RNA Sequencing Reveals Effects of a New Complex Bath Additive on Cultured Cells
Abstract
1. Introduction
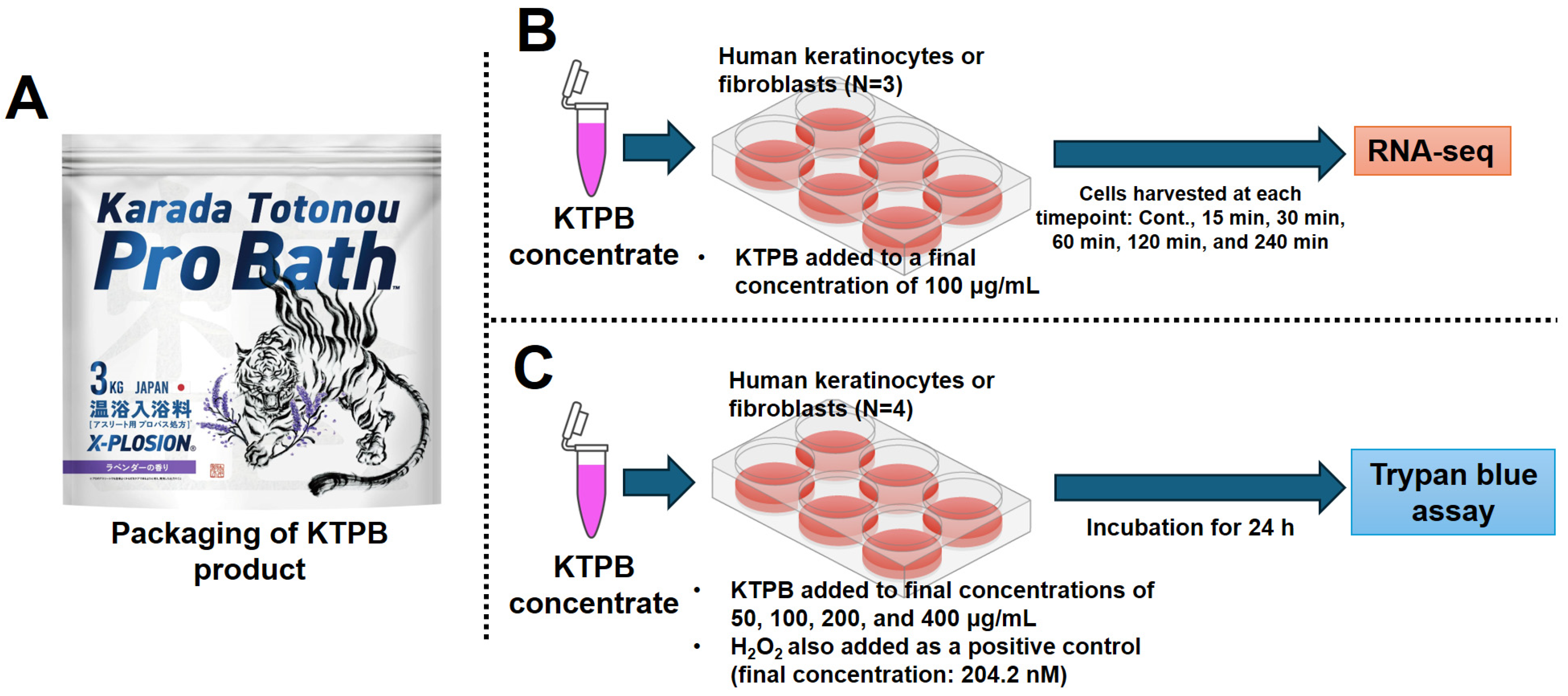
2. Materials and Methods
2.1. Cell Culture Experiments
2.2. RNA-Seq
2.3. Bioinformatic Analysis
2.4. Statistical Analyses
3. Results
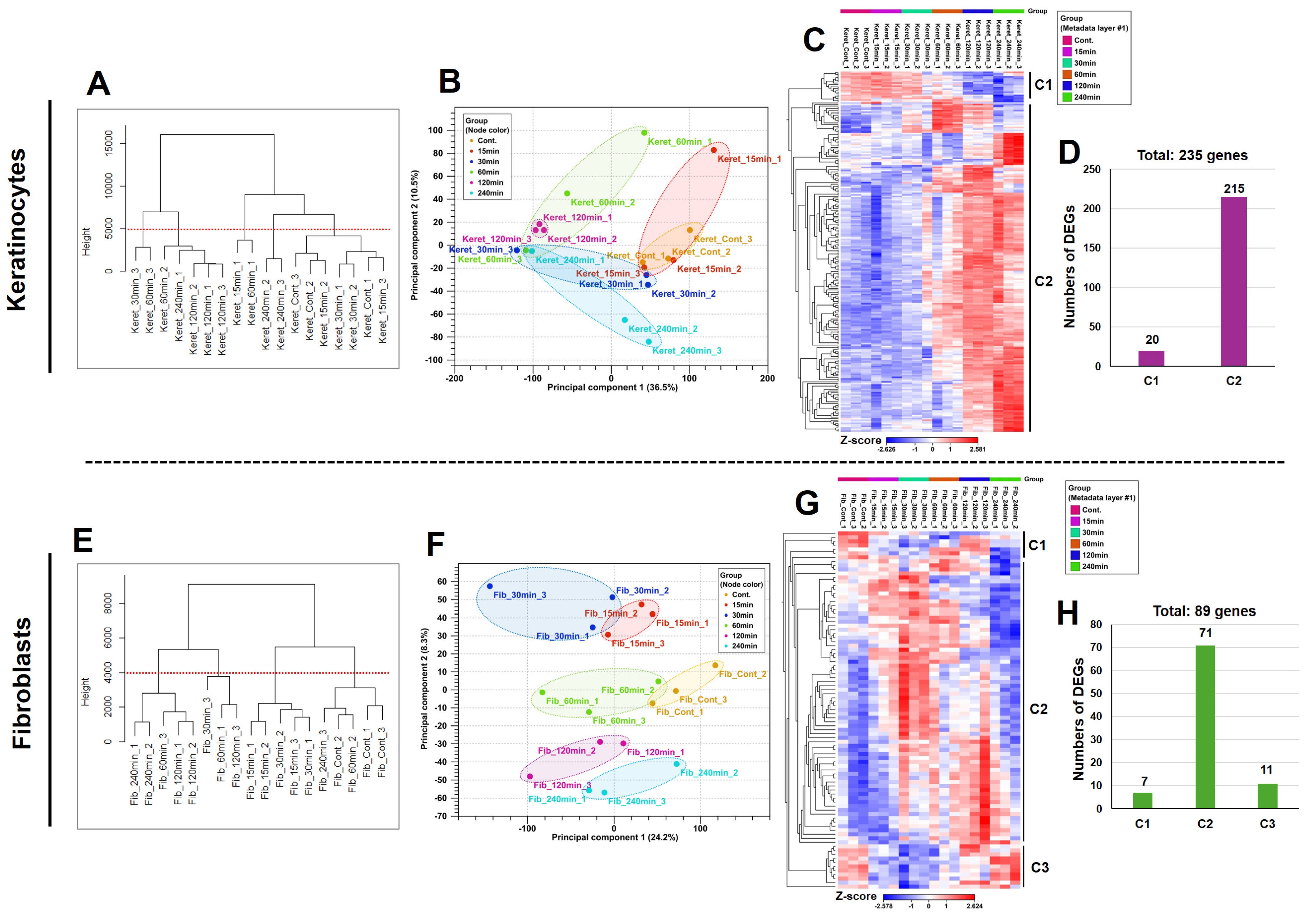
3.1. KTPB Affects the Expression of Many Genes Depending on the Time Course
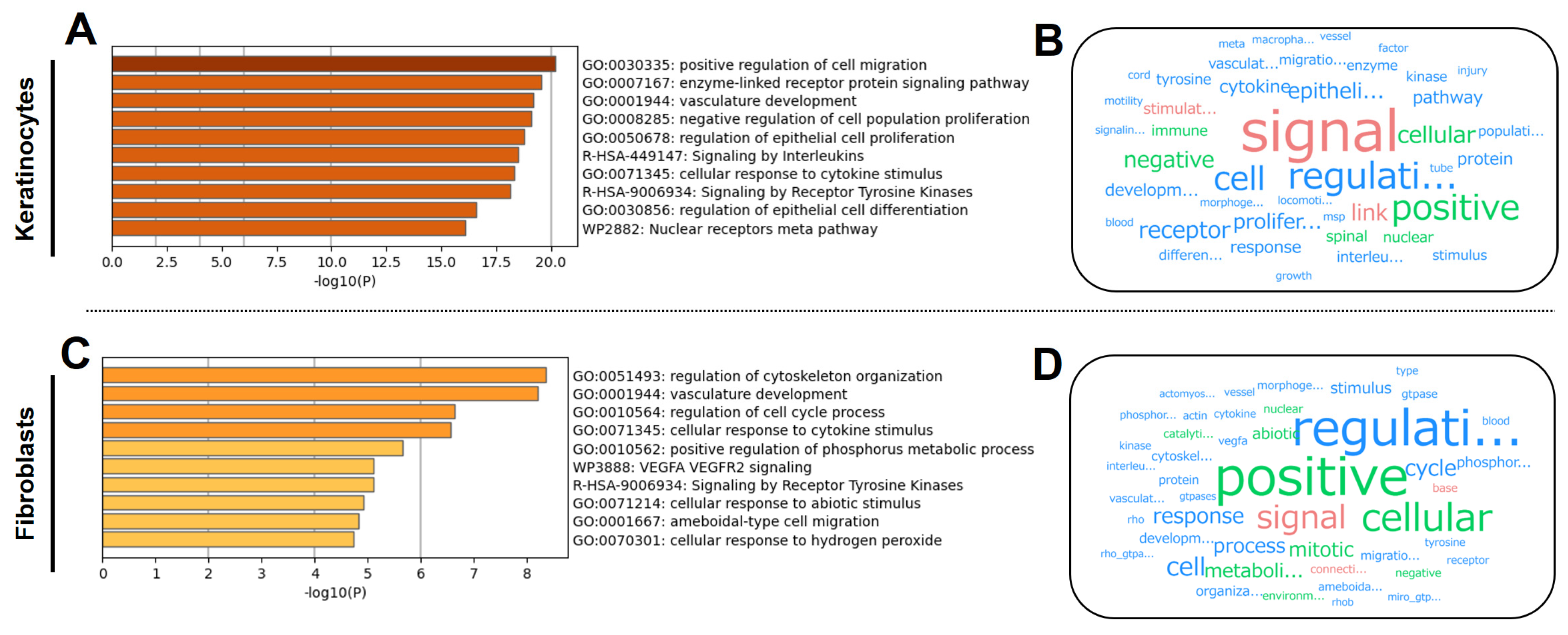
3.2. KTPB Affects Cell Activation
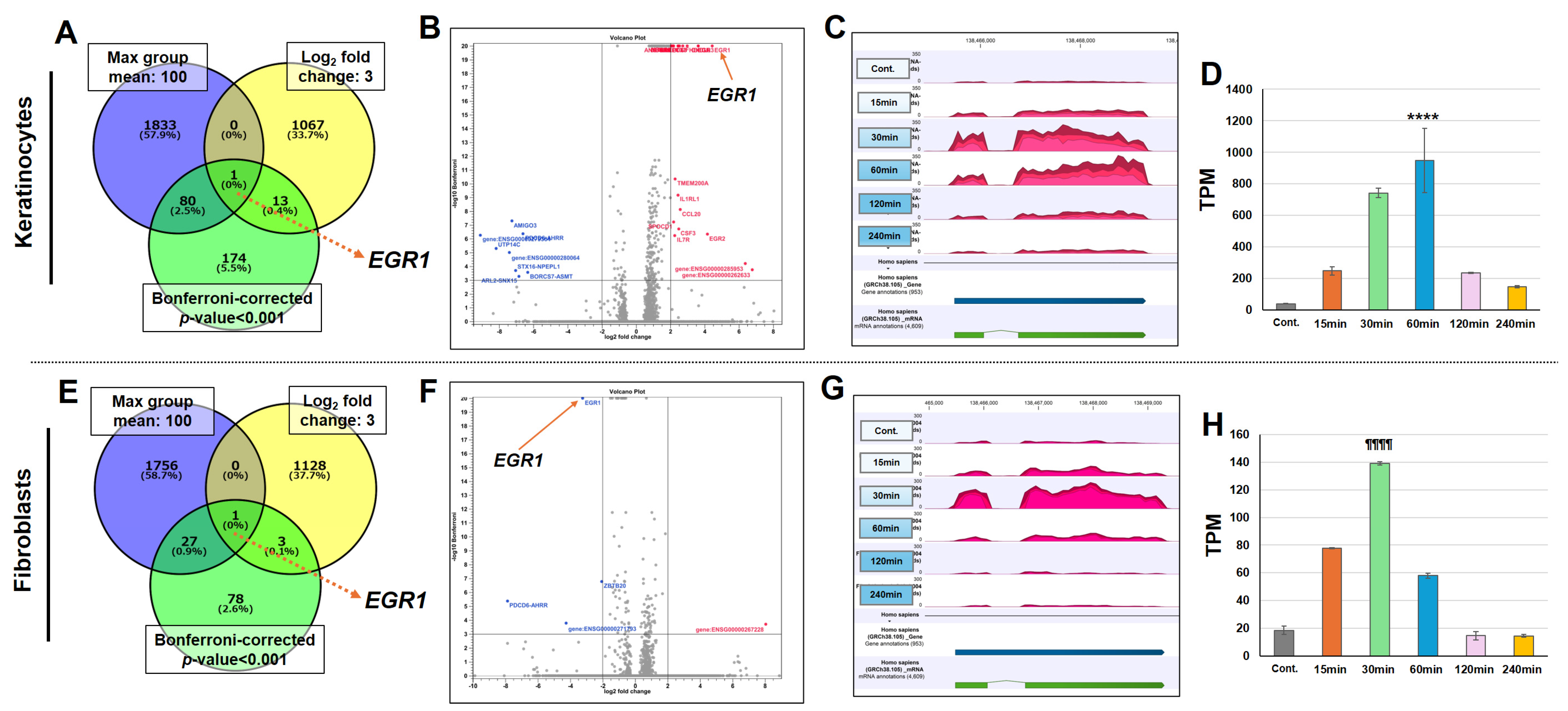
3.3. KTPB Significantly Increases EGR1 Gene Expression
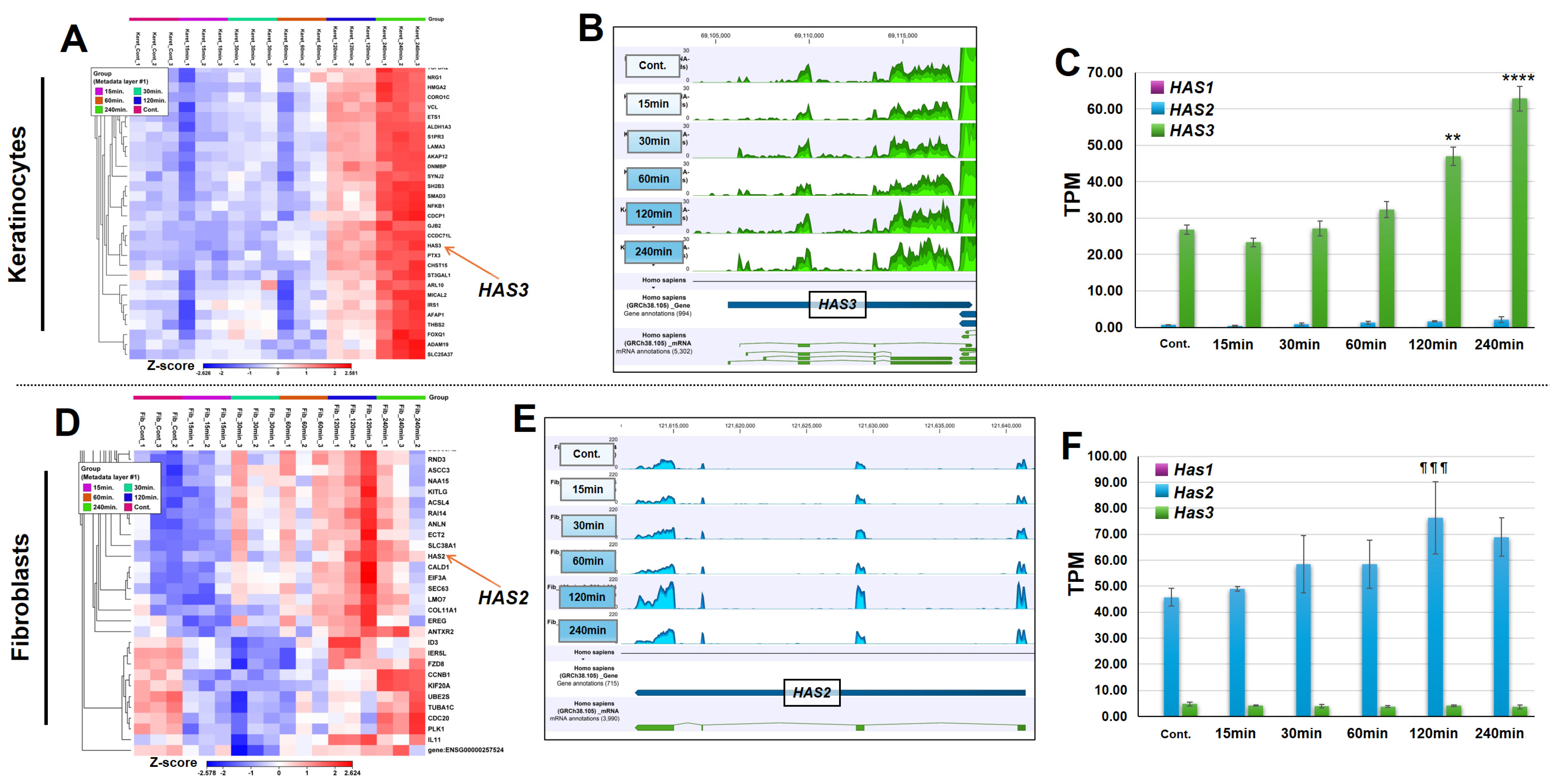
3.4. KTPB Increases the Expression of HASs Without Increasing the Expression of HYLAs
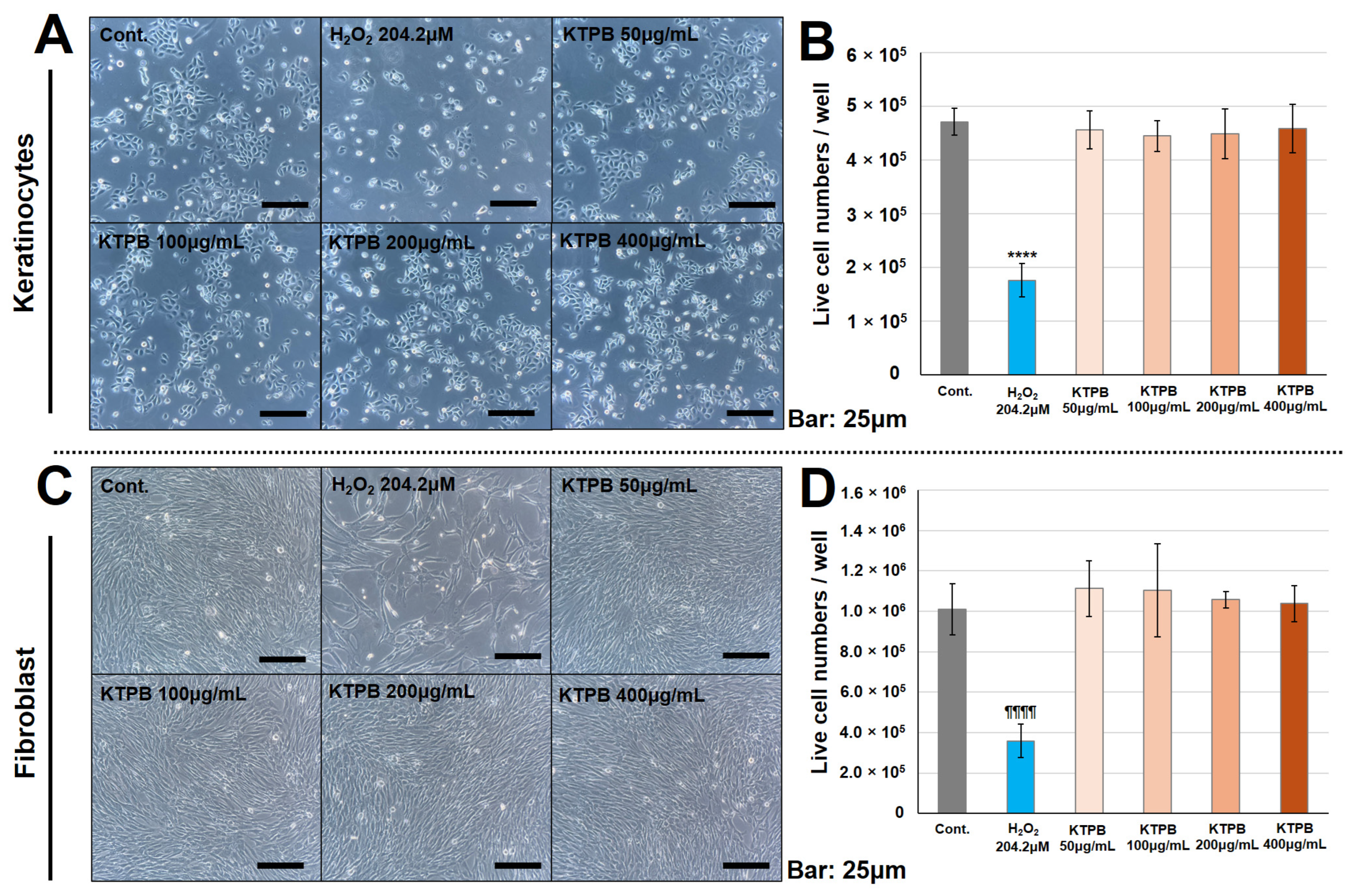
3.5. KTPB Did Not Result in Cytotoxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bath Salt Market. Available online: https://www.hpc-news.co.jp/media/feature/a1246 (accessed on 17 February 2025).
- Bath Salts Market by Product Type. Available online: https://www.360iresearch.com/library/intelligence/bath-salts (accessed on 12 May 2025).
- Watanabe, S.; Ishizawa, T.; Tsunakawa, M. The Current State and Prospects of Bath Additive. Jpn. J. Biometeorol. 2020, 56, 121–131. [Google Scholar] [CrossRef]
- Kouzuki, M.; Kitao, S.; Kaju, T.; Urakami, K. Evaluation of the Effect of Aroma Oil as a Bath Salt on Cognitive Function. Psychogeriatrics 2020, 20, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Web of Science. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 17 February 2025).
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 17 February 2025).
- Peinemann, F.; Harari, M.; Peternel, S.; Chan, T.; Chan, D.; Labeit, A.M.; Gambichler, T. Indoor Balneophototherapy for Chronic Plaque Psoriasis: Abridged Cochrane Review. Dermatol. Ther. 2021, 34, e14588. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Rapp, S.; Senger, E.; Altmeyer, P.; Hoffmann, K. Balneophototherapy of Psoriasis: Highly Concentrated Salt Water versus Tap Water—A Randomized, One-Blind, Right/Left Comparative Study. Photodermatol. Photoimmunol. Photomed. 2001, 17, 22–25. [Google Scholar] [CrossRef]
- Pagliaro, M.; Pecoraro, L.; Stefani, C.; Pieropan, S.; Piacentini, G.; Pietrobelli, A. Bathing in Atopic Dermatitis in Pediatric Age: Why, How and When. Pediatr. Rep. 2024, 16, 57–68. [Google Scholar] [CrossRef]
- Gittler, J.K.; Wang, J.F.; Orlow, S.J. Bathing and Associated Treatments in Atopic Dermatitis. Am. J. Clin. Dermatol. 2017, 18, 45–57. [Google Scholar] [CrossRef]
- Sherman, G.; Zeller, L.; Avriel, A.; Friger, M.; Harari, M.; Sukenik, S. Intermittent Balneotherapy at the Dead Sea Area for Patients with Knee Osteoarthritis. Isr. Med. Assoc. J. 2009, 11, 88–93. [Google Scholar]
- Sekiya, M.; Kainoh, K.; Sugasawa, T.; Yoshino, R.; Hirokawa, T.; Tokiwa, H.; Nakano, S.; Nagatoishi, S.; Tsumoto, K.; Takeuchi, Y.; et al. The Transcriptional Corepressor CtBP2 Serves as a Metabolite Sensor Orchestrating Hepatic Glucose and Lipid Homeostasis. Nat. Commun. 2021, 12, 6315. [Google Scholar] [CrossRef]
- Ronchi, C.; Haider, S.; Brisken, C. EMBER Creates a Unified Space for Independent Breast Cancer Transcriptomic Datasets Enabling Precision Oncology. NPJ Breast Cancer 2024, 10, 56. [Google Scholar] [CrossRef]
- Watson, B.R.; Paul, B.; Rahman, R.U.; Amir-Zilberstein, L.; Segerstolpe, Å.; Epstein, E.T.; Murphy, S.; Geistlinger, L.; Lee, T.; Shih, A.; et al. Spatial Transcriptomics of Healthy and Fibrotic Human Liver at Single-Cell Resolution. Nat. Commun. 2025, 16, 319. [Google Scholar] [CrossRef]
- Sugasawa, T.; Hirokawa, A.; Otani, N.; Kanki, Y.; Nguyen, K.D.; Takemasa, T.; Watanabe, K.; Takeuchi, Y.; Yahagi, N.; Takahashi, Y. Detection Method for Gene Doping in a Mouse Model Expressing Human Erythropoietin from Adeno-Associated Virus Vector-9. Genes 2024, 15, 709. [Google Scholar] [CrossRef] [PubMed]
- Sugasawa, T.; Kanki, Y.; Komine, R.; Watanabe, K.; Takekoshi, K. Identification of RNA Markers in Red Blood Cells for Doping Control in Autologous Blood Transfusion. Genes 2022, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Sugasawa, T.; Komine, R.; Manevich, L.; Tamai, S.; Takekoshi, K.; Kanki, Y. Gene Expression Profile Provides Novel Insights of Fasting-Refeeding Response in Zebrafish Skeletal Muscle. Nutrients 2022, 14, 2239. [Google Scholar] [CrossRef] [PubMed]
- Sugasawa, T.; Nakano, T.; Fujita, S.-I.; Matsumoto, Y.; Ishihara, G.; Aoki, K.; Yanazawa, K.; Ono, S.; Tamai, S.; Manevich, L.; et al. Proof of Gene Doping in a Mouse Model with a Human Erythropoietin Gene Transferred Using an Adenoviral Vector. Genes 2021, 12, 1249. [Google Scholar] [CrossRef]
- bcl2fastq2 Conversion Software v2.20. Available online: https://jp.support.illumina.com/sequencing/sequencing_software/bcl2fastq-conversion-software.htmL (accessed on 17 February 2025).
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- AI Text Mining by User Local. Available online: https://textmining.userlocal.jp/ (accessed on 17 February 2025).
- Venny ver.2.1.0. Available online: https://bioinfogp.cnb.csic.es/tools/venny/ (accessed on 17 February 2025).
- Younesi, F.S.; Miller, A.E.; Barker, T.H.; Rossi, F.M.V.; Hinz, B. Fibroblast and Myofibroblast Activation in Normal Tissue Repair and Fibrosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 617–638. [Google Scholar] [CrossRef]
- Akhtari, N.; Ahmadi, M.; Kiani Doust Vaghe, Y.; Asadian, E.; Behzad, S.; Vatanpour, H.; Ghorbani-Bidkorpeh, F. Natural Agents as Wound-Healing Promoters. Inflammopharmacology 2024, 32, 101–125. [Google Scholar] [CrossRef]
- Zomer, H.D.; Trentin, A.G. Skin Wound Healing in Humans and Mice: Challenges in Translational Research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and Molecular Mechanisms of Skin Wound Healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Hegde, A.; Ananthan, A.S.; Kashyap, C.; Ghosh, S. Wound Healing by Keratinocytes: A Cytoskeletal Perspective. J. Indian Inst. Sci. 2021, 101, 73–80. [Google Scholar] [CrossRef]
- Ong, H.T.; Dilley, R.J. Novel Non-Angiogenic Role for Mesenchymal Stem Cell-Derived Vascular Endothelial Growth Factor on Keratinocytes during Wound Healing. Cytokine Growth Factor Rev. 2018, 44, 69–79. [Google Scholar] [CrossRef]
- Serra, M.B.; Barroso, W.A.; da Silva, N.N.; Silva, S.d.N.; Borges, A.C.R.; Abreu, I.C.; Borges, M.O.d.R. From Inflammation to Current and Alternative Therapies Involved in Wound Healing. Int. J. Inflam. 2017, 2017, 3406215. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.M.; Stuart, G.S.; Jones, N.C.; Fleming, S.B.; Mercer, A.A. Orf Virus IL-10 and VEGF-E Act Synergistically to Enhance Healing of Cutaneous Wounds in Mice. J. Clin. Med. 2020, 9, 1085. [Google Scholar] [CrossRef]
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical Vascular Endothelial Growth Factor Accelerates Diabetic Wound Healing through Increased Angiogenesis and by Mobilizing and Recruiting Bone Marrow-Derived Cells. Am. J. Pathol. 2004, 164, 1935–1947. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.-Y.; Pan, Z.-W.; Li, Q.-Q.; Sun, L.-H.; Li, X.; Gong, M.-Y.; Yang, X.-W.; Wang, Y.-Y.; Li, H.-D.; et al. GDF11 Promotes Wound Healing in Diabetic Mice via Stimulating HIF-1α-VEGF/SDF-1α-Mediated Endothelial Progenitor Cell Mobilization and Neovascularization. Acta Pharmacol. Sin. 2023, 44, 999–1013. [Google Scholar] [CrossRef]
- Woodson, C.M.; Kehn-Hall, K. Examining the Role of EGR1 during Viral Infections. Front. Microbiol. 2022, 13, 1020220. [Google Scholar] [CrossRef]
- Havis, E.; Duprez, D. EGR1 Transcription Factor Is a Multifaceted Regulator of Matrix Production in Tendons and Other Connective Tissues. Int. J. Mol. Sci. 2020, 21, 1664. [Google Scholar] [CrossRef]
- Wu, M.; Melichian, D.S.; de la Garza, M.; Gruner, K.; Bhattacharyya, S.; Barr, L.; Nair, A.; Shahrara, S.; Sporn, P.H.S.; Mustoe, T.A.; et al. Essential Roles for Early Growth Response Transcription Factor Egr-1 in Tissue Fibrosis and Wound Healing. Am. J. Pathol. 2009, 175, 1041–1055. [Google Scholar] [CrossRef]
- Feng, X.; Feng, W.; Ji, Y.; Jin, T.; Li, J.; Guo, J. Transforming Growth Factor-Β1 Negatively Regulates SOCS7 via EGR1 during Wound Healing. Cell Commun. Signal. 2022, 20, 86. [Google Scholar] [CrossRef]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jiang, X.; Li, H.; Zhang, C.; Zhang, Z.; Wu, C.; Zhang, J.; Hu, J.; Zhang, J. The Role of Mesenchymal Stem Cell-Derived EVs in Diabetic Wound Healing. Front. Immunol. 2023, 14, 1136098. [Google Scholar] [CrossRef] [PubMed]
- Finsterwald, C.; Alberini, C.M. Stress and Glucocorticoid Receptor-Dependent Mechanisms in Long-Term Memory: From Adaptive Responses to Psychopathologies. Neurobiol. Learn. Mem. 2014, 112, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Chourpiliadis, C.; Aeddula, N.R. Physiology, Glucocorticoids. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tiganescu, A.; Hupe, M.; Uchida, Y.; Mauro, T.; Elias, P.M.; Holleran, W.M. Increased Glucocorticoid Activation during Mouse Skin Wound Healing. J. Endocrinol. 2014, 221, 51–61. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A. Glucocorticoids Inhibit Wound Healing: Novel Mechanism of Action. J. Investig. Dermatol. 2017, 137, 1012–1014. [Google Scholar] [CrossRef]
- Bablok, M.; Gellisch, M.; Brand-Saberi, B.; Morosan-Puopolo, G. Local Glucocorticoid Administration Impairs Embryonic Wound Healing. Biomedicines 2022, 10, 3125. [Google Scholar] [CrossRef]
- Jozic, I.; Vukelic, S.; Stojadinovic, O.; Liang, L.; Ramirez, H.A.; Pastar, I.; Tomic Canic, M. Stress Signals, Mediated by Membranous Glucocorticoid Receptor, Activate PLC/PKC/GSK-3β/β-Catenin Pathway to Inhibit Wound Closure. J. Investig. Dermatol. 2017, 137, 1144–1154. [Google Scholar] [CrossRef]
- Carolina, E.; Kato, T.; Khanh, V.C.; Moriguchi, K.; Yamashita, T.; Takeuchi, K.; Hamada, H.; Ohneda, O. Glucocorticoid Impaired the Wound Healing Ability of Endothelial Progenitor Cells by Reducing the Expression of CXCR4 in the PGE2 Pathway. Front. Med. 2018, 5, 276. [Google Scholar] [CrossRef]
- Hill, E.E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A.C. Exercise and Circulating Cortisol Levels: The Intensity Threshold Effect. J. Endocrinol. Investig. 2008, 31, 587–591. [Google Scholar] [CrossRef]
- Popovic, D.; Plecas-Solarovic, B.; Pesic, V.; Petrovic, M.; Vujisic-Tesic, B.; Popovic, B.; Ignjatovic, S.; Ristic, A.; Damjanovic, S.S. How Does Stress Possibly Affect Cardiac Remodeling? Peptides 2014, 57, 20–30. [Google Scholar] [CrossRef]
- Ushiki, K.; Tsunekawa, K.; Shoho, Y.; Martha, L.; Ishigaki, H.; Matsumoto, R.; Yanagawa, Y.; Nakazawa, A.; Yoshida, A.; Nakajima, K.; et al. Assessment of Exercise-Induced Stress by Automated Measurement of Salivary Cortisol Concentrations within the Circadian Rhythm in Japanese Female Long-Distance Runners. Sports Med. Open 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The Anti-Inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Nikolaidis, P.T. Physiology and Pathophysiology in Ultra-Marathon Running. Front. Physiol. 2018, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the Regulation of Immune Functions. Prog. Mol. Biol. Transl. Sci. 2015, 135, 355–380. [Google Scholar] [CrossRef]
- Nieman, D.C. Exercise Immunology: Nutritional Countermeasures. Can. J. Appl. Physiol. 2001, 26, S45–S55. [Google Scholar] [CrossRef]
- Yousef, H.; Alhajj, M.; Fakoya, A.O.; Sharma, S. Anatomy, Skin (Integument), Epidermis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hewitt, N.J.; Grégoire, S.; Cubberley, R.; Duplan, H.; Eilstein, J.; Ellison, C.; Lester, C.; Fabian, E.; Fernandez, J.; Géniès, C.; et al. Measurement of the Penetration of 56 Cosmetic Relevant Chemicals into and through Human Skin Using a Standardized Protocol. J. Appl. Toxicol. 2020, 40, 403–415. [Google Scholar] [CrossRef]
- Shiseido Co., Ltd. Visualization of the Distribution of Useful Ingredients That Have Penetrated the Skin at the Cellular Level to Accelerate Functionality and Safety. Available online: https://corp.shiseido.com/jp/news/detail.htmL?n=00000000003768 (accessed on 17 February 2025).
- Shrestha, R.; Krishan, K.; Ishaq, H.; Kanchan, T. Abrasion. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- HAS1 Hyaluronan Synthase 1 [Homo sapiens (human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/3036 (accessed on 17 February 2025).
- Matías, F.L. HAS2 (Hyaluronan Synthase 2). Available online: https://atlasgeneticsoncology.org/gene/412/has2-%28hyaluronan-synthase (accessed on 17 February 2025).
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Dermatoendocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Shang, L.; Li, M.; Xu, A.; Zhuo, F. Recent Applications and Molecular Mechanisms of Hyaluronic Acid in Skin Aging and Wound Healing. Med. Nov. Technol. Devices 2024, 23, 100320. [Google Scholar] [CrossRef]
- Discovered That the Metabolic Balance of Dermal Hyaluronic Acid Is Disrupted in Wrinkled and Sagging Areas, Kao Corporation. Available online: https://www.kao.com/jp/newsroom/news/release/2017/20170607-001/ (accessed on 17 February 2025).
- Zhou, R.; Yu, M. The Effect of Local Hyaluronic Acid Injection on Skin Aging: A Systematic Review and Meta-Analysis. J. Cosmet. Dermatol. 2025, 24, e16760. [Google Scholar] [CrossRef]
- Immediate and Long-Term Effects of a Topical Serum with Five Forms of Hyaluronic Acid on Facial Wrinkles and Intrinsic Skin Moisture Content. J. Am. Acad. Dermatol. 2016, 74, AB18. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otani, N.; Nguyen, K.D.M.; Wan, J.; Kanki, Y.; Sugasawa, T. RNA Sequencing Reveals Effects of a New Complex Bath Additive on Cultured Cells. Cosmetics 2025, 12, 98. https://doi.org/10.3390/cosmetics12030098
Otani N, Nguyen KDM, Wan J, Kanki Y, Sugasawa T. RNA Sequencing Reveals Effects of a New Complex Bath Additive on Cultured Cells. Cosmetics. 2025; 12(3):98. https://doi.org/10.3390/cosmetics12030098
Chicago/Turabian StyleOtani, Norihiro, Kieu D. M. Nguyen, Jiawei Wan, Yasuharu Kanki, and Takehito Sugasawa. 2025. "RNA Sequencing Reveals Effects of a New Complex Bath Additive on Cultured Cells" Cosmetics 12, no. 3: 98. https://doi.org/10.3390/cosmetics12030098
APA StyleOtani, N., Nguyen, K. D. M., Wan, J., Kanki, Y., & Sugasawa, T. (2025). RNA Sequencing Reveals Effects of a New Complex Bath Additive on Cultured Cells. Cosmetics, 12(3), 98. https://doi.org/10.3390/cosmetics12030098






