Abstract
One of the most important functions of the skin, i.e., protection from mechanical damage, is ensured by collagen fibers and their interaction with other elements in the extracellular matrix. Collagen fiber turnover is a complex multi-stage process. At each stage, a disruption may occur, leading to a decrease in the mechanical properties of the connective tissue. Clinically, collagen formation disorders manifest themselves as increased flabbiness and looseness of the skin and as early signs of facial aging. In addition to the clinical picture, it is important for cosmetologists and dermatologists to understand the etiology and pathogenesis of collagenopathies. In our review, we summarized and systematized the available information concerning the role of genetic and epigenetic factors in skin collagen fiber turnover. Furthermore, we focused on the functions of different types of collagens present in the skin. Understanding the etiology of impaired collagen formation can allow doctors to prescribe pathogenetically based treatments, achieve the most effective results, and minimize adverse reactions.
1. Introduction
To prescribe pathogenetically grounded therapy for aesthetic skin imperfections, it is important to understand the physiological and pathological processes in the skin and, on this basis, prescribe a set of measures aimed at restoring the skin’s physiological properties [1]. It is thus necessary to perform an in-depth study of collagen fiber turnover, including the genetic aspects of collagen formation. The synthesis of the existing data concerning the genes that encode key proteins and enzymes at all stages of skin collagen fiber turnover can help develop new predictive strategies in medical cosmetology (aesthetic medicine).
Collagen makes up 25% (in dry weight) of all proteins in the human body, constituting the basis of connective tissue, including skin [2]. Many modern methods of aesthetic medicine are aimed at improving or stimulating the synthesis of collagen fibers in the skin [3]. Furthermore, various companies have undertaken clinical trials and histological studies and have suggested that particular techniques bring significant results. However, in practice, we are far from achieving consistent and significant clinical effects in all patients.
In the context of obtaining diverse results in our patients, we often speak of “individual characteristics” of a particular person, but what lies at the core of such individual, personal, characteristics?
There are two groups of factors that can influence the synthesis of collagen in the skin: external and internal [4]. External factors include the type of diet (the completeness of the intake of nutrients necessary for collagen synthesis) and the impact of environmental factors. Internal factors include the state of the hormonal background, the inherent genetic sequences encoding the structural elements of the skin, and epigenetic regulation of the activity of genes encoding key proteins and enzymes of collagen formation [5].
The extracellular matrix of the skin can regulate cell morphology, proliferation, migration, and gene expression [6]. Disruption of collagen fiber turnover can lead to damage to skin architectonics, reduction regeneration, a response to aesthetic procedures, and the formation of pathological fibrosis [7]. Moreover, pathological “vicious circles” occur when a disruption in the state of the extracellular matrix leads to a decrease in the synthetic activity of fibroblasts and an even greater disruption of the extracellular matrix structure; thickening of collagen fibers (in the case of pathological fibrosis) leads to the differentiation of fibroblasts into α-smooth muscle actin-positive contractile myofibroblasts, resulting in an even greater severity of pathological fibrosis [8,9]. Thus, understanding and taking into account the genetic predictors of the metabolism of collagen fibers in the skin underlie the personalized management of patients by dermatologists and cosmetologists [10].
The genetic aspects of collagen fiber turnover (synthesis, functioning, and degradation) and their role in health and disease are under active investigation. The majority of studies are devoted to collagen in bone tissue and internal organs. The number of studies concerning genetic predictors of collagen formation in the skin has been increasing in recent years, but there is a need to systematize the existing data.
2. Collagen Fibers in the Skin
One of the most important functions of the skin, protection from mechanical damage, is realized through the reversible deformation of the structure, and this is made possible, to a large extent, due to collagen fibers [11]. There are ethnic features relating to the structure of collagen fibers and the cellular composition of the dermis, but with age, the skin becomes thinner, more rigid, less tense, and less elastic [12]. Changes also occur in the structure of collagen fibers. Specifically, with age, collagen fibers become more disorganized; in the papillary dermis, the diameter of collagen fibers decreases. In the reticular layer, the diameter of collagen fibers increases in people up to 45 years of age, and then it decreases and the collagen fibers become coarser and more rigid [13].
Collagen fibers make up most of the dermis and form a highly organized three-dimensional scaffold that surrounds cells. Between them, there are a large number of various macromolecules that bind water (glycosaminoglycans, fibronectin, tenascin, fibronectin, epimorphin, and others) [14]. Collagen fibers have a uniform orientation and provide passive tension, which causes internal skin tension along Langer’s lines [15]. Furthermore, collagen bundles are connected by elastic fibers, which perform the function of adaptation to deformation (returning collagen fibers to their original state after the termination of the load) [16].
In addition to its mechanical function, collagen plays a key role in the regulation of cell migration and differentiation, and it has a signaling function as proteins of the cell surface bind to it [17]. Collagen’s interaction with cell surface proteins can be carried out through receptors that recognize amino acid sequences on the collagen molecule. Moreover, certain proteins can bind to both collagen and integrins, promoting cell adhesion and proliferation [18,19]. When collagen fibers disintegrate, peptide regulatory factors are released that affect further regeneration [20].
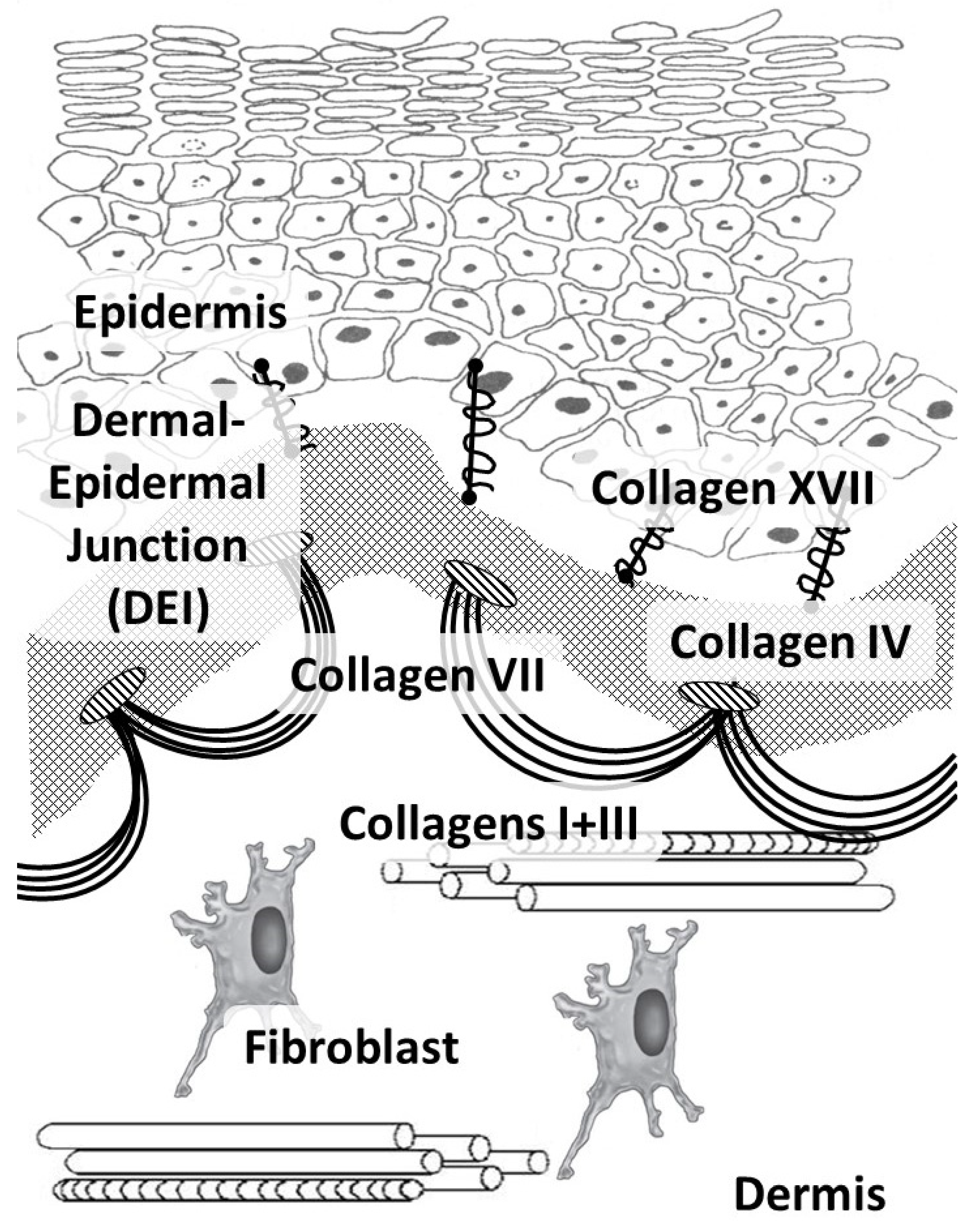
In humans, like in other vertebrates, 29 types of collagens have currently been described, encoded by at least 45 different genes [21]. The composition of collagen fibers varies in different organs depending on the functions of the corresponding organ [22]. The skin, which bears mechanical stress, is dominated by fibrillar collagens (including a large amount of type I collagen and a small amount of types III and V) (Figure 1). The activity related to the synthesis of different skin collagen types can be estimated by RPKM (reads per kilobase per million mapped reads) (Table 1).
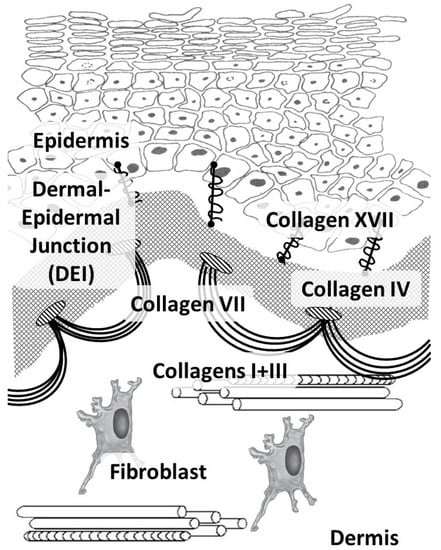
Figure 1.
Main skin collagens: dermal (types I and III) and dermal-epidermal junction (types IV, VII, and XVII) collagens.
Collagens of types I, III, and V belong to the group of fibrillar collagens. Moreover, collagen type I is distributed in many tissues, such as the skin, bone tissue, the cornea and sclera of the eye, and the walls of blood vessels. Collagen type I, in addition to its mechanical function, has a signaling function and is involved in the organization of the extracellular matrix, which, in turn, affects the organization of the epidermis and dermis [23]. Cells are able to directly bind to collagen monomers through integrins α1β1, α2β1, α10β1, and α11β1. Furthermore, this complex is involved in cell signaling, cell adhesion, cell migration, and remodeling of the collagen matrix [24].
Collagen type III is the main collagen in the skin of the fetus, while less is found in the adult skin. Furthermore, it is present in the reticular organs and the walls of blood vessels, and it is often found in fibers together with type I collagen fibrils [25]. Collagen type III is the most important in hollow organs, but it also interacts with platelets during blood coagulation (through specific glycoproteins and non-integrin receptors) and plays an important role as a signaling molecule in tissue regeneration (by participating in cellular adhesion, migration, proliferation, and differentiation through interaction with receptors on the cell surface, including integrins) [26]. Throughout a person’s life, the ratio of collagen types I and III fibers shifts towards an increase in collagen type I [27].
The COL3A1 gene is tail-to-tail with another fibrillar collagen gene, COL5A2; hence they are believed to have evolved from the same ancestor [28].
Collagen type V plays a valuable regulatory role and is found in soft tissues, placenta, blood vessels, and chorion. Without collagen type V, the assembly of the fibrillar type I collagen fiber is not possible. Collagen type V is located in the region of the N-terminal domain on the fibril surface and is therefore believed to determine the site of the beginning of fibril assembly in vivo [29].
Collagen types IV and VII and laminin form the basis of the epidermal basement membrane, providing anchoring sites (anchoring of endothelial cells and keratinocytes) and performing barrier functions in the epidermis [30]. Collagen type IV belongs to the group of network-forming collagens and is part of the basement membrane and lens capsule [31]. It belongs to collagens that form filament beads and can be found in microfibrils in soft tissues and cartilage [32].
Collagen type VII belongs to the group of collagens that form anchor fibrils located in the dermo-epidermal junction and is responsible for the strength of this junction [33]. Collagen type XIV belongs to fibril-associated collagens and is common for various soft tissues; it interacts with the surface of fibrils, regulating fibrillogenesis [34]. Collagen type XVII belongs to transmembrane collagens and is located on the surface of epidermal cells. It is a component of hemidesmosomes (multi-protein complexes located on the basement membrane), which mediate the attachment of keratinocytes to the underlying membrane [35].

Table 1.
Genes responsible for the structure of collagen fibers, their activity, and diseases caused by their mutations (adapted from [36]).
Table 1.
Genes responsible for the structure of collagen fibers, their activity, and diseases caused by their mutations (adapted from [36]).
| Gene (Protein) | Chromosome Localization | Clinical Manifestations of Mutation/Polymorphism | Expression in the Skin (RPKM) |
|---|---|---|---|
| Fibrillar Collagens | |||
| COL1A1 (α1 chain of collagen type I) | 17q21.33 (51 exons) | Osteogenesis imperfecta, classic type of Ehlers-Danlos syndrome, Caffey disease, idiopathic osteoporosis | 164.508 ± 48.747 |
| COL1A2 (α2 chain of collagen type I) | 17q21.3 (52 exons) | Osteogenesis imperfecta, type VII B of Ehlers-Danlos syndrome, idiopathic osteoporosis, atypical Marfan syndrome | 190.333 ± 32.009 |
| COL3A1 (α1 chain of collagen type III) | 2q32.2 (51 exons) | Ehlers-Danlos syndrome type IV, aortic and arterial aneurysms | 168.586 ± 46.57 |
| COL5A1 (α1 chain of collagen type V) | 9q34.3 (67 exons) | Ehlers-Danlos syndrome types I and II | 7.679 ± 0.808 |
| COL5A2 (α2 chain of collagen type V) | 2q32.2 (55 exons) | Ehlers-Danlos syndrome types I and II | 6.217 ± 1.778 |
| Networking Collagens | |||
| COL4A1 (α1 chain of collagen type IV) | 13q34 (54 exons) | Cerebrovascular diseases, kidney and muscle pathology | 1.798 ± 0.45 |
| COL4A2 (6 subunits of collagen type IV) | 13q34 (48 exons) | Cerebrovascular diseases, kidney and muscle pathology | 5.245 ± 1.325 |
| Collagens Forming Filament Beads | |||
| COL6A1 (α1 chain of collagen type VI) | 21q22.3 (35 exons) | Bethlem myopathy | 65.872 ± 35.541 |
| COL6A2 (α2 chain of collagen type VI) | 21q22.3 (30 exons) | Bethlem myopathy, Ullrich scleroatonic muscular dystrophy Bethlem myopathy | 68.022 ± 43.357 |
| COL6A3 (α3 chain of collagen type VI) | 2q37.3 (50 exons) | Ullrich scleroatonic muscular dystrophy, autosomal dominant proximal myopathy | 68.022 ± 43.357 |
| COL6A5 (a protein that can interact with the α1 and α2 chains of type VI collagen to form a trimer) | 3q22.1 (44 exons) | Eczema | 1.896 ± 0.958 |
| COL6A6 (a protein that regulates the interaction of epithelial cells with fibronectin) | 3q22.1 (44 exons) | Dermatoses (eczema) | 0.183 ± 0.082 |
| Collagens Forming Anchor Fibrils | |||
| COL7A1 (α3 chain of collagen type VII) | 3q21.1 (120 exons) | Dystrophic epidermolysis bullosa | 62.83 ± 31.474 |
| Fibril-Associated Collagens | |||
| COL14A1 (α-chain of collagen type XIV) | 8q24.12 (50 exons) | Increased risk of carcinogenesis | 3.173 ± 1.431 |
| Transmembrane Collagens | |||
| COL17A1 (α1 chain of collagen type XVII) | 10q25.1 (56 exons) | Generalized atrophic epidermolysis and epidermolysis bullosa | 284.358 ± 48.16 |
The data on the role of collagen genes under physiological conditions presented in Table 1 confirm that genes encoding fibrillar collagens (collagen types I and III) and collagen type XVII have the highest expression in the skin. In the case of skin damage and activation of regeneration processes in fibroblasts, the highest expression of the COL1A1, COL1A2, and COL3A1 genes is also observed [37]. The gene expression of other types of collagens has only been detected in trace amounts, since they are not structural proteins of the skin. These genes are responsible for the primary polypeptide sequence of amino acids in the collagen molecule, and their mutations, single nucleotide variants (SNVs), and polymorphisms can lead to a disruption in the amino acid sequence of collagen molecules with subsequent disruption of protein function.
Not all mutations and/or SNVs lead to the formation of a pathological protein [38], but for each candidate gene, there are databases of pathological mutations, SNVs, and their contribution to the development of various human diseases [39].
Monogenic diseases caused by mutations in the collagen genes (Table 1) have a relatively low population frequency, while multi-factorial pathologies are widespread [40]. In these pathologies, additional adverse environmental factors are required for the genetic defect in a candidate gene (SNVs or polymorphisms) to be manifested as a pathological phenotype. In Russia, such collagen fiber pathologies are known as a group of connective tissue dysplasias. In contrast, elsewhere in the world, there is no single term; different studies show the contribution of SNVs or polymorphisms to the development of various (in terms of clinical course and prognosis) multi-factorial syndromes of collagen formation impairment, including in the skin.
3. Collagen Molecule Structure
In the extracellular matrix, two main classes of macromolecules are distinguished: glycoproteins (fibronectin, proteoglycans, and laminin) and fibrous proteins (collagen and elastin). The extracellular matrix proteins are called “matrisomes” [41]. The collagen molecule is a fibrillar glycoprotein characterized by versatility in the construction of various tissues. The natural form of collagen fibers provides the necessary mobility when stretching the skin. However, in scar tissue, the fibers are straighter and thinner, and, consequently, the tensile strength of the collagen fiber decreases [42].
Depending on the type of collagen, its supramolecular structure can be fibrillar or non-fibrillar. Among the 29 types of collagens in the skin, fibrillar collagens of types I, III, and V are the most important, while non-fibrillar collagens (type IV, located in the basement membrane, and types VI, VII, XIV, and XVII) are considered to be less important.
All collagens, at least partially, are left-handed supercoils consisting of three polypeptide chain helices [43]. These polypeptide chains can have the same sequence of amino acid residues (in this case, the collagen molecule is known as homomeric) or a different sequence (a heteromeric collagen molecule) [44].
Specifically, the dominant form of collagen type I is a heterotrimer. The homotrimeric form is found in fetal tissues, tumors, and in some fibrotic lesions of various tissues; this form is more resistant to the influence of collagenases [45]. However, collagen type III is most often found in the form of a homotrimer; its fiber diameter is smaller than that of type I collagen. Nevertheless, when collagen type I appears together with collagen type III, the latter regulates the diameter of the collagen fiber [46].
The collagen molecule consists of repeating triads (XY-Gly)n, where Gly is the amino acid glycine, and X and Y are any other amino acids, the most frequent among them being proline or hydroxyproline [47]. Glycine is the smallest of the amino acids; its lateral hydrogen is always in the center of the helix. This amino acid contributes to winding the collagen molecule helix and ensures that collagen is tightly packed into a helix [48,49]. Accordingly, gene mutations leading to the replacement of glycine with another amino acid cause a change in the structure of the helix and thus a disruption to the function of the protein.
For example, more than 650 mutations of the COL3A1 gene, encoding the alpha-1 helix of procollagen type III, have been identified, among which the most common are missense mutations replacing glycine with a bulkier amino acid. Most glycine substitutions lead to the formation of a more thermolabile protein with a greater sensitivity to proteinases [50].
Most patients with such mutations are heterozygous and can produce both normal and abnormal α-chains of procollagen type III, so they can have both normal and mutant homotrimers and triple chains containing one or two abnormal chains [51].
4. Collagen Synthesis
Fibroblasts are the main producers of extracellular matrix components, including collagen. Collagen fiber synthesis is a complex multistep process that begins with the transcription of a gene encoding collagen in the cell nucleus and ends with the assembly of collagen fibers in the extracellular space [52].
The genes that contribute to the formation of a complete fiber have been identified for all stages (Figure 2). At the primary stage, these are genes responsible for the structure of the polypeptide chain. Moreover, the role of epigenetic regulation can be observed here. In the next stages of assembly (post-translational changes), the roles of genes responsible for collagen fiber spatial organization, which affects the functionality of these fibers, are important [53].
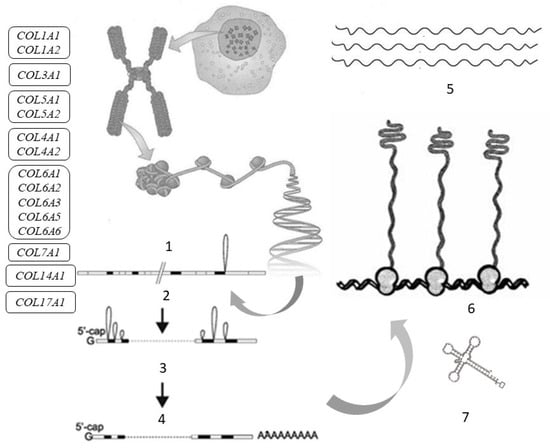
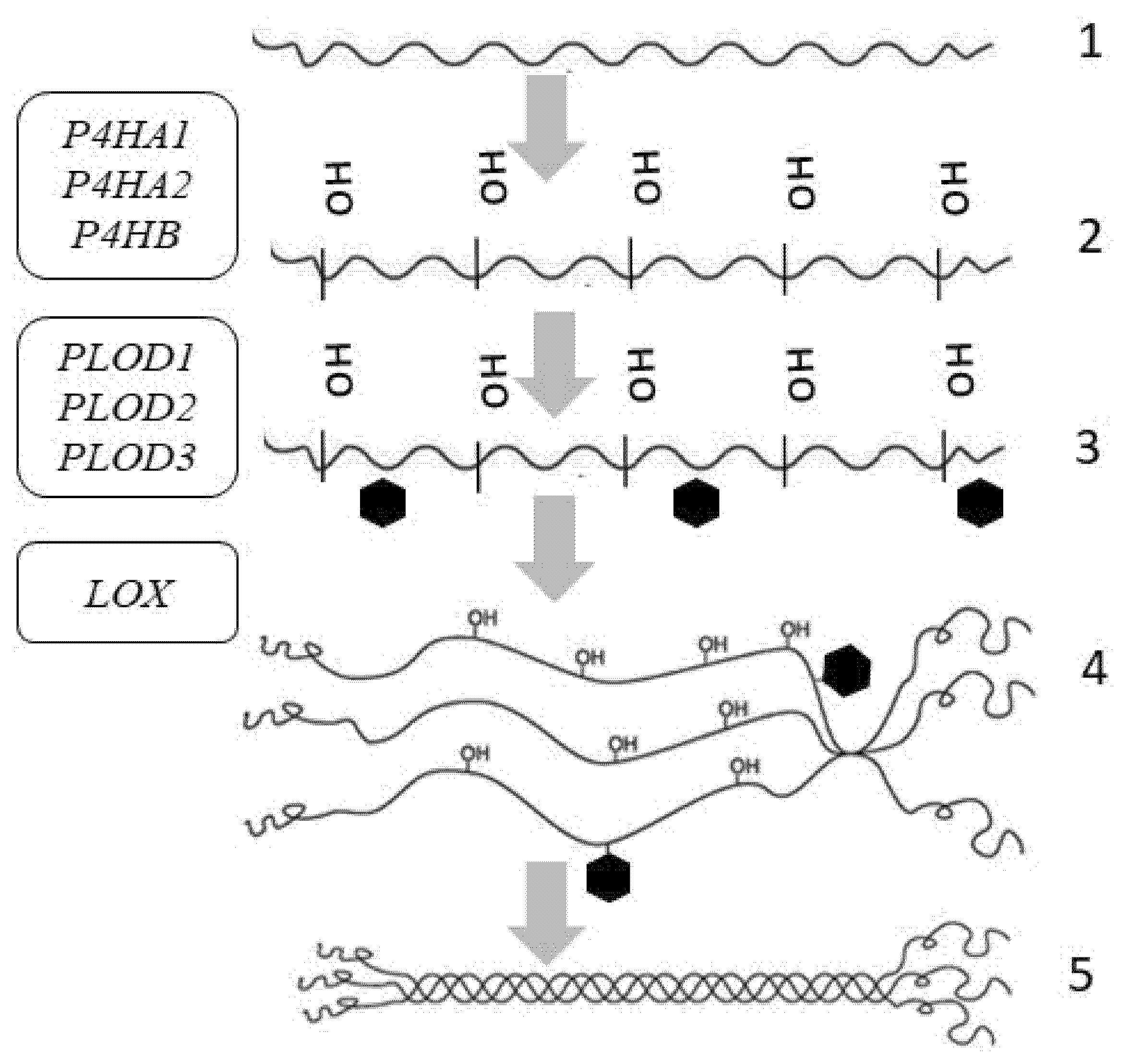
Figure 2.
Tropocollagen synthesis and the genes responsible for the synthesis of procollagen α-chains in the skin: COL1A1, COL1A2—genes encoding collagen type I; COL3A1—gene encoding collagen type III; COL4A1, COL4A2—genes encoding collagen type IV; COL5A1, COL5A2—genes encoding collagen type V; COL6A1, COL6A2, COL6A3, COL6A5, COL6A6—genes encoding collagen type VI; COL7A1—gene encoding collagen type VII; COL14A1—gene encoding collagen type XIV; COL17A1—gene encoding collagen type XVII. Symbols: 1—gene; 2—transcription; 3—splicing and processing; 4—messenger ribonucleic acid (mRNA); 5—collagen α-chains; 6—ribosomes; 7—transfer ribonucleic acid (tRNA).
The assembly of the collagen polypeptide chain occurs on the ribosomes, where information is read from the messenger ribonucleic acid (mRNA) and the polypeptide chain is assembled (translated) from amino acids with the participation of transfer RNA (tRNA).
The primary polypeptide chain of collagen consists of three domains: including N-propeptide, triple-helical (which makes up 95% of the molecule), and C-propeptide. These domains are transported to the endoplasmic reticulum, where they undergo subsequent post-translational modification [54].
The key stage in collagen formation is the formation of a triple supercoil, or trimerization, which begins at the C-terminus, in the position of the disulfide bonds, and proceeds with lightning speed towards the N-end of the molecule. Each individual polypeptide chain folds into a left-handed helix. Then, all three chains are folded together into a right-handed helix.
Before the supercoil assembly, post-translational changes occur in each of the chains, including hydroxylation, glycosylation, and oxidative deamination. All these changes occur inside the cell (Figure 3) [55].
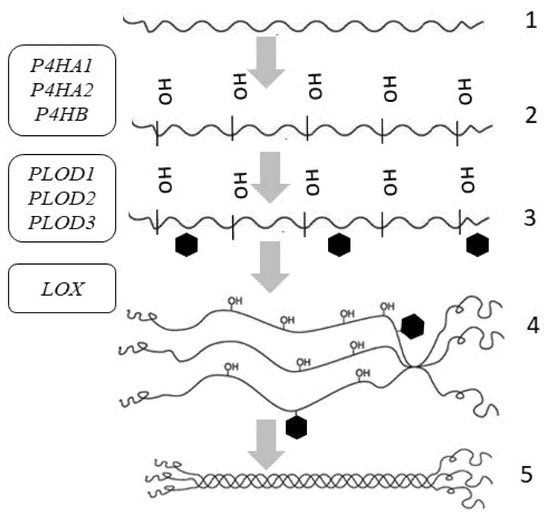
Figure 3.
Intracellular post-translational modifications of tropocollagen and the genes involved in post-translational changes of skin procollagen: P4HA1, P4HA2, P4HB—genes encoding prolyl-4-hydroxylase; PLOD1, PLOD2, PLOD3—genes encoding procollagen lysine,2-oxoglutarate-5-dioxygenase; LOX—gene encoding lysyl hydroxylase. Symbols: 1—α-chain of collagen; 2—hydroxylation; 3—glycosylation; 4—interchain bond formation at C-terminal domains; 5—procollagen triple helix formation.
For the thermal stability of collagen, some residues in the triple-helical domain are hydroxylated to 4-hydroxyproline by prolyl-4-hydroxylase encoded by the genes P4HA1, P4HA2, P4HB, P4HA3 (Table 1) [56]. To assure collagen reticulation, some of the lysine residues are hydroxylated by procollagen-lysine, 2-oxoglutarate-5-dioxygenase encoded by the PLOD gene, and then glycosylated [57,58]. Under physiological conditions, PLOD1 and PLOD3 are highly expressed in the skin (Table 2). Hydroxylation requires the presence of oxygen, iron ions (in the active site of enzymes), vitamin C (for the reduction of iron ions), and α-ketoglutarate [59]. Ascorbic acid (vitamin C) is a cofactor of prolyl hydroxylases and lysyl hydroxylases, which are involved in collagen biosynthesis [60].

Table 2.
Genes encoding enzymes involved in post-translational changes in collagen fiber (adapted from [36]).
Other lysine and hydroxylysine residues in N- and C-telopeptides undergo oxidative deamination by lysyl oxidase (LOX), thus forming reactive aldehydes capable of forming covalent intramolecular and intermolecular crosslinks [61].
Trimerization is facilitated by chaperone proteins and occurs in the endoplasmic reticulum. The folding of the procollagen molecule begins only after the translation of the entire protein molecule is completed, with the autonomous folding of the C-propeptide domain on each monomeric strand. After folding, the cysteine-rich C-propeptide is stabilized by disulfide bonds. After the formation of folds, individual C-propeptide domains “recognize” each other and assemble together; in fibrillar proteins, this process is mediated by Ca2+ and intermolecular disulfide bonds [62]. The assembled C-propeptide trimer then initiates almost instantaneous folding of the triple-helical domain, which is rich in proline and glycine, with preliminary isomerization of proline peptide bonds into the trans-configuration [45] (Figure 4).
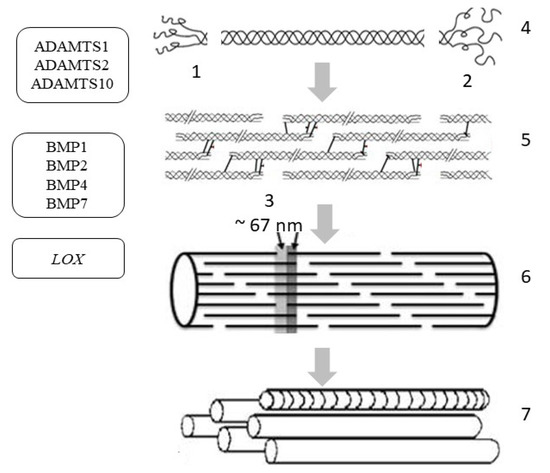
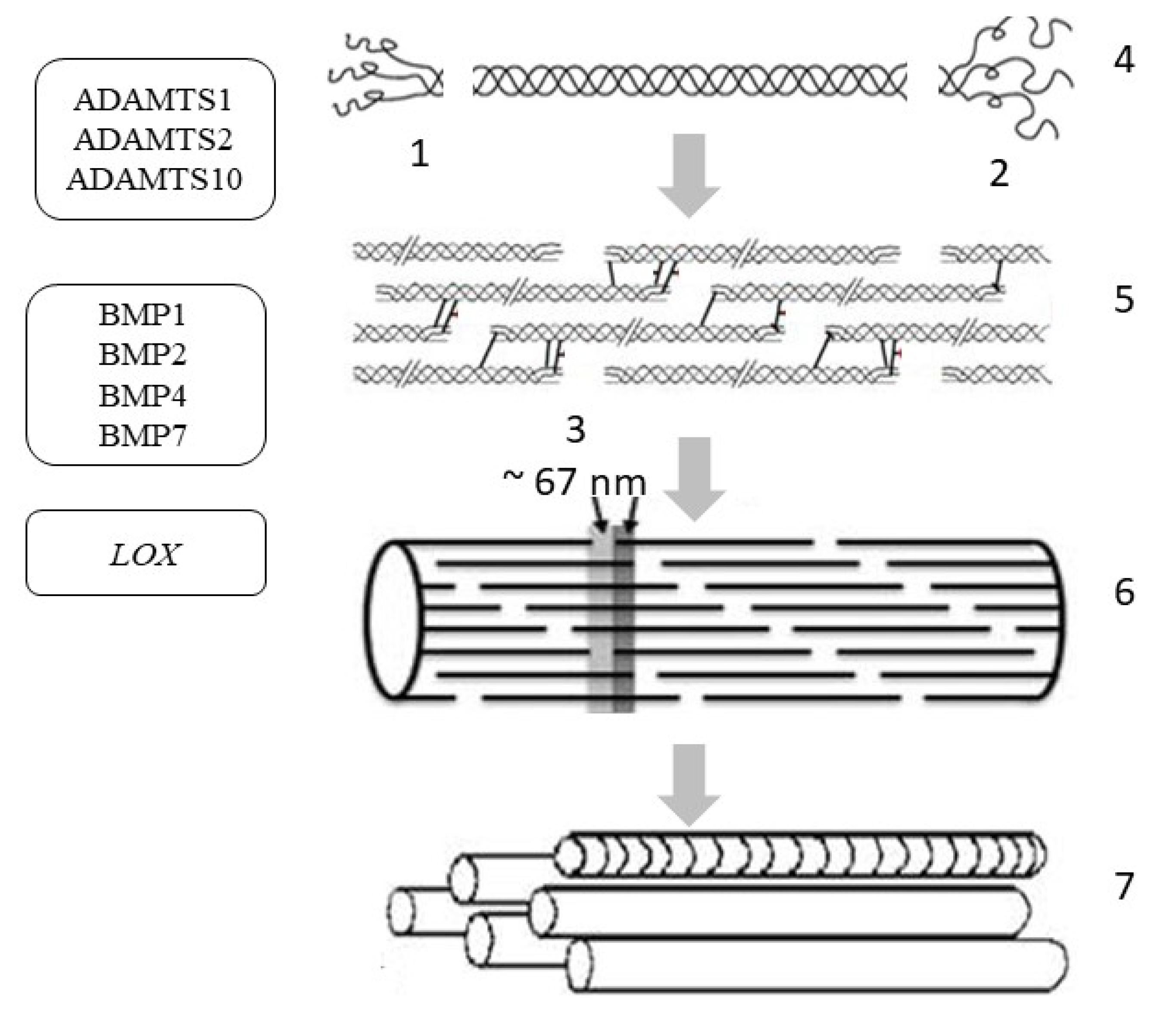
Figure 4.
Extracellular post-translational changes in tropocollagen and the genes involved in post-translational changes in skin procollagen: ADAMTS1, ADAMTS2, ADAMTS10—genes encoding the enzyme called A disintegrin and metalloproteinase with thrombospondin motifs; BMP1, BMP2, BMP4, BMP7—genes encoding bone morphogenetic proteins/tolloid-like proteinase; LOX—gene encoding lysyl oxidase. Symbols: 1—N-propeptide; 2—C-propeptide; 3—D-period; 4—cleavage of terminal propeptides; 5—assembly of microfibrils; 6—collagen fibril; 7—aggregation of fibrils into collagen fiber.
In the resulting triple helix, further hydroxylation of procollagen is weakened and preparation for protein secretion begins (in a non-canonical way). After the formation of the supercoil, large globular domains are removed from both sides of the molecule to produce tropocollagen. N-terminal propeptides are removed by zinc-dependent proteases belonging to the ADAMTS group (A disintegrin and metalloproteinase with thrombospondin motifs), and the C-terminal propeptides of collagen are cleaved off by a group of metalloproteases belonging to BMP1/tolloid-like (bone morphogenetic protein 1/tolloid-like proteinase) (Table 2) [63].
Under physiological conditions in the skin, the expression of the ADAMTS1, ADAMTS2, and ADAMTS10 genes is approximately the same, while the rest of the enzymes of the ADAMTS group exhibit only low-level gene expression (Table 2). However, the key enzyme involved in the cleavage of the N-terminal propeptide is the N-protease, which is encoded by the ADAMTS2 gene. In the BMP gene group, BMP7 and BMP1 are most expressed in the skin under physiological conditions, but BMP-1/tolloid-like C-protease plays a key role in the cleavage of the C-terminal propeptide of procollagen in the skin [64]. The assembly of a collagen molecule is spatially organized depending on the type of collagen and is enzymatically supported by additional molecular organizers, such as fibronectin, integrins, and minor collagens [65]. First, the supramolecular structures of 4–5 molecules (protofibrils) are assembled from the tropocollagen molecule; then, microfibrils are produced, from which a fibril (10 to 300 nm in diameter) is aggregated with the participation of proteoglycans [66]. Proteoglycans on the surface of the fibrils create a kind of shell; then, during autogenesis, the fibrils form a collagen fiber, which also includes glycosaminoglycans, glycoproteins, and non-collagen proteins.
Fibrillogenesis is a spontaneous process (self-assembly), which is evidenced by the spontaneous formation of fibrils by collagen fibers in vitro. However, in vivo fibrillogenesis of collagen type I is controlled by cellular mechanisms; i.e., it occurs only in the presence of collagen type V, fibronectin, and integrins (fibronectin binding and collagen binding) [67]. Furthermore, it is believed that collagen type V is important for the nucleation of type I collagen fibrils, while fibronectin and integrins are needed during its assembly.
The tissue specificity of collagen fiber is determined by the final composition of various collagens in heterotypic fibrils, and this composition is influenced by various signaling molecules involved in fibrillogenesis [68]. When both ends are enzymatically removed, collagen reticulation occurs, which means that crosslinks are formed between some lysine and hydroxylysine residues [69]. Reticulated collagen is resistant to enzymatic and chemical degradation. The creation of intrahelical and interhelical bonds is carried out through two mechanisms: specific (enzymatically controlled) and non-specific (spontaneous). The first leads to specific bivalent products, which then spontaneously react and form more complex, stable crosslinks. Thus, the oxidation of lysine by lysyl oxidase occurs in addition to the formation of aldimines. Then, a reaction with histidine occurs to form a chemically stable histidino-hydroxylysinonorleucine [70]. Lysyl oxidase, which hydroxylates the lysyl residues of collagens of types I and II, is encoded by the LOX gene [71]. The second mechanism may include a multitude of non-specific reactions with glucose and its oxidation products, resulting in the formation of the end products of glycation. This mechanism is especially important in aging and in diseases such as diabetes. Carbohydrates and oxidized carbohydrates react with arginine, lysine, and hydroxylysine to form a glycated protein.
5. Regulation of Collagen Synthesis
The synthesis and assembly of collagen fiber is influenced by many signaling molecules and proteins. Some of the most important of these are N-propeptides of collagen type I, fibronectin, lysyl oxidase, tenascin-X, thrombospondin, matrilins, perlecan, decorin, biglycan, fibromodulin, and lumican. For example, a mutation of the gene encoding tenascin-X leads to the development of Ehlers-Danlos syndrome. In this syndrome, collagen fibrils are of the usual size and shape, with a lower packing density. As a result, the total collagen content in the skin is reduced by 30% [72]. In addition, collagen and N-propeptides inhibit further procollagen synthesis through negative feedback regulation.
One of the most common extracellular matrix glycoproteins is fibronectin, playing an important role in development, cell growth, differentiation, adhesion, and cell migration by means of integrin-mediated signaling [73].
For the fibrillation of collagen type I, the presence of collagen type V is required because it acts as a central nucleus in the formation of collagen type I fibers.
Transforming growth factor β1 (TGF-β1) also plays a role in the regulation of collagen gene expression [74]. It binds to the extracellular matrix through binding to latent TGF-β binding protein 1, which is associated with fibronectin 1 and fibrillin microfibrils [75]. However, it should be noted that TGFβ1 stimulates myofibroblast differentiation, resulting in pathological fibrosis (scarring) during tissue regeneration. An additional factor in the differentiation of myofibroblasts is mechanical tension (stiffness) of tissues, which supports profibrotic activation [76].
Various cytokines may be involved in suppressing TGFβ1 activity, including interferon-gamma (IFNγ), interleukin-1 (IL-1), and basic fibroblast growth factors (bFGF, FGF-2). As a result of their action, collagen deposition decreases, and apoptosis is induced [77].
Hypoxia can lead to a decrease in the level of mRNA and collagen type III protein in chondrocytes and, on the contrary, to their increase in the lungs, leading to alveolar fibrosis. In response to hypoxia, trauma, or metabolic stress, adenosine and purine, which are formed from ATP and ADP, are released in the skin. In fibroblasts, adenosine, acting through its receptors, activates the expression of the COL3A1 gene [78].
Epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) also increase the expression of COL3A1 mRNA and protein in human skin fibroblasts through MAPK signaling [79].
6. Epigenetic Regulation of Collagen Synthesis
Epigenetics studies the inherited changes in protein synthesis that are not due to changes in the nucleotide sequence. Typically, such changes are caused by regulators of protein synthesis—methylation and demethylation of DNA, acetylation and deacetylation of histones, phosphorylation and dephosphorylation of transcription factors, and the action of regulatory microRNA (miRNA)—and other intracellular mechanisms. Modification of DNA and histones (which are involved in DNA packaging in the nucleus cells) alters the histone-histone and histone-DNA interactions, regulating the availability of transcription factors and influencing gene transcription [80]. Among other factors, the modification of epigenetic mechanisms underlies the mechanisms of skin and collagen fiber aging.
The roles of DNA and histone methylation and histone acetylation are the most studied [81]. In particular, DNA methylation results in transcription repression and the long-term maintenance of genome stability; however, in some sporadic cases, DNA methylation leads to the activation of genes in several types of cells [82]. Demethylation of DNA is facilitated by the influence of certain external and internal factors. Maintaining methylated DNA is important for the preservation of progenitor cells and skin self-renewal [83].
With skin aging, so-called epigenetic drift accumulates, and, as a result, both hypomethylated and hypermethylated DNA regions accumulate. Furthermore, ultraviolet (UV) radiation makes a large contribution to DNA hypomethylation, and the degree of hypomethylation correlates with clinical indicators of skin photoaging [84]. An example of epigenetic changes is a decrease in the regulation of the gene encoding LOX in old fibroblasts, as a result of which the mechanical properties of the skin are reduced [85].
Methylation of histones, depending on which site is modified, can lead to the activation or suppression of transcription.
The acetylation (deacetylation) of histone tails has the opposite effect of methylation (demethylation). Specifically, acetylation leads to the relaxation of chromatin and the activation of transcription. Deacetylation, on the contrary, leads to denser curling of chromatin and inhibition of transcription.
As a result of their participation in histone acetylation, specific NAD+-dependent enzymes (sirtuins) (SIRTs) play a key role in epigenetic regulation and facilitate transcription. Moreover, they are involved in the control of energy metabolism and oxidative stress, cell survival, response to UV damage, DNA repair, tissue regeneration, and inflammation [86]. In the dermis, sirtuins can inhibit collagen degradation, regulate DNA repair, and increase the activity of collagen type I synthesis by fibroblasts. The activity of sirtuins decreases with age and under oxidative stress conditions [87].
7. Collagen Degradation
The physiological process of renewal of collagen fibers takes 40 to 60 days on average. Collagen degradation occurs in two stages: in the first stage, collagen fibers and fibrils are fragmented; in the second, phagocytosis caused by macrophages and fibroclasts occurs, with subsequent cleavage of fragments in the lysosomes to amino acids and peptide sequences [88].
Non-cellular fragmentation of collagens is mediated by proteinases. However, in general, mature collagen fiber is quite resistant to the action of most proteinases, except for certain matrix metalloproteinases (MMPs), including collagenases, stromelysin, cysteine cathepsins, and serine proteases (plasmin) [35]. MMPs act at neutral pH, and they recognize specific cleavage sites on target molecules [89]. They are a large family of zinc-dependent endopeptidases involved in the renewal (remodeling) of tissues, cleavage of membrane receptors, activation or deactivation of cytokines and chemokines, and other important cellular functions due to the generation of paracrine bioactive products [90]. They are initially secreted as inactive zymogens with a propeptide domain that must be removed for activation.
A total of 24 MMPs have been described in humans. All of them have two domains: substrate-binding and catalytic. Based on the activity and structure of their preferred substrate, MMP are divided into six groups: collagenases (MMP 1, 8, and 13); stromelysins (MMPs 3, 10, and 11); gelatinases (MMPs 2 and 9); matrilysins (MMPs 7 and 26); membrane-type MMP (MT-MMP) and others [91].
Under normal conditions, collagenases can quite often attach to the collagen fiber, but this does not always lead to its degradation, because the spatial structure of the collagen fiber does not allow the catalytic domain to be activated. With aging, pathology, or damage, the fiber structure becomes more accessible to the catalytic domain.
Various MMPs are involved in the release of regulatory proteins; for example, MMP-9 and MMP-14 release transforming growth factor β (TGF-β) from the complex with integrin ανβ, while MMP-1 and MMP-3 release tumor necrosis factor α (TNFα). When collagen is cleaved, its biologically active fragments (peptides) are formed. These are matrins, the inhibitors of which are complementary peptides. The most studied collagen tripeptide is Pro-Gly-Pro (PGP).
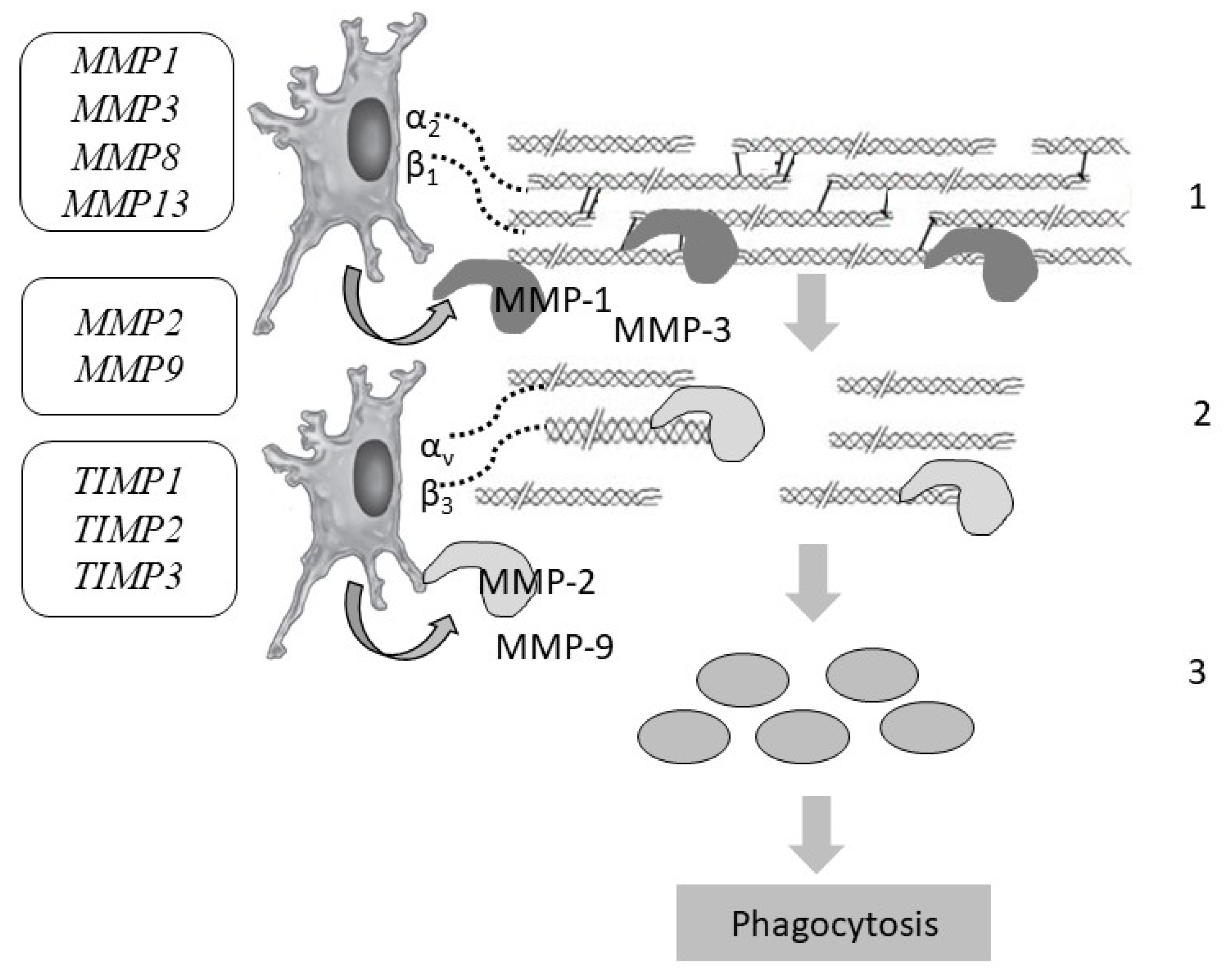
Various components of the extracellular matrix (laminin, elastin, fibronectin) can be cleaved by most MMPs, and fibrillar collagens (types I, II, and III) are cleaved mainly by collagenases (peptidases), i.e., MMPs 1, 8, and 13. Stromelysins cannot usually break down the triple-helical regions of fibrillar collagens. In this case, MMP-3 is a critical activator of procollagenase. In addition, MMP-3 is capable of binding to fibrillar proteins, thus being retained in tissues and influencing the renewal of the collagen and neocollagen matrix. Studies suggest that MMP-3 also promotes the degradation of collagen types III and IV in the skin. After that, the cleaved fragments are denatured, forming gelatin, which is cleaved by gelatinases (MMPs 2 and 9). In addition, gelatinase breaks down non-fibrillar collagens. When collagenase initiates the cleavage of fibrillar collagen, the bonds between peptides in the polypeptide chain inside the central triple helix are cleaved. Hence, large fragments are formed that denature into gelatin and are cleaved by gelatinase. Interstitial collagens (types I, II, and III) are hydrolyzed by “classical” collagenases (MMPs 1, 8, and 13) into two fragments along the length of the molecule, i.e., at ¼ and ¾. However, their relative activity differs in relation to different types of collagens: MMP-1 breaks down collagen type III more effectively, MMP-8 breaks down type I collagen more effectively, and MMP-13 breaks down type II collagen more effectively.
MMP-3 can attach to the native helix of collagen type I, but it breaks it down only after its destabilization of MMP-1 [92] (Figure 5). The main sources of MMPs in the skin are epidermal keratinocytes and dermal fibroblasts. To a lesser extent, MMPs are produced by epithelial and immune cells.
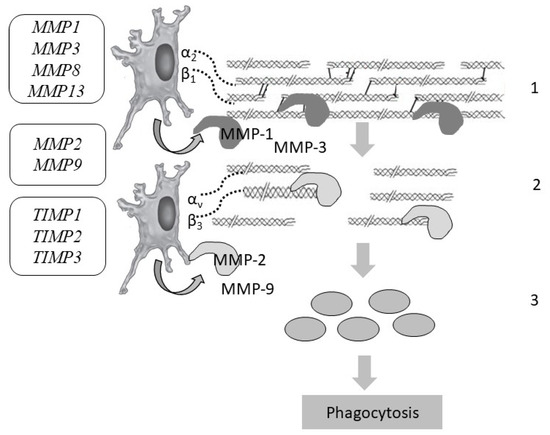
Figure 5.
Collagen degradation and the genes responsible for the stages of skin collagen degradation: MMP1, MMP2, MMP3, MMP8, MMP9, MMP13—genes encoding matrix metalloproteinases; TIMP1, TIMP2, TIMP3—genes encoding matrix metalloproteinase inhibitors. Designations: 1—cleavage of intact bonds in the collagen helix (MMP-1); 2—denaturation of gelatin (MMP-2, MMP-9); 3—phagocytosis of collagen residues by fibroblasts and macrophages.
The fibrillar form of collagen is more resistant to general hydrolysis, and true collagenase is considered to be an enzyme capable of breaking down intact bonds in the triple helix of collagen fiber. Furthermore, certain collagens, such as collagen type III, are more sensitive to hydrolysis by various proteases, since they have more flexible potential cleavage sites. Stromyelysins are capable of cleaving fibronectin, laminin, collagens type IV and VII, cell surface proteins, and other molecules that affect cell differentiation and proliferation [93].
Under physiological conditions in the skin, there is a low level of expression of genes encoding MMPs (Table 3). Three MMPs have been most studied in the skin, including interstitial collagenase (MMP-1), which is mainly present in fibroblasts and endothelial cells; stromelysin 1 (MMP-3); and gelatinase (MMP-9). MMPs are involved in physiological (tissue remodeling, morphogenesis, migration, adhesion) and pathological processes, but their functions are still being investigated and are not fully understood [94]. For example, the expression of MMP9 increases during tissue remodeling, and inflammation and in the disruption of the orientation of collagen fibers [95].

Table 3.
Genes responsible for the structure of enzymes involved in the degradation of collagen fibers, their activity, and the diseases caused by mutations in them (adapted from [36]).
MMP activity is regulated at different levels of regulation. Cellular regulation is carried out through surface receptors such as integrins, which transmit information about the protein and the required type of enzyme to the cell. For example, in the case of skin damage, basal keratinocytes induce enzymes when they are detached from the basement membrane and interact with collagen type I in the wound. An increase in the number of MMP-1 leads to an increase in the degradation of type I collagen, which reduces the signaling through integrins by collagen, the migration of keratinocytes, and the production of MMP-1. MMP-3 is considered a regulator of healthy and pathological tissue remodeling [96].
With inflammation in the skin, an increased breakdown of collagen fibers is possible, because TNFα can activate MMP-1, promoting collagenolysis. Presumably, the activation of MMP-1 occurs indirectly through the activation of MMP-3.
Indirect cleavage of collagen can occur when plasminogen is activated to become plasmin. The enzyme proMMP-2 (gelatinase A) is activated by plasmin to become MMP-2. The activated MMP-2 can destroy collagen, fibronectin, elastin, and gelatin (a denatured form of collagen) [97].
Extracellular collagen fragments undergo phagocytosis by fibroblasts and macrophages via the lysosomal pathway. Lysosomes that are fused together to form large structures with fragments of collagen and extracellular matrix are subjected to enzymatic digestion by cysteine cathepsins. As a result of the proteolytic cleavage of collagen, biologically active fragments, i.e., matrikines, are formed.
The level of MMP activity is strictly controlled by specific enzymes, tissue MMP inhibitors (1, 2, 3, and 4), the activity of which, in turn, is regulated by cytokines and hormones. MMP inhibitors can regulate the activity of most MMPs, but preferences persist. Specifically, MMP-1 inhibitors (TIMP1) mainly control MMP-1, MMP-3, and MMP-9, while MMP-2 inhibitors (TIMP2) are selective for MMP-14 [98]. In the skin, three TIMPs are best described, i.e., tissue MMP inhibitors of MMPs 1, 2, and 3. Furthermore, a certain level of expression of genes encoding these MMP inhibitors is noted in the skin under physiological conditions (Table 3).
Moreover, reactive oxygen species, which can be released from phagocytic immune cells during inflammation, can have an inhibitory effect on MMPs. The glycosylation of collagen, by disrupting the contraction of the collagen network, inhibits the production and activation of MMP types 1 and 2 by fibroblasts through a reduction in lattice compression [99].
Thus, the biomechanical properties of the skin are influenced by the balance between the synthesis of collagen fibers, the state of MMP activity, and the activity of MMP. An increase in the rate of collagen catabolism can be caused by internal factors (genetic mutations, inflammation, aging) or external factors (physicochemical stress, UV radiation).
Internal factors that affect the balance of collagen metabolism include the processes of internal aging, which leads to a decrease in the functional activity of fibroblasts, and, consequently, to a decrease in the synthesis of collagen fibers, with a simultaneous gradual increase in MMP activity.
The main external factor that causes damage to collagen fibers is UV irradiation. UVA has the greatest damaging effect, as it can penetrate the hypodermis and damage skin cells, collagen, elastic fibers, and other proteins in the extracellular matrix.
UV irradiation is more easily absorbed by hydrophobic amino acids (aromatic tryptophan, tyrosine, and phenylalanine), which are hidden inside the structure of the molecule under physiological conditions. With sunburn, protein denaturation occurs, as a result of which hydrophobic amino acids become more open to UV absorption by aromatic acids, and the collagen protein molecule is damaged. Furthermore, UV rays damage the chaperone proteins that control the assembly of the protein molecule, leading to abnormal assembly of the collagen molecule. The presence of damaged and abnormal collagen molecules increases the sensitivity of proteinases, and, as a result of all these processes, “weak” collagen is formed.
An additional factor involved in UV radiation damage to collagen molecules is the activation of lipid peroxidation in cell membranes, which causes a decrease in the activity of antioxidant systems and has a destructive effect on the structure of the collagen molecule.
Changes in the regulation of extracellular matrix remodeling can affect the course of several pathological processes, including fibrosis, skin diseases, and malignant neoplasms [100]. Various studies clearly show the role of polymorphism in the development of pathology in all the aforementioned processes due to the variety of MMPs (a defect in one is compensated by the activity of another); they are interchangeable with varying degrees of effectiveness. Moreover, studies show the role of polymorphism of the MMP2, MMP7, and MMP9 genes in the development of depression, atherosclerosis, and autoimmune diseases.
8. Disruption of Collagen Fiber Degradation
As mentioned above, disruption of the natural process of collagen fiber exchange can be caused by a decrease in MMP activity, excessive destruction (for example, resulting from pronounced activation of MMP after UV irradiation, part of the collagen fibers being denatured without complete degradation, or the formation of “lumps” of pathological collagen), or reticulation. After the completion of collagen synthesis and the formation of collagen fiber, changes continue to occur. Additional crosslinks inside the fiber are formed and the qualitative characteristics of the collagen fiber change, leading to an increase in the rigidity of collagen fibers and their resistance to degradation by enzymes. Among these mechanisms, non-enzymatic glycation is the most significant one [101].
This mechanism involves the attachment of reduced sugars (glucose, ribose, etc.) to the collagen molecule. Glycation leads to the formation of so-called AGEs (advanced glycation end-products) [102]. Glycated collagen exhibits increased rigidity, resistance to the action of proteinases, and impaired interaction with cells and other components of the extracellular matrix [103].
Glycation is a complex, multi-step process characterized by the formation of glucose carbonyl groups and then Schiff bases and ketoamine, which are further oxidized to reactogenic products (such as methylglyoxal, glyoxal), which, in turn, form crosslinks with protein molecules. Oxidative stress can increase protein glycation. The accumulation of glycated (ballast) collagen occurs with age and may also depend on the action of external factors (UV radiation, smoking, eating habits) and genetic factors.
The damaging effect of advanced glycation end products (AGE) can occur through specific receptors (RAGE) located on the cell surface. Stimulation of RAGE, among other things, causes the activation of the transcription factor nuclear factor kappa B. Therefore, in the presence of skin glycation, in addition to the deterioration of its mechanical properties (increased rigidity, decreased elasticity, dullness), an active inflammatory process also occurs [104].
In the skin, collagen types I and IV and fibronectin are more susceptible to glycation. Glycated collagen appears in the skin at the age of about 20 years, reaching 20–50% at the age of 80 years. Collagen glycation disrupts its functions; i.e., the intermolecular bonds between adjacent fibers change its biomechanical properties, leading to stiffness, decreased flexibility, and increased susceptibility to mechanical stress [105]. Changes in charge during glycation and the formation of AGEs on the side chains affect the ability of collagen to contact cells and other matrix components. Moreover, its organization into a triple helix and the connection with laminin in collagen type IV located in the basement membrane can be affected. The altered collagen is resistant to the action of MMP, and it is difficult to remove and replace it with a new synthesized and functionally active collagen. Thus, tissue permeability and fluidity are impaired [106].
Other less-studied receptors, i.e., AGER types 1, 2, and 3, regulate endocytosis and degradation of AGEs. They counteract the oxidative stress induced by AGEs and inhibit RAGE signaling [107]. Glyoxalase types 1 and 2 (GloI and GloII) play key roles in protection against glycation as they catalyze glyoxal and methylglyoxal into the less toxic D-lactate.
9. Conclusions
A large number of genetic and epigenetic factors affect the functioning of collagen fibers and, accordingly, the mechanical properties of the skin. Mutations of the genes that encode collagen proteins, MMP, and/or glycosaminoglycans, also the enzymes involved in post-translational collagen modifications, are causes of various forms of collagenopathies in humans [45].
The functioning and degeneration processes of collagen fibers in the skin are genetically determined. Therefore, mutations in fibrillar skin collagens lead to hereditary diseases, such as osteogenesis imperfecta, Ehlers-Danlos syndrome, Kaffi’s disease, idiopathic osteoporosis, etc. Furthermore, mutations in non-fibrillar skin collagens lead to cerebrovascular diseases, kidney and muscle pathologies, and epidermolysis bullosa. In Russian medicine, the terms differentiated and undifferentiated hereditary connective tissue dysplasias were previously proposed. The introduction of modern methods of molecular genetic diagnostics indicates that the most common hereditary (“differentiated”) collagenopathies include osteogenesis imperfecta, Ehlers-Danlos syndrome, Kaffi’s disease, and Marfan syndrome [60], which should be taken into account by doctors of aesthetic medicine. These are monogenic syndromes of Mendelian inheritance, caused by causal (pathogenic) gene mutations, in which the contribution of the environment is minimal or absent. For example, the genes involved in the development of Ehlers-Danlos syndrome include COL5A1, COL5A2, COL3A1, PLOD1, COL1A1, COL1A2, ADAMTS2, TNXB, FMNA, CHST14, SLC39A13, B4GALT7, and FKBP14 [25].
However, the number of associative genetic studies concerning multi-factorial (“undifferentiated”) collagenopathies is increasing. In these diseases, both the carriage of polymorphisms in candidate collagen genes and the influence of environmental factors are important. This is the reason for the higher incidence of multi-factorial collagenopathies in the population as compared with monogenic collagenopathies, many of which are rare (orphan). Investigations of the contribution of SNVs and polymorphisms to the development of multi-factorial connective tissue diseases, in general, and to the development of human skin collagen pathologies, in particular [39], are relevant.
However, associative genetic studies concerning the genes responsible for collagen fiber function are currently insufficient to compile a complete and clear personalized algorithm for the management of such patients by cosmetologists and dermatologists. Therefore, doctors, to a greater extent, are guided by the clinical picture, i.e., increased flabbiness, hyper-elasticity, early manifestation of aging, and other signs indirectly indicating a pathology in the collagen link. Therefore, only on the basis of the clinical picture can’t doctors form a treatment plan aimed at protecting and improving the synthesis of collagen fibers. Such recommendations, based on external and internal factors, might include, for example, lifestyle changes, additional intake of nutrients (vitamins, minerals), and mesotherapy (bio-revitalization) with amino acids and cofactors necessary for collagen synthesis. Implementing the results of molecular genetic diagnostics of monogenic and multi-factorial collagenopathies and their translation into real cosmetic, dermatologic, and plastic surgery practice will increase the efficiency and safety of local and general therapies for normal and pathological skin aging processes. The importance of translating the results of basic research into real clinical practice has been confirmed by research in recent years. For example, various studies [35,39,108,109,110] demonstrated the contribution of diverse factors related to the skin, opening up potential opportunities for therapeutic interventions with various cosmetic ingredients.
Author Contributions
Conceptualization, O.B.B.; methodology, E.I.K. and N.A.S.; software, O.B.B. and O.M.D.; validation, N.N.P., N.A.S. and M.M.P.; formal analysis, M.M.P.; data curation, G.V.M. and T.E.P.; writing—original draft preparation, O.B.B.; writing—review and editing, N.A.S., O.A.G. and G.V.M.; visualization, O.B.B. and V.V.T.; supervision, N.N.P.; project administration, N.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kapuler, O.; Selskaya, B.; Galeeva, A.; Kamilo, F. Metabolism of collagen fibers in the presence of age-related changes. Vrach 2015, 8, 64–69. [Google Scholar]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Manturova, N.E.; Stenko, A.G.; Petinati, Y.A.; Chaikovskaya, E.A.; Bolgarina, A.A. Injectable collagen in correction of age-related skin changes: Experimental and clinical parallels. Bull. RSMU 2019, 1, 79–85. [Google Scholar] [CrossRef]
- Blair, M.J.; Jones, J.D.; Woessner, A.E.; Quinn, K.P. Skin structure-function relationships and the wound healing response to intrinsic aging. Adv. Wound Care (New Rochelle) 2020, 9, 127–143. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.L.; Holmes, D.F. Collagenous extracellular matrix biomaterials for tissue engineering: Lessons from the common sea urchin tissue. Int. J. Mol. Sci. 2017, 18, 901. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.R.; Chen, X.; Ling, L.; Song, Y.H.; Shimpi, A.A.; Choi, S.; Gonzalez, J.; Sapudom, J.; Wang, K.; Andresen Eguiluz, R.C.; et al. Collagen microarchitecture mechanically controls myofibroblast differentiation. Proc. Natl. Acad. Sci. USA 2020, 117, 11387–11398. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jung, W.H.; Pittman, M.; Chen, J.; Chen, Y. The effects of stiffness, viscosity, and geometry of microenvironment in Homeostasis, Aging and Diseases. J. Biomech. Eng. 2020, 142, 100804. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Yuan, L.; Lee, Y.; Bharti, A.; Mitra, A.; Shivashankar, G.V. Fibroblast rejuvenation by mechanical reprogramming and redifferentiation. Proc. Natl. Acad. Sci. USA 2020, 117, 10131–10141. [Google Scholar] [CrossRef]
- Litman, T. Personalized medicine-concepts, technologies, and applications in inflammatory skin diseases. APMIS 2019, 127, 386–424. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Lelonkiewicz, M.; Wieczorowski, M. Age-dependent biomechanical properties of the skin. Postepy Dermatol. Alergol. 2013, 30, 302–306. [Google Scholar] [CrossRef]
- Vashi, N.A.; de Castro Maymone, M.B.; Kundu, R.V. Aging differences in ethnic skin. J. Clin. Aesthetic Dermatol. 2016, 9, 31–38. [Google Scholar]
- Lynch, B.; Bonod-Bidaud, C.; Ducourthial, G.; Affagard, J.S.; Bancelin, S.; Psilodimitrakopoulos, S.; Ruggiero, F.; Allain, J.M.; Schanne-Klein, M.C. How aging impacts skin biomechanics: A multiscale study in mice. Sci. Rep. 2017, 7, 13750. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Qin, Z.; Alexander Wilks, J.; Balimunkwe, R.M.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Physical properties of the photodamaged human skin dermis: Rougher collagen surface and stiffer/harder mechanical properties. Exp. Dermatol. 2019, 28, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sherman, V.R.; Gludovatz, B.; Schaible, E.; Stewart, P.; Ritchie, R.O.; Meyers, M.A. On the tear resistance of skin. Nat. Commun. 2015, 6, 6649. [Google Scholar] [CrossRef]
- Mostaço-Guidolin, L.; Rosin, N.L.; Hackett, T.L. Imaging Collagen in Scar Tissue: Developments in Second Harmonic Generation Microscopy for Biomedical Applications. Int. J. Mol. Sci. 2017, 18, 1772. [Google Scholar] [CrossRef]
- de Wild, M.; Pomp, W.; Koenderink, G.H. Thermal memory in self-assembled collagen fibril networks. Biophys. J. 2013, 105, 200–210. [Google Scholar] [CrossRef]
- Terzi, A.; Storelli, E.; Bettini, S.; Sibillano, T.; Altamura, D.; Salvatore, L.; Madaghiele, M.; Romano, A.; Siliqi, D.; Ladisa, M.; et al. Effects of processing on structural, mechanical and biological properties of collagen-based substrates for regenerative medicine. Sci. Rep. 2018, 8, 1429. [Google Scholar] [CrossRef]
- Kang, M.C.; Yumnam, S.; Kim, S.Y. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int. J. Mol. Sci. 2018, 19, 3551. [Google Scholar] [CrossRef]
- Söderhäll, C.; Marenholz, I.; Kerscher, T.; Rüschendorf, F.; Esparza-Gordillo, J.; Worm, M.; Gruber, C.; Mayr, G.; Albrecht, M.; Rohde, K.; et al. Variants in a novel epidermal collagen gene (COL29A1) are associated with atopic dermatitis. PLoS Biol. 2007, 5, e242. [Google Scholar] [CrossRef]
- Manka, S.W.; Bihan, D.; Farndale, R.W. Structural studies of the MMP-3 interaction with triple-helical collagen introduce new roles for the enzyme in tissue remodeling. Sci. Rep. 2019, 9, 18785. [Google Scholar] [CrossRef]
- Ouyang, M.; Yu, J.Y.; Chen, Y.; Deng, L.; Guo, C.L. Cell-extracellular matrix interactions in the fluidic phase direct the topology and polarity of self-organized epithelial structures. Cell Prolif. 2021, 54, e13014. [Google Scholar] [CrossRef] [PubMed]
- Musiime, M.; Chang, J.; Hansen, U.; Kadler, K.E.; Zeltz, C.; Gullberg, D. Collagen Assembly at the Cell Surface: Dogmas Revisited. Cells 2021, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Kuivaniemi, H.; Tromp, G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, E.; Jusman, S.W.; Moenadjat, Y.; Jusuf, A.A.; Sadikin, M. Expressions of collagen I and III in hypoxic keloid tissue. Kobe J. Med. Sci. 2016, 62, E58–E69. [Google Scholar] [PubMed]
- Kehlet, S.N.; Willumsen, N.; Armbrecht, G.; Dietzel, R.; Brix, S.; Henriksen, K.; Karsdal, M.A. Age-related collagen turnover of the interstitial matrix and basement membrane: Implications of age- and sex-dependent remodeling of the extracellular matrix. PLoS ONE 2018, 13, e0194458. [Google Scholar] [CrossRef]
- Niu, K.; Chen, X.; Lu, Y. COL3A1 rs1800255 polymorphism is associated with pelvic organ prolapse susceptibility in Caucasian individuals: Evidence from a meta-analysis. PLoS ONE 2021, 16, e0250943. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Z.; Zan, T. A case of Ehlers-Danlos syndrome presenting with widened atrophic scars of forehead, elbow, knee, and pretibial area: A case report. Medicine (Baltimore) 2019, 98, e17138. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Naranjo, J.D.; Londono, R.; Badylak, S.F. Biologic Scaffolds. Cold Spring Harb. Perspect. Med. 2017, 7, a025676. [Google Scholar] [CrossRef]
- Brown, K.L.; Cummings, C.F.; Vanacore, R.M.; Hudson, B.G. Building collagen IV smart scaffolds on the outside of cells. Protein Sci. 2017, 26, 2151–2161. [Google Scholar] [CrossRef]
- Fidler, A.L.; Boudko, S.P.; Rokas, A.; Hudson, B.G. The triple helix of collagens—An ancient protein structure that enabled animal multicellularity and tissue evolution. J. Cell Sci. 2018, 131, jcs203950. [Google Scholar] [CrossRef]
- Gatseva, A.; Sin, Y.Y.; Brezzo, G.; Van Agtmael, T. Basement membrane collagens and disease mechanisms. Essays Biochem. 2019, 63, 297–312. [Google Scholar] [CrossRef]
- Li, L.; Sun, Z.; Chen, J.; Zhang, Y.; Shi, H.; Zhu, L. Genetic polymorphisms in collagen-related genes are associated with pelvic organ prolapse. Menopause 2020, 27, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef]
- NCBI. Genes & Expression. Gene. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 23 April 2021).
- Wietecha, M.S.; Pensalfini, M.; Cangkrama, M.; Müller, B.; Jin, J.; Brinckmann, J.; Mazza, E.; Werner, S. Activin-mediated alterations of the fibroblast transcriptome and matrisome control the biomechanical properties of skin wounds. Nat. Commun. 2020, 11, 2604. [Google Scholar] [CrossRef] [PubMed]
- Demina, O.M.; Karpova, E.I.; Borzykh, O.B. Modern aspects of medical genetics. Russ. J. Clin. Dermatol. Venereol. 2021, 20, 124–134. [Google Scholar] [CrossRef]
- Borzykh, O.B.; Petrova, M.M.; Shnayder, N.A.; Nasyrova, R.F. Problems of implementation of personalized medicine in medical cosmetology in Russia. Sib. Med. Rev. 2021, 2, 12–22. [Google Scholar] [CrossRef]
- Yeo, J.; Jung, G.; Tarakanova, A.; Martín-Martínez, F.J.; Qin, Z.; Cheng, Y.; Zhang, Y.W.; Buehler, M.J. Multiscale modeling of keratin, collagen, elastin and related human diseases: Perspectives from atomistic to coarse-grained molecular dynamics simulations. Extreme Mech. Lett. 2018, 20, 112–124. [Google Scholar] [CrossRef]
- Arseni, L.; Lombardi, A.; Orioli, D. From structure to phenotype: Impact of collagen alterations on human health. Int. J. Mol. Sci. 2018, 19, 1407. [Google Scholar] [CrossRef]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. Hypertrophic scars and keloids: Overview of the evidence and practical guide for differentiating between these abnormal scars. Exp. Dermatol. 2021, 30, 146–161. [Google Scholar] [CrossRef]
- Fertala, A. Three Decades of research on recombinant collagens: Reinventing the wheel or developing new biomedical products? Bioengineering (Basel) 2020, 7, 155. [Google Scholar] [CrossRef]
- Sharma, U.; Carrique, L.; Vadon-Le Goff, S.; Mariano, N.; Georges, R.N.; Delolme, F.; Koivunen, P.; Myllyharju, J.; Moali, C.; Aghajari, N.; et al. Structural basis of homo- and heterotrimerization of collagen I. Nat. Commun. 2017, 8, 14671. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, S.; Wang, Y.; Ren, X.; Han, J. Molecular mechanisms and clinical manifestations of rare genetic disorders associated with type I collagen. Intractable Rare Dis. Res. 2019, 8, 98–107. [Google Scholar] [CrossRef]
- Asgari, M.; Latifi, N.; Heris, H.K.; Vali, H.; Mongeau, L. In vitro fibrillogenesis of tropocollagen type III in collagen type I affects its relative fibrillar topology and mechanics. Sci. Rep. 2017, 7, 1392. [Google Scholar] [CrossRef]
- Rowley, A.T.; Nagalla, R.R.; Wang, S.W.; Liu, W.F. Extracellular matrix-based strategies for immunomodulatory biomaterials engineering. Adv. Healthc. Mater. 2019, 8, e1801578. [Google Scholar] [CrossRef] [PubMed]
- San Antonio, J.D.; Jacenko, O.; Fertala, A.; Orgel, J.P.R.O. Collagen Structure-Function Mapping Informs Applications for Regenerative Medicine. Bioengineering (Basel) 2020, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish collagen: Extraction, characterization, and applications for biomaterials engineering. Polymers (Basel) 2020, 12, 2230. [Google Scholar] [CrossRef]
- Wan, T.; Ye, J.; Wu, P.; Cheng, M.; Jiang, B.; Wang, H.; Li, J.; Ma, J.; Wang, L.; Huang, X. Recurrent pneumothorax and intrapulmonary cavitary lesions in a male patient with vascular Ehlers-Danlos syndrome and a novel missense mutation in the COL3A1 gene: A case report. BMC Pulm. Med. 2020, 20, 149. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Toda, M.; Kyoyama, H.; Nishimura, H.; Kojima, A.; Kuwabara, Y.; Kobayashi, Y.; Kikuchi, S.; Hirata, Y.; Moriyama, G.; et al. Vascular Ehlers-Danlos syndrome with a novel missense mutation in COL3A1: A man in His 50s with aortic dissection after interventional treatment for hemothorax as the first manifestation. Intern. Med. 2019, 58, 3441–3447. [Google Scholar] [CrossRef]
- Rajan, A.M.; Ma, R.C.; Kocha, K.M.; Zhang, D.J.; Huang, P. Dual function of perivascular fibroblasts in vascular stabilization in zebrafish. PLoS Genet. 2020, 16, e1008800. [Google Scholar] [CrossRef]
- Merl-Pham, J.; Basak, T.; Knüppel, L.; Ramanujam, D.; Athanason, M.; Behr, J.; Engelhardt, S.; Eickelberg, O.; Hauck, S.M.; Vanacore, R.; et al. Quantitative proteomic profiling of extracellular matrix and site-specific collagen post-translational modifications in an in vitro model of lung fibrosis. Matrix Biol. Plus 2019, 1, 100005. [Google Scholar] [CrossRef]
- Wong, M.Y.; Shoulders, M.D. Targeting defective proteostasis in the collagenopathies. Curr. Opin. Chem. Biol. 2019, 50, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Sipilä, K.H.; Drushinin, K.; Rappu, P.; Jokinen, J.; Salminen, T.A.; Salo, A.M.; Käpylä, J.; Myllyharju, J.; Heino, J. Proline hydroxylation in collagen supports integrin binding by two distinct mechanisms. J. Biol. Chem. 2018, 293, 7645–7658. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xu, R. Roles of PLODs in Collagen Synthesis and Cancer Progression. Front. Cell Dev. Biol. 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-dependent regulation of collagen metabolism. Cell. Mol. Life Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.J.; Lindert, U.; Opitz, L.; Hausser, I.; Rohrbach, M.; Giunta, C. Transcriptome profiling of primary skin fibroblasts reveal distinct molecular features between PLOD1- and FKBP14-kyphoscoliotic Ehlers-Danlos syndrome. Genes (Basel) 2019, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Castori, M. Ehlers-Danlos syndrome, hypermobility type: An underdiagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations. ISRN Dermatol. 2012, 2012, 751768. [Google Scholar] [CrossRef] [PubMed]
- DiChiara, A.S.; Li, R.C.; Suen, P.H.; Hosseini, A.S.; Taylor, R.J.; Weickhardt, A.F.; Malhotra, D.; McCaslin, D.R.; Shoulders, M.D. A cysteine-based molecular code informs collagen C-propeptide assembly. Nat. Commun. 2018, 9, 4206. [Google Scholar] [CrossRef]
- Caviness, P.; Bauer, R.; Tanaka, K.; Janowska, K.; Roeser, J.R.; Harter, D.; Sanders, J.; Ruth, C.; Matsushita, O.; Sakon, J. Ca2+-induced orientation of tandem collagen binding domains from clostridial collagenase ColG permits two opposing functions of collagen fibril formation and retardation. FEBS J. 2018, 285, 3254–3269. [Google Scholar] [CrossRef]
- Heumüller, S.E.; Talantikite, M.; Napoli, M.; Armengaud, J.; Mörgelin, M.; Hartmann, U.; Sengle, G.; Paulsson, M.; Moali, C.; Wagener, R. C-terminal proteolysis of the collagen VI α3 chain by BMP-1 and proprotein convertase (s) releases endotrophin in fragments of different sizes. J. Biol. Chem. 2019, 294, 13769–13780. [Google Scholar] [CrossRef]
- Graham, J.; Raghunath, M.; Vogel, V. Fibrillar fibronectin plays a key role as nucleator of collagen I polymerization during macromolecular crowding-enhanced matrix assembly. Biomater. Sci. 2019, 7, 4519–4535. [Google Scholar] [CrossRef]
- Goldbloom-Helzner, L.; Hao, D.; Wang, A. Developing regenerative treatments for developmental defects, injuries, and diseases using extracellular matrix collagen-targeting peptides. Int. J. Mol. Sci. 2019, 20, 4072. [Google Scholar] [CrossRef]
- Hoop, C.L.; Zhu, J.; Nunes, A.M.; Case, D.A.; Baum, J. Revealing accessibility of cryptic protein binding sites within the functional collagen fibril. Biomolecules 2017, 7, 76. [Google Scholar] [CrossRef]
- McKay, T.B.; Priyadarsini, S.; Karamichos, D. Mechanisms of collagen crosslinking in diabetes and keratoconus. Cells 2019, 8, 1239. [Google Scholar] [CrossRef] [PubMed]
- Rosell-Garcia, T.; Rodriguez-Pascual, F. Enhancement of collagen deposition and cross-linking by coupling lysyl oxidase with bone morphogenetic protein-1 and its application in tissue engineering. Sci. Rep. 2018, 8, 10780. [Google Scholar] [CrossRef] [PubMed]
- Rosell-García, T.; Paradela, A.; Bravo, G.; Dupont, L.; Bekhouche, M.; Colige, A.; Rodriguez-Pascual, F. Differential cleavage of lysyl oxidase by the metalloproteinases BMP1 and ADAMTS2/14 regulates collagen binding through a tyrosine sulfate domain. J. Biol. Chem. 2019, 294, 11087–11100. [Google Scholar] [CrossRef] [PubMed]
- Harlow, C.R.; Wu, X.; van Deemter, M.; Gardiner, F.; Poland, C.; Green, R.; Sarvi, S.; Brown, P.; Kadler, K.E.; Lu, Y.; et al. Targeting lysyl oxidase reduces peritoneal fibrosis. PLoS ONE 2017, 12, e0183013. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Xiong, X.; Kong, X.; Xie, J. The role of the lysyl oxidases in tissue repair and remodeling: A concise review. Tissue Eng. Regen. Med. 2017, 14, 15–30. [Google Scholar] [CrossRef]
- Petersen, J.W.; Douglas, J.Y. Tenascin-X, collagen, and Ehlers-Danlos syndrome: Tenascin-X gene defects can protect against adverse cardiovascular events. Med. Hypotheses 2013, 81, 443–447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hielscher, A.; Ellis, K.; Qiu, C.; Porterfield, J.; Gerecht, S. Fibronectin deposition participates in extracellular matrix assembly and vascular morphogenesis. PLoS ONE 2016, 11, e0147600. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kim, J.S.; Choi, E.K.; Kim, J.; Kim, K.M.; Seo, H.R. TGF-β-independent CTGF induction regulates cell adhesion mediated drug resistance by increasing collagen I in HCC. Oncotarget 2017, 8, 21650–21662. [Google Scholar] [CrossRef] [PubMed]
- Spada, S.; Tocci, A.; Di Modugno, F.; Nisticò, P. Fibronectin as a multiregulatory molecule crucial in tumor matrisome: From structural and functional features to clinical practice in oncology. J. Exp. Clin. Cancer Res. 2021, 40, 102. [Google Scholar] [CrossRef]
- Duong, T.E.; Hagood, J.S. Epigenetic regulation of myofibroblast phenotypes in fibrosis. Curr. Pathobiol. Rep. 2018, 6, 79–96. [Google Scholar] [CrossRef]
- Wang, P.; Shu, B.; Xu, Y.; Zhu, J.; Liu, J.; Zhou, Z.; Chen, L.; Zhao, J.; Liu, X.; Qi, S.; et al. Basic fibroblast growth factor reduces scar by inhibiting the differentiation of epidermal stem cells to myofibroblasts via the Notch1/Jagged1 pathway. Stem Cell Res. Ther. 2017, 8, 114. [Google Scholar] [CrossRef]
- Gómez-Leduc, T.; Desancé, M.; Hervieu, M.; Legendre, F.; Ollitrault, D.; de Vienne, C.; Herlicoviez, M.; Galéra, P.; Demoor, M. Hypoxia is a critical parameter for chondrogenic differentiation of human umbilical cord blood mesenchymal stem cells in type I/III collagen sponges. Int. J. Mol. Sci. 2017, 18, 1933. [Google Scholar] [CrossRef]
- Zeng, F.; Harris, R.C. Epidermal growth factor, from gene organization to bedside. Semin Cell Dev. Biol. 2014, 28, 2–11. [Google Scholar] [CrossRef]
- Yang, I.V. Epigenomics of idiopathic pulmonary fibrosis. Epigenomics 2012, 4, 195–203. [Google Scholar] [CrossRef]
- Perdigoto, C.N.; Valdes, V.J.; Bardot, E.S.; Ezhkova, E. Epigenetic regulation of epidermal differentiation. Cold Spring Harb. Perspect. Med. 2014, 4, a015263. [Google Scholar] [CrossRef]
- Stoll, S.; Wang, C.; Qiu, H. DNA methylation and histone modification in hypertension. Int. J. Mol. Sci. 2018, 19, 1174. [Google Scholar] [CrossRef]
- Vandiver, A.R.; Irizarry, R.A.; Hansen, K.D.; Garza, L.A.; Runarsson, A.; Li, X.; Chien, A.L.; Wang, T.S.; Leung, S.G.; Kang, S.; et al. Age and sun exposure-related widespread genomic blocks of hypomethylation in nonmalignant skin. Genome Biol. 2015, 16, 80. [Google Scholar] [CrossRef]
- Moulin, L.; Cenizo, V.; Antu, A.N.; André, V.; Pain, S.; Sommer, P.; Debret, R. Methylation of LOXL1 promoter by DNMT3A in aged human skin fibroblasts. Rejuvenation Res. 2017, 20, 103–110. [Google Scholar] [CrossRef]
- Ghosh, K.; O’Neil, K.; Capell, B.C. Histone modifiers: Dynamic regulators of the cutaneous transcriptome. J. Dermatol. Sci. 2018, 89, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Peterson, L.M.; Wilking-Busch, M.J.; Ndiaye, M.A.; Philippe, C.G.A.; Setaluri, V.; Ahmad, N. Sirtuins in skin and skin cancers. Skin Pharmacol. Physiol. 2017, 30, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Carlomosti, F.; D’Agostino, M.; Beji, S.; Torcinaro, A.; Rizzi, R.; Zaccagnini, G.; Maimone, B.; Di Stefano, V.; De Santa, F.; Cordisco, S.; et al. Oxidative stress-induced miR-200c disrupts the regulatory loop among SIRT1, FOXO1, and eNOS. Antioxid. Redox Signal. 2017, 27, 328–344. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and function of human matrix metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Tan, Y. Different roles of matrix metalloproteinase 2 in osteolysis of skeletal dysplasia and bone metastasis (Review). Mol. Med. Rep. 2021, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864 Pt A, 1940–1951. [Google Scholar] [CrossRef]
- Durmanova, V.; Javor, J.; Parnicka, Z.; Minarik, G.; Ocenasova, A.; Vaseckova, B.; Reznakova, V.; Kralova, M.; Hromadka, T.; Shawkatova, I. Impact of MMP2 rs243865 and MMP3 rs3025058 Polymorphisms on Clinical Findings in Alzheimer’s Disease Patients. Mediat. Inflamm. 2021, 2021, 5573642. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, Y.; Huang, W.; Zeng, Y.; Li, X. Association between matrix metalloproteinase-1 (MMP-1) protein level and the risk of rheumatoid arthritis and osteoarthritis: A meta-analysis. Braz. J. Med. Biol. Res. 2020, 54, e10366. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.C.; Puzzi, M.B. The effect of aging in primary human dermal fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef]
- Loffek, S.; Schilling, O.; Franzke, C.W. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef]
- Mirastschijski, U.; Lupše, B.; Maedler, K.; Sarma, B.; Radtke, A.; Belge, G.; Dorsch, M.; Wedekind, D.; McCawley, L.J.; Boehm, G.; et al. Matrix metalloproteinase-3 is key effector of TNF-α-induced collagen degradation in skin. Int. J. Mol. Sci. 2019, 20, 5234. [Google Scholar] [CrossRef]
- Ren, X.; Lamb, G.D.; Murphy, R.M. Distribution and activation of matrix metalloproteinase-2 in skeletal muscle fibers. Am. J. Physiol.-Cell Physiol. 2019, 317, 613–625. [Google Scholar] [CrossRef]
- Liu, J.; Khalil, R.A. Matrix metalloproteinase inhibitors as investigational and therapeutic tools in unrestrained tissue remodeling and pathological disorders. Prog. Mol. Biol. Transl. Sci. 2017, 148, 355–420. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care (New Rochelle) 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Velez, D.O.; Ranamukhaarachchi, S.K.; Kumar, A.; Modi, R.N.; Lim, E.W.; Engler, A.J.; Metallo, C.M.; Fraley, S.I. 3D collagen architecture regulates cell adhesion through degradability, thereby controlling metabolic and oxidative stress. Integr. Biol. 2019, 11, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Gardelli, C.; Russo, L.; Cipolla, L.; Moro, M.; Andriani, F.; Rondinone, O.; Nicotra, F.; Sozzi, G.; Bertolini, G.; Roz, L. Differential glycosylation of collagen modulates lung cancer stem cell subsets through β1 integrin-mediated interactions. Cancer Sci. 2021, 112, 217–230. [Google Scholar] [CrossRef]
- Hoy, R.C.; D’Erminio, D.N.; Krishnamoorthy, D.; Natelson, D.M.; Laudier, D.M.; Illien-Jünger, S.; Iatridis, J.C. Advanced glycation end products cause RAGE-dependent annulus fibrosus collagen disruption and loss identified using in situ second harmonic generation imaging in mice intervertebral disk in vivo and in organ culture models. JOR Spine 2020, 3, e1126. [Google Scholar] [CrossRef]
- Bansode, S.; Bashtanova, U.; Li, R.; Clark, J.; Müller, K.H.; Puszkarska, A.; Goldberga, I.; Chetwood, H.H.; Reid, D.G.; Colwell, L.J.; et al. Glycation changes molecular organization and charge distribution in type I collagen fibrils. Sci. Rep. 2020, 10, 3397. [Google Scholar] [CrossRef]
- Pennacchi, P.C.; de Almeida, M.E.; Gomes, O.L.; Faião-Flores, F.; de Araújo Crepaldi, M.C.; Dos Santos, M.F.; de Moraes Barros, S.B.; Maria-Engler, S.S. Glycated reconstructed human skin as a platform to study the pathogenesis of skin aging. Tissue Eng. Part A 2015, 21, 2417–2425. [Google Scholar] [CrossRef]
- Tsamis, A.; Krawiec, J.T.; Vorp, D.A. Elastin and collagen fiber microstructure of the human aorta in aging and disease: A review. J. R. Soc. Interface 2013, 10, 20121004. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, J.; Chen, Q.; Lei, C.; Qiao, B.; Liu, Q. SPP1 and AGER as potential prognostic biomarkers for lung adenocarcinoma. Oncol. Lett. 2018, 15, 7028–7036. [Google Scholar] [CrossRef]
- Nyström, A.; Thriene, K.; Mittapalli, V.; Kern, J.S.; Kiritsi, D.; Dengjel, J.; Bruckner-Tuderman, L. Losartan ameliorates dystrophic epidermolysis bullosa and uncovers new disease mechanisms. EMBO Mol. Med. 2015, 7, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, G.; Zingkou, E.; Pampalakis, G. Redirecting drug repositioning to discover innovative cosmeceuticals. Exp. Dermatol. 2021, 30, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, N.A.; Dyuzhakova, A.V.; Vaiman, E.E.; Nikitina, E.I.; Borzykh, O.B.; Nasyrova, R.F. The role of genetic factors of endogenous hyaluronic acid metabolism in maintaining skin homeostasis. Bull. Dermatol. Venerol. 2021, 97, 24–38. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).