Neurocosmetics and Aromatherapy Through Neurocutaneous Receptors and Their Functional Implications in Cosmetics
Abstract
1. Introduction
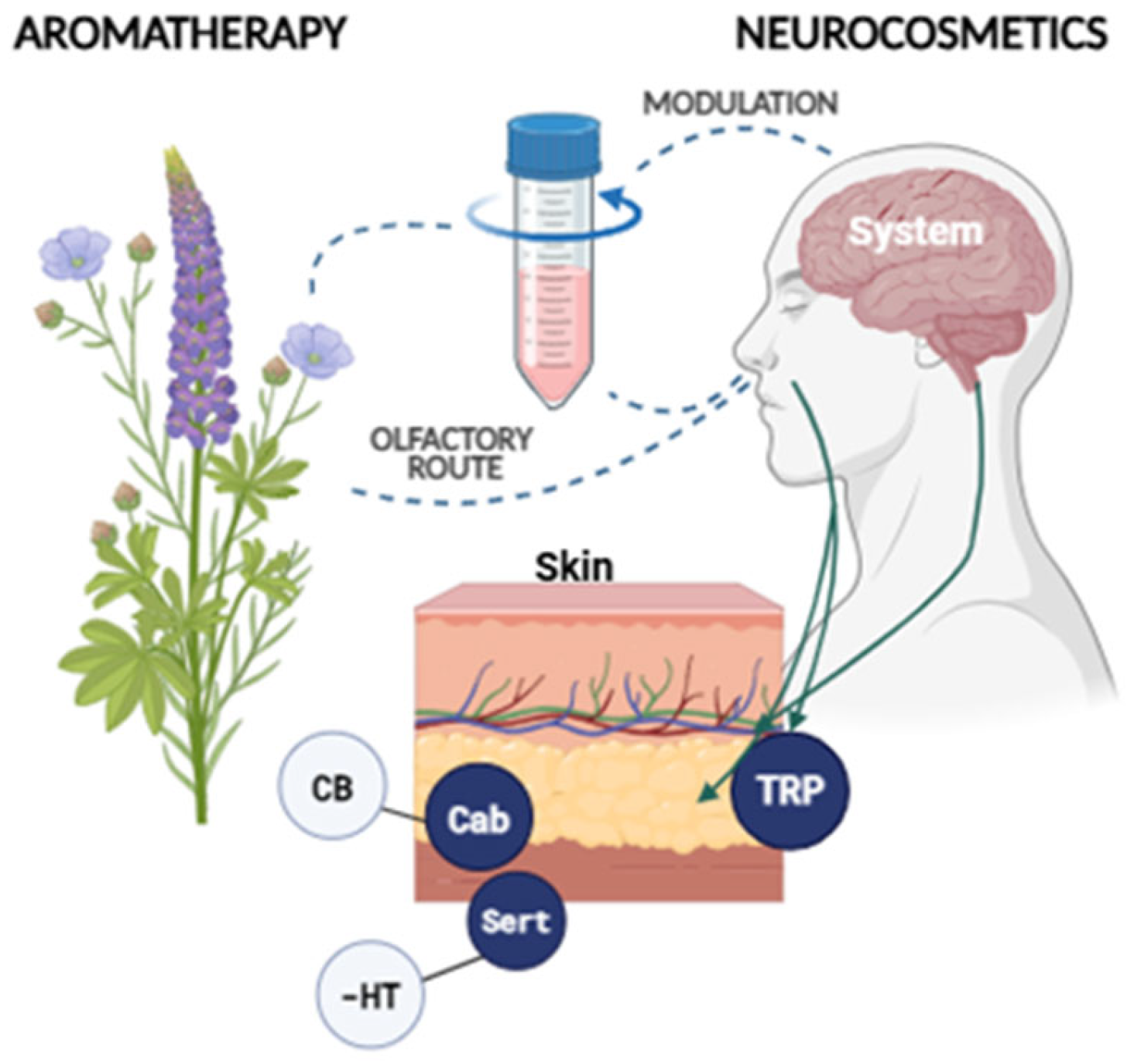
1.1. The Skin as a Neuroendocrine–Sensory Organ: Functional Basis
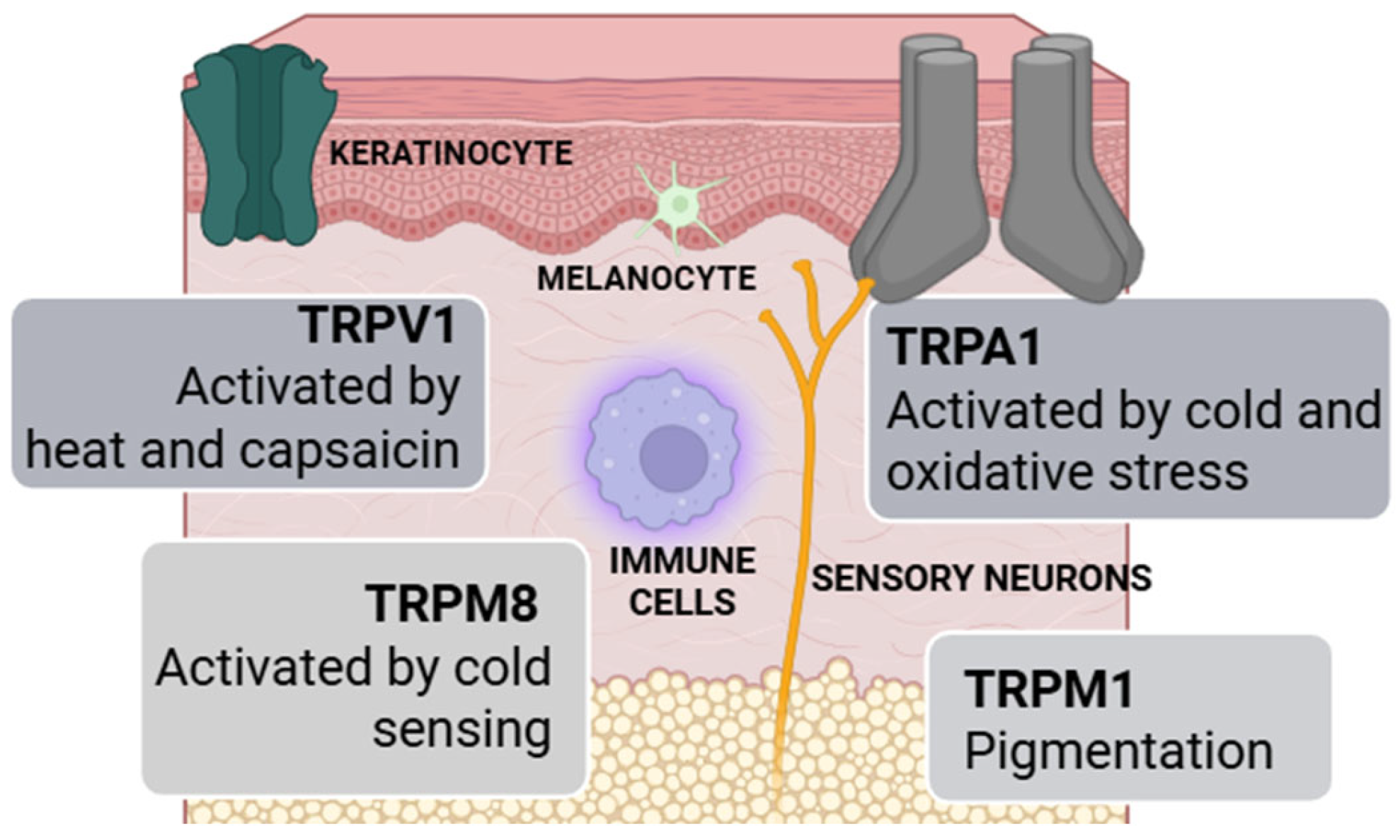
1.2. Neurocutaneous Receptors and Their Functional Implications
1.3. Neuroactive Ingredients
| Entry | Neurocosmetic Active | Receptor/Target | Cutaneous Effect | Emotional/Sensory Effect | References |
|---|---|---|---|---|---|
| 1 | Palmitoyl tetrapeptide-7 | Cytokine/pro-inflammatory receptors | Reduces inflammation, improves firmness | Sensation of relief and comfort | [167] |
| 2 | Niacinamide | GPR109A, immune receptors | Enhances skin barrier, reduces hyperpigmentation | Decreases discomfort in sensitive skin | [168] |
| 3 | Cannabidiol (CBD) | Cannabinoid receptors CB1, CB2 | Regulates sebum, anti-inflammatory | Promotes relaxation, reduces anxiety | [169] |
| 4 | Menthol | TRPM8 ion channels | Cooling, soothing effect | Induces freshness, immediate local relief | [170] |
| 5 | Capsaicin | TRPV1 ion channels | Stimulates microcirculation, enhances skin tone | Mild warming sensation, invigorating effect | [171,172,173] |
| 6 | Botulinum-like peptides | Neuronal receptors at neuromuscular junction | Smoothes expression lines | Sensation of facial relaxation and skin smoothing | [162,174,175] |
| 7 | Ylang-ylang essential oil | Limbic system via olfactory route | Antioxidant, skin toning properties | Promotes relaxation, stress relief | [53,176,177] |
| 8 | Lavender essential oil | GABA receptors/limbic system | Calms irritation, improves sleep quality | Deep relaxation, anxiety reduction | [178,179,180] |
| 9 | Citral compounds | Olfactory/limbic receptors | Brightens and revitalizes skin | Enhances mood, induces optimistic and energizing sensations | [181,182,183] |
| 10 | Enkephalins | Cutaneous opioid receptors | Pain relief, increases skin comfort | Pleasant sensation, supports general emotional well-being | [184,185,186,187] |
1.4. Biomimetic Peptides: An Advanced Molecular Strategy in Neurocosmetics
- Signaling peptides: Encourage the production of collagen and elastin and other extracellular matrix (ECM) proteins.
- Carrier peptides: Enhance the penetration of vital trace elements (copper and manganese).
- Enzyme inhibitory peptides: Inhibit enzymes that break down the extracellular matrix.
- Neurotransmitter-inhibiting peptides: Reduce the action of facial muscle contraction, also helping to diminish dynamic wrinkles.
1.5. Challenges of Neurocosmetics and Essential Oils
2. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armstrong, T.; Detweiler-Bedell, B. Beauty as an Emotion: The Exhilarating Prospect of Mastering a Challenging World. Rev. Gen. Psychol. 2008, 12, 305–329. [Google Scholar] [CrossRef]
- Haase, F.-A. Beauty and Esthetics. Meanings of an Idea and Concept of the Senses. An Introduction to an Esthetic Communication Concept Facing the Perspectives of Its Theory, History, and Cultural Traditions of the Beautiful; Universität Tübingen: Tübingen, Germany, 2008. [Google Scholar]
- Démuthová, S.; Selecká, L.; Démuth, A. Human Facial Attractiveness in Psychological Research; Peter Lang Verlag: Berlin, Germany, 2019; ISBN 978-3-631-80097-3. [Google Scholar]
- Haykal, D.; Berardesca, E.; Kabashima, K.; Dréno, B. Beyond Beauty: Neurocosmetics, the Skin-Brain Axis, and the Future of Emotionally Intelligent Skincare. Clin. Dermatol. 2025, 43, 523–527. [Google Scholar] [CrossRef]
- Eissa, M.E. Olfactory interventions for sleep enhancement: A review. Univers. J. Pharm. Res. 2024, 9, 51–58. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Quintero-Rincón, P.; Olivero-Verbel, J. Aromatherapy and Essential Oils: Holistic Strategies in Complementary and Alternative Medicine for Integral Wellbeing. Plants 2025, 14, 400. [Google Scholar] [CrossRef]
- Reddy, B.H.V.; Hussain, S.M.S.; Hussain, M.S.; Kumar, R.N.; Gupta, J. Essential Oils in Cosmetics: Antioxidant Properties and Advancements through Nanoformulations. Pharmacol. Res.-Nat. Prod. 2025, 6, 100192. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Hatvate, N.T.; Naren, P.; Khan, S.; Chavda, V.P.; Balar, P.C.; Gandhi, J.; Khatri, D.K. Essential Oils for Clinical Aromatherapy: A Comprehensive Review. J. Ethnopharmacol. 2024, 330, 118180. [Google Scholar] [CrossRef]
- Wu, J.-J.; Cui, Y.; Yang, Y.-S.; Kang, M.-S.; Jung, S.-C.; Park, H.K.; Yeun, H.-Y.; Jang, W.J.; Lee, S.; Kwak, Y.S.; et al. Modulatory Effects of Aromatherapy Massage Intervention on Electroencephalogram, Psychological Assessments, Salivary Cortisol and Plasma Brain-Derived Neurotrophic Factor. Complement. Ther. Med. 2014, 22, 456–462. [Google Scholar] [CrossRef]
- Afghan, R.; Heysieattalab, S.; Zangbar, H.S.; Ebrahimi-Kalan, A.; Jafari-Koshki, T.; Samadzadehaghdam, N. Lavender Essential Oil Inhalation Improves Attentional Shifting and Accuracy: Evidence from Dynamic Changes of Cognitive Flexibility and Power Spectral Density of Electroencephalogram Signals. J. Med. Signals Sens. 2024, 14, 12. [Google Scholar] [CrossRef]
- Soares, G.A.B.e.; Bhattacharya, T.; Chakrabarti, T.; Tagde, P.; Cavalu, S. Exploring Pharmacological Mechanisms of Essential Oils on the Central Nervous System. Plants 2022, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring Pharmacological Mechanisms of Lavender (Lavandula angustifolia) Essential Oil on Central Nervous System Targets. Front. Pharmacol. 2017, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, H.; Mardani, A.; Basirinezhad, M.H.; Hamidzadeh, A.; Eskandari, F. The Effects of Lavender and Chamomile Essential Oil Inhalation Aromatherapy on Depression, Anxiety and Stress in Older Community-Dwelling People: A Randomized Controlled Trial. EXPLORE 2022, 18, 272–278. [Google Scholar] [CrossRef]
- Emadikhalaf, M.; Ghods, A.A.; Sotodeh-asl, N.; Mirmohamadkhani, M.; Vaismoradi, M. Effects of Rose and Lavender Scents on Nurses’ Job Stress: A Randomized Controlled Trial. EXPLORE 2023, 19, 371–375. [Google Scholar] [CrossRef]
- Angulo, S.M.; Occhieppo, V.B.; Moya, C.; Crespo, R.; Bregonzio, C. Anxiolytic-like Effect Characterization of Essential Oil from Local Lavender Cultivation. Pharmaceuticals 2025, 18, 624. [Google Scholar] [CrossRef]
- Chambali, Z.A.S.P.; Algristian, H. Lavender Essential Oil as an Adjuvant Therapy for Anxiety Disorders. J. Health Lit. Qual. Res. 2025, 5, 32–50. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Iahtisham-Ul-Haq; Khan, S.; Sohail, M.; Iqbal, M.J.; Awan, K.A.; Nayik, G.A. Chapter 20-Tea Tree Essential Oil. In Essential Oils; Nayik, G.A., Ansari, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 479–500. ISBN 978-0-323-91740-7. [Google Scholar]
- Baraka, A.A.E. Effect of Peppermint Aroma on Physiological Parameters of Mechanically Ventilated Patients: Randomized Placebo Controlled Trial. Clin. Epidemiol. Glob. Health 2025, 33, 102009. [Google Scholar] [CrossRef]
- Cetin, N.; Kose, G.; Gokbel, A. Examining the Effect of Peppermint Oil on Postoperative Nausea After Cervical Surgery. J. Neurosci. Nurs. 2024, 56, 203. [Google Scholar] [CrossRef] [PubMed]
- Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horčinová Sedláčková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.P.; Lipok, J. Mentha Piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products. Molecules 2023, 28, 7444. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint Essential Oil: Its Phytochemistry, Biological Activity, Pharmacological Effect and Application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Lau, B.K.; Karim, S.; Goodchild, A.K.; Vaughan, C.W.; Drew, G.M. Menthol Enhances Phasic and Tonic GABAA Receptor-Mediated Currents in Midbrain Periaqueductal Grey Neurons. Br. J. Pharmacol. 2014, 171, 2803–2813. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Okello, E.; Chazot, P.; Howes, M.-J.; Ohiomokhare, S.; Jackson, P.; Haskell-Ramsay, C.; Khan, J.; Forster, J.; Wightman, E. Volatile Terpenes and Brain Function: Investigation of the Cognitive and Mood Effects of Mentha × Piperita L. Essential Oil with In Vitro Properties Relevant to Central Nervous System Function. Nutrients 2018, 10, 1029. [Google Scholar] [CrossRef] [PubMed]
- Surbhi; Kumar, A.; Singh, S.; Kumari, P.; Rasane, P. Eucalyptus: Phytochemical Composition, Extraction Methods and Food and Medicinal Applications. Adv. Tradit. Med. 2023, 23, 369–380. [Google Scholar] [CrossRef]
- Adesina, A.A.; Dona, D.U.; Wheto, B.M.; Lamina, B.R. Modelling of the Effect of Pretreatment Methods, and Time on the Yield of Eucalyptus Essential Oil Using Response Surface Methodology. Eur. J. Appl. Sci. Eng. Technol. 2025, 3, 287–294. [Google Scholar] [CrossRef]
- Qneibi, M.; Bdir, S.; Maayeh, C.; Bdair, M.; Sandouka, D.; Basit, D.; Hallak, M. A Comprehensive Review of Essential Oils and Their Pharmacological Activities in Neurological Disorders: Exploring Neuroprotective Potential. Neurochem. Res. 2024, 49, 258–289. [Google Scholar] [CrossRef] [PubMed]
- Akinyede, K.A.; Oyewusi, H.A.; Oladipo, O.O.; Tugbobo, O.S.; Akinyede, K.A.; Oyewusi, H.A.; Oladipo, O.O.; Tugbobo, O.S. Essential Oils and Their Antioxidant Importance: The In Vitro and In Vivo Treatment and Management of Neurodegenerative Diseases with New Delivery Applications. In Essential Oils-Recent Advances, New Perspectives and Applications; IntechOpen: London, UK, 2023; ISBN 978-0-85014-205-1. [Google Scholar]
- Agarwal, P.; Sebghatollahi, Z.; Kamal, M.; Dhyani, A.; Shrivastava, A.; Singh, K.K.; Sinha, M.; Mahato, N.; Mishra, A.K.; Baek, K.-H. Citrus Essential Oils in Aromatherapy: Therapeutic Effects and Mechanisms. Antioxidants 2022, 11, 2374. [Google Scholar] [CrossRef]
- Komori, T.; Fujiwara, R.; Tanida, M.; Nomura, J. Potential Antidepressant Effects of Lemon Odor in Rats. Eur. Neuropsychopharmacol. 1995, 5, 477–480. [Google Scholar] [CrossRef]
- A Systematic Review of the Therapeutic Properties of Lemon Essential Oil-ClinicalKey. Available online: https://www.clinicalkey.es/#!/content/playContent/1-s2.0-S2212958824001356?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2212958824001356%3Fshowall%3Dtrue&referrer= (accessed on 3 July 2025).
- Xiao, Z.; Li, Q.; Niu, Y.; She, Y.; Sun, Z.; Zhang, J.; Wang, Z.; Zhou, R.; Zhu, J. Mechanism of the Interaction between Olfactory Receptors and Characteristic Aroma Compounds in Sweet Orange Juice. LWT 2024, 207, 116660. [Google Scholar] [CrossRef]
- Nascimento, J.C.; dos S.Gonçalves, V.S.; Souza, B.R.S.; de C. Nascimento, L.; de Carvalho, B.M.R.; Nogueira, P.C.L.; Santos, J.P.S.; Borges, L.P.; Goes, T.C.; de Souza, J.B.; et al. Effectiveness of Aromatherapy with Sweet Orange Oil (Citrus sinensis L.) in Relieving Pain and Anxiety during Labor. EXPLORE 2025, 21, 103081. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Wang, L.; Li, F.; Weschler, L.B.; Huang, J.; Zhang, Y. Potential Benefits of Short-Term Indoor Exposure to Sweet Orange Essential Oil for Relaxation during Mental Work Breaks. J. Build. Eng. 2023, 78, 107602. [Google Scholar] [CrossRef]
- Erdal, S.; Harman Özdoğan, M.; Yildirim, D.; Kuni, A.; Selçuk, S.; Güneri, A.; Arslan, E.N. Effects of Orange Oil Aromatherapy on Pain and Anxiety During Invasive Interventions in Patients with Hematopoietic Stem Cell Transplants. J. Infus. Nurs. 2024, 47, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhu, J.; Niu, Y.; Zhang, J.; Xiao, Z.; Zhao, L. Identification of Characteristic Compounds of Sweet Orange Oil and Their Sweetening Effects on the Sucrose Solution with Sweetness Meter, Sensory Analysis, Electronic Tongue, and Molecular Dynamics Simulation. Food Chem. 2024, 461, 140815. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Sheu, S.-R.; Hsu, S.-C.; Tsai, Y.-H. Determination of Bactericidal Efficacy of Essential Oil Extracted from Orange Peel on the Food Contact Surfaces. Food Control 2010, 21, 1710–1715. [Google Scholar] [CrossRef]
- Valduga, A.T.; Gonçalves, I.L.; Magri, E.; Delalibera Finzer, J.R. Chemistry, Pharmacology and New Trends in Traditional Functional and Medicinal Beverages. Food Res. Int. 2019, 120, 478–503. [Google Scholar] [CrossRef]
- Pourshaikhian, M.; Moghadamnia, M.T.; Kazemnezhad Leyli, E.; Shafiei Kisomi, Z. Effects of Aromatherapy with Matricaria Chamomile Essential Oil on Anxiety and Hemodynamic Indices in Patients with Acute Coronary Syndrome, 2021: A Randomized Controlled Trial. BMC Complement. Med. Ther. 2024, 24, 17. [Google Scholar] [CrossRef]
- Bahrami, F.; Hanifi, N.; Mardani, A. Comparison of the Effects of Aromatherapy with Damask Rose and Chamomile Essential Oil on Preoperative Pain and Anxiety in Emergency Orthopedic Surgery: A Randomized Controlled Trial. J. Perianesth. Nurs. 2024, 39, 583–588. [Google Scholar] [CrossRef]
- Deepa, Y.; Vijay, A.; Nivethitha, L.; Nandhakumar, G.; Sathiya, S.; Mooventhan, A. Effects of Chamomile Oil Inhalation on Sleep Quality in Young Adults with Insomnia: A Randomized Controlled Trial. Int. J. Psychiatry Med. 2024, 60, 533–542. [Google Scholar] [CrossRef]
- Saadatmand, S.; Zohroudi, F.; Tangestani, H. The Effect of Oral Chamomile on Anxiety: A Systematic Review of Clinical Trials. Clin. Nutr. Res. 2024, 13, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Alahmady, N.F.; Alkhulaifi, F.M.; Abdullah Momenah, M.; Ali Alharbi, A.; Allohibi, A.; Alsubhi, N.H.; Ahmed Alhazmi, W. Biochemical Characterization of Chamomile Essential Oil: Antioxidant, Antibacterial, Anticancer and Neuroprotective Activity and Potential Treatment for Alzheimer’s Disease. Saudi J. Biol. Sci. 2024, 31, 103912. [Google Scholar] [CrossRef]
- Yeddes, W.; Ouerghemmi, I.; Hammami, M.; Gadhoumi, H.; Affes, T.G.; Nait Mohamed, S.; Aidi-Wannes, W.; Witrowa-Rajchert, D.; Saidani-Tounsi, M.; Nowacka, M. Optimizing the Method of Rosemary Essential Oils Extraction by Using Response Surface Methodology (RSM)-Characterization and Toxicological Assessment. Sustainability 2022, 14, 3927. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Therapeutic Effects of Rosemary (Rosmarinus officinalis L.) and Its Active Constituents on Nervous System Disorders. Iran. J. Basic. Med. Sci. 2020, 23, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Xu, L.; Qiao, M.; Wang, Y. The Anti-Depression Effect and Mechanism of Harmonious Rosemary Essential Oil and Its Application in Microcapsules. Mater. Today Bio 2025, 31, 101546. [Google Scholar] [CrossRef]
- Bengana, K.; Serseg, T.; Benarous, K.; Mermer, A.; Şirin, Y.; Kaouka, A. Antilipase Activities of Cultivated Peppermint and Rosemary Essential Oils: In Vitro and in Silico Studies. Turk. J. Biol. 2025, 49, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Chetouani, M.; Arabi, M.; Belasri, L.; Mharchi, S.; Alaoui, K. Effect of Salt Stress on the Essential Oil Content of Rosemary at Juvenile and Adult Stages Under Greenhouse Conditions. E3S Web Conf. 2025, 632, 03005. [Google Scholar] [CrossRef]
- Khan, F.; Rashan, L. Phytochemical Analysis and Pharmaceutical Applications of Monoterpenoids Present in the Essential Oil of Boswellia sacra (Omani Luban). Adv. Pharmacol. Pharm. Sci. 2025, 2025, 3536898. [Google Scholar] [CrossRef]
- Ng, F.; Thong, A.; Basri, N.; Wu, W.; Chew, W.; Dharmawan, J. Profiling of Aroma-Active Compounds in Ylang-Ylang Essential Oils by Aroma Extract Dilution Analysis (AEDA) and Chemometric Methods. J. Agric. Food Chem. 2022, 70, 260–266. [Google Scholar] [CrossRef]
- Mrani, S.A.; Zejli, H.; Azzouni, D.; Fadili, D.; Alanazi, M.M.; Hassane, S.O.S.; Sabbahi, R.; Kabra, A.; Moussaoui, A.E.; Hammouti, B.; et al. Chemical Composition, Antioxidant, Antibacterial, and Hemolytic Properties of Ylang-Ylang (Cananga odorata) Essential Oil: Potential Therapeutic Applications in Dermatology. Pharmaceuticals 2024, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
- Karabatak, N.; Gök, B.; Budama-kılınc, Y. Development of Nanoemulsion Formulation Containing Ylang Ylang Essential Oil for Topical Applications, Evaluation of In Vitro Cytotoxicity and ADMET Profile. J. Turk. Chem. Soc. Sect. Chem. 2024, 11, 1181–1196. [Google Scholar] [CrossRef]
- Alam, P.; Imran, M.; Ali, A.; Majid, H. Cananga Odorata (Ylang-Ylang) Essential Oil Containing Nanoemulgel for the Topical Treatment of Scalp Psoriasis and Dandruff. Gels 2024, 10, 303. [Google Scholar] [CrossRef]
- Tan, L.T.H.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Kadir, H.A.; Chan, K.G.; Goh, B.H. Traditional Uses, Phytochemistry, and Bioactivities of Cananga odorata (Ylang-Ylang). Evid.-Based Complement. Altern. Med. 2015, 2015, 896314. [Google Scholar] [CrossRef]
- Sharma, K.; Lanzilotto, A.; Yakubu, J.; Therkelsen, S.; Vöegel, C.D.; Du Toit, T.; Jørgensen, F.S.; Pandey, A.V. Effect of Essential Oil Components on the Activity of Steroidogenic Cytochrome P450. Biomolecules 2024, 14, 203. [Google Scholar] [CrossRef]
- Faris, A.; Edder, Y.; Louchachha, I.; Lahcen, I.A.; Azzaoui, K.; Hammouti, B.; Merzouki, M.; Challioui, A.; Boualy, B.; Karim, A.; et al. From Himachalenes to Trans-Himachalol: Unveiling Bioactivity through Hemisynthesis and Molecular Docking Analysis. Sci. Rep. 2023, 13, 17653. [Google Scholar] [CrossRef]
- Bozova, B.; Gölükcü, M.; Giuffrè, A.M. The Effect of Different Hydrodistillation Times on the Composition and Yield of Bergamot (Citrus bergamia Risso) Peel Essential Oil and a Comparison of the Cold-Pressing Method. Flavour. Fragr. J. 2024, 39, 263–270. [Google Scholar] [CrossRef]
- Chang, J.; Yang, H.; Shan, X.; Zhao, L.; Li, Y.; Zhang, Z.; Abankwah, J.K.; Zhang, M.; Bian, Y.; Guo, Y. Bergamot Essential Oil Improves CUMS-Induced Depression-like Behaviour in Rats by Protecting the Plasticity of Hippocampal Neurons. J. Cell. Mol. Med. 2024, 28, e18178. [Google Scholar] [CrossRef]
- Zhu, M.-Y.; Dong, W.-Y.; Guo, J.-R.; Huang, J.-Y.; Cheng, P.-K.; Yang, Y.; Liu, A.; Yang, X.-L.; Zhu, X.; Zhang, Z.; et al. A Neural Circuit for Bergamot Essential Oil-Induced Anxiolytic Effects. Adv. Sci. 2025, 12, 2406766. [Google Scholar] [CrossRef]
- Gogoi, R.; Begum, T.; Sarma, N.; Tamang, R.; Chanda, S.K.; Lal, M.; Perveen, K.; Khan, F.; Marinković, J. Pelargonium graveolens L., (Geranium) Essential Oil from Northeast India: Chemical Composition, Pharmacology and Genotoxicity Study. J. Essent. Oil Bear. Plants 2024, 27, 135–151. [Google Scholar] [CrossRef]
- Pandur, E.; Major, B.; Rák, T.; Sipos, K.; Csutak, A.; Horváth, G. Linalool and Geraniol Defend Neurons from Oxidative Stress, Inflammation, and Iron Accumulation in In Vitro Parkinson’s Models. Antioxidants 2024, 13, 917. [Google Scholar] [CrossRef]
- Topor, G.; Buzia, O.D.; Huzum, R.M.; Maftei, M.I.; Monica, D.; Serban, C. Dermopreparations with antiacneic action. formulation and pharmacotechnical evaluation. Rom. J. Oral Rehabil. 2024, 16, 629–643. [Google Scholar] [CrossRef]
- Hashimoto, M.; Takahashi, K.; Unno, T.; Ohta, T. Linalyl Acetate Exerts Analgesic Effects by Inhibiting Nociceptive TRPA1 in Mice. Biomed. Res. 2024, 45, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Grigore-Gurgu, L.; Dumitrașcu, L.; Aprodu, I. Aromatic Herbs as a Source of Bioactive Compounds: An Overview of Their Antioxidant Capacity, Antimicrobial Activity, and Major Applications. Molecules 2025, 30, 1304. [Google Scholar] [CrossRef] [PubMed]
- Anjali; Garg, D.; Pragi; Kumar, V.; Dimple; Monika, R. A Review of Therapeutic Properties and Uses of Salvia Officinalis: Review Article. J. Pharma Insights Res. 2024, 2, 146–154. [Google Scholar] [CrossRef]
- Weshahi, H.A.; Akhtar, M.S.; Tobi, S.S.A.; Hossain, A.; Khan, S.A.; Akhtar, A.B.; Said, S.A. Evaluation of Acute Plant Toxicity, Antioxidant Activity, Molecular Docking and Bioactive Compounds of Lemongrass Oil Isolated from Omani Cultivar. Toxicol. Rep. 2025, 14, 101888. [Google Scholar] [CrossRef]
- Hotimah, U.H.; Rahmadhena, M.P. The Effectiveness of Lemongrass Aromatherapy in Reducing Nausea and Vomiting in Pregnant Women. Indones. J. Glob. Health Res. 2024, 6, 95–102. [Google Scholar] [CrossRef]
- Khasanah, L.U.; Praseptiangga, D.; Purwanto, E.; Ariviani, S. Bioactive Components and Bioactivity of Essential Oils, Hydrosol and Water Steam Distillation Solvents of Lemongrass Leaves (Cymbopogon citratus). IOP Conf. Ser. Earth Environ. Sci. 2024, 1377, 012059. [Google Scholar] [CrossRef]
- Tazi, A.; Zinedine, A.; Rocha, J.M.; Errachidi, F. Review on the Pharmacological Properties of Lemongrass (Cymbopogon citratus) as a Promising Source of Bioactive Compounds. Pharmacol. Res.-Nat. Prod. 2024, 3, 100046. [Google Scholar] [CrossRef]
- Nour, A.H.; Modather, R.H.; Yunus, R.M.; Elnour, A.A.M.; Ismail, N.A. Characterization of Bioactive Compounds in Patchouli Oil Using Microwave-Assisted and Traditional Hydrodistillation Methods. Ind. Crops Prod. 2024, 208, 117901. [Google Scholar] [CrossRef]
- Singh, S.; Agrawal, N. Exploring the Pharmacological Potential and Bioactive Components of Pogostemon cablin (Blanco) Benth, Traditional Chinese Medicine. Pharmacol. Res.-Mod. Chin. Med. 2024, 10, 100382. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, N.; Chanotiya, C.S.; Lal, R.K. The Pharmacological Potential and the Agricultural Significance of the Aromatic Crop Patchouli (Pogostemon cablin Benth.): A Review. Ecol. Front. 2024, 44, 1109–1118. [Google Scholar] [CrossRef]
- Varaprasad Rao, V.; Rajitha, T.; Ankatwar, G. Role of Jasmine Flowers in Stress Relief and Advantages and Disadvantages of Jasmine Plants. IJFMR-Int. J. Multidiscip. Res. 2025, 7, 2582-2160. [Google Scholar] [CrossRef]
- Rescigno, A.; Zucca, P.; Peddio, S.; Srikanth, S.; Kaushik, N.P.; Kumar, N.V.A.; Leyva-Gómez, G.; Kregiel, D.; Abu-Reidah, I.M.; Sen, S.; et al. Harnessing Jasminum Bioactive Compounds: Updated Insights for Therapeutic and Food Preservation Innovations. Food Front. 2025, 6, 1093–1128. [Google Scholar] [CrossRef]
- Feng, P.; Chen, J.; Chen, X.; Tang, M.; Song, N.; Zhang, L.; He, T. Comparing Effects of Aromatherapy with Five Herbs Essential Oils on PCPA-Induced Insomnia Mice. J. Microbiol. Biotechnol. 2025, 35, e2409021. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.R.O. Aromatherapy Today: A Science of Integration and Evidence-Based Practice. Braz. J. Health Aromather. Essent. Oil 2025, 2, bjhae20. [Google Scholar] [CrossRef]
- Yan, X.; David, S.D.; Du, G.; Li, W.; Liang, D.; Nie, S.; Ge, M.; Wang, C.; Qiao, J.; Li, Y.; et al. Biological Properties of Sandalwood Oil and Microbial Synthesis of Its Major Sesquiterpenoids. Biomolecules 2024, 14, 971. [Google Scholar] [CrossRef] [PubMed]
- El Hachlafi, N.; Benkhaira, N.; Mssillou, I.; Touhtouh, J.; Aanniz, T.; Chamkhi, I.; El Omari, N.; Khalid, A.; Abdalla, A.N.; Aboulagras, S.; et al. Natural Sources and Pharmacological Properties of Santalenes and Santalols. Ind. Crops Prod. 2024, 214, 118567. [Google Scholar] [CrossRef]
- Kang, S.; Li, S.; Han, S.; Peng, L. Microencapsulation of Sandalwood Essential Oil with Melamine Formaldehyde and β-Cyclodextrin: Analysis of Study on Aroma Profile Components and Antibacterial Properties. ChemistrySelect 2025, 10, e202405908. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, D.-S.; Jo, S.M.; Yoon, S.; Jeong, H.; Yoon, M.Y.; Kim, J.K.; Kim, Y.J.; Shin, E.-C. Exploration of Basil (Ocimum basilicum) Essential Oil Profiles Using E-Nose and GC–MS Combined with GC-O and Inhalation Effects on the Human EEG Topography and Tomography (s-LORETA) and Blood Pressure. J. Funct. Foods 2024, 112, 105918. [Google Scholar] [CrossRef]
- Mulugeta, S.M.; Gosztola, B.; Radácsi, P. Diversity in Morphology and Bioactive Compounds among Selected Ocimum Species. Biochem. Syst. Ecol. 2024, 114, 104826. [Google Scholar] [CrossRef]
- Ezeorba, T.P.C.; Chukwuma, I.F.; Asomadu, R.O.; Ezeorba, W.F.C.; Uchendu, N.O. Health and Therapeutic Potentials of Ocimum Essential Oils: A Review on Isolation, Phytochemistry, Biological Activities, and Future Directions. J. Essent. Oil Res. 2024, 36, 271–290. [Google Scholar] [CrossRef]
- Dmitriev, L.B.; Dmitrieva, V.L.; Trukhachev, V.I.; Sushkova, L.O. Biologically Active Substances of Plants of the Cupressaceae Family of the Genus Thuya and the Genus Juniperus for Phyto- and Aromatherapy. BIO Web Conf. 2024, 82, 01012. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Chen, R.; Huang, J.; Yu, P.; Wei, Q.; Wang, Y.; Jin, Q. Function Analysis of Essential Oils as Environmental Scents for Improving Undergraduate Students Emotional State. Ind. Crops Prod. 2025, 232, 121200. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Lin, C.-L.; Chang, L.-Y. The Effects of Aromatherapy Massage on Sleep Quality of Nurses on Monthly Rotating Night Shifts. Evid.-Based Complement. Altern. Med. 2017, 2017, 3861273. [Google Scholar] [CrossRef]
- Amaghnouje, A.; Mechchate, H.; Es-safi, I.; Alotaibi, A.A.; Noman, O.M.; Nasr, F.A.; Al-zharani, M.; Cerruti, P.; Calarco, A.; EL Fatemi, H.; et al. Anxiolytic, Antidepressant-Like Proprieties and Impact on the Memory of the Hydro-Ethanolic Extract of Origanum majorana L. on Mice. Appl. Sci. 2020, 10, 8420. [Google Scholar] [CrossRef]
- Jung, H.-N.; Choi, H.-J. Effects of Origanum Majorana Essential Oil Aroma on the Electroencephalograms of Female Young Adults with Sleep Disorders. J. Life Sci. 2012, 22, 1077–1084. [Google Scholar] [CrossRef]
- Hammoudi Halat, D.; Krayem, M.; Khaled, S.; Younes, S. A Focused Insight into Thyme: Biological, Chemical, and Therapeutic Properties of an Indigenous Mediterranean Herb. Nutrients 2022, 14, 2104. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Sheikhlar, A.; Raissy, M.; Chaharmahali, F.H.; Maneepitaksanti, W.; Faheem, M.; Van Doan, H. The Effects of Dietary Thyme Oil (Thymus vulgaris) Essential Oils for Common Carp (Cyprinus carpio): Growth Performance, Digestive Enzyme Activity, Antioxidant Defense, Tissue and Mucus Immune Parameters, and Resistance against Aeromonas Hydrophila. Aquac. Nutr. 2022, 2022, 7942506. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Deng, H.; Deng, Y.; Song, T.; Pang, L.; Zhu, S.; Ren, Z.; Guo, H.; Xu, Z.; Zhu, L.; Geng, Y.; et al. Evaluation of the Activity and Mechanisms of Oregano Essential Oil against PRV in Vivo and in Vitro. Microb. Pathog. 2024, 194, 106791. [Google Scholar] [CrossRef] [PubMed]
- Thao, C.B.; Tran, T.T.; Tran, T.K.N.; Mai, H.C. Extraction and Volatile Compounds in Ginger Essential Oil (Zingiber officinale Roscoe) at Laboratory Scale. Asian J. Chem. 2023, 35, 3066–3070. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Abd El-Kareem, M.S.M.; Rabbih, M.A.; Rashad, A.M.; EL-Hefny, M. Essential Oils from Fennel Plants as Valuable Chemical Products: Gas Chromatography–Mass Spectrometry, FTIR, Quantum Mechanical Investigation, and Antifungal Activity. Biomass Convers. Biorefin. 2025, 15, 9173–9191. [Google Scholar] [CrossRef]
- Naaz, S.; Ahmad, N.; Qureshi, M.I.; Hashmi, N.; Akhtar, M.S.; A Khan, M.M. Antimicrobial and Antioxidant Activities of Fennel Oil. Bioinformation 2022, 18, 795–800. [Google Scholar] [CrossRef]
- Abdullah; Alam, W.; Hussain, Y.; Ahmad, S.; Khan, F.; Ali, A.; Khan, H. Chapter 12-Neuroprotective Effect of Essential Oils. In Phytonutrients and Neurological Disorders; Khan, H., Aschner, M., Mirzaei, H., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 305–333. ISBN 978-0-12-824467-8. [Google Scholar]
- Zhang, L.-L.; Chen, Y.; Li, Z.-J.; Li, X.; Fan, G. Bioactive Properties of the Aromatic Molecules of Spearmint (Mentha spicata L.) Essential Oil: A Review. Food Funct. 2022, 13, 3110–3132. [Google Scholar] [CrossRef]
- Chávez-Delgado, E.L.; Gastélum-Estrada, A.; Pérez-Carrillo, E.; Ramos-Parra, P.A.; Estarrón-Espinosa, M.; Reza-Zaldívar, E.E.; Hernández-Brenes, C.; Mora-Godínez, S.; de los Santos, B.E.; Guerrero-Analco, J.A.; et al. Bioactive Properties of Spearmint, Orange Peel, and Baby Sage Oleoresins Obtained by Supercritical CO2 Extraction and Their Integration into Dark Chocolate. Food Chem. 2025, 463, 141306. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, X.; Tian, Y.; Yan, S.; Liu, J.; Xie, J.; Zhang, F.; Yao, C.; Hao, E. Therapeutic Potential of Cinnamon Oil: Chemical Composition, Pharmacological Actions, and Applications. Pharmaceuticals 2024, 17, 1700. [Google Scholar] [CrossRef]
- Nguyen, L.T.H.; Nguyen, N.P.K.; Tran, K.N.; Shin, H.-M.; Yang, I.-J. Anxiolytic-like Effect of Inhaled Cinnamon Essential Oil and Its Main Component Cinnamaldehyde in Animal Models. Molecules 2022, 27, 7997. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R. Effects of Essential Oils on Central Nervous System: Focus on Mental Health. Phytother. Res. 2021, 35, 657–679. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Wasef, L.; Teibo, J.O.; Shaheen, H.M.; Zakariya, A.M.; Akinfe, O.A.; Teibo, T.K.A.; Al-kuraishy, H.M.; Al-Garbee, A.I.; Alexiou, A.; et al. Commiphora Myrrh: A Phytochemical and Pharmacological Update. Naunyn. Schmiedebergs Arch. Pharmacol. 2023, 396, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Hanus, L.O.; Rezanka, T.; Dembitsky, V.M.; Moussaieff, A. Myrrh--Commiphora Chemistry. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2005, 149, 3–27. [Google Scholar] [CrossRef]
- Cao, B.; Wei, X.-C.; Xu, X.-R.; Zhang, H.-Z.; Luo, C.-H.; Feng, B.; Xu, R.-C.; Zhao, S.-Y.; Du, X.-J.; Han, L.; et al. Seeing the Unseen of the Combination of Two Natural Resins, Frankincense and Myrrh: Changes in Chemical Constituents and Pharmacological Activities. Molecules 2019, 24, 3076. [Google Scholar] [CrossRef] [PubMed]
- Scandurra, C.; Mezzalira, S.; Cutillo, S.; Zapparella, R.; Statti, G.; Maldonato, N.M.; Locci, M.; Bochicchio, V. The Effectiveness of Neroli Essential Oil in Relieving Anxiety and Perceived Pain in Women during Labor: A Randomized Controlled Trial. Healthcare 2022, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Kang, P.; Lee, H.S.; Seol, G.H. Effects of Inhalation of Essential Oil of Citrus aurantium L. var. amara on Menopausal Symptoms, Stress, and Estrogen in Postmenopausal Women: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2014, 2014, 796518. [Google Scholar] [CrossRef]
- Borba, C.A.; Fernandes, G.V.; Campos, J.C.; da Silva, T.B.; Gonzaga, R.V. Potential Action on the Central Nervous System of Neroli Oil Extracted from Citrus Aurantium. Res. Soc. Dev. 2021, 10, e418101321447. [Google Scholar] [CrossRef]
- Glumac, M.; Jažo, Z.; Paštar, V.; Golemac, A.; Čikeš Čulić, V.; Bektić, S.; Radan, M.; Carev, I. Chemical Profiling and Bioactivity Assessment of Helichrysum italicum (Roth) G. Don. Essential Oil: Exploring Pure Compounds and Synergistic Combinations. Molecules 2023, 28, 5299. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Cruciani, S.; Garroni, G.; Sarais, G.; Kavak, F.F.; Satta, R.; Montesu, M.A.; Floris, M.; Ventura, C.; Maioli, M. Effect of Helichrysum italicum in Promoting Collagen Deposition and Skin Regeneration in a New Dynamic Model of Skin Wound Healing. Int. J. Mol. Sci. 2024, 25, 4736. [Google Scholar] [CrossRef]
- Silori, G.K.; Kushwaha, N.; Kumar, V. Essential Oils from Pines: Chemistry and Applications. In Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Malik, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 275–297. ISBN 978-3-030-16546-8. [Google Scholar]
- Nikolic, M.; Andjic, M.; Bradic, J.; Kocovic, A.; Tomovic, M.; Samanovic, A.M.; Jakovljevic, V.; Veselinovic, M.; Capo, I.; Krstonosic, V.; et al. Topical Application of Siberian Pine Essential Oil Formulations Enhance Diabetic Wound Healing. Pharmaceutics 2023, 15, 2437. [Google Scholar] [CrossRef]
- Chandharakool, S.; Koomhin, P.; Sinlapasorn, J.; Suanjan, S.; Phungsai, J.; Suttipromma, N.; Songsamoe, S.; Matan, N.; Sattayakhom, A. Effects of Tangerine Essential Oil on Brain Waves, Moods, and Sleep Onset Latency. Molecules 2020, 25, 4865. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Capdevila, L. Aromatherapy Improves Work Performance Through Balancing the Autonomic Nervous System. J. Altern. Complement. Med. 2017, 23, 214–221. [Google Scholar] [CrossRef]
- Dyer, J.; Cleary, L.; McNeill, S.; Ragsdale-Lowe, M.; Osland, C. The Use of Aromasticks to Help with Sleep Problems: A Patient Experience Survey. Complement. Ther. Clin. Pract. 2016, 22, 51–58. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef]
- Park, J.-S. Effects of Juniper Essential Oil on the Activity of Autonomic Nervous System. Biomed. Sci. Lett. 2017, 23, 286–289. [Google Scholar] [CrossRef]
- Albrecht, U.W.; Madisch, A. Therapeutic Potentials Associated with Biological Properties of Juniper Berry Oil (Juniperus communis L.) and Its Therapeutic Use in Several Diseases—A Review. Bioact. Compd. Health Dis. 2022, 5, 174–185. [Google Scholar] [CrossRef]
- Sugawara, Y.; Hino, Y.; Kawasaki, M.; Hara, C.; Tamura, K.; Sugimoto, N.; Yamanishi, Y.; Miyauchi, M.; Masujima, T.; Aoki, T. Alteration of Perceived Fragrance of Essential Oils in Relation to Type of Work: A Simple Screening Test for Efficacy of Aroma. Chem. Senses 1999, 24, 415–421. [Google Scholar] [CrossRef]
- Dilrukshi, E.A.C.; Nishiyama, Y.; Ito, K.; Nomura, S. Alleviation of Acute Stress Response by Black Pepper Aroma Administration. J. Physiol. Anthropol. 2024, 43, 3. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar, A.; Sachan, N.; Chandra, P. Anxiolytic and Antidepressant-like Effects of Essential Oil from the Fruits of Piper nigrum Linn. (Black Pepper) in Mice: Involvement of Serotonergic but Not GABAergic Transmission System. Heliyon 2021, 7, e06884. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Azam, S.; Kim, I.-S.; Choi, D.-K. Neuroprotective Effects of Black Pepper and Its Bioactive Compounds in Age-Related Neurological Disorders. Aging Dis. 2023, 14, 750–777. [Google Scholar] [CrossRef]
- Sadraei, H.; Ghannadi, A.; Malekshahi, K. Relaxant Effect of Essential Oil of Melissa officinalis and Citral on Rat Ileum Contractions. Fitoterapia 2003, 74, 445–452. [Google Scholar] [CrossRef]
- Mathews, I.M.; Eastwood, J.; Lamport, D.J.; Cozannet, R.L.; Fanca-Berthon, P.; Williams, C.M. Clinical Efficacy and Tolerability of Lemon Balm (Melissa officinalis L.) in Psychological Well-Being: A Review. Nutrients 2024, 16, 3545. [Google Scholar] [CrossRef]
- Lotfi, A.; Mohtashami, J.; Khangholi, Z.A.; Shirmohammadi-Khorram, N. The Efficacy of Aromatherapy with Lemon Balm (Melissa officinalis L.) on Sleep Quality in Cardiac Patients: A Randomized Controlled Trial. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Alimoradi, Z.; Jafari, E.; Abdi, F.; Griffiths, M.D. Therapeutic Applications of Lemon Balm (Melissa officinalis) for Obstetrics and Gynecological Health Issues: A Systematic Review. J. Herb. Med. 2023, 42, 100751. [Google Scholar] [CrossRef]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Fantasma, F.; Samukha, V.; Aliberti, M.; Colarusso, E.; Chini, M.G.; Saviano, G.; De Felice, V.; Lauro, G.; Casapullo, A.; Bifulco, G.; et al. Essential Oils of Laurus nobilis L.: From Chemical Analysis to In Silico Investigation of Anti-Inflammatory Activity by Soluble Epoxide Hydrolase (sEH) Inhibition. Foods 2024, 13, 2282. [Google Scholar] [CrossRef]
- Xia, X.; Cheng, G.; Pan, Y.; Xia, Z.H.; Kong, L.D. Behavioral, Neurochemical and Neuroendocrine Effects of the Ethanolic Extract from Curcuma longa L. in the Mouse Forced Swimming Test. J. Ethnopharmacol. 2007, 110, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.F.; Kong, L.D.; Chen, Y. Antidepressant Activity of Aqueous Extracts of Curcuma longa in Mice. J. Ethnopharmacol. 2002, 83, 161–165. [Google Scholar] [CrossRef]
- Jaiswal, S.G.; Naik, S.N. Turmeric Oil: Composition, Extraction, Potential Health Benefits and Other Useful Applications. Avicenna J. Med. Biochem. 2021, 9, 93–106. [Google Scholar] [CrossRef]
- Sayowan, W.; Siripornpanich, V.; Piriyapunyaporn, T.; Hongratanaworakit, T.; Kotchabhakdi, N.; Ruangrungsi, N. The Harmonizing Effects of Citronella Oil on Mood States and Brain Activities. J. Health Res. 2012, 26, 69–75. [Google Scholar]
- Kasmirah; Zulliati; Kusvitasari, H.; Yuliantie, P. Effectiveness of Citronella Oil Aromatherapy in Enhancing Appetite among Stunted Toddlers. Health Sci. Int. J. 2025, 3, 1–14. [Google Scholar] [CrossRef]
- Batubara, I.; Suparto, I.H.; Sa’diah, S.; Matsuoka, R.; Mitsunaga, T. Effects of Inhaled Citronella Oil and Related Compounds on Rat Body Weight and Brown Adipose Tissue Sympathetic Nerve. Nutrients 2015, 7, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Cuchet, A.; Jame, P.; Anchisi, A.; Schiets, F.; Oberlin, C.; Lefèvre, J.-C.; Carénini, E.; Casabianca, H. Authentication of the Naturalness of Wintergreen (Gaultheria Genus) Essential Oils by Gas Chromatography, Isotope Ratio Mass Spectrometry and Radiocarbon Assessment. Ind. Crops Prod. 2019, 142, 111873. [Google Scholar] [CrossRef]
- Michel, P.; Olszewska, M.A. Phytochemistry and Biological Profile of Gaultheria Procumbens L. and Wintergreen Essential Oil: From Traditional Application to Molecular Mechanisms and Therapeutic Targets. Int. J. Mol. Sci. 2024, 25, 565. [Google Scholar] [CrossRef]
- da Silva, F.R.O.; Ripardo, N.A.; de Jesus, R.A.; de Oliveira Barros e Silva Camargo, J. Copaiba Essential Oil: Composition, Therapeutic Actions, and Methods of Use for Health and Well-Being. Braz. J. Health Aromather. Essent. Oil 2024, 1, bjhae18. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, J.; Dong, W.; Yao, L. The Effect of Copaiba Oil Odor on Anxiety Relief in Adults under Mental Workload: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2022, 2022, 3874745. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Ghahfarokhi, B.B.; Ghahfarokhi, S.A.M.; Malekpoor, F. Chemical Composition of Essential Oils from the Aerial Parts and Underground Parts of Iranian Valerian Collected from Different Natural Habitats. Ind. Crops Prod. 2015, 63, 147–151. [Google Scholar] [CrossRef]
- Shinjyo, N.; Waddell, G.; Green, J. Valerian Root in Treating Sleep Problems and Associated Disorders—A Systematic Review and Meta-Analysis. J. Evid.-Based Integr. Med. 2020, 25, 2515690X20967323. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Guo, Q.; Li, H.; Wang, Z.; Li, J.; Li, T.; Tang, T.; Wang, Y.; Jia, Y.; et al. Valerian Essential Oil for Treating Insomnia via the Serotonergic Synapse Pathway. Front. Nutr. 2022, 9, 927434. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, C.T.P. Evaluation of the Effects of Essential Oils on the Reduction of Stress: A Rapid Narrative Review. Braz. J. Health Aromather. Essent. Oil 2024, 1, bjhae4. [Google Scholar] [CrossRef]
- Yousef, H.; Alhajj, M.; Fakoya, A.O.; Sharma, S. Anatomy, Skin (Integument), Epidermis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470464 (accessed on 8 June 2025).
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The Applied Anatomy of Human Skin: A Model for Regeneration. Wound Med. 2020, 28, 100179. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Slominski, A.T. Neuroendocrinology of the Skin. Dermato-Endocrinology 2011, 3, 3–10. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.-C.; Wong, J.K. The Dynamic Anatomy and Patterning of Skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.A.; Uitto, J. Structure and Function of the Skin. In Rook’s Textbook of Dermatology; Barker, J., Griffiths, C., Bleiker, T., Simpson, R., Hussain, W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2024. [Google Scholar] [CrossRef]
- González-Ramírez, R.; Chen, Y.; Liedtke, W.B.; Morales-Lázaro, S.L. TRP Channels and Pain. In Neurobiology of TRP Channels; Emir, T.L.R., Ed.; Frontiers in Neuroscience; CRC Press; Taylor & Francis: Boca Raton, FL, USA, 2017; ISBN 978-1-315-15283-7. [Google Scholar]
- Caterina, M.J.; Pang, Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals 2016, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Rogers, T.J.; Lichtman, A.H. Turning Over a New Leaf: Cannabinoid and Endocannabinoid Modulation of Immune Function. J. Neuroimmune Pharmacol. 2015, 10, 193–203. [Google Scholar] [CrossRef]
- Simankowicz, P.; Stępniewska, J. The Role of Endocannabinoids in Physiological Processes and Disease Pathology: A Comprehensive Review. J. Clin. Med. 2025, 14, 2851. [Google Scholar] [CrossRef]
- Cabral, G.A.; Griffin-Thomas, L. Emerging Role of the Cannabinoid Receptor CB2 in Immune Regulation: Therapeutic Prospects for Neuroinflammation. Expert Rev. Mol. Med. 2009, 11, e3. [Google Scholar] [CrossRef]
- Recognition of Melanocytes in Immuno-Neuroendocrinology and Circadian Rhythms: Beyond the Conventional Melanin Synthesis. Available online: https://www.mdpi.com/2073-4409/11/13/2082 (accessed on 11 June 2025).
- Slominski, A.; Wortsman, J.; Tobin, D.J. The Cutaneous Serotoninergic/Melatoninergic System: Securing a Place under the Sun. FASEB J. 2005, 19, 176–194. [Google Scholar] [CrossRef]
- Channer, B.; Matt, S.M.; Nickoloff-Bybel, E.A.; Pappa, V.; Agarwal, Y.; Wickman, J.; Gaskill, P.J. Dopamine, Immunity, and Disease. Pharmacol. Rev. 2023, 75, 62–158. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Marzo, V.D.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; Stelt, M. van der Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023, 75, 885–958. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-Y.; Menuz, K.; Carlson, J.R. Olfactory Perception: Receptors, Cells, and Circuits. Cell 2009, 139, 45–59. [Google Scholar] [CrossRef]
- Franco, R.; Garrigós, C.; Capó, T.; Serrano-Marín, J.; Rivas-Santisteban, R.; Lillo, J. Olfactory Receptors in Neural Regeneration in the Central Nervous System. Neural Regen. Res. 2025, 20, 2480. [Google Scholar] [CrossRef] [PubMed]
- Stougiannou, T.M.; Christodoulou, K.C.; Karangelis, D. Olfactory Receptors and Aortic Aneurysm: Review of Disease Pathways. J. Clin. Med. 2024, 13, 7778. [Google Scholar] [CrossRef]
- Sun, K.; Ray, S.; Gupta, N.; Aldworth, Z.; Stopfer, M. Olfactory System Structure and Function in Newly Hatched and Adult Locusts. Sci. Rep. 2024, 14, 2608. [Google Scholar] [CrossRef]
- Mika, K.; Benton, R. Olfactory Receptor Gene Regulation in Insects: Multiple Mechanisms for Singular Expression. Front. Neurosci. 2021, 15, 738088. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Cosma, P. Neurocosmetics in Skincare—The Fascinating World of Skin–Brain Connection: A Review to Explore Ingredients, Commercial Products for Skin Aging, and Cosmetic Regulation. Cosmetics 2021, 8, 66. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the Skin1. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Slominski, R.M.; Raman, C.; Chen, J.Y.; Athar, M.; Elmets, C. Neuroendocrine Signaling in the Skin with a Special Focus on the Epidermal Neuropeptides. Am. J. Physiol.-Cell Physiol. 2022, 323, 1757–1776. [Google Scholar] [CrossRef]
- Vieira, D.; Duarte, J.; Vieira, P.; Gonçalves, M.B.S.; Figueiras, A.; Lohani, A.; Veiga, F.; Mascarenhas-Melo, F. Regulation and Safety of Cosmetics: Pre- and Post-Market Considerations for Adverse Events and Environmental Impacts. Cosmetics 2024, 11, 184. [Google Scholar] [CrossRef]
- Rajagopal, S.; Sivanathan, G.; Mahadevaswamy, G.; Angamuthu, G.; Dhandapani, N.V. Neurocosmetics: An extensive overview. Int. J. Appl. Pharm. 2025, 17, 31–38. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Ferreira, M.S.; Sousa-Lobo, J.M.; Sousa, E.; Almeida, I.F. Usage of Synthetic Peptides in Cosmetics for Sensitive Skin. Pharmaceuticals 2021, 14, 702. [Google Scholar] [CrossRef]
- Marques, C.; Hadjab, F.; Porcello, A.; Lourenço, K.; Scaletta, C.; Abdel-Sayed, P.; Hirt-Burri, N.; Applegate, L.A.; Laurent, A. Mechanistic Insights into the Multiple Functions of Niacinamide: Therapeutic Implications and Cosmeceutical Applications in Functional Skincare Products. Antioxidants 2024, 13, 425. [Google Scholar] [CrossRef]
- Kuzumi, A.; Yoshizaki-Ogawa, A.; Fukasawa, T.; Sato, S.; Yoshizaki, A. The Potential Role of Cannabidiol in Cosmetic Dermatology: A Literature Review. Am. J. Clin. Dermatol. 2024, 25, 951–966. [Google Scholar] [CrossRef]
- Kumar, S.; Acharya, T.K.; Halder, R.R.; Mahapatra, P.; Chang, Y.-T.; Goswami, C. Menthol Causes Mitochondrial Ca2+-Influx, Affects Structure-Function Relationship and Cools Mitochondria. Life Sci. 2023, 331, 122032. [Google Scholar] [CrossRef] [PubMed]
- Peppin, J.F.; Pappagallo, M. Capsaicinoids in the Treatment of Neuropathic Pain: A Review. Ther. Adv. Neurol. Disord. 2014, 7, 22–32. [Google Scholar] [CrossRef]
- Alalami, K.; Goff, J.; Grimson, H.; Martin, O.; McDonald, E.; Mirza, T.; Mistry, D.; Ofodile, A.; Raja, S.; Shaker, T.; et al. Does Topical Capsaicin Affect the Central Nervous System in Neuropathic Pain? A Narrative Review. Pharmaceuticals 2024, 17, 842. [Google Scholar] [CrossRef]
- Tyagi, S.; Shekhar, N.; Thakur, A.K. Protective Role of Capsaicin in Neurological Disorders: An Overview. Neurochem. Res. 2022, 47, 1513–1531. [Google Scholar] [CrossRef]
- Kim, H.R.; Jung, Y.; Shin, J.; Park, M.; Kweon, D.-H.; Ban, C. Neuron-Recognizable Characteristics of Peptides Recombined Using a Neuronal Binding Domain of Botulinum Neurotoxin. Sci. Rep. 2022, 12, 4980. [Google Scholar] [CrossRef]
- Lupin, M.; Bjerring, P.; Andriessen, A.; Chantrey, J.; Fabi, S.G.; Liew, S.; McDonald, C.; Xiaolei, Q.; White, S. Real-World Clinical Experience with a Neuro-Peptide Serum in Combination with Botulinum Toxin Type-A Injections. J. Drugs Dermatol. 2024, 23, 43661s3–43661s14. [Google Scholar]
- Hongratanaworakit, T.; Buchbauer, G. Evaluation of the Harmonizing Effect of Ylang-Ylang Oil on Humans after Inhalation. Planta Med. 2004, 70, 632–636. [Google Scholar] [CrossRef]
- Moss, M.; Hewitt, S.; Moss, L.; Wesnes, K. Modulation of cognitive performance and mood by aromas of peppermint and ylang-ylang. Int. J. Neurosci. 2008, 118, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Bavarsad, N.H.; Bagheri, S.; Kourosh-Arami, M.; Komaki, A. Aromatherapy for the Brain: Lavender’s Healing Effect on Epilepsy, Depression, Anxiety, Migraine, and Alzheimer’s Disease: A Review Article. Heliyon 2023, 9, e18492. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and Anti-Aging Potentials of Essential Oils from Aromatic and Medicinal Plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Koulivand, P.H.; Ghadiri, M.K.; Gorji, A. Lavender and the Nervous System. Evid.-Based Complement. Altern. Med. 2013, 2013, 681304. [Google Scholar] [CrossRef]
- Leong, E.J.; Tan, L.F.; Yap, V.L.; Rajagopal, M.; Chandran, R. Cosmetological Applications of Citrus Limon: A Mini-Review. Indian J. Nat. Prod. Resour. IJNPR Former. Nat. Prod. Radiance NPR 2024, 15, 286–293. [Google Scholar] [CrossRef]
- Ali, A.; Chaudhary, A.; Sharma, A.; Siddiqui, N.; Anurag; Parihar, V.K. Exploring Role of Citrus Fruits in Comorbid Neurodegenerative Disorders Associated with Psoriasis. Metab. Brain Dis. 2024, 40, 62. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.G.; Malik, M.M.A.H.; Connell, E.; Müller, M.; Vauzour, D. Citrus Polyphenols in Brain Health and Disease: Current Perspectives. Front. Neurosci. 2021, 15, 640648. [Google Scholar] [CrossRef]
- Belluzzi, J.D.; Stein, L. Enkephalin May Mediate Euphoria and Drive-Reduction Reward. Nature 1977, 266, 556–558. [Google Scholar] [CrossRef]
- Cullen, J.M.; Cascella, M. Physiology, Enkephalin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557764 (accessed on 2 July 2025).
- Proteau-Gagné, A.; Bournival, V.; Rochon, K.; Dory, Y.L.; Gendron, L. Exploring the Backbone of Enkephalins to Adjust Their Pharmacological Profile for the δ-Opioid Receptor. ACS Chem. Neurosci. 2010, 1, 757–769. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, M. Enkephalins and Pain Modulation: Mechanisms of Action and Therapeutic Perspectives. Biomolecules 2024, 14, 926. [Google Scholar] [CrossRef]
- Pintea, A.; Manea, A.; Pintea, C.; Vlad, R.-A.; Bîrsan, M.; Antonoaea, P.; Rédai, E.M.; Ciurba, A. Peptides: Emerging Candidates for the Prevention and Treatment of Skin Senescence: A Review. Biomolecules 2025, 15, 88. [Google Scholar] [CrossRef]
- Lima, T.N.; Pedriali Moraes, C.A. Bioactive Peptides: Applications and Relevance for Cosmeceuticals. Cosmetics 2018, 5, 21. [Google Scholar] [CrossRef]
- Gazitaeva, Z.I.; Drobintseva, A.O.; Chung, Y.; Polyakova, V.O.; Kvetnoy, I.M. Cosmeceutical Product Consisting of Biomimetic Peptides: Antiaging Effects in Vivo and in Vitro. Clin. Cosmet. Investig. Dermatol. 2017, 10, 11–16. [Google Scholar] [CrossRef]
- Michalek, I.M.; Lelen-Kaminska, K.; Caetano dos Santos, F.L. Peptides Stimulating Synthesis of Extracellular Matrix Used in Anti-Ageing Cosmetics: Are They Clinically Tested? A Systematic Review of the Literature. Australas. J. Dermatol. 2019, 60, e267–e271. [Google Scholar] [CrossRef]
- Veiga, E.; Ferreira, L.; Correia, M.; Pires, P.C.; Hameed, H.; Araújo, A.R.T.S.; Cefali, L.C.; Mazzola, P.G.; Hamishehkar, H.; Veiga, F.; et al. Anti-Aging Peptides for Advanced Skincare: Focus on Nanodelivery Systems. J. Drug Deliv. Sci. Technol. 2023, 89, 105087. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Yi, E.-J.; Jin, X.; Zheng, Q.; Park, S.-J.; Yi, G.-S.; Yang, S.-J.; Yi, T.-H. Sustainable Dynamic Wrinkle Efficacy: Non-Invasive Peptides as the Future of Botox Alternatives. Cosmetics 2024, 11, 118. [Google Scholar] [CrossRef]
- Viper Venom and Synthetic Peptides: Emerging Active Ingredients in Anti-Ageing Cosmeceuticals. Available online: https://www.mdpi.com/2076-3417/15/8/4501 (accessed on 13 June 2025).
- Dragomirescu, A.O.; Andoni, M.; Ionescu, D.; Andrei, F. The Efficiency and Safety of Leuphasyl—A Botox-Like Peptide. Cosmetics 2014, 1, 75–81. [Google Scholar] [CrossRef]
- Zdrada-Nowak, J.; Surgiel-Gemza, A.; Szatkowska, M. Acetyl Hexapeptide-8 in Cosmeceuticals—A Review of Skin Permeability and Efficacy. Int. J. Mol. Sci. 2025, 26, 5722. [Google Scholar] [CrossRef]
- Olsson, S.E.; Sreepad, B.; Lee, T.; Fasih, M.; Fijany, A. Public Interest in Acetyl Hexapeptide-8: Longitudinal Analysis. JMIR Dermatol. 2024, 7, e54217. [Google Scholar] [CrossRef]
- Raikou, V.; Kalogria, E.; Varvaresou, A.; Tsirivas, E.; Panderi, I. Quantitation of Acetyl Hexapeptide-8 in Cosmetics by Hydrophilic Interaction Liquid Chromatography Coupled to Photo Diode Array Detection. Separations 2021, 8, 125. [Google Scholar] [CrossRef]
- Kachooeian, M.; Mousivand, Z.; Sharifikolouei, E.; Shirangi, M.; Firoozpour, L.; Raoufi, M.; Sharifzadeh, M. Matrixyl Patch vs Matrixyl Cream: A Comparative In Vivo Investigation of Matrixyl (MTI) Effect on Wound Healing. ACS Omega 2022, 7, 24695–24704. [Google Scholar] [CrossRef]
- Li, F.; Chen, H.; Chen, D.; Zhang, B.; Shi, Q.; He, X.; Zhao, H.; Wang, F. Clinical Evidence of the Efficacy and Safety of a New Multi-Peptide Anti-Aging Topical Eye Serum. J. Cosmet. Dermatol. 2023, 22, 3340–3346. [Google Scholar] [CrossRef] [PubMed]
- Gok, B.; Budama-Kilinc, Y.; Kecel-Gunduz, S. Anti-Aging Activity of Syn-Ake Peptide by in Silico Approaches and in Vitro Tests. J. Biomol. Struct. Dyn. 2024, 42, 5015–5029. [Google Scholar] [CrossRef]
- Robinson, L.R.; Fitzgerald, N.C.; Doughty, D.G.; Dawes, N.C.; Berge, C.A.; Bissett, D.L. Topical Palmitoyl Pentapeptide Provides Improvement in Photoaged Human Facial Skin. Int. J. Cosmet. Sci. 2005, 27, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Schagen, S.K. Topical Peptide Treatments with Effective Anti-Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Hoppel, M.; Reznicek, G.; Kählig, H.; Kotisch, H.; Resch, G.P.; Valenta, C. Topical Delivery of Acetyl Hexapeptide-8 from Different Emulsions: Influence of Emulsion Composition and Internal Structure. Eur. J. Pharm. Sci. 2015, 68, 27–35. [Google Scholar] [CrossRef]
- Kraeling, M.E.K.; Zhou, W.; Wang, P.; Ogunsola, O.A. In Vitro Skin Penetration of Acetyl Hexapeptide-8 from a Cosmetic Formulation. Cutan. Ocul. Toxicol. 2015, 34, 46–52. [Google Scholar] [CrossRef]
- Moy, M.; Diaz, I.; Lesniak, E.; Giancola, G. Peptide-pro Complex Serum: Investigating Effects on Aged Skin. J. Cosmet. Dermatol. 2023, 22, 267–274. [Google Scholar] [CrossRef]
- Shariati Pour, S.R.; Oddis, S.; Barbalinardo, M.; Ravarino, P.; Cavallini, M.; Fiori, J.; Giuri, D.; Tomasini, C. Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels. Molecules 2023, 28, 2528. [Google Scholar] [CrossRef] [PubMed]
- WO2016090252A1-Topical Skin Care Composition Comprising Trifluoroacetyl Tripeptide-2-Google Patents. Available online: https://patents.google.com/patent/WO2016090252A1/en (accessed on 7 July 2025).
- The Mechanism and Function of Acetyl Tetrapeptide-2 in Human Skin-Creative Peptides. Available online: https://www.creative-peptides.com/article/the-mechanism-and-function-of-acetyl-tetrapeptide-2-in-human-skin-149.html (accessed on 7 July 2025).
- Kobiela, T.; Milner-Krawczyk, M.; Pasikowska-Piwko, M.; Bobecka-Wesołowska, K.; Eris, I.; Święszkowski, W.; Dulinska-Molak, I. The Effect of Anti-Aging Peptides on Mechanical and Biological Properties of HaCaT Keratinocytes. Int. J. Pept. Res. Ther. 2018, 24, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, N.; Ozols, M.; Bell, M.; Bradley, E.; Gilmore, A.; Debelle, L.; Sherratt, M.J. Matrikines as Mediators of Tissue Remodelling. Adv. Drug Deliv. Rev. 2022, 185, 114240. [Google Scholar] [CrossRef]
- Raikou, V.; Varvaresou, A.; Panderi, I.; Papageorgiou, E. The Efficacy Study of the Combination of Tripeptide-10-Citrulline and Acetyl Hexapeptide-3. A Prospective, Randomized Controlled Study. J. Cosmet. Dermatol. 2017, 16, 271–278. [Google Scholar] [CrossRef]
- Puig, A.; Antón, J.M.G.; Mangues, M. A new decorin-like tetrapeptide for optimal organization of collagen fibres. Int. J. Cosmet. Sci. 2008, 30, 97–104. [Google Scholar] [CrossRef]
- Yuan, M.; Niu, J.; Li, F.; Ya, H.; Liu, X.; Li, K.; Fan, Y.; Zhang, Q. Dipeptide-1 Modified Nanostructured Lipid Carrier-Based Hydrogel with Enhanced Skin Retention and Topical Efficacy of Curcumin. RSC Adv. 2023, 13, 29152–29162. [Google Scholar] [CrossRef] [PubMed]
- Topouzidou, N.; Miliotou, A.N.; Nodaraki, D.; Galatou, E.; Petrou, C.; Sarigiannis, Y. Unraveling the Molecular Mechanisms of Synthetic Acetyl Hexapeptide in E-Cadherin Activation for Tissue Rejuvenation. Cosmetics 2025, 12, 48. [Google Scholar] [CrossRef]
- Hrenak, J.; Paulis, L.; Simko, F. N-Acetyl-Seryl-Aspartyl-Lysyl-Proline (Ac-SDKP): Potential Target Molecule in Research of Heart, Kidney and Brain. Curr. Pharm. Des. 2015, 21, 5135–5143. [Google Scholar] [CrossRef]
- Kanasaki, K. N-acetyl-seryl-aspartyl-lysyl-proline Is a Valuable Endogenous Antifibrotic Peptide for Kidney Fibrosis in Diabetes: An Update and Translational Aspects. J. Diabetes Investig. 2020, 11, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Hajem, N.; Chapelle, A.; Bignon, J.; Pinault, A.; Liu, J.-M.; Salah-Mohellibi, N.; Lati, E.; Wdzieczak-Bakala, J. The regulatory role of the tetrapeptide AcSDKP in skin and hair physiology and the prevention of ageing effects in these tissues—A potential cosmetic role. Int. J. Cosmet. Sci. 2013, 35, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Tripeptide-1, Hexapeptide-12, Their Metal Salts and Fatty Acyl Derivatives, and Palmitoyl Tetrapeptide-7 as Used in Cosmetics. Int. J. Toxicol. 2018, 37, 90S–102S. [Google Scholar] [CrossRef]
- Hostynek, J.J.; Dreher, F.; Maibach, H.I. Human Skin Retention and Penetration of a Copper Tripeptide in Vitro as Function of Skin Layer towards Anti-Inflammatory Therapy. Inflamm. Res. 2010, 59, 983–988. [Google Scholar] [CrossRef] [PubMed]
- CN113461774A-Preparation Method of Palmitoyl Tripeptide-1-Google Patents. Available online: https://patents.google.com/patent/CN113461774A/en (accessed on 7 July 2025).
- Mondon, P.; Hillion, M.; Peschard, O.; Andre, N.; Marchand, T.; Doridot, E.; Feuilloley, M.G.; Pionneau, C.; Chardonnet, S. Evaluation of Dermal Extracellular Matrix and Epidermal–Dermal Junction Modifications Using Matrix-assisted Laser Desorption/Ionization Mass Spectrometric Imaging, in Vivo Reflectance Confocal Microscopy, Echography, and Histology: Effect of Age and Peptide Applications. J. Cosmet. Dermatol. 2015, 14, 152–160. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; ISBN 978-0-7020-5434-1. [Google Scholar]
- Burfield, T. Safety of Essential Oils. Int. J. Aromather. 2000, 10, 16–29. [Google Scholar] [CrossRef]
- Sindle, A.; Martin, K. Art of Prevention: Essential Oils-Natural Products Not Necessarily Safe. Int. J. Womens Dermatol. 2021, 7, 304–308. [Google Scholar] [CrossRef]


| Entry | Essential Oil | Main Function in Aromatherapy | Some of the Main Active Compounds | References |
|---|---|---|---|---|
| 1 | Lavender | Relaxing, anti-anxiety | Linalool, Linalyl acetate | [12,13,14,15,16] |
| 2 | Tea Tree | Antiseptic, immune support | Terpinen-4-ol, α-Terpinene | [17,18] |
| 3 | Peppermint | Stimulating, decongestant, neuroprotective effect | Menthol, Menthone | [19,20,21,22,23,24] |
| 4 | Eucalyptus | Respiratory relief, antibacterial, central nervous system | 1,8-Cineole, α-Pinene | [25,26,27,28] |
| 5 | Lemon | Uplifting, refreshing, stress-related disorders/mood disorders | Limonene, Citral | [29,30,31] |
| 6 | Sweet Orange | Mood enhancer, calming, pain and anxiety | Limonene, Linalol, Myrcene | [32,33,34,35,36,37] |
| 7 | Chamomile | Anti-inflammatory, soothing, anxiety | Chamazulene, Bisabolol, Matricine | [38,39,40,41,42,43] |
| 8 | Rosemary | Mental clarity, energizing | 1,8-Cineole, Camphor, α-Pinene | [44,45,46,47,48] |
| 9 | Frankincense | Grounding, stress relief | α-Pinene, Incensole acetate | [6,49] |
| 10 | Ylang-Ylang | Balancing, mood enhancing | Linalool, Geranyl acetate | [50,51,52,53,54] |
| 11 | Cedarwood | Calming, sedative | Cedrol, Thujopsene | [55,56] |
| 12 | Bergamot | Stress reduction, mood enhancer | Linalyl acetate, Limonene | [57,58,59] |
| 13 | Geranium | Balancing hormones, skincare | Geraniol, Citronellol | [60,61,62] |
| 14 | Clary Sage | Hormonal balance, antidepressant | Linalyl acetate, Sclareol | [6,63,64,65] |
| 15 | Lemongrass | Detoxifying, purifying | Citral, Geraniol | [66,67,68,69] |
| 16 | Patchouli | Earthy, aphrodisiac | Patchoulol, Norpatchoulenol | [70,71,72] |
| 17 | Jasmine | Euphoric, soothing | Benzyl acetate, Jasmonates | [73,74,75] |
| 18 | Sandalwood | Grounding, meditative | Santalol, β-Santalene | [76,77,78,79] |
| 19 | Basil | Mental alertness, fatigue | Linalool, Methyl chavicol | [80,81,82] |
| 20 | Cypress | Circulation booster, calming | α-Pinene, Limonene | [83,84] |
| 21 | Marjoram | Muscle relaxation, sleep aid | Terpinen-4-ol, Sabinene | [85,86,87] |
| 22 | Thyme | Immune support, purifying | Thymol, Carvacrol | [88,89,90] |
| 23 | Oregano | Antiviral, immune booster | Carvacrol, Thymol | [91,92] |
| 24 | Ginger | Warming, anti-nausea | Zingiberene, β-Bisabolene | [93,94] |
| 25 | Fennel | Digestive aid, calming | Anethole, Fenchone | [95,96,97] |
| 26 | Spearmint | Mental clarity, digestive support | Carvone, Limonene | [97,98,99] |
| 27 | Cinnamon | Stimulant, warming, mental clarity | Cinnamaldehyde, Eugenol | [100,101,102] |
| 28 | Myrrh | Soothing, skincare | Furanosesquiterpenoids, Curzerene | [103,104,105] |
| 29 | Neroli | Nervous tension, uplifting, anxiety | Nerolidol, Linalool | [102,106,107,108] |
| 30 | Helichrysum | Skin regeneration, wound healing | Italidione, Geranyl acetate, α-Cedrene | [109,110] |
| 31 | Pine | Respiratory support, energizing | α-Pinene, β-Pinene | [84,111,112] |
| 32 | Tangerine | Cheering, emotional balance | Limonene, γ-Terpinene | [113] |
| 33 | Petitgrain | Nervous system calming | Linalyl acetate, Geraniol | [114,115,116] |
| 34 | Juniper Berry | Reduce stress and instill calm | α-Pinene, β-Myrcene | [117,118,119] |
| 35 | Black Pepper | Pain relief, neuroprotective, anxiolytic | Piperine, Piperlonguminine | [120,121,122] |
| 36 | Melissa (Lemon Balm) | Calming, antidepressant | Citral, β-Caryophyllene | [123,124,125,126] |
| 37 | Bay Laurel | Respiratory and lymphatic support | 1,8-Cineole, Sabinene, Linalool | [127,128] |
| 38 | Turmeric | Mental disorders, including depression | Curcumin, Turmerone | [129,130,131] |
| 39 | Citronella | Mood states and brain activities | Citronellal, Geraniol | [132,133,134] |
| 40 | Wintergreen | Analgesic, anti-inflammatory | Methyl salicylate | [135,136] |
| 41 | Copaiba | Reducing anxiety | β-Caryophyllene, α-Humulene | [137,138] |
| 42 | Valerian | Reduction in stress and sleep problems | Valerenic acid, Bornyl acetate | [139,140,141,142] |
| Entry | Commercial Name | Peptide Name | Amino Acid Sequence | Target Receptor/Pathway | Emotional/Sensory Effect | References |
|---|---|---|---|---|---|---|
| 1 | Argireline® | Acetyl Hexapeptide-8 | Ac-Glu-Glu-Met-Gln-Arg-Arg-NH2 | SNARE complex | Reduces muscle contraction signals | [196,197,198] |
| 2 | Matrixyl® 3000 | Palmitoyl Tetrapeptide-7 + Palmitoyl Oligopeptide | Gly-Gln-Pro-Arg + Pal-Gly-Gly-Gly | TGF-β pathway | Supports cell communication and repair | [199,200] |
| 3 | Syn®-Ake | Dipeptide Diaminobutyroyl Benzylamide Diacetate | H-D-Ala-D-But-OH derivative | Nicotinic acetylcholine receptor | Neuromuscular modulation | [193,201] |
| 4 | Syn®-Coll | Palmitoyl Tripeptide-5 | Pal-Lys-Val-Lys | TGF-β receptor | Improves dermal matrix via signal transduction | [202,203] |
| 5 | Snap-8 | Acetyl Octapeptide-3 | Ac-Glu-Glu-Met-Gln-Arg-Arg-Glu-Lys | SNAP-25 cascade | Inhibits facial tension | [204,205] |
| 6 | Leuphasyl® | Pentapeptide-18 | Tyr-D-Ala-Gly-Phe-Leu | SNARE complex | Modulates synaptic contraction | [195,206] |
| 7 | Progeline™ | Trifluoroacetyl Tripeptide-2 | TFA-Val-Tyr-Val | MMPs and progerin | Improves firmness by reducing cell aging | [207,208] |
| 8 | Thymulen™ | Acetyl Tetrapeptide-2 | Ac-Tyr-Arg-Lys-Lys-NH2 | ECM regulation | Improves firmness and elasticity | [209,210,211] |
| 9 | Decorinyl® | Decorin-mimetic peptide | Not disclosed | Decorin interaction | Strengthens skin via ECM support | [212,213] |
| 10 | Idealift™ | Acetyl dipeptide-1 cetyl ester | Ac-Tyr-Arg-NH2 (with cetyl ester) | Elastic fiber proteins | Improves tone by elastic fiber reinforcement | [167,214] |
| 11 | Melitane™ | Acetyl Hexapeptide-1 | Ac-Tyr-Arg-Ser-Arg-Lys-NH2 | MC1-R receptor | Stimulates melanin and mood-linked peptides | [215] |
| 12 | Ac-SDKP | N-acetyl-seryl-aspartyl-lysyl-proline | Ac-Ser-Asp-Lys-Pro | Angiotensin II/TGF-β signaling modulation | Promotes cutaneous comfort and relaxation | [216,217,218] |
| 13 | GHK-Cu | Copper Tripeptide-1 | Gly-His-Lys + Cu2+ ion | Copper transporters | Promotes healing via neurotrophic pathways | [219,220] |
| 14 | SpecPed® PT1P | Palmitoyl Tripeptide-1 | Pal-Gly-His-Lys | Collagen biosynthesis enzymes | Stimulates collagen linked to nerve stimulation | [167,192,221] |
| 15 | Rigin™ | Palmitoyl Tetrapeptide-7 | Pal-Gly-Gln-Pro-Arg | Cytokine receptors | Reduces inflammation mediated by neuropeptides | [203,219,222] |
| 16 | Neutrazen™ | Palmitoyl Tripeptide-8 | Pal-Gly-Gln-Pro-Arg | Inflammatory neuropeptide receptors | Calms via neuroimmune modulation | [167] |
| Term | Definition |
|---|---|
| Neurocosmetics | Topical products that modulate the skin’s neuro-immuno-endocrine system to influence cutaneous function and emotional states. |
| Neurocosmetic Active | A functional ingredient (e.g., biomimetic peptide, TRP modulator, cannabinoid ligand, ectopic olfactory receptor ligand) that alters neurocutaneous signaling to produce measurable skin and/or neurosensory effects. |
| Neurocosmetic Formulation | A topical product designed to deliver one or more neurocosmetic actives at safe, effective levels using suitable delivery systems, sensory/olfactory design, and evidence-based claims within cosmetic regulations. |
| Aromatherapy | Use of volatile compounds (essential oils) via the olfactory route to modulate limbic activity and psychophysiological responses. |
| Skin–Brain Axis | Bidirectional communication between skin and CNS through neuroendocrine, immune, and sensory pathways. |
| TRP Channels (e.g., TRPV1, TRPM8, TRPA1) | Sensory ion channels responding to heat/cold/irritants; regulate pain, itch, inflammation, and pigmentation—key neurocosmetic targets. |
| Cannabinoid Receptors (CB1/CB2) | GPCRs in skin and immune cells that modulate inflammation, nociception, and sebum; CB1 is more neuronal, CB2 is more immune-linked. |
| Cutaneous Serotonergic/Dopaminergic Receptors | Neuroreceptors in skin cells implicated in proliferation, pigmentation, inflammation, and sensory comfort. |
| Ectopic Olfactory Receptors (e.g., OR2AT4) | Olfactory receptors expressed in skin and other tissues; activation can influence regeneration, inflammation, and cell migration. |
| Limbic System | Brain network (e.g., amygdala, piriform/entorhinal cortex) mediating emotion and memory; activated by olfactory inputs. |
| HPA Axis | Hypothalamic–pituitary–adrenal stress pathway; commonly monitored via salivary cortisol in psychoderm studies. |
| BDNF | Brain-derived neurotrophic factor; supports neuroplasticity and can change with olfactory/aromatherapy interventions. |
| Psychophysiological Biomarkers | Objective measures (e.g., cortisol, HRV, EEG/neuroimaging) used to validate neurocosmetic effects. |
| EEG/Power Spectral Density (PSD) | Electrophysiological methods quantifying brain activity across frequency bands to assess arousal/attention states. |
| HRV (Heart Rate Variability) | Autonomic biomarker of stress and emotional regulation; useful in cosmetic/psychoderm endpoints. |
| Transdermal Delivery | Penetration of actives across the epidermal barrier; a central challenge for efficacy in neurocosmetics. |
| Advanced Delivery Systems | Technologies (e.g., nanocarriers, lipid vehicles, controlled release) that enhance penetration, targeting, and bioavailability. |
| Placebo and Subjective Outcomes | Expectation effects and self-reported endpoints that add variability; motivate inclusion of objective measures. |
| Borderline Products (EU Reg. 1223/2009) | Products at the cosmetic–drug/device interface; classification depends on site, mechanism, and intended purpose. |
| Photosensitization | Light-triggered adverse reaction (common with some essential oils) leading to erythema or hyperpigmentation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Peña, M.J.; Magallón-Chávez, O.; Rivas-Loaiza, J.A. Neurocosmetics and Aromatherapy Through Neurocutaneous Receptors and Their Functional Implications in Cosmetics. Cosmetics 2025, 12, 179. https://doi.org/10.3390/cosmetics12050179
Sánchez-Peña MJ, Magallón-Chávez O, Rivas-Loaiza JA. Neurocosmetics and Aromatherapy Through Neurocutaneous Receptors and Their Functional Implications in Cosmetics. Cosmetics. 2025; 12(5):179. https://doi.org/10.3390/cosmetics12050179
Chicago/Turabian StyleSánchez-Peña, María Judith, Odessa Magallón-Chávez, and Juan Antonio Rivas-Loaiza. 2025. "Neurocosmetics and Aromatherapy Through Neurocutaneous Receptors and Their Functional Implications in Cosmetics" Cosmetics 12, no. 5: 179. https://doi.org/10.3390/cosmetics12050179
APA StyleSánchez-Peña, M. J., Magallón-Chávez, O., & Rivas-Loaiza, J. A. (2025). Neurocosmetics and Aromatherapy Through Neurocutaneous Receptors and Their Functional Implications in Cosmetics. Cosmetics, 12(5), 179. https://doi.org/10.3390/cosmetics12050179






