Recognition of Melanocytes in Immuno-Neuroendocrinology and Circadian Rhythms: Beyond the Conventional Melanin Synthesis
Abstract
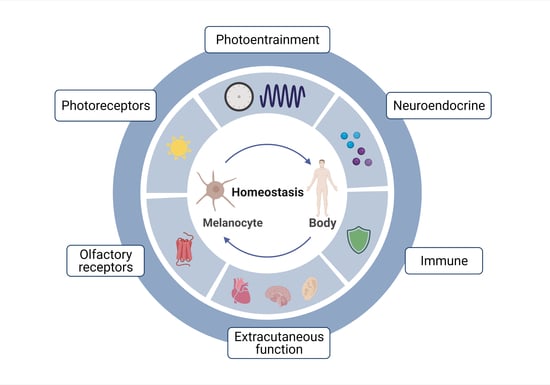
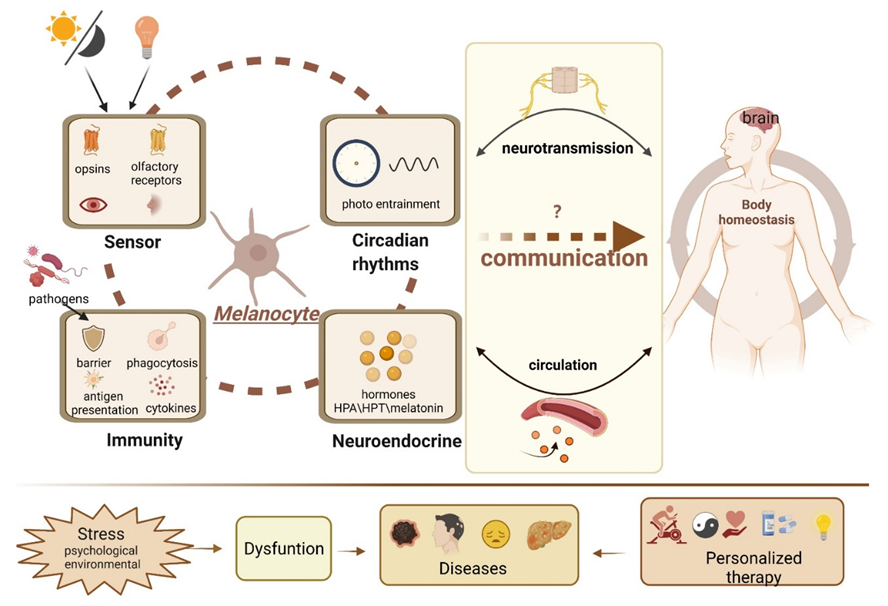
1. Introduction
2. Melanocytes and the Endocrine System
2.1. Hypothalamic-Pituitary-Adrenal (HPA) Axis Homolog
2.2. Hypothalamic-Pituitary-Thyroid Axis (HPT) Homolog
2.3. Serotoninergic/Melatoninergic System
2.4. Other Neuroendocrine Activities
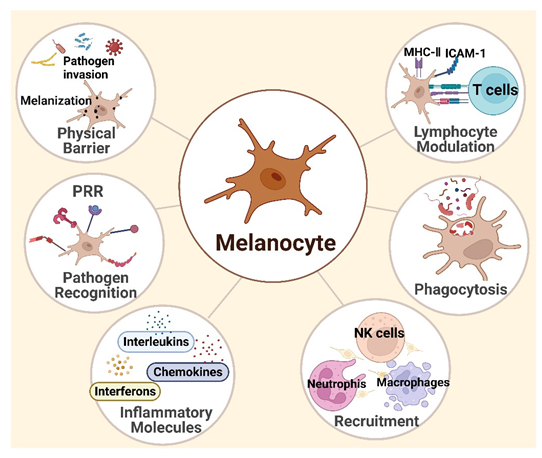
3. Melanocytes and Skin Immunity
3.1. Melanocytes Are Functional Immune Sentinel Cells in the Skin
3.2. Innate Immune Responses
3.3. Adaptive Immunity
4. Melanocytes and the Sensory System
4.1. The Melanocyte Photosensory System
4.1.1. Phototransduction
4.1.2. Circadian Photoentrainment
4.2. The Melanocyte Olfactory System
5. Melanocytes Outside the Skin
5.1. Heart
5.2. Ear
5.3. Other Organs
6. Challenges and Future Perspectives
6.1. Neuroendocrine Functions of Melanocytes in Health and Disease
6.2. Multi-Directional Communication Network and Signaling
6.3. Neuroendocrine-Immune Interactions
6.4. Circadian Rhythms and Neuroendocrine Pathways
6.5. The Intertwining of Light and Hormones
6.6. Light Pollution, Skin Light-Receptions, and Modern Diseases
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Sulaimon, S.S.; Kitchell, B.E. The biology of melanocytes. Vet. Dermatol. 2003, 14, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.J.B.; Casadevall, A. Melanin. Curr. Biol. 2020, 30, R142–R143. [Google Scholar] [CrossRef]
- ElObeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological Properties of Melanin and its Function in Health. Basic Clin. Pharmacol. Toxicol. 2017, 120, 515–522. [Google Scholar] [CrossRef]
- Plonka, P.M.; Passeron, T.; Brenner, M.; Tobin, D.J.; Shibahara, S.; Thomas, A.; Slominski, A.; Kadekaro, A.L.; Hershkovitz, D.; Peters, E.; et al. What are melanocytes really doing all day long…? Exp. Dermatol. 2009, 18, 799–819. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–115. [Google Scholar] [CrossRef]
- Slominski, A.; Paus, R.; Schadendorf, D. Melanocytes as “sensory” and regulatory cells in the epidermis. J. Theor. Biol. 1993, 164, 103–120. [Google Scholar] [CrossRef]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tyminska, A. Skin melanocytes: Biology and development. Postepy Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef]
- Slominski, A. Neuroendocrine activity of the melanocyte. Exp. Dermatol. 2009, 18, 760–763. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Chrousos, G.P. Stress-related skin disorders. Rev. Endocr. Metab. Disord. 2016, 17, 295–304. [Google Scholar] [CrossRef]
- Phan, T.S.; Schink, L.; Mann, J.; Merk, V.M.; Zwicky, P.; Mundt, S.; Simon, D.; Kulms, D.; Abraham, S.; Legler, D.F.; et al. Keratinocytes control skin immune homeostasis through de novo-synthesized glucocorticoids. Sci. Adv. 2021, 7, eabe0337. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Arck, P. Neuroendocrine perspectives in alopecia areata: Does stress play a role? J. Investig. Dermatol. 2009, 129, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Kotb El-Sayed, M.I.; Abd El-Ghany, A.A.; Mohamed, R.R. Neural and Endocrinal Pathobiochemistry of Vitiligo: Comparative Study for a Hypothesized Mechanism. Front. Endocrinol. 2018, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ma, S.; Rachmin, I.; He, M.; Baral, P.; Choi, S.; Goncalves, W.A.; Shwartz, Y.; Fast, E.M.; Su, Y.; et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 2020, 577, 676–681. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Neuroendocrine Aspects of Skin Aging. Int. J. Mol. Sci. 2019, 20, 2798. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Stewart, J.M.; Taracanova, A.; Conti, P.; Zouboulis, C.C. Neuroendocrinology of the skin. Rev. Endocr. Metab. Disord. 2016, 17, 287–294. [Google Scholar] [CrossRef]
- Leis, K.; Mazur, E.; Jablonska, M.J.; Kolan, M.; Galazka, P. Endocrine systems of the skin. Postepy Dermatol. Alergol. 2019, 36, 519–523. [Google Scholar] [CrossRef]
- Takeda, K.; Takahashi, N.H.; Shibahara, S. Neuroendocrine functions of melanocytes: Beyond the skin-deep melanin maker. Tohoku J. Exp. Med. 2007, 211, 201–221. [Google Scholar] [CrossRef]
- Rousseau, K.; Kauser, S.; Pritchard, L.E.; Warhurst, A.; Oliver, R.L.; Slominski, A.; Wei, E.T.; Thody, A.J.; Tobin, D.J.; White, A. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J. 2007, 21, 1844–1856. [Google Scholar] [CrossRef]
- Spencer, J.D.; Schallreuter, K.U. Regulation of pigmentation in human epidermal melanocytes by functional high-affinity beta-melanocyte-stimulating hormone/melanocortin-4 receptor signaling. Endocrinology 2009, 150, 1250–1258. [Google Scholar] [CrossRef]
- Slominski, A. Identification of beta-endorphin, alpha-MSH and ACTH peptides in cultured human melanocytes, melanoma and squamous cell carcinoma cells by RP-HPLC. Exp. Dermatol. 1998, 7, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Bhm, M.; Metze, D.; Schulte, U.; Becher, E.; Luger, T.A.; Brzoska, T. Detection of melanocortin-1 receptor antigenicity on human skin cells in culture and in situ. Exp. Dermatol. 2010, 8, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, J.Z.; Medrano, E.E.; Abdel-Malek, Z.; Nordlund, J. The expression of proopiomelanocortin and various POMC-derived peptides in mouse and human skin. Ann. N. Y. Acad. Sci. 1993, 680, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Ermak, G.; Hwang, J.; Chakraborty, A.; Mazurkiewicz, J.E.; Mihm, M. Proopiomelanocortin, corticotropin releasing hormone and corticotropin releasing hormone receptor genes are expressed in human skin. FEBS Lett. 1995, 374, 113–116. [Google Scholar] [CrossRef]
- Slominski, A.; Baker, J.; Ermak, G.; Chakraborty, A.; Pawelek, J. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS Lett. 1996, 399, 175–176. [Google Scholar] [CrossRef]
- Kono, M.; Nagata, H.; Umemura, S.; Kawana, S.; Osamura, R.Y. In situ expression of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skin. FASEB J. 2001, 15, 2297–2299. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, B.; Szczesniewski, A.; Semak, I.; Kaminski, J.; Sweatman, T.; Wortsman, J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E701–E706. [Google Scholar] [CrossRef]
- Ramot, Y.; Bohm, M.; Paus, R. Translational Neuroendocrinology of Human Skin: Concepts and Perspectives. Trends Mol. Med. 2021, 27, 60–74. [Google Scholar] [CrossRef]
- Skobowiat, C.; Dowdy, J.C.; Sayre, R.M.; Tuckey, R.C.; Slominski, A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: Regulation by ultraviolet radiation. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E484–E493. [Google Scholar] [CrossRef]
- Skobowiat, C.; Nejati, R.; Lu, L.; Williams, R.W.; Slominski, A.T. Genetic variation of the cutaneous HPA axis: An analysis of UVB-induced differential responses. Gene 2013, 530, 1–7. [Google Scholar] [CrossRef]
- Slominski, A.T.; Slominski, R.M.; Raman, C. UVB stimulates production of enkephalins and other neuropeptides by skin-resident cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2020425118. [Google Scholar] [CrossRef] [PubMed]
- Jozic, I.; Stojadinovic, O.; Kirsner, R.S.F.; Tomic-Canic, M. Skin under the (Spot)-Light: Cross-Talk with the Central Hypothalamic-Pituitary-Adrenal (HPA) Axis. J. Investig. Dermatol. 2015, 135, 1469–1471. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Nagashima, Y.; Chrousos, G.P.; Ichihashi, M.; Funasak, Y. The expression of corticotropin-releasing hormone in melanoma. Pigment. Cell Res. 2002, 15, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Cho, D.; Kim, H.J.; Chong, S.J.; Lee, K.H.; Yu, D.S.; Park, C.J.; Lee, J.Y.; Cho, B.K.; Park, H.J. Investigation of the corticotropin-releasing hormone-proopiomelanocortin axis in various skin tumours. Br. J. Dermatol. 2006, 155, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Eberle, A.N.; Rout, B.; Qi, M.B.; Bigliardi, P.L. Synthetic Peptide Drugs for Targeting Skin Cancer: Malignant Melanoma and Melanotic Lesions. Curr. Med. Chem. 2017, 24, 1797–1826. [Google Scholar] [CrossRef]
- Kingo, K.; Aunin, E.; Karelson, M.; Philips, M.A.; Ratsep, R.; Silm, H.; Vasar, E.; Soomets, U.; Koks, S. Gene expression analysis of melanocortin system in vitiligo. J. Dermatol. Sci. 2007, 48, 113–122. [Google Scholar] [CrossRef]
- Shaker, O.G.; Eltahlawi, S.M.; Tawfic, S.O.; Eltawdy, A.M.; Bedair, N.I. Corticotropin-releasing hormone (CRH) and CRH receptor 1 gene expression in vitiligo. Clin. Exp. Dermatol. 2016, 41, 734–740. [Google Scholar] [CrossRef]
- Spencer, J.; Gibbons, N.; Rokos, H.; Peters, E.; Wood, J.; Schallreuter, K. Oxidative stress via hydrogen peroxide affects proopiomelanocortin peptides directly in the epidermis of patients with vitiligo. J. Investig. Dermatol. 2007, 127, 411–420. [Google Scholar] [CrossRef]
- Mancino, G.; Miro, C.; Di Cicco, E.; Dentice, M. Thyroid hormone action in epidermal development and homeostasis and its implications in the pathophysiology of the skin. J. Endocrinol. Investig. 2021, 44, 1571–1579. [Google Scholar] [CrossRef]
- Bodo, E.; Kany, B.; Gaspar, E.; Knuver, J.; Kromminga, A.; Ramot, Y.; Biro, T.; Tiede, S.; van Beek, N.; Poeggeler, B.; et al. Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology 2010, 151, 1633–1642. [Google Scholar] [CrossRef]
- Baldini, E.; Odorisio, T.; Sorrenti, S.; Catania, A.; Tartaglia, F.; Carbotta, G.; Pironi, D.; Rendina, R.; D’Armiento, E.; Persechino, S.; et al. Vitiligo and Autoimmune Thyroid Disorders. Front. Endocrinol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Kohn, L.; Ain, K.B.; Venkataraman, G.M.; Pisarchik, A.; Chung, J.H.; Giuliani, C.; Thornton, M.; Slugocki, G.; et al. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J. Investig. Dermatol. 2002, 119, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, E.; Nguyen-Thi, K.T.; Hardenbicker, C.; Tiede, S.; Plate, C.; Bodo, E.; Knuever, J.; Funk, W.; Biro, T.; Paus, R. Thyrotropin-releasing hormone selectively stimulates human hair follicle pigmentation. J. Investig. Dermatol. 2011, 131, 2368–2377. [Google Scholar] [CrossRef]
- Sandru, F.; Carsote, M.; Albu, S.E.; Dumitrascu, M.C.; Valea, A. Vitiligo and chronic autoimmune thyroiditis. J. Med. Life 2021, 14, 127–130. [Google Scholar] [CrossRef]
- Liu, M.; Murphy, E.; Amerson, E.H. Rethinking screening for thyroid autoimmunity in vitiligo. J. Am. Acad. Dermatol. 2016, 75, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liang, G.; Calderone, R.; Bellanti, J.A. Vitiligo and Hashimoto’s thyroiditis: Autoimmune diseases linked by clinical presentation, biochemical commonality, and autoimmune/oxidative stress-mediated toxicity pathogenesis. Med. Hypotheses 2019, 128, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ellerhorst, J.A.; Naderi, A.A.; Johnson, M.K.; Pelletier, P.; Prieto, V.G.; Diwan, A.H.; Johnson, M.M.; Gunn, D.C.; Yekell, S.; Grimm, E.A. Expression of thyrotropin-releasing hormone by human melanoma and nevi. Clin. Cancer Res. 2004, 10, 5531–5536. [Google Scholar] [CrossRef]
- Ellerhorst, J.A.; Sendi-Naderi, A.; Johnson, M.K.; Cooke, C.P.; Dang, S.M.; Diwan, A.H. Human melanoma cells express functional receptors for thyroid-stimulating hormone. Endocr. Relat. Cancer 2006, 13, 1269–1277. [Google Scholar] [CrossRef]
- Ursu, H. Functional TSH Receptors, Malignant Melanomas and Subclinical Hypothyroidism. Eur. Thyroid. J. 2012, 1, 208. [Google Scholar] [CrossRef][Green Version]
- Hou, P.; Liu, D.; Ji, M.; Liu, Z.; Engles, J.M.; Wahl, R.L.; Xing, M. Induction of thyroid gene expression and radioiodine uptake in melanoma cells: Novel therapeutic implications. PLoS ONE 2009, 4, e6200. [Google Scholar] [CrossRef]
- Scheau, C.; Draghici, C.; Ilie, M.A.; Lupu, M.; Caruntu, C. Neuroendocrine Factors in Melanoma Pathogenesis. Cancers 2021, 13, 2277. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, A.; Chéret, J.; Slominski, R.M.; Slominski, A.T.; Paus, R. Revisiting the role of melatonin in human melanocyte physiology: A skin context perspective. J. Pineal Res. 2022, 72, e12790. [Google Scholar] [CrossRef] [PubMed]
- Rusanova, I.; Martinez-Ruiz, L.; Florido, J.; Rodriguez-Santana, C.; Guerra-Librero, A.; Acuna-Castroviejo, D.; Escames, G. Protective Effects of Melatonin on the Skin: Future Perspectives. Int. J. Mol. Sci. 2019, 20, 4948. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Kleszczynski, K.; Janjetovic, Z.; Sweatman, T.; Lin, Z.; Li, W.; Reiter, R.J.; Fischer, T.W.; Slominski, A.T. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013, 27, 2742–2755. [Google Scholar] [CrossRef]
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J.; Szczesniewski, A.; Slugocki, G.; McNulty, J.; Kauser, S.; Tobin, D.J.; et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002, 16, 896–898. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Kleszczynski, K.; Semak, I.; Janjetovic, Z.; Sweatman, T.; Skobowiat, C.; Steketee, J.D.; Lin, Z.; Postlethwaite, A.; et al. Characterization of serotonin and N-acetylserotonin systems in the human epidermis and skin cells. J. Pineal Res. 2020, 68, e12626. [Google Scholar] [CrossRef]
- Slominski, A.; Pisarchik, A.; Johansson, O.; Jing, C.; Semak, I.; Slugocki, G.; Wortsman, J. Tryptophan hydroxylase expression in human skin cells. Biochim. Biophys. Acta—Mol. Basis Dis. 2003, 1639, 80–86. [Google Scholar] [CrossRef]
- Kim, T.K.; Lin, Z.; Tidwell, W.J.; Li, W.; Slominski, A.T. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol. Cell Endocrinol. 2015, 404, 1–8. [Google Scholar] [CrossRef]
- Slominski, A.; Semak, I.; Pisarchik, A.; Sweatman, T.; Szczesniewski, A.; Wortsman, J. Conversion ofL-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002, 511, 102–106. [Google Scholar] [CrossRef]
- Skobowiat, C.; Brozyna, A.A.; Janjetovic, Z.; Jeayeng, S.; Oak, A.S.W.; Kim, T.K.; Panich, U.; Reiter, R.J.; Slominski, A.T. Melatonin and its derivatives counteract the ultraviolet B radiation-induced damage in human and porcine skin ex vivo. J. Pineal Res. 2018, 65, e12501. [Google Scholar] [CrossRef]
- Kleszczynski, K.; Kim, T.K.; Bilska, B.; Sarna, M.; Mokrzynski, K.; Stegemann, A.; Pyza, E.; Reiter, R.J.; Steinbrink, K.; Bohm, M.; et al. Melatonin exerts oncostatic capacity and decreases melanogenesis in human MNT-1 melanoma cells. J. Pineal Res. 2019, 67, e12610. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, J.; Quintana, C.; Gonzalez, I.; Hernandez, I.; Rubio, S.; Loro, J.F.; Reiter, R.J.; Estevez, F.; Quintana, J. Melatonin Induces Melanogenesis in Human SK-MEL-1 Melanoma Cells Involving Glycogen Synthase Kinase-3 and Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 4970. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Nahmias, Z.P.; Hanna, S.; Jarrett, S.G.; Kim, T.K.; Reiter, R.J.; Slominski, A.T. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J. Pineal Res. 2014, 57, 90–102. [Google Scholar] [CrossRef]
- Janjetovic, Z.; Jarrett, S.G.; Lee, E.F.; Duprey, C.; Reiter, R.J.; Slominski, A.T. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci Rep. 2017, 7, 1274. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Goyarts, E.; Rella, A.; Pelle, E.; Wong, Y.H.; Pernodet, N. Age Associated Decrease of MT-1 Melatonin Receptor in Human Dermal Skin Fibroblasts Impairs Protection Against UV-Induced DNA Damage. Int. J. Mol. Sci. 2020, 21, 326. [Google Scholar] [CrossRef] [PubMed]
- Gillbro, J.M.; Marles, L.K.; Hibberts, N.A.; Schallreuter, K.U. Autocrine catecholamine biosynthesis and the beta-adrenoceptor signal promote pigmentation in human epidermal melanocytes. J. Investig. Dermatol. 2004, 123, 346–353. [Google Scholar] [CrossRef]
- Sivamani, R.K.; Porter, S.M.; Isseroff, R.R. An epinephrine-dependent mechanism for the control of UV-induced pigmentation. J. Investig. Dermatol. 2009, 129, 784–787. [Google Scholar] [CrossRef]
- Choi, M.E.; Yoo, H.; Lee, H.R.; Moon, I.J.; Lee, W.J.; Song, Y.; Chang, S.E. Carvedilol, an Adrenergic Blocker, Suppresses Melanin Synthesis by Inhibiting the cAMP/CREB Signaling Pathway in Human Melanocytes and Ex Vivo Human Skin Culture. Int. J. Mol. Sci. 2020, 21, 8796. [Google Scholar] [CrossRef]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment. Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef]
- Ono, K.; Viet, C.T.; Ye, Y.; Dang, D.; Hitomi, S.; Toyono, T.; Inenaga, K.; Dolan, J.C.; Schmidt, B.L. Cutaneous pigmentation modulates skin sensitivity via tyrosinase-dependent dopaminergic signalling. Sci. Rep. 2017, 7, 9181. [Google Scholar] [CrossRef]
- Mackintosh, J.A. The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin. J. Theor. Biol. 2001, 211, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Gasque, P.; Jaffar-Bandjee, M.C. The immunology and inflammatory responses of human melanocytes in infectious diseases. J. Infect. 2015, 71, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J. Organization of the Skin Immune System and Compartmentalized Immune Responses in Infectious Diseases. Clin. Microbiol. Rev. 2019, 32, e00034-18. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Schroder, J.M.; Glaser, R. The skin surface as antimicrobial barrier: Present concepts and future outlooks. Exp. Dermatol. 2013, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, D.; Ning, W.; Xu, A. Cytosolic dsDNA triggers apoptosis and pro-inflammatory cytokine production in normal human melanocytes. Exp. Dermatol. 2015, 24, 298–300. [Google Scholar] [CrossRef]
- Iverson, M.V. Hypothesis: Vitiligo virus. Pigment Cell Res. 2000, 13, 281–282. [Google Scholar] [CrossRef]
- Erf, G.F.; Bersi, T.K.; Wang, X.; Sreekumar, G.P.; Smyth, J.R., Jr. Herpesvirus connection in the expression of autoimmune vitiligo in Smyth line chickens. Pigment Cell Res. 2001, 14, 40–46. [Google Scholar] [CrossRef]
- Kawamura, T.; Ogawa, Y.; Aoki, R.; Shimada, S. Innate and intrinsic antiviral immunity in skin. J. Dermatol. Sci. 2014, 75, 159–166. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Coates, M.; Blanchard, S.; MacLeod, A.S. Innate antimicrobial immunity in the skin: A protective barrier against bacteria, viruses, and fungi. PLoS Pathog. 2018, 14, e1007353. [Google Scholar] [CrossRef]
- Ahn, J.H.; Park, T.J.; Jin, S.H.; Kang, H.Y. Human melanocytes express functional Toll-like receptor 4. Exp. Dermatol. 2008, 17, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Tapia, C.; Falconer, M.; Tempio, F.; Falcón, F.; López, M.; Fuentes, M.; Alburquenque, C.; Amaro, J.; Bucarey, S.; Di Nardo, A. Melanocytes and melanin represent a first line of innate immunity against Candida albicans. Med. Mycol. 2014, 52, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Tam, I.; Dzierzega-Lecznar, A.; Stepien, K. Differential expression of inflammatory cytokines and chemokines in lipopolysaccharide-stimulated melanocytes from lightly and darkly pigmented skin. Exp. Dermatol. 2019, 28, 551–560. [Google Scholar] [CrossRef]
- Tam, I.; Stępień, K. Secretion of proinflammatory cytokines by normal human melanocytes in response to lipopolysaccharide. Acta Biochim. Pol. 2011, 58, 507–511. [Google Scholar] [CrossRef]
- Song, H.; Lee, S.; Choi, G.; Shin, J. Repeated ultraviolet irradiation induces the expression of Toll-like receptor 4, IL-6, and IL-10 in neonatal human melanocytes. Photodermatol. Photoimmunol. Photomed. 2018, 34, 145–151. [Google Scholar] [CrossRef]
- Koike, S.; Yamasaki, K. Melanogenesis Connection with Innate Immunity and Toll-Like Receptors. Int. J. Mol. Sci. 2020, 21, 9769. [Google Scholar] [CrossRef]
- Vavricka, C.J.; Christensen, B.M.; Li, J. Melanization in living organisms: A perspective of species evolution. Protein Cell 2010, 1, 830–841. [Google Scholar] [CrossRef]
- D’Alba, L.; Shawkey, M.D. Melanosomes: Biogenesis, Properties, and Evolution of an Ancient Organelle. Physiol. Rev. 2019, 99, 1–19. [Google Scholar] [CrossRef]
- Tolleson, W.H. Human melanocyte biology, toxicology, and pathology. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2005, 23, 105–161. [Google Scholar] [CrossRef]
- Montefiori, D.C.; Zhou, J. Selective antiviral activity of synthetic soluble l-tyrosine and l-dopa melanins against human immunodeficiency virus in vitro. Antivir. Res. 1991, 15, 11–25. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, R.; Schurer, N.Y.; Shoo, B.A.; Celli, A.; Hachem, J.P.; Crumrine, D.; Sirimanna, G.; Feingold, K.R.; Mauro, T.M.; Elias, P.M. pH-regulated mechanisms account for pigment-type differences in epidermal barrier function. J. Investig. Dermatol. 2009, 129, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Menon, G.; Wetzel, B.K.; Williams, J. Barrier requirements as the evolutionary “driver” of epidermal pigmentation in humans. Am. J. Hum. Biol. 2010, 22, 526–537. [Google Scholar] [CrossRef]
- Lin, T.K.; Man, M.Q.; Abuabara, K.; Wakefield, J.S.; Sheu, H.M.; Tsai, J.C.; Lee, C.H.; Elias, P.M. By protecting against cutaneous inflammation, epidermal pigmentation provided an additional advantage for ancestral humans. Evol. Appl. 2019, 12, 1960–1970. [Google Scholar] [CrossRef]
- Jablonski, N.G.; Chaplin, G. Colloquium paper: Human skin pigmentation as an adaptation to UV radiation. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. S2), 8962–8968. [Google Scholar] [CrossRef]
- Man, M.Q.; Lin, T.K.; Santiago, J.L.; Celli, A.; Zhong, L.; Huang, Z.M.; Roelandt, T.; Hupe, M.; Sundberg, J.P.; Silva, K.A.; et al. Basis for enhanced barrier function of pigmented skin. J. Investig. Dermatol. 2014, 134, 2399–2407. [Google Scholar] [CrossRef]
- Liu, J.; Man, W.Y.; Lv, C.Z.; Song, S.P.; Shi, Y.J.; Elias, P.M.; Man, M.Q. Epidermal permeability barrier recovery is delayed in vitiligo-involved sites. Skin Pharmacol. Physiol. 2010, 23, 193–200. [Google Scholar] [CrossRef]
- Koike, S.; Yamasaki, K.; Yamauchi, T.; Inoue, M.; Shimada-Ohmori, R.; Tsuchiyama, K.; Aiba, S. Toll-like receptors 2 and 3 enhance melanogenesis and melanosome transport in human melanocytes. Pigment Cell Melanoma Res. 2018, 31, 570–584. [Google Scholar] [CrossRef]
- Sun, L.; Pan, S.; Yang, Y.; Sun, J.; Liang, D.; Wang, X.; Xie, X.; Hu, J. Toll-like receptor 9 regulates melanogenesis through NF-κB activation. Exp. Biol. Med. 2016, 241, 1497–1504. [Google Scholar] [CrossRef]
- Le Poole, I.C.; van den Wijngaard, R.M.; Westerhof, W.; Verkruisen, R.P.; Dutrieux, R.P.; Dingemans, K.P.; Das, P.K. Phagocytosis by normal human melanocytes in vitro. Exp. Cell Res. 1993, 205, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Smit, N.; Le Poole, I.; van den Wijngaard, R.; Tigges, A.; Westerhof, W.; Das, P. Expression of different immunological markers by cultured human melanocytes. Arch. Dermatol. Res. 1993, 285, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Le Poole, I.; Mutis, T.; van den Wijngaard, R.; Westerhof, W.; Ottenhoff, T.; de Vries, R.; Das, P. A novel, antigen-presenting function of melanocytes and its possible relationship to hypopigmentary disorders. J. Immunol. 1993, 151, 7284–7292. [Google Scholar] [PubMed]
- Lu, Y.; Zhu, W.Y.; Tan, C.; Yu, G.H.; Gu, J.X. Melanocytes are potential immunocompetent cells: Evidence from recognition of immunological characteristics of cultured human melanocytes. Pigment Cell Res. 2002, 15, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Dalesio, N.M.; Barreto Ortiz, S.F.; Pluznick, J.L.; Berkowitz, D.E. Olfactory, Taste, and Photo Sensory Receptors in Non-sensory Organs: It Just Makes Sense. Front. Physiol. 2018, 9, 1673. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.N.; de Assis, L.V.M.; Provencio, I.; Castrucci, A.M.L. Opsins outside the eye and the skin: A more complex scenario than originally thought for a classical light sensor. Cell Tissue Res. 2021, 385, 519–538. [Google Scholar] [CrossRef]
- Do, M.T.H. Melanopsin and the Intrinsically Photosensitive Retinal Ganglion Cells: Biophysics to Behavior. Neuron 2019, 104, 205–226. [Google Scholar] [CrossRef]
- Lee, S.J.; Depoortere, I.; Hatt, H. Therapeutic potential of ectopic olfactory and taste receptors. Nat. Rev. Drug Discov. 2019, 18, 116–138. [Google Scholar] [CrossRef]
- Cheret, J.; Bertolini, M.; Ponce, L.; Lehmann, J.; Tsai, T.; Alam, M.; Hatt, H.; Paus, R. Olfactory receptor OR2AT4 regulates human hair growth. Nat. Commun. 2018, 9, 3624. [Google Scholar] [CrossRef]
- Buscone, S.; Mardaryev, A.N.; Raafs, B.; Bikker, J.W.; Sticht, C.; Gretz, N.; Farjo, N.; Uzunbajakava, N.E.; Botchkareva, N.V. A new path in defining light parameters for hair growth: Discovery and modulation of photoreceptors in human hair follicle. Lasers Surg. Med. 2017, 49, 705–718. [Google Scholar] [CrossRef]
- Mignon, C.; Botchkareva, N.V.; Uzunbajakava, N.E.; Tobin, D.J. Photobiomodulation devices for hair regrowth and wound healing: A therapy full of promise but a literature full of confusion. Exp. Dermatol. 2016, 25, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Pellicena, I.; Uzunbajakava, N.E.; Mignon, C.; Raafs, B.; Botchkarev, V.A.; Thornton, M.J. Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing? Lasers Surg. Med. 2019, 51, 370–382. [Google Scholar] [CrossRef]
- Portillo, M.; Mataix, M.; Alonso-Juarranz, M.; Lorrio, S.; Villalba, M.; Rodriguez-Luna, A.; Gonzalez, S. The Aqueous Extract of Polypodium leucotomos (Fernblock((R))) Regulates Opsin 3 and Prevents Photooxidation of Melanin Precursors on Skin Cells Exposed to Blue Light Emitted from Digital Devices. Antioxidants 2021, 10, 400. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, Y.; Lu, H. Opsin 3 is a key regulator of ultraviolet A-induced photoageing in human dermal fibroblast cells. Br. J. Dermatol. 2020, 182, 1228–1244. [Google Scholar] [CrossRef]
- Lan, Y.; Zeng, W.; Dong, X.; Lu, H. Opsin 5 is a key regulator of ultraviolet radiation-induced melanogenesis in human epidermal melanocytes. Br. J. Dermatol. 2021, 185, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Massberg, D.; Hatt, H. Human Olfactory Receptors: Novel Cellular Functions Outside of the Nose. Physiol. Rev. 2018, 98, 1739–1763. [Google Scholar] [CrossRef]
- Suh, S.; Choi, E.H.; Atanaskova Mesinkovska, N. The expression of opsins in the human skin and its implications for photobiomodulation: A Systematic Review. Photodermatol. Photoimmunol. Photomed. 2020, 36, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.Y.; Montell, C. Unconventional Roles of Opsins. Annu. Rev. Cell Dev. Biol. 2017, 33, 241–264. [Google Scholar] [CrossRef]
- Buhr, E.D.; Yue, W.W.; Ren, X.; Jiang, Z.; Liao, H.W.; Mei, X.; Vemaraju, S.; Nguyen, M.T.; Reed, R.R.; Lang, R.A.; et al. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc. Natl. Acad. Sci. USA 2015, 112, 13093–13098. [Google Scholar] [CrossRef]
- Olinski, L.E.; Lin, E.M.; Oancea, E. Illuminating insights into opsin 3 function in the skin. Adv. Biol. Regul. 2020, 75, 100668. [Google Scholar] [CrossRef]
- Provencio, I.; Jiang, G.; De Grip, W.J.; Hayes, W.P.; Rollag, M.D. Melanopsin: An opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. USA 1998, 95, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Isoldi, M.C.; Rollag, M.D.; Castrucci, A.M.; Provencio, I. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc. Natl. Acad. Sci. USA 2005, 102, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Bertolesi, G.E.; McFarlane, S. Seeing the light to change colour: An evolutionary perspective on the role of melanopsin in neuroendocrine circuits regulating light-mediated skin pigmentation. Pigment Cell Melanoma Res. 2018, 31, 354–373. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, J.; Takeo, M.; Hashikawa, K.; Komori, T.; Tsuji, T.; Terashi, H.; Sakakibara, S. OPN4 belongs to the photosensitive system of the human skin. Genes Cells 2020, 25, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, Y.; Moriya, T.; Kubota, T.; Yamada, K.; Asami, K. Expression of opsin molecule in cultured murine melanocyte. J. Investig. Dermatol. Symp. Proc. 2001, 6, 54–57. [Google Scholar] [CrossRef]
- Denda, M.; Fuziwara, S. Visible radiation affects epidermal permeability barrier recovery: Selective effects of red and blue light. J. Investig. Dermatol. 2008, 128, 1335–1336. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Ikeyama, K.; Denda, S.; Nakanishi, J.; Fuziwara, S.; Aoki, H.; Denda, M. Expressions of rod and cone photoreceptor-like proteins in human epidermis. Exp. Dermatol. 2009, 18, 567–570. [Google Scholar] [CrossRef]
- Wicks, N.L.; Chan, J.W.; Najera, J.A.; Ciriello, J.M.; Oancea, E. UVA phototransduction drives early melanin synthesis in human melanocytes. Curr. Biol. 2011, 21, 1906–1911. [Google Scholar] [CrossRef]
- Haltaufderhyde, K.; Ozdeslik, R.N.; Wicks, N.L.; Najera, J.A.; Oancea, E. Opsin expression in human epidermal skin. Photochem. Photobiol. 2015, 91, 117–123. [Google Scholar] [CrossRef]
- Hu, Q.M.; Yi, W.J.; Su, M.Y.; Jiang, S.; Xu, S.Z.; Lei, T.C. Induction of retinal-dependent calcium influx in human melanocytes by UVA or UVB radiation contributes to the stimulation of melanosome transfer. Cell Prolif. 2017, 50, e12372. [Google Scholar] [CrossRef]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.K.; De Donatis, G.M.; Chignon-Sicard, B.; Rocchi, S.; Passeron, T. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. J. Investig. Dermatol. 2018, 138, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ozdeslik, R.N.; Olinski, L.E.; Trieu, M.M.; Oprian, D.D.; Oancea, E. Human nonvisual opsin 3 regulates pigmentation of epidermal melanocytes through functional interaction with melanocortin 1 receptor. Proc. Natl. Acad. Sci. USA 2019, 116, 11508–11517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lan, Y.; Lu, H. Opsin3 Downregulation Induces Apoptosis of Human Epidermal Melanocytes via Mitochondrial Pathway. Photochem. Photobiol. 2020, 96, 83–93. [Google Scholar] [CrossRef] [PubMed]
- de Assis, L.V.M.; Moraes, M.N.; Magalhaes-Marques, K.K.; Castrucci, A.M.L. Melanopsin and rhodopsin mediate UVA-induced immediate pigment darkening: Unravelling the photosensitive system of the skin. Eur. J. Cell Biol. 2018, 97, 150–162. [Google Scholar] [CrossRef]
- Jin, H.; Zou, Z.; Chang, H.; Shen, Q.; Liu, L.; Xing, D. Photobiomodulation therapy for hair regeneration: A synergetic activation of β-CATENIN in hair follicle stem cells by ROS and paracrine WNTs. Stem Cell Rep. 2021, 16, 1568–1583. [Google Scholar] [CrossRef]
- Le Duff, F.; Fontas, E.; Guardoli, D.; Lacour, J.; Passeron, T. HeaLED: Assessment of skin healing under light-emitting diode (LED) exposure-A randomized controlled study versus placebo. Lasers Surg. Med. 2022, 54, 342–347. [Google Scholar] [CrossRef]
- Diogo, M.; Campos, T.; Fonseca, E.; Pavani, C.; Horliana, A.; Fernandes, K.; Bussadori, S.; Fantin, F.; Leite, D.; Yamamoto, Â.; et al. Effect of Blue Light on Acne Vulgaris: A Systematic Review. Sensors 2021, 21, 6943. [Google Scholar] [CrossRef]
- Spinella, A.; de Pinto, M.; Galluzzo, C.; Testoni, S.; Macripò, P.; Lumetti, F.; Parenti, L.; Magnani, L.; Sandri, G.; Bajocchi, G.; et al. Photobiomodulation Therapy: A New Light in the Treatment of Systemic Sclerosis Skin Ulcers. Rheumatol. Ther. 2022, 9, 891–905. [Google Scholar] [CrossRef]
- Kemény, L.; Varga, E.; Novak, Z. Advances in phototherapy for psoriasis and atopic dermatitis. Expert Rev. Clin. Immunol. 2019, 15, 1205–1214. [Google Scholar] [CrossRef]
- Allada, R.; Bass, J. Circadian Mechanisms in Medicine. N. Engl. J. Med. 2021, 384, 550–561. [Google Scholar] [CrossRef]
- Tanioka, M.; Yamada, H.; Doi, M.; Bando, H.; Yamaguchi, Y.; Nishigori, C.; Okamura, H. Molecular clocks in mouse skin. J. Investig. Dermatol. 2009, 129, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Sandu, C.; Dumas, M.; Malan, A.; Sambakhe, D.; Marteau, C.; Nizard, C.; Schnebert, S.; Perrier, E.; Challet, E.; Pevet, P.; et al. Human skin keratinocytes, melanocytes, and fibroblasts contain distinct circadian clock machineries. Cell Mol. Life Sci. 2012, 69, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Andersen, B. Skin as a window to body-clock time. Proc. Natl. Acad. Sci. USA 2018, 115, 12095–12097. [Google Scholar] [CrossRef] [PubMed]
- Upton, B.A.; Diaz, N.M.; Gordon, S.A.; Van Gelder, R.N.; Buhr, E.D.; Lang, R.A. Evolutionary Constraint on Visual and Nonvisual Mammalian Opsins. J. Biol. Rhythm. 2021, 36, 109–126. [Google Scholar] [CrossRef]
- Buhr, E.D.; Vemaraju, S.; Diaz, N.; Lang, R.A.; Van Gelder, R.N. Neuropsin (OPN5) Mediates Local Light-Dependent Induction of Circadian Clock Genes and Circadian Photoentrainment in Exposed Murine Skin. Curr. Biol. 2019, 29, 3478–3487.e4. [Google Scholar] [CrossRef]
- de Assis, L.V.; Moraes, M.N.; da Silveira Cruz-Machado, S.; Castrucci, A.M. The effect of white light on normal and malignant murine melanocytes: A link between opsins, clock genes, and melanogenesis. Biochim. Biophys. Acta 2016, 1863, 1119–1133. [Google Scholar] [CrossRef]
- de Assis, L.V.M.; Mendes, D.; Silva, M.M.; Kinker, G.S.; Pereira-Lima, I.; Moraes, M.N.; Menck, C.F.M.; Castrucci, A.M.L. Melanopsin mediates UVA-dependent modulation of proliferation, pigmentation, apoptosis, and molecular clock in normal and malignant melanocytes. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118789. [Google Scholar] [CrossRef]
- Moraes, M.N.; de Assis, L.V.M.; Magalhaes-Marques, K.K.; Poletini, M.O.; de Lima, L.; Castrucci, A.M.L. Melanopsin, a Canonical Light Receptor, Mediates Thermal Activation of Clock Genes. Sci. Rep. 2017, 7, 13977. [Google Scholar] [CrossRef]
- Solessio, E.; Engbretson, G.A. Antagonistic chromatic mechanisms in photoreceptors of the parietal eye of lizards. Nature 1993, 364, 442–445. [Google Scholar] [CrossRef]
- Okano, T.; Yoshizawa, T.; Fukada, Y. Pinopsin is a chicken pineal photoreceptive molecule. Nature 1994, 372, 94–97. [Google Scholar] [CrossRef]
- Zhao, X.; Haeseleer, F.; Fariss, R.N.; Huang, J.; Baehr, W.; Milam, A.H.; Palczewski, K. Molecular cloning and localization of rhodopsin kinase in the mammalian pineal. Vis. Neurosci. 1997, 14, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, C.A.; Shanahan, T.L.; Klerman, E.B.; Martens, H.; Brotman, D.J.; Emens, J.S.; Klein, T.; Rizzo, J.F., 3rd. Suppression of melatonin secretion in some blind patients by exposure to bright light. N. Engl. J. Med. 1995, 332, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.S.; Murphy, P.J. Extraocular circadian phototransduction in humans. Science 1998, 279, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Busse, D.; Kudella, P.; Gruning, N.M.; Gisselmann, G.; Stander, S.; Luger, T.; Jacobsen, F.; Steinstrasser, L.; Paus, R.; Gkogkolou, P.; et al. A synthetic sandalwood odorant induces wound-healing processes in human keratinocytes via the olfactory receptor OR2AT4. J. Investig. Dermatol. 2014, 134, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Pavan, B.; Dalpiaz, A. Odorants could elicit repair processes in melanized neuronal and skin cells. Neural Regen Res. 2017, 12, 1401–1404. [Google Scholar] [CrossRef]
- Spehr, M.; Gisselmann, G.; Poplawski, A.; Riffell, J.A.; Wetzel, C.H.; Zimmer, R.K.; Hatt, H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 2003, 299, 2054–2058. [Google Scholar] [CrossRef]
- Griffin, C.A.; Kafadar, K.A.; Pavlath, G.K. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev. Cell 2009, 17, 649–661. [Google Scholar] [CrossRef]
- Weber, L.; Al-Refae, K.; Ebbert, J.; Jagers, P.; Altmuller, J.; Becker, C.; Hahn, S.; Gisselmann, G.; Hatt, H. Activation of odorant receptor in colorectal cancer cells leads to inhibition of cell proliferation and apoptosis. PLoS ONE 2017, 12, e0172491. [Google Scholar] [CrossRef]
- Sondersorg, A.C.; Busse, D.; Kyereme, J.; Rothermel, M.; Neufang, G.; Gisselmann, G.; Hatt, H.; Conrad, H. Chemosensory information processing between keratinocytes and trigeminal neurons. J. Biol. Chem. 2014, 289, 17529–17540. [Google Scholar] [CrossRef]
- Tsai, T.; Veitinger, S.; Peek, I.; Busse, D.; Eckardt, J.; Vladimirova, D.; Jovancevic, N.; Wojcik, S.; Gisselmann, G.; Altmuller, J.; et al. Two olfactory receptors-OR2A4/7 and OR51B5-differentially affect epidermal proliferation and differentiation. Exp. Dermatol. 2017, 26, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Gelis, L.; Jovancevic, N.; Veitinger, S.; Mandal, B.; Arndt, H.D.; Neuhaus, E.M.; Hatt, H. Functional Characterization of the Odorant Receptor 51E2 in Human Melanocytes. J. Biol. Chem. 2016, 291, 17772–17786. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, S.; Weidinger, D.; Stander, S.; Luger, T.; Hatt, H.; Jovancevic, N. Functional characterization of the extranasal OR2A4/7 expressed in human melanocytes. Exp. Dermatol. 2018, 27, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Adameyko, I.; Lallemend, F. Glial versus melanocyte cell fate choice: Schwann cell precursors as a cellular origin of melanocytes. Cell Mol. Life Sci. 2010, 67, 3037–3055. [Google Scholar] [CrossRef] [PubMed]
- Lerner, M.R.; Reagan, J.; Gyorgyi, T.; Roby, A. Olfaction by melanophores: What does it mean? Proc. Natl. Acad. Sci. USA 1988, 85, 261–264. [Google Scholar] [CrossRef]
- Karlsson, J.O.; Svensson, S.P.; Martensson, L.G.; Odman, S.; Elwing, H.; Lundstrom, K.I. Effects of odorants on pigment aggregation and cAMP in fish melanophores. Pigment. Cell Res. 1994, 7, 61–64. [Google Scholar] [CrossRef]
- Gelis, L.; Jovancevic, N.; Bechara, F.G.; Neuhaus, E.M.; Hatt, H. Functional expression of olfactory receptors in human primary melanoma and melanoma metastasis. Exp. Dermatol. 2017, 26, 569–576. [Google Scholar] [CrossRef]
- Ranzani, M.; Iyer, V.; Ibarra-Soria, X.; Del Castillo Velasco-Herrera, M.; Garnett, M.; Logan, D.; Adams, D.J. Revisiting olfactory receptors as putative drivers of cancer. Wellcome Open Res. 2017, 2, 9. [Google Scholar] [CrossRef]
- Suzuki, T.; Tomita, Y. Recent advances in genetic analyses of oculocutaneous albinism types 2 and 4. J. Dermatol. Sci. 2008, 51, 1–9. [Google Scholar] [CrossRef]
- Nichols, S.E., Jr.; Reams, W.M., Jr. The occurrence and morphogenesis of melanocytes in the connective tissues of the PET/MCV mouse strain. J. Embryol. Exp. Morphol. 1960, 8, 24–32. [Google Scholar]
- Mjaatvedt, C.H.; Kern, C.B.; Norris, R.A.; Fairey, S.; Cave, C.L. Normal distribution of melanocytes in the mouse heart. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005, 285, 748–757. [Google Scholar] [CrossRef]
- Brito, F.C.; Kos, L. Timeline and distribution of melanocyte precursors in the mouse heart. Pigment. Cell Melanoma Res. 2008, 21, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Yajima, I.; Larue, L. The location of heart melanocytes is specified and the level of pigmentation in the heart may correlate with coat color. Pigment. Cell Melanoma Res. 2008, 21, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Pina, J.; Lorenzale, M.; Fernandez, M.C.; Duran, A.C.; Sans-Coma, V.; Fernandez, B. Pigmentation of the aortic and pulmonary valves in C57BL/6J x Balb/cByJ hybrid mice of different coat colours. Anat. Histol. Embryol. 2019, 48, 429–436. [Google Scholar] [CrossRef]
- Balani, K.; Brito, F.C.; Kos, L.; Agarwal, A. Melanocyte pigmentation stiffens murine cardiac tricuspid valve leaflet. J. R. Soc. Interface 2009, 6, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, F.; Kruithof, B.P.; Balani, K.; Agarwal, A.; Gaussin, V.; Kos, L. Relationships between melanocytes, mechanical properties and extracellular matrix composition in mouse heart valves. J. Long Term Eff. Med. Implant. 2015, 25, 17–26. [Google Scholar] [CrossRef]
- Levin, M.D.; Lu, M.M.; Petrenko, N.B.; Hawkins, B.J.; Gupta, T.H.; Lang, D.; Buckley, P.T.; Jochems, J.; Liu, F.; Spurney, C.F.; et al. Melanocyte-like cells in the heart and pulmonary veins contribute to atrial arrhythmia triggers. J. Clin. Investig. 2009, 119, 3420–3436. [Google Scholar] [CrossRef][Green Version]
- Hwang, H.; Liu, F.; Levin, M.D.; Patel, V.V. Isolating primary melanocyte-like cells from the mouse heart. J. Vis. Exp. 2014, 91, 4357. [Google Scholar] [CrossRef]
- Hwang, H.; Liu, F.; Petrenko, N.B.; Huang, J.; Schillinger, K.J.; Patel, V.V. Cardiac melanocytes influence atrial reactive oxygen species involved with electrical and structural remodeling in mice. Physiol. Rep. 2015, 3, e12559. [Google Scholar] [CrossRef]
- Tsai, W.C.; Chan, Y.H.; Hsueh, C.H.; Everett, T.H., IV; Chang, P.C.; Choi, E.K.; Olaopa, M.A.; Lin, S.F.; Shen, C.; Kudela, M.A.; et al. Small conductance calcium-activated potassium current and the mechanism of atrial arrhythmia in mice with dysfunctional melanocyte-like cells. Heart Rhythm. 2016, 13, 1527–1535. [Google Scholar] [CrossRef]
- Tachibana, M. Sound needs sound melanocytes to be heard. Pigment. Cell Res. 1999, 12, 344–354. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, M.; Fridberger, A.; Hassan, A.; Degagne, J.; Neng, L.; Zhang, F.; He, W.; Ren, T.; Trune, D.; et al. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc. Natl. Acad. Sci. USA 2012, 109, 10388–10393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Dai, M.; Neng, L.; Zhang, J.H.; Zhi, Z.; Fridberger, A.; Shi, X. Perivascular macrophage-like melanocyte responsiveness to acoustic trauma--a salient feature of strial barrier associated hearing loss. FASEB J. 2013, 27, 3730–3740. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Song, J.; He, C.; Cai, X.; Yuan, K.; Mei, L.; Feng, Y. Genetic insights, disease mechanisms, and biological therapeutics for Waardenburg syndrome. Gene Ther. 2021. [Google Scholar] [CrossRef]
- Andrade, A.; Pithon, M. Alezzandrini syndrome: Report of a sixth clinical case. Dermatology 2011, 222, 8–9. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, G.A.; Rao, N.A. Vogt-Koyanagi-Harada disease. Surv. Ophthalmol. 2017, 62, 1–25. [Google Scholar] [CrossRef]
- Lee, T.L.; Lin, P.H.; Chen, P.L.; Hong, J.B.; Wu, C.C. Hereditary Hearing Impairment with Cutaneous Abnormalities. Genes 2020, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Anbar, T.S.; El-Badry, M.M.; McGrath, J.A.; Abdel-Azim, E.S. Most individuals with either segmental or non-segmental vitiligo display evidence of bilateral cochlear dysfunction. Br. J. Dermatol. 2015, 172, 406–411. [Google Scholar] [CrossRef]
- Ertugrul, G.; Ertugrul, S.; Soylemez, E. Investigation of hearing and outer hair cell function of the cochlea in patients with vitiligo. Dermatol. Ther. 2020, 33, e13724. [Google Scholar] [CrossRef]
- Genedy, R.; Assal, S.; Gomaa, A.; Almakkawy, B.; Elariny, A. Ocular and auditory abnormalities in patients with vitiligo: A case-control study. Clin. Exp. Dermatol. 2021, 46, 1058–1066. [Google Scholar] [CrossRef]
- Mujica-Mota, M.A.; Schermbrucker, J.; Daniel, S.J. Eye color as a risk factor for acquired sensorineural hearing loss: A review. Hear. Res. 2015, 320, 1–10. [Google Scholar] [CrossRef]
- Wrzesniok, D.; Beberok, A.; Otreba, M.; Buszman, E. Gentamicin affects melanogenesis in normal human melanocytes. Cutan. Ocul. Toxicol. 2015, 34, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Wrzesniok, D.; Rok, J.; Beberok, A.; Rzepka, Z.; Respondek, M.; Pilawa, B.; Zdybel, M.; Delijewski, M.; Buszman, E. Kanamycin induces free radicals formation in melanocytes: An important factor for aminoglycosides ototoxicity. J. Cell Biochem. 2019, 120, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Cuesta, S.; Contreras, J.; Zurita, E.; Cediel, R.; Cantero, M.; Varela-Nieto, I.; Montoliu, L. Melanin precursors prevent premature age-related and noise-induced hearing loss in albino mice. Pigment Cell Melanoma Res. 2010, 23, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, H.; Watanabe, R.; Maeno, A.; Ichimura, K.; Tamura, M.; Wakana, S.; Shiroishi, T.; Ohba, K.; Takeda, K.; Tomita, H.; et al. Melanocytes contribute to the vasculature of the choroid. Genes Genet. Syst. 2018, 93, 51–58. [Google Scholar] [CrossRef]
- Gudjohnsen, S.; Atacho, D.; Gesbert, F.; Raposo, G.; Hurbain, I.; Larue, L.; Steingrimsson, E.; Petersen, P. Meningeal Melanocytes in the Mouse: Distribution and Dependence on Mitf. Front. Neuroanat. 2015, 9, 149. [Google Scholar] [CrossRef]
- Randhawa, M.; Huff, T.; Valencia, J.C.; Younossi, Z.; Chandhoke, V.; Hearing, V.J.; Baranova, A. Evidence for the ectopic synthesis of melanin in human adipose tissue. FASEB J. 2009, 23, 835–843. [Google Scholar] [CrossRef]
- Page, S.; Chandhoke, V.; Baranova, A. Melanin and melanogenesis in adipose tissue: Possible mechanisms for abating oxidative stress and inflammation? Obes. Rev. 2011, 12, e21–e31. [Google Scholar] [CrossRef]
- Jehs, T.; Faber, C.; Udsen, M.S.; Jager, M.J.; Clark, S.J.; Nissen, M.H. Induction of Chemokine Secretion and Monocyte Migration by Human Choroidal Melanocytes in Response to Proinflammatory Cytokines. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6568–6579. [Google Scholar] [CrossRef]
- Nag, T.C. Ultrastructural changes in the melanocytes of aging human choroid. Micron 2015, 79, 16–23. [Google Scholar] [CrossRef]
- Jang, Y.H.; Kim, S.L.; Lee, J.S.; Kwon, K.Y.; Lee, S.J.; Kim, D.W.; Lee, W.J. Possible existence of melanocytes or melanoblasts in human sebaceous glands. Ann. Dermatol. 2014, 26, 469–473. [Google Scholar] [CrossRef]
- Vandamme, N.; Berx, G. From neural crest cells to melanocytes: Cellular plasticity during development and beyond. Cell Mol. Life Sci. 2019, 76, 1919–1934. [Google Scholar] [CrossRef] [PubMed]
- Adameyko, I.; Lallemend, F.; Aquino, J.B.; Pereira, J.A.; Topilko, P.; Muller, T.; Fritz, N.; Beljajeva, A.; Mochii, M.; Liste, I.; et al. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell 2009, 139, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Tatarakis, D.; Cang, Z.; Wu, X.; Sharma, P.P.; Karikomi, M.; MacLean, A.L.; Nie, Q.; Schilling, T.F. Single-cell transcriptomic analysis of zebrafish cranial neural crest reveals spatiotemporal regulation of lineage decisions during development. Cell Rep. 2021, 37, 110140. [Google Scholar] [CrossRef] [PubMed]
- Kaucka, M.; Szarowska, B.; Kavkova, M.; Kastriti, M.E.; Kameneva, P.; Schmidt, I.; Peskova, L.; Joven Araus, A.; Simon, A.; Kaiser, J.; et al. Nerve-associated Schwann cell precursors contribute extracutaneous melanocytes to the heart, inner ear, supraorbital locations and brain meninges. Cell Mol. Life Sci. 2021, 78, 6033–6049. [Google Scholar] [CrossRef]
- Bonnamour, G.; Soret, R.; Pilon, N. Dhh-expressing Schwann cell precursors contribute to skin and cochlear melanocytes, but not to vestibular melanocytes. Pigment Cell Melanoma Res. 2021, 34, 648–654. [Google Scholar] [CrossRef]
- Slominski, A.; Brożyna, A.; Tuckey, R. Cutaneous Glucocorticoidogenesis and Cortisol Signaling Are Defective in Psoriasis. J. Investig. Dermatol. 2017, 137, 1609–1611. [Google Scholar] [CrossRef]
- Paus, R.; Theoharides, T.; Arck, P. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006, 27, 32–39. [Google Scholar] [CrossRef]
- Martins, A.; Ascenso, A.; Ribeiro, H.; Marto, J. The Brain-Skin Connection and the Pathogenesis of Psoriasis: A Review with a Focus on the Serotonergic System. Cells 2020, 9, 796. [Google Scholar] [CrossRef]
- Slominski, A. Ultraviolet radiation (UVR) activates central neuro-endocrine-immune system. Photodermatol. Photoimmunol. Photomed. 2015, 31, 121–123. [Google Scholar] [CrossRef]
- Fell, G.; Robinson, K.; Mao, J.; Woolf, C.; Fisher, D. Skin β-endorphin mediates addiction to UV light. Cell 2014, 157, 1527–1534. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, N.; Yao, L.; Chen, Q.; Zhang, R.; Qian, J.; Hou, Y.; Guo, W.; Fan, S.; Liu, S.; et al. Moderate UV Exposure Enhances Learning and Memory by Promoting a Novel Glutamate Biosynthetic Pathway in the Brain. Cell 2018, 173, 1716–1727.e17. [Google Scholar] [CrossRef] [PubMed]
- Sancar, A.; Van Gelder, R. Clocks, cancer, and chronochemotherapy. Science 2021, 371, eabb0738. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, M.; Boudreau, P.; Cermakian, N.; Boivin, D. Rapid resetting of human peripheral clocks by phototherapy during simulated night shift work. Sci. Rep. 2017, 7, 16310. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Yuan, X.; Eltzschig, H. Circadian rhythm as a therapeutic target. Nat. Rev. Drug Discov. 2021, 20, 287–307. [Google Scholar] [CrossRef]
| Opsin | Species | Potential Effect | Reference |
|---|---|---|---|
| OPN1 | Mouse | Not shown | [146] |
| Human | Not shown | [127,129] | |
| OPN2 | Mouse | Mediate UVA-induced immediate pigment darkening | [126,135,147] |
| Regulate clock genes and melanogenesis responding to white light | |||
| Human | Mediate UVR-induced early melanin synthesis | [127,128,129] | |
| OPN3 | Human | Sense blue light and regulate long-lasting hyperpigmentation | [129,130,131,132,133] |
| Negatively regulate pigmentation through interaction with MC1R | |||
| Regulate the survival of melanocytes | |||
| OPN4 | Mouse | Mediate UVA-induced immediate pigment darkening | [146,147,148] |
| Regulate clock genes and melanogenesis responding to white light | |||
| Mediate UVA-related proliferation and apoptosis | |||
| Mediate thermal activation of clock genes | |||
| Human | Photoreceptor of blue light | [124] | |
| OPN5 | Mouse | Local circadian photoentrainment | [145] |
| Human | Regulate UVR-induced melanogenesis | [129,130] |
| Location | Function | Reference |
|---|---|---|
| Heart | Support the stiffness and mechanical properties of the cardiac valves | [172,173,174,175,176] |
| Reduce ROS | ||
| Regulate electrical and structural remodel | ||
| Maintain the endolymphatic potential | ||
| Regulate cochlear development | ||
| Stabilize the intrastriatal fluid–blood barrier | ||
| Protect from noise and ototoxic | ||
| Inner ear | Reduce ROS | [180,181,182,190,191,193] |
| Eye | Eye pigmentation and protection against UV | [189,194,198,199] |
| Support the normal vasculature of the choroid | ||
| Induce chemokine secretion and monocyte Migration | ||
| Sebaceous glands | May be a source of melanocyte stem cells | [200] |
| Brain | Neuroendocrine and detoxification | [195] |
| Adipose | Abate oxidative stress and inflammation | [196,197] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-Y.; Liu, L.-P.; Zhou, H.; Zheng, Y.-W.; Li, Y.-M. Recognition of Melanocytes in Immuno-Neuroendocrinology and Circadian Rhythms: Beyond the Conventional Melanin Synthesis. Cells 2022, 11, 2082. https://doi.org/10.3390/cells11132082
Chen Y-Y, Liu L-P, Zhou H, Zheng Y-W, Li Y-M. Recognition of Melanocytes in Immuno-Neuroendocrinology and Circadian Rhythms: Beyond the Conventional Melanin Synthesis. Cells. 2022; 11(13):2082. https://doi.org/10.3390/cells11132082
Chicago/Turabian StyleChen, Yan-Yan, Li-Ping Liu, Hang Zhou, Yun-Wen Zheng, and Yu-Mei Li. 2022. "Recognition of Melanocytes in Immuno-Neuroendocrinology and Circadian Rhythms: Beyond the Conventional Melanin Synthesis" Cells 11, no. 13: 2082. https://doi.org/10.3390/cells11132082
APA StyleChen, Y.-Y., Liu, L.-P., Zhou, H., Zheng, Y.-W., & Li, Y.-M. (2022). Recognition of Melanocytes in Immuno-Neuroendocrinology and Circadian Rhythms: Beyond the Conventional Melanin Synthesis. Cells, 11(13), 2082. https://doi.org/10.3390/cells11132082