Abstract
Power conversion efficiency (PCE) of single-junction perovskite solar cells (PSCs) has already soared from 3.8% to more than 26%. Their potential for application in tandem architecture with silicon and other established solar technologies has been deemed the future of low-cost solar technology. However, the commercialization of this technology is critically limited by instability under operational and environmental stress. The instability in the PSCs stems from both internal mechanisms including ion migration, defect formation, and electrode or charge transport layer (CTL)-induced degradation as well as external stressors such as moisture, oxygen, heat, and illumination. A complete understanding of both the internal and external stimuli-induced degradation and their mitigation strategies is essential for improving the device’s longevity. This review aims to provide a complete overview of the degradation mechanisms and steps taken to mitigate the degradation issues. The first half of the review discusses the degradation mechanism caused by internal degradation factors and provides strategies to mitigate them, while the second half focuses on the external stressors and the approaches developed by the perovskite community to overcome them. The commercialization of PSCs will depend on a holistic approach that simultaneously ensures both intrinsically as well as extrinsically stable devices.
1. Introduction
The trend in today’s solar industry is towards producing devices with high reliability, reduced cost, and enhanced performance. Perovskite-based photovoltaic (PV) technology has a promising performance with low cost and low pollution during both manufacturing and disposal, despite the existence of a small amount of lead in the thin perovskite layer [1]. Perovskites have formula ABX3 with a cubic unit cell shown in Figure 1a. The A-site species can be an organic, e.g., methylammonium MA+ or formamidinium FA+ , or an inorganic, e.g., cesium (Cs+) or a mixture of them [2]. Additions of guanidinium (Gu+), and Rubidium (Rb+) have also been reported [3,4]. The B-site has a metal cation, which is usually lead (Pb2+) or tin (Sn2+). The X-site contains a halide anion, which is usually iodide (I−), bromide (Br−), or chloride (Cl−) or a combination of them [5]. The use of perovskites in solar cells was first reported in 2009 with the power conversion efficiency (PCE) of 3.8% [6], which has increased to over 26% to date [7]. However, due to the intrinsic instabilities of the perovskite absorber layer, as well as the external degradants, the stability of the perovskite-based devices limits their adoption in the photovoltaic market.
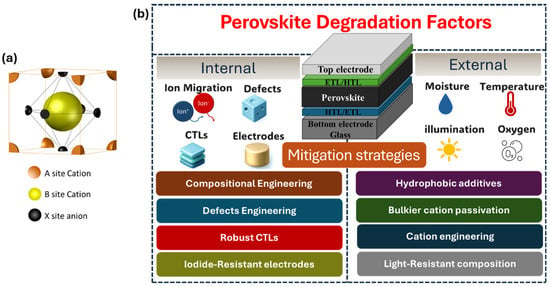
Figure 1.
(a) Cubic structure of a perovskite unit cell (b) Graphical representation of degradation factors and mitigation strategies of a perovskite solar cell. The abbreviations CTLs, HTL, and ETL refer to charge transport layers, hole transport layer and electron transport layer, respectively.
A reliable solar cell has a high probability of performing its intended function for its design life of 25 to 30 years, under certain operating conditions [8]. Accelerated stress tests are developed to assess the reliability of PV modules; however, specific industrial qualification tests are not well-defined for the perovskite solar cells (PSCs). The stability of the PSC devices is often assessed using thermal cycling tests (200 cycles between −40 °C/85 °C) and damp heat test (1000 h at 85 °C and a relative humidity of 85%) as outlined in the International Electrotechnical Committee (IEC) 61646 guidelines. Additionally, International Summit on Organic PV Stability (ISOS) protocols are also employed to evaluate the durability of the perovskite PV cells [9]. While the inherent instability in the perovskites stems from their brittleness, mechanical fragility with low cohesion energy [10], and the ion-migration driven phase segregation, the heat and light-induced structural deformation further accelerate the internal degradation of the perovskite material [11]. At the same time, external degradants such as moisture, oxygen, light illumination including ultra-violet (UV) radiation, and thermal stress can cause the decomposition of the perovskite, thereby leading to performance deterioration over time [12]. A holistic approach that addresses both the internal and external degradation factors is a requisite for prospering the perovskite technology. Several reviews have previously summarized the stability challenges and mitigation strategies for perovskite stability including degradation mechanisms and remedies [13,14,15]. This review extends and complements these prior works by offering an updated survey of the literature through 2025. Moreover, this work provides a comprehensive discussion of stability testing based on methodologies beyond the standard IEC qualification tests. In addition to IEC 61646, we summarize and compare the ISOS testing series, lifetime evaluations, light soaking, and accelerated stress protocols that have been widely employed in recent research to assess long-term device stability. In the subsequent sections of this article, we shall discuss the internal and external degradation factors responsible for the instability caused in the perovskite solar cells (PSCs) and explore the measures the perovskite community has adopted to overcome the degradation (Figure 1b).
2. Internal Degradation Pathways and Mitigation Strategies
2.1. Ion-Migration-Induced Degradation
Ion migration is the movement of the ionic species within the perovskite bulk, or to the interfaces, or even to other layers within the PSCs. This phenomenon is primarily attributed to the soft lattice structure of the perovskite with weak bonding, which gives rise to the ionic defects within the perovskite, thereby generating the mobile ions [16]. The low activation energies for the halide anions (0.08–0.58 eV) as well as organic cations (0.46–0.84 eV) make them susceptible to migration in ambient conditions which further exacerbates in the presence of external stimuli such as temperature, electric bias, and light illumination [17,18,19]. Additionally, the polycrystalline nature of perovskite films gives rise to the defects and grain boundaries, which behave as dominating channels for the ion migration [20]. Although the ion migration within the perovskite bulk is considered reversible, they can cause a serious irreversible degradation of the PSCs if the ions move to other functional layers [21,22]. Thus, ion migration has been considered one of the prominent causes for decreasing the operational and long-term stability of the PSCs.
The ion migration leads to charge accumulation at the interfaces, which gives rise to unbalanced charge transport towards the ETL and HTL of a PSC device, thereby causing a mismatch in the forward and reverse current density-voltage (JV) scan of the PSC device called hysteresis [23]. The hysteresis would thus lead to inaccurate performance measurements and indicate the underlying instability in the PSC devices. Another effect of ion migration is the phase segregation observed in the multi-halide perovskites. Hoke et al., while studying MAPbBrxI3−x perovskite, found that Iodine-rich and Iodine-poor perovskite phases were formed within the perovskite film upon light illumination [24]. The photoluminescence (PL) characterization of the light soaked MAPb(BrxI1−x)3 film with 0.2 < x < 1 revealed an additional PL peak at 1.68 eV, which appeared in all the mixed-halide samples, irrespective of the halide composition and bandgap, see Figure 2a. This PL peak which appeared in all the light soaked mixed-halide composition samples corresponded to that of x = 0.2 perovskite phase, disappeared, however, after the samples were left in the dark for some time. They attribute this reversible peak shift to the ion-migration-induced segregation of halides which led to the formation of larger and smaller bandgap perovskites within the film, promoting the formation of trap states, thereby decreasing performance.
The ion migration has also been identified as a major degradation factor for photo-induced losses in the PSCs. A recent finding by Thiesbrummel et al. points out that the increased mobile halide ions during aging screens the built-in electric field, thereby leading to the accumulation of electronic charges (both holes and electrons) near the hole-selective contact (Figure 2b) [25]. This results in the increased recombination at the HTL–perovskite interface, causing significant decrease in the current density and the fill factor of the device. Likewise, the ion migration could give rise to unbalanced stoichiometric polarization in the perovskite absorber layer, enhancing the formation of detrimental anti-sites and metal interstitial defects [26,27]. These defects are prone to changing the photoactive perovskite α phase to photoinactive δ phase, causing the overall perovskite instability [28]. Furthermore, the mobile ions can enter the adjacent charge transport layers, thereby altering their properties and leading to the device instability. It has been observed that the penetration of mobile halide ions to commonly used hole transport layer such as 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamine)-9,9-spiro-bifluorene (spiro-OMeTAD) could prevent its oxidation, thereby reducing its electrical conductivity [21]. Also, the migration of halides from the active perovskite layer to the metal electrodes facilitates the formation of metal halides, causing irreversible degradation of the electrodes [22,29].
Strain has a significant effect on the stability of perovskite films and hence the PSC devices. In perovskite films, studies have shown that compressive strain reduces the bandgap of the perovskite material while increasing carrier mobility and the activation energy for iodide vacancy formation [30]. This helps to suppress ion migration and improve the stability of the device. In contrast, tensile strain has the opposite effect, promoting ion migration and faster degradation. However, excessive compression can cause the perovskite layer to delaminate from the substrate, while tension can lead to cracking. During the high-temperature annealing process, perovskite films often experience strong tensile strain due to the mismatch in thermal expansion coefficients between the perovskite and the glass substrate [31]. This strain, which can reach up to 1.6%, significantly affects the long-term stability of the film.
Mitigation Strategies
Various strategies have been adopted for minimizing the effect of ion migration in the PSCs. The incorporation of alkali cations in the perovskite layer and their interstitial occupancy leads to the suppression of ion migration [32,33]. The interstitial alkali cations can act as physical hinderances for the ions that tend to migrate through the interstitial channels. Furthermore, they could electrostatically bind with the halide anions, which are the most prominent mobile ions in the perovskite crystal, at their crystal lattice sites, thereby strengthening the perovskite crystal structure [34]. Also, the electrostatic binding of these cations with undercoordinated halide ions in the grain boundaries and surfaces neutralize and immobilize the dangling halides. For instance, Cao et al. found that the incorporation of potassium ion (K+) in interstitial sites increased the diffusion barriers for all ionic diffusion paths of the iodide ion by 0.48 eV, thereby mitigating the ionic migrations (Figure 2c) [32]. In addition to the incorporation of cations, the incorporation of anions has also led to increased migration barriers in PSCs. The interstitial occupancy of fluoride (F−) anions has led to more p-type doping in the perovskite material, which increases the formation energy of halide vacancies that ultimately lowers the halide ion migration in the perovskite layer. The incorporation of interstitial F− anion resulted in increased activation energy for iodide ions, thereby increasing the migration barrier by 0.26 eV [35]. As a result of this, unencapsulated CsPbI2Br-based PSCs retained 94% of their initial PCE even after 1000 h of continuous 1-sun illumination. However, a recent finding from Liang et al. pointed out that the B-site doping of the perovskites with multiple cations from alkaline earth and lanthanide groups increases the ion migration barrier by internally stabilizing the octahedra and the perovskite lattice, thereby outperforming the A and X-site substitutions as well as interstitial doping strategies (Figure 2d) [36]. They observed that the interstitial iodide ion diffusivity for pristine perovskites reduced from 6.4 × 10−8 cm2/s to 9.2 × 10−13 cm2/s upon doping the B-site with Eu–Yb–Ca (europium, ytterbium, and calcium) multiple dopants.
Compositional engineering of the A-site cation in perovskites with the introduction of larger dopants to create a steric hinderance for reducing the halide ion migration has been another effective strategy to improve the stability of the PSCs [4,37]. Tan et al. substituted MA cation in the perovskites partially by acetamidinium (CH3C(NH2)2+), which introduced size-mismatch-induced lattice distortions in the crystal, thereby sterically hindering the iodide migration with a higher activation energy of 0.68 eV compared to 0.45 eV for pristine MAPbI3 [38]. This led to an impressive T80 of more than 2000 h under continuous 1-sun illumination at open-circuit condition in ambient environment. Furthermore, the passivation of perovskite surface has proven to be another effective technique for the mitigation of the ion-migration-induced instability in the PSCs. The post treatment of perovskite films with appropriate cationic or anionic species can help passivate the defects and imperfections present at the perovskite/CTL interface which would otherwise act as a path for the ionic migration from perovskite towards the CTL and subsequently towards the electrodes. The use of ammonium salts for surface passivation has been a common strategy which can effectively mitigate surface defect states as well as impede the negative effect of excess lead iodide (PbI2). For instance, dimethylammonium trifluoroacetate (DMATFA) has been applied to the perovskite film surface in which DMA+ and TFA− ions synergistically managed the interfacial lead-based imperfections like Pb0, Vpb, or undercoordinated Pb2+ which resulted in increased activation energy of the iodide ion’s migration from 0.526 eV to 0.604 eV [39]. This led to the enhanced stability of the perovskite solar cell where the DMATFA-passivated device retained 91% of its initial PCE compared to 64% for the control device under maximum power point (MPP) and the cyclic J-V operational stability test. Similarly polymeric interfacial layers between perovskites and CTLs also effectively suppress the ion migration in perovskite solar cells to enhance the stability of the devices. In a recent study, Lin et al. introduced a multifunctional polymeric buffer layer made of magnetic endohedral metallofullerene (Nd@C32) dispersed in a PMMA polymer matrix [40]. This layer enhanced electron extraction and provided encapsulation in inverted PSCs, effectively suppressing ion migration. The electromagnetic-coupled channel ensured balanced carrier extraction between the hole and electron transport layers. As a result, ion interdiffusion is reduced and band alignment is improved, achieving certified efficiencies of 26.29% (0.08 cm2) and 23.08% (16 cm2 module). The unencapsulated devices maintained 82% of their initial efficiency after 2500 h of continuous operation under 1-sun illumination at 65 °C. Likewise, Li et al. evaporated a 2 nm layer of polytetrafluoroethylene (PTFE) between the perovskite absorber and C60 [41]. This layer encapsulated the perovskite and suppressed ion diffusion across the interface, leading to reduced non-radiative recombination. As a result, the device showed a slight improvement in power conversion efficiency, reaching 25.49%. More importantly, the PTFE-based devices retained 92.6% of their initial efficiency after 1500 h of air exposure. Another effective strategy to inhibit the ion-migration-induced instability in the PSCs is the incorporation of ultrathin inorganic oxide layer in the interfaces between perovskite and CTL and/or the CTL and electrode [42,43]. These oxide layers act as both a physical barrier preventing the mobile ions from migrating into adjacent CTLs or the electrode, and as chemical passivators that neutralize vacancy defects or undercoordinated ions in the perovskite surface, thereby lowering the migration pathways for the mobile ions. For instance, Artuk et al. utilized the thin AlOx interlayer between the perovskite absorber and the electron transport layer (ETL) which greatly improved the operational stability of the device with T80 lifetime improved from 80 h to 530 h [44]. The improvement in the lifetime of the device is attributed to inhibited ionic species (MA+, FA+, I−) removal from the perovskite absorber and the alumina layer slowing down the ingress of external moisture and oxygen. In another work, Chen et al. explored ytterbium oxide (YbOx) as a buffer layer between the ETL and the electrode, which acted as a barrier inhibiting the interdiffusion of harmful species into or outside the perovskite layer [45].
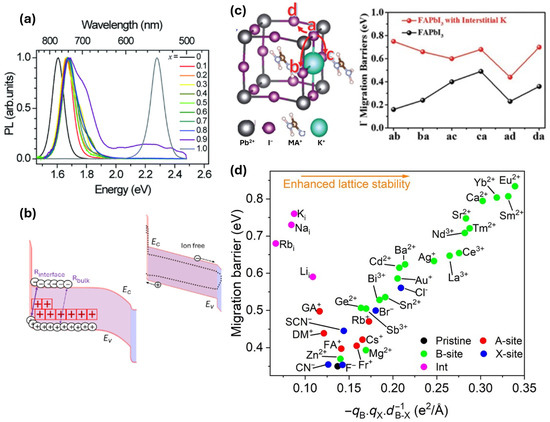
Figure 2.
(a) Normalized photoluminescence spectra of light-soaked MAPb(BrxI1−x)3 perovskite film with different compositions of iodide and bromide anions [24] (CC BY 3.0). (b) Schematic band diagram of HTL/Perovskite/ETL stack showcasing cation vacancy-induced electrons and hole accumulation at the HTL/Perovskite interface (inset: Ion free case); Ec and Ev are energy levels for the conduction and valence bands [25] (CC BY 4.0). (c) Illustration of iodide ion’s diffusion pathways in FAPbI3 perovskite with K+ ion at an interstitial site and comparison plot of iodide ion’s migration barriers with and without interstitial K+ ions [32] (reproduced with permission from John Wiley and Sons, copyright 2018) and (d) Interstitial iodide migration barrier as a function of doping different sites in perovskite crystal. Red, green, blue, magenta, and black dots denote substitutionally doped at A, B, X-site, interstitial doped and pristine perovskite, respectively [36] (Reproduced from Liang et al., Science Advances, 10.1126/sciadv.ads7054, 2025, AAAS.
The reduction in strain has been proven to reduce the ion migration in perovskite solar cells. Thus, strategies for reducing strain have been discussed in subsequent paragraphs. One effective way to reduce residual tensile strain in perovskite films is to lower the processing temperature. Park et al. demonstrated that the crystallization of FAPbI3 can be initiated at room temperature by adding volatile alkylammonium chlorides such as methylammonium chloride (MACl), propylammonium chloride (PACl), and butylammonium chloride (BACl) [46]. These additives promote the formation of intermediate phases that start organizing into a crystalline structure without introducing thermal strain. This early-stage ordering minimizes the need for high-temperature annealing, allowing final crystallization at a lower temperature. As a result, the devices achieved a certified PCE of 26.08% and retained 95% of their initial performance after 1000 h of continuous illumination under MPPT conditions.
Additives in the perovskite precursor can also help relieve thermal strain. For example, trimethylolpropane triacrylate (TMTA) undergoes in situ crosslinking during annealing to form a polymer scaffold at the film surface, where lattice distortion is most severe [47]. This scaffold acts as a mechanical brace, restricting lattice relaxation and anchoring the upper-film region during crystallization. It limits differential shrinkage between the surface and the bulk, converting residual tensile strain into slight compressive strain. Consequently, both phase stability and mechanical integrity are improved. Devices using this approach reached a PCE of 22.39% and maintained 95% of their initial efficiency after 4000 h of storage and 80% after 1248 h of continuous light soaking.
Introducing an intermediate layer is another effective strategy to mitigate thermal mismatch between the perovskite and adjacent layers. Octylammonium iodide (OAI) has been shown to reduce residual tensile stress from 77.7 MPa to 45 MPa [31]. Besides stress relaxation, OAI also passivates surface defects and suppresses non-radiative recombination, contributing to enhanced device performance and stability [48].
2.2. Defects-Induced Degradation
Defect states in the perovskite absorber layer are characterized by theoretical studies using first principal density functional theory. In a commonly used MAPbI3 perovskite structure, three vacancy defects, VMA, VPb, and VI, three interstitial defects, MAi, Pbi, and Ii, and six anti-site defects, MAPb, MAI, PbMA, PbI, IMA, and IPb, constitute a total of 12 intrinsic point defects (Figure 3a) [49]. The defects with low formation energy, which readily form during the crystallization of perovskites, namely VMA, VPb, VI, Ii, MAPb, MAI, and PbMA, form trap states very near to the conduction/valence band, acting as shallow defects [49]. As these defects do not form in the mid-gap states, they do not act as non-radiative recombination centers; instead, they could contribute to the ionic conduction-related issues in the device. Other defects with deep-level trap states such as IMA, IPb, Pbi, and PbI have higher formation energy, thus are less likely to form [49]. Thus, these intrinsic point defects do not act detrimental to the charge carriers generated within the perovskites. However, migration of the shallow charged point defects during the operational condition of a PSC could cause their accumulation at the interfaces inducing unintentional doping, thereby causing local band bending, hysteresis in JV-curves, phase segregation, and consequently degradation of the perovskites [1,50]. Also, apart from these intrinsic point defects, extrinsic defects like grain boundaries present in the polycrystalline perovskite films give rise to the trap states associated with lattice mismatch and disturbances in the regular arrangement of atoms [51]. A polycrystalline MAPbI3 with more grain boundaries has trap densities in the range from 1016 to 1017 cm−3, much higher than that of single crystal MAPbI3 (109–1010 cm−3) [52,53]. Another extrinsic defect that plays a major role in creating the trap states in the perovskite film is the surface. Surfaces give rise to dangling bonds, uncoordinated atoms, dislocations, etc., which act as recombination centers. This can increase the surface trap densities (~6 1017 cm−3) to two orders of magnitude higher than the bulk trap states (~5.8 1015 cm−3) [54].
The defect states in the perovskites can significantly degrade the performance as well as stability of the PSCs. They act as a charge carrier recombination center, thereby impeding the carrier collection. The mobile ionic species in the perovskite can migrate through the defects easily, so the defects trigger the ion-migration-induced degradation in the PSCs [55]. The degradation caused by external stimuli like moisture and oxygen is aggravated in the defective perovskite surface (Figure 3b) [56]. Tian et al. theoretically investigated the diffusion of water molecules in MAPbI3 perovskite using density functional theory (DFT) and found that water diffusion through grain boundaries and defects is easier compared to bulk perovskite [57]. The loose atomic packing fraction and large free volume at grain boundaries support the diffusion of water molecules, initially forming a loose covalent interaction between the undercoordinated Pb atom in perovskite surface and O atom in water. The weak and easily breakable Pb–O covalent bond offers little resistance to the inward diffusion of water molecule into the crystal forming H···O hydrogen bonds with the organic cation, eventually leading to crystal degradation. In contrast, the formation of Pb–O covalent bond by the dissociation of the Pb–I inorganic framework in the bulk perovskite is limited by very high energy barriers, thereby resisting moisture infiltration under similar conditions. The diffusion energy barrier for water molecules was as low as 0.07 eV for grain boundaries, 0.24 eV for diffusion from grain boundaries to the bulk, and 0.7 eV for diffusion in the pristine bulk. This lower barrier signifies that the grain boundaries are detrimental for water ingress and degradation of the PSCs. Likewise, the defects can act as the site for O2 adsorption [58] which can form a superoxide (O2−) on capturing a photoexcited electron during illumination. The perovskite films composed of smaller crystallites show a higher yield of superoxide which verifies the role of grain boundaries with defects towards the superoxide formation. Furthermore, theoretical calculations also reveal that the most favorable site for superoxide formation is the iodide vacancy site. For instance, the calculated formation energies of superoxide at different sites in the perovskite crystal are as follows: : −1.94 eV; the surface site, where the superoxide interacts with four neighboring iodide ions: −1.19 eV; : +0.29 eV; and : −0.23 eV [59]. These results further corroborate the role of grain boundaries and surfaces in oxygen-induced deterioration of perovskite films. The superoxide can react with the organic component of the perovskite absorber, thereby initiating the irreversible degradation of the absorber [60]. The defect states can also cause the transition of perovskites from photoactive alpha phase to inactive delta phase (Figure 3c) [61]. The iodine vacancy defects, which are easily generated during film deposition and device operation, cause the reduction in energy barrier for the phase transition, thereby triggering the alpha to delta phase transition.
Mitigation Strategies
Multiple approaches for lowering the extrinsic defects and immobilizing the internal unavoidable intrinsic point defects have been implemented to mitigate the defect-induced instabilities in the PSCs. The use of excess precursor material has been adopted for the reduction in the defect density as well as for the passivation of the grain boundaries. Park et al. studied the effect of excess lead iodide in the perovskite precursor and unraveled the mechanism of improved performance and stability [62]. They concluded that the excess PbI2 containing perovskite is less susceptible to defect formation during cleaving or lab air exposure. The excess PbI2 maintains higher I− ion activity during the crystallization of halide perovskites and thus shifts the reaction equilibrium towards the formation of halide perovskite with fewer iodide vacancies, which helps to lower the iodide vacancies’ formation during crystallization. They further found that excess PbI2 surrounds the perovskite grains rather than being confined to specific locations at the grain boundaries, which facilitates better charge transfer, reduces hysteresis, and leads to a more stable device. However, the photolysis of excess lead iodide could also give rise to metallic lead and iodine as decomposition products, which could possibly induce the degradation of the PSCs [63,64]. Thus, the use of excess lead iodide should be carefully optimized to balance its passivation benefits over the potential stability issues. Similar to excess lead iodide, an excess of organic precursor powder has also led to improvement in the performance and stability of the perovskite device. For instance, Son et al., using a high-resolution transmission electron microscope (HRTEM), showed that excess methylammonium iodide (excess 6 molar %) added in the precursor resided around the grain boundary suppressing the non-radiative recombination and facilitating the electron and hole extraction from the grain boundaries [65].
Another successful strategy for reducing the defect-induced instability in the PSCs is the cationic or anionic doping of the perovskite precursor. Alkali metal ion doping to the perovskite has resulted in significant improvement in grain size, thereby reducing the trap states [66]. Moreover, the Na+ and K+-ion doping led to a reduced trap-filled voltage to 0.28 V and 0.3 V, respectively, which is significantly lower than 0.48 V of undoped perovskite, thereby confirming the reduction in the trap states in the device. Furthermore, Zhao et al. explored the doping of trace amounts of multivalent cations such as Nd3+ and Ca2+ instead of high-dosage monovalent cations [67]. The interaction energies between the defects and Nd3+ were the highest, followed by those for Ca2+ and Na+ (Figure 3d). Trace amount of Nd3+ dopant (0.08 molar %) caused less strain in the perovskite crystal compared to high-dosage Na+ dopant (0.45 molar %), while increasing the energy barrier for VI migration to 2.8 eV (Ca+: 0.81 eV and Na+: 0.43 eV) from 0.37 eV for undoped perovskite film. The thermal stability of the devices greatly improved such that the unencapsulated devices in nitrogen environment at 85 °C maintained 86.4%, 72.7%, and 60.3% for Nd3+, Ca2+, and Na+ respectively after 2002 h (Figure 3d). Likewise, tetrafluoroborate (B) pseudohalide anion doped to Sn-Pb perovskite exhibited higher binding capacity with Sn2+/Pb2+ cations, which resulted in the lowered VI formation. This improved the light soaking stability of the devices; PSCs doped with the B anion maintained 88% of initial PCE until 1000 h of light illumination at maximum power point tracking (MPPT) measurement condition [68]. In another study, Liu et al. doped the perovskite with P anion, which controlled the crystallization via the formation of fully coordinated intermediate groups in the solution, ultimately decreasing the halide vacancies [69]. This strategy led to long-term stable devices with T80 at 4000 h under ambient storage (40–50% RH) and T80 at 1000 h under continuous illumination.
The Lewis acids and bases have the capability to accept and donate electron pairs to the uncoordinated charged species in the perovskite bulk or surface, making them suitable to mitigate the defects inside the perovskites. Different studies on the application of these materials for the enhancement of performance as well as stability of the PSC devices have been carried out. For instance, Ding et al. utilized Lewis base ionic liquid such as 1,3-bis(cyanomethyl)imidazolium chloride combined with methylammonium chloride as additive to the perovskite precursor and found that the nitrile group could passivate the uncoordinated Pb2+ sites and the chloride ion could occupy the halide vacancy sites [70]. By the use of 1H NMR spectroscopy, they further found that the additives slowed the decomposition of the organic MA+/FA+ cations. The PSCs retained 94.7% of their initial PCE after 1000 h of continuous 1-sun illumination, and maintained 84.5% of their initial PCE when subjected to continuous 1-sun illumination at 65 °C, demonstrating excellent stability metrics. Another study by Liang et al. made use of a low Lewis basic solvent divinyl sulfone (DVS) and treated the perovskite film with glycerinum to trigger the co-polymerization at the perovskite surface and grain boundary [71]. This strategy retarded the crystal growth rate from 0.073 S−1 to 0.061 S−1, thereby reducing the formation of defect states during the crystallization. Grazing incidence XRD performed at 50 nm near the surface and 200 nm in the bulk part of the film revealed that the tensile stress, which was present in the near surface region of the control sample, had diminished for the co-polymerized samples. This resulted in the excellent operational stability at elevated temperature (MPP, 65 °C, 50% RH, T98 at 1350 h) and room temperature (MPP, 60%RH, T90 at 1800 h) as well as thermal stability in the dark (85 °C, N2, T95 at 1560 h) (Figure 3e).
Recent efforts to reduce deep-level defects in perovskite films have focused on bulk passivation using tailored molecular agents. Liu et al. developed sulfonium-based passivators that can penetrate the perovskite lattice and directly interact with bulk defects, suppressing non-radiative recombination and improving charge transport [72]. These passivators increased power conversion efficiency from about 23.5% to 24.8%, with clear improvements in open-circuit voltage and fill factor. The treated devices also maintained over 90% of their initial efficiency after 1000 h of continuous illumination and thermal stress.
Apart from the bulk defects, interfacial defects also play a crucial role for the device longevity. The passivation of interfacial defects has been an important strategy not only to reduce the interfacial non-radiative recombination centers, but also to facilitate the charge transfer from the perovskite layer to the charge transport layers. The use of two-dimensional (2D) perovskite forming materials for the passivation of the perovskite surface has been a successful strategy to overcome the interface-related issues. For instance, Zhang et al. utilized guanidine halides (GuCl and GuI) for the passivation of bulk and surface defects of the perovskites [73]. From first principle theoretical calculation, they identified GuCl as suitable candidate for the bulk passivation, which could slow the crystal growth kinetics, giving rise to bigger grains and less defect state density, while GuI having a bulkier iodide group was deemed beneficial for the surface. It led to the formation of 2D Gu2PbI4, which passivated the interface for efficient hole transport, thereby enhancing the performance and stability of the device. In another study, Wang et al. designed a bilayer interface engineering strategy, implying phenyl ethyl ammonium iodide (PEAI) to form a 2D/3D heterojunction at the perovskite surface for interfacial defect passivation and a piperazinium iodide (PI) layer on top of the 2D/3D interface to induce surface dipoles for fine tuning the energy level alignment between the perovskite and the ETL [74]. This strategy led to increased n-type characteristic of the perovskite film, thereby facilitating the electron extraction, which led to better performance and the stability of the device. Furthermore, the presence of bulkier hydrophobic organic spacer cations along with stronger binding between the layers in 2D perovskites impart onto them better moisture as well as thermal stability. Application of these robust 2D perovskite layers atop the fragile 3D perovskite layer protects the 3D counterparts from the moisture ingress and maintains the structural integrity of the 3D perovskite device under elevated temperature [75,76]. Also, the application of a 2D perovskite layer on the 3D perovskites help minimize the ion migration issue, which has been a key challenge in the 3D perovskite’s stability. These properties of 2D perovskites thus impart long-term stability and higher performance in the 3D perovskite-based solar cells. Liu’s group, however, pointed out that the cations could migrate between 3D and 2D perovskite layers, resulting in octahedra distortion, and hence chose 2D perovskitoids instead of 2D perovskites for the surface passivation of their perovskite films [77]. The 2D perovskitoids help protect the 3D perovskite layer by reducing the cation diffusion within the heterostructure, thanks to extra π–π interactions between the flat, and well-aligned spacer molecules in the 2D perovskitoids layer. The 2D perovskitoids such as (A6BfP)8Pb7I22 (A6Bfp: n-aminohexyl-benz[f]-pthalamide) (Figure 3f) benefit from having higher stability compared to 2D perovskites such as (PEA)2PbI4 and provide the better defect passivating capacity. The thermal stability of the devices with 2D perovskitoids was greatly enhanced, which retained 85% of their initial PCE after 1250 h of continuous operation under 1-sun at 85 °C while the 2D perovskite devices had only 52% PCE retention.
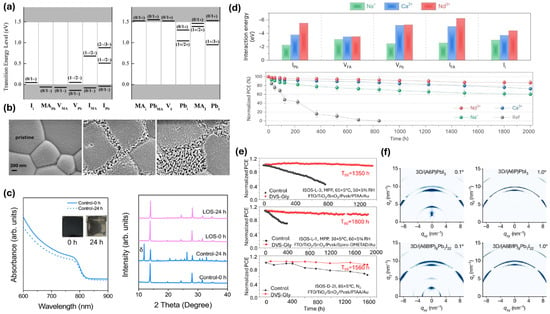
Figure 3.
(a) Transition energy levels of intrinsic point defects in MAPbI3 perovskite [49] (reproduced with permission from AIP publishing, copyright 2014). (b) SEM image of Lead acetate (PbAc2) films showing oxygen-induced degradation initiating from the grain boundaries [56] (reproduced with permission from John Wiley and Sons, copyright 2017). (c) UV-Vis spectra of FAPbI3 perovskite film indicating transformation from alpha to delta phase (Inset: visual pictures of the tested film) and XRD spectra of the perovskite films depicting the transformation from alpha to delta phase [61] (CC bY 4.0). (d) Interaction energies of Nd3+, Ca2+ and Na+ cations incorporated in perovskite lattice with different negatively charged defects and thermal stability of those cations doped unencapsulated PSC [67] (reproduced with permission from Nature Springer, copyright 2022). (e) Stability enhancement in the divinyl sulfone (DVS) and glycerinum-treated PSCs tested under different conditions [71] (reproduced with permission from Nature Springer, copyright 2025) and (f) GIWAXS patterns of the 1D (A6P)PbI3/3D perovskite (top) and 2D (A6BfP)8Pb7I22/3D perovskite (bottom) heterostructure films at incident angles of 0.1° (left) and 1.0° (right) [77] (reproduced with permission from Nature Springer, copyright 2024).
2.3. Charge-Transport-Layer-Induced Degradation
Charge transfer layers (CTL) are necessary for the efficient separation of the photogenerated carriers from the perovskite absorber layer. A Perovskite absorber layer is often embedded between n-type and p-type semiconducting materials, which are termed as electron transport layer (ETL) and hole transport layer (HTL), respectively. Depending on which CTL is deposited first on the transparent conducting electrode, the perovskite solar cells are classified into regular (n-i-p) structure and inverted (p-i-n) structure. Different electron, hole transfer layers, and processing conditions are applicable for those different architectures. Different organic materials like spiro OMeTAD, poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] (PTAA), poly(3,4-ethylenedioxythiophene)polystyrene sulfonate (Pedot:PSS), poly(3-hexylthiophene) (P3HT), self-assembled monolayer (SAM) molecules like [2-(3,6-dimethoxy-9H-carbazol-9-yl)ethyl]phosphonic acid (MeO-2PACZ), [2-(3,6-dimethoxy-9H-carbazol-9-yl)butyl]phosphonic acid (MeO-4PACZ), etc., and inorganic materials like nickel oxide (NiOx), copper oxide (Cu2O), copper thiocyanate (CuSCN), molybdenum disulfide (MoS2), etc., have been used as HTL by the perovskite community [78,79,80,81,82,83,84,85]. Likewise, ETLs used in perovskite solar cells can also be divided into organic and inorganic categories. Carbon-based ETLs like fullerene (C-60), [6,6]-phenyl-C61-butyric acid methyl ester (PCBM), indene-C60 bisadduct (ICBA), pyrelene diimides (PDI), napthelene diimides (NDI), etc., are the common organic ETLs while titanium dioxide (TiO2), tin oxide (SnO2), zinc oxide (ZnO), ferric oxide (Fe2O3), tungsten trioxide (WO3), cerium (IV) oxide (CeO2), etc., are the common inorganic ETLs [86,87,88,89,90,91,92,93,94,95,96]. In this section, instability caused by the most commonly used CTLs and their mitigation strategies shall be discussed.
The most commonly used HTL in the high-performance n-i-p PSCs, spiro-OMeTAD, is intrinsically less conductive with lower hole mobility. It is doped with lithium bis(trifluoromethane)sulfonimide (LiTFSI) and 4-tert-butylpyridine (t-BP) for improving its conductivity [97]. Oxidation of the LiTFSI is essential to achieve improved conductivity; however, its hygroscopic nature allows it to absorb moisture during this process, leading to degradation of the underlying perovskite layer [98]. Additionally, the diffusion of Li+ ion from the HTL to the perovskite absorber layer has been observed, which lowers the hole conductivity of spiro-OMeTAD as well as induces instability in the absorber layer [99]. Furthermore, time of flight secondary ion mass spectroscopy (TOF SIMS) analysis has revealed the migration of t-BP or its fragments towards the absorber layer, which can induce perovskite degradation forming a PbI2–t-BP complex [100]. Another common HTL, PTAA, is also p-doped using LiTFSI and t-BP, similar to spiro-OMeTAD; thus, a similar instability mechanism as induced by spiro, can also occur in PTAA devices [101]. Similarly, PEDOT: PSS, another common HTL, introduces instability due to its acidity and high hygroscopicity, both of which can deteriorate device performance over time [102,103]. The most common inorganic HTL used in p-i-n PSC devices, NiOx, could induce degradation in the perovskite layer owing to the presence of undercoordinated metal cation sites (Ni3+) on its surface (Figure 4a) [104]. This cation can induce the formation of A-site-deficient PbI2 only layer at the interface between HTL and the absorber, which not only limits the performance but also the stability of the device over time. Likewise, Cheng et al. observed that the sol-gel-processed NiOx surface exhibited dipole character, which induced the adhesion of precursor ions on the NiOx surface during the film formation, thereby creating vacancy defect states in the interface which proved detrimental to the device stability [105].
Similarly to HTLs, ETLs, which are used for efficient electron extraction from the perovskite layer, could also induce instability in the PSC devices. A commonly utilized ETL for n-i-p regular structure perovskite solar cells, TiO2, is susceptible to inducing degradation in the perovskite layer due to its photocatalytic action in the presence of UV light (Figure 4b) [5]. Holes generated due to UV irradiation can extract electrons from organic materials as well as iodides, thereby causing the perovskite crystal decomposition [106]. Similarly, in a widely used inorganic ETL such as SnO2, increasing the concentration of oxygen vacancies is beneficial for enhancing electron mobility [107,108]. However, this also results in a higher positive charge on the Sn atoms, which can lead to an increased Pb–I bond length at the SnO2/perovskite interface, which can induce iodide interstitials, potentially accelerating device degradation [109]. Furthermore, high oxygen vacancies could deprotonate the organic cations, followed by their hydrolysis to form amides, which cause the decomposition of perovskites. Likewise, most common ETLs such as PCBM for p-i-n type solar cells strongly facilitate the photo-induced decomposition of perovskite films by absorbing the organic components (methylammonium halide) of the perovskite degradation products [110]. The cavity between the carbon spheres can easily accommodate those degradation products. Such intercalation of these products and their diffusion towards metal electrodes deteriorate the device stability.
Mitigation Strategies
The mitigation strategies employed to reduce CTL-induced degradation in PSCs are tailored to the specific degradation pathways caused by the CTLs. For example, in spiro-OMeTAD-based PSCs, degradation typically begins due to its prolonged oxidation time [69]. To address this, various oxidation-accelerating materials, such as vanadium oxide (V2O5) and alkylthiol additives (e.g., 1-dodecanethiol, DDT), have been introduced, which help to rapidly oxidize spiro-OMeTAD, thereby preventing degradation [111,112]. In addition to reducing oxidation time, the coordination between DDT and LiTFSI in the spiro-OMeTAD layer helps minimize the formation of pinholes and enhances the hydrophobicity of the layer (Figure 4c). This results in significantly improved device stability [84]. Likewise, the doping of spiro-OMeTAD to enhance the hydrophobicity of the layer has been used as another strategy to minimize the degradation [113,114]. For instance, the use of fluorinated spiro-OMeTAD as HTLs created kinetic barriers slowing the intrusion of O2 and moisture in the perovskite layer, maintaining its integrity (Figure 4d: XRD of perovskite films deposited on control and fluorinated spiro) [114]. This resulted in much-enhanced stability of the PSC devices. Another strategy is the use of dopant-free spiro-derived HTLs, which could oxidize without air assistance, thereby enhancing the stability of the devices [115,116,117]. The surface-induced degradation in another common inorganic HTL, NiOx, has been mitigated using various strategies. Boyd et al. utilized an excess A-site cation salt in the perovskite precursor to inhibit the formation of PbX2 hole extraction barrier at the perovskite–NiOx interface, which in turn enhanced both the performance as well as stability of the inverted PSCs [104]. Likewise, Mahmoudi et al. developed an innovative way of ensuring long-term stability and efficiency of perovskite devices by introducing p-type NiOx and highly conductive graphene into perovskite layer and inserting another NiOx interface layer between perovskite and the electron transporting layer. The strong chemical interaction and bonding between perovskite, NiOx, and graphene effectively reduces the side reaction between perovskite and oxygen and moisture, which resulted in the much-enhanced long-term stability, retaining 97–100% of the initial performance after 310 days under ambient condition (25–30 °C, RH = 45–55%) [118]. Passivation of the NiOx surface by oxide layers has also been implemented to enhance the device stability. One recent finding highlights the use of an ultrathin, ALD (atomic layer deposition)-coated alumina layer sandwiched between NiOx and perovskite which resulted in negative fixed charge passivation of the interface, which led to ultra-stable PSCs [119]. The passivated devices under 1-sun illumination at MPPT exhibited no loss in performance until 2000 h of measurement (Figure 4f). The use of SAM as HTL in inverted PSCs has led to enhanced performance; however, their instabilities often compromise the long-term stability of the devices. In a recent study, Jiang et al. introduced 3-azidopropyl phosphonic acid into 4-(7H-dibenzo[c,g]carbazol-7-yl)butyl phosphonic acid (CbzNaph) SAM to form a cross-linkable co-SAM, which improved the toughness and conformational stability of hole-selective interfaces [120]. This enhances interfacial adhesion and mechanical resilience. In addition, they used EDAI2 to reduce ion migration and non-radiative recombination. Together, these strategies enabled a power conversion efficiency of 26.17%. Stability tests following the ISOS-L2 protocol showed negligible PCE loss after 1000 h of MPPT and 98% retention after 700 thermal cycles.
The photocatalytic degradation in PSCs induced by TiO2-based ETLs in high-performing devices has been minimized using different approaches, like using UV absorption or downshifting layers [121,122,123], using different inorganic and organic materials as an interfacial layer between the ETL and perovskites [124,125,126,127], using alternative non-UV-sensitive ETL materials [128,129], etc. Qiu et al. utilized 4-aminobutyric acid (CA) and 3-amino-1propanesulfonic acid (SA) to construct an interfacial dipole between the titania and perovskite surface (Figure 4e), which helped in the reduction of interfacial defects, thereby enhancing UV stability of the CsPbI3 device [127]. Likewise, Wang et al. used 4-chloro-3-sulfamoylbenzoic acid (CSBA), which connected the TiO2 top surface and perovskite bottom surface, forming a molecular bridge at the TiO2/Perovskite interface which greatly reduced the interfacial degradation, thereby improving the ambient as well as UV stability of the devices (Figure 4g) [126]. Koo et al. utilized mesoporous MoS2 as an ETL alternative to TiO2 and obtained an impressive performance (>25% PCE) as well as photostability (1-sun illumination/2000 h/T90) of their PSCs [128]. Also, the use of surface-modified SnO2 as the ETL in PSC devices resulted in enhanced environmental stability compared to the TiO2–ETL devices [129,130].
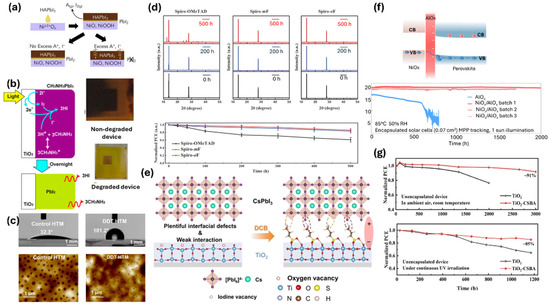
Figure 4.
(a) Schematics of formation of lead halide (PbX2) hole extraction barrier at the NiOx/perovskite interface and strategic addition of excess A-site precursor to overcome the issue (PbI2 is the lead iodide layer between NiOx and perovskite) [104] (Reproduced with permission from Elsevier, Copyright 2020). (b) Schematics of TiO2-induced photo degradation of perovskite absorber layer [5] (Reproduced with permission from American Chemical Society, Copyright 2014). (c) Contact angle measurement on the spiro-ometad and 1-dodecanethiol (DDT)-doped spiro-ometad surfaces and corresponding atomic force microscopy measurement images. (d) Time evolution of XRD patterns of perovskite film with control and fluorinated spiro-ometad and the stability of corresponding PSCs [114] (Reproduced from Jeong et al., Science, 10.1126/science.abb7167 [2020], AAAS). (e) Schematic of interfacial dipole formation by using 4-aminobutyric acid and 3-amino-1-propanesulfonic acid molecules between TiO2 and perovskite layer [127] (Reproduced with permission from John Wiley and Sons, Copyright 2024). (f) Schematics of electronic structure and carrier distribution in conduction and valence band (CB, VB) of perovskite/NiOx interface passivated by ultrathin aluminum oxide (AlOx) and the corresponding device performances showing long-term operational stability [119] (Reproduced with permission from Springer Nature, Copyright 2023) and (g) enhanced UV and ambient stability of perovskite solar cells with titanium dioxide (TiO2) electron transport layer passivated by 4-chloro-3-sulfamoylbenzoic acid (CSBA) molecule [126] (Reproduced with permission from John Wiley and Sons, Copyright 2024).
2.4. Electrode-Material-Induced Degradation
Perovskite solar cells are typically fabricated starting with fluorine-doped tin oxide (FTO) or indium-doped tin oxide (ITO)-coated glass as the bottom electrode, onto which the active material is deposited between appropriate charge transport layers (CTLs) and finished with top electrode deposition [131]. The top electrode, which is near the atmosphere, must be robust enough to maintain its own stability while also protecting the underlying layers [132]. Generally, noble metals like silver (Ag) and gold (Au) are employed as top electrodes in PSCs, owing to their resistance to oxidation and corrosion. However, instabilities in the PSC devices related to the use of those electrodes have been observed, which shall be discussed in this section. Also, we shall provide the schemes adopted to overcome those instability issues.
The migration of the top contact electrode to the active perovskite layer as well as the CTLs has been found to degrade the stability of PSCs. The degradation of perovskite material by the penetration of Au from top contact to the perovskite layer through spiro-OMeTAD has been reported by Domanski et al. [133]. The elemental depth profile using TOF-SIMS and inductively coupled plasma mass spectrometry (ICPMS) of the perovskite active layer revealed the presence of large amount of Au in 70 °C/15 h/1-sun MPPT-aged samples. The presence of Au in the active layer resulted in shunting of the device and introduced deep-level trap states in the perovskite active layer, leading to instability in the device. Although Au is deemed more stable against chemical degradation compared to other electrodes like Ag and Al, studies have pointed out that highly reactive polyiodide melts (MAI-nI2), that are released upon UV exposure from the iodide containing perovskite films, could chemically interact with Au, thereby forming a (MA)2Au2I6 phase at the Au/HTL interface, inducing intrinsic instability in the device [134]. Another study by Boyd et al. made use of different metal electrodes (Ag, Au and Cu) in their PSC devices and found thermal-induced degradation for all of them when the devices were heated at 85 °C (Figure 5a) [135]. They speculated that the metals diffused to the perovskite layer, thereby reacting with it to degrade the performance of the PSC over time. They further pointed out that the metal electrodes could also act as a sink for iodide migration from the perovskite, inducing the degradation of the PSC devices. Likewise, Kato et al. suggested that the pinholes in the spiro-OMeTAD layer make the perovskite vulnerable to moisture ingress, leading to the release of volatile iodine-containing species such as MAI and HI [136]. These species can then diffuse toward the top Ag layer, where they react to form a nonconductive AgI layer at the HTL/Ag interface (Figure 5b). Interestingly, the degradation of the perovskite active layer can also be initiated at the grain boundaries, driven by the thermodynamic tendency to form AgI within the Ag electrode, even in the absence of moisture. This process results in the release of mobile MA+ and I− ions, which can diffuse through the CTL and accumulate at the inner surface of the electrode, ultimately forming a highly detrimental AgI layer [137].
Copper can be a promising electrode material for the PSCs because of its excellent conductivity and low cost; however, the stability of the copper-based PSCs is also not satisfying. They can readily oxidize in air, forming CuO or Cu2O which reduces the conductivity and work function alignment with underlying layers. Also, the reactivity of copper with the perovskite constituents like the organic cations or the halide anions could result in degradation of both the perovskite and the copper electrode itself. Such reactions could be initiated by the diffusion of copper ions into the perovskite or through the diffusion of ions from perovskite to the copper electrode [138]. Such degradation of PSCs due to electrode degradation caused by the origination of mobile ions in the perovskite layer has also been studied for low-work-function metal electrodes like aluminum [139]. A comparison of different electrodes (Ag, Al, Au and Ni) for PSC revealed the most rapid degradation of Al-based PSC, exhibiting both the perovskite decomposition as well as the electrode’s corrosion.
Mitigation Strategies
Different approaches have been adopted to minimize the degradation of electrode materials to enhance the PSC longevity. One of them is the use of a physical barrier in between the HTL and the electrode layer. For instance, Domanski et al. utilized a chromium metal interlayer between the HTL and the gold electrode. The deposited chromium layer acted as a diffusion barrier between the gold electrode and the heat-stressed, crack-prone spiro-OMeTAD, effectively preventing gold migration across the HTL to the perovskite, thereby improving the device’s long-term operational stability [133]. Likewise, the use of semi-metal bismuth (Bi) as an interlayer between the HTL–electrode interface resulted in the inhibition of iodide-induced corrosion of the electrode as well as the penetration of electrode materials to the perovskite bulk via the CTL [140]. This strategy significantly improved the long-term storage stability of the target devices. The unencapsulated devices, with the Bi interlayer, retained 88% of their initial PCE after 6000 h, compared to only 33% PCE for the device without the Bi interlayer after just 1000 h under similar storage conditions (ambient air/dark). The chemical inertness and low penetration of compact bismuth on the perovskite layer during thermal aging not only inhibited the ion migration but also prevented the permeation of oxygen and water towards the perovskite film, thereby enhancing the stability of the PSC device. Another study by Lin et al. utilized chemical-vapor-deposited graphene as a bilayer on both sides of their copper–nickel alloy electrode [141]. The outer-layered graphene blocked the external degradants such as moisture and oxygen, while the inner graphene layer suppressed the ion migration and metal diffusion from and towards the perovskite layer. The graphene-composited electrode displayed a higher self-corrosion potential (Figure 5c), representing higher resistance to electrode corrosion. This resulted in the retention of more than 95% of initial PCE after 5000 h of continuous 1-sun illumination at MPPT (Figure 5d) [141].
Another successful approach for the mitigation of electrode corrosion and electrode-induced degradation in the perovskite active layer is using materials, which form a chemical anti-corrosion layer with the electrode material. This anti-corrosion layer inhibits the migration of perovskite ions as well as electrode atoms through them. Different materials such as benzotriazole, 2-mercaptobenzothiazole, 2,2′-(1,3-phenylene)-bis [5-(4-tert-butylphenyl)-1,3,4-oxadiazole], etc., have been found to bind with electrodes to form the corrosion-resistant layer, which greatly enhances the stability of the PSCs [142,143,144]. For instance, The benzotriazole (BTA) chemically coordinates with the Cu electrode and forms an insoluble and polymeric BTA–Cu film, suppressing corrosion of the Cu electrode and the reaction between the perovskite and the electrode (Figure 5e), which resulted in enhanced moisture stability of the device (Figure 5f) [142]. The incorporation of thiazole and thiol-based compounds to modify the bathocuproine (BCP) layer in p-i-n-type PSCs has also been reported, which facilitates chemical coordination between the additives and electrode atoms, leading to the formation of an anti-corrosion layer on the electrode’s bottom surface, significantly enhancing the stability of the PSC devices [145,146]. Likewise, Gong et al. incorporated a bipyridine derivative, 4,4′-dicyano-2,2′-bipyridine (DCBP) into PCBM in their p-i-n PSCs, enabling nitrogen coordination with the Ag electrode and facilitating charge transfer between surface iodide ions on the perovskite and the cyano groups in DCBP [147]. This dual interaction effectively blocked Ag diffusion and prevented iodide intrusion into the electrode (Figure 5g), which led to very stable devices (95% PCE retention, 2500 h, 1-sun MPPT and >90% PCE retention, 1500 h, 85 °C/85% RH).
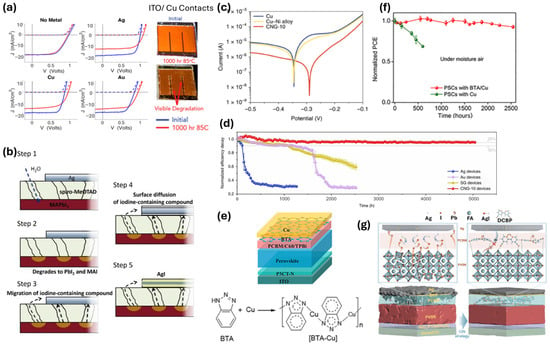
Figure 5.
(a) Performance degradation of the thermally treated (85 °C) FA0.83Cs0.17Pb(I0.83Br0.17)3 perovskite solar cells with different top electrodes (no metal, silver, copper, and gold), blue curves and red curves correspond before and after thermal treatment, dashed and solid curves represent the dark and light JV curves respectively, and the inset picture shows visual degradation of Cu-based solar cells where degradation originates along the metal fingers [135] (Reproduced with permission from American Chemical Society, Copyright 2018). (b) Schematics representing the formation of silver iodide (AgI), thereby hindering the long-term stability of the perovskite-based solar cell device [136] (Reproduced with permission from John Wiley and Sons, Copyright 2015). (c) Tafel curves of the copper (Cu), copper–nickel (Cu–Ni alloy) and copper–nickel–graphene (CNG)-based electrodes showing enhanced electrochemical corrosion stability upon graphene coating [141] (Reproduced with permission from Springer Nature, Copyright 2022). (d) Operational stability of the encapsulated perovskite PV devices with silver (Ag), gold (Au), sprayed graphene (SG) and copper–nickel–graphene (CNG)-based electrodes [141] (Reproduced with permission from Springer Nature, Copyright 2022). (e) Device structure of perovskite solar cells implementing copper (Cu) as top electrode and benzotriazole (BTA) interlayer beneath it and chemical reaction depicting the formation of polymeric BTA–Cu film (Reproduced from [Xiaodong Li] et al., Science, 10.1126/sciadv.abd1580 [2020], AAAS). (f) Enhanced moisture stability of the solar cell device with polymeric BTA–Cu film [142] (Reproduced from [Xiaodong Li] et al., Science, 10.1126/sciadv.abd1580 [2020], AAAS) and (g) schematics illustrating the nitrogen coordination between the top silver (Ag) electrode atoms and 4,4′-dicyano-2,2′-bipyridine (DCBP) dopants in PCBM, which facilitates the stability of the perovskite solar cells [147] (CC BY 4.0).
The incorporation of carbon as an electrode material has significantly enhanced the stability of PSCs. Its hydrophobic nature and chemical inertness help protect the perovskite layer from external contaminants while also suppressing electrode-induced degradation [148,149]. This has led to highly stable devices, exhibiting no performance degradation even after one year of storage [150,151]. However, these carbon-based PSCs are limited by their PCEs, due to poor crystallinity of applicable perovskite materials and energy band mismatch between the perovskite and the carbon electrode [152,153]. Efforts to maximize the PCEs of the carbon-based PSCs have been conducted to benefit from their durability. Li et al. utilized a moisture-induced secondary growth strategy to improve the crystallinity and optimize the energy levels of the perovskite films and obtain remarkably high-performing carbon-based PSCs. Likewise, Tang et al. utilized an appropriately long alkylammonium spacer cation to form a 2D layer on top of 3D perovskite which balanced the defect passivation, interface charge separation, and charge transfer between the perovskite and carbon electrode in their HTL-free PSCs. This led to a record PCE of about 20% for carbon-based PSCs. Furthermore, no loss in PCE of the device after 1500 h storage in 50% RH/room temperature condition signifies the better stability of the carbon-based PSC.
3. External Degradation Pathways and Mitigation Strategies
3.1. Moisture-Induced Degradation
Atmospheric conditions have been observed to induce a very rapid degradation in the perovskites [154]. The degradation path can be associated with the volatility of hygroscopic nature of perovskite material and hydrophilicity of organic cations, which trigger the formation of defect states. Those defects accelerate the non-radiative recombination, diminish the charge–carrier lifetime, and consequently pave the way for the moisture or oxygen ingress as well as the ion migration [155]. Water acts as a catalyst that triggers the chemical decomposition of perovskites [156]. The decomposition of the perovskite active phase into its constituent components, namely PbI2 and CH3NH3I, is an irreversible process that compromises the integrity of the device [157,158]. The CH3NH3I further breaks down into CH3NH2 and HI, which, upon exposure to oxygen, undergo secondary reactions forming I2 and H2O [156], thereby further accelerating the degradation. Equations (1)–(6), presented below, detail the water-induced degradation mechanism in MAPbI3 perovskites.
An investigation into the moisture-induced degradation of MAPbI3 single crystals and thin films revealed that, in the early stages of water exposure, a monohydrate perovskite phase forms. This phase is reversible and can fully revert to the original perovskite structure upon exposure to a dry atmosphere. However, prolonged exposure to moisture leads to the formation of a dihydrate phase, resulting in irreversible degradation and the formation of PbI2 within the device [157]. In another study, to understand the degradation mechanism, Song et al. utilized the laser-beam-induced current (LBIC) imaging method to investigate the spatial and temporal evolution of the external quantum efficiency (EQE) of PSCs under controlled humidity [158]. The temporal EQE curve reveals four distinct degradation stages (Figure 6a). In the first stage, an initial increase in EQE was observed, attributed to the passivation of interfacial traps at the perovskite–HTL interface. This was followed by a gradual decline in the second stage, associated with bulk changes in the doping level of the HTL and a reduction in hole collection efficiency within the HTL. The third stage was marked by a sudden drop in EQE, caused by the interaction of water molecules with the perovskite, leading to the formation of low-dimensional hydrated perovskite phases as described in Equations (1) and (2) above. Finally, the fourth stage resulted in irreversible degradation of the perovskite layer, driven by water-catalyzed decomposition into PbI2 and volatile by-products (Equations (3)–(6)). Similarly, Wang et al. investigated the influence of perovskite crystal quality on water-induced degradation [159]. Their study revealed that degradation initiates at amorphous and defect-rich grain boundaries and progressively propagates into the grain interiors (Figure 6b). Furthermore, they demonstrated that increasing the grain size effectively delays moisture-induced degradation, with the results exhibiting a linear dependence between grain size and degradation resistance.
Compared to MAPbI3, the FAPbI3 are more resistant to water-induced degradation. Yun et al. reported that FAPbI3 remains stable under ambient condition with relative humidity up to 30% at room temperature. However, when RH increases to 50%, decomposition occurs by the change in the phase, from the perovskite phase into a non-perovskite phase, which has a yellow color and a bandgap of 2.43 eV. The grain boundaries are assumed to serve as moisture pathways, which initiate the transition from the black perovskite phase to the yellow non-perovskite phase (Figure 6c) [160].
Mitigtation Strategies
Although the external moisture-induced degradation can be reduced significantly by a robust encapsulation, enhancing the internal moisture stability of the perovskite, along with interfacial layers, further aids in gaining better stability against moisture. Various strategies have been investigated to enhance the internal moisture resistance of perovskite devices, and this subsection provides a brief overview of these efforts.
Compositional engineering of the perovskite precursor has been one of the pioneering strategies towards increasing the moisture stability of the PSC devices. Partial replacement of iodides in the perovskite precursor by smaller bromides or chlorides can lead to enhanced Pb–X bonds and compact crystal lattice, which could provide better moisture resistance to the perovskite films. It has also been observed that chloride ion does not fully incorporate into perovskite lattice but acts as a crystal growth modulator promoting larger grain growth with fewer grain boundaries, thereby minimizing the moisture ingress. For instance, Buin et al. utilized both experimental as well as theoretical calculations to determine the stability of different MAPbX3 (X = I, Br, Cl) and found that the chloride perovskites were most stable [161]. Since chloride-based perovskites exhibit significantly lower power conversion efficiencies (PCEs) than their iodide counterparts due to their wider bandgaps, mixed-halide perovskites have been investigated to enhance device stability without compromising the efficiency. Noh et al. investigated the moisture stability of MAPb(I1−xBrx)3 perovskite and found that the cells with were more stable in the humid condition (Figure 6d) [162]. Moreover, the replacement of halides with pseudohalides has also led to enhanced moisture stability of the devices. Pseudohalides could potentially form stronger bonding in the perovskite crystal that lowers the probability of bond cleavage upon moisture exposure. Tai et al. investigated the use of thiocyanate (SCN−) anion in their MAPbI3−x(SCN)x PSC devices and found that the incorporation of (SCN−) not only boosted the moisture stability of their devices but also enhanced the perovskite film preparation in humid environment (70% R.H.) [163]. Recently, Tian et al. incorporated bis(trifluoromethanesulfonyl)imide (TFSI) pseudohalide in their perovskite precursors and discovered synergistic enhancement in both performance as well as moisture stability of the devices (ambient air/RH-(20–30)%/unencapsulated/T92-2400 h) [164]. Like anion engineering, cation site engineering has also led to significant improvement in the moisture stability of the PSC devices. Formamidinium (FA) cations, having more protons compared to the MA cations, can form enhanced hydrogen bonds with I− anion, thereby stabilizing the perovskite crystal structure, which in turn can enhance moisture stability to the device [165]. Incorporation of some cesium into the FA-based perovskite has been found to increase performance [2] and moisture stability [166]. Incorporation of small inorganic cations suppresses unfavorable phase transitions and reduces the lattice strain as well as defect density in the perovskite crystal, thereby imparting moisture stability to the PSC devices. For instance, Lee et al. fabricated FA0.9Cs0.1PbI3-based PSCs and compared their moisture stability (RH-40%) to pristine FAPbI3 and found that cesium incorporation enhanced the moisture stability by a great extent [167]. Moreover, the incorporation of triple cation FA, MA, and Cs in the perovskite devices resulted in higher stability to the moisture (RH (60–80)%, 15 days, 85% PCE retention) compared to the pristine MA-only PSC devices (40% PCE retention) [168].
Another strategy for enhancing the moisture stability of the perovskite material internally is by the passivation of the grain boundaries and defects in the bulk by using different hydrophobic moieties. Since the initiation of moisture ingress and degradation of the perovskites start from the grain boundaries, enhancement in the grain size and passivation of grain boundaries by hydrophobic entities can reduce the moisture-induced degradation in the PSCs [169]. For example, Gao et al. used amphiphilic fluorinated additive, 1,1,1-trifluoroethylammonium iodide, to their MAPbI3 perovskite which enhanced the perovskite morphology and uniformity. Furthermore, the hydrophobic fluorine entity led to enhanced moisture stability of the device (ambient storage, T92 = 120 days) [170]. Likewise, Yang et al. mixed fluorinated pyrelenediimide (F-PDI) into their perovskite film, which not only enhanced the crystallization of the films resulting in larger grain size (Figure 6e) but also increased the hydrophobicity of the grains by filling at the grain boundaries. This improved the moisture stability of the resulting devices by a great extent [171]. Recently, Hu et al. incorporated 1,1′-methylenbispyridinium dichloride which increased the hydrophobicity of the perovskite film significantly, evidenced by the moisture resistance of up to 180 s on completely water-immersed perovskite films. This resulted in the enhanced continuous operational stability (20 ± 5 °C, 60 ± 10% RH, T80 = 2000 h) of unencapsulated devices [172]. In addition to this, the incorporation of polymeric compound additives in the perovskite precursor has also brought a significant moisture stability in the perovskite devices [173,174,175,176]. The polymers which often contain aromatic rings with long chains are intrinsically hydrophobic, thereby forming a moisture-barrier layer around the perovskite grains. This barrier can act as moisture repellant which slows down the infusion of water molecules inside the perovskite lattice, thereby providing durability against moisture. For instance, Zheng et al. utilized a conductive polymer, polyaniline additive to their perovskite films, which significantly increased the hydrophobicity of the film resulting in the moisture stability of the unencapsulated devices (50% RH, T86 = 1600 h) [173]. Similarly, a research group recently led by Li et al. designed and synthesized a hyperbranched dopamine polymer adhesive (HPDA) which could form a stereoscopic network at the interfaces as well as the bulk of the whole device (Figure 6f), thereby increasing the toughness as well as moisture resistivity of their flexible PSC devices [176]. This strategy improved the bending durability of the flexible PSC devices at higher moisture conditions (bending radius = 3 mm, cycles = 1000, 65% RH, PCE retention = 94.7%). Also, the incorporation of hydrophobic, bulkier cations capable of forming 2D perovskites for grain boundary passivation has been explored for mitigating the moisture-induced instability in the PSC devices [177,178,179,180]. For instance, different diammonium spacer cation-based molecules were mixed with the perovskite precursor, resulting in enhanced crystallization as well as improved moisture resistance [179]. 2,2′-(ethylenedioxy)bis(ethylamine) (EDOEA) spacer cation resulted in Dion–Jacobson type 2D/3D perovskite (Figure 6g), which gave the best results with improved device performance as well as the moisture stability of the device (RH 50 ± 5%, T82 = 1560 h) [179]. In a more recent study, Zhang et al. tested different aromatic spacer cations in the perovskite precursors, among which the pyrenylbutanamine (PyBa) exhibited superior performance [180]. Due to the higher conjugation of spacer cations, the moisture stability of the device has been greatly improved (unencapsulated PSC, 80% RH, T80 = 1600 h). Along with hydrophobicity, the abilities of defect passivation and suppression of ion migration on the grain boundaries of 3D perovskites by the 2D perovskite layer help in mitigating moisture-induced degradation in the PSCs.
The perovskite surface is particularly susceptible to moisture ingress, which can degrade the film and compromise device stability. Thus, surface passivation has also emerged as an effective strategy to enhance the moisture resistance of perovskite solar cells. In addition to improving environmental stability, surface passivation reduces defect densities and promotes better interfacial contact with the upper CTL, facilitating more efficient charge extraction and contributing to higher PCE. Different organic as well as inorganic materials have been utilized for achieving the passivation of the perovskite surface to improve the performance and longevity. For instance, Jiang et al. used 4-fluorophenethylammonium iodide on the perovskite surface without further annealing, and the device showed improved performance as well as stability (ambient air 40% RH storage, T90 = 720 h) [181]. Further heat treatment of such alkylammonium salts after deposition on the perovskite surface leads to the formation of a 2D–3D interface, which might further enhance the moisture stability of the device. Liang et al. employed n-butylamine acetate instead of traditional spacer n-butylamine iodide, by which the strong ionic coordination between the spacer and perovskite framework is favorable for the formation of phase-pure layered perovskite with microscale vertically oriented grains [182]. The devices showed <10% efficiency degradation after storage under RH of 65 ± 10% for 4680 h, under operation at 85 °C for 558 h, or under continuous light illumination for 1100 h. Likewise, the use of sulfonium cations instead of alkylammonium cations has also resulted in improvements in the longevity of perovskite devices. Sulfonium cations exhibit stronger electrostatic interactions with the halide anions and, unlike alkylammonium cations, lack the hydrogen-bond-forming N-H bond, thereby providing greater hydrophobic protection against moisture. One such example is a study by Suo et al. where they make use of sulfonium-based molecules, dimethylphenylsulfonium iodide (DMPESI) for treating the perovskite surface, which resulted in excellent stability of the PSC devices. The devices show less than 1% PCE loss after 4500 h at MPPT, and less than 5% PCE loss under 1050 h of damp heat test (85 °C, 85% RH) as well as thermal cycling test (Figure 6h) [183]. Inorganic materials have stronger chemical bonds and stable chemistry, so they tend to make robust passivation layers as compared to the organic counterparts. Different inorganic metal oxides like AlOx, MoOx, ZrOx, etc., have been utilized for the passivation of the perovskite layer for enhancing the longevity of the devices [184,185,186]. As an example, Choi et al. used both an organic passivation layer (octylammonium iodide) and an ALD-coated thin inorganic AlOx layer for perovskite surface passivation. The solar cell devices with the combined organic and inorganic passivation retained 90% of their initial PCE after 1872 h under 85 °C and 85 % RH [187].
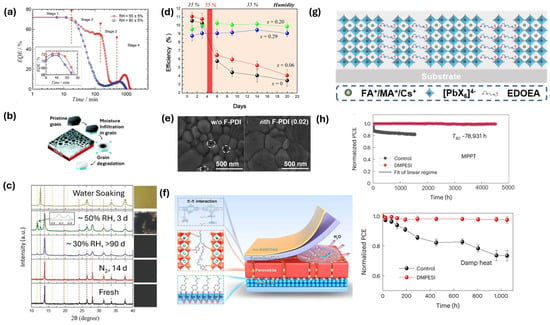
Figure 6.
(a) Laser-beam-induced current-external quantum efficiency (LBIC-EQE) of a perovskite solar cell as a function of time after humidity exposure [158] (Reproduced with permission from John Wiley and Sons, Copyright 2016). (b) Schematics illustrating water-induced degradation of perovskites starting from the grain boundaries [159] (Reproduced with permission from Royal Society of Chemistry, Copyright 2017). (c) XRD pattern of fresh, nitrogen (N2) stored and moisture-exposed (different levels) perovskite solar cells and corresponding pictures of the films are displayed on the side [160] (Reproduced with permission from John Wiley and Sons, Copyright 2018). (d) Moisture stability of the MAPb(I1−xBrx)3 perovskite solar cells [162] (Reproduced with permission from John American Chemical Society Copyright 2013). (e) Top-view scanning electron microscopy (SEM) image of the perovskite surface containing fluorinated pyrelenediimide (F-PDI), the dashed circles show the defective grain boundary in the control perovskite film [171] (Reproduced with permission from John Wiley and Sons, Copyright 2019). (f) Schematics showing the interaction of hyperbranched dopamine polymer adhesive (HPDA)with all other functional layers in the solar cell stack for improved moisture stability [176] (CC BY 4.0). (g) Schematic illustrating the arrangement of 2-dimensional (2D) Dion–Jacobson perovskite grown using large spacer cation 2,2′-(ethylenedioxy)bis(ethylamine) (EDOEA) in 3-dimensional perovskite precursor [179] (Reproduced with permission from John Wiley and Sons, Copyright 2021) and (h) long-term stability in the dimethylphenylsulfonium iodide (DMPESI) surface-treated perovskite solar cells in both maximum power point tracking (MPPT) measurement and damp heat test conditions [183] (CC BY 4.0).
3.2. Temperature-Induced Degradation
Photovoltaic devices are meant to be deployed in direct sunlight for harvesting solar energy. During regular operation, they often reach temperatures 20–30 °C above the ambient environment [188]. Therefore, under operating conditions, PV devices can experience significant temperature increases above ambient levels. These temperature rises are influenced by a combination of environmental factors, PV system design and installation parameters, and intrinsic thermal properties of the PV materials. To ensure their durability and performance, international standards, such as the IEC 61646 climate chamber tests, specify long-term stability testing at 85 °C. Therefore, addressing thermal instability is crucial for the widespread adoption of perovskite technology. In this section, we examine the impact of thermal stress on perovskite materials and discuss the strategies developed to mitigate temperature-induced degradation mechanisms.
Conings et al. utilized an ITO/TiO2/Perovskite device structure to study the effect of heat on MAPbI3 perovskite. The samples were heated at 85 °C for 24 h in ambient, nitrogen and oxygen environment and the resulting degradations were mapped using conductive atomic force microscopy (C-AFM) (Figure 7a). The ambient, N2, and O2 environment samples reveal the formation of PbI2 as a degradation product of the perovskite film [189]. This reflects the temperature-induced degradation of perovskites, even in the absence of oxygen and water. Although external encapsulation can protect the PSC device from oxygen- and water-induced degradation, the degradation caused from heat stress might still hinder the device stability. A study by Kim et al. utilized in situ grazing incidence XRD (GIXRD), high resolution X-ray photoelectron spectroscopy (HR-XPS) and near edge X-ray absorption fine structure (NEXAFS) techniques to investigate the thermal degradation in MAPbI3 perovskite and revealed that the device degraded even from moderate heat (85 °C), despite being well encapsulated (Figure 7b). The degradation initiated from the perovskite/CTL interface resulted in the preliminary formation of intermediate degraded phases and eventual conversion to PbI2 [190]. The temperature-induced degradation mechanism of MAPbI3 has been outlined in Equations (7) and (8) below [191,192].
Another commonly used perovskite material, FAPbI3, is deemed more thermally stable as compared to MAPbI3 [177]. However, a study by Juarez-Perez et al. revealed the degradation of the FAPbI3 perovskite above 95 °C with the formation of sym-triazine, formamidine, and hydrogen cyanide (HCN) [193]. Compared to organic cations, the inorganic Cs+ cations containing perovskites are stable to thermal degradation. Akbultov et al. studied photo-induced thermal degradation of different organic and inorganic cations containing halide perovskites by exposing the 100 mW/cm2 white light to the samples heated at 70 °C and found the thermal stability of different perovskites to be in the following order: MAPbBr3 < MAPbI3 < FAPbI3 < FAPbBr3 < CsPbI3 < CsPbBr3 [194].
Mitigation Strategies
In this section, we discuss various approaches such as cation substitution, 2D–3D dimensional stacking, vacuum deposition, additives, etc., which have been employed for increasing the thermal stability of the perovskite-based devices. One of the most used cations, methylammonium (MA), is not thermally stable and decomposes upon prolonged heat exposure [195]. Substitution of this cation by more thermally stable cations such as formamidinium (FA) or inorganic cation: cesium (Cs) can significantly increase the thermal stability of the PSC devices [50,196]. For example, Niu et al. studied the effect of Cs substitution in MAPbI3 perovskite’s thermal stability and found that 9% Cs-substituted perovskites showed enhanced performance and thermal stability [197]. The devices underwent 85 °C thermal treatment in air without encapsulation for 60 min, where the Cs-containing sample (80% PCE retention) outperformed the control sample (40% PCE retention). Likewise, Schwenzer et al. utilized MA, FA, Cs, and their mixtures as cations in the PSC devices to investigate their effect on the thermal stability of the devices [198]. They found that the decomposition was more pronounced in MA-based perovskite as compared to FA-only and FA–Cs-based PSC devices (Figure 7c). Furthermore, in a study by Cheng et al., it was revealed that the substitution of MA cations by FA cations in 2D perovskites brought an enhancement in the thermal stability of the devices [199]. The FA-based, PDA(FA)3Pb4I13-based device exhibited exceptional thermal stability compared to its MA counterpart (Figure 7d). Also, a stacking layer of 2D–3D perovskite materials in the PSCs have brought a significant improvement in the thermal and moisture stability in the devices [75,200,201]. The thermal decomposition in PSCs usually starts from surfaces and grain boundaries; thus, the passivation of those surfaces and grain boundaries by temperature-resistant 2D perovskite material can bring significant thermal stability. For instance, Azmi et al. utilized Oleylamine (OA)-based 2D perovskite layers on the surface of 3D perovskite and tested the treatment of the deposited film in ambient and high temperature. They found that at room temperature, a gradient of 2D stackings was formed on the top of 3D perovskite film (Figure 7e), which increased the performance as well as stability of the PSC device, and the devices maintained more than 95% of their initial PCE after more than 1000 h of damp heat test [75].
The vacuum deposition technique such as thermal evaporation has proven efficient at producing perovskite films with larger grain sizes, smooth surfaces, and minimal defects such as pinholes [202]. Moreover, vacuum deposition is carried out at relatively lower temperatures, resulting in the formation of perovskites with reduced thermal stress [203]. These advantages can enhance the thermal stability of the vacuum-deposited PSC devices compared to their solution processed counterparts. Arivazanghan et al. utilized a tetrafluoro-tetracyanoquinodimethane (F4-TCNQ)/CuPc-based HTL in their all vacuum-deposited MAPbI3-based perovskite solar cells and obtained superior thermal stability in their devices (85 °C, 250 h plus, 95% PCE retention) [204]. Later on, Yuan et al. utilized the same CuPc-based HTL in their all vacuum-deposited FACsPbI3-based PSC devices and found much-enhanced long-term thermal stability. No observable degradation in the PCE of the device was reported until 3700 h of thermal stressing at 85 °C [205]. Dewi et al. compared the performance of all vacuum-deposited and solution-processed MAPbI3-based PSCs against thermal aging at 85 °C and found that the vacuum-deposited devices maintained over 80% of their initial PCE after 3600 h, while the solution-processed devices completely degraded after 500 h (Figure 7f) [203]. Another strategy for enhancing the thermal stability of the PSC devices has been additive engineering. The additives can induce strong interaction with the perovskite cations and anions, thereby enhancing the crystallinity and grain size. Furthermore, the additives can reside at grain boundaries, thereby mitigating the defects as well as inhibiting the ion migration. These defect passivation activities can lead to better thermal stability of the perovskite-based devices. For example, the use of caffeine as an additive to perovskite precursor imparted an excellent thermal stability to the device (85 °C, T86 = 1300 h) compared to the control device (85 °C, T60 = 175 h) [206]. In another work, Yang et al. used thermotropic liquid crystals with a melting point below the perovskite-processing temperature to enhance the diffusion of additives during the perovskite crystallization [207]. This caused homogeneous spatial distribution of the additives within the perovskite bulk, resulting in enhanced defect mitigation. This strategy led to enhanced thermal stability of the device (Figure 7g). Likewise, Zhang et al. introduced acrylamides into perovskite precursor to enable in situ polymerization around the grain boundaries of perovskites, which not only reduced the defect density but converted tensile stress to compressive stress, which significantly improved the thermal stability of the device (Figure 7h) [208].
Figure 7.
(a) Conductive atomic force microscopy (C-AFM) images of pristine and thermally treated MAPbI3 perovskite films under different conditions (N2: nitrogen, O2: oxygen, and ambient) [189] (Reproduced with permission from John Wiley and Sons, Copyright 2015). (b) In situ grazing incidence wide-angle X-ray diffraction (GIWAXD) patterns of MAPbI3 perovskite films thermally treated under different conditions [190] (CC BY 4.0). (c) Thermal stability comparison of different A-site cations containing perovskite solar cells (FAPI: formamidinium lead iodide, MAPI: methylammonium lead iodide, FA: formamidinium, MA: methylammonium, Cs: cesium) [198] (Reproduced with permission from American Chemical Society, Copyright 2021). (d) Stability enhancement in the formamidinium based 2D-perovskite-based devices compared to methylammonium-based counterparts [199] (Reproduced with permission from John Wiley and Sons, Copyright 2020). (e) Schematic illustration of the passivation of perovskite surface by thermally treated and room-temperature-treated 2D perovskites [75] (Reproduced from [Randi Azmi] et al., Science, 10.1126/science.abm5784 [2022], AAAS. (f) Stability comparison for all vacuum-processed and solution-processed MAPbI3 perovskite solar cells under elevated temperature (85 °C) [203] (Reproduced with permission from John Wiley and Sons, Copyright 2021). (g) Enhanced stability of 3,4,5-trifluoro-4′-(trans-4-propylcyclohexyl)biphenyl (TFPCBP) liquid-crystal-doped perovskite solar cell against pristine cell under humid condition [207] (Reproduced with permission from Springer Nature, Copyright 2024) and (h) schematics showing enhanced thermal stability by acrylamide in situ polymerization outside the perovskite grains [208] (Reproduced with permission from American Chemical Society, Copyright 2025).
3.3. Illumination and Oxygen-Induced Degradation
The degradation of PSC devices under light exposure is a serious issue, as PSCs are always operated under solar irradiation. While external degradation factors like moisture can be effectively mitigated through proper encapsulation, photo-induced degradation requires a deeper understanding of photon–material interaction to address its detrimental impact on perovskite stability. Photo-induced degradation often worsens in the presence of oxygen, making it appropriate to consider illumination- and oxygen-induced degradation under a unified framework. Therefore, in this section, we will discuss both photo-induced and photo-oxygen-induced degradation mechanisms in perovskite solar cells (PSCs) and explore relevant countermeasures to inhibit these degradation pathways.
Light-induced degradation in perovskites has been observed to have both a reversible as well as an irreversible effect. While light-induced ion migration and phase segregation in mixed-halide perovskites can be reversed back by dark storage, the decomposition of perovskites themselves upon light exposure leads to complete failure of the devices [209,210,211]. For instance, the light exposure in the perovskite bulk invokes the generation of extra negative and positive ions, which act as recombination centers for the light-generated electrons and holes, thereby causing a reduction in the current generated by the device. However, this effect is reversible, and device performance is typically restored after a period in the dark [209]. Similarly, light-induced formation of metastable deep-level trap states in the perovskite absorber layer has been linked to photocurrent degradation in perovskite solar cells (PSCs) [212]. These trap states are not permanent and tend to dissipate quickly, often within one minute of dark storage or when operated at low temperatures (e.g., 0 °C), allowing the device to regain its original steady-state performance (Figure 8a) [212]. However, a study by Khenkin et al. highlights that light-induced degradation in perovskite devices can be either reversible or irreversible, depending on the duration of light exposure [213]. They found that, in the early stages, degradation is primarily due to the formation of bulk trap states within the absorber layer, which is completely reversible, but prolonged exposure leads to the development of shallow interfacial trap states that create charge extraction barriers at the interfaces, resulting in irreversible degradation. Likewise, Ruan et al. used Raman spectroscopy to show that phase segregation occurs in mixed-halide (I, Br) perovskites under low irradiation intensity, with the segregation quickly reversing after illumination, whereas at high irradiation, iodine-rich domains begin to decompose, leading to irreversible degradation [210]. Also, light illumination induces C–N bond dissociation, causing the decomposition of organic cations and the reduction of lead cations to metallic lead, leading to complete and irreversible degradation of the perovskite [214].
Light irradiation not only degrades the perovskite layer, but can also induce degradations in the CTLs, the CTL–perovskite interfaces, and the top electrode [135,215,216]. For example, the photocatalytic action of TiO2 ETL upon UV light absorption is detrimental to the perovskite–TiO2 interface, thereby inducing degradation in the high-performing PSCs [5]. Likewise, the interface between NiOx HTL and the perovskite has also been found to degrade upon prolonged light exposure. For instance, compared to bare FAMAPbI3, a stronger iodine signal as well as higher partial pressures of FA, MA, and free protons observed for the FAMAPbI3/NiOx signify the deprotonation and oxidation reaction happening between the FAI/MAI and NiOx contact surfaces [216]. Furthermore, reduction in surface potential caused by light irradiation in the spiro-OMeTAD/Au interface resulted in breaking of the Au–O bond between the HTL and Au electrode, demonstrating that the photo-induced degradation originated as an HTL/electrode interface [215]. Also, light irradiation can initiate the polyiodide formations in the perovskites, which migrate through the HTL to the Au electrode, where they react with the electrode material, leading to irreversible degradation [135].
The presence of oxygen often worsens light-induced degradation in perovskites. A study by Aristidou et al. highlighted that the MAPbI3 perovskite decomposes into PbI2, I2, and methylamine when exposed to both light and molecular oxygen [60]. They report that under illumination, photogenerated electrons can reduce the molecular oxygen to form superoxide radicals (). The superoxide radical being highly reactive initiates irreversible degradation of both organic cations as well as lead–halide octahedra, thereby causing irreversible degradation of the MAPbI3 perovskite as below [60]:
Also, Liu et al. studied the degradation of MAPbI3 under UV light and oxygen exposure, finding that superoxide ions (O2−) are generated, which react with CH3NH3+ and cause rapid degradation of MAPbI3 into CH3NH2, PbI2, and I2 [217]. Their research also highlighted the significant impact of the perovskite–electron transport layer (ETL) interface on UV stability. In their study, cells with different ETL materials were exposed to UV light (365 nm, 60 mW/cm2, exceeding 13 suns). Tests were conducted under nitrogen gas to isolate the effects of moisture and oxygen. They demonstrated that TiO2, as an ETL, acts as a photocatalyst, accelerating degradation, while SiO2 offers improved stability, though degradation remains significant. In contrast, inverted PSCs with PCBM ETL exhibited negligible degradation, outperforming both TiO2 and SiO2. Also, the degradation of perovskites upon exposure to light and oxygen has been studied by exposing perovskite films to different conditions: with and without light, in presence or absence of oxygen. The degradation of films in the presence of both light and oxygen (Figure 8b) explains the catalytic action of light for oxygen-induced degradation in PSCs [154].
Mitigation Strategies
Strategies to mitigate light-induced degradation have been tailored based on the specific degradation pathways triggered by light within the perovskite device structure. One such degradation pathway is light-induced halide segregation (LIHS), which is known to impair both the performance and stability of wide-bandgap mixed-halide perovskites. This issue can be mitigated through various strategies, including cation and anion engineering of the perovskite composition [218,219,220], the use of additives, etc., [221,222]. For instance, Xu et al. utilized a triple halide combination in their wide-bandgap perovskite composition which greatly suppressed the LIHS in the perovskite films even at 100-sun illumination. The tripe halide composition enhanced the Pb–X bond strength and reduced lattice strain, which resulted in lattice stabilization as well as reduced local distortions responsible for halide segregation. This resulted in excellent illumination stability of the PSC devices, with less than 4% degradation after 1000 h of MPPT operation at 60 °C [220]. Likewise, Wang et al. found that the use of rubidium in cesium-based wide-bandgap mixed-halide perovskite causes lattice distortion, thereby decreasing the interatomic distance between the A-site cation and iodide, which increased the ion-migration energy barrier. This resulted in the suppression of LIHS in the perovskite devices [219]. Recently, Ghorai et al. made use of 1-dodecanthion ligand as additives in the perovskite precursor which suppressed the vacancy or defect states in the film, thereby mitigating the unwanted LIHS in the devices [221]. Furthermore, Yang et al. investigated different organic additives such as (4-cyclopentene-1,3-dione, maleimide, and 3,4-dibromo-1H-pyrrole-2,5(2H,5H)-dione) in the wide-bandgap CsPbIBr2 perovskite precursor and found that the chemical interactions between additives and iodide ions, as well as undercoordinated Pb2+ through coordination bonds, hydrogen bonds, and ionic bonds, greatly reduced the illumination stability of the devices [222].
Another major issue with the illumination-induced degradation in PSCs is the presence of high-energy UV spectrum in the solar spectrum. The UV-light-induced degradation might initiate at the bottom-charge-transport-layer–perovskite interface or in the perovskite layer itself. Various approaches like the use of UV absorption or downshifting layers, modification of perovskite–bottom-CTL interface, UV-resistant additives to the bulk perovskite, etc., have been employed for enhancing the UV stability of the PSCs. Zhang et al. employed colloidal carbon quantum dots both on the exterior front side of the PSC as an optical layer as well as the interfacial layer between the ETL and perovskite and were able to mitigate UV-induced degradation in their devices [223]. The carbon-quantum-dots-employed PSCs could maintain 94% of their initial PCE after 100 h under continuous UV illumination (5 mW cm−2). Likewise, Kim et al. employed a UV-absorbing polyimide substrate integrated with a nanostructured photonic sticker that functioned as a UV-to-visible downshifter for their flexible PSCs, resulting in a significant reduction in UV-induced device degradation [224,225]. To minimize the UV-induced interfacial photocatalysis between TiO2 and perovskite layer, Zhu et al. employed a UV absorber interlayer composed of 4,4′-oxybisbenzoic acid, which not only filtered the harmful UV light but also eliminated oxygen vacancies in TiO2 surface [225]. This reduced the photocatalytic degradation of perovskite and, hence, the modified devices maintained almost 70% of their initial PCE after 72 h of extreme UV exposure (275 nm, 10 mW cm−2). Furthermore, Fei et al. utilized a phosphonic acid, [2-(9-ethyl-9H-carbazol-3-yl)ethyl]phosphonic acid, to modify the PTAA–perovskite interface in their PSCs and prevent the UV-light-induced degradation of the buried interface, which led to the excellent UV stability of the device (Figure 8c) [226]. The champion minimodule was tested in an outdoor environment and it was sustained efficiently even after 29 weeks of testing (Figure 8d). The use of UV-absorbing or converting molecules in the perovskite precursor itself has also proved beneficial for enhancing the UV stability of the PSC devices [227,228,229]. For instance, Luo et al. utilized a functionalized UV conversion molecule, Coumarin 153, into a perovskite precursor which not only downshifted the UV-to-visible spectrum but also passivated the perovskite defects as shown in Figure 8e [229]. As a result, the unencapsulated devices retained over 90% of their efficiency after 380 h of continuous UV exposure at 50 mW cm−2 (Figure 8f).
Additionally, the use of interfacial layers between CTL and perovskite in the buried interface [230,231], as well as application of additives in the perovskite bulk [232,233,234], has helped mitigate the illumination-induced perovskite degradation. For example, Yang et al. introduced ordonezite (ZnSb2O6−x) as an electron transport layer (ETL) modifier between SnO2 and the perovskite absorber layer [231]. This interlayer effectively captured migrating oxygen species from SnO2 that would otherwise induce cation deprotonation at the A-site of the perovskite, thereby accelerating its degradation. This strategy resulted in much-enhanced illumination stability of the perovskite device where the target device maintained 90.62% of its original efficiency after 1000 h, while the control device showed a rapid performance decline, retaining only 90.40% after just 300 h when tested under ISOS-D-2 protocol [231]. Another research group used amber acid as an additive to their tin-lead perovskite precursor, which resulted in the inhibition of photo-induced iodide migration and subsequent formation of I2 in the devices [234]. This resulted in a much-enhanced photostability of the perovskite film (Figure 8g), which resulted in significant stability of the device under MPP tracking (81.35% PCE retention, 350 h) (Figure 8h).
Figure 8.
(a) Schematic of the formation of deep-level trap states in perovskites under illumination causing photocurrent degradation and subsequent recovery in the dark [212] (CC BY 4.0). (b) Absorption spectra of MAPbI3 films on glass under different aging conditions (Inset: picture of the respective perovskite films before and after aging) [154] (CC BY 3.0). (c) Stability of perovskite solar cells with different hole transporting layers under light emitting phosphor (LEP) lamp with 3.5% UV light soaking (EtCz3EPA refers to [2-(9-ethyl-9H-carbazol-3-yl)ethyl]phosphonic acid, PTAA: polytriarylamine and BCP: bathocuproin) and (d) outdoor stability testing setup for mini modules [226] (Reproduced from [Chengbin Fei] et al., Science, 10.1126/science.adi4531 [2024], AAAS). (e) Schematic of UV down conversion as well as perovskite defect passivation by coumarin 153 (C153) and (f) enhancement in the long-term stability of the C153-containing device [229] (Reproduced with permission from John Wiley and Sons, Copyright 2024). (g) Scanning electron microscopy (SEM) images for control and amber acid (AA)-modified perovskite surfaces at different illumination time intervals and (h) device stability for control and thiomalic acid (TA)-modified devices [234] (Reproduced with permission from John Wiley and Sons, Copyright 2024).
4. Conclusions and Outlook
Perovskite solar cells (PSCs) have shown remarkable progress in efficiency, but their commercial potential is limited by the long-term stability issues. In this review, we examined both intrinsic instability factors, such as ion migration and defect formation, and extrinsic environmental stressors, such as moisture and heat affecting perovskite solar cells. Intrinsic mechanisms contribute to critical issues like phase segregation, current-voltage hysteresis, and the presence of defective grain boundaries that act as degradation pathways. Additionally, metal electrode diffusion under thermal or photo-induced stress can cause irreversible damage to the device. These internal challenges are further exacerbated by external factors such as moisture and elevated temperatures, which promote grain boundary degradation and accelerate the decomposition of perovskite films, collectively undermining device performance and stability. Various strategies to address both the intrinsic and extrinsic factors include material and device engineering approaches such as cation substitution, mixed-halide formulations, and advanced surface passivation techniques that enhance stability and suppress defect formation. In addition, advancements in charge of transport layer and electrode design, UV-stable interfaces, and chemically inert electrodes have been found to play a crucial role in extending device lifetimes. Future research must adopt a holistic, system-level approach to truly commercialize PSCs. Firstly, stable perovskite compositions that maintain performance under combined stressors (light, heat, oxygen, and moisture) should be prioritized. This could be implemented using different strategies like compositional engineering on all the A-, B- and X-sites of perovskite crystals and controlled crystallization achieved through solvent engineering or the use of additives. Along with bulk stabilization, future efforts should focus on interfacial engineering through the application of multifunctional layers that combine physical blocking as well as chemical passivation of defective surfaces. The application of 2D-perovskite-forming materials should be optimized further for their structural and electronic coupling with 3D perovskites, maintaining a balance between charge transfer efficiency and durability. Multifunctional passivation materials capable of simultaneously addressing ion migration, defect passivation, and moisture protection will be essential. Along with improving perovskite quality, attention should be given to developing stable, dopant-free, and hydrophobic HTLs, as well as robust UV-stable ETLs achieved through surface modification or by using inherently UV-inert materials. These layers help prevent degradation at the CTL–perovskite interfaces, maintain efficient charge transport, and enhance the overall operational stability of perovskite solar cells. Likewise, interface engineering should be explored more to create chemically and structurally robust contact layers that resist ion diffusion and preserve proper energy band alignment. Furthermore, efforts towards making the inert electrode material by inserting physical or chemical barriers between the perovskite and metal electrode layer represent a promising route to inhibit both electrode diffusion as well as halide-induced corrosion, but the long-term chemical and thermal stability of these interlayers under combined stressors requires further exploration. The use of carbon as an electrode material has already provided excellent stability to the perovskite devices, owing to its chemical inertness; however, the performance of these devices lags when compared to their metallic electrode counterparts. Future research should focus on improving the charge extraction and achieving better energy level alignment for this electrode to enhance the overall efficiency of the devices without compromising their long-term durability. Also, the use of artificial intelligence and machine learning has brought about a significant improvement in the power conversion efficiency of the perovskite devices. The automations implemented with machine learning algorithms have accelerated the analysis of large experimental datasets over multi-scale features which overcomes the limitations of small, specialized datasets, providing a generalizable platform for predicting performance and gaining mechanistic insights [235]. These automation tools could be further implemented to predict the best materials, additives, and interface layers to reduce ion migration, moisture damage, and heat effects, which can improve both the efficiency and long-term durability of perovskite solar cells.
Moreover, robust and scalable encapsulation and barrier technologies are needed to protect devices in real-world environments without adding excessive cost or complexity. The development of in situ and operando characterization tools, coupled with predictive modeling of degradation under realistic operating conditions, will enable a deeper understanding of failure mechanisms. Also, the lack of standardized testing protocols for assessing the long-term stability of perovskite-based devices has led to disparity in the testing conditions, making it unreliable for cross-comparison between different studies. Adherence to already established protocols for testing the device reliability such as ISOS (international summit of organic photovoltaic stability) and IEC 61215, with even more stringent testing conditions, are deemed essential for consistent lifetime benchmarking of perovskite-based devices. Furthermore, alongside the accelerated degradation tests, continuous performance monitoring in outdoor environments can reveal synergistic degradation mechanisms. Progress in material chemistry, device architecture, and process engineering must converge to deliver PSCs that combine high efficiency with the long-term stability required for commercial solar panel deployment.
Author Contributions
The manuscript draft was written by B.R. and K.M.A.; A.L.K. and Z.C. reviewed, edited, and added recent developments in stability work on perovskites; S.L. reviewed the manuscript and provided perspectives on stability and accelerated degradation testing; T.P.D. reviewed and edited manuscript and formulated the overall structure of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported in part by the National Science Foundation under Grant Numbers 1751946, 2432832 and 2331447.
Data Availability Statement
No data was generated for this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leijtens, T.; Eperon, G.E.; Noel, N.K.; Habisreutinger, S.N.; Petrozza, A.; Snaith, H.J. Stability of Metal Halide Perovskite Solar Cells. Adv. Energy Mater. 2015, 5, 1500963. [Google Scholar] [CrossRef]
- Rajbhandari, P.P.; Dhakal, T.P. Limit of Incorporating Cesium Cations into Formamidinium-Methylammonium Based Mixed Halide Perovskite Solar Cells. Nanotechnology 2020, 31, 135406. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of Rubidium Cations into Perovskite Solar Cells Improves Photovoltaic Performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef]
- Jodlowski, A.D.; Roldán-Carmona, C.; Grancini, G.; Salado, M.; Ralaiarisoa, M.; Ahmad, S.; Koch, N.; Camacho, L.; De Miguel, G.; Nazeeruddin, M.K. Large Guanidinium Cation Mixed with Methylammonium in Lead Iodide Perovskites for 19% Efficient Solar Cells. Nat. Energy 2017, 2, 972–979. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, S.; Bae, S.; Cho, K.; Chung, T.; Mundt, L.E.; Lee, S.; Park, S.; Park, H.; Schubert, M.C.; et al. UV Degradation and Recovery of Perovskite Solar Cells. Sci. Rep. 2016, 6, 38150. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart | Photovoltaic Research | NREL. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 23 February 2025).
- Jordan, D.C.; Kurtz, S.R. Photovoltaic Degradation Rates—An Analytical Review. Prog. Photovolt. Res. Appl. 2013, 21, 12–29. [Google Scholar] [CrossRef]
- Roesch, R.; Faber, T.; Von Hauff, E.; Brown, T.M.; Lira-Cantu, M.; Hoppe, H. Procedures and Practices for Evaluating Thin-Film Solar Cell Stability. Adv. Energy Mater. 2015, 5, 1501407. [Google Scholar] [CrossRef]
- Rolston, N.; Printz, A.D.; Tracy, J.M.; Weerasinghe, H.C.; Vak, D.; Jia Haur, L.; Priyadarshi, A.; Mathews, N.; Slotcavage, D.J.; McGehee, M.D.; et al. Effect of Cation Composition on the Mechanical Stability of Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1702116. [Google Scholar] [CrossRef]
- Chowdhury, T.A.; Bin Zafar, M.A.; Sajjad-Ul Islam, M.; Shahinuzzaman, M.; Islam, M.A.; Khandaker, M.U. Stability of Perovskite Solar Cells: Issues and Prospects. RSC Adv. 2023, 13, 1787–1810. [Google Scholar] [CrossRef]
- Zhang, D.; Li, D.; Hu, Y.; Mei, A.; Han, H. Degradation Pathways in Perovskite Solar Cells and How to Meet International Standards. Commun. Mater. 2022, 3, 58. [Google Scholar] [CrossRef]
- Zhu, H.; Teale, S.; Lintangpradipto, M.N.; Mahesh, S.; Chen, B.; McGehee, M.D.; Sargent, E.H.; Bakr, O.M. Long-Term Operating Stability in Perovskite Photovoltaics. Nat. Rev. Mater. 2023, 8, 569–586. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.H.; Jiang, Y.; Hu, J.S. Instability of Solution-Processed Perovskite Films: Origin and Mitigation Strategies. Mater. Futures 2023, 2, 012102. [Google Scholar] [CrossRef]
- Mazumdar, S.; Zhao, Y.; Zhang, X. Stability of Perovskite Solar Cells: Degradation Mechanisms and Remedies. Front. Electron. 2021, 2, 712785. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, S.; Zhang, X.; Wang, A.; Wu, C.; Hao, F.; Zhu, W.; Wang, S.; Zhang, X.; Wang, A.; et al. Ion Migration in Organic–Inorganic Hybrid Perovskite Solar Cells: Current Understanding and Perspectives. Small 2022, 18, 2105783. [Google Scholar] [CrossRef]
- Haruyama, J.; Sodeyama, K.; Han, L.; Tateyama, Y. First-Principles Study of Ion Diffusion in Perovskite Solar Cell Sensitizers. J. Am. Chem. Soc. 2015, 137, 10048–10051. [Google Scholar] [CrossRef]
- Azpiroz, J.M.; Mosconi, E.; Bisquert, J.; De Angelis, F. Defect Migration in Methylammonium Lead Iodide and Its Role in Perovskite Solar Cell Operation. Energy Environ. Sci. 2015, 8, 2118–2127. [Google Scholar] [CrossRef]
- Eames, C.; Frost, J.M.; Barnes, P.R.F.; O’regan, B.C.; Walsh, A.; Saiful Islam, M. Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Commun. 2015, 6, 7497. [Google Scholar] [CrossRef]
- Xiao, Z.; Yuan, Y.; Shao, Y.; Wang, Q.; Dong, Q.; Bi, C.; Sharma, P.; Gruverman, A.; Huang, J. Giant Switchable Photovoltaic Effect in Organometal Trihalide Perovskite Devices. Nat. Mater. 2014, 14, 193–198. [Google Scholar] [CrossRef]
- Kim, S.; Bae, S.; Lee, S.W.; Cho, K.; Lee, K.D.; Kim, H.; Park, S.; Kwon, G.; Ahn, S.W.; Lee, H.M.; et al. Relationship between Ion Migration and Interfacial Degradation of CH3NH3PbI3 Perovskite Solar Cells under Thermal Conditions. Sci. Rep. 2017, 7, 1200. [Google Scholar] [CrossRef]
- Besleaga, C.; Abramiuc, L.E.; Stancu, V.; Tomulescu, A.G.; Sima, M.; Trinca, L.; Plugaru, N.; Pintilie, L.; Nemnes, G.A.; Iliescu, M.; et al. Iodine Migration and Degradation of Perovskite Solar Cells Enhanced by Metallic Electrodes. J. Phys. Chem. Lett. 2016, 7, 5168–5175. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H.J.; Abate, A.; Ball, J.M.; Eperon, G.E.; Leijtens, T.; Noel, N.K.; Stranks, S.D.; Wang, J.T.W.; Wojciechowski, K.; Zhang, W. Anomalous Hysteresis in Perovskite Solar Cells. J. Phys. Chem. Lett. 2014, 5, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Hoke, E.T.; Slotcavage, D.J.; Dohner, E.R.; Bowring, A.R.; Karunadasa, H.I.; McGehee, M.D. Reversible Photo-Induced Trap Formation in Mixed-Halide Hybrid Perovskites for Photovoltaics. Chem. Sci. 2014, 6, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Thiesbrummel, J.; Shah, S.; Gutierrez-Partida, E.; Zu, F.; Peña-Camargo, F.; Zeiske, S.; Diekmann, J.; Ye, F.; Peters, K.P.; Brinkmann, K.O.; et al. Ion-Induced Field Screening as a Dominant Factor in Perovskite Solar Cell Operational Stability. Nat. Energy 2024, 9, 664–676. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Hu, J.; Huang, B.; Sun, M.; Dong, B.; Zheng, G.; Huang, Y.; Chen, Y.; Li, L.; et al. A Eu3+-Eu2+ Ion Redox Shuttle Imparts Operational Durability to Pb-I Perovskite Solar Cells. Science 2019, 363, 265–270. [Google Scholar] [CrossRef]
- McGovern, L.; Futscher, M.H.; Muscarella, L.A.; Ehrler, B. Understanding the Stability of MAPbBr3 versus MAPbI3: Suppression of Methylammonium Migration and Reduction of Halide Migration. J. Phys. Chem. Lett. 2020, 11, 7127–7132. [Google Scholar] [CrossRef]
- Bi, E.; Song, Z.; Li, C.; Wu, Z.; Yan, Y. Mitigating Ion Migration in Perovskite Solar Cells. Trends Chem. 2021, 3, 575–588. [Google Scholar] [CrossRef]
- Rivkin, B.; Fassl, P.; Sun, Q.; Taylor, A.D.; Chen, Z.; Vaynzof, Y. Effect of Ion Migration-Induced Electrode Degradation on the Operational Stability of Perovskite Solar Cells. ACS Omega 2018, 3, 10042–10047. [Google Scholar] [CrossRef]
- Zhang, H.; Park, N.G. Strain Control to Stabilize Perovskite Solar Cells. Angew. Chem. Int. Ed. 2022, 61, e202212268. [Google Scholar] [CrossRef]
- Dailey, M.; Li, Y.; Printz, A.D. Residual Film Stresses in Perovskite Solar Cells: Origins, Effects, and Mitigation Strategies. ACS Omega 2021, 6, 30214–30223. [Google Scholar] [CrossRef]
- Cao, J.; Tao, S.X.; Bobbert, P.A.; Wong, C.P.; Zhao, N. Interstitial Occupancy by Extrinsic Alkali Cations in Perovskites and Its Impact on Ion Migration. Adv. Mater. 2018, 30, e1707350. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandari, P.P.; Rijal, B.; Chen, Z.; Choudhary, A.; Efstathiadis, H.; Dhakal, T.P. Performance Enhancement of Inverted Perovskite Solar Cells through Lithium-Ion Diffusion from the Nickel Oxide Hole Transport Layer to the Perovskite Absorber. Energy Adv. 2025. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Y.; Liu, K.; Liu, J.; Zhang, H.; Khan, Q.U.; Dai, S.; Qian, W.; Liu, R.; Wang, Y.; et al. Understanding the Decoupled Effects of Cations and Anions Doping for High-Performance Perovskite Solar Cells. Nanomicro Lett. 2025, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hu, Z.; Su, J.; Gong, Z.; Guo, X.; Chen, X.; Yang, Y.; Lin, Z.; Ding, L.; Hao, Y.; et al. Inhibiting Ion Migration and Stabilizing Crystal-Phase in Halide Perovskite via Directly Incorporated Fluoride Anion. Angew. Chem. 2025, 137, e202413550. [Google Scholar] [CrossRef]
- Liang, Y.; Li, F.; Cui, X.; Stampfl, C.; Ringer, S.P.; Yang, X.; Huang, J.; Zheng, R. Multiple B-Site Doping Suppresses Ion Migration in Halide Perovskites. Sci. Adv. 2025, 11, eads7054. [Google Scholar] [CrossRef]
- Pering, S.R.; Deng, W.; Troughton, J.R.; Kubiak, P.S.; Ghosh, D.; Niemann, R.G.; Brivio, F.; Jeffrey, F.E.; Walker, A.B.; Islam, M.S.; et al. Azetidinium Lead Iodide for Perovskite Solar Cells. J. Mater. Chem. A Mater. 2017, 5, 20658–20665. [Google Scholar] [CrossRef]
- Tan, S.; Yavuz, I.; De Marco, N.; Huang, T.; Lee, S.-J.; Choi, C.S.; Wang, M.; Nuryyeva, S.; Wang, R.; Zhao, Y.; et al. Steric Impediment of Ion Migration Contributes to Improved Operational Stability of Perovskite Solar Cells. Adv. Mater. 2020, 32, 1906995. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Li, R.; Zhuang, X.; Wang, C.; Shang, X.; He, D.; Chen, J.; Chen, C. Suppressing Ion Migration by Synergistic Engineering of Anion and Cation toward High-Performance Inverted Perovskite Solar Cells and Modules. Adv. Mater. 2024, 36, 2313860. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Z.; Lv, S.; Shui, Y.; Zhu, W.; Zhang, Z.; Yang, W.; Zhao, J.; Gu, H.; Xia, J.; et al. A Nd@C82–Polymer Interface for Efficient and Stable Perovskite Solar Cells. Nature 2025, 642, 78–84. [Google Scholar] [CrossRef]
- Li, W.; Bao, X.; Zhu, A.; Gu, H.; Mao, Y.; Wang, B.; Wang, G.; Guo, J.; Li, Y.; Xing, G. Internal Encapsulation Enables Efficient and Stable Perovskite Solar Cells. Adv. Funct. Mater. 2025, 35, 2414004. [Google Scholar] [CrossRef]
- Das, C.; Kot, M.; Hellmann, T.; Wittich, C.; Mankel, E.; Zimmermann, I.; Schmeisser, D.; Khaja Nazeeruddin, M.; Jaegermann, W. Atomic Layer-Deposited Aluminum Oxide Hinders Iodide Migration and Stabilizes Perovskite Solar Cells. Cell Rep. Phys. Sci. 2020, 1, 100112. [Google Scholar] [CrossRef]
- Rajbhandari, P.P.; Dhakal, T.P.; Phys Lett, A.; Dhakal, T.P. Low Temperature ALD Growth Optimization of ZnO, TiO2, and Al2O3 to Be Used as a Buffer Layer in Perovskite Solar Cells. J. Vac. Sci. Technol. A 2020, 38, 32406. [Google Scholar] [CrossRef]
- Artuk, K.; Turkay, D.; Mensi, M.D.; Steele, J.A.; Jacobs, D.A.; Othman, M.; Yu Chin, X.; Moon, S.J.; Tiwari, A.N.; Hessler-Wyser, A.; et al. A Universal Perovskite/C60 Interface Modification via Atomic Layer Deposited Aluminum Oxide for Perovskite Solar Cells and Perovskite–Silicon Tandems. Adv. Mater. 2024, 36, 2311745. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, Y.; Hu, J.; Li, S.; Luo, D.; Su, R.; Caprioglio, P.; Kaienburg, P.; Jia, X.; Chen, N.; et al. Multifunctional Ytterbium Oxide Buffer for Perovskite Solar Cells. Nature 2024, 625, 516–522. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Yun, H.S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S. Il Controlled Growth of Perovskite Layers with Volatile Alkylammonium Chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Qin, M.; Ren, Z.; Liu, K.; Huang, J.; Shen, D.; Wu, Z.; Zhang, Y.; Hao, J.; et al. Multifunctional Crosslinking-Enabled Strain-Regulating Crystallization for Stable, Efficient α-FAPbI3-Based Perovskite Solar Cells. Adv. Mater. 2021, 33, 2008487. [Google Scholar] [CrossRef]
- Sahayaraj, S.; Starowicz, Z.; Ziółek, M.; Socha, R.; Major, Ł.; Góral, A.; Gawlińska-Nęcek, K.; Palewicz, M.; Sikora, A.; Piasecki, T.; et al. Synergistic Effect of Precursor and Interface Engineering Enables High Efficiencies in FAPbI3 Perovskite Solar Cells. Materials 2023, 16, 5352. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.J.; Shi, T.; Yan, Y. Unusual Defect Physics in CH3NH3PbI3 Perovskite Solar Cell Absorber. Appl. Phys. Lett. 2014, 104, 063903. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-Containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Xu, Y.; Wang, M.; Zhu, G.; Jin, Z.; Lei, Y.; Xu, Y.; Wang, M.; Jin, Z.; Zhu, G. Origin, Influence, and Countermeasures of Defects in Perovskite Solar Cells. Small 2021, 17, 2005495. [Google Scholar] [CrossRef]
- DeQuilettes, D.W.; Vorpahl, S.M.; Stranks, S.D.; Nagaoka, H.; Eperon, G.E.; Ziffer, M.E.; Snaith, H.J.; Ginger, D.S. Impact of Microstructure on Local Carrier Lifetime in Perovskite Solar Cells. Science 2015, 348, 683–686. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Lim, S.S.; Yantara, N.; Liu, X.; Sabba, D.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Low-Temperature Solution-Processed Wavelength-Tunable Perovskites for Lasing. Nat. Mater. 2014, 13, 476–480. [Google Scholar] [CrossRef]
- Wu, B.; Nguyen, H.T.; Ku, Z.; Han, G.; Giovanni, D.; Mathews, N.; Fan, H.J.; Sum, T.C. Discerning the Surface and Bulk Recombination Kinetics of Organic–Inorganic Halide Perovskite Single Crystals. Adv. Energy Mater. 2016, 6, 1600551. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, J.; Wang, X.; Liu, Y.; Cai, Q.; Tan, L.; Chen, Y. Inhibition of Ion Migration for Highly Efficient and Stable Perovskite Solar Cells. Adv. Mater. 2023, 35, 2302552. [Google Scholar] [CrossRef]
- Sun, Q.; Fassl, P.; Becker-Koch, D.; Bausch, A.; Rivkin, B.; Bai, S.; Hopkinson, P.E.; Snaith, H.J.; Vaynzof, Y.; Sun, Q.; et al. Role of Microstructure in Oxygen Induced Photodegradation of Methylammonium Lead Triiodide Perovskite Films. Adv. Energy Mater. 2017, 7, 1700977. [Google Scholar] [CrossRef]
- Tian, F.; Feng, W.; Xing, B.; He, X.; Saidi, W.A.; Zhang, L. Grain Boundaries in Methylammonium Lead Halide Perovskites Facilitate Water Diffusion. Adv. Energy Sustain. Res. 2021, 2, 2100087. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, X.; Xie, D.; Tian, Y. Mechanisms of Oxygen Passivation on Surface Defects in MAPbI3 Revealed by First-Principles Study. J. Phys. Chem. C 2020, 124, 3731–3737. [Google Scholar] [CrossRef]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Saiful Islam, M.; Haque, S.A. Fast Oxygen Diffusion and Iodide Defects Mediate Oxygen-Induced Degradation of Perovskite Solar Cells. Nat. Commun. 2017, 8, 15218. [Google Scholar] [CrossRef]
- Aristidou, N.; Sanchez-Molina, I.; Chotchuangchutchaval, T.; Brown, M.; Martinez, L.; Rath, T.; Haque, S.A. The Role of Oxygen in the Degradation of Methylammonium Lead Trihalide Perovskite Photoactive Layers. Angew. Chem. Int. Ed. Engl. 2015, 54, 8208–8212. [Google Scholar] [CrossRef]
- Chen, T.; Xie, J.; Wen, B.; Yin, Q.; Lin, R.; Zhu, S.; Gao, P. Inhibition of Defect-Induced α-to-δ Phase Transition for Efficient and Stable Formamidinium Perovskite Solar Cells. Nat. Commun. 2023, 14, 6125. [Google Scholar] [CrossRef]
- Park, B.W.; Kedem, N.; Kulbak, M.; Lee, D.Y.; Yang, W.S.; Jeon, N.J.; Seo, J.; Kim, G.; Kim, K.J.; Shin, T.J.; et al. Understanding How Excess Lead Iodide Precursor Improves Halide Perovskite Solar Cell Performance. Nat. Commun. 2018, 9, 3301. [Google Scholar] [CrossRef]
- Holovský, J.; Peter Amalathas, A.; Landová, L.; Dzurňák, B.; Conrad, B.; Ledinský, M.; Hájková, Z.; Pop-Georgievski, O.; Svoboda, J.; Yang, T.C.J.; et al. Lead Halide Residue as a Source of Light-Induced Reversible Defects in Hybrid Perovskite Layers and Solar Cells. ACS Energy Lett. 2019, 4, 3011–3017. [Google Scholar] [CrossRef]
- Tumen-Ulzii, G.; Qin, C.; Klotz, D.; Leyden, M.R.; Wang, P.; Auffray, M.; Fujihara, T.; Matsushima, T.; Lee, J.-W.; Lee, S.-J.; et al. Detrimental Effect of Unreacted PbI2 on the Long-Term Stability of Perovskite Solar Cells. Adv. Mater. 2020, 32, 1905035. [Google Scholar] [CrossRef]
- Son, D.Y.; Lee, J.W.; Choi, Y.J.; Jang, I.H.; Lee, S.; Yoo, P.J.; Shin, H.; Ahn, N.; Choi, M.; Kim, D.; et al. Self-Formed Grain Boundary Healing Layer for Highly Efficient CH3NH3PbI3 Perovskite Solar Cells. Nat. Energy 2016, 1, 16081. [Google Scholar] [CrossRef]
- Zhao, W.; Yao, Z.; Yu, F.; Yang, D.; Liu, S.F. Alkali Metal Doping for Improved CH3NH3PbI3 Perovskite Solar Cells. Adv. Sci. 2017, 5, 1700131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yavuz, I.; Wang, M.; Weber, M.H.; Xu, M.; Lee, J.H.; Tan, S.; Huang, T.; Meng, D.; Wang, R.; et al. Suppressing Ion Migration in Metal Halide Perovskite via Interstitial Doping with a Trace Amount of Multivalent Cations. Nat. Mater. 2022, 21, 1396–1402. [Google Scholar] [CrossRef]
- Wang, J.; Uddin, M.A.; Chen, B.; Ying, X.; Ni, Z.; Zhou, Y.; Li, M.; Wang, M.; Yu, Z.; Huang, J. Enhancing Photostability of Sn-Pb Perovskite Solar Cells by an Alkylammonium Pseudo-Halogen Additive. Adv. Energy Mater. 2023, 13, 2204115. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Lu, M.; Shi, Z.; Yu, W.W.; Hu, J.; Bai, X.; Zhang, Y. Ionic Additive Engineering for Stable Planar Perovskite Solar Cells with Efficiency > 22%. Chem. Eng. J. 2021, 426, 130841. [Google Scholar] [CrossRef]
- Ding, B.; Ding, Y.; Peng, J.; Romano-deGea, J.; Frederiksen, L.E.K.; Kanda, H.; Syzgantseva, O.A.; Syzgantseva, M.A.; Audinot, J.N.; Bour, J.; et al. Dopant-Additive Synergism Enhances Perovskite Solar Modules. Nature 2024, 628, 299–305. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, K.; Han, Y.; Xia, H.; Ren, Z.; Li, D.; Zhu, T.; Cheng, L.; Wang, Z.; Zhu, C.; et al. Highly Stable Perovskite Solar Cells with 0.30 Voltage Deficit Enabled by a Multi-Functional Asynchronous Cross-Linking. Nat. Commun. 2025, 16, 190. [Google Scholar] [CrossRef]
- Liu, L.Y.; Tay, D.J.J.; Furuhashi, T.; Xue, K.; Li, Y.; Xiao, X.; Abuzeid, H.R.; Paul Erinjeri, J.; Sum, T.C.; Mathews, N. Enhancing Perovskite Solar Cell Stability and Performance via Bulk Passivation with Sulfonium-Based Passivators. ChemSusChem 2025, 18, e202500931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, W.; Chen, X.; Chen, Y.; Li, X.; Wang, M.; Zhou, Y.; Yan, H.; Zheng, Z.; Zhang, Y. Dual Optimization of Bulk and Surface via Guanidine Halide for Efficient and Stable 2D/3D Hybrid Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2201105. [Google Scholar] [CrossRef]
- Wang, J.; Bi, L.; Huang, X.; Feng, Q.; Liu, M.; Chen, M.; An, Y.; Jiang, W.; Lin, F.R.; Fu, Q.; et al. Bilayer Interface Engineering through 2D/3D Perovskite and Surface Dipole for Inverted Perovskite Solar Modules. eScience 2024, 4, 100308. [Google Scholar] [CrossRef]
- Azmi, R.; Ugur, E.; Seitkhan, A.; Aljamaan, F.; Subbiah, A.S.; Liu, J.; Harrison, G.T.; Nugraha, M.I.; Eswaran, M.K.; Babics, M.; et al. Damp Heat-Stable Perovskite Solar Cells with Tailored-Dimensionality 2D/3D Heterojunctions. Science 2022, 376, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, A.A.; Szostak, R.; Drigo, N.; Queloz, V.I.E.; Marchezi, P.E.; Germino, J.C.; Tolentino, H.C.N.; Nazeeruddin, M.K.; Nogueira, A.F.; Grancini, G. In Situ Analysis Reveals the Role of 2D Perovskite in Preventing Thermal-Induced Degradation in 2D/3D Perovskite Interfaces. Nano Lett. 2020, 20, 3992–3998. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Chen, H.; Spanopoulos, I.; Bati, A.S.R.; Gilley, I.W.; Chen, J.; Maxwell, A.; Vishal, B.; Reynolds, R.P.; et al. Two-Dimensional Perovskitoids Enhance Stability in Perovskite Solar Cells. Nature 2024, 633, 359–364. [Google Scholar] [CrossRef]
- Lou, Q.; Lou, G.; Guo, H.; Sun, T.; Wang, C.; Chai, G.; Chen, X.; Yang, G.; Guo, Y.; Zhou, H. Enhanced Efficiency and Stability of N-i-p Perovskite Solar Cells by Incorporation of Fluorinated Graphene in the Spiro-OMeTAD Hole Transport Layer. Adv. Energy Mater. 2022, 12, 2201344. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Liu, T.; Zeng, Q.; Cao, D.; Pan, H.; Xing, G. Interfacial Engineering of PTAA/Perovskites for Improved Crystallinity and Hole Extraction in Inverted Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 3284–3292. [Google Scholar] [CrossRef]
- Reza, K.M.; Gurung, A.; Bahrami, B.; Mabrouk, S.; Elbohy, H.; Pathak, R.; Chen, K.; Chowdhury, A.H.; Rahman, M.T.; Letourneau, S.; et al. Tailored PEDOT:PSS Hole Transport Layer for Higher Performance in Perovskite Solar Cells: Enhancement of Electrical and Optical Properties with Improved Morphology. J. Energy Chem. 2020, 44, 41–50. [Google Scholar] [CrossRef]
- De Rossi, F.; Renno, G.; Taheri, B.; Yaghoobi Nia, N.; Ilieva, V.; Fin, A.; Di Carlo, A.; Bonomo, M.; Barolo, C.; Brunetti, F. Modified P3HT Materials as Hole Transport Layers for Flexible Perovskite Solar Cells. J. Power Sources 2021, 494, 229735. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Z.; Zhang, Y.; Chen, M.; Liu, T.; Xiao, C.; Gao, D.; Patel, J.B.; Kuciauskas, D.; Magomedov, A.; et al. Co-Deposition of Hole-Selective Contact and Absorber for Improving the Processability of Perovskite Solar Cells. Nat. Energy 2023, 8, 462–472. [Google Scholar] [CrossRef]
- Arjun, V.; Muthukumaran, K.P.; Ramachandran, K.; Nithya, A.; Karuppuchamy, S. Fabrication of Efficient and Stable Planar Perovskite Solar Cell Using Copper Oxide as Hole Transport Material. J. Alloys Compd. 2022, 923, 166285. [Google Scholar] [CrossRef]
- Liang, J.W.; Firdaus, Y.; Azmi, R.; Faber, H.; Kaltsas, D.; Kang, C.H.; Nugraha, M.I.; Yengel, E.; Ng, T.K.; De Wolf, S.; et al. Cl2-Doped CuSCN Hole Transport Layer for Organic and Perovskite Solar Cells with Improved Stability. ACS Energy Lett. 2022, 7, 3139–3148. [Google Scholar] [CrossRef]
- Fahsyar, P.N.A.; Ludin, N.A.; Ramli, N.F.; Sepeai, S.; Suait, M.S.; Ibrahim, M.A.; Teridi, M.A.; Sopian, K. Correlation of Simulation and Experiment for Perovskite Solar Cells with MoS2 Hybrid-HTL Structure. Appl. Phys. A Mater. Sci. Process 2021, 127, 383. [Google Scholar] [CrossRef]
- Said, A.A.; Aydin, E.; Ugur, E.; Xu, Z.; Deger, C.; Vishal, B.; Vlk, A.; Dally, P.; Yildirim, B.K.; Azmi, R.; et al. Sublimed C60 for Efficient and Repeatable Perovskite-Based Solar Cells. Nat. Commun. 2024, 15, 708. [Google Scholar] [CrossRef]
- Zhong, Y.; Hufnagel, M.; Thelakkat, M.; Li, C.; Huettner, S.; Zhong, Y.; Li, C.; Huettner, S.; Hufnagel, M.; Thelakkat, M. Role of PCBM in the Suppression of Hysteresis in Perovskite Solar Cells. Adv. Funct. Mater. 2020, 30, 1908920. [Google Scholar] [CrossRef]
- Lee, M.; Kim, D.; Lee, Y.K.; Koo, H.; Lee, K.T.; Chung, I. Indene-C60Bisadduct Electron-Transporting Material with the High LUMO Level Enhances Open-Circuit Voltage and Efficiency of Tin-Based Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 5581–5588. [Google Scholar] [CrossRef]
- Ran, X.; Ali, M.A.; Hu, Z.; Li, P.; Xia, Y.; Zhang, H.; Yang, L.; Chen, Y. State-of-the-Art Techniques on Asymmetrical Perylene Diimide Derivatives: Efficient Electron-Transport Materials for Perovskite Solar Cells. J. Phys. Chem. C 2023, 127, 5114–5124. [Google Scholar] [CrossRef]
- Jameel, M.A.; Yang, T.C.J.; Wilson, G.J.; Evans, R.A.; Gupta, A.; Langford, S.J. Naphthalene Diimide-Based Electron Transport Materials for Perovskite Solar Cells. J. Mater. Chem. A Mater. 2021, 9, 27170–27192. [Google Scholar] [CrossRef]
- Fatima, Q.; Haidry, A.A.; Zhang, H.; El Jery, A.; Aldrdery, M. A Critical Review on Advancement and Challenges in Using TiO2 as Electron Transport Layer for Perovskite Solar Cell. Mater. Today Sustain. 2024, 27, 100857. [Google Scholar] [CrossRef]
- Altinkaya, C.; Aydin, E.; Ugur, E.; Isikgor, F.H.; Subbiah, A.S.; De Bastiani, M.; Liu, J.; Babayigit, A.; Allen, T.G.; Laquai, F.; et al. Tin Oxide Electron-Selective Layers for Efficient, Stable, and Scalable Perovskite Solar Cells. Adv. Mater. 2021, 33, 2005504. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, G.; Huang, W.; Wang, B.; Ke, W.; Leigh Logsdon, J.; Wang, H.; Wang, Z.; Zhu, W.; Yu, J.; et al. Combustion Synthesized Zinc Oxide Electron-Transport Layers for Efficient and Stable Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1900265. [Google Scholar] [CrossRef]
- Gu, B.; Du, Y.; Fang, S.; Chen, X.; Li, X.; Xu, Q.; Lu, H. Fabrication of UV-Stable Perovskite Solar Cells with Compact Fe2O3 Electron Transport Layer by FeCl3 Solution and Fe3O4 Nanoparticles. Nanomaterials 2022, 12, 4415. [Google Scholar] [CrossRef]
- Mahjabin, S.; Haque, M.M.; Bashar, M.S.; Shahiduzzaman, M.; Hossain, M.I.; Gantumur, M.; Jamal, M.S.; Abdur, R.; Shakel, M.S.; Muhammad, G.; et al. Boosting Perovskite Solar Cell Stability through a Sputtered Mo-Doped Tungsten Oxide (WOx) Electron Transport Layer. Energy Fuels 2023, 37, 19860–19869. [Google Scholar] [CrossRef]
- Al-Mousoi, A.K.; Mohammed, M.K.A.; Pandey, R.; Madan, J.; Dastan, D.; Ravi, G.; Sakthivel, P.; Anandha Babu, G. Simulation and Analysis of Lead-Free Perovskite Solar Cells Incorporating Cerium Oxide as Electron Transporting Layer. RSC Adv. 2022, 12, 32365–32373. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Leijtens, T.; Pathak, S.; Teuscher, J.; Avolio, R.; Errico, M.E.; Kirkpatrik, J.; Ball, J.M.; Docampo, P.; Mcpherson, I.; et al. Lithium Salts as “Redox Active” p-Type Dopants for Organic Semiconductors and Their Impact in Solid-State Dye-Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2013, 15, 2572. [Google Scholar] [CrossRef]
- Tan, B.; Raga, S.R.; Chesman, A.S.R.; Fürer, S.O.; Zheng, F.; McMeekin, D.P.; Jiang, L.; Mao, W.; Lin, X.; Wen, X.; et al. LiTFSI-Free Spiro-OMeTAD-Based Perovskite Solar Cells with Power Conversion Efficiencies Exceeding 19%. Adv. Energy Mater. 2019, 9, 1901519. [Google Scholar] [CrossRef]
- Kim, S.G.; Le, T.H.; de Monfreid, T.; Goubard, F.; Bui, T.T.; Park, N.G. Capturing Mobile Lithium Ions in a Molecular Hole Transporter Enhances the Thermal Stability of Perovskite Solar Cells. Adv. Mater. 2021, 33, 2007431. [Google Scholar] [CrossRef]
- Bastos, J.P.; Paetzold, U.W.; Gehlhaar, R.; Qiu, W.; Cheyns, D.; Surana, S.; Spampinato, V.; Aernouts, T.; Poortmans, J. Light-Induced Degradation of Perovskite Solar Cells: The Influence of 4-Tert-Butyl Pyridine and Gold. Adv. Energy Mater. 2018, 8, 1800554. [Google Scholar] [CrossRef]
- Rombach, F.M.; Haque, S.A.; Macdonald, T.J. Lessons Learned from Spiro-OMeTAD and PTAA in Perovskite Solar Cells. Energy Environ. Sci. 2021, 14, 5161–5190. [Google Scholar] [CrossRef]
- Neophytou, M.; Griffiths, J.; Fraser, J.; Kirkus, M.; Chen, H.; Nielsen, C.B.; McCulloch, I. High Mobility, Hole Transport Materials for Highly Efficient PEDOT:PSS Replacement in Inverted Perovskite Solar Cells. J. Mater. Chem. C Mater. 2017, 5, 4940–4945. [Google Scholar] [CrossRef]
- Labban, A.E.; Chen, H.; Kirkus, M.; Barbe, J.; Del Gobbo, S.; Neophytou, M.; McCulloch, I.; Eid, J. Improved Efficiency in Inverted Perovskite Solar Cells Employing a Novel Diarylamino-Substituted Molecule as PEDOT:PSS Replacement. Adv. Energy Mater. 2016, 6, 1502101. [Google Scholar] [CrossRef]
- Boyd, C.C.; Shallcross, R.C.; Moot, T.; Kerner, R.; Bertoluzzi, L.; Onno, A.; Kavadiya, S.; Chosy, C.; Wolf, E.J.; Werner, J.; et al. Overcoming Redox Reactions at Perovskite-Nickel Oxide Interfaces to Boost Voltages in Perovskite Solar Cells. Joule 2020, 4, 1759–1775. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, M.; Liu, X.; Cheung, S.H.; Chandran, H.T.; Li, H.W.; Xu, X.; Xie, Y.M.; So, S.K.; Yip, H.L.; et al. Impact of Surface Dipole in NiOx on the Crystallization and Photovoltaic Performance of Organometal Halide Perovskite Solar Cells. Nano Energy 2019, 61, 496–504. [Google Scholar] [CrossRef]
- Ito, S.; Tanaka, S.; Manabe, K.; Nishino, H. Effects of Surface Blocking Layer of Sb2S3 on Nanocrystalline TiO2 for CH3NH3PbI3 Perovskite Solar Cells. J. Phys. Chem. C 2014, 118, 16995–17000. [Google Scholar] [CrossRef]
- Yu, H.; Yeom, H.-I.; Woo Lee, J.; Lee, K.; Hwang, D.; Yun, J.; Ryu, J.; Lee, J.; Bae, S.; Keun Kim, S.; et al. Superfast Room-Temperature Activation of SnO2 Thin Films via Atmospheric Plasma Oxidation and Their Application in Planar Perovskite Photovoltaics. Adv. Mater. 2018, 30, 1704825. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Dong, W.; Cai, H.; Zu, C.; Yue, W.; Li, H.; Zhao, J.; Huang, F.; Cheng, Y.B.; Zhong, J. Electrochemical Reduction and Ion Injection of Annealing-Free SnO2 for High Performance Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2300491. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Liu, S.; Chu, Y.; Ye, T.; Qiu, C.; Qiu, Z.; Wang, X.; Wang, Y.; Su, Y.; et al. Oxygen Vacancy Management for High-Temperature Mesoporous SnO2 Electron Transport Layers in Printable Perovskite Solar Cells. Angew. Chem. 2022, 134, e202202012. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Frolova, L.A.; Griffin, M.P.; Gearba, I.R.; Dolocan, A.; Vanden Bout, D.A.; Tsarev, S.; Katz, E.A.; Shestakov, A.F.; Stevenson, K.J.; et al. Effect of Electron-Transport Material on Light-Induced Degradation of Inverted Planar Junction Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1700476. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, B.; Shi, L.; Zhou, S.; Xu, J.; Liu, Z.; Yun, J.S.; Choi, E.; Zhang, M.; Lv, Y.; et al. Perovskite Solar Cells Based on Spiro-OMeTAD Stabilized with an Alkylthiol Additive. Nat. Photonics 2022, 17, 96–105. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Yang, Y.; Liu, X.; Guo, Q.; Song, Z.; Li, G.; Lan, Z.; Huang, M. High Performance and Stable Perovskite Solar Cells Using Vanadic Oxide as a Dopant for Spiro-OMeTAD. J. Mater. Chem. A Mater. 2019, 7, 13256–13264. [Google Scholar] [CrossRef]
- Ma, S.; Pang, S.; Dong, H.; Xie, X.; Liu, G.; Dong, P.; Liu, D.; Zhu, W.; Xi, H.; Chen, D.; et al. Stability Improvement of Perovskite Solar Cells by the Moisture-Resistant PMMA:Spiro-OMeTAD Hole Transport Layer. Polymers 2022, 14, 343. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Choi, I.W.; Go, E.M.; Cho, Y.; Kim, M.; Lee, B.; Jeong, S.; Jo, Y.; Choi, H.W.; Lee, J.; et al. Stable Perovskite Solar Cells with Efficiency Exceeding 24.8% and 0.3-V Voltage Loss. Science 2020, 369, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, T.; Chen, W.; Wu, Y.; Guo, X.; Shen, Y.; Ding, C.; Chen, X.; Chen, H.; Ding, J.; et al. Iodonium Initiators: Paving the Air-Free Oxidation of Spiro-OMeTAD for Efficient and Stable Perovskite Solar Cells. Angew. Chem. Int. Ed. 2024, 63, e202316183. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Fu, Q.; Sun, L.; Liu, Y.; Sun, Z.; Xue, S.; Liu, Y.; Liang, M. Conjugation Engineering of Spiro-Based Hole Transport Materials for Efficient and Stable Perovskite Solar Cells. ACS Energy Lett. 2022, 7, 2667–2676. [Google Scholar] [CrossRef]
- Liu, X.; Wang, K.; Li, Y.; You, S.; Liu, T.; Lv, Y.; Li, Y.; Wang, Y.; He, H.; Li, Y.; et al. Mesoscale Ordering 3D Mosaic Self-Assembly of Dopant-Free Hole Transport Material for Perovskite Solar Cells. ACS Energy Lett. 2024, 9, 2446–2455. [Google Scholar] [CrossRef]
- Mahmoudi, T.; Wang, Y.; Hahn, Y.B. Highly Stable Perovskite Solar Cells Based on Perovskite/NiO-Graphene Composites and NiO Interface with 25.9 MA/Cm2 Photocurrent Density and 20.8% Efficiency. Nano Energy 2021, 79, 105452. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y. Fixed Charge Passivation in Perovskite Solar Cells. Nat. Energy 2024, 9, 16–17. [Google Scholar] [CrossRef]
- Jiang, W.; Qu, G.; Huang, X.; Chen, X.; Chi, L.; Wang, T.; Wong, C.T.; Lin, F.R.; Yang, C.; Jiang, Q.; et al. Toughened Self-Assembled Monolayers for Durable Perovskite Solar Cells. Nature 2025, 646, 95–101. [Google Scholar] [CrossRef]
- Sun, Y.; Fang, X.; Ma, Z.; Xu, L.; Lu, Y.; Yu, Q.; Yuan, N.; Ding, J. Enhanced UV-Light Stability of Organometal Halide Perovskite Solar Cells with Interface Modification and a UV Absorption Layer. J. Mater. Chem. C Mater. 2017, 5, 8682–8687. [Google Scholar] [CrossRef]
- Chen, C.; Li, H.; Jin, J.; Chen, X.; Cheng, Y.; Zheng, Y.; Liu, D.; Xu, L.; Song, H.; Dai, Q. Long-Lasting Nanophosphors Applied to UV-Resistant and Energy Storage Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1700758. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, S.; Zhu, X.; Li, S.; Zheng, Z.; Zhao, K.; Ji, L.; Li, R.; Liu, Y.; Liu, C.; et al. Inverted Perovskite Solar Cells with over 2,000 h Operational Stability at 85 °C Using Fixed Charge Passivation. Nat. Energy 2024, 9, 37–46. [Google Scholar] [CrossRef]
- Chen, T.; Xie, J.; Gao, P. Ultraviolet Photocatalytic Degradation of Perovskite Solar Cells: Progress, Challenges, and Strategies. Adv. Energy Sustain. Res. 2022, 3, 2100218. [Google Scholar] [CrossRef]
- Su, H.; Xu, Z.; He, X.; Yao, Y.; Zheng, X.; She, Y.; Zhu, Y.; Zhang, J.; Liu, S. Surface Energy Engineering of Buried Interface for Highly Stable Perovskite Solar Cells with Efficiency Over 25%. Adv. Mater. 2024, 36, 2306724. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, H.; Wang, M.; Lan, Z.; Cui, P.; Du, S.; Yang, Y.; Yan, L.; Zhang, Q.; Qu, S.; et al. Oriented Molecular Bridge Constructs Homogeneous Buried Interface for Perovskite Solar Cells with Efficiency Over 25.3%. Adv. Mater. 2024, 36, 2310710. [Google Scholar] [CrossRef]
- Qiu, J.; Mei, X.; Zhang, M.; Wang, G.; Zou, S.; Wen, L.; Huang, J.; Hua, Y.; Zhang, X. Dipolar Chemical Bridge Induced CsPbI3 Perovskite Solar Cells with 21.86% Efficiency. Angew. Chem. Int. Ed. 2024, 63, e202401751. [Google Scholar] [CrossRef]
- Koo, D.; Choi, Y.; Kim, U.; Kim, J.; Seo, J.; Son, E.; Min, H.; Kang, J.; Park, H. Mesoporous Structured MoS2 as an Electron Transport Layer for Efficient and Stable Perovskite Solar Cells. Nat. Nanotechnol. 2024, 20, 75–82. [Google Scholar] [CrossRef]
- Zhao, M.; Gu, W.-M.; Jiang, K.-J.; Jiao, X.; Gong, K.; Li, F.; Zhou, X.; Song, Y. 2,2′-Bipyridyl-4,4′-Dicarboxylic Acid Modified Buried Interface of High-Performance Perovskite Solar Cells. Angew. Chem. 2025, 137, e202418176. [Google Scholar] [CrossRef]
- Panyathip, R.; Sucharitakul, S.; Hongsith, K.; Bumrungsan, W.; Yarangsi, V.; Phaduangdhitidhada, S.; Chanlek, N.; Choopun, S. Surface Modification of SnO2 Electron Transporting Layer by Graphene Quantum Dots for Performance and Stability Improvement of Perovskite Solar Cells. Ceram. Int. 2024, 50, 34840–34848. [Google Scholar] [CrossRef]
- Príncipe, J.; Duarte, V.C.M.; Mendes, A.; Andrade, L. Influence of the Transparent Conductive Oxide Type on the Performance of Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2023, 6, 12442–12451. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.K.; Xue, J.; Yang, Y. A Review of Perovskites Solar Cell Stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
- Domanski, K.; Correa-Baena, J.P.; Mine, N.; Nazeeruddin, M.K.; Abate, A.; Saliba, M.; Tress, W.; Hagfeldt, A.; Grätzel, M. Not All That Glitters Is Gold: Metal-Migration-Induced Degradation in Perovskite Solar Cells. ACS Nano 2016, 10, 6306–6314. [Google Scholar] [CrossRef] [PubMed]
- Shlenskaya, N.N.; Belich, N.A.; Grätzel, M.; Goodilin, E.A.; Tarasov, A.B. Light-Induced Reactivity of Gold and Hybrid Perovskite as a New Possible Degradation Mechanism in Perovskite Solar Cells. J. Mater. Chem. A Mater. 2018, 6, 1780–1786. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Bush, K.A.; Prasanna, R.; Leijtens, T.; McGehee, M.D. Barrier Design to Prevent Metal-Induced Degradation and Improve Thermal Stability in Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 1772–1778. [Google Scholar] [CrossRef]
- Kato, Y.; Ono, L.K.; Lee, M.V.; Wang, S.; Raga, S.R.; Qi, Y. Silver Iodide Formation in Methyl Ammonium Lead Iodide Perovskite Solar Cells with Silver Top Electrodes. Adv. Mater. Interfaces 2015, 2, 1500195. [Google Scholar] [CrossRef]
- Li, J.; Dong, Q.; Li, N.; Wang, L. Direct Evidence of Ion Diffusion for the Silver-Electrode-Induced Thermal Degradation of Inverted Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1602922. [Google Scholar] [CrossRef]
- Svanström, S.; García-Fernández, A.; Jacobsson, T.J.; Bidermane, I.; Leitner, T.; Sloboda, T.; Man, G.J.; Boschloo, G.; Johansson, E.M.J.; Rensmo, H.; et al. The Complex Degradation Mechanism of Copper Electrodes on Lead Halide Perovskites. ACS Mater. Au 2022, 2, 301–312. [Google Scholar] [CrossRef]
- Sirotinskaya, S.; Schmechel, R.; Benson, N. Influence of the Cathode Microstructure on the Stability of Inverted Planar Perovskite Solar Cells. RSC Adv. 2020, 10, 23653–23661. [Google Scholar] [CrossRef]
- Wu, S.; Chen, R.; Zhang, S.; Babu, B.H.; Yue, Y.; Zhu, H.; Yang, Z.; Chen, C.; Chen, W.; Huang, Y.; et al. A Chemically Inert Bismuth Interlayer Enhances Long-Term Stability of Inverted Perovskite Solar Cells. Nat. Commun. 2019, 10, 1161. [Google Scholar] [CrossRef]
- Lin, X.; Su, H.; He, S.; Song, Y.; Wang, Y.; Qin, Z.; Wu, Y.; Yang, X.; Han, Q.; Fang, J.; et al. In Situ Growth of Graphene on Both Sides of a Cu–Ni Alloy Electrode for Perovskite Solar Cells with Improved Stability. Nat. Energy 2022, 7, 520–527. [Google Scholar] [CrossRef]
- Li, X.; Fu, S.; Zhang, W.; Ke, S.; Song, W.; Fang, J. Chemical Anti-Corrosion Strategy for Stable Inverted Perovskite Solar Cells. Sci. Adv. 2020, 6, eabd1580. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Li, X.; Zhang, W.; Yang, H.; Guo, X.; Lu, C.; Yuan, H.; Ou-Yang, W.; Fang, J. Ag Electrode Anticorrosion in Inverted Perovskite Solar Cells. Adv. Funct. Mater. 2024, 34, 2307310. [Google Scholar] [CrossRef]
- Liu, N.; Xiong, J.; He, Z.; Yuan, C.; Dai, J.; Zhang, Y.; Zhou, C.; Zhang, X.; Li, L.; Wang, D.; et al. Multifunctional Anti-Corrosive Interface Modification for Inverted Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2300025. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Zhu, R.; Chen, X.; Wang, T.; Pu, X.; Chen, H.; Cao, Q.; Li, X. Enhanced Corrosion Resistance of Ag Electrode Through Ionized 2-Mercaptobenzothiazole in Inverted Perovskite Solar Cells. Adv. Funct. Mater. 2025, 35, 2413245. [Google Scholar] [CrossRef]
- Yang, J.; Cao, Q.; Wang, T.; Yang, B.; Pu, X.; Zhang, Y.; Chen, H.; Tojiboyev, I.; Li, Y.; Etgar, L.; et al. Inhibiting Metal-Inward Diffusion-Induced Degradation through Strong Chemical Coordination toward Stable and Efficient Inverted Perovskite Solar Cells. Energy Environ. Sci. 2022, 15, 2154–2163. [Google Scholar] [CrossRef]
- Gong, C.; Li, H.; Wang, H.; Zhang, C.; Zhuang, Q.; Wang, A.; Xu, Z.; Cai, W.; Li, R.; Li, X.; et al. Silver Coordination-Induced n-Doping of PCBM for Stable and Efficient Inverted Perovskite Solar Cells. Nat. Commun. 2024, 15, 4922. [Google Scholar] [CrossRef]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y.; et al. A Hole-Conductor-Free, Fully Printable Mesoscopic Perovskite Solar Cell with High Stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, Y.; Dong, Q.; Zhang, H.; Xing, Y.; Wang, K.; Du, Y.; Ma, T. Hole-Conductor-Free, Metal-Electrode-Free TiO2/CH3NH3PbI3 Heterojunction Solar Cells Based on a Low-Temperature Carbon Electrode. J. Phys. Chem. Lett. 2014, 5, 3241–3246. [Google Scholar] [CrossRef]
- Mei, A.; Sheng, Y.; Ming, Y.; Hu, Y.; Rong, Y.; Zhang, W.; Luo, S.; Na, G.; Tian, C.; Hou, X.; et al. Stabilizing Perovskite Solar Cells to IEC61215:2016 Standards with over 9,000-h Operational Tracking. Joule 2020, 4, 2646–2660. [Google Scholar] [CrossRef]
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M.; et al. One-Year Stable Perovskite Solar Cells by 2D/3D Interface Engineering. Nat. Commun. 2017, 8, 15684. [Google Scholar] [CrossRef]
- Zouhair, S.; Clegg, C.; Valitova, I.; March, S.; Jailani, J.M.; Pecunia, V. Carbon Electrodes for Perovskite Photovoltaics: Interfacial Properties, Meta-Analysis, and Prospects. Sol. RRL 2024, 8, 2300929. [Google Scholar] [CrossRef]
- Don, M.F.; Ekanayake, P.; Jennings, J.R.; Nakajima, H.; Kumar, D.U.; Lim, C.M. Influence of Metal Salts (Al, Ca, and Mg) on the Work Function and Hole Extraction at Carbon Counter Electrodes in Perovskite Solar Cells. Heliyon 2023, 9, e17748. [Google Scholar] [CrossRef]
- Bryant, D.; Aristidou, N.; Pont, S.; Sanchez-Molina, I.; Chotchunangatchaval, T.; Wheeler, S.; Durrant Ab, J.R.; Haque, S.A. Light and Oxygen Induced Degradation Limits the Operational Stability of Methylammonium Lead Triiodide Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 1655. [Google Scholar] [CrossRef]
- Akman, E.; Akin, S.; Akman, E.; Akin, S. Poly(N,N′-Bis-4-Butylphenyl-N,N′-Bisphenyl)Benzidine-Based Interfacial Passivation Strategy Promoting Efficiency and Operational Stability of Perovskite Solar Cells in Regular Architecture. Adv. Mater. 2021, 33, 2006087. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Guo, X.; Wang, L. Review of Recent Progress in Chemical Stability of Perovskite Solar Cells. J. Mater. Chem. A Mater. 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; Van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible Hydration of CH3NH3PbI3 in Films, Single Crystals, and Solar Cells. Chem. Mater. 2015, 27, 3397–3407. [Google Scholar] [CrossRef]
- Song, Z.; Abate, A.; Watthage, S.C.; Liyanage, G.K.; Phillips, A.B.; Steiner, U.; Graetzel, M.; Heben, M.J. Perovskite Solar Cell Stability in Humid Air: Partially Reversible Phase Transitions in the PbI2-CH3NH3I-H2O System. Adv. Energy Mater. 2016, 6, 1600846. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Liu, Y.; Deng, Y.; Bai, Y.; Dong, Q.; Huang, J. Scaling Behavior of Moisture-Induced Grain Degradation in Polycrystalline Hybrid Perovskite Thin Films. Energy Environ. Sci. 2017, 10, 516–522. [Google Scholar] [CrossRef]
- Yun, J.S.; Kim, J.; Young, T.; Patterson, R.J.; Kim, D.; Seidel, J.; Lim, S.; Green, M.A.; Huang, S.; Ho-Baillie, A. Humidity-Induced Degradation via Grain Boundaries of HC(NH2)2PbI3 Planar Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1705363. [Google Scholar] [CrossRef]
- Buin, A.; Comin, R.; Xu, J.; Ip, A.H.; Sargent, E.H. Halide-Dependent Electronic Structure of Organolead Perovskite Materials. Chem. Mater. 2015, 27, 4405–4412. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S. Il Chemical Management for Colorful, Efficient, and Stable Inorganic-Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Tai, Q.; You, P.; Sang, H.; Liu, Z.; Hu, C.; Chan, H.L.W.; Yan, F. Efficient and Stable Perovskite Solar Cells Prepared in Ambient Air Irrespective of the Humidity. Nat. Commun. 2016, 7, 11105. [Google Scholar] [CrossRef]
- Tian, C.; Wu, T.; Zhao, Y.; Zhou, X.; Li, B.; Han, X.; Li, K.; Hou, C.; Li, Y.; Wang, H.; et al. Anion-Stabilized Precursor Inks Toward Efficient and Reproducible Air-Processed Perovskite Solar Cells. Adv. Energy Mater. 2024, 14, 2303666. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, Z.; Li, T.; Chen, Y.; Huang, W. Stability of Perovskite Solar Cells: A Prospective on the Substitution of the A Cation and X Anion. Angew. Chem. Int. Ed. 2017, 56, 1190–1212. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, D.H.; Kim, H.S.; Seo, S.W.; Cho, S.M.; Park, N.G. Formamidinium and Cesium Hybridization for Photo- and Moisture-Stable Perovskite Solar Cell. Adv. Energy Mater. 2015, 5, 1501310. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, D.; Wang, Y.; Zhang, T.; Gu, X.; Zhang, P.; Wu, J.; Chen, Z.D.; Zhao, Y.; Li, S. Theoretical Lifetime Extraction and Experimental Demonstration of Stable Cesium-Containing Tri-Cation Perovskite Solar Cells with High Efficiency. Electrochim. Acta 2018, 265, 98–106. [Google Scholar] [CrossRef]
- Azmi, R.; Zhumagali, S.; Bristow, H.; Zhang, S.; Yazmaciyan, A.; Pininti, A.R.; Utomo, D.S.; Subbiah, A.S.; De Wolf, S. Moisture-Resilient Perovskite Solar Cells for Enhanced Stability. Adv. Mater. 2024, 36, 2211317. [Google Scholar] [CrossRef]
- Bi, D.; Gao, P.; Scopelliti, R.; Oveisi, E.; Luo, J.; Grätzel, M.; Hagfeldt, A.; Nazeeruddin, M.K.; Bi, D.; Hagfeldt, A.; et al. High-Performance Perovskite Solar Cells with Enhanced Environmental Stability Based on Amphiphile-Modified CH3NH3PbI3. Adv. Mater. 2016, 28, 2910–2915. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Cai, C.; Hu, X.; Huang, Z.; Duan, X.; Meng, X.; Yuan, Z.; Tan, L.; Chen, Y. High-Performance Perovskite Solar Cells with Excellent Humidity and Thermo-Stability via Fluorinated Perylenediimide. Adv. Energy Mater. 2019, 9, 1900198. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, L.; Sun, T.; Shi, H.; Xu, Y.; Huang, J.; Li, X.; Shen, Y.; Wang, M. Moisture-Resistant Perovskite Solar Cells: The Role of 1,1′-Methylenebispyridinium Dichloride in Enhancing Stability and Performance. J. Mater. Chem. A Mater. 2025, 13, 4167–4175. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, X.; Xu, S.; Liu, G.; Chen, S.; Zhang, X.; Chen, T.; Pan, X. The Multiple Effects of Polyaniline Additive to Improve the Efficiency and Stability of Perovskite Solar Cells. J. Mater. Chem. C Mater. 2019, 7, 4441–4448. [Google Scholar] [CrossRef]
- Choi, Y.W.; Jeon, Y.S.; Lee, D.N.; Park, N.G. Microencapsulation of Grain Boundaries for Moisture-Stable Perovskite Solar Cells. ACS Energy Lett. 2024, 9, 3754–3765. [Google Scholar] [CrossRef]
- Sun, R.; Chen, S.; He, Q.; Yang, P.; Gao, X.; Wu, M.; Wang, J.; Zhong, C.; Zhao, X.; Li, M.; et al. A Stepwise Melting-Polymerizing Molecule for Hydrophobic Grain-Scale Encapsulated Perovskite Solar Cell. Adv. Mater. 2025, 37, 2410395. [Google Scholar] [CrossRef]
- Li, Z.; Jia, C.; Wan, Z.; Cao, J.; Shi, J.; Xue, J.; Liu, X.; Wu, H.; Xiao, C.; Li, C.; et al. Boosting Mechanical Durability under High Humidity by Bioinspired Multisite Polymer for High-Efficiency Flexible Perovskite Solar Cells. Nat. Commun. 2025, 16, 1771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, Q.; Chmiel, F.P.; Sakai, N.; Herz, L.M.; Snaith, H.J. Efficient Ambient-Air-Stable Solar Cells with 2D-3D Heterostructured Butylammonium-Caesium-Formamidinium Lead Halide Perovskites. Nat. Energy 2017, 2, 17135. [Google Scholar] [CrossRef]
- Liu, T.; Guo, J.; Lu, D.; Xu, Z.; Fu, Q.; Zheng, N.; Xie, Z.; Wan, X.; Zhang, X.; Liu, Y.; et al. Spacer Engineering Using Aromatic Formamidinium in 2D/3D Hybrid Perovskites for Highly Efficient Solar Cells. ACS Nano 2021, 15, 7811–7820. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, G.; Su, Y.; Sheng, W.; Gong, L.; Zhang, J.; Tan, L.; Chen, Y. Diammonium Molecular Configuration-Induced Regulation of Crystal Orientation and Carrier Dynamics for Highly Efficient and Stable 2D/3D Perovskite Solar Cells. Angew. Chem. Int. Ed. 2022, 61, e202114588. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, L.; Liu, T.; Tian, B.; Chu, W.; Sun, X.; Nie, R.; Zhang, W.; Zhang, Z.; Zhao, X.; et al. Engineering Spacer Conjugation for Efficient and Stable 2D/3D Perovskite Solar Cells and Modules. Angew. Chem. 2025, 137, e202413303. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, S.; Li, Y.; Zhang, L.; Shen, N.; Zhang, G.; Du, J.; Fu, N.; Xu, B. Direct Surface Passivation of Perovskite Film by 4-Fluorophenethylammonium Iodide toward Stable and Efficient Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 2558–2565. [Google Scholar] [CrossRef]
- Liang, C.; Gu, H.; Xia, Y.; Wang, Z.; Liu, X.; Xia, J.; Zuo, S.; Hu, Y.; Gao, X.; Hui, W.; et al. Two-Dimensional Ruddlesden–Popper Layered Perovskite Solar Cells Based on Phase-Pure Thin Films. Nat. Energy 2020, 6, 38–45. [Google Scholar] [CrossRef]
- Suo, J.; Yang, B.; Mosconi, E.; Bogachuk, D.; Doherty, T.A.S.; Frohna, K.; Kubicki, D.J.; Fu, F.; Kim, Y.J.; Er-Raji, O.; et al. Multifunctional Sulfonium-Based Treatment for Perovskite Solar Cells with Less than 1% Efficiency Loss over 4,500-h Operational Stability Tests. Nat. Energy 2024, 9, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Yang, M.; Han, D.; Yang, L.; Fan, L.; Sui, Y.; Sun, Y.; Liu, X.; Meng, X.; et al. Interface Dipole Induced Field-effect Passivation for Achieving 21.7% Efficiency and Stable Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2008052. [Google Scholar] [CrossRef]
- Loiudice, A.; Saris, S.; Oveisi, E.; Alexander, D.T.L.; Buonsanti, R. CsPbBr3 QD/AlOx Inorganic Nanocomposites with Exceptional Stability in Water, Light, and Heat. Angew. Chem. Int. Ed. 2017, 56, 10696–10701. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tan, Y.; Cao, M.; Hu, H.; Wu, L.; Yu, X.; Wang, L.; Sun, B.; Zhang, Q. Fabricating CsPbX3-Based Type I and Type II Heterostructures by Tuning the Halide Composition of Janus CsPbX3/ZrO2 Nanocrystals. ACS Nano 2019, 13, 5366–5374. [Google Scholar] [CrossRef]
- Choi, E.; Lee, J.W.; Anaya, M.; Mirabelli, A.; Shim, H.; Strzalka, J.; Lim, J.; Yun, S.; Dubajic, M.; Lim, J.; et al. Synergetic Effect of Aluminum Oxide and Organic Halide Salts on Two-Dimensional Perovskite Layer Formation and Stability Enhancement of Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2301717. [Google Scholar] [CrossRef]
- Appelbaum, J.; Maor, T. Dependence of PV Module Temperature on Incident Time-Dependent Solar Spectrum. Appl. Sci. 2020, 10, 914. [Google Scholar] [CrossRef]
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Kim, N.K.; Min, Y.H.; Noh, S.; Cho, E.; Jeong, G.; Joo, M.; Ahn, S.W.; Lee, J.S.; Kim, S.; Ihm, K.; et al. Investigation of Thermally Induced Degradation in CH3NH3PbI3 Perovskite Solar Cells Using In-Situ Synchrotron Radiation Analysis. Sci. Rep. 2017, 7, 4645. [Google Scholar] [CrossRef]
- Dualeh, A.; Gao, P.; Seok, S.I.; Nazeeruddin, M.K.; Grätzel, M. Thermal Behavior of Methylammonium Lead-Trihalide Perovskite Photovoltaic Light Harvesters. Chem. Mater. 2014, 26, 6160–6164. [Google Scholar] [CrossRef]
- Williams, A.E.; Holliman, P.J.; Carnie, M.J.; Davies, M.L.; Worsley, D.A.; Watson, T.M. Perovskite Processing for Photovoltaics: A Spectro-Thermal Evaluation. J. Mater. Chem. A Mater. 2014, 2, 19338–19346. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Qi, Y. Thermal Degradation of Formamidinium Based Lead Halide Perovskites into Sym-Triazine and Hydrogen Cyanide Observed by Coupled Thermogravimetry-Mass Spectrometry Analysis. J. Mater. Chem. A Mater. 2019, 7, 16912–16919. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Frolova, L.A.; Dremova, N.N.; Zhidkov, I.; Martynenko, V.M.; Tsarev, S.A.; Luchkin, S.Y.; Kurmaev, E.Z.; Aldoshin, S.M.; Stevenson, K.J.; et al. Light or Heat: What Is Killing Lead Halide Perovskites under Solar Cell Operation Conditions? J. Phys. Chem. Lett. 2020, 11, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Ava, T.T.; Al Mamun, A.; Marsillac, S.; Namkoong, G. A Review: Thermal Stability of Methylammonium Lead Halide Based Perovskite Solar Cells. Appl. Sci. 2019, 9, 188. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional Engineering of Perovskite Materials for High-Performance Solar Cells. Nature 2014, 517, 476–480. [Google Scholar] [CrossRef]
- Niu, G.; Li, W.; Li, J.; Liang, X.; Wang, L. Enhancement of Thermal Stability for Perovskite Solar Cells through Cesium Doping. RSC Adv. 2017, 7, 17473–17479. [Google Scholar] [CrossRef]
- Schwenzer, J.A.; Hellmann, T.; Nejand, B.A.; Hu, H.; Abzieher, T.; Schackmar, F.; Hossain, I.M.; Fassl, P.; Mayer, T.; Jaegermann, W.; et al. Thermal Stability and Cation Composition of Hybrid Organic-Inorganic Perovskites. ACS Appl. Mater. Interfaces 2021, 13, 15292–15304. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, Z.; Li, S.; Zhai, Y.; Wang, X.; Qiao, Z.; Xu, Q.; Meng, K.; Zhu, Z.; Chen, G. Highly Thermostable and Efficient Formamidinium-Based Low-Dimensional Perovskite Solar Cells. Angew. Chem. 2021, 133, 869–877. [Google Scholar] [CrossRef]
- Lin, Y.; Bai, Y.; Fang, Y.; Chen, Z.; Yang, S.; Zheng, X.; Tang, S.; Liu, Y.; Zhao, J.; Huang, J. Enhanced Thermal Stability in Perovskite Solar Cells by Assembling 2D/3D Stacking Structures. J. Phys. Chem. Lett. 2018, 9, 654–658. [Google Scholar] [CrossRef]
- Yue, T.; Li, K.; Li, X.; Ahmad, N.; Kang, H.; Cheng, Q.; Zhang, Y.; Yue, Y.; Jing, Y.; Wang, B.; et al. A Binary Solution Strategy Enables High-Efficiency Quasi-2D Perovskite Solar Cells with Excellent Thermal Stability. ACS Nano 2023, 17, 14632–14643. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Simchi, A.; Mo, X.; Fan, Z. High-Quality Organohalide Lead Perovskite Films Fabricated by Layer-by-Layer Alternating Vacuum Deposition for High Efficiency Photovoltaics. Mater. Chem. Front. 2017, 1, 1520–1525. [Google Scholar] [CrossRef]
- Arianita Dewi, H.; Li, J.; Wang, H.; Chaudhary, B.; Mathews, N.; Mhaisalkar, S.; Bruno, A.; Dewi, H.A.; Li, J.; Wang, H.; et al. Excellent Intrinsic Long-Term Thermal Stability of Co-Evaporated MAPbI3 Solar Cells at 85 °C. Adv. Funct. Mater. 2021, 31, 2100557. [Google Scholar] [CrossRef]
- Arivazhagan, V.; Hang, P.; Parvathi, M.M.; Tang, Z.; Khan, A.; Yang, D.; Yu, X. All-Vacuum Deposited and Thermally Stable Perovskite Solar Cells with F4-TCNQ/CuPc Hole Transport Layer. Nanotechnology 2019, 31, 065401. [Google Scholar] [CrossRef]
- Yuan, Q.; Lohmann, K.B.; Oliver, R.D.J.; Ramadan, A.J.; Yan, S.; Ball, J.M.; Christoforo, M.G.; Noel, N.K.; Snaith, H.J.; Herz, L.M.; et al. Thermally Stable Perovskite Solar Cells by All-Vacuum Deposition. ACS Appl. Mater. Interfaces 2023, 15, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xue, J.; Meng, L.; Lee, J.W.; Zhao, Z.; Sun, P.; Cai, L.; Huang, T.; Wang, Z.; Wang, Z.K.; et al. Caffeine Improves the Performance and Thermal Stability of Perovskite Solar Cells. Joule 2019, 3, 1464–1477. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, C.; Ding, Y.; Ding, B.; Xu, J.; Liu, A.; Yu, J.; Grater, L.; Zhu, H.; Hadke, S.S.; et al. A Thermotropic Liquid Crystal Enables Efficient and Stable Perovskite Solar Modules. Nat. Energy 2024, 9, 316–323. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Wang, K.; Peng, C.; Sun, X.; Zhao, Q.; Gao, K.; Ji, H.; Yan, X.; Wang, X.; et al. An In Situ Polymerization Strategy to Enhance Thermal Stability of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2025, 17, 28173–28180. [Google Scholar] [CrossRef]
- Joshi, P.H.; Zhang, L.; Hossain, I.M.; Abbas, H.A.; Kottokkaran, R.; Nehra, S.P.; Dhaka, M.; Noack, M.; Dalal, V.L. The Physics of Photon Induced Degradation of Perovskite Solar Cells. AIP Adv. 2016, 6, 115114. [Google Scholar] [CrossRef]
- Ruan, S.; Surmiak, M.A.; Ruan, Y.; McMeekin, D.P.; Ebendorff-Heidepriem, H.; Cheng, Y.B.; Lu, J.; McNeill, C.R. Light Induced Degradation in Mixed-Halide Perovskites. J. Mater. Chem. C Mater. 2019, 7, 9326–9334. [Google Scholar] [CrossRef]
- Xu, R.P.; Li, Y.Q.; Jin, T.Y.; Liu, Y.Q.; Bao, Q.Y.; O’Carroll, C.; Tang, J.X. In Situ Observation of Light Illumination-Induced Degradation in Organometal Mixed-Halide Perovskite Films. ACS Appl. Mater. Interfaces 2018, 10, 6737–6746. [Google Scholar] [CrossRef]
- Nie, W.; Blancon, J.C.; Neukirch, A.J.; Appavoo, K.; Tsai, H.; Chhowalla, M.; Alam, M.A.; Sfeir, M.Y.; Katan, C.; Even, J.; et al. Light-Activated Photocurrent Degradation and Self-Healing in Perovskite Solar Cells. Nat. Commun. 2016, 7, 11574. [Google Scholar] [CrossRef]
- Khenkin, M.V.; Anoop, K.M.; Visoly-Fisher, I.; Kolusheva, S.; Galagan, Y.; Di Giacomo, F.; Vukovic, O.; Patil, B.R.; Sherafatipour, G.; Turkovic, V.; et al. Dynamics of Photoinduced Degradation of Perovskite Photovoltaics: From Reversible to Irreversible Processes. ACS Appl. Energy Mater. 2018, 1, 799–806. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wang, C.; Ecker, B.; Yang, J.; Huang, J.; Gao, Y. Light-Induced Degradation of CH3NH3PbI3 Hybrid Perovskite Thin Film. J. Phys. Chem. C 2017, 121, 3904–3910. [Google Scholar] [CrossRef]
- Wei, D.; Wang, T.; Ji, J.; Li, M.; Cui, P.; Li, Y.; Li, G.; Mbengue, J.M.; Song, D. Photo-Induced Degradation of Lead Halide Perovskite Solar Cells Caused by the Hole Transport Layer/Metal Electrode Interface. J. Mater. Chem. A Mater. 2016, 4, 1991–1998. [Google Scholar] [CrossRef]
- Wu, T.; Ono, L.K.; Yoshioka, R.; Ding, C.; Zhang, C.; Mariotti, S.; Zhang, J.; Mitrofanov, K.; Liu, X.; Segawa, H.; et al. Elimination of Light-Induced Degradation at the Nickel Oxide-Perovskite Heterojunction by Aprotic Sulfonium Layers towards Long-Term Operationally Stable Inverted Perovskite Solar Cells. Energy Environ. Sci. 2022, 15, 4612–4624. [Google Scholar] [CrossRef]
- Liu, R.; Wang, L.; Fan, Y.; Li, Z.; Pang, S. UV Degradation of the Interface between Perovskites and the Electron Transport Layer. RSC Adv. 2020, 10, 11551–11556. [Google Scholar] [CrossRef] [PubMed]
- Safdari, M.; Kim, D.; Balvanz, A.; Kanatzidis, M.G. Mitigation of Halide Segregation by Cation Composition Management in Wide Bandgap Perovskites. ACS Energy Lett. 2024, 9, 3400–3408. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, L.; Zhu, T.; Chen, H.; Chen, B.; Kubicki, D.J.; Balvanz, A.; Li, C.; Maxwell, A.; Ugur, E.; et al. Suppressed Phase Segregation for Triple-Junction Perovskite Solar Cells. Nature 2023, 618, 74–79. [Google Scholar] [CrossRef]
- Xu, J.; Boyd, C.C.; Yu, Z.J.; Palmstrom, A.F.; Witter, D.J.; Larson, B.W.; France, R.M.; Werner, J.; Harvey, S.P.; Wolf, E.J.; et al. Triple-Halide Wide-Band Gap Perovskites with Suppressed Phase Segregation for Efficient Tandems. Science 2020, 367, 1097–1104. [Google Scholar] [CrossRef]
- Ghorai, A.; Singh, S.; Roy, B.; Bose, S.; Mahato, S.; Mukhin, N.; Jha, P.; Ray, S.K. Suppression of Light-Induced Phase Segregations in Mixed Halide Perovskites through Ligand Passivation. J. Phys. Chem. Lett. 2025, 16, 1760–1768. [Google Scholar] [CrossRef]
- Yang, J.; Gan, Y.; Han, M.; Wang, S.; Li, P.; Zhang, Y.; Li, G.; Song, Y. Suppression of Light-Induced Phase Segregation in All-Inorganic Wide-Bandgap Perovskite Solar Cells via Molecular Interaction Design. J. Energy Chem. 2025, 108, 550–557. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, Z.; Vlaic, S.; Xin, C.; Pons, S.; Billot, L.; Aigouy, L.; Chen, Z. Synergetic Exterior and Interfacial Approaches by Colloidal Carbon Quantum Dots for More Stable Perovskite Solar Cells Against UV. Small 2024, 20, 2401505. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, S.; Lee, N.K.; Cho, H.E.; Park, S.J.; Kim, J.H.; Lee, N.; Kim, S.K.; Cho, S.H.; Lee, S.M. Ultraviolet-Resistant Flexible Perovskite Solar Cells with Enhanced Efficiency Through Attachable Nanophotonic Downshifting and Light Trapping. Small 2025, 21, 2501374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, X.; Liu, Z.; Guo, M.; Zhang, Y.; Li, Y.; Li, J.; Wei, M. UV-Robust and Efficient Perovskite Solar Cells Enabled by Interfacial Photocatalysis Suppression and Defect Passivation. J. Mater. Chem. A Mater. 2023, 11, 14959–14970. [Google Scholar] [CrossRef]
- Fei, C.; Kuvayskaya, A.; Shi, X.; Wang, M.; Shi, Z.; Jiao, H.; Silverman, T.J.; Owen-Bellini, M.; Dong, Y.; Xian, Y.; et al. Strong-Bonding Hole-Transport Layers Reduce Ultraviolet Degradation of Perovskite Solar Cells. Science 2024, 384, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Valencia, A.; Zhu, Y.; Zhang, X.; Li, W.; Daoud, W.A. Melatonin Treatment as an Anti-Aging Therapy for UV-Related Degradation of Perovskite Solar Cells. J. Mater. Chem. A Mater. 2024, 12, 11986–11994. [Google Scholar] [CrossRef]
- Zhi, C.; Li, C.; Wan, Z.; Liu, C.; Jiang, Z.; Zunair, H.; Du, L.; Zhang, S.; Li, Z.; Shi, J.; et al. Boosting Efficiency and UV Resistance in Perovskite Solar Cells via Sunscreen Ingredient Octinoxate. Adv. Funct. Mater. 2024, 34, 2403321. [Google Scholar] [CrossRef]
- Luo, W.; Wen, H.; Guo, Y.; Yin, T.; Tan, H.; Zhang, Z.; Si, S.; Zhang, Z.; Wu, H.; Huang, S. Simultaneous Ultraviolet Conversion and Defect Passivation Stabilize Efficient and Operational Durable Perovskite Solar Cells. Adv. Funct. Mater. 2024, 34, 2400474. [Google Scholar] [CrossRef]
- Seo, J.; Shin, Y.S.; Lee, D.G.; Lee, J.; Roe, J.; Son, J.G.; Lee, W.; Lee, Y.; Lee, D.; Song, J.W.; et al. Stabilized Intermediate Phase Via Pseudo-Halide Anions Toward Highly Efficient and Light-Soaking Stable Perovskite Solar Cells. Adv. Funct. Mater. 2025, 35, 2413390. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, H.; Jiang, Z.; Shen, H.; Gong, X. Mobile Oxygen Capture Enhances Photothermal Stability of Perovskite Solar Cells Under ISOS Protocols. Adv. Mater. 2025, 37, 2500268. [Google Scholar] [CrossRef]
- Tuo, B.; Wang, Z.; Ren, Z.; Zhang, H.; Lu, X.; Zhang, Y.; Zang, S.; Song, Y. A Novel Radical-Reaction Interruption Strategy for Enhancing the Light Stability of Perovskite Solar Cells. Energy Environ. Sci. 2024, 17, 2945–2955. [Google Scholar] [CrossRef]
- Lu, X.; Sun, K.; Wang, Y.; Liu, C.; Meng, Y.; Lang, X.; Xiao, C.; Tian, R.; Song, Z.; Zhu, Z.; et al. Dynamic Reversible Oxidation-Reduction of Iodide Ions for Operationally Stable Perovskite Solar Cells under ISOS-L-3 Protocol. Adv. Mater. 2024, 36, 2400852. [Google Scholar] [CrossRef]
- Yang, M.; Bai, Y.; Meng, Y.; Tian, R.; Sun, K.; Lu, X.; Pan, H.; Wang, J.; Zhou, S.; Zhang, J.; et al. Sn-Pb Perovskite with Strong Light and Oxygen Stability for All-Perovskite Tandem Solar Cells. Adv. Mater. 2025, 37, 2415627. [Google Scholar] [CrossRef]
- Ye, X.; Yuan, W.; Fu, P.; Yang, X.; Chu, X.; Bai, Y.; Sun, Y.; Cheng, H.M. A Full-Process Artificial Intelligence Framework for Perovskite Solar Cells. Sci. China Mater. 2025, 68, 2526–2535. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).