Abstract
Endocrine disruptors (EDs) are molecules capable of mimicking the natural hormones of the body and interfering with the endocrine system in both humans and wildlife. Cosmetic products are one source of EDs; these include an extensive variety of personal care and beauty products designed for the skin and hair, as well as makeup. The widespread use of such products has raised concerns about the presence of EDs within them. In this study, we highlight the issue of EDs and analyze the functioning of the EU regulatory framework for chemicals, specifically those which act as EDs in cosmetic products. We also highlight issues related to the interface between science and policy in the critical area of risk regulation within the EU. In addition, we investigate how chemical substances that act as EDs are identified based on specific criteria and conditions, a process which involves the production and adoption of particular scientific opinions. Finally, we assess the efficiency, suitability, and effectiveness of the regulatory framework in this sensitive area of human exposure to chemicals, especially those that function as EDs.
1. Introduction
Endocrine disruptors (EDs) are not a new phenomenon in the EU. In recent years, they have been a source of concern to the public and to stakeholders who have sought to identify these chemical substances, which are found in many everyday products such as pesticides, biocides, cosmetics, and children’s toys [1,2]. EDs cause serious disruptions to the endocrine system, with equally serious implications for human health and overall well-being [3]. Long-term exposure to these substances, especially during the embryonic period, is believed to contribute to the development of various diseases, including fertility disorders, certain cancers, neurological disorders, obesity, and reproductive problems. It should be emphasized that the majority of chemicals used have not been adequately assessed for their safety [4]. It is essential to evaluate the potential adverse impacts of EDs within the context of regulating hormone synthesis, secretion, and functionality, while also accounting for the variations in these regulatory processes throughout the various stages of life. An important factor to emphasize is the developmental stage during which exposure to EDs takes place, as this plays a pivotal role in comprehending outcomes [3,4].
Absorption of chemicals from cosmetics is possible, with the skin being the main route of exposure. The health of the skin represents a critical determinant in the efficiency of the skin’s protective barrier. Exposure is also possible through inhalation and through ingestion. The scientific debate regarding the appropriate criteria for identifying chemical substances as EDs continues unabated, with strong disagreements within the scientific community. The operation of the EU regulatory framework for EDs, as part of the broader field of EU risk regulation, is primarily characterized by the intersection of science and policy [5]. Within this framework, however, it can be a challenging task to determine where science ends and policy begins, as the boundaries are often blurred. Another key aspect of this framework which is characterized by scientific uncertainty is the wide discretionary power of EU institutions in the decision-making process [6]. Specifically, concerning EDs, the method and procedure for determining chemical substances with ED properties has become a matter of particular importance.
A major research issue which the scientific community concerns the question of whether the regulations in this sensitive area of human exposure to chemicals, especially those that act as EDs, serve the EU’s goal of providing a high level of environmental and human health protection [7]. The EU directed its attention towards EDs quite early on. In 1999, the European Commission published a strategy for EDs, outlining specific EU actions of a short-term (research and international cooperation), medium-term (testing methods), and long-term (regulatory measures) nature, all of which were aimed at reducing exposure to the lowest possible levels [8,9].
Since then, in addition to funding research [10], the EU has adopted regulatory measures to identify these substances, enabling a reduction in exposure in various sectors such as water [11], cosmetics [12], and chemical products [13]. Under the REACH Regulation, ECHA included substances with endocrine-disrupting properties in the “substances of very high concern” list which were subject to authorization [14]. Finally, it was stipulated that the Commission would initiate “a review to assess, taking into account the evolution of scientific knowledge, whether to broaden the scope” of the more stringent procedure (i.e., authorization) to include EDs [15].
2. Risk Regulation
Science plays a dual role as both the primary creator of environmental problems and as the essential tool for identifying and solving them. This dual role reflects the absolute dependence of our societies on science on the one hand, and skepticism towards it on the other, leading to scientific uncertainty [16].
The identification of a chemical substance as an ED by the Commission is indeed based on scientific data. However, due to existing scientific uncertainty, a significant role is given to the precautionary principle, which is a fundamental principle in EU risk governance [17]. In the EU, the precautionary principle is a cornerstone legal and policy principle with significant application in the field of chemical substances [18].
Specifically, it is at the core of the institutional architecture of EU risk regulation, and it is applied at both the risk assessment stage and the risk management stage. During the risk management stage, in line with this principle, the decision-making political body is obligated to establish measures aimed at achieving a high level of environmental and human health protection. This principle allows for action to be taken even in cases where scientific evidence is uncertain or incomplete in order to prevent potential harm to the environment and/or human health [19]. It emphasizes the importance of acting cautiously when there are indications of potential harm, even in the absence of conclusive scientific evidence [20]. In particular, it allows for the adoption of precautionary measures when scientific data concerning risk to the environment or human health is uncertain and not definitive [21]. This means that in cases where the assessment of the risk level (risk assessment) for certain substances with regard to human health or the environment is uncertain, precautionary actions can and should be taken to mitigate potential risks [22].
Risk regulation is a process that involves a set of parameters which include the legislative framework, regulatory provisions of the administration, scientific knowledge, and specific policy objectives. The apparent complexity of risk regulation, the core of which is interdisciplinarity, is the reason for the difficulty in scientific or political analysis [23]. The regulatory framework of risk analysis or risk regulation is a decision-making process under conditions of uncertainty that includes three stages, as listed in Table 1 [24].

Table 1.
Risk regulation parameters [25].
3. Risk Assessment

Table 2.
The four steps of risk assessment [26,27].
4. The Stages of Risk Assessment
Despite the fact that the general approach to quantitative risk assessment (QRA) has remained largely unchanged since the early 1980s, it is continually evolving in various forms, and its fields of application have significantly expanded. Several organizations and research groups have developed or adopted systematic reviews in the assessment of chemical substances. Moreover, there is a growing body of research which focuses on dynamic risk assessment and risk management, rather than static or traditional risk assessment [33]. The use of systematic reviews can identify differences in how questions are formulated, searches are conducted, or studies are evaluated [34]. The application of these methods can lead to improved transparency, objectivity, and communication in risk assessment [35].On the other hand, the process also involves disadvantages, mainly uncertainty resulting from contentious comparative results, such as the assumption that exposure to high doses applies equally to low doses, and that short-term exposures apply equally to long-term ones. Additionally, it often disregards the synergy of multiple sources of exposure (e.g., chemical substances and their mixtures in real-life exposures). Factors such as these which lead to uncertainty in the risk assessment process are illustrated in Figure 1 [36,37]. While scientific knowledge is essential, it is not always adequate for the assessment of risks. In any case, the management of risks falls under the jurisdiction of the competent political bodies of the community, which determine whether a risk is acceptable or not [38].
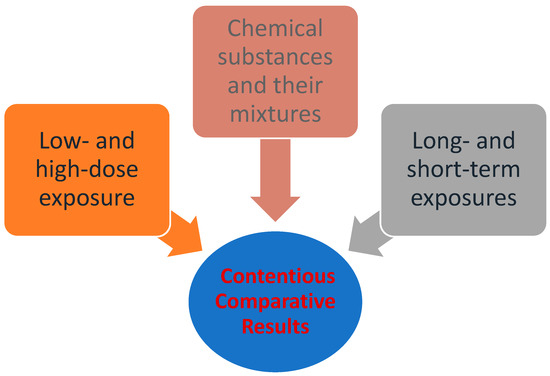
Figure 1.
Factors that lead to uncertainty in the risk assessment process [39] (modified by Dr. P.Kalofiri).
5. The Criteria for Determining ED
The criteria for identifying and determining EDs can be found in the regulations for Biocides 2100/2017 [40] and Plant Protection Products 2018/605 [41], and in the REACH Regulation [42]. Substances that pose endocrine disruption risks should not be placed on the market.
The criteria for determining the properties of EDs in humans are different from those that apply to non-target organisms. Both sets of criteria are further subdivided into two sections: one section for defining an ED, and one section regarding the information that must be taken into account for the determination of the properties of EDs [43].
In March 2019, the European Parliament published a study that examined the scientific evidence related to the concept of endocrine disruption, the extent of exposure, the relevant health impacts, and the associated costs [44]. It called on the EU to establish regulations governing all types of chemicals that cause endocrine disruptions in order to minimize human exposure. The conclusions included several recommendations to EU political bodies regarding goals, the definition of endocrine disruptors, guidance documents, test development and requirements, the management of endocrine disruptors in specific sectors and various areas, production, use, exposure to endocrine disruptors, and research priorities. The Endocrine Society praised the report. It stated that “the report demonstrates that chemicals causing endocrine disruption pose a serious threat to the health of current and future generations and highlight the need for additional action by policymakers in the EU to address this issue” [45].
In October 2020, a significant milestone was reached when the European Commission initiated the process of revising the requirements related to the identification of endocrine-disrupting substances. This important development was achieved by amending the Biocidal Products Regulation (Regulation 2021/525) [46]. The modifications take into account the “need to reduce testing on vertebrate animals and the need of a testing strategy and methods for the determination of endocrine disrupting properties of substances” [47].
In the context of the regulatory procedure, if the Regulatory Committee does not agree with the draft decision submitted by the Commission, the matter is referred to the Council where, if a majority is not achieved, the decision is ultimately taken by the Commission [48]. In this process, there are advantages in avoiding deadlock, but there are also disadvantages. The first is that the right of each member state to determine the level of protection it desires is disregarded; the second is that achieving a majority in the Council is difficult, especially in highly sensitive political issues, so that political decisions are finally made by a body which is not democratically legitimized, such as the Commission. While Regulatory Committees are not considered political committees, in practice they often take on a political character because the delineation between political decisions and techno science assessments is ambiguous [49]. A notable example is the case of EDs, in which many legal, political, and ethical controversies arise.
The absence of a universally accepted definition makes the risk assessment of EDs more challenging. Relevant public authorities, stakeholders, and the public should all examine the extent of uncertainty as well as its sources and nature, and consider whether it is due to inherent random occurrences or a lack of knowledge [50]. Although consultation and public participation play a crucial role in clarifying elements of uncertainty or ignorance, and lead to more informed decisions, the established criteria are highly restrictive and make it very difficult if not impossible to prove that a substance disrupts the endocrine system, as the high degree of uncertainty does not allow for complete proof. Because of the greater burden of proof of harm, more products will remain on the market, resulting in citizens being exposed to dangerous substances and creating a significant burden on the public health budget [51]. In other words, the definition requires such a high level of evidence that it ultimately leads to very few substances being considered EDs [52].
6. Cosmetics
Cosmetics have been used for centuries, not always with safe ingredients. Even in ancient times, when all ingredients were natural, the toxicity of cosmetics was often high, especially when ingredients such as mercury and lead were used. In addition, there was ignorance about their effects [53]. Today, Regulation 1223/2009 for Cosmetics defines a “cosmetic product” as “any substance or mixture intended to come into contact with various external parts of the human body (skin, hair, scalp, nails, lips, and external genital organs) or with the teeth and the mucous membranes of the oral cavity, with the exclusive or main purpose of cleaning them, perfuming them, changing their appearance, and/or correcting body odors and/or protecting them or keeping them in good condition” [54]. Cosmetic products contain active substances, preservatives, and also so-called “fragrances” [55].
6.1. Exposure Pathways
Human exposure to EDs occurs through ingestion of food, dust, and water, inhalation of airborne particles, dermal contact, and maternal exposure [56,57]. The primary exposure route for cosmetics is the skin; most cosmetic products are applied directly to the skin, and their ingredients, or molecules, can penetrate the skin barrier and enter the systemic circulation through various pathways [58]. Additionally, exposure can occur through inhalation, as in the case of fragrances and hair sprays, as well as through ingestion, as in the case of lipsticks [59]. These means of exposure are illustrated in Figure 2. Furthermore, though the environmental risk is not fully understood, it is nonetheless known to exist. Many substances found in cosmetics end up in natural ecosystems through wastewater treatment facilities, water used for personal hygiene, and seawater used by swimmers using sunscreen, as well as through sanitary landfills where residues from cosmetic product packaging may enter the soil [60]. The potential for cosmetic ingredients to bioaccumulate in the fatty tissue of fish [61], the presence of such ingredients in marine mussels [62], and the ability of UV filters to cause coral bleaching [63] are all important environmental concerns [64]. However, there are limitations in scientific evidence, and it is challenging to establish absolute certainty, as some EDs can have adverse effects even at low doses. Moreover, establishing a causal relationship between exposure and the outcome of a disease is nearly impossible to confirm [65,66].
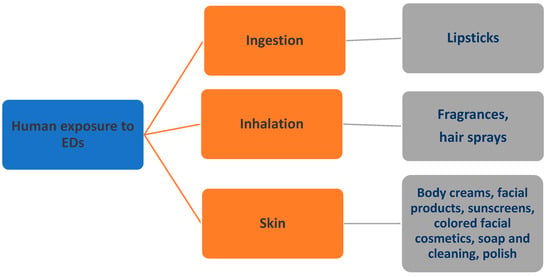
Figure 2.
Human exposure to EDs in cosmetics (modified by Dr. P.Kalofiri) [56,57].
The use of alternative natural substances should not be considered absolutely safe. Many of these substances have estrogenic activity and can also function as EDs [67].
The absence of a systematic method for integrating data to help identify risks from EDs has recently led to the recognition of ten key characteristics (KCs) of EDs. These are known as “Key Characteristics (KCs) of EDs”, and provide a framework for searching and organizing relevant literature regarding the mechanistic information required to support the assessment of an ED [68]. Given the incomplete nature of knowledge, the suggested KCs for EDs can methodically pinpoint areas of missing data. As a result, they can guide the establishment of research priorities during the risk identification process [69,70].
6.2. Approval Process for Ingredients in Cosmetic Products
Certain categories of ingredients (colorants, preservatives, and UV filters) can only be used in cosmetic products if they have been approved through their inclusion in the relevant positive lists set out in cosmetics regulations [71]. Other ingredients can be used in cosmetic products without a permit, provided they are safe for human health [72,73].
Before substances are included in these annexes, a scientific risk assessment is carried out by an independent scientific committee, the Scientific Committee on Consumer Safety (SCCS). During the risk assessment process for substances used as ingredients in cosmetics, the SCCS examines exposure assessments for specific vulnerable groups, such as children and pregnant women [74]. This is crucial because cosmetic products are consumer goods used on a wide scale by citizens every day [54].
For substances classified as carcinogenic, mutagenic, or toxic to reproduction (SVHC—Substances of Very High Concern) [75], specific rules apply for labeling and packaging of substances and mixtures. Furthermore, according to Regulation 1272/2008, under Part 3 of Annex VI, the use of SVHC substances of category 1A or 1B and category 2 in cosmetic products is prohibited, based on the SVHC classification of these substances due to their hazardous properties [76]. The European Commission assigns the SCCS to conduct safety assessments of substances used in cosmetic products, including SVHC substances and nanomaterials [77]. A chemical substance may be proposed as an SVHC substance by a member state or by ECHA if it meets the specified criteria [78]. Different regulatory approaches exist in different legislative acts for substances recognized as EDs [79].
As mentioned in the REACH Regulation, chemicals with endocrine-disrupting properties are included. Such substances may be used in cosmetic products and are therefore subject to the REACH Regulation. Under the REACH Regulation, nine substances have been identified as endocrine disruptors with adverse effects on the environment [80].
In the Cosmetic Regulation, there are no specific provisions for EDs. To address potential risks to human health, the Commission may introduce measures that prohibit or restrict the use of substances. When a substance that is characterized or considered a possible ED has also been classified as a CMR substance (carcinogenic, mutagenic, or toxic for reproduction), then Article 15 of the Cosmetic Regulation applies, and the substance is prohibited unless an exemption from this prohibition is granted under the strict conditions specified in Article 15, paragraphs 1 and 2 (Compliance with the food safety requirements of the general food legislation, restriction of use to a specific category of products, lack of suitable alternative substances, evaluation and positive opinion by the SCCS regarding the safe use of the substance in cosmetic products) [81].
For identified or potential endocrine disruptors that are not classified as CMR substances, their use in cosmetics is governed by the general provisions of Article 31 of the Cosmetic Regulation, which require a scientific opinion from SCCS [54].
In the Memorandum on Endocrine Disruptors (SCCS/1544/14) dated 16 December 2014, the SCCS established the criteria for safety assessment of endocrine disruptors. The SCCS has evaluated cosmetic ingredients suspected of having endocrine-disrupting properties, and has issued scientific opinions on these ingredients, as summarized in Table 3 [82].

Table 3.
Substances with endocrine-disrupting properties.
Other substances include phthalates (plasticizers, lubricants, solvents etc.) [93,94,95], aluminum salts(antiperspirant agents) [96], perfluorinated chemicals [97], and nanoparticles [98,99].
These opinions indicate that substances with endocrine-disrupting properties should be investigated based on the safety assessment by the SCCS, taking into account the limitations associated with the bans on animal testing, and considering data from in vitro and in vivo studies [100]. The SCCS has conducted case-by-case safety assessments for various categories of EDs. While it has confirmed the safety of certain types of endocrine disruptors, it has not ruled out the risk to human health from other categories(isopropylparaben, isobutylparaben, phenylparaben, benzylparaben, and pentylparaben) [101]. Some parabens have been banned in the cosmetic industry, particularly those intended for use in the diaper region of infants and children under three years of age. Other parabens, including methyl, ethyl, propyl, and butyl parabens, are generally regarded as safe, provided that the combined paraben concentration in cosmetics does not exceed 0.4% for individual parabens or 0.8% for mixtures of various parabens. It is worth noting that parabens can alter the pharmacokinetics of bisphenol A [44]. Substances identified as endocrine disruptors are subject to the general safety assessment by the SCCS. They are treated like other substances that raise concerns for human health and are subject to case-by-case regulatory measures based on the general requirements of legislation [102].
It is important to emphasize that an analysis was conducted on 51 substances used as cosmetic ingredients. This analysis showed that the majority of these substances did not exhibit potential endocrine-disrupting properties based on the applied methodology [103,104,105]. However, this result may be due to a lack of data regarding the presence of endocrine-disrupting properties in the subset of the 51 substances examined, rather than evidence of the absence of such properties [54].
7. Discussion
In light of the above, it is evident that the cornerstone of the existing legislative framework is the scientific risk assessment of cosmetic ingredients conducted by the SCCS [106]. The SCCS can assess the safety of cosmetic ingredients with respect to their endocrine-disrupting activity, with the exception of limitations imposed by the ban on animal testing for cosmetics [107]. The most important knowledge gaps derive from the non availability of a validated set of tests for EDs, as well as an inadequate understanding of the mechanisms of action of EDs, exposure and effects during sensitive developmental periods, and the validated dose response relationship for the outcomes induced by ED [108]. These knowledge gaps form the basis for the ongoing debate concerning the need for regulating EDs [109].
Specifically, the existing framework for the detection of EDs needs to be further developed to ensure the detection of effects on human health [110,111]. For example, it is impossible to prove that a pathology is related to the action of a substance to which the population was exposed years ago. The aforementioned restrictive approach to the criteria for determination has served as the basis for criticism against the European Commission, which has even been accused of serving the interests of the chemical industry and not protecting European citizens from certain substances considered potentially harmful to health [110]. Specifically, the above criticism is rooted in the fact that the Regulation proposes the exclusion only of substances with a known and fully proven ability to cause endocrine disruptions, and disregards the existing database of potential endocrine disruptors, thus excluding only a small subset of hazardous substances [112]. In order to cover a greater number of substances, such a large amount of evidence is required that it is practically impossible to achieve. Furthermore, the Regulation ignores substances for which there are only indications of possible endocrine action and therefore does not ensure the desired levels of safety for health and the environment, as previously stipulated in earlier legal texts [113]. The above concerns and criticisms are related to the fundamental question of whether risk assessment is a transparent process. Under the current regulatory framework of the EU, the applicant company (which benefits from the approval of its chemical product) is the primary supplier of the scientific studies that will be evaluated by EFSA and ECHA [114]. Moreover, the risk assessment studies conducted by the industry are not available to the public, with the excuse of preserving commercial confidentiality [115]. Thus, risk assessments carried out by companies are considered more important than an open and transparent scientific evaluation of the documents. Such a choice in the way knowledge is generated is problematic and raises suspicions of serving vested interests [116]. The issue of scientific credibility has also been raised by European citizens who, through the ECI (European Citizens’ Initiative), have called on the Commission to make reforms to ensure that the scientific assessment of pesticides for EU regulatory approval is based solely on published studies conducted by competent public authorities, rather than the pesticide industry(The European Citizens’ Initiative (ECI) is a mechanism in the European Union (EU) that allows citizens to directly participate in the EU legislative process by proposing new laws or changes to existing ones) [117].
8. Conclusions
As is evident from the above, EU legislation does not provide sufficient regulatory approaches for the effective management of EDs, and this has raised serious concerns among European citizens. Moreover, no unified assessment has yet been carried out covering all the different (vertical and horizontal) aspects of EDs [118]. Therefore, there is a need to take action at both European and national levels with four objectives: (a) reducing exposure to endocrine disruptors, with accompanying further development of relevant scientific knowledge and the dissemination of appropriate information to society; (b) protecting the environment and health as a single goal, including all data related to the presentation of EDs; (c) strengthening and deepening scientific assessment and the search for substitutes for these substances to reduce exposure to hazardous substances through the enforcement of regulatory regulations; (d) involvement of stakeholders in the decision-making process regarding the designation of a chemical substance as an ED.
Author Contributions
Conceptualization: P.K. and F.B.; methodology: P.K. and V.K.; software: E.S. and N.T.; validation: F.B. and E.R.; formal analysis: P.K. and E.S.; investigation, F.B. and P.K.; resources, V.K.; data curation F.B.; writing—original draft preparation, P.K.; writing—review and editing, E.R. and V.K.; visualization: N.T. and V.K.; supervision: E.R.; and project administration, P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gonsioroski, A.; Mourikes, V.E.; Flaws, J.A. Endocrine Disruptors in Water and Their Effects on the Reproductive System. Int. J. Mol. Sci. 2020, 21, 1929. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, C.; Meslin, M.; Berman, T.; Woutersen, M.; Bil, W.; Wildeman, J.; Chaudhry, Q. Using Human Biomonitoring Data to Support Risk Assessment of Cosmetic Ingredients—A Case Study of Benzophenone-3. Toxics 2022, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sinha, J.K.; Ghosh, S.; Vashisth, K.; Han, S.; Bhaskar, R. Microplastics as an Emerging Threat to the Global Environment and Human Health. Sustainability 2023, 15, 10821. [Google Scholar] [CrossRef]
- Ahn, C.; Jeung, E.B. Endocrine-Disrupting Chemicals and Disease Endpoints. Int. J. Mol. Sci. 2023, 24, 5342. [Google Scholar] [CrossRef]
- Gustafsson, M.; Vacaflor, A.C.; Lenschow, A. The politics of supply chain regulations: Towards foreign corporate accountability in the area of human rights and the environment? Regul. Gov. 2023, 17, 853–869. [Google Scholar] [CrossRef]
- Cavoski, A. Science and Lawin Environmental Law and Policy: The Case of the European Commission. Transnatl. Environ. Law 2020, 9, 263–295. [Google Scholar] [CrossRef]
- Robinson, C.; Portier, C.J.; Čavoski, A.; Mesnage, R.; Roger, A.; Clausing, P.; Whaley, P.; Muilerman, H.; Lyssimachou, A. Achieving a High Level of Protection from Pesticidesin Europe: Problems with the Current Risk Assessment Procedure and Solutions. Eur. J. Risk Regul. 2020, 11, 450–480. [Google Scholar] [CrossRef]
- European Parliament. Communication from the Commission to the Council and the European Parliament. Community Strategy for Endocrine Disrupters a Range of Substances Suspected of Interfering with the Hormone Systems of Humans and Wildlife; European Parliament: Brussels, Belgium, 1999; COM(1999)706. [Google Scholar]
- Decision No 1386/2013/EU of the European Parliament and of the Council of 20 November 2013 on a General Union Environment Action Programme to 2020 ‘Living Well, within the Limits of Our Planet’. Official Journal of the European Union. pp. 16–30. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32013D1386 (accessed on 14 September 2023).
- European Commission. Communication from the Commission to the European Parliament and the Council on Endocrine Disruptors and the Draft Commission Acts Setting out Scientific Criteria for Their Determination in the Context of the EU Legislation on Plant Protection Products and Biocidal Products; European Commission: Brussels, Belgium, 2016; COM (2016)350; p. 10. [Google Scholar]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy, Official Journal of the European Communities, 22.12.2000. pp. 1–70. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32000L0060 (accessed on 14 September 2023).
- Regulation 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products, Official Journal of the European Union, Article 15, Annex 4. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009R1223 (accessed on 14 September 2023).
- REACH Regulation 1907/2006 of the European Parliament and of the Council of 18 December 2006 on the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) and on the Establishment of the European Chemicals Agency and Amending the Directive 1999/45/EC and Repealing Regulation (EEC) No. 793/93 of the Council and Regulation (EC) no. 1488/94 of the Commission as Well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC, Official Journ al of the European Union. Available via EUR-LEX. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1907 (accessed on 24 July 2023).
- uropean Chemicals Agency, Causal impacts of the REACH Authorisation Process on the Use of Substances of Very High Concern in the EU 2021. Available via ECHA. Available online: https://echa.europa.eu/documents/10162/17231/causal_analysis_reach_authorisation_en.pdf/ (accessed on 25 July 2023). [CrossRef]
- Slama, R.; Bourguignon, J.P.; Demeneix, B.; Ivell, R.; Panzica, G.; Kortenkamp, A.; Zoeller, R.T. Scientific Issues Relevant to Setting Regulatory Criteria to Identify Endocrine-Disrupting Substances in the European Union. Environ. Health Perspect. 2016, 124, 10. [Google Scholar] [CrossRef]
- WHO. Strategic Toolkit for Assessing Risks: A Comprehensive Toolkit for All-Hazards Health Emergency Risk Assessment; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Hanna, C.; White, I.; Glavovic, B. The Uncertainty Contagion: Revealing the Interrelated, Cascading Uncertainties of Managed Retreat. Sustainability 2020, 12, 736. [Google Scholar] [CrossRef]
- EPRS. On the Precautionary Principle in the Plant Protection Products Context See Also Regulation (EC)1107/2009 on the Placing of Plant Protection Products on the Market, European Implementation Assessment; EPRS: Strasbourg, France, 2018. [Google Scholar]
- More, S.J.; Bampidis, V.; Benford, D.; Bennekou, S.H.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Koutsoumanis, K.; Naegeli, H.; Schlatter, J.R.; et al. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J. 2019, 17, 5634. [Google Scholar]
- Balias, G. Environmental Impact Assessment in the EU; Papazisi Publications: Athens, Greece, 2018; pp. 179–190. [Google Scholar]
- Bourguignon, D. The Precautionary Principle: Definitions, Applications and Governance; European Parliamentary Service: Strasbourg, France, 2015. [Google Scholar]
- EPRS. European Parliamentary Research Service, Endocrine Disruptors: An Over view of Latest Developments at European Level in the Context of Plant Protection Products; EPRS: Strasbourg, France, 2019; p. 18. [Google Scholar]
- Vogel, D. The Politics of Precaution: Regulating Health, Safety and Envinronmental Risks in Europeand the US; Princeton University Press: Princeton, NJ, USA, 2012; pp. 1315–1317. [Google Scholar]
- FAO; WHO. International Programmeon Chemical Safety, Environmental Health Criteria 240 Principles and Methods for the Risk Assessment of Chemicals in Food; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2009; p. 5. [Google Scholar]
- U.S. Environmental Protection Agency. Risk Characterization Handbook; U.S. Environmental Protection Agency: Washington, DC, USA, 2000.
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety, Official Journal of the European Communities, Article 3, Paragraph 11. Available via EUR-LEX. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002R0178 (accessed on 28 July 2023).
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Moy, G.G. Risk Analysis of Hazards in Food: An Overview. In Encyclopedia of Food Safety, Reference Module in Food Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 261–267. [Google Scholar] [CrossRef]
- Cameron, I.; Sam, Μ.; Németh, Ε.; Park, S.; Pasman, H.; Rogers, W.; Seligmann, B. Process hazard analysis, hazard identification and scenario definition: Are the conventional tools sufficient, or should and can we do much better? Process Saf. Environ. Prot. 2017, 110, 53–70. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations. Environmental Health Criteria 240, Principles and Methods for the Risk Assessment of Chemicals in Food; Ch. 4. Hazard Identification and Characterization: Toxicological and Human Studies; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2009; pp. 1–190. [Google Scholar]
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Memorandum on the Use of the Scientific Literature for Human Health Risk Assessment Purposes—Weighing of Evidence and Expression of Uncertainty. Available online: http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_s_001.pdf2012 (accessed on 30 July 2023).
- Canavez, A.D.P.M.; de Oliveira Prado Corrêa, G.; Isaac, V.L.B.; Schuck, D.C.; Lorencini, M. Integrated approaches to testing and assessment as a tool for the hazard assessment and risk characterization of cosmetic preservatives. J. Appl. Toxicol. 2021, 41, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Flage, R.; Aven, T. Emerging risk—Conceptual definition and are lation to black swan types of events. Reliab. Eng. Syst. Saf. 2015, 144, 61–67. [Google Scholar] [CrossRef]
- Chartres, N.; Bero, L.A.; Norris, S.L. Are view of methods used for hazard identification and risk assessment of environmental hazards. Environ. Int. 2019, 123, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Rooney, A.A.; Cooper, G.S.; Jahnke, G.D.; Lam, J.; Morgan, R.L.; Boyles, A.L.; Ratcliffe, J.M.; Kraft, A.D.; Schunemann, H.J.; Schwingl, P.; et al. How credible are the study results? Evaluating and applying internal validity tools to literature-based assessments of environmental health hazards. Environ. Int. 2016, 92–93 (Suppl. C), 617–629. [Google Scholar] [CrossRef]
- Villa, V.; Paltrinieri, N.; Khan, F.; Cozzani, V. Towards dynamic risk analysis: A review of the risk assessment approach and its limitations in the chemical process industry. Saf. Sci. 2016, 89, 77–93. [Google Scholar] [CrossRef]
- Duh-Leong, C.; Maffini, M.V.; Kassotis, C.D.; Vandenberg, L.N.; Trasande, L. The regulation of endocrine-disrupting chemicals to minimize their impact on health. Nat. Rev. Endocrinol. 2023, 19, 600–614. [Google Scholar] [CrossRef]
- Lee, M. EU Environmental Law, Governance and Decision-Making; Hart Publishing: Oxford, UK, 2014. [Google Scholar]
- Kalofiri, P.; Balias, G.; Tekos, F. The EU Endocrine Disruptors’ Regulation and the Glyphosate Controversy. Toxicol. Rep. 2021, 8, 1193–1199. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2017/2100 of 4 September 2017 Establishing Scientific Criteria for the Determination of Endocrine Disruptor Properties Pursuant to Regulation (EU) No. 528/2012 of the European Parliament and of the Council, Official Journal of the European Union. Available via EUR-LEX. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32017R2100 (accessed on 28 July 2023).
- Commission Regulation (EU) 2018/605 of 19 April 2018 Amending Annex II to Regulation (EC) No. 1107/2009 Establishing Scientific Criteria for the Determination of Endocrine Disrupting Properties, Official Journal of the European Union. Available via EUR-LEX. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018R0605 (accessed on 28 July 2023).
- Regulation (EC) No 1272/2008 of the European Parliament and the Council of 16 December 2008 on classification, labelling and packaging of substances andmixtures, amending and repealing Directives 67/548/EEC and1999/45/EC, and Amending Regulation (EC) No 1907/2006 Annex 7, Number 3, Case B, Risk Classes 3.1–3.6, 3.7 Adverse Effects on Sexual Function and Fertility or on Development. Available via EUR-LEX. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1272 (accessed on 28 July 2023).
- EFSA. Guidance for the Identification of Endocrine Disruptors in the Context of Regulations (EU) No.528/2012 and (EC) No.1107/2009. EFSA J. 2018, 16, e05311. [Google Scholar]
- European Parliament. Endocrine Disruptors: From Scientific Evidence to Human Health Protection Policy, Policy Department for Citizens’, Rights and Constitutional Affairs; European Parliament: Strasbourg, France, 2019. [Google Scholar]
- Endocrine Society. Endocrine Society Praises European Parliament Report’s Call to Regulate Endocrine-Disrupting Chemicals, 21 March 2019; Endocrine Society: Washington, DC, USA, 2019. [Google Scholar]
- Commission Delegated Regulation (EU) 2021/525 of 19 October 2020 amending Annexes II and III, Page 1, to Regulation (EU) No. 528/2012 of the European Parliament and of the Council Concerning the Making Available on the Market and Use of Biocidal Products. Available via EUR-LEX. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?toc=OJ%3AL%3A2021%3A106%3ATOC&uri=uriserv%3AOJ.L_.2021.106.01.0003.01.ENG (accessed on 29 July 2023).
- Robitaille, J.; Denslow, N.D.; Escher, B.I.; Kurita-Oyamada, H.K.; Marlatt, V.; Martyniuk, C.J.; Navarro-Martín, L.; Prosser, R.; Sanderson, T.; Yargeau, V.; et al. Towards regulation of Endocrine Disrupting chemicals (EDCs) in water resources using bioassays– A guide to developing a testing strategy. Environ. Res. 2022, 205, 112483. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 219/2009 of the European Parliament and the Council of 11 March 2009 Adapting a Number of Instruments Subject to the Procedure Referred to in Article 251 of the Treaty to Council Decision 1999/468/EC with Regard to the Regulatory Procedure with Scrutiny Adaptation to the Regulatory Procedure with Scrutiny—Part Two, Article 5(6) of Council Decision 1999/468/EC. Available via EUR-LEX. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R0219 (accessed on 23 July 2023).
- Brown, M.B. Politicizing science: Conceptions of politics in science and technology studies. Soc. Stud. Sci. 2015, 45, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Attina, T.M.; Hauser, R.; Sathyanarayana, S.; Hunt, P.A.; Bourguignon, J.-P.; Myers, J.P.; Di Gangi, J.; Zoeller, R.T.; Trasande, L. Exposure to endocrine- disrupting chemicals in the USA: Apopulation-baseddisease burden and cost analysis. Lancet Diabetes Endocrinol. 2016, 4, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- European Commission Horizon EuropeWork Programme 2023–2024, Health, European Commission Decision C(2023) 2178 of 31 March 2023. pp. 44–48. Available online: https://www.kowi.de/Portaldata/2/Resources/HEU/wp/Work_Programme_Climate.pdf (accessed on 14 September 2023).
- Olson, K. Cosmetics in romanantiquity: Substance, remedy, poison. Class. World 2009, 102, 291–310. [Google Scholar] [CrossRef]
- Sarkar, S.; Pandey, A.; Pant, A.B. Regulatory Requirements for Safety/Toxicity Assessment of Cosmetics/Nanocosmetic Products: Challenges and Opportunities. In Skin 3-D Models and Cosmetics Toxicity; Springer: Berlin/Heidelberg, Germany, 2023; pp. 149–176. [Google Scholar] [CrossRef]
- Regulation (E.C.) No 1223/2009 of the European Parliament and the Council of 30 November 2009 on Cosmetic Products, Article 2(a). p. 6. Available online: https://health.ec.europa.eu/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf (accessed on 28 July 2023).
- Steinemann, A.C. Fragranced consumer products and undiscloseding redients. Environ. Impact Assess. 2009, 29, 32–38. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP); World Health Organization (WHO). State of the Science of Endocrine Disrupting Chemicals; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2012. [Google Scholar]
- WHO. State of the Science of Endocrine Disrupting Chemicals; WHO: Geneva, Switzerland, 2012; p. 12. [Google Scholar]
- Archer, C.B. Functions of the skin. In Rook’s Textbook of Dermatology; Burns, T., Breathnach, S., Cox, N., Griffiths, C., Eds.; Blackwell Publishing Ltd.: Singapore, 2010; Volume 4, pp. 1–11. [Google Scholar]
- Api, A.M.; Barraj, L.M.; Burdick, J.; Dressler, W.E.; Gettings, S.D.; Hsu, H.H.; Pan, Y.H.L.; Re, T.A.; Renskers, K.J.; Rothenstein, A.; et al. Exposuredataforcosmeticproducts: Lipstick, body lotion, andfacecream. Food Chem. Toxicol. 2005, 43, 279–291. [Google Scholar]
- Feng, M.; He, Q.; Meng, L.; Zhang, X.; Sun, P.; Wang, Z. Evaluation of single and joint toxicity of perfluorooctane sulfonate, perfluorooctanoic acid, and copper to Carassius auratus using oxidative stress biomarkers. Aquat. Toxicol. 2015, 161, 108–116. [Google Scholar] [CrossRef]
- Haman, C.; Dauchy, X.; Rosin, C.; Munoz, J.F. Occurrence, fate and behavior of parabens in aquatic environments: A review. Water Res. 2015, 68, 1–11. [Google Scholar] [CrossRef]
- Groz, M.P.; Bueno, M.J.M.; Rosain, D.; Fenet, H.; Casellas, C.; Pereira, C.; Maria, V.; Bebianno, M.J.; Gomez, E. Detection of emerging contaminants (UVfilters, UVstabilizers and musks) in marine mussels from Portuguese coast by QuEChER Sex traction and GC–MS/MS. Sci. Total Environ. 2014, 493, 162–169. [Google Scholar] [CrossRef]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause cora lbleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Luc, H.; Annie, J.S. Cosmetics as endocrine disruptors: Are they a health risk? Rev. Endocr. Metab. Disord. 2015, 16, 373–383. [Google Scholar] [CrossRef]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS ONE 2013, 8, e55387. [Google Scholar] [CrossRef]
- Plunk, E.C.; Richards, S.M. Epigenetic modifications due to environment, ageing, nutrition, andendocrine disrupting chemicals and their effects on the endocrine system. Internet J. Endocrinol. 2020, 2020, 9251980. [Google Scholar]
- Poluzzi, E.; Piccinni, C.; Raschi, E.; Rampa, A.; Recanatini, M.; De Ponti, F. Phytoestrogens in postmenopause: The state of the art from a chemical, pharmacological and regulatory perspective. Curr. Med.Chem. 2014, 21, 417–436. [Google Scholar] [CrossRef]
- Villar-Pazos, S.; Martinez-Pinna, J.; Castellano-Munoz, M.; Alonso-Magdalena, P.; Marroqui, L.; Quesada, I.; Gustafsson, J.A.; Nadal, A. Molecular mechanisms in volvedin the non-monotonic effect of bisphenol-a on ca2þentry in mouse pancreaticb-cells. Sci. Rep. 2017, 7, 11770. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 46–57. [Google Scholar] [CrossRef]
- Guyton, K.Z.; Rusyn, I.; Chiu, W.A.; Corpet, D.E.; van den Berg, M.; Ross, M.K.; Christiani, D.C.; Beland, F.A.; Smith, M.T. Application of the key characteristics of carcinogens in cancer hazard identification. Carcinogenesis 2018, 39, 614–622. [Google Scholar] [CrossRef]
- Cano, R.; Pérez, J.L.; Dávila, L.A.; Ortega, Á.; Gómez, Y.; Valero-Cedeño, N.J.; Parra, H.; Manzano, A.; Véliz Castro, T.I.; Albornoz, M.P.D.; et al. Role of Endocrine-Disrupting Chemicals in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 4807. [Google Scholar] [CrossRef]
- Regulation (EU) 2022/1176 of 7 July 2022 amending Regulation (EC) No 1223/2009 of the European Parliament and of the Council as Regardsthe Use of Certain UV Filters in Cosmetic Products. Available via EUR-LEX. Available online: https://eur-lex.europa.eu/eli/reg/2022/1176 (accessed on 29 July 2023).
- Gautam, P.; Ghanghas, D.; Mittal, P.; Gupta, V.; Kanika, D. Regulatory framework of cosmetic in the European Union. Toxicol. Commun. 2022, 4, 13. [Google Scholar] [CrossRef]
- Bialas, I.; Zelent-Kraciuk, S.; Jurowski, K. The Skin Sensitisation of Cosmetic Ingredients: Review of Actual Regulatory Status. Toxics 2023, 11, 392. [Google Scholar] [CrossRef]
- Regulation (EC) No. 1272/2008 of the European Parliament and of the Council, of 16 December 2008, on the Classification, Labeling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC and Amendment of Regulation (EC) No. 1907/2006 (OJ L 353 of 31.12.2008); p. 1. Available via EUR-LEX. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022R1176 (accessed on 24 July 2023).
- Pistollato, F.; Madia, F.; Corvi, R.; Munn, S.; Grignard, E.; Paini, A.; Worth, A.; Bal-Price, A.; Prieto, P.; Casati, S.; et al. Current EU regulatory requirements for the assessment of chemicals and cosmetic products: Challenges and opportunities for introducing new approach methodologies. Arch. Toxicol. 2021, 95, 1867–1897. [Google Scholar] [CrossRef]
- Bitsch, A.; Blume, A.; Delamaar, C.; Hahn, S. Exposure to Substances by Use of Consumer Products. InThe Practice of Consumer Exposure Assessment; Heinemeyer, G., Jantunen, M., Hakkinen, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 361–479. [Google Scholar] [CrossRef]
- REACH Regulation, Article 57.Corrigendum to Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishinga European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No793/93 and Commission Regulation (EC) No 1488/94 as Well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC(Official Journal of the European Union L 396 of 30 December 2006). Available via EUR-LEX. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:136:0003:0280:en:PDF (accessed on 24 July 2023).
- Article 1(4) of Regulation 1107/2009 of the European Parliament and the Council of 21 October 2009 concerning the placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EECand Article 1(1) of Regulation 528/2012F of the European Parliament and the Council of 22 May 2012 Concerning the Making Available on the Market and Use of Biocidal Products. Available via EUR-LEX. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:309:0001:0050:en:PDF (accessed on 24 July 2023).
- Available online: https://echa.europa.eu/el/substances-restricted-under-reach/-/dislist/details/0b0236e1807e2038 (accessed on 5 August 2023).
- Bour, A.; Christensen, T.B.; Hunka, A.D.; Palmqvist, A.; Skjold, E.; Syberg, K. Implications of circular textile policies for the future regulation of hazardous substances in textiles in the European Union. Sci. Total Environ. 2023, 896, 165153. [Google Scholar] [CrossRef]
- SCCS/1544/14; Scientific Committee on Consumer Safety, SCCS, Memorandum on Endocrine Disruptors. European Union: Brussels, Belgium, 2014.
- Karolina, N.; Ratajczak–Wrona, W.; EwaJabłońska, G.M. Parabens and the ireffects on the endocrine system. Mol. Cell. Endocrinol. 2018, 474, 238–251. [Google Scholar]
- Honda, M.; Robinson, M.; Kurunthachalam, K. Parabens in human urine from several Asian countries, Greece, and the United States. Chemosphere 2018, 201, 13–19. [Google Scholar] [CrossRef]
- Bernauer, U.; Bodin, L.; Chaudhry, Q.; Coenraad, P.J.; Dusinska, M. SCIENTIFIC ADVICE ON the safety of Homosalate (CAS No 118-56-9, EC No 204-260-8) as a UV-Filter in Cosmetic Products—SCCS/1638/21—Scientific Advice. Scientific Committee for Consumer Safety (SCCS, EC). 2021, Scientific Committee for Consumer Safety (SCCS, EC). ⟨hal-03464662⟩. Available online: https://health.ec.europa.eu/publications/scientific-advice-safety-homosalate-cas-no-118-56-9-ec-no-204-260-8-uv-filter-cosmetic-products_en (accessed on 14 September 2023).
- Mustieles, V.; Balogh, R.K.; Axelstad, M.; Montazeri, P.; Márquez, S.; Vrijheid, M.; Draskau, M.K.; Taxvig, C.; Peinado, F.M.; Berman, T.; et al. Benzophenone-3: Comprehensive review of the toxicological and human evidence with meta-analysis of human biomonitoring studies. Environ. Int. 2023, 173, 107739. [Google Scholar] [CrossRef]
- Bernauer, U.; Bodin, L.; Chaudhry, Q.; Coenraad, P.J.; Dusinska, M.; Ezendam, J.; Gaffet, E.; Galli, C.L.; Panteri, E.; Rogiers, V.; et al. SCCS OPINION on Benzyl Salicylate (CAS No. 118-58-1, EC No. 204-262-9)—SCCS/1656/23–Final Opinion. Scientific Committee for Consumer Safety (SCCS, EC). 2023, Scientific Committee for Consumer Safety (SCCS, EC). ⟨hal-04273028⟩SCCS. Available online: https://health.ec.europa.eu/publications/benzyl-salicylate-cas-no-118-58-1-ec-no-204-262-9_en#:~:text=Conclusion%20of%20the%20opinion%3A&text=Based%20on%20the%20assessment%20of,Table%201%20of%20this%20Opinion (accessed on 14 September 2023).
- Nitulescu, G.; Lupuliasa, D.; Adam-Dima, I.; Nitulescu, G.M. Ultraviolet Filtersfor Cosmetic Applications. Cosmetics 2023, 10, 101. [Google Scholar] [CrossRef]
- Gorman, M.R. Temporal organization of pineal melatonin signal in gin mammals. Mol. Cell. Endocrinol. 2020, 503, 110687. [Google Scholar] [CrossRef]
- SCCS OPINION on Resorcinol—SCCS/1619/20—Final Opinion. 2021.SCCS OPINION on Resorcinol-SCCS/1619/20—Final Opinion. Scientific Committee for Consumer Safety (European Commission). 2021, Scientific Committee for Consumer Safety (European Commission). ⟨hal-03199391⟩. Available online: https://health.ec.europa.eu/system/files/2022-08/sccs_o_241.pdf (accessed on 14 September 2023).
- Pasquier, E.; Viguié, C.; Fini, J.B.; Mhaouty-Kodja, S.; Michel-Caillet, C. Limits of the regulatory evaluation of resorcinol as a thyroid disruptor: When limited experimental data challenge established effects in humans. Environ. Res. 2023, 222, 115330. [Google Scholar] [CrossRef]
- Darbre, P.D.; Harvey, P.W. Chapter 22—Regulatory Considerations for Dermal Application of Endocrine Disrupters in Personal Care Products. In Endocrine Disruption and Human Health(Second Edition); Darbre, P.D., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 463–484. [Google Scholar]
- Huang, P.C.; Liou, S.H.; Ho, I.K.; Chiang, H.C.; Huang, H.I.; Wang, S.L. Phthalatesexposureandendocrinaleffects: An epidemiological review. J. Food Drug Anal. 2012, 20, 719–733. [Google Scholar]
- Philippat, C.; Bennett, D.H.; Krakowiak, P.; Rose, M.; Hwang, H.M.; Hertz-Picciotto, I. Phthalate concentrations in house dust in relation to autism spectrum disorder and developmental delay in the Childhood Autism Risks from Genetics and the Environment (CHARGE) study. Environ. Health 2015, 14, 56. [Google Scholar] [CrossRef]
- Huang, P.C.; Liao, K.W.; Chang, J.W.; Chan, S.H.; Lee, C.C. Characterization of phthalates exposure and risk for cosmetics and perfume sales clerks. Environ. Pollut. 2018, 233, 577–587. [Google Scholar] [CrossRef]
- Darbre, P.D.; Mannello, F.; Exley, C. Aluminium and breast cancer: Sources of exposure, tissue measurements and mechanisms of toxicological actions on breast biology. J. Inorg. Biochem. 2013, 128, 257–261. [Google Scholar] [CrossRef]
- Kouji, F.; Harada, H.; Koizumi, A. Occurrence of perfluorinated carboxylic acids (PFCAs) in personal care products and compound ingagents. Chemosphere 2013, 93, 538–544. [Google Scholar]
- Lu, P.J.; Fang, W.S.; Cheng, W.L.; Huang, S.C.; Huang, M.C.; Cheng, H.F. Characterization of titanium dioxide and zinc oxide nanoparticles in sunscreen powder by comparing different measurement methods. J. Food Drug Anal. 2018, 26, 1192–1200. [Google Scholar] [CrossRef]
- Lu, P.J.; Huang, S.C.; Chen, Y.P.; Chiueh, L.C.; Shih, D.H.Y. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef]
- Iavicoli, I.; Fontana, L.; Leso, V.; Bergamaschi, A. The Effects of Nanomaterials as Endocrine Disruptors. Int. J. Mol. Sci. 2013, 14, 16732–16801. [Google Scholar] [CrossRef]
- SCCS Members. Opinion of the Scientific Committee on Consumer Safety (SCCS)—Final Opinion on propylparaben (CASNo.94-13-3, ECNo.202-307-7). Regul. Toxicol. Pharmacol. 2021, 125, 105005. [Google Scholar] [CrossRef]
- European Commission. Review of Regulation (EC) No. 1223/2009 of the European Parliament and of the Council on Cosmetic Products with Regard to Substances with Endocrine Disrupting Properties; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- European Commission. Selection of Chemical Substances to be Screened in the Contex to fthe Impact Assessment on Criteria to Identify Endocrine Disruptor. 2013, pp. 1–23. Available online: https://ec.europa.eu/health//sites/health/files/endocrine_disruptors/docs/impactassessment_chemicalsubstancesselection_en.pdf (accessed on 16 August 2023).
- Johnson, P.I.; Favela, C.; Jarin, J.; Le, A.M.; Clark, P.I.; Fu, L.; Gillis, A.D.; Morga, N.; Nguyen, C.; Harley, K.G. Chemicals of concern in personal care products used by women of color in three communities of California. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 864–876. [Google Scholar] [CrossRef]
- The Endocrine Disruption Exchange, Inc. (TEDX). List of Potential Endocrine Disruptors. 2018. Available online: https://endocrinedisruption.org/interactive-tools/tedx-list-of-potential-endocrine-disruptors/search-the-tedx-list (accessed on 18 August 2023).
- Bernauer, U.; Bodin, L.; Chaudhry, Q.; Coenraads, P.J.; Dusinska, M.; Ezendam, J.; Gaffet, E.; Galli, C.L.; Granum, B.; Panteri, E.; et al. The SCCS Notes of Guidance for the testing of cosmetic ingredients and their safety evaluation, 11th revision, SCCS/1628/21. Regul. Toxicol. Pharmacol. 2021, 127, 105052. [Google Scholar] [CrossRef]
- Berggren, E. Current trends in safety assessment of cosmetics ingredients. Regul. Toxicol. Pharmacol. 2022, 134, 105228. [Google Scholar] [CrossRef]
- EFSA. Scientific Committee, Scientific Opinion on the hazard assessment of endocrine disruptors: Scientific criteria for identification of endocrine disruptors and appropriateness of existing test methods for assessing effects mediated by these substances on human health and the environment. EFSA J. 2013, 11, 3132. [Google Scholar]
- Grignard, E.; Jesus, K.; Humbert, P. Regulatory Testing for Endocrine Disruptors; Need for Validated Methods and Integrated Approaches. Front. Toxicol. 2022, 3, 821736. [Google Scholar] [CrossRef]
- Ravel, C.; Kah, O. Perturbateurs endocriniens: Vers une regulation insatisfaisante. Arch. Toxicol. 2018, 91, 1001–1006. [Google Scholar] [CrossRef]
- Fabienne, E. Endocrine Disruptors: EU Definition Contains “Too Many Exemptions and Loopholes”. 11 July 2017, Euractiv. Available online: https://www.euractiv.com/section/endocrine-disruptors/interview/endocrine-disruptors-eu-definition-contains-too-many-exemptions-and-loopholes/ (accessed on 18 August 2023).
- Kassotis, C.D.; Trasande, L. Chapter One—Endocrine Disruptor Global Policy. In Advances in Pharmacology; Vandenberg, L.N., Turgeon, J.L., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 92, pp. 1–34. [Google Scholar]
- Savitz, D.A.; Hattersley, A.M. Evaluating Chemical Mixtures in Epidemiological Studies to Inform Regulatory Decisions. Environ. Health Perspect. 2023, 131, 045001. [Google Scholar] [CrossRef]
- Maack, G.; Arning, J.; Frische, T.; Kehrer-Berger, A.; Mordziol, C. Chapter 23—Assessment of Endocrine Disruptors under European Regulations. In Neuroendocrine Regulation of Animal Vocalization; Rosenfeld, C.S., Hoffmann, F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 355–362. [Google Scholar]
- Vandenberg, L.N.; Rayasam, S.D.G.; Axelrad, D.A.; Bennett, D.H.; Brown, P.; Carignan, C.C.; Chartres, N.; Diamond, M.L.; Joglekar, R.; Shamasunder, B.; et al. Addressing systemic problems with exposure assessments to protect the public’s health. Environ. Health 2023, 21, 121. [Google Scholar] [CrossRef]
- Arcuri, A.; Hendlin, Y.H. The Chemical Anthropocene: Glyphosateasa Case Study of Pesticide Exposures. King’s Law J. 2019, 30, 234–253. [Google Scholar] [CrossRef]
- Tosum, J.; Varone, F. Politicizing the Use of Glyphosate in Europe: Comparing Policy Issue Link age across Advocacy Organizations and Countries. J. Comp. Policy Anal. 2021, 23, 607–624. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic ad the Social Committee Ante Committee of the Regions. S, towards a Comprehensive European Union Framework on Endocrine Disruptors; European Commission: Brussels, Belgium, 2018; COM(2018)734. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).