Abstract
There is little scientific evidence for many of the medical benefits attributed to aromatherapy and neurocosmetics; however, they have been shown to be useful in the management of symptoms such as pain, nausea, general well-being, anxiety, depression, stress, and insomnia through various mechanisms, including the olfactory pathway and activation of TRPV and CBD receptors. This review therefore aims to compile the most relevant literature on active ingredients proven effective in neurocosmetics and aromatherapy, as well as the mechanisms responsible for their function, in order to highlight how they can be synergistically integrated into a new generation of multifunctional formulations forming the basis of neuro-functional skin care.
1. Introduction
Aesthetic beauty is the holistic interrelation of mind and body. Over the past few decades, the concept of beauty has taken a new course in which focus on physical appearance has given way to the notion of greater emotional well-being. This developing vision recognizes that the essence of beauty and skin health in the individual also depends upon emotional, neuropsychological, and sensory parameters [1,2,3].
Within this framework, neurocosmetics are defined as products capable of modulating the activity of the neuro-immuno-cutaneous system at the epidermal level. These products have gained increasing relevance as a transdisciplinary field of study, focused on the complex and dynamic biochemistry of skin–nerve interactions, and are establishing themselves as a cutting-edge area within current cosmetic science [4].
From a neurophysiological perspective, the skin is viewed as a very specialized neuroendocrine and immunological organ, closely linked with the central nervous system through a sophisticated network of sensory receptors, nerve endings, neuropeptides, and neurotransmitters.
This subtle system lets some active ingredients—such as biomimetic peptides, botanical extracts, or volatile compounds—target molecular pathways that not only modulate the key processes at work in the skin (such as inflammation, aging, and tissue renewal) but also affect emotional states by either calming stress and anxiety or fostering a general sense of well-being. In parallel, aromatherapy has attracted renewed interest from the scientific community, as it has the power to modulate the limbic system through odors [5,6,7,8].
The olfactory pathway has a unique anatomical connection to the limbic system, unlike other sensory systems that require prior relay through the thalamus. This distinctive feature allows olfactory stimuli—such as essential oils used in aromatherapy—to trigger immediate emotional and physiological responses (Figure 1). Structurally, this sensory pathway begins in the olfactory epithelium of the nasal cavity, where aromatic molecules bind to specific receptor proteins on olfactory neurons. These signals are transmitted via the olfactory nerve to the olfactory bulb and then through the olfactory tract to key brain regions within the limbic system, including the amygdala, piriform cortex, and entorhinal cortex. This intimate connection between smell, emotion, and memory explains why certain scents can evoke vivid recollections or elicit strong emotional states [8].
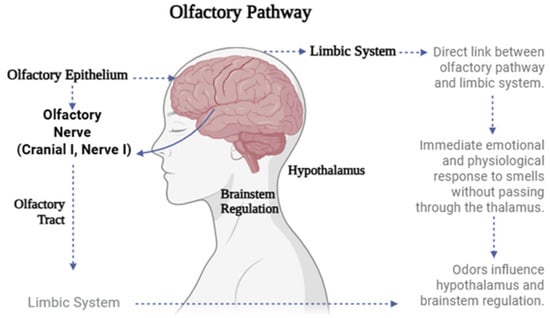
Figure 1.
Schematic representation of the olfactory pathway and its connection to the limbic system.
Several studies have shown that aromatherapy not only modulates brain activity but also exerts beneficial effects on mental health. For instance, a clinical trial conducted at the Jeju National University Hospital evaluated the impact of an essential oil massage program on neurobiological and psychological variables in women who were caregivers of children with attention-deficit/hyperactivity disorder (ADHD). Participants were randomly assigned to either an experimental group (n = 13), which received a 40 min massage with lavender and geranium essential oils twice a week for four weeks, or a control group with no treatment (n = 12). The results revealed a significant reduction in levels of anxiety, depression, and stress, accompanied by a decrease in salivary cortisol and an increase in plasma levels of brain-derived neurotrophic factor (BDNF). These findings suggest that the intervention exerted a regulatory effect on the hypothalamic–pituitary–adrenal (HPA) axis, as well as a potential neuroprotective mechanism mediated by olfactory stimulation with essential oils [9].
Olfactory stimulation has also been shown to influence various brain functions, particularly those related to cognition and mental flexibility. In a recent study, the effects of inhaling lavender essential oil on brain activity dynamics and cognitive performance were assessed in 20 healthy adults. Participants completed a set-shifting task under two conditions: without aromatic stimulation and with olfactory exposure to lavender oil. Using electroencephalographic (EEG) recordings and normalized power spectral density (PSD) analysis across five frequency bands, researchers observed that lavender inhalation significantly increased theta, alpha, and beta wave activity—associated with relaxation, focus, and cognitive agility—while delta wave activity, linked to unconscious states, was reduced. Behaviorally, participants demonstrated greater response accuracy and shorter reaction times, suggesting improved cognitive flexibility. These findings provide neurophysiological evidence for the potential of aromatherapy as a non-invasive strategy to enhance mental performance in contexts requiring adaptability and sustained attention [10,11].
Table 1 summarizes some essential oils used in aromatherapy, highlighting their main therapeutic functions, relevant active compounds, and corresponding references. This compilation reveals the remarkable diversity of chemical profiles and associated sensory and physiological effects of essential oils, supporting their increasing integration into neurocosmetic formulations.

Table 1.
Some essential oils used in aromatherapy, their main functions, and their active compounds.
Essential oils such as lavender (Entry 1), bergamot (Entry 12), ylang-ylang (Entry 10), neroli (Entry 29), and melissa (Entry 36) share anxiolytic and relaxing activity, generally attributed to the presence of monoterpenes such as linalool, linalyl acetate, or citral. These compounds have been shown to modulate central nervous system activity by acting on GABAergic and serotonergic receptors, which justifies their use for inducing calm, improving sleep, and reducing stress. In contrast, oils like peppermint (Entry 3), rosemary (Entry 8), basil (Entry 19), or cinnamon (Entry 27) exhibit stimulating and mentally clarifying effects due to components such as menthol, 1,8-cineole, or methyl chavicol. These molecules can enhance alertness, focus, and cognitive performance through noradrenergic and dopaminergic pathways.
Several oils present dual cutaneous and emotional functions, making them particularly attractive for neurocosmetic development. For example, jasmine (Entry 17) and rose (not listed in the table but commonly cited with geraniol and citronellol) not only induce euphoria and well-being but also possess anti-inflammatory and skin-regenerative properties mediated by compounds such as benzyl acetate or geraniol. Similarly, helichrysum (Entry 30) promotes skin regeneration, while turmeric (Entry 38) and ginger (Entry 24) have been explored for their neuroprotective potential, linked to their systemic antioxidant and anti-inflammatory effects.
It is important to highlight that several of these oils—such as thyme (Entry 22), oregano (Entry 23), tea tree (Entry 2), and cinnamon (Entry 27)—possess immunomodulatory or antimicrobial properties, enabling their use in cosmetics with protective or purifying functions. The synergy between sensory (olfactory) impact and topical activity reinforces the skin–brain axis hypothesis as a therapeutic target in modern neurocosmetics.
Finally, some oils, such as valerian (Entry 42), vetiver (not included in this table), or wintergreen (Entry 40), show specific applications in the management of pain, inflammation, or sleep disorders, extending their utility beyond the cosmetic realm and positioning them as potential adjuvants in the treatment of psychophysiological conditions.
Altogether, the functional and chemical diversity of essential oils supports their potential as multifunctional ingredients in neurocosmetic formulations—aimed not only at improving the skin’s physical appearance but also at eliciting positive emotional states through sensory and neurocutaneous pathways.
This multidimensional action of essential oils—combining emotional modulation with cutaneous effects—aligns with the growing understanding of the skin as more than a passive barrier. Instead, it functions as an active neuro-immuno-endocrine interface capable of perceiving, responding to, and even generating biochemical signals that influence both local and systemic physiology. This conceptual framework forms the foundation of neurocosmetics, where the interaction between the skin and the nervous system is leveraged to promote not only dermatological health but also emotional well-being through targeted sensory pathways.
1.1. The Skin as a Neuroendocrine–Sensory Organ: Functional Basis
The skin is not only a physical barrier, but it is a specialized neuroendocrine and immune organ with complex signaling to the central nervous system [143,144]. This network consists of peripheral nerve fibers and their endings, free nerve endings, and immunocompetent cells, together with molecular receptors for the transduction and detection of internal and external stimuli. Furthermore, several (epidermal) cell types, i.e., keratinocytes, melanocytes, and Langerhans cells, have the ability to synthesize and release neuromediators and neurohormones, like substance P, β-endorphin, gamma-aminobutyric acid (GABA), and melatonin. These compounds are integral to the maintenance of local balance in vital humoral reactions such as inflammation, tissue repair, pigmentation, nociception, and cutaneous comfort sensation [145,146,147].
1.2. Neurocutaneous Receptors and Their Functional Implications
The skin’s neuroactive functionality is supported by the expression of a wide range of specialized receptors that mediate responses to physicochemical and neurosensory stimuli. Among the most relevant are the following:
Transient Receptor Potential (TRP) Channels [148,149]: Transient Receptor Potential (TRP) channels are sensory ion channels in the skin that respond to physical and chemical stimuli like heat, cold, pH changes, and irritants. Found in keratinocytes, sensory neurons, melanocytes, and immune cells, they help regulate pain, itch, inflammation, pigmentation, and wound healing. Dysfunctions in these channels are linked to skin conditions such as dermatitis, vitiligo, alopecia, and chronic itch. Key TRP types include TRPV1 (heat, capsaicin), TRPA1 (cold, oxidative stress), TRPM8 (cold sensing), and TRPM1 (pigmentation). As mediators between environmental stimuli and skin responses, TRP channels are promising targets for dermatological and neurocosmetic treatments (Figure 2).
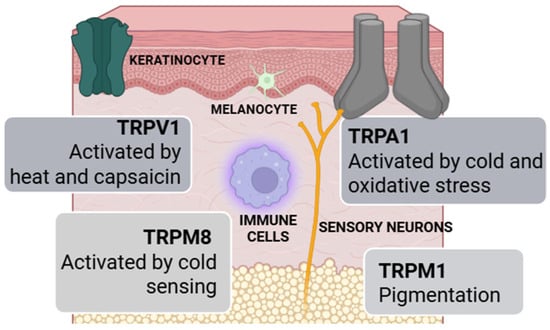
Figure 2.
Cellular distribution and functions of TRP channels in human skin.
Cannabinoid Receptors (CB1 and CB2) [150,151,152]: Involved in skin homeostasis, these receptors modulate crucial functions such as inflammation, nociception, sebum production, and immune regulation. Activation of these receptors by both phytocannabinoids and endogenous ligands has stimulated interest in the clinical use of cosmeceuticals.
Cannabinoid receptors CB1 and CB2 are the primary receptor components of the endocannabinoid system and belong to the family of G protein-coupled receptors (GPCRs), characterized by the presence of seven transmembrane domains. These receptors can be activated by both phytocannabinoids (such as Δ9-tetrahydrocannabinol [THC] and cannabidiol [CBD]) and endogenous ligands (anandamide and 2-arachidonoylglycerol [2-AG]), which has driven extensive research into their therapeutic potential, including applications in the field of cosmeceuticals.
CB1 receptors are mainly expressed in the central nervous system, although they have also been identified in peripheral tissues. Their activation is directly associated with the psychoactive effects of THC and is involved in the regulation of various physiological processes, including nociception, stress response, appetite, lipogenesis, and immune modulation. However, CB1 stimulation can also lead to adverse effects such as cognitive impairment, anxiety, and the potential for dependency.
In contrast, CB2 receptors are predominantly expressed in immune cells, including B lymphocytes, macrophages, NK cells, and microglia. Their activation significantly modulates inflammatory and immune responses without inducing psychotropic effects, making them an attractive therapeutic target for autoimmune diseases, neuroinflammatory disorders, and transplant rejection prevention.
In the skin, both receptors have been shown to play an active role in maintaining cutaneous homeostasis by modulating key processes such as inflammation, peripheral nociception, sebum production, and local immune responses. The functional presence of these receptors in skin cells has sparked growing interest in developing cosmeceutical formulations that leverage their regulatory effects for the treatment of dermatological conditions and the promotion of skin health.
Figure 3 presents a schematic comparison of CB1 and CB2 cannabinoid receptors, highlighting their cellular localization and key physiological functions. The CB1 receptor is primarily located in the central and peripheral nervous systems, including the skin, where it mediates the psychoactive effects of THC and regulates pain, stress, inflammation, nociceptive perception, and sebum production. Conversely, the CB2 receptor is predominantly expressed in immune cells such as B lymphocytes, NK cells, macrophages, and microglia, where it contributes to immune response modulation, inflammation control, and physiological processes related to transplant response.
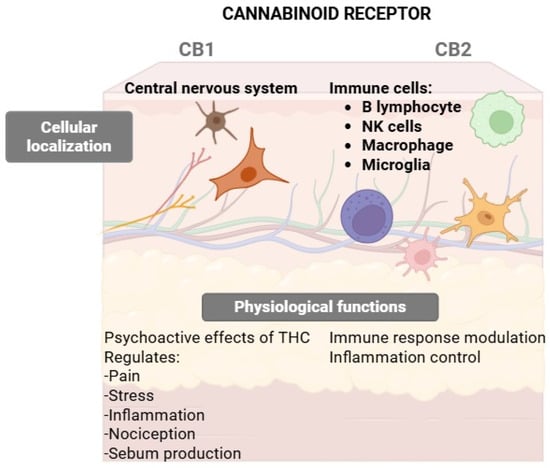
Figure 3.
CB1 vs. CB2 Receptors: localization and functional roles in skin and immune cells.
This differential distribution suggests complementary roles for both receptors in neuroimmune-cutaneous homeostasis, positioning the endocannabinoid system as a relevant therapeutic target in both dermatology and immunomodulation.
Serotonergic and Dopaminergic Receptors [153,154,155]: Both expressed in keratinocytes and melanocytes, it is speculated that they could be involved in cell proliferation, pigmentation, and mood regulation by the use of the same signaling cascades used by the nervous system.
In recent years, an integrative view of the skin has emerged, recognizing it as a highly specialized neuro-immuno-endocrine organ, capable of perceiving, processing, and responding to environmental stimuli through a complex network of cellular communication. Within this network, melanocytes are no longer regarded solely as pigment-producing cells but rather as multifunctional neuroectodermal sensors. These cells not only synthesize melanin for photoprotection but also secrete neurotransmitters, neuropeptides, and hormones and express ectopic sensory systems similar to those found in the retina and olfactory mucosa. Through these capabilities, melanocytes actively participate in light detection, local circadian rhythm regulation, inflammation, wound healing, and epidermal immune responses [153].
In parallel, a functional intrinsic serotoninergic/melatoninergic system has been identified in the skin, operating independently of the central axis, with the capacity to synthesize and metabolize these neuromodulators locally. Key enzymes such as TPH1, AANAT, and HIOMT have been detected in melanocytes, keratinocytes, fibroblasts, and cutaneous tumor cells. Moreover, the presence of specific receptors for serotonin (5-HT1A, 2A, 2B, 7) and melatonin (MT1) in various skin layers suggests a complex signaling system that regulates proliferation, pigmentation, inflammation, and oxidative stress. Functional evidence from cell and animal models has demonstrated that these compounds modulate the hair cycle, skin homeostasis, and the response to environmental stressors, consolidating their relevance as promising targets for new therapeutic and cosmetic interventions [154].
Simultaneously, dopamine—classically known for its role in central neurotransmission—has emerged as a significant immunomodulator in both the central nervous system and peripheral tissues, including the skin. Various immune cells express dopaminergic receptors and enzymes involved in dopamine synthesis, degradation, and transport, enabling them to respond through autocrine and paracrine mechanisms. Depending on the receptor subtype activated, dopamine may either promote or inhibit inflammatory processes, influencing functions such as phagocytosis, cytokine secretion, and cell migration. These context-dependent actions position dopamine as a key regulator at the neuroimmune interface, and its dysregulation may contribute to inflammatory, neurodegenerative, and autoimmune diseases. Investigating its role in the cutaneous environment represents an emerging avenue for understanding skin pathophysiology and developing novel therapeutic strategies [156].
Cutaneous Olfactory Receptors [5,157,158,159,160]: Recently found on epidermal cells, these receptors allow the perception of volatile molecules applied to the skin; the process is uncoupled from the nasal olfactory system. Activation of these channels elicits intracellular responses that may modulate regeneration, inflammation, and sensation.
Taken together, the skin emerges as a bona fide peripheral sensory organ that participates in emotional cognition of and reaction to the environment. This physiological model forms the scientific basis for the effect of neuroactive actives in high-tech cosmetics.
Olfactory systems in animals have evolved a sophisticated architecture to detect, discriminate, and interpret a vast diversity of chemical signals that are fundamental to survival and social behavior. In both insects and mammals, olfactory receptor neurons (ORNs)—which typically express one or a few specific receptors—project their axons toward highly organized glomeruli within structures such as the antennal lobe or olfactory bulb, where sensory processing begins through mechanisms such as lateral inhibition, non-linear amplification, and interneuron-mediated modulation [161]. This processing results in spatiotemporal neural representations that are decoded by higher brain centers, including the piriform cortex and mushroom bodies, enabling an efficient combinatorial coding that integrates memory, emotion, and behavior. In parallel, the concept of “olfactory spaces” has been proposed to link the chemical structure of odorants with their neural and perceptual correlates, opening new avenues for modeling and predicting olfactory perception.
Complementarily, recent studies have revealed that olfactory receptors (ORs), beyond their traditional sensory function, are ectopically expressed in non-olfactory tissues such as the skin, gut, immune system, and central nervous system (CNS), where they fulfill specific physiological roles [159]. In the CNS, ORs have been detected in neurons, astrocytes, and microglia within neurogenic niches like the dentate gyrus of the hippocampus and the subventricular zone, where they may modulate neuronal differentiation, synaptic plasticity, and cell survival. In vitro assays using odorants such as β-ionone and sandalore have shown activation of specific ORs, such as OR2AT4, leading to enhanced cell proliferation, migration, and differentiation.
Furthermore, olfactory ensheathing glia (OEG)—which naturally support regeneration of the olfactory epithelium—have demonstrated the ability to promote axonal growth, angiogenesis, and remyelination in spinal cord injury models.
It has also been proposed that ectopic ORs can be activated by a broad range of endogenous ligands, including short-chain fatty acids, microbiota-derived metabolites, and steroid hormones, expanding their role as functional sensors in diverse physiopathological contexts [158]. The interaction of ORs with other signaling proteins, such as β-adrenergic receptors, suggests a complex network of intracellular regulation with potential therapeutic applications.
Collectively, current evidence supports the study of ectopic olfactory receptors as key elements in neuroregenerative processes and highlights their potential as molecular targets for the treatment of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and spinal cord injuries.
1.3. Neuroactive Ingredients
Neurocosmetic actives act on specific receptors present on the skin, modifying sensory and physiological responses. These bioactive chemicals can act on pathways associated with inflammation, neurosensory perception, sebum production, pigmentation, and tissue repair. Their effect is not just limited to the skin, since they participate in the modulation of emotional states via skin–brain communication [162].
In recent years, the development of neurocosmetics has transformed the traditional concept of skin care, promoting an integrative approach that considers both cutaneous physiology and the individual’s emotional state. This emerging category of cosmetic products arises from the recognition that the skin is not merely a physical barrier but a highly sensory neuroendocrine and immunological organ, capable of perceiving, processing, and responding to environmental stimuli. Its common embryological origin with the nervous system explains the complex bidirectional communication network between the skin and the brain, known as the skin–brain axis.
The skin contains numerous neurosensory receptors, including TRPV1, serotoninergic, dopaminergic, and melatoninergic receptors, as well as POMC-derived peptides such as ACTH, α-MSH, and β-endorphins. These systems play crucial roles in regulating processes such as inflammation, pigmentation, itching, sensitivity, and stress response [163]. When these pathways are disrupted—such as during chronic stress, aging, or persistent inflammation—visible skin dysfunctions may occur. Neurocosmetic strategies aim to restore this balance through specific molecular mechanisms.
One of the most relevant targets is the TRPV1 channel, which is involved in neurosensory sensitivity, inflammation, and thermally induced aging. Its modulation helps soothe sensitive skin and reduce redness. Another key target is the enzyme 11β-HSD1, which converts cortisone into cortisol and contributes to structural skin damage. Inhibiting 11β-HSD1 reduces local cortisol overload, improving skin firmness and elasticity.
Recent advances in skin biology have highlighted the importance of neuroendocrine signaling and longevity-associated pathways in maintaining skin homeostasis and delaying visible aging. Among the central molecular regulators, FOXO (Forkhead box O) and KLOTHO have emerged as critical players in cellular repair, oxidative stress resistance, and tissue longevity. FOXO transcription factors promote DNA repair and antioxidant defense, while KLOTHO exerts anti-inflammatory, anti-senescent, and metabolic effects. Together, they contribute synergistically to skin resilience and healthy aging [164].
Simultaneously, the activation of the POMC (proopiomelanocortin) pathway has gained recognition for its role in the neurocutaneous axis. Stimulation of this pathway induces the release of β-endorphins, endogenous opioids that bind to skin receptors and enhance the perception of comfort and well-being. This dual mechanism—structural protection via FOXO/KLOTHO and sensory modulation via β-endorphins—underscores the integrative potential of neurocosmetics, which aim to address both the physiological and emotional dimensions of skin health.
A wide variety of active ingredients have demonstrated efficacy through in vitro, ex vivo, and even clinical studies, consolidating the scientific foundation of this new generation of multifunctional topical products.
Table 2 presents representative examples of ingredients used in neurocosmetics, along with their molecular targets, physiological effects on the skin, and their perceived sensory or emotional impact. These actives range from biotechnological compounds and biomimetic peptides to essential oils and natural molecules, all capable of modulating specific receptors in the skin or the peripheral nervous system. This interaction results not only in objective skin improvements—such as reduced inflammation, regulated sebum production, enhanced microcirculation, or a strengthened epidermal barrier—but also in measurable subjective sensory effects, including relaxation, freshness, comfort, or mood enhancement. This multidimensional approach reflects the emerging paradigm of functional neurocosmetics, which aims to act simultaneously on the skin–brain–emotion axis to promote holistic well-being.
The table offers an integrated view of how different neuroactive ingredients act on well-characterized molecular receptors. For instance, palmitoyl tetrapeptide-7 targets inflammatory pathways, reducing the expression of proinflammatory cytokines, which improves skin firmness and induces a sense of relief and comfort. Similarly, niacinamide interacts with receptors such as GPR109A and immunological pathways, enhancing the skin barrier, decreasing hyperpigmentation, and alleviating discomfort in sensitive skin.
Another notable example is cannabidiol (CBD), which binds to CB1 and CB2 cannabinoid receptors to regulate sebum production, reduce inflammation, and produce emotionally relaxing effects. Menthol, through the activation of TRPM8 ion channels, generates an immediate sensation of freshness and local relief, while capsaicin, acting on TRPV1, stimulates microcirculation and produces a warming, revitalizing effect.
Botulinum-like peptides, designed to mimic botulinum toxin’s action at neuromuscular junctions, visibly reduce expression lines and induce a subjective sensation of facial relaxation—highly valued in anti-aging treatments.
Likewise, natural ingredients such as ylang-ylang and lavender essential oils act via the olfactory pathway, modulating the limbic system: the former with antioxidant and toning properties, and the latter promoting deep relaxation and improved sleep quality through GABA receptor interactions.
Citral-rich compounds, found in lemon or verbena essential oils, also act on olfactory receptors associated with the limbic system, evoking sensations of optimism, vitality, and freshness, while providing toning and revitalizing effects on the skin. Lastly, enkephalins—small endogenous opioid peptides—bind to cutaneous opioid receptors, delivering pain relief, increased comfort, and a pleasant sensory experience that fosters emotional well-being.
Together, these examples illustrate the wide array of mechanisms by which neurocosmetic actives can modulate both skin functions and neurosensory responses. This biological-perceptual synergy forms the foundation of modern neurocosmetics, capable of addressing both physical and emotional well-being through innovative topical formulations.
The use of these functional ingredients has enabled the development of neurocosmetics indicated for sensitive, aging, inflamed, or pigmented skin. Notably, clinical and preclinical studies have demonstrated benefits such as reduction in signs of cutaneous stress, restoration of barrier function, increased sensory resilience, improved skin texture and tone, and enhanced subjective perceptions of comfort and well-being.
However, this innovative approach also poses significant regulatory challenges. According to European Regulation (EC) No. 1223/2009, a cosmetic product must be intended for application to the external parts of the human body, and its effects must remain limited to surface-level actions, without significantly altering physiological functions [165]. Nevertheless, many neurocosmetics interact with molecular pathways that could be interpreted as pharmacological, creating ambiguity regarding their classification. As a result, the category of “borderline products” has emerged—those whose nature may fall between cosmetic, medicinal, or medical device, depending on application site, absorption route, active ingredient concentration, and mechanism of action [166].
To address this issue, the European Commission has published the Borderline Products Manual, a guiding document to help determine on a case-by-case basis whether a product qualifies as a cosmetic. It relies on three criteria: type of product, site of application, and intended cosmetic purpose. For example, an anti-wrinkle product will be considered a cosmetic if it acts on external appearance, but not if it significantly alters physiological functions like collagen synthesis through pharmacological pathways. In the case of neurocosmetics, this distinction is especially relevant, as many ingredients act at the dermal or neurosensory level, requiring detailed evaluation and strong scientific evidence to support their claims.
Moreover, international regulation remains heterogeneous: while Europe has clear regulatory frameworks, in other markets such as the United States or China, classification of these products is still uncertain, which limits their global positioning.

Table 2.
Examples of neurocosmetic actives and their cutaneous/sensory effects.
Table 2.
Examples of neurocosmetic actives and their cutaneous/sensory effects.
| Entry | Neurocosmetic Active | Receptor/Target | Cutaneous Effect | Emotional/Sensory Effect | References |
|---|---|---|---|---|---|
| 1 | Palmitoyl tetrapeptide-7 | Cytokine/pro-inflammatory receptors | Reduces inflammation, improves firmness | Sensation of relief and comfort | [167] |
| 2 | Niacinamide | GPR109A, immune receptors | Enhances skin barrier, reduces hyperpigmentation | Decreases discomfort in sensitive skin | [168] |
| 3 | Cannabidiol (CBD) | Cannabinoid receptors CB1, CB2 | Regulates sebum, anti-inflammatory | Promotes relaxation, reduces anxiety | [169] |
| 4 | Menthol | TRPM8 ion channels | Cooling, soothing effect | Induces freshness, immediate local relief | [170] |
| 5 | Capsaicin | TRPV1 ion channels | Stimulates microcirculation, enhances skin tone | Mild warming sensation, invigorating effect | [171,172,173] |
| 6 | Botulinum-like peptides | Neuronal receptors at neuromuscular junction | Smoothes expression lines | Sensation of facial relaxation and skin smoothing | [162,174,175] |
| 7 | Ylang-ylang essential oil | Limbic system via olfactory route | Antioxidant, skin toning properties | Promotes relaxation, stress relief | [53,176,177] |
| 8 | Lavender essential oil | GABA receptors/limbic system | Calms irritation, improves sleep quality | Deep relaxation, anxiety reduction | [178,179,180] |
| 9 | Citral compounds | Olfactory/limbic receptors | Brightens and revitalizes skin | Enhances mood, induces optimistic and energizing sensations | [181,182,183] |
| 10 | Enkephalins | Cutaneous opioid receptors | Pain relief, increases skin comfort | Pleasant sensation, supports general emotional well-being | [184,185,186,187] |
These neuroactive ingredients serve as a model for which state-of-the-art cosmetic compositions can address cutaneous health and emotional status for functional, sensorially driven skin care. In this context, their potential to act via topical-/olfactory-mediated administration also broadens their utility in holistic formulations that aim to moderate the skin–brain axis.
1.4. Biomimetic Peptides: An Advanced Molecular Strategy in Neurocosmetics
This review will focus, among other relevant actives of neurocosmetics, on biomimetic peptides, which can modulate in an anodyne way the crucial cellular processes, imitating properly the natural function of the growth factor [167,188,189,190]. These synthetic oligopeptides, usually 10–15 amino acids in length, possess several advantages over natural peptides, such as increased chemical stability, low molecular weight, superior skin penetration, low toxicity, and lower cost of production.
In cosmetic dermatology, biomimetic peptides find broad use in anti-aging, depigmenting, hair-growth-stimulating, and anti-inflammatory formulations [191,192]. They work by fighting against the degradation of the extracellular matrix, which is caused by intrinsic (e.g., oxidative stress and cellular metabolism) and extrinsic factors (e.g., UV radiation and air pollution).
Biomimetic peptides can be functionally divided into four main groups (Figure 1):
- Signaling peptides: Encourage the production of collagen and elastin and other extracellular matrix (ECM) proteins.
- Carrier peptides: Enhance the penetration of vital trace elements (copper and manganese).
- Enzyme inhibitory peptides: Inhibit enzymes that break down the extracellular matrix.
- Neurotransmitter-inhibiting peptides: Reduce the action of facial muscle contraction, also helping to diminish dynamic wrinkles.
The latter group (neurotransmitter-inhibiting peptides) includes compounds such as acetyl hexapeptide-3 (also known as Argireline®) and acetyl octapeptide-3 (also known as Snap-8), since they block acetylcholine release by hindering the formation of the SNARE complex and therefore all work at the same mechanism of botulinum toxins but with a better safety profile [188,193]. Others, like Vialox® or Syn-Ake® [194], act through antagonizing the postsynaptic receptors, whereas opioid-like peptides like Leuphasyl® [195] and Peptilift® [192] induce muscle relaxation through stimulation of cutaneous opioid receptors.
Altogether, biomimetic peptides are bridging a critical gap in the cosmetic industry, enabling the rational design of next-generation products with targeted activity at both structural and neurosensory levels. However, some of these compounds still require independent clinical validation to confirm their individual efficacy within complex, multi-ingredient formulations.
Table 3 compiles a representative selection of cosmetic peptides currently used in commercial formulations, highlighting their INCI names, amino acid sequences (when available), molecular targets, and associated emotional or sensory effects. These peptides act primarily through modulation of neuroreceptors, cellular signaling pathways, and extracellular matrix (ECM) components, contributing not only to anti-aging and skin repair effects but also to neurocosmetic outcomes such as relaxation, comfort, or muscle relaxation. Their mechanisms of action include SNARE complex interference (Entries 1, 5, 6), TGF-β signaling (Entries 2, 4, 12), neuromodulation of acetylcholine or neuropeptides (Entries 3, 15, 16), and interaction with ECM-related proteins (Entries 7–10). Some peptides, such as Ac-SDKP (Entry 12) and Copper Tripeptide-1 (Entry 13), also demonstrate anti-inflammatory or neurotrophic activity, reinforcing their dual functionality in both cutaneous and emotional modulation. This convergence of dermatological efficacy and sensory perception modulation supports the growing relevance of peptides in neurocosmetic science.

Table 3.
Bioactive peptides used in cosmetic formulations and their neurocosmetic potential.
The versatility of these biomimetic peptides positions them as key elements in modern cosmetic innovation. Their high molecular specificity, efficacy at low concentrations, and excellent skin tolerance make them especially valuable in formulations for sensitive, aging, or neuroreactive skin. Moreover, their capacity to function as peripheral neuromodulators or sensory messengers opens the door to a new generation of functional cosmetic products—ones that aim not only to enhance external appearance but also to promote emotional and sensory balance.
Thus, biomimetic peptides represent an advanced tool for designing integrative cosmetic treatments that combine science, perception, and well-being.
1.5. Challenges of Neurocosmetics and Essential Oils
These findings support the emergence of neurocosmetics and aromatherapy as pioneering therapeutic strategies that simultaneously target skin health and emotional balance through the modulation of the skin–brain axis. Both approaches interact with neurocutaneous and neurosensory receptors, although through distinct routes: neurocosmetic actives act topically via cutaneous receptors—such as TRP channels, cannabinoid receptors, and serotonergic pathways—whereas aromatic compounds exert their effects through the olfactory system, ultimately modulating central structures like the limbic system (Figure 4).
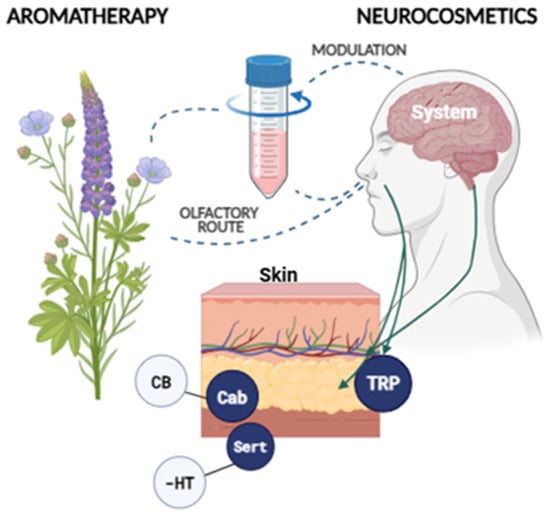
Figure 4.
Olfactory pathway linking aromatherapy and neurocosmetics.
However, despite their appeal as an emerging field aimed at modulating both emotional and cutaneous responses via topically applied neuroactive ingredients, neurocosmetics still face significant scientific, technological, and clinical limitations that hinder their full integration into evidence-based dermatology.
Firstly, most neurocosmetic formulations remain at the preclinical or early clinical trial stage, with limited validation in human subjects through robust methodologies. Much of the current evidence is derived from in vitro models or animal studies, restricting translational applicability. Additionally, human clinical trials frequently rely on subjective outcome measures such as self-reported mood, stress perception, or quality-of-life indices, introducing variability and complicating the establishment of clear causal links between active ingredients and psychophysiological effects.
Moreover, the inherently subjective nature of emotional responses, combined with the pronounced placebo effect commonly observed in cosmetic studies, further complicates result interpretation. Interindividual variability in neurocutaneous signaling, skin type, microbiome composition, emotional baseline, and sensory sensitivity also challenges the generalizability of findings across diverse populations.
From a methodological perspective, a notable gap exists in the integration of objective biomarkers of emotional state—such as salivary cortisol, heart rate variability, or functional neuroimaging—into neurocosmetic research. The absence of unified regulatory frameworks and international consensus guidelines for evaluating psychodermatological outcomes contributes to inconsistencies in validation and commercialization standards.
Finally, the effective transdermal delivery of neuroactive ingredients remains a critical formulation challenge, as many compounds possess physicochemical properties that limit epidermal penetration. Innovations such as controlled-release systems, nanocarriers, or lipid-based delivery vehicles are needed to ensure adequate bioavailability at neurocutaneous receptor sites.
From a safety standpoint, although fatal outcomes from dermal absorption of essential oils have not been documented to date, cases of non-fatal systemic toxicity have been reported. The most common adverse effects are photosensitization and contact dermatitis. Given the uncertain true incidence of these reactions and the wide variability in published estimates, essential oils should be used cautiously, adhering to safe concentration ranges and minimizing cumulative exposure [223,224,225].
Altogether, these limitations underscore the urgent need for longitudinal, multicenter studies employing rigorous methodological designs and incorporating multimodal assessments and validated psychophysiological biomarkers. Only then will it be possible to definitively establish the efficacy, safety, and therapeutic relevance of neurocosmetics in modern dermatological practice and neuroscience-based functional skincare.
2. Conclusions
The convergence of neurocosmetics and aromatherapy represents a promising frontier in functional skincare, offering a dual approach that targets both the physiological and emotional dimensions of cutaneous health. As scientific understanding of the skin–brain axis deepens, it becomes increasingly evident that the skin is not only a sensory interface but also an active participant in neuroendocrine and immune signaling. By leveraging bioactive compounds—whether topically applied or olfactorily delivered—formulations can modulate neurosensory pathways, promote homeostasis, and evoke perceptible emotional benefits such as relaxation, comfort, and improved mood.
This review underscores the potential of neuroactive ingredients, including biomimetic peptides, essential oils, cannabinoids, and neurotransmitter modulators, to influence skin structure, sensory perception, and emotional well-being through defined molecular targets such as TRP channels, CB1/CB2, serotonin and dopamine receptors, and cutaneous opioid systems. Furthermore, the integration of aromatherapeutic compounds via olfactory-limbic stimulation offers a complementary mechanism for enhancing psychodermatological outcomes. Table 4 provides a concise glossary of the key terms.

Table 4.
Short glossary of key terms.
However, despite encouraging preclinical and emerging clinical evidence, significant challenges remain—particularly regarding standardized methodologies, objective biomarker validation, and regulatory classification. Future progress will depend on robust interdisciplinary research, longitudinal human trials, and the development of advanced delivery systems that ensure efficacy and safety.
Ultimately, neurocosmetics and aromatherapy embody a shift toward a more holistic, evidence-based paradigm in cosmetics—one that embraces not only the aesthetic and structural integrity of the skin but also the subjective and emotional experience of the user. This integrative approach holds great promise for the development of next-generation cosmetic formulations that promote both skin health and emotional resilience.
Author Contributions
M.J.S.-P.: conceptualization, writing—review and editing. O.M.-C.: supervision, editing, visualization. J.A.R.-L.: investigation, writing—original draft, validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The author thanks DMX Dermocosméticos for their support and motivation in composing this review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Armstrong, T.; Detweiler-Bedell, B. Beauty as an Emotion: The Exhilarating Prospect of Mastering a Challenging World. Rev. Gen. Psychol. 2008, 12, 305–329. [Google Scholar] [CrossRef]
- Haase, F.-A. Beauty and Esthetics. Meanings of an Idea and Concept of the Senses. An Introduction to an Esthetic Communication Concept Facing the Perspectives of Its Theory, History, and Cultural Traditions of the Beautiful; Universität Tübingen: Tübingen, Germany, 2008. [Google Scholar]
- Démuthová, S.; Selecká, L.; Démuth, A. Human Facial Attractiveness in Psychological Research; Peter Lang Verlag: Berlin, Germany, 2019; ISBN 978-3-631-80097-3. [Google Scholar]
- Haykal, D.; Berardesca, E.; Kabashima, K.; Dréno, B. Beyond Beauty: Neurocosmetics, the Skin-Brain Axis, and the Future of Emotionally Intelligent Skincare. Clin. Dermatol. 2025, 43, 523–527. [Google Scholar] [CrossRef]
- Eissa, M.E. Olfactory interventions for sleep enhancement: A review. Univers. J. Pharm. Res. 2024, 9, 51–58. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Quintero-Rincón, P.; Olivero-Verbel, J. Aromatherapy and Essential Oils: Holistic Strategies in Complementary and Alternative Medicine for Integral Wellbeing. Plants 2025, 14, 400. [Google Scholar] [CrossRef]
- Reddy, B.H.V.; Hussain, S.M.S.; Hussain, M.S.; Kumar, R.N.; Gupta, J. Essential Oils in Cosmetics: Antioxidant Properties and Advancements through Nanoformulations. Pharmacol. Res.-Nat. Prod. 2025, 6, 100192. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Hatvate, N.T.; Naren, P.; Khan, S.; Chavda, V.P.; Balar, P.C.; Gandhi, J.; Khatri, D.K. Essential Oils for Clinical Aromatherapy: A Comprehensive Review. J. Ethnopharmacol. 2024, 330, 118180. [Google Scholar] [CrossRef]
- Wu, J.-J.; Cui, Y.; Yang, Y.-S.; Kang, M.-S.; Jung, S.-C.; Park, H.K.; Yeun, H.-Y.; Jang, W.J.; Lee, S.; Kwak, Y.S.; et al. Modulatory Effects of Aromatherapy Massage Intervention on Electroencephalogram, Psychological Assessments, Salivary Cortisol and Plasma Brain-Derived Neurotrophic Factor. Complement. Ther. Med. 2014, 22, 456–462. [Google Scholar] [CrossRef]
- Afghan, R.; Heysieattalab, S.; Zangbar, H.S.; Ebrahimi-Kalan, A.; Jafari-Koshki, T.; Samadzadehaghdam, N. Lavender Essential Oil Inhalation Improves Attentional Shifting and Accuracy: Evidence from Dynamic Changes of Cognitive Flexibility and Power Spectral Density of Electroencephalogram Signals. J. Med. Signals Sens. 2024, 14, 12. [Google Scholar] [CrossRef]
- Soares, G.A.B.e.; Bhattacharya, T.; Chakrabarti, T.; Tagde, P.; Cavalu, S. Exploring Pharmacological Mechanisms of Essential Oils on the Central Nervous System. Plants 2022, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring Pharmacological Mechanisms of Lavender (Lavandula angustifolia) Essential Oil on Central Nervous System Targets. Front. Pharmacol. 2017, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, H.; Mardani, A.; Basirinezhad, M.H.; Hamidzadeh, A.; Eskandari, F. The Effects of Lavender and Chamomile Essential Oil Inhalation Aromatherapy on Depression, Anxiety and Stress in Older Community-Dwelling People: A Randomized Controlled Trial. EXPLORE 2022, 18, 272–278. [Google Scholar] [CrossRef]
- Emadikhalaf, M.; Ghods, A.A.; Sotodeh-asl, N.; Mirmohamadkhani, M.; Vaismoradi, M. Effects of Rose and Lavender Scents on Nurses’ Job Stress: A Randomized Controlled Trial. EXPLORE 2023, 19, 371–375. [Google Scholar] [CrossRef]
- Angulo, S.M.; Occhieppo, V.B.; Moya, C.; Crespo, R.; Bregonzio, C. Anxiolytic-like Effect Characterization of Essential Oil from Local Lavender Cultivation. Pharmaceuticals 2025, 18, 624. [Google Scholar] [CrossRef]
- Chambali, Z.A.S.P.; Algristian, H. Lavender Essential Oil as an Adjuvant Therapy for Anxiety Disorders. J. Health Lit. Qual. Res. 2025, 5, 32–50. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Iahtisham-Ul-Haq; Khan, S.; Sohail, M.; Iqbal, M.J.; Awan, K.A.; Nayik, G.A. Chapter 20-Tea Tree Essential Oil. In Essential Oils; Nayik, G.A., Ansari, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 479–500. ISBN 978-0-323-91740-7. [Google Scholar]
- Baraka, A.A.E. Effect of Peppermint Aroma on Physiological Parameters of Mechanically Ventilated Patients: Randomized Placebo Controlled Trial. Clin. Epidemiol. Glob. Health 2025, 33, 102009. [Google Scholar] [CrossRef]
- Cetin, N.; Kose, G.; Gokbel, A. Examining the Effect of Peppermint Oil on Postoperative Nausea After Cervical Surgery. J. Neurosci. Nurs. 2024, 56, 203. [Google Scholar] [CrossRef] [PubMed]
- Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horčinová Sedláčková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.P.; Lipok, J. Mentha Piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products. Molecules 2023, 28, 7444. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint Essential Oil: Its Phytochemistry, Biological Activity, Pharmacological Effect and Application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Lau, B.K.; Karim, S.; Goodchild, A.K.; Vaughan, C.W.; Drew, G.M. Menthol Enhances Phasic and Tonic GABAA Receptor-Mediated Currents in Midbrain Periaqueductal Grey Neurons. Br. J. Pharmacol. 2014, 171, 2803–2813. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Okello, E.; Chazot, P.; Howes, M.-J.; Ohiomokhare, S.; Jackson, P.; Haskell-Ramsay, C.; Khan, J.; Forster, J.; Wightman, E. Volatile Terpenes and Brain Function: Investigation of the Cognitive and Mood Effects of Mentha × Piperita L. Essential Oil with In Vitro Properties Relevant to Central Nervous System Function. Nutrients 2018, 10, 1029. [Google Scholar] [CrossRef] [PubMed]
- Surbhi; Kumar, A.; Singh, S.; Kumari, P.; Rasane, P. Eucalyptus: Phytochemical Composition, Extraction Methods and Food and Medicinal Applications. Adv. Tradit. Med. 2023, 23, 369–380. [Google Scholar] [CrossRef]
- Adesina, A.A.; Dona, D.U.; Wheto, B.M.; Lamina, B.R. Modelling of the Effect of Pretreatment Methods, and Time on the Yield of Eucalyptus Essential Oil Using Response Surface Methodology. Eur. J. Appl. Sci. Eng. Technol. 2025, 3, 287–294. [Google Scholar] [CrossRef]
- Qneibi, M.; Bdir, S.; Maayeh, C.; Bdair, M.; Sandouka, D.; Basit, D.; Hallak, M. A Comprehensive Review of Essential Oils and Their Pharmacological Activities in Neurological Disorders: Exploring Neuroprotective Potential. Neurochem. Res. 2024, 49, 258–289. [Google Scholar] [CrossRef] [PubMed]
- Akinyede, K.A.; Oyewusi, H.A.; Oladipo, O.O.; Tugbobo, O.S.; Akinyede, K.A.; Oyewusi, H.A.; Oladipo, O.O.; Tugbobo, O.S. Essential Oils and Their Antioxidant Importance: The In Vitro and In Vivo Treatment and Management of Neurodegenerative Diseases with New Delivery Applications. In Essential Oils-Recent Advances, New Perspectives and Applications; IntechOpen: London, UK, 2023; ISBN 978-0-85014-205-1. [Google Scholar]
- Agarwal, P.; Sebghatollahi, Z.; Kamal, M.; Dhyani, A.; Shrivastava, A.; Singh, K.K.; Sinha, M.; Mahato, N.; Mishra, A.K.; Baek, K.-H. Citrus Essential Oils in Aromatherapy: Therapeutic Effects and Mechanisms. Antioxidants 2022, 11, 2374. [Google Scholar] [CrossRef]
- Komori, T.; Fujiwara, R.; Tanida, M.; Nomura, J. Potential Antidepressant Effects of Lemon Odor in Rats. Eur. Neuropsychopharmacol. 1995, 5, 477–480. [Google Scholar] [CrossRef]
- A Systematic Review of the Therapeutic Properties of Lemon Essential Oil-ClinicalKey. Available online: https://www.clinicalkey.es/#!/content/playContent/1-s2.0-S2212958824001356?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2212958824001356%3Fshowall%3Dtrue&referrer= (accessed on 3 July 2025).
- Xiao, Z.; Li, Q.; Niu, Y.; She, Y.; Sun, Z.; Zhang, J.; Wang, Z.; Zhou, R.; Zhu, J. Mechanism of the Interaction between Olfactory Receptors and Characteristic Aroma Compounds in Sweet Orange Juice. LWT 2024, 207, 116660. [Google Scholar] [CrossRef]
- Nascimento, J.C.; dos S.Gonçalves, V.S.; Souza, B.R.S.; de C. Nascimento, L.; de Carvalho, B.M.R.; Nogueira, P.C.L.; Santos, J.P.S.; Borges, L.P.; Goes, T.C.; de Souza, J.B.; et al. Effectiveness of Aromatherapy with Sweet Orange Oil (Citrus sinensis L.) in Relieving Pain and Anxiety during Labor. EXPLORE 2025, 21, 103081. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Wang, L.; Li, F.; Weschler, L.B.; Huang, J.; Zhang, Y. Potential Benefits of Short-Term Indoor Exposure to Sweet Orange Essential Oil for Relaxation during Mental Work Breaks. J. Build. Eng. 2023, 78, 107602. [Google Scholar] [CrossRef]
- Erdal, S.; Harman Özdoğan, M.; Yildirim, D.; Kuni, A.; Selçuk, S.; Güneri, A.; Arslan, E.N. Effects of Orange Oil Aromatherapy on Pain and Anxiety During Invasive Interventions in Patients with Hematopoietic Stem Cell Transplants. J. Infus. Nurs. 2024, 47, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhu, J.; Niu, Y.; Zhang, J.; Xiao, Z.; Zhao, L. Identification of Characteristic Compounds of Sweet Orange Oil and Their Sweetening Effects on the Sucrose Solution with Sweetness Meter, Sensory Analysis, Electronic Tongue, and Molecular Dynamics Simulation. Food Chem. 2024, 461, 140815. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Sheu, S.-R.; Hsu, S.-C.; Tsai, Y.-H. Determination of Bactericidal Efficacy of Essential Oil Extracted from Orange Peel on the Food Contact Surfaces. Food Control 2010, 21, 1710–1715. [Google Scholar] [CrossRef]
- Valduga, A.T.; Gonçalves, I.L.; Magri, E.; Delalibera Finzer, J.R. Chemistry, Pharmacology and New Trends in Traditional Functional and Medicinal Beverages. Food Res. Int. 2019, 120, 478–503. [Google Scholar] [CrossRef]
- Pourshaikhian, M.; Moghadamnia, M.T.; Kazemnezhad Leyli, E.; Shafiei Kisomi, Z. Effects of Aromatherapy with Matricaria Chamomile Essential Oil on Anxiety and Hemodynamic Indices in Patients with Acute Coronary Syndrome, 2021: A Randomized Controlled Trial. BMC Complement. Med. Ther. 2024, 24, 17. [Google Scholar] [CrossRef]
- Bahrami, F.; Hanifi, N.; Mardani, A. Comparison of the Effects of Aromatherapy with Damask Rose and Chamomile Essential Oil on Preoperative Pain and Anxiety in Emergency Orthopedic Surgery: A Randomized Controlled Trial. J. Perianesth. Nurs. 2024, 39, 583–588. [Google Scholar] [CrossRef]
- Deepa, Y.; Vijay, A.; Nivethitha, L.; Nandhakumar, G.; Sathiya, S.; Mooventhan, A. Effects of Chamomile Oil Inhalation on Sleep Quality in Young Adults with Insomnia: A Randomized Controlled Trial. Int. J. Psychiatry Med. 2024, 60, 533–542. [Google Scholar] [CrossRef]
- Saadatmand, S.; Zohroudi, F.; Tangestani, H. The Effect of Oral Chamomile on Anxiety: A Systematic Review of Clinical Trials. Clin. Nutr. Res. 2024, 13, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Alahmady, N.F.; Alkhulaifi, F.M.; Abdullah Momenah, M.; Ali Alharbi, A.; Allohibi, A.; Alsubhi, N.H.; Ahmed Alhazmi, W. Biochemical Characterization of Chamomile Essential Oil: Antioxidant, Antibacterial, Anticancer and Neuroprotective Activity and Potential Treatment for Alzheimer’s Disease. Saudi J. Biol. Sci. 2024, 31, 103912. [Google Scholar] [CrossRef]
- Yeddes, W.; Ouerghemmi, I.; Hammami, M.; Gadhoumi, H.; Affes, T.G.; Nait Mohamed, S.; Aidi-Wannes, W.; Witrowa-Rajchert, D.; Saidani-Tounsi, M.; Nowacka, M. Optimizing the Method of Rosemary Essential Oils Extraction by Using Response Surface Methodology (RSM)-Characterization and Toxicological Assessment. Sustainability 2022, 14, 3927. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Therapeutic Effects of Rosemary (Rosmarinus officinalis L.) and Its Active Constituents on Nervous System Disorders. Iran. J. Basic. Med. Sci. 2020, 23, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Xu, L.; Qiao, M.; Wang, Y. The Anti-Depression Effect and Mechanism of Harmonious Rosemary Essential Oil and Its Application in Microcapsules. Mater. Today Bio 2025, 31, 101546. [Google Scholar] [CrossRef]
- Bengana, K.; Serseg, T.; Benarous, K.; Mermer, A.; Şirin, Y.; Kaouka, A. Antilipase Activities of Cultivated Peppermint and Rosemary Essential Oils: In Vitro and in Silico Studies. Turk. J. Biol. 2025, 49, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Chetouani, M.; Arabi, M.; Belasri, L.; Mharchi, S.; Alaoui, K. Effect of Salt Stress on the Essential Oil Content of Rosemary at Juvenile and Adult Stages Under Greenhouse Conditions. E3S Web Conf. 2025, 632, 03005. [Google Scholar] [CrossRef]
- Khan, F.; Rashan, L. Phytochemical Analysis and Pharmaceutical Applications of Monoterpenoids Present in the Essential Oil of Boswellia sacra (Omani Luban). Adv. Pharmacol. Pharm. Sci. 2025, 2025, 3536898. [Google Scholar] [CrossRef]
- Ng, F.; Thong, A.; Basri, N.; Wu, W.; Chew, W.; Dharmawan, J. Profiling of Aroma-Active Compounds in Ylang-Ylang Essential Oils by Aroma Extract Dilution Analysis (AEDA) and Chemometric Methods. J. Agric. Food Chem. 2022, 70, 260–266. [Google Scholar] [CrossRef]
- Mrani, S.A.; Zejli, H.; Azzouni, D.; Fadili, D.; Alanazi, M.M.; Hassane, S.O.S.; Sabbahi, R.; Kabra, A.; Moussaoui, A.E.; Hammouti, B.; et al. Chemical Composition, Antioxidant, Antibacterial, and Hemolytic Properties of Ylang-Ylang (Cananga odorata) Essential Oil: Potential Therapeutic Applications in Dermatology. Pharmaceuticals 2024, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
- Karabatak, N.; Gök, B.; Budama-kılınc, Y. Development of Nanoemulsion Formulation Containing Ylang Ylang Essential Oil for Topical Applications, Evaluation of In Vitro Cytotoxicity and ADMET Profile. J. Turk. Chem. Soc. Sect. Chem. 2024, 11, 1181–1196. [Google Scholar] [CrossRef]
- Alam, P.; Imran, M.; Ali, A.; Majid, H. Cananga Odorata (Ylang-Ylang) Essential Oil Containing Nanoemulgel for the Topical Treatment of Scalp Psoriasis and Dandruff. Gels 2024, 10, 303. [Google Scholar] [CrossRef]
- Tan, L.T.H.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Kadir, H.A.; Chan, K.G.; Goh, B.H. Traditional Uses, Phytochemistry, and Bioactivities of Cananga odorata (Ylang-Ylang). Evid.-Based Complement. Altern. Med. 2015, 2015, 896314. [Google Scholar] [CrossRef]
- Sharma, K.; Lanzilotto, A.; Yakubu, J.; Therkelsen, S.; Vöegel, C.D.; Du Toit, T.; Jørgensen, F.S.; Pandey, A.V. Effect of Essential Oil Components on the Activity of Steroidogenic Cytochrome P450. Biomolecules 2024, 14, 203. [Google Scholar] [CrossRef]
- Faris, A.; Edder, Y.; Louchachha, I.; Lahcen, I.A.; Azzaoui, K.; Hammouti, B.; Merzouki, M.; Challioui, A.; Boualy, B.; Karim, A.; et al. From Himachalenes to Trans-Himachalol: Unveiling Bioactivity through Hemisynthesis and Molecular Docking Analysis. Sci. Rep. 2023, 13, 17653. [Google Scholar] [CrossRef]
- Bozova, B.; Gölükcü, M.; Giuffrè, A.M. The Effect of Different Hydrodistillation Times on the Composition and Yield of Bergamot (Citrus bergamia Risso) Peel Essential Oil and a Comparison of the Cold-Pressing Method. Flavour. Fragr. J. 2024, 39, 263–270. [Google Scholar] [CrossRef]
- Chang, J.; Yang, H.; Shan, X.; Zhao, L.; Li, Y.; Zhang, Z.; Abankwah, J.K.; Zhang, M.; Bian, Y.; Guo, Y. Bergamot Essential Oil Improves CUMS-Induced Depression-like Behaviour in Rats by Protecting the Plasticity of Hippocampal Neurons. J. Cell. Mol. Med. 2024, 28, e18178. [Google Scholar] [CrossRef]
- Zhu, M.-Y.; Dong, W.-Y.; Guo, J.-R.; Huang, J.-Y.; Cheng, P.-K.; Yang, Y.; Liu, A.; Yang, X.-L.; Zhu, X.; Zhang, Z.; et al. A Neural Circuit for Bergamot Essential Oil-Induced Anxiolytic Effects. Adv. Sci. 2025, 12, 2406766. [Google Scholar] [CrossRef]
- Gogoi, R.; Begum, T.; Sarma, N.; Tamang, R.; Chanda, S.K.; Lal, M.; Perveen, K.; Khan, F.; Marinković, J. Pelargonium graveolens L., (Geranium) Essential Oil from Northeast India: Chemical Composition, Pharmacology and Genotoxicity Study. J. Essent. Oil Bear. Plants 2024, 27, 135–151. [Google Scholar] [CrossRef]
- Pandur, E.; Major, B.; Rák, T.; Sipos, K.; Csutak, A.; Horváth, G. Linalool and Geraniol Defend Neurons from Oxidative Stress, Inflammation, and Iron Accumulation in In Vitro Parkinson’s Models. Antioxidants 2024, 13, 917. [Google Scholar] [CrossRef]
- Topor, G.; Buzia, O.D.; Huzum, R.M.; Maftei, M.I.; Monica, D.; Serban, C. Dermopreparations with antiacneic action. formulation and pharmacotechnical evaluation. Rom. J. Oral Rehabil. 2024, 16, 629–643. [Google Scholar] [CrossRef]
- Hashimoto, M.; Takahashi, K.; Unno, T.; Ohta, T. Linalyl Acetate Exerts Analgesic Effects by Inhibiting Nociceptive TRPA1 in Mice. Biomed. Res. 2024, 45, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Grigore-Gurgu, L.; Dumitrașcu, L.; Aprodu, I. Aromatic Herbs as a Source of Bioactive Compounds: An Overview of Their Antioxidant Capacity, Antimicrobial Activity, and Major Applications. Molecules 2025, 30, 1304. [Google Scholar] [CrossRef] [PubMed]
- Anjali; Garg, D.; Pragi; Kumar, V.; Dimple; Monika, R. A Review of Therapeutic Properties and Uses of Salvia Officinalis: Review Article. J. Pharma Insights Res. 2024, 2, 146–154. [Google Scholar] [CrossRef]
- Weshahi, H.A.; Akhtar, M.S.; Tobi, S.S.A.; Hossain, A.; Khan, S.A.; Akhtar, A.B.; Said, S.A. Evaluation of Acute Plant Toxicity, Antioxidant Activity, Molecular Docking and Bioactive Compounds of Lemongrass Oil Isolated from Omani Cultivar. Toxicol. Rep. 2025, 14, 101888. [Google Scholar] [CrossRef]
- Hotimah, U.H.; Rahmadhena, M.P. The Effectiveness of Lemongrass Aromatherapy in Reducing Nausea and Vomiting in Pregnant Women. Indones. J. Glob. Health Res. 2024, 6, 95–102. [Google Scholar] [CrossRef]
- Khasanah, L.U.; Praseptiangga, D.; Purwanto, E.; Ariviani, S. Bioactive Components and Bioactivity of Essential Oils, Hydrosol and Water Steam Distillation Solvents of Lemongrass Leaves (Cymbopogon citratus). IOP Conf. Ser. Earth Environ. Sci. 2024, 1377, 012059. [Google Scholar] [CrossRef]
- Tazi, A.; Zinedine, A.; Rocha, J.M.; Errachidi, F. Review on the Pharmacological Properties of Lemongrass (Cymbopogon citratus) as a Promising Source of Bioactive Compounds. Pharmacol. Res.-Nat. Prod. 2024, 3, 100046. [Google Scholar] [CrossRef]
- Nour, A.H.; Modather, R.H.; Yunus, R.M.; Elnour, A.A.M.; Ismail, N.A. Characterization of Bioactive Compounds in Patchouli Oil Using Microwave-Assisted and Traditional Hydrodistillation Methods. Ind. Crops Prod. 2024, 208, 117901. [Google Scholar] [CrossRef]
- Singh, S.; Agrawal, N. Exploring the Pharmacological Potential and Bioactive Components of Pogostemon cablin (Blanco) Benth, Traditional Chinese Medicine. Pharmacol. Res.-Mod. Chin. Med. 2024, 10, 100382. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, N.; Chanotiya, C.S.; Lal, R.K. The Pharmacological Potential and the Agricultural Significance of the Aromatic Crop Patchouli (Pogostemon cablin Benth.): A Review. Ecol. Front. 2024, 44, 1109–1118. [Google Scholar] [CrossRef]
- Varaprasad Rao, V.; Rajitha, T.; Ankatwar, G. Role of Jasmine Flowers in Stress Relief and Advantages and Disadvantages of Jasmine Plants. IJFMR-Int. J. Multidiscip. Res. 2025, 7, 2582-2160. [Google Scholar] [CrossRef]
- Rescigno, A.; Zucca, P.; Peddio, S.; Srikanth, S.; Kaushik, N.P.; Kumar, N.V.A.; Leyva-Gómez, G.; Kregiel, D.; Abu-Reidah, I.M.; Sen, S.; et al. Harnessing Jasminum Bioactive Compounds: Updated Insights for Therapeutic and Food Preservation Innovations. Food Front. 2025, 6, 1093–1128. [Google Scholar] [CrossRef]
- Feng, P.; Chen, J.; Chen, X.; Tang, M.; Song, N.; Zhang, L.; He, T. Comparing Effects of Aromatherapy with Five Herbs Essential Oils on PCPA-Induced Insomnia Mice. J. Microbiol. Biotechnol. 2025, 35, e2409021. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.R.O. Aromatherapy Today: A Science of Integration and Evidence-Based Practice. Braz. J. Health Aromather. Essent. Oil 2025, 2, bjhae20. [Google Scholar] [CrossRef]
- Yan, X.; David, S.D.; Du, G.; Li, W.; Liang, D.; Nie, S.; Ge, M.; Wang, C.; Qiao, J.; Li, Y.; et al. Biological Properties of Sandalwood Oil and Microbial Synthesis of Its Major Sesquiterpenoids. Biomolecules 2024, 14, 971. [Google Scholar] [CrossRef] [PubMed]
- El Hachlafi, N.; Benkhaira, N.; Mssillou, I.; Touhtouh, J.; Aanniz, T.; Chamkhi, I.; El Omari, N.; Khalid, A.; Abdalla, A.N.; Aboulagras, S.; et al. Natural Sources and Pharmacological Properties of Santalenes and Santalols. Ind. Crops Prod. 2024, 214, 118567. [Google Scholar] [CrossRef]
- Kang, S.; Li, S.; Han, S.; Peng, L. Microencapsulation of Sandalwood Essential Oil with Melamine Formaldehyde and β-Cyclodextrin: Analysis of Study on Aroma Profile Components and Antibacterial Properties. ChemistrySelect 2025, 10, e202405908. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, D.-S.; Jo, S.M.; Yoon, S.; Jeong, H.; Yoon, M.Y.; Kim, J.K.; Kim, Y.J.; Shin, E.-C. Exploration of Basil (Ocimum basilicum) Essential Oil Profiles Using E-Nose and GC–MS Combined with GC-O and Inhalation Effects on the Human EEG Topography and Tomography (s-LORETA) and Blood Pressure. J. Funct. Foods 2024, 112, 105918. [Google Scholar] [CrossRef]
- Mulugeta, S.M.; Gosztola, B.; Radácsi, P. Diversity in Morphology and Bioactive Compounds among Selected Ocimum Species. Biochem. Syst. Ecol. 2024, 114, 104826. [Google Scholar] [CrossRef]
- Ezeorba, T.P.C.; Chukwuma, I.F.; Asomadu, R.O.; Ezeorba, W.F.C.; Uchendu, N.O. Health and Therapeutic Potentials of Ocimum Essential Oils: A Review on Isolation, Phytochemistry, Biological Activities, and Future Directions. J. Essent. Oil Res. 2024, 36, 271–290. [Google Scholar] [CrossRef]
- Dmitriev, L.B.; Dmitrieva, V.L.; Trukhachev, V.I.; Sushkova, L.O. Biologically Active Substances of Plants of the Cupressaceae Family of the Genus Thuya and the Genus Juniperus for Phyto- and Aromatherapy. BIO Web Conf. 2024, 82, 01012. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Chen, R.; Huang, J.; Yu, P.; Wei, Q.; Wang, Y.; Jin, Q. Function Analysis of Essential Oils as Environmental Scents for Improving Undergraduate Students Emotional State. Ind. Crops Prod. 2025, 232, 121200. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Lin, C.-L.; Chang, L.-Y. The Effects of Aromatherapy Massage on Sleep Quality of Nurses on Monthly Rotating Night Shifts. Evid.-Based Complement. Altern. Med. 2017, 2017, 3861273. [Google Scholar] [CrossRef]
- Amaghnouje, A.; Mechchate, H.; Es-safi, I.; Alotaibi, A.A.; Noman, O.M.; Nasr, F.A.; Al-zharani, M.; Cerruti, P.; Calarco, A.; EL Fatemi, H.; et al. Anxiolytic, Antidepressant-Like Proprieties and Impact on the Memory of the Hydro-Ethanolic Extract of Origanum majorana L. on Mice. Appl. Sci. 2020, 10, 8420. [Google Scholar] [CrossRef]
- Jung, H.-N.; Choi, H.-J. Effects of Origanum Majorana Essential Oil Aroma on the Electroencephalograms of Female Young Adults with Sleep Disorders. J. Life Sci. 2012, 22, 1077–1084. [Google Scholar] [CrossRef]
- Hammoudi Halat, D.; Krayem, M.; Khaled, S.; Younes, S. A Focused Insight into Thyme: Biological, Chemical, and Therapeutic Properties of an Indigenous Mediterranean Herb. Nutrients 2022, 14, 2104. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Sheikhlar, A.; Raissy, M.; Chaharmahali, F.H.; Maneepitaksanti, W.; Faheem, M.; Van Doan, H. The Effects of Dietary Thyme Oil (Thymus vulgaris) Essential Oils for Common Carp (Cyprinus carpio): Growth Performance, Digestive Enzyme Activity, Antioxidant Defense, Tissue and Mucus Immune Parameters, and Resistance against Aeromonas Hydrophila. Aquac. Nutr. 2022, 2022, 7942506. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Deng, H.; Deng, Y.; Song, T.; Pang, L.; Zhu, S.; Ren, Z.; Guo, H.; Xu, Z.; Zhu, L.; Geng, Y.; et al. Evaluation of the Activity and Mechanisms of Oregano Essential Oil against PRV in Vivo and in Vitro. Microb. Pathog. 2024, 194, 106791. [Google Scholar] [CrossRef] [PubMed]
- Thao, C.B.; Tran, T.T.; Tran, T.K.N.; Mai, H.C. Extraction and Volatile Compounds in Ginger Essential Oil (Zingiber officinale Roscoe) at Laboratory Scale. Asian J. Chem. 2023, 35, 3066–3070. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Abd El-Kareem, M.S.M.; Rabbih, M.A.; Rashad, A.M.; EL-Hefny, M. Essential Oils from Fennel Plants as Valuable Chemical Products: Gas Chromatography–Mass Spectrometry, FTIR, Quantum Mechanical Investigation, and Antifungal Activity. Biomass Convers. Biorefin. 2025, 15, 9173–9191. [Google Scholar] [CrossRef]
- Naaz, S.; Ahmad, N.; Qureshi, M.I.; Hashmi, N.; Akhtar, M.S.; A Khan, M.M. Antimicrobial and Antioxidant Activities of Fennel Oil. Bioinformation 2022, 18, 795–800. [Google Scholar] [CrossRef]
- Abdullah; Alam, W.; Hussain, Y.; Ahmad, S.; Khan, F.; Ali, A.; Khan, H. Chapter 12-Neuroprotective Effect of Essential Oils. In Phytonutrients and Neurological Disorders; Khan, H., Aschner, M., Mirzaei, H., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 305–333. ISBN 978-0-12-824467-8. [Google Scholar]
- Zhang, L.-L.; Chen, Y.; Li, Z.-J.; Li, X.; Fan, G. Bioactive Properties of the Aromatic Molecules of Spearmint (Mentha spicata L.) Essential Oil: A Review. Food Funct. 2022, 13, 3110–3132. [Google Scholar] [CrossRef]
- Chávez-Delgado, E.L.; Gastélum-Estrada, A.; Pérez-Carrillo, E.; Ramos-Parra, P.A.; Estarrón-Espinosa, M.; Reza-Zaldívar, E.E.; Hernández-Brenes, C.; Mora-Godínez, S.; de los Santos, B.E.; Guerrero-Analco, J.A.; et al. Bioactive Properties of Spearmint, Orange Peel, and Baby Sage Oleoresins Obtained by Supercritical CO2 Extraction and Their Integration into Dark Chocolate. Food Chem. 2025, 463, 141306. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, X.; Tian, Y.; Yan, S.; Liu, J.; Xie, J.; Zhang, F.; Yao, C.; Hao, E. Therapeutic Potential of Cinnamon Oil: Chemical Composition, Pharmacological Actions, and Applications. Pharmaceuticals 2024, 17, 1700. [Google Scholar] [CrossRef]
- Nguyen, L.T.H.; Nguyen, N.P.K.; Tran, K.N.; Shin, H.-M.; Yang, I.-J. Anxiolytic-like Effect of Inhaled Cinnamon Essential Oil and Its Main Component Cinnamaldehyde in Animal Models. Molecules 2022, 27, 7997. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R. Effects of Essential Oils on Central Nervous System: Focus on Mental Health. Phytother. Res. 2021, 35, 657–679. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Wasef, L.; Teibo, J.O.; Shaheen, H.M.; Zakariya, A.M.; Akinfe, O.A.; Teibo, T.K.A.; Al-kuraishy, H.M.; Al-Garbee, A.I.; Alexiou, A.; et al. Commiphora Myrrh: A Phytochemical and Pharmacological Update. Naunyn. Schmiedebergs Arch. Pharmacol. 2023, 396, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Hanus, L.O.; Rezanka, T.; Dembitsky, V.M.; Moussaieff, A. Myrrh--Commiphora Chemistry. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2005, 149, 3–27. [Google Scholar] [CrossRef]
- Cao, B.; Wei, X.-C.; Xu, X.-R.; Zhang, H.-Z.; Luo, C.-H.; Feng, B.; Xu, R.-C.; Zhao, S.-Y.; Du, X.-J.; Han, L.; et al. Seeing the Unseen of the Combination of Two Natural Resins, Frankincense and Myrrh: Changes in Chemical Constituents and Pharmacological Activities. Molecules 2019, 24, 3076. [Google Scholar] [CrossRef] [PubMed]
- Scandurra, C.; Mezzalira, S.; Cutillo, S.; Zapparella, R.; Statti, G.; Maldonato, N.M.; Locci, M.; Bochicchio, V. The Effectiveness of Neroli Essential Oil in Relieving Anxiety and Perceived Pain in Women during Labor: A Randomized Controlled Trial. Healthcare 2022, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Kang, P.; Lee, H.S.; Seol, G.H. Effects of Inhalation of Essential Oil of Citrus aurantium L. var. amara on Menopausal Symptoms, Stress, and Estrogen in Postmenopausal Women: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2014, 2014, 796518. [Google Scholar] [CrossRef]
- Borba, C.A.; Fernandes, G.V.; Campos, J.C.; da Silva, T.B.; Gonzaga, R.V. Potential Action on the Central Nervous System of Neroli Oil Extracted from Citrus Aurantium. Res. Soc. Dev. 2021, 10, e418101321447. [Google Scholar] [CrossRef]
- Glumac, M.; Jažo, Z.; Paštar, V.; Golemac, A.; Čikeš Čulić, V.; Bektić, S.; Radan, M.; Carev, I. Chemical Profiling and Bioactivity Assessment of Helichrysum italicum (Roth) G. Don. Essential Oil: Exploring Pure Compounds and Synergistic Combinations. Molecules 2023, 28, 5299. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Cruciani, S.; Garroni, G.; Sarais, G.; Kavak, F.F.; Satta, R.; Montesu, M.A.; Floris, M.; Ventura, C.; Maioli, M. Effect of Helichrysum italicum in Promoting Collagen Deposition and Skin Regeneration in a New Dynamic Model of Skin Wound Healing. Int. J. Mol. Sci. 2024, 25, 4736. [Google Scholar] [CrossRef]
- Silori, G.K.; Kushwaha, N.; Kumar, V. Essential Oils from Pines: Chemistry and Applications. In Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Malik, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 275–297. ISBN 978-3-030-16546-8. [Google Scholar]
- Nikolic, M.; Andjic, M.; Bradic, J.; Kocovic, A.; Tomovic, M.; Samanovic, A.M.; Jakovljevic, V.; Veselinovic, M.; Capo, I.; Krstonosic, V.; et al. Topical Application of Siberian Pine Essential Oil Formulations Enhance Diabetic Wound Healing. Pharmaceutics 2023, 15, 2437. [Google Scholar] [CrossRef]
- Chandharakool, S.; Koomhin, P.; Sinlapasorn, J.; Suanjan, S.; Phungsai, J.; Suttipromma, N.; Songsamoe, S.; Matan, N.; Sattayakhom, A. Effects of Tangerine Essential Oil on Brain Waves, Moods, and Sleep Onset Latency. Molecules 2020, 25, 4865. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Capdevila, L. Aromatherapy Improves Work Performance Through Balancing the Autonomic Nervous System. J. Altern. Complement. Med. 2017, 23, 214–221. [Google Scholar] [CrossRef]
- Dyer, J.; Cleary, L.; McNeill, S.; Ragsdale-Lowe, M.; Osland, C. The Use of Aromasticks to Help with Sleep Problems: A Patient Experience Survey. Complement. Ther. Clin. Pract. 2016, 22, 51–58. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef]
- Park, J.-S. Effects of Juniper Essential Oil on the Activity of Autonomic Nervous System. Biomed. Sci. Lett. 2017, 23, 286–289. [Google Scholar] [CrossRef]
- Albrecht, U.W.; Madisch, A. Therapeutic Potentials Associated with Biological Properties of Juniper Berry Oil (Juniperus communis L.) and Its Therapeutic Use in Several Diseases—A Review. Bioact. Compd. Health Dis. 2022, 5, 174–185. [Google Scholar] [CrossRef]
- Sugawara, Y.; Hino, Y.; Kawasaki, M.; Hara, C.; Tamura, K.; Sugimoto, N.; Yamanishi, Y.; Miyauchi, M.; Masujima, T.; Aoki, T. Alteration of Perceived Fragrance of Essential Oils in Relation to Type of Work: A Simple Screening Test for Efficacy of Aroma. Chem. Senses 1999, 24, 415–421. [Google Scholar] [CrossRef]
- Dilrukshi, E.A.C.; Nishiyama, Y.; Ito, K.; Nomura, S. Alleviation of Acute Stress Response by Black Pepper Aroma Administration. J. Physiol. Anthropol. 2024, 43, 3. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar, A.; Sachan, N.; Chandra, P. Anxiolytic and Antidepressant-like Effects of Essential Oil from the Fruits of Piper nigrum Linn. (Black Pepper) in Mice: Involvement of Serotonergic but Not GABAergic Transmission System. Heliyon 2021, 7, e06884. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Azam, S.; Kim, I.-S.; Choi, D.-K. Neuroprotective Effects of Black Pepper and Its Bioactive Compounds in Age-Related Neurological Disorders. Aging Dis. 2023, 14, 750–777. [Google Scholar] [CrossRef]
- Sadraei, H.; Ghannadi, A.; Malekshahi, K. Relaxant Effect of Essential Oil of Melissa officinalis and Citral on Rat Ileum Contractions. Fitoterapia 2003, 74, 445–452. [Google Scholar] [CrossRef]
- Mathews, I.M.; Eastwood, J.; Lamport, D.J.; Cozannet, R.L.; Fanca-Berthon, P.; Williams, C.M. Clinical Efficacy and Tolerability of Lemon Balm (Melissa officinalis L.) in Psychological Well-Being: A Review. Nutrients 2024, 16, 3545. [Google Scholar] [CrossRef]
- Lotfi, A.; Mohtashami, J.; Khangholi, Z.A.; Shirmohammadi-Khorram, N. The Efficacy of Aromatherapy with Lemon Balm (Melissa officinalis L.) on Sleep Quality in Cardiac Patients: A Randomized Controlled Trial. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Alimoradi, Z.; Jafari, E.; Abdi, F.; Griffiths, M.D. Therapeutic Applications of Lemon Balm (Melissa officinalis) for Obstetrics and Gynecological Health Issues: A Systematic Review. J. Herb. Med. 2023, 42, 100751. [Google Scholar] [CrossRef]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Fantasma, F.; Samukha, V.; Aliberti, M.; Colarusso, E.; Chini, M.G.; Saviano, G.; De Felice, V.; Lauro, G.; Casapullo, A.; Bifulco, G.; et al. Essential Oils of Laurus nobilis L.: From Chemical Analysis to In Silico Investigation of Anti-Inflammatory Activity by Soluble Epoxide Hydrolase (sEH) Inhibition. Foods 2024, 13, 2282. [Google Scholar] [CrossRef]
- Xia, X.; Cheng, G.; Pan, Y.; Xia, Z.H.; Kong, L.D. Behavioral, Neurochemical and Neuroendocrine Effects of the Ethanolic Extract from Curcuma longa L. in the Mouse Forced Swimming Test. J. Ethnopharmacol. 2007, 110, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.F.; Kong, L.D.; Chen, Y. Antidepressant Activity of Aqueous Extracts of Curcuma longa in Mice. J. Ethnopharmacol. 2002, 83, 161–165. [Google Scholar] [CrossRef]
- Jaiswal, S.G.; Naik, S.N. Turmeric Oil: Composition, Extraction, Potential Health Benefits and Other Useful Applications. Avicenna J. Med. Biochem. 2021, 9, 93–106. [Google Scholar] [CrossRef]
- Sayowan, W.; Siripornpanich, V.; Piriyapunyaporn, T.; Hongratanaworakit, T.; Kotchabhakdi, N.; Ruangrungsi, N. The Harmonizing Effects of Citronella Oil on Mood States and Brain Activities. J. Health Res. 2012, 26, 69–75. [Google Scholar]
- Kasmirah; Zulliati; Kusvitasari, H.; Yuliantie, P. Effectiveness of Citronella Oil Aromatherapy in Enhancing Appetite among Stunted Toddlers. Health Sci. Int. J. 2025, 3, 1–14. [Google Scholar] [CrossRef]
- Batubara, I.; Suparto, I.H.; Sa’diah, S.; Matsuoka, R.; Mitsunaga, T. Effects of Inhaled Citronella Oil and Related Compounds on Rat Body Weight and Brown Adipose Tissue Sympathetic Nerve. Nutrients 2015, 7, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Cuchet, A.; Jame, P.; Anchisi, A.; Schiets, F.; Oberlin, C.; Lefèvre, J.-C.; Carénini, E.; Casabianca, H. Authentication of the Naturalness of Wintergreen (Gaultheria Genus) Essential Oils by Gas Chromatography, Isotope Ratio Mass Spectrometry and Radiocarbon Assessment. Ind. Crops Prod. 2019, 142, 111873. [Google Scholar] [CrossRef]
- Michel, P.; Olszewska, M.A. Phytochemistry and Biological Profile of Gaultheria Procumbens L. and Wintergreen Essential Oil: From Traditional Application to Molecular Mechanisms and Therapeutic Targets. Int. J. Mol. Sci. 2024, 25, 565. [Google Scholar] [CrossRef]
- da Silva, F.R.O.; Ripardo, N.A.; de Jesus, R.A.; de Oliveira Barros e Silva Camargo, J. Copaiba Essential Oil: Composition, Therapeutic Actions, and Methods of Use for Health and Well-Being. Braz. J. Health Aromather. Essent. Oil 2024, 1, bjhae18. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, J.; Dong, W.; Yao, L. The Effect of Copaiba Oil Odor on Anxiety Relief in Adults under Mental Workload: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2022, 2022, 3874745. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Ghahfarokhi, B.B.; Ghahfarokhi, S.A.M.; Malekpoor, F. Chemical Composition of Essential Oils from the Aerial Parts and Underground Parts of Iranian Valerian Collected from Different Natural Habitats. Ind. Crops Prod. 2015, 63, 147–151. [Google Scholar] [CrossRef]
- Shinjyo, N.; Waddell, G.; Green, J. Valerian Root in Treating Sleep Problems and Associated Disorders—A Systematic Review and Meta-Analysis. J. Evid.-Based Integr. Med. 2020, 25, 2515690X20967323. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Guo, Q.; Li, H.; Wang, Z.; Li, J.; Li, T.; Tang, T.; Wang, Y.; Jia, Y.; et al. Valerian Essential Oil for Treating Insomnia via the Serotonergic Synapse Pathway. Front. Nutr. 2022, 9, 927434. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, C.T.P. Evaluation of the Effects of Essential Oils on the Reduction of Stress: A Rapid Narrative Review. Braz. J. Health Aromather. Essent. Oil 2024, 1, bjhae4. [Google Scholar] [CrossRef]
- Yousef, H.; Alhajj, M.; Fakoya, A.O.; Sharma, S. Anatomy, Skin (Integument), Epidermis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470464 (accessed on 8 June 2025).
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The Applied Anatomy of Human Skin: A Model for Regeneration. Wound Med. 2020, 28, 100179. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Slominski, A.T. Neuroendocrinology of the Skin. Dermato-Endocrinology 2011, 3, 3–10. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.-C.; Wong, J.K. The Dynamic Anatomy and Patterning of Skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.A.; Uitto, J. Structure and Function of the Skin. In Rook’s Textbook of Dermatology; Barker, J., Griffiths, C., Bleiker, T., Simpson, R., Hussain, W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2024. [Google Scholar] [CrossRef]
- González-Ramírez, R.; Chen, Y.; Liedtke, W.B.; Morales-Lázaro, S.L. TRP Channels and Pain. In Neurobiology of TRP Channels; Emir, T.L.R., Ed.; Frontiers in Neuroscience; CRC Press; Taylor & Francis: Boca Raton, FL, USA, 2017; ISBN 978-1-315-15283-7. [Google Scholar]
- Caterina, M.J.; Pang, Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals 2016, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Rogers, T.J.; Lichtman, A.H. Turning Over a New Leaf: Cannabinoid and Endocannabinoid Modulation of Immune Function. J. Neuroimmune Pharmacol. 2015, 10, 193–203. [Google Scholar] [CrossRef]
- Simankowicz, P.; Stępniewska, J. The Role of Endocannabinoids in Physiological Processes and Disease Pathology: A Comprehensive Review. J. Clin. Med. 2025, 14, 2851. [Google Scholar] [CrossRef]
- Cabral, G.A.; Griffin-Thomas, L. Emerging Role of the Cannabinoid Receptor CB2 in Immune Regulation: Therapeutic Prospects for Neuroinflammation. Expert Rev. Mol. Med. 2009, 11, e3. [Google Scholar] [CrossRef]
- Recognition of Melanocytes in Immuno-Neuroendocrinology and Circadian Rhythms: Beyond the Conventional Melanin Synthesis. Available online: https://www.mdpi.com/2073-4409/11/13/2082 (accessed on 11 June 2025).
- Slominski, A.; Wortsman, J.; Tobin, D.J. The Cutaneous Serotoninergic/Melatoninergic System: Securing a Place under the Sun. FASEB J. 2005, 19, 176–194. [Google Scholar] [CrossRef]
- Channer, B.; Matt, S.M.; Nickoloff-Bybel, E.A.; Pappa, V.; Agarwal, Y.; Wickman, J.; Gaskill, P.J. Dopamine, Immunity, and Disease. Pharmacol. Rev. 2023, 75, 62–158. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Marzo, V.D.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; Stelt, M. van der Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023, 75, 885–958. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-Y.; Menuz, K.; Carlson, J.R. Olfactory Perception: Receptors, Cells, and Circuits. Cell 2009, 139, 45–59. [Google Scholar] [CrossRef]
- Franco, R.; Garrigós, C.; Capó, T.; Serrano-Marín, J.; Rivas-Santisteban, R.; Lillo, J. Olfactory Receptors in Neural Regeneration in the Central Nervous System. Neural Regen. Res. 2025, 20, 2480. [Google Scholar] [CrossRef] [PubMed]
- Stougiannou, T.M.; Christodoulou, K.C.; Karangelis, D. Olfactory Receptors and Aortic Aneurysm: Review of Disease Pathways. J. Clin. Med. 2024, 13, 7778. [Google Scholar] [CrossRef]
- Sun, K.; Ray, S.; Gupta, N.; Aldworth, Z.; Stopfer, M. Olfactory System Structure and Function in Newly Hatched and Adult Locusts. Sci. Rep. 2024, 14, 2608. [Google Scholar] [CrossRef]
- Mika, K.; Benton, R. Olfactory Receptor Gene Regulation in Insects: Multiple Mechanisms for Singular Expression. Front. Neurosci. 2021, 15, 738088. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Cosma, P. Neurocosmetics in Skincare—The Fascinating World of Skin–Brain Connection: A Review to Explore Ingredients, Commercial Products for Skin Aging, and Cosmetic Regulation. Cosmetics 2021, 8, 66. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the Skin1. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Slominski, R.M.; Raman, C.; Chen, J.Y.; Athar, M.; Elmets, C. Neuroendocrine Signaling in the Skin with a Special Focus on the Epidermal Neuropeptides. Am. J. Physiol.-Cell Physiol. 2022, 323, 1757–1776. [Google Scholar] [CrossRef]
- Vieira, D.; Duarte, J.; Vieira, P.; Gonçalves, M.B.S.; Figueiras, A.; Lohani, A.; Veiga, F.; Mascarenhas-Melo, F. Regulation and Safety of Cosmetics: Pre- and Post-Market Considerations for Adverse Events and Environmental Impacts. Cosmetics 2024, 11, 184. [Google Scholar] [CrossRef]
- Rajagopal, S.; Sivanathan, G.; Mahadevaswamy, G.; Angamuthu, G.; Dhandapani, N.V. Neurocosmetics: An extensive overview. Int. J. Appl. Pharm. 2025, 17, 31–38. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Ferreira, M.S.; Sousa-Lobo, J.M.; Sousa, E.; Almeida, I.F. Usage of Synthetic Peptides in Cosmetics for Sensitive Skin. Pharmaceuticals 2021, 14, 702. [Google Scholar] [CrossRef]
- Marques, C.; Hadjab, F.; Porcello, A.; Lourenço, K.; Scaletta, C.; Abdel-Sayed, P.; Hirt-Burri, N.; Applegate, L.A.; Laurent, A. Mechanistic Insights into the Multiple Functions of Niacinamide: Therapeutic Implications and Cosmeceutical Applications in Functional Skincare Products. Antioxidants 2024, 13, 425. [Google Scholar] [CrossRef]
- Kuzumi, A.; Yoshizaki-Ogawa, A.; Fukasawa, T.; Sato, S.; Yoshizaki, A. The Potential Role of Cannabidiol in Cosmetic Dermatology: A Literature Review. Am. J. Clin. Dermatol. 2024, 25, 951–966. [Google Scholar] [CrossRef]
- Kumar, S.; Acharya, T.K.; Halder, R.R.; Mahapatra, P.; Chang, Y.-T.; Goswami, C. Menthol Causes Mitochondrial Ca2+-Influx, Affects Structure-Function Relationship and Cools Mitochondria. Life Sci. 2023, 331, 122032. [Google Scholar] [CrossRef] [PubMed]
- Peppin, J.F.; Pappagallo, M. Capsaicinoids in the Treatment of Neuropathic Pain: A Review. Ther. Adv. Neurol. Disord. 2014, 7, 22–32. [Google Scholar] [CrossRef]
- Alalami, K.; Goff, J.; Grimson, H.; Martin, O.; McDonald, E.; Mirza, T.; Mistry, D.; Ofodile, A.; Raja, S.; Shaker, T.; et al. Does Topical Capsaicin Affect the Central Nervous System in Neuropathic Pain? A Narrative Review. Pharmaceuticals 2024, 17, 842. [Google Scholar] [CrossRef]
- Tyagi, S.; Shekhar, N.; Thakur, A.K. Protective Role of Capsaicin in Neurological Disorders: An Overview. Neurochem. Res. 2022, 47, 1513–1531. [Google Scholar] [CrossRef]
- Kim, H.R.; Jung, Y.; Shin, J.; Park, M.; Kweon, D.-H.; Ban, C. Neuron-Recognizable Characteristics of Peptides Recombined Using a Neuronal Binding Domain of Botulinum Neurotoxin. Sci. Rep. 2022, 12, 4980. [Google Scholar] [CrossRef]
- Lupin, M.; Bjerring, P.; Andriessen, A.; Chantrey, J.; Fabi, S.G.; Liew, S.; McDonald, C.; Xiaolei, Q.; White, S. Real-World Clinical Experience with a Neuro-Peptide Serum in Combination with Botulinum Toxin Type-A Injections. J. Drugs Dermatol. 2024, 23, 43661s3–43661s14. [Google Scholar]
- Hongratanaworakit, T.; Buchbauer, G. Evaluation of the Harmonizing Effect of Ylang-Ylang Oil on Humans after Inhalation. Planta Med. 2004, 70, 632–636. [Google Scholar] [CrossRef]
- Moss, M.; Hewitt, S.; Moss, L.; Wesnes, K. Modulation of cognitive performance and mood by aromas of peppermint and ylang-ylang. Int. J. Neurosci. 2008, 118, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Bavarsad, N.H.; Bagheri, S.; Kourosh-Arami, M.; Komaki, A. Aromatherapy for the Brain: Lavender’s Healing Effect on Epilepsy, Depression, Anxiety, Migraine, and Alzheimer’s Disease: A Review Article. Heliyon 2023, 9, e18492. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and Anti-Aging Potentials of Essential Oils from Aromatic and Medicinal Plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Koulivand, P.H.; Ghadiri, M.K.; Gorji, A. Lavender and the Nervous System. Evid.-Based Complement. Altern. Med. 2013, 2013, 681304. [Google Scholar] [CrossRef]
- Leong, E.J.; Tan, L.F.; Yap, V.L.; Rajagopal, M.; Chandran, R. Cosmetological Applications of Citrus Limon: A Mini-Review. Indian J. Nat. Prod. Resour. IJNPR Former. Nat. Prod. Radiance NPR 2024, 15, 286–293. [Google Scholar] [CrossRef]
- Ali, A.; Chaudhary, A.; Sharma, A.; Siddiqui, N.; Anurag; Parihar, V.K. Exploring Role of Citrus Fruits in Comorbid Neurodegenerative Disorders Associated with Psoriasis. Metab. Brain Dis. 2024, 40, 62. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.G.; Malik, M.M.A.H.; Connell, E.; Müller, M.; Vauzour, D. Citrus Polyphenols in Brain Health and Disease: Current Perspectives. Front. Neurosci. 2021, 15, 640648. [Google Scholar] [CrossRef]
- Belluzzi, J.D.; Stein, L. Enkephalin May Mediate Euphoria and Drive-Reduction Reward. Nature 1977, 266, 556–558. [Google Scholar] [CrossRef]
- Cullen, J.M.; Cascella, M. Physiology, Enkephalin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557764 (accessed on 2 July 2025).
- Proteau-Gagné, A.; Bournival, V.; Rochon, K.; Dory, Y.L.; Gendron, L. Exploring the Backbone of Enkephalins to Adjust Their Pharmacological Profile for the δ-Opioid Receptor. ACS Chem. Neurosci. 2010, 1, 757–769. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, M. Enkephalins and Pain Modulation: Mechanisms of Action and Therapeutic Perspectives. Biomolecules 2024, 14, 926. [Google Scholar] [CrossRef]
- Pintea, A.; Manea, A.; Pintea, C.; Vlad, R.-A.; Bîrsan, M.; Antonoaea, P.; Rédai, E.M.; Ciurba, A. Peptides: Emerging Candidates for the Prevention and Treatment of Skin Senescence: A Review. Biomolecules 2025, 15, 88. [Google Scholar] [CrossRef]
- Lima, T.N.; Pedriali Moraes, C.A. Bioactive Peptides: Applications and Relevance for Cosmeceuticals. Cosmetics 2018, 5, 21. [Google Scholar] [CrossRef]
- Gazitaeva, Z.I.; Drobintseva, A.O.; Chung, Y.; Polyakova, V.O.; Kvetnoy, I.M. Cosmeceutical Product Consisting of Biomimetic Peptides: Antiaging Effects in Vivo and in Vitro. Clin. Cosmet. Investig. Dermatol. 2017, 10, 11–16. [Google Scholar] [CrossRef]
- Michalek, I.M.; Lelen-Kaminska, K.; Caetano dos Santos, F.L. Peptides Stimulating Synthesis of Extracellular Matrix Used in Anti-Ageing Cosmetics: Are They Clinically Tested? A Systematic Review of the Literature. Australas. J. Dermatol. 2019, 60, e267–e271. [Google Scholar] [CrossRef]
- Veiga, E.; Ferreira, L.; Correia, M.; Pires, P.C.; Hameed, H.; Araújo, A.R.T.S.; Cefali, L.C.; Mazzola, P.G.; Hamishehkar, H.; Veiga, F.; et al. Anti-Aging Peptides for Advanced Skincare: Focus on Nanodelivery Systems. J. Drug Deliv. Sci. Technol. 2023, 89, 105087. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Yi, E.-J.; Jin, X.; Zheng, Q.; Park, S.-J.; Yi, G.-S.; Yang, S.-J.; Yi, T.-H. Sustainable Dynamic Wrinkle Efficacy: Non-Invasive Peptides as the Future of Botox Alternatives. Cosmetics 2024, 11, 118. [Google Scholar] [CrossRef]
- Viper Venom and Synthetic Peptides: Emerging Active Ingredients in Anti-Ageing Cosmeceuticals. Available online: https://www.mdpi.com/2076-3417/15/8/4501 (accessed on 13 June 2025).
- Dragomirescu, A.O.; Andoni, M.; Ionescu, D.; Andrei, F. The Efficiency and Safety of Leuphasyl—A Botox-Like Peptide. Cosmetics 2014, 1, 75–81. [Google Scholar] [CrossRef]
- Zdrada-Nowak, J.; Surgiel-Gemza, A.; Szatkowska, M. Acetyl Hexapeptide-8 in Cosmeceuticals—A Review of Skin Permeability and Efficacy. Int. J. Mol. Sci. 2025, 26, 5722. [Google Scholar] [CrossRef]
- Olsson, S.E.; Sreepad, B.; Lee, T.; Fasih, M.; Fijany, A. Public Interest in Acetyl Hexapeptide-8: Longitudinal Analysis. JMIR Dermatol. 2024, 7, e54217. [Google Scholar] [CrossRef]
- Raikou, V.; Kalogria, E.; Varvaresou, A.; Tsirivas, E.; Panderi, I. Quantitation of Acetyl Hexapeptide-8 in Cosmetics by Hydrophilic Interaction Liquid Chromatography Coupled to Photo Diode Array Detection. Separations 2021, 8, 125. [Google Scholar] [CrossRef]
- Kachooeian, M.; Mousivand, Z.; Sharifikolouei, E.; Shirangi, M.; Firoozpour, L.; Raoufi, M.; Sharifzadeh, M. Matrixyl Patch vs Matrixyl Cream: A Comparative In Vivo Investigation of Matrixyl (MTI) Effect on Wound Healing. ACS Omega 2022, 7, 24695–24704. [Google Scholar] [CrossRef]
- Li, F.; Chen, H.; Chen, D.; Zhang, B.; Shi, Q.; He, X.; Zhao, H.; Wang, F. Clinical Evidence of the Efficacy and Safety of a New Multi-Peptide Anti-Aging Topical Eye Serum. J. Cosmet. Dermatol. 2023, 22, 3340–3346. [Google Scholar] [CrossRef] [PubMed]
- Gok, B.; Budama-Kilinc, Y.; Kecel-Gunduz, S. Anti-Aging Activity of Syn-Ake Peptide by in Silico Approaches and in Vitro Tests. J. Biomol. Struct. Dyn. 2024, 42, 5015–5029. [Google Scholar] [CrossRef]
- Robinson, L.R.; Fitzgerald, N.C.; Doughty, D.G.; Dawes, N.C.; Berge, C.A.; Bissett, D.L. Topical Palmitoyl Pentapeptide Provides Improvement in Photoaged Human Facial Skin. Int. J. Cosmet. Sci. 2005, 27, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Schagen, S.K. Topical Peptide Treatments with Effective Anti-Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Hoppel, M.; Reznicek, G.; Kählig, H.; Kotisch, H.; Resch, G.P.; Valenta, C. Topical Delivery of Acetyl Hexapeptide-8 from Different Emulsions: Influence of Emulsion Composition and Internal Structure. Eur. J. Pharm. Sci. 2015, 68, 27–35. [Google Scholar] [CrossRef]
- Kraeling, M.E.K.; Zhou, W.; Wang, P.; Ogunsola, O.A. In Vitro Skin Penetration of Acetyl Hexapeptide-8 from a Cosmetic Formulation. Cutan. Ocul. Toxicol. 2015, 34, 46–52. [Google Scholar] [CrossRef]
- Moy, M.; Diaz, I.; Lesniak, E.; Giancola, G. Peptide-pro Complex Serum: Investigating Effects on Aged Skin. J. Cosmet. Dermatol. 2023, 22, 267–274. [Google Scholar] [CrossRef]
- Shariati Pour, S.R.; Oddis, S.; Barbalinardo, M.; Ravarino, P.; Cavallini, M.; Fiori, J.; Giuri, D.; Tomasini, C. Delivery of Active Peptides by Self-Healing, Biocompatible and Supramolecular Hydrogels. Molecules 2023, 28, 2528. [Google Scholar] [CrossRef] [PubMed]
- WO2016090252A1-Topical Skin Care Composition Comprising Trifluoroacetyl Tripeptide-2-Google Patents. Available online: https://patents.google.com/patent/WO2016090252A1/en (accessed on 7 July 2025).
- The Mechanism and Function of Acetyl Tetrapeptide-2 in Human Skin-Creative Peptides. Available online: https://www.creative-peptides.com/article/the-mechanism-and-function-of-acetyl-tetrapeptide-2-in-human-skin-149.html (accessed on 7 July 2025).
- Kobiela, T.; Milner-Krawczyk, M.; Pasikowska-Piwko, M.; Bobecka-Wesołowska, K.; Eris, I.; Święszkowski, W.; Dulinska-Molak, I. The Effect of Anti-Aging Peptides on Mechanical and Biological Properties of HaCaT Keratinocytes. Int. J. Pept. Res. Ther. 2018, 24, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, N.; Ozols, M.; Bell, M.; Bradley, E.; Gilmore, A.; Debelle, L.; Sherratt, M.J. Matrikines as Mediators of Tissue Remodelling. Adv. Drug Deliv. Rev. 2022, 185, 114240. [Google Scholar] [CrossRef]
- Raikou, V.; Varvaresou, A.; Panderi, I.; Papageorgiou, E. The Efficacy Study of the Combination of Tripeptide-10-Citrulline and Acetyl Hexapeptide-3. A Prospective, Randomized Controlled Study. J. Cosmet. Dermatol. 2017, 16, 271–278. [Google Scholar] [CrossRef]
- Puig, A.; Antón, J.M.G.; Mangues, M. A new decorin-like tetrapeptide for optimal organization of collagen fibres. Int. J. Cosmet. Sci. 2008, 30, 97–104. [Google Scholar] [CrossRef]
- Yuan, M.; Niu, J.; Li, F.; Ya, H.; Liu, X.; Li, K.; Fan, Y.; Zhang, Q. Dipeptide-1 Modified Nanostructured Lipid Carrier-Based Hydrogel with Enhanced Skin Retention and Topical Efficacy of Curcumin. RSC Adv. 2023, 13, 29152–29162. [Google Scholar] [CrossRef] [PubMed]
- Topouzidou, N.; Miliotou, A.N.; Nodaraki, D.; Galatou, E.; Petrou, C.; Sarigiannis, Y. Unraveling the Molecular Mechanisms of Synthetic Acetyl Hexapeptide in E-Cadherin Activation for Tissue Rejuvenation. Cosmetics 2025, 12, 48. [Google Scholar] [CrossRef]
- Hrenak, J.; Paulis, L.; Simko, F. N-Acetyl-Seryl-Aspartyl-Lysyl-Proline (Ac-SDKP): Potential Target Molecule in Research of Heart, Kidney and Brain. Curr. Pharm. Des. 2015, 21, 5135–5143. [Google Scholar] [CrossRef]
- Kanasaki, K. N-acetyl-seryl-aspartyl-lysyl-proline Is a Valuable Endogenous Antifibrotic Peptide for Kidney Fibrosis in Diabetes: An Update and Translational Aspects. J. Diabetes Investig. 2020, 11, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Hajem, N.; Chapelle, A.; Bignon, J.; Pinault, A.; Liu, J.-M.; Salah-Mohellibi, N.; Lati, E.; Wdzieczak-Bakala, J. The regulatory role of the tetrapeptide AcSDKP in skin and hair physiology and the prevention of ageing effects in these tissues—A potential cosmetic role. Int. J. Cosmet. Sci. 2013, 35, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Tripeptide-1, Hexapeptide-12, Their Metal Salts and Fatty Acyl Derivatives, and Palmitoyl Tetrapeptide-7 as Used in Cosmetics. Int. J. Toxicol. 2018, 37, 90S–102S. [Google Scholar] [CrossRef]
- Hostynek, J.J.; Dreher, F.; Maibach, H.I. Human Skin Retention and Penetration of a Copper Tripeptide in Vitro as Function of Skin Layer towards Anti-Inflammatory Therapy. Inflamm. Res. 2010, 59, 983–988. [Google Scholar] [CrossRef] [PubMed]
- CN113461774A-Preparation Method of Palmitoyl Tripeptide-1-Google Patents. Available online: https://patents.google.com/patent/CN113461774A/en (accessed on 7 July 2025).
- Mondon, P.; Hillion, M.; Peschard, O.; Andre, N.; Marchand, T.; Doridot, E.; Feuilloley, M.G.; Pionneau, C.; Chardonnet, S. Evaluation of Dermal Extracellular Matrix and Epidermal–Dermal Junction Modifications Using Matrix-assisted Laser Desorption/Ionization Mass Spectrometric Imaging, in Vivo Reflectance Confocal Microscopy, Echography, and Histology: Effect of Age and Peptide Applications. J. Cosmet. Dermatol. 2015, 14, 152–160. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; ISBN 978-0-7020-5434-1. [Google Scholar]
- Burfield, T. Safety of Essential Oils. Int. J. Aromather. 2000, 10, 16–29. [Google Scholar] [CrossRef]
- Sindle, A.; Martin, K. Art of Prevention: Essential Oils-Natural Products Not Necessarily Safe. Int. J. Womens Dermatol. 2021, 7, 304–308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).