Stimulation of the Fibrillar Collagen and Heat Shock Proteins by Nicotinamide or Its Derivatives in Non-Irradiated or UVA Radiated Fibroblasts, and Direct Anti-Oxidant Activity of Nicotinamide Derivatives
Abstract
:1. Introduction
2. Results
2.1. Stimulation of Type I Collagen, Type I Collagen Promoter, Type III Collagen, and Type V Collagen Protein by Nicotinamide, 2,6-Dihydroxynicotinamide, 2,4,5,6-Tetrahydroxynicotinamide, and 3-Hydroxypicolinamide in Non-Irradiated Fibroblasts
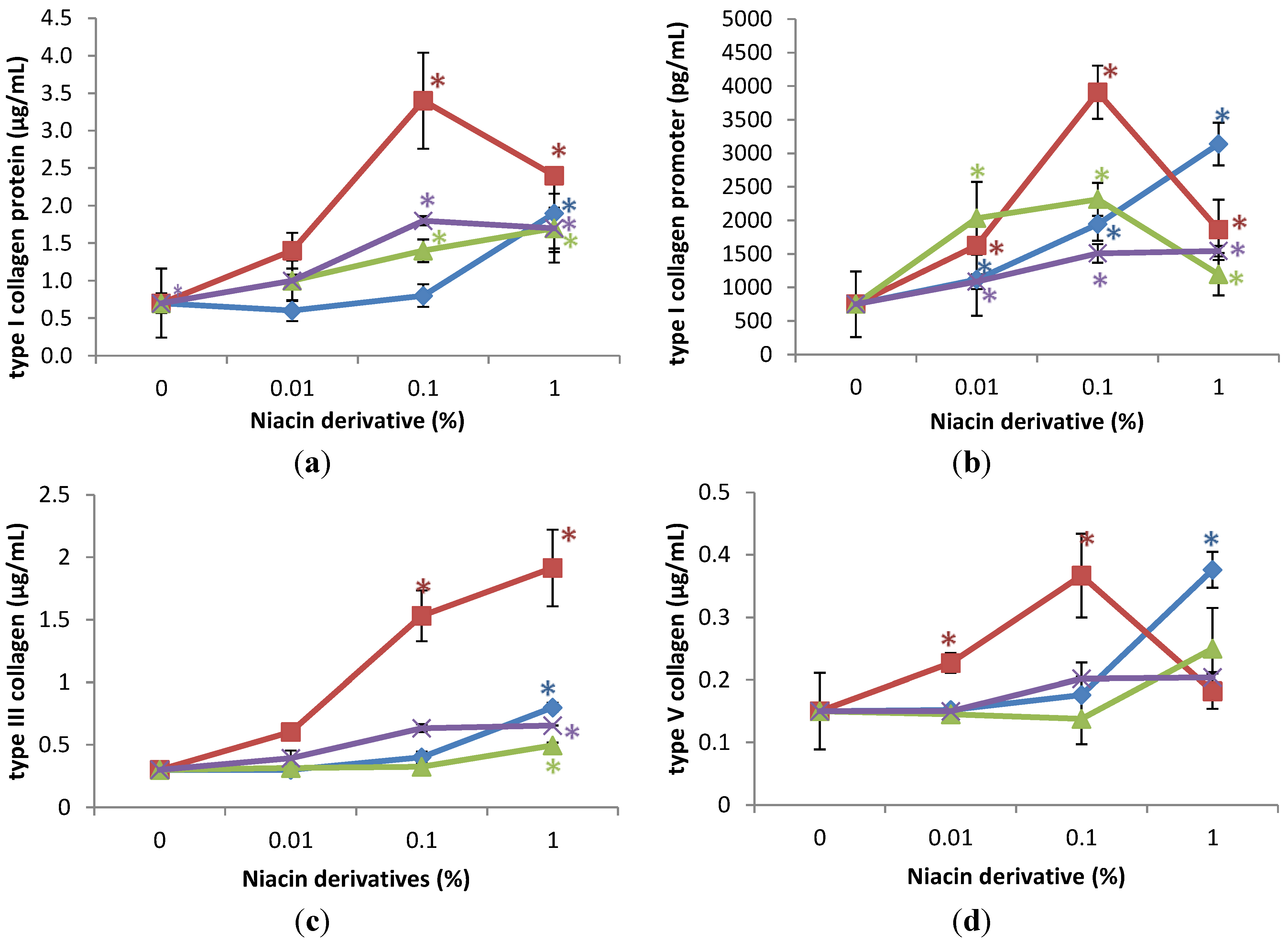
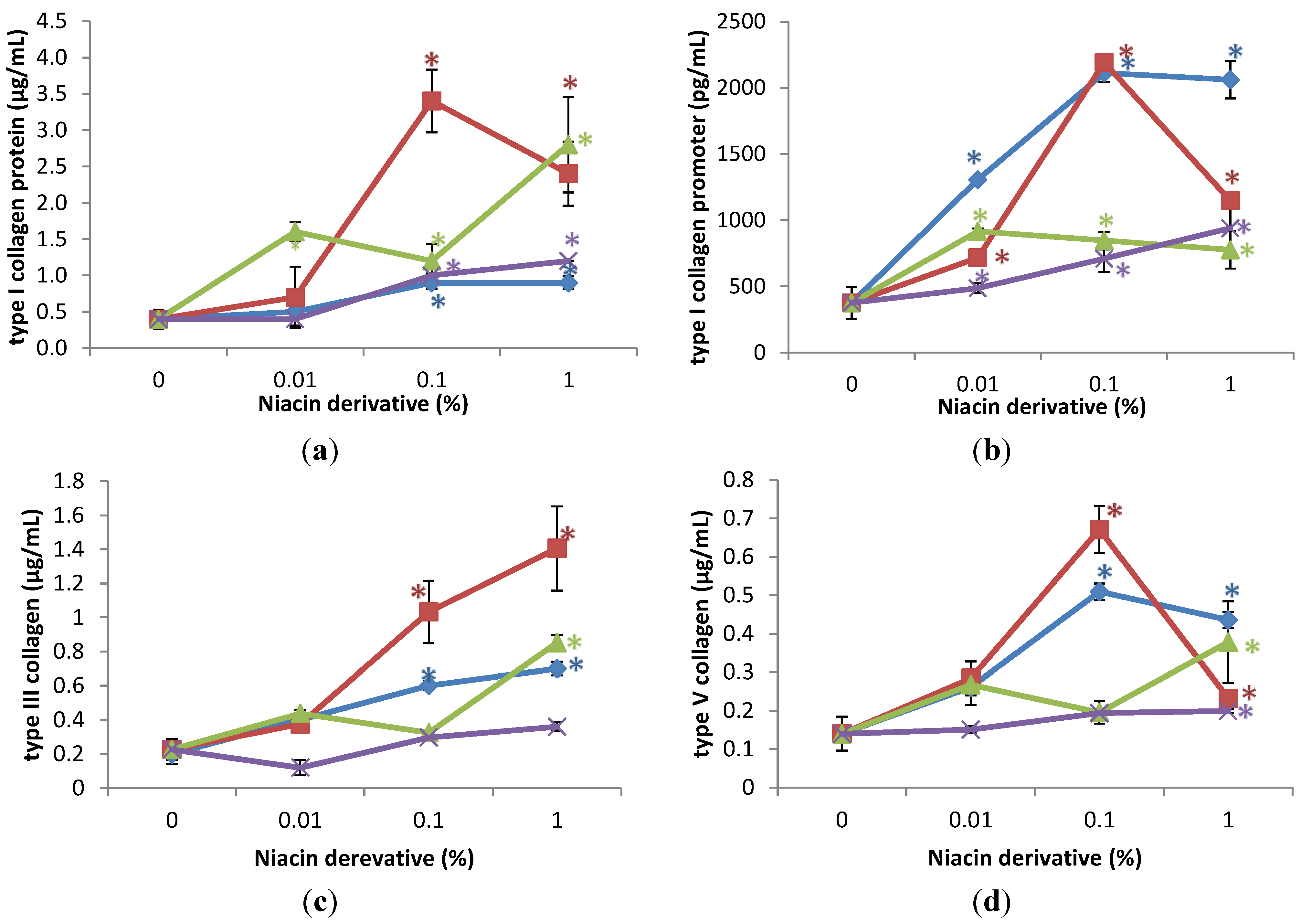
2.2. Stimulation of Expression of Type I Collagen, Type I Collagen Promoter, Type III Collagen, and Type V Collagen Protein by Nicotinamide, 2,6-Dihydroxynicotinamide, 2,4,5,6-Tetrahydroxynicotinamide, and 3-Hydroxypicolinamide in UVA Radiated Fibroblasts
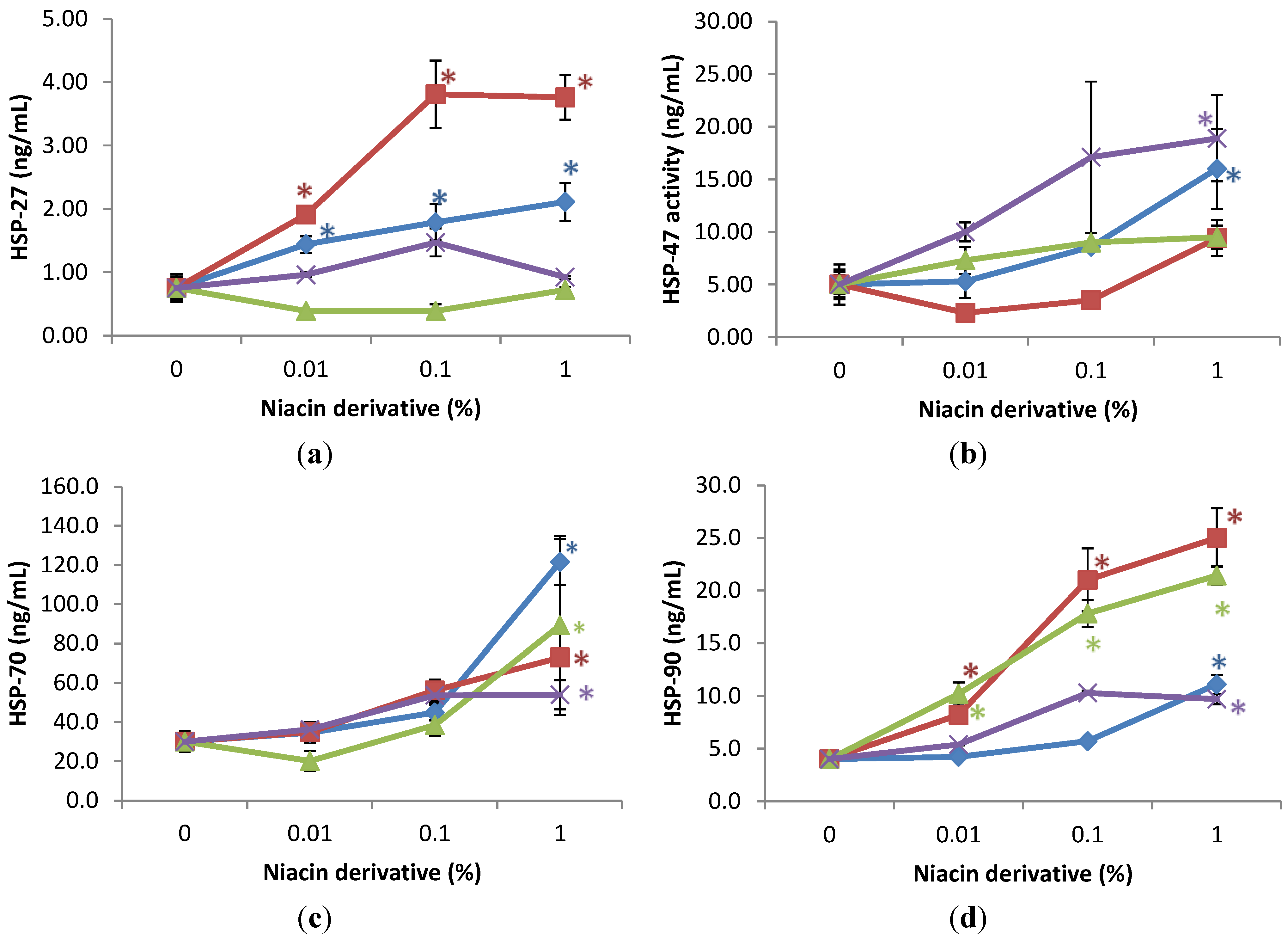
2.3. Stimulation of Heat Shock Protein-27, Heat Shock Protein-47, Heat Shock Protein-70, and Heat Shock Protein-90 by Nicotinamide, 2,6-Dihydroxynicotinamide, 2,4,5,6-Tetrahydroxynicotinamide, and 3-Hydroxypicolinamide in Non-Irradiated Fibroblasts
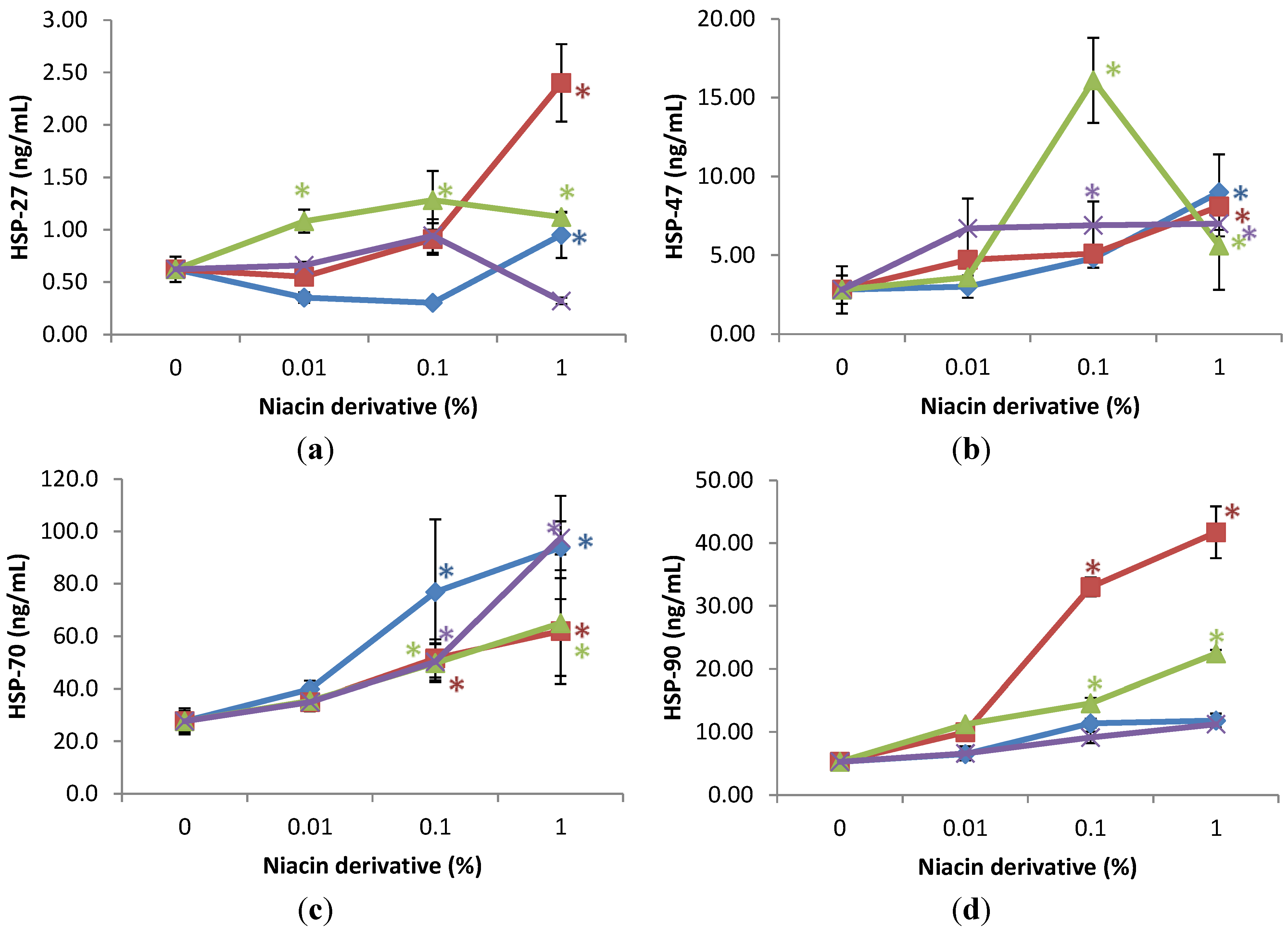
2.4. Stimulation of Heat Shock Protein-27, Heat Shock Protein-47, Heat Shock Protein-70, and Heat Shock Protein-90 by Nicotinamide, 2,6-Dihydroxynicotinamide, 2,4,5,6-Tetrahydroxynicotinamide, and 3-Hydroxypicolinamide Nicotinamide in UVA Radiated Fibroblasts
2.5. Direct Inhibition of ABTS Oxidation by Nicotinamide and Its Derivatives
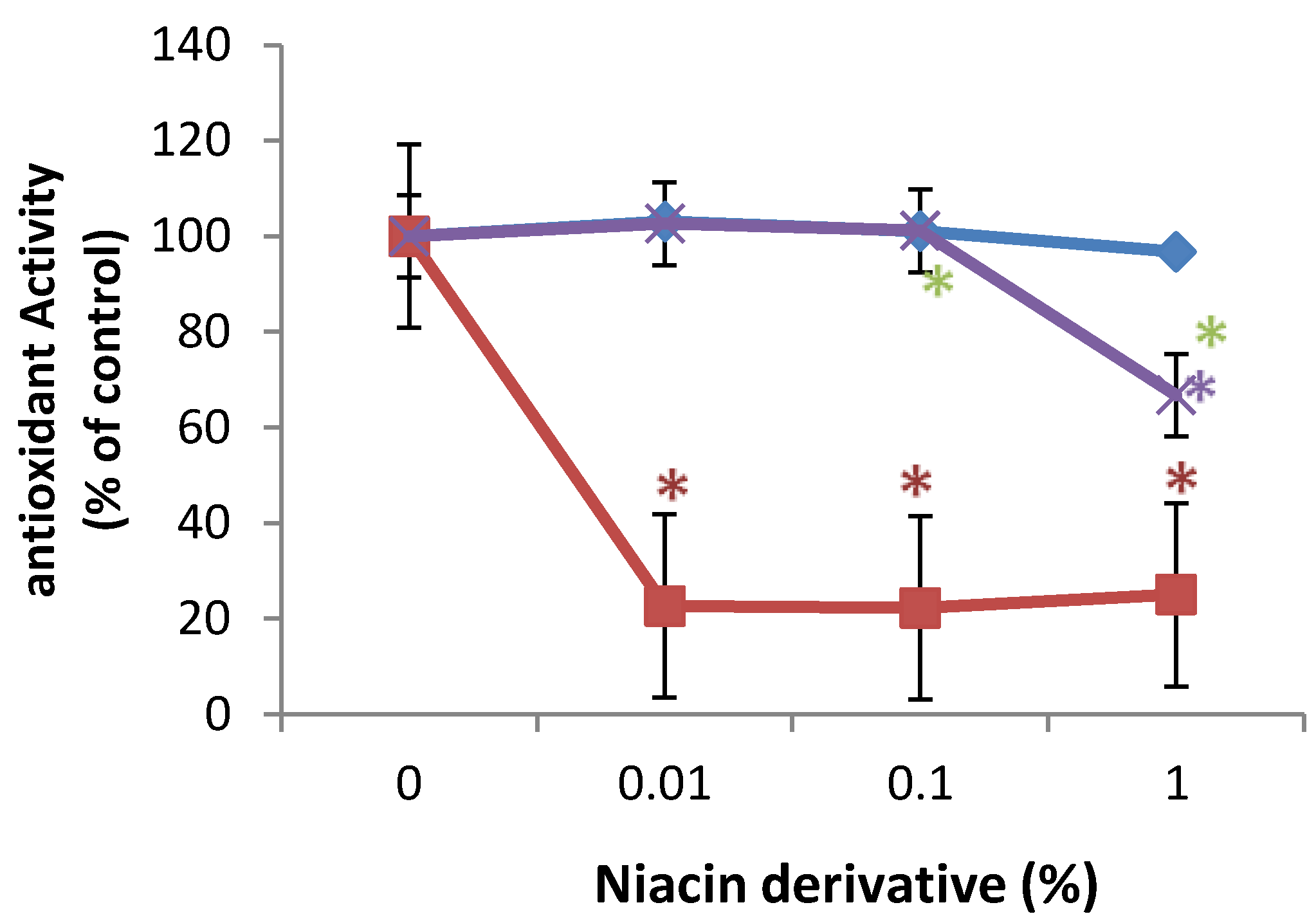
3. Discussion
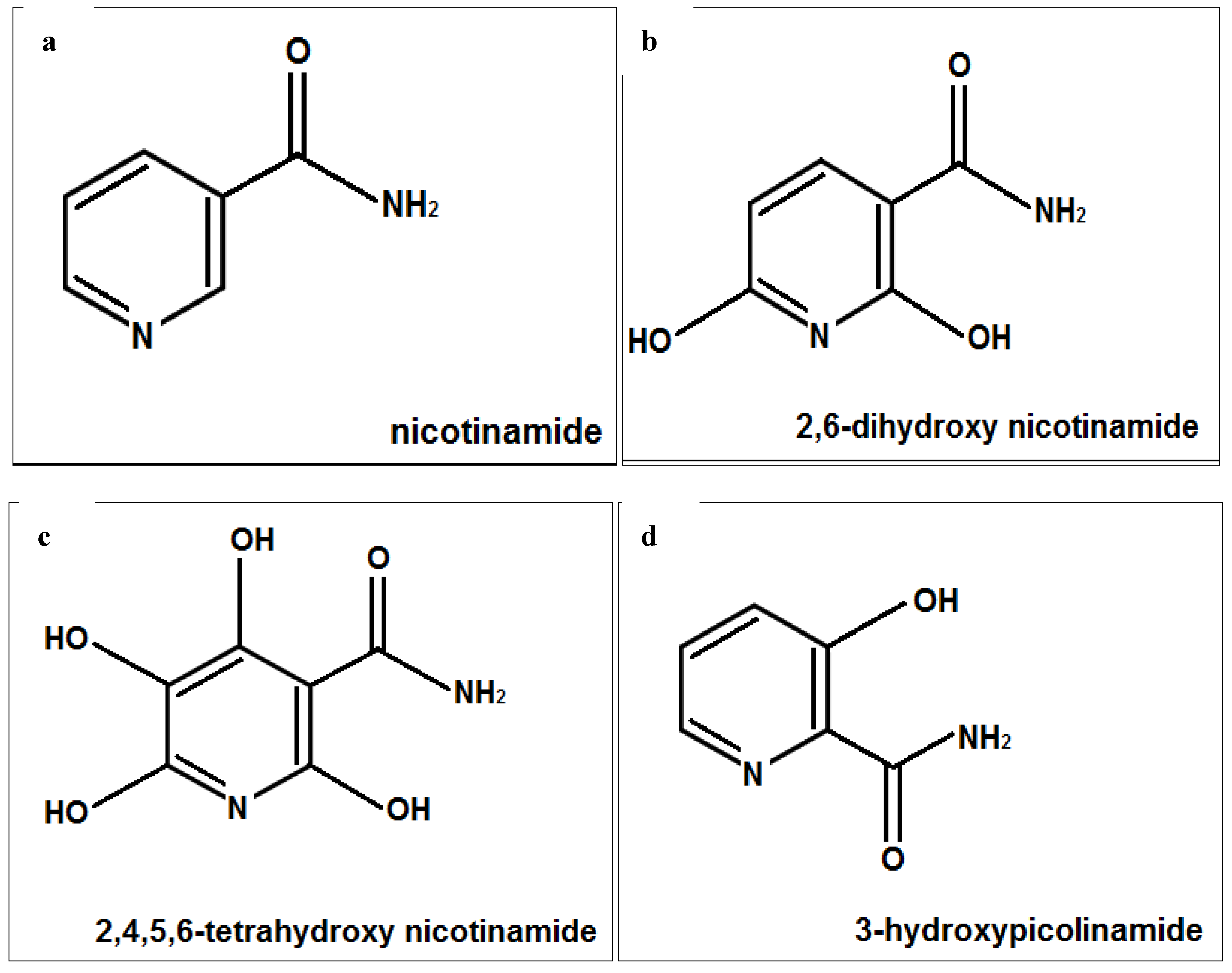
4. Materials and Methods
4.1. Cell Culture and Dosing
4.2. Collagen (Types I, III, V), and HSP (27, 47. 70, 90) Protein Levels
4.3. Type I Collagen Promoter Activity
4.4. Antioxidant Activity
4.5. Data Analysis
5. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Philips, N.; Samuel, M.; Arena, R.; Chen, Y.; Conte, J.; Natrajan, P.; Haas, G.; Gonzalez, S. Direct inhibition of elastase and matrixmetalloproteinases, and stimulation of biosynthesis of fibrillar collagens, elastin and fibrillins by xanthohumol. J. Cosmet. Sci. 2010, 61, 125–132. [Google Scholar] [PubMed]
- Philips, N.; Smith, J.; Keller, T.; Gonzalez, S. Predominant effects of Polypodium leucotomos on membrane integrity, lipid peroxidation, and expression of elastin and matrixmetalloproteinase-1 in ultraviolet radiation exposed fibroblasts, and keratinocytes. J. Dermatol. Sci. 2003, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Conte, J.; Chen, Y.; Natrajan, P.; Taw, M.; Keller, T.; Givant, J.; Tuason, M.; Dulaj, L.; Leonardi, D.; et al. Beneficial regulation of matrixmetalloproteinases and its inhibitors, fibrillar collagens and transforming growth factor-β by P. leucotomos, directly or in dermal fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells. Arch. Dermatol. Res. 2009, 301, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Keller, T.; Hendrix, C.; Hamilton, S.; Arena, R.; Tuason, M.; Gonzalez, S. Regulation of the extracellular matrix remodeling by lutein in dermal fibroblasts, melanoma cells, and ultraviolet radiation exposed fibroblasts. Arch. Dermatol. Res. 2007, 299, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Siomyk, H.; Bynum, D.; Gonzalez, S. Skin cancer, polyphenols, and oxidative stress. In Cancer: Oxidative Stress and Dietary Antioxidants; Academic Press: Waltham, MA, USA, 2014. [Google Scholar]
- Philips, N.; Samuel, M.; Parakandi, H.; Siomyk, H.; Gopal, S.; Jia, H.; Shahin, H. Vitamins in the therapy of inflammatory and oxidative disease. In Frontiers in Clinical Drug Research-Anti Allery Agents; Bentham Science Publishers: Bussum, The Netherlands, 2013; Volume 1. [Google Scholar]
- Philips, N.; Samuel, P.; Siomyk, H.; Parakandi, H.; Jia, H.; Gopal, S.; Shahin, H. Improved cell metabolism and strengthening of the extracellular matrix by nicotinamide, and copper for anti-skin aging. In Skin Aging: New Research; Nova Science: Hauppauge, NY, USA, 2012. [Google Scholar]
- Philips, N.; Samuel, P.; Parakandi, H.; Gopal, S.; Siomyk, H.; Ministro, A.; Thompson, T.; Borkow, G. Beneficial regulation of fibrillar collagens, heat shock protein-47, elastin fiber components, transforming growth factor-β1 vascular endothelial growth factor and oxidative stress effects by copper in dermal fibroblasts. Connect. Tissue Res. 2012, 53, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Khorramizadeh, M.R.; Tredget, E.E.; Telasky, C.; Shen, Q.; Ghahary, A. Aging differentially modulates the expression of collagen and collagenase in dermal fibroblasts. Mol. Cell. Biochem. 1999, 194, 99–108. [Google Scholar] [CrossRef]
- Arrigo, A.P.; Virot, S.; Chaufour, S.; Firdaus, W.; Kretz-Remy, C.; Diaz-Latoud, C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid. Redox Signal. 2005, 7, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Hoshino, T.; Yamashita, Y.; Tanaka, K.; Maji, D.; Sato, K.; Adachi, H.; Sobue, G.; Ihn, H.; Funasaka, Y.; et al. Prevention of UVB radiation-induced epidermal damage by expression of heat shock protein 70. J. Biol. Chem. 2010, 285, 5848–5858. [Google Scholar] [CrossRef] [PubMed]
- Gutsmann-Conrad, A.; Heydari, A.R.; You, S.; Richardson, A. The expression of heat shock protein 70 decreases with cellular senescence in vitro and in cells derived from young and old human subjects. Exp. Cell Res. 1998, 241, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Guan, S.; Fan, J.; Cheng, C.F.; Bright, A.M.; Chinn, C.; Chen, M.; Woodley, D.T. Extracellular heat shock protein-90α: Linking hypoxia to skin cell motility and wound healing. EMBO J. 2007, 26, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.X.; Tang, J.J.; Luo, H.; Jin, X.L.; Dai, F.; Yang, J.; Qian, Y.P.; Li, X.Z.; Zhou, B. Antioxidant and antiproliferative activities of hydroxyl-substituted Schiff bases. Bioorg. Med. Chem. Lett. 2010, 20, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Chan, P.T.; Ho, K.Y.; Fung, K.P.; Wang, J. Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem. Phys. Lipids 1996, 79, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gazák, R.; Sedmera, P.; Vrbacký, M.; Vostálová, J.; Drahota, Z.; Marhol, P.; Walterová, D.; Kren, V. Molecular mechanisms of silybin and 2,3-dehydrosilybin antiradical activity—Role of individual hydroxyl groups. Free Radic. Biol. Med. 2009, 46, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Moon, K.Y.; Lee, J.; Kim, Y.S. Downregulation of NF-kappaB activation in human keratinocytes by melanogenic inhibitors. J. Dermatol. Sci. 2003, 31, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Grange, P.A.; Raingeaud, J.; Calvez, V.; Dupin, N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathways. J. Dermatol. Sci. 2009, 56, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Damian, D.L.; Patterson, C.R.S.; Stapelberg, M.; Park, J.; Barnetson, R.S.; Halliday, G.M. Ultraviolet radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J. Investig. Dermatol. 2008, 128, 447–454. [Google Scholar] [PubMed]
- Maiese, K.; Chong, Z.Z.; Hou, J.; Shang, Y.C. The vitamin nicotinamide: Translating nutrition into clinical care. Molecules 2009, 14, 3446–3485. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Halliday, G.M.; Surjana, D.; Damian, D.L. Nicotinamide prevents ultraviolet radiation-induced cellular energy loss. Photochem. Photobiol. 2010, 86, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.C.; Halliday, G.M.; Damian, D.L. Nicotinamide enhances repair of arsenic and UVR-induced DNA damage in HaCaT keratinocytes and ex vivo human skin. PLoS ONE 2015, 10, e0117491. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Oblong, J.E.; Berge, C.A. Niacinamide: AB vitamin that improves aging facial skin appearance. Dermatol. Surg. 2005, 31, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, E.; Bertona, M.; Altabas, K.; Altabas, V.; Alessandrinin, G. Anti-inflammatory effect of a topical preparation containing nicotinamide, retinol, and 7-dehydrocholesterol in patients with acne: a gene expression study. Clin. Cosmet. Invest. Dermatol. 2012, 5, 33–37. [Google Scholar] [CrossRef]
- Kawada, A.; Konishi, N.; Oiso, N.; Kawara, S.; Date, A. Evaluation of anti-wrinkle effects of a novel cosmetic containing niacinamide. J. Dermatol. 2008, 35, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Pinkas-Sarafova, A.; Markova, N.G.; Simon, M. Dynamic changes in nicotinamide pyridine dinucleotide content in normal human epidermal keratinocytes and their effect on retinoic acid biosynthesis. Biochem. Biophys. Res. Commun. 2005, 336, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Namazi, M.R. Nicotinamide-containing sunscreens for use in Australasian countries and cancer-provoking conditions. Med. Hypotheses 2003, 60, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Oblong, J.E. The evolving role of the NAD+/nicotinamide metabolome in skin homeostasis, cellular bioenergetics, and aging. DNA Repair 2014, 23, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.J.; Hillebrand, G.G.; Raleigh, P.; Li, J.; Marmor, M.J.; Bertucci, V.; Grimes, P.E.; Mandy, S.H.; Perez, M.I.; Weinkle, S.H.; et al. A randomized, controlled comparative study of the wrinkle reduction benefits of a cosmetic niacinamide/peptide/retinyl propionate product regimen vs. a prescription 0.02% tretinoin product regimen. Br. J. Dermatol. 2010, 162, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.L.; Filho, P.C.; Neri, C.R.; Serra, O.A.; de Figueiredo, A.T.; Varela, J.A.; Longo, E. Synthesis and study of the photophysical properties of a new Eu3+ complex with 3-hydroxypicolinamide. J. Fluoresc. 2011, 21, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Imakubo, T.; Ichikawa, M.; Taniguchi, Y. A photoluminescent six-coordinated zinc(II) complex with hydroxides as axial ligands, [Zn(Hhpa)2(OH)2] (Hhpa = 3-hydroxypicolinamide). Dalton Trans. 2006, 21, 881–883. [Google Scholar] [CrossRef]
- Largeron, M.; Fleury, M.B. Acid-base properties of pristinamycin IA and related compounds. J. Pharm. Sci. 1992, 81, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Wessels, Q.; Pretorius, E.; Smith, C.M.; Nel, H. The potential of a niacinamide dominated cosmeceutical formulation on fibroblast activity and wound healing in vitro. Int. Wound J. 2014, 11, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Niforou, K.; Cheimonidou, C.; Trougakos, I.P. Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol. 2014, 2, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, anti-oxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Bachelor, M.A.; Bowden, G.T. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin. Cancer Biol. 2004, 14, 131–138. [Google Scholar] [CrossRef] [PubMed]
- DiGiovanni, J. Multistage carcinogenesis in mouse skin. Pharmacol. Ther. 1992, 54, 63–128. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F.R. Photocarcinogenesis: UVA vsx. UVB. Methods Enzymol. 2000, 319, 359–366. [Google Scholar]
- Krutmann, J. The role of UVA rays in skin aging. Eur. J. Dermatol. 2001, 11, 170–171. [Google Scholar] [PubMed]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef] [PubMed]
- DiPalma, J.R.; Thayer, W.S. Use of niacin as a drug. Annu. Rev. Nutr. 1991, 11, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Benavente, C.A.; Jacobson, M.K.; Jacobson, E.L. NAD in skin: Therapeutic approaches for niacin. Curr. Pharm. Des. 2009, 15, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Thappa, D.M. Pellagra and skin. Int. J. Dermatol. 2002, 41, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chong, Z.Z.; Maiese, K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front. Biosci 2004, 9, 2500–2520. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.M.; Rawling, J.M.; Roebuck, B.D.; Kirkland, J.B. Large supplements of nicotinic acid and nicotinamide increase tissue NAD+ and poly(ADP-ribose) levels but do not affect diethylnitrosamine-induced altered hepatic foci in Fischer-344 rats. J. Nutr. 1995, 125, 1455–1461. [Google Scholar] [PubMed]
- Yui, R.; Matsuura, E.T. Detection of deletions flanked by short direct repeats in mitochondrial DNA of aging Drosophila. Mutat. Res. 2006, 594, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Wang, D.; Hwang, C.P.; Liu, H.W.; Wei, J.; Lee, R.P.; Chen, H.I. The protective effect of niacinamide on ischemia-reperfusion-induced liver injury. J. Biomed. Sci. 2001, 8, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Traister, A.; Breitman, I.; Bar-Lev, E.; Zvibel, I.; Harel, A.; Halpern, Z.; Oren, R. Nicotinamide induces apoptosis and reduces collagen I and pro-inflammatory cytokines expression in rat hepatic stellate cells. Scand. J. Gastroenterol. 2005, 40, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z. Nicotinamide: Necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol. Sci. 2003, 24, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.L.; Boyer, J.L. Hepatic toxicity from large doses of vitamin-B3 (Nicotinamide). N. Engl. J. Med. 1973, 289, 1180–1182. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philips, N.; Chalensouk-Khaosaat, J.; Gonzalez, S. Stimulation of the Fibrillar Collagen and Heat Shock Proteins by Nicotinamide or Its Derivatives in Non-Irradiated or UVA Radiated Fibroblasts, and Direct Anti-Oxidant Activity of Nicotinamide Derivatives. Cosmetics 2015, 2, 146-161. https://doi.org/10.3390/cosmetics2020146
Philips N, Chalensouk-Khaosaat J, Gonzalez S. Stimulation of the Fibrillar Collagen and Heat Shock Proteins by Nicotinamide or Its Derivatives in Non-Irradiated or UVA Radiated Fibroblasts, and Direct Anti-Oxidant Activity of Nicotinamide Derivatives. Cosmetics. 2015; 2(2):146-161. https://doi.org/10.3390/cosmetics2020146
Chicago/Turabian StylePhilips, Neena, Jovinna Chalensouk-Khaosaat, and Salvador Gonzalez. 2015. "Stimulation of the Fibrillar Collagen and Heat Shock Proteins by Nicotinamide or Its Derivatives in Non-Irradiated or UVA Radiated Fibroblasts, and Direct Anti-Oxidant Activity of Nicotinamide Derivatives" Cosmetics 2, no. 2: 146-161. https://doi.org/10.3390/cosmetics2020146
APA StylePhilips, N., Chalensouk-Khaosaat, J., & Gonzalez, S. (2015). Stimulation of the Fibrillar Collagen and Heat Shock Proteins by Nicotinamide or Its Derivatives in Non-Irradiated or UVA Radiated Fibroblasts, and Direct Anti-Oxidant Activity of Nicotinamide Derivatives. Cosmetics, 2(2), 146-161. https://doi.org/10.3390/cosmetics2020146




