Effect of SMART DNA Therapy Retix.C Application on Skin Microbiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Cosmetic Products Used in the Study
- Retix.C SMART DNA AOX Peel (brand: Retix.C, manufacturer: URGO Sp. z o.o., Warsaw, Poland)—chemical peel containing ferulic acid as the main active ingredient (exact concentration is proprietary information of the manufacturer). Product purchased directly from the manufacturer.
- Retix.C SMART DNA Meso Peptide Cocktail (Retix.C, URGO Sp. z o.o., Warsaw, Poland)—sterile solution for microinjection containing a proprietary blend of peptides and ferulic acid (exact concentration is proprietary information of the manufacturer). Product purchased from the manufacturer.
- Ferulic Triple-C SPF Cream (Retix.C, URGO Sp. z o.o., Warsaw, Poland)—antioxidant sunscreen containing vitamin C derivatives and ferulic acid (exact concentration is proprietary information of the manufacturer). Provided for home use.
2.2. Study Design
2.3. Selection of Study Group
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Treatment Schedule
2.5. Effectiveness Evaluation
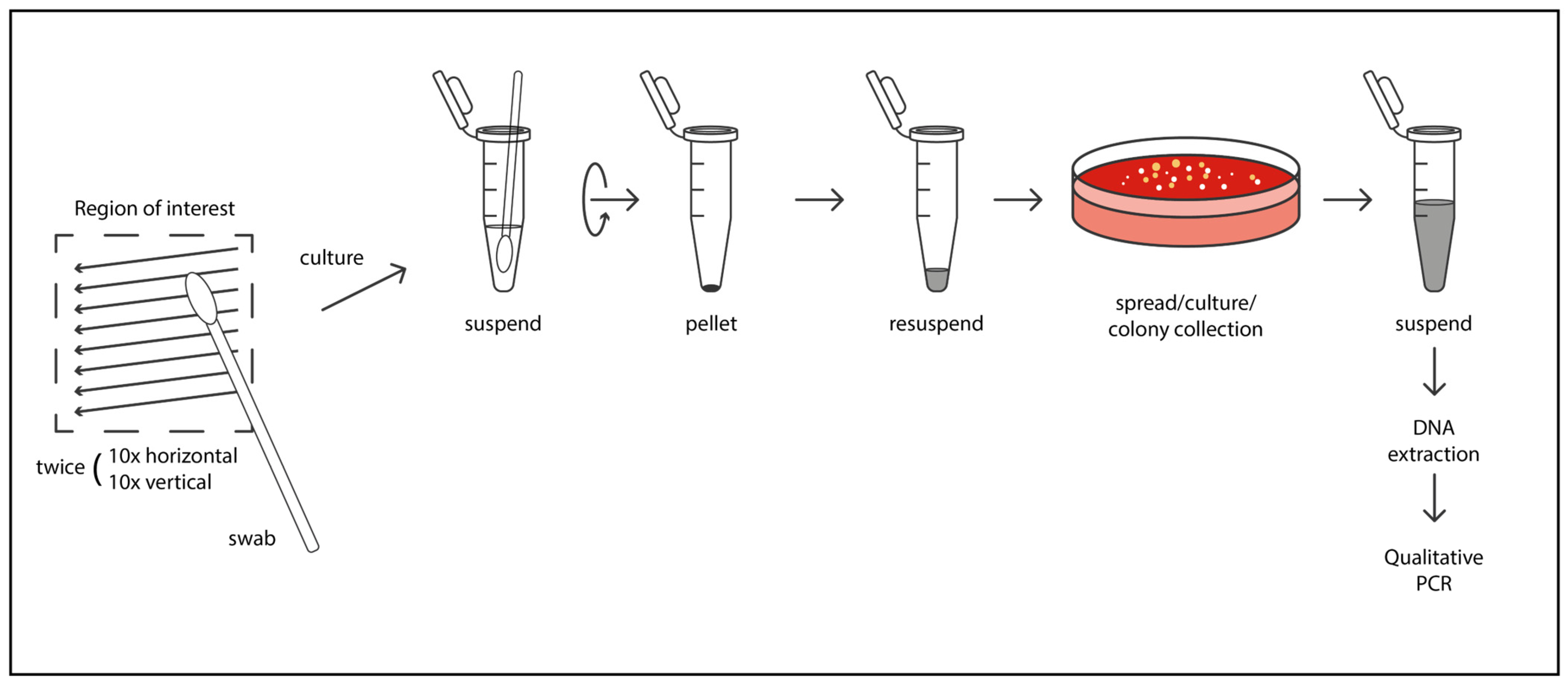
Microbiological Sampling and Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMP | antimicrobial peptide |
| FOXO3 | forkhead box O3 |
| PCR | polymerase chain reaction |
| SPF | sun protection factor |
| TGF-β2 | transforming growth factor β2 |
References
- Smythe, P.; Wilkinson, H.N. The skin microbiome: Current landscape and future opportunities. Int. J. Mol. Sci. 2023, 24, 3950. [Google Scholar] [CrossRef]
- Zhang, X.E.; Zheng, P.; Ye, S.Z.; Ma, X.; Liu, E.; Pang, Y.-B.; He, Q.-Y.; Zhang, Y.-X.; Li, W.-Q.; Zeng, J.-H.; et al. Microbiome: Role in inflammatory skin diseases. J. Inflamm. Res. 2024, 17, 1057–1082. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M. Skin barrier function and the microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.J.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two major sentinels of skin microbiota and the influence of cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef]
- Joshi, A.A.; Vocanson, M.; Nicolas, J.F.; Wolf, P.; Patra, V. Microbial derived antimicrobial peptides as potential therapeutics in atopic dermatitis. Front. Immunol. 2023, 14, 1125635. [Google Scholar] [CrossRef]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Myers, T.; Bouslimani, A.; Huang, S.; Hansen, S.T.; Clavaud, C.; Azouaoui, A.; Ott, A.; Gueniche, A.; Bouez, C.; Zheng, Q.; et al. A multi-study analysis enables identification of potential microbial features associated with skin aging signs. Front. Aging 2024, 4, 1304705. [Google Scholar] [CrossRef]
- Woo, Y.R.; Kim, H.S. Interaction between the microbiota and the skin barrier in aging skin: A comprehensive review. Front. Physiol. 2024, 15, 1322205. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- SanMiguel, A.J.; Meisel, J.S.; Horwinski, J.; Zheng, Q.; Grice, E.A. Topical antimicrobial treatments can elicit shifts to resident skin bacterial communities and reduce colonization by Staphylococcus aureus competitors. Antimicrob. Agents Chemother. 2017, 61, e00774-17. [Google Scholar] [CrossRef]
- Hwang, B.K.; Lee, S.; Myoung, J.; Hwang, S.J.; Lim, J.M.; Jeong, E.T.; Park, S.G.; Youn, S.H. Effect of the skincare product on facial skin microbial structure and biophysical parameters: A pilot study. Microbiologyopen 2021, 10, e1236. [Google Scholar] [CrossRef]
- Rendon, M.I.; Berson, D.S.; Cohen, J.L.; Roberts, W.E.; Starker, I.; Wang, B. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J. Clin. Aesthet. Dermatol. 2010, 3, 32–41. [Google Scholar]
- Baldwin, H.E.; Bhatia, N.C.; Friedman, A.; Prunty, T.; Martin, R.; Seite, S. The role of cutaneous microbiota harmony in maintaining a functional skin barrier. J. Drugs Dermatol. 2017, 16, 12–18. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin wound healing: An update on the current knowledge and concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Schagen, S.K. Topical peptide treatments with effective anti-aging results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 2023, 21, 97–111. [Google Scholar] [CrossRef]
- Nakamizo, S.; Egawa, G.; Honda, T.; Nakajima, S.; Belkaid, Y.; Kabashima, K. Commensal bacteria and cutaneous immunity. Semin. Immunopathol. 2015, 37, 73–80. [Google Scholar] [CrossRef]
- Azzimonti, B.; Ballacchino, C.; Zanetta, P.; Cucci, M.A.; Monge, C.; Grattarola, M.; Dianzani, C.; Barrera, G.; Pizzimenti, S. Microbiota, oxidative stress, and skin cancer: An unexpected triangle. Antioxidants 2023, 12, 546. [Google Scholar] [CrossRef]
- Pastar, I.; O’Neill, K.; Padula, L.; Head, C.R.; Burgess, J.L.; Chen, V.; Garcia, D.; Stojadinovic, O.; Hower, S.; Plano, G.V.; et al. Staphylococcus epidermidis boosts innate immune response by activation of gamma delta T cells and induction of perforin-2 in human skin. Front. Immunol. 2020, 11, 550946. [Google Scholar] [CrossRef]
- Nguyen, H.L.T.; Trujillo-Paez, J.V.; Umehara, Y.; Yue, H.; Peng, G.; Kiatsurayanon, C.; Chieosilapatham, P.; Song, P.; Okumura, K.; Ogawa, H.; et al. Role of antimicrobial peptides in skin barrier repair in individuals with atopic dermatitis. Int. J. Mol. Sci. 2020, 21, 7607. [Google Scholar] [CrossRef]
- Simanski, M.; Erkens, A.S.; Rademacher, F.; Harder, J. Staphylococcus epidermidis-induced interleukin-1 beta and human beta-defensin-2 expression in human keratinocytes is regulated by the host molecule A20 (TNFAIP3). Acta Derm. Venereol. 2019, 99, 181–187. [Google Scholar] [CrossRef]
- Chessa, C.; Bodet, C.; Jousselin, C.; Wehbe, M.; Lévêque, N.; Garcia, M. Antiviral and immunomodulatory properties of antimicrobial peptides produced by human keratinocytes. Front. Microbiol. 2020, 11, 1155. [Google Scholar] [CrossRef]
- Leech, J.M.; Dhariwala, M.O.; Lowe, M.M.; Chu, K.; Merana, G.R.; Cornuot, C.; Weckel, A.; Ma, J.M.; Leitner, E.G.; Gonzalez, J.R.; et al. Toxin-triggered interleukin-1 receptor signaling enables early-life discrimination of pathogenic versus commensal skin bacteria. Cell Host Microbe 2019, 26, 795–809. [Google Scholar] [CrossRef]
- Singh, T.P.; Amorim, C.F.; Lovins, V.M.; Bradley, C.W.; Carvalho, L.P.; Carvalho, E.M.; Grice, E.A.; Scott, P. Regulatory T cells control Staphylococcus aureus and disease severity of cutaneous leishmaniasis. J. Exp. Med. 2023, 220, e20230035. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Butcher, A.M.; Trzoss, L.L.; Nam, S.-J.; Shirakawa, K.T.; Zhou, W.; Oh, J.; Otto, M.; Fenical, W.; et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 2018, 4, eaao4502. [Google Scholar] [CrossRef]
- Dréno, B.; Araviiskaia, E.; Berardesca, E.; Gontijo, G.; Sanchez Viera, M.; Xiang, L.F.; Martin, R.; Bieber, T. Microbiome in healthy skin, update for dermatologists. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2038–2047. [Google Scholar] [CrossRef]


| Patient | Before Treatment | After Treatment |
|---|---|---|
| 1 forehead | S. epidermidis—numerous S. hominis subsp. hominis—moderately numerous S. aureus—a few C. acnes—a few Candida albicans—a few | S. epidermidis—numerous S. hominis subsp. hominis—numerous Candida albicans—a few |
| 1 cheek | S. epidermidis—numerous S. hominis subsp. hominis—moderately numerous C. acnes—a few | S. epidermidis—numerous S. hominis subsp. hominis—numerous |
| 2 forehead | S. epidermidis—numerous S. hominis subsp. hominis—moderately numerous Micrococcus spp.—moderately numerous | S. epidermidis—numerous S. hominis subsp. hominis—numerous Micrococcus spp.—moderately numerous |
| 2 cheek | S. epidermidis—numerous S. hominis subsp. hominis—moderately numerous Micrococcus spp.—moderately numerous | S. epidermidis—numerous S. hominis subsp. hominis—numerous |
| 3 forehead | S. epidermidis—numerous S. hominis subsp. hominis—moderately numerous Corynebacterium spp.—numerous | S. epidermidis—numerous S. hominis subsp. hominis—numerous Corynebacterium spp.—a few |
| 3 cheek | S. epidermidis—numerous S. hominis subsp. hominis—moderately numerous Corynebacterium spp.—moderately numerous | S. epidermidis—numerous S. hominis subsp. hominis—moderately numerous Corynebacterium spp.—moderately numerous |
| 4 forehead | S. hominis subsp. hominis—moderately numerous Dermacoccus spp.—a few Micrococcus spp.—a few | S. epidermidis—moderately numerous S. hominis subsp. hominis—moderately numerous Dermacoccus spp.—a few |
| 4 cheek | S. epidermidis—numerous Dermacoccus spp.—a few | S. epidermidis—numerous |
| 5 forehead | C. tuberculostearicum—a few C. amycolatum—a few | S. capitis—moderately numerous C. amycolatum—a few |
| 5 cheek | S. capitis—a few S. mitis—a few Micrococcus spp.—a few | S. capitis—numerous Micrococcus spp.—a few |
| 6 forehead | S. epidermidis—numerous Streptococcus spp.—a few C. acnes—a few | S. epidermidis—numerous Streptococcus spp.—moderately numerous C. albicans—a few |
| 6 cheek | S. epidermidis—numerous Streptococcus spp.—a few C. albicans—a few | S. epidermidis—numerous S. lugdunensis—numerous C. albicans—a few |
| 7 forehead | S. epidermidis—numerous | S. epidermidis—numerous |
| 7 cheek | S. epidermidis—numerous P. aeruginosa—a few | S. epidermidis—numerous |
| 8 forehead | S. epidermidis—numerous D. hominis—moderately numerous | S. epidermidis—numerous D. hominis—a few |
| 8 cheek | S. epidermidis—numerous | S. epidermidis—numerous |
| 9 forehead | S. epidermidis—numerous S. intermedius—a few C. acnes—moderately numerous | S. epidermidis—numerous S. intermedius—numerous |
| 9 cheek | S. epidermidis—numerous S. intermedius—a few | S. epidermidis—numerous S. intermedius—moderately numerous |
| 10 forehead | K. kristinae—moderately numerous M. luteus—a few C. striatum—numerous M. restricta—a few | S. epidermidis—numerous K. kristinae—moderately numerous M. luteus—a few C. striatum—a few |
| 10 cheek | S. epidermidis —moderately numerous M. luteus—a few C. striatum—numerous M. restricta—a few | S. epidermidis—numerous K. kristinae—moderately numerous M. luteus—a few |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolewska-Sztychny, D.; Wódz, K.; Lesiak, A. Effect of SMART DNA Therapy Retix.C Application on Skin Microbiome. Cosmetics 2025, 12, 178. https://doi.org/10.3390/cosmetics12050178
Sobolewska-Sztychny D, Wódz K, Lesiak A. Effect of SMART DNA Therapy Retix.C Application on Skin Microbiome. Cosmetics. 2025; 12(5):178. https://doi.org/10.3390/cosmetics12050178
Chicago/Turabian StyleSobolewska-Sztychny, Dorota, Karolina Wódz, and Aleksandra Lesiak. 2025. "Effect of SMART DNA Therapy Retix.C Application on Skin Microbiome" Cosmetics 12, no. 5: 178. https://doi.org/10.3390/cosmetics12050178
APA StyleSobolewska-Sztychny, D., Wódz, K., & Lesiak, A. (2025). Effect of SMART DNA Therapy Retix.C Application on Skin Microbiome. Cosmetics, 12(5), 178. https://doi.org/10.3390/cosmetics12050178







