Photoprotective Effect of Hydroxychloroquine on Human Keratinocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Treatment with HEVL and HCQ
2.3. Cell Viability Assay
2.4. Reactive Oxygen Species (ROS) Production Assay
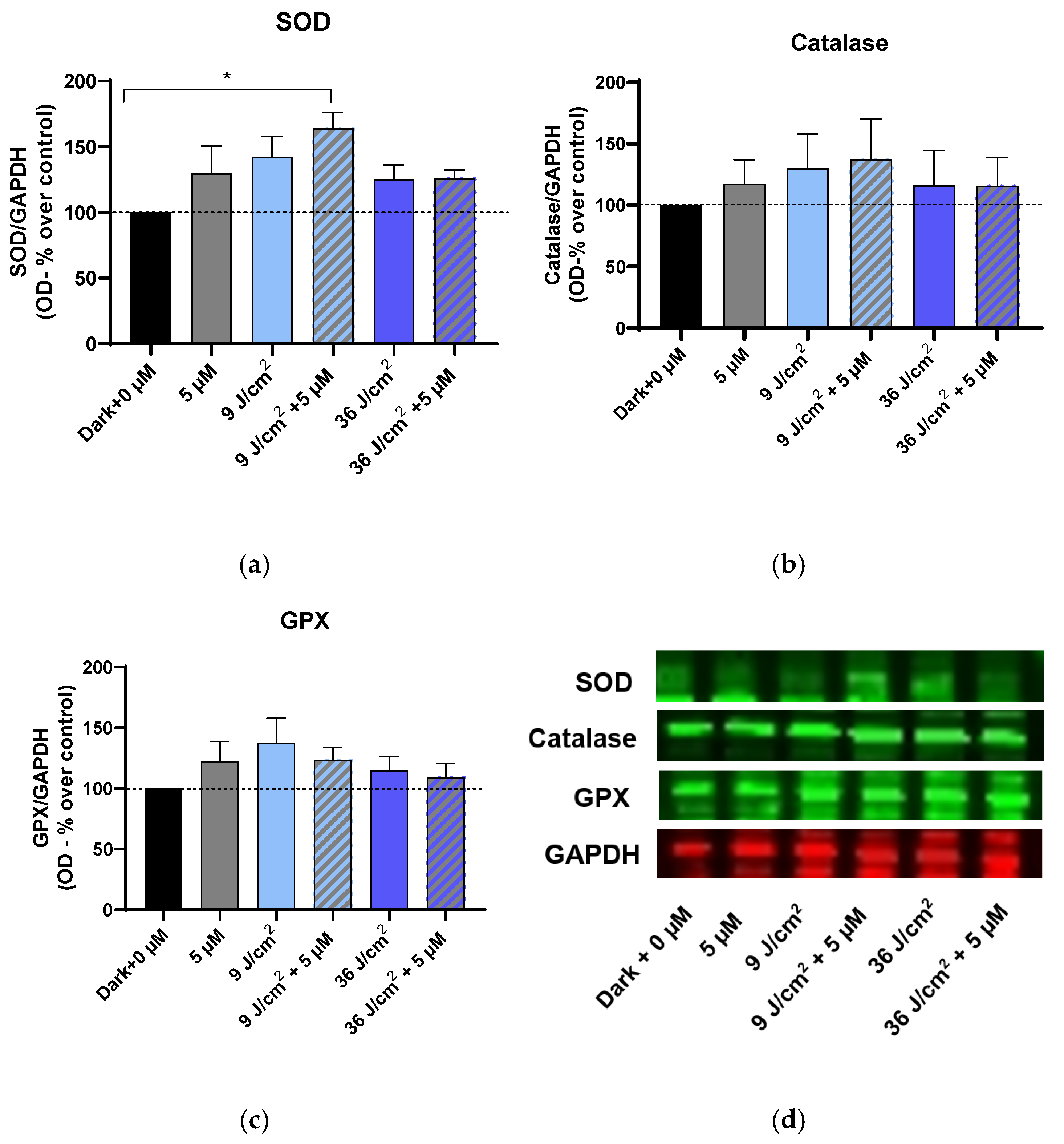
2.5. Immunoblotting for the Enzymes Superoxide Dismutase (SOD), Catalase, and Glutathione Peroxidase (GPX)
2.6. Statistical Analysis
3. Results
3.1. Effects on Cell Viability of HEVL and HCQ
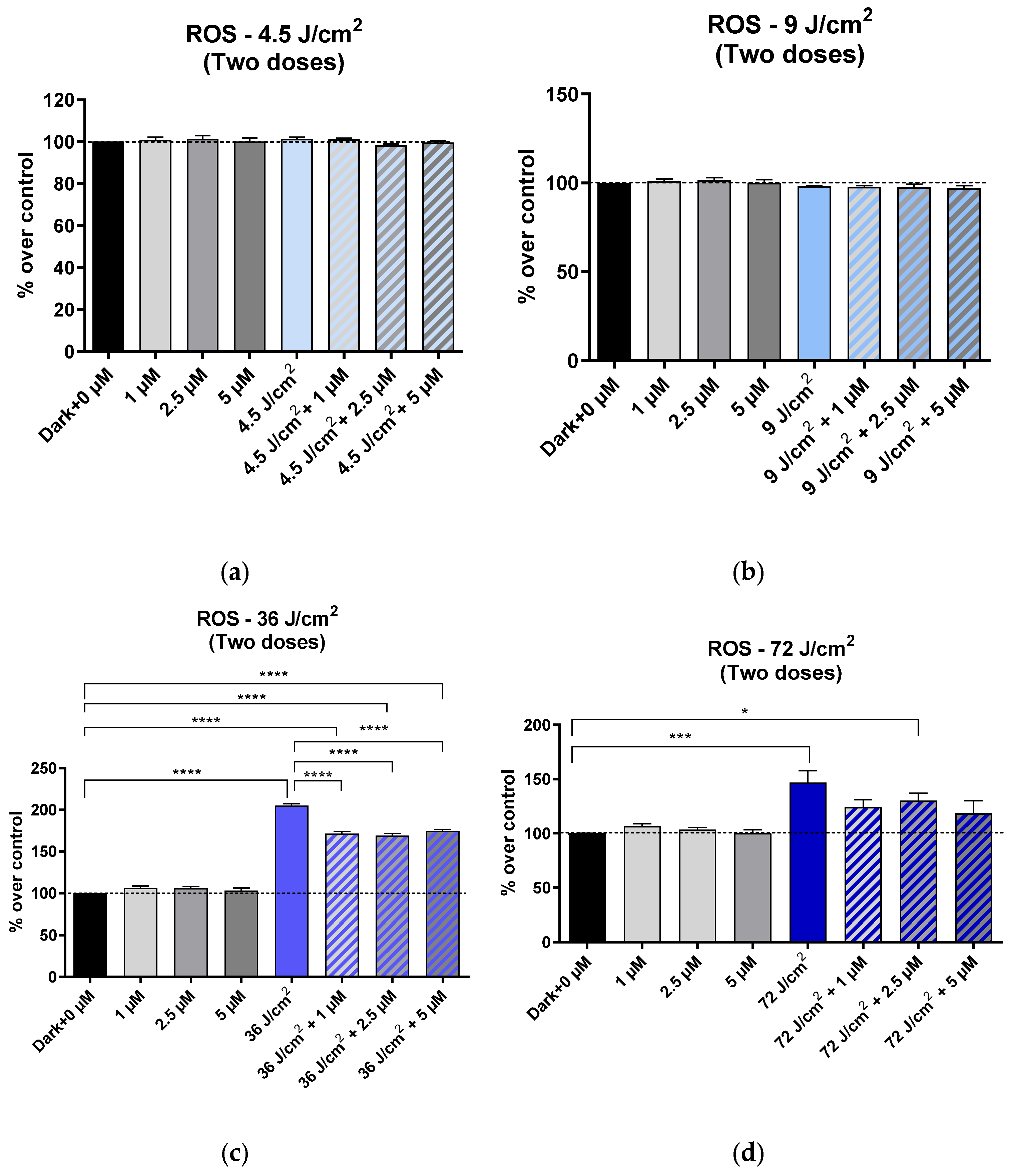
3.2. Effects on ROS Production
3.2.1. Effects on ROS Production After One Exposure to HEVL, with or Without HCQ
3.2.2. Effects on ROS Production After Two Exposures to HEVL with or Without HCQ
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez-Caruncho, C.; Bielsa Marsol, I. Antipalúdicos en dermatología: Mecanismo de acción, indicaciones y efectos secundarios. Actas Dermo-Sifiliográficas 2014, 105, 243–252. [Google Scholar] [CrossRef]
- Sardana, K.; Sinha, S.; Sachdeva, S. Hydroxychloroquine in Dermatology and Beyond: Recent Update. Indian Dermatol. Online J. 2020, 11, 453–464. [Google Scholar] [CrossRef]
- Barrat, F.J.; Crow, M.K.; Ivashkiv, L.B. Interferon Target-Gene Expression and Epigenomic Signatures in Health and Disease. Nat. Immunol. 2019, 20, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Nocturne, G.; Mariette, X. Interferon Signature in Systemic Autoimmune Diseases: What Does It Mean? RMD Open 2022, 8, e002687. [Google Scholar] [CrossRef] [PubMed]
- Willis, R.; Seif, A.; McGwin, G.; Martinez-Martinez, L.; González, E.; Dang, N.; Papalardo, E.; Liu, J.; Vilá, L.; Reveille, J.; et al. Effect of Hydroxychloroquine Treatment on Pro-Inflammatory Cytokines and Disease Activity in SLE Patients: Data from LUMINA (LXXV), a Multiethnic US Cohort. Lupus 2012, 21, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.P.; Zabaleta, M.E.; Di Giulio, C.; Charris, J.E.; Mijares, M.R. The Role of Chloroquine and Hydroxychloroquine in Immune Regulation and Diseases. Curr. Pharm. Des. 2020, 26, 4467–4485. [Google Scholar] [CrossRef]
- Fox, R. Anti-Malarial Drugs: Possible Mechanisms of Action in Autoimmune Disease and Prospects for Drug Development. Lupus 1996, 5 (Suppl. S1), S4–S10. [Google Scholar] [CrossRef]
- Fox, R.I.; Kang, H.I. Mechanism of Action of Antimalarial Drugs: Inhibition of Antigen Processing and Presentation. Lupus 1993, 2 (Suppl. S1), S9–S12. [Google Scholar] [CrossRef]
- Solomon, D.H.; Garg, R.; Lu, B.; Todd, D.J.; Mercer, E.; Norton, T.; Massarotti, E. Effect of Hydroxychloroquine on Insulin Sensitivity and Lipid Parameters in Rheumatoid Arthritis Patients Without Diabetes Mellitus: A Randomized, Blinded Crossover Trial. Arthritis Care Res. 2014, 66, 1246–1251. [Google Scholar] [CrossRef]
- Siqueira-Neto, J.L.; Wicht, K.J.; Chibale, K.; Burrows, J.N.; Fidock, D.A.; Winzeler, E.A. Antimalarial Drug Discovery: Progress and Approaches. Nat. Rev. Drug Discov. 2023, 22, 807–826. [Google Scholar] [CrossRef]
- Fang, Q.-Q.; Wang, X.-F.; Zhao, W.-Y.; Ding, S.-L.; Shi, B.-H.; Xia, Y.; Yang, H.; Wu, L.-H.; Li, C.-Y.; Tan, W.-Q. Angiotensin-Converting Enzyme Inhibitor Reduces Scar Formation by Inhibiting Both Canonical and Noncanonical TGF-Β1 Pathways. Sci. Rep. 2018, 8, 3332. [Google Scholar] [CrossRef]
- Vlahopoulos, S.; Critselis, E.; Voutsas, I.; Perez, S.; Moschovi, M.; Baxevanis, C.; Chrousos, G. New Use for Old Drugs? Prospective Targets of Chloroquines in Cancer Therapy. CDT 2014, 15, 843–851. [Google Scholar] [CrossRef]
- Hsu, S. Comprehensive Dermatologic Drug Therapy. Arch. Dermatol. 2002, 138, 703. [Google Scholar] [CrossRef]
- Ezekwe, N.; Maghfour, J.; Kohli, I. Visible Light and the Skin. Photochem. Photobiol. 2022, 98, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Narla, S.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Visible Light in Photodermatology. Photochem. Photobiol. Sci. 2020, 19, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Pourang, A.; Tisack, A.; Ezekwe, N.; Torres, A.E.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Effects of Visible Light on Mechanisms of Skin Photoaging. Photodermatol. Photoimmunol. Photomed. 2022, 38, 191–196. [Google Scholar] [CrossRef]
- Lenane, P.; Murphy, G. Sunscreens and the Photodermatoses. J. Dermatol. Treat. 2001, 12, 53–57. [Google Scholar] [CrossRef]
- Neubert, E.; Bach, K.M.; Busse, J.; Bogeski, I.; Schön, M.P.; Kruss, S.; Erpenbeck, L. Blue and Long-Wave Ultraviolet Light Induce in Vitro Neutrophil Extracellular Trap (NET) Formation. Front. Immunol. 2019, 10, 2428. [Google Scholar] [CrossRef]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil Extracellular Traps Enriched in Oxidized Mitochondrial DNA Are Interferogenic and Contribute to Lupus-like Disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Bacqueville, D.; Jacques-Jamin, C.; Dromigny, H.; Boyer, F.; Brunel, Y.; Ferret, P.J.; Redoulès, D.; Douki, T.; Bessou-Touya, S.; Duplan, H. Phenylene Bis-Diphenyltriazine (TriAsorB), a New Sunfilter Protecting the Skin against Both UVB + UVA and Blue Light Radiations. Photochem. Photobiol. Sci. 2021, 20, 1475–1486. [Google Scholar] [CrossRef]
- Métayer, I.; Balguerie, X.; Courville, P.; Lauret, P.; Joly, P. Photodermatosis induced by hydroxychloroquine: 4 cases. Ann. Dermatol. Venereol. 2001, 128, 729–731. [Google Scholar]
- Miyachi, Y.; Yoshioka, A.; Imamura, S.; Niwa, Y. Antioxidant Action of Antimalarials. Ann. Rheum. Dis. 1986, 45, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Catalano, R.; Rocca, R.; Juli, G.; Costa, G.; Maruca, A.; Artese, A.; Caracciolo, D.; Tagliaferri, P.; Alcaro, S.; Tassone, P.; et al. A Drug Repurposing Screening Reveals a Novel Epigenetic Activity of Hydroxychloroquine. Eur. J. Med. Chem. 2019, 183, 111715. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.; Capra, J.D.; Sontheimer, R.D. 4-Aminoquinoline Antimalarials Enhance UV-B Induced c–Jun Transcriptional Activation. Lupus 1998, 7, 148–153. [Google Scholar] [CrossRef]
- Campiche, R.; Curpen, S.J.; Lutchmanen-Kolanthan, V.; Gougeon, S.; Cherel, M.; Laurent, G.; Gempeler, M.; Schuetz, R. Pigmentation Effects of Blue Light Irradiation on Skin and How to Protect against Them. Int. J. Cosmet. Sci. 2020, 42, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yang, X.; Luo, F.; Yuan, X.; Xiong, H.; Ma, W.; Yao, X. Hydroxychloroquine-Induced Hyperpigmentation of the Skin and Bull’s-Eye Maculopathy in Rheumatic Patients: A Case Report and Literature Review. Front. Immunol. 2024, 15, 1383343. [Google Scholar] [CrossRef]
- Ramser, B.; Kokot, A.; Metze, D.; Weiß, N.; Luger, T.A.; Böhm, M. Hydroxychloroquine Modulates Metabolic Activity and Proliferation and Induces Autophagic Cell Death of Human Dermal Fibroblasts. J. Investig. Dermatol. 2009, 129, 2419–2426. [Google Scholar] [CrossRef]
- de Gálvez, E.N.; Aguilera, J.; Solis, A.; de Gálvez, M.V.; de Andrés, J.R.; Herrera-Ceballos, E.; Gago-Calderon, A. The Potential Role of UV and Blue Light from the Sun, Artificial Lighting, and Electronic Devices in Melanogenesis and Oxidative Stress. J. Photochem. Photobiol. B Biol. 2022, 228, 112405. [Google Scholar] [CrossRef]
- Pérez González, L.A.; Martínez-Pascual, M.A.; Toledano-Macías, E.; Jara-Laguna, R.C.; Fernández-Guarino, M.; Hernández-Bule, M.L. Effect of Combination of Blue and Red Light with Terbinafine on Cell Viability and Reactive Oxygen Species in Human Keratinocytes: Potential Implications for Cutaneous Mycosis. Int. J. Mol. Sci. 2024, 25, 12145. [Google Scholar] [CrossRef]
- Hernández-Bule, M.L.; Trillo, M.A.; Cid, M.A.; Leal, J.; Ubeda, A. In Vitro Exposure to 0.57-MHz Electric Currents Exerts Cytostatic Effects in HepG2 Human Hepatocarcinoma Cells. Int. J. Oncol. 2007, 30, 583–592. [Google Scholar] [CrossRef][Green Version]
- Suitthimeathegorn, O.; Yang, C.; Ma, Y.; Liu, W. Direct and Indirect Effects of Blue Light Exposure on Skin: A Review of Published Literature. Ski. Pharmacol. Physiol. 2022, 35, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Banchelli, M.; Cherchi, F.; Coppi, E.; Fraccalvieri, M.; Rossi, M.; Tatini, F.; Pugliese, A.M.; Rossi Degl’Innocenti, D.; Alfieri, D.; et al. Experimental Study on Blue Light Interaction with Human Keloid-Derived Fibroblasts. Biomedicines 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Cherchi, F.; Banchelli, M.; Tatini, F.; Nardini, P.; Guasti, D.; Coppi, E.; Pugliese, A.M.; Fraccalvieri, M.; Bacci, S.; et al. Effects of Short-Wavelength Blue Light on Fibroblasts, Experimental Evidence in Wound Healing and Cutaneous Fibrosis. In Proceedings of the 2nd International Electronic Conference on Biomedicines, Online, 1–15 December 2023; p. 27. [Google Scholar]
- Magni, G.; Cherchi, F.; Banchelli, M.; Tatini, F.; Nardini, P.; Pugliese, A.M.; Guasti, D.; Bacci, S.; Cavigli, L.; Pini, R.; et al. Experimental Evidence on Photobiomodulation Induced by Short Wavelengths Blue LED Light. In Proceedings of the Mechanisms of Photobiomodulation Therapy XVIII; Carroll, J.D., Liebert, A., Lyons, J.-A., Eds.; SPIE: San Francisco, CA, USA, 2024; p. 7. [Google Scholar]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Bonina, F.; Cardile, V. Hydroxytyrosol from Olive Fruits Prevents Blue-light-induced Damage in Human Keratinocytes and Fibroblasts. J. Cell. Physiol. 2019, 234, 9065–9076. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J.Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Garza, Z.C.F.; Born, M.; Hilbers, P.A.J.; Van Riel, N.A.W.; Liebmann, J. Visible Blue Light Therapy: Molecular Mechanisms and Therapeutic Opportunities. CMC 2019, 25, 5564–5577. [Google Scholar] [CrossRef]
- McNish, H.; Mathapathi, M.S.; Figlak, K.; Damodaran, A.; Birch-Machin, M.A. The Effect of Blue Light on Mitochondria in Human Dermal Fibroblasts and the Potential Aging Implications. FASEB J. 2025, 39, e70675. [Google Scholar] [CrossRef]
- Habib, L.; Michael-Jubeli, R.; Abboud, M.; Lteif, R.; Tfayli, A. Impact of Blue Light on Cutaneous Barrier Structures and Properties: NPLC/HR-MSn and Raman Analyses. Analyst 2024, 149, 5693–5703. [Google Scholar] [CrossRef]
- Chamayou-Robert, C.; DiGiorgio, C.; Brack, O.; Doucet, O. Blue Light Induces DNA Damage in Normal Human Skin Keratinocytes. Photodermatol. Photoimmunol. Photomed. 2022, 38, 69–75. [Google Scholar] [CrossRef]
- Tang, S.-C.; Lu, C.-T.; Ko, J.-L.; Lin, C.-H.; Hsiao, Y.-P. Hydroxychloroquine Repairs Burn Damage through the Wnt/β-Catenin Pathway. Chem. Biol. Interact. 2023, 370, 110309. [Google Scholar] [CrossRef]
- Klouda, C.B.; Stone, W.L. Oxidative Stress, Proton Fluxes, and Chloroquine/Hydroxychloroquine Treatment for COVID-19. Antioxidants 2020, 9, 894. [Google Scholar] [CrossRef]
- Ekstein, S.F.; Hylwa, S. Sunscreens: A Review of UV Filters and Their Allergic Potential. Dermatitis 2023, 34, 176–190. [Google Scholar] [CrossRef]
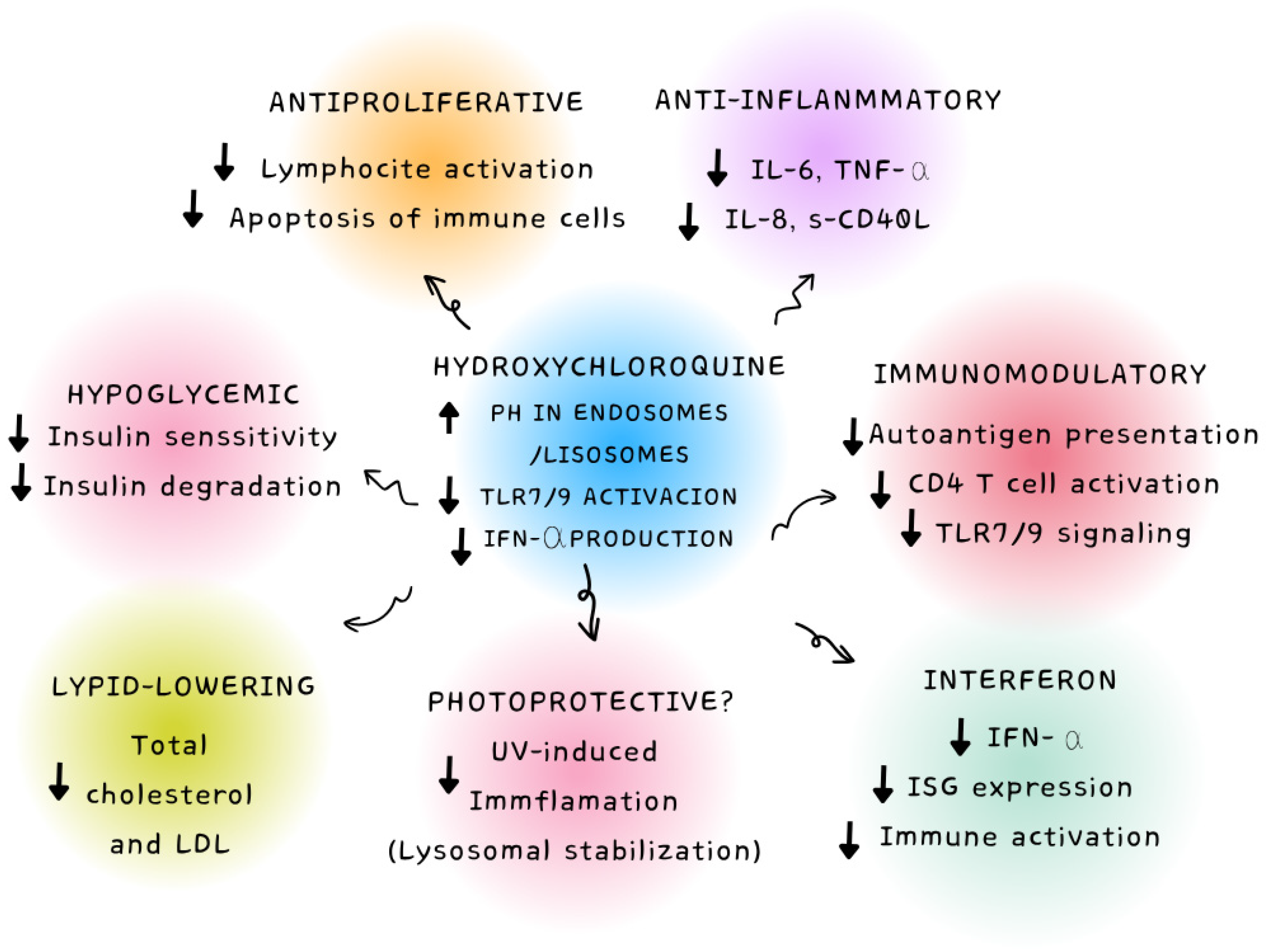


| Clinical Indication (HCQ) | Pharmacological/Clinical Rationale |
|---|---|
| Systemic lupus erythematosus (SLE) | Cornerstone therapy for mucocutaneous, musculoskeletal, and constitutional symptoms; reduces disease activity and flare rates. |
| Cutaneous lupus erythematosus | Effective in discoid and subacute cutaneous variants; reduces photosensitivity and cutaneous inflammation. |
| Rheumatoid arthritis (RA) | Conventional synthetic DMARD with immunomodulatory effects; indicated in mild-to-moderate disease often in combination therapy. |
| Primary Sjögren’s syndrome | Provides symptomatic relief for arthralgias, fatigue, and extraglandular features; limited impact on sicca symptoms. |
| Antiphospholipid syndrome (APS) | May confer antithrombotic and endothelial-protective effects; used adjunctively with anticoagulation. |
| Dermatomyositis/polymyositis (cutaneous forms) | Useful for photosensitive cutaneous lesions; minimal effect on muscle involvement. |
| Porphyria cutanea tarda | Low-dose regimens decrease hepatic porphyrin deposits and photosensitivity; alternative in patients unsuitable for phlebotomy. |
| Cutaneous sarcoidosis | May ameliorate granulomatous cutaneous lesions in refractory cases. |
| COVID-19 (investigational; not recommended) | Initially explored for antiviral and immunomodulatory potential; randomized trials show no clinical benefit—currently not recommended. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez González, L.A.; Pascual, M.A.M.; Toledano Macías, E.; Jara Laguna, R.C.; Fernández Guarino, M.; Bacci, S.; Naharro Rodriguez, J.; Hernández Bule, M.L. Photoprotective Effect of Hydroxychloroquine on Human Keratinocytes. Cosmetics 2025, 12, 213. https://doi.org/10.3390/cosmetics12050213
Pérez González LA, Pascual MAM, Toledano Macías E, Jara Laguna RC, Fernández Guarino M, Bacci S, Naharro Rodriguez J, Hernández Bule ML. Photoprotective Effect of Hydroxychloroquine on Human Keratinocytes. Cosmetics. 2025; 12(5):213. https://doi.org/10.3390/cosmetics12050213
Chicago/Turabian StylePérez González, Luis Alfonso, María Antonia Martínez Pascual, Elena Toledano Macías, Rosa Cristina Jara Laguna, Montserrat Fernández Guarino, Stefano Bacci, Jorge Naharro Rodriguez, and María Luisa Hernández Bule. 2025. "Photoprotective Effect of Hydroxychloroquine on Human Keratinocytes" Cosmetics 12, no. 5: 213. https://doi.org/10.3390/cosmetics12050213
APA StylePérez González, L. A., Pascual, M. A. M., Toledano Macías, E., Jara Laguna, R. C., Fernández Guarino, M., Bacci, S., Naharro Rodriguez, J., & Hernández Bule, M. L. (2025). Photoprotective Effect of Hydroxychloroquine on Human Keratinocytes. Cosmetics, 12(5), 213. https://doi.org/10.3390/cosmetics12050213








