Preparation of Readily-to-Use Stilbenoids Extract from Morus alba Callus Using a Natural Deep Eutectic Solvent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Preparation of NADES
2.4. Extraction
2.5. Stability Study
2.6. Determination of Stilbenoids by HPLC Analysis
2.7. Anti-Melanogenesis Activity
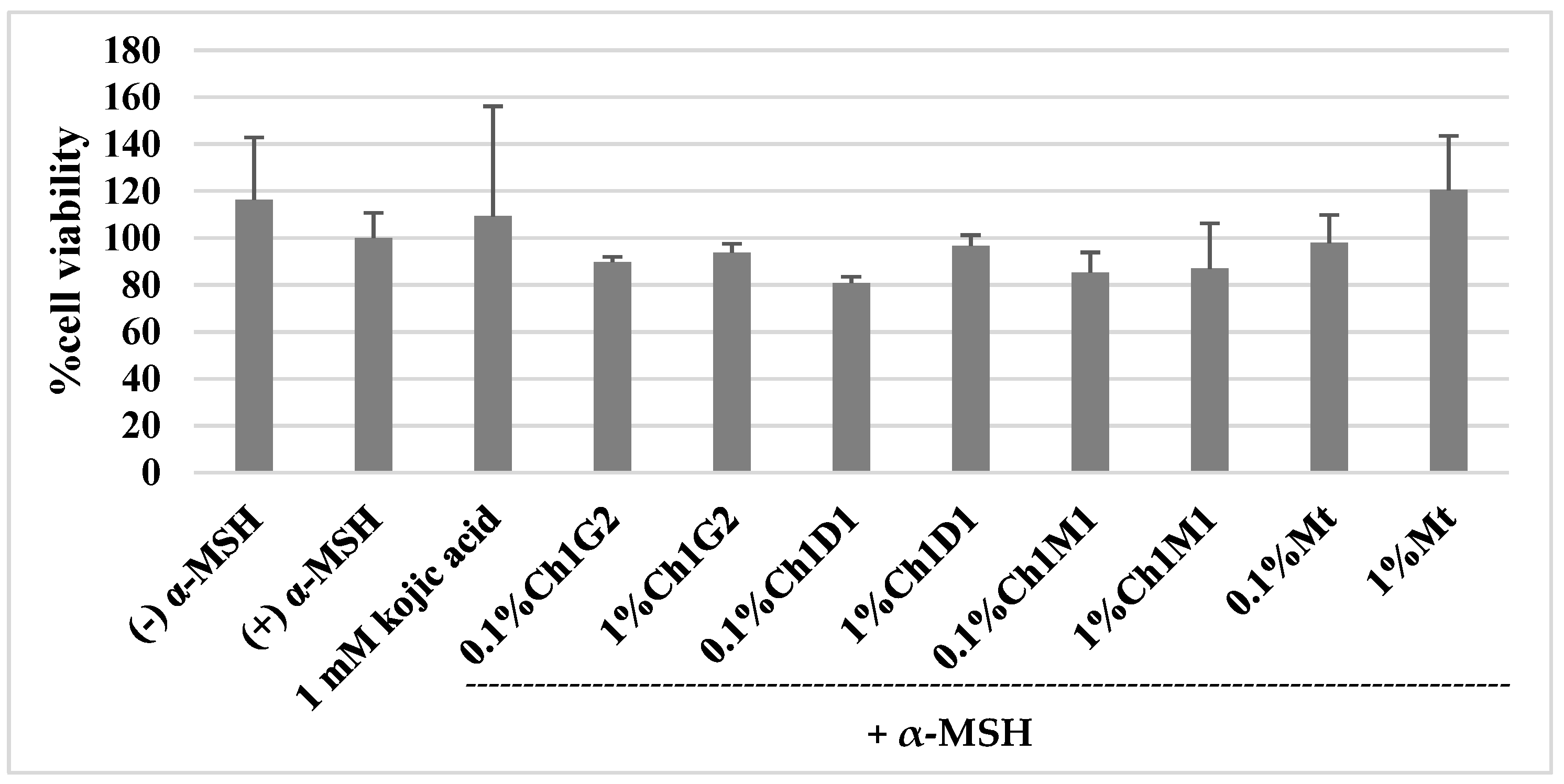
2.7.1. Cytotoxicity Assay
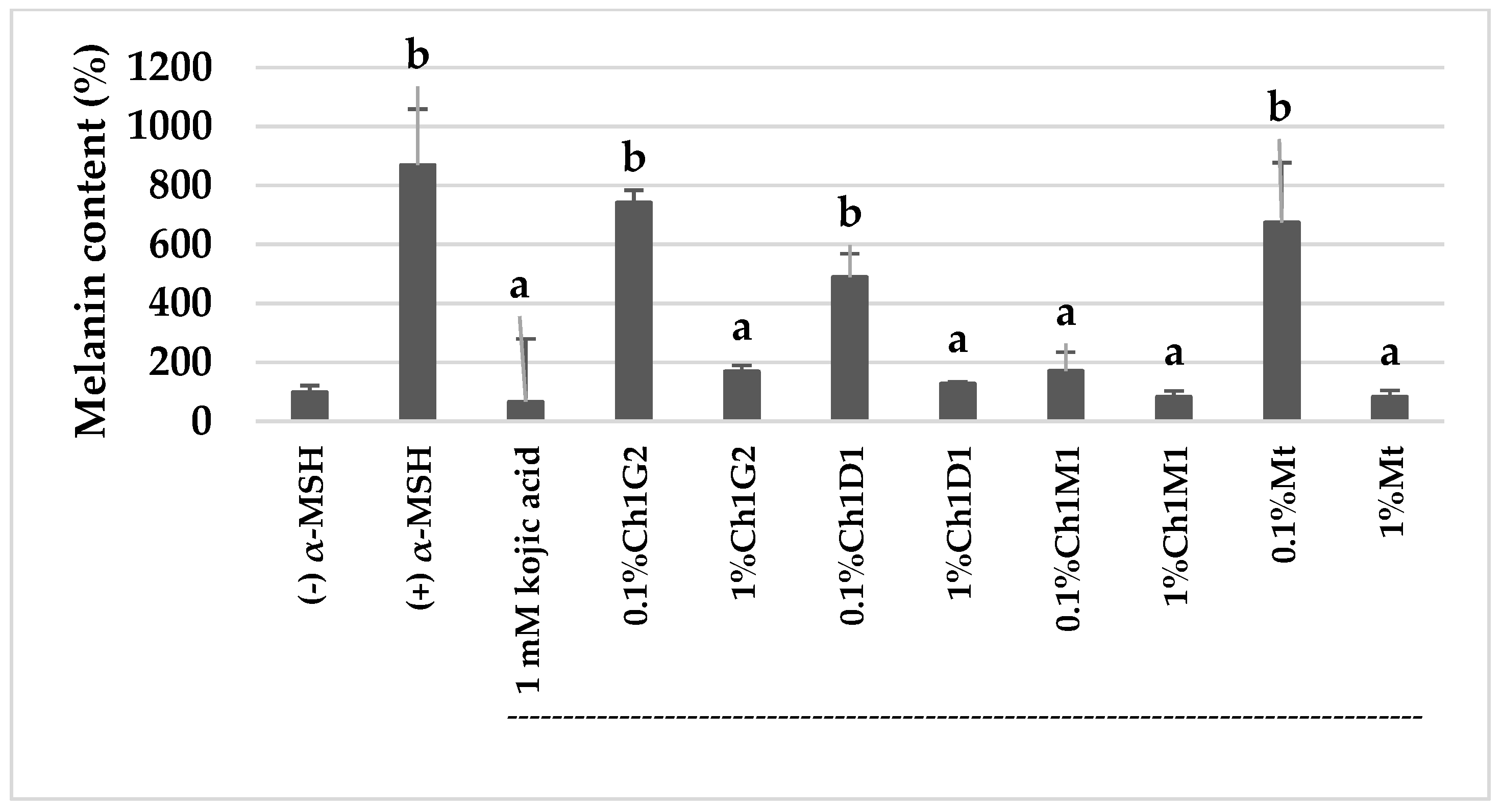
2.7.2. Inhibitory Activity in Extracellular Melanin Production
2.8. Skin Irritation Test
2.9. Statistical Analysis
3. Results
4. Discussion
4.1. Capacity of NADES for M. alba Callus Extraction
4.2. Stilbenoids Content and Anti-Melanogenesis Activity
4.3. Stilbenoids Stability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 7, 70–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.L.Á.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef]
- González, C.G.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents for the “green”extraction of vanillin from vanilla pods. Flavour Fragr. J. 2018, 33, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Doldolova, K.; Bener, M.; Lalikoğlu, M.; Aşçı, Y.S.; Arat, R.; Apak, R. Optimization and modeling of microwave-assisted extraction of curcumin and antioxidant compounds from turmeric by using natural deep eutectic solvents. Food Chem. 2021, 353, 129337. [Google Scholar] [CrossRef]
- Sun, B.; Zheng, Y.L.; Yang, S.K.; Zhang, J.R.; Cheng, X.Y.; Ghiladi, R.; Ma, Z.; Wang, J.; Deng, W.W. One-pot method based on deep eutectic solvent for extraction and conversion of polydatin to resveratrol from Polygonum cuspidatum. Food Chem. 2021, 343, 128498. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, J.; Zhao, Y.; Yu, L.; He, Y.; Wan, H.; Li, C. Screening, optimization, and bioavailability research of natural deep eutectic solvent extracts from Radix Pueraria. Molecules 2021, 26, 729. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, X.; Yang, G.; Bi, Y.; Liu, W. Green and efficient extraction of resveratrol from peanut roots using deep eutectic solvents. J. Chem. 2018, 4091930, 1–9. [Google Scholar]
- Komaikul, J.; Kitisripanya, T.; Likhitwitayawuid, K.; Sritularak, B.; Tanaka, H.; Putalun, W. Improvement of stilbenoid production by 2-hydroxypropyl-β-cyclodextrin in white mulberry (Morus alba L.) callus cultures. Nat. Prod. Res. 2019, 33, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Park, K.C.; Park, J.H.; Lee, C.G.; Ye, S.K.; Park, J.Y. Inhibition of tyrosinase activity and melanin production by the chalcone derivative 1-(2-cyclohexylmethoxy-6-hydroxy-phenyl)-3-(4-hydroxymethyl-phenyl)-propenone. Biochem. Biophys. Res. Commun. 2016, 480, 648–654. [Google Scholar] [CrossRef]
- Tanticharakunsiri, W.; Mangmool, S.; Wongsariya, K.; Ochaikul, D. Characteristics and upregulation of antioxidant enzymes of kitchen mint and oolong tea kombucha beverages. J. Food Biochem. 2021, 45, e13574. [Google Scholar] [CrossRef]
- OECD. Guideline for the Testing of Chemicals, No.439, In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. Available online: https://www.oecd-ilibrary.org/docserver/9789264242845-en.pdf?expires=1628777920&id=id&accname=guest&checksum=338F044AE7A8325B23AA0416C2D14217 (accessed on 12 August 2021).
- Ivanović, M.; Albreht, A.; Krajnc, P.; Vovk, I.; Islamčević Razboršek, M. Sustainable ultrasound-assisted extraction of valuable phenolics from inflorescences of Helichrysum arenarium L. using natural deep eutectic solvents. Ind. Crops Prod. 2021, 160, 113102. [Google Scholar] [CrossRef]
- Islamčević Razboršek, M.; Ivanović, M.; Krajnc, P.; Kolar, M. Choline chloride based natural deep eutectic solvents as extraction media for extracting phenolic compounds from chokeberry (Aronia melanocarpa). Molecules 2020, 25, 1619. [Google Scholar] [CrossRef] [Green Version]
- Alishlah, T.; Mun’im, A.; Jufri, M. Optimization of urea-glycerin based nades-uae for oxyresveratrol extraction from Morus alba roots for preparation of skin whitening lotion. J. Young Pharm. 2019, 11, 155–160. [Google Scholar] [CrossRef]
- Usuki, A.; Ohashi, A.; Sato, H.; Ochiai, Y.; Ichihashi, M.; Funasaka, Y. The inhibitory effect of glycolic acid and lactic acid on melanin synthesis in melanoma cells. Exp. Dermatol. 2003, 12 (Suppl. 2), 43–50. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Ivanović, M.; Islamčević Razboršek, M.; Kolar, M. Innovative extraction techniques using deep eutectic solvents and analytical methods for the isolation and characterization of natural bioactive compounds from plant material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.J.; Chen, L.X.; Kang, N.; Qiu, F. Simultaneous determination of five characteristic stilbene glycosides in root bark of Morus albus L. (Cortex Mori) using high-performance liquid chromatography. Phytochem. Anal. 2011, 22, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Komaikul, J.; Kitisripanya, T.; Inyai, C.; Likhitwitayawuid, K.; Sritularak, B.; Tanaka, H.; Putalun, W. Phytostilbenoid production in white mulberry (Morus alba L.) cell culture using bioreactors and simple deglycosylation by endogenous enzymatic hydrolysis. Vitro Cell. Dev. Biol. —Plant 2019, 55, 199–208. [Google Scholar] [CrossRef]
- Mohamed, M. Green, environment-friendly, analytical tools give insights in pharmaceuticals and cosmetics analysis. Trends Anal. Chem. 2015, 66, 176–192. [Google Scholar] [CrossRef]
- Yi, X.; Guo, D.; Deng, X.; Li, B.; Fan, X.; Zhu, J. Determination of methanol in cosmetics by headspace and multidimensional gas chromatography with mass spectrometric detection. J. AOAC Int. 2011, 94, 655–659. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Szulc-Musioł, B.; Sarecka-Hujar, B. The use of micro-and nanocarriers for resveratrol delivery into and across the skin in different skin diseases—A literature review. Pharmaceutics 2021, 13, 451. [Google Scholar] [CrossRef]

| Abbreviation | Hydrogen Bonding Acceptor | Hydrogen Bonding Donor | Molar Ratio |
|---|---|---|---|
| Ch1G2 | Choline chloride | Glycerol | 1:2 |
| Ch1G1 | Choline chloride | Glycerol | 1:1 |
| Ch1D1 | Choline chloride | Dextrose | 1:1 |
| Ch1S1 | Choline chloride | Sorbitol | 1:1 |
| Ch1C1 | Choline chloride | Citric acid | 1:1 |
| Ch1O1 | Choline chloride | Oxalic acid | 1:1 |
| Ch1M1 | Choline chloride | Malic acid | 1:1 |
| NADES | (mg/g Callus Dry Weight) | ||
|---|---|---|---|
| Mulberroside A | Oxyresveratrol | Stilbenoids Content | |
| Ch1G2 | 4.93 ± 0.04 b | 0.13 ± 0.01 a | 5.06 ± 0.05 a,b |
| Ch1G1 | 2.20 ± 0.04 e | n.d. | 2.20 ± 0.04 c,d |
| Ch1D1 | 3.87 ± 0.16 c | n.d. | 3.87 ± 0.16 c,d |
| Ch1S1 | 1.67 ± 0.11 f | 0.08 ± 0.00 b | 1.74 ± 0.11 d |
| Ch1C1 | 2.95 ± 0.07 d | n.d. | 2.95 ± 0.07 c |
| Ch1O1 | 0.89 ± 0.04 g | 0.05 ± 0.01 b | 0.94 ± 0.05 d |
| Ch1M1 | 3.82 ± 0.08 c | 0.13 ± 0.01 a | 3.95 ± 0.08 b,c |
| Ethanol | 0.81 ± 0.04 g | n.d. | 0.81 ± 0.04 d |
| Methanol | 6.14 ± 0.37 a | 0.17 ± 0.04 a | 6.32 ± 0.40 a |
| Solvents | Ch1G2 | Ch1M1 | Methanol | |
|---|---|---|---|---|
| 4 °C | mg/g callus dry weight | |||
| Mulberroside A (mg/g) | 0 month 1 month 6 months | 4.93 ± 0.04 4.74 ± 0.06 4.65 ± 0.09 * | 3.82 ± 0.08 2.62 ± 0.17 * 2.94 ± 0.03 * | 6.14 ± 0.37 5.32 ± 0.16 * 5.28 ± 0.03 * |
| Oxyresveratrol (mg/g) | 0 month 1 month 6 months | 0.13 ± 0.01 0.09 ± 0.00 * 0.05 ± 0.01 * | 0.13 ± 0.01 n.d. n.d. | 0.17 ± 0.04 0.11 ± 0.00 0.07 ± 0.00 * |
| 30 °C | ||||
| Mulberroside A (mg/g) | 0 month 1 month 6 months | 4.93 ± 0.04 4.75 ± 0.12 2.15 ± 0.00 * | 3.82 ± 0.08 2.71 ± 0.11 * 2.32 ± 0.02 * | - - - |
| Oxyresveratrol (mg/g) | 0 month 1 month 6 months | 0.13 ± 0.01 n.d. n.d. | 0.13 ± 0.01 n.d. n.d. | - - - |
| 40 °C | ||||
| Mulberroside A (mg/g) | 0 month 1 month 6 months | 4.93 ± 0.04 5.15 ± 0.31 3.18 ± 0.03 * | 3.82 ± 0.08 1.74 ± 0.07 * n.d. | - - - |
| Oxyresveratrol (mg/g) | 0 month 1 month 6 months | 0.13 ± 0.01 n.d. n.d. | 0.13 ± 0.01 n.d. n.d. | - - - |
| DES | Ch1G2 | Ch1M1 | Methanol | |
|---|---|---|---|---|
| 4 °C | µg/mL (Percentage of residue content) | |||
| Mulberroside A | 1 month 6 months | 24.80 ± 0.48 (99.20%) 23.08 ± 0.80 (92.32%) | 20.20 ± 0.96 (80.80%) 16.88 ± 0.40 (67.52%) | 24.92 ± 1.80 (99.68%) 24.24 ± 1.08 (96.96%) |
| Oxyresveratrol (µg/mL) | 1 month 6 months | 17.52 ± 0.24 (70.08%) 17.56 ± 0.64 (70.24%) | 15.80 ± 0.52 (63.20%) 13.12 ± 0.32 (52.48%) | 19.68 ± 0.92 (78.72%) 17.84 ± 0.40 (71.36%) |
| Resveratrol (µg/mL) | 1 month 6 months | 16.08 ± 0.44 (64.2%) 17.96 ± 0.72 (71.84%) | 16.00 ± 1.52 (64.00%) 13.88 ± 0.52 (55.52%) | 23.40 ± 1.48 (93.60%) 20.00 ± 1.60 (80.00%) |
| 30 °C | ||||
| Mulberroside A (µg/mL) | 1 month 6 months | 20.12 ± 0.24 (80.48%) 22.24 ± 0.48 (88.96%) | 13.76 ± 0.88 (55.04%) 10.76 ± 0.52 (43.04%) | - - |
| Oxyresveratrol (µg/mL) | 1 month 6 months | 15.04 ± 0.28 (60.16%) 7.68 ± 0.08 (30.72%) | 13.32 ± 0.44 (53.28%) 3.36 ± 0.04 (13.44%) | - - |
| Resveratrol (µg/mL) | 1 month 6 months | 15.80 ± 1.20 (63.20%) 14.84 ± 0.60 (59.36%) | 14.08 ± 0.88 (56.32%) 9.80 ± 0.08 (39.20%) | - - |
| 40 °C | ||||
| Mulberroside A (µg/mL) | 1 month 6 months | 23.68 ± 1.28 (94.72%) 23.56 ± 0.28 (94.24%) | 8.96 ± 0.44 (35.84%) n.d. - | - - |
| Oxyresveratrol (µg/mL) | 1 month 6 months | 17.08 ± 0.76 (68.32%) n.d. - | 9.80 ± 0.16 (39.20%) n.d. - | - - |
| Resveratrol (µg/mL) | 1 month 6 months | 17.28 ± 1.00 (69.12%) 5.76 ± 0.12 (23.04%) | 12.56 ± 1.04 (50.24%) n.d. - | - - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komaikul, J.; Mangmool, S.; Putalun, W.; Kitisripanya, T. Preparation of Readily-to-Use Stilbenoids Extract from Morus alba Callus Using a Natural Deep Eutectic Solvent. Cosmetics 2021, 8, 91. https://doi.org/10.3390/cosmetics8030091
Komaikul J, Mangmool S, Putalun W, Kitisripanya T. Preparation of Readily-to-Use Stilbenoids Extract from Morus alba Callus Using a Natural Deep Eutectic Solvent. Cosmetics. 2021; 8(3):91. https://doi.org/10.3390/cosmetics8030091
Chicago/Turabian StyleKomaikul, Jukrapun, Supachoke Mangmool, Waraporn Putalun, and Tharita Kitisripanya. 2021. "Preparation of Readily-to-Use Stilbenoids Extract from Morus alba Callus Using a Natural Deep Eutectic Solvent" Cosmetics 8, no. 3: 91. https://doi.org/10.3390/cosmetics8030091
APA StyleKomaikul, J., Mangmool, S., Putalun, W., & Kitisripanya, T. (2021). Preparation of Readily-to-Use Stilbenoids Extract from Morus alba Callus Using a Natural Deep Eutectic Solvent. Cosmetics, 8(3), 91. https://doi.org/10.3390/cosmetics8030091






