Abstract
Catalytically active titanium dioxide is conventionally used as a white pigment for cosmetics, but undesirably induces a certain degree of decomposition of sebum on the skin on exposure to ultraviolet radiation in sunlight. In this work, titanium phosphates were prepared as a novel white pigment for cosmetics using titanium sulfate and phosphoric acid at various temperatures, with/without ultrasonic treatment. The chemical composition, powder properties, photocatalytic activity, color phase, moisture retention, and smoothness of the phosphates were evaluated. These titanium phosphates had less photocatalytic activity than titanium dioxide, which should be beneficial for protecting sebum on the skin. Samples prepared with ultrasonic treatment had lower visible light absorption than those not subjected to ultrasonication. The sample prepared at 40 °C with ultrasonic treatment had higher moisture retention capacity than those prepared under other conditions. Samples prepared at 40 °C had lower slipping resistance than samples prepared at 7 °C.
1. Introduction
Titanium dioxide is used as a white pigment for cosmetic applications [1]. This oxide is well known to exhibit photocatalytic activity; therefore, in its presence, a certain amount of sebum on the skin is decomposed by the ultraviolet radiation in sunlight. Several techniques have been investigated and applied in order to repress this effect. One such technique involves the use of composite particles with silicon dioxide [2]. However, these particle materials are too abrasive for use on sensitive human skin, where the use of mild materials is required. It has been reported that microfine titanium dioxide is adsorbed through the skin [3], and thus a novel white pigment that is not adsorbed is desirable.
Phosphates have been used for ceramic materials, catalysts, adsorbents, fluorescent materials, dielectric substances, biomaterials, for metal surface treatment, as fertilizers, detergents, food additives, in fuel cells, pigments, and in other applications [4,5]. Phosphate materials are recognized as being well tolerated by living organisms. Therefore, as a novel white pigment, phosphates are expected to be useful in cosmetics.
In earlier studies [6,7], we prepared a titanium phosphate pigment from titanium chloride that, desirably, had no catalytic activity. However, the use of titanium chloride is hampered by the formation of smoke and undesirable precipitates. Generally, raw materials influence the formation and properties of the resultant materials. In the group of titanium compounds, titanium sulfate is another important compound for generating titanium oxide as well as titanium chloride. Furthermore, the preparation temperature was also found to influence the particle shape and size of phosphate materials [8], and ultrasonic treatment was found to be a useful method of controlling the particle shape [9,10]. In previous work, we studied the effects of ultrasonic treatment on the synthesis of titanium phosphate from titanium chloride. Spherical particles of titanium phosphate were obtained from the ultrasonic treatment [6].
Herein, titanium phosphates are prepared from titanium sulfate and phosphoric acid at 7 and 40 °C with/without ultrasonic treatment. The respective chemical compositions, powder properties, photocatalytic activity, color phases, moisture retention, and smoothness of the obtained precipitates and their thermal products are evaluated for application to cosmetics.
2. Experimental Section
Exactly 0.1 mol/L of titanium sulfate solution was mixed with 0.1 mol/L of phosphoric acid solution at either 7 or 40 °C for more than 1 h using a Ti/P ratio of 3/4. This Ti/P ratio was decided based on the chemical composition of Ti3(PO4)4. The mixed solutions were subjected to ultrasonic treatment for 10 min (26 W, CITIZEN SW5800, CITIZEN Co. Ltd., Nishi-tokyo, Japan). For comparison, samples not exposed to ultrasound were also prepared. The precipitates were filtered off, washed with water, and dried. All chemicals were of commercial purity from Wako Chemical Industries Ltd. (Osaka, Japan) and were used without further purification.
A part of the precipitates was dissolved in sulfuric acid solution for inductively coupled plasma (ICP; SPS1500VR, Seiko Instruments, Inc., Chiba, Japan) analysis, and the ratios of phosphorus and titanium in the precipitates were calculated based on these results. The chemical compositions of these materials were analyzed using X-ray diffraction (XRD). The XRD patterns were recorded on an X-ray diffractometer (MiniFlex; Rigaku Corp., Akishima, Japan) using monochromated CuKα radiation. Samples were heated at 100 °C in air and the thermal products were also analyzed by recording the XRD patterns.
The particle shapes and sizes of the precipitates, as well as their thermal products at 100 °C, were evaluated using scanning electron microscopy (SEM) images and particle size distributions. The SEM images of the titanium phosphates were observed using a JGM-5510LV, JEOL instrument (Akishima, Japan). The particle size distributions of these materials were measured using a particle size distribution analyzer employing photo-sedimentation with gravitational and centrifugal acceleration (SA-CP3L, Shimadzu Corp., Kyoto, Japan).
The cosmetic properties were estimated on the basis of the photocatalytic activity, color phase, and smoothness. The photocatalytic activity of the samples was estimated based on the decomposition of methylene blue upon 365 nm irradiation [11,12]. A 0.01 g portion of the sample was placed in 4 mL of methylene blue solution (1.0 × 10−5 mol/L); this solution was then irradiated. The decrease in the absorption at about 660 nm was evaluated over the course of 120 min. The absorbance of the phosphate pigments was evaluated using the ultraviolet–visible (UV–Vis) reflectance spectra (UV2100; Shimadzu Corp.). The whiteness was also estimated using a TES135 plus color analyzer (TES Electrical Electronic Corp., Taipei, Taiwan). For moisture retention analysis of the samples, 0.3 g of each sample was mixed with 0.1 g of water, and the weight loss was then evaluated at 50 °C (MS-70 Moisture Analyzer, A and D Instruments Co. Ltd., Tokyo, Japan). The same weight loss over a longer time indicated higher water retention of the samples. The particle smoothness was measured on artificial leather with a KES-SE objective for evaluation of the surface friction property (Kato Tech Co. Ltd., Kyoto, Japan). The values of MIU (average value of μ at a distance of 20 mm) and MMD (fluctuations of average frictional coefficient) respectively represent the slipping resistance (friction coefficient) and roughness (fluctuations of average frictional coefficient) of the powders. Sample powders were initially spread on the leather, and then a sensor was run over these powders. The values of MIU and MMD were respectively calculated based on the power required to move the sensor and the pitching of the sensor. The values of MIU and MMD are dimensionless because these values are related to the coefficient of friction and scattering, respectively.
3. Results and Discussion
3.1. Chemical Composition and Powder Properties of Precipitates
Table 1 shows the Ti/P ratios of the samples prepared under various conditions. The Ti/P ratio of all the samples was ca. 1.1. This ratio is higher than the Ti/P ratio of 3/4 in Ti3(PO4)4. This difference results from the formation of titanium dioxide. Figure 1 presents the XRD patterns of the samples prepared under various conditions. All samples were in the amorphous state based on the XRD analyses. The samples heated at 100 °C were also amorphous (data not shown). These results were the same as those obtained using titanium chloride as the starting material [7]. Titanium phosphates are readily generated in the amorphous state.

Table 1.
Ti/P ratio of samples prepared under various conditions.
| Sample | Temperature/°C | Ultrasound/min | Ti/P ratio |
|---|---|---|---|
| A | 7 | 0 | 1.07 |
| B | 7 | 10 | 1.09 |
| C | 40 | 0 | 1.19 |
| D | 40 | 10 | 1.12 |
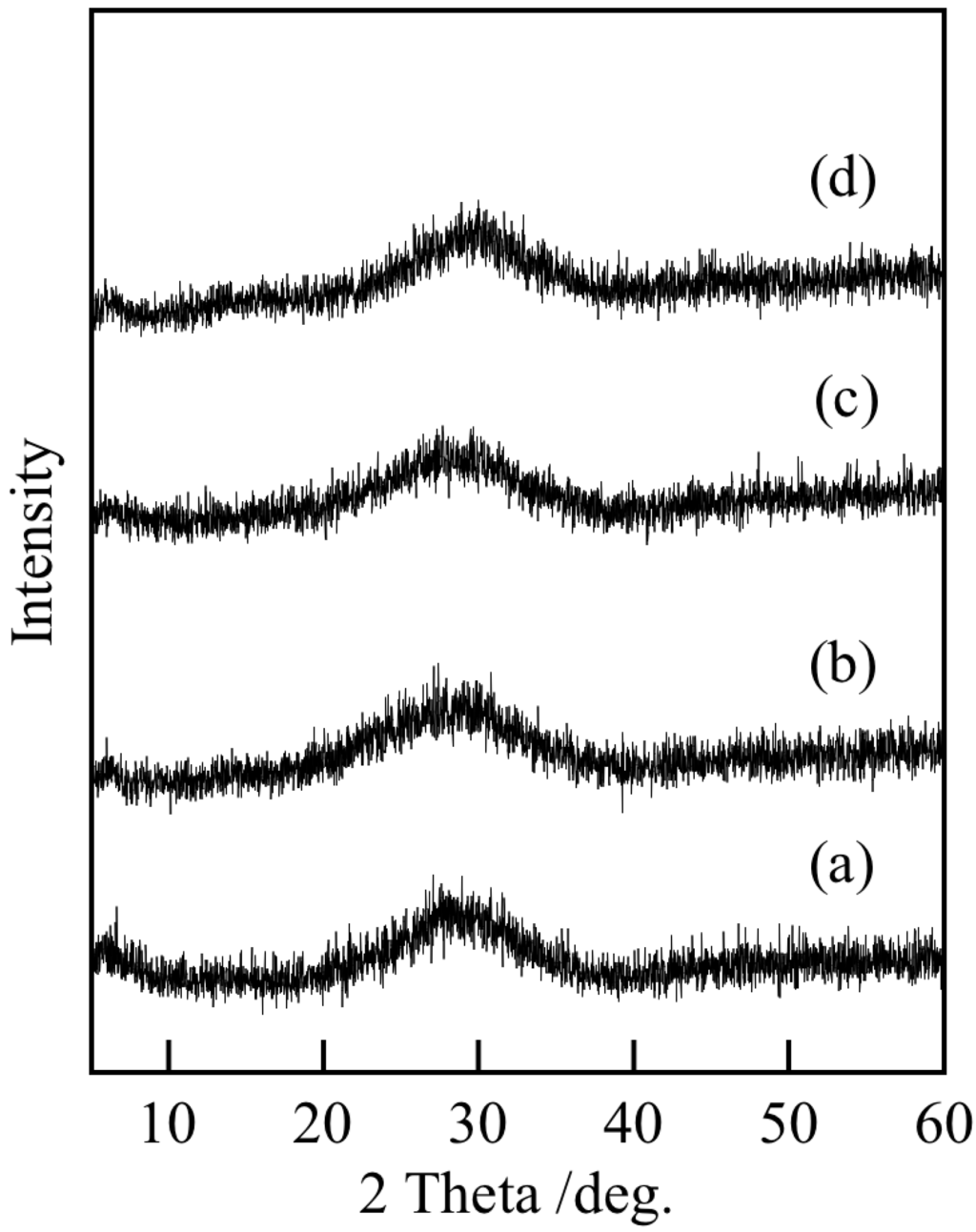
Figure 1.
X-ray diffraction (XRD) patterns of samples prepared under various conditions: (a) temperature: 7 °C, ultrasound: 0 min; (b) 7 °C, 10 min; (c) 40 °C, 0 min and (d) 40 °C, 10 min.
From the morphological perspective, spherical particles are more suitable for cosmetic applications for ease of spread-ability on the skin. Figure 2 shows the SEM images of the samples prepared under various conditions. No defined shape was observed for any of the samples. The preparation temperature and ultrasonic treatment had little influence on the shape of the titanium phosphate particles. Figure 3 presents the particle size distribution of the samples prepared under various conditions. For all samples, a concentration of particles of 15 μm was present in the particle size distribution. For cosmetic applications, small and homogeneous particles are suitable. However, overly small particles may undesirably enter pores in the skin [3]. The standard size of the white pigment for cosmetics is difficult to determine because the skin pore size is affected by factors such as age, gender, and climate. Furthermore, overly large particles are inappropriate as they lead to cracking of the coating on the skin. Thus, it is important to control the pigment particle size.

Figure 2.
Scanning electron microscopy (SEM) images of samples prepared under various conditions: (a) temperature: 7 °C, ultrasound: 0 min; (b) 7 °C, 10 min; (c) 40 °C, 0 min and (d) 40 °C, 10 min.
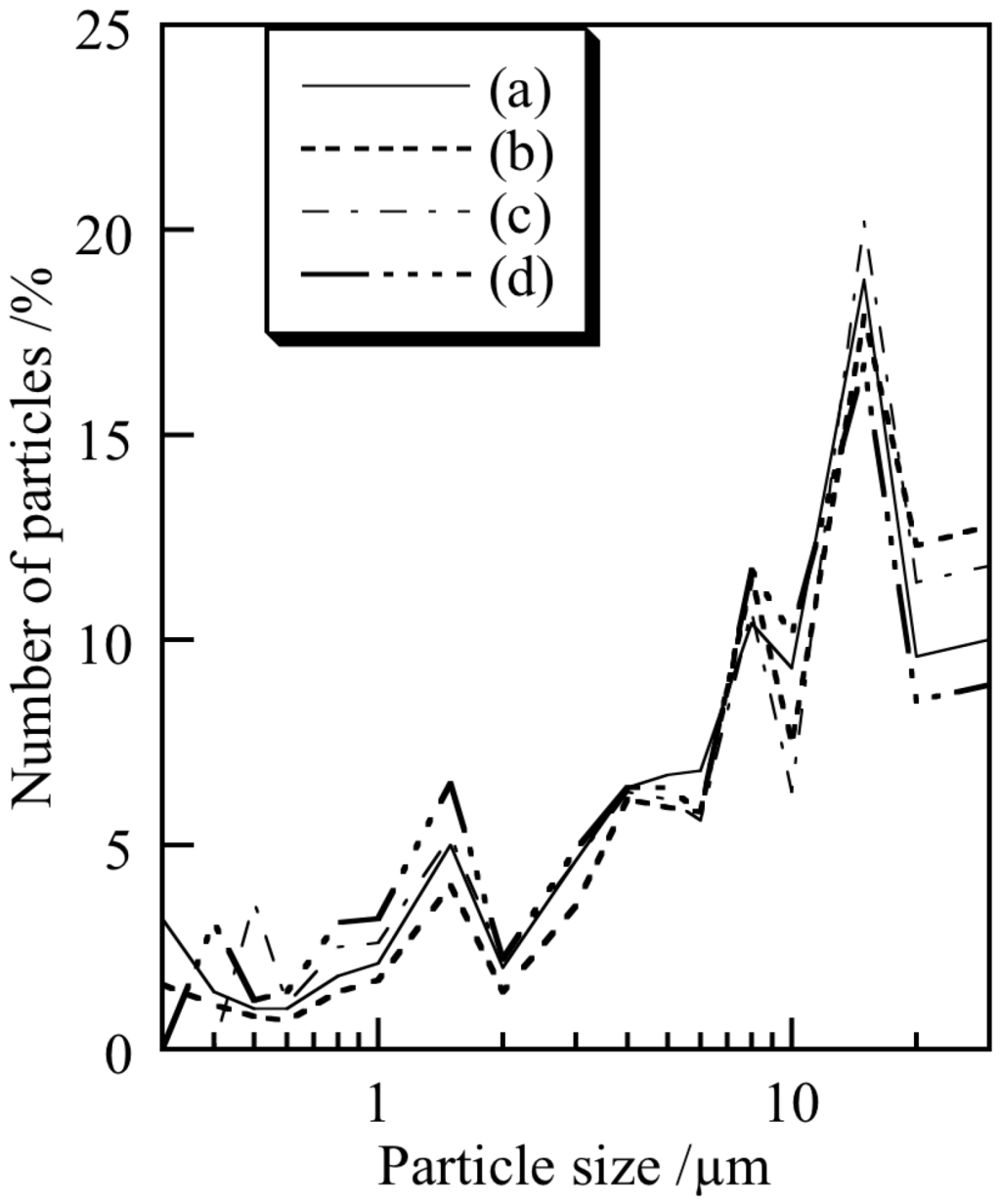
Figure 3.
Particle size distribution of samples prepared under various conditions: (a) temperature: 7 °C, ultrasound: 0 min; (b) 7 °C, 10 min; (c) 40 °C, 0 min and (d) 40 °C, 10 min.
3.2. Cosmetic Properties of Titanium Phosphates
Figure 4 shows the respective photocatalytic activities of the samples prepared under various conditions based on the temporal evolution of the residual ratio. Because titanium dioxide is used as a white pigment in cosmetics, this compound was evaluated for comparison with titanium phosphate [1]. For comparison, methylene blue was decomposed with titanium dioxide using UV irradiation (Figure 4b). Titanium phosphate had little photocatalytic activity for decomposition of methylene blue regardless of the preparation temperature and ultrasonic treatment conditions employed (Figure 4c–f). Thus, titanium phosphate is a sufficiently mild material that should not induce photodecomposition of the sebum on the skin.
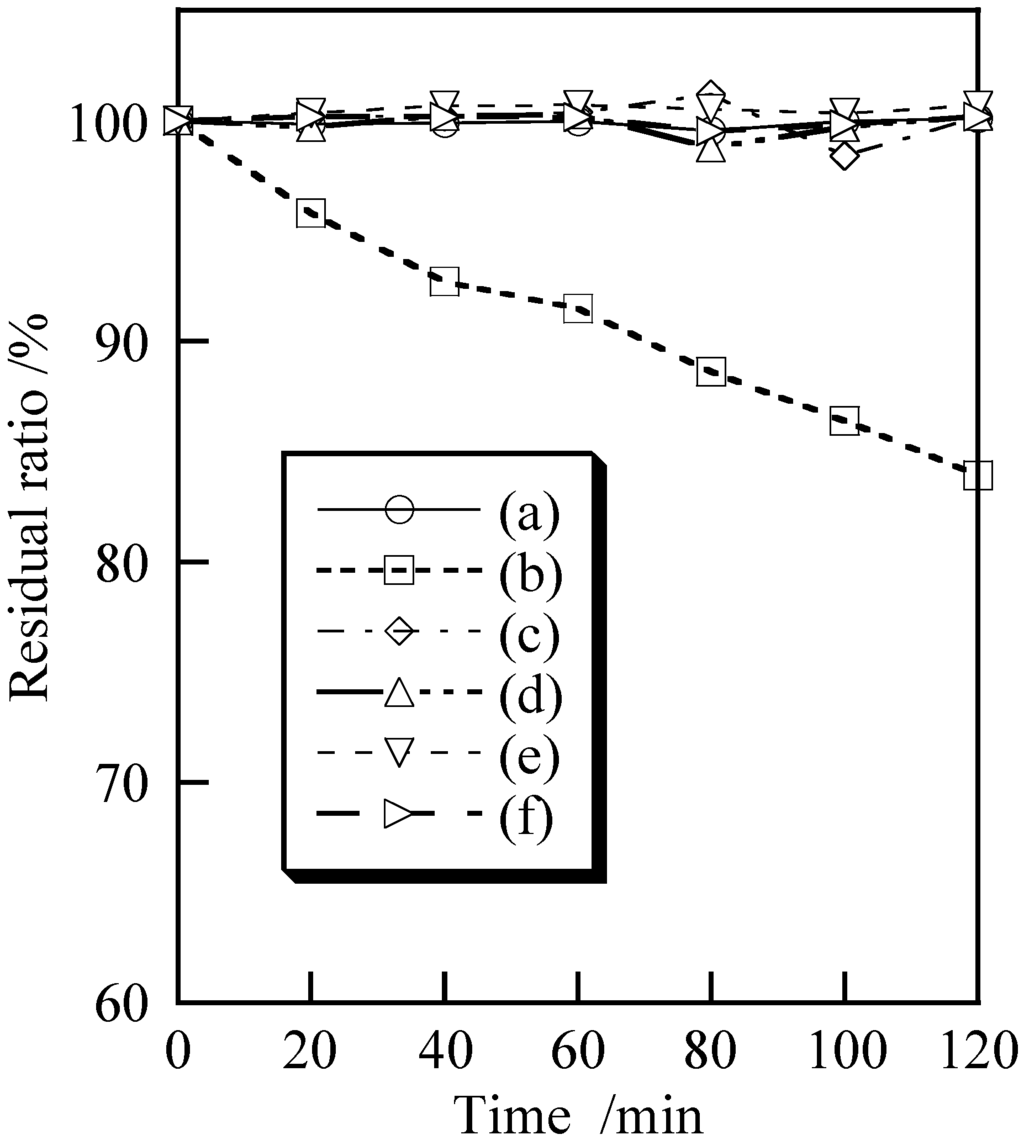
Figure 4.
Photocatalytic activity of samples prepared under various conditions: (a) blank; (b) TiO2; (c) temperature: 7 °C, ultrasound: 0 min; (d) 7 °C, 10 min; (e) 40 °C, 0 min and (f) 40 °C, 10 min.
Figure 5 shows the UV–Vis reflectance spectra of the samples prepared under various conditions. All samples exhibited high reflectance in the range of visible light. Samples prepared with ultrasonic treatment had higher reflectance than those not subject to ultrasound. Samples heated at 100 °C also exhibited high reflectance in this range (data not shown). White powders were obtained whether samples were not heated or heated at 100 °C. Table 2 shows the L* value in L*a*b* color space of the samples prepared under the various conditions. All samples were characterized by high brightness regardless of the preparation temperature and ultrasonic treatment conditions. Samples prepared with ultrasonic treatment were characterized by higher whiteness than those without ultrasound, and samples that were not heated had higher brightness than the samples heated at 100 °C. This color difference is generally related to the surface of the particles, the particle size, crystalline structure, and defects in the crystalline structure. Herein, the particle size changed marginally due to heating. Because the samples that were not heated and those heated at 100 °C both had amorphous structures based on the XRD patterns, the crystalline structure and their defects were not clear. Therefore, it is difficult to pinpoint the origin of the slight darkening of the samples.
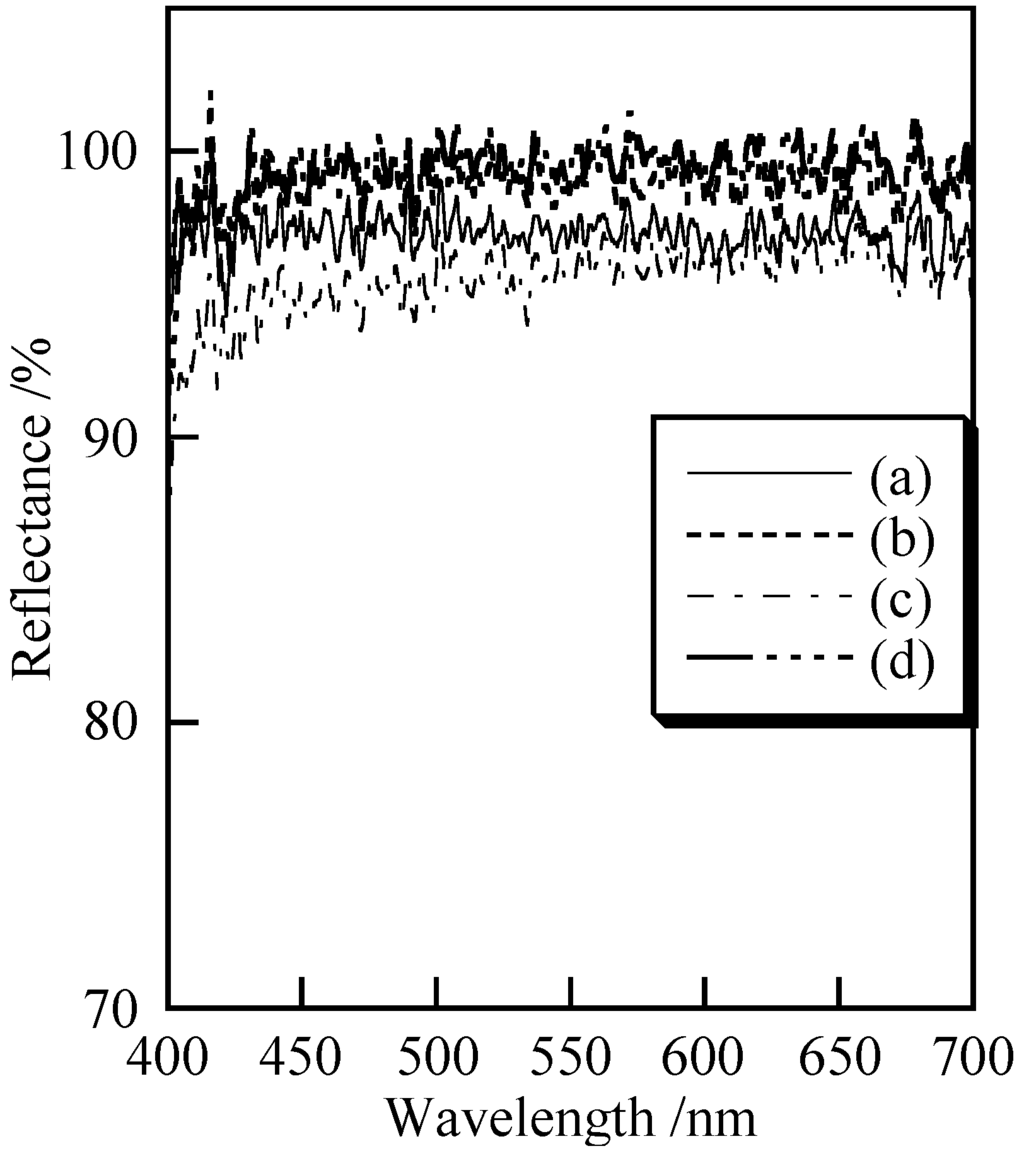
Figure 5.
Ultraviolet–visible (UV–Vis) reflectance spectra of samples prepared under various conditions: (a) temperature: 7 °C, ultrasound: 0 min; (b) 7 °C, 10 min; (c) 40 °C, 0 min and (d) 40 °C, 10 min.

Table 2.
L* value in L*a*b* color space of samples prepared under various conditions, determined using color analyzer.
| Sample | Temperature/°C | Ultrasound/min | L* Value | |
|---|---|---|---|---|
| R.T. | 100 °C | |||
| A | 7 | 0 | 95.0 | 89.8 |
| B | 7 | 10 | 98.5 | 91.0 |
| C | 40 | 0 | 93.2 | 86.1 |
| D | 40 | 10 | 99.6 | 88.9 |
Moisture helps to prevent itchiness and damage to skin. Thus, it is important for pigments used in cosmetics to retain the moisture on the skin [12]. Figure 6 shows the moisture retention of the samples prepared under various conditions. In this analysis, the theoretical weight loss corresponding to total loss of water is 25% (sample: 0.3 g, water: 0.1 g). However, for some samples the weight loss exceeded 25%. Similar weight loss at a later time is indicative of higher moisture retention. For example, 20% weight loss was achieved at 10.1 min for the sample prepared at 40 °C without ultrasonic treatment, whereas for the sample prepared at 40 °C with ultrasonic treatment, similar weight loss was observed at 17.7 min. The highest moisture retention was achieved with the sample prepared at 40 °C with ultrasonic treatment. No influence of the ultrasonic treatment was observed in the moisture retention analysis of the samples prepared at 7 °C.
As described above, pigments with high smoothness spread well on the skin. The powder smoothness is also important for cosmetics [13]. Table 3 shows the smoothness of the samples prepared under various conditions. Generally, for cosmetic application, the appropriate MIU and MMD values are less than 0.6 and less than 0.04, respectively. The MIU and MMD values of titanium dioxide were 1.278 and 0.019, respectively. The MIU and MMD values of all the titanium phosphate samples obtained in this work were within the acceptable range. Samples prepared at 40 °C had lower MIU values than samples prepared at 7 °C. The ultrasonic treatment had little influence of the smoothness of the samples.
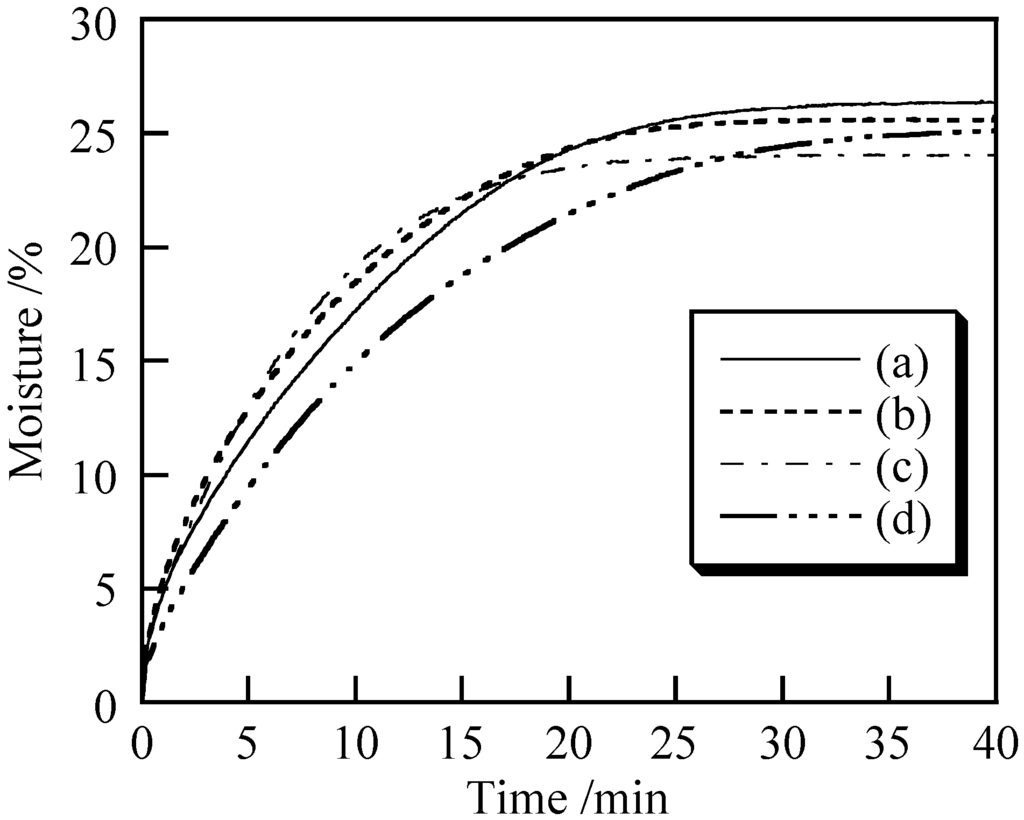
Figure 6.
Moisture retention of samples prepared under various conditions: (a) temperature: 7 °C, ultrasound: 0 min; (b) 7 °C, 10 min; (c) 40 °C, 0 min and (d) 40 °C, 10 min.

Table 3.
Smoothness of samples prepared under various conditions.
| Sample | Temperature/°C | Ultrasound/min | MIU | MMD |
|---|---|---|---|---|
| A | 7 | 0 | 0.340 | 0.008 |
| B | 7 | 10 | 0.358 | 0.010 |
| C | 40 | 0 | 0.290 | 0.010 |
| D | 40 | 10 | 0.300 | 0.009 |
4. Conclusions
Titanium phosphates were prepared from titanium sulfate and phosphoric acid at 7 and 40 °C with/without ultrasonic treatment. These titanium phosphates exhibited no photocatalytic activity based on methylene blue decomposition, and thus should protect the sebum on the skin from undesirable photocatalytic decomposition encountered with titanium dioxide. Ultrasonic treatment was effective for enhancing the whiteness relative to samples not subjected to ultrasonication. Preparation at 40 °C with ultrasonic treatment gave rise to the highest moisture retention capacity, and a preparation temperature of 40 °C resulted in lower slipping resistance values than that of samples prepared at 7 °C.
Acknowledgments
The authors are grateful to Dr. Takeshi Toyama, Nihon University, Japan, for performing smoothness measurements.
Author Contributions
The study design was undertaken by Hiroaki Onoda and Syohei Fujikado, while experimental work was performed by Syohei Fujikado with the support of Hiroaki Onoda. The analysis and interpretation were conducted by Hiroaki Onoda and Syohei Fujikado. The manuscript was written by Hiroaki Onoda.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Senzui, M.; Tamura, T.; Miura, K.; Ikarashi, Y.; Watanabe, Y.; Fujii, M. Study on penetration of titanium dioxide (TiO2) nanoparticles into intact and damaged skin in vitro. J. Toxicol. Sci. 2010, 35, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gamer, A.O.; Leibold, E.; van Ravenzwaay, B. The in vitro absorption of microfine zinc oxide and titanium dioxide through porcine skin. Toxicol. Vitro 2006, 20, 301–307. [Google Scholar] [CrossRef]
- Jones, D.J.; Aptel, G.; Brandhorst, M.; Jacquin, M.; Jimenez-Jimenez, J.; Jimenez-Lopez, A.; Maireles-Torres, P.; Piwonski, I.; Rodrigues-Castellon, E.; Zajac, J.; et al. High surface area mesoporous titanium phosphate: Synthesis and surface acidity determination. J. Mater. Chem. 2000, 10, 1957–1963. [Google Scholar]
- Bhaumik, A.; Inagaki, S. Titanium Phosphate Molecular Sieves with Ion-Exchange Capacity. J. Am. Chem. Soc. 2001, 123, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Onoda, H.; Yamaguchi, T. Influence of ultrasonic treatment on preparation and powder properties of titanium phosphates. J. Mater. Chem. 2012, 22, 19826–19830. [Google Scholar] [CrossRef]
- Onoda, H.; Yamaguchi, T.; Takenaka, A. Synthesis and pigmental properties of titanium phosphates with the addition of urea. Int. J. Cosmet. Sci. 2012, 34, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Izaki, M.; Sasaki, S.; Mohamad, F.B.; Shinagawa, T.; Ohta, T.; Watase, S.; Sasano, J. Effects of preparation temperature on optical and electrical characteristics of (111)-oriented Cu2O films electrodeposited on (111)-Au film. Thin Solid Films 2012, 520, 1779–1783. [Google Scholar] [CrossRef]
- Cai, Y.; Pan, H.; Xu, X.; Hu, Q.; Li, L.; Tang, R. Ultrasonic Controlled Morphology Transformation of Hollow Calcium Phosphate Nanospheres: A Smart and Biocompatible Drug Release System. Chem. Mater. 2007, 19, 3081–3083. [Google Scholar] [CrossRef]
- Jung, S.H.; Oh, E.; Lim, H.; Shim, D.S.; Cho, S.; Lee, K.H.; Jeong, S.H. Shape-Selective Fabrication of Zinc Phosphate Hexagonal Bipyramids via a Disodium Phosphate-Assisted Sonochemical Route. Cryst. Growth Des. 2009, 9, 3544–3547. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Jagtap, N.B.; Vijayanand, S.; Bhange, D.S.; Awati, P.S. Photocatalytic decomposition of methylene blue on nanocrystalline titania prepared by different methods. Mater. Res. Bull. 2008, 43, 1145–1152. [Google Scholar] [CrossRef]
- Du, P.; Bueno-Lopez, A.; Verbaas, M.; Almeida, A.R.; Makkee, M.; Moulijn, J.A.; Mui, G. The effect of surface OH-population on the photocatalytic activity of rare earth-doped P25-TiO2 in methylene blue degradation. J. Catal. 2008, 260, 75–80. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Yuen, C.W.M.; Kan, C.W.; Cheuk, K.K.L.; Tang, J.C.O.; Li, S.Y. A comprehensive study of silicone-based cosmetic textile agent. Fibers Polym. 2009, 10, 132–140. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).