Abstract
The European Society for Laser Dermatology (ELSD) has established recommendations for safe and effective photo epilation; however, short-term common adverse effects occur as a result of laser treatment, such as edema and perifollicular erythema. Post-inflammatory hyperpigmentation also appears in certain skin types. Very few clinical studies have been conducted on the topical application of cosmetic skin care products aimed at decreasing the adverse effects on the skin epidermis following laser-assisted epilation procedures. Stem cells are found in plant and animal organisms and are responsible for the growth and restoration of damaged tissues. Plant stem cells divide throughout the life of the plant, creating new plant parts. Our aim was to develop a new cosmetic cream to decrease the intensity of some of the side effects of laser epilation and thus reduce the administration of topical medication. We developed a formulation with the active substance Olea europaea (Olive) Callus Culture Lysate (OLEA VITAE™ 02), which is derived from plant stem cells of the Mediterranean wild variety of Olea europaea, for application following laser epilation with an Nd:YAG 1064 nm laser. The new skin care cream was tested for its physicochemical and microbiological stability, according to the European Pharmacopoeia. The impacts of this substance on the potential side effects of Nd:YAG 1064 nm application, i.e., trans-epidermal water loss, keratin hydration, melanin, erythema, and skin elasticity, in comparison with the appropriate placebo, were investigated using biophysical measurements and a self-assessment questionnaire. Skin biopsies were also performed to evaluate the influence of the procedure and the application of the products on the epidermis and papillary dermis thickness. According to our findings, the incorporation of the plant stem cell extract of Olea europaea into our cream resulted in a stable cream with an appealing texture. Furthermore, the activity of erythema and hyperpigmentation was decreased when the cream was applied after Nd:YAG 1064 nm laser epilation.
1. Introduction
Laser technology is advancing quickly due to the increased demand for laser epilation. Ruby, alexandrite, diode, and neodymium–yttrium–aluminum–garnet (Nd:YAG 1064 nm) lasers, as well as intense pulsed light (IPL) sources, are the devices that are currently available and in use. Better results with epilation have been promised by Nd:YAG and alexandrite lasers [1]. Although there are well-established guidelines for photo epilation from the European Society for Laser Dermatology (ELSD) [2], short-term side effects such as edema and perifollicular erythema are inevitable with laser epilation. However, these consequences are essential components of laser photo thermolysis and therefore cannot be regarded as side effects. It is acknowledged that there are unfavorable consequences to laser epilation, with most resulting from epidermal damage [3]. Pain, burns, folliculitis, leukotrichia, paradoxical hypertrichosis, pigmentary abnormalities, nevi alterations, pili bigemini, herpes infection, hyperhidrosis, bromhidrosis, Fox–Fordyce syndrome, and frostbite from the cooling system are among the cutaneous consequences. These unfavorable consequences are generally associated with light devices, predetermined parameters, skin types, and body areas. It has been determined that intense pulsed light devices cause more pain than diode lasers but less discomfort than alexandrite lasers; however, Nd:YAG 1064 nm lasers cause the most pain. Cutaneous problems are typically associated with patient features and errors made by laser operators [4]. Short-wavelength lasers have a higher incidence of pigmentary modifications (up to 19%), especially in darker skin types. Neodymium–yttrium–aluminum–garnet lasers have a lower incidence of pigmentary alterations (2–4%).
Very few clinical studies have been conducted on the topical application of skin care products to decrease the adverse effects following laser-assisted epilation procedures on the skin epidermis, i.e., pain, erythema, purpura, blisters, edema, crusts, bleeding, and temporary hypo- and hyperpigmentation. These events are mostly transient in nature and heal without scars [5,6]. Most studies refer to the application of a mixture of local anesthetics prior to laser epilation [7,8]. Topical corticosteroids can be prescribed to reduce erythema and swelling if a patient’s skin is prone to prolonged redness [2].
Stem cells are found in plant and animal organisms and are responsible for the growth and restoration of damaged tissues. Plant stem cells divide throughout the life of the plant, creating new plant parts. All of the parts of a plant are formed by connective tissue cells located in the upper part of the stem. In cosmetic products, plant stem cells are used in the form of oily extracts, water-soluble extracts, ester extract powder (with maltodextrin), liposomes, nano molecules, and suspensions [9]. Plant stem cells, which are used in the biomedical sciences, are reproduced using the method of micro-replication, which includes in vitro cell culture. Plant stem cells are used in dermato-cosmetic science as sustainable anti-aging ingredients. Moreover, plant stem cells have been proposed as cosmetic ingredients for their antioxidant, collagen-promoting, and anti-melanogenic activities [10,11,12].
The active substance Olea europaea (Olive) Callus Culture Lysate (OLEA VITAE™ 02) is derived from plant stem cells of the Mediterranean wild variety of Olea europaea of wild olive seed shoots. Olive seeds contain lipids, proteins, and the main phenolics hydroxytyrosol and tyrosol. Polyphenols are phenyl rings with several hydroxyl (-OH) groups attached. The antioxidant capacity of (poly)phenolic compounds is associated with their structure [13]. Flavonoids, a sub-category of polyphenols, consist of two fused aromatic rings, named diphenylpropanes. The fused aromatic system bears at least one phenolic group. Flavonoids possess antiradical activity; they scavenge reactive oxygen species (ROS) that are formed during plant stress responses. Both polyphenols and flavonoids are natural products and have anti-inflammatory properties, possibly correlated with their antioxidant and antiradical activity, with several applications in the cosmetic and pharmaceutical industries [13,14].
Using special technology, the lipid product is extracted from stem cells of the cell membrane. This creates the first generation of biomimetic lipids of membrane stem cells called phyto-lipid fractions (phyto-lipidic fractions, PLFs). According to in vitro experiments, PLFs protect, interfere with mitochondrial function, increase the available energy of skin cells, and increase the production of skin proteins. OLEA VITAE™ 02 is a potent anti-aging and rejuvenating agent for older skin using a novel mechanism of action to combat energy aging. It is GMO-, BSE-, listed CMR-, VOC-, heavy metal-, allergen-, paraben-, phenoxyethanol-, aflatoxin–pesticide-, and pollutant-free. Cellular oil mimics the action of cellular lipids, protects and optimizes the energy of skin cells, and promotes the creation of structural proteins. The active substance has important anti-wrinkle, constrictive, and anaplastic properties [15].
Our target was to develop a new personal care cosmetic cream to decrease the intensity of some of the short-term adverse effects that often occur following laser epilation, i.e., erythema, pain, sense of burning, or itching, and thus reduce the requirement for pharmaceutical regimes. We developed a dermato-cosmetic cream with the active substance OLEA VITAE™ 02 for application following laser epilation with a Nd:YAG 1064 nm laser on the arm of human volunteers. The cream was assessed for its ability to reduce potential adverse effects, and its efficacy was compared with a placebo cream. The new skin care cream was tested for its physicochemical and microbiological stability according to the European Pharmacopoeia. The impacts of this substance on the potential adverse effects of Nd:YAG 1064 nm applications were investigated using biophysical measurements and a self-assessment questionnaire. Skin biopsies were also performed to evaluate the influence of the procedure and the tested products on the epidermis and papillary dermis thickness.
According to our findings, the incorporation of the extract of plant stem cells of Olea europaea in our formulation resulted in a stable cream with an appealing texture and hydrating qualities that decreased erythema and hyperpigmentation activity when applied following Nd:YAG 1064 nm laser epilation. Moreover, the cream could be used for the relief of adverse effects following other aesthetic procedures that can cause temporary pain, itching, feelings of dryness, and discomfort.
2. Materials and Methods
2.1. Formulation
The creams under study were O/W emulsions prepared in the Laboratory of Chemistry–Biochemistry–Cosmetic Science, Department of Biomedical Sciences, University of West Attica. Cream 1 with the active ingredient Olea Europaea (Olive) Callus Culture Lysate (OLEA VITAE™ 02), used at a concentration of 2% w/w, also contained aqua, xanthan gum, lecithin, sclerotium gum, pullulan, glycerin, C12-20 acid PEG-8 ester, glyceryl stearate, PEG-100 stearate, cetearyl alcohol, caprylic/capric triglyceride, ethylhexyl palmitate, dimethicone, cyclopentasiloxame, cycloexasiloxane, butylated hydroxytoluene, phenoxyethanol, ethylhexylglycerin, cyamopsis tetragonoloba (guar) gum, citric acid, tocopherol, and perfume (International Nomenclature of Cosmetic Ingredients (INCI) terminology). Cream 2, the placebo, contained the same ingredients at the same concentrations as Cream 1, with OLEA VITAE™ 02 being replaced by an equal concentration of aqua.
2.2. Quality Control of the Cosmetic Creams
2.2.1. Physicochemical Stability of Cosmetic Creams
All the accelerated and long-term stability tests were performed according to ICH Q1A (R2) stability testing. The quality control of the developed formulations consisted of the following tests: physicochemical control; organoleptic testing (appearance, color, and odor); measurement of pH (Ιnolab pH meter) and viscosity (Brookfield DVI+, spindle F, rpm 6 and T:20 °C); and determination of preservatives such as phenoxyethanol and butylated hydroxytoluene using the high-performance liquid chromatography (HPLC) Shimadzu Prominence System with an autosampler adjusted to inject 20 μL and a DD SPD-M20A UV/visible (UV/Vis) detector with the LC Solution software Shimadzu.
Determination of Phenoxyethanol in the Cream with OLEA VITAE™ 02
A total of 0.5 g of the cream was weighed into a 25 mL volumetric flask and diluted to a volume with methanol. The mixture was stirred and placed in an ultrasonic bath for 15 min. Then, the mixture was filtered through a 0.45 μm PET membrane and placed in an automatic sampling vial for product analysis. The mobile phase was filtered through 0.45 μm PTFE membrane filters (Membrane Solution) and degassed under vacuum prior to use. The conditions are presented in Table 1.

Table 1.
Methodology for the determination of phenoxyethanol and butylated hydroxytoluene.
Determination of Butylated Hydroxytoluene in the Cream with OLEA VITAE™ 02
A total of 0.1 g of the sample was weighed into a 50 mL volumetric flask and diluted to a volume with methanol. The sample was stirred and then placed in an ultrasonic bath for 30 min. A total of 0.5 mL of this solution was diluted in a 10 mL volumetric flask with methanol. The mixture was filtered through a 0.45 μm PET membrane and placed in an automatic sampling vial for product analysis. The mobile phase was filtered through 0.45 μm PTFE membrane filters (Membrane Solution) and degassed under vacuum prior to use (Table 1).
2.2.2. Microbiological Control and Assessment of the Effectiveness of Preservation
The EU Cosmetic Products Regulation (EC) No. 1223/2009 states that cosmetics should demonstrate preservation efficacy because cosmetic preservative systems should ensure product robustness and consumer safety throughout product life cycles. Preservative efficacy testing (PET), also known as challenge testing, is the appropriate tool to substantiate antimicrobial preservation efficacy against microbial contamination. Cosmetic challenge testing methodology may follow pharmacopoeias, international standards, industry, or in-house developed protocols. In this study, antimicrobial protection was evaluated for the developed formulation using standard procedures: enumeration of total aerobic microbial count, yeast, and molds and detection of Staphylococcus aureus, Candida albicans, Escherichia coli, Pseudomonas aeruginosa, and Aspergillus brasiliensis (Eur. Pharm 11.0).
2.3. Evaluation Methods
Laser epilation, application of the tested creams, and evaluation methods were performed 15 cm from the wrist joint οn the right or left arms of the volunteers.
The quantitative variables of the efficacy of the tested creams were evaluated with biophysical techniques able to quantify biophysical parameters of the skin for the claim substantiation according to the guidelines of Cosmetics Europe. Additionally, a self-assessment questionnaire was completed by the volunteers at the end of the treatment in which product satisfaction and tolerability of the two tested creams were examined. Skin biopsies were also performed in order to evaluate dermal alterations resulting from the procedure (Table 2).

Table 2.
Evaluation protocol in each visit.
2.3.1. Biophysical Measurements
The following equipment was used for the measurement of the biophysical skin parameters: Tewameter MPA-5 Courage + Khazaka electronic GmbH (Germany) was used for trans-epidermal water loss (TEWL); Corneometer CM 825 Courage + Khazaka electronic GmbH (Germany) was used for keratin hydration; Mexameter MPA-5 Courage + Khazaka electronic GmbH (Germany) was used for melanin and skin erythema; and Cutometer MPA 580 Courage + Khazaka electronic GmbH (Germany) was used for the following skin elasticity parameters: R2: visco-elasticity as a percentage (resistance to mechanical force versus ability of recovery), R5: net elasticity as a percentage: Ur/Ue = elastic part of the suction phase vs. immediate recovery during relaxation phase, and R7: Ur/Uf proportion of the immediate recovery compared with the amplitude after suction as a percentage. All measurements were repeated three times, and the average was obtained.
2.3.2. Skin Biopsies
Skin puncture biopsies (2.5–3.0 mm) were obtained from the forearm of each volunteer. The samples were obtained from all 12 volunteers before treatment (pre-treatment), D0, and 14 days following laser and product application (post-treatment), D14. The skin was cleaned with a providone–iodine solution before taking the biopsy, and the area was anesthetized with a xylocaine solution with epinephrine. A special cylindrical tool was used, which was rotated through the skin, to remove a small part of the skin, including deeper layers. The incision depth was up to the dermis. The biopsies were placed in numbered vials with formalin solution and then fixed for observation under the optical microscope. The goal was to observe the possible increase in the thickness of the epidermis, or the dermis, as well as morphological observation of the structure of the dermis in terms of collagen and elastin fibers. The dermis’ thickness was measured from the start of the dermo-epidermal synapse to the end of the slice, whereas the thickness of the epidermis was measured from the start of the granular layer to the dermo-epidermal synapse.
Hematoxylin/Eosin Stain and Epidermis Depth Calculations
Skin tissue from punch biopsies was formalin-fixed and paraffin-embedded. Representative 4-μm serial tissue sections were de-paraffinized in xylene, rehydrated in graded ethanol, and stained with hematoxylin and eosin (H/E). Images stained for H/E were imported in the Amscope software (Version x64, 4.11.18573.20210303), and epidermis depth was manually calculated in random positions after calibration.
Masson Trichrome Stain and Quantification
Skin tissue from punch biopsies was formalin-fixed and paraffin-embedded. Representative 4-μm serial tissue sections were de-paraffinized in xylene, rehydrated in graded ethanol, and stained for collagen identification with the Masson trichrome Goldner kit (Bio-Optica, Cat.no 04-011802) based on the manufacturer’s instructions. Masson trichrome staining was semi-quantitively assessed for intensity by a specialized pathologist on an arbitrary scale of 1 to 3 (low-, medium-, and high-staining intensity).
2.3.3. Self-Assessment Questionnaire
Finally, forty-five female volunteers aged between 30 and 60 (mean age: 45 ± 5 years), belonging to phototypes II and III according to Fitzpatrick classification, were included in the self-assessment questionnaire study after signing the appropriate informed consent form. This study was conducted during the years 2022–2023. The area treated with OLEA VITAETM 02 cream or the placebo was the left or right arm, which was randomly assigned (Figure 1). This study was approved by the Research Ethics Committee of the University of West Attica, Greece (Decision Number 37942/11-05-2021).
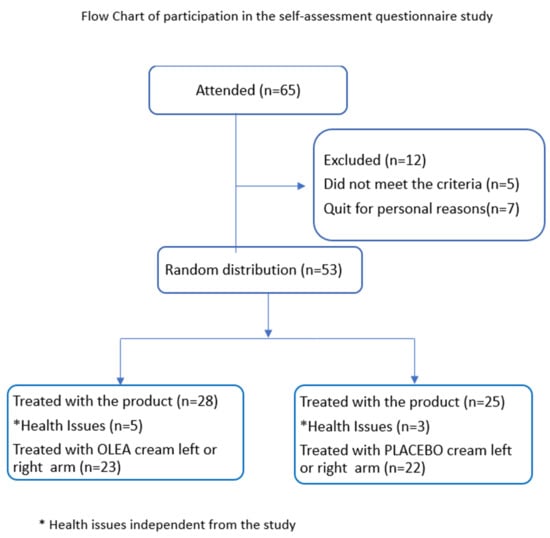
Figure 1.
CONSORT 2010 FLOW DIAGRAM 1. Flow chart of participation in the self-assessment questionnaire study. * Health issues independent from the study.
- Self-assessment questionnaire completed by the enrolled volunteers
- Regarding the therapy
- Date
- Session number
- Product code number
- Regarding the volunteers
- 4.
- Sex (male/female/other)
- 5.
- Age
- 6.
- Body area to be treated
- 7.
- Is your hirsutism hormonally induced?
- 8.
- What is your hair type?
- 9.
- How would you characterize your skin?
- 10.
- Is this your first session of laser epilation? If not, in what area have you had laser before?
- Regarding the subjective evaluation of the cream applied after laser epilation
- 11.
- Did you notice your pain being relieved after being treated with the product?
- 12.
- Did you notice redness of the skin after being treated with the product?
- 13.
- Did you notice your skin healing faster after being treated with the product?
- 14.
- Did you notice the product has anti-inflammatory action?
- 15.
- Did you notice your skin is more hydrated after being treated with the product?
- 16.
- Did you notice an oily sensation after application of the product?
- 17.
- Did you notice the product reduces redness of the skin?
- 18.
- Did you feel an itching effect after application of the product?
2.3.4. Clinical Evaluation
All volunteers with Fitzpatrick phototypes I–IV were treated with the Nd:YAG 1064 nm laser (fluence: 20 J/cm2, pulse duration: 15 ms, spot: 8 mm, and pulse frequency: 1 Hz). We were able to treat darker phototypes (and not exclude them from the trial) with the Nd:YAG 1064 nm laser due to the longer wavelength of this laser. Longer wavelengths reduce the absorption of laser light by the epidermal melanin; the epidermal melanin of darker skin types competes as a significant chromophore and may lead to excessive heating of the surrounding tissue. Therefore, a better margin of safety was ensured with the Nd:YAG 1064 nm laser. External skin cooling was coupled with the Nd:YAG 1064 nm laser handpiece. Informed consent was obtained from all volunteers, in agreement with the Declaration of Helsinki. This study was approved by the Research Ethics Committee of the University of West Attica, Greece (Decision Number 37942/11-05-2021). Female volunteers aged between 30 and 60 (mean age: 45 ± 5 years), belonging to phototypes II and III according to Fitzpatrick classification, were finally included in this study after signing the appropriate informed consent form. The volunteers were selected according to the following inclusion criterion: discontinuation of using any product for skin care during this study. Pregnancy was an exclusion criterion; moreover, the participants were required to avoid medication such as steroid hormone replacement, birth control pills, and vitamin supplements during the study period. At the baseline visit, the participants were instructed to not apply their test cream in the previous twelve hours. Members of our group (pharmacists and chemists) assigned participants to the appropriate treatment. Finally, a total of twelve volunteers enrolled in this study, with six using the OLEA VITAE™ 02 cream and six using the placebo (Figure 2). This study was conducted during the period from 2022–2023. Coded vessels with 70 g of cream were distributed at the first visit to volunteers for use twice daily, without indicating whether or not the formulations contained active ingredients. The subjects were randomly assigned to one of two treatment groups. At each visit, the used vessels were collected in order to allow calculation of the amount of cream used. The participants rested for a 20 min acclimatizing period before the measurements at a room temperature ranging between 19 and 21 °C and RH between 50 and 60%.
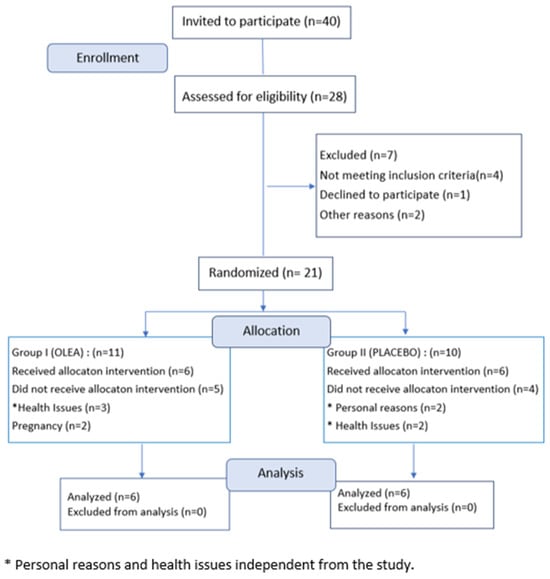
Figure 2.
CONSORT 2010 FLOW DIAGRAM 2. Flow chart of participation in biophysical measurements and skin biopsies. * Personal reasons and health issues independent from the study.
2.3.5. Statistical Analysis of the Biophysical Measurements and Skin Biopsies
Data are expressed as the mean ± standard deviation (SD). The Shapiro–Wilk test examined the normality of the variables’ distribution. We used the two-way mixed ANOVA model using ‘intervention’ (between-group) and ‘time’ (within-group) as comparison factors for the analysis of variables using Bonferroni correction for all pairwise comparisons. Sensitivity analysis of variables concerning the baseline balance between the groups was performed using the following two methods: (a) The mean percentage change from baseline after 14 days, where comparison of percentage changes between the groups was compared, was analyzed using the independent samples t-test, and Mann–Whitney tests were used in the case of a violation of normality. (b) The absolute change from baseline after 14 days was analyzed using the analysis of covariance model (ANCOVA) considering the absolute change from baseline to 14 days as the dependent variable, the group (placebo vs. OLEA VITAE™ 02) as a factor, and the baseline value of the variables as a covariate. All tests were two-sided, and statistical significance was set at p < 0.05. All analyses were carried out using the statistical package SPSS vr. 21.00 (IBM Corporation, Somers, NY, USA).
2.3.6. Statistical Analysis for the Self-Assessment Questionnaire
The Kolmogorov–Smirnov test was used to assess the normality of the distribution of the data. The comparison for homogeneity between the intervention groups concerning demographic and clinical indices was conducted using the Chi-square test and Fisher’s exact test. The t-test for independent samples was used for the comparison between the intervention groups regarding the effectiveness indicators and the Mann–Whitney test was used if the data did not follow a normal distribution. All statistical analyses were conducted using the SPSS statistical package version 21.00. (IBM Corporation, Somers, NY, USA). All tests were two-sided. p-values of <0.05 were considered to indicate statistically significant results.
3. Results
3.1. Quality Control
Stability testing is crucial for the determination of the suggested storage conditions, retesting frequency, and shelf-life. Stability data regarding the cream with OLEA VITAE™ 02 are presented in Table 3. The cream proved stable regarding visual appearance, pH, viscosity, and stability of the dispersion of the phases at different temperatures and periods of time. The concentrations of phenoxyethanol and butylated hydroxytoluene remained between 0.81 and 0.99 w/w and 0.09 and 0.11 w/w under the tested circumstance.

Table 3.
Physicochemical stability of the OLEA VITAE™ 02 cream.
As presented in Figure 3, the OLEA VITAE™ 02 cream satisfied criterion A for Gram-positive bacteria, Gram-negative bacteria, and fungi. The placebo cream was also satisfactory regarding criterion A for all of the microorganisms tested.
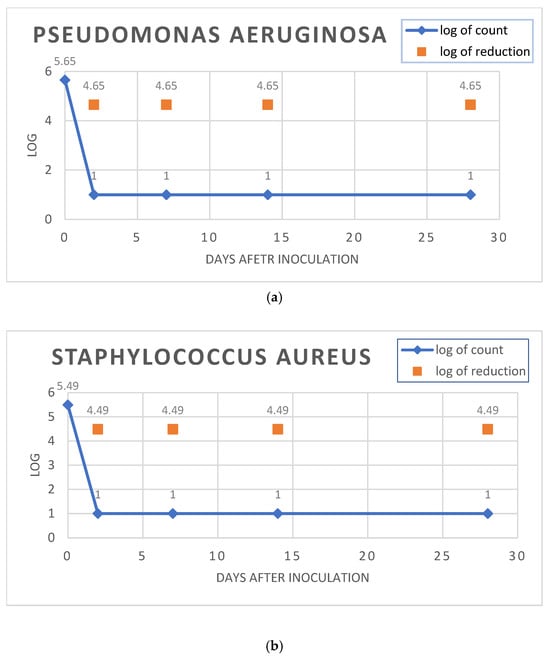
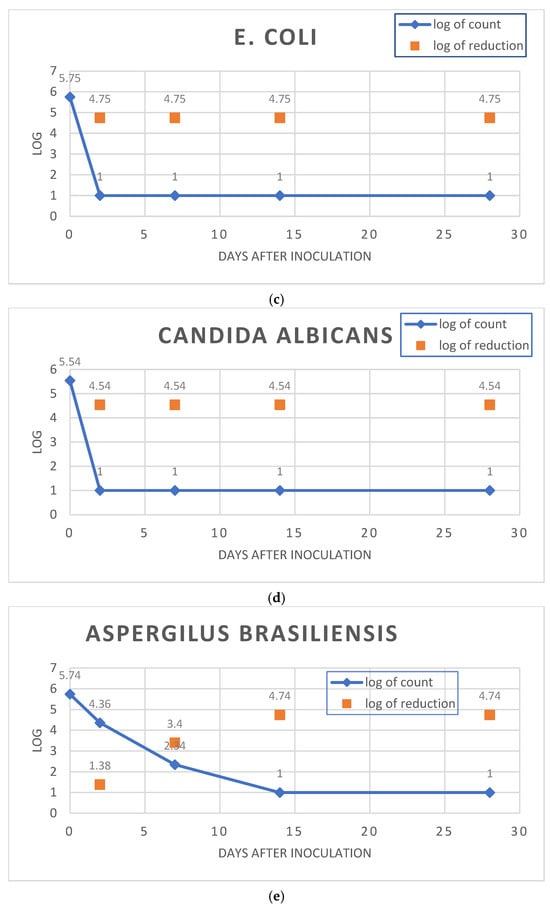
Figure 3.
Test for the efficacy of antimicrobial evaluation according to Eur. Pharm. for the OLEA VITAE™ 02 cream. Subfigures (a–e): log reduction charts for (a). Staphylococcus aureus, (b). Escherichia coli, (c). Pseudomonas aeruginosa, (d). Candida albicans, (e). Aspergillus brasiliensis.
3.2. Biophysical Measurements
The finding that there were no significant differences between the two groups for all recorded variables indicates that they were sufficiently homogeneous, thus eliminating the influence of external factors that could affect the results of the received interventions. The measured values of the trans-epidermal water loss, keratin hydration, melanin index, erythema index, and R2, R5, and R7 elasticity indices at D0 and D14 are presented in Table 4. The mean percentage changes in the measurements are shown in Figure 4.

Table 4.
Changes in the variables measured biophysically (D0–D14) for the tested creams OLEA VITAE™ 02 (referred to as OLEA in Table 4) and the placebo.
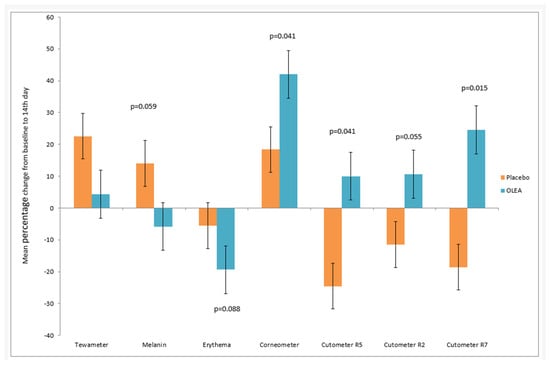
Figure 4.
Mean percentage change in the measured values (D0–D14) for the tested creams OLEA VITAE™ 02 (referred to as OLEA in Figure 4) and the placebo.
3.2.1. Trans-Epidermal Water Loss (TEWL)
There was no statistically significant change in the Tewameter from baseline to D14 for the placebo group (14.02 ± 6.63 vs. 16.64 ± 9.96, p = 0.318) and the OLEA VITAE™ 02 group (13.10 ± 3.05 vs. 14.20 ± 7.45, p = 0.668). There was no statistically significant difference between the absolute change from D0 to D14 between the groups (p = 0.735, Supplementary Materials). There was no statistically significant difference in the percentage change from D0 to D14 between the groups (p = 0.462) However, we noticed the following trend: the increase in trans-epidermal water loss in the placebo group was greater in comparison with the OLEA VITAE™ 02 group.
3.2.2. Keratin Hydration
A statistically significant increase in keratin hydration was observed during the 14-day treatment period for both the placebo group and the OLEA VITAE™ 02 group. The increase in hydration for the OLEA VITAE™ 02 group was higher (45.79 ± 16.90 vs. 64.76 ± 23.39, p < 0.005) in comparison with the placebo group (43.97 ± 16.28 vs. 49.97 ± 13.18, p < 0.05). Hydration was statistically significantly greater at D14 compared with D0 for the placebo group (p = 0.052) and the OLEA VITAE™ 02 group (p < 0.005). There was a statistically significant difference in percentage change from D0 to D14 between the groups (p = 0.030). There was a statistically significant difference between the absolute change from baseline to D14 of the adjusted index between the groups (p = 0.011). The keratin hydration showed a statistically significant improvement with the use of both the placebo and the OLEA VITAE™ 02 creams. Therefore, the use of OLEA VITAE™ 02 leads to a greater increase in keratin hydration.
3.2.3. Melanin Index (MI)
The melanin index in the placebo group was statistically significantly increased from D0 to D14 (147.67 ± 45.59 vs. 165.50 ± 49.00, p = 0.029), whereas in the OLEA VITAE™ 02 group, the MI was decreased, although it was not statistically significant (p = 0.229). A statistically significant difference in the percentage change in MI from D0 to D14 was detected between the groups (placebo: 14.00% ± 26.79 vs. OLEA VITAE™ 02 −5.82% ± 9.60, p = 0.059). A statistically significant difference in the absolute change in MI from D0 to D14 was detected between the groups (placebo: 18.04 ± 7.39 vs. OLEA VITAE™ 02 8.04 ± 7.39; p = 0.035, Supplementary Materials). It seems that hyperpigmentation of the skin in the area of application of the laser epilation device may occur, which seems to be prevented by the application of the preparation with OLEA VITAE™ 02.
3.2.4. Erythema Index (EI)
The erythema index was statistically significantly lower at D14 (p = 0.004) compared with D0 for the OLEA VITAE™ 02 group, while there was no change in the placebo group (p = 0.271). There was no statistically significant difference between the absolute change from D0 to D14 between the groups (p = 0.173, Supplementary Materials). There was a marginally statistically significant difference in percentage change from D0 to D14 of the EI between the groups parametrically (p = 0.088). Τhe use of both creams, the placebo and OLEA VITAE™ 02, led to a reduction in after-epilation erythema, with the OLEA VITAE™ 02 cream being more effective, demonstrating statistical significance.
Elasticity Index—R2
There was a statistically significant difference between the groups at D14 (p < 0.0.005). There was no statistically significant change in the elasticity R2 index from D0 to D14, neither for the placebo group (p = 0.076) nor for the OLEA VITAE™ 02 (p = 0.152) group. A statistically significant difference in the elasticity R2 index in the absolute change adjusted for D0 was observed between the groups from D0 to D14 (p < 0.005). There was a statistically significant percentage change from D0 to D14 in the Cutometer R2 index between the groups parametrically (p = 0.033). Moreover, there was a decrease in the elasticity R2 index in the laser area application, which did not seem to be reversed with the use of the placebo. On the contrary, the OLEA VITAE™ 02 cream appeared to have a positive effect on the elasticity R2 index.
Elasticity Index—R5
The elasticity R5 index was statistically significantly lower for the placebo group at D14 in comparison to D0 (0.68 ± 0.22 vs. 0.50 ± 0.18, p = 0.0450), while the increase in the index for the OLEA VITAE™ 02 group (0.73 ± 0.33 vs. 0.77 ± 0.32, p = 0.651) was not statistically significant. There was a statistically significant difference between the absolute change in the elasticity R5 index adjusted for the baseline from D0 to D14 between the groups (p = 0.055 marginally). There was a statistically significant increase in the percentage change in the elasticity R5 index between the groups parametrically from D0 to D14 (p = 0.046). It seems that there was a decrease in the elasticity of the area where the laser epilation was applied, which might have been prevented by the application of the OLEA VITAE™ 02 plant stem cell extract.
Elasticity Index—R7
The elasticity R7 index was statistically significantly lower at D14 (p = 0.031) compared with D0 for the placebo group, while it was statistically significantly higher for the OLEA VITAE™ 02 group (p = 0.041). There was no statistically significant difference between the groups at baseline (p = 0.884), while there was one at D14 (p = 0.043). There was a statistically significant difference in the absolute change from D0 to D14 for the elasticity R7 index adjusted for the D0 value between the groups (p = 0.003). There was a statistically significant difference in the percentage change from baseline at D14 of the elasticity R7 index between the parametric groups (p = 0.010).
3.3. Analysis of Skin Biopsy Results
Two variables of the images were analyzed, namely, the distance expressing the keratin thickness and the color intensity (Masson trichrome staining), as potential representatives of collagenogenesis (Figure 5). Τhe average increase in the keratin thickness was higher in the OLEA VITAE™ 02 group in comparison with the placebo group. The greatest increase in the mean color intensity value (Masson staining) was also in the OLEA VITAE™ 02 group. Although the results from the skin biopsies were found to be not statistically significant, they correlated well with the statistically significant measurements of the elasticity variables R2, R5, and R7.
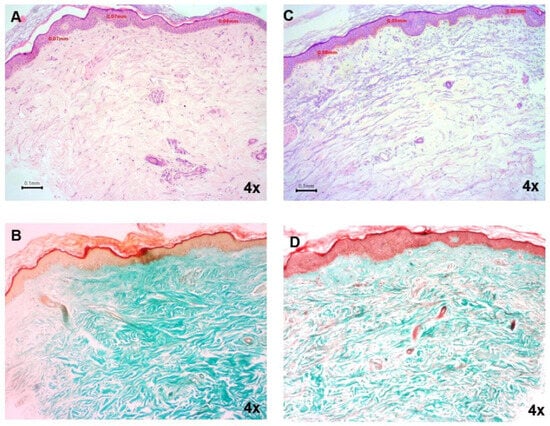
Figure 5.
Representative hematoxylin/eosin-stained skin tissue punch biopsies from the placebo and OLEA VITAE™ 02 cream groups, respectively (A,C). Representative Masson trichrome-stained skin tissue punch biopsies from untreated and treated volunteers (B,D). Images (A,B) represent the same case (case, coded VG10.1) as (C,D) (case, coded VG7.2). All magnifications are 4×. Scale bars of 0.1 mm are shown. Epidermis depth was calculated in multiple random positions and is shown in (A,C).
3.4. Analysis of Self-Assessment Questionnaire Results
The self-assessment questionnaire completed by volunteers at the end of this study reflected that the OLEA VITAE™ 02 cream showed better scores in comparison with the placebo group (Figure 6) regarding the tested variables: pain relief (p < 0.005), treatment of redness (p < 0.005), faster healing (p < 0.005), anti-inflammatory action (p < 0.005), hydration (p < 0.005), oiliness (p < 0.005), redness (p < 0.005 ), and itching (p < 0.005). The active cream showed anti-irritant and anti-inflammatory effects, which is consistent with the statistically significant measurements of the erythema index. Improved hydration was noticed by the volunteers using the OLEA VITAE™ 02 cream, which is also in correlation with the biophysical measurement. Additionally, volunteers were satisfied with the texture of the cream.
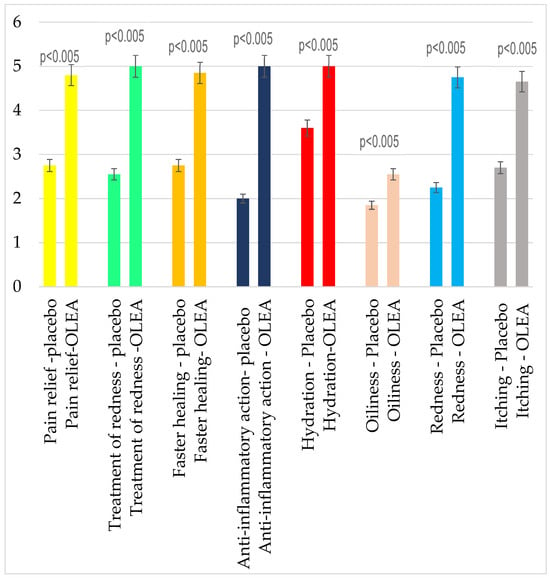
Figure 6.
Self-assessment questionnaire: Comparison of the effectiveness between the groups.
4. Discussion
The finding that there had been no significant differences in the biophysical measurements between the two groups at D0 indicates that they were sufficiently homogeneous, thus eliminating the influence of external factors that could affect the results of the received treatment.
Trans-Epidermal Water Loss (TEWL): There is no mention in the bibliography of a clear long-term change in trans-epidermal water loss (TEWL) using the Nd:YAG 1064 nm laser, perhaps because the Nd:YAG 1064 nm laser penetrates the skin and is thus not absorbed by it. In a study by Dang Y et al. on experimental animals, there is mention of a significant increase in the TEWL immediately following the application of the Nd:YAG 1064 nm laser. The increase in TEWL decreased until the 35th day [16]. In our own study, TEWL was measured at 30 min after the application of the laser in both groups (D0) but not immediately after to avoid overly burdening the volunteers. The measurement was repeated at the end of the treatment (D14). No statistically significant increase, nor any statistically significant percentage increase, was observed in either of the two groups from D0 to D14. However, a larger increase was observed in the group that used the placebo compared with the OLEA VITAE™ 02 group, but it was not statistically significant. Moreover, no statistically significant difference was observed between the groups on D14.
Keratin Hydration: The keratin hydration caused by the placebo cream that contained the traditional humectants, such as glycerin and the emollients glyceryl stearate, ethylhexyl palmitate, and cyclomethicone, showed a statistically significant increase over the length of the 14-day treatment (43.97 ± 16.28 vs. 49.97 ± 13.18, p < 0.05), while during the same period, a greater increase was observed under a statistical significance (45.79 ± 16.90 vs. 64.76 ± 23.39, p < 0.005) in the OLEA VITAE™ 02 group. The percentage change in the OLEA VITAE™ 02 group was greater compared with the placebo group, while the difference in the percentage increase in hydration between the two groups was also statistically significant. It seems that the extract of the plant stem cells positively affects the hydration of keratin, although no such claim has been made so far [16].
Melanin (MI): Pigmentary alterations, such as post-inflammatory hyperpigmentation (PIH) and hypopigmentation, may occur after laser application due to the deposition of melanin caused by the thermal effects of a laser, particularly in the higher phototypes [17]. On the other hand, hypopigmentation or even depigmentation may be observed following the application of Nd:YAG 1064 nm Q-switched laser sessions, mainly in dark-colored phototypes [18]. The mechanism of this side effect is most probably due to the destruction of the epidermal melanocytes; however, it is not necessary to associate this side effect with high fluence, as has been reported with low fluence laser toning for the treatment of melasma. This can be explained by cumulative melanocyte toxicity, which can result in melanocyte destruction after multiple sessions [19]. According to our study, the MI showed a statistically significant increase in the placebo group (147.67 ± 45.59 vs. 165.50 ± 49.00, p = 0.029). A reduction in the OLEA VITAE™ 02 group was observed (136.00 ± 43.97 vs. 128.17 ± 43.29, p = 0.229), although it was not statistically significant. Furthermore, a statistically significant difference in the percentage change in MI from D0 to D14 was detected between the groups (placebo: 14.00% ± 26.79 vs. OLEA VITAE™ 02–5.82% ± 9.60; p = 0.059).
Hyperpigmentation of the skin in the area of application of the laser seems to be prevented by the application of the cream containing OLEA VITAE™ 02. However, the marginal anti-melanogenic effect of OLEA VITAE™ 02 cannot be attributed to the antioxidant effects of the leaves of Olea Europaea or the Olea stem cells. Although melanocytes are sensitive to oxidative stress, there is no evidence that the Nd:YAG 1064 nm Q switch laser may result in the creation of reactive oxygen species (ROS). Additionally, the Nd:YAG 1064 nm Q switch laser is used for the management of oxidative stress caused by UV-B irradiation [20]. Further investigation into the potential anti-melanogenic activity of Olea is required.
Erythema: No clear reduction in the erythema was observed under the application of the placebo (270.83 ± 37.58 vs. 253.83 ± 38.63, p = 0.271). On the contrary, the Olea application led to a statistically significant reduction in erythema (290.83 ± 27.73 vs. 235.50 ± 45.09, p = 0.004). The difference in the percentage change between the two groups was marginally statistically significant (p = 0.088). According to the study by Dang Y., erythema was observed immediately following the application of the Nd:YAG 1064 nm laser on laboratory animals; the erythema began to subside seven days after application. In the recent literature, it is mentioned that erythema occurs in 79–100% of cases immediately following the application of the Nd:YAG 1064 nm laser. The erythema is attributed to the fact that the Nd:YAG 1064 nm laser penetrates the epidermis, is absorbed by oxyhemoglobin, and transfers energy to the tissues, raising their temperature. This increase in temperature causes the dilation of blood vessels and increased blood flow to the area, resulting in erythema, which then decreases in a satisfactory timeframe [17].
In our study, measurements were performed both before the application of the laser and 14 days after the laser application, as well as after the application of the placebo or OLEA VITAE™ 02 product, respectively. Seemingly, the laser application led to a non-statistically significant appearance of erythema, which, however, did not particularly decrease from the application of the placebo cream (270.83 ± 37.58 vs. 253.83 ± 38.63, p = 0.271). On the other hand, a statistically significant reduction in erythema was observed with the use of OLEA VITAE™ 02 in the corresponding measurements (290.83 ± 27.73 vs. 235.50 ± 45.09, p = 0.004). A marginal statistically significant difference was also observed in the percentage change from D0 to D14 for the erythema index between the groups (p = 0.088). The impact of Olea Europeae L. leaves on UV-B-induced erythema is reported in the literature [21]. The improvement in the erythema could also be related to the extract of Olea stem cells. The anti-inflammatory effects of plant stem cells, i.e., Apple Malus domestica L., on UVB-induced damaged skin have been proven in vivo [22].
Elasticity: The literature mentions a study regarding the simultaneous application of the Nd:YAG 1064 nm Q switch laser, plant stem cells, and N-acetylglucosamine for the enhancement of collagenogenesis and the anti-aging effect. In the study by Beri K. and Milgraum S.S., women participants with a mean average age of 56 years +/− 11 years were separated into two groups: group 1—laser application and group 2—modern laser application, plant stem cells, and N-acetyl glucosamine. The measurements on the volunteers of groups 1 and 2 were carried out 4 and 8 weeks after the implementation of the two different protocols in groups 1 and 2. A better impact was observed in the group that followed the combined therapy (group 2), which may possibly be attributed to plant stem cells since the N-acetylglucosamine has a surface action [23]. In our study, a decrease in R2 in the placebo group during the 14 days of therapy was observed (0.71 ± 0.04 vs. 0.62 ± 0.08, p = 0.076), whereas an increase was observed in the OLEA group (0.75 ± 0.11 vs. 0.82 ± 0.06, p = 0.152). A statistically significant percentage change from the baseline of R2 between the two groups was noticed (p = 0.033). The elasticity R5 index was statistically significantly lower for the placebo group at D14 in comparison with the baseline (0.68 ± 0.22 vs. 0.50 ± 0.18, p = 0.0450), while the increase in the index for the OLEA group (0.73 ± 0.33 vs. 0.77 ± 0.32, p = 0.651) was not statistically significant. A statistically significant percentage change from D0 in the elasticity R5 index between the two groups was also noticed (p = 0.046). The elasticity R7 index was statistically significantly lower at D14 (p = 0.031) in relation to D0 for the placebo group, while it was statistically significantly larger for the OLEA group (p = 0.041). A statistically significant difference was observed in the percentage change from D0 to D14 in the Cutometer R7 index between the groups (p = 0.010).
Although the placebo formulation was rich in moisturizing ingredients, it did not manage to restore the area. In the literature, a reduction in skin elasticity has also been reported after the application of another type of laser, the alexandrite laser. The mechanism of action of Olea stem cell extract, through the activation of mitochondrial function, may be responsible for the statistically significant improvement in elasticity and restoration of the tested skin area. The positive effect of OLEA VITAE™ 02 on skin elasticity could be useful for additional conditions of reduced elasticity, i.e., scars. Skin scarring can result from burns, injuries, stretch marks, and acne; the application of the formulation of OLEA VITAE™ 02 could improve scarring. Currently, our laboratory is conducting a study on the application of Olea in scars, where elasticity is reduced, as well as the application of the ingredient in a group of postmenopausal women, where mitochondrial function is impaired.
The self-assessment results seem to align with the biophysical measurements. The participants who received the plant stem cell extract had statistically significantly better scores in anti-inflammatory activity and in erythema, as well as in the sensation of hydration, in comparison with the placebo group. Additionally, a statistically significant better score was observed in the case of the OLEA VITAE™ 02 cream regarding the parameters of pain relief, faster healing, and itching, as well as the feeling of better texture.
5. Conclusions
A physicochemical and microbiological O/W stable cream with the plant stem cell extract OLEA VITAE™ 02 and a placebo cream were developed with an aim to be used following laser epilation to reduce adverse effects that often appear, such as erythema, feeling of burning, and immediate or post-inflammatory hyperpigmentation, especially in skin prone to sensitization. A randomized double-blind study was conducted using biophysical measurements, self-assessment questionnaires, and a collection of skin biopsies. The results clearly revealed that the application of the OLEA VITAE™ 02 cream does not disturb the skin barrier, as confirmed by a previous clinical study [16]. The extracts of plant stem cells in the OLEA VITAE™ 02 formulation had a positive effect on the hydration of keratin after laser epilation, although such a claim has not been made thus far. The hypopigmentation effect of Olea stem cells after laser epilation leads us to the conclusion that the active ingredient in OLEA VITAE™ 02 could help the skin to heal without complications and maintain the tone it had before medium peeling procedures, where post-inflammatory hyperpigmentation can arise. The product proved to provide anti-irritant and anti-inflammatory action after laser epilation. As a result, the formulation could lead to clinical benefits and relief for patients who undergo medium peeling procedures. The active ingredient in the OLEA VITAE™ 02 formulation contributes to the increase in skin elasticity after the Nd:YAG 1064 nm laser epilation treatment of the skin. This could be useful for additional conditions of reduced elasticity, i.e., scars. Skin scarring can result from burns, injuries, stretch marks, and acne. The influence of the OLEA VITAE™ 02 formulation on skin elasticity could also have a clinical application on the decrease in adverse events of other aesthetic procedures, i.e., fibrosis and scaring after microblading [24]. Furthermore, OLEA VITAE™ 02 does not cause ocular damage or irritation and therefore could be proposed as an anti-irritant and anti-inflammatory regimen after blepharoplasty [25]. The results of skin biopsies are encouraging regarding the possible influence of OLEA VITAE™ 02 on collagenogenesis. Moreover, the results of the self-assessment questionnaires are in agreement with the biophysical measurements and skin biopsies.
To further elucidate and expand our findings, immunohistochemical markers could be employed to identify fibroblast activations in the stroma of our treated samples. The proliferation marker ki-67 may be employed for measuring actively proliferating fibroblasts, while FGFR (fibroblast growth factor receptor) immunostaining could identify fibroblastic cells that actively produce extracellular matrix components.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics12010002/s1.
Author Contributions
Conceptualization, A.V. and V.G.; methodology, A.V., V.G., S.P., P.P. and E.R.; software, V.G. and P.P.; validation, A.V., V.G., S.P. and D.C.; formal analysis, V.G. and A.P.; investigation, A.V., V.G., S.P. and E.P.; resources, A.V. and S.P.; data curation, V.G., A.V., P.P. and D.C.; writing—original draft preparation, A.V., V.G. and P.P.; writing—review and editing, V.G., A.V. and P.P.; visualization, A.V., V.G., and P.P.; supervision, AV.; project administration, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by the Special Account for Research Grants, University of West Attica, 28 Ag. Spyridonos Str., Panepistimioupolis Egaleo Park, GR-12243, Greece.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee, University of West Attica, Athens, Greece (Decision Number 37942/11-05-2021). According to the EU Cosmetic Products Regulation No. 1223/2009, the cosmetic product must not cause damage to human health when applied under normal or reasonably foreseeable conditions of use and must be assessed for its safety of use before human subjects are exposed to it, and as such, further ethical approval was not required. OLEA VITAE™ 02 and all the ingredients of the formulation are characterized as cosmetic ingredients (CosIng) https://ec.europa.eu/). This study was conducted in the Chemistry–Biochemistry–Cosmetic Science Laboratory, Department of Biomedical Science, University of West Attica, Greece, during the period from 2022–23 (specifically, this study ended at the end of 2022–beginning of 2023).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in this study. The anonymity of the volunteers was secured by codes as requested by the Code of Ethics and Conduct of Research, University of West Attica, Greece (https://research-ethics-comittee.uniwa.gr/kodikas-deontologias/, accessed on 8 October 2024).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
We thank Pagkalos Vassilios, Plastic Surgeon, for the collection of skin biopsies. We thank Galanos Antonios for his contribution to the statistical work. We would also like to thank Associate Professor Anastasios Papanastasiou and Emeritus Professor Frangiski Anthouli-Anagnostopoulou for the histopathological examination and scoring of tissue slides, as well as for providing valuable insights into this study. We thank Krallis S.A. for the kind provision of the active ingredient Olea Europaea (Olive) Callus Culture Lysate (OLEA VITAE™ 02).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zappia, E.; Federico, S.; Volpe, C.; Scali, E.; Nisticò, S.P.; Bennardo, L. Alexandrite and Nd:YAG Laser vs. IPL in the Management of Facial Hirsutism: A Retrospective Study. Photonics 2023, 10, 572. [Google Scholar] [CrossRef]
- Drosner, M.; Adatto, M.; European Society for Laser Dermatology. Photo-epilation: Guidelines for care from the European Society for Laser Dermatology (ESLD). J. Cosmet. Laser Ther. 2005, 7, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P.; Lanigan, S.W. A review of the adverse effects of laser hair removal. Lasers Med. Sci. 2006, 21, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Mallat, F.; Chaaya, C.; Aoun, M.; Soutou, B.; Helou, J. Adverse Events of Light-Assisted Epilation: An Updated Review. J. Cutan. Med. Surg. 2023, 27, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Kautz, G. (Ed.) Energy for the Skin; Kardoff Bernd; Springer: Cham, Switzerland, 2018; ISBN 978-3-030-90679-5/978-3-030-90680-1. [Google Scholar] [CrossRef]
- Atta-Motte, M.; Załęska, I. Diode Laser 805 Hair Removal Side Effects in Groups of Various Ethnicities—Cohort Study Results. J. Lasers Med. Sci. 2020, 11, 132–137. [Google Scholar] [CrossRef]
- Lerner, R.P.; Lee, E. EMLA-induced methemoglobinemia after laser-assisted hair removal procedure. Am. J. Emerg. Med. 2019, 37, e1–e2119. [Google Scholar] [CrossRef] [PubMed]
- Alster, T.; Garden, J.; Fitzpatrick, R.; Rendon, M.; Sarkany, M.; Adelglass, J. Lidocaine/tetracaine peel in topical anesthesia prior to a laser-assisted epilation: Phase-II and Phase-III study results. J. Dermatol. Treat. 2014, 25, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Hermosaningtyas, A.A.; Chanaj-Kaczmarek, J.; Kikowska, M.; Gornowicz-Porowska, J.; Budzianowska, A.; Pawlaczyk, M. Potential of Plant Stem Cells as Helpful Agents for Skin Disorders—A Narrative Review. Appl. Sci. 2024, 14, 7402. [Google Scholar] [CrossRef]
- Miastkowska, M.; Sikora, E. Anti-Aging Properties of Plant Stem Cell Extracts. Cosmetics 2018, 5, 55. [Google Scholar] [CrossRef]
- Marchev, A.S.; Georgiev, M.I. Plant In Vitro Systems as a Sustainable Source of Active Ingredients for Cosmeceutical Application. Molecules 2020, 25, 2006. [Google Scholar] [CrossRef]
- Wangthong, S.; Palaga, T.; Rengpipat, S.; Wanichwecharungruang, S.P.; Chanchaisak, P.; Heinrich, M. Biological activities and safety of Thanaka (Hesperethusa crenulata) stem bark. J. Ethnopharmacol. 2010, 132, 466–472. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Abdel Rahman, M.F.; Elhawary, E.; Hafez, A.M.; Capanoglu, E.; Fang, Y.; Farag, M.A. How does olive seed chemistry, health benefits and action mechanisms compare to its fruit oil? A comprehensive review for valorization purposes and maximizing its health benefits. Food Biosci. 2024, 59, 104017. [Google Scholar] [CrossRef]
- Available online: https://www.vytrus.com/natural-active/olea-vitae/ (accessed on 8 October 2024).
- Dang, Y.; Ren, Q.; Li, W.; Yang, Q.; Zhang, J. Comparison of biophysical properties of skin measured by using non-invasive techniques in the KM mice following 595 nm pulsed dye, 1064 nm Q-Switched Nd:YAG and 1320 nm Nd:YAG laser non-ablative rejuvenation. Skin Res. Technol. 2006, 12, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Modena, D.A.O.; Miranda, A.C.G.; Grecco, C.; Liebano, R.E.; Cordeiro, R.C.T.; Guidi, R.M. Efficacy and safety of ND:YAG 1064 nm lasers for photoepilation: A systematic review. Lasers Med. Sci. 2020, 35, 797–806. [Google Scholar] [CrossRef] [PubMed]
- ALOmair, I.A.; Ghobara, Y.A.; AlTalhab, S.; Alissa, A.; AlJasser, M.I. Hypopigmented macules following quality-switched 1064 nm laser epilation: A retrospective study. J. Dermatol. Dermatol. Surg. 2020, 24, 47–50. [Google Scholar] [CrossRef]
- Chan, N.P.; Ho, S.G.; Shek, S.Y.; Yeung, C.K.; Chan, H.H. A case series of facial depigmentation associated with low fluence Q-switched 1,064 nm ND YAG 1064 laser for skin rejuvenation and melasma. Lasers Surg. Med. 2010, 42, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Jia, X.; Duan, X.; Xu, Q.; Zhang, R.; He, Y.; Yang, Z. Q-switched1064 nm Nd: YAG laser restores skin photoageing by activating autophagy by TGFβ1 and ITGB1. Exp. Dermatol. 2024, 33, e15006. [Google Scholar] [CrossRef] [PubMed]
- Machała, P.; Liudvytska, O.; Kicel, A.; Heir, A.; Olszewska, M.A.; Zbikowska, H.M. Valorization of the Photo-Protective Potential of the Phytochemicals Standardized Olive (Olea europaea L.) Leaf Extract in UVA-Irradiated Humans Skin Fibroblasts. Molecules 2022, 27, 5144. [Google Scholar] [CrossRef]
- Khayatan, D.; Nilforoushzadeh, M.A.; Ashtiani, H.R.A.; Hashemian, F. Effect of Apple (Malus domestica) Stem Cells on UVB-Induced Damage Skin with Anti-Inflammatory Properties: An In Vivo Study. Adv. Mater. Sci. Eng. 2022, 2022, 2417766. [Google Scholar] [CrossRef]
- Mekas, M.; Chwalek, J.; MacGregor, J.; Chapas, A. An Evaluation of Efficacy and Tolerability of Novel Enzyme Exfoliation Versus Glycolic Acid in Photodamage Treatment. J. Drugs Dermatol. 2015, 14, 1306–1319. [Google Scholar] [PubMed]
- Serup, J.; Ercegovac, M.; Bennoun, I.; Hvas, D. (Eds.) Cosmetic and Medical Tattoos: Technique and Application; S. Karger AG: Basel, Switzerland, 2023; pp. 225–244. ISBN 978-3-318-07039-2. [Google Scholar] [CrossRef]
- Safety Data Sheet OLEA VITAE 02. Available online: https://www.vytrus.com/wp-content/uploads/MSDS-OLEA-VITAE_02_EN_v6-2.pdf (accessed on 8 October 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).