Abstract
Chialvo is one of the two-dimensional map-based neural models. In this paper, a memristor is added to this model to consider the electromagnetic induction’s effects. The memristor is defined based on a hyperbolic tangent function. The dynamical variations are analyzed by obtaining the bifurcation diagrams and Lyapunov spectra. It is shown that the most effective parameters on the dynamics are the magnetic strength and the injected current. The memristive Chialvo can exhibit different neural behaviors. It is also proven that, like the primary Chialvo model, the memristive version has coexisting attractors; an oscillating state coexists with a fixed point. In addition, to understand how memristive neurons behave in a network, two memristive Chialvo models are coupled with electrochemical synapses. By connecting two neurons and calculating the synchronization error, we can determine the system’s synchronizability. It is indicated that the electrical coupling is essential for the occurrence of complete synchronization in the network of memristive Chialvo, and the sole chemical coupling does not lead to synchronization.
1. Introduction
Introducing mathematical models for neurons facilitates computational analysis of brain behavior. Computational neuroscience has evolved significantly since Hodgkin and Huxley determined the contribution of sodium and potassium ions in a neuron’s axon and investigated its action potential in 1952 [1]. After the 4D Hodgkin and Huxley model, various mathematical models have also been introduced, and their electrical circuits have been implemented [2,3]. The 3D Hindmarsh–Rose [4], the 2D Morris–Lecar [5], and the 2D FitzHugh–Nagumo [6] models are examples of them in the continuous time domain. There are also other neural models in the discrete-time domain, such as the Rulkov map [7], the Zandi neuron map [8], the Cazelles–Courbage–Rabinovich map [9], and the Chialvo map [10] models. Not only are map-based models fast, but they are also easy to use. In a continuous model with dimensions lower than three, the chaotic oscillation of the neuron cannot be modeled. In contrast, discrete models do not follow a similar rule to illustrate chaotic oscillations. Hence, the networks of continuous neural models are time-consuming, especially when coupled in a high-dimensional network or higher-order one [11,12]. Even though some reduction methods reduce the cost of analyzing a high-dimensional network of oscillations [13], map-based models are generally faster and more efficient for analyzing large networks.
By studying neurons in a network of oscillations, one can analyze different types of synchronization, including partial synchronization [14], phase synchronization [15,16], lag synchronization [17], and complete synchronization [18,19]. A complete synchronization is a state where all the neurons in the network oscillate at the same dynamics simultaneously. Synchronization plays an essential role in the brain’s general functioning, including working memory and motor planning [20,21,22,23]. A variety of types of synchronization have also been observed in patients with epileptic seizures [24], Parkinson’s disease [25], schizophrenia [26], and other brain disorders.
The brain transfers information between neurons via synapses, where neural interactions occur. As the axon fires, the presynaptic neuron releases neurotransmitters in the synaptic cleft (the gap between two synapses). Each neurotransmitter can activate its receptors in the postsynaptic neuron. Then, based on involved ions, the postsynaptic neuron can be either hyperpolarized or depolarized. These neurotransmitters can be electrical or chemical. Also, in some cases, both neurotransmitters can exist simultaneously in the same synapse. As well as chemical and electrical synapses, an axon can receive feedback from itself via autapses [27]. Autapses are self-synapses in the neuron that are common throughout the brain [28], both excitatory and inhibitory [29,30].
Neurons can also be affected by external stimuli, such as temperature [31], noise [32], or electromagnetic changes [33]. The neuron model should be added with a memristor to study the effect of electrical changes on magnetic flux. Prof. Chua first introduced the concept of a memristor or a memory resistor as a fourth fundamental electrical component in addition to resistance, inductance, and capacitance [34]. Meanwhile, the field of studying memristor-based systems became very popular after Strukov et al. implemented it physically in 2008 [35]. By introducing continuous and discrete memristors, many advances were accomplished in chaotic systems [36,37,38] and nonlinear electronic circuits [39,40,41].
Because of being nonlinear and having hysteresis loops, memristors are applicable in various scientific fields. Memristor-based neural networks and analog memristor arrays have lately been employed in machine learning or pattern recognition approaches [42,43,44] and also in image encryption [45]. Numerous studies have also been conducted on the memristive model of both continuous and discrete mathematical neural models. Memristive Hindmarsh–Rose [46,47,48], memristive Hodgkin–Huxley [49], memristive Rulkov [50,51,52], and memristive Zandi [53] are some examples. The neuronal models can be used to simulate neuronal populations and reproduce their behaviors, which are relevant to many brain disorders and cognitive tasks. However, most proposed models are flow-based, resulting in a high computational cost. The map-based models can decrease this cost considerably and are a good substitute for flow-based models, especially when investigating neuronal networks. Therefore, improving the map-based models by adding the memristor can be helpful for this purpose. Hence, this paper investigates the effect of an external electromagnetic field on the Chialvo map-based model.
Here, we consider the hyperbolic tangent function as the memductance function. The electromagnetic effects are mainly examined through the bifurcation diagram and Lyapunov exponents for different parameters. It is shown that magnetic strength significantly influences the neuron’s dynamics. The effects of the initial condition are also examined, and coexisting attractors are shown. Moreover, by coupling two memristive Chialvo models, the synchronous and asynchronous behaviors of the model are analyzed by computing the synchronization error. Both electrical and chemical synapses are investigated to see how each coupling affects its synchronizability. The dependence of the coupled neurons’ dynamics on their initial conditions is also revealed.
The dynamics of the memristive Chialvo are studied in Section 2, consisting of the system’s fixed points and stability. The effect of different system parameters (especially the magnetic strength) on the system dynamics is also investigated through numerically simulating bifurcation diagrams and Lyapunov exponents, the neuron time series, and phase space. The effects of initial conditions in the system are also considered through the basin of attraction diagram. In Section 3, the synchronizability of the system is examined when two memristive neurons are chemically and electrically coupled. Computing synchronization errors shows that chemical coupling strength is essential for complete synchronization to occur. Finally, the conclusion of the paper is provided in Section 4.
2. Neuron Dynamics
Take n as the time step, the 2D map model of Chialvo is described as follows [10]:
where represents the activation variable and represents the recovery variable. The four remaining parameters, i.e., , , , and , are, respectively, the injected ion current, the time of recovery (), the activation dependence of the recovery process (), and the offset. The model can illustrate both chaotic and periodic behaviors of the neuron. Here, to analyze the electromagnetic effects on this map-based neuron, the parameter in Equation (1) is added with a discrete memristor. A memristor with hyperbolic tangent flux controls, which is bounded, smooth, and nonlinear, is described in Equation (2) [50].
In Equation (2), is the flux variable, is the time scale factor for generating the induced electromotive force in finite transients, and is the voltage of the memristor. Accordingly, the modified 3D memristive Chialvo model (3D-MCM) can be formulated as follows:
in which is the magnetic strength parameter and is the scale factor of flux.
The fixed point of the 3D-MCM is the point that all state variables () satisfy , which leads to the following:
Considering the parameters in Table 1, the fixed point obtained by solving Equation (4) is . To analyze the stability of the model equilibria, the Jacobian of the 3D-MCM is calculated as follows:

Table 1.
The value of parameters for 3D-MCM.
The fixed point of the 3D-MCM is stable when all Jacobean eigenvalues at it are less than unity. The eigenvalues of at the fixed point () are , , showing that is stable. The stability analysis of other fixed points can be conducted similarly.
To demonstrate the dynamics of the 3D-MCM as a result of the variation of four system parameters (, , , and ), the bifurcation diagrams (Figure 1 and Figure 2) and Lyapunov exponents (Figure 3) are obtained. As shown in Figure 1, two initial conditions and represent the bursting (purple) and quiescent (blue) state of the system, respectively, indicating that the 3D-MCM has coexisting attractors when the same parameter and different initial conditions are chosen. The straight blue line in the bifurcation diagrams shows the stable fixed-point attractor , which does not bifurcate by changing the parameters. Chaotic behavior and several periodic windows can be observed at in Figure 1a, where the largest periodic window is approximately . For , the system has two different fixed points, while for , both initial conditions lead to the single fixed point . In Figure 1b for , in Figure 1c for , and in Figure 1d for , chaotic behavior with periodic windows can be seen. Both initial conditions lead to one fixed point in Figure 1c for and Figure 1b for . Additionally, in Figure 1d for and , a single fixed point is obtained, whereas coexisting fixed points are evident for , which both satisfy Equation (3), based on the parameters. To have better observation, the bifurcation diagrams are plotted with respect to two bifurcation parameters, and the system’s dynamics are plotted with particular colors in Figure 2 with (1.0, 0.8, 0.2) as the initial condition. The purple color shows the chaotic oscillation, the red one is the periodic oscillation, and the blue regions show the quiescent mode of the 3D-MCM. Figure 2a depicts the system bifurcation in the I-k plane in which some periodic oscillation (red region) can be observed in the chaotic zone (purple regions), while there is no quiescent mode (blue region) in the chaotic zone. In Figure 2b, which illustrates the system bifurcation in the r-ϵ plane, chaotic and quiescent zones are somehow separated by periodic regions.
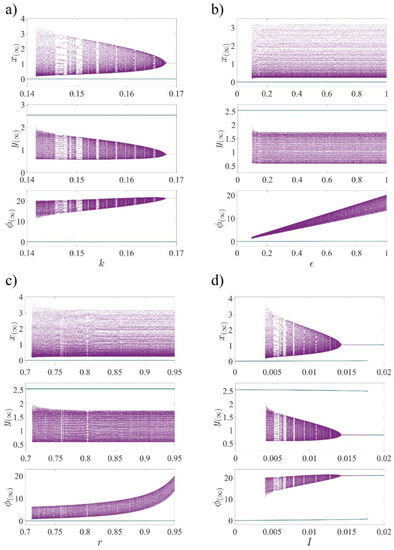
Figure 1.
The bifurcation diagram of 3D-MCM versus system parameters: (a) the magnetic strength, ; (b) the scale factor of time, ; (c) the scale factor of flux, ; and (d) the injected ion current, . Excluding each bifurcation parameter in the panels, the other parameters are set, as mentioned in Table 1. The diagram in purple is plotted with the initial conditions , and the one in blue is plotted with the initial conditions , indicating that the 3D-MCM has coexistence of an oscillating attractor and a fixed-point attractor.
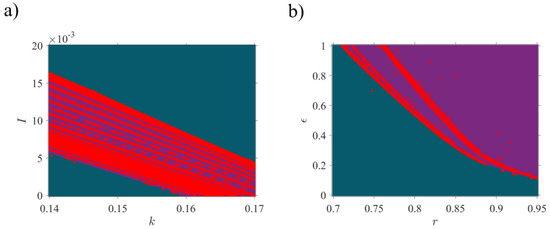
Figure 2.
Two-parameter bifurcation diagram of 3D-MCM versus system parameters: (a) the magnetic strength, , and the injected ion current, ; (b) the scale factor of flux, , and the scale factor of time, . Excluding each bifurcation parameter in the panels, the other parameters are set, as mentioned in Table 1. Both diagrams are plotted for .
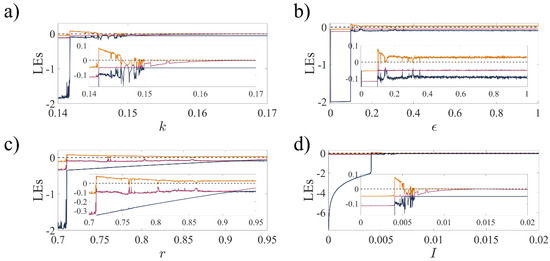
Figure 3.
The Lyapunov exponents (LEs) of 3D-MCM as a function of system parameters: (a) the magnetic strength, ; (b) the scale factor of time, ; (c) the scale factor of flux, ; and (d) the injected ion current, . The parameters are the same as in Figure 1. The initial condition is . In each panel, the largest exponent is displayed in yellow, while the two smaller ones are displayed in red and dark blue.
The Lyapunov exponents corresponding to each panel of Figure 1 are presented in Figure 3. Using as the initial condition, chaotic ranges with at least one positive LE indicate aperiodic behavior in the mentioned ranges.
Figure 4 illustrates the periodic and chaotic dynamics of the 3D-MCM for the parameters in Table 1 and two different values for in the time domain and phase space. Figure 4a shows the periodic orbits of the 3D-MCM at , and Figure 4b shows the chaotic dynamics at with the initial condition . Throughout the figures, the transient parts of the 3D-MCM are represented by the bright gray points, while dark blue ones indicate the steady states.
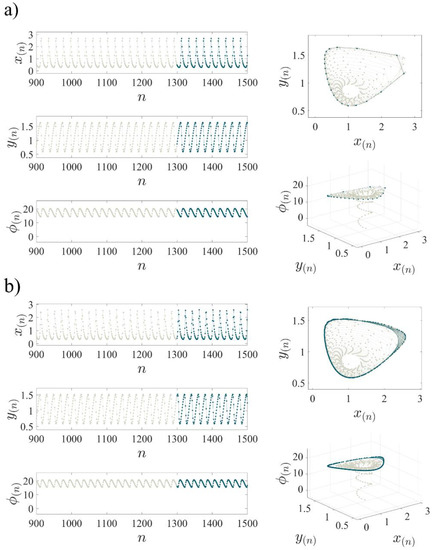
Figure 4.
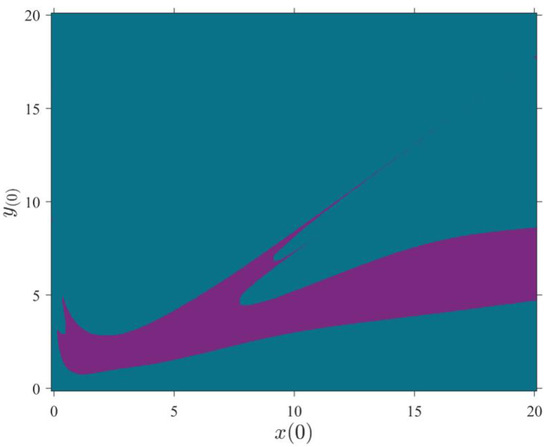
Time series of , , and (Left column) and 2D state space projections of 3D-MCM in the - plane and 3D state space projections in the -- plane (right column) with the initial conditions . The periodic oscillation occurs for (a), and the chaotic oscillation occurs for k = 0.152 (b). The other parameters are set, as mentioned in Table 1. The 3D-MCM’s basin of attraction in the - plane is illustrated in Figure 5, where the initial condition leading to chaotic bursting (quiescent) mode is illustrated in purple (blue). The system’s parameters are set, as mentioned in Table 1. According to the diagram, selecting different initial conditions with the same parameters results in different attractors.
By analyzing 3D-MCM dynamics, it has been shown that the model demonstrates a wide range of neuronal behaviors, including quiescence, periodic oscillation, and chaotic oscillation. The map-based behaviors are more efficient than those of other neuron models, especially in large-scale networks. As the 3D-MCM is a memristor-based model with memory, it can be used in learning approaches or other fields needing memory. The proposed 3D-MCM is compared to some other memristor-based and non-memristor-based neuron models in Table 2. Most studies have demonstrated that memristor resistance has a significant impact on system dynamics.

Table 2.
Comparison of the proposed model with some other memristive neuron models.
3. Synchronizability of the 3D-MCM
An analysis of synchronizability can be done by coupling at least two neurons. Hence, the effect of flux on the collective behavior of 3D-MCM synchronization is investigated by connecting a pair of neurons with electrochemical synapses, as follows:
In Equation (6), which shows the two coupled 3D-MCM, is the states of -th neuron and denotes the other neuron states, where . Also, and are, respectively, electrical, and chemical coupling. Here, the coupled model parameters are mentioned in Table 3.

Table 3.
The value of parameters for two coupled 3D-MCM.
The synchronizability of 3D-MCM is determined by the synchronization error, which is defined as:
in which indicates the mean over samples. The effects of chemical and electrical coupling strengths on synchronizability are examined through error. First, the error is calculated concerning only one coupling to investigate how each coupling strength affects the coupled neurons’ synchronizability. Then, the effect of both chemical and electrical coupling strengths on its synchronizability is analyzed.
Figure 6a shows the error in the absence of chemical coupling () and varying , while Figure 6b shows the error for the opposite condition ( and varying ) when the initial conditions are chosen as and . In Figure 6a, error indicates that the neurons are synchronous when , while in Figure 6b, the error is always positive, which shows that the neurons cannot be synchronized in the absence of .
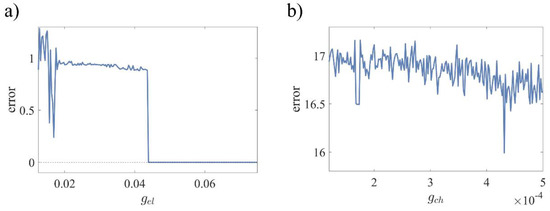
Figure 6.
Synchronization error of two 3D-MCMs as a function of only one coupling strength, while the other one equals zero when parameters are set, as mentioned in Table 3, with and as initial conditions. (a) Error in the absence of as a function of and (b) error in the absence of as a function of .
The synchronization error of the network is also calculated when both chemical and electrical couplings vary. Black regions in Figure 7a indicate zero error, hence the complete synchronization of two neurons. As increases, the synchronization error gets lower. It confirms that the network cannot be fully synchronized without the parameter. Figure 7b indicates the error in the - plane when . According to Figure 1a, in this diagram, is chosen in the chaotic range of a single 3D-MCM. The black spots in the error plane, where error , illustrate that by adjusting chemical coupling strength, two neurons can be synchronized whether the single neuron is in its quiescent mode or oscillates chaotically or periodically. The error in Figure 7a indicates that if electrochemical coupling in the synapses is considered, for larger , larger is required for full synchronization. In addition, in Figure 7b, it is observed that as increases, the dynamics of the system become more ordered and smaller is needed to achieve zero synchronization error.
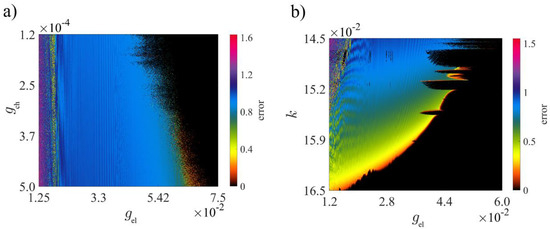
Figure 7.
The 2D synchronization error of two 3D-MCMs in the (a) – plane when , and (b) – plane when . The other system parameters are set, as mentioned in Table 3, with initial conditions and .
Figure 8 shows four time series of two MCMs with different coupling strengths. In Figure 8a, the chemical coupling is absent, but complete synchronization has occurred; however, in Figure 8b, when the electrical coupling is absent, synchrony in neurons does not occur; one neuron oscillates chaotically, and the other is placed in the quiescent state. In addition, two different patterns of asynchronous oscillation are illustrated in Figure 8c,d.
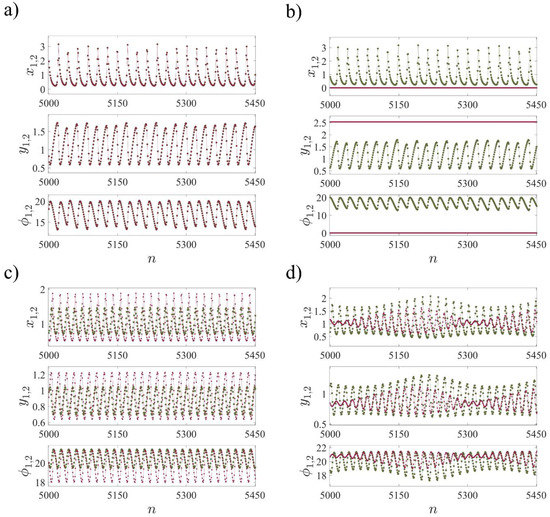
Figure 8.
, , and variables of the network in the time domain for different chemical and electrical couplings when parameters are set, as mentioned in Table 3, with and as initial conditions. (a) and ; (b) and ; (c) and ; and (d) and . In the absence of , synchronization can be seen; while in the absence of , no synchronization occurs.
The network basin of attraction is also plotted for coupled 3D-MCMs. Figure 9 shows the oscillators’ dynamics with initial conditions and . The initial conditions in the purple region are and which leads the networks to oscillate chaotically but not necessarily synchronously. The ones in the blue region lead to the quiescent state. The system parameters are set as mentioned in Table 3.
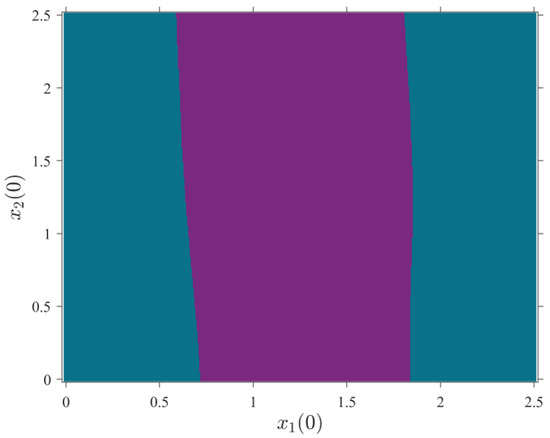
Figure 9.
Basin of attraction of two 3D-MCMs in the - plane for and . Purple region: chaotic oscillation; blue region: quiescent. The system parameters are set as mentioned in Table 3.
4. Discussion and Conclusions
The Chialvo neuron map model under electromagnetic fluctuation was studied, as described in this paper. A hyperbolic tangent version of the memristor was added to the activation variable of the 2D Chialvo map, and its dynamics were analyzed. The fixed point of the memristive Chialvo was calculated, and its stability was demonstrated. Moreover, the effects of model parameters were examined, revealing that the magnetic strength can mainly cause the model to bifurcate. Also, the occurrence of chaotic behavior by adjusting parameters was evident through plotting the bifurcation diagram and calculating Lyapunov exponents. Additionally, the memristive model’s time series and phase space were plotted to demonstrate the periodic and chaotic patterns of the neuron. Based on the initial condition, the system has coexisting attractors (bursting or quiescent mode). The synchronizability of the memristive Chialvo model was also examined by coupling two identical neurons with electrochemical synapses. The computation of synchronization error showed that in the absence of electrical coupling, complete synchronization cannot arise; however, the electrical coupling can individually synchronize the two coupled neurons without chemical coupling. In the end, the effect of different coupling strengths on the neurons’ dynamics was illustrated in the time domain.
Author Contributions
Conceptualization, Y.M., K.R. and S.J.; methodology, Y.M., K.R. and S.J.; software, G.V. and H.N.; validation, Y.M. and K.R.; investigation, H.N.; writing—original draft preparation, G.V. and H.N.; writing—review and editing, Y.M., K.R. and S.J.; visualization, K.R.; supervision, S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Centre for Nonlinear Systems, Chennai Institute of Technology, India, vide funding number CIT/CNS/2023/RP/004.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500. [Google Scholar] [CrossRef] [PubMed]
- Khanday, F.A.; Dar, M.R.; Kant, N.A.; Rossello, J.L.; Psychalinos, C. 0.65 V integrable electronic realisation of integer-and fractional-order Hindmarsh–Rose neuron model using companding technique. IET Circuit. Devices Syst. 2018, 12, 696–706. [Google Scholar] [CrossRef]
- Khanday, F.A.; Kant, N.A.; Dar, M.R.; Zulkifli, T.Z.A.; Psychalinos, C. Low-voltage low-power integrable CMOS circuit implementation of integer-and fractional–order FitzHugh–Nagumo neuron model. IEEE Trans. Neural Netw. Learn. Syst. 2018, 30, 2108–2122. [Google Scholar] [CrossRef] [PubMed]
- Hindmarsh, J.; Rose, R. A model of the nerve impulse using two first-order differential equations. Nature 1982, 296, 162–164. [Google Scholar] [CrossRef]
- Morris, C.; Lecar, H. Voltage oscillations in the barnacle giant muscle fiber. Biophys. J. 1981, 35, 193–213. [Google Scholar] [CrossRef] [PubMed]
- FitzHugh, R. Impulses and physiological states in theoretical models of nerve membrane. Biophys. J. 1961, 1, 445–466. [Google Scholar] [CrossRef] [PubMed]
- Rulkov, N.F. Modeling of spiking-bursting neural behavior using two-dimensional map. Phys. Rev. E 2002, 65, 041922. [Google Scholar] [CrossRef]
- Zandi-Mehran, N.; Panahi, S.; Hosseini, Z.; Golpayegani, S.M.R.H.; Jafari, S. One dimensional map-based neuron model: A phase space interpretation. Chaos Solitons Fractals 2020, 132, 109558. [Google Scholar] [CrossRef]
- Cazelles, B.; Courbage, M.; Rabinovich, M. Anti-phase regularization of coupled chaotic maps modelling bursting neurons. Europhys. Lett. 2001, 56, 504. [Google Scholar] [CrossRef]
- Chialvo, D.R. Generic excitable dynamics on a two-dimensional map. Chaos Solitons Fractals 1995, 5, 461–479. [Google Scholar] [CrossRef]
- Majhi, S.; Perc, M.; Ghosh, D. Dynamics on higher-order networks: A review. J. R. Soc. Interface 2022, 19, 20220043. [Google Scholar] [CrossRef] [PubMed]
- Parastesh, F.; Mehrabbeik, M.; Rajagopal, K.; Jafari, S.; Perc, M. Synchronization in Hindmarsh–Rose neurons subject to higher-order interactions. Chaos 2022, 32, 013125. [Google Scholar] [CrossRef]
- Naseri, N.; Parastesh, F.; Ghassemi, F.; Jafari, S.; Schöll, E.; Kurths, J. Converting high dimensional complex networks to lower dimensional ones preserving synchronization features. Europhys. Lett. 2022, 140, 21001. [Google Scholar] [CrossRef]
- Schöll, E. Partial synchronization patterns in brain networks. EPL (Europhys. Lett.) 2022, 126, 18001. [Google Scholar] [CrossRef]
- Pikovsky, A.; Rosenblum, M.; Kurths, J. Phase synchronization in regular and chaotic systems. Int. J. Bifurc. Chaos 2000, 10, 2291–2305. [Google Scholar] [CrossRef]
- Sun, X.; Perc, M.; Kurths, J. Effects of partial time delays on phase synchronization in Watts-Strogatz small-world neuronal networks. Chaos 2017, 27, 053113. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Chowdhury, A.R. Dual-anticipating, dual and dual-lag synchronization in modulated time-delayed systems. Phys. Lett. A 2010, 374, 3425–3436. [Google Scholar] [CrossRef]
- Kang, J.; Ramadoss, J.; Wang, Z.; Ali, A.M.A. Complete synchronization analysis of neocortical network model. Eur. Phys. J. Spec. Top. 2022, 231, 4037–4048. [Google Scholar] [CrossRef]
- Rakshit, S.; Majhi, S.; Ghosh, D. Synchronization in complex networks with long-range interactions. J. Phys. A Math. Theor. 2020, 53, 154002. [Google Scholar] [CrossRef]
- Bahmani, Z.; Daliri, M.R.; Merrikhi, Y.; Clark, K.; Noudoost, B. Working memory enhances cortical representations via spatially specific coordination of spike times. Neuron 2018, 97, 967–979.e966. [Google Scholar] [CrossRef]
- Merrikhi, Y.; Clark, K.; Albarran, E.; Parsa, M.; Zirnsak, M.; Moore, T.; Noudoost, B. Spatial working memory alters the efficacy of input to visual cortex. Nat. Commun. 2017, 8, 15041. [Google Scholar] [CrossRef] [PubMed]
- Merrikhi, Y.; Shams-Ahmar, M.; Karimi-Rouzbahani, H.; Clark, K.; Ebrahimpour, R.; Noudoost, B. Dissociable contribution of extrastriate responses to representational enhancement of gaze targets. J. Cognit. Neurosci. 2021, 33, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Merrikhi, Y.; Clark, K.; Noudoost, B. Concurrent influence of top-down and bottom-up inputs on correlated activity of Macaque extrastriate neurons. Nat. Commun. 2018, 9, 5393. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhu, L.; Cai, L.; Wang, J.; Liu, C.; Shi, N.; Liu, J. Variation of functional brain connectivity in epileptic seizures: An EEG analysis with cross-frequency phase synchronization. Cogn. Neurodyn. 2020, 14, 35–49. [Google Scholar] [CrossRef]
- Rubchinsky, L.L.; Park, C.; Worth, R.M. Intermittent neural synchronization in Parkinson’s disease. Nonlinear Dyn. 2012, 68, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, D.; Miyakoshi, M.; Joshi, Y.B.; Molina, J.L.; Tanaka-Koshiyama, K.; Sprock, J.; Braff, D.L.; Swerdlow, N.R.; Light, G.A. A distributed frontotemporal network underlies gamma-band synchronization impairments in schizophrenia patients. Neuropsychopharmacology 2020, 45, 2198–2206. [Google Scholar] [CrossRef]
- Ikeda, K.; Bekkers, J.M. Autapses. Curr. Biol. 2006, 16, R308. [Google Scholar] [CrossRef]
- Wang, H.-T.; Chen, Y. Firing dynamics of an autaptic neuron. Chin. Phys. B 2015, 24, 128709. [Google Scholar] [CrossRef]
- Saada-Madar, R.; Miller, N.; Susswein, A.J. Autaptic muscarinic self-excitation and nitrergic self-inhibition in neurons initiating Aplysia feeding are revealed when the neurons are cultured in isolation. J. Mol. Histol. 2012, 43, 431–436. [Google Scholar] [CrossRef]
- Yilmaz, E.; Ozer, M.; Baysal, V.; Perc, M. Autapse-induced multiple coherence resonance in single neurons and neuronal networks. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Q.; Li, T.; Yu, D.; Jia, Y. Effect of temperature on synchronization of scale-free neuronal network. Nonlinear Dyn. 2022, in press. [Google Scholar] [CrossRef]
- Faisal, A.A.; Selen, L.P.; Wolpert, D.M. Noise in the nervous system. Nat. Rev. Neurosci. 2008, 9, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jia, Y.; Ma, J.; Alsaedi, A.; Ahmad, B. Synchronization between neurons coupled by memristor. Chaos Solitons Fractals 2017, 104, 435–442. [Google Scholar] [CrossRef]
- Chua, L. Memristor-the missing circuit element. IEEE Trans. Circuit Theory 1971, 18, 507–519. [Google Scholar] [CrossRef]
- Strukov, D.B.; Snider, G.S.; Stewart, D.R.; Williams, R.S. The missing memristor found. Nature 2008, 453, 80–83. [Google Scholar] [CrossRef]
- Peng, Y.; He, S.; Sun, K. A higher dimensional chaotic map with discrete memristor. AEU Int. J. Electron. Commun. 2021, 129, 153539. [Google Scholar] [CrossRef]
- Rajagopal, K.; Kacar, S.; Wei, Z.; Duraisamy, P.; Kifle, T.; Karthikeyan, A. Dynamical investigation and chaotic associated behaviors of memristor Chua’s circuit with a non-ideal voltage-controlled memristor and its application to voice encryption. AEU Int. J. Electron. Commun. 2019, 107, 183–191. [Google Scholar] [CrossRef]
- Wu, H.; Ye, Y.; Bao, B.; Chen, M.; Xu, Q. Memristor initial boosting behaviors in a two-memristor-based hyperchaotic system. Chaos Solitons Fractals 2019, 121, 178–185. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, J.; Chen, M.; Xu, Q.; Bao, B. DC-offset induced asymmetry in memristive diode-bridge-based Shinriki oscillator. Chaos Solitons Fractals 2022, 154, 111624. [Google Scholar] [CrossRef]
- Chen, M.; Wang, A.; Wang, C.; Wu, H.; Bao, B. DC-offset-induced hidden and asymmetric dynamics in Memristive Chua’s circuit. Chaos Solitons Fractals 2022, 160, 112192. [Google Scholar] [CrossRef]
- Itoh, M.; Chua, L.O. Dynamics of memristor circuits. Int. J. Bifurc. Chaos 2014, 24, 1430015. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Song, W.; Rao, M.; Belkin, D.; Li, Y.; Yan, P.; Jiang, H.; Lin, P.; Hu, M. Reinforcement learning with analogue memristor arrays. Nat. Electron. 2019, 2, 115–124. [Google Scholar] [CrossRef]
- Wang, C.; Xiong, L.; Sun, J.; Yao, W. Memristor-based neural networks with weight simultaneous perturbation training. Nonlinear Dyn. 2019, 95, 2893–2906. [Google Scholar] [CrossRef]
- Sun, J.; Han, J.; Liu, P.; Wang, Y. Memristor-based neural network circuit of pavlov associative memory with dual mode switching. AEU Int. J. Electron. Commun. 2021, 129, 153552. [Google Scholar] [CrossRef]
- Lai, Q.; Lai, C.; Zhang, H.; Li, C. Hidden coexisting hyperchaos of new memristive neuron model and its application in image encryption. Chaos Solitons Fractals 2022, 158, 112017. [Google Scholar] [CrossRef]
- Bao, B.; Hu, A.; Bao, H.; Xu, Q.; Chen, M.; Wu, H. Three-Dimensional Memristive Hindmarsh–Rose Neuron Model with Hidden Coexisting Asymmetric Behaviors. Complexity 2018, 2018, 3872573. [Google Scholar] [CrossRef]
- Min, F.; Rui, Z. Boundary dynamics of a non-smooth memristive Hindmarsh–Rose neuron system. Chaos 2022, 32, 103117. [Google Scholar] [CrossRef]
- Fan, W.; Wu, H.; Li, Z.; Xu, Q. Synchronization and chimera in a multiplex network of Hindmarsh–Rose neuron map with flux-controlled memristor. Eur. Phys. J. Spec. Top. 2022, 231, 4131–4141. [Google Scholar] [CrossRef]
- Hu, X.; Liu, C. Dynamic property analysis and circuit implementation of simplified memristive Hodgkin–Huxley neuron model. Nonlinear Dyn. 2019, 97, 1721–1733. [Google Scholar] [CrossRef]
- Li, K.; Bao, H.; Li, H.; Ma, J.; Hua, Z.; Bao, B. Memristive Rulkov neuron model with magnetic induction effects. IEEE Trans. Ind. Inf. 2021, 18, 1726–1736. [Google Scholar] [CrossRef]
- Mehrabbeik, M.; Parastesh, F.; Ramadoss, J.; Rajagopal, K.; Namazi, H.; Jafari, S. Synchronization and chimera states in the network of electrochemically coupled memristive Rulkov neuron maps. Math. Biosci. Eng. 2021, 18, 9394–9409. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, H.; Krejcar, O.; Namazi, H. Synchronization in a network of map-based neurons with memristive synapse. Eur. Phys. J. Spec. Top. 2022, 231, 4057–4064. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Mehrabbeik, M.; Parastesh, F.; Rajagopal, K.; Jafari, S. A New Memristive Neuron Map Model and Its Network’s Dynamics under Electrochemical Coupling. Electronics 2022, 11, 153. [Google Scholar] [CrossRef]
- Fan, W.; Chen, X.; Wu, H.; Li, Z.; Xu, Q. Firing patterns and synchronization of Morris-Lecar neuron model with memristive autapse. AEU Int. J. Electron. Commun. 2023, 158, 154454. [Google Scholar] [CrossRef]
- Liu, L.J.; Qin, Y.H. Dynamics of discrete memristor-based Rulkov neuron. IEEE Access 2022, 10, 72051–72056. [Google Scholar] [CrossRef]
- Li, R.; Ding, R. A simple time-delay memristor and its application in 2D HR neuron model. Int. J. Mod. Phys. B 2021, 35, 2150166. [Google Scholar] [CrossRef]
- Kafraj, M.S.; Parastesh, F.; Jafari, S. Firing patterns of an improved Izhikevich neuron model under the effect of electromagnetic induction and noise. Chaos Solitons Fractals 2020, 137, 109782. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).