining the screening process.
3.1. Population Characteristics
In the included studies, the majority [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49] of participants belonged to the elderly age group, with mean ages ranging from 58 [33,34] to 86 years [42]. Most of the studies had an equal distribution of participants among the sexes [33,35,37,40,41,42,43,48], while some studies had a male-predominant population [31,32,34,38,39,49]. The characteristics of the population in the included studies are presented in Table 1. The umbrella review includes ten systematic reviews and meta-analyses; their characteristics are described in Table 2.

Table 1.
Population characteristics of the studies included in the review.

Table 2.
Characteristics of the included systematic reviews and meta-analyses.
3.1.1. Effect of SGLT2i Intervention on Hospitalised COVID-19 Patients (Trials)
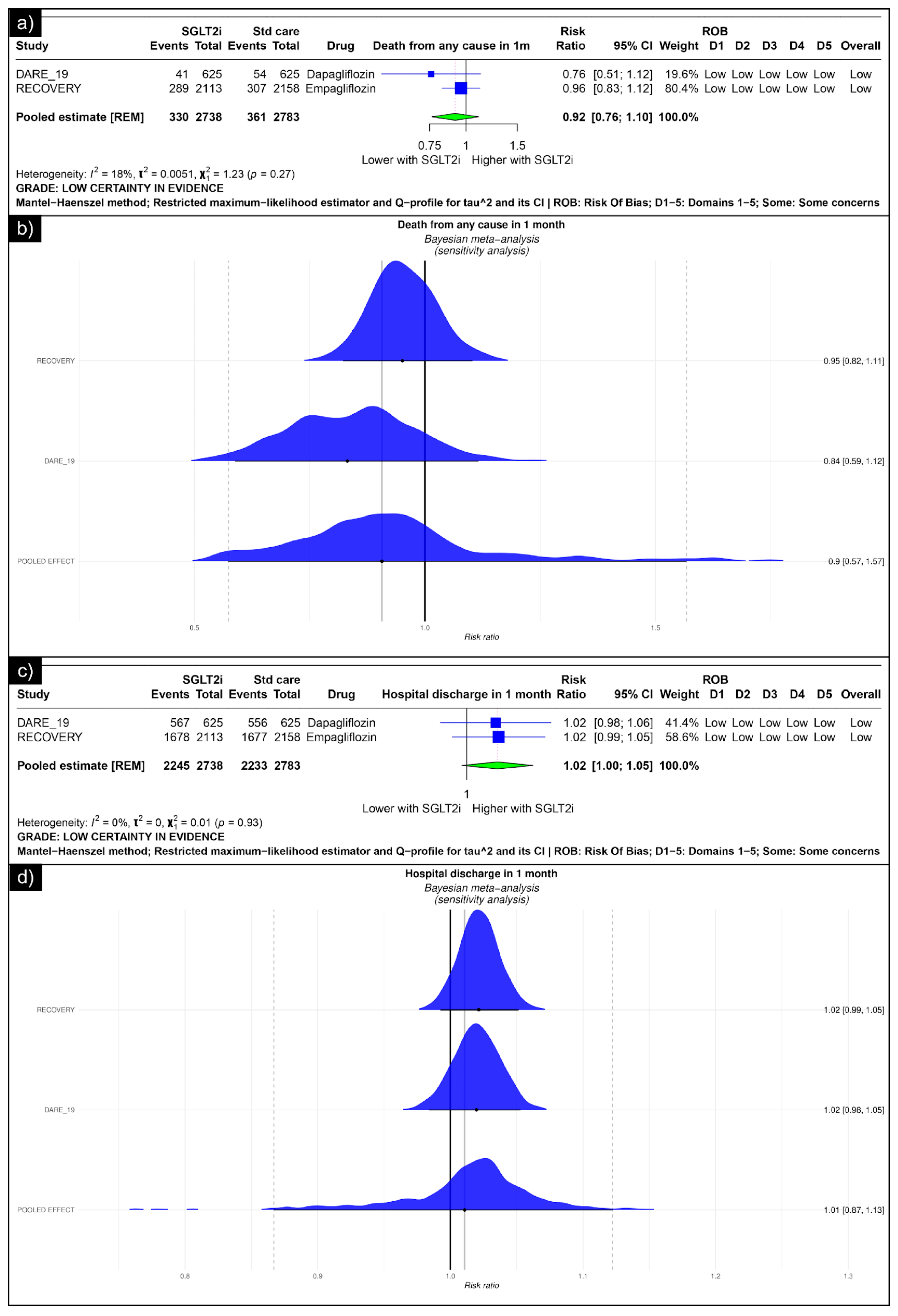
The analysis of death from any cause revealed an estimated pooled risk ratio of 0.92 (CI: 0.76, 1.10, I2 = 18%) using the random-effects model (Figure 2a). The Bayesian sensitivity analysis of the trials yielded insignificant results (Figure 2b). The analysis of hospital discharge in one month revealed an estimated pooled risk ratio of 1.02 (CI: 1.00; 1.05, I2 = 0%) using the random-effects model (Figure 2c). The Bayesian sensitivity analysis of the trials yielded insignificant results (Figure 2d).
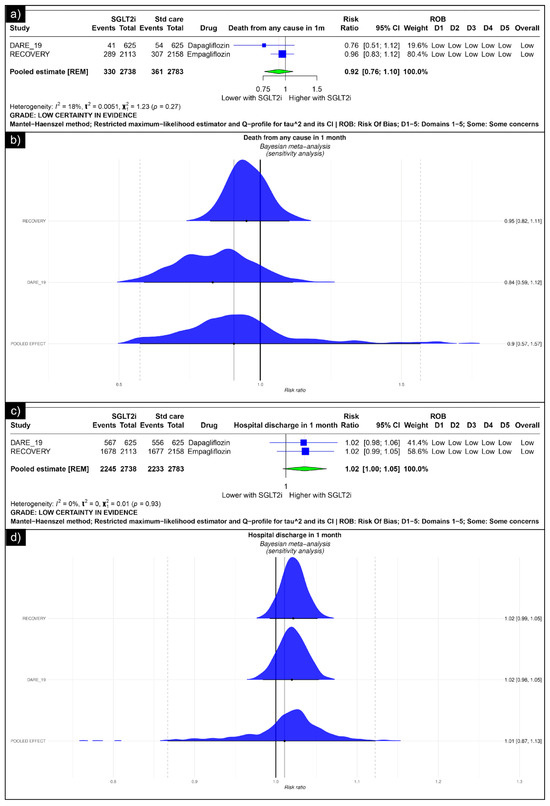
Figure 2.
Forest plot of primary outcomes reported in trials: (a) Forest plot of “mortality” outcome reported in trials. (b) Bayesian Meta-Analysis (Sensitivity Analysis) of “mortality” outcomes reported in trials. (c) Forest plot of “hospitalisation” outcomes reported in the trials. (d) Bayesian Meta-Analysis (Sensitivity Analysis) of “hospitalisation” outcomes reported in the trials.
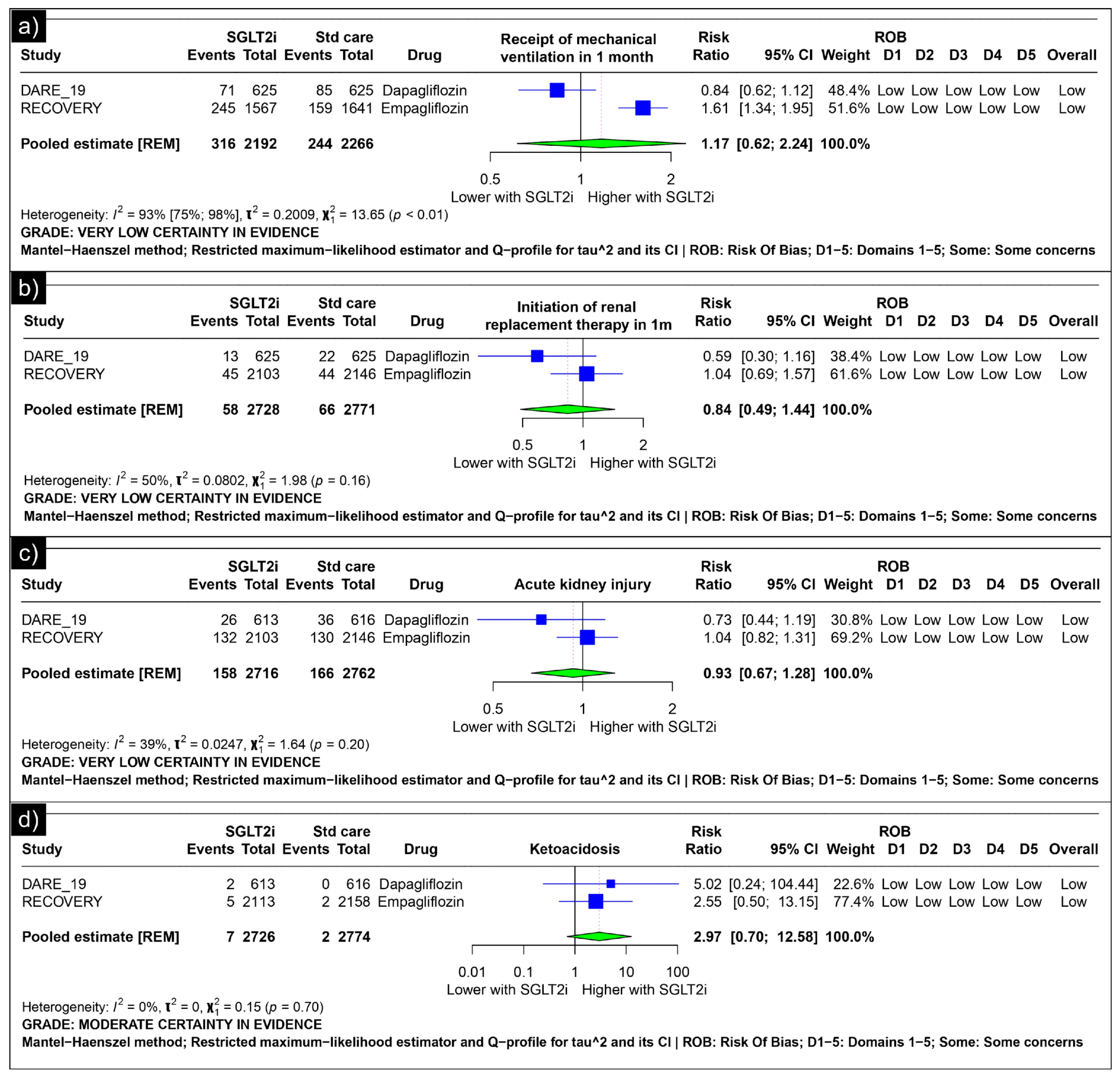
Analysis of mechanical ventilation within one month revealed an estimated pooled risk ratio of 1.17 (CI: 0.62; 2.24, I2 = 93%) using the random-effects model (Figure 3a). The analysis of renal replacement therapy within one month revealed an estimated pooled risk ratio of 0.84 (CI: 0.49; 1.44, I2 = 50%) using the random-effects model (Figure 3b). The analysis of acute kidney injury revealed an estimated pooled risk ratio of 0.93 (CI: 0.67, 1.28, I2 = 39%) using the random-effects model (Figure 3c). The analysis of ketoacidosis revealed an estimated pooled risk ratio of 2.97 (CI: 0.70; 12.58, I2 = 0%) using the random-effects model (Figure 3d).
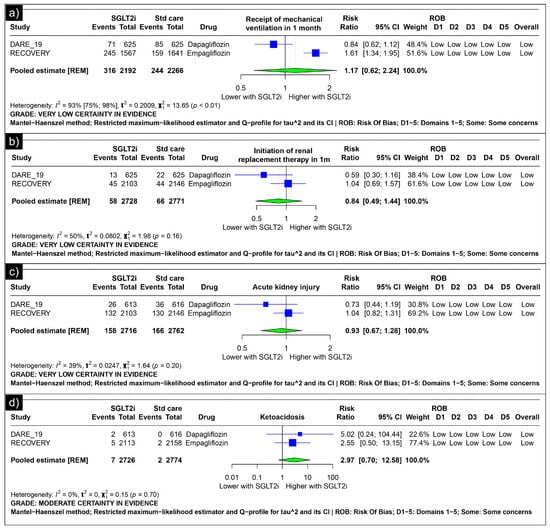
Figure 3.
Forest plot of secondary outcomes reported in the trials. (a) Forest plot of “mechanical ventilation” outcomes reported in trials. (b) Forest plot of “renal replacement therapy” outcomes reported in trials. (c) Forest plot of “acute kidney injury” outcomes reported in trials. (d) Forest plot of “ketoacidosis” outcomes reported in trials.
3.1.2. Prior SGLT2i Use Among COVID-19 Patients in Observational Studies
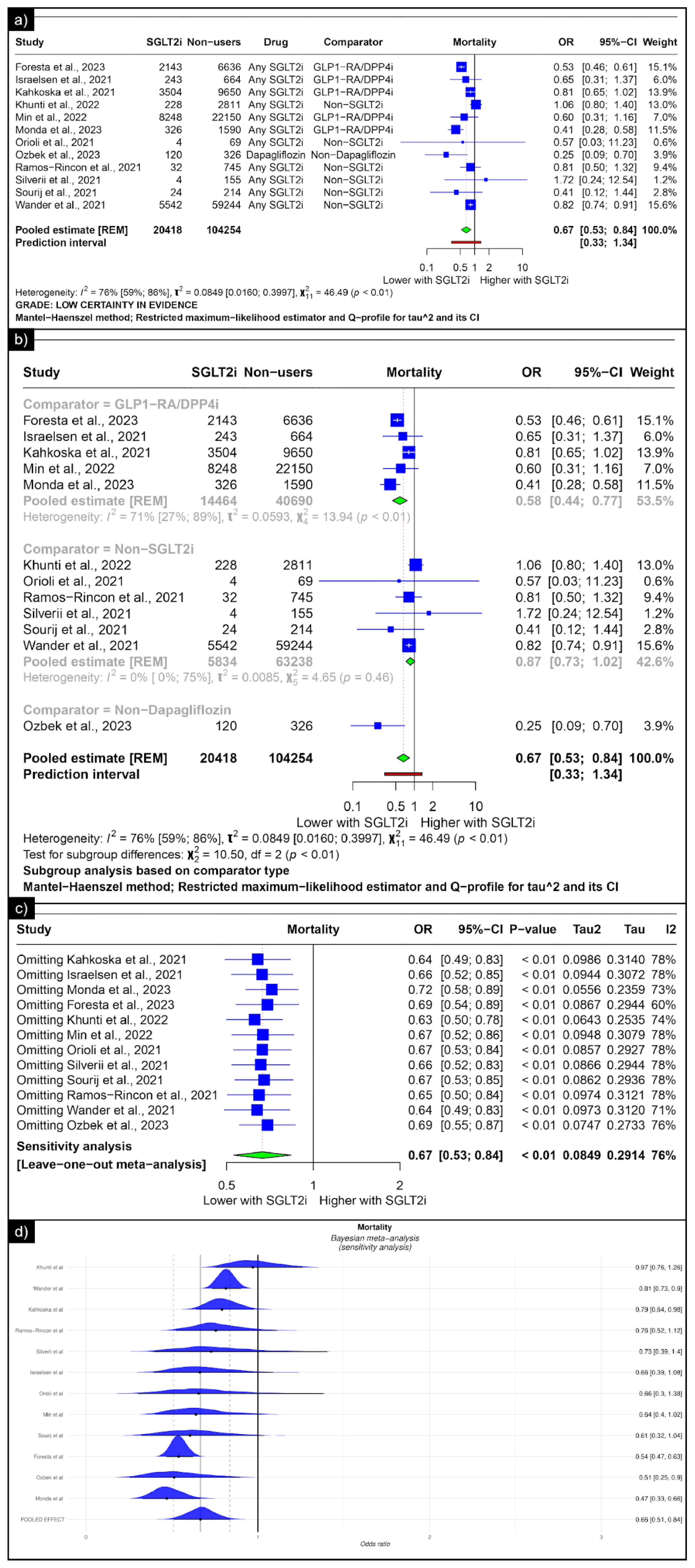
The analysis of mortality revealed an estimated pooled odds ratio of 0.67 (CI: 0.53; 0.84, I2 = 76%) using the random-effects model (Figure 4a). Subgroup analysis was conducted based on the comparators used in the studies. In comparisons between SGLT-2 inhibitors and GLP-1 receptor agonists/Dipeptidyl Peptidase 4 inhibitors concerning mortality, the pooled odds ratio was 0.58 (CI: 0.44; 0.77, I2 = 71%) (Figure 4b). When compared with non-SGLT2is overall, the pooled odds ratio was 0.87 (CI: 0.73; 1.02, I2 = 0%), and in comparison with non-dapaglifozin, it showed a pooled odds ratio of 0.25 (CI: 0.53; 0.84) (Figure 4b). The sensitivity analysis showed that no single study significantly affected the overall primary outcome (mortality). Removing any individual studies did not affect the significance of the results (Figure 4c). The Bayesian sensitivity analysis, as depicted in Figure 4d, indicated that, upon excluding specific studies, notable changes occurred in the outcomes.
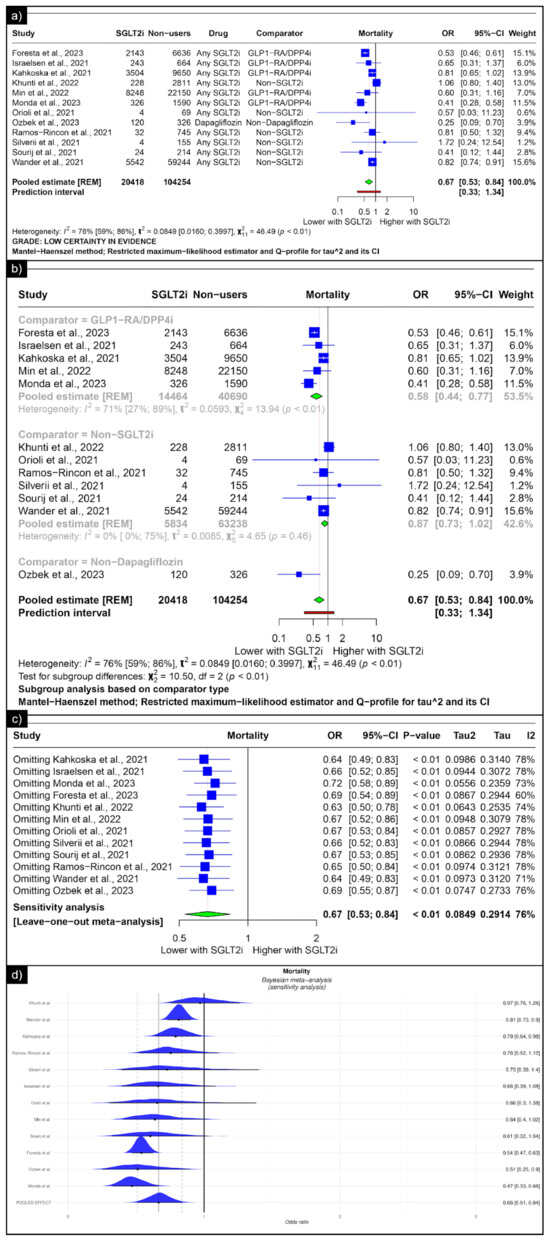
Figure 4.
Forest plot of primary outcomes reported in observational studies: (a) Forest plot of “mortality” outcome reported in observational studies. (b) Sub-group analysis of “mortality” outcomes with SGLT2i use compared with GLP1-RA/DPP4i, non-SGLT2i users, and non-dapagliflozin users reported in observational studies. (c) Sensitivity Analysis (leave one out meta-analysis) of “mortality” outcome reported in observational studies. (d) Bayesian Meta-Analysis (Sensitivity Analysis) of “mortality” outcomes reported in observational studies [33,34,35,36,38,39,41,42,43,44,47,49].
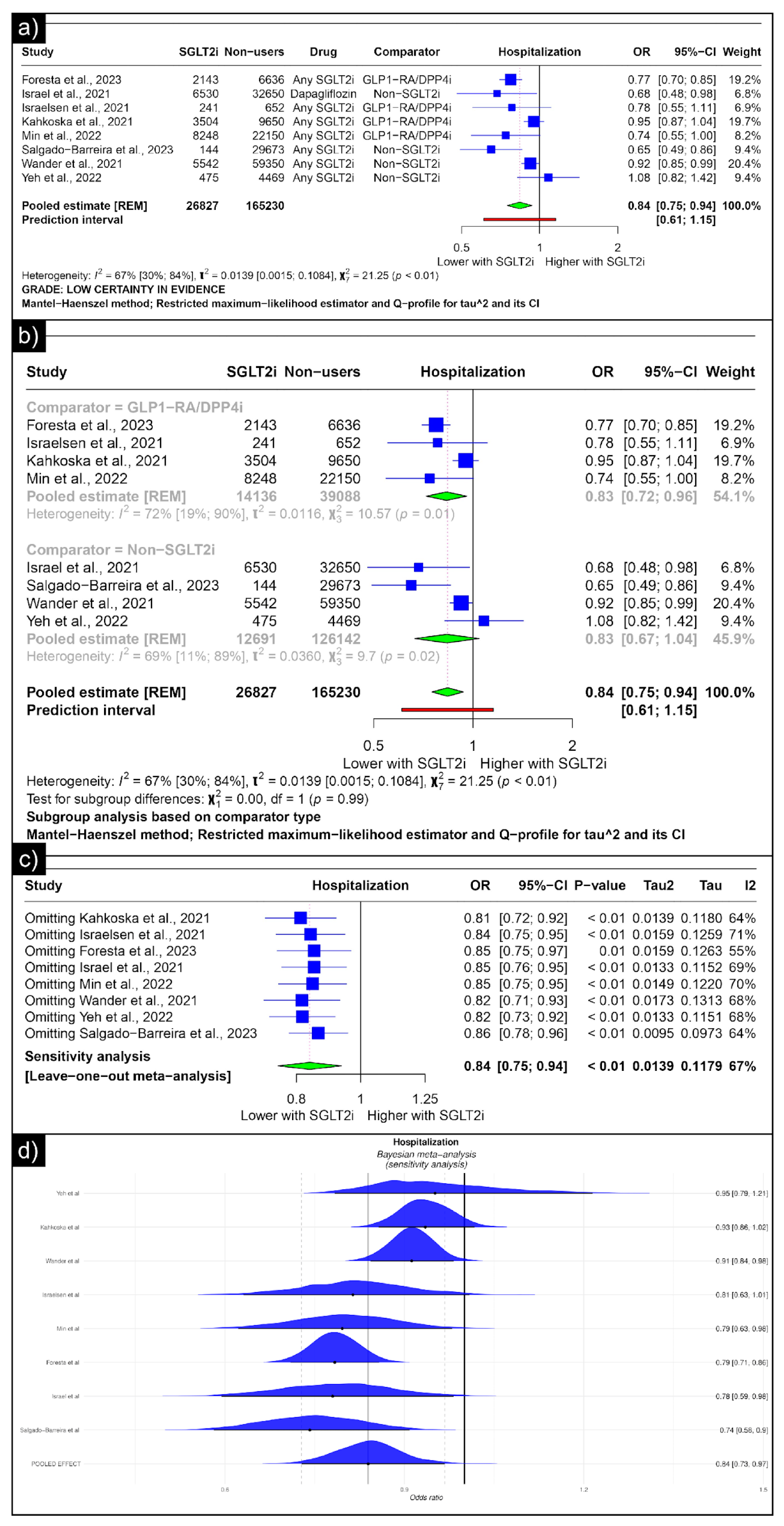
The analysis of hospitalisation revealed an estimated pooled odds ratio of 0.84 (CI: 0.75; 0.94, I2 = 67%) utilising the random-effects model (Figure 5a). A subgroup analysis was conducted based on the comparators used in the studies. In comparisons between SGLT-2 inhibitors and GLP-1 receptor agonists/Dipeptidyl Peptidase 4 inhibitors concerning hospitalisation, the pooled odds ratio was 0.83 (CI: 0.72; 0.96, I2 = 72%) (Figure 5b). When compared with non-SGLT2is overall, the pooled odds ratio was 0.83 (CI: 0.67; 1.04, I2 = 69%). No single study significantly affected the sensitivity analysis’s overall outcome (hospitalisation) (Figure 5c). No individual study affected the significance of the results. Bayesian sensitivity analysis, however, revealed that the exclusion of certain studies led to significant shifts in the outcomes (Figure 5d).
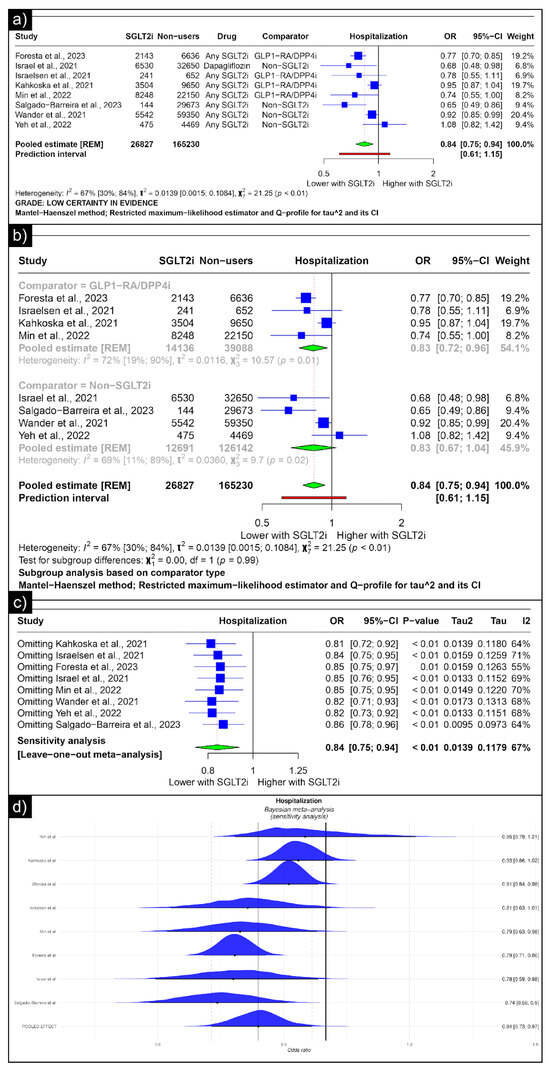
Figure 5.
Forest plot of primary outcomes reported in observational studies: (a) Forest plot of “hospitalisation” outcome reported in observational studies. (b) Sub-group analysis of “hospitalisation” outcomes with SGLT2i use compared with GLP1-RA/DPP4i and non-SGLT2i use reported in observational studies. (c) Sensitivity Analysis (leave-one-out meta-analysis) of “hospitalisation” outcomes reported in observational studies. (d) Bayesian Meta-Analysis (Sensitivity Analysis) of “hospitalisation” outcomes reported in observational studies [33,34,37,38,40,41,44,48].
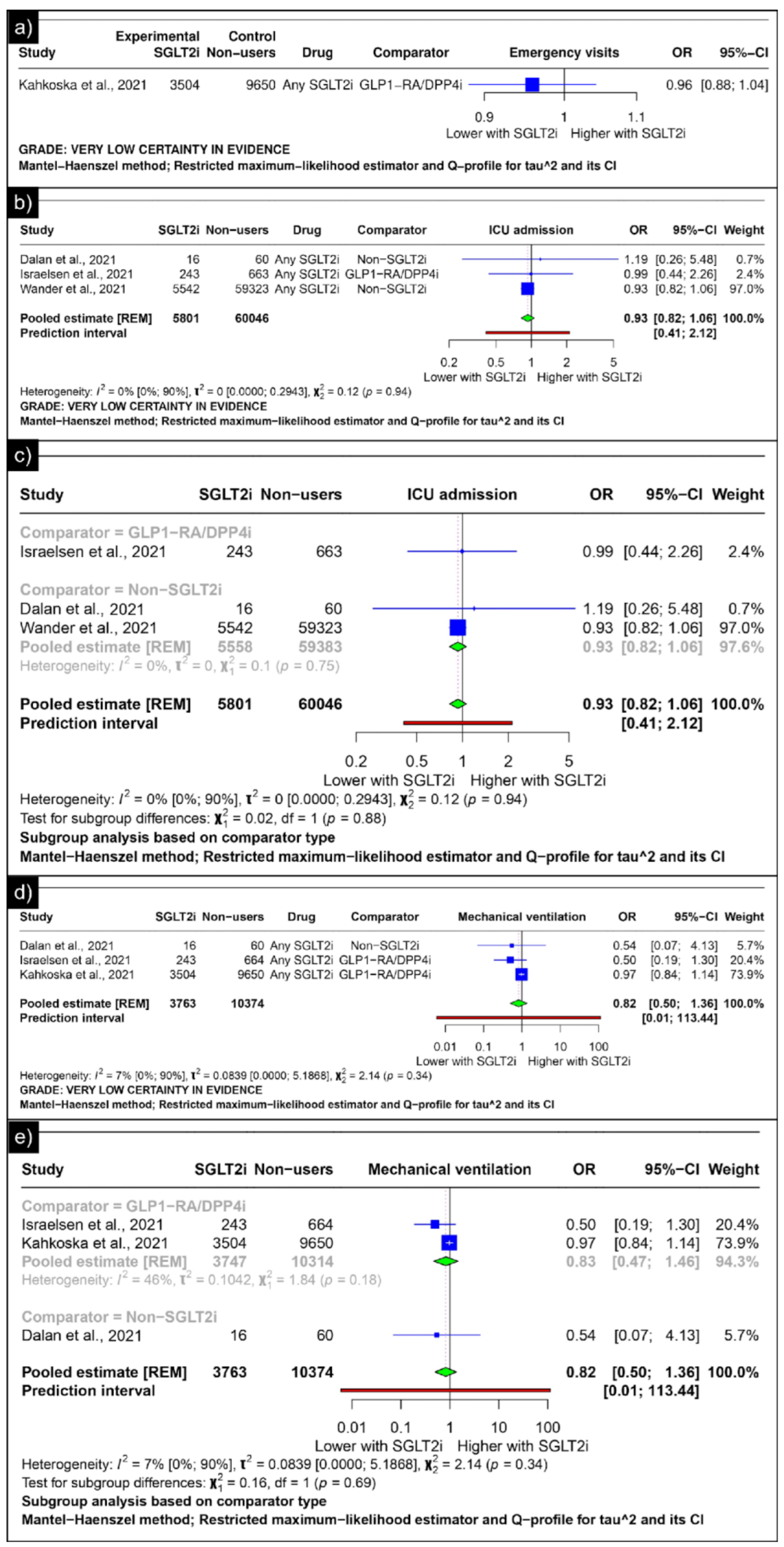
Only Kahkoska et al. reported the outcomes of emergency visits, revealing an odds ratio of 0.96 (CI: 0.88;1.04) (Figure 6a). The analysis of ICU admission revealed an estimated pooled odds ratio of 0.93 (CI: 0.82; 1.06, I2 = 0%) using the random-effects model (Figure 6b). A subgroup analysis was conducted based on the comparators used in the studies. In comparisons between SGLT-2 inhibitors and GLP-1 receptor agonists/Dipeptidyl Peptidase 4 inhibitors concerning ICU admission, the odds ratio reported by Israelsen et al. was 0.99 (CI: 0.44; 2.26) (Figure 6c). When compared with non-SGLT2is overall, the estimated pooled odds ratio was 0.93 (CI: 0.82; 1.06, I2 = 0%) (Figure 6c).
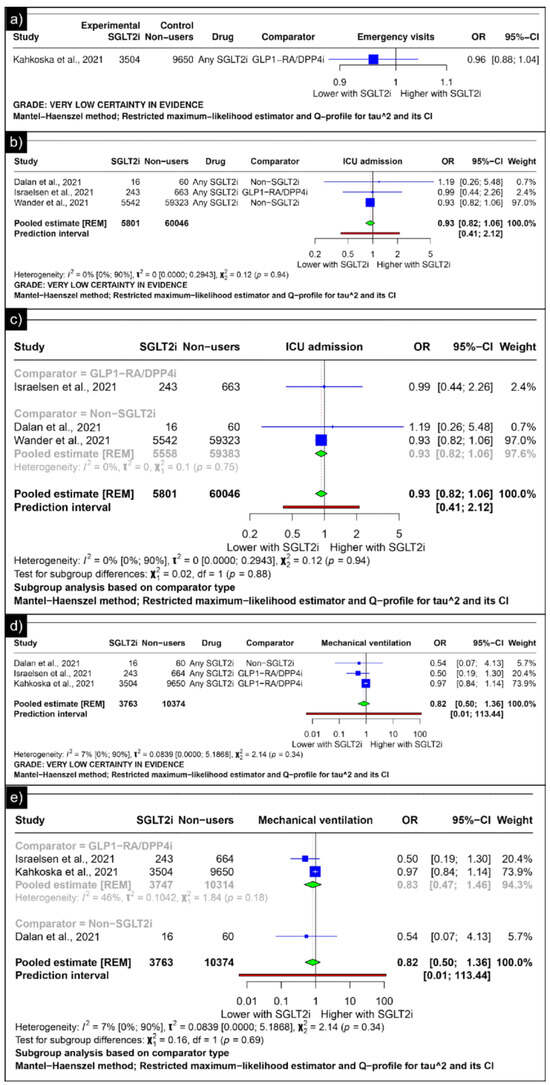
Figure 6.
Forest plot of secondary outcomes reported in observational studies: (a) Forest plot of “emergency visits” outcome reported in observational studies. (b) Forest plot of “ICU admission” outcome reported in observational studies. (c) Subgroup analysis of “ICU admission” outcomes with SGLT2i use compared with GLP1-RA/DPP4i use and non-SGLT2i users reported in observational studies. (d) Forest plot of “mechanical ventilation” outcome reported in observational studies. (e) A sub-group analysis of the “mechanical ventilation” outcome with SGLT2i use was compared with GLP1-RA/DPP4i use, and non-SGLT2i users were reported in observational studies [33,34,44,46].
The analysis of mechanical ventilation revealed an estimated pooled odds ratio of 0.82 (CI: 0.50; 1.36, I2 = 7%) utilising the random-effects model (Figure 6d). A subgroup analysis was conducted based on the comparators used in the studies. In comparisons between SGLT-2 inhibitors and GLP-1 receptor agonists/Dipeptidyl Peptidase 4 inhibitors concerning mechanical ventilation, the estimated pooled odds ratio was 0.83 (CI: 0.47; 1.46, I2 = 46%) (Figure 6e). When compared with non-SGLT2is, Dalan et al. reported an odds ratio of 0.54 (CI: 0.07; 4.13) (Figure 6e).
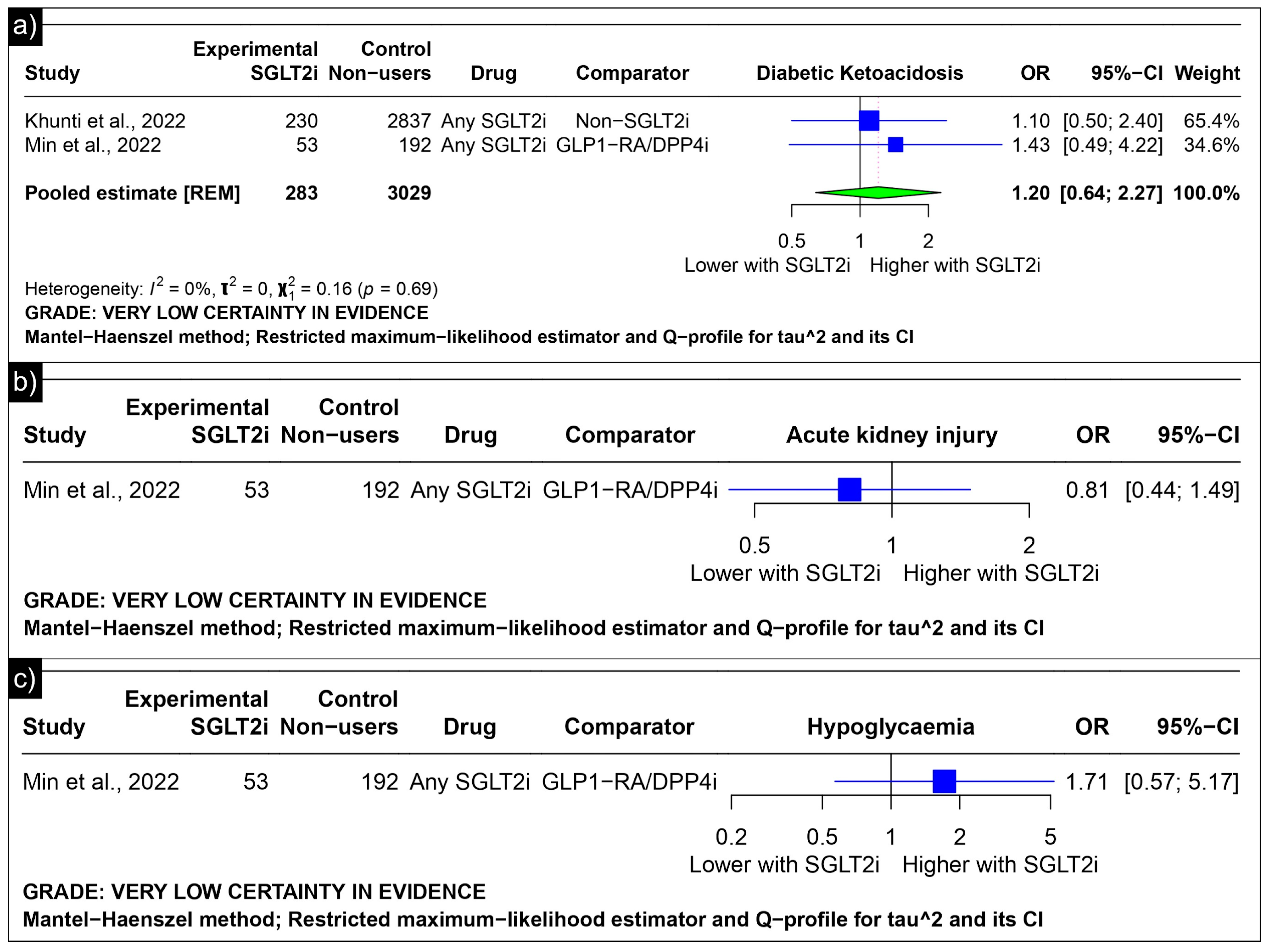
The analysis of diabetic ketoacidosis revealed an estimated pooled odds ratio of 1.20 (CI: 0.64; 2.27, I2 = 0%) using the random-effects model (Figure 7a). Min et al. reported odds ratios of 0.81 (CI: 0.44 to 1.49) for acute kidney injury and 1.71 (CI: 0.57 to 5.17) for hypoglycaemia as compared to GLP-1 receptor agonists/Dipeptidyl Peptidase 4 inhibitors (Figure 7b,c).
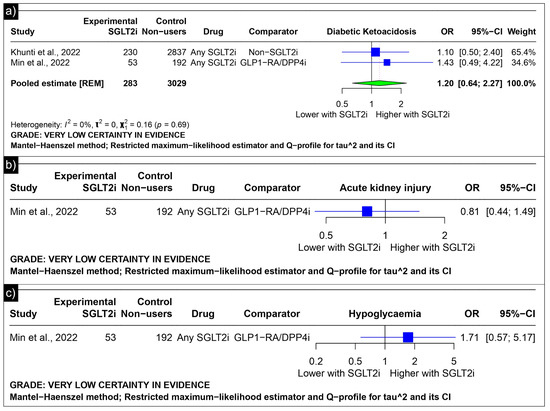
Figure 7.
Forest plot of adverse outcomes reported in observational studies: (a) Forest plot of “Diabetic Ketoacidosis” outcome reported in observational studies. (b) Forest plot of “Acute Kidney Injury” outcome reported in observational studies. (c) Forest plot of “Hypoglycaemia” outcome reported in observational studies [39,41].
3.2. Risk of Bias Analysis
Risk of bias analysis for the trials was performed using ROB 2.0, with both trials being categorised as having “low bias” (Supplementary Figure S1). The risk of bias in the observational studies was assessed using the ROBINS-I tool, as depicted in Supplementary Table S4. The risk of bias in the systematic reviews, assessed using the JBI tool, is shown in Supplementary Table S5.
3.3. Publication Bias
Publication bias was assessed for the outcomes “hospitalisation” and “mortality,” as other outcomes lacked sufficient studies for evaluation. The Doi plot displayed visual asymmetry (Supplementary Figure S2), and the LFK index was −3.88, suggesting publication bias in studies reporting hospitalisation. However, the trim-and-fill contour-enhanced funnel plot with Egger’s regression revealed a p-value of 0.55, indicating no evidence of publication bias or small-study effects for studies that reported the outcome “mortality”.
3.4. GRADE Assessment
The GRADE assessment table outlines the evaluation of SGLT2 inhibitor use in COVID-19 (Supplementary Table S6). The certainty of evidence varied from low (hospitalisation: odds ratio 0.84, CI 0.75–0.94; mortality: odds ratio 0.67, CI 0.53–0.84) to very low (ICU admission: odds ratio 0.93, CI 0.82–1.06; mechanical ventilation: odds ratio 0.82, CI 0.50–1.36; emergency visits: odds ratio 0.96, CI 0.88–1.04; diabetic ketoacidosis: odds ratio 1.20, CI 0.64–2.27; and acute kidney injury: odds ratio 0.81, CI 0.44–1.49). Despite the critical importance of these outcomes in COVID-19 management, the uncertainty of the data persists due to limitations in the study design, risk of bias, inconsistency, and imprecision observed in non-randomised studies.
4. Discussion
Our meta-analysis aimed to comprehensively review the utility of SGLT-2 inhibitors in COVID-19 patients. Focusing on their role as an intervention for hospitalised COVID-19 patients, our analysis centred on the following two randomised controlled trials: the RECOVERY and DARE-19 trials [31,32]. The analysis revealed no significant differences between the two groups in terms of mortality, hospital discharge, the need for mechanical ventilation, renal replacement therapy, acute kidney injury, or ketoacidosis associated with SGLT2 inhibitor use. Both the RECOVERY and DARE-19 trials reached similar conclusions, although their inclusion criteria varied [31,32]. The RECOVERY trial did not include patients who had diabetes at the time of recruitment, but did include patients with a history of diabetes [31]. In contrast, the DARE-19 trial included participants exhibiting cardiometabolic risk factors, including type 2 diabetes, heart failure, and chronic kidney disease [32]. The presence of comorbidities, particularly diabetes, heart failure, and chronic kidney disease, may have influenced the impact of the drugs on disease progression, considering the established efficacy of SGLT2is in these conditions [2,59]. However, in the RECOVERY trial, a post hoc subgroup analysis was performed between patients with and without diabetes, yielding insignificant results. Despite the given evidence, trials are unlikely to derive a definitive conclusion. Our analysis of hospital discharge within one month yielded a pooled risk ratio of 1.02 (CI: 1.00; 1.05), with the lower limit of the confidence interval intersecting 1, underscoring the necessity for further trials to establish a conclusive inference.
We performed a meta-analysis of eligible observational studies to further understand the effects of SGLT-2 inhibitors in COVID-19 patients with a history of SGLT-inhibitor use. Among the 17 observational studies analysed, 12 focused on mortality outcomes. These 12 studies collectively included 20,418 COVID-19 patients with prior SGLT2i use and 104,254 COVID-19 patients without prior SGLT2i exposure. The analysis revealed a significant 33% reduction in mortality odds among individuals with prior SGLT2i use compared to non-SGLT2i users. Except for the study by Kahkoska et al., all studies in the mortality analysis specifically included populations with pre-existing diabetes, potentially introducing bias with regard to our research question [33,34,35,36,38,39,41,42,43,44,47,49]. This suggests that the effects of medication could be attributed to its impact on diabetes rather than solely on COVID-19. However, drawing a definitive conclusion regarding this association remains challenging because of the inherent limitations of the available data.
Similarly, studies by Khunti et al., Shestakova et al., and Solerte et al. reported favourable outcomes in COVID-19 patients with T2DM taking SGLT2is [39,45,60]. Khunti et al.’s retrospective analysis involving nearly 3 million individuals showed a reduced COVID-19-related mortality risk (adjusted hazard ratio: 0.82) for SGLT2i users compared to those not on glucose-lowering medications [39,45,60]. The study of Shestakova et al. highlighted a significantly lower fatality rate among T2DM patients on SGLT2is before infection [45]. Solerte et al.’s recent research indicated lower death rates in COVID-19 patients with diabetes receiving SGLT2i therapy upon hospital admission [60].
A comparison of these medications provides insights into their relative efficacy. Shestakova et al.’s study showed varied mortality outcomes between different glucose-lowering therapies among patients with T2DM. Specifically, glucagon-like peptide-1 receptor agonists exhibited a lower case fatality rate (CFR) than SGLT2is [45]. In contrast, Israelsen et al. reported comparable 30-day mortality rates between users of GLP-1 receptor agonists (GLP-1 RAs) and SGLT-2 inhibitors (3.3% vs. 3.7%), and both were lower than those for DPP-4i users (8.6%) [34]. Khunti et al., however, concluded that there was little overall variation in outcomes for all glucose-lowering treatments, including SGLT2is, and that these differences were probably confounded by indication [39]. Our subgroup analysis confirmed that SGLT2is had a better outcome than GLP-1 RA or DPP-4i, which aligns with all observational studies analysed.
The assessment of hospitalisation risk in COVID-19 patients across observational studies revealed a 16% reduction in odds. When SGLT2is were directly compared with GLP-1 RA/DDP-i, there was a 17% decrease in odds. Israelsen et al., however, reported that hospital admission risks were generally comparable for GLP-1 RA and DPP-4i users and SGLT2i users [34]. However, their study had a smaller sample size than those analysed for this outcome. The meta-analysis of ICU admission and mechanical ventilation across studies yielded statistically insignificant results, indicating no significant differences in odds ratios between the various treatments. These findings suggest that, while SGLT2is offer a potential advantage in reducing the risk of hospitalisation, they do not seem to confer additional benefits concerning ICU admission. It is plausible that patients requiring ICU care might already be in a more critical health state, where improvement with these oral hypoglycaemic agents becomes less discernible or less effective. This implies that medication might be more influential in mitigating less severe COVID-19 cases.
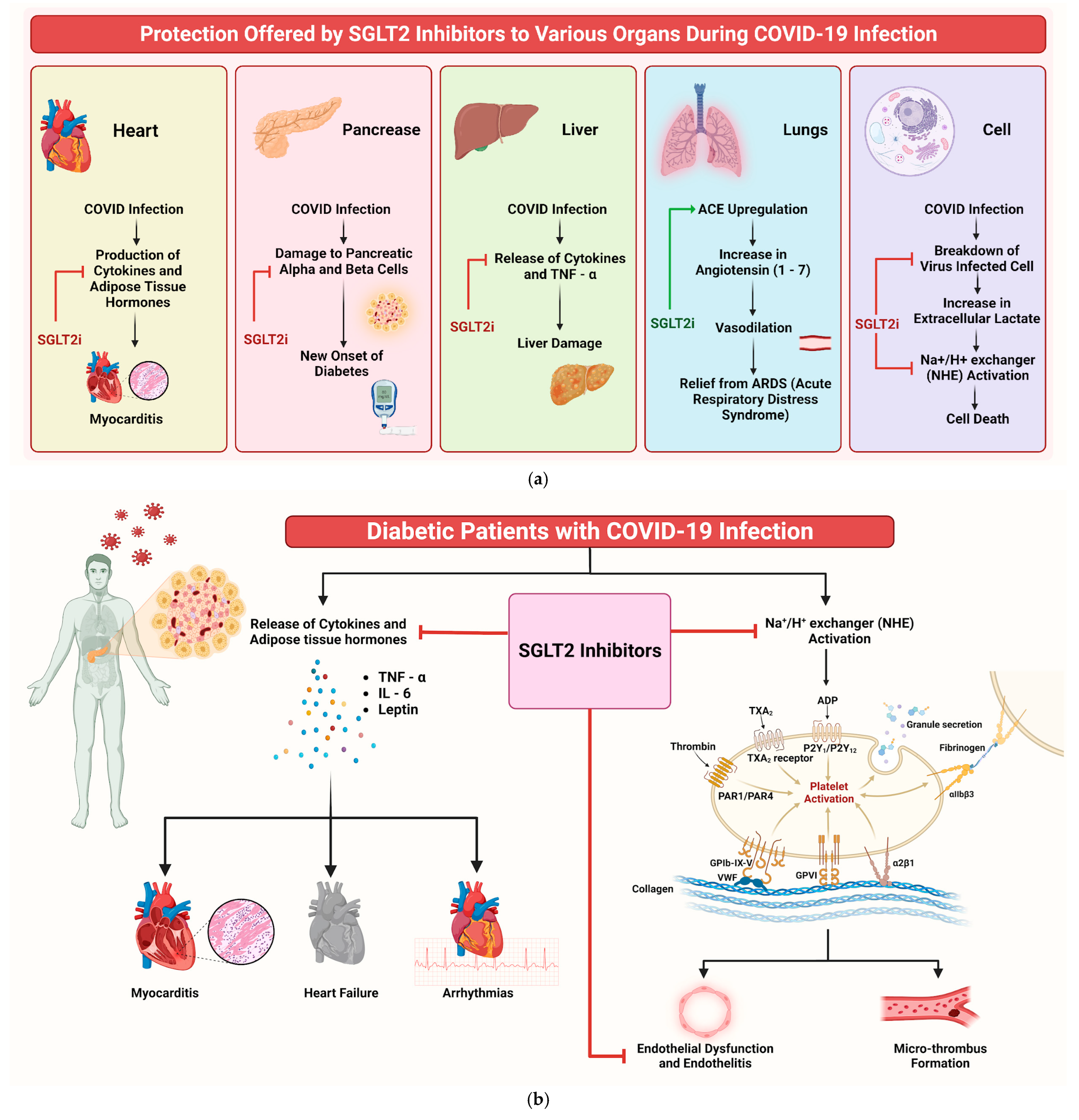
Understanding the potential efficacy of SGLT2is in addressing COVID-19 infection and its complications is crucial. Despite their primary use in diabetes management, drugs such as dapagliflozin show additional beneficial properties beyond glucose control. These medications have pleiotropic effects, including anti-inflammatory actions, enhanced myocardial function, and improved oxygen delivery, as documented in previous studies [61,62]. SGLT2is’ role in COVID-19 can be understood through multiple mechanisms (Figure 8a). One of them that emerges prominently is their influence on cellular pH levels. The binding of SARS-CoV-2 to the angiotensin-converting enzyme 2 (ACE2) is facilitated in acidic environments [50]. SGLT2is indirectly regulate this pH balance, potentially reducing the viral load. Additionally, SGLT2is upregulate ACE2 receptors, increasing Ang 1–7 levels, which are protective against acute respiratory distress syndrome (ARDS) caused by COVID-19 [63,64,65].
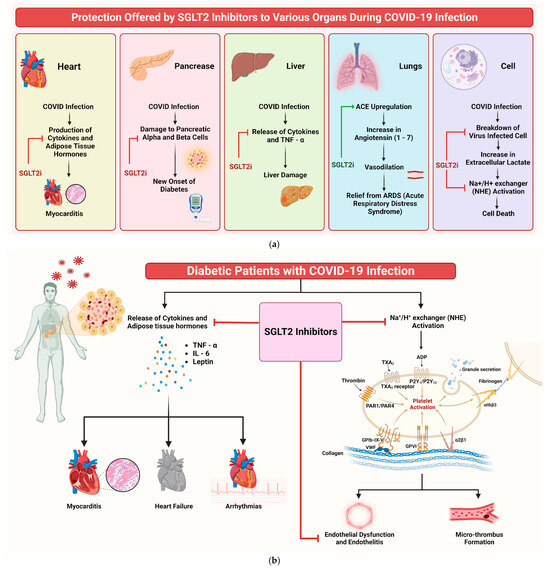
Figure 8.
(a) Mechanism of protection offered by SGLT2is to various organs during COVID-19 infection. The red arrow indicates inhibition, while the green arrow indicates stimulation. (b) Infographic illustrating potential mechanisms of SGLT2is in benefiting COVID-19 patients.
SGLT2is also significantly reduce inflammation and oxidative stress, two key factors in COVID-19’s pathogenesis [66]. By lowering proinflammatory cytokines such as IL-6 and TNF-a, SGLT2is could mitigate the cytokine storm associated with severe COVID-19 cases [67]. Furthermore, these inhibitors impact myocardial and endothelial function, reducing the likelihood of complications such as myocarditis and heart failure [68,69]. SGLT2is also induce metabolic alterations that influence the disease. For instance, they decrease lactate production and modulate the activity of the lactate/H+-symporter and Na+/H+ exchanger (NHE), reducing cellular swelling and death [70,71]. This mechanism is important, because SARS-CoV-2 infection often leads to tissue hypoxia and heightened lactate production [72]. Of note, dapagliflozin has been shown to lower the incidence of new-onset diabetes in COVID-19 patients [73]. This finding holds significance, as new-onset diabetes is a recognised complication of COVID-19, possibly attributed to viral-induced damage to pancreatic cells [74,75]. Moreover, SGLT2is might have a protective role against COVID-19-related renal complications. Acute kidney injury (AKI) is a common complication in COVID-19 patients [76,77], and SGLT2is’ nephroprotective properties could prove beneficial in these cases [77,78,79,80]. Figure 8b shows an illustrative diagram depicting the potential mechanisms through which SGLT2is can benefit COVID-19 patients.
SGLT2is have been shown to have significant beneficial effects on circulating insulinemia and may also influence the course of COVID-19 through their impact on insulin levels and related metabolic processes. SGLT2is lower blood glucose levels by promoting renal glucose excretion rather than directly stimulating insulin secretion. This mechanism leads to decreased insulin levels in the circulation, as the body requires less insulin to effectively manage its blood glucose levels [81]. They have also been associated with weight loss and reductions in body fat, which contribute to an improved insulin sensitivity [82]. Furthermore, SGLT2is can enhance glucagon levels, which may help to balance insulin secretion [81]. Reductions in visceral fat are also closely linked to insulin resistance [83]. Elevated insulin levels can exacerbate inflammation, contributing to the cytokine storm observed in severe COVID-19 cases. By lowering insulin levels, SGLT2is may help to reduce inflammation and improve outcomes for patients suffering from COVID-19 [84]. Moreover, they may reduce adipose tissue inflammation, which is a known contributor to severe COVID-19 complications [84]. Improved glycaemic control can also enhance immune function and reduce the risk of severe disease progression, particularly in patients with pre-existing conditions like diabetes [81]. SGLT2is have been shown to provide cardiovascular protection and improve lung function by reducing interstitial lung oedema and enhancing oxygen utilisation, which can be particularly beneficial for COVID-19 patients who experience respiratory distress or hypoxia [83,84].
However, it is crucial to consider the balance between these advantages and the potential risks associated with SGLT2is. SGLT2is can increase the likelihood of diabetic ketoacidosis (DKA), especially regarding COVID-19 infection, which can trigger DKA [85]. Additionally, they may exacerbate low blood pressure in severe COVID-19 cases and increase the risk of urogenital fungal infections, particularly when combined with treatments such as dexamethasone and antibiotics commonly used in COVID-19 [86,87,88,89,90]. However, our analysis of diabetic ketoacidosis did not show a statistically significant association, suggesting that the observed risk of DKA with SGLT2is in the context of COVID-19 requires further investigation.
The umbrella review part of our study comprised the following ten reviews: one systematic review of case reports [51], six systematic reviews and meta-analyses [7,8,53,54,57,58], one meta-analysis [56], and two network meta-analyses [4,65]. The review population consisted of patients with T2DM who contracted COVID-19 and were on SGLT-2 inhibitors either before or after their hospitalisation or the onset of infection. The reviews primarily assessed outcomes such as the severity of COVID-19 infection [7,53,57], the need for Intensive Care Unit (ICU) admission, invasive and non-invasive mechanical ventilation [4,7,8,53,57], Disseminated Intravascular Coagulation (DIC) [57], Euglycemic Diabetic Ketoacidosis (Eu-DKA) [51,54,58], and in-hospital mortality [4,7,8,53,54,56,57,58,65].
The reviews suggested that the prior use of SGLT2is was associated with reduced adverse outcomes related to COVID-19 and notably lowered mortality rates among individuals with diabetes [7,8,54,56,57,65]. It was also noted that there was a decreased risk of hospitalisation [50] and a reduced risk of requiring non-invasive or invasive mechanical ventilation [69]. While a review of case reports suggested a possible increase in the risk of euglycemic diabetic ketoacidosis [51] with SGLT2is, the level of evidence was deemed non-significant. A systematic review and meta-analysis supported the notion that SGLT-2 inhibitors reduce mortality in COVID-19 without increasing the risk of DKA [54]. Conversely, another review found that prior SGLT-2 inhibitor treatment, when associated with euglycemic diabetic ketoacidosis, might protect renal function in COVID-19 patients with pre-existing ketotic states [58]. The reviews mostly fell into the Class II level of evidence, three were non-significant, and two were classified as having a Class IV level of evidence (Table 2). Our analysis of both observational studies and trials showed no significant increase in the odds of developing DKA. Based on this collective assessment, it appears that SGLT2is may not inherently lead to DKA.
Although SGLT2is such as dapagliflozin offer promising therapeutic avenues to manage COVID-19 and its complications, carefully considering their risks and benefits is essential. Their role in modulating pH levels, reducing inflammation and oxidative stress, and protecting against metabolic, cardiac, and renal complications positions them as valuable pharmacological agents for COVID-19 management. However, their utilisation requires careful consideration, particularly in severely ill COVID-19 patients.
5. Conclusions
The prophylactic use of SGLT2is reduces mortality rates and hospitalisation in COVID-19 patients, particularly in patients with diabetes. However, their utility as interventions after hospitalisation remains uncertain, highlighting the need for larger randomised controlled trials. The medication does not seem to have any significantly associated adverse events. The efficacy of SGLT2is appears to be limited when administered to critically ill patients. The presence of comorbidities and varying trial inclusion criteria underscores the need for more focused investigations. Despite their potential, individualised considerations are essential before integrating SGLT2is into COVID-19 management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diseases13030067/s1, Supplementary Table S1: The PICO (Population, Intervention, Comparator, Outcomes) elements of the research questions of the review. Supplementary Table S2: PRISMA Checklist. Supplementary Table S3: Search strategy. Supplementary Table S4: Critical Appraisal for observational studies using the ROBINS-I tool for non-randomised studies of interventions. Supplementary Table S5: Critical Appraisal Tool for the Risk of Bias in Systematic Reviews from the Joanna Briggs Institute for Systematic Reviews and Research Syntheses [91]. Supplementary Table S6: GRADE Assessment of SGLT2 Inhibitor Effects on COVID-19 Outcomes. Supplementary Figure S1. Risk of Bias assessment of trials using the ROB 2.0. Supplementary Figure S2: (a) Publication Bias of “Hospitalisation” outcome reported in Observational Studies. (b) Publication Bias of “Mortality” Outcome Reported in Observational Studies.
Author Contributions
Conceptualisation, V.S. (Vinay Suresh), M.A.S. and M.B.; methodology, V.G., T.D., M.J., A.V., V.S. (Vinay Suresh) and P.R.; software, M.A.S., M.B. and V.S (Vinay Suresh).; validation, P.R., V.G., T.D., A.V. and M.J.; formal analysis, M.A.S.; investigation, P.R., V.G, M.B., T.D. and A.V.; resources, M.B.; data curation, V.G., T.D., M.J., A.V. and V.S. (Vinay Suresh); writing—original draft preparation, V.S. (Vivek Sanker), M.A.S., M.B., V.G. and T.D.; writing—review and editing, M.B. and P.R.; visualisation, V.S. (Vivek Sanker) and M.A.S.; supervision, M.B.; project administration, M.B.; funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval was not required for this systematic review, as it did not involve human or animal subjects.
Data Availability Statement
The data supporting the findings of this article are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| T2DM | Type 2 Diabetes Mellitus |
| SGLT2i | Sodium-Glucose Cotransporter 2 Inhibitor |
| ACE-2 | Angiotensin-Converting Enzyme 2 |
| IL-6 | Interleukin-6 |
| DPP-4 i | Dipeptidyl Peptidase-4 inhibitor |
| GLP-1 RA | Glucagon-Like Peptide-1 Receptor Agonist |
| TZD | Thiazolidinediones |
| CKD | Chronic Kidney Disease |
| ROB-2 | Risk Of Bias Tool 2.0 |
| ROBINS-I | Risk Of Bias In Non-Randomised Studies - of Interventions |
| AMSTAR 2 | Assessing the Methodological Quality of Systematic Reviews |
| ICU | Intensive Care Unit |
| ARDS | Acute Respiratory Distress Syndrome |
| DIC | Disseminated Intravascular Coagulation |
| Eu-DKA | Euglycemic Diabetic Ketoacidosis |
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 12 December 2023).
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. 2020, 14, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, S.; Yu, H.; Wang, P.; Zhang, Y.; Chen, Z.; Li, Y.; Cheng, L.; Li, W.; Jia, H.; et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: A systematic review and meta-analysis. Aging 2020, 12, 12493–12503. [Google Scholar] [CrossRef]
- Azhar, A.; Khan, W.H.; Al-hosaini, K.; Zia, Q.; Kamal, M.A. Crosstalk between SARS-CoV-2 Infection and Type II Diabetes. Comb. Chem. High. Throughput Screen. 2022, 25, 2429–2442. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Jiang, Z.; Fang, X.; Yang, S.; Jia, H.; Li, L.; Cao, G.; Zhang, K.; Ma, X. Short- and long-term prognosis of glycemic control in COVID-19 patients with type 2 diabetes. QJM Mon. J. Assoc. Physicians 2022, 115, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; She, Z.-G.; Cheng, X.; Qin, J.-J.; Zhang, X.-J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077.e3. [Google Scholar] [CrossRef]
- Katsiki, N.; Ferrannini, E. Anti-inflammatory properties of antidiabetic drugs: A “promised land” in the COVID-19 era? J. Diabetes Complicat. 2020, 34, 107723. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Kurniawan, A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes. Med. 2020, 19, 100290. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, Z.; Zhang, J. DPP-4 inhibitors may improve the mortality of coronavirus disease 2019: A meta-analysis. PLoS ONE 2021, 16, e0251916. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Intan, D.; Hananto, J.E.; Putri, C.; Kurniawan, A. Pre-admission glucagon-like peptide-1 receptor agonist (GLP-1RA) and mortality from coronavirus disease 2019 (COVID-19): A systematic review, meta-analysis, and meta-regression. Diabetes Res. Clin. Pract. 2021, 179, 109031. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Lugito, N.P.H.; Yanto, T.A.; Siregar, J.I.; Kurniawan, A. Insulin Therapy and Outcome of Coronavirus Disease 2019 (COVID-19): A Systematic Review, Meta-Analysis, and Meta-Regression. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 481–489. [Google Scholar] [CrossRef] [PubMed]
- van der Aart-van der Beek, A.B.; de Boer, R.A.; Heerspink, H.J.L. Kidney and heart failure outcomes associated with SGLT2 inhibitor use. Nat. Rev. Nephrol. 2022, 18, 294–306. [Google Scholar] [CrossRef]
- Koufakis, T.; Pavlidis, A.N.; Metallidis, S.; Kotsa, K. Sodium-glucose co-transporter 2 inhibitors in COVID-19: Meeting at the crossroads between heart, diabetes and infectious diseases. Int. J. Clin. Pharm. 2021, 43, 764–767. [Google Scholar] [CrossRef] [PubMed]
- PubMed PubReMiner: A Tool for PubMed Query Building and Literature Mining. Available online: https://hgserver2.amc.nl/cgi-bin/miner/miner2.cgi (accessed on 7 December 2023).
- Bain, P. Research Guides: Systematic Reviews and Meta Analysis: Observational Designs. Available online: https://guides.library.harvard.edu/c.php?g=309982&p=2079546 (accessed on 7 December 2023).
- Furlan, A.D.; Irvin, E.; Bombardier, C. Limited search strategies were effective in finding relevant nonrandomized studies. J. Clin. Epidemiol. 2006, 59, 1303–1311. [Google Scholar] [CrossRef]
- Lefebvre, C.; Glanville, J.; Briscoe, S.; Littlewood, A.; Marshall, C.; Metzendorf, M.-I.; Noel-Storr, A.; Rader, T.; Shokraneh, F.; Thomas, J.; et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane, 2021. Available online: https://training.cochrane.org/handbook/current/chapter-04 (accessed on 7 December 2023).
- Salvador-Oliván, J.A.; Marco-Cuenca, G.; Arquero-Avilés, R. Development of an efficient search filter to retrieve systematic reviews from PubMed. J. Med. Libr. Assoc. JMLA 2021, 109, 561. [Google Scholar] [CrossRef] [PubMed]
- Robins, J.; Breslow, N.; Greenland, S. Estimators of the Mantel-Haenszel variance consistent in both sparse data and large-strata limiting models. Biometrics 1986, 42, 311–323. [Google Scholar] [CrossRef] [PubMed]
- IntHout, J.; Ioannidis, J.P.A.; Rovers, M.M.; Goeman, J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016, 6, e010247. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M. How to understand and report heterogeneity in a meta-analysis: The difference between I-squared and prediction intervals. Integr. Med. Res. 2023, 12, 101014. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Bias and Efficiency of Meta-Analytic Variance Estimators in the Random-Effects Model. J. Educ. Behav. Stat. 2005, 30, 261–293. [Google Scholar] [CrossRef]
- McNeish, D. On Using Bayesian Methods to Address Small Sample Problems. Struct. Equ. Model. Multidiscip. J. 2016, 23, 750–773. [Google Scholar] [CrossRef]
- Shamim, M.A.; Gandhi, A.P.; Dwivedi, P.; Padhi, B.K. How to perform meta-analysis in R: A simple yet comprehensive guide. Evidence 2023, 1, 93–113. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Radua, J. Ten simple rules for conducting umbrella reviews. Evid. Based Ment. Health 2018, 21, 95–100. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Empagliflozin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet Diabetes Endocrinol. 2023, 11, 905–914. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Esterline, R.; Furtado, R.H.M.; Oscarsson, J.; Gasparyan, S.B.; Koch, G.G.; Martinez, F.; Mukhtar, O.; Verma, S.; Chopra, V.; et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021, 9, 586–594. [Google Scholar] [CrossRef]
- Kahkoska, A.R.; Abrahamsen, T.J.; Alexander, G.C.; Bennett, T.D.; Chute, C.G.; Haendel, M.A.; Klein, K.R.; Mehta, H.; Miller, J.D.; Moffitt, R.A.; et al. Association Between Glucagon-Like Peptide 1 Receptor Agonist and Sodium-Glucose Cotransporter 2 Inhibitor Use and COVID-19 Outcomes. Diabetes Care 2021, 44, 1564–1572. [Google Scholar] [CrossRef]
- Israelsen, S.B.; Pottegård, A.; Sandholdt, H.; Madsbad, S.; Thomsen, R.W.; Benfield, T. Comparable COVID-19 outcomes with current use of GLP-1 receptor agonists, DPP-4 inhibitors or SGLT-2 inhibitors among patients with diabetes who tested positive for SARS-CoV-2. Diabetes Obes. Metab. 2021, 23, 1397–1401. [Google Scholar] [CrossRef]
- Monda, V.M.; Voci, C.; Strollo, F.; Passaro, A.; Greco, S.; Monesi, M.; Bigoni, R.; Porcellati, F.; Piani, D.; Satta, E.; et al. Protective Effects of Home T2DM Treatment with Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Co-transporter-2 Inhibitors Against Intensive Care Unit Admission and Mortality in the Acute Phase of the COVID-19 Pandemic: A Retrospective Observational Study in Italy. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2023, 14, 2127–2142. [Google Scholar] [CrossRef]
- Ozbek, O.; Can, M.M. The effect of dapagliflozin use on cardiovascular outcomes in type 2 diabetic patients hospitalized with COVID-19. Int. J. Diabetes Dev. Ctries. 2023, 44, 477–485. [Google Scholar] [CrossRef]
- Salgado-Barreira, A.; Seijas-Amigo, J.; Rodriguez-Mañero, M.; Piñeiro-Lamas, M.; Eiras, S.; Cordero, A.; Gonzalez-Juanatey, J.R.; Figueiras, A. Effect of dapagliflozin on COVID-19 infection and risk of hospitalization. J. Antimicrob. Chemother. 2023, 78, 2335–2342. [Google Scholar] [CrossRef]
- Foresta, A.; Ojeda-Fernandez, L.; Macaluso, G.; Roncaglioni, M.C.; Tettamanti, M.; Fortino, I.; Leoni, O.; Genovese, S.; Baviera, M. Dipeptidyl Peptidase-4 Inhibitors, Glucagon-like Peptide-1 Receptor Agonists, and Sodium-Glucose Cotransporter-2 Inhibitors and COVID-19 Outcomes. Clin. Ther. 2023, 45, e115–e126. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Ruan, Y.; Davies, J.; Field, B.C.; Harris, S.; Kosiborod, M.; Nagi, D.; Narendran, P.; Patel, D.; Ryder, R.E.; et al. Association Between SGLT2 Inhibitor Treatment and Diabetic Ketoacidosis and Mortality in People with Type 2 Diabetes Admitted to Hospital with COVID-19. Diabetes Care 2022, 45, 2838–2843. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Schäffer, A.A.; Cicurel, A.; Cheng, K.; Sinha, S.; Schiff, E.; Feldhamer, I.; Tal, A.; Lavie, G.; Ruppin, E. Identification of drugs associated with reduced severity of COVID-19—A case-control study in a large population. eLife 2021, 10, e68165. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Simmons, W.; Banerjee, S.; Wang, F.; Williams, N.; Zhang, Y.; Reese, A.B.; Mushlin, A.I.; Flory, J.H. Association between antidiabetic drug use and the risk of COVID-19 hospitalization in the INSIGHT Clinical Research Network in New York City. Diabetes Obes. Metab. 2022, 24, 1402–1405. [Google Scholar] [CrossRef]
- Ramos-Rincon, J.M.; Perez-Belmonte, L.M.; Carrasco-Sanchez, F.J.; Jansen-Chaparro, S.; De-Sousa-Baena, M.; Bueno-Fonseca, J.; Perez-Aguilar, M.; Arevalo-Canas, C.; Cebrian, M.B.; Mendez-Bailon, M.; et al. Cardiometabolic Therapy and Mortality in Very Old Patients with Diabetes Hospitalized due to COVID-19. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, e102–e109. [Google Scholar] [CrossRef]
- Orioli, L.; Servais, T.; Belkhir, L.; Laterre, P.-F.; Thissen, J.-P.; Vandeleene, B.; Maiter, D.; Yombi, J.C.; Hermans, M.P. Clinical characteristics and short-term prognosis of in-patients with diabetes and COVID-19: A retrospective study from an academic center in Belgium. Diabetes Metab. Syndr. 2021, 15, 149–157. [Google Scholar] [CrossRef]
- Wander, P.L.; Lowy, E.; Beste, L.A.; Tulloch-Palomino, L.; Korpak, A.; Peterson, A.C.; Kahn, S.E.; Boyko, E.J. Prior Glucose-Lowering Medication Use and 30-Day Outcomes Among 64,892 Veterans with Diabetes and COVID-19. Diabetes Care 2021, 44, 2708–2713. [Google Scholar] [CrossRef] [PubMed]
- Shestakova, M.V.; Vikulova, O.K.; Elfimova, A.R.; Deviatkin, A.A.; Dedov, I.I.; Mokrysheva, N.G. Risk factors for COVID-19 case fatality rate in people with type 1 and type 2 diabetes mellitus: A nationwide retrospective cohort study of 235,248 patients in the Russian Federation. Front. Endocrinol. 2022, 13, 909874. [Google Scholar] [CrossRef]
- Dalan, R.; Ang, L.W.; Tan, W.Y.T.; Fong, S.-W.; Tay, W.C.; Chan, Y.-H.; Renia, L.; Ng, L.F.P.; Lye, D.C.; Chew, D.E.K.; et al. The association of hypertension and diabetes pharmacotherapy with COVID-19 severity and immune signatures: An observational study. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, e48–e51. [Google Scholar] [CrossRef] [PubMed]
- Silverii, G.A.; Monami, M.; Cernigliaro, A.; Vigneri, E.; Guarnotta, V.; Scondotto, S.; Allotta, V.A.; Conti, M.; Giordano, C.; Mannucci, E. Are diabetes and its medications risk factors for the development of COVID-19? Data from a population-based study in Sicily. Nutr. Metab. Cardiovasc. Dis. NMCD 2021, 31, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-C.; Kraschnewski, J.L.; Kong, L.; Lehman, E.B.; Heilbrunn, E.S.; Williams, P.; Poger, J.M.; Francis, E.; Bryce, C.L. Hospitalization and mortality in patients with COVID-19 with or at risk of type 2 diabetes: Data from five health systems in Pennsylvania and Maryland. BMJ Open Diabetes Res. Care 2022, 10, e002774. [Google Scholar] [CrossRef] [PubMed]
- Sourij, H.; Aziz, F.; Bräuer, A.; Ciardi, C.; Clodi, M.; Fasching, P.; Karolyi, M.; Kautzky-Willer, A.; Klammer, C.; Malle, O.; et al. COVID-19 fatality prediction in people with diabetes and prediabetes using a simple score upon hospital admission. Diabetes Obes. Metab. 2021, 23, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, Y.; Wang, G. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020, 92, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Khedr, A.; Al Hennawi, H.; Khan, M.K.; Eissa, A.; Mir, M.; Rauf, I.; Nitesh, J.; Surani, S.; Khan, S.A. Sodium-glucose cotransporter-2 inhibitor-associated euglycemic diabetic ketoacidosis in COVID-19-infected patients: A systematic review of case reports. World J. Clin. Cases. 2023, 11, 5700–5709. [Google Scholar] [CrossRef] [PubMed]
- Zhan, K.; Weng, L.; Qi, L.; Wang, L.; Lin, H.; Fang, X.; Jia, H.; Ma, X. Effect of Antidiabetic Therapy on Clinical Outcomes of COVID-19 Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Ann. Pharmacother. 2023, 57, 776–786. [Google Scholar] [CrossRef]
- Nassar, M.; Abosheaishaa, H.; Singh, A.K.; Misra, A.; Bloomgarden, Z. Noninsulin-based antihyperglycemic medications in patients with diabetes and COVID-19: A systematic review and meta-analysis. J. Diabetes 2023, 15, 86–96. [Google Scholar] [CrossRef]
- Permana, H.; Audi Yanto, T.; Ivan Hariyanto, T. Pre-admission use of sodium glucose transporter-2 inhibitor (SGLT-2i) may significantly improves COVID-19 outcomes in patients with diabetes: A systematic review, meta-analysis, and meta-regression. Diabetes Res. Clin. Pract. 2023, 195, 110205. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, L.; Wang, Y.; Liu, C.; Ke, T. Effects of novel glucose-lowering drugs on the COVID-19 patients with diabetes: A network meta-analysis of clinical outcomes. Int. J. Diabetes Dev. Ctries. 2024, 44, 426–436. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Ho, D.S.; Nguyen, H.S.; Ho, D.K.N.; Li, H.-Y.; Lin, C.-Y.; Chiu, H.-Y.; Chen, Y.-C. Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis. Metabolism 2022, 131, 155196. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Ma, S.; Sun, C.; Zhang, H.; Qu, G.; Chen, Y.; Cheng, C.; Chen, E.L.; Ahmed, M.A.; Kim, K.Y.; et al. Association Between Antidiabetic Agents and Clinical Outcomes of COVID-19 in Patients with Diabetes: A Systematic Review and Meta-Analysis. Arch. Med. Res. 2022, 53, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.P.; Koutroulos, M.-V.; Zikoudi, D.-G.; Bakola, S.-A.; Avramidou, P.; Touzlatzi, N.; Filippou, D.K. Diabetes-related acute metabolic emergencies in COVID-19 patients: A systematic review and meta-analysis. Diabetol. Int. 2021, 12, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Callender, L.A.; Curran, M.; Bates, S.M.; Mairesse, M.; Weigandt, J.; Betts, C.J. The Impact of Pre-existing Comorbidities and Therapeutic Interventions on COVID-19. Front. Immunol. 2020, 11, 1991. [Google Scholar] [CrossRef]
- Solerte, S.B.; D’addio, F.; Trevisan, R.; Lovati, E.; Rossi, A.; Pastore, I.; Dell’acqua, M.; Ippolito, E.; Scaranna, C.; Bellante, R.; et al. Sitagliptin Treatment at the Time of Hospitalization Was Associated with Reduced Mortality in Patients with Type 2 Diabetes and COVID-19: A Multicenter, Case-Control, Retrospective, Observational Study. Diabetes Care 2020, 43, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Braunwald, E. Cardiac and Renal Effects of Sodium-Glucose Co-Transporter 2 Inhibitors in Diabetes. J. Am. Coll. Cardiol. 2018, 72, 1845–1855. [Google Scholar] [CrossRef]
- Sano, M.; Goto, S. Possible Mechanism of Hematocrit Elevation by Sodium Glucose Cotransporter 2 Inhibitors and Associated Beneficial Renal and Cardiovascular Effects. Circulation 2019, 139, 1985–1987. [Google Scholar] [CrossRef]
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of Biological Peptides by Human Angiotensin-converting Enzyme-related Carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, D.; Matoba, K.; Takeda, Y.; Nagai, Y.; Akamine, T.; Yokota, T.; Sango, K.; Utsunomiya, K. SGLT2 Inhibitors as a Therapeutic Option for Diabetic Nephropathy. Int. J. Mol. Sci. 2017, 18, 1083. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lv, X.; Lin, S.; Arshad, M.; Dai, M. The Association Between Antidiabetic Agents and Clinical Outcomes of COVID-19 Patients with Diabetes: A Bayesian Network Meta-Analysis. Front. Endocrinol. 2022, 13, 895458. [Google Scholar] [CrossRef] [PubMed]
- Gohari, S.; Ismail-Beigi, F.; Mahjani, M.; Ghobadi, S.; Jafari, A.; Ahangar, H.; Gohari, S. The effect of sodium-glucose co-transporter-2 (SGLT2) inhibitors on blood interleukin-6 concentration: A systematic review and meta-analysis of randomized controlled trials. BMC Endocr. Disord. 2023, 23, 257. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Scheen, A.J. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: The potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018, 44, 457–464. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Das, L.; Dutta, P. SGLT2 inhibition and COVID-19: The road not taken. Eur. J. Clin. Invest. 2020, 50, e13339. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, Q.; Bai, X.Y.; Zhou, Y.F.; Zhou, Q.L.; Zhang, M. Effect of dapagliflozin on obstructive sleep apnea in patients with type 2 diabetes: A preliminary study. Nutr. Diabetes 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Hovde, M.J.; Bolland, D.E.; Armand, A.; Pitsch, E.; Bakker, C.; Kooiker, A.J.; Provost, J.J.; Vaughan, R.A.; Wallert, M.A.; Foster, J.D. Sodium hydrogen exchanger (NHE1) palmitoylation and potential functional regulation. Life Sci. 2022, 288, 120142. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Storti, E.; Belliato, M.; Serrano, R. Clinical phenotypes of SARS-CoV-2: Implications for clinicians and researchers. Eur. Respir. J. 2020, 55, 2001028. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Docherty, K.F.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Solomon, S.D.; Verma, S.; Bělohlávek, J.; et al. Dapagliflozin and the Incidence of Type 2 Diabetes in Patients with Heart Failure and Reduced Ejection Fraction: An Exploratory Analysis From DAPA-HF. Diabetes Care 2021, 44, 586–594. [Google Scholar] [CrossRef]
- Rubino, F.; Amiel, S.A.; Zimmet, P.; Alberti, G.; Bornstein, S.; Eckel, R.H.; Mingrone, G.; Boehm, B.; Cooper, M.E.; Chai, Z.; et al. New-Onset Diabetes in COVID-19. N. Engl. J. Med. 2020, 383, 789–790. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Chan, L.; Chaudhary, K.; Saha, A.; Chauhan, K.; Vaid, A.; Zhao, S.; Paranjpe, I.; Somani, S.; Richter, F.; Miotto, R.; et al. AKI in Hospitalized Patients with COVID-19. J. Am. Soc. Nephrol. 2021, 32, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Lee, H.H.; Kim, A.J.; Ro, H.; Kim, H.S.; Chang, J.H.; Chung, W.; Jung, J.Y. Renin-Angiotensin-Aldosterone System Blockade in Critically Ill Patients Is Associated with Increased Risk for Acute Kidney Injury. Tohoku J. Exp. Med. 2016, 238, 17–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, C.; Ye, Y.; Yu, M.; Qu, X. Prospect of Sodium-Glucose Co-transporter 2 Inhibitors Combined with Insulin for the Treatment of Type 2 Diabetes. Front. Endocrinol. 2020, 11, 190. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Ogawa, W. SGLT2 inhibitors for genetic and acquired insulin resistance: Considerations for clinical use. J. Diabetes Investig. 2020, 11, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.; Herat, L.; Schlaich, M.P.; Matthews, V. The Impact of SGLT2 Inhibitors in the Heart and Kidneys Regardless of Diabetes Status. Int. J. Mol. Sci. 2023, 24, 14243. [Google Scholar] [CrossRef]
- Scheen A., J. SGLT2 inhibition during the COVID-19 epidemic: Friend or foe? Diabetes Metab. 2020, 46, 343–344. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Chen, J.; Zuo, X.; Zhang, H.; Deng, A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes. Metab. 2020, 22, 1935–1941. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.M.; Berard, L.D.; Cheng, A.Y.; Gilbert, J.D.; Verma, S.; Woo, V.C.; Yale, J.-F. SGLT2 Inhibitor–associated Diabetic Ketoacidosis: Clinical Review and Recommendations for Prevention and Diagnosis. Clin. Ther. 2016, 38, 2654–2664.e1. [Google Scholar] [CrossRef] [PubMed]
- Hirji, I.; Andersson, S.W.; Guo, Z.; Hammar, N.; Gomez-Caminero, A. Incidence of genital infection among patients with type 2 diabetes in the UK General Practice Research Database. J. Diabetes Complicat. 2012, 26, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, I.R.; Rose, S.C.P.; Freire, N.B.; Patrocínio, M.S.; Pierdoná, N.; Bittencourt, R.J. Use of sodium-glucose cotransporter-2 inhibitors and urinary tract infections in type 2 diabetes patients: A systematic review. Rev. Assoc. Médica Bras. 2019, 65, 246–252. [Google Scholar] [CrossRef]
- Brossart, P.; Kotthoff, P. Dexamethasone Promotes Fungal Infection By Inhibition of APC Activation with Beta-Glucans Via STAT-3 and NF-κb. Blood 2016, 128, 3710. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).