Abstract
Melasma, also known as chloasma and the “mask of pregnancy”, is a common acquired pigmentary disorder characterized by irregular, hyperpigmented brown-to-grey patches primarily situated on the face. It typically affects women of reproductive age, especially those exhibiting Fitzpatrick skin types III to V. The precise etiopathogenesis of melasma is complex and has not been fully elucidated; however, ultraviolet radiation, hormonal factors, and genetic predispositions significantly contribute to the melanin production increase associated with this condition. Due to the multifactorial aetiology, resistance to various therapeutic options, and high recurrence rate, treating melasma is challenging. Hydroquinone has long been considered a gold standard in melasma treatment due to its ability to inhibit tyrosinase; however, it has faced scrutiny after concerns about its adverse effects. Current treatment strategies include various topical and systemic therapies, procedural interventions, as well as combinations of these methods. For optimal results, both photoprotection and a treatment plan that targets different pathogenic mechanisms should be used. Additionally, treatment should be tailored to patient characteristics, such as skin type, the severity of the condition, and compliance. This review summarises current treatment options, focusing on long-term therapy and the latest advancements in managing this challenging condition.
1. Introduction
Melasma is a common acquired and relapsing pigmentary disorder presenting with irregular, hyperpigmented brown-to-grey patches symmetrically distributed on sun-exposed areas, primarily the face. It typically affects women of reproductive age, especially those exhibiting Fitzpatrick skin types III to V [1]. The prevalence of this condition varies considerably across different populations, ranging from 1.5% to 33% [2]. It is predominantly observed in individuals of East Asian, Middle Eastern, African, and Latin American descent [3]. While hyperpigmentation resulting from inflammation or sun exposure naturally fades after the triggering factor is removed, the pigmentation in melasma tends to persist [4].
The precise etiopathogenesis of melasma is complex and has not been fully elucidated; however, ultraviolet radiation, hormonal factors, and genetic predispositions significantly contribute to melanin production increase. Ultraviolet (UV) radiation upregulates melanocyte-stimulating hormone (MSH) receptors on melanocytes, thereby increasing hormone binding and subsequently enhancing melanin production. Furthermore, prolonged exposure to UV radiation induces dermal inflammation and triggers fibroblast activation. The activated fibroblasts subsequently secrete stem cell factor (SCF), which binds to the upregulated c-kit (stem cell growth factor receptor) in the epidermis. This interaction leads to the activation of the tyrosine kinase pathway, initiating melanogenesis. Additionally, UV-induced changes in the basement membrane, solar elastosis, mast cell count increase, and hypervascularization underlie the condition [5,6] (Figure 1). Hormonal influence in melasma pathogenesis has been evidenced by its increased occurrence in pregnant women and those on hormone replacement therapy (HRT) and oral contraceptive pills (OCP). In fact, 10–20% of patients taking OCT develop melasma [7]. Since melasma is a common finding in pregnant women, it is often deemed the “mask of pregnancy”, with studies reporting a prevalence ranging from 36.4% to 70% [8]. During pregnancy, elevated levels of oestrogen, progesterone, and melanocyte-stimulating hormone (MSH) promote melanogenesis through various regulatory pathways [9]. It has been shown that the epidermal layer of melasma lesions contains a greater number of progesterone receptors (PR), whereas the dermal layer exhibits a greater abundance of oestrogen receptors (ER). The interaction of oestrogen with its receptors on keratinocytes and melanocytes activates tyrosinase and thereby promotes melanogenesis. In addition, oestrogen can upregulate the expression of α-melanocyte-stimulating hormone (α-MSH) and PDZ domain protein kidney 1 (PDZK1), thereby boosting tyrosinase synthesis and promoting melanin production [5]. The impact of progesterone requires further clarification [3]. However, sex steroid hormones are unable to induce hyperpigmentation alone; instead, they work synergistically with UVB radiation [6]. This “mask of pregnancy” or chloasma gravidarum, typically resolves spontaneously within a year following delivery. Nevertheless, it may persist permanently in around 30% of women [8]. The genetic predisposition for developing melasma is highlighted by a positive family history in a subset of patients. In fact, according to studies, 55–64% of patients with melasma report family members affected by the condition [10]. Along with sun exposure, a positive family history is the most prevalent risk factor identified in men, and in women, it is pregnancy [2]. Familial melasma is characterized by a longer duration and is less likely to be triggered by hormonal contraceptives [4].
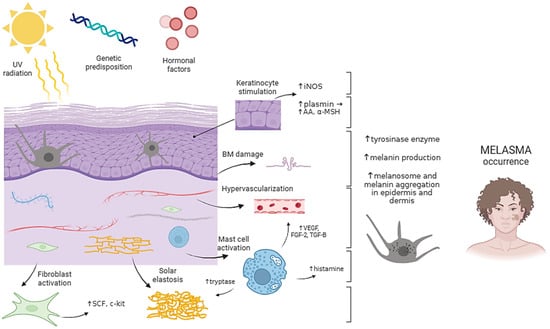
Figure 1.
Pathogenesis of melasma. UV radiation, genetic predisposition, and hormonal factors are key contributors to melasma development. Chronic sun exposure in susceptible individuals triggers a complex interplay between dermal and epidermal cells, ultimately leading to increased melanin production and hyperpigmented patches characteristic of melasma. Abbreviations: AA—arachidonic acid; BM—basement membrane; FGF-2—fibroblast growth factor 2; iNOS—inducible nitric oxide synthase; SCF—stem cell factor; TGF-B—transforming growth factor B; UV—ultraviolet; α-MSH—alpha-melanocyte stimulating hormone. Created with Biorender.com.
According to the regions affected by melasma, there are three primary clinical patterns: centrofacial, malar, and mandibular. Centrofacial melasma is the most common type, and it affects the nose, cheeks, forehead, chin, and upper lip, while sparing the nasolabial folds and philtrum. Malar melasma is confined to the malar cheek area, while the mandibular type appears along the jawline [2]. Therefore, symmetrically distributed and irregularly shaped macules and patches of melasma primarily affect the face, with less frequent occurrences on the neck and forearms [11].
Given its localization, chronic course, and tendency to recur, melasma may significantly negatively impact patients’ emotional and social well-being, though the correlation between the severity of melasma and quality of life is inconsistent across studies [12,13].
Due to the multifactorial aetiology, resistance to various therapeutic options, and high recurrence rate, treating melasma is challenging and often unsatisfactory. Sun protection using broad-spectrum sunscreen and protective clothing is a crucial component of melasma treatment. Ideally, sunscreens containing physical blockers such as zinc oxide and titanium dioxide are recommended. Treatment should be tailored to individual characteristics such as skin type, severity, and patient compliance. There are numerous approaches available, and in order to achieve optimal results, a treatment plan should target different pathogenic mechanisms [10,11]. Hydroquinone has long been considered a gold standard for treating melasma due to its ability to inhibit tyrosinase; however, it has faced scrutiny after concerns about its adverse effects. Current treatment strategies include different topical and systemic therapies, procedural interventions such as microneedling, chemical peels, and laser treatments, as well as combinations of these approaches [10]. To determine the severity of the disease and the efficacy of therapeutic options, several scales are used, with the most commonly applied being the Melasma Area and Severity Index (MASI), modified Melasma Area and Severity Index (mMASI), Melasma Severity Scale (MSS), and Melasma Severity Index (MSI) (Table 1) [14].

Table 1.
Commonly used scoring systems for melasma.
This review focuses on different therapeutic options for melasma, with the emphasis on the challenges when maintaining therapeutic effects after the initial therapy, and long-term efficacy and safety of future treatment strategies.
2. Topical Therapy
Topical preparations, including photoprotection, generally serve as the first-line treatment for melasma. A combination of different topical therapies with different mechanisms of action is favoured over monotherapy.
2.1. Photoprotection
Irrespective of the chosen therapeutic approach, photoprotection is essential for melasma treatment. Prolonged and rigorous photoprotection is essential to avoid the progression of existing hyperpigmentation, prevent the formation of new patches, and reduce the risk of relapses [15]. Photoprotection entails reducing sun exposure (especially during the middle of the day), wearing protective clothing, hats, and sunglasses, finding shade, and applying a broad-spectrum sunscreen with a Sun Protection Factor ≥ 30, with reapplications every 2 h when outside [16]. Since long-wave UVA radiation and high-energy visible light have recently been shown to contribute to the pathogenesis of melasma, sunscreens containing inorganic filters and iron compounds are recommended. However, since physical sunscreens often leave white residues, they may be cosmetically unappealing, which could result in reduced usage. Therefore, water-based and easy-to-apply formulas are more likely to ensure better compliance [17]. Additionally, tinted sunscreens that contain pigmentary iron oxides can provide camouflage, positively affecting patients’ quality of life [18]. Personalized photoprotection is an important approach that takes into account genetic factors, skin phototype, personal preferences in terms of sunscreen formulation, lifestyle, pollution levels, geographical location, work setting, and underlying skin conditions [19]. In order to achieve adherence, patient counselling and education are necessary.
2.2. Hydroquinone and Triple-Combination Therapy
Hydroquinone (HQ) is a topical depigmentation agent that has long been the mainstay of the treatment of melasma. It blocks melanin synthesis by inhibiting tyrosinase, an enzyme responsible for the hydroxylation of L-tyrosine into 3,4-dihydroxyphenylalanine (L-DOPA) and the oxidation of L-DOPA to dopaquinone, both necessary steps for forming eumelanin and pheomelanin [20]. Additionally, HQ contributes to melanosome and melanocyte degradation [21]. This hydroxyphenolic compound has typically been used at concentrations of 2–5% [16]. However, a more effective formulation, known as Kligman’s formula, has emerged. It uses a triple-combination therapy with HQ, a retinoid and a corticosteroid, usually consisting of HQ (4%), tretinoin (0.05%), and fluocinolone acetonide (0.01%). The effectiveness of the triple-combination therapy has been demonstrated to be slightly superior compared to using HQ alone at 4% or in dual combination with either of the other agents [22]. This therapy may show improvements or achieve clearance in up to 60–80% of melasma patients [16]; therefore, it is a first-line treatment option by many practitioners [23]. HQ faced controversies due to the side-effect profile of the drug. Namely, both irritant and allergic contact dermatitis, colloid milium, guttate hypomelanosis, changes in nail colour, paradoxical post-hyperpigmentation, and corneal degeneration have been described [24]. Moreover, a cutaneous condition known as exogenous ochronosis appeared as a complication of long-term HQ therapy. This disorder marked by black-bluish pigmentation, although rare, was even observed at low concentrations of HQ (1–2%) [24,25]. As a result, due to concerns regarding exogenous ochronosis and proposed carcinogenic risks in animal studies, the US Food and Drug Administration (FDA) banned over-the-counter hydroquinone. Since 2020, HQ is only available through prescribed formulations or newly approved medications [16,24].
2.3. Azelaic Acid
Azelaic acid (AZA) is a saturated dicarboxylic acid used in topical treatments for various dermatological conditions, such as acne, rosacea, and hyperpigmentation disorders. It is found in both over-the-counter and prescription products in the form of gels and creams containing 5% to 20% AZA. Besides its antioxidant and anti-inflammatory properties, AZA’s inhibition of tyrosinase makes it beneficial in treating melasma [26,27]. AZA may be incorporated into a treatment plan either as monotherapy or in combination with other medications like oral tranexamic acid (TXA) [28]. A randomized controlled trial by Tehrani et al. [29] compared AZA 20% with HQ 5% to HQ 5% alone, showing superior efficacy of combination treatment in melasma reduction.
A meta-analysis by Albzea et al. [30] compared AZA to HQ in terms of efficacy and safety in melasma treatment. According to their results, AZA was more effective than HQ in improving Melasma Area and Severity Index (MASI) scores. However, there was no significant difference in pigmentation reduction, and both treatments had similar rates of reported adverse events, including stinging, burning, erythema, and scaling [27].
2.4. Retinoids
Retinoids such as tretinoin, adapalene, tazarotene, and isotretinoin act as depigmenting agents by accelerating keratinocyte turnover, inhibiting tyrosinase, and decreasing melanosome transfer [21,24]. Additionally, their ability to alter the stratum corneum and permeability barrier may enhance transepidermal penetration of other depigmenting agents [31]. Tretinoin or all-trans-retinoic acid (ATRA) at 0.05–1% is a widely used retinoid for treating melasma. It may be used as monotherapy; however, noticeable results require almost 6 months of regular application [16]. Studies evaluating the efficacy of tretinoin 0.1% cream versus placebo in Caucasian and African American patients with melasma showed significant improvement in depigmentation in the Caucasian group after 24 weeks of tretinoin treatment. In contrast, this improvement was only marginal in the African American group [32,33]. Another randomized controlled trial assessed the efficacy of a combination of topical retinoids compared to placebo. The study showed a significant (70%) improvement in MASI scores on the treated side, similar to the improvement typically seen with HQ [34]. A combination of ATRA and a low concentration of HQ improves the effectiveness of HQ and reduces the unpleasant adverse effects that are associated with higher concentrations of HQ [35].
2.5. Corticosteroids
Corticosteroids inhibit cytokines like endothelin-1 (ET-1) and granulocyte-macrophage colony-stimulating factor (GM-CSF), as well as prostaglandins, all of which promote melanin production [16]. In addition, their anti-metabolic effect leads to decreased turnover of the epidermis, thereby producing mild depigmentation [36]. However, topical corticosteroids are rarely used alone in melasma treatment because of their potential adverse effects, including skin thinning, acne-like eruptions, facial hypertrichosis, and telangiectasias [36,37]. Compared to other corticosteroids, fluticasone exhibits lower atrophogenic properties. Fluocinolone acetonide, hydrocortisone, dexamethasone, fluticasone, and mometasone furoate are still preferred components of triple-combination therapy [38].
2.6. L-Ascorbic Acid
Ascorbic acid, widely known as vitamin C, is an antioxidant found in various cosmeceuticals, and it is well known for its depigmentation properties. Its effectiveness in skincare formulations is limited by its low permeability and susceptibility to rapid oxidation [39]. Therefore, esterified forms of ascorbic acid, such as magnesium ascorbyl-2-phosphate (MAP), are often used because of their greater stability and lipophilic nature, which consequently leads to better penetration through the stratum corneum [40]. Ascorbic acid prevents UV-induced pigmentation by inhibiting tyrosinase through interaction with copper at the enzyme’s active site and blocking the oxidative polymerization of melanin precursors [41]. Furthermore, it stimulates collagen synthesis and provides photoprotection by preventing the absorption of UV radiation [42,43]. Trials comparing ascorbic acid to HQ showed somewhat superior results of HQ in melasma treatment, although treatment with ascorbic acid still showed improvement in pigmentation and had minimal side effects [44]. The use of ascorbic acid in melasma treatment has been effective, especially when combined with Q-switched Nd:YAG laser therapy, as it has been shown to boost its effects [45]. Also, combinations with mesotherapy, iontophoresis, vitamin E, tranexamic acid, or fractional Q-switched ruby laser show greater treatment success than using vitamin C alone [46,47,48,49,50]. Adverse effects of topical vitamin C therapy may involve contact dermatitis but are rarely reported [39].
2.7. Kojic Acid
Kojic acid (KA) is a naturally derived fungal metabolite that has shown benefits in melasma treatment because of its skin-lightening properties. In addition to its antioxidant properties, KA chelates copper ions and inhibits tyrosinase activity [51]. It is typically available in concentrations ranging from 1 to 4%, and optimal results are often achieved by combining KA with other depigmentation products [52]. A double-blind comparison demonstrated that the combination of 2% KA, 2% HQ, and 10% glycolic acid led to greater melasma improvement than 2% HQ and 10% glycolic acid alone [53]. KA as monotherapy may not be as effective. In a comparative study, KA 0.75% was inferior to HQ 4% cream in achieving depigmentation [54]. A similar result was obtained when comparing KA 2% cream to modified Kligman’s formula [55]. However, it is a useful agent in patients who are either intolerant or respond poorly to first-line therapies. The cosmetic use of KA is generally associated with few adverse effects, with irritant contact dermatitis (seen mostly in the sensitive skin types), being the main one [52].
2.8. Tranexamic Acid
Tranexamic acid (TXA) is a synthetic derivative of lysine that has become widely used to treat melasma via topical, oral, and injectable preparations. Treatment success typically depends on TXA formulation and concentration. Liposomal formulations containing 2–5% TXA are typically used, and their effects are evident after 2 to 3 months of continuous application [38,56]. Recent studies comparing TXA 5% cream to different concentrations of HQ 2–4% showed similar reductions in MASI with TXA and HQ after 12 weeks of treatment. However, patients in the TXA group reported higher satisfaction levels and fewer adverse effects [57,58,59]. Despite positive outcomes, the topical form of TXA is not suitable for monotherapy and is less successful than its oral and injectable counterparts [10,60]. Cosmeceuticals containing a combination of depigmenting agents such as TXA, KA, and niacinamide are safe therapeutic options with very high patient satisfaction [61].
2.9. Niacinamide
Niacinamide, the amide form of vitamin B3, has recently been studied in the treatment of various dermatologic conditions due to its anti-inflammatory and antioxidant properties [62]. The mechanism by which niacinamide regulates pigmentation is not entirely understood. It is proposed that niacinamide and its metabolites are involved in melanosome transfer signalling [63]. The effect of skin depigmentation using 4% niacinamide cream is nearly comparable to HQ 4% cream [64]. Besides photoprotection, combination with other topical agents or methods boosts niacinamide’s effectiveness [61,63]. It is typically a well-tolerated agent, but prolonged use may lead to pruritus, erythema, and a mild burning sensation [64].
A recent study found that changes in the nicotinamide nucleotide transhydrogenase (NNT) could influence melanin production in the skin. Specifically, the inhibition of NNT activity may lead to increased skin pigmentation, a mechanism that is independent of UVB radiation [65]. Newly recognized pathways may pave the way for new therapeutic interventions.
Further research is needed to specifically investigate pure niacinamide for treating pigmentation in melasma [66].
2.10. Arbutin
Arbutin is a compound made of D-glucose bound to HQ that is naturally found in different plant species. Due to its skin-lightening properties, it is a component of various cosmeceuticals [67,68]. Most studies suggest that arbutin competitively inhibits tyrosinase or irreversibly inactivates it, which blocks the production of melanin [68]. Arbutin has a similar inhibitory effect on tyrosinase to HQ; however, it is less efficacious than KA. Additionally, it prevents melanosome maturation [69]. In comparison to HQ, arbutin is less toxic to melanocytes [70]. Deoxyarbutin (dA) is a synthetic form of arbutin that is more stable and more potent than natural arbutin. A recent randomized controlled study compared a 2% dA to a 4% HQ serum over 12 weeks and showed a similar decrease in the melanin index and therefore, similar depigmenting properties in both groups. However, there is a concern about dA metabolizing to HQ, which could potentially lead to toxicity [71]. Lastly, although arbutin may be more effective at higher concentrations, there is a risk of paradoxical hyperpigmentation [40].
3. Oral Therapy
3.1. Oral Tranexamic Acid
Oral TXA is safe, effective, and a convenient therapeutic option for melasma that may be used alone or in combination with other treatments. Its efficacy has been documented in a number of studies [56,60]. TXA is a valuable antifibrinolytic agent as it inhibits the conversion of plasminogen to plasmin (a molecule responsible for fibrin degradation). Besides its use in surgical settings, it is beneficial in blocking keratinocyte–melanocyte interactions. Essentially, UV radiation and hormones activate the plasmogen activator system in keratinocytes and epidermal basal cells, leading to plasmin formation. This, in turn, produces inflammatory mediators such as arachidonic acid (AA) and prostaglandins that increase melanocyte tyrosinase activity. Additionally, plasmin-induced increase in α-melanocyte stimulating hormone (α-MSH) and fibroblast growth factor (FGF) further contributes to melanin synthesis [69,70]. Therefore, TXA reduces melanocyte response to UV radiation. Furthermore, by inhibiting vascular endothelial growth factor (VEGF), TXA also reduces angiogenesis that is implicated in the pathogenesis of melasma [72]. A recent meta-analysis by Feng et al. [73] revealed significant reductions in MASI scores following oral TXA treatment, suggesting that TXA as monotherapy or adjuvant therapy may be superior to standard treatment. However, no significant differences were detected in the melasma index (MI) and erythema index (EI) between standard treatment methods and TXA. The study also suggested that oral TXA may provide better results than topical or intradermal injections of TXA. To date, there is no official guideline for TXA use in melasma [74]. Oral TXA at a dosage of 250 mg twice daily appears to be a good treatment option, while being much lower than the dose used for haemostasis. Interestingly, studies that employed higher doses of oral TXA showed similar results in MASI and MI scores as those with lower doses [73]. One study compared a combination of oral TXA and 3% topical TXA versus oral TXA and 20% AZA. Both groups had a good therapeutic response, but the combination of oral and topical TXA showed a better improvement in the mean MASI score [75]. Furthermore, the effect of oral TXA is enhanced when paired with other procedures, such as Q-switched Nd:YAG laser [76].
A minimum of 3 months may be necessary in order to see the results of oral TXA. Further research is required to assess the ideal length of treatment. According to expert opinions, oral TXA may be taken for up to 6 months [74]. It is a promising option for moderate-to-severe recurrent melasma and for refractory melasma [77,78].
The most commonly reported adverse effects of oral TXA are gastrointestinal discomfort and menstrual irregularities. Before initiating treatment, patients should be evaluated for any risk factors for thrombosis or thromboembolism [79,80].
3.2. Glutathione
Glutathione (GSH) is a tripeptide consisting of glycine, cysteine, and glutamate. It is a valuable antioxidant in aerobic organisms. GSH is becoming regarded as a skin-lightening agent due to its inhibition of tyrosinase and the ability to shift melanogenesis from eumelanin to pheomelanin [15]. It may be administered as a topical, oral, or intravenous agent. However, despite a recent increase in publicity regarding intravenous GSH, there is no evidence to prove its benefits and it may, on the contrary, be associated with life-threatening reactions such as anaphylaxis and Stevens–Johnson syndrome (SJS) [81,82]. Regarding oral GSH, a randomized controlled trial in 60 young participants showed that 250 mg of GSH twice daily over 4 weeks reduced the MI and the development of lentigines in the GSH group. This suggests that GSH might influence new melanin production rather than existing pigment [83]. Another trial that assessed a combination of topical and oral GSH showed a superior skin-lightening effect with the two agents than with monotherapy [84].
Topical and oral GSH have no significant side effects and are usually well tolerated. Further studies are needed to assess the benefits of GSH in melasma treatment [82].
The aforementioned topical and oral treatment options are presented in Table 2.

Table 2.
Selected clinical results of the currently available topical and oral treatment strategies in melasma.
4. Procedural Therapy
Procedural interventions serve as a second-line treatment option for patients unresponsive to topical therapy or as an adjunct to topical treatments when these alone are insufficient. While procedural therapies offer promising results, maintenance is crucial for sustained improvement. Although effective, procedures may cause some pain and discomfort, temporary redness, swelling, irritation, post-inflammatory hyperpigmentation, and on rare occasions, allergic reactions and infections.
4.1. Platelet-Rich Plasma
Treatments with platelet-rich plasma (PRP) have gained popularity in recent decades due to their regenerative potential. The use of an autologous serum containing increased concentrations of platelets and growth factors helps regenerate stem cells and remodel soft tissues. PRP’s applications in dermatology are wide and include hair restoration, scar and striae treatment, and skin rejuvenation [85]. Furthermore, it has recently been introduced as a treatment option for melasma [86]. There are two reported mechanisms by which PRP improves melasma: one is increased synthesis of extracellular components that leads to increased skin volume; and the other is decreased melanin synthesis [87]. Furthermore, the anti-inflammatory properties of PRP may enhance this effect [85]. Treatment is usually achieved by delivering PRP through microneedling or intradermal injections.
A meta-analysis by Zhao et al. [88] showed the highest satisfaction rate among participants treated with the combination of PRP and microneedling. Thus, it outperformed both PRP as monotherapy and intradermally administered PRP. Nevertheless, an analysis of all included studies revealed that the mean modified MASI (mMASI) score decreased by 1.18 after treatment.
One recent study compared intradermal TXA to intradermal PRP in 40 participants by injecting TXA into one side of the face, and PRP into the other. Although both treatments resulted in a significant reduction in the mMASI score, this was more prominent on the PRP-treated side, showing that intradermal PRP may have superior efficacy in treating melasma [89].
Furthermore, a study by Gamea et al. [90] showed that intradermal injections of PRP enhanced the effect of topical TXA in a liposome-based cream. However, another study reported no added advantage of PRP in patients who received topical TXA treatment [91].
Adverse effects of PRP therapy are minor; a small number of patients may experience temporary redness, local congestion, discoloration, and hyperpigmentation [88].
Interpreting studies on PRP treatment is challenging; there is a lack of standardized preparation protocols for PRP, different clinical endpoints are used, and PRP is often employed in combination with other methods [92]. Finally, PRP is a safe option with high patient satisfaction. Since its use in melasma is a new concept, additional research has yet to determine its regular application.
4.2. Intralesional Tranexamic Acid
Intralesional delivery of TXA is achieved through two routes: transepidermal (using microneedling) and intradermal (using localized microinjections). This method offers controlled administration of therapy, avoids systemic adverse effects of oral TXA, and provides better availability in the skin compared to topical TXA [38,93]. Most commonly, solutions prepared for intralesional administration use TXA at a concentration of 4 mg/mL [38].
Microneedling is performed at a usual depth of 1.5 mm using a dermapen or a dermaroller, which therefore allows better absorption of topical TXA into the dermal layer. It is usually well tolerated and has a limited adverse effect profile. Some patients report erythema, pain, pruritus, and a burning sensation after the treatment, all of which typically subside within a few hours to a few days. Studies have shown that although microneedling on its own is beneficial in patients with melasma, the addition of TCA in the treatment regimen leads to a greater reduction in MASI scores [94,95]. On the contrary, results of some studies suggested no additional benefit of adding microneedling with TXA to a 4% HQ regimen [96,97]. Nevertheless, despite discrepancies in clinical trials, a recent meta-analysis supports the use of microneedling with TCA over topical TCA alone [73].
Intradermal injections, or mesotherapy, uses small needles to deliver TXA into the dermis, with injections placed 1 cm apart. Studies have shown that intradermal TXA leads to a significant improvement in MASI score, especially if combined with other treatments [98,99]. Additionally, higher concentrations of TXA are not superior to injections with 4 mg/mL TXA [100]. Due to a high rate of recurrence in melasma, it may not be sufficient to use intradermal TCA solely for maintenance therapy; combination with another therapeutic option (along with photoprotection) may be necessary [101]. In conclusion, intralesional TXA is an effective method for improving melasma. It acts directly on the affected skin region, and it is safe and minimally invasive. Patients may experience burning pain at the injection site, along with local erythema and swelling, which generally resolves within 1–2 h after the injection. Additionally, minimal pain, bruising, and irritation may occur [98,99,100,101]. Long-term 48-week administration of 4 mg/mL TXA demonstrated only local adverse effects, with systemic adverse effects being avoided due to the TXA dose being considerably lower than the antifibrinolytic dose [101]. However, there are no standardized treatment intervals, as the sessions are still being determined by the treating physician [93].
4.3. Chemical Peels
Chemical peels use exfoliative agents to induce skin regeneration by increasing the turnover of epidermal keratinocytes. They are commonly employed for both cosmetic and therapeutic purposes, such as acne and acne scars, pigmentation disorders, and signs of skin ageing. Chemical peels can be categorized into three groups based on their depth of penetration into the skin layers, namely superficial, medium-depth, and deep peels [101,102,103].
When considering melasma, they are usually not the primary treatment option and are mainly used as an additional therapy. Agents that are frequently used are 15% or 20% trichloroacetic acid (TCA), 30%, 50%, or 70% glycolic acid (GA), 20% or 30% salicylic acid (SA), and Jessner’s solution [15]. They are generally safe, effective, and can even achieve results faster than topical agents [104,105]. However, conventional exfoliative agents may potentially cause irritation, epidermal necrosis, and post-inflammatory hyperpigmentation (PIH) [106]. It is important to note that post-inflammatory hyperpigmentation is more common in individuals with darker skin types, the same group that suffers from melasma more often [107]. Pigmentary complications arising after chemical peels in darker-skinned patients are usually associated with TCA. Furthermore, the use of TCA in this group is less preferred due to the risk of scarring [108]. Moreover, a limitation of alpha hydroxy peels is the need for neutralization, and it may be challenging to determine the exact timing for it [109]. Newly introduced agents like amino fruit acid and phytic acid may overcome the disadvantages of traditional chemical peels, including overpeeling, burning sensation, and the need for neutralization [38,109].
4.4. Laser and Light-Based Therapy
Laser and light-based therapies use light energy to treat various clinical and cosmetic skin conditions. To achieve a clinical effect, laser light must use the appropriate wavelength in order to be absorbed by the chromophores in the skin. The endogenous chromophore melanin has a broad absorption spectrum, ranging from about 630 nm to 1100 nm [110]. Therefore, various devices with appropriate wavelengths have been studied in hyperpigmentary disorders, including melasma. They accelerate melanin removal, rather than target its production [11]. Laser treatments are one of the newer methods used for melasma treatment; however, they are usually not recommended as first-line therapy and may offer benefit to resistant and recurrent cases, as well as in combination treatments [110,111]. Laser therapy may be associated with erythema, scaling, burning sensation, oedema, and in patients with darker skin types, PIH and hypopigmentation [16,112]. To date, there is still no consensus on the laser treatment regimen and the required number of sessions.
Intense pulsed light (IPL) is a light-based device that, unlike lasers, emits noncoherent and noncollimated light pulses of different wavelengths, ranging from 515 to 1200 nm. This allows for selective absorption in the melanosomes and simultaneous targeting of multiple layers of the epidermis and dermis [16,110]. Additionally, IPL’s pulse duration is measured in milliseconds, which allows for a wider heat distribution and reduces the risk of PIH. A recent meta-analysis reported high patient satisfaction and a significant reduction in MASI following combined therapy with IPL [113]. A randomized controlled trial that evaluated the effectiveness of IPL therapy in combination with triple-combination therapy (TCC) versus TCC alone showed considerable improvement in the IPL/TCC group compared to the controls. In the IPL/TCC group, there was a 49.4% reduction in MASI scores at 6 months, which persisted as a 44.9% reduction at 12 months [114]. Another recent retrospective study on fifty patients with melasma, who were treated exclusively with IPL, demonstrated a statistically significant correlation between IPL treatments and reductions in MASI scores [115]. Therefore, IPL may be beneficial in patients with refractory melasma when combined with effective topical therapy, preferentially in lighter skin types and in epidermal melasma [116]. Typical side effects include minor tingling sensation and erythema, both of which usually subside within a day. In a small number of patients, increased energy levels may cause mild exfoliation of the skin, though it generally resolves without leaving scars in a week [113].
Q-switched lasers are among the most extensively studied lasers in melasma treatment. Their beams are highly effective at targeting melanin and exist in different wavelengths, namely at 694 nm (Q-switched ruby laser), 755 nm (Q-switched alexandrite laser), and 532 nm or 1064 nm (Q-switched Nd:YAG laser) [11]. At present, the most commonly utilized Q-switched laser is low-fluence Q-switched (LFQS) Nd:YAG laser, also known as laser toning [117,118]. This technique selectively heats and destroys melanosomes and melanin within keratinocytes while preserving the cell membrane and the nucleus [119]. Treatments with LFQS Nd:YAG laser are effective, especially when combined with other topical agents, oral TXA, or chemical peels [77]. In order to achieve a good clinical effect, multiple treatments should be performed within a short period of time, such as weekly [116]. A recent network meta-analysis suggests that LFQS Nd:YAG laser paired with topical medications is superior to all other laser treatments [120]. Nevertheless, this laser carries a risk of mottled hypopigmentation and melasma relapse three months after treatment. Although combined treatments avoid recurrence, long-term studies are needed [121].
Fractional lasers include non-ablative (NAFL) and ablative fractional lasers (AFL). Their beams are absorbed by water-containing tissues and create columns of thermal injury that are intertwined with unaffected skin zones. Most commonly employed are fractional ablative 2940 nm Er:YAG laser, fractional ablative CO2 laser, and fractional non-ablative 1550/1540 nm laser [110]. NAFL laser with the highest water absorption coefficient, the 1927 NAFL thulium laser, shows the best response after a single treatment and is effective in treating patients with darker skin types [122]. Generally, NAFLs are favoured in darker skin types because they carry a lower risk of PIH than other laser treatments [123]. AFLs are usually not advised in melasma treatment due to the high incidence of both adverse effects and relapses. Typical adverse effects include a burning sensation, temporary erythema, swelling, and superficial crust formation. A small number of patients may experience reversible PIH, acne, and oral herpes [124]. If a specialist opts for an AFL treatment, low-fluence CO2 lasers are preferred, typically in combination therapy [110,124].
Recent studies on procedural treatments for melasma are presented in Table 3.

Table 3.
Selected recent clinical results of the currently available procedural treatments in melasma.
The treatment modalities for melasma discussed in the text are illustrated in Figure 2.
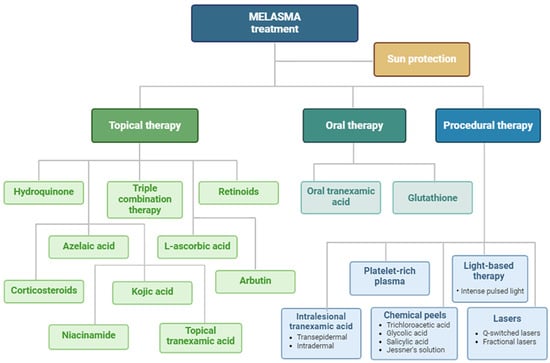
Figure 2.
A schematic representation of described therapeutic options in melasma. Created with Biorender.com.
5. Novel Strategies in Melasma Treatment
Due to the ongoing challenges in melasma treatment, innovative approaches such as nanotechnology aim to improve the absorption of topical agents and increase their efficacy in the skin. Nanoparticles (NPs) facilitate better availability, stability, and controlled release of prescribed treatments [130].
Lipid nanoparticles are new drug delivery systems that include solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs). They may improve skin hydration and elasticity, enhance drug delivery and protect against its degradation [131]. Studies have demonstrated that HQ-loaded SLNs yielded greater drug accumulation in the skin than conventional HQ. Additionally, HQ encapsulation in SLNs reduces systemic absorption, consequently diminishing adverse effects [132]. Similarly, HQ-loaded NLCs and AZA-loaded NLCs showed beneficial prolonged drug release and reduced skin irritation [133,134].
Nanoemulsions and microemulsions are colloidal delivery systems that are able to transport both hydrophilic and lipophilic molecules through the skin layers [135]. A recent study showed that nanoparticles loaded with AZA and hyaluronic acid may improve drug deposition, inhibit tyrosinase, and lower cytotoxicity [136]. Another study revealed increased drug release and stability of HQ-loaded microemulsions compared to conventional HQ [137].
Liposomes are vesicles composed of lipid bilayers that easily interact with the cell membrane and distribute both hydrophilic and hydrophobic drugs [131]. In a study on patients with melasma, a liposomal serum containing AZA, retinol, and 4-n-butylresorcinol led to substantial improvements in their MASI scores [138]. On the contrary, the results of another study suggested that although liposomal HQ produced a good therapeutic effect, no advantage over conventional HQ was noted [139].
Niosomes are unilamellar or multilamellar vesicles composed of nonionic surfactants. They are characterized by a rigid membrane bilayer, offering several advantages over liposomes, such as better chemical stability, drug containment, and affordability [131,140]. Niosomal formulations of KA and HQ demonstrate a gradual and more consistent drug release [141].
Transferosomes are highly deformable vesicles composed of a lipid bilayer and membrane-softening components. This recent innovation enables transferosomes to enter the stratum corneum with ease and improves transepidermal drug delivery [142]. Therefore, depigmenting agents encapsulated in transferosomes seem to inhibit melanogenesis more efficiently while also being safe [143].
Finally, the application of nanotechnology has gained considerable attention over the last decades, particularly due to its wide use in the cosmetic industry. NPs have been scrutinized due to their complex chemical and physical properties, potential interactions, and questionable toxicological profiles [144]. The ability of NPs to form reactive oxygen species (ROS) when exposed to UV light has raised fears about their possible long-term toxicity [130]. Additionally, the effects on health and the environment, such as potential transport through sewage systems and biomagnification, have been questioned [145]. These concerns resulted in several regulations published by the European Commission, with the final regulation issued in 2009 [146]. It integrated the latest technological advancements in the cosmetic industry, including those related to nanomaterials. This ensured further research data on nano-enhanced products, improved transparency for customers, and rigorous safety standards. However, there remains a need for additional regulations concerning lipid-based nanoparticles, as they are currently researched and marketed more liberally [134]. Nowadays, various coatings are applied to NPs to minimize reactions that result in ROS formation [130]. Ultimately, nanotechnology offers better penetration through the stratum corneum, greater stability of active ingredients, and reduced toxicities through controlled drug release. However, its long-term efficacy and advantages over first-line treatment options have yet to be fully determined [134].
6. Maintenance Therapy
Since melasma shows a pronounced tendency to recur, maintaining the therapeutic effect after the initial treatment is a real challenge. Furthermore, topical lightening agents are often associated with irritative dermatitis, which can result in post-inflammatory hyperpigmentation.
The cornerstone of maintenance therapy is strict photoprotection. A broad-spectrum sunscreen with SPF 50+ that shields against UVA, UVB, and visible light, preferably with iron oxides for enhanced protection, should be used. Sunscreen must be applied to the entire face every day, regardless of the season, and a broad-brimmed hat should be worn outdoors to further shield the skin from the sun. Exposure to heat sources at work and home should be reduced, as heat can exacerbate melasma. Any known triggers that worsen the condition should be identified and avoided [147].
Non-hydroquinone bleaching agents such as azelaic acid, topical retinoids, niacinamide, and kojic acid are recommended for maintenance therapy. A hydroquinone 2% cream can be applied intermittently for a limited time, considering the potential risk of irritative dermatitis and ochronosis [148]. A limited number of studies support the intermittent application of a triple-combination (TC) cream, consisting of hydroquinone (4%), tretinoin (0.05%), and fluocinolone acetonide (0.01%) (twice weekly). One notable study conducted by Arellano et al. aimed to evaluate the effectiveness of two different 6-month maintenance regimens in preventing the recurrence of melasma after an initial 8-week treatment with a TC cream. One regimen involved applying the TC cream twice weekly, and the other one involved applying the TC cream once weekly combined with the daily application of a broad-spectrum sunscreen. Both maintenance regimens effectively sustained the improvements achieved during the initial treatment phase. Additionally, both regimens were generally well tolerated by patients, with some experiencing mild, manageable irritation that did not lead to discontinuation of the treatment [149].
7. Conclusions
Melasma is a distressing dermatologic condition that frequently requires a comprehensive approach to control its chronic and relapsing nature. Treatment of this pigmentary disorder should be tailored to each patient and take into account factors such as skin type, personal medical history, melasma severity, and patient preferences. Various therapeutic options are available, including topical and oral depigmenting agents, chemical peels, and laser and light-based therapies. Moreover, different procedural interventions and innovative methods for drug delivery are continuously being developed.
However, melasma is quite often resistant to therapy. It is more challenging to treat in individuals with Fitzpatrick skin types III–V, a genetic predisposition, or a family history of the condition. Other factors that worsen the prognosis include having the condition for over two years without improvement, long-term use of topical steroids, developing ochronosis from extended hydroquinone use, undergoing multiple treatments like lasers or microneedling, seeing multiple doctors indicating stubborn disease, and having mixed-type melasma [147].
Irrespective of the chosen therapeutic approach, photoprotection is essential for preventing the worsening of the condition, recurrence following therapy, and the emergence of new lesions.
First-line treatment usually includes a topical 4% HQ cream on its own or as part of a TC alongside a retinoid and a corticosteroid. However, prolonged use may be associated with adverse effects, including atrophy, perioral dermatitis, and ochronosis. Other depigmenting agents such as TXA, AZA, and KA are becoming increasingly popular due to their effectiveness in managing melasma. Nevertheless, the greatest benefits are observed when agents are combined with other drugs or methods such as microneedling, microinjections, energy-based devices, and other skin-lightening and resurfacing techniques. Recent advancements in nanotechnology may secure the improved efficacy of applied treatment and reduce adverse effects. The mentioned therapeutic options often yield equivocal results in clinical trials. Therefore, further research is needed to refine treatment protocols for long-term control of this condition.
Author Contributions
Conceptualization, E.P. and Z.B.M.; methodology, Z.B.M.; software, E.P.; validation, E.P. and Z.B.M.; formal analysis E.P. and Z.B.M.; investigation, E.P. and Z.B.M.; resources, Z.B.M.; data curation, E.P. and Z.B.M.; writing—original draft preparation, E.P.; writing—review and editing, Z.B.M.; visualization, E.P.; supervision, Z.B.M.; project administration, E.P. and Z.B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arrowitz, C.; Schoelermann, A.M.; Mann, T.; Jiang, L.I.; Weber, T.; Kolbe, L. Effective Tyrosinase Inhibition by Thiamidol Results in Significant Improvement of Mild to Moderate Melasma. J. Investig. Dermatol. 2019, 139, 1691–1698.e6. [Google Scholar] [CrossRef]
- Majid, I.; Aleem, S. Melasma: Update on Epidemiology, Clinical Presentation, Assessment, and Scoring. J. Skin. Stem Cell. 2022, 8, e120283. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Q.; Xia, Y. New Mechanistic Insights of Melasma. Clin. Cosmet. Investig. Dermatol. 2023, 16, 429–442. [Google Scholar] [CrossRef]
- Espósito, A.C.C.; Cassiano, D.P.; da Silva, C.N.; Lima, P.B.; Dias, J.A.F.; Hassun, K.; Bagatin, E.; Miot, L.D.B.; Miot, H.A. Update on Melasma—Part I: Pathogenesis. Dermatol. Ther. 2022, 12, 1967–1988. [Google Scholar] [CrossRef] [PubMed]
- Rajanala, S.; Maymone, M.B.d.C.; Vashi, N.A. Melasma Pathogenesis: A Review of the Latest Research, Pathological Findings, and Investigational Therapies. Dermatol. Online J. 2019, 25, 10. [Google Scholar] [CrossRef]
- Artzi, O.; Horovitz, T.; Bar-Ilan, E.; Shehadeh, W.; Koren, A.; Zusmanovitch, L.; Mehrabi, J.N.; Salameh, F.; Isman Nelkenbaum, G.; Zur, E.; et al. The Pathogenesis of Melasma and Implications for Treatment. J. Cosmet. Dermatol. 2021, 20, 3432–3445. [Google Scholar] [CrossRef]
- Böhm, M. Disorders of Melanin Pigmentation. In Braun-Falco’s Dermatology, 4th ed.; Plewig, G., French, L., Ruzicka, T., Kaufmann, R., Hertl, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1245–1279. [Google Scholar] [CrossRef]
- Türkmen, H.; Yörük, S. Risk Factors of Striae Gravidarum and Chloasma Melasma and Their Effects on Quality of Life. J. Cosmet. Dermatol. 2023, 22, 603–612. [Google Scholar] [CrossRef]
- Filoni, A.; Mariano, M.; Cameli, N. Melasma: How Hormones Can Modulate Skin Pigmentation. J. Cosmet. Dermatol. 2019, 18, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Ogbechie-Godec, O.A.; Elbuluk, N. Melasma: An Up-to-Date Comprehensive Review. Dermatol. Ther. 2017, 7, 305–318. [Google Scholar] [CrossRef]
- Piętowska, Z.; Nowicka, D.; Szepietowski, J.C. Understanding Melasma-How Can Pharmacology and Cosmetology Procedures and Prevention Help to Achieve Optimal Treatment Results? A Narrative Review. Int. J. Environ. Res. Pub. Health 2022, 19, 12084. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, X.; Ying, J.; Cai, Y.; Qiu, Y.; Xiang, W. Evaluating the quality of life among melasma patients using the MELASQoL scale: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0262833. [Google Scholar] [CrossRef]
- Sheth, V.M.; Pandya, A.G. Melasma: A comprehensive update: Part II. J. Am. Acad. Dermatol. 2011, 65, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Heidemeyer, K.; Cazzaniga, S.; Feldmeyer, L.; Imstepf, V.; Adatto, M.; Lehmann, M.; Rammlmair, A.; Pelloni, L.; Seyed Jafari, S.M.; Bossart, S. Skin hyperpigmentation index in melasma: A complementary method to classic scoring systems. J. Cosmet. Dermatol. 2023, 22, 3405–3412. [Google Scholar] [CrossRef] [PubMed]
- Cassiano, D.P.; Espósito, A.C.C.; Da Silva, C.N.; Lima, P.B.; Dias, J.A.F.; Hassun, K.; Miot, L.D.B.; Miot, H.A.; Bagatin, E. Update on Melasma—Part II: Treatment. Dermatol. Ther. 2022, 12, 1989–2012. [Google Scholar] [CrossRef]
- Jiryis, B.; Toledano, O.; Avitan-Hersh, E.; Khamaysi, Z. Management of Melasma: Laser and Other Therapies—Review Study. J. Clin. Med. 2024, 13, 1468. [Google Scholar] [CrossRef]
- Morgado-Carrasco, D.; Piquero-Casals, J.; Granger, C.; Trullàs, C.; Passeron, T. Melasma: The need for tailored photoprotection to improve clinical outcomes. Photodermatol. Photoimmunol. Photomed. 2022, 38, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.R.; Alexis, A.F.; Elbuluk, N.; Grimes, P.E.; Weiss, J.; Hamzavi, I.H.; Taylor, S.C. Best practices in the treatment of melasma with a focus on patients with skin of color. J. Am. Acad. Dermatol. 2024, 90, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.P.; Taylor, S.C.; Lim, H.W. Personalized photoprotection: Commentary on “Adjusting best practices in the treatment of melasma with a focus on patients with skin of color”. J. Am. Acad. Dermatol. 2023, 89, 635–636. [Google Scholar] [CrossRef]
- Schwartz, C.; Jan, A.; Zito, P.M. Hydroquinone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539693/ (accessed on 28 May 2024).
- Jimbow, K.; Obata, H.; Pathak, M.A.; Fitzpatrick, T.B. Mechanism of Depigmentation by Hydroquinone. J. Investig. Dermatol. 1974, 62, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Park, K.C.; Lee, M.H.; Lee, E.-S.; Chang, S.E.; Leow, Y.H.; Tay, Y.-K.; Legarda-Montinola, F.; Tsai, R.-Y.; Tsai, T.-H.; et al. A Randomized Controlled Trial of the Efficacy and Safety of a Fixed Triple Combination (Fluocinolone Acetonide 001, Hydroquinone 4, Tretinoin 005) Compared with Hydroquinone 4 Cream in Asian Patients with Moderate to Severe Melasma. Br. J. Dermatol. 2008, 159, 697–703. [Google Scholar] [CrossRef]
- Austin, E.; Nguyen, J.K.; Jagdeo, J. Topical Treatments for Melasma: A Systematic Review of Randomized Controlled Trials. J. Drugs Dermatol. 2019, 18, S1545961619P1156X. [Google Scholar]
- González-Molina, V.; Martí-Pineda, A.; González, N. Topical Treatments for Melasma and Their Mechanism of Action. J. Clin. Aesthet. Dermatol. 2022, 15, 19–28. [Google Scholar] [PubMed]
- Lazar, M.; De La Garza, H.; Vashi, N.A. Exogenous Ochronosis: Characterizing a Rare Disorder in Skin of Color. J. Clin. Med. 2023, 12, 4341. [Google Scholar] [CrossRef]
- Sauer, N.; Oślizło, M.; Brzostek, M.; Wolska, J.; Lubaszka, K.; Karłowicz-Bodalska, K. The Multiple Uses of Azelaic Acid in Dermatology: Mechanism of Action, Preparations, and Potential Therapeutic Applications. Postepy. Dermatol. Alergol. 2023, 40, 716–724. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. The Versatility of Azelaic Acid in Dermatology. J. Dermatol. Treat. 2022, 33, 722–732. [Google Scholar] [CrossRef]
- Akl, E.M. Liposomal Azelaic Acid 20% Cream vs Hydroquinone 4% Cream as Adjuvant to Oral Tranexamic Acid in Melasma: A Comparative Study. J. Dermatol. Treat. 2022, 33, 2008–2013. [Google Scholar] [CrossRef]
- Tehrani, S.; Tehrani, S.; Esmaili-Azad, M.; Vaezi, M.; Saljoughi, N. Efficacy and Safety of Azelaic Acid 20% plus Hydroquinone 5% in the Management of Melasma. Iran. J. Dermatol. 2012, 2012, 11–14. [Google Scholar]
- Albzea, W.; AlRashidi, R.; Alkandari, D.; Sadan, M.; Alkandari, A.; Alkanderi, J.J.; AlHajri, M.T.; Almutairi, S.N.; Alenzi, A.; Alanazi, S.; et al. Azelaic Acid Versus Hydroquinone for Managing Patients with Melasma: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cureus 2023, 15, e41796. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, J.-P. Retinoid Therapy of Pigmentary Disorders. Dermatol. Ther. 2006, 19, 280–288. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Finkel, L.J.; Ditre, C.M.; Hamilton, T.A.; Ellis, C.N.; Voorhees, J.J. Topical Tretinoin (Retinoic Acid) Improves Melasma. A Vehicle-Controlled, Clinical Trial. Br. J. Dermatol. 1993, 129, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough-Green, C.K.; Griffiths, C.E.; Finkel, L.J.; Hamilton, T.A.; Bulengo-Ransby, S.M.; Ellis, C.N.; Voorhees, J.J. Topical Retinoic Acid (Tretinoin) for Melasma in Black Patients. A Vehicle-Controlled Clinical Trial. Arch. Dermatol. 1994, 130, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Truchuelo, M.T.; Jiménez, N.; Jaén, P. Assessment of the Efficacy and Tolerance of a New Combination of Retinoids and Depigmenting Agents in the Treatment of Melasma. J. Cosmet. Dermatol. 2014, 13, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.A.; Fitzpatrick, T.B.; Kraus, E.W. Usefulness of Retinoic Acid in the Treatment of Melasma. J. Am. Acad. Dermatol. 1986, 15, 894–899. [Google Scholar] [CrossRef]
- Menter, A. Rationale for the Use of Topical Corticosteroids in Melasma. J. Drugs Dermatol. 2004, 3, 169–174. [Google Scholar]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The Treatment of Melasma: A Review of Clinical Trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, V.K.; Patil, A.; Blicharz, L.; Kassir, M.; Konnikov, N.; Gold, M.H.; Goldman, M.P.; Galadari, H.; Goldust, M. Medical Therapies for Melasma. J. Cosmet. Dermatol. 2022, 21, 3707–3728. [Google Scholar] [CrossRef]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic Acid in Skin Health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef]
- Ebanks, J.; Wickett, R.; Boissy, R. Mechanisms Regulating Skin Pigmentation: The Rise and Fall of Complexion Coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef]
- De Dormael, R.; Bastien, P.; Sextius, P.; Gueniche, A.; Ye, D.; Tran, C.; Chevalier, V.; Gomes, C.; Souverain, L.; Tricaud, C. Vitamin C Prevents Ultraviolet-Induced Pigmentation in Healthy Volunteers: Bayesian Meta-Analysis Results from 31 Randomized Controlled versus Vehicle Clinical Studies. J. Clin. Aesthet. Dermatol. 2019, 12, E53–E59. [Google Scholar]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef] [PubMed]
- Darr, D.; Combs, S.; Dunston, S.; Manning, T.; Pinnell, S. Topical Vitamin C Protects Porcine Skin from Ultraviolet Radiation-Induced Damage. Br. J. Dermatol. 1992, 127, 247–253. [Google Scholar] [CrossRef]
- Espinal-Perez, L.E.; Moncada, B.; Castanedo-Cazares, J.P. A Double-blind Randomized Trial of 5% Ascorbic Acid vs. 4% Hydroquinone in Melasma. Int. J. Dermatol. 2004, 43, 604–607. [Google Scholar] [CrossRef]
- Correia, G.; Magina, S. Efficacy of Topical Vitamin C in Melasma and Photoaging: A Systematic Review. J. Cosmet. Dermatol. 2023, 22, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.B.; Yanaki, J.S.; Draper, D.O.; Shurtz, J.C.; Coglianese, M. Successful Short-Term and Long-Term Treatment of Melasma and Postinflammatory Hyperpigmentation Using Vitamin C with a Full-Face Iontophoresis Mask and a Mandelic/Malic Acid Skin Care Regimen. J. Drugs Dermatol. 2013, 12, 45–50. [Google Scholar] [PubMed]
- Zhou, H.L.; Hu, B.; Zhang, C. Efficacy of 694-Nm Fractional Q-Switched Ruby Laser (QSRL) Combined with Sonophoresis on Levorotatory Vitamin C for Treatment of Melasma in Chinese Patients. Lasers Med. Sci. 2016, 31, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Balevi, A.; Ustuner, P.; Özdemir, M. Salicylic Acid Peeling Combined with Vitamin C Mesotherapy versus Salicylic Acid Peeling Alone in the Treatment of Mixed Type Melasma: A Comparative Study. J. Cosmet. Laser Ther. 2017, 19, 294–299. [Google Scholar] [CrossRef]
- Speeckaert, R.; Bulat, V.; Speeckaert, M.M.; Van Geel, N. The Impact of Antioxidants on Vitiligo and Melasma: A Scoping Review and Meta-Analysis. Antioxidants 2023, 12, 2082. [Google Scholar] [CrossRef] [PubMed]
- Pazyar, N.; Molavi, S.N.; Hosseinpour, P.; Hadibarhaghtalab, M.; Parvar, S.Y.; Dezfuly, M.B. Efficacy of Intradermal Injection of Tranexamic Acid and Ascorbic Acid versus Tranexamic Acid and Placebo in the Treatment of Melasma: A Split-face Comparative Trial. Health Sci. Rep. 2022, 5, e537. [Google Scholar] [CrossRef]
- Chib, S.; Jamwal, V.L.; Kumar, V.; Gandhi, S.G.; Saran, S. Fungal Production of Kojic Acid and Its Industrial Applications. Appl. Microbiol. Biotechnol. 2023, 107, 2111–2130. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic Acid Applications in Cosmetic and Pharmaceutical Preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Lim, J.T.E. Treatment of Melasma Using Kojic Acid in a Gel Containing Hydroquinone and Glycolic Acid. Dermatol. Surg. 1999, 25, 282–284. [Google Scholar] [CrossRef]
- Monteiro, R.C.; Kishore, B.N.; Bhat, R.M.; Sukumar, D.; Martis, J.; Ganesh, H.K. A Comparative Study of the Efficacy of 4% Hydroquinone vs 0.75% Kojic Acid Cream in the Treatment of Facial Melasma. Indian. J. Dermatol. 2013, 58, 157. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, P.V.; Manangi, S.; Dani, A.; Kudligi, C. Efficacy and Safety of 2% Kojic Acid Containing Formulation versus Modified Kligman’s Formula in Melasma—A Comparative Study. J. Pak. Assoc. Dermatol. 2017, 26, 183–187. [Google Scholar]
- Maeda, K. Mechanism of Action of Topical Tranexamic Acid in the Treatment of Melasma and Sun-Induced Skin Hyperpigmentation. Cosmetics 2022, 9, 108. [Google Scholar] [CrossRef]
- Atefi, N.; Dalvand, B.; Ghassemi, M.; Mehran, G.; Heydarian, A. Therapeutic Effects of Topical Tranexamic Acid in Comparison with Hydroquinone in Treatment of Women with Melasma. Dermatol. Ther. 2017, 7, 417–424. [Google Scholar] [CrossRef]
- Janney, M.; Subramaniyan, R.; Dabas, R.; Lal, S.; Das, N.; Godara, S. A Randomized Controlled Study Comparing the Efficacy of Topical 5% Tranexamic Acid Solution versus 3% Hydroquinone Cream in Melasma. J. Cutan. Aesthet. Surg. 2019, 12, 63. [Google Scholar] [CrossRef]
- El-Husseiny, R.; Rakha, N.; Sallam, M. Efficacy and Safety of Tranexamic Acid 5% Cream vs Hydroquinone 4% Cream in Treating Melasma: A Split-face Comparative Clinical, Histopathological, and Antera 3D Camera Study. Dermatol. Ther. 2020, 33. [Google Scholar] [CrossRef]
- Thieman, T.; Swali, R.; Adams, J. 33031 Use of Topical Tranexamic Acid in Patients with Melasma: A Narrative Review. J. Am. Acad. Dermatol. 2022, 87, AB126. [Google Scholar] [CrossRef]
- Desai, S.; Ayres, E.; Bak, H.; Manco, M.; Lynch, S.; Raab, S.; Du, A.; Green, D.; Skobowiat, C.; Wangari-Talbot, J.; et al. Effect of a Tranexamic Acid, Kojic Acid, and Niacinamide Containing Serum on Facial Dyschromia: A Clinical Evaluation. J. Drugs Dermatol. 2019, 18, 454–459. [Google Scholar]
- Madaan, P.; Sikka, P.; Malik, D.S. Cosmeceutical Aptitudes of Niacinamide: A Review. Recent. Adv. Antiinfect. Drug Discov. 2021, 16, 196–208. [Google Scholar] [CrossRef]
- Boo, Y.C. Mechanistic Basis and Clinical Evidence for the Applications of Nicotinamide (Niacinamide) to Control Skin Aging and Pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Solís, J.; Castanedo-Cázares, J.P.; Torres-Álvarez, B.; Oros-Ovalle, C.; Fuentes-Ahumada, C.; González, F.J.; Martínez-Ramírez, J.D.; Moncada, B. A Double-Blind, Randomized Clinical Trial of Niacinamide 4% versus Hydroquinone 4% in the Treatment of Melasma. Dermatol. Res. Pract. 2011, 2011, 1–5. [Google Scholar] [CrossRef]
- Allouche, J.; Rachmin, I.; Adhikari, K.; Pardo, L.M.; Lee, J.H.; McConnell, A.M.; Kato, S.; Fan, S.; Kawakami, A.; Suita, Y.; et al. NNT Mediates Redox-Dependent Pigmentation via a UVB- and MITF-Independent Mechanism. Cell 2021, 184, 4268–4283. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, A.G.; Furtado, G.R.D.; Barbosa, K.L. Niacinamide for the Treatment of Melasma: An Integrative Review of Randomized Clinical Trials. Res. Soc. Dev. 2022, 11, e198111133581. [Google Scholar] [CrossRef]
- Saeedi, M.; Khezri, K.; Seyed Zakaryaei, A.; Mohammadamini, H. A Comprehensive Review of the Therapeutic Potential of A-arbutin. Phytoth. Res. 2021, 35, 4136–4154. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Arbutin as a Skin Depigmenting Agent with Antimelanogenic and Antioxidant Properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. The Top 10 Cosmeceuticals for Facial Hyperpigmentation. Dermatol. Ther. 2020, 33, e14095. [Google Scholar] [CrossRef]
- Hamed, S.H.; Sriwiriyanont, P.; deLong, M.A.; Visscher, M.O.; Wickett, R.R.; Boissy, R.E. Comparative Efficacy and Safety of Deoxyarbutin, a New Tyrosinase-Inhibiting Agent. J. Cosmet. Sci. 2006, 57, 291–308. [Google Scholar]
- Anwar, A.I.; Asmarani, Y.; Madjid, A.; Patellongi, I.; Adriani, A.; As’ad, S.; Kurniadi, I. Comparison of 2% Deoxyarbutin and 4% Hydroquinone as a Depigmenting Agent in Healthy Individuals: A Double-blind Randomized Controlled Clinical Trial. J. Cosmet. Dermatol. 2021, 20, 3953–3959. [Google Scholar] [CrossRef]
- Nguyen, J.; Rajgopal Bala, H.; Ross, A.; Wong, C.C.; Paul, E.; Rodrigues, M. Effect of Oral Tranexamic Acid on Erythema Index in Patients with Melasma. Aust. J. Dermatol. 2021, 62, 206–209. [Google Scholar] [CrossRef]
- Feng, X.; Su, H.; Xie, J. Efficacy and Safety of Tranexamic Acid in the Treatment of Adult Melasma: An Updated Meta-analysis of Randomized Controlled Trials. J. Clin. Pharm. Ther. 2021, 46, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Godse, K.; Sarkar, R.; Mysore, V.; Shenoy, M.M.; Chatterjee, M.; Damisetty, R.; Shah, S.; Vedamurthy, M.; Aurangabadkar, S.; Srinivas, C.; et al. Oral Tranexamic Acid for the Treatment of Melasma: Evidence and Experience-Based Consensus Statement from Indian Experts. Indian J. Dermatol. 2023, 68, 178–185. [Google Scholar] [CrossRef]
- Malik, F.; Hanif, M.; Mustafa, G. Combination of Oral Tranexamic Acid with Topical 3% Tranexamic Acid versus Oral Tranexamic Acid with Topical 20% Azelaic Acid in the Treatment of Melasma. J. Coll. Physicians Surg. Pak. 2019, 29, 502–504. [Google Scholar] [CrossRef]
- Agamia, N.; Apalla, Z.; Salem, W.; Abdallah, W. A Comparative Study between Oral Tranexamic Acid versus Oral Tranexamic Acid and Q-Switched Nd-YAG Laser in Melasma Treatment: A Clinical and Dermoscopic Evaluation. J. Dermatol. Treat. 2021, 32, 819–826. [Google Scholar] [CrossRef]
- McKesey, J.; Tovar-Garza, A.; Pandya, A.G. Melasma Treatment: An Evidence-Based Review. Am. J. Clin. Dermatol. 2020, 21, 173–225. [Google Scholar] [CrossRef]
- Yao, H.; Shen, S.; Gao, X.; Feng, J.; Song, X.; Xiang, W. Definition of Refractory Melasma and Its Treatment: A Review. Lasers Med. Sci. 2024, 39, 118. [Google Scholar] [CrossRef] [PubMed]
- Konisky, H.; Balazic, E.; Jaller, J.A.; Khanna, U.; Kobets, K. Tranexamic Acid in Melasma: A Focused Review on Drug Administration Routes. J. Cosmet. Dermatol. 2023, 22, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-Y.; Li, Y.; Sun, Q.-N.; Takada, A.; Kawada, A. Analysis of the Effect of Different Doses of Oral Tranexamic Acid on Melasma: A Multicentre Prospective Study. Eur. J. Dermatol. 2019, 29, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Sonthalia, S.; Daulatabad, D.; Sarkar, R. Glutathione as a Skin Whitening Agent: Facts, Myths, Evidence and Controversies. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 262. [Google Scholar] [CrossRef]
- Grimes, P.E.; Ijaz, S.; Nashawati, R.; Kwak, D. New Oral and Topical Approaches for the Treatment of Melasma. Int. J. Womens Dermatol. 2019, 5, 30–36. [Google Scholar] [CrossRef]
- Arjinpathana, N.; Asawanonda, P. Glutathione as an Oral Whitening Agent: A Randomized, Double-Blind, Placebo-Controlled Study. J. Dermatol. Treat. 2012, 23, 97–102. [Google Scholar] [CrossRef]
- Wahab, S.; Anwar, A.I.; Zainuddin, A.N.; Hutabarat, E.N.; Anwar, A.A.; Kurniadi, I. Combination of Topical and Oral Glutathione as a Skin-whitening Agent: A Double-blind Randomized Controlled Clinical Trial. Int. J. Dermatol. 2021, 60, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Vladulescu, D.; Scurtu, L.G.; Simionescu, A.A.; Scurtu, F.; Popescu, M.I.; Simionescu, O. Platelet-Rich Plasma (PRP) in Dermatology: Cellular and Molecular Mechanisms of Action. Biomedicines 2023, 12, 7. [Google Scholar] [CrossRef]
- Acar, A.; Ozturk, A.; Sokmen, N.; Unal, I.; Ertam Sagduyu, I. Evaluation of Platelet-Rich Plasma Efficacy in Melasma. J. Cosmet. Laser Ther. 2022, 24, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Hofny, E.R.M.; Abdel-Motaleb, A.A.; Ghazally, A.; Ahmed, A.M.; Hussein, M.R.A. Platelet-Rich Plasma Is a Useful Therapeutic Option in Melasma. J. Dermatol. Treat. 2019, 30, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, M.; Xiao, Q.; Zhou, R.; Li, Y.; Xiong, L.; Li, L. Efficacy and Safety of Platelet-Rich Plasma in Melasma: A Systematic Review and Meta-Analysis. Dermatol. Ther. 2021, 11, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Abd Elraouf, I.G.; Obaid, Z.M.; Fouda, I. Intradermal Injection of Tranexamic Acid versus Platelet-Rich Plasma in the Treatment of Melasma: A Split-Face Comparative Study. Arch. Dermatol. Res. 2023, 315, 1763–1770. [Google Scholar] [CrossRef]
- Gamea, M.M.; Kamal, D.A.; Donia, A.A.; Hegab, D.S. Comparative Study between Topical Tranexamic Acid Alone versus Its Combination with Autologous Platelet Rich Plasma for Treatment of Melasma. J. Dermatol. Treat. 2022, 33, 798–804. [Google Scholar] [CrossRef]
- Sarkar, R.; Gupta, M. Platelet-Rich Plasma in Melasma—A Systematic Review. Dermatol. Surg. 2022, 48, 131–134. [Google Scholar] [CrossRef]
- Pixley, J.N.; Cook, M.K.; Singh, R.; Larrondo, J.; McMichael, A.J. A Comprehensive Review of Platelet-Rich Plasma for the Treatment of Dermatologic Disorders. J. Dermatol. Treat. 2023, 34, 2142035. [Google Scholar] [CrossRef]
- Batra, J.; Brar, B.; Kumar, S.; Arora, H. Tranexamic Acid in Melasma: Comparative Evaluation of Therapeutic Efficacy of Oral Tranexamic Acid versus Its Transepidermal Administration. J. Cutan. Aesthet. Surg. 2022, 15, 394. [Google Scholar] [CrossRef]
- Saleh, F.; Abdel-Azim, E.; Ragaie, M.; Guendy, M. Topical Tranexamic Acid with Microneedling versus Microneedling Alone in Treatment of Melasma: Clinical, Histopathologic, and Immunohistochemical Study. J. Egypt. Womens Dermatol. Soc. 2019, 16, 89. [Google Scholar] [CrossRef]
- Kaur, A.; Bhalla, M.; Pal Thami, G.; Sandhu, J. Clinical Efficacy of Topical Tranexamic Acid With Microneedling in Melasma. Dermatol. Surg. 2020, 46, e96–e101. [Google Scholar] [CrossRef]
- Zaky, M.S.; Obaid, Z.M.; Khalil, E.A.; Elsaie, M.L. Microneedling-assisted Topical Tranexamic Acid Solution versus 4% Hydroquinone for Treating Melasma: A Split-face Randomized Study. J. Cosmet. Dermatol. 2021, 20, 4011–4016. [Google Scholar] [CrossRef]
- Shamsi Meymandi, S.; Mozayyeni, A.; Shamsi Meymandi, M.; Aflatoonian, M. Efficacy of Microneedling plus Topical 4% Tranexamic Acid Solution vs 4% Hydroquinone in the Treatment of Melasma: A Single-blind Randomized Clinical Trial. J. Cosmet. Dermatol. 2020, 19, 2906–2911. [Google Scholar] [CrossRef]
- Kaleem, S.; Ghafoor, R.; Khan, S. Comparison of Efficacy of Tranexamic Acid Mesotherapy versus 0.9% Normal Saline for Melasma; A Split Face Study in a Tertiary Care Hospital of Karachi. Pak. J. Med. Sci. 2020, 36, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Tehranchinia, Z.; Saghi, B.; Rahimi, H. Evaluation of Therapeutic Efficacy and Safety of Tranexamic Acid Local Infiltration in Combination with Topical 4% Hydroquinone Cream Compared to Topical 4% Hydroquinone Cream Alone in Patients with Melasma: A Split-Face Study. Dermatol. Res. Pract. 2018, 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pazyar, N.; Yaghoobi, R.; Zeynalie, M.; Vala, S. Comparison of the Efficacy of Intradermal Injected Tranexamic Acid vs Hydroquinone Cream in the Treatment of Melasma. Clin. Cosmet. Investig. Dermatol. 2019, 12, 115–122. [Google Scholar] [CrossRef]
- Lueangarun, S.; Sirithanabadeekul, P.; Wongwicharn, P.; Namboonlue, C.; Pacharapakornpong, S.; Juntongjin, P.; Tempark, T. Intradermal Tranexamic Acid Injection for the Treatment of Melasma: A Pilot Study with 48-Week Follow-Up. J. Clin. Aesthet. Dermatol. 2020, 13, 36–39. [Google Scholar]
- Conforti, C.; Zalaudek, I.; Vezzoni, R.; Retrosi, C.; Fai, A.; Fadda, S.; Di Michele, E.; Dianzani, C. Chemical Peeling for Acne and Melasma: Current Knowledge and Innovations. G. Ital. Dermatol. Venereol. 2020, 155, 280–285. [Google Scholar] [CrossRef]
- Samargandy, S.; Raggio, B.S. Chemical Peels for Skin Resurfacing. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547752/ (accessed on 3 June 2024).
- Sarkar, R.; Lakhani, R. Chemical Peels for Melasma: A Systematic Review. Dermatol. Surg. 2024, 50, 656–661. [Google Scholar] [CrossRef]
- Prasad, N.; Singh, M.; Malhotra, S.; Singh, N.; Tyagi, A.; Tyagi, S. Comparative Efficacy of Chemical Peeling Agents in the Treatment of Melasma. Cureus 2023, 15, e47312. [Google Scholar] [CrossRef] [PubMed]
- Basit, H.; Godse, K.V.; Al Aboud, A.M. Melasma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459271/ (accessed on 5 June 2024).
- Ho, S.G.Y.; Chan, H.H.L. The Asian Dermatologic Patient: Review of Common Pigmentary Disorders and Cutaneous Diseases. Am. J. Clin. Dermatol. 2009, 10, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Fanous, N.; Zari, S. Universal Trichloroacetic Acid Peel Technique for Light and Dark Skin. JAMA Facial Plast. Surg. 2017, 19, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Bansal, S.; Garg, V.K. Chemical peels for melasma in dark-skinned patients. J. Cutan. Aesthet. Surg. 2012, 5, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Aurangabadkar, S.; Salim, T.; Das, A.; Shah, S.; Majid, I.; Singh, M.; Ravichandran, G.; Godse, K.; Arsiwala; et al. Lasers in Melasma: A Review with Consensus Recommendations by Indian Pigmentary Expert Group. Indian J. Dermatol. 2017, 62, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Neagu, N.; Conforti, C.; Agozzino, M.; Marangi, G.F.; Morariu, S.H.; Pellacani, G.; Persichetti, P.; Piccolo, D.; Segreto, F.; Zalaudek, I.; et al. Melasma Treatment: A Systematic Review. J. Dermatol. Treat. 2022, 33, 1816–1837. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Zhou, S.; Cheng, S.; Liu, H.; Cui, Y. Laser Therapy in the Treatment of Melasma: A Systematic Review and Meta-Analysis. Lasers Med. Sci. 2022, 37, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Hong, T.; Zeng, H.; Li, P.; Li, P.; Wang, S.; Chen, J.; Li, P.; Zhou, J. A Meta-Analysis-Based Assessment of Intense Pulsed Light for Treatment of Melasma. Aesth. Plast. Surg. 2020, 44, 947–952. [Google Scholar] [CrossRef]
- Figueiredo Souza, L.; Trancoso Souza, S. Single-session intense pulsed light combined with stable fixed-dose triple combination topical therapy for the treatment of refractory melasma. Dermatol. Ther. 2012, 25, 477–480. [Google Scholar] [CrossRef]
- Ertam Sagduyu, I.; Dirican, F.; Acar, A.; Unal, I. Efficacy of intense pulsed light therapy for melasma. J. Cosmet. Laser Ther. 2019, 21, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Yang, F.C.; Cho, B.K. A Review of Laser and Light Therapy in Melasma. Int. J. Womens Dermatol. 2017, 3, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Aurangabadkar, S. Optimizing Q-Switched Lasers for Melasma and Acquired Dermal Melanoses. Indian. J. Dermatol. Venereol. Leprol. 2019, 85, 10. [Google Scholar] [CrossRef] [PubMed]
- Micek, I.; Pawlaczyk, M.; Kroma, A.; Seraszek-Jaros, A.; Urbańska, M.; Gornowicz-Porowska, J. Treatment of Melasma with a Low-fluence 1064 Nm Q-switched Nd:YAG Laser: Laser Toning in Caucasian Women. Lasers Surg. Med. 2022, 54, 366–373. [Google Scholar] [CrossRef]
- Shah, S.; Aurangabadkar, S. Laser Toning in Melasma. J. Cutan. Aesthet. Surg. 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Gao, Q.; Liu, J.; Zhong, X.; Xu, T.; Wu, Q.; Cheng, Z.; Luo, N.; Hao, P. Efficacy and Safety of Laser-related Therapy for Melasma: A Systematic Review and Network Meta-analysis. J. Cosmet. Dermatol. 2023, 22, 2910–2924. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, Y.J.; Lee, J.M.; Han, T.Y.; Lee, J.H.; Choi, J.E. The Low-Fluence Q-Switched Nd:YAG Laser Treatment for Melasma: A Systematic Review. Medicina 2022, 58, 936. [Google Scholar] [CrossRef]
- Wanitphakdeedecha, R.; Sy-Alvarado, F.; Patthamalai, P.; Techapichetvanich, T.; Eimpunth, S.; Manuskiatti, W. The Efficacy in Treatment of Facial Melasma with Thulium 1927-Nm Fractional Laser-Assisted Topical Tranexamic Acid Delivery: A Split-Face, Double-Blind, Randomized Controlled Pilot Study. Lasers Med. Sci. 2020, 35, 2015–2021. [Google Scholar] [CrossRef]
- Aggarwal, I.; Rossi, M.; Puyana, C.; Tsoukas, M. Review of Fractional Nonablative Lasers for the Treatment of Dermatologic Conditions in Darker Skin Phototypes. Dermatol. Surg. 2024, 50, 459–466. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, M.; Lai, X.; Yan, Y. Efficacy and Safety of Ablative Fractional Laser in Melasma: A Meta-Analysis and Systematic Review. Lasers Med. Sci. 2024, 39, 71. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, T.; Shen, N. Effect of platelet-rich plasma combined with tranexamic acid in the treatment of melasma and its effect on the serum levels of vascular endothelial growth factor, endothelin-1 and melatonin. Pak. J. Med. Sci. 2022, 38, 2163–2168. [Google Scholar] [CrossRef]
- Tuknayat, A.; Thami, G.P.; Bhalla, M.; Sandhu, J.K. Autologous intralesional platelet rich plasma improves melasma. Dermatol. Ther. 2021, 34, e14881. [Google Scholar] [CrossRef]
- Adel, S.; Serri, A.; Abd El-Raheem, T. Study of autologous platelet-rich-plasma versus its combination with intense pulsed light in treatment of melasma. Dermatol. Ther. 2021, 34, e15008. [Google Scholar] [CrossRef]
- Sirithanabadeekul, P.; Dannarongchai, A.; Suwanchinda, A. Platelet-rich plasma treatment for melasma: A pilot study. J. Cosmet. Dermatol. 2020, 19, 1321–1327. [Google Scholar] [CrossRef]
- Ertam Sagduyu, I.; Marakli, O.; Oraloglu, G.; Bulut Okut, E.; Unal, I. Comparison of 1064 nm Q-switched Nd:YAG laser and Jessner peeling in melasma treatment. Dermatol Ther. 2022, 35, e15970. [Google Scholar] [CrossRef]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The Emerging Role of Nanotechnology in Skincare. Adv. Colloid. Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sharma, N.; Zahoor, I.; Behl, T.; Antil, A.; Gupta, S.; Anwer, M.K.; Mohan, S.; Bungau, S.G. Decrypting the Potential of Nanotechnology-Based Approaches as Cutting-Edge for Management of Hyperpigmentation Disorder. Molecules 2022, 28, 220. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, S.; Hariri, R.; Kouhsoltani, M.; Shokri, J.; Javadzadeh, Y.; Hamishehkar, H. Enhanced Stability and Dermal Delivery of Hydroquinone Using Solid Lipid Nanoparticles. Colloids Surf. B. Biointerfaces 2015, 136, 1004–1010. [Google Scholar] [CrossRef]
- Wu, P.-S.; Lin, C.-H.; Kuo, Y.-C.; Lin, C.-C. Formulation and Characterization of Hydroquinone Nanostructured Lipid Carriers by Homogenization Emulsification Method. J. Nanomater. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Tangau, M.J.; Chong, Y.K.; Yeong, K.Y. Advances in Cosmeceutical Nanotechnology for Hyperpigmentation Treatment. J. Nanopart. Res. 2022, 24, 155. [Google Scholar] [CrossRef]
- Nikolaev, B.; Yakovleva, L.; Fedorov, V.; Li, H.; Gao, H.; Shevtsov, M. Nano- and Microemulsions in Biomedicine: From Theory to Practice. Pharmaceutics 2023, 15, 1989. [Google Scholar] [CrossRef]
- Tomić, I.; Juretić, M.; Jug, M.; Pepić, I.; Cetina Čižmek, B.; Filipović-Grčić, J. Preparation of in Situ Hydrogels Loaded with Azelaic Acid Nanocrystals and Their Dermal Application Performance Study. Int. J. Pharm. 2019, 563, 249–258. [Google Scholar] [CrossRef]
- Çağlar, E.Ş.; Pekcan, A.N.; Okur, M.E.; Ayla, Ş.; Üstündağ Okur, N. Preparation, Optimization and in Vivo Anti-Inflammatory Evaluation of Hydroquinone Loaded Microemulsion Formulations for Melasma Treatment. J. Res. Pharm. 2019, 23, 662–670. [Google Scholar] [CrossRef]
- Kusumawardani, A.; Paramitasari, A.R.; Dewi, S.R.; Betaubun, A.I. The Application of Liposomal Azelaic Acid, 4-n Butyl Resorcinol and Retinol Serum Enhanced by Microneedling for Treatment of Malar Pattern Melasma: A Case Series. Dermatol. Rep. 2019. [Google Scholar] [CrossRef]
- Taghavi, F.; Banihashemi, M.; Zabolinejad, N.; Salehi, M.; Jaafari, M.R.; Marhamati, H.; Golnouri, F.; Dorri, M. Comparison of Therapeutic Effects of Conventional and Liposomal Form of 4% Topical Hydroquinone in Patients with Melasma. J. Cosmet. Dematol. 2019, 18, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Moacă, E.-A.; Péter, F. Niosomes: Composition, Formulation Techniques, and Recent Progress as Delivery Systems in Cancer Therapy. Pharmaceutics 2024, 16, 223. [Google Scholar] [CrossRef] [PubMed]
- Divanbeygikermani, M.; Pardakhty, A.; Amanatfard, A. Kojic Acid and Hydroquinone Non-Ionic Surfactant Vesicles for Topical Application. Int. Pharm. Acta 2018, 1, 110. [Google Scholar] [CrossRef]
- Fernández-García, R.; Lalatsa, A.; Statts, L.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Transferosomes as Nanocarriers for Drugs across the Skin: Quality by Design from Lab to Industrial Scale. Int. J. Pharm. 2020, 573, 118817. [Google Scholar] [CrossRef]
- Li, J.; Duan, N.; Song, S.; Nie, D.; Yu, M.; Wang, J.; Xi, Z.; Li, J.; Sheng, Y.; Xu, C.; et al. Transfersomes Improved Delivery of Ascorbic Palmitate into the Viable Epidermis for Enhanced Treatment of Melasma. Int. J. Pharm. 2021, 608, 121059. [Google Scholar] [CrossRef]
- Karamanidou, T.; Bourganis, V.; Gatzogianni, G.; Tsouknidas, A. A Review of the EU’s Regulatory Framework for the Production of Nano-Enhanced Cosmetics. Metals 2021, 11, 455. [Google Scholar] [CrossRef]
- Raj, S.; Jose, S.; Sumod, U.S.; Sabitha, M. Nanotechnology in cosmetics: Opportunities and challenges. J. Pharm. Bioallied Sci. 2012, 4, 186–193. [Google Scholar] [CrossRef]
- EUR-Lex. Access to European Union Law. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. OJ L 342/59, 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009R1223 (accessed on 27 July 2024).
- Doolan, B.J.; Gupta, M. Melasma. Aust. J. Gen. Pract. 2021, 50, 880–885. [Google Scholar] [CrossRef]
- Shankar, K.; Godse, K.; Aurangabadkar, S.; Lahiri, K.; Mysore, V.; Ganjoo, A.; Vedamurty, M.; Kohli, M.; Sharad, J.; Kadhe, G.; et al. Evidence-based treatment for melasma: Expert opinion and a review. Dermatol. Ther. 2014, 4, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Arellano, I.; Cestari, T.; Ocampo-Candiani, J.; Azulay-Abulafia, L.; Bezerra Trindade Neto, P.; Hexsel, D.; Machado-Pinto, J.; Muñoz, H.; Rivitti-Machado, M.C.; Sittart, J.A.; et al. Preventing melasma recurrence: Prescribing a maintenance regimen with an effective triple combination cream based on long-standing clinical severity. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 611–618. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).